Abstract
Background
An intervention to potentiate hypoxic pulmonary vasoconstriction may reduce intrapulmonary shunt and hypoxemia during one-lung ventilation. Previous animal studies reported that repeated intermittent hypoxic stimuli potentiated hypoxic pulmonary vasoconstriction, but no clinical study has examined the effects of this intervention on hypoxemia during one-lung ventilation. We thus performed a single-center, parallel-group, double-blind, randomized controlled trial to investigate whether repeated intermittent hypoxic stimuli to the operative lung reduce hypoxemia during the subsequent one-lung ventilation for thoracoscopic surgery.
Methods
Patients undergoing one-lung ventilation were randomized into two groups (n = 68 each). Before one-lung ventilation, in the intermittent hypoxia group, the nondependent lung was not ventilated for 2 min and then ventilated for 2 min while the dependent lung was continuously ventilated. This was repeated five times. In the continuous normoxia group, both lungs were ventilated for 20 min. We measured SpO2, PaO2, FiO2, PaCO2, SaO2, and central venous oxygen saturation during one-lung ventilation. The primary outcome was the number of patients with hypoxemia defined as a SpO2 <95% during one-lung ventilation, which was analyzed with a chi-squared test.
Results
Hypoxemia was less frequent in the intermittent hypoxia group than in the continuous normoxia group during OLV [6/68 (8.8%) vs 17/68 (25.0%), risk ratio (95% CI) 0.35 (0.15–0.84), p = 0.012]. The PaO2 (p = 0.008 for 30 min and 0.007 for 60 min) and PaO2/FiO2 (p = 0.008 for both) were higher 30 and 60 min after starting one-lung ventilation, and the alveolar-arterial pressure gradient (p = 0.010) and shunt index (p = 0.008) were lower 30 min after starting one-lung ventilation in the intermittent hypoxia group than in the continuous normoxia group. Postoperative adverse events did not differ significantly between groups.
Conclusions
Repeated intermittent hypoxic stimuli to the operative lung seemed to potentiate hypoxic pulmonary vasoconstriction, and thus reduced hypoxemia during the subsequent one-lung ventilation.
Introduction
To improve the surgical conditions for pulmonary resection, the operative lung is deflated while the contralateral lung is ventilated. During the one-lung ventilation (OLV), because the deflated lung is still perfused, the intrapulmonary shunt impairs arterial oxygenation [1–4]. However, alveolar hypoxia developed in the deflated lung leads to the constriction of the ipsilateral resistance pulmonary arteries [5–9]. This hypoxic pulmonary vasoconstriction (HPV) diverts blood flow from the non-ventilated lung to the ventilated lung, minimizing ventilation-perfusion mismatch and improving arterial oxygenation [5–7, 9–11]. Therefore, an intervention to potentiate HPV may reduce the intrapulmonary shunt and hypoxemia during OLV [12–15].
Several animal studies reported that repeated intermittent hypoxic stimuli potentiated the subsequent HPV [12–15]. This intervention was also used in an observational pediatric study [16], a non-randomized study about biomarkers of lung injury [17], and a randomized study that only examined a PaO2/FiO2 30 min after OLV and postoperative outcomes [18]. However, no study has examined the effects of this intervention on intraoperative hypoxemia, even though 7–48% of thoracic surgical patients show hypoxemia during OLV [2, 10, 19–21]. We thus conducted a prospective randomized trial to test the hypothesis that repeated intermittent hypoxic stimuli to the operative lung reduce hypoxemia during the subsequent OLV for thoracoscopic surgery in the lateral decubitus position.
Materials and methods
Patients
This prospective, single-center, parallel-group, double-blind, randomized controlled trial was approved by the Institutional Review Board (IRB #1705-090-855) of Seoul National University Hospital (Seoul, Korea) and was registered prior to patient enrollment at ClinicalTrials.gov (NCT03282032, Principal investigator: Jeong-Hwa Seo, Date of registration: 8 September 2017). After obtaining written informed consent, we enrolled patients aged 20–70 yr with ASA physical status I–III, and undergoing elective thoracoscopic surgery under OLV using a left-sided double-lumen tube in the lateral decubitus position between November 2017 and October 2018. We excluded patients with severe cardiopulmonary diseases, history of pulmonary resection, planned bilateral lung surgery, irregular cardiac rhythm, anemia, pregnancy, and body mass index >35 kg m-2.
Patients were randomized into two groups to receive five intermittent hypoxic stimuli (intermittent hypoxia group) or one continuous normoxic stimulus (continuous normoxia group) to the operative lung before OLV. An assistant unrelated to the study assigned the group in a 1:1 ratio using a complete randomization procedure without stratification, blocking, and terminal balance, and concealed the allocation sequence in serially-numbered, sealed, and opaque envelopes. After induction of general anesthesia, the attending anesthesiologist, who was not the investigator of this study, opened the envelope and conducted the experimental intervention according to the group assignment. Investigators did not enter the operating room during the experimental protocol for blinding of group assignment. The manuscript adheres to the applicable Consolidated Standards of Reporting Trials guidelines. No changes to the protocol are made after trial commencement, and the trial ended after recruiting all participants without interim analyses. The trial protocol is provided in the (S1 and S2 Protocols).
Anesthesia and experimental protocol
Patients were monitored with non-invasive blood pressure, pulse oximetry, electrocardiography, bispectral index (A-2000 XP; Aspect Medical Systems, Newton, MA, USA), and acceleromyography (TOF-watch Sx; Organon, Dublin, Ireland). Propofol (Fresofol MCT 2%; Fresenius Kabi, Homburg, Germany) and remifentanil (Ultiva; GlaxoSmithKline, Brentford, Middlesex, UK) were administered intravenously with effect-site target-controlled infusion (Orchestra; Fresenius Kabi, Brézins, France). The initial target concentration was 3–5 μg ml-1 for propofol, and 3–5 ng ml-1 for remifentanil.
