Abstract
Background
Pleomorphic xanthoastrocytomas (PXA) are circumscribed gliomas that typically have a favorable prognosis. Limited studies have revealed factors affecting survival outcomes in PXA. Here, we analyzed the largest PXA dataset in the literature and identify factors associated with outcomes.
Methods
Using the Surveillance, Epidemiology, and End Results (SEER) 18 Registries database, we identified histologically confirmed PXA patients between 1994 and 2016. Overall survival (OS) was analyzed using Kaplan-Meier survival and multivariable Cox proportional hazard models.
Results
In total, 470 patients were diagnosed with PXA (males = 53%; median age = 23 years [14-39 years]), the majority were Caucasian (n = 367; 78%). The estimated mean OS was 193 months [95% CI: 179-206]. Multivariate analysis revealed that greater age at diagnosis (≥39 years) (3.78 [2.16-6.59], P < .0001), larger tumor size (≥30 mm) (1.97 [1.05-3.71], P = .034), and postoperative radiotherapy (RT) (2.20 [1.31-3.69], P = .003) were independent predictors of poor OS. Pediatric PXA patients had improved survival outcomes compared to their adult counterparts, in which chemotherapy (CT) was associated with worse OS. Meanwhile, in adults, females and patients with temporal lobe tumors had an improved survival; conversely, tumor size ≥30 mm and postoperative RT were associated with poor OS.
Conclusions
In PXA, older age and larger tumor size at diagnosis are risk factors for poor OS, while pediatric patients have remarkably improved survival. Postoperative RT and CT appear to be ineffective treatment strategies while achieving GTR confer an improved survival in male patients and remains the cornerstone of treatment. These findings can help optimize PXA treatment while minimizing side effects. However, further studies of PXAs with molecular characterization are needed.
Keywords: gender, pleomorphic xanthoastrocytoma, PXA, SEER, survival
Pleomorphic xanthoastrocytoma (PXA) is a rare circumscribed primary glial tumor that accounts for less than 1% of astrocytic tumors, most commonly affecting children and young adults.1–4 The rare nature of PXA has resulted in a sparsity of literature with most studies being represented by case reports or series with limited sample size.3 Molecular characterization of PXAs has consistently demonstrated the presence of the BRAF p.V600E in a high proportion of cases (up to ~80%).3–12 PXAs without the BRAF p.V600E mutation show other MAPK pathway gene alterations.11,12 Additionally, PXAs show coexisting BRAF p.V600E and CDKN2A/B loss in up to 94% of cases.9–12 Therefore, PXA diagnosis in the absence of these mutations should prompt careful re-evaluation to avoid misdiagnosis. Additionally, methylation profiling of PXA has been shown to distinguish this tumor from other brain tumors in a precise manner.12 Despite these advances in the molecular landscape of PXA, our understanding of the factors affecting survival outcomes has been limited by its relatively low incidence.
Surgical resection remains the cornerstone of PXA treatment; however, there are conflicting studies on the impact of extent of resection on survival, with the largest series reporting improved progression-free survival (PFS), but no statistically significant differences in overall survival (OS) for patients undergoing gross total resection (GTR).1–3,6,13–16 Even though the role of adjuvant radiotherapy (RT) and chemotherapy (CT) is not well established in the literature,1–4,6,14,17 both are routinely utilized in patients with incomplete surgical resection, tumor recurrence, or high-grade features (ie, anaplastic PXA, WHO grade III).1,3,4,8,17
Given the relative paucity of data in the literature exploring factors associated with outcomes in PXA, we utilized the large, publicly available SEER database, to examine the demographic and clinical characteristics of PXA patients to identify factors that predict OS.
Methods
Study Population
Patients with a diagnosis of PXA were identified and selected from the SEER 18-Registry, from 1994 to 2016 using the International Classification of Diseases for Oncology, third edition (ICD-O-3), with code 9424/3. This period was selected, as PXA was not included until the 1993 WHO classification.18 Only histologically confirmed cases with available survival data were included in the study. The SEER database provides data on cancer statistics, including incidence and mortality, and collects demographic, clinicopathologic, and treatment data from cancer patients in the United States. The SEER 18-Registry contains data from approximately 28% of the US cancer cases.
Study Design
Demographic characteristics included age at diagnosis, gender, race, and ethnicity. Clinicopathologic characteristics included the year of diagnosis, tumor size, location, and laterality. Tumor size reported, as a size range (eg, 0-1 cm), was assigned the midpoint of the range (eg, 5 mm). About 209 (44%) records did not have available tumor size data; these were categorized as unknown tumor size. Tumor laterality was categorized as left, right, or not a paired site. A supratentorial tumor location was defined by topography codes C70.0 and C70.2-C71.4, and an infratentorial location by topography codes C71.6-C71.7.
