Abstract
Tumor hypoxia in head-and-neck squamous cell carcinoma (HNSCC) leads to an immunosuppressive microenvironment and reduces the response to radiotherapy. In this prospective imaging trial, we investigated potential interactions between functional hypoxia imaging and infiltrating lymphocyte levels as a potential predictor for treatment response in HNSCC patients. Methods: In total, 49 patients receiving definitive chemoradiation for locally advanced HNSCCs underwent pretherapeutic biopsies and peritherapeutic hypoxia imaging using 18F-misonidazole PET at weeks 0, 2, and 5 during chemoradiation. Hematoxylin–eosin and immunohistochemical stainings for tumor-infiltrating lymphocytes, tissue-based hypoxia, and microvascular markers were analyzed and correlated with the longitudinal hypoxia dynamics and patient outcomes. Results: High levels of tumor-infiltrating total lymphocytes correlated with superior locoregional control (LRC) (hazard ratio [HR], 0.279; P = 0.011) and progression-free survival (PFS) (HR, 0.276; P = 0.006). Similarly, early resolution of 18F-misonidazole PET–detected tumor hypoxia quantified by 18F-misonidazole dynamics between weeks 0 and 2 of chemoradiation was associated with improved LRC (HR, 0.321; P = 0.015) and PFS (HR, 0.402; P = 0.043). Outcomes in the favorable early hypoxia resolution subgroup significantly depended on infiltrating lymphocyte counts, with patients who showed both an early hypoxia response and high lymphocyte infiltration levels exhibiting significantly improved LRC (HR, 0.259; P = 0.036) and PFS (HR, 0.242; P = 0.017) compared with patients with an early hypoxia response but low lymphocyte counts. These patients exhibited oncologic results comparable to those of patients with no hypoxia response within the first 2 wk of chemoradiation. Conclusion: This analysis established a clinical hypoxia-immune score that predicted treatment responses and outcomes in HNSCC patients undergoing chemoradiation and may help to devise novel concepts for biology-driven personalization of chemoradiation.
Keywords: head-and-neck cancer, hypoxia, FMISO PET, chemoradiation, immune system
Head-and-neck squamous cell carcinomas (HNSCCs) are common malignancies, with 650,000 new diagnoses each year (1). Radiotherapy, often combined with systemic therapies, constitutes a standard treatment for HNSCC, and 5-y overall survival (OS) ranges between 40% and 70% depending on tumor stage and localization (2).
Tumor-associated hypoxia is a key factor influencing treatment outcomes of HNSCC patients, and hypoxia increases resistance of HNSCCs to ionizing radiation both in vitro and clinically (3–6). Different methods of detecting and monitoring tumor-associated hypoxia during radiotherapy have been investigated, including histopathologic analyses, blood- and tissue-based biomarkers, and different imaging modalities (7–13). 18F misonidazole tracer uptake corresponds well with invasive tissue oxygen measurements, and PET using 18F-misonidazole has been established as the imaging gold standard for longitudinal noninvasive hypoxia monitoring (14). Several analyses have investigated the role of hypoxia dynamics during radiotherapy, and early hypoxia dynamics rather than individual measurements seem to predict treatment response (15,16).
Similarly, HNSCC immunogenicity has been previously investigated, and tumor infiltration by lymphocytes and individual lymphocyte subpopulations has been linked to radiotherapy response and patient outcomes (17). Pretreatment biopsies enable baseline histopathologic lymphocyte analyses, but to date, no noninvasive procedures for monitoring HNSCC immune dynamics during radiotherapy are available.
There is increasing evidence of an interplay between tumor-associated hypoxia and the immune response within a tumor’s microenvironment, but clinical data are lacking (18). For instance, most studies showed that hypoxia promotes M2 polarization of macrophages, supports the protumorigenic effects of myeloid-derived suppressor cells and induces the differentiation of CD4-positive T lymphocytes into immunosuppressive regulatory T cells (19). Although an in-depth understanding of the interplay between lymphocyte infiltration and hypoxia dynamics may be crucial to predict radiation responses and to personalize treatment concepts, no data are available yet.
This prospective trial investigated the predictive roles of tumor-associated hypoxia and tumor-infiltrating lymphocytes both individually and regarding their interplay in a homogeneous cohort of HNSCC patients undergoing chemoradiation.
MATERIALS AND METHODS
Patient Treatment
This trial was registered with the German Clinical Trial Register (DRKS00003830) and was approved in advance by the Independent Ethics Committee of the University of Freiburg (reference no. 479/12). In total, 49 patients with histologically confirmed locally advanced HNSCC undergoing definitive chemoradiation were enrolled in this prospective trial. Exclusion criteria were disorders interfering with the patients’ compliance with the study protocol (e.g., dementia), a Karnofsky performance status of 70% or less, claustrophobia or other MRI contraindications, previous malignancies, prior HNSCC resection, distant metastases, and pregnancy. The patient characteristics are detailed in Table 1. The patients received definitive chemoradiation using intensity-modulated radiotherapy to a cumulative dose of 70 Gy in 35 fractions. Concomitant systemic treatment was administered with up to 3 cycles of cisplatin (100 mg/m2 of body surface area in weeks 1, 4, and 7). Locoregional control (LRC), progression-free survival (PFS), and OS were calculated from the initiation of chemoradiation. All patients provided written informed consent, and all aspects of this trial were performed in accordance with the Declaration of Helsinki.