After intravenous administration of rocuronium 0.6–0.8 mg kg-1, train-of-four (TOF) counts were monitored at the adductor pollicis muscle every 15 s. At a TOF count = 0 and bispectral index <60, a double-lumen tube (Mallinckrodt endobronchial tube; Covidien, Mansfield, MA, USA) was placed into the left mainstem bronchus by direct or video laryngoscopy. The 32, 35, 37, or 39-Fr double-lumen tubes were selected based on the left bronchial diameter measured on the preoperative chest computed tomography, or sex and height of the patient.
With fiberoptic bronchoscopy (LF-DP or LF-GP; Olympus Optical Co., Tokyo, Japan), we positioned the bronchial cuff of the double-lumen tube into the left bronchus below the carina without herniation, and the bronchial tip above the left lobar bronchi without obstruction. If the tube was malpositioned into the right bronchus, it was repositioned into the left bronchus with bronchoscopic guidance. The tracheal and bronchial cuff pressures were adjusted to <25 cm H2O using a portable manometer (VBM Medizintechnik GmbH, Sulz am Neckar, Germany).
A 20-gauge catheter was inserted into the radial artery and was connected to a transducer for arterial waveform analysis (FloTrac, version 4.0; Edwards Life Sciences, Irvine, CA, USA). An 8.5-Fr central venous oximetry catheter (PreSep; Edwards Lifesciences, Irvine, CA, USA) was inserted into the right internal jugular vein, and its tip was positioned at the junction of the superior vena cava and right atrium with sonographic guidance (Vivid i; GE Healthcare, Chicago, IL, USA) [22]. The PreSep catheter was calibrated in vivo with hemoglobin, hematocrit, and oxygen saturation (ScvO2) values measured in the central venous blood. The arterial and venous pressure transducers were placed at the right atrium level and were exposed to atmospheric pressure for zeroing.
After moving the patient to the lateral decubitus position during surgical preparation, we adjusted the positions of the double-lumen tube and pressure transducers. In the intermittent hypoxia group, the nondependent lung was not ventilated for 2 min and then ventilated for 2 min while the dependent lung was continuously ventilated (S1 Fig). The non-ventilation and ventilation of the nondependent lung was repeated five times. In the continuous normoxia group, both lungs were ventilated for 20 min. In both groups, the anesthetic machine (Primus; Dräger, Lübeck, Germany) was set to an FiO2 1.0 and tidal volume 6 ml kg-1 of predicted body weight (PBW) during non-ventilation of the dependent lung, and to an FiO2 0.5 and tidal volume 8 ml kg-1 of PBW during ventilation of both lungs. The PBW was calculated as 50.0+0.905×(height-152.4) for men, and 45.5+0.905×(height-152.4) for women [23]. A respiratory rate 12 breaths min-1, PEEP 5 cm H2O, inspiratory: expiratory (I:E) ratio 1:2, and fresh gas flow 2 liter min-1 were applied during the experimental protocol in both groups.
After the protocol, OLV (defined as the ventilation of only the dependent lung before or after starting operation) was initiated with an FiO2 0.8, PEEP 5 cm H2O, I:E ratio 1:2, tidal volume 4–8 ml kg-1 of PBW, and respiratory rate 12–20 breaths min-1 to maintain an SpO2 ≥95%, PaO2 13.3–33.3 kPa (100–250 mm Hg), PaCO2 4.7–6.7 kPa (35–50 mm Hg), and peak inspiratory pressure <30 cm H2O. When a PaO2 was ≥33.3 kPa (250 mm Hg), the FiO2 was decreased to 0.6; and when a PaO2 was <13.3 kPa (100 mm Hg), the FiO2 and PEEP were increased to 1.0 and 8 mm Hg, respectively. If SpO2 decreased to <95%, we applied alveolar recruitment maneuvers to the dependent ventilated lung with an FiO2 1.0, end-inspiratory pressure 30 cm H2O, PEEP 10 cm H2O, and I:E ratio 1:1 until SpO2 increased to ≥98% after checking the double-lumen tube position with fiberoptic bronchoscopy. If SpO2 dropped to <90%, we asked the surgeon to stop the operation, and ventilated both lungs manually with an FiO2 1.0 until SpO2 increased to ≥98%. The effect-site concentrations of propofol and remifentanil were titrated for a bispectral index 30–60, and rocuronium 0.2–0.3 mg kg-1 was intermittently administered for a TOF count = 0. Ephedrine 5–10 mg, phenylephrine 30–50 μg, or plasmalyte 100–200 ml were administered intravenously to manage a mean blood pressure <60 mm Hg, central venous pressure <4 mm Hg, stroke volume variation >13%, or urine output <0.5 ml kg-1 h-1 according to the attending anesthesiologist’s discretion, when one or more conditions were met. A packed red blood cell was transfused to maintain a hematocrit level >20%.
After pulmonary resection, the nondependent operative lung was inflated and then bilateral lungs were ventilated with an FiO2 0.5. A chest tube was inserted into the operative hemithorax through the incision for the thoracoscopic port. Intravenous patient-controlled analgesia (Automed 3200; Ace Medical, Seoul, Korea) was started with a 100-ml mixture of fentanyl 1000–2000 μg, morphine 40–80 mg, ramosetron 0.3–0.6 mg, and saline at an infusion of 1 ml h-1, bolus of 0.5 ml, and lockout time of 10 min.
After surgery, patients were turned to the supine position and secretions were suctioned from both lungs. The double-lumen tube was removed after administration of sugammadex 4 mg kg-1 when the patient had spontaneous breathing, responses to verbal commands, and a TOF ratio >0.9. If mechanical ventilation was required after surgery, the double-lumen tube was replaced with a plain tube without the administration of sugammadex. Patients were transferred to post-anesthesia or intensive care units.
