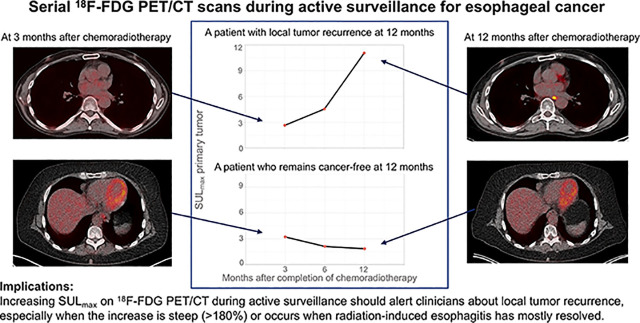
Visual Abstract
Keywords: esophageal neoplasms, neoadjuvant therapy, watchful waiting, PET, local neoplasm recurrence
Abstract
Active surveillance for patients with esophageal cancer and a clinically complete response (cCR) after neoadjuvant chemoradiotherapy (nCRT) is being studied. Active surveillance requires accurate clinical response evaluations. 18F-FDG PET/CT might be able to detect local tumor recurrence after nCRT as soon as the esophagus recovers from radiation-induced esophagitis. The aims of this study were to assess the value of serial 18F-FDG PET/CT scans for detecting local recurrence in patients beyond 3 mo after nCRT and to determine when radiation-induced esophagitis has resolved. Methods: This retrospective multicenter study included patients who had cCR after nCRT, who initially declined surgery, and who subsequently underwent active surveillance. Clinical response evaluations included 18F-FDG PET/CT, endoscopic biopsies, and endoscopic ultrasound with fine-needle aspiration at regular intervals. SUVmax normalized for lean body mass (SULmax) was measured at the primary tumor site. The percentage change in SULmax (Δ%SULmax) between the last follow-up scan and the scan at 3 mo after nCRT was calculated. Tumor recurrence was defined as biopsy-proven vital tumor at the initial tumor site. Results: Of 41 eligible patients, 24 patients had recurrent disease at a median of 6.5 mo after nCRT and 17 patients remained cancer free during a median follow-up of 24 mo after nCRT. Five of 24 patients with tumor recurrence had sudden intense SULmax increases of greater than 180%. In 19 of 24 patients with tumor recurrence, SULmax gradually increased (median Δ%SULmax, +18%), whereas SULmax decreased (median Δ%SULmax, −12%) in patients with ongoing cCR (P < 0.001, independent-samples t test). In patients with ongoing cCR, SULmax was lowest at 11 mo after nCRT. Conclusion: Serial 18F-FDG PET/CT might be a useful tool for detecting tumor recurrence during active surveillance. In patients with ongoing cCR, the lowest SULmax was reached at 11 mo after nCRT, suggesting that radiation-induced esophagitis had mostly resolved by that time. These findings warrant further evaluation in a larger cohort.
Neoadjuvant chemoradiotherapy (nCRT) followed by esophagectomy is emerging as a standard treatment for locally advanced esophageal cancer. This approach is largely based on the results of the ChemoRadiotherapy for Oesophageal cancer followed by Surgery Study, which showed improved survival with multimodality treatment compared with surgery alone (1,2). In that trial, the surgical resection specimens from 29% of patients treated with nCRT showed no evidence of residual tumor (1). These patients may not have benefited from surgery, since surgery is tied to an increased risk of mortality, postoperative morbidity, and decreased quality of life (1,3,4). For this reason, the feasibility and efficacy of active surveillance for patients with a clinically complete response (cCR) to nCRT are being investigated (5). Active surveillance implies that surgery is offered only when locoregional tumor is detected in the absence of distant metastases. Clinical response evaluations (CREs) are needed to select patients who can safely undergo active surveillance and to monitor disease recurrence. The optimal set of diagnostics was investigated previously and comprises endoscopy with bite-on-bite biopsies, endoscopic ultrasound with fine-needle aspiration of suspect lymph nodes, and 18F-FDG PET/CT (6).
Detection of local residual tumor by qualitative and quantitative assessments of a single 18F-FDG PET/CT scan at 3 mo after nCRT alone is inaccurate because of persistent 18F-FDG uptake probably due to postradiation esophagitis (7). Thus, after nCRT, 18F-FDG PET/CT is primarily being performed to detect regional lymph node metastases and hematogenous metastases (6). In the context of an active surveillance strategy, however, the efficacy of 18F-FDG PET/CT for the detection of local tumor recurrence is unclear.
We hypothesize that the inflammatory response in the esophagus will diminish beyond 3 mo after nCRT as the esophagus continues to recover from radiotherapy (7). Accordingly, increasing 18F-FDG uptake over time could well be a sensitive parameter for detecting local residual tumor regrowth during active surveillance. SUVmax corrected for lean body mass (SULmax)—a quantification of 18F-FDG uptake—might serve as an imaging biomarker for monitoring disease recurrence from the lowest value observed, which is defined as the so-called “nadir” (8).
