Abstract
On March 2020, hydroxychloroquine (HCQ) was recommended as a treatment for COVID-19 high risk patients. Following the massive and widespread use of HCQ worldwide, a discernible high quantity is anticipated to end-up through the sewage systems in marine coastal areas. A closed microcosm study was undertaken herein for 30 days where meiobenthic nematodes were exposed to a range of HCQ concentrations (3.162, 31.62 and 63.24 μg.ml−1). After one month of exposure in HCQ, the total abundances and Shannon-Wiener index of the assemblages decreased, whereas the individual mass and the Trophic Diversity Index increased at the highest concentrations. Overall, a numerical negative impact was observed for the epistrate feeders and non-selective deposit feeders, however, this benefited to the omnivores-carnivores, and particularly to the Oncholaimids. Such responses of the nematodes 2B and the corresponding taxa are bioindicative of current- or post-COVID-19 crisis risks in relation with the bioaccumulation of HCQ in seafood.
Keywords: COVID-19 crisis, Hydroxychloroquine, Ecotoxicity, Free-living marine nematodes, Seafood
1. Introduction
Coronavirus Infectious Disease 2019 (COVID-19) is caused by the Severe Acute Respiratory Syndrome COronaVirus strain 2 (SARS-CoV-2) (Whitworth, 2020). It was first recorded in Wuhan, China, in December 2019 and since then; the disease became universal and led to a pandemic, with severe efects on global health and economy. As of March 29, 2021, 127,258,173 cases and 2,785,286 deaths are documented globally (https://gisanddata.maps.arcgis.com/apps/opsdashboard/index.html?fbclid=IwAR0-JhqnSy-s-gfnCAw0hvBFOqSEirVVdeDW38bCQWEx7ovbITkYmdDaReo#/bda7594740fd40299423467b48e9ecf6), and the records of diagnosed cases with COVID-19 worldwide are expected to increase. It should be also noted that two thirds of these cases are being recorded in only 10 countries, five European ones (France, Russia, Spain, UK and Italy), four in America (USA, Brazil, Argentina and Colombia), and one (India) in Asia.
Hydroxychloroquine (HCQ), has been used for a long time for the therapy and control of malaria and autoimmune diseases, and was the most talked about treatment of COVID-19 since the beginning of the pandemic (Risch, 2020). In September 2020, Dr. Harvey A. Risch, of Yale University, published a scientific meta-analysis on the efficacy of hydroxychloroquine (Ladapo et al., 2020). In this study, randomized studies involving in toto 5 clinical trials with a sample of 5577 patients between the United States, Canada and Spain were analysed. Through the analyses, the researchers concluded that early use of the medicine by non-hospitalized individuals for prophylaxis or early treatment of COVID-19 produced a statistically significant 24% reduction in the infectious compound, hospitalization or death. Treatment is more effective if the progression of the disease is just beginning.
The U.S. Food and Drug Administration (FDA) issued on March 28th, 2020, an Emergency Use Authorization (EUA) to allow hydroxychloroquine sulfate (HCQ) to be distributed and recommended in hospitalized high-risk outpatients. The effectiveness of HCQ against COVID-19 thus, more or less, was accepted worldwide and was used as a promising treatment in numerous countries (https://www.mediterranee-infection.com/coronavirus-pays-ou-lhydroxychloroquine-est-recommandee/). As other pharmaceutical substanses consumed by humans, HCQ would finally reach the marine coastal environment through sewage systems, and would increase suddenly. This abrupt increase poses serious questions, inquiries or concerns related with short- and long-term ecotoxic effects on aquatic organisms and the enviroment.
To explore short-term ecotoxicity of HCQ during and after the current COVID-19 pandemic, four main criteria should be taken into consideration: (1) results must be statistically reliable and deriving from quick bioassays due to the widespread and excessive use of the drug within a short-term and the extremely high need of data during such global health crises; (2) experiments should be based on small-sized taxa, and a significant position in the ‘small food web’ (protists, bacteria, and meiofauna) so as to provide early detections of ecotoxic effects of the drug use; (3) organisms with simple and short life cycles, sedentary lifestyle and without planktonic larvae stages that would depict clearly the actual intensity of drud-related pollution; and (4) models applicable to a wider georgraphical spectrum in order to apply the methodology and results worldwide.
Among marine taxa, all the above-mentioned criteria are met in meiobenthic nematodes (Semprucci et al., 2018). Indeed, these small invertebrates (1–5 mm in average length) are the most abundant and diverse component of the marine benthos what makes data statistically reliable (Moens and Vincx, 1997). Morover, abundances of up to several million individuals per square meter (Moens and Vincx, 1997) can be reached and descriptions of more than 7000 species are available in Nemys database (Bezerra et al., 2020). Nematode assemblages are ideal for experimental studies as these animals spend their short life cycle of days to weeks (egg to egg-laying parent) in the sediment and are easy cultured in controlled laboratory conditions (Suderman and Thistle, 2003). Numerous studies have also supported the efficacy of the use of marine nematodes as bioindicators due to their susceptibility to several toxic compounds (e.g. Hedfi et al., 2007; Beyrem et al., 2010; Hedfi et al., 2013; Boufahja and Semprucci, 2015; Lacoste et al., 2020; Wakkaf et al., 2020). Their use also reduces the size of the enclosures without these tiny animals losing their ability to respond very quickly to environmental disturbances. Indeed, meiobenthic taxa are known to be cosmopolitan due to their passive and active dispersal, which is commonly recognised as the “meiofauna paradox” (Boeckner et al., 2009).
The results obtained for free-living marine nematodes are of extreme importance for the human health (Beyrem et al., 2011; Boufahja et al., 2011). They make up a considerable portion of the food source of many macrobenthic organisms, that serve as seafood, like crabs (Fitzhugh and Fleeger, 1985), fishes (Albertini-Berhaut, 1974) and shrimps (Wilcox and Jeffries, 1979; Allouche et al., 2021). Consequently, nematodes as bioindicators would provide the scientific community an effective and timely indicator of the probable risks deriving from the consumption of seafood from coastal countries where HCQ was recommended and used as treatment of COVID-19. In order to make the application of such approaches feasible, nematode species that could act as indicators, due to their high tolerance to HCQ that is found and assimilated from their surrounding environment, should be identified.
In this work, it was presented the results of a microcosm experiment conducted to assess whether the eventual massive use of HCQ as an anti-COVID-19 drug treatment will be harmful for a community of free-living nematodes from Bizerte bay, Tunisia. It should be noted here, that HCQ has not been used in the country for COVID-19 treatment and hence the communities were not previously exposed to the substance. Our investigation focussed on comparing the quantitative and qualitative data from control microcosms and those contaning a range of three different HCQ concentrations.
2. Materials and methods
2.1. Sampling
Sediment with its natural meiofauna was collected from Bizerte bay on August 6th, 2020 (7 A.M) at a pristine coastal site (37°15′07.34”N, 9°56′26.75″E). Hand-cores with a 10 cm2 section and 3.6 cm inner diameter (Coull and Chandler, 1992) were deployed at a depth of 10 cm before transferring their contents into plastic buckets. The sampling was restricted to 10 cm in the top of sediment because, in most coastal areas, more than 90% of the meiofauna are found in the surface 1–2 cm (Coull and Chandler, 1992). On the sampling day, four parameters were measured in the sediment-water interface: temperature, salinity, pH and dissolved oxygen. Measurements were conducted by means of a temperature/conductivity meter (WTW LF 196, Weilheim, Germany), pH meter (WTW, model pH 330 / SET-1), and Oximeter (WTW OXI 330/SET, WTW, Weilheim, Germany).
