Abstract
COVID-19 has recently become one of the most challenging pandemics of the last century with deadly outcomes and a high rate of reproduction number. It emphasizes the critical need for the designing of efficient vaccines to prevent virus infection, early and fast diagnosis by the high sensitivity and selectivity diagnostic kits, and effective antiviral and protective therapeutics to decline and eliminate the viral load and side effects derived from tissue damages. Therefore, non-toxic antiviral nanoparticles (NPs) have been under development for clinical application to prevent and treat COVID-19. NPs showed great promise to provide nano vaccines against viral infections. Here, we discuss the potentials of NPs that may be applied as a drug itself or as a platform for the aim of drug and vaccine repurposing and development. Meanwhile, the advanced strategies based on NPs to detect viruses will be described with the goal of encouraging scientists to design effective and cost-benefit nanoplatforms for prevention, diagnosis, and treatment.
Keywords: COVID-19, Nanoparticle, Drug repurposing, Vaccine, Nanocarrier, Coronavirus, Drug delivery, Nanomaterials, Nanotechnology, Cells, Preclinical, Clinical
COVID-19; Nanoparticle; Drug repurposing; Vaccine; Nanocarrier; Coronavirus; Drug delivery; Nanomaterials; Nanotechnology; Cells; Preclinical; Clinical.
1. Introduction
1.1. Coronavirus and its viral advanture in the body: Corona
COVID-19 is caused by the SARS-CoV-2 virus and it has recently become one of the most challenging pandemics of the last century with deadly outcomes and a high rate of reproduction number and transmission. Since late 2002, coronaviruses have become a serious epidemic problem with severe acute respiratory syndrome coronavirus (SARS) and then with the onset of the Middle East respiratory syndrome (MERS) in late 2012. The enveloped ssRNA+ virus with an average particle size of 120–160 nm belongs to the subfamily, family, order, and the realm of Orthocoronavirinae, Coronaviridae, Nidovirales, and Riboviria, respectively [1]. Coronaviruses encode some structural and non-structural proteins that might be considered as an anti-viral target, including phosphorylated nucleocapsid, envelope, membrane glycoproteins, spike, helicase, papain-like protease and 3-chymotrypsin-like protease [2]. Although several of clinical trials are underway, currently, there is no clinically effective protective vaccine or therapeutic drug for COVID-19 patients in the world. Due to the urgent need for the treatment of COVID-19 patients and the desperation of uncertainly of any new vaccine, drug repurposing has been considered as a promising strategy. Therefore, the crucial step in drug repurposing is to have a thorough and accurate understanding and knowledge of structural differences of the coronaviruses and RNA (Ribonucleic acid) viruses and their involved receptors in a host.
Both SARS and COVID-19 were started from China during the spring festival with the same animal source, bat, and eventually pangolin in COVID-19 [3]. To the best of our knowledge, the coronavirus responsible for SARS and COVID-19 attached to the receptor-binding site of angiotensin-converting enzyme 2 (ACE2). However, MERS was started from Saudi Arabia with the animal source of camel and dipeptidyl peptidase-4 (DPP4) receptor involvement. The age of involvement in MERS and COVID-19 is middle age and occurs mostly in patients with the underlying disease for 2–14 days post-exposure, unlike the SARS in young individuals.
As a view of clinical features, these viruses share some similarities and differences. They all appear to have common clinical symptoms such as fever, cough, and general symptoms of the common cold. Pathologically, ACE2 a cell surface receptor in human is the main receptor of the SARS-CoV-2 virus. It is over-expressed in the lung, heart, kidney, testis, intestine, and brain. Therefore, we expect these organs to experience cell and tissue damages in part due to cytokine storm. However, CD147 (Basigin) as an extracellular matrix metalloproteinase inducer is considered as the other receptor for SARS-CoV-2 on the surface of lots of cells, including epithelial cells, endothelial cells, leukocytes, red blood cells, etc. It is the receptors of plasmodium falciparum on red blood cells, and also it has rules in spermatogenesis, the responsiveness of lymphocytes, etc. [2]. However, the disruption of the CD147-C-type lectin leads to the induction of prostate cancer [4]. The point is related to the eventual influence of prostate tissue by SARS-CoV-2 through the involvement of CD-147. Clinically, MERS and COVID-19 cause higher cardiovascular problems than the SARS, while SARS and COVID-19 patients more frequently than MERS experienced renal and neurological problems.
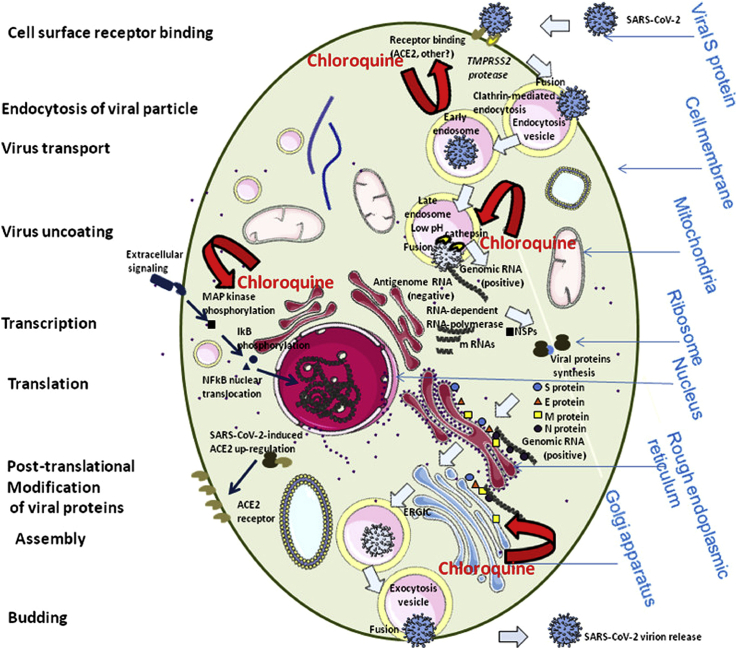
The mechanism underlying the COVID-19 disease is in part related to the decline of ACE2 (angiotensin-converting enzyme 2) owing to the entrance of the virus by its spike glycoprotein resulting in spike cleavage by transmembrane protease TMPRSS2. It triggers a cascade that leads to inflammation, TNF-α production, and tissue damage through the C-terminal cleavage of ACE2 by the TNF-alpha-converting enzyme (TACE). The sodium-dependent neutral amino acid transporter (SLC6A19 or B0AT1) occupies the C-terminal cleavage site and inhibits the effects of TACE on ACE2. The inhibition of TACE, renin-angiotensin pathway, C-terminal cleavage of ACE2, TNF-α, and inflammatory inhibitors will decrease the level of virus infection and tissue damage. As depicted in Figure 1, a virus is entered by binding to the ACE2 receptor on the cell surface of target cells and infects host cells [100]. However, chloroquine has the potential to interfere at different points of this cycle. Furthermore, chloroquine has a possible inhibitory effect on the MAPK kinase activity and hence a downregulation of certain proinflammatory responses.
Figure 1.
Schematic representation of the life cycle of SARS-CoV-2 in the face to the host cells and in interaction with chloroquine. SARS-CoV-2 binds to ACE2 using its spike and then the spike undergoes cleavage by TMPRSS2 protease resulting in inhibition of antibody neutralization. S protein further cleaves by the cathepsin L resulting in a fusion of endosomal membrane with the viral envelope to lead the release of the viral genome into the cell cytoplasm, transcription and translation of positive RNA and proteins. The packed final virion is released from the host cells. Chloroquine influences on the life cycle of SARS-CoV-2 through the disturbance in ACE2 receptor glycosylation, the biosynthesis of sialic acids, autophagosome formation, virus replication and immune system. Rights and permissions obtained from [100].
From the other side, the interaction of the virus by CD147 will cause other parts of pathogenesis in patients [5]. However, one of the most crucial pathogenesis mechanisms of SARS-CoV and MERS- CoV is through delay interferon, TNF-α, and pro-inflammatory responses that lead to the decrement of innate immunity in the host [6].
In the present paper, we discuss the diagnostic and therapeutic strategies for the other viruses which have similar mechanisms of action with SARS-CoV-2. The aim was to repurpose some strategies to diagnosis and cure the COVID-19 based on successfulness in similar diseases aiming to reduce the time and cost of diagnosis and treatment.
2. What strategy been taking for the treatment of COVID-19
To design and evaluate a successful drug and vaccine repurposing, it is necessary to know how much SARS-CoV-2 is close to the MERS-CoV and SARS-CoV as a genotype and structure (Table 1). Nucleotide sequence identity among the human coronaviruses, SARS-CoV, and SARS-CoV-2, are varying from 99.99-79.7% and 82% in COVID-19 patients. The sequence homology of SARS-CoV and MERS-CoV with SARS-CoV-2 are 70–77.5% and 50%, respectively [7, 8]. The proteins of the envelope, membrane, nucleocapsid, S1, and S2 have 95–96, 91, 89.6–94, 70 and 99% similarity in SARS-CoV and SARS-CoV-2, respectively [7, 9, 10]. Noteworthy, the spike of MERS-CoV and SARS-CoV-2 just have a 31.9% amino acid identity that makes it unfavourable for drug repurposing in COVID-19 therapy. Furthermore, receptor-binding domain (RBD) of spike and ORF3b, ORF8 in SARS-CoV-2, just have 40, 32 and 40% amino acid identity with SARS-CoV [10, 11]. Moreover, SARS-CoV-2 has some mutations such as replacing serine instead of glycine and proline instead of isoleucine in the position of 723 and 1010 of ORF1ab compared to SARS-CoV, respectively [12]. ORF1ab is involved in the synthesis of papain-like protease: nsp3, chymotrypsin-like: nsp5, RNA dependent RNA polymerase: nsp12 (96%), helicase (100%): nsp13 [10]. The function of every non-structural protein (nsp) and its sequence identity with SARS-CoV has been mentioned in Table 1. Based on these pieces of information, for the drug repurposing, we can select the drugs and vaccines that share antiviral efficacy among the SARS and COVID-19 by the targeting of the envelope, membrane, nucleocapsid, S2 spike, RdRp, helicase, etc. [10] (Table 1).
Table 1.