Outcomes
We collected data for characteristics of patients, surgery, and anesthesia. We continuously monitored SpO2 during OLV and defined mild hypoxemia as 90%≤ SpO2 <95% and severe hypoxemia as SpO2 <90% [2, 3, 7, 10, 21, 24–27]. During OLV, if patients experienced more than one episode of mild hypoxemia or severe hypoxemia, we recorded the time of the first episode. If patients had both mild and severe hypoxemia, it was counted as severe hypoxemia. Immediately before and 30, 60, 90, and 120 min after starting OLV, we measured PaO2, PaCO2, SaO2, and hematocrit with a blood gas analyzer (GEM premier 3000, Model 5700; Instrumentation Laboratory, Lexington, MA, USA); FiO2, PEEP, I:E ratio, EtCO2, tidal volume, respiratory rate, and peak or plateau airway pressures, with the Primus anesthetic machine; mean arterial pressure, heart rate, and cardiac index with the FloTrac sensor; and ScvO2 with the PreSep catheter. Some variables were calculated as follows: PaO2/FiO2; alveolar-arterial oxygen pressure gradient [P(A-a)O2] = 95×FiO2-PaCO2/0.8-PaO2 [6, 9, 27, 28]; shunt index = (100-SaO2)/(100-ScvO2) ×100 [29]; minute ventilation = tidal volume×respiratory rate; alveolar dead space = tidal volume× (PaCO2-EtCO2)/PaCO2 [30]; static pulmonary compliance = tidal volume/(plateau pressure-PEEP) [31]; and dynamic pulmonary compliance = tidal volume/(peak inspiratory pressure-PEEP) [31].
At 30 min after surgery, PaO2, PaCO2, and hematocrit were checked while oxygen was supplied via a facemask at a rate of 5 liter min-1. The chest tube was removed when the drainage was less than 200–250 ml, and no air leak was detected. We recorded lengths of stay in the post-anesthesia or intensive care units and in the hospital. Patients were discharged from the post-anesthesia care unit when the modified Aldrete score was ≥9; from the intensive care unit when they had no cardiorespiratory problems and did not require inotropes or oxygen therapy; and from the hospital when they were able to ambulate, cough, and clear sputum without severe pain, fever, and complications. We recorded any perioperative adverse events.
The primary outcome was the number of patients with SpO2 <95% during the entire period of OLV. Secondary outcomes were the number of episodes and the number of patients with mild or severe hypoxemia during OLV; PaO2, PaO2/FiO2, P(A-a)O2, shunt index, and respiratory or hemodynamic variables including minute ventilation, alveolar dead space, static and dynamic pulmonary compliances, FiO2, PEEP, I:E ratio, PaCO2, hematocrit, mean arterial pressure, heart rate, and cardiac index immediately before (baseline) and 30, 60, 90, and 120 min after starting OLV; duration of the chest tube drainage and lengths of stay in the postoperative care units and hospital after surgery; and perioperative adverse events.
Statistical analysis
Continuous variables were summarized as mean (SD) and were compared with unpaired t-tests (duration of the chest tube drainage; and lengths of stay in the postoperative care units and hospital after surgery). Repeatedly measured longitudinal data were analyzed with linear mixed effects models (PaO2, PaO2/FiO2, P(A-a)O2, shunt index, and respiratory or hemodynamic variables before and 30, 60, 90, and 120 min after starting OLV). A first-order autoregressive correlation structure was assumed to model the within-subject correlation over time. The fixed effects were the time (as a categorical variable), group, and interaction between the time and group. If the interaction was significant, pairwise comparisons between groups were performed at each time point. If no interaction was detected, the main effect collapsed over time was reported. In within-group comparisons, the measurement at each time point after starting OLV was compared with the baseline (before OLV).
Categorical variables were presented as the number of patients and were compared with a chi-squared test (number of patients with SpO2 <95% during OLV, primary outcome) or Fisher’s exact tests (number of patients with mild or severe hypoxemia during OLV, and perioperative adverse events).
All the statistical analyses were two-sided and a significance criterion was P <0.05. Risk ratios or mean differences with 95% CIs were calculated for the primary outcome and secondary outcomes as appropriate. All analyses were conducted in an intention-to-treat manner. STATA (Special Edition 14.2; Stata Corporation, College Station, Texas, USA) was used for statistical comparisons between and within groups, power analyses, and group randomization.
In our pilot study (n = 10), three patients (30%) showed hypoxemia with SpO2 <95% during OLV when no pretreatment had been applied before OLV. To detect a 20% difference in the number of hypoxemic patients using a chi-squared test, 62 patients were needed in each group with an α of 0.05 and power of 0.8 for two-sided analysis. We planned to enroll 68 patients in each group considering the dropout rate of 10%.
Results
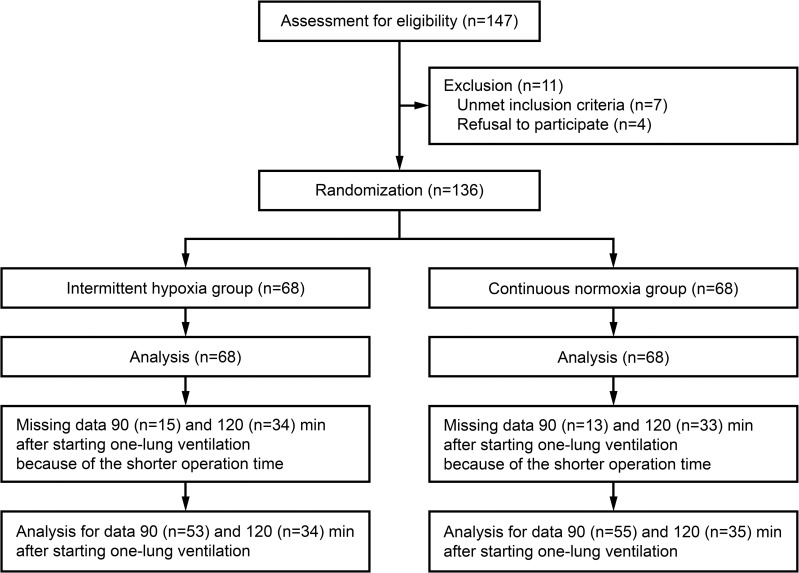
After screening 147 patients, 136 were randomized to the intermittent hypoxia or continuous normoxia groups (n = 68 each; Fig 1). All the 136 patients were included for all the statistical analyses, but some data were missing 90 and 120 min after starting OLV because of the shorter operation time (Fig 1). No clinically relevant differences were observed between groups in patient characteristics (Table 1).