The SEER database provides data on the first course of treatment, including surgical resection, RT, and CT. However, the reason for a patient not undergoing a certain treatment is not captured in the SEER registry data. Surgical procedures were identified following the two-digit site-specific surgery codes (1994-1997) and the SEER Program Coding and Staging Manual 2018 (1998+) and were categorized as GTR (codes 30, 50 [1994-1997] and 30, 55 [1998+]) and non-GTR (codes 00, 01, 02, 03, 04, 05, 06, 10, 20, 40, 80, 90 [1983-1997] and 00, 10, 20, 21, 22, 40, 90 [1998+]). The use of CT and RT was categorized as yes or no/unknown. Postoperative RT was defined using the available field regarding radiation sequence with surgery. WHO grade was not included as a variable, as WHO grade III PXA was added on the 2016 WHO central nervous system (CNS) classification update, the year in which our study period ended.19
Statistical Analysis
Continuous variables were reported as medians and were compared using the Wilcoxon rank-sum test and discrete variables were analyzed using Fisher’s exact test. In this cohort, age and year of diagnosis did not follow a normal distribution. Thus, the quartiles of these variables were utilized as cutoffs to be defined as ordinal variables as follows: age (0-13, 14-22, 23-38, and ≥39) and year of diagnosis (1994-2000, 2001-2005, 2006-2010, and 2011-2016). Tumor size was grouped into 3 categories: 0-29 mm, ≥30 mm, and unknown.
The primary endpoint of the study was OS. Survival estimates of OS were calculated using the Kaplan-Meier method and compared by the univariable log-rank test. Multivariable Cox regression model was utilized to assess the effect of gender, adjusting for the following set of covariates: age at diagnosis, female gender, White race, Hispanic ethnicity, year of diagnosis, tumor location, size, and laterality, GTR, postoperative RT, and CT. We then examined the effect of gender on OS in pediatric and adult patients, separately, in multivariable analysis adjusting for a set of covariates, as mentioned above.
Data preprocessing and statistical analyses were performed using STATA (14.0, StataCorp, College Station, TX). Results are given as median (IQR) and hazard ratio (HR) reported with 95% CI (HR [95% CI]) unless otherwise specified. All P-values were two-sided and considered statistically significant as P ≤ .05.
Ethics Statement
This study adheres to the SEER data use policies. Access to the SEER database was requested and granted to one of the authors (V.L.) after the execution of a data use agreement. Ethical approval was waived by the Committee for the Protection of Human Subjects as this study does not involve human subjects research.
Results
Patients’ Demographics
The cohort consisted of 470 patients diagnosed with PXA between 1994 and 2016, of which 250 (53%) were male and 220 (47%) were female (Table 1). Among patients in the cohort, the median age was 23 years (14-39 years), 78% were White, and 23% were Spanish or Hispanic or Latino. Two-thirds of patients (68%) were diagnosed between 2006 and 2016, and in nearly all patients, the tumor was located in the supratentorial compartment (97%), of which the majority were found in the temporal lobe (37%), followed by the frontal lobe (20%). The tumor size median was 32 mm (20-50 mm). As part of their first course of treatment, 57% of patients were treated with GTR, 26% with postoperative RT, and 17% with CT. As shown in Table 1, demographic, clinicopathological, and treatment characteristics of male patients were comparable to those of female patients.
Table 1.