The nuclear shuttle protein (NSP) function and amino acid identity between COVID-19 and bat-SL-CoVZXC21 and SARS [10]. Keys: ACIB, Amino acid identity; btw, between; Fm, formulation.
| NSPs and other parts of coronaviruses | Putative function | Amino acid position | Putative cleave site | AC identity btw COVID-19 & bat-SL-CoVZXC21 | AC identity btw COVID-19 & SARS |
|---|---|---|---|---|---|
| NSP1 | suppress antiviral host response | M1 – G180 | LNGG'AYTR | 96 | 84 |
| NSP2 | unknown | A181 – G818 | LKGG'APTK | 96 | 68 |
| NSP3 | putative PL-pro domain | A819 – G2763 | LKGG'KIVN | 93 | 76 |
| NSP4 | complex with nsp3 and 6: DMV Fm | K2764 – Q3263 | AVLQ'SGFR | 96 | 80 |
| NSP5 | 3CL-pro domain | S3264 – Q3569 | VTFQ'SAVK | 99 | 96 |
| NSP6 | complex with nsp3 and 4: DMV Fm | S3570 – Q3859 | ATVQ'SKMS | 98 | 88 |
| NSP7 | complex with nsp8: primase | S3860 – Q3942 | ATLQ'AIAS | 99 | 99 |
| NSP8 | complex with nsp7: primase | A3943 – Q4140 | VKLQ'NNEL | 96 | 97 |
| NSP9 | RNA/DNA binding activity | N4141 – Q4253 | VRLQ'AGNA | 96 | 97 |
| NSP10 | complex with nsp14: replication | A4254 – Q4392 | PMLQ'SADA | 98 | 97 |
| NSP11 | short peptide at the end of orf1a | S4393 – V4405 | end of orf1a | 85 | 85 |
| NSP12 | RNA-dependent RNA polymerase | S4393 – Q5324 | TVLQ'AVGA | 96 | 96 |
| NSP13 | helicase | A5325 – Q5925 | ATLQ'AENV | 99 | 100 |
| NSP14 | ExoN: 3′–5′ exonuclease | A5926 – Q6452 | TRLQ'SLEN | 95 | 95 |
| NSP15 | XendoU: poly(U)-specific endoribonuclease | A5926 – Q6452 | PKLQ'SSQA | 88 | 89 |
| NSP16 | 2′-O-MT: 2′-O-ribose methyltransferase | S6799 – N7096 | end of orf1b | 98 | 93 |
| Spike | Host cell attachment and entry | - | - | 80 | 76 |
| Orf3a | - | - | 92 | 72 | |
| Orf3b | - | - | 32 | 32 | |
| Envelope | protection | - | - | 100 | 95 |
| Membrane | fusion | - | - | 99 | 91 |
| Orf6 | - | - | 94 | 69 | |
| Orf7a | - | - | 89 | 85 | |
| Orf7b | - | - | 93 | 81 | |
| Orf8a/8b | - | - | 94 | 40 | |
| Nucleoprotein | Viral genome and proteins | - | - | 94 | 94 |
| Orf9b | - | - | 73 | 73 | |
| Envelope | protection | - | - | 100 | 95 |
| Membrane | fusion | - | - | 99 | 91 |
| Orf6 | - | - | 94 | 69 | |
| Orf7a | - | - | 89 | 85 | |
| Orf7b | - | - | 93 | 81 | |
| Orf8a/8b | - | - | 94 | 40 | |
| Nucleoprotein | Viral genome and proteins | - | - | 94 | 94 |
| Orf9b | - | - | 73 | 73 |
Zhou et al. [9] found 47 human proteins in the HCoV–host interactome and as a targeting biomolecules for targeting in drug development such as STAT3, BCL2, SMAD3, PARP1, GSK3B, etc. They found several HCoV–host interactions by network proximity analyses in a human interactome and suggested repurposing of some drugs for COVID-19 treatment [9]. The targeting of these proteins by the pre-exist drugs may overcome the obstacles from the COVID-19. However, the encapsulation and decoration of drugs into/on nanoparticles will enhance their efficacy.
3. Diagnosis
The first stage of diagnosis in COVID-19 patients is based on a traveling and communication history of patients. Then Chest X-Ray and CT are recommended to detect ground-glass opacity and patchy bilateral shadows in the lungs. Other analyses on blood serum are differential CBC, CRP, LDH, AST/AL, procalcitonin, troponin I. Lymphopenia in patients indicated a bad prognosis of the disease. To detect the virus in specimens, Real-time RT-PCR is recommended. However, the diagnostic kit based on the RT-PCR technique has varied negative results. The PCR positive results are valuable, and the negative results do not reject the possible diseases. A systematic review indicated that the CT scan is a valuable diagnostic method for the symptomatic and hospitalized patient, however, at the early stage PCR method is recommended [13]. The biodistribution of SARS-CoV-2 in bronchoalveolar lavage fluid, sputum, nasal, fibre-bronchoscope brush biopsy, pharyngeal, stool, blood and urine, and their biodistribution were 93, 72, 63, 46, 32, 29, 1 and 0%, respectively, during three days of hospitalization [14].
Accurate and early detection plays a critical role in limiting COVID-19 spread and prevents future epidemics. Nanotechnology, based on molecular techniques and specific pathogens targeting, has emerged as a promising strategy in the early and fast COVID-19 detection. In the field of NPs applications for SARS-CoV-2 detection, RNA might be extracted using high-affinity silica-coated iron oxide NPs. Recently [15] has been reviewed diagnostic strategies for SARS-CoV-2, focusing on nanotechnology. Notably, a molecular-based technique to assess the RNA virus has superior sensitivity compared to antibody-based methods and serology. However, a lateral flow antigen detection based on colloidal gold NPs is under investigation [16] and it seems that since virus infection is started from the lung, the rapid (3–15 min) and early (three days post-infection) serology methods by colloidal gold NPs are superior to PCR methods for the detection antibodies against virus instead of RNA virus [17, 18]. These findings related to the sampling, time point of sample collection, active infection versus post-infection seroconversion explains the false negative results of PCR methods. This point of care testing has exhibited a clinical sensitivity, specificity, and accuracy of 57%, 100%, and 69% for IgM and 81%, 100%, and 86% for IgG, respectively while showing a clinical sensitivity of 82% for detecting the both IgM and IgG. For example, the gold NPs that quickly detect IgM and IgG antibodies derived from SARS-CoV-2 virus [19]. Furthermore, Ahmed et al. [20] reported an ultrasensitive chiro-immunosensor for viruses, including coronavirus based on chiral AuNPs (CAu NPs)-quantum dot (QDs) nanocomposites which showed significantly enhanced sensitivity in viral detection based on exciton–plasmon interactions. Singaporean scientists developed an RNA stabilization kit that stabilizes RNA of the virus at room temperature to prevent its degradation up to one week storage [19].
As depicted in Table 1, it was shown some protein and structural similarities and differences between SARS-CoV, MERS-CoV and SARS-CoV-2 and then in Table 2, it was summarized the recent advancements in nanoplatforms for the detection and treatment of SARS-CoV-2 with regards to its similarities and differences with two other sisters. The other Nano platforms for the diagnosis of coronaviruses are polymeric NPs [21], chaperone-mediated ferritin nanoparticles [22], Nanobodies [23, 24, 25, 26, 27], Self-Assembling Protein Nanoparticle (SAPN) [28], an adenoviral vector encoding Ad5 [29], spike protein nanoparticles [30], VLPs [31], AuNPs [32], AgNPs [33], Lumazine synthase NPs [34, 35], Microneedle array (MNA) delivered SARS-CoV-2 S1 subunit vaccines [36]. The based on the structure and protein similarities between SARS-CoV, MERS-CoV and SARS-CoV-2, it may be proposed a diagnostic method with less time and cost to design a new kit.
Table 2.
Nano-based approach for viral diagnosis and treatment.
| Nanoparticles | Virus | Mechanism of Antiviral Action | Outcome | Ref. |
|---|---|---|---|---|
| Chiral AuNPs-quantum dot nanocomposites | coronavirus | Chiral plasmon–exciton systems | Viral detection | [20] |
| Nanoparticulate vacuolar ATPase blocker (diphyllin) poly(ethylene glycol)-block-poly(lactide-coglycolide) (PEG-PLGA) | Feline coronavirus | Improved safety profile and enhanced inhibitory activity against virus infection | Exhibits potent host-targeted antiviral activity | [21] |
| chaperone-mediated ferritin nanoparticles | MERS-CoV | Targeting the RBD, inhibited RBD binding to hDPP4 receptor protein | Induce MERS-CoV RBD-specific antibodies (IgG, IgG1, IgG2a, IgG2b, IgA) in mice Elicit MERS-CoV RBD-specific T-cell responses (IFN-γ, TNF-α) in mouse splenocytes |
[22] |
| Oligomeric Nanobodies | MERS-CoV | RBD–receptor binding inhibition | Targeting the Receptor-Binding Domain dimeric Nb (Di-Nb) and trimeric Nb (Tri-Nb) had higher ability to bind MERS-CoV RBD proteins | [23] |
| MERS-CoV-specific VHHs or nanobodies | MERS-CoV | VHHs bind with exceptionally high affinity to the receptor-binding domain of the viral spike protein | Efficiently blocked virus entry at picomolar concentrations. | [24] |
| Nanobody (NbMS10) Nanobody (NbMS10) and human-Fc-fused version (NbMS10-Fc) |
MERS-CoV | Targeting the MERS-CoV spike protein receptor-binding domain (RBD) | Vigorous Cross-Neutralizing Activity and Protective Efficacy against MERS-CoV | [25] |
| VHHs | viral infections | Binding to viral coat proteins or blocking interactions with cell-surface receptors | immunotherapeutic agents, inhibitors of viral entry |
[26] |
| Nanobodies® ALX-0171 |
for lung diseases | Inhaled biotherapeutics | in clinical development for the treatment of respiratory syncytial virus (RSV) infection | [27] |
| enVision Lab-on-Chip platform integrating PCR and microarray |
SARS-CoV-2 | Potential commercial rapid diagnostic kits | Rapid detection | [47, 48] |
| Self-Assembling Protein Nanoparticle (SAPN) | Infectious bronchitis virus (IBV) the genus gamma coronavirus | The second heptad repeat (HR2) region of IBV spike proteins | Vaccine High antibody responses |
[28] |
| adenoviral vector encoding Ad5 and spike protein nanoparticles |
MERS-CoV | Stimulation of both Th1 and Th2 responses against MERS | Heterologous prime-boost vaccination (heterologous immunization by priming with Ad5/MERS and boosting with spike protein nanoparticles) | [29] |
| spike nanoparticles | MERS-CoV | Targeting the S protein | high titer anti-S neutralizing antibody and protective immunity | [30] |
| VLPs | Human coronavirus NL63 | Selectively transduce cells expressing the ACE2 protein | Development of a highly specific delivery platform for displaying narrow tissue tropism. | [31] |
| AuNPs | MERS-CoV | Color changes of AuNPs | Label-free colorimetric detection | [32] |
| AgNPs | MERS-CoV | color change | paper-based colorimetric assay for DNA detection | [33] |
| Lumazine synthase NPs | MERS-CoV | The MERS-CoV spike protein selectively binds to sialic acid (Sia), and cell-surface sialoglycoconjugates can serve as an attachment factor | Investigation of viral transmission and pathogenesis | [34] |
| Lumazine synthase NPs | MERS-CoV | The importance of the S1 domain in MERS-CoV infection and tropism | Investigation of viral transmission and pathogenesis, species-specific colocalization of MERS-CoV entry and attachment receptors | [35] |
| Microneedle array (MNA) delivered SARS-CoV-2 S1 subunit vaccines | SARS-CoV-2 | Targeting the S protein | Strong and long-lasting antigen-specific antibody responses | [36] |
4. Properties of NPs for COVID-19 treatment
Nanomedicine, the application of nanomaterials to medicine, is used in vectors, biosensors, drug and gene delivery. Nanotechnology, by taking off its unique physicochemical characteristics, leads to improved therapeutic efficacy through enhanced drug bioavailability, overcome to the low Log P (hydrophobicity or insolubility in water), specific delivery to target sites in cellular and intracellular levels and reduces side effects; it can also overcome the drug resistance, which is beneficial for viral treatment. Nanomaterials that are scientifically engineered are used to help pharmaceutical companies; they help to enhance the effectiveness of therapy and target specific dysfunctional organs or decaying body cells. The emergence of nanomaterials in recent years is swiftly transforming the scientific landscape of fields as diverse as aerospace, military, and medicine.