Fig 1. CONSORT diagram.
Table 1. Characteristics of patients, surgery, and anesthesia.
| Intermittent hypoxia group (n = 68) | Continuous normoxia group (n = 68) | |
|---|---|---|
| Age | 60 (8) | 59 (10) |
| Female | 37 | 38 |
| Height (cm) | 162 (9) | 162 (9) |
| Weight (kg) | 63 (10) | 62 (10) |
| Predicted body weight (kg) | 56 (10) | 56 (10) |
| Body mass index (kg m-2) | 24.1 (3.0) | 23.8 (3.1) |
| ASA physical status (II/III) | 25/8 | 26/6 |
| Diagnosis (malignancy/benign) | 58/10 | 62/6 |
| Comorbidity (hypertension/diabetes/angina/stroke/hepatitis) | 15/10/4/3/1 | 16/10/4/2/1 |
| Smoker | 12 | 11 |
| Forced expiratory volume in 1 s (liter) | 2.6 (0.6) | 2.6 (0.7) |
| Forced vital capacity (liter) | 3.5 (0.8) | 3.4 (0.9) |
| Forced expiratory volume in 1 s/Forced vital capacity (%) | 75.3 (7.3) | 77.0 (7.6) |
| Diffusing capacity of lung for carbon monoxide (ml kPa-1 min-1) | 17.9 (4.3) | 18.0 (4.4) |
| PaO2 (kPa, room air) | 13.6 (1.8) | 13.7 (2.5) |
| PaCO2 (kPa, room air) | 5.4 (0.5) | 5.4 (0.5) |
| Hematocrit (%) | 39.9 (4.1) | 39.2 (3.1) |
| Thoracoscopic procedure (wedge resection/segmentectomy/lobectomy) | 5/6/57 | 4/6/58 |
| Side of surgery (left/right) | 19/49 | 25/43 |
| Amount of anesthetic drugs and a fluid | ||
| Propofol (mg) | 1184 (358) | 1257 (438) |
| Remifentanil (μg) | 1383 (421) | 1461 (531) |
| Rocuronium (mg) | 100 (18) | 103 (27) |
| Plasmalyte (ml) | 602 (286) | 584 (267) |
| Inotropic requirement | 22 | 21 |
| Estimated blood loss (ml) | 89 (77) | 81 (68) |
| Duration of intervention | ||
| One-lung ventilation (min) | 105 (35) | 111 (41) |
| Surgery (min) | 131 (43) | 133 (50) |
| Anesthesia (min) | 182 (41) | 186 (53) |
Data are mean (standard deviation) or number of patients. ASA, American Society of Anesthesiologists.
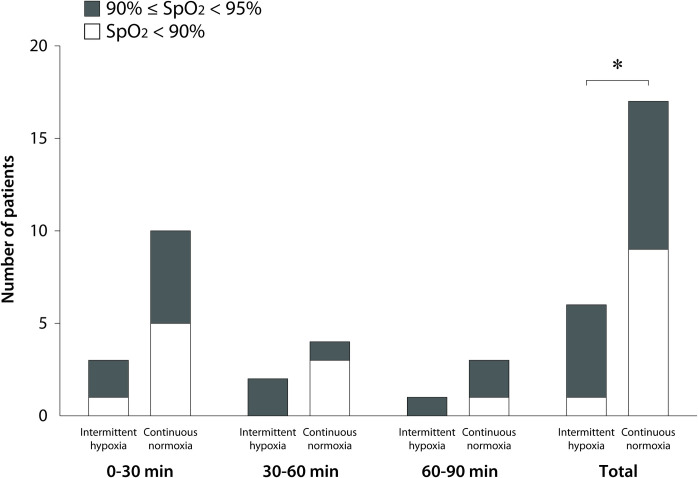
During the experimental protocol for 20 min before OLV, SpO2 was 98–100% in both groups. During OLV, the number of hypoxemic patients with SpO2 <95% [primary outcome; 6/68 (8.8%) vs 17/68 (25.0%), risk ratio (95% CI) 0.35 (0.15–0.84), P = 0.012 by chi-squared test] and severely hypoxemic patients with SpO2 <90% [1/68 (1.5%) vs 9/68 (13.2%), risk ratio (95% CI) 0.11 (0.01–0.85), P = 0.017 by Fisher’s exact test] were fewer in the intermittent hypoxia group than in the continuous normoxia group (Fig 2). Out of 23 hypoxemic patients in both groups, hypoxemia was observed within 30 min of starting OLV in 13 patients (52.5%), and within 60 min of starting OLV in 19 patients (82.6%). Hypoxemia was observed with an FiO2 0.8, PEEP 5 cm H2O, and I:E ratio 1:2 in both groups (n = 20) except for one patient in the intermittent hypoxia group and two patients in the continuous normoxia group with an FiO2 0.6, PEEP 5 cm H2O, and I:E ratio 1:2 at 60–90 min after starting OLV. No patient experienced more than one episode of hypoxemia.
Fig 2. Number of hypoxemic patients with SpO2 <95% for 0–30, 30–60, and 60–90 min after starting one-lung ventilation.
*6/68 (8.8%) vs 17/68 (25.0%), risk ratio (95% CI) 0.35 (0.15–0.84), P = 0.012 by chi-squared test for SpO2 <95%; 1/68 (1.5%) vs 9/68 (13.2%), risk ratio (95% CI) 0.11 (0.01–0.85), P = 0.017 by Fisher’s exact test for SpO2 <90%.