The primary aim of this retrospective study was to assess the value of serial 18F-FDG PET/CT scans for identifying local tumor recurrence in patients undergoing active surveillance beyond 3 mo after nCRT. The secondary aim was to determine the lowest value of SULmax (nadir-SULmax) during follow-up of patients with ongoing cCR as an indicator of the time point at which radiation-induced esophagitis has mostly resolved.
MATERIALS AND METHODS
Study Design
The present study is a retrospective observational cohort study using data obtained from the prospective diagnostic pre-Surgery As Needed in Oesophageal cancer (preSANO) trial (www.trialregister.nl identifier: NTR4834), a local prospectively maintained database, and the surgery arm of the ongoing therapeutic Surgery As Needed in Oesophageal cancer (SANO) trial (www.trialregister.nl identifier: NTR6803) (5,6,9). The multicenter preSANO trial assessed the accuracy of a set of diagnostic modalities for detecting substantial residual tumor (>10% residual tumor). The multicenter SANO trial was initiated to assess the effectiveness and cost-effectiveness of active surveillance compared with immediate surgery. All patients included in the present study underwent nCRT with the intention to undergo immediate surgery after nCRT. The data in the present study were obtained from 3 Dutch hospitals: the Erasmus University Medical Center, the Zuyderland Medical Center, and the Catharina Hospital Eindhoven. The trials were approved by the medical–ethical committee of the Erasmus University Medical Center (MEC-2013-211 and MEC-2017-392). All patients provided informed consent.
Patients
Patients had been diagnosed with potentially curable esophageal cancer and received neoadjuvant treatment consisting of 5 weekly cycles of carboplatin (area under the curve, 2 mg/mL/min) and paclitaxel (50 mg/m2) on day 1 in combination with a total radiotherapy dose of 41.4 Gy delivered in 23 daily fractions of 1.8 Gy 5 d/wk. At 1.5 mo after nCRT, the first CRE (CRE-1) was performed with endoscopy and biopsies of the primary tumor site. If no histologic evidence of vital tumor was detected, then a second CRE (CRE-2) was performed at 3 mo after nCRT. To exclude disseminated disease before the scheduled surgery, at CRE-2, an 18F-FDG PET/CT scan also was performed. Moreover, patients underwent endoscopy with biopsies and endoscopic ultrasound with fine-needle aspiration of suspect lymph nodes.
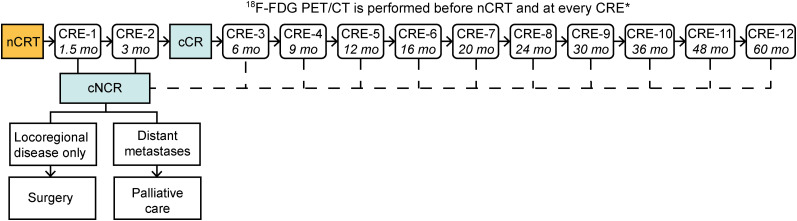
Patients were eligible for this study if they had cCR without signs of distant metastases at CRE-2 but had declined surgery for various reasons or had become unfit for surgery because of a deteriorating physical condition. cCR at CRE-2 was defined as the absence of residual tumor on biopsies and negative fine-needle aspiration of suspect lymph nodes. Instead of surgery, patients were offered an active surveillance protocol with frequent CREs, similar to the active surveillance arm of the SANO trial (5). After CRE-2, additional CREs (i.e., CRE-3, CRE-4, and so on) were scheduled every 3 mo in the first year, every 4 mo in the second year, every 6 mo in the third year, and yearly thereafter, up to a 5-y follow-up period in total (Fig. 1). If, during active surveillance, the regrowth of tumor was histologically proven or highly suspected (e.g., because of nontraversable tumor at endoscopy), then patients were referred to either immediate surgery or palliative care (Fig. 1). Patients with tumors that were not 18F-FDG avid before the start of nCRT were excluded from analysis.
FIGURE 1.
Time line of CREs during active surveillance in accordance with the SANO trial protocol. *At CRE-1, 18F-FDG PET/CT was performed in case of clinically noncomplete response (cNCR) to exclude distant metastases. mo = months after nCRT.