Demographics, Clinical Characteristics, and Treatments of Patients with Pleomorphic Xanthoastrocytoma (N = 470)
| Variables | All (N = 470) | Male (N = 250) | Female (N = 220) | P-value |
|---|---|---|---|---|
| Age at diagnosis (years), median [IQR] | 23 [14-39] | 23 [13-40] | 23 [15-39] | .4943 |
| Race, % | .453 | |||
| White | 367 (78%) | 201 (80%) | 166 (75%) | |
| Black | 55 (12%) | 27 (11%) | 28 (13%) | |
| Asian or Pacific Islander | 35 (7%) | 14 (6%) | 21 (10%) | |
| Other/unknown | 13 (3%) | NA | NA | |
| Spanish or Hispanic or Latino, % | 106 (23%) | 50 (20%) | 56 (25%) | .184 |
| Year of diagnosis, % | .112 | |||
| 1994-2000 | 61 (13%) | 27 (11%) | 34 (15%) | |
| 2001-2005 | 90 (19%) | 41 (17%) | 49 (22%) | |
| 2006-2010 | 124 (26%) | 70 (28%) | 54 (25%) | |
| 2011-2016 | 195 (42%) | 112 (45%) | 82 (38%) | |
| Tumor laterality, % | .277 | |||
| Left | 163 (35%) | 84 (34%) | 97 (36%) | |
| Right | 156 (33%) | 91 (36%) | 84 (30%) | |
| Not a paired site | 151 (32%) | 75 (30%) | 85 (35%) | |
| Supratentorial location, % | 457 (97%) | 245 (98%) | 212 (96%) | .399 |
| Tumor location, % | .941 | |||
| Frontal lobe | 93 (20%) | 51 (20%) | 42 (19%) | |
| Temporal lobe | 173 (37%) | 92 (37%) | 81 (37%) | |
| Parietal lobe | 71 (15%) | 37 (15%) | 34 (15%) | |
| Occipital lobe | 36 (8%) | 21 (8%) | 15 (7%) | |
| Other location | 97 (21%) | 49 (20%) | 48 (22%) | |
| Tumor size (mm), median [IQR] | 32 [20-50] | 32 [20-50] | 30 [20-48] | .6208 |
| Tumor size, categorical, % | .729 | |||
| ≥ 30 mm | 118 (25%) | 62 (25%) | 56 (25%) | |
| 0-29 mm | 143 (30%) | 80 (32%) | 63 (29%) | |
| Other/Unknown | 209 (44%) | 108 (43%) | 101 (46%) | |
| Gross total resection, % | 266 (57%) | 148 (59%) | 118 (54%) | .131 |
| Postoperative radiotherapy, % | 123 (26%) | 63 (25%) | 60 (27%) | .674 |
| Chemotherapy, % | 82 (17%) | 45 (18%) | 37 (17%) | .808 |
Factors Associated with Overall Survival
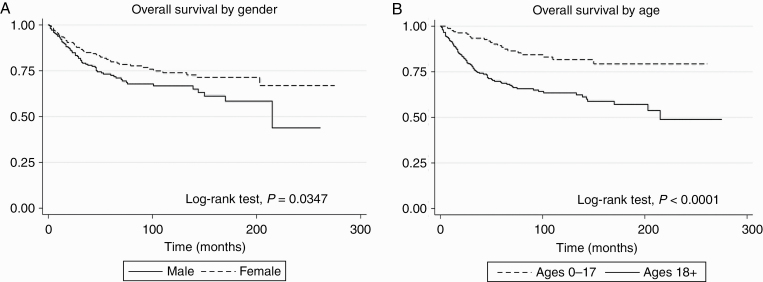
The estimated mean survival time of patients in the cohort was 193 months [95% CI: 179-206]. Twenty-five percent (25%) of male patients in the cohort died by 50 months, whereas 25% of female patients by 120 months. As shown in Figure 1A, the univariable analysis revealed a better survival among female patients compared to male patients (log-rank test, P = .0347). Unadjusted proportional hazards regression analysis (Table 2) revealed that female patients experienced an improved survival (HR: 0.67 [0.47-0.97]; P = .036) compared to male patients; however, this finding was non-significant in multivariable analysis (HR: 0.68 [0.47-1.01]; P = .057). In unadjusted analysis, increasing age, tumor size ≥30 mm, use of postoperative RT, and CT were associated with worse survival in patients with a PXA. Multivariable analysis confirmed that age ≥39 years (HR: 3.78 [2.16-6.59], P < .0001), tumor size ≥30 mm (HR: 1.97 [1.05-3.71], P = .034), and postoperative RT (HR: 2.20 [1.32-3.69], P = .003) were associated with worse OS. Meanwhile, the unadjusted analysis demonstrated that temporal lobe location PXA patients had an improved survival (HR: 0.60 [0.39-0.90], P = .013), this was not significant after multivariable adjustment (Table 2).
Figure 1.
Kaplan-Meier curves of survival outcomes estimates of pleomorphic xanthoastrocytoma (PXA) by gender, age, extent of resection (n = 470). A, Kaplan-Meier curve of survival outcome estimate of PXA by gender, in which female (n = 220) patients had improved survival compared to male (n = 250) patients (P = .0347). B, Kaplan-Meier curve of survival outcome estimate of PXA by age, in which pediatric (n = 174) patients had improved survival compared to adults (n = 296) patients (P < .0001).
Table 2.