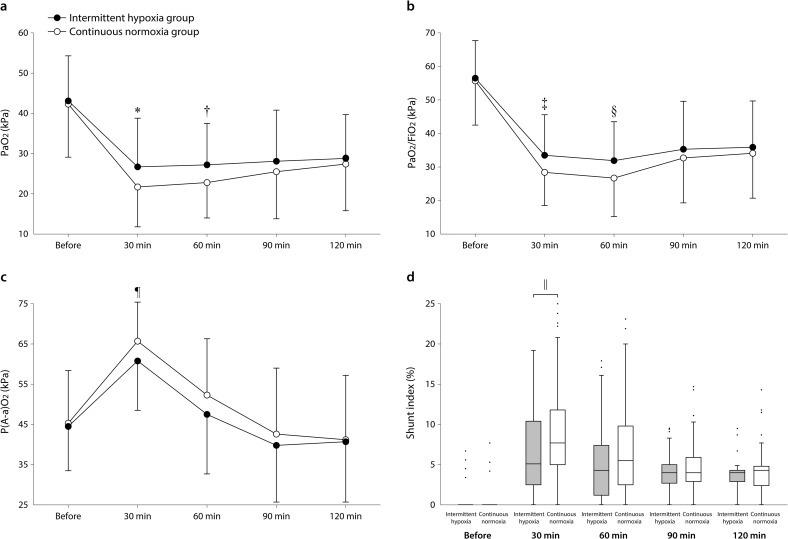
The interactions between time and group were significant in the PaO2, PaO2/FiO2, P(A-a)O2, and shunt index (P<0.001). In within-group comparisons, compared with the baseline, the PaO2 and PaO2/FiO2 were lower, and the shunt index was higher 30, 60, 90, and 120 min after starting OLV; and P(A-a)O2 was greater 30 and 60 min after starting OLV (Fig 3; all P<0.001). In between-group comparisons, the PaO2 and PaO2/FiO2 were higher 30 and 60 min after starting OLV, and the P(A-a)O2 and shunt index were lower 30 min after starting OLV in the intermittent hypoxia group than in the continuous normoxia group (Fig 3). There were no significant interactions between time and group and no significant differences between groups during OLV in the minute ventilation (P = 0.263 by linear mixed effects model), alveolar dead space (P = 0.845), static (P = 0.723) and dynamic (P = 0.800) pulmonary compliances, FiO2 (P = 0.991), PEEP (P = 0.993), I:E ratio (P = 0.997), PaCO2 (P = 0.926), hematocrit (P = 0.785), mean arterial pressure (P = 0.688), heart rate (P = 0.445), and cardiac index (P = 0.332).
Fig 3.
PaO2 (a), PaO2/FiO2 (b), alveolar-arterial oxygen pressure gradient [P(A-a)O2; c], and shunt index (d) before and 30, 60, 90, and 120 min after starting one-lung ventilation. The circles and error bars are mean and standard deviation. *mean difference (95% CI) 5.0 (1.3–8.8) kPa, P = 0.008; †4.4 (1.2–7.6) kPa, P = 0.007; ‡5.0 (1.3–8.8) kPa, P = 0.008; §5.3 (1.3–9.2) kPa, P = 0.008; ¶4.9 (-8.8 to -1.2) kPa, P = 0.010; ║median (interquartile range) 5.1% (2.5–10.4%) vs 7.7% (5.0–11.8%), P = 0.008.
Postoperative adverse events did not differ significantly between groups (Table 2). No patient received intraoperative transfusion and postoperative mechanical ventilation.
Table 2. Postoperative outcomes.
| Intermittent hypoxia group (n = 68) | Continuous normoxia group (n = 68) | P-value | |
|---|---|---|---|
| PaO2 (kPa)* | 24.1 (9.6) | 25.2 (8.5) | 0.502 |
| PaCO2 (kPa)* | 6.7 (0.9) | 6.7 (0.8) | 0.930 |
| Hematocrit (%) | 39.1 (4.4) | 39.0 (4.5) | 0.875 |
| Duration of chest tube drainage (day) | 3.7 (2.3) | 4.0 (3.7) | 0.620 |
| Length of stay | |||
| Post-anesthesia care unit (min) | 45.0 (16.1) (n = 49) | 44.1 (14.1) (n = 51) | 0.751 |
| Intensive care unit (h) | 22.5 (7.9) (n = 19) | 25.9 (13.2) (n = 17) | 0.347 |
| Hospital (day) | 5.0 (2.9) | 5.3 (3.7) | 0.610 |
| Adverse events | 0.507 | ||
| Atelectasis | 1 | 1 | |
| Pneumothorax | 1 | 1 | |
| Pleural effusion | 1 | 4 | |
| Prolonged air leak | 1 | 1 | |
| Atrial fibrillation | 0 | 2 | |
| Chylothorax | 0 | 1 |
Data are mean (standard deviation) or number of patients. Unpaired t-tests were used to compare PaO2, PaCO2, Hematocrit, duration of chest tube drainage, and length of stay; and a Fisher’s exact test was used to compare adverse events between groups.
*Measured while O2 was supplied via a facemask at a rate of 5 liter min-1.
Discussion
In previous human [1] and animal studies [32, 33], HPV seemed to be maximized 30–60 min after the onset of hypoxia. Thus, the risk of hypoxemia may be high until HPV has been maximized and the intrapulmonary shunt has been minimized after starting OLV [7, 10, 12]. In both groups of our study, 82.6% of hypoxemic patients with SpO2 <95% were found within 60 min of starting OLV. However, the incidence of hypoxemia was lower during OLV, and the PaO2/FiO2 were higher 30 and 60 min after starting OLV in the intermittent hypoxia group than in the continuous normoxia group.
During OLV, hypoxemia can develop not only by the intrapulmonary shunt in the non-ventilated lung but also by the ventilation-perfusion mismatch in the ventilated lung [9, 21] or hemodynamic instability [4]. In our study, no differences were found between groups in the respiratory or hemodynamic variables during OLV. However, the P(A-a)O2 and shunt index were lower 30 min after starting OLV in the intermittent hypoxia group than in the continuous normoxia group. Because the two parameters are known to be positively associated with the amount of right-to-left shunt [9, 29], these findings may suggest that the intrapulmonary shunt was smaller in the intermittent hypoxia group than in the continuous normoxia group 30 min after starting OLV.