Definition of Tumor Recurrence
Local tumor recurrence was defined as histologically proven vital tumor located at the initial tumor site. This definition ignores the locoregional lymph node status because the present study relates changes in 18F-FDG uptake in the esophagus—at the primary tumor site—to corresponding histopathology. Histopathologic assessment was performed on tissue from biopsies or on the resection specimen. The primary tumor in the resection specimen was assessed by means of the modified tumor regression grade (TRG) system according to Chirieac et al. (10): TRG1 (0% residual carcinoma), TRG2 (1%–10% residual carcinoma), TRG3 (11%–50% residual carcinoma), and TRG4 (>50% residual carcinoma). Ongoing cCR was defined as no histologic evidence of recurrence of tumor at the initial tumor site at the time of analysis.
18F-FDG PET/CT Acquisition and Processing
18F-FDG PET/CT scans were acquired in 3 different centers that applied a scanning protocol similar to that used in the SANO trial (5). In brief, scanning was performed in accordance with European Association Research Limited qualifications for qualitative SUV measurements (11). The 18F-FDG PET/CT acquisition was started 60 ± 5 min after the injection of 18F-FDG at 2.3 MBq/kg. All follow-up scans were performed on the same scanners under the same conditions.
18F-FDG PET/CT Analysis
On every follow-up 18F-FDG PET/CT scan, regions of interest were manually drawn over the primary tumor site determined from the baseline 18F-FDG PET/CT scan (OsiriX MD v.7.5; Pixmeo SARL). The placement of regions of interest was independently reviewed by an experienced nuclear medicine physician. If this investigator disagreed with the placement of the region of interest by the first investigator, then a consensus between the 2 investigators was established. Regions of interest were also placed at the normal esophagus, blood pool, and liver to obtain internal reference measurements. At the regions of interest, SULmax was measured. Lean body mass was calculated in accordance with the James equation (11).
The absolute and percentage change in SULmax during active surveillance (ΔSULmax × and Δ%SULmax) was calculated from the SULmax of the scan at 3 mo after nCRT and the last follow-up scan in active surveillance. In patients who developed local tumor recurrence, the last follow-up scan corresponded to the moment when local recurrence was histologically proven. In patients with ongoing cCR, the last follow-up scan corresponded to the most recent scan performed during active surveillance at the moment of analysis. If active surveillance had been stopped in patients with ongoing cCR at the primary tumor site because of distant or lymph node metastases, then the last follow-up scan corresponded to the moment of the last histopathologic evaluation of the initial tumor with biopsies.
In patients with ongoing cCR, the nadir-SULmax was determined (8). This nadir-SULmax served to determine the moment when 18F-FDG uptake caused by radiation-induced esophagitis was supposed to have normalized.
Statistical Analysis
Continuous data are presented as the median and interquartile range (IQR). ΔSULmax and Δ%SULmax were analyzed between groups using the parametric independent-samples t test for normally distributed data or the nonparametric Mann–Whitney U test for nonnormally distributed data. Extreme outliers of Δ%SULmax were identified by data visualization with box plots and are described separately. The extreme outliers were removed from the statistical tests for comparison of means and medians because we expected that these outliers would distort the assessment of clinically relevant subtle differences in ΔSULmax between patients with and patients without local tumor recurrence. To indicate the precision of results, 95% CIs were used. A 2-sided P value of less than 0.05 was considered statistically significant. Because this study was exploratory, sample size calculation was not performed. Statistical analysis was performed using R-3.6.1 for MacOS (The R Project for Statistical Computing).
RESULTS
Study Group
Between March 2013 and July 2019, 43 patients who had 18F-FDG–avid tumors and cCR at CRE-2, who declined planned surgery, and who underwent active surveillance off-protocol were identified from the prospective database of 278 patients (15%) who underwent nCRT with the intention to undergo immediate surgery thereafter. Baseline characteristics are shown in Table 1. The 18F-FDG PET/CT scan at CRE-2 was performed at a median of 11.6 wk (IQR, 10.4 to 12.3) after the completion of nCRT.
TABLE 1.
Baseline Patient (n = 43) and Tumor Characteristics
| Variable | Data* |
| Male | 33 (77) |
| Median age in years† | 70 (62–74) |
| Histology | |
| Squamous cell carcinoma | 11 (26) |
| Adenocarcinoma | 31 (72) |
| Adenosquamous carcinoma | 1 (2) |
| cT‡ | |
| cT1 | 0 (0) |
| cT2 | 11 (26) |
| cT3 | 28 (65) |
| cT4 | 1 (2) |
| cTx | 1 (2) |
| Missing | 2 (5) |
| cN‡ | |
| cN0 | 17 (40) |
| cN1 | 11 (26) |
| cN2 | 12 (28) |
| cNx | 1 (2) |
| Missing | 2 (5) |
| Differentiation grade | |
| Good to moderate | 16 (37) |
| Poor | 10 (23) |
| Missing | 17 (40) |
Data are reported as number of patients, with percentage of patients in parentheses, unless otherwise indicated.
Values in parentheses are interquartile range.