Univariable and Multivariable Cox Hazard Proportional Regression Model for Overall Survival in the Cohort (n = 470)
| Factor | Univariable | Multivariable | ||
|---|---|---|---|---|
| HR [95% CI] | P-value | HR [95% CI] | P-value | |
| Age at diagnosis, 0-13 | Ref | - | Ref | - |
| 14-22 | 0.78 [0.38-1.59] | .495 | 0.94 [0.45-1.94] | .862 |
| 23-38 | 1.87 [1.03-3.78] | .039 | 1.68 [0.91-3.11] | .095 |
| 39+ | 4.90 [2.89-8.34] | <.0001 | 3.78 [2.16-6.59] | <.0001 |
| Female gender | 0.67 [0.47-0.97] | .036 | 0.68 [0.47-1.01] | .057 |
| White race | 0.92 [0.59-1.41] | .693 | 1.00 [0.63-1.58] | .994 |
| Hispanic ethnicity | 0.63 [0.38-1.05] | .074 | 0.76 [0.44-1.30] | .313 |
| Year of diagnosis, 1994-2000 | Ref | - | Ref | - |
| 2001-2005 | 0.93 [0.54-1.63] | .810 | 1.67 [0.90-3.10] | .100 |
| 2006-2010 | 0.87 [0.50-1.51] | .623 | 1.47 [0.78-2.77] | .229 |
| 2011-2016 | 1.04 [0.59-1.86] | .872 | 1.37 [0.59-3.18] | .458 |
| Tumor size, 0-29 mm | Ref | - | Ref | - |
| ≥30 mm | 2.68 [1.46-4.95] | .002 | 1.97 [1.05-3.71] | .034 |
| Other/unknown | 2.37 [1.32-4.25] | .004 | 2.66 [1.35-5.27] | .005 |
| Temporal lobe location | 0.60 [0.39-0.90] | .013 | 0.73 [0.48-1.12] | .155 |
| Left laterality | 0.93 [0.62-1.39] | .728 | 1.02 [0.66-1.59] | .919 |
| Gross total resection | 0.77 [0.54-1.12] | .172 | 0.75 [0.51-1.09] | .128 |
| Postoperative radiotherapy | 4.34 [3.00-6.27] | <.0001 | 2.20 [1.31-3.69] | .003 |
| Chemotherapy | 3.98 [2.71-5.86] | <.0001 | 1.60 [0.95-2.70] | .076 |
Bold values are the statistically significant variables.
Additionally, a subanalysis of RT and CT by the extent of resection demonstrated that patients treated with adjuvant therapies had a worse survival even when stratified by the extent of resection (see Supplementary Figure 1A and B).
Multivariable Cox Hazard Regression Model for Overall Survival by Gender and Age Group
We examined the factors influencing survival among male and female patients. Multivariable analysis (Table 3) revealed that male patients undergoing GTR had improved survival. Meanwhile, increasing age and treatment with CT were related to poor survival in male patients. Conversely, GTR did not improve survival in the female cohort and CT did not worsen the OS in female patients. However, age was associated with increased mortality.
Table 3.
Multivariable Cox Hazard Regression Model for Overall Survival in Male and Female Patients
| Factor | Male (n = 250) | Female (n = 220) | ||
|---|---|---|---|---|
| HR [95% CI] | P-value | HR [95% CI] | P-value | |
| Age at diagnosis, 0-13 | Ref | - | Ref | - |
| 14-22 | 0.74 [0.22-2.50] | .627 | 1.08 [0.41-2.8] | .881 |
| 23-38 | 2.58 [1.12-5.95] | .026 | 1.04 [0.39-2.82] | .934 |
| 39+ | 4.66 [2.16-10.06] | <.001 | 3.55 [1.54-8.18] | .003 |
| White race | 1.14 [0.58-2.23] | .701 | 0.72 [0.36-1.44] | .351 |
| Hispanic ethnicity | 0.88 [0.40-1.92] | .753 | 0.5 [0.22-1.14] | .100 |
| Year of diagnosis, 1994-2000 | Ref | - | Ref | - |
| 2001-2005 | 0.65 [0.26-1.64] | .361 | 4.64 [1.81-11.92] | .001 |
| 2006-2010 | 0.7 [0.29-1.68] | .42 | 3.5 [1.27-9.67] | .016 |
| 2011-2016 | 0.73 [0.23-2.31] | .588 | 3.48 [0.93-12.97] | .063 |
| Tumor size, 0-29 mm | Ref | - | Ref | - |
| ≥30 mm | 1.69 [0.75-3.77] | .205 | 2.1 [0.71-6.16] | .177 |
| Other/unknown | 1.11 [0.42-2.96] | .829 | 6.55 [2.26-18.98] | .001 |
| Temporal lobe location | 0.81 [0.47-1.39] | .440 | 0.64 [0.31-1.30] | .215 |
| Left laterality | 1.19 [0.66-2.15] | .569 | 0.88 [0.43-1.80] | .733 |
| Gross total resection | 0.58 [0.35-0.96] | .036 | 0.92 [0.51-1.66] | .778 |
| Postoperative radiotherapy | 1.52 [0.71-3.25] | .280 | 2.1 [0.93-4.75] | .074 |
| Chemotherapy | 2.56 [1.21-5.43] | .014 | 1.43 [0.64-3.21] | .387 |
Bold values are the statistically significant variables.
