Abstract
Background
Cognitive deficits have been frequently assessed in brain tumor patients. However, self-reported cognitive complaints have received little attention so far. Cognitive complaints are important as they often interfere with participation in society. In this study, cognitive complaints were systematically assessed in brain tumor patients. As patients’ experiences and relatives’ estimations may vary, the level of agreement was investigated.
Methods
Brain tumor outpatients (n = 47) and relatives (n = 42) completed the inventory Cognitive Complaints—Participation, assessing cognitive complaints across 10 daily life activities and cognitive domains (total, memory, executive, attention). Cognitive complaints scores were compared between patients with different clinical characteristics (tumor type, number of treatments, the absence/presence of epilepsy). Complaints difference scores in patient-relative pairs were calculated to explore the level of agreement using intraclass correlations (ICC). Furthermore, we explored whether the level of agreement was related to (1) the magnitude of cognitive complaints in patient-relative pairs and (2) patients’ cognitive functioning (assessed with the Montreal Cognitive Assessment).
Results
Patients and relatives reported most cognitive complaints during work/education (100%) and social contacts (88.1%). Patients with different clinical characteristics reported comparable cognitive complaints scores. Overall, the level of agreement in patient-relative pairs was moderate-good (ICC 0.73-0.86). Although in 24% of the pairs, there was a substantial disagreement. The level of agreement was not related to the magnitude of complaints in patient-relative pairs or patients’ cognitive functioning.
Conclusion
Both the perspectives of brain tumor patients and their relatives’ on cognitive complaints are important. Clinicians could encourage communication to reach mutual understanding.
Keywords: brain tumor, cognitive complaints, neuro-oncology, patient-relative agreement
Cognitive deficits—as assessed with neuropsychological tests—have frequently been examined in brain tumor patients.1–4 Cognitive complaints received some, yet little attention so far. Earlier research in various patient groups showed that cognitive complaints and cognitive deficits are not always related.3,5–7 Cognitive complaints are defined as subjective cognitive difficulties patients encounter in daily life.8 Cognitive complaints are usually assessed by interviewing patients and occasionally with the use of self-reported questionnaires (eg, patient-reported outcome measures).9 The presence of cognitive problems is strongly associated with participation restrictions such as returning to work.10,11 Experiencing cognitive complaints have earlier also been associated with problems of participation in society in patients after surgery for cerebral meningioma.12 Maintaining daily life functioning is considered at least as important as prolonged survival by brain tumor patients.13,14 It is therefore important to further investigate cognitive complaints in brain tumor patients, regardless of the tumor type and received treatments.
Questionnaires on cognitive complaints can also be completed by close relatives, so that the patient’s report may be compared with relative’s estimations. We know from earlier research with an acquired brain injury that disagreement in cognitive complaints can occur between patients and their relatives.15–18 This is especially important as disagreement can likely lead to stressful situations in which patients and relatives do not understand each other. Stressful situations are unwanted as psychological distress in both brain tumor patients and relatives has been linked to a reduced quality of life.19 Furthermore, impaired insight into one’s cognitive strengths and weaknesses (ie, self-awareness) is common in patients with cognitive deficits.20 Beyond patients’ potential reduced self-awareness, different perspectives in terms of first vs third point of view may also contribute to discordance in patient-relative pairs.
In the current study, cognitive complaints were assessed in brain tumor patients to provide insight into the severity of cognitive complaints with respect to both daily life activities (eg, work) and cognitive domains (eg, attention), and to investigate potential associations with different clinical characteristics regarding tumor etiology (eg, tumor type) and treatment characteristics (eg, use of anti-epileptic drugs). Next, the estimated cognitive complaints of patients by their relatives and the level of agreement between patients’ and relatives’ reports were examined. Finally, we explored whether the level of agreement was related to the magnitude of cognitive complaints in patient-relative pairs and/or patients’ cognitive functioning. The current study gives insight into the severity of cognitive complaints in brain tumor patients, relatives’ perspectives and creates awareness for clinicians of potential discordance in patient-relative pairs.
Materials and Methods
Participants
In this cross-sectional study, brain tumor patients who (formerly) received outpatient rehabilitation care at the University Medical Center Utrecht, Utrecht, the Netherlands, between May 2017 and March 2020 were invited to participate. Close relatives of patients (ie, this could be anyone who observed the patient during daily life activities regularly) were invited to participate as well. To participate in the study, patients and relatives were required to (a) be ≥18 years old and (b) have sufficient comprehension of the Dutch language to complete the questionnaire. All participants gave written informed consent. The research was performed in accordance with the Declaration of Helsinki. The research protocol was approved by the medical ethics committee of the University Medical Center Utrecht (registration number 18-404).