Clinical tumor staging was in accordance with seventh edition of International Union Against Cancer TNM classification (23).
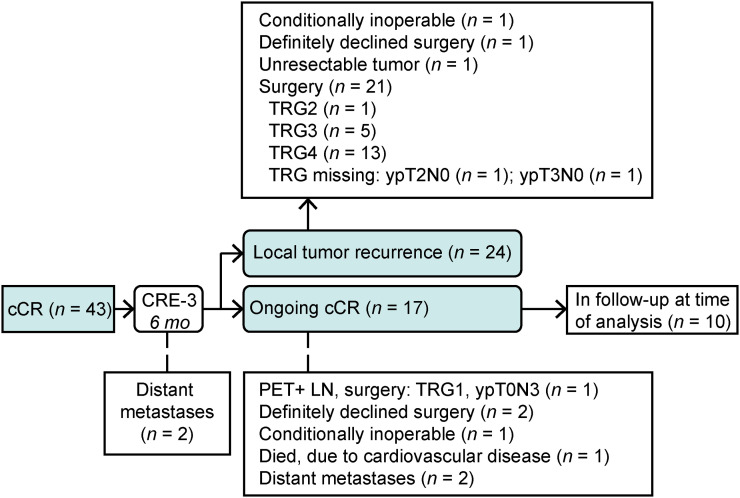
The flowchart of the study is shown in Figure 2. Two of the 43 patients had clinically manifested distant metastases at 3 mo after nCRT and did not undergo further analysis of the primary tumor with endoscopy and biopsies. Since the histologic status of the primary tumor was therefore unknown, these patients were excluded from further analysis. Thus, data for 41 patients were eligible for analysis of serial 18F-FDG PET/CT scans during active surveillance.
FIGURE 2.
Flowchart of study patients with cCR at 3 mo after nCRT. mo = months after nCRT; PET+ LN = positive lymph nodes detected with 18F-FDG PET/CT.
At a median follow-up of 6.5 mo after the completion of nCRT (IQR, 5.9 to 11), the primary tumor had recurred in 24 of 41 patients (59%). In most cases, local tumor recurrence was seen at CRE-3 (15/24; 63%). Esophagectomy was performed for 21 of 24 patients; 20 of them had biopsy-proven local tumor recurrence, and 1 had nontraversable tumor at endoscopy with TRG4 in the resection specimen. Three of 24 patients did not undergo esophagectomy for the following reasons: unfit for surgery, definitely declined surgery, and unresectable tumor (Fig. 2).
During a median follow-up of 24 mo after nCRT (IQR, 12 to 25), no biopsy-proven recurrence of the primary tumor was found in 17 of 41 patients (41%) (i.e., ongoing cCR). Ten of these 17 patients were in active surveillance at the time of analysis. Active surveillance had been ended for 7 of the 17 patients with cCR at the time of analysis; of these 7 patients, 1 patient underwent esophagectomy because of a solitary lymph node recurrence without biopsy-proven tumor at the primary tumor site (ypT0N3; TRG1), 2 patients definitely declined surgery after CRE-3, 1 patient was conditionally inoperable at CRE-3, 1 patient died because of cardiovascular disease, and 2 patients had distant metastases after CRE-3 and CRE-4 (Fig. 2).
For all patients with either local tumor recurrence or ongoing cCR, the individual courses of SULmax at the primary tumor site and the SUL at the reference regions are shown in Supplemental Tables 1 and 2 (supplemental materials are available at http://jnm.snmjournals.org).
SULmax in Patients with Local Tumor Recurrence
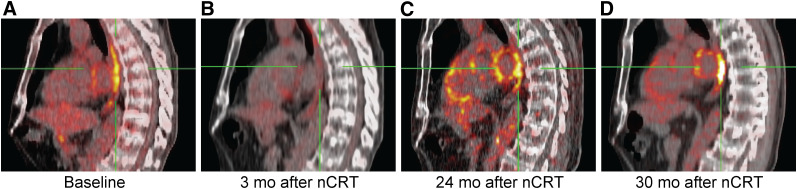
Two different patterns of 18F-FDG uptake indicative of local recurrence were observed. Five of 24 patients had sudden intense increases in SULmax, all greater than 180% (i.e., extreme outliers). In these patients, the median Δ%SULmax was +283% (IQR, 262 to 316) and the absolute ΔSULmax was +6.1 (IQR, 5.6 to 8.3). These increases took place at the following times after nCRT: between 3 and 6 mo (n = 2); between 6 and 9 mo (n = 1); between 12 and 16 mo (n = 1); and between 24 and 30 mo, after a first increase between 20 and 24 mo (n = 1) (Fig. 3).
FIGURE 3.