Finally, we examined the influence of age on survival and identified factors associated with outcome in pediatric (n = 174) and adult patients (n = 296). As shown in Figure 1B, pediatric patients had lower mortality compared to adult patients (log-rank test, P < .0001). In adjusted proportional hazards regression analysis (Table 4), pediatric patients treated with CT had worse survival compared to those who did not receive CT. Female patients had a similar survival compared to male patients in the pediatric population. However, we noted that female patients experienced a decrease in mortality in the adult population (HR: 0.59 [0.38-0.91], P = .017). Moreover, in the adult population, patients with tumor size ≥30 mm (HR: 2.06 [1.08-3.95], P = .029) and treated with postoperative RT (HR: 2.51 [1.43-4.41], P = .001) had poor survival. Meanwhile, patients with a tumor located in the temporal lobe had an improved survival (HR: 0.61 [0.37-0.99], P = .050). Interestingly, GTR did not improve the survival outcomes in the pediatric (HR: 0.77, P = .620).
Table 4.
Multivariable Cox Hazard Regression Model for Overall Survival in Pediatric and Adult Patients
| Factor | Pediatric (n = 174) | Adult (n = 296) | ||
|---|---|---|---|---|
| HR [95% CI] | P-value | HR [95% CI] | P-value | |
| Female gender | 0.51 [0.19-0.1.36] | .177 | 0.59 [0.38-0.91] | .017 |
| White race | 0.42 [0.13-1.41] | .162 | 1.00 [0.94-2.63] | .087 |
| Hispanic ethnicity | 1.91 [0.67-5.48] | .227 | 0.55 [0.29-1.08] | .082 |
| Year of diagnosis, 1994-2000 | Ref | - | Ref | - |
| 2001-2005 | 0.91 [0.25-3.35] | .883 | 2.07 [1.00-4.28] | .050 |
| 2006-2010 | 0.50 [0.08-2.89] | .440 | 2.298 [1.12-4.69] | .024 |
| 2011-2016 | NA | - | 2.04 [0.83-5.02] | .119 |
| Tumor size, 0-29 mm | Ref | - | Ref | - |
| ≥30 mm | - | - | 2.06 [1.08-3.95] | .029 |
| Other/unknown | - | - | 3.17 [1.55-6.46] | .001 |
| Temporal lobe location | 1.62 [0.63-4.22] | .319 | 0.61 [0.37-0.99] | .050 |
| Left laterality | 0.42 [0.12-1.51] | .187 | 1.05 [0.65-1.69] | .851 |
| Gross total resection | 0.77 [0.28-2.15] | .620 | 0.71 [0.46-1.09] | .118 |
| Postoperative radiotherapy | 3.06 [0.93-10.11] | .065 | 2.51 [1.43-4.41] | .001 |
| Chemotherapy | 5.28 [1.42-19.55] | .013 | 1.19 [0.65-2.15] | .573 |
Bold values are the statistically significant variables.
Discussion
Due to the rarity of PXA, most studies in the literature are case studies, small case series, or literature reviews. The epidemiologic perspective on PXA has been limited, with the only PXA study using the Surveillance, Epidemiology, and End Results (SEER) reporting the outcome of 214 patients between 1981 and 2007; however, this study comprehends a period in which PXA was not a WHO diagnosis entity.14 Meanwhile, the Central Brain Tumor Registry of the United States (CBTRUS), classified PXA under the umbrella term “unique astrocytic variants.” 1,20 In the present study of 470 patients between 1994 and 2016, we analyzed the largest dataset of PXA patients described in the literature. We observed that older age (≥39 years), tumor size ≥30 mm, postoperative RT, and CT-treated patients had a poor outcome, while GTR showed improved survival in male patients. Also, in adults, female patients showed an improved survival, but this was not observed in the pediatric population.
Factors Associated with Overall Survival
Previously, older age has been shown to correlate to poor survival outcomes. Perkins et al.14 identified in a cohort of 214 patients using the SEER database that increasing age was associated with worse OS. Moreover, Mallick et al.4 identified in a meta-analysis of 167 patients with grade II PXA that patients >30 years of age had a worse outcome, although the reason for choosing that cutoff is unclear and their results were only adjusted for EOR. Also, their study lacked a more in-depth analysis of other factors that could impact prognosis. Recently, a study showed that patients >65 years have worse survival than younger patients.21 However, the reason for choosing that cutoff is unclear and the median age of the patient population was 9 years older than the present study. In our study, we divided the patients into quartiles, in which patients ≥39 years presented a strong association with decreased OS. Given our results and previous literature reporting the association between age and survival, we propose that older PXA patients, especially, those ≥39 years should be considered as high risk and more aggressive treatment should be pursued. However, multi-institutional prospective studies are needed to identify the ideal age cutoff for risk stratification.