In order to evaluate HPV and to calculate the shunt fraction, a pulmonary artery catheter is needed to measure the pulmonary artery pressure, mixed venous oxygen content, and cardiac output [4, 5, 11, 14, 28]. However, we did not use the catheter because of its invasiveness [34]. Instead of the shunt fraction requiring the mixed venous oxygen content [9, 27, 28], we calculated the shunt index using ScvO2 measured in the superior vena cava. The trend over time is known to be similar in the shunt fraction and the shunt index under comparable FiO2, PaCO2, and hematocrit levels, although their absolute values differ [29]. In addition, the arterial waveform analysis can estimate the cardiac output reliably in stable hemodynamic status with regular cardiac rhythms as in our study [35]. In our study, no differences were found between groups in the FiO2, PEEP, I:E ratio, PaCO2, hematocrit, and cardiac index during OLV. Therefore, the fewer hypoxemic patients and higher PaO2/FiO2 might be due to a smaller amount of the intrapulmonary shunt and greater HPV response in the intermittent hypoxia group than in the continuous normoxia group.
Previous human [36] and animal [5] studies reported that the HPV response was initiated within 2 min of the onset of hypoxia. However, the offset of HPV seems to take longer when normoxia is restored [7, 8, 37]. Therefore, if hypoxic and normoxic stimuli are applied for the same duration, the pulmonary arteries may be in a more constricted state than the baseline state, so the subsequent vasoconstriction can be augmented by the next hypoxic stimulus [7, 8]. However, previous animal studies reported that HPV was no longer augmented after 4–5 cycles of hypoxic and normoxic stimuli [14, 15]. Based on these findings, we designed the experimental protocol that the operative lung was not ventilated for 2 min and then ventilated for 2 min five times. Because this protocol only takes 20 min, we were able to apply it during surgical preparation without delaying the operation.
The effects of repeated intermittent hypoxic stimuli on HPV was studied in various experimental settings and showed inconsistent findings. Several animal studies reported that repeated hypoxic stimuli potentiated HPV in two lungs [15], one lung [13], and one lobe [12, 14]. On the other hand, in another human [6, 27] and animal studies [11], HPV was maximized by the first hypoxic stimulus for 30–60 min and was not potentiated by the subsequent hypoxic stimuli. However, out of 23 hypoxemic patients in our study, 13 (56.5%) were found within 30 min of starting OLV, whereas no patient showed hypoxemia during the experimental protocol for 20 min. Therefore, although we compared five intermittent hypoxic stimuli only with one continuous normoxic stimulus but not with one continuous hypoxic stimulus, it may be more reliable to apply multiple short-term hypoxic stimuli than one long-term hypoxic stimulus for patient safety. However, our study does not suggest the most effective and safest protocol to prevent hypoxemia during OLV.
Hypoxemia is generally defined as a PaO2 less than 8.0–10.7 kPa (60–80 mmHg) [10, 19, 21]. However, PaO2 is not continuously monitored although hypoxemia should be immediately managed during OLV. Based on previous studies [21, 24–26], we thus defined hypoxemia as a SpO2 <95% and severe hypoxemia as a SpO2 <90%, which are approximated with a PaO2 <10.0 kPa (75 mm Hg) and <8.0 kPa (60 mm Hg) in the oxyhemoglobin dissociation curve, respectively [10, 21, 26].
Before OLV, the alveolar recruitment strategy is another method to prevent intraoperative hypoxemia by reducing atelectasis and alveolar dead space [38]. This strategy can be used both for thoracic and non-thoracic surgery, but the high airway pressure may lead to ventilator-induced lung injury and hemodynamic instability [39]. Our intermittent hypoxic stimulating method is unlikely to cause these complications, although our method can be applied only before thoracic surgery with OLV.
Our study has limitations. We changed the FiO2, PEEP, and I:E ratio according to a PaO2 and SpO2 in order to provide adequate oxygenation during OLV. This was likely to confound the observed treatment effect although these parameters did not differ significantly between groups. In addition, patients with lower cardiopulmonary reserve are more vulnerable to intraoperative hypoxemia [10], but our patients were relatively healthy. We also only studied thoracic surgical patients receiving intravenous anesthesia with propofol and remifentanil in the lateral decubitus position. Because the HPV response can be affected by the type of anesthetic drugs or patient position [3, 7, 8], our findings cannot be extrapolated to patients with poor cardiopulmonary functions or with inhalational anesthesia in the supine position. Furthermore, as well as intraoperative hypoxemia, postoperative lung injury is a critical complication associated with thoracic surgery [10, 40]. However, we did not investigate it although previous studies [17, 18] suggested that repeated intermittent hypoxic stimuli would reduce lung injury by the similar preventive mechanism of remote ischemic preconditioning [41, 42]. In addition, for alveolar hypoxia, the operative lung was passively deflated but was not actively ventilated with inert gases such as nitrogen [6, 12–15, 27], in order to make a clinically applicable protocol. Out study also has potential sources of attrition and reporting biases because of missing data in the several secondary outcomes and missing outcomes in the clinical trial registry (NCT03282032), and this may decrease the validity of our study.
Conclusions
In conclusion, repeated intermittent hypoxic stimuli to the operative lung seemed to potentiate HPV. As a result, it improved oxygenation and reduced hypoxemia during the subsequent OLV for thoracoscopic surgery in the lateral decubitus position.