Sagittal view of patient who developed local tumor recurrence during active surveillance. (A) Baseline scan. (B) Normalized 18F-FDG uptake in esophagus at 3 mo after nCRT. (C) From 20 to 24 mo after nCRT, SULmax increased 20% without histologic evidence for recurrence of tumor. (D) From 24 to 30 mo after nCRT, SULmax increased 51% and local tumor recurrence was diagnosed by biopsies. Esophagectomy was performed at 30 mo after nCRT (TRG3; ypT1bN0).
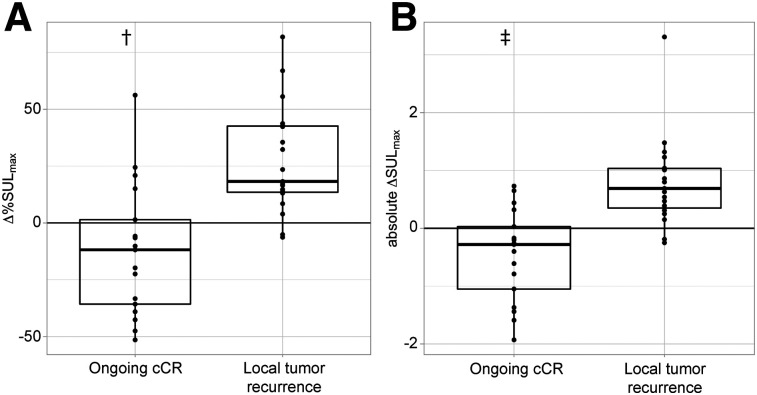
In the remaining 19 of 24 patients with local tumor recurrence, a gradual increase in the median Δ%SULmax of +18% (IQR, 14 to 43) was seen. In contrast, the median Δ%SULmax was −12% (IQR, −36 to 1.4) in the 17 patients with ongoing cCR. The mean difference in the Δ%SULmax between these groups was statistically significant (P < 0.001; 95% CI, 21%–58%; independent-samples t test) (Fig. 4). In patients with local tumor recurrence, the median absolute ΔSULmax was +0.69 (IQR, 0.35 to 1.0); in patients with ongoing cCR, this value was −0.28 (IQR, −1.1 to 0.30; P < 0.001; 95% CI, 0.65–1.69; Mann–Whitney U test) (Fig. 4).
FIGURE 4.
Box plots of Δ%SULmax (A) and absolute ΔSULmax (B) of primary tumor site in patients with ongoing cCR vs. patients who developed local tumor recurrence. Five outliers with extremely high Δ%SULmax of >180% are not shown and are described separately in text. †P < 0.001 (independent-samples t test). ‡P < 0.001 (Mann–Whitney U test).
Patients’ tumor characteristics, separated by the different 18F-FDG uptake patterns, are shown in Supplemental Table 3.
SULmax in Patients with Ongoing cCR
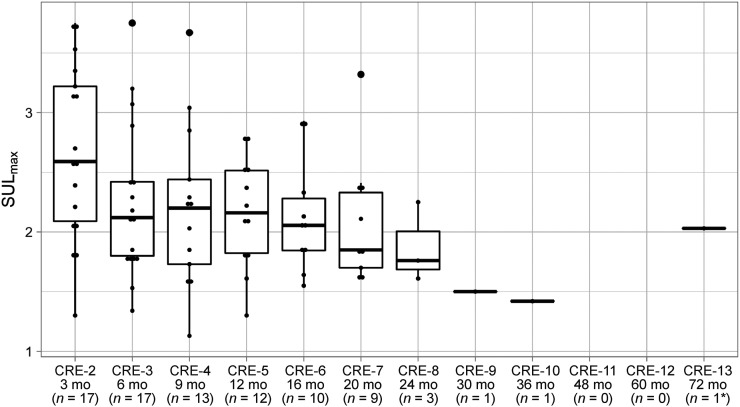
In patients with ongoing cCR, the nadir-SULmax was found at a median time of 11 mo (IQR, 5.9 to 18) after nCRT. The median nadir-SULmax was 1.80 (IQR, 1.4 to 2.1). At CRE-2, the median SULmax was 2.6 (IQR, 2.1 to 3.2); the values at CRE-3, CRE-4, and CRE-5 were 2.1 (IQR, 1.8 to 2.4), 2.2 (IQR, 1.7 to 2.4), and 2.2 (IQR, 1.8 to 2.5), respectively (Fig. 5).
FIGURE 5.
Box plots representing median and interquartile range of SULmax in patients with ongoing cCR. *This patient had no scans performed between CRE-6 and CRE-13. mo = months after nCRT.