TABLE 1.
Patient and Tumor Characteristics
| Tumor localization | n | % |
| Oral cavity | 2 | 4.1 |
| Oropharynx | 19 | 38.8 |
| Hypopharynx | 12 | 24.5 |
| Larynx | 7 | 14.3 |
| Multilevel | 9 | 18.4 |
| T stage | ||
| T1 | 1 | 2.0 |
| T2 | 4 | 8.2 |
| T3 | 14 | 28.6 |
| T4 | 30 | 61.2 |
| N stage | ||
| N0 | 4 | 8.2 |
| N1 | 1 | 2.0 |
| N2a | 0 | 0.0 |
| N2b | 9 | 18.4 |
| N2c | 35 | 71.4 |
| Human papillomavirus | ||
| Positive | 9 | 18.4 |
| Negative | 40 | 81.6 |
Imaging
All patients underwent initial CT and MRI scans as well as PET imaging with 18F-FDG and 18F-misonidazole tracers before treatment. Further 18F-misonidazole PET imaging was performed in weeks 2 and 5 during chemoradiation (Fig. 1). PET imaging was performed for the first 15 patients on an ECAT EXACT 921 PET scanner (Siemens) and for the consecutive patients on a Gemini TrueFlight PET/CT scanner (Philips). 18F-misonidazole was administered at 3.7 MBq/kg to a maximum activity of 370 MBq; hypoxia imaging was performed in radiotherapy position using a thermoplastic head immobilization mask at 150 min after tracer administration (3 frames at 10 min each, followed by a transmission scan for 5 min). PET images were coregistered to the corresponding planning CT scans, and quantitative analyses were performed using a sum of 3 attenuation-corrected frames as described previously (16).
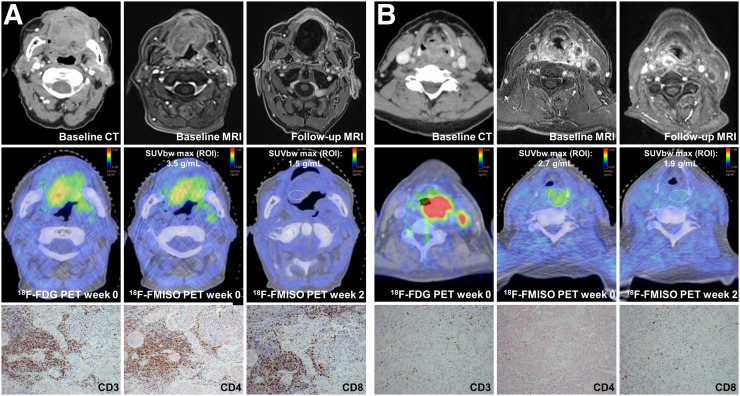
FIGURE 1.
Lymphocyte levels and hypoxia dynamics during chemoradiation. (A) HNSCC patient with hypoxia response and high intratumoral lymphocyte levels. Pretherapeutic CT and MRI show oral cavity carcinoma. Follow-up MRI after 43 mo shows LRC. Pretherapeutic 18F-FDG PET and 18F-misonidazole PET at weeks 0 and 2 are representative images of immunohistochemical stainings for CD3, CD4, and CD8. (B) HNSCC patient with hypoxia response but low intratumoral lymphocyte levels. Pretherapeutic CT and MRI scans show oropharyngeal and hypopharyngeal carcinoma. Follow-up MRI demonstrates locoregional relapse after 5 mo. Representative pretherapeutic 18F-FDG PET and 18F-misonidazole PET at weeks 0 and 2 are depicted; pretherapeutic immunohistochemical staining for CD3, CD4, and CD8 shows low lymphocyte infiltration levels.
Gross tumor volumes were manually delineated using the 18F-FDG PET/MRI coregistered images with a PET threshold of 40% of the SUVmax within the tumor (16). All gross tumor volumes were individually cross-validated with the coregistered planning CT scans by 2 experienced physicians. Hypoxic subvolumes were defined as all voxels within the gross tumor volume with a ratio of 18F-misonidazole SUV to SUVmean in the contralateral sternocleidomastoideus muscles above 1.4. The ratio was based on published thresholds and has previously been validated in this trial cohort (16,20).
Imaging parameters investigated in this analysis comprised the 18F-misonidazole uptake–defined hypoxic subvolumes and the SUV index (normalized 18F-misonidazole SUVmax) as the ratio between the tumor SUVmax and the SUVmean in the contralateral sternocleidomastoid muscle at weeks 0, 2, and 5. Hypoxia dynamics during the course of treatment (ΔSUV index) were quantified by subtracting the SUV index at baseline from the values of weeks 2 or 5 and calculating the percentage change.
Immunohistochemistry
Pretreatment biopsies were performed for all patients to confirm diagnosis. Tumor samples were formalin-fixed and embedded in paraffin according to institutional histopathology protocols. Tumors were sectioned to 2 μm and mounted on coated glass slides before deparaffinization and rehydration through descending graded ethanol concentrations. Antigens were retrieved by heat induction, and endogenous peroxidase activity was blocked using H2O2. Antigen complexes were visualized by a mouse linker (Dako) and Envision Flex Kit using horseradish peroxidase–diaminobenzidine reaction (Dako) as chromogen. Details of primary antibodies and antigen retrieval are outlined in Supplemental Table 1 (supplemental materials are available at http://jnm.snmjournals.org).