Also, we identified that patients with a tumor size ≥30 mm had significantly worse survival compared to 0-29 mm of diameter. This significance persisted after multivariable analysis even though 44% of our cohort had an unknown tumor diameter. Unfortunately, no previous studies correlating tumor diameter cutoff and survival have been performed in PXA to validate our results. However, tumor diameter has been previously studied in other CNS malignancies, in which larger tumor diameters have been usually associated with worse outcomes and are considered as a high-risk group.22,23 Therefore, tumor diameter may be a useful way to stratify risk in patients with PXA that should be explored in future studies.
Female patients with PXA have been previously reported to have improved survival compared to males.14 In our study, female patients had improved survival in the unadjusted analysis but not in multivariable adjustment. It is unknown if biological factors as estrogen and aromatase expression might account for potential differences between genders and PXA and should be explored in more detail.
Despite conflicting reports on the role of GTR in patients with PXA,1–3,6,13–16,21 maximal safe surgical resection continues to be the standard of care for PXA patients.1 While our study did not identify a significant increase in survival benefit all patients, we observed improved survival in males undergoing GTR. Prior studies have observed an improvement in PFS or univariable analysis of OS in PXA patients treated with GTR but not after multivariable analysis.3,14 Mallick et al.4 identified in a meta-analysis of 167 patients with grade II PXA that STR was associated with worse survival, although their analysis was adjusted only for age and lacked a more robust analysis. Larger studies taking into consideration the current classification, molecular characteristics, and volumetric analysis of PXA patients are needed to identify if a threshold of improved OS exists.
Data on RT and systemic CT utilization in large PXA databases are scant and these therapies are used without definitive proof of their efficacy. In this study, patients with postoperative RT and CT treatment experience a poor outcome even after multivariable adjustment. These results might be explained by the fact that sicker patients with early recurrence, non-gross total resected tumors, and anaplastic PXA are more commonly treated with these therapeutic strategies.3 Importantly, most studies evaluating therapeutic outcomes (GTR, RT, and CT) in PXA have included patients diagnosed prior to the recognition of anaplastic PXA grade III and might be cataloged as grade II PXA with anaplastic features. Currently, anaplastic PXAs are considered more aggressive and therefore are classified as grade III, according to the 2016 WHO classification.19 A study has shown that anaplastic PXAs with high mitotic count (≥5/10 HPF) and necrosis have a worse survival in univariable analysis. However, the number of patients studied was insufficient to evaluate its independent significance.3 Moreover, a recent study evaluating 67 PXA cases (14 of which were anaplastic PXA) observed that grade was the strongest predictor of worse outcome in univariable analysis.12 The grading difference in survival might explain the lack of benefit and worse survival observed with RT and CT, as anaplastic PXA is more commonly treated with adjuvant therapy. In the present study, we were unable to evaluate the effect of grading on survival, as our study period ended in 2016, the year in which anaplastic PXA grade III was formally codified in the WHO classification.19 Therefore, studies evaluating therapy outcomes in molecular defined PXA and anaplastic PXA are warranted.
Due to the lack of proven efficacy of RT and CT in PXA despite the prior stated limitations and the knowledge provided by the molecular characterization of PXA, targeted therapy for BRAF and CDKN2A/B, the most common mutations in PXA, should be evaluated. Clinical trials using the BRAF inhibitor, Vemurafenib, have observed clinical benefit in a cohort of 7 BRAF-mutant PXA patients, in which complete and partial responses were achieved in 57% of patients, and stable disease in the remaining 43% of patients.24 Moreover, 86% and 43% of the patients treated had a previous failure to RT and CT, respectively.24,25 Even though this clinical trial included a small number of PXA patients, it serves as a proof of concept that BRAF inhibitors can be used in BRAF p.V600E mutant PXAs. CDK4/6 inhibitors are being investigated for several cancer types including CNS malignancies,26 the role that they might have in PXA patients that have failed other therapies warrants further study.