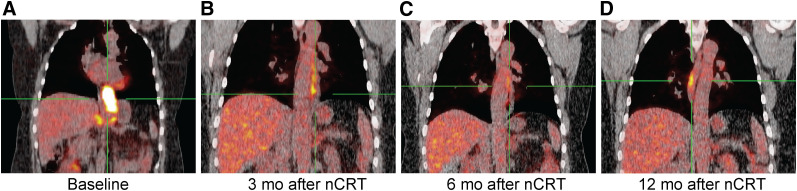
Figure 6 shows 18F-FDG PET/CT scans of a patient with ongoing cCR of the distal esophagus, illustrating a pattern of SULmax increase at a location different from the location of the primary tumor. Approximately 1 year after nCRT, linear 18F-FDG uptake developed cranially to the initial tumor site, and the cause was unknown. At the primary tumor site in the distal esophagus, SULmax remained comparable to the background 18F-FDG activity level. No histologically proven recurrence of tumor was found during all CREs.
FIGURE 6.
Coronal view of patient with ongoing cCR. (A) Baseline scan. (B and C) Normalization of 18F-FDG uptake in esophagus until 6 mo after nCRT. (D) Development of linear 18F-FDG uptake at 12 mo after nCRT cranially to initial tumor site and of unknown cause. No histologically proven recurrence of tumor was found during all CREs.
DISCUSSION
The present study identified 2 patterns of SULmax increases (Δ%SULmax) in patients with local tumor regrowth beyond 3 mo after nCRT. Some patients showed a pattern of a sudden increase in 18F-FDG metabolism (Δ%SULmax of >180%), which was indicative of residual disease in all. Most patients with local tumor regrowth, however, had an insidious gradual increase in Δ%SULmax. In contrast, patients with ongoing cCR had stable or decreasing Δ%SULmax. These findings suggest that 18F-FDG PET/CT can be used during active surveillance after nCRT, not only to detect distant metastases or to guide endoscopic ultrasound with fine-needle aspiration of suspect lymph nodes but also to monitor local tumor recurrence. These findings apply to patients with cCR who, like in the present cohort, choose to refrain from surgery after nCRT. They would also become relevant for patients who will undergo active surveillance if that strategy becomes a standard alternative treatment to immediate surgery in patients with cCR (5,9,12–14). This policy is currently being investigated in the ongoing therapeutic Dutch SANO trial and the French ESOSTRATE trial (5,15).
To our knowledge, this is the first study that describes repeated 18F-FDG PET/CT scans in an active surveillance setting for esophageal cancer patients with cCR. For rectal carcinoma, serial 18F-FDG PET/CT scans were used in a watch-and-wait protocol for patients with cCR after nCRT (16). In that study, complete responses on 18F-FDG PET/CT corresponded to negative clinical and endoscopic examinations. Moreover, for squamous cell head and neck cancer, surveillance with 18F-FDG PET/CT was shown to be cost-effective for guiding the decision to perform surgery after nCRT (17).
Response assessment with a single 18F-FDG PET/CT scan at 3 mo after the completion of nCRT is not accurate, partly because of persisting postradiation inflammation (7). In the present study, 18F-FDG uptake decreased at 3 mo after nCRT and further normalized at 6 mo after nCRT and onward; this finding was supported by a median nadir-SULmax of 1.80 (IQR, 1.4 to 2.1) at 11 mo (IQR, 5.9 to 18) after nCRT. These findings indicate an ongoing recovery from esophagitis beyond 3 mo after nCRT, presumably reaching stability within 1 year.
Increased 18F-FDG uptake after the completion of nCRT, as shown in Figure 6, should be interpreted carefully with respect to its distribution and location. A linear pattern of 18F-FDG uptake located outside the initial tumor site suggests benign inflammatory conditions, such as Candida esophagitis or gastroesophageal reflux disease, whereas focal 18F-FDG uptake at the initial tumor site suggests recurrent tumor (18).
A major strength of the present study is that 18F-FDG PET/CT data were prospectively and systematically obtained. This approach allowed comparison of serial SULmax measurements with histologic biopsies at all CREs. Nevertheless, there were several limitations. First, the cohort size was too small to define a cutoff value for ΔSULmax that reliably discriminates between a clinically manifested recurrence and ongoing cCR. Hypothetically, a cutoff value for ΔSULmax could be formulated in a manner similar to the definition of biochemical failure in prostate cancer (i.e., a certain increase higher than the nadir prostate-specific antigen value) (8). Such a cutoff value incorporates the information about the course of SULmax over time, rather than 1 moment in time. Second, the nadir-SULmax for defining the moment at which radiation-induced esophagitis has resolved may change when a larger number of patients is analyzed (i.e., larger than the number analyzed in the present study). Third, regions of interest were manually placed on the initial tumor site. An automatic registration of regions of interest in multiple scans might improve the robustness of serial SULmax measurements. Fourth, the cohort of patients included in the present study might be a highly selected group, imposing selection bias on the results (e.g., the median age of 70 y in our cohort as opposed to the median age of 66 y in patients in the preSANO trial, although the other baseline characteristics were relatively similar) (6). Fifth, to optimize the accuracy of serial 18F-FDG PET/CT scans for detection of tumor recurrence after nCRT, adherence to scanning protocols should even become more strict. Fluctuations of SULmax in patients with ongoing cCR (Supplemental Table 2) may be partially attributed to variations in scanning parameters apart from physiologic causes. Performing scanning under exactly the same circumstances every time might further improve the signal-to-noise ratio.