Hypoxia markers carbonic anhydrase IX (CAIX) and hypoxia-inducible factor 1-α (HIF1α) were assessed semiquantitatively by the H-score. Staining intensity (0 = no staining, 1 = weak staining, 2 = moderate staining, 3 = strong staining) was assessed in all viable tumor cells, and the H-score (range, 0–300) was quantified as the sum of the tumor cell percentages of the different staining intensities multiplied by their specific intensity scores.
Microvessel density was investigated by CD34 staining and divided into 3 categories (1 = only larger vessels in the stroma, no contact to tumor cells; 2 = smaller vessels in the stroma with contact to tumor cells, no vessels intermingled in the tumor-stroma border; 3 = small vessels intermingling with tumor cells at the tumor-stroma border).
Lymphocyte infiltration was quantified per high-power field and was dichotomized into low infiltration levels (<100 cells per high-power field) and high infiltration levels (>100 cells per high-power field). Lymphocytes were scored both for the intraepithelial and for the stromal compartments. Although total lymphocyte scoring was based on hematoxylin–eosin stainings, quantification of total T lymphocytes, CD4-positive lymphocytes, and CD8-positive T lymphocytes depended on the immunohistochemical stainings for CD3, CD4, and CD8, respectively. Masked samples were used for histologic analyses, and one section of the same paraffin block was analyzed for each marker.
Statistical Analyses
Statistical analyses were performed to assess potential correlations between individual tissue-based variables and between tumor tissue variables and imaging variables. The statistical significance of variable pairs was tested by the Pearson product-moment correlation. Clinical outcome parameters were investigated using the Kaplan–Meier method, and correlations between individual tumor tissue variables and clinical outcome parameters were analyzed using the log-rank test and the Cox proportional-hazards model. Hazard ratios (HR) are indicated together with the corresponding 95% CI, and statistical significance was assumed for a P value of less than 0.05.
RESULTS
High Levels of Tumor-Infiltrating Lymphocytes Are Associated with Improved LRC and Survival
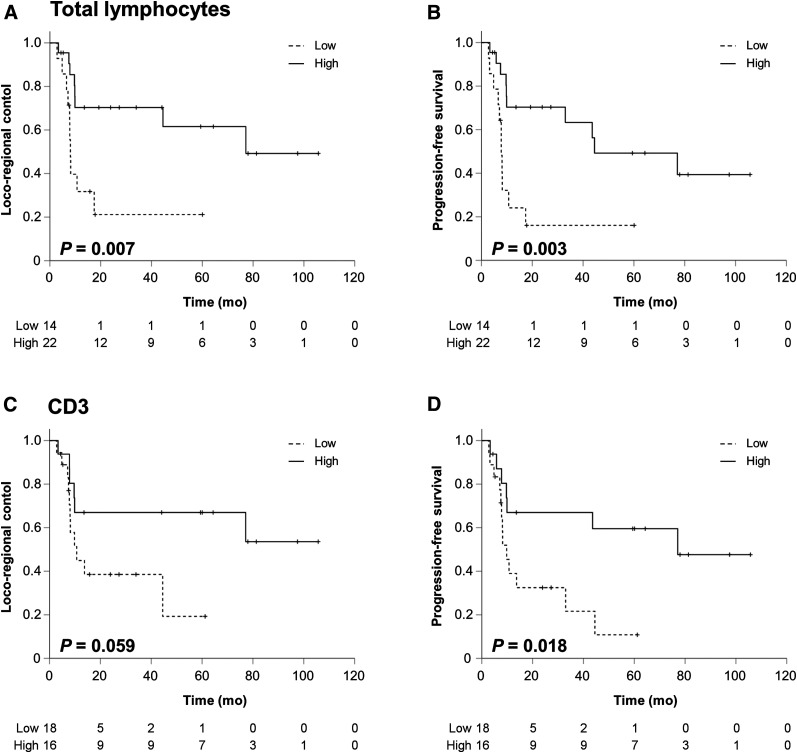
High numbers of total infiltrating total lymphocytes correlated with a significantly improved LRC (HR, 0.279 [0.104–0.748]; P = 0.011) and PFS (HR, 0.276 [95% CI, 0.110–0.690]; P = 0.006) (Fig. 2). Also for the subgroup of human papillomavirus–negative HNSCC patients, LRC (HR, 0.296 [95% CI, 0.104–0.842]; P = 0.022) and PFS (HR, 0.250 [95% CI, 0.089–0.700]; P = 0.008) were superior for patients with high tumor-infiltrating lymphocyte levels (Supplemental Fig. 1).
FIGURE 2.
High lymphocyte levels correspond to increased LRC and PFS rates in HNSCC patients. (A and B) LRC (A) and PFS (B) rates stratified by total tissue lymphocyte levels in tumor biopsies. (C and D) LRC (C) and PFS (D) rates divided by high and low levels of CD3-positive lymphocytes.