2.2. Experiment setup
Upon return to the laboratory, the sediment was manually homogenized with a large spatula prior to the set-up of the microcosms. The sediment was first sieved using a 63 μm mesh size to determine proportions of silt/clay (< 63 μm) and coarse fractions (≥ 63 μm) (Buchanan, 1971). The mean grain size was determined after plotting cumulative curves for the sandy particles (Buchanan, 1971). Following, it was dried at 45 °C until reaching a constant mass (Fabiano and Danovaro, 1994) and the total organic matter was evaluated after heating the sediment to 450 °C for 6 h (Fabiano and Danovaro, 1994). Then several sub-samples of filtered seawater (1 μm filtration) from the sampling site were contaminated with appropriate amounts of hydroxychloroquine, so as to create three final concentrations corresponding to low (HCQ1 = 3.162 μg.ml−1), medium (HCQ2 = 31.62 μg.ml−1) and high (HCQ3 = 63.24 μg.ml−1) concentrations. These concentrations were selected based on the work of Ramesh et al. (2018) who report that the median lethal concentration of chloroquine, a close pharmaceutical to HCQ, for the freshwater fish Cyprinus carpio was 31.62 mg.ml−1 after 96 h of exposure. Indeed, in order to standardize the HCQ concentrations to be tested, prior to the initiation of the experiment, a pre-experiment was set-up and nematofauna from the same site in Bizerte bay, Tunisia (37°15′07.34”N, 9°56′26.75″E) was used. For this purpose, the 1/1000 of the median lethal concentration of chloroquine identified by Ramesh et al. (2018) was used as a reference (31.62 μg.ml−1), and apart from that one, two more concentrations were also tested: a lower one corresponding to the 1/10 of the median lethal value (i.e. test-low = 3.162 μg.ml−1), and a high tenfold concentration (i.e. test-high = 316.62 μg.ml−1). Results obtained showed the absence of nematodes in the sediment contaminated by the highest concentration (316.62 μg.ml−1). Following, the experiment was repeated by multiplying by two (i.e. 63.24 μg.ml−1) and by five (i.e. 158.1 μg.ml−1) times the 1/1000 of the median lethal value. Once again, the concentration of 158.1 μg.ml−1 resulted in an azoic sediment. Thus, for the purposes of this study, three concentrations were selected and tested: low (HCQ1 = 3.162 μg.ml−1), medium (HCQ2 = 31.62 μg.ml−1) and high (HCQ3 = 63.24 μg.ml−1).
Before the installation of the microcosms, the treated water with HCQ was stored in darkness for a week at 5 °C for stabilization (Mahmoudi et al., 2005, Mahmoudi et al., 2007; Dabic et al., 2019). The microcosms consisted of 2-l-glass bottles. All but the controls were filled, first with 300 g of natural sediment and then with 500 ml of filtered water (1 μm filtration). In control microcosms, 300 g of natural sediment was topped up by untreated water. Each microcosm works as a closed system with continuous aeration by means of an aquarium pump. The reliability of comparable experimental devices has been verified by several authors (Austen et al., 1994; Schratzberger and Warwick, 1998; Mahmoudi et al., 2005; Beyrem et al., 2010, Beyrem et al., 2011; Boufahja et al., 2015; Nasri et al., 2016, Nasri et al., 2020; Hedfi et al., 2021). In total, four types of microcosms were used; including one control (C) and three treated ones (HCQ1, HCQ2, and HCQ3). For each microcosm type, three replicates were deployed. All experiments were completed after one month in darkness under a constant temperature of 29 °C and the sediment of each microcosm was preserved in 4% formaldehyde for meiofaunal study. The above-mentioned average temperature was estimated from meteorological data published in http://www.infoclimat.fr from July 5th to August 5th, 2020 for the city of Bizerte.
2.3. Meiofauna study
Meiofauna, made up of organisms between 1 and 0.04 mm in size, was extracted with the levigation-tamisage technique (Mahmoudi et al., 2005; Lacoste et al., 2020; Allouche et al., 2021). The 1 mm mesh sieve is intended to withhold macrofauna and large sedimentary particles, and that of 40 μm to retain the meiofauna. The refusal of the 40 μm sieve, was coloured for 24 h in Rose Bengal (0.2 g.l−1; Guo et al., 2001), and then poured into a Dollfus chamber with a gridded bottom for counting and sorting of nematodes under a 50× stereomicroscope (Model WildHeerbrugg M5A). The gradual penetration of glycerin into organisms according to the technique described by Seinhorst (1959) prevents the deformation of the nematode cuticle before being mouted on microscopic slides. According to this author, nematodes were firstly treated with an aqueous solution of 1% glycerin ethanol, secondly with 5% glycerin ethanol, and finally with pure glycerin.
A Nikon DS-Fi2 camera coupled with a Nikon microscope (Image Software NIS Elements Analysis Version 4.0 Nikon 4.00.07–build 787–64 bit), allowed the morphological observations (cuticle appearance, amphide shape and position, buccal cavity type, structure of male copulatory apparatus, etc.) as well as morphometrics (total length, tail length, length of pharynx, length of spicules in males, maximum width, etc.) necessary for the taxonomic determination. The generic identification was possible through pictorial keys of Platt and Warwick, 1983, Platt and Warwick, 1988 and Warwick et al. (1998), coupled with the Nemys database (Bezerra et al., 2020).
The dimensions of nematodes were used to determine their body volume (V, in nanolilers) following the formula of Somerfield and Warwick (2013), V = 530 × L × W2, where L indicates the body length (in mm), measured excluding the flagella, W the maximum width (in mm), measured at the middle of the body for larvae and males, and at the vulva level for females. The volume of nematodes is a prerequisite for the estimation of their dry mass (in μg) after multiplying by the specific gravity of 1.13 μg.nl−1 (Wieser, 1960) and the ratio of 0.25 relating dry and wet masses (Vanaverbeke et al., 1997).
To assess the taxonomic diversity of the nematode assemblages exposed or not to HCQ, the Shannon-Wiener index was used. Four feeding categories of nematodes were distinguished based on the structure of their mouth (Wieser, 1953): (1) selective deposit-feeders (1A) with absent or virtual buccal cavity, the animal swallowing small particles by the sucking abilities of its pharynx; (2) non-selective deposit-feeders (1B) with conical/cylindrical buccal cavity without teeth; individuals can swallow particles large enough as diatoms if necessary; (3) epistrate feeders (2A) with small teeth, physiological liquid is scraped or sucked after perforation of the diatom frustules; and (4) omnivores-carnivores (2B) with a large buccal cavity, most often with large, powerful teeth. For the latter feeding group, the freshly dead preys are fully sucked or perforated to suck their inner liquid. The feeding richness was estimated through the Trophic Diversity Index (TDI) of Heip et al. (1985) and equals the sum of squares of proportions of each feeding category, its value being higher when a given diet predominates.
2.4. Data processing
Data processing followed the standard methods for community study, as described by Clarke (1993) and Clarke and Clarke and Warwick (2001). Prior to the statistical analyses, numerical series were checked for Guassian distribution (Kolmogorov-Smirnov test) and homogeneity of variance (Bartlett test). If necessary, data were log-transformed to fulfill the needed parametric requirements (Clarke, 1993; Clarke and Gorley, 2001). The one-way ANOVA was used to detect significant overall differences between treatments for all univariate indices considered. Multiple comparisons were carried out using Tukey's HSD test. Effects of HCQ on the feeding structure (i.e., proportions of buccal cavity categories) were evaluated based on separate chi-square randomization tests in the Ecosim version 7 (Gotelli and Entsminger, 2005). Significant differences were detected at p-values less than 0.05.
Multivariate analyses were performed with the software PRIMER v.5.0 (Clarke and Gorley, 2001). The ordination of species or feeding groups using the nMDS (non-parametric multi-dimensional scaling) method, carried out by considering the squareroot-transformed abundances and the Bray-Curtis similarity matrix, enable data exploration and indicate if there is a trend after exposure to HCQ. The Correspondence Analysis was performed in order to process the taxonomic and feeding data obtained and to describe efficiently the responses of nematode species and feeding groups in hydroxychloroquine-contaminated microcosms.
The ANOSIM (analysis of similarity) was performed to highlight possible significant differences between the stucture of assemblages from controls and those treated with hydroxychloroquine. The SIMPER (similarity percentage) procedure was used to determine the contribution of each species or feeding group to the average dissimilarity between treatments.