Differences in Overall Survival by Gender
In the present study, we explored the differences in clinical characteristics and survival among the different genders. We did not observe any demographic, clinical, or histological characteristics that could explain the differences in survival between genders. However, we identified that different clinical characteristics affect OS in male and female patients. Age had a stronger effect on the survival of male patients, as patients ≥22 years presented a worse survival, while females ≥39 years showed worse survival. Interestingly, GTR was a predictor of improved survival in males only. Also, males treated with CT showed significantly worse outcomes. How gender influences the response to the different therapeutic regimens in PXA needs further investigation.
Differences in Overall Survival in Pediatric and Adult Patients
We investigated the different predictors of survival in pediatric and adult patients. Remarkably, most of the factors associated with survival were observed in the adult population, as tumor size ≥30 mm and postoperative RT patients had worse survival. Meanwhile, females and temporal lobe PXA patients had improved outcomes. None of these findings were observed in the pediatric population, in which only CT-treated patients were observed to have worse survival. These findings might be related to better outcome seen in younger patients. Additionally, RT and CT worse outcomes might be related to more aggressive tumor biology. Differences in pediatric and adult PXAs need further investigation, as characterization of infiltrating gliomas has shown that pediatric and adult tumors can have different genetic drivers.10,27 Genetic alterations are a crucial factor that should be taken into account in future studies, as it has recently been demonstrated that BRAF p.V600E mutant PXA had a worse PFS than BRAF-wild-type PXA.10,12
Limitations
This study has some limitations, including the use of non-randomized observational data, lack of indication for RT and CT, the absence of size for half of the patients as these variables were incorporated into SEER later in the study period. Grade was not utilized as a variable in the study, as anaplastic PXA WHO grade III was introduced until 2016.19 Also, the SEER database does not record the cause of death of patients consistently, and not all death among patients with PXA are likely to be due to tumor progression. The lack of molecular information that guide to precision diagnosis is a major limitation of this and previous manuscripts, as the inclusion of potentially misdiagnosed cases will impact the results. Moreover, the SEER database does not capture the reasons behind treatment selection and the granular details of adjuvant therapy, which could influence our results. Despite its limitations, the study presents the largest PXA dataset, thus comprehensive statistical analyses were employed to provide high-quality reliable data on clinical and tumor characteristics, management, and survival, for this rare entity. Future multicentric studies assessing the relationship between tumor grade and genetics with clinical and demographic characteristics are needed to explore future adjuvant treatment and the role of targeted therapy for the treatment of PXA.
Conclusions
In patients with PXA, older age (≥39 years) and larger tumor size (≥30 mm) at diagnosis are risk factors for poor survival, while pediatric patients had a significantly improved OS. In terms of treatments, postoperative RT and CT appear to be ineffective treatment strategies. GTR improved the survival of male patients and remains the cornerstone of treatment when feasible. However, the molecular era has re-defined the diagnosis of PXA. Therefore, future studies evaluating treatment modalities in molecularly defined tumors by grade are needed. Additionally, new therapeutic avenues are warranted, especially BRAF inhibitors, which could be more effective than CT or RT.
Supplementary Material
Funding
No funding to disclose.
Conflict of interest statement. The authors declared no conflict of interest.
References
- 1. Shaikh N, Brahmbhatt N, Kruser TJ, et al. . Pleomorphic xanthoastrocytoma: a brief review. CNS Oncol. 2019;8(3):CNS39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lim S, Kim JH, Kim SA, et al. . Prognostic factors and therapeutic outcomes in 22 patients with pleomorphic xanthoastrocytoma. J Korean Neurosurg Soc. 2013;53(5):281–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ida CM, Rodriguez FJ, Burger PC, et al. . Pleomorphic xanthoastrocytoma: natural history and long-term follow-up. Brain Pathol. 2015;25(5):575–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mallick S, Benson R, Melgandi W, et al. . Grade II pleomorphic xanthoastrocytoma; a meta-analysis of data from previously reported 167 cases. J Clin Neurosci. 2018;54:57–62. [DOI] [PubMed] [Google Scholar]