Nanoparticles (NPs) possessing unique physicochemical properties showed virucidal activities [37]. Small size, large surface area, targetability, and stimulus-responsive characteristics, make NPs the effective antiviral agents. NPs such as iron oxide, zinc oxide, silver, carbon-based and AVNP2 are considered as the promising agents to modulate the viral infection process. Metal NPs through their localized surface plasmon resonance (LSPR) effect may act as the theranostic agents for the diagnostic and therapeutic applications [38] while polymeric NPs do not have LSPR effect. They can inactivate viruses, including H1N1 and H5N1 influenza [39], poliovirus [40], NIPAH virus [41], respiratory syncytial virus, herpes simplex virus, human papillomavirus, dengue, and lentivirus [42]. It has been shown that nitric oxide (NO) nanoparticles through the supplementation of NO for endothelial cells and also, inhibition of RNA replication by peroxynitrite residue derived from NO prevent SARS-CoV infection and virulence [43]. Nanomaterials such as AuNPs can be used to bind the coronavirus and interrupt its structure through infrared light emission, and then the virus is deactivated by nanomaterials. In addition to the antiviral properties, NPs are determined as promising antiviral drugs carriers. They increase the activity of the drugs and improve their bioavailability. For example, chitosan NPs in the form of an aerosol (Novochizol) encapsulate several drugs and adhere to epithelial cells and can release the drug up to 3h. Therefore, drug dilution in body fluid does not cause reduced drug effectiveness [19].
Currently, only a few unspecific anti-coronavirus agents with serious adverse effects are available such as Kaletra (Lopinavir/Ritonavir), hydroxychloroquine, oseltamivir phosphate, Azithromycin, etc. Whereas acute respiratory distress syndrome (ARDS) derived from cytokine release syndrome (CRS), especially interleukin (IL)-6, will worsen the patient's fate. Therefore, the blocking of IL-6 and its receptor with Siltuximab and Tocilizumab are under clinical trial studies [37]. However, the role of lymphocytic apoptosis is not negligible in viral spreading by macrophages in COVID-19 patients [44]. Although there is no specific antiviral therapeutics for COVID-19, some unspecific medicines cured the patients. Nanostructures enhance the efficacy of these therapeutic agents.
Furthermore, siRNA, microRNA, and shRNA regulating viral gene expression will be developed as novel therapeutic agents against COVID-19 [45]. NPs cover the noncoding RNA and protect them from enzymes to improve their functions [46]. Nanodiamond particles functionalized and encapsulated with octadecylamine and dexamethasone show promising results dose dependency on declining of TNF-α, iNOS, and macrophage infiltration in mice [47]. However, the immunosuppression must be targeted to specific immune cells to not leading to sepsis in patients; extracorporeal perfusion of cytokines at the early stages using mesoporous carbon absorbents and graphene [48] makes enough time to host eliminate SARS-CoV-2 and limits mortality in patients derived from cytokine storm, etc.
Noteworthy, combination therapy seems to combat pandemic diseases. Co-delivery systems facilitate combination strategies [49]. Thus, nanocarriers can be applied for the co-encapsulation of the candidate drugs such as Remdesivir and hydroxychloroquine to treat COVID-19.
5. Preclinical assessment of NPs used for COVID-19 therapy
Some studies demonstrated the applications of NPs against coronavirus. Fortunately, airborne nanomaterials due to a small size and their tuneable physicochemical properties suited to penetrate alveolar epithelial type II cells (AECII) in the deep lung. The nanomaterial may be considered as a favourable candidate to deliver therapeutic agents in COVID-19 patients [37]. The most important point is related to the finding of some similar characteristics in viruses to repurpose medications such as attachment of virus attachment ligand (VAL) to heparan sulphate proteoglycans (HSPG) or sialic acids (SA) on the surface of host cells. The occupation of ligand sites on the cell surfaces, the viral attachment will be diminished [37]. However, clearance and dilution of nanomaterials upon virus replication have to be kept in mind.
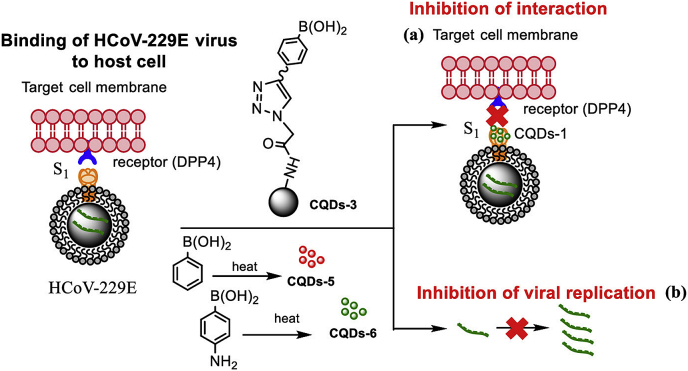
Recently, different types of carbon dots have investigated for the treatment of human coronavirus HCoV-229E infection. These nanoscale materials derived from ethylenediamine/citric acid and modified boronic acid ligands. The carbon nanomaterials inhibit and inactivate HCoV-229E entry in a concentration-dependent manner [50]. The functional groups of these carbon quantum dots interacting with HCoV-229E entry receptors and inhibit viral replication (Figure 2).
Figure 2.
Binding of HCoV-229E virus to host cell. The virus binds to DPP4 cell surface receptors via spike glycoprotein while carbon quantum dot, prepared by hydrothermal carbonization, inhibits its interaction with cell surface receptor and viral replication. Rights and permissions obtained from [50].
In the meantime, gold nanorods (AuNRs) have been applied to increase the inactivation of MERS-CoV. Conjugation of the AuNRs with an HR1 peptide inhibitor, pregnancy-induced hypertension (PIH), exhibits inhibitory effects and effectively prevents membrane fusion in MERS-CoV infections. These stable PIH-AuNRs complex represented as a new antiviral agent. These PIH-AuNRs possessing biocompatibility may provide fast clinical translation for MERS and eventually COVID-19 treatment [51]. Moreover, a molecular docking study proposed that Fe2O3 NPs strongly interacts to S1-RBD of the SARS-CoV-2 and eventually through the viral protein conformational change promotes antiviral efficacy [52]. Another strategy is to grab the virus by nano-sponges. They have made nanosponges comprising the cell membrane of human macrophages and lung epithelial type II cells and decorated them with the host receptors of SARS-CoV-2. Viruses are trapped into the nanosponges and neutralized [53].
Currently, some repurposing antiviral drugs have been suggested in the treatment protocol of COVID-19, including IFN-α, Lopinavir/ritonavir, Ribavirin, Chloroquine phosphate, and Arbidol [54, 55]. Chloroquine shows antiviral efficacy through inhibition of endosome–lysosome fusion by endosomal pH enhancement, reduction of viral load and virus entrance to host cells, and suppression of PICALM involve in endocytosis [56] Therefore encapsulation of the hydroxychloroquine into the nanocarriers that deliver cargo to the respiratory system and decline systemic administration side effects such as retinopathy, myopathy, and heart diseases is valuable. The nanocarriers can be liposomes, polymeric NPs same as albumin NPs, micelles, etc [57].
However, as mentioned earlier, CD147 is a common receptor for plasmodium falciparum, a parasite of malaria, and SARS-CoV-2. The interaction of CD147 on RBCs and RAP2 from the plasmodium falciparum is necessary for a parasite invasion. Humanized CD147 antibody of HP6H8 completely inhibits this interaction [58]. However, owing to chloroquine resistance in malaria patients, it has been successfully substituted with artemisinin.