Supporting information
(DOC)
(DOCX)
(DOCX)
(XLSX)
(TIF)
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Gong Q, Yang Z, Wei W. The changes of pulmonary blood flow in non-ventilated lung during one lung ventilation. J Clin Monit Comput. 2010; 24: 407–12. 10.1007/s10877-010-9262-0 [DOI] [PubMed] [Google Scholar]
- 2.Ishikawa S, Lohser J. One-lung ventilation and arterial oxygenation. Curr Opin Anaesthesiol. 2011; 24: 24–31. 10.1097/ACO.0b013e3283415659 [DOI] [PubMed] [Google Scholar]
- 3.Karzai W, Schwarzkopf K. Hypoxemia during one-lung ventilation: prediction, prevention, and treatment. Anesthesiology. 2009; 110: 1402–11. 10.1097/ALN.0b013e31819fb15d [DOI] [PubMed] [Google Scholar]
- 4.McMullen MC, Girling LG, Graham MR, Mutch WA. Biologically variable ventilation improves oxygenation and respiratory mechanics during one-lung ventilation. Anesthesiology. 2006; 105: 91–7. 10.1097/00000542-200607000-00017 [DOI] [PubMed] [Google Scholar]
- 5.Bennie RE, Packer CS, Powell DR, Jin N, Rhoades RA. Biphasic contractile response of pulmonary artery to hypoxia. Am J Physiol. 1991; 261: L156–63. 10.1152/ajplung.1991.261.2.L156 [DOI] [PubMed] [Google Scholar]
- 6.Carlsson AJ, Bindslev L, Santesson J, Gottlieb I, Hedenstierna G. Hypoxic pulmonary vasoconstriction in the human lung: the effect of prolonged unilateral hypoxic challenge during anaesthesia. Acta Anaesthesiol Scand. 1985; 29: 346–51. 10.1111/j.1399-6576.1985.tb02212.x [DOI] [PubMed] [Google Scholar]
- 7.Lumb AB, Slinger P. Hypoxic pulmonary vasoconstriction: physiology and anesthetic implications. Anesthesiology. 2015; 122: 932–46. 10.1097/ALN.0000000000000569 [DOI] [PubMed] [Google Scholar]
- 8.Nagendran J, Stewart K, Hoskinson M, Archer SL. An anesthesiologist’s guide to hypoxic pulmonary vasoconstriction: implications for managing single-lung anesthesia and atelectasis. Curr Opin Anaesthesiol. 2006; 19: 34–43. 10.1097/01.aco.0000192777.09527.9e [DOI] [PubMed] [Google Scholar]
- 9.Sarkar M, Niranjan N, Banyal PK. Mechanisms of hypoxemia. Lung India. 2017; 34: 47–60. 10.4103/0970-2113.197116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Slinger PD, Campos JH. Anesthesia for thoracic surgery. In: Miller RD. Miller’s Anesthesia. Eighth edition. Philadelphia: Elsevier Saunders, 2015; 1942–2004. [Google Scholar]
- 11.Chen L, Miller FL, Williams JJ, Alexander CM, Domino KB, Marshall C, et al. Hypoxic pulmonary vasoconstriction is not potentiated by repeated intermittent hypoxia in closed chest dogs. Anesthesiology. 1985; 63: 608–10. 10.1097/00000542-198512000-00009 [DOI] [PubMed] [Google Scholar]
- 12.Benumof JL. Intermittent hypoxia increases lobar hypoxic pulmonary vasoconstriction. Anesthesiology. 1983; 58: 399–404. 10.1097/00000542-198305000-00001 [DOI] [PubMed] [Google Scholar]
- 13.Miller MA, Hales CA. Stability of alveolar hypoxic vasoconstriction with intermittent hypoxia. J Appl Physiol Respir Environ Exerc Physiol. 1980; 49: 846–50. 10.1152/jappl.1980.49.5.846 [DOI] [PubMed] [Google Scholar]
- 14.Pirlo AF, Benumof JL, Trousdale FR. Potentiation of lobar hypoxic pulmonary vasoconstriction by intermittent hypoxia in dogs. Anesthesiology 1981; 55: 226–30. 10.1097/00000542-198109000-00007 [DOI] [PubMed] [Google Scholar]
- 15.Unger M, Atkins M, Briscoe WA, King TK. Potentiation of pulmonary vasoconstrictor response with repeated intermittent hypoxia. J Appl Physiol Respir Environ Exerc Physiol. 1977; 43: 662–7. 10.1152/jappl.1977.43.4.662 [DOI] [PubMed] [Google Scholar]
- 16.Zhang GQ, Ye J, Chen JY, Liu W, Cai KC. Effect of one lung ventilation preconditioning on oxygenation during pediatric video-assisted thoracoscopic surgery. Nan Fang Yi Ke Da Xue Xue Bao. 2015; 35: 1492–6. [PubMed] [Google Scholar]
- 17.Cheng YD, Gao Y, Zhang H, Duan CJ, Zhang CF. Effects of OLV preconditioning and postconditioning on lung injury in thoracotomy. Asian J Surg. 2014; 37: 80–5. 10.1016/j.asjsur.2013.09.003 [DOI] [PubMed] [Google Scholar]
- 18.Zhang W, Chen M, Li H, Yuan J, Li J, Wu F, et al. Hypoxia preconditioning attenuates lung injury after thoracoscopic lobectomy in patients with lung cancer: a prospective randomized controlled trial. BMC anesthesiol. 2019; 19: 209. 10.1186/s12871-019-0854-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guenoun T, Journois D, Silleran-Chassany J, Frappier J, D’Attellis N, Salem A, et al. Prediction of arterial oxygen tension during one-lung ventilation: analysis of preoperative and intraoperative variables. J Cardiothorac Vasc Anesth. 2002; 16: 199–203. 10.1053/jcan.2002.31067 [DOI] [PubMed] [Google Scholar]
- 20.Kim SH, Jung KT, An TH. Effects of tidal volume and PEEP on arterial blood gases and pulmonary mechanics during one-lung ventilation. J Anesth. 2012; 26: 568–73. 10.1007/s00540-012-1348-z [DOI] [PubMed] [Google Scholar]
- 21.Park HP, Yoon MJ, Jeon YT, Kang JM, Hwang JW, Oh YS. Which Predictable Variables Identify Patients at Risk of Arterial Hypoxemia during One-Lung Ventilation?: Analysis of Preoperative and Intraoperative Variables. Korean J Anesthesiol. 2005; 49: 167–71. [Google Scholar]
- 22.Vezzani A, Brusasco C, Palermo S, Launo C, Mergoni M, Corradi F. Ultrasound localization of central vein catheter and detection of postprocedural pneumothorax: an alternative to chest radiography. Crit Care Med. 2010; 38: 533–8. 10.1097/CCM.0b013e3181c0328f [DOI] [PubMed] [Google Scholar]