3. Results
3.1. Abiotic variables
Ιn August 6th 2020 when sampling was conducted, at site, the depth was 42 cm, wherease the salinity reached 39.6. The remaining parameters measured for the water were as follows: temperature = 28.1 °C, pH = 8.23, and dissolved oxygen = 5.8 mg.l−1. The sediment, with a mean grain size of 0.36 ± 0.02 mm and a water content of 12.4 ± 0.95%, composed mainly of coarse particles (94.42 ± 1.11%), and its proportion of total organic matter was 0.91 ± 0.02%.
3.2. Quantitative parameters
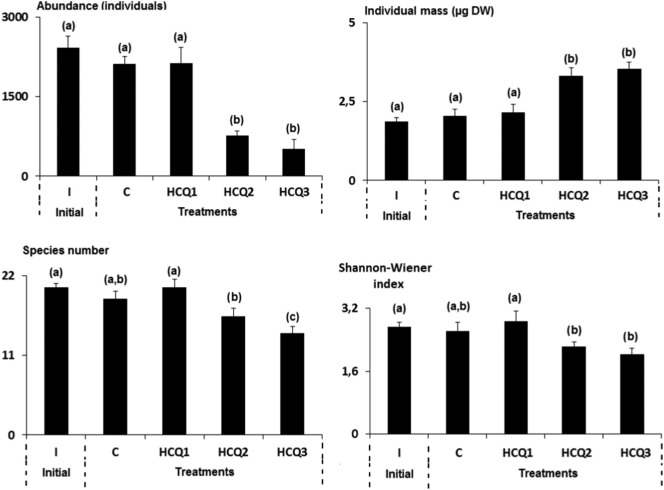
The untreated microcosms ‘C' and the initial ‘I' ones were similarly populated in nematodes (p-value = 0.070). Average nematode abundances have been reduced following the pattern of hydroxychloroquine (HCQ) sedimentary enrichment, with lowest HCQ consentrations affecting the least the animal assemblages (Fig. 1 ). It is notworthy that, the average nematode community has dropped from 2071 ± 142 individuals in untreated microcosms to 464 ± 178 individuals in replicates treated with the maximal concentration of HCQ applied (HCQ3) (Fig. 1). The results of one-way ANOVA supported the observed numerical decline after exposure to HCQ (Fig. 1). Multiple comparisons of combinations between controls and treated assemblages showed significant differences in average abundances in the case of HCQ2 and HCQ3 (Tukey's HSD test, Fig. 1). Under these latter treatments with HCQ, a significant increase in the individual body mass of nematodes was also recorded (Tukey's HSD test, Fig. 1). Finally, it should be noted that no significant difference was observed when individual biomasses of the community collected on from the field and controls were compared (Tukey's HSD test, p-value = 0.245).
Fig. 1.
Values of univariate indices (mean ± SD) for nematode assemblages from the initial assemblage (I), the control one (C), and those treated with hydroxychloroquine (HCQ1, HCQ 2 and HCQ 3). Different letters indicate significant differences (log-transformed data, Tukey's HSD test, p ≤ 0.05).
3.3. Taxonomic features and diversity
Twenty-two species of marine nematodes, belonging to 22 genera, 12 families, and 5 orders, were identified in the initial and all treated microcosms. It should be noted that, compared with each other, the initial and control nematode assemblages shared approximately the same faunistic list (Table 1 ). Twenty-two taxa were listed in the control microcosms (C), 21 under the low concentration (HCQ1), 20 in the moderately enriched one (HCQ2), and only 16 in HCQ3 (Table 1). Accordingly, Shannon-Wiener index has reached its lowest average value under the highest level of the contamination (HCQ3) (Tukey's HSD test, Fig. 1).
Table 1.
Relative abundances (± SD) of nematode taxa identified in the initial assemblage, the control one (C), and those exposed to hydroxychloroquine (HCQ1, HCQ2 and HCQ3). Sensitive and tolerant species to HCQ were marked with gray and back, respectively. Feeding groups according to Wieser Wieser (1953) (FG): selective deposit-feeders (1A); non-selective deposit-feeders (1B); epistrate-feeders (2A), omnivores-carnivores (2B). Species absent (−). Bold values indicate dominant species (≥ 10% according to Engelmann (1978)).
| Species | Order | Family | FG | I | C | HCQ1 | HCQ2 | HCQ3 |
|---|---|---|---|---|---|---|---|---|
| Halalaimus gracilis | Enoplida | Oxystominidae | 1A | 1.33 ± 0.58 | 1.67 ± 1.53 | 0.67 ± 1.15 | 1.33 ± 1.53 | 6.00 ± 2.00 |
| Bathylaimus sp. | Enoplida | Tripyloididae | 1B | 1.00 ± 1.00 | 0.67 ± 1.15 | – | – | – |
| Daptonema fallax | Monhysterida | Xyalidae | 4.00 ± 2.65 | 6.00 ± 2.65 | 4.00 ± 2.00 | 0.33 ± 0.58 | – | |
| Paramonohystera proteus | Monhysterida | Xyalidae | 14.00 ± 5.29 | 13.67 ± 2.08 | 11.00 ± 2.65 | 1.00 ± 1.73 | 1.67 ± 2.89 | |
| Sabatiera punctata | Araeolaimida | Comesomatidae | 4.00 ± 1.00 | 3.00 ± 1.00 | 1.00 ± 1.00 | 0.33 ± 0.58 | 1.33 ± 1.15 | |
| Trichotheristus mirabilis | Monhysterida | Xyalidae | 7.67 ± 2.08 | 7.67 ± 4.73 | 6.67 ± 3.06 | – | – | |
| Calomicrolaimus honestus | Desmodorida | Microlaimidae | 2A | 10.67 ± 1.15 | 12.33 ± 3.79 | 4.33 ± 2.08 | 0.67 ± 1.15 | 1.00 ± 1.73 |
| Longicyatholaimus longigicandatus | Chromadorida | Cyatholaimidae | 7.33 ± 2.52 | 7.00 ± 1.73 | 7.67 ± 2.31 | 0.67 ± 0.58 | – | |
| Microlaimus cyatholaimoïdes | Desmodorida | Microlaimidae | 5.00 ± 1.00 | 5.00 ± 2.65 | 4.67 ± 2.52 | 1.33 ± 1.53 | – | |
| Pomponema elegans | Chromadorida | Cyatholaimidae | 7.33 ± 1.15 | 8.00 ± 1.73 | 7.33 ± 2.08 | 1.00 ± 0.00 | – | |
| Prochromadorella longicaudata | Chromadorida | Chromadoridae | 9.67 ± 2.08 | 10.00 ± 4.36 | 3.00 ± 2.65 | 0.67 ± 1.15 | – | |
| Ptycholaimellus ponticus | Chromadorida | Chromadoridae | 8.67 ± 0.58 | 9.00 ± 2.65 | 5.00 ± 2.00 | 1.00 ± 1.00 | 2.00 ± 1.73 | |
| Enoploides longispiculosus | Enoplida | Thoracostomopsidae | 2B | 1.00 ± 1.00 | 3.00 ± 1.00 | 2.67 ± 1.53 | 6.33 ± 2.52 | 8.67 ± 1.53 |