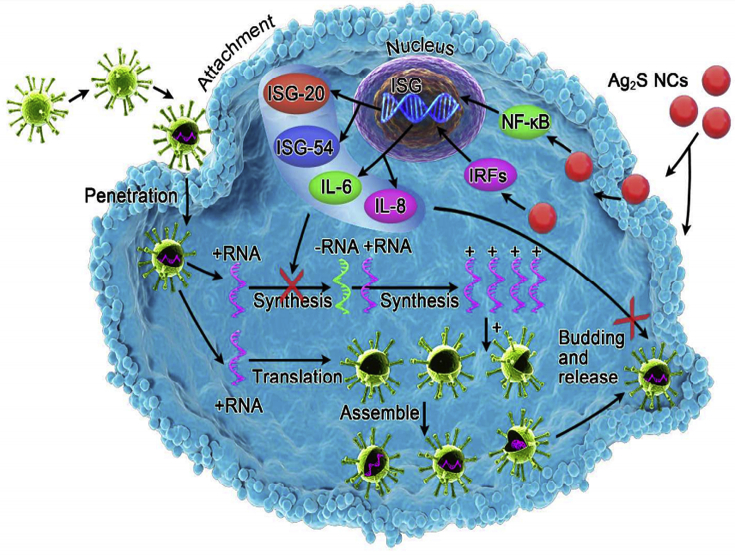
Regarding the antiviral drug limitations, applying available nano-platforms is suggested. Recent it has been surveyed antiviral drugs nanocarriers, and some of them would be suggested for the treatment of COVID-19. These include; selenium@oseltamivir and nano-formulations of INFs-α. Moreover, liver targeted delivery of INFs-α decorated by hybrid NPs such as human serum albumin and copolymers of maleic anhydride/alkyl vinyl ethers of oligo (ethylene glycol) is a valuable nanoplatforms. It can be functionalized by digalactosyl diacyl glycerol, a natural glycolipid to selectively interact with the asialofetuin receptor of liver hepatocytes. Other examples are PegIntron® and Pegasys® as two FDA approved polymeric NPs containing PEG-interferon alfa-2b and PEG-interferon alfa-2a, respectively and chitosan NPs for the oral delivery of IFNα [56, 59]; combination therapy of lopinavir/ritonavir/tenofovir by lipid nanoparticles composed of DSPC + MPEG + DSPE also introduced, although, a combination of Lopinavir/ritonavir has been suggested [60]. Combination therapy using nanomedicine not only increase the therapeutic effects induced by synergistic effects of drugs but also minimized the effective dose of drugs. In another antiviral study, lopinavir/ritonavir loaded PLGA NPs showed potent antiviral activity while reducing the effective dose [61]. Glutathione-capped Ag2S nanoclusters were also potent nanomaterial for coronavirus suppressing. They significantly prevent the RNA synthesis and budding and upregulate the INF-stimulating genes (ISGs) expression (Figure 3) [62]. Kerry and et al [41], also reviewed the nano-based approaches against viral infection in which noticed the antiviral activity of graphene oxide (GO) with Ag NPs study against Feline coronavirus (FCoV). GO-Ag NPs could efficiently suppress the FCoV through lipid membrane rupture mediated interaction of GO with lipid membrane and then, Ag NPs absorb the –SH groups of viral proteins. Furthermore, phage capsid NPs functionalized with multivalent ligands in an arrangement in which it matches the geometry of the spike protein binding site is promising. These NPs provide a collective strength of multiple binding sites and cover the entire virus envelop, thereby suppress the influenza virus entry [63]. The authors announced that they immediately take this approach to combat coronaviruses [64]. Another strategy is to rapidly eliminate pro-inflammatory cytokines from the bloodstream before the further adverse effect in tissues. It seems that extracorporeal perfusion through graphene and porous carbons is promising to quickly remove the cytokines from the blood circulation [48, 65]. However, a theranostic platform to detect and treat the virus infection is of interest. In this regards, organic, inorganic and virus-like NPs such as lipid NPs, dendrimer, magnetic and gold NPs have been reported [66]. A clinical trial phase 1 has been recently designed to investigate the safety and efficiency of aerosol inhalation of the exosomes derived from allogeneic adipose mesenchymal stem cells in patients with severe COVID-19 [67]. There are several nano-strategies to combat SARS-CoV-2 by repurposing from pharmaceutics for other diseases with similar symptoms including TNF-α inhibitors and interferon producers. TNF-α is accounted as the one of major cytokines involved in cytokine storm and its inhibition will be promising to decline tissue damage thereof. To this aim, TNF-α-siRNA will be helpful especially when are encapsulated into the NPs to protect them from biodegradation and enhance their bio-distribution and transfer to the inflamed tissues. Targeted delivery to inflamed tissues may provide with acid-sensitive NPs such as acid-sensitive sheddable PEGylated solid-lipid NPs (SLN). The TNF-α-siRNA- SLN had high encapsulation efficacy up to 90% with a 5% burst release of siRNA. However, they showed superior anti-inflammatory efficacy than free-methotrexate, as well [68]. Other nanoplatforms that inhibit TNF-α are surface charge tuneable (PEG5K-b-PLGA10K) NPs [69], gold NPs [70], nanodiamonds [47], Chitosan-Tripolyphosphate NPs [71], Polycaprolactone NPs [72], Magnetic nanocomposite particles [73], Zein NPs [74], Melatonin/Polydopamine Nanostructures [75], Polyurethane-chitosan copolymer [76], Nanocrystalline suspension [77], Lyophilized non-aggregated NP of Irbesartan (IBS-NPs) [78, 79]and Solid lipid NPs [80].
Figure 3.
The anti-viral effect of Ag2S nanoclusters. They inhibit the replication of the viral genome and budding. However, they upregulate the INF-stimulating genes (ISGs) expression. Rights and permissions obtained from [62].
Besides, it has been proven that melatonin inhibits TNF-α production and also, it has beneficial potential to decline COVID-19 symptoms. One clinical trial on the efficacy of melatonin in COVID-19 patients has proven its efficacy [81]. In other words, melatonin modulates the immune system by acting on T cells and inhibiting of TNF-α [82].
It has been proven the general administration of interferon-α needs a high dose of the parental regime and several severe side effects, therefore; encapsulation of the drug into a nanocarrier is of interest. For example, encapsulated interferon-α into chitosan NPs with mucoadhesive potential is promising to orally deliver the cargo. The NPs had a particle size of 36 nm without the decline of interferon-α activity into the carrier. Interferon-α -chitosan NP appear 1 h after the oral administration in plasma of mice while the commercial interferon-alpha was not detected. This finding showed that encapsulation of interferon-alpha into a NP remains its activity and enhances its biodistribution. However, the patients are more compatible with the oral administration of the injection [56]. Some other nanoplatforms that have been applied to produce interferon are a derivate of chitosan NPs [25, 56, 59, 83], Fe3O4 NPs [84], Disulfide-based PEGylatednanogels [85], CD44-targeted glutathione-sensitive hyaluronic acid-mercaptopurine prodrug (HA-GS-MP) [86],Nanoliposomes [87], Sirolimus nanocomposites [88], Sirolimus Nanocrystals [89] and Calcium phosphate (CaP) NP-based vaccine carrier functionalized with CpG and viral peptides [90]. These NPs can encapsulate biomaterials and drugs that induce interferon-α.
Hydroxychloroquine is another drug that uses in COVID-19 patients through reduction of viral load and virus entrance to host cells. Therefore, encapsulation of the hydroxychloroquine into the nanocarriers that deliver cargo to the respiratory system and decline systemic administration side effects such as retinopathy, myopathy, and heart diseases is valuable. The nanocarriers can be liposomes, polymeric NPs same as albumin NPs, micelles, etc [57].
Safe, and effective vaccines are crucial for disrupting the COVID-19 transmission chain, and nanotechnology plays a growing potent role in vaccine development [91]. Novavax announced the development of a novel virus-like particle (VLP; recombinant NP) formulation with the Matrix-M™ adjuvant (RN 1235341-17-9) vaccine against at least one trimer of an S protein in COVID-19. The vaccine elicits a neutralizing antibody response in administered animals with a single prophylactic injection [45, 92]. A novel development of vaccine [93] reported a self-assembling peptide NP with deposition of N- and C-termini and containing NSP10, a viral transcription factor, of the human SARS-CoV as an antigen presentation platform.
A promising strategy in vaccine development is taking benefits of mRNA owing to mimicking the natural infection and the ability to combine multiple mRNAs into a single vaccine that resulted in the stimulation of a more potent immune response. For example, mRNA vaccines encapsulated in cationic lipid NPs that encode the full-length of S, S1, or S2 proteins from SARS-CoV and MERS-CoV lead to more amplified neutralizing antibody responses than mRNA encoding the S2 subunit in mice with high efficacy. Recently, Moderna announced [94] the release of the first mRNA-1273 vaccine formulated in cationic lipid NPs against the S protein of COVID-19 for human application. Others patented vaccines containing mRNA encoding at least one antigen from MERS, including an S protein or an S protein subunit (S1), an envelope protein (E), a membrane protein (M), or a nucleocapsid protein (N), all of which caused to significant specific protective immunity. Notably, intradermal injection of lipid NP (LNP)-loaded mRNA combination encoding S proteins of MERS stimulated the humoral immune responses in mice. Briefly, two companies developed nanoparticulate vaccines for SARS-CoV-2, including LNP-encapsulated mRNA form the Moderna (NCT04405076) and 3LNP-mRNAs from BioNTech/Fosun Pharma/Pfizer (2020-001038-36). These vaccines are under clinical trial studies. Some other vaccines are under preclinical investigations including LNP-mRNA from Translate Bio/Sanofi Pasteur, LNP-encapsulated mRNA encoding RBD and LNP-encapsulated mRNA cocktail encoding VLP from Fudan University/Shanghai JiaoTong University/RNACure Biopharma, LNP-encapsulated mRNA from University of Tokyo/Daiichi-Sankyo, Liposome-encapsulated mRNA from BIOCAD and LNP-mRNA from CanSino Biologics/Precision NanoSystems. Please see the recent review on vaccines on clinical trials and their mode of action for immunity against the virus [55].