Similarly, high levels of CD3-positive T lymphocytes revealed a trend toward higher LRC (HR, 0.370 [95% CI, 0.126–1.082]; P = 0.058) and significantly improved PFS (HR, 0.320 [95% CI, 0.118–0.866]; P = 0.019), whereas OS was increased, with borderline significance (HR, 0.260 [95% CI, 0.067–1.006]; P = 0.051) (Supplemental Fig. 2). For human papillomavirus–negative HNSCCs, higher levels of tumor-infiltrating CD3-positive T lymphocytes led to a trend toward increased PFS (HR, 0.361 [95% CI, 0.126–1.040]; P = 0.059) but not LRC (HR, 0.468 [95% CI, 0.158–1.387]; P = 0.171). Neither LRC (HR, 2.094 [95% CI, 0.679–6.456]; P = 0.215) nor PFS (HR, 2.389 [95% CI, 0.839–6.806]; P = 0.117) differed between patients with high and low numbers of CD4-positive T lymphocytes (Supplemental Fig. 3), and CD8-positive lymphocyte levels also did not correlate with LRC (HR, 0.578 [95% CI, 0.128–2.609]; P = 0.448), PFS (HR, 0.679 [95% CI, 0.193–2.387]; P = 0.531) or OS (HR, 0.578 [95% CI, 0.128–2.609]; P = 0.448) (Supplemental Figs. 2 and 3).
Lymphocyte Infiltration Does Not Correlate with Tissue-Based Hypoxia Biomarkers
Pairwise correlations were performed between expression levels of immune and hypoxia biomarkers, as well as p16 status, using the Pearson correlation coefficient. Numbers of infiltrating lymphocyte subgroups did not correlate with the tested tissue-based hypoxia biomarkers HIF1α or CAIX (Supplemental Table 2). However, strong expression of the microvascular protein CD34 as an indirect surrogate marker for tissue hypoxia corresponded with significantly increased levels of total infiltrating lymphocytes (r = 0.476; P = 0.003) and a trend toward high numbers of CD3-positive (r = 0.313; P = 0.059) and CD8-positive (r = 0.275; P = 0.099) T lymphocytes.
Early Resolution of Tumor-Associated Hypoxia During Chemoradiation Is Associated with Improved LRC and Survival
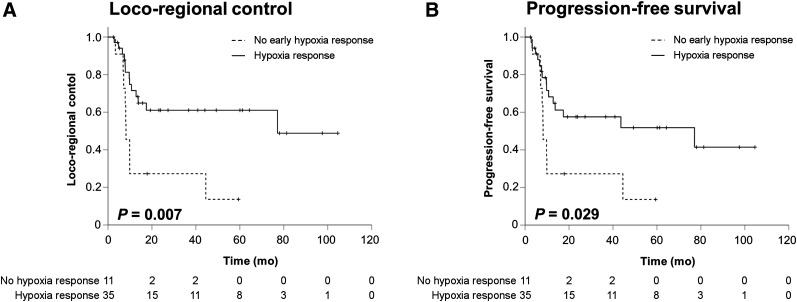
The dynamics of tumor-associated hypoxia during chemoradiation were assessed by the SUV index in weeks 0, 2, and 5 during treatment, as well as differences between the different time points. In total, 29 patients demonstrated an early decrease in their SUV indices between weeks 0 and 2, and all but 1 patient demonstrated a reduction in tumor-associated hypoxia between weeks 2 and 5. Early hypoxia resolution as indicated by a negative ΔSUV index between weeks 0 and 2 resulted in significantly improved LRC (HR, 0.321 [95% CI, 0.134–0.770]; P = 0.015) and PFS (HR, 0.402 [95% CI, 0.173–0.936]; P = 0.043), compared with an increase in hypoxia during the first 2 treatment weeks, but no effect on OS (HR, 0.785 [95% CI, 0.275–2.242]; P = 0.657) (Fig. 3). Using the SUV index cutoff of 1.4 in week 2, patients with no or low tumor hypoxia had considerably enhanced LRC (HR, 0.314 [95% CI, 0.103–0.953]; P = 0.041) and PFS (HR, 0.273 [95% CI, 0.091–0.820]; P = 0.021) (Supplemental Table 3). For the subgroup of human papillomavirus–negative HNSCC patients, early hypoxia response resulted in a trend toward improved LRC (HR, 0.465 [95% CI, 0.189–1.143]; P = 0.095) and PFS (HR, 0.555 [95% CI, 0.229–1.344]; P = 0.095) (Supplemental Fig. 1).
FIGURE 3.
Early hypoxia resolution during chemoradiation is prognosticator for improved LRC and PFS. (A and B) LRC (A) and PFS (B) in HNSCC patients undergoing chemoradiation stratified by hypoxia resolution assessed by 18F-misonidazole PET imaging between weeks 0 and 2.