The results of the present study have potential implications for clinical decision making. As shown in Figure 3, an increase in SULmax at the initial tumor site after a relatively stable signal at more than 2 y in active surveillance might be more suggestive of residual tumor than of physiologic fluctuations or other benign causes, such as reflux esophagitis. If such a deviation takes place without confirmation by biopsy-proven recurrence, then shortening of the interval to the next CRE should be considered. Alternatively, one could even decide to proceed to surgery without further delay.
Before such clinical implications can be accepted, the results require validation with a larger group of patients randomly allocated to active surveillance, such as the experimental active surveillance arm of the ongoing SANO trial (5). Furthermore, new techniques for response assessment should be explored as well. Integrated PET/MRI seems promising because it could provide additional anatomic and functional value over PET/CT (19,20). However, visualization of the esophagus with PET/MRI is still challenging because of the cardiorespiratory motion in the mediastinum (21). Additionally, complex imaging features could be explored by radiomics. For instance, radiomic features are able to describe shape characteristics or heterogeneity of a tumor (22). Theoretically, changes in radiomic features may reveal early tissue changes within an active surveillance setting.
CONCLUSION
The results of this explorative study showed that serial 18F-FDG PET/CT scans might be useful for distinguishing recurrence of tumor from physiologic SULmax fluctuations in complete responders during active surveillance. A steep increase in 18F-FDG activity over a short period of time should be a warning sign for recurrent local tumor. Furthermore, a gradual increase in 18F-FDG activity over time should also alert clinicians about the recurrence of tumor. Radiotherapy-induced esophagitis will usually have resolved at 11 mo after the completion of chemoradiotherapy.
DISCLOSURE
The preSANO trial was funded by the Dutch Cancer Foundation (project no. EMCR-2014-7430). The SANO trial is currently funded by ZonMW (project no. 843004104) and the Dutch Cancer Foundation (project no. 10825). No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: Is serial 18F-FDG PET/CT a valuable tool for monitoring local esophageal cancer recurrence in patients who undergo active surveillance after nCRT?
PERTINENT FINDINGS: In this retrospective cohort study, some 19 of 24 patients who developed biopsy-proven tumor during active surveillance had a gradual increase in SULmax at the primary tumor site compared to the start of active surveillance, whereas 5 of 24 patients had sudden SULmax increases of greater than 180%. In contrast, SULmax decreased in 17 patients without local tumor recurrence; in these patients, the lowest SULmax was observed at 11 mo after nCRT.
IMPLICATIONS FOR PATIENT CARE: Increasing SULmax on 18F-FDG PET/CT during active surveillance should alert clinicians about local tumor recurrence, especially when the increase is steep (>180%) or occurs when radiation-induced esophagitis has mostly resolved.