- 23.Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, Wheeler A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000; 342: 1301–8. 10.1056/NEJM200005043421801 [DOI] [PubMed] [Google Scholar]
- 24.Jung DM, Ahn HJ, Jung SH, Yang M, Kim JA, Shin SM, et al. Apneic oxygen insufflation decreases the incidence of hypoxemia during one-lung ventilation in open and thoracoscopic pulmonary lobectomy: A randomized controlled trial. J Thorac Cardiovasc Surg. 2017; 154: 360–6. 10.1016/j.jtcvs.2017.02.054 [DOI] [PubMed] [Google Scholar]
- 25.Liu SS, Chisholm MF, Ngeow J, John RS, Shaw P, Ma Y, et al. Postoperative hypoxemia in orthopedic patients with obstructive sleep apnea. HSS J. 2011; 7: 2–8. 10.1007/s11420-010-9165-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vegh T, Juhasz M, Szatmari S, Enyedi A, Sessler DI, Szegedi LL, et al. Effects of different tidal volumes for one-lung ventilation on oxygenation with open chest condition and surgical manipulation: a randomised cross-over trial. Minerva Anestesiol. 2013; 79: 24–32. [PubMed] [Google Scholar]
- 27.Bindslev L, Jolin A, Hedenstierna G, Baehrendtz S, Santesson J. Hypoxic pulmonary vasoconstriction in the human lung: effect of repeated hypoxic challenges during anesthesia. Anesthesiology. 1985; 62: 621–5. 10.1097/00000542-198505000-00014 [DOI] [PubMed] [Google Scholar]
- 28.el-Fiky MM, Taggart DP, Carter R, Stockwell MC, Maule BH, Wheatley DJ. Respiratory dysfunction following cardiopulmonary bypass: verification of a non-invasive technique to measure shunt fraction. Respir Med. 1993; 87: 193–8. 10.1016/0954-6111(93)90091-d [DOI] [PubMed] [Google Scholar]
- 29.Carroll GC. A continuous monitoring technique for management of acute pulmonary failure. Chest. 1987; 92: 467–9. 10.1378/chest.92.3.467 [DOI] [PubMed] [Google Scholar]
- 30.Helmholz HF Jr. The abbreviated alveolar air equation. Chest. 1979; 75: 748. 10.1378/chest.75.6.748 [DOI] [PubMed] [Google Scholar]
- 31.Gommers D, Vilstrup C, Bos JA, Larsson A, Werner O, Hallllappel E, et al. Exogenous surfactant therapy increases static lung compliance, and cannot be assessed by measurements of dynamic compliance alone. Crit Care Med. 1993; 21: 567–74. 10.1097/00003246-199304000-00019 [DOI] [PubMed] [Google Scholar]
- 32.Dipp M, Nye PC, Evans AM. Hypoxic release of calcium from the sarcoplasmic reticulum of pulmonary artery smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2001; 281: L318–25. 10.1152/ajplung.2001.281.2.L318 [DOI] [PubMed] [Google Scholar]
- 33.Glasser SA, Domino KB, Lindgren L, Parcella P, Marshall C, Marshall BE. Pulmonary blood pressure and flow during atelectasis in the dog. Anesthesiology. 1983; 58: 225–31. 10.1097/00000542-198303000-00004 [DOI] [PubMed] [Google Scholar]
- 34.Rajaram SS, Desai NK, Kalra A, Gajera M, Cavanaugh SK, Brampton W, et al. Pulmonary artery catheters for adult patients in intensive care. Cochrane Database Syst Rev. 2013: Cd003408. 10.1002/14651858.CD003408.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Button D, Weibel L, Reuthebuch O, Genoni M, Zollinger A, Hofer CK. Clinical evaluation of the FloTrac/Vigileo system and two established continuous cardiac output monitoring devices in patients undergoing cardiac surgery. Br J Anaesth. 2007; 99: 329–36. 10.1093/bja/aem188 [DOI] [PubMed] [Google Scholar]
- 36.Morrell NW, Nijran KS, Biggs T, Seed WA. Magnitude and time course of acute hypoxic pulmonary vasoconstriction in man. Respir Physiol. 1995; 100: 271–81. 10.1016/0034-5687(95)00002-u [DOI] [PubMed] [Google Scholar]
- 37.Ohe M, Mimata T, Haneda T, Takishima T. Time course of pulmonary vasoconstriction with repeated hypoxia and glucose depletion. Respir Physiol. 1986; 63: 177–86. 10.1016/0034-5687(86)90112-x [DOI] [PubMed] [Google Scholar]
- 38.Unzueta C, Tusman G, Suarez-Sipmann F, Böhm S, Moral V. Alveolar recruitment improves ventilation during thoracic surgery: a randomized controlled trial. Br J Anaesth. 2012; 108: 517–524. 10.1093/bja/aer415 [DOI] [PubMed] [Google Scholar]
- 39.Park SH, Jeon YT, Hwang JW, Do SH, Kim JH, Park HP. A preemptive alveolar recruitment strategy before one-lung ventilation improves arterial oxygenation in patients undergoing thoracic surgery: a prospective randomised study. Eur J Anaesthesiol. 2011; 28: 298–302. [PubMed] [Google Scholar]
- 40.Licker M, Fauconnet P, Villiger Y, Tschopp JM. Acute lung injury and outcomes after thoracic surgery. Curr Opin Anaesthesiol. 2009; 22: 61–7. 10.1097/ACO.0b013e32831b466c [DOI] [PubMed] [Google Scholar]
- 41.Li C, Xu M, Wu Y, Li YS, Huang WQ, Liu KX. Limb remote ischemic preconditioning attenuates lung injury after pulmonary resection under propofol-remifentanil anesthesia: a randomized controlled study. Anesthesiology. 2014; 121: 249–59. 10.1097/ALN.0000000000000266 [DOI] [PubMed] [Google Scholar]
- 42.García-de-la-Asunción J, Bruno L, Perez-Griera J, Galan G, Morcillo A, Wins R, et al. Remote ischemic preconditioning decreases oxidative lung damage after pulmonary lobectomy: a single-center randomized, double-Blind, controlled trial. Anesth analg. 2017; 125: 499–506. 10.1213/ANE.0000000000002065 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(DOCX)
(DOCX)
(XLSX)
(TIF)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.