High Lymphocyte Levels Determine the Response of Hypoxia-Resolving and Nonresolving HNSCCs to Radiotherapy
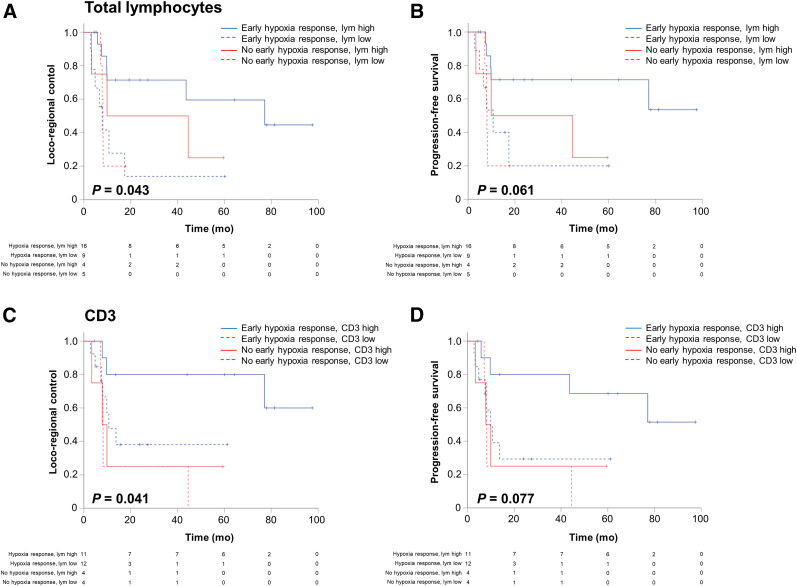
To assess the individual values of lymphocyte infiltration and hypoxia dynamics as potential predictors for the response to chemoradiation, patients were divided into 4 groups according to their status of early hypoxia resolution and their levels of infiltrating lymphocytes at initial biopsy. Even within the group of patients that demonstrated an early resolution of tumor-associated hypoxia, infiltrating lymphocyte levels significantly determined outcomes, and patients with high numbers of total lymphocytes demonstrated superior LRC (HR, 0.259 [95% CI, 0.072–0.926]; P = 0.036) and PFS (HR, 0.242 [95% CI, 0.075–0.775]; P = 0.017), compared with patients with low lymphocyte counts (Fig. 4). Considering only the human papillomavirus–negative HNSCC patients, the favorable subgroup (negative SUV index dynamics between weeks 0 and 2 and high tumor-infiltrating lymphocyte levels) still had significantly increased LRC (HR, 0.262 [95% CI, 0.072–0.947]; P = 0.041) and PFS (HR, 0.202 [95% CI, 0.058–0.706]; P = 0.012) compared with patients displaying early hypoxia response but low lymphocyte levels.
FIGURE 4.
High lymphocyte levels determine response of hypoxia-resolving and -nonresolving HNSCCs to chemoradiation. (A and B) Kaplan–Meier curves showing LRC (A) and PFS (B) depending on early hypoxia resolution and total tumor lymphocyte levels. (C and D) LRC (C) and PFS (D) divided by early hypoxia resolution and CD3-positive lymphocyte levels.
In contrast, patients with low total infiltrating lymphocyte numbers exhibited only nonsignificantly improved LRC (HR, 0.359 [95% CI, 0.080–1.615]; P = 0.201) and PFS (HR, 0.462 [95% CI, 0.110–1.946]; P = 0.313) in case of an early hypoxia resolution, compared with patients with no resolution. In turn, patients with high counts of CD3-positive T lymphocytes showed superior LRC (HR, 0.246 [95% CI, 0.051–1.191]; P = 0.054) and PFS (HR, 0.256 [95% CI, 0.064–1.017]; P = 0.038) within the favorable patient subgroup with an early PET-detected hypoxia response. Levels of CD4- or CD8-positive lymphocytes were not able to discriminate between outcomes for patients with an early resolution or increase in tumor-associated hypoxia (Supplemental Fig. 4). Pairwise correlations between lymphocyte levels and tumor hypoxia during chemoradiation were conducted to reveal potential associations between immune cell infiltration and hypoxia levels; however, we did not find any significant correlations (Table 2).
TABLE 2.
Pairwise Correlations Between Immune Biomarkers and Tumor Hypoxia
| SUV |
ΔSUV |
|||||||
| Parameter | Pearson correlation | Tumor volume | Hypoxic subvolume | Week 0 | Week 2 | Week 5 | Weeks 0–2 | Weeks 2–5 |
| Lymphocytes | r | −0.247 | −0.023 | −0.033 | 0.008 | 0.018 | 0.061 | 0.002 |
| p | 0.135 | 0.887 | 0.844 | 0.961 | 0.915 | 0.718 | 0.990 | |
| CD3 | r | 0.059 | 0.050 | 0.015 | −0.007 | 0.233 | −0.062 | 0.056 |
| p | 0.728 | 0.770 | 0.931 | 0.968 | 0.184 | 0.724 | 0.753 | |
| CD4 | r | 0.303 | −0.120 | −0.092 | −0.171 | 0.225 | −0.093 | 0.145 |
| p | 0.068 | 0.480 | 0.588 | 0.327 | 0.200 | 0.594 | 0.414 | |
| CD8 | r | 0.232 | 0.057 | −0.116 | −0.110 | 0.091 | −0.010 | 0.137 |
| p | 0.168 | 0.738 | 0.495 | 0.531 | 0.609 | 0.957 | 0.441 | |
DISCUSSION
The increasing availability of tumor-specific biologic and functional information may enable a better response prediction for radiotherapy, which in turn will form the basis for developing individualized and personalized treatment concepts. Both molecular analyses and functional imaging modalities have provided novel insights into HNSCCs over the last few years, but clear correlations between the tumor biology and the corresponding imaging information are largely unknown. However, these may be of great importance, as an in-depth biologic analysis commonly requires invasively harvested tissue samples, preventing longitudinal evaluations, whereas imaging enables noninvasive and repetitive monitoring and early response assessment of tumors during treatment. In this prospective imaging trial, we found that both an early hypoxia response within the first 2 wk of chemoradiation and high levels of infiltrating lymphocytes resulted in improved LRC and PFS in HNSCC patients. Additionally, we demonstrated that the relevance of an early hypoxia response depended on the levels of infiltrating lymphocytes, and only patients with both hypoxia response and elevated numbers of intratumoral lymphocytes had superior LRC and PFS, whereas patients with a hypoxia response but low lymphocyte counts exhibited significant deteriorations in their outcomes that were comparable to patients with no hypoxia response.