- 5. Zou H, Duan Y, Wei D, et al. . Molecular features of pleomorphic xanthoastrocytoma. Hum Pathol. 2019;86:38–48. [DOI] [PubMed] [Google Scholar]
- 6. Oh T, Kaur G, Madden M, Bloch O, Parsa AT. Pleomorphic xanthoastrocytomas: institutional experience of 18 patients. J Clin Neurosci. 2014;21(10):1767–1772. [DOI] [PubMed] [Google Scholar]
- 7. Schindler G, Capper D, Meyer J, et al. . Analysis of BRAF V600E mutation in 1320 nervous system tumors reveals high mutation frequencies in pleomorphic xanthoastrocytoma, ganglioglioma and extra-cerebellar pilocytic astrocytoma. Acta Neuropathol. 2011;121(3):397–405. [DOI] [PubMed] [Google Scholar]
- 8. Ma C, Feng R, Chen H, et al. . BRAF V600E, TERT, and IDH2 mutations in pleomorphic xanthoastrocytoma: observations from a large case-series study. World Neurosurg. 2018;120:e1225–e1233. [DOI] [PubMed] [Google Scholar]
- 9. Vaubel RA, Caron AA, Yamada S, et al. . Recurrent copy number alterations in low-grade and anaplastic pleomorphic xanthoastrocytoma with and without BRAF V600E mutation. Brain Pathol. 2018;28(2):172–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ryall S, Zapotocky M, Fukuoka K, et al. . Integrated molecular and clinical analysis of 1000 pediatric low-grade gliomas. Cancer Cell. 2020;37(4):569–583.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Phillips JJ, Gong H, Chen K, et al. . The genetic landscape of anaplastic pleomorphic xanthoastrocytoma. Brain Pathol. 2019;29(1):85–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vaubel R, Zschernack V, Tran QT, et al. . Biology and grading of pleomorphic xanthoastrocytoma - what have we learned about it? Brain Pathol. 2020;1–13. doi: 10.1111/bpa.12874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Byun J, Hong SH, Kim YH, et al. . Peritumoral edema affects the prognosis in adult pleomorphic xanthoastrocytoma: retrospective analysis of 25 patients. World Neurosurg. 2018;114:e457–e467. [DOI] [PubMed] [Google Scholar]
- 14. Perkins SM, Mitra N, Fei W, et al. . Patterns of care and outcomes of patients with pleomorphic xanthoastrocytoma: a SEER analysis. J Neurooncol. 2012;110(1):99–104. [DOI] [PubMed] [Google Scholar]
- 15. Giannini C, Scheithauer BW, Burger PC, et al. . Pleomorphic xanthoastrocytoma: what do we really know about it? Cancer. 1999;85(9):2033–2045. [PubMed] [Google Scholar]
- 16. Fouladi M, Jenkins J, Burger P, et al. . Pleomorphic xanthoastrocytoma: favorable outcome after complete surgical resection. Neuro Oncol. 2004;3(3):184–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rutkowski MJ, Oh T, Niflioglu GG, et al. . Pleomorphic xanthoastrocytoma with anaplastic features: retrospective case series. World Neurosurg. 2016;95:368–374. [DOI] [PubMed] [Google Scholar]
- 18. Kleihues P, Burger PC, Scheithauer BW. The new WHO classification of brain tumours. Brain Pathol. 1993;3(3):255–268. [DOI] [PubMed] [Google Scholar]
- 19. Louis DN, Perry A, Reifenberger G, et al. . The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–820. [DOI] [PubMed] [Google Scholar]
- 20. Ostrom QT, Cioffi G, Gittleman H, et al. . CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2012–2016. Neuro Oncol. 2019;21(5):v1–v100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Khalafallah A, Rakovec M, Mukherjee D. Association between adjuvant radiation therapy and overall survival in pleomorphic xanthoastrocytoma. Clin Neurol Neurosurg. 2020;196:106042. [DOI] [PubMed] [Google Scholar]
- 22. Pignatti F, van den Bent M, Curran D, et al. ; European Organization for Research and Treatment of Cancer Brain Tumor Cooperative Group; European Organization for Research and Treatment of Cancer Radiotherapy Cooperative Group. Prognostic factors for survival in adult patients with cerebral low-grade glioma. J Clin Oncol. 2002;20(8):2076–2084. [DOI] [PubMed] [Google Scholar]
- 23. Baumert BG, Hegi ME, van den Bent MJ, et al. . Temozolomide chemotherapy versus radiotherapy in high-risk low-grade glioma (EORTC 22033-26033): a randomised, open-label, phase 3 intergroup study. Lancet Oncol. 2016;17(11):1521–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kaley T, Touat M, Subbiah V, et al. . BRAF inhibition in BRAFV600-mutant gliomas: results from the VE-BASKET study. J Clin Oncol. 2018;36(35):3477–3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hyman DM, Puzanov I, Subbiah V, et al. . Vemurafenib in multiple nonmelanoma cancers with BRAF V600 mutations. N Engl J Med. 2015;373(8):726–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. O’Leary B, Finn RS, Turner NC. Treating cancer with selective CDK4/6 inhibitors. Nat Rev Clin Oncol. 2016;13(7):417–430. [DOI] [PubMed] [Google Scholar]
- 27. Molinaro AM, Taylor JW, Wiencke JK, et al. . Genetic and molecular epidemiology of adult diffuse glioma. Nat Rev Neurol. 2019;15(7):405–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.