6. Remark
Overall, nanotechnology has emerged as a potent strategy for the designing of antiviral therapeutics. However, translation of extensive researches from bench to clinic is an urgent priority to control COVID-19. Noteworthy, we offer the repurposing of their nano-platforms. These nanosystems have been summarized in Table 3. Most recent R&D on coronavirus vaccines has been dedicated to RNA and protein vaccines containing the S RNA and protein subunits and explicitly targeting the receptor-binding domain (RBD) of the S1 subunit of the viral S protein. Modern vaccines composed of antigenic protein subunits, including viral S protein subunits, present higher neutralizing antibody and more protective immunity compared to a live-attenuated virus, full-lengtth S protein, and DNA-based S protein vaccines. Therefore, spike RNA and protein segments are the preferred targets for SARS/MERS vaccine development, which can take advantage of the same approach in developing COVID-19 nano-vaccines. From the other side, NPs are ideal carriers and therapeutics for pulmonary delivery [58]. In this point of view, blocking the S- ACE2 receptor interaction by biocompatible NPs would be promising; especially when the theranosticNPs are designed for early and fast detection and effective treatment, Nanomaterials can act as an adjuvant to enhance immunogenicity and antigen protection from biodegradation. These immune-targeted nanotherapeutics show dose spare, modulation of type of immunity, vast antibody responses, more rapid and durable immunity in vaccinated individuals [95]. Their responsiveness is size-dependent and, in some cases, may increase the immunity responses 100 folds higher than the soluble antigens. The NPs with 10–100 nm may be considered as a favourable particle size that can be deposited in the alveolar region, and lesser [95] than this size may be filtrated from the kidney. Nano-vaccines should act as a delivering and immunomodulatory system. To reach this goal, functionalization of nanomaterials with the ligands of TLR4 and NLRP3 and also dendritic cells' receptors such as ligands of Fc receptor, mannose receptor and CD11c/CD18 receptor, are promising. However, the cellular uptake may improve by the active targeting of target cells, cell-penetrating peptides (CPPs), cationic nanoparticles (branched PEI), etc. The candidate's nanomaterials are MF59 (165 nm nanoemulsion), pH-sensitive or redox-sensitive nanomaterials, liposomes, gold nanoparticles, polymerosome, virosomes, virus-like nanoparticles (VLNs) (15–30 nm), cationic nanoparticles such as chitosan nanoparticles, the polymeric nanoparticle of PLGA, poly(ethyleneglycol)-b-poly(L-lysine)-b-poly(L-leucine) (PEG-PLL-PLLeu) micelles, dendrimers, etc. [95]. Although two developed SARS-CoV-2 nano-vaccines in clinical trials are encapsulated RNA, the most preclinical vaccines focus on recombinant spike protein vaccine, one reason my related to non-exciting of RNA vaccine in the market. Therefore, the functionalization of NPs, such as liposomes with versatile biomolecules and motifs that target SARS-CoV-2 would be effective. However, functionalized graphene, carbon nanotubes, nanodiamonds, and polystyrene particles provoke the immune system. For example, amino-graphene oxide same as alum and AS01 and AS03 (conventional adjuvant) activate the inflammasome sensor NLRP3 and interferon pathways of STAT1/IRF1, respectively resulting in macrophage/Th1 polarization and T cell chemoattractants [37, 96]. However, graphene and fullerene are both candidates of photodynamic therapy [97]
Table 3.
The potential nano-based drugs repurposing for the treatment of COVID-19.
| Nanoparticles Formulation | Therapeutic agent | Indication | Ref | |
|---|---|---|---|---|
| TNF-α inhibitors | Lipid NPs | TNF-α siRNA | rheumatoid arthritis | [68] |
| Surface charge tunable (PEG5K-b-PLGA10K) nanoparticles | TNF-α siRNA | ulcerative colitis | [69] | |
| Gold NPs (AuNGs) | - | collagen-induced arthritic (CIA) | [70] | |
| Octadecylamine-functionalized nanodiamond (ND-ODA) & dexamethasone (Dex)-adsorbed ND-ODA (ND-ODA–Dex) | ND-ODA and dexamethasone | rheumatoid arthritis | [47] | |
| Chitosan-Tripolyphosphate NPs |
Melatonin | Attenuating Etoposide-Induced Genotoxicity |
[71] | |
| Polycaprolactone NPs | Melatonin | Glioblastoma | [72] | |
| Magnetic nanocomposite particles | Melatonin | breast cancer | [73] | |
| Zein NPs | Melatonin | - | [74] | |
| Melatonin/Polydopamine Nanostructures | Melatonin | Parkinson's Disease | [75] | |
| Polyurethane-chitosan copolymer | Mesalamine | An anti-inflammatory drug for the treatment of inflammatory bowel disease | [76] | |
| Nanocrystalline suspension | Irbesartan (IBS) | angiotensin receptor blocker | [77] | |
| Lyophilized non-aggregated NP of Irbesartan (IBS-NPs) | Irbesartan (IBS) | antihypertensive agent | [78] | |
| IBS NPs | Irbesartan (IBS) | angiotensin II receptor antagonist | [79] | |
| Solid lipid NPs | Irbesartan (IBS) | angiotensin receptor blocker | [80] | |
| Interferon inducers | Chitosan NPs | Interferon-alpha | viral infections and cancer diseases | [56, 59] |
| Chitosan NPs | 6-Mercaptopurine | immunosuppressant and anti-cancer drug | [101] | |
| hydroxypropyl-β-cyclodextrin (HP-β-CD) inclusion complex loaded chitosan (CS) NPs | mesalazine (MSZ) | anti-inflammation activity | [83] | |
| Folate Receptor-Targeted and GSH-Responsive Carboxymethyl Chitosan NPs | 6-mercaptopurine (6-MP) | Leukemia | [25] | |
| PVA-coating- Fe3O4 NPs | 6-Mercaptopurine (6-MP) | Leukemia Therapy | [84] | |
| Disulfide-based PEGylatednanogels | 6-mercaptopurine and methotrexate | breast cancer | [85] | |
| CD44-targeted glutathione-sensitive hyaluronic acid-mercaptopurine prodrug (HA-GS-MP) | mercaptopurine prodrug | Leukemia | [86] | |
| Nanoliposomes (Nanolimus) | Sirolimus known as rapamycin | Peripheral artery disease (PAD) | [87] | |
| Sirolimus nanocomposites | Sirolimus | macrocyclic lactone immunosuppressant, anti-rejection reaction after organ transplantation | [88] | |
| Sirolimus Nanocrystals |
Sirolimus | the immunosuppressive agent also used to treat inflammation, cancer | [89] | |
| Calcium phosphate (CaP) NP-based vaccine carrier functionalized with CpG and viral peptides | - | chronic retroviral infection |
[90] |
7. Barriers to overcome
Recently, due to the importance of limiting the virus pandemic in the world, the results of the effectiveness of a new drug are announced every day, which seems to be understandable, but it should be reviewed more wisely. Nano drugs, meanwhile, acts as a double-edged sword. From one side, there are some concerns about their toxicities, and on the other hand, are promising to enhance the efficacy of drugs or act as a nano drug to inhibit virus attachment, fusion, replication, and infection or even restrain the inflammatory and damaging cascade following virus infection in patients. There are several reports associated with mutagenicity, tumorgenicity, producing free radicals, penetration into the brain, etc. of NPs, especially metallic NPs. The caution should be considered related to toxicity and size dose dependency, the route of administration, biodistribution, and biodegradability of NPs [38, 98]. Another critical point is associated with the cell death mechanisms provoke by NPs and cross-reaction with the efficacy of drugs against a virus such as autophagy, ferroptosis, etc. [49, 99]. Translation of in vitro studies to clinical investigations without definitive studies that carefully cover NPs’ biocompatibility resulted in irreparable damages.
8. Future perspective
Medical nanotechnology has established a new and mighty era in the diagnosis and treatment of viruses, including COVID-19. However, the safety, dose-response, and size-efficacy of nanoparticles are their concern to translate to clinical applications. Designing nanoplatforms to diagnose and treat the COVID-19 and limit triggering of tissue damage cascade in human cells is necessary. To this aim, an accurate understanding of lethal death mechanisms of NPs in cells and viruses, occupying of the receptors binding sites of the virus in human cells using NPs and strategies to manipulate virus structures and their enzymes by NPs (Virus-NP interaction) is crucial. Meanwhile, designing of the NPs with enhanced circulation time, drug entrapment efficacy, and biodistribution that limit drug metabolization and release in unwanted tissue and organs are of interest. Noteworthy, to enhance the effectiveness of antiviral drug delivery by the NPs, they can be functionalized with the specific antibodies and ligands for active targeting. However, NPs based on their small size exhibit passive targeting potential. Advanced methods such as molecular dynamic and molecular docking, stochastic, and micro-fluidic predicting the involvement of viruses’ structures with NPs and Virus-NPs interaction will be valuable to achieve this goal. Therefore, it appears that NPs themselves and also as a combination therapy system or carriers for the aim of drug repurposing and development will be promising in designing rapid test diagnosis methods and therapeutic agents.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data included in article/supp. material/referenced in article.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Contributor Information
Shima Tavakol, Email: shima.tavakol@yahoo.com.
Alexander Seifalian, Email: a.seifalian@gmail.com.