| Enoplolaimus longicaudatus | Enoplida | Thoracostomopsidae | 1.33 ± 1.15 | 1.67 ± 1.15 | 6.00 ± 2.65 | 11.00 ± 2.00 | 7.00 ± 4.36 | |
| Halichoanolaimus dolichurus | Chromadorida | Selachinematidae | 2.00 ± 1.00 | 3.00 ± 1.73 | 3.33 ± 1.15 | 8.00 ± 1.00 | 6.67 ± 1.15 | |
| Mesacanthion monhystera | Enoplida | Thoracostomopsidae | 1.00 ± 1.00 | 0.33 ± 0.58 | 2.33 ± 0.58 | 7.00 ± 2.00 | 4.00 ± 1.73 | |
| Metoncholaimus pristiurus | Enoplida | Oncholaimidae | 1.33 ± 1.15 | 1.33 ± 1.53 | 4.67 ± 1.53 | 10.67 ± 2.31 | 9.67 ± 1.53 | |
| Oncholaimellus calvadosicus | Enoplida | Oncholaimidae | 2.33 ± 2.08 | 0.67 ± 1.15 | 5.00 ± 1.73 | 11.33 ± 3.51 | 10.67 ± 2.08 | |
| Oncholaimus campylocercoïdes | Enoplida | Oncholaimidae | 3.00 ± 3.61 | 1.67 ± 2.08 | 6.33 ± 4.04 | 8.33 ± 4.93 | 9.00 ± 2.65 | |
| Parasphaerolaimus paradoxus | Monhysterida | Sphaerolaimidae | 2.67 ± 1.53 | 2.00 ± 2.00 | 4.67 ± 2.08 | 10.67 ± 3.06 | 10.00 ± 5.57 | |
| Synonchiella edax | Chromadorida | Selachinematidae | 1.67 ± 1.53 | 0.67 ± 1.15 | 1.67 ± 1.53 | 4.00 ± 1.00 | 7.00 ± 2.00 | |
| Thoonchus inermis | Enoplida | Enchelidiidae | 1.67 ± 1.15 | 1.33 ± 1.15 | 5.00 ± 2.00 | 5.00 ± 3.00 | 8.00 ± 2.00 | |
| Viscosia cobbi | Enoplida | Oncholaimidae | 1.33 ± 0.58 | 0.33 ± 0.58 | 3.00 ± 0.00 | 9.33 ± 3.2 | 7.33 ± 0.58 |
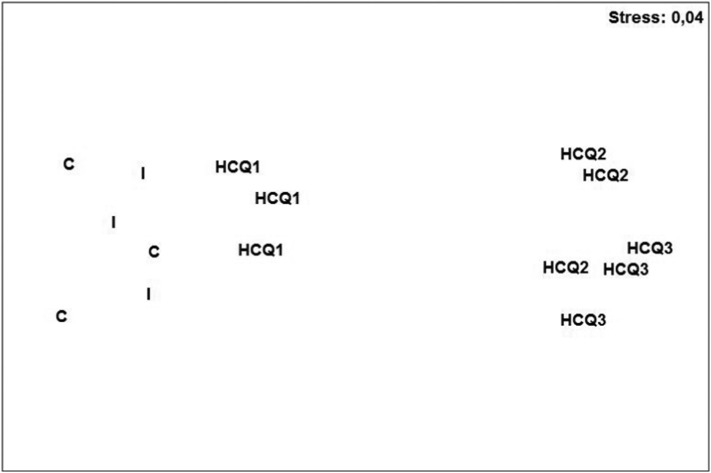
The ordination results (nMDS) of the initial community and those exposed to treatments (Fig. 2 ) was associated to a discernible stress value (= 0.04). The majority of nematodes exposed to HCQ2 and HCQ3 have been placed in the extreme right of the ordination graph, separately and far from the the initial nematode assemblage, the control one and that exposed to HCQ1. The results of the analysis of similarity (ANOSIM) confirmed those results of nMDS (p-value = 0.001). In fact, the average Bray-Curtis dissimilarity between the control nematode assemblage and those populating treated sediments HCQ1–3 (26.11% to 61.48%) desplayed a clear gradiend that followed the hydroxychloroquine concentrations (Table 2 ). The community exposed to HCQ1 was the least disturbed at the taxonomic level (R-value = 0.741) while HCQ2 and HCQ3 were more affected (R-value = 1) (Table 2).
Fig. 2.
Non-metric MDS ordination of nematode assemblages based on square-root transformed abundances of species from the initial nematofauna (I), the control one (C), and those treated with hydroxychloroquine (HCQ1, HCQ 2 and HCQ 3).
Table 2.
ANOSIM analysis and nematode species responsible for average dissimilarities (A.D.) between the initial assemblage (I), the control one and those exposed to hydroxychloquine (HCQ1, HCQ2 and HCQ3) based on similarity percentages (SIMPER) analysis. More abundant (+); less abundant (−); stable abundance (st); elimination (ø). Species accounting for about 50% of the overall dissimilarity between treatment pairs are ranked in order of importance of their contribution to this dissimilarity.
| Comparisons | I vs. C | C vs. HCQ1 | C vs. HCQ2 | C vs. HCQ3 |
|---|---|---|---|---|
| R statistics | −0.407 | 0.741 | 1 | 1 |
| A.D. (%) | 18.70 | 26.11 | 57.70 | 61.48 |
| Species |
Oncholaimus campylocercoides 7.60% (−) Oncholaimellus calvadocicus 7.21% (−) Enoploides longispicolosus 6.02% (+) Synonchiella edax 5.87% (−) Parasphaerolaimus paradoxus 5.39% (−) Viscosia cobbi 5.28% (−) Metoncholaimus pristiurus 5.14% (st) Bathylaimus sp. 4.87% (−) Daptonema fallax 4.75% (+) |
Oncholaimellus calvadocicus 8.10% (+) Prochromadorella longicaudata 7.94% (−) Oncholaimus campylocercoides 7.15% (+) Calomicrolaimus honestus 6.72% (−) Viscosia cobbi 6.45% (+) Thoonchus inermis 5.87% (+) Metoncholaimus pristiurus 5.73% (+) Mesacanthion monystera 5.47% (+) |
Paramonohystera proteus 7.04% (−) Calomicrolaimus honestus 6.77% (−) Oncholaimus campylocercoides 6.50% (+) Viscosia cobbi 6.06% (+) Trichotheristus mirabilis 6.05% (ø) Prochromadorella longicaudata 5.99% (−) Metoncholaimus pristiurus 5.31% (+) Mesacanthion monystera 5.15% (+) Ptycholaimellus ponticus 4.91% (−) |
Prochromadorella longicaudata 6.70% (ø) Paramonohystera proteus 6.34% (−) Calomicrolaimus honestus 6.27% (−) Pomponema elegans 6.04% (−) Oncholaimellus calvadocicus 5.99% (+) Trichotheristus mirabilis 5.77% (ø) Longicyatholaimus longicaudatus 5.64% (ø) Daptonema fallax 5.18% (ø) Viscosia cobbi 5.08% (+) |
The results of SIMPER (Table 2) showed that the difference between the control nematofauna and the HCQ1 could be attributed to the decrease in relative abundance of two species, namely Prochromadorella longicaudata and Calomicrolaimus honestus. In return, six other species (i.e. Oncholaimellus calvadocicus, Oncholaimus campylocercoides, Viscosia cobbi, Thoonchus inermis, Metoncholaimus pristiurus, and Mesacanthion monhystera) showed higher relative abundances. The increase in HCQ concentrations in sediment was followed by the enhance in terms of the number of defavorized species (↓) associated with a reduction in that of those favored (↑) (Table 2): (HCQ1: 6 species ↑, 2↓) → (HCQ2: 4↑,5↓) → (HCQ3: 2↑, 7↓).