Supplementary Material
REFERENCES
- 1.van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366:2074–2084. [DOI] [PubMed] [Google Scholar]
- 2.Shapiro J, van Lanschot JJB, Hulshof M, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol. 2015;16:1090–1098. [DOI] [PubMed] [Google Scholar]
- 3.O’Connell L, Coleman M, Kharyntiuk N, Walsh TN. Quality of life in patients with upper GI malignancies managed by a strategy of chemoradiotherapy alone versus surgery. Surg Oncol. 2019;30:33–39. [DOI] [PubMed] [Google Scholar]
- 4.Noordman BJ, Verdam MGE, Lagarde SM, et al. Impact of neoadjuvant chemoradiotherapy on health-related quality of life in long-term survivors of esophageal or junctional cancer: results from the randomized CROSS trial. Ann Oncol. 2018;29:445–451. [DOI] [PubMed] [Google Scholar]
- 5.Noordman BJ, Wijnhoven BPL, Lagarde SM, et al. Neoadjuvant chemoradiotherapy plus surgery versus active surveillance for oesophageal cancer: a stepped-wedge cluster randomised trial. BMC Cancer. 2018;18:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Noordman BJ, Spaander MCW, Valkema R, et al. Detection of residual disease after neoadjuvant chemoradiotherapy for oesophageal cancer (preSANO): a prospective multicentre, diagnostic cohort study. Lancet Oncol. 2018;19:965–974. [DOI] [PubMed] [Google Scholar]
- 7.Valkema MJ, Noordman BJ, Wijnhoven BPL, et al. Accuracy of 18F-FDG PET/CT in predicting residual disease after neoadjuvant chemoradiotherapy for esophageal cancer. J Nucl Med. 2019;60:1553–1559. [DOI] [PubMed] [Google Scholar]
- 8.Cornford P, Bellmunt J, Bolla M, et al. EAU-ESTRO-SIOG guidelines on prostate cancer. Part II: treatment of relapsing, metastatic, and castration-resistant prostate cancer. Eur Urol. 2017;71:630–642. [DOI] [PubMed] [Google Scholar]
- 9.van der Wilk BJ, Noordman BJ, Neijenhuis LKA, et al. Active surveillance versus immediate surgery in clinically complete responders after neoadjuvant chemoradiotherapy for esophageal cancer: a multicenter propensity matched study. Ann Surg. October 4, 2019 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 10.Chirieac LR, Swisher SG, Ajani JA, et al. Posttherapy pathologic stage predicts survival in patients with esophageal carcinoma receiving preoperative chemoradiation. Cancer. 2005;103:1347–1355. [DOI] [PubMed] [Google Scholar]
- 11.Aide N, Lasnon C, Veit-Haibach P, Sera T, Sattler B, Boellaard R. EANM/EARL harmonization strategies in PET quantification: from daily practice to multicentre oncological studies. Eur J Nucl Med Mol Imaging. 2017;44:17–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Castoro C, Scarpa M, Cagol M, et al. Complete clinical response after neoadjuvant chemoradiotherapy for squamous cell cancer of the thoracic oesophagus: is surgery always necessary? J Gastrointest Surg. 2013;17:1375–1381. [DOI] [PubMed] [Google Scholar]
- 13.Taketa T, Correa AM, Suzuki A, et al. Outcome of trimodality-eligible esophagogastric cancer patients who declined surgery after preoperative chemoradiation. Oncology. 2012;83:300–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taketa T, Xiao L, Sudo K, et al. Propensity-based matching between esophagogastric cancer patients who had surgery and who declined surgery after preoperative chemoradiation. Oncology. 2013;85:95–99. [DOI] [PubMed] [Google Scholar]
- 15.clinicaltrials.gov. Comparison of systematic surgery versus surveillance and rescue surgery in operable oesophageal cancer with a complete clinical response to radiochemotherapy (Esostrate). clinicaltrials.gov website. https://clinicaltrials.gov/ct2/show/NCT02551458. Accessed March 1, 2021.
- 16.Perez RO, Habr-Gama A, Gama-Rodrigues J, et al. Accuracy of positron emission tomography/computed tomography and clinical assessment in the detection of complete rectal tumor regression after neoadjuvant chemoradiation: long-term results of a prospective trial (National Clinical Trial 00254683). Cancer. 2012;118:3501–3511. [DOI] [PubMed] [Google Scholar]
- 17.Mehanna H, Wong WL, McConkey CC, et al. PET-CT surveillance versus neck dissection in advanced head and neck cancer. N Engl J Med. 2016;374:1444–1454. [DOI] [PubMed] [Google Scholar]
- 18.Bural GG, Kumar R, Mavi A, Alavi A. Reflux esophagitis secondary to chemotherapy detected by serial FDG-PET. Clin Nucl Med. 2005;30:182–183. [DOI] [PubMed] [Google Scholar]
- 19.Fang P, Musall BC, Son JB, et al. Multimodal imaging of pathologic response to chemoradiation in esophageal cancer. Int J Radiat Oncol Biol Phys. 2018;102:996–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heethuis SE, Goense L, van Rossum PSN, et al. DW-MRI and DCE-MRI are of complementary value in predicting pathologic response to neoadjuvant chemoradiotherapy for esophageal cancer. Acta Oncol. 2018;57:1201–1208. [DOI] [PubMed] [Google Scholar]
- 21.Peerlings J, Paulis L, Mitea C, et al. Performing clinical 18F-FDG-PET/MRI of the mediastinum optimising a dedicated, patient-friendly protocol. Nucl Med Commun. 2019;40:815–826. [DOI] [PubMed] [Google Scholar]
- 22.Lambin P, Leijenaar RTH, Deist TM, et al. Radiomics: the bridge between medical imaging and personalized medicine. Nat Rev Clin Oncol. 2017;14:749–762. [DOI] [PubMed] [Google Scholar]
- 23.International Union Against Cancer (UICC) . TNM Classification of Malignant Tumours. 7th ed. Sobin LH, Gospodarowicz MK, Wittekind Ch, eds. Wiley & Sons; 2011. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.