References
- 1.Sexton N.R., Smith E.C., Blanc H., Vignuzzi M., Peersen O.B., Denison M.R. Homology-based identification of a mutation in the coronavirus RNA-dependent RNA polymerase that confers resistance to multiple mutagens. J. Virol. 2016;90(16):7415–7428. doi: 10.1128/JVI.00080-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ulrich H., Pillat M.M. CD147 as a target for COVID-19 treatment: suggested effects of azithromycin and stem cell engagement. Stem Cell Rev. Rep. 2020:1–7. doi: 10.1007/s12015-020-09976-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu P., Jiang J.-Z., Hua Y., Wang X., Hou F., Wan X.-F., Chen J., Zou J., Chen J. bioRxiv; 2020. Are Pangolins the Intermediate Host of the 2019 Novel Coronavirus (2019-nCoV)? [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rodriguez-Teja M., Gronau J.H., Minamidate A., Darby S., Gaughan L., Robson C., Mauri F., Waxman J., Sturge J. Survival outcome and EMT suppression mediated by a lectin domain interaction of Endo180 and CD147. Mol. Canc. Res. 2015;13(3):538–547. doi: 10.1158/1541-7786.MCR-14-0344-T. [DOI] [PubMed] [Google Scholar]
- 5.Wang K., Chen W., Zhou Y.-S., Lian J.-Q., Zhang Z., Du P., Gong L., Zhang Y., Cui H.-Y., Geng J.-J. BioRxiv; 2020. SARS-CoV-2 invades host cells via a novel route: CD147-spike protein. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lau S.K., Lau C.C., Chan K.-H., Li C.P., Chen H., Jin D.-Y., Chan J.F., Woo P.C., Yuen K.-Y. Delayed induction of proinflammatory cytokines and suppression of innate antiviral response by the novel Middle East respiratory syndrome coronavirus: implications for pathogenesis and treatment. J. Gen. Virol. 2013;94(12):2679–2690. doi: 10.1099/vir.0.055533-0. [DOI] [PubMed] [Google Scholar]
- 7.Gralinski L.E., Menachery V.D. Return of the coronavirus: 2019-nCoV. Viruses. 2020;12(2):135. doi: 10.3390/v12020135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoffmann Markus, Kleine-Weber Hannah, Schroeder Simon, Müller Marcel A., Drosten Christian, Pöhlmann Stefan. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020 doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou Y., Hou Y., Shen J., Huang Y., Martin W., Cheng F. Network-based drug repurposing for novel coronavirus 2019-nCoV/SARS-CoV-2. Cell Discov. 2020;6(1):1–18. doi: 10.1038/s41421-020-0153-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chan J.F.-W., Kok K.-H., Zhu Z., Chu H., To K.K.-W., Yuan S., Yuen K.-Y. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg. Microb. Infect. 2020;9(1):221–236. doi: 10.1080/22221751.2020.1719902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cascella Marco, Rajnik Michael, Cuomo Arturo, Scott C., Dulebohn, Napoli R.D. StatPearls Publishing; Treasure Island (FL): 2010. Features, Evaluation and Treatment Coronavirus (COVID-19) [PubMed] [Google Scholar]
- 12.Angeletti S., Benvenuto D., Bianchi M., Giovanetti M., Pascarella S., Ciccozzi M. COVID-2019: the role of the nsp2 and nsp3 in its pathogenesis. J. Med. Virol. 2020 doi: 10.1002/jmv.25719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poortahmasebi V., Zandi M., Soltani S., Jazayeri S.M. Clinical performance of RT-PCR and chest CT scan for covid-19 diagnosis; a systematic review. Adv. J. Emer. Med. 2020;4(2s):e57. e57. [Google Scholar]
- 14.Wang W., Xu Y., Gao R., Lu R., Han K., Wu G., Tan W. Detection of SARS-CoV-2 in different types of clinical specimens. Jama. 2020 doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Udugama B., Kadhiresan P., Kozlowski H.N., Malekjahani A., Osborne M., Li V.Y.C., Chen H., Mubareka S., Gubbay J., Chan W.C.W. Diagnosing COVID-19: the disease and tools for detection. ACS Nano. 2020 doi: 10.1021/acsnano.0c02624. [DOI] [PubMed] [Google Scholar]
- 16.Xiang J., Yan M., Li H., Liu T., Lin C., Huang S., Shen C. medRxiv; 2020. Evaluation of Enzyme-Linked Immunoassay and Colloidal Gold-Immunochromatographic Assay Kit for Detection of Novel Coronavirus (SARS-Cov-2) Causing an Outbreak of Pneumonia (COVID-19) [Google Scholar]
- 17.Foundation T.W.N. 2020. COVID-19 Colloidal Gold Method Antibody Test. [Google Scholar]
- 18.Qiu G., Gai Z., Tao Y., Schmitt J., Kullak-Ublick G.A., Wang J. Dual-functional plasmonic photothermal biosensors for highly accurate severe acute respiratory syndrome coronavirus 2 detection. ACS Nano. 2020;14(5):5268–5277. doi: 10.1021/acsnano.0c02439. [DOI] [PubMed] [Google Scholar]
- 19.Cavalcanti I.D.L., Nogueira MCdBL. Pharmaceutical nanotechnology: which products are been designed against COVID-19? J. Nanoparticle Res. 2020;22(9):1–11. doi: 10.1007/s11051-020-05010-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahmed S.R., Nagy É., Neethirajan S. Self-assembled star-shaped chiroplasmonic gold nanoparticles for an ultrasensitive chiro-immunosensor for viruses. RSC Adv. 2017;7(65):40849–40857. [Google Scholar]
- 21.Hu C.-M.J., Chang W.-S., Fang Z.-S., Chen Y.-T., Wang W.-L., Tsai H.-H., Chueh L.-L., Takano T., Hohdatsu T., Chen H.-W. Nanoparticulate vacuolar ATPase blocker exhibits potent host-targeted antiviral activity against feline coronavirus. Sci. Rep. 2017;7(1):1–11. doi: 10.1038/s41598-017-13316-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim Y.-S., Son A., Kim J., Kwon S.B., Kim M.H., Kim P., Kim J., Byun Y.H., Sung J., Lee J. Chaperna-mediated assembly of ferritin-based Middle East respiratory syndrome-coronavirus nanoparticles. Front. Immunol. 2018;9:1093. doi: 10.3389/fimmu.2018.01093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He L., Tai W., Li J., Chen Y., Gao Y., Li J., Sun S., Zhou Y., Du L., Zhao G. Enhanced ability of oligomeric nanobodies targeting MERS coronavirus receptor-binding domain. Viruses. 2019;11(2):166. doi: 10.3390/v11020166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raj V.S., Okba N.M., Gutierrez-Alvarez J., Drabek D., van Dieren B., Widagdo W., Lamers M.M., Widjaja I., Fernandez-Delgado R., Sola I. Chimeric camel/human heavy-chain antibodies protect against MERS-CoV infection. Sci. Adv. 2018;4(8) doi: 10.1126/sciadv.aas9667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao G., He L., Sun S., Qiu H., Tai W., Chen J., Li J., Chen Y., Guo Y., Wang Y. A novel nanobody targeting Middle East respiratory syndrome coronavirus (MERS-CoV) receptor-binding domain has potent cross-neutralizing activity and protective efficacy against MERS-CoV. J. Virol. 2018;92(18) doi: 10.1128/JVI.00837-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilken L., McPherson A. Application of camelid heavy-chain variable domains (VHHs) in prevention and treatment of bacterial and viral infections. Int. Rev. Immunol. 2018;37(1):69–76. doi: 10.1080/08830185.2017.1397657. [DOI] [PubMed] [Google Scholar]
- 27.Van Heeke G., Allosery K., De Brabandere V., De Smedt T., Detalle L., de Fougerolles A. Nanobodies® as inhaled biotherapeutics for lung diseases. Pharmacol. Therapeut. 2017;169:47–56. doi: 10.1016/j.pharmthera.2016.06.012. [DOI] [PubMed] [Google Scholar]
- 28.Li J., Helal Z.H., Karch C.P., Mishra N., Girshick T., Garmendia A., Burkhard P., Khan M.I. A self-adjuvanted nanoparticle based vaccine against infectious bronchitis virus. PloS One. 2018;13(9) doi: 10.1371/journal.pone.0203771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jung S.-Y., Kang K.W., Lee E.-Y., Seo D.-W., Kim H.-L., Kim H., Kwon T., Park H.-L., Kim H., Lee S.-M. Heterologous prime–boost vaccination with adenoviral vector and protein nanoparticles induces both Th1 and Th2 responses against Middle East respiratory syndrome coronavirus. Vaccine. 2018;36(24):3468–3476. doi: 10.1016/j.vaccine.2018.04.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coleman C.M., Venkataraman T., Liu Y.V., Glenn G.M., Smith G.E., Flyer D.C., Frieman M.B. MERS-CoV spike nanoparticles protect mice from MERS-CoV infection. Vaccine. 2017;35(12):1586–1589. doi: 10.1016/j.vaccine.2017.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Naskalska A., Dabrowska A., Nowak P., Szczepanski A., Jasik K., Milewska A., Ochman M., Zeglen S., Rajfur Z., Pyrc K. Novel coronavirus-like particles targeting cells lining the respiratory tract. PloS One. 2018;13(9) doi: 10.1371/journal.pone.0203489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim H., Park M., Hwang J., Kim J.H., Chung D.-R., Lee K-s, Kang M. Development of label-free colorimetric assay for MERS-CoV using gold nanoparticles. ACS Sens. 2019;4(5):1306–1312. doi: 10.1021/acssensors.9b00175. [DOI] [PubMed] [Google Scholar]