3.4. Feeding traits and diversity
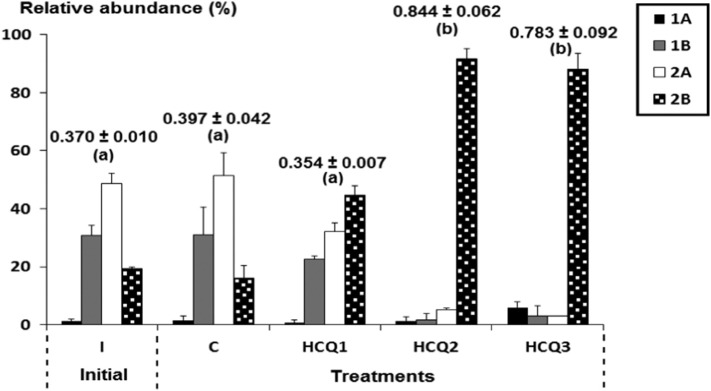
For initial and control nematode assemblages, with low average dissimilarity (= 6.45%), epistrate feeders (2A) ranked commonly first and followed by non-selective deposit feeders (1B) and omnivores-carnivores (2B). The main difference between these two nematode assemblages came from the presence of deposit feeders, selective and not. The ANOSIM analysis showed a significant difference (p-value = 0.001). The microcosms weakly contaminated with HCQ were characterized by a more or less balanced presence of three feeding groups: 2B ˃ 2A ˃ 1B. It should be noted that the selective detritivores (1A) were underrepresented in assemblages I, C and HCQ1 (Fig. 3 ). The medium- and highest contaminated sdiments with hydroxychloroquine were clearly dominated by omnivores-carnivores with average percentages of 91.67 ± 3.51% and 88 ± 5.56%, respectively (chi-square test, p < 0.0001). These treatments were also characterized by a more remarkable presence of 1A nematodes (chi-square test, p < 0.01). In contrast, the remaining feeding groups (i.e. 2A and 1B) were significantly lesser represented compared with I, C and HCQ1 (chi-square test, p < 0.001). Results of SIMPER analysis (Table 3 ) supported that the effect of HCQ was mainly expressed by an increase in the relative abundance of the omnivores-carnivores and a decrease of that of the epistrate-feeders. Finally, the average values of TDI ranged significantly from a minima in I (0.370), C (0.397) and HCQ1 (0.354) to a maxima in HCQ2 (0.844) and HCQ3 (0.783) (Fig. 3).
Fig. 3.
Variation in relative abundances of nematode feeding groups in the initial assemblage (I), the control one (C), and those treated with hydroxychloroquine (HCQ1, HCQ 2 and HCQ 3). Selective deposit feeders (1A); non-selective deposit feeders (1B); epigrowth feeders (2A); omnivores-carnivores (2B). Different letters indicate significant differences (log-transformed data, Tukey's HSD test, p ≤ 0.05). Values above histograms correspond to the Trophic Diversity Index.
Table 3.
ANOSIM analysis and feeding groups responsible for average dissimilarities (A.D.) between the initial assemblage (I), the control one and those exposed to hydroxychloquine (HCQ1, HCQ2 and HCQ3) based on similarity percentages (SIMPER) analysis. More abundant (+); less abundant (−). Feeding groups accounting for about 50% of the overall dissimilarity between treatment pairs are ranked in order of importance of their contribution to this dissimilarity. Selective deposit-feeders (1A); non-selective deposit-feeders (1B); epistrate-feeders (2A), omnivores-carnivores (2B).
| Comparisons | I vs. C | C vs. HCQ1 | C vs. HCQ2 | C vs. HCQ3 |
|---|---|---|---|---|
| R statistics | −0.037 | 0.963 | 1 | 1 |
| A.D. (%) | 6.45 | 16.63 | 50.44 | 50.53 |
| Feedfing groups | 1A 29.36% (+) 1B 27.69% (+) |
2B 46.23% (+) 2A 25.64% (−) |
2B 35.41% (+) 2A 30.63% (−) |
2A 33.02% (−) 2B 32.96% (+) |
4. Discussion
Numerous works started to explore effects of HCQ on humans after it was proposed as an early treatment of COVID-19 patients (Million et al., 2020). Several of them suggested short-term (days to weeks) toxicity at cardiac (Doyno et al., 2020), cardiovascular (Stevenson et al., 2020), and ocular (Al Adel et al., 2020) tissues. According to Doyno et al. (2020), deaths from overdoses of HCQ most often result from cardiovascular collapse. Several other effects have been reported by McChesney (1983) in association with the response of mammals after HCQ exposure. The data support a short-, medium and long-term testing on a wide range of mammals (mice, rats, dogs, rabbits and monkeys).
In the absence of an effective and approved vaccine, the excessive, non-rigorous and world-wide usage of HCQ can lead to environmental impacts during or after the current COVID-19 pandemic. The ecotoxic effects of HCQ in the aquatic environment needs to be assessed urgently, as there is a continuous and serious concern worldwide regarding the COVID-19 pandemic, alongside with potential environmental impact which is very little documented.
Until present, the effects of HCQ on aquatic taxa have not been studied. Only few data are available on the effects of chloroquine on these organisms, and eventualy human health through the food web. Zurita et al. (2005) examined the sensitivity towards chloroquine from bacteria to zooplankton as well as the values of NOAEL (Non-Observed Adverse Effect Levels). Their results indicated a decreasing sensitivity of the following pattern: Daphnia magna > Chlorella vulgaris > Poeciliopsis lucida cells > Vibrio fischeri, and chloroquine's NOAEL values of 2.5, 15, 10 and 50 μM were noted, respectively. In another study, Rendal et al. (2011) reported that the toxicity to chloroquine of Salix viminalis and Daphnia magna increased with increasing pH. Previous studies on fish, have shown contradictory results, with mortality for salmon at 20 μM (MacPhee and Ruelle, 1969) and just behavioural modifications in rainbow trout at 388 μM (Tojo et al., 1993). Moreover, the toxicity of chloroquine in the freshwater fish Cyprinus carpio was invetigated by Ramesh et al. (2018) on enzymological and histopathological levels (gills, liver and kidney). After acute exposure (96 h) to chloroquine the median lethal concentration corresponded to 31.62 mg.ml−1. During the acute treatment, Glutamate oxaloacetate transaminase and glutamate pyruvate transaminase activities increased in blood plasma when compare to controls. However, a significant decrease was noted for lactate dehydrogenase activity decreased.
Although the results of the abovementioned scientific surveys are of high interest to the scientific community, they mainly examine the effects of HCQ within a narrow range of certain freshwater species that might lack global distribution, and as such the obtained results might be of reduced applicability at a larger scale. In the results presented from this bioassay, could be globally exploited by scientists since (i) free-living nematodes are the most dominant and richiest group in species among marine organisms (Boufahja et al., 2015); (ii) they are known to be extremely ubiquitous and tolerators even in the worst conditions (Heip et al., 1985); and (iii) taxa are characterized by their cosmopolitan geographical distribution, which is commonly called the “meiofauna paradox” (Boeckner et al., 2009).
The current study supported that traits of free-living nematodes have been greatly affected both quantitatively and qualitatively by the enrichment of the environment in HCQ. Particularly, it has been noted that the highest the concentration tested the more significant the reduction in abundances and species richness of the nematode community. Moreover, the largest morphotypes were able to survive at those high concentrations (HCQ2 and HCQ3). Multivariate analysis results were related to the predominance of species that were poorly represented in the controls and became highly represented in treatments with HCQ (Fig. 2 and Table 2).
SIMPER results confirmed that HCQ was harmful to 8 species, namely Prochromadorella longicaudata, Calomicrolaimus honestus, Paramonohystera proteus, Trichotheristus mirabilis, Ptycholaimellus ponticus, Pomponema elegans, Longicyatholaimus longicaudatus, and Daptonema fallax. Extirpation of the aforementioned species could be considered as bioindicator of a bad environmental condition with respect to HCQ concentrations. The cascading effect of HCQ was that the empty niches will be occupied by those species (i.e. Oncholaimellus calvadocicus, Oncholaimus campylocercoides, Viscosia cobbi, Thoonchus inermis, Metoncholaimus pristiurus, and Mesacanthion monhystera.) that appeared advantaged probably because of their high tolerance to HCQ and/or opportunistic features.
It is well acknowledged that, the communities display a resistance threshold that may lead to a shift in the community, as a response to contaminants during chronic stressor (for details see Clements and Rohr, 2009). Yet, in the frame of this study, the aim was to explore the direct and short-term ecotoxicity of HCQ and therefore initial responses of a community (i.e. that of nematodes) to exposure to HCQ, deriving from the massive and large scale usage of the substance induced by the pandemic. Thus, the initial source of the community was a pristine one, and nematodes that have not been previously imposed to the chronic stress induced by HQC, ensuring that no previous shifts in community structure have taken place.