Several papers have shown that the immune pattern and especially the level of tumor-infiltrating lymphocytes correlate with the prognosis of HNSCC patients and may help to predict response to radiation treatment (17,21). For definitive chemoradiation, increased numbers of tumor-infiltrating CD3- and CD8-positive T lymphocytes corresponded with improved LRC and OS, whereas for adjuvant chemoradiation, only CD8-positive lymphocytes led to improved survival rates (17,21). In our study, the levels of CD3-positive but not CD8-positive T lymphocytes correlated with improved survival rates after definitive chemoradiation. The fact that we did not confirm the correlation between the levels of CD8-positive T lymphocytes and survival rates may be related to small biopsies and limited patient numbers. However, we observed increased infiltration of CD8-positive T lymphocytes in p16-positive HNSCC tumors, which is consistent with previous reports (22,23).
Tumor hypoxia is known to increase resistance to ionizing radiation, and 18F-misonidazole PET imaging has been established as a standard measure to monitor hypoxia during radiotherapy (14). It has been demonstrated that LRC and survival of HNSCC patients are superior for tumors with rapid resolution of tumor-associated hypoxia during the first weeks of radiotherapy (15,16,24). Therefore, several approaches have been considered to improve oncologic outcomes in HNSCC patients with reduced hypoxia resolution during chemoradiation. For instance, the DAHANCA-33 trial is currently investigating the impact of dose escalation in combination with cisplatin and the hypoxic modifier nimorazole for patients with locally advanced and hypoxic tumors (25). In another phase II trial, dose escalation with 77 Gy delivered in 35 fractions to the hypoxic subvolumes was feasible, with acceptable toxicity (24). A metaanalysis including almost 5,000 patients demonstrated that hypoxia modification administered concomitantly with radiotherapy in HNSCC patients improved LRC and OS (26).
As both hypoxic modifiers and checkpoint inhibitors show activity in HNSCC, and hypoxia leads to an immunosuppressive microenvironment, one may hypothesize that combination of hypoxic modifiers with checkpoint inhibitors could further improve the outcome compared with each treatment alone. Although clinical trials combining hypoxia modification with checkpoint inhibitors are missing and no data are available for HNSCC yet, at least mouse models of prostate cancer have shown that a reduction of tumor hypoxia restores T-cell infiltration and sensitizes cancer cells to checkpoint inhibitors (27).
Recently, a hypoxia-transcriptional classifier was proposed and validated, showing potential to discriminate 3 patient subgroups with different hypoxia–immune phenotypes, namely a low-hypoxia/high-immune cell phenotype, a high-hypoxia/low-immune cell phenotype, and a mixed phenotype (28). The authors demonstrated that each of the 3 subtypes exhibited distinct biologic characteristics with varying activated pathways, for which potentially effective targeted therapies exist. In this dataset, the low-hypoxia/high-immunity phenotype was associated with markedly improved survival rates. In line with these data, we have now shown for the first time in a prospective clinical trial the superior survival of patients exhibiting early hypoxia resolution and high intratumoral lymphocyte infiltration.
Although the exact underlying mechanism for the observed interaction is to be elucidated, we hypothesize that preexisting lymphocyte infiltration of the tumor tissue may be required to exploit the disappearance of the immune-suppressing hypoxic microenvironment during chemoradiation. In this respect, HNSCC patients with insufficient numbers of tumor-infiltrating T lymphocytes may not be able to derive benefit from the resolving tumor hypoxia environment. In turn, the omission of the immune-suppressing hypoxic microenvironment during chemoradiation, which allows tumor-infiltrating T lymphocytes to exert their antitumor effects, may be an important mechanism by which radiotherapy-induced hypoxia resolution exerts its beneficial effects. Because hypoxia upregulates TGF-β secretion, increases checkpoint protein expression, and directly impairs T cells’ antitumor activities, hypoxia resolution could restore T cells’ antitumor functions (29). Combinations of hypoxia imaging and immuno-PET imaging in order to monitor T-cell localization, thereby investigating the interaction between tumor hypoxia and the tumor immune microenvironment in real time and noninvasively, could verify our hypotheses in the future (30,31). Recently, a first-in-humans study showed the feasibility and safety of targeting CD8-positive T lymphocytes using the radiolabeled minibody 89Zr-IAB22M2C (32). The potential advantages of functional immuno-PET imaging compared with tumor biopsies include the avoidance of invasive procedures, the possibility for real-time measurements, and the coverage of the whole tumor, including distant metastases, addressing the well-known heterogeneity of HNSCC. For instance, we did not observe a correlation between tumor-infiltrating lymphocyte levels and the hypoxia markers HIF1α and CAIX, which may be related to the spatial distribution of tumor hypoxia that cannot be adequately addressed using small tissue samples.