- 33.Teengam P., Siangproh W., Tuantranont A., Vilaivan T., Chailapakul O., Henry C.S. Multiplex paper-based colorimetric DNA sensor using pyrrolidinyl peptide nucleic acid-induced AgNPs aggregation for detecting MERS-CoV, MTB, and HPV oligonucleotides. Anal. Chem. 2017;89(10):5428–5435. doi: 10.1021/acs.analchem.7b00255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li W., Hulswit R.J., Widjaja I., Raj V.S., McBride R., Peng W., Widagdo W., Tortorici M.A., Van Dieren B., Lang Y. Identification of sialic acid-binding function for the Middle East respiratory syndrome coronavirus spike glycoprotein. Proc. Natl. Acad. Sci. Unit. States Am. 2017;114(40):E8508–E8517. doi: 10.1073/pnas.1712592114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Widagdo W., Okba N.M., Li W., De Jong A., De Swart R.L., Begeman L., Van Den Brand J.M., Bosch B.-J., Haagmans B.L. Species-specific colocalization of Middle East respiratory syndrome coronavirus attachment and entry receptors. J. Virol. 2019;93(16) doi: 10.1128/JVI.00107-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim E., Erdos G., Huang S., Kenniston T.W., Balmert S.C., Carey C.D., Raj V.S., Epperly M.W., Klimstra W.B., Haagmans B.L. Microneedle array delivered recombinant coronavirus vaccines: immunogenicity and rapid translational development. EBioMedicine. 2020;55:102743. doi: 10.1016/j.ebiom.2020.102743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weiss C., Carriere M., Fusco L., Capua I., Regla-Nava J.A., Pasquali M., Scott J.A., Vitale F., Unal M.A., Mattevi C. Toward nanotechnology-enabled approaches against the COVID-19 pandemic. ACS Nano. 2020 doi: 10.1021/acsnano.0c03697. [DOI] [PubMed] [Google Scholar]
- 38.Tavakol S., Hoveizi E., Kharrazi S., Tavakol B., Karimi S., Rezayat Sorkhabadi S.M. Organelles and chromatin fragmentation of human umbilical vein endothelial cell influence by the effects of zeta potential and size of silver nanoparticles in different manners. Artif. Cells Nanomed. Biotechnol. 2017;45(4):817–823. doi: 10.1080/21691401.2016.1178132. [DOI] [PubMed] [Google Scholar]
- 39.Lisuzzo L., Cavallaro G., Parisi F., Milioto S., Fakhrullin R., Lazzara G. Core/shell gel beads with embedded halloysite nanotubes for controlled drug release. Coatings. 2019;9(2):70. [Google Scholar]
- 40.Huy T.Q., Thanh N.T.H., Thuy N.T., Van Chung P., Hung P.N., Le A.-T., Hanh N.T.H. Cytotoxicity and antiviral activity of electrochemical–synthesized silver nanoparticles against poliovirus. J. Virol Methods. 2017;241:52–57. doi: 10.1016/j.jviromet.2016.12.015. [DOI] [PubMed] [Google Scholar]
- 41.Kerry R.G., Malik S., Redda Y.T., Sahoo S., Patra J.K., Majhi S. Nano-based approach to combat emerging viral (NIPAH virus) infection. Nanomed. Nanotechnol. Biol. Med. 2019;18:196–220. doi: 10.1016/j.nano.2019.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cagno V., Andreozzi P., D’Alicarnasso M., Silva P.J., Mueller M., Galloux M., Le Goffic R., Jones S.T., Vallino M., Hodek J. Broad-spectrum non-toxic antiviral nanoparticles with a virucidal inhibition mechanism. Nat. Mater. 2018;17(2):195–203. doi: 10.1038/nmat5053. [DOI] [PubMed] [Google Scholar]
- 43.Åkerström S., Mousavi-Jazi M., Klingström J., Leijon M., Lundkvist Å., Mirazimi A. Nitric oxide inhibits the replication cycle of severe acute respiratory syndrome coronavirus. J. Virol. 2005;79(3):1966–1969. doi: 10.1128/JVI.79.3.1966-1969.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Feng Z., Diao B., Wang R., Wang G., Wang C., Tan Y., Liu L., Wang C., Liu Y., Liu Y. The novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) directly decimates human spleens and lymph nodes. MedRxiv. 2020 [Google Scholar]
- 45.Liu C., Zhou Q., Li Y., Garner L.V., Watkins S.P., Carter L.J., Smoot J., Gregg A.C., Daniels A.D., Jervey S. ACS Publications; 2020. Research and Development on Therapeutic Agents and Vaccines for COVID-19 and Related Human Coronavirus Diseases. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sohrab S.S., El-Kafrawy S.A., Mirza Z., Kamal M.A., Azhar E.I. Design and delivery of therapeutic siRNAs: application to MERS-Coronavirus. Curr. Pharmaceut. Des. 2018;24(1):62–77. doi: 10.2174/1381612823666171109112307. [DOI] [PubMed] [Google Scholar]
- 47.Pentecost A., Kim M.J., Jeon S., Ko Y.J., Kwon I.C., Gogotsi Y., Kim K., Spiller K.L. Immunomodulatory nanodiamond aggregate-based platform for the treatment of rheumatoid arthritis. Regen. Biomater. 2019;6(3):163–174. doi: 10.1093/rb/rbz012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Seredych M., Haines B., Sokolova V., Cheung P., Meng F., Stone L., Mikhalovska L., Mikhalovsky S., Mochalin V.N., Gogotsi Y. Graphene-based materials for the fast removal of cytokines from blood plasma. ACS App. Bio Mater. 2018;1(2):436–443. doi: 10.1021/acsabm.8b00151. [DOI] [PubMed] [Google Scholar]
- 49.Tavakol S., Ashrafizadeh M., Deng S., Azarian M., Abdoli A., Motavaf M., Poormoghadam D., Khanbabaei H., Ghasemipour Afshar E., Mandegary A. Autophagy modulators: mechanistic aspects and drug delivery systems. Biomolecules. 2019;9(10):530. doi: 10.3390/biom9100530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Łoczechin A., Séron K., Barras A., Giovanelli E., Belouzard S., Chen Y.-T., Metzler-Nolte N., Boukherroub R., Dubuisson J., Szunerits S. Functional carbon quantum dots as medical countermeasures to human coronavirus. ACS Appl. Mater. Interfaces. 2019;11(46):42964–42974. doi: 10.1021/acsami.9b15032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huang X., Li M., Xu Y., Zhang J., Meng X., An X., Sun L., Guo L., Shan X., Ge J. Novel gold nanorod-based HR1 peptide inhibitor for Middle East respiratory syndrome coronavirus. ACS Appl. Mater. Interfaces. 2019;11(22):19799–19807. doi: 10.1021/acsami.9b04240. [DOI] [PubMed] [Google Scholar]
- 52.Abo-Zeid Y., Ismail N.S., McLean G.R., Hamdy N.M. A molecular docking study repurposes FDA approved iron oxide nanoparticles to treat and control COVID-19 infection. Eur. J. Pharmaceut. Sci. 2020;153:105465. doi: 10.1016/j.ejps.2020.105465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang Q., Honko A., Zhou J., Gong H., Downs S.N., Vasquez J.H., Fang R.H., Gao W., Griffiths A., Zhang L. Cellular nanosponges inhibit SARS-CoV-2 infectivity. Nano Lett. 2020;20(7):5570–5574. doi: 10.1021/acs.nanolett.0c02278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dong L., Hu S., Gao J. Discovering drugs to treat coronavirus disease 2019 (COVID-19) Drug Discov. Therapeut. 2020;14(1):58–60. doi: 10.5582/ddt.2020.01012. [DOI] [PubMed] [Google Scholar]
- 55.Lu H. Drug treatment options for the 2019-new coronavirus (2019-nCoV) Biosci. Trends. 2020;14(1):69–71. doi: 10.5582/bst.2020.01020. [DOI] [PubMed] [Google Scholar]
- 56.Cánepa C., Imperiale J.C., Berini C.A., Lewicki M., Sosnik A., Biglione M.M. Development of a drug delivery system based on chitosan nanoparticles for oral administration of interferon-α. Biomacromolecules. 2017;18(10):3302–3309. doi: 10.1021/acs.biomac.7b00959. [DOI] [PubMed] [Google Scholar]
- 57.Cavalcanti I.D.L., Ramos dos Santos Medeiros SMdF., Macêdo DCdS., Cavalcanti I.M.F., Lira Nogueira MCdB. Nanocarriers in the delivery of hydroxychloroquine to the respiratory system: an alternative to COVID-19. Curr. Drug Deliv. 2020 doi: 10.2174/1567201817666200827110445. [DOI] [PubMed] [Google Scholar]
- 58.Zhang M.-Y., Zhang Y., Wu X.-D., Zhang K., Lin P., Bian H.-J., Qin M.-M., Huang W., Wei D., Zhang Z. Disrupting CD147-RAP2 interaction abrogates erythrocyte invasion by Plasmodium falciparum. Blood. 2018;131(10):1111–1121. doi: 10.1182/blood-2017-08-802918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Imperiale J.C., Schlachet I., Lewicki M., Sosnik A., Biglione M.M. Oral pharmacokinetics of a chitosan-based nano-drug delivery system of interferon alpha. Polymers. 2019;11(11):1862. doi: 10.3390/polym11111862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cojocaru F.-D., Botezat D., Gardikiotis I., Uritu C.-M., Dodi G., Trandafir L., Rezus C., Rezus E., Tamba B.-I., Mihai C.-T. Nanomaterials designed for antiviral drug delivery transport across biological barriers. Pharmaceutics. 2020;12(2):171. doi: 10.3390/pharmaceutics12020171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Abou-El-Naga I.F., El Kerdany E.D., Mady R.F., Shalaby T.I., Zaytoun E.M. The effect of lopinavir/ritonavir and lopinavir/ritonavir loaded PLGA nanoparticles on experimental toxoplasmosis. Parasitol. Int. 2017;66(6):735–747. doi: 10.1016/j.parint.2017.08.007. [DOI] [PubMed] [Google Scholar]
- 62.Du T., Liang J., Dong N., Lu J., Fu Y., Fang L., Xiao S., Han H. Glutathione-capped Ag2S nanoclusters inhibit coronavirus proliferation through blockage of viral RNA synthesis and budding. ACS Appl. Mater. Interfaces. 2018;10(5):4369–4378. doi: 10.1021/acsami.7b13811. [DOI] [PubMed] [Google Scholar]
- 63.Lauster D., Klenk S., Ludwig K., Nojoumi S., Behren S., Adam L., Stadtmüller M., Saenger S., Zimmler S., Hönzke K. Phage capsid nanoparticles with defined ligand arrangement block influenza virus entry. Nat. Nanotechnol. 2020:1–7. doi: 10.1038/s41565-020-0660-2. [DOI] [PubMed] [Google Scholar]
- 64.Braunstein G.D. Safety of testosterone treatment in postmenopausal women. Fertil. Steril. 2007;88(1):1–17. doi: 10.1016/j.fertnstert.2007.01.118. [DOI] [PubMed] [Google Scholar]
- 65.Presser V., Yeon S.H., Vakifahmetoglu C., Howell C.A., Sandeman S.R., Colombo P., Mikhalovsky S., Gogotsi Y. Hierarchical porous carbide-derived carbons for the removal of cytokines from blood plasma. Adv. Healthcare Mater. 2012;1(6):796–800. doi: 10.1002/adhm.201200044. [DOI] [PubMed] [Google Scholar]
- 66.Itani R., Tobaiqy M., Al Faraj A. Optimizing use of theranostic nanoparticles as a life-saving strategy for treating COVID-19 patients. Theranostics. 2020;10(13):5932. doi: 10.7150/thno.46691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fratev F. bioRxiv; 2020. The SARS-CoV-2 S1 Spike Protein Mutation N501Y Alters the Protein Interactions with Both hACE2 and Human Derived Antibody: A Free Energy of Perturbation Study. [DOI] [PubMed] [Google Scholar]