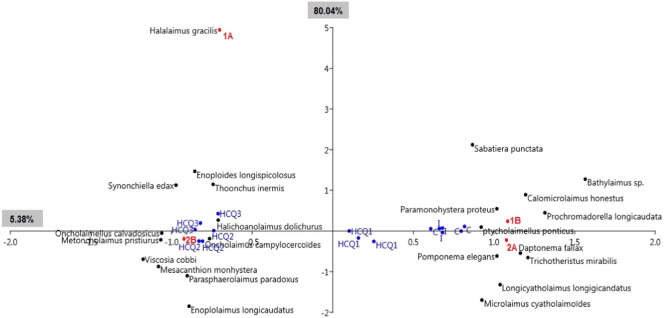
The differential reactions of the 14 species mentioned above, following their exposure to HCQ, revealed that the buccal cavity type and the diet were capital in shaping the composition of the nematofauna. Such a taxonomic restructuring was mainly the result of the decrease in the relative abundances of non-selective deposit feeders and epistrate feeders in one side and the increase in those of omnivores-carnivores in the other one (Fig. 4 ). This explains why average values of the Trophic Diversity Index augmented with increasing HCQ concentrations in the microcosms. It seems that contaminated microcosms were increasingly populated with the corpses of sensitive nematodes, a fact that was confirmed by the numerical decrease. The bacterial decomposition of these bodies was evident only at HCQ3. At the lower concentrations, no sufficient detritus was available to feed nematodes 1B, and they probably were preyed by omnivores-carnovores. The members of this latter trophic category are known to consume freshly dead preys (Moens and Vincx, 1997; Semprucci et al., 2018) and being tolerant of extreme conditions, as they are able of developing detoxification mechanisms and thus withstand exposure to xenobiotics (Cobb, 1931; Allouche et al., 2020a, Allouche et al., 2020b, Allouche et al., 2020c). In the case of nematodes belonging to the feeding type 2A, feeding principally on benthic diatoms, their sensitivity towards HCQ seems to be closely related to their diet. This hypothesis is further supported by the findings of Zurita et al. (2005) that report a more than eight-fold increase in the mean Effective Concentration (EC50) from 24 (258.4 μM) to 72 h (30.6 μM) of exposure to chloroquine in the inhibition of the growth in the unicellular alga Chlorella vulgaris.
Fig. 4.
Correspondence Analysis (CA) 2D plot based on the abundance of of nematode species from the initial assemblage, the control one (C), and those treated with hydroxychloroquine (HCQ1, HCQ 2 and HCQ 3). Selective deposit feeders (1A); non-selective deposit feeders (1B); epigrowth feeders (2A); omnivores-carnivores (2B). Percentage values next to axes indicate their contributions to the inertia.
Based on our results nematodes 2B were extremely tolerant in the high concentrations HCQ2 and HCQ3. In fact, this trophic group is known (1) to show a diet oscillating between facultative predation and deposit feeding (Moens and Vincx, 1997), and (2) for its easy mobility into the sedimentary column (Allouche et al., 2020a, Allouche et al., 2020b, Allouche et al., 2020c) which makes it succeptible to a wider contamination potential. As a result, fishes and other seafood sources such as shrimps, lobsters, prawns, and crabs may be easily contaminated by HCQ, through bioaccumulation, due to their feeding habits that might include in their diets nematodes in certain points of their ontogeny (Wilcox and Jeffries, 1979; Albertini-Berhaut, 1974; Fitzhugh and Fleeger, 1985). The results of Zurita et al. (2005) prove that chloroquine is bioaccumulable through the marine food web, from producers to consumers. Bioaccumulation is also anticipated for chemical we used in this study, HCQ, mainly due to its high solubility (86 mg.ml−1; Dabic et al., 2019). Consequently, the coastal areas located in front of COVID-19 epicenters, especially those of the semi-closed Mediterranean Sea, should be cautiously monitored, as seafood sources fished there might contain high concentrations of HCQ. Yet, this hypothesis needs to be tested, before actions towards food safety are taken.
5. Conclusions
Since the begining of the novel coronavirus disease (COVID-19) in December 2019, researchers across the world are testing the efficacy of numerous medication combinations as an effective cure. Starting from March 2020, numerous countries have recommended HCQ, an anti-malarial medicine, as efficient for treating patients in early stages. Thus, significant amounts of this chemical are predicted to be deposited into the sea, especially semi-closed areas. The present study concluded that HCQ may affect significatly the community of meiobenthic nematodes both quantitatively and qualitatively, particularly at the two highest concentrations tested, and that these changes could act as descriptors of the impacts of HCQ use in the marine environment. The use of nematodes in investigating the effects of the immediate and massive use of HCQ as a COVID-19 treatment is mainly based on their ubiquism and cosmopolitan species distribution. The results found that, compared to controls, during a short-term ecotoxicity there was a significant decline in abundance and species richness diversity, followed by the prevailance of large morphotypes of the feeding type 2B (omnivores-carnivores). The taxa belonging to this functional group are tolerant to toxicity, and are supposed to bioaccumulate HCQ released in coastal areas at COVID-19 epicenter countries. At these locations, seafood that includes meiofauna in their diet, in various stages of their ontogeny, might be associated to high health risks for consumers.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the Tunisian Ministry of the High Education and Scientific Research. The authors extend their appreciation to the deanship of scientific research for funding this article by Taif University Research Supporting Project number (TURSP-2020/225), Taif University, Taif, Saudi arabia.
References
- Al Adel F., Shoughy S.S., Tabbara K.F. Hydroxychloroquine dosing and toxicity: a real-world experience in Saudi Arabia of 63 patients. Saudi J. Ophthalmol. 2020 doi: 10.1016/j.sjopt.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albertini-Berhaut J. Biologie des stades juvéniles des Téléostéens Mugilidae Mugil auratus R 1810 Mugil capito C 1829 et Mugil saliens R 1810. II. Modification du régime alimentaire en relation avec la taille. Aquaculture. 1974;4:13–27. [Google Scholar]
- Allouche M., Nasri A., Harrath A.H., Mansour L., Beyrem H., Boufahja F. Migratory behavior of free-living marine nematodes surrounded by sediments experimentally contaminated by mixtures of polycyclic aromatic hydrocarbons. J. King Saud Univ. Sci. 2020;32:1339–1345. [Google Scholar]
- Allouche M., Hamdi I., Nasri A., Harrath A.H., Mansour L., Beyrem H., Boufahja F. Laboratory bioassay exploring the effects of anti-aging skincare products on free-living marine nematodes: a case study of collagen. Env. Sci. Pollut. Res. 2020;27:11403–11412. doi: 10.1007/s11356-020-07655-1. [DOI] [PubMed] [Google Scholar]
- Allouche M., Nasri A., Harrath A.H., Mansour L., Alwasel S., Beyrem H., Bourioug M., Geret F., Boufahja F. New protocols for the selection and rearing of Metoncholaimus pristiurus and the first evaluation of oxidative stress biomarkers in meiobenthic nematodes. Envon. Pollut. 2020;263(B):114529. doi: 10.1016/j.envpol.2020.114529. [DOI] [PubMed] [Google Scholar]