Although our trial was prospective, with comprehensive longitudinal hypoxia imaging and homogeneous treatment characteristics, our exploratory analysis has some limitations, including the limited sample size; therefore, the data from our analysis require confirmation in larger prospective trials. Furthermore, we did not perform repeat biopsies during treatment because of ethical concerns; therefore, no data are available about longitudinal correlations between imaging and histology. It is well known that tumors exhibit intratumoral heterogeneity; tumor biopsies may not be able to represent the complete tumor.
CONCLUSION
This dataset demonstrated that high pretherapeutic tumor lymphocyte levels determine the response of HNSCC to definitive chemoradiation depending on their hypoxia dynamics: patients with high levels of tumor-infiltrating lymphocytes combined with early hypoxia resolution assessed by 18F-misonidazole PET imaging had excellent LRC and PFS rates, whereas HNSCC patients exhibiting low tumor lymphocyte levels did not benefit from early hypoxia resolution. Our findings might help to devise novel concepts for personalized cancer treatment, in particular for HNSCC patients.
DISCLOSURE
The trial was funded by the German Cancer Consortium (DKTK) and registered with the German Clinical Trial Register (DRKS00003830). No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: How do intratumoral lymphocyte levels influence the favorable outcomes of HNSCC patients with early hypoxia resolution during definitive chemoradiation?
PERTINENT FINDINGS: In this prospective imaging trial, patients received pretherapeutic biopsies and longitudinal hypoxia imaging using 18F-misonidazole PET at weeks 0, 2, and 5 during chemoradiation. We showed that outcomes in the favorable early-hypoxia-resolution subgroup were dependent on tumor-infiltrating lymphocyte levels: patients exhibiting both an early hypoxia response and high lymphocyte infiltration levels exhibited significantly improved LRC and PFS rates, compared with patients with a hypoxia response but low lymphocyte counts.
IMPLICATIONS FOR PATIENT CARE: Understanding of the interplay between the immune system and tumor-associated hypoxia dynamics during chemoradiation may help to identify patients who might benefit from personalized treatment strategies, including dose escalation to hypoxic subvolumes or combination with hypoxia modifiers and checkpoint inhibitors.
Supplementary Material
Acknowledgments
Wes thank Andrei Bunea, Hatice Bunea, and Raluca Stoian for their help with patient recruitment.
REFERENCES
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 2.Blanchard P, Baujat B, Holostenco V, et al. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): a comprehensive analysis by tumour site. Radiother Oncol. 2011;100:33–40. [DOI] [PubMed] [Google Scholar]
- 3.Baumann R, Depping R, Delaperriere M, Dunst J. Targeting hypoxia to overcome radiation resistance in head & neck cancers: real challenge or clinical fairytale? Expert Rev Anticancer Ther. 2016;16:751–758. [DOI] [PubMed] [Google Scholar]
- 4.Overgaard J, Eriksen JG, Nordsmark M, Alsner J, Horsman MR. Plasma osteopontin, hypoxia, and response to the hypoxia sensitiser nimorazole in radiotherapy of head and neck cancer: results from the DAHANCA 5 randomised double-blind placebo-controlled trial. Lancet Oncol. 2005;6:757–764. [DOI] [PubMed] [Google Scholar]
- 5.Mortensen LS, Johansen J, Kallehauge J, et al. FAZA PET/CT hypoxia imaging in patients with squamous cell carcinoma of the head and neck treated with radiotherapy: results from the DAHANCA 24 trial. Radiother Oncol. 2012;105:14–20. [DOI] [PubMed] [Google Scholar]
- 6.Vaupel P, Thews O, Hoeckel M. Treatment resistance of solid tumors: role of hypoxia and anemia. Med Oncol. 2001;18:243–259. [DOI] [PubMed] [Google Scholar]
- 7.Zips D, Zophel K, Abolmaali N, et al. Exploratory prospective trial of hypoxia-specific PET imaging during radiochemotherapy in patients with locally advanced head-and-neck cancer. Radiother Oncol. 2012;105:21–28. [DOI] [PubMed] [Google Scholar]
- 8.Wiedenmann N, Bunea H, Rischke HC, et al. Effect of radiochemotherapy on T2* MRI in HNSCC and its relation to FMISO PET derived hypoxia and FDG PET. Radiat Oncol. 2018;13:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grosu AL, Souvatzoglou M, Röper B, et al. Hypoxia imaging with FAZA-PET and theoretical considerations with regard to dose painting for individualization of radiotherapy in patients with head and neck cancer. Int J Radiat Oncol Biol Phys. 2007;69:541–551. [DOI] [PubMed] [Google Scholar]
- 10.Stadler P, Feldmann HJ, Creighton C, Kau R, Molls M. Changes in tumor oxygenation during combined treatment with split-course radiotherapy and chemotherapy in patients with head and neck cancer. Radiother Oncol. 1998;48:157–164. [DOI] [PubMed] [Google Scholar]
- 11.Kwon OJ, Park JJ, Ko GH, et al. HIF-1alpha and CA-IX as predictors of locoregional control for determining the optimal treatment modality for early-stage laryngeal carcinoma. Head Neck. 2015;37:505–510. [DOI] [PubMed] [Google Scholar]