- 68.Aldayel A.M., O'Mary H.L., Valdes S.A., Li X., Thakkar S.G., Mustafa B.E., Cui Z. Lipid nanoparticles with minimum burst release of TNF-α siRNA show strong activity against rheumatoid arthritis unresponsive to methotrexate. J. Contr. Release. 2018;283:280–289. doi: 10.1016/j.jconrel.2018.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Iqbal S., Du X., Wang J., Li H., Yuan Y., Wang J. Surface charge tunable nanoparticles for TNF-α siRNA oral delivery for treating ulcerative colitis. Nano Res. 2018;11(5):2872–2884. [Google Scholar]
- 70.Khan M.A., Khan M.J. Nano-gold displayed anti-inflammatory property via NF-kB pathways by suppressing COX-2 activity. Artif. Cells Nanomed. Biotechnol. 2018;46(sup1):1149–1158. doi: 10.1080/21691401.2018.1446968. [DOI] [PubMed] [Google Scholar]
- 71.Shokrzadeh M., Ghassemi-Barghi N. Melatonin loading chitosan-tripolyphosphate nanoparticles: application in attenuating etoposide-induced genotoxicity in HepG2 cells. Pharmacology. 2018;102(1-2):74–80. doi: 10.1159/000489667. [DOI] [PubMed] [Google Scholar]
- 72.de Oliveira Junior E.R., Nascimento T.L., Salomão M.A., da Silva A.C.G., Valadares M.C., Lima E.M. Increased nose-to-brain delivery of melatonin mediated by polycaprolactone nanoparticles for the treatment of glioblastoma. Pharmaceut. Res. 2019;36(9):131. doi: 10.1007/s11095-019-2662-z. [DOI] [PubMed] [Google Scholar]
- 73.Xie W., Gao Q., Wang D., Wang W., Yuan J., Guo Z., Yan H., Wang X., Sun X., Zhao L. Melatonin potentiates “inside-out” nano-thermotherapy in human breast cancer cells: a potential cancer target multimodality treatment based on melatonin-loaded nanocomposite particles. Int. J. Nanomed. 2017;12:7351. doi: 10.2147/IJN.S148520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li S., Zhao Y. Preparation of melatonin-loaded Zein nanoparticles using supercritical CO2 antisolvent and in vitro release evaluation. Int. J. Food Eng. 2017;13(11) [Google Scholar]
- 75.Srivastava A.K., Choudhury S.R., Karmakar S. Melatonin/polydopamine nanostructures for collective neuroprotection-based Parkinson's disease therapy. Biomater. Sci. 2020 doi: 10.1039/c9bm01602c. [DOI] [PubMed] [Google Scholar]
- 76.Mirabbasi F., Dorkoosh F.A., Moghimi A., Shahsavari S., Babanejad N., Seifirad S. Preparation of mesalamine nanoparticles using a novel polyurethane-chitosan graft copolymer. Pharm. Nanotechnol. 2017;5(3):230–239. doi: 10.2174/2211738505666171103120026. [DOI] [PubMed] [Google Scholar]
- 77.Meruva S., Thool P., Shah S., Karki S., Bowen W., Ghosh I., Kumar S. Formulation and performance of Irbesartan nanocrystalline suspension and granulated or bead-layered dried powders–Part I. Int. J. Pharm. 2019;568:118189. doi: 10.1016/j.ijpharm.2019.03.007. [DOI] [PubMed] [Google Scholar]
- 78.Saleem M.A., Nazar M.F., Siddique M.Y., Khan A.M., Ashfaq M., Hussain S.Z., Khalid M.R., Yameen B. Soft-templated fabrication of antihypertensive nano-Irbesartan: structural and dissolution evaluation. J. Mol. Liq. 2019;292:111388. [Google Scholar]
- 79.Zhang Z., Ji J. Large-scale preparation of stable irbesartan nanoparticles by high-gravity liquid antisolvent precipitation technique. Powder Technol. 2017;305:546–552. [Google Scholar]
- 80.Soma D., Attari Z., Reddy M.S., Damodaram A., Koteshwara K.B.G. Solid lipid nanoparticles of irbesartan: preparation, characterization, optimization and pharmacokinetic studies. Brazil. J. Pharmaceut. Sci. 2017;53(1) [Google Scholar]
- 81.Kleszczyński K., Slominski A.T., Steinbrink K., Reiter R.J. Clinical trials for use of melatonin to fight against COVID-19 are urgently needed. Nutrients. 2020;12(9):2561. doi: 10.3390/nu12092561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Di Stefano A., Paulesu L. Inhibitory effect of melatonin on production of IFNγ or TNFα in peripheral blood mononuclear cells of some blood donors. J. Pineal Res. 1994;17(4):164–169. doi: 10.1111/j.1600-079x.1994.tb00128.x. [DOI] [PubMed] [Google Scholar]
- 83.Tang P., Sun Q., Zhao L., Pu H., Yang H., Zhang S., Gan R., Gan N., Li H. Mesalazine/hydroxypropyl-β-cyclodextrin/chitosan nanoparticles with sustained release and enhanced anti-inflammation activity. Carbohydr. Polym. 2018;198:418–425. doi: 10.1016/j.carbpol.2018.06.106. [DOI] [PubMed] [Google Scholar]
- 84.Moustafa M., Amin A., Magdi Y. Cytotoxicity of 6-mercaptopurine via loading on PVA-coated magnetite nanoparticles delivery system: a new era of leukemia therapy. J. Nanomed. Nanotechnol. 2018;9(521):2. [Google Scholar]
- 85.Nezhad-Mokhtari P., Ghorbani M., Mahmoodzadeh F. Smart co-delivery of 6-mercaptopurine and methotrexate using disulphide-based PEGylated-nanogels for effective treatment of breast cancer. New J. Chem. 2019;43(30):12159–12167. [Google Scholar]
- 86.Qiu J., Cheng R., Zhang J., Sun H., Deng C., Meng F., Zhong Z. Glutathione-sensitive hyaluronic acid-mercaptopurine prodrug linked via carbonyl vinyl sulfide: a robust and CD44-targeted nanomedicine for leukemia. Biomacromolecules. 2017;18(10):3207–3214. doi: 10.1021/acs.biomac.7b00846. [DOI] [PubMed] [Google Scholar]
- 87.Wu W., Guo L., Liang Z., Liu Y., Yao Z. Lnc-SNHG16/miR-128 axis modulates malignant phenotype through WNT/β-catenin pathway in cervical cancer cells. J. Canc. 2020;11(8):2201. doi: 10.7150/jca.40319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shen Y., Li X., Le Y. Amorphous nanoparticulate formulation of sirolimus and its tablets. Pharmaceutics. 2018;10(3):155. doi: 10.3390/pharmaceutics10030155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kong J., Wu K., Ji Y., Chen K., Zhang J., Sun H., Liang Y., Liang W., Chang Y., Cheng J. Enhanced bioavailability by orally administered sirolimus Nanocrystals. ACS App. Bio Mater. 2019;2(10):4612–4621. doi: 10.1021/acsabm.9b00695. [DOI] [PubMed] [Google Scholar]
- 90.Knuschke T., Rotan O., Bayer W., Kollenda S., Dickow J., Sutter K., Hansen W., Dittmer U., Lang K.S., Epple M. Induction of type I interferons by therapeutic nanoparticle-based vaccination is indispensable to reinforce cytotoxic CD8+ T cell responses during chronic retroviral infection. Front. Immunol. 2018;9:614. doi: 10.3389/fimmu.2018.00614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tavakol S., Alavijeh M.S., Seifalian A.M. Current pharmaceutical design; 2020. COVID-19 Vaccines in Clinical Trials and Their Mode of Action for Immunity against the Virus. [DOI] [PubMed] [Google Scholar]
- 92.Kuba K., Zhang L., Imai Y., Arab S., Chen M., Maekawa Y., Leschnik M., Leibbrandt A., Markovic M., Schwaighofer J. Impaired heart contractility in Apelin gene–deficient mice associated with aging and pressure overload. Circ. Res. 2007;101(4):e32–e42. doi: 10.1161/CIRCRESAHA.107.158659. [DOI] [PubMed] [Google Scholar]
- 93.Carter D.C., Wright B., Jerome W.G., Rose J.P., Wilson E. A unique protein self-assembling nanoparticle with significant advantages in vaccine development and production. J. Nanomater. 2020:2020. [Google Scholar]
- 94.Jando J., Camargo S.M., Herzog B., Verrey F. Expression and regulation of the neutral amino acid transporter B0AT1 in rat small intestine. PloS One. 2017;12(9) doi: 10.1371/journal.pone.0184845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhu M., Wang R., Nie G. Applications of nanomaterials as vaccine adjuvants. Hum. Vaccines Immunother. 2014;10(9):2761–2774. doi: 10.4161/hv.29589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ma J., Liu R., Wang X., Liu Q., Chen Y., Valle R.P., Zuo Y.Y., Xia T., Liu S. Crucial role of lateral size for graphene oxide in activating macrophages and stimulating pro-inflammatory responses in cells and animals. ACS Nano. 2015;9(10):10498–10515. doi: 10.1021/acsnano.5b04751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wiehe A., O'Brien J.M., Senge M.O. Trends and targets in antiviral phototherapy. Photochem. Photobiol. Sci. 2019;18(11):2565–2612. doi: 10.1039/c9pp00211a. [DOI] [PubMed] [Google Scholar]
- 98.Tavakol S., Kiani V., Tavakol B., Derakhshan M.A., Joghataei M.T., Rezayat S.M. Elsevier; 2017. Toxicity Concerns of Nanocarriers. Nanotechnology-Based Approaches for Targeting and Delivery of Drugs and Genes; pp. 453–484. [Google Scholar]
- 99.Mohammadinejad R., Moosavi M.A., Tavakol S., Vardar D.Ö., Hosseini A., Rahmati M., Dini L., Hussain S., Mandegary A., Klionsky D.J. Necrotic, apoptotic and autophagic cell fates triggered by nanoparticles. Autophagy. 2019;15(1):4–33. doi: 10.1080/15548627.2018.1509171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Devaux C.A., Rolain J.-M., Colson P., Raoult D. New insights on the antiviral effects of chloroquine against coronavirus: what to expect for COVID-19? Int. J. Antimicrob. Agents. 2020:105938. doi: 10.1016/j.ijantimicag.2020.105938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Govindappa P.K., Joladarashi D., Hallur R.L.S., Sanganal J.S., Phani A.R. Toxicity evaluation of 6-mercaptopurine-Chitosan nanoparticles in rats. Saudi Pharmaceut. J. 2020;28(1):147–154. doi: 10.1016/j.jsps.2019.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supp. material/referenced in article.