- Allouche M., Nasri A., Harrath A.H., Mansour L., Alwasel S., Beyrem H., Plăvan G., Rohal-Lupher M., Boufahja F. Do presence of gray shrimp Crangon crangon larvae influence meiobenthic features? Assessment with a focus on traits of nematodes. Environ. Sci. Pollut. Res. doi. 2021 doi: 10.1007/s11356-020-12069-0. [DOI] [PubMed] [Google Scholar]
- Austen M.C., McEvoy A.J., Warwick R.M. The specificity of meiobenthiccommunity responses to different pollutants: results from microcosm experiments. Mar. Pollut. Bull. 1994;28:557–563. [Google Scholar]
- Beyrem H., Louati H., Essid N., Aïssa P., Mahmoudi E. Effects of two lubricant oils on marine nematode assemblages in a laboratory microcosm experiment. Mar. Environ. Res. 2010;69:248–253. doi: 10.1016/j.marenvres.2009.10.018. [DOI] [PubMed] [Google Scholar]
- Beyrem H., Boufahja F., Hedfi A., Essid N., Aïssa P., Mahmoudi E. Laboratory study on individual and combined effects of cobalt- and zinc-spiked sediment on meiobenthic nematodes. Biol. Trace Elem. Res. 2011;144:790–803. doi: 10.1007/s12011-011-9032-y. [DOI] [PubMed] [Google Scholar]
- Bezerra T.N., Decraemer W., Eisendle-Flockner U., Hodda M., Holovachov O., Leduc D., Miljutin D., Mokievsky V., Pena Santiago R., Sharma J., Smol N., Tchesunov A., Enekey V., Zhao Z., Vanreusel A. In: Nemys: World Database of Nematodes. Bezerra T.N., editor. Ghent University; 2020. http://nemys.ugent.be/ edited by. available at: [Google Scholar]
- Boeckner M.J., Sharma J., Proctor H.C. Revisiting the meiofauna paradox: dispersal and colonization of nematodes and other meiofaunal organisms in low- and high-energy environments. Hydrobiologia. 2009;624:91–106. [Google Scholar]
- Boufahja F., Semprucci F. Stress-induced selection of a single species from an entire meiobenthic nematode assemblage: is it possible using iron enrichment and does pre-exposure affect the ease of the process? Environ. Sci. Poll. Res. 2015;22:1979–1998. doi: 10.1007/s11356-014-3479-2. [DOI] [PubMed] [Google Scholar]
- Boufahja F., Hedfi A., Amorri J., Aïssa P., Beyrem H., Mahmoudi E. An assessment of the impact of chromium-amended sediment on a marine nematode assemblage using microcosm bioassays. Biol. Trace Elem. Res. 2011;142:242–255. doi: 10.1007/s12011-010-8762-6. [DOI] [PubMed] [Google Scholar]
- Boufahja F., Ismaїly S., Beyrem H. Experimental evidence of effects of an antimitotic agent, colchicine, on a nematode community through a microcosm approach. Cah. Biol. Mar. 2015;56:39–48. [Google Scholar]
- Buchanan J.B. In: Methods for the Study of Marine Benthos. International Biological Programme Handbook No. 16. Holme N.A., McIntyre A.D., editors. Blackwell Scientific Publications; Oxford: 1971. Measurement of the physical and chemical environment: Sediments; p. 334. [Google Scholar]
- Clarke K.R. Non-parametric multivariate analyses of changes in community structure. Aust. J. Ecol. 1993;18:117–143. [Google Scholar]
- Clarke K.R., Gorley R.N. PRIMER-E; Plymouth, UK: 2001. PRIMER v5: User Manual/Tutorial. 91 p. [Google Scholar]
- Clarke K.R., Warwick R.M. 2nd edition. PRIMER-E; Polymouth, UK: 2001. Changes in Marine Communities: An Approach to Statistical Analysis and Interpretation. 164 p. [Google Scholar]
- Clements W.H., Rohr J.R. Community responses to contaminants: using basic ecological principles to predict ecotoxicological effects. Environ. Toxicol. Chem. 2009;28(9):1789–1800. doi: 10.1897/09-140.1. [DOI] [PubMed] [Google Scholar]
- Cobb N.A. The use of live nemas (Metoncholaimus pristiurus) in zoological courses in schools and colleges. Science. 1931;74:489–490. doi: 10.1126/science.74.1924.489. [DOI] [PubMed] [Google Scholar]
- Coull B.C., Chandler G.T. Pollution and meiofauna: field, laboratory and mesocosm studies. Oceanogr. Mar. Biol. 1992;30:191–271. [Google Scholar]
- Dabic D., Babic S., Skoric I. The role of photodegradation in the environmental fate of hydroxychloroquine. Chemosphere. 2019;230:268e277. doi: 10.1016/j.chemosphere.2019.05.032. [DOI] [PubMed] [Google Scholar]
- Doyno C., Sobieraj D.M., Baker W.L. Toxicity of chloroquine and hydroxychloroquine following therapeutic use or overdose. Clin. Toxicol. 2020 doi: 10.1080/15563650.2020.1817479. [DOI] [PubMed] [Google Scholar]
- Engelmann H.D. Zur Dominanzklassifizierung von Bodenarthropoden. Pedobiologia. 1978;18:378–380. [Google Scholar]
- Fabiano M., Danovaro R. Composition of organic matter in sediment facing a river estuary (Tyrrhenian Sea): relationships with bacteria and microphytobenthic biomass. Hydrobiologia. 1994;277:71–84. [Google Scholar]
- Fitzhugh G.R., Fleeger J.W. Goby (Pisces: Gobiidae) interactions with meiofauna and small macrofauna. Bull. Mar. Sci. 1985;36(3):436–444. [Google Scholar]
- Gotelli N.J., Entsminger G.L. Acquired Intelligence Inc, & Kesey-Bear; 2005. Ecosim: Null Models Software for Ecology. Version 7.72. [Google Scholar]
- Guo Y., Somerfield P.J., Warwick R.M., Zhang Z. Large-scale patterns in the community structure and biodiversity of free living nematodes in the Bohai Sea. China. J. Mar. Biol. Ass. U.K. 2001;81:755–763. [Google Scholar]
- Hedfi A., Mahmoudi E., Boufahja F., Beyrem H., Aïssa P. Effects of increasing levels of nickel contamination on structure of offshore nematode communities in experimental microcosms. Bull. Environ. Contam. Toxicol. 2007;79:345–349. doi: 10.1007/s00128-007-9261-0. [DOI] [PubMed] [Google Scholar]
- Hedfi A., Boufahja F., Ben Ali M., Aïssa P., Mahmoudi E., Beyrem H. Do trace metals (chromium, copper and nickel) influence toxicity of diesel fuel for free-living marine nematodes? Environ. Sci. Pollut. Res. 2013;20(6):3760–3770. doi: 10.1007/s11356-012-1305-2. [DOI] [PubMed] [Google Scholar]
- Hedfi A., Ben Ali M., Hassan M.M., Albogami B., Al-Zahrani S.S., Mahmoudi E., Karachle P.K., Rohal-Lupher M., Boufahja F. Nematode traits after separate and simultaneous exposure to polycyclic aromatic hydrocarbons (anthracene, pyrene and benzo[a]pyrene) in closed and open microcosms. Env. Pollut. 2021;276:116759. doi: 10.1016/j.envpol.2021.116759. [DOI] [PubMed] [Google Scholar]
- Heip C., Vincx M., Vranken G. The ecology of marine nematodes. Oceanogr. Mar. Biol. 1985;23:399–489. [Google Scholar]
- Lacoste E., Boufahja F., Pelaprat C., Le Gall P., Berteaux T., Messiaen G., Mortreux S., Oheix J., Ouisse V., d’Orbcastel E.R., Gaertner-Mazouni N., Richard M. First simultaneous assessment of macro- and meiobenthic community response to juvenile shellfish culture in a Mediterranean coastal lagoon (Thau, France) Ecol. Indic. 2020;115:106462. [Google Scholar]
- Ladapo J.A., McKinnon J.E., McCullough P.A., Risch H. Randomized controlled trials of early ambulatory hydroxychloroquine in the prevention of COVID-19 infection, hospitalization, and death: meta-analysis. medRxiv – Infect. Dis. 2020 doi: 10.1101/2020.09.30.20204693. [DOI] [Google Scholar]