- 12.Wiedenmann N, Grosu AL, Buchert M, et al. The utility of multiparametric MRI to characterize hypoxic tumor subvolumes in comparison to FMISO PET/CT: consequences for diagnosis and chemoradiation treatment planning in head and neck cancer. Radiother Oncol. 2020;150:128–135. [DOI] [PubMed] [Google Scholar]
- 13.Nicolay NH, Wiedenmann N, Mix M, et al. Correlative analyses between tissue-based hypoxia biomarkers and hypoxia PET imaging in head and neck cancer patients during radiochemotherapy: results from a prospective trial. Eur J Nucl Med Mol Imaging. 2020;47:1046–1055. [DOI] [PubMed] [Google Scholar]
- 14.Rajendran JG, Schwartz DL, O’Sullivan J, et al. Tumor hypoxia imaging with [F-18] fluoromisonidazole positron emission tomography in head and neck cancer. Clin Cancer Res. 2006;12:5435–5441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Löck S, Perrin R, Seidlitz A, et al. Residual tumour hypoxia in head-and-neck cancer patients undergoing primary radiochemotherapy, final results of a prospective trial on repeat FMISO-PET imaging. Radiother Oncol. 2017;124:533–540. [DOI] [PubMed] [Google Scholar]
- 16.Wiedenmann NE, Bucher S, Hentschel M, et al. Serial [18F]-fluoromisonidazole PET during radiochemotherapy for locally advanced head and neck cancer and its correlation with outcome. Radiother Oncol. 2015;117:113–117. [DOI] [PubMed] [Google Scholar]
- 17.Balermpas P, Michel Y, Wagenblast J, et al. Tumour-infiltrating lymphocytes predict response to definitive chemoradiotherapy in head and neck cancer. Br J Cancer. 2014;110:501–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Noman MZ, Hasmim M, Messai Y, et al. Hypoxia: a key player in antitumor immune response—a review in the theme: cellular responses to hypoxia. Am J Physiol Cell Physiol. 2015;309:C569–C579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Colegio OR, Chu NQ, Szabo AL, et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature. 2014;513:559–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bittner MI, Wiedenmann N, Bucher S, et al. Exploratory geographical analysis of hypoxic subvolumes using 18F-MISO-PET imaging in patients with head and neck cancer in the course of primary chemoradiotherapy. Radiother Oncol. 2013;108:511–516. [DOI] [PubMed] [Google Scholar]
- 21.Balermpas P, Rodel F, Rodel C, et al. CD8+ tumour-infiltrating lymphocytes in relation to HPV status and clinical outcome in patients with head and neck cancer after postoperative chemoradiotherapy: a multicentre study of the German Cancer Consortium Radiation Oncology Group (DKTK-ROG). Int J Cancer. 2016;138:171–181. [DOI] [PubMed] [Google Scholar]
- 22.Nguyen N, Bellile E, Thomas D, et al. Tumor infiltrating lymphocytes and survival in patients with head and neck squamous cell carcinoma. Head Neck. 2016;38:1074–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jung AC, Guihard S, Krugell S, et al. CD8-alpha T-cell infiltration in human papillomavirus-related oropharyngeal carcinoma correlates with improved patient prognosis. Int J Cancer. 2013;132:E26–E36. [DOI] [PubMed] [Google Scholar]
- 24.Welz S, Monnich D, Pfannenberg C, et al. Prognostic value of dynamic hypoxia PET in head and neck cancer: results from a planned interim analysis of a randomized phase II hypoxia-image guided dose escalation trial. Radiother Oncol. 2017;124:526–532. [DOI] [PubMed] [Google Scholar]
- 25.Saksø M, Andersen E, Bentzen J, et al. A prospective, multicenter DAHANCA study of hyperfractionated, accelerated radiotherapy for head and neck squamous cell carcinoma. Acta Oncol. 2019;58:1495–1501. [DOI] [PubMed] [Google Scholar]
- 26.Overgaard J. Hypoxic modification of radiotherapy in squamous cell carcinoma of the head and neck: a systematic review and meta-analysis. Radiother Oncol. 2011;100:22–32. [DOI] [PubMed] [Google Scholar]
- 27.Jayaprakash P, Ai M, Liu A, et al. Targeted hypoxia reduction restores T cell infiltration and sensitizes prostate cancer to immunotherapy. J Clin Invest. 2018;128:5137–5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brooks JM, Menezes AN, Ibrahim M, et al. Development and validation of a combined hypoxia and immune prognostic classifier for head and neck cancer. Clin Cancer Res. 2019;25:5315–5328. [DOI] [PubMed] [Google Scholar]
- 29.Ohta A. Oxygen-dependent regulation of immune checkpoint mechanisms. Int Immunol. 2018;30:335–343. [DOI] [PubMed] [Google Scholar]
- 30.Seo JW, Tavare R, Mahakian LM, et al. CD8(+) T-cell density imaging with 64Cu-labeled Cys-diabody informs immunotherapy protocols. Clin Cancer Res. 2018;24:4976–4987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tavaré R, McCracken MN, Zettlitz KA, et al. Immuno-PET of murine T cell reconstitution postadoptive stem cell transplantation using anti-CD4 and anti-CD8 Cys-diabodies. J Nucl Med. 2015;56:1258–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pandit-Taskar N, Postow MA, Hellmann MD, et al. First-in-humans imaging with 89Zr-Df-IAB22M2C anti-CD8 minibody in patients with solid malignancies: preliminary pharmacokinetics, biodistribution, and lesion targeting. J Nucl Med. 2020;61:512–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.