- MacPhee C., Ruelle R. University of Idaho Forest; Moscow, ID: 1969. Lethal Effects of 1888 Chemicals upon Four Species of Fish from Western North America, Wild L. Range Exp. Station Bull., No.3; p. 112. [Google Scholar]
- Mahmoudi E., Essid N., Beyrem H., Hedfi A., Boufahja F., Vitiello P., Aïssa P. Effects of hydrocarbon contamination on a free-living marine nematode community: results from microcosm experiments. Mar. Pollut. Bull. 2005;50:1197–1204. doi: 10.1016/j.marpolbul.2005.04.018. [DOI] [PubMed] [Google Scholar]
- Mahmoudi E., Essid E., Beyrem H., Hedfi A., Boufahja F., Vitiello P., Aïssa P. Individual and combined effects of lead and zinc of a free living marine nematode community: results from microcosm experiments. J. Exp. Mar. Biol. Ecol. 2007;343:217–226. [Google Scholar]
- McChesney E.W. Animal toxicity and hydroxychloroquine. Am. J. Med. 1983;75(1A):8–11. doi: 10.1016/0002-9343(83)91265-2. [DOI] [PubMed] [Google Scholar]
- Million M., Lagier J.-C., Gautret P., Colson P., Fournier P.-E., Amrane S., Hocquart M., Mailhe M., Esteves-Vieira V., Doudier B., Aubry C., Correard F., Giraud-Gatineau A., Roussel Y., Berenger C., Cassir N., Seng P., Zandotti C., Dhiver C., Ravaux I., Tomei C., Eldin C., Tissot-Dupont H., Honoré S., Stein A., Jacquier A., Deharo J.C., Chabrière E., Levasseur A., Fenollar F., Rolain J.-M., Obadia Y., Brouqui P., Drancourt M., La Scola B., Parola P., Raoult D. Early treatment of COVID-19 patients with hydroxychloroquine and azithromycin: a retrospective analysis of 1061 cases in Marseille. France. Travel Med. Infect. Di. 2020;35:101738. doi: 10.1016/j.tmaid.2020.101738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moens T., Vincx M. Observations on the feeding ecology of estuarine nematodes. J. Mar. Biol. Ass. U.K. 1997;77:211–227. [Google Scholar]
- Nasri A., Jouili S., Boufahja F., Hedfi A., Saidi I., Mahmoudi E., Aïssa P., Essid N., Hamouda B. Trophic restructuring (Wieser 1953) of free-living nematode in marine sediment experimentally enriched to increasing doses of pharmaceutical penicillin G. Ecotoxicology. 2016;25:1160–1169. doi: 10.1007/s10646-016-1670-6. [DOI] [PubMed] [Google Scholar]
- Nasri A., Allouche M., Hannachi A., Harrath A.H., Aldahmash W., Alwasel S., Mahmoudi E., Beyrem H., Boufahja F. Restructuring of a meiobenthic assemblage after sediment contamination with an antibacterial compound: case study of ciprofloxacin. Ecotoxicol. Environ. Saf. 2020;205:111084. doi: 10.1016/j.ecoenv.2020.111084. [DOI] [PubMed] [Google Scholar]
- Platt H.M., Warwick R.M. Cambridge University Press; Cambridge: 1983. Free Living Marine Nematodes. Part I. British Enoplids. Synopses of the British Fauna no 28. 314 p. [Google Scholar]
- Platt H.M., Warwick R.M. 1988. Free living Marine Nematodes. Part II. British Chromadorids. Synopses of the British Fauna No 38. E.J Brill, Leiden. 502 p. [Google Scholar]
- Ramesh M., Anitha S., Poopal R.K., Shobana C. Evaluation of acute and sublethal effects of chloroquine (C18H26CIN3) on certain enzymological and histopathological biomarker responses of a freshwater fish Cyprinus carpio. Toxicol. Rep. 2018;5:18–27. doi: 10.1016/j.toxrep.2017.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendal C., Kusk K.O., Trapp S. The effect of pH on the uptake and toxicity of the bivalent weak base chloroquine tested on Salix viminalis and Daphnia magna. Environ. Toxicol. Chem. 2011;30(2):354–359. doi: 10.1002/etc.391. [DOI] [PubMed] [Google Scholar]
- Risch H.A. Early outpatient treatment of symptomatic, high-risk COVID-19 patients that should be ramped up immediately as key to the pandemic crisis. Am. J. Epidemiol. 2020;189(11):1218–1226. doi: 10.1093/aje/kwaa093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schratzberger M., Warwick R.M. Effects of intensity and frequency of organic enrichment on two estuarine nematode communities. Mar. Ecol. Prog. Ser. 1998;164:83–94. [Google Scholar]
- Seinhorst J.W. A rapid method for the transfer of nematodes from fixative to anhydrous glycerin. Nematologica. 1959;4:67–69. [Google Scholar]
- Semprucci F., Cesaroni L., Guidi L., Balsamo M. Do the morphological and functional traits of free-living marine nematodes mirror taxonomicalal diversity? Mar. Env. Res. 2018;135:114–122. doi: 10.1016/j.marenvres.2018.02.001. [DOI] [PubMed] [Google Scholar]
- Somerfield P.J., Warwick R.M. In: Methods for the Study of Marine Benthos. 4th edn. Eleftheriou A., editor. Wiley-Blackwell Ltd; Oxford: 2013. Meiofauna techniques. Chapter 6; pp. 253–284. [Google Scholar]
- Stevenson A., Kirresh A., Conway S., White L., Ahmad M., Little C. Hydroxychloroquine use in COVID-19: is the risk of cardiovascular toxicity justified? Open Heart. 2020;7 doi: 10.1136/openhrt-2020-001362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suderman K., Thistle D. Spills of fuel oil #6 and Orimulsion can have indistinguishable effects on the benthic meiofauna. Mar. Poll. Bull. 2003;46(1):49–55. doi: 10.1016/s0025-326x(02)00235-7. [DOI] [PubMed] [Google Scholar]
- Tojo J., Santamarina M.T., Ubeira F.M., Leiro J., Sanmartin M.L. Efficacy of antiprotozoal drugs against gyrodactilosis in rainbow trout (Oncorhynchus mykiss) Bull. Eur. Assoc. Fish Pathol. 1993;13:79–82. [Google Scholar]
- Vanaverbeke J., Martinez Arbizu P., Dahms H.U., Schminke H.K. The metazoan meiobenthos along a depth gradient in the Arctic Laptev Sea with special attentions to nematode communities. Polar Biol. 1997;18:391–401. [Google Scholar]
- Wakkaf T., Allouche M., Harrath A.H., Mansour L., Alwasel S., Ansari K.G.M.T., Beyrem H., Sellami B., Boufahja F. The individual and combined effects of cadmium, polyvinyl chloride (PVC) microplastics and their polyalkylamines modified forms on meiobenthic features in a microcosm. Environ. Pollut. 2020;266:115263. doi: 10.1016/j.envpol.2020.115263. [DOI] [PubMed] [Google Scholar]
- Warwick R.M., Platt H.M., Somerfield P.J. 1998. Free-living marine nematodes. Part III. British Monohysterids. Synopsis of British Fauna (New Series) No. 53, Field Studies Council, Shrewsbury. [Google Scholar]
- Whitworth J. COVID-19: a fast evolving pandemic. Trans. R. Soc. Trop. Med. Hyg. 2020;114(4):241–248. doi: 10.1093/trstmh/traa025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieser Die Beziehung zwischen Mundhoehlengestalt, Ernaehrugsweise und Vorkommen bei freilebenden marinen Nematoden. Arkiv. Fur. Zoologi. 1953;4:439–484. [Google Scholar]
- Wieser W. Benthic studies in Buzzards Bay. II. The meiofauna. Limnol. Oceanogr. 1960;5:121–137. [Google Scholar]
- Wilcox J.R., Jeffries H.P. Feeding habits of the sand shrimp Crangon septemspinosa. Biol. Bull. 1979;146:424–434. doi: 10.2307/1540689. [DOI] [PubMed] [Google Scholar]
- Zurita J.L., Jos A., del Peso A., Salguero M.A., Lopez-Artiguez M., Repetto G. Ecotoxicological evaluation of the antimalarial drug chloroquine. Aquat. Toxicol. 2005;75:97–107. doi: 10.1016/j.aquatox.2005.07.009. [DOI] [PubMed] [Google Scholar]