Procedure
Patients who met the inclusion criteria received written information and an invitation to participate by post. The invitation was followed up with a phone call from the researcher to make sure that the invitation had been arrived well and any further questions were answered. After showing interest and discussing possible questions with the researcher, patients were included. Patients were asked to fill out a questionnaire regarding cognitive complaints (ie, the Cognitive Complaints—Participation [CoCo-P] inventory) which was sent by post or email. An appointment was scheduled at the medical center to administer a cognitive screener (ie, Montreal Cognitive Assessment [MoCA]). If willing to participate, relatives completed the relative version of the questionnaire about the patient (from a third-person point of view). These were relative-reported subjective estimations of patients’ cognitive complaints.
Outcome Measures
Demographical characteristics were collected on sex, age, and level of education. Level of education was assessed using a Dutch classification system,21 consisting of 7 levels ([1] less than primary education; [2] primary education; [3] primary education and less than 2 years of low-level secondary education; [4] low-level secondary education; [5] average-level secondary education; [6] high-level secondary education; and [7] academic degree). These levels were converted into 3 categories for analysis: low (level 1-4), average (level 5), and high (level 6-7). We extracted the following clinical characteristics from medical files: tumor type (glioma, meningioma, other), days since diagnosis, type of treatment (resection, radiotherapy, chemotherapy), the tumor grade according to the World Health Organization (WHO) grade I-IV,22 and the presence of epilepsy (ie, taking anti-epileptic drugs [yes, no]) as this can contribute to cognitive difficulties.3 In addition, the percentage of patients working/studying before the diagnosis was reported.
Regarding relatives, sex, age, and relationship to the patient (eg, partner, parent) were administered.
Cognitive complaints were assessed with the use of the CoCo-P inventory.16 This inventory includes 38 items on memory, executive functions, or attention, divided over 10 daily life activities (ie, work/education, leisure activities, travel, driving, finances, use of medication, family life, social contacts, cooking, and grocery shopping). Response options were “independently without effort” (0), “independently with effort” (1), “with help” (2), “not possible” (3), or “not applicable” (eg, not able to drive a car as patients have no driver license). Furthermore, the level of fatigue was reported per daily life activity, on a Visual Analogue Scale (VAS) with a range of 0-10. Output scores were first of all categorized per daily life activity. Per daily activity, patients reporting complaints were presented in percentages. Output scores could also be categorized as cognitive complaints scores. Only items that were applicable to the specific cognitive domain (ie, memory, executive, and attention) were included. The total cognitive complaints score is the sum score based on all items as a global indication of cognitive complaints. Overall, 7 items were related to memory, 20 items to executive functions, and 11 to attention. Cognitive complaints score ranged from 0 to 100. A higher complaints score indicated more cognitive complaints. Patients were labeled as restricted when they reported to perform the task independently but with effort (response option 1) on ≥1 item within that activity. Similarly, patients were labeled as dependent when help was needed (response option 2) on ≥1 item within that activity. Patients were labeled as incapable when they reported being incapable of doing the task (response option 3) on ≥1 item within that activity. Only items that were applicable for participants were included for analysis. Relatives filled out identical questions as patients on cognitive complaints that patients may have during daily life situations (from a third-person point of view). Face validity of the CoCo-P was considered adequate in patients with acquired brain injury.16
The Dutch version 8.1 of the MoCA23,24 was administered to screen for cognitive deficits. The MoCA was found feasible to administer in brain tumor patients.25 A level of sensitivity of 0.44 for the MoCA was found in brain tumor patients.26 The total score of the MoCA ranges from 0 to 30 points, with a lower score indicating a higher risk for cognitive deficits. A score of <26 indicated cognitive deficits.
Statistical Analysis
Cognitive complaints reported by patients
Per daily life activity, the percentages of patients reporting cognitive complaints were presented, by distinguishing between no complaints, restricted, dependent, or incapable. In addition, we compared the complaints scores between the domains (ie, memory, executive, attention) within the patient group with a Wilcoxon signed-rank test (2 related samples) (adjusted P for 3 tests = .017).
Patients’ cognitive complaints related to clinical characteristics
Patients were classified according to tumor type (glioma, meningioma, or other tumors), number of treatment (received 1, 2, or 3 of the following treatments: resection, radiotherapy, or chemotherapy), and the absence/presence of epilepsy (taking anti-epileptic drugs [yes, no]). Cognitive complaints scores (ie, total, memory, attention, and executive) were compared by using a Kruskal-Wallis (3 groups) and a Mann-Whitney U test (2 groups; adjusted P for 12 tests = .004).
Estimated cognitive complaints of patients reported by relatives
Per daily life activity, the percentages of relatives reporting estimated cognitive complaints of patients were presented, by distinguishing between no complaints, restricted, dependent, or incapable. Next, we compared the complaints scores between the domains (ie, memory, attention, and executive) within the relatives' group with a Wilcoxon signed-rank test (2 related samples) (adjusted P for 3 tests = .017).
Level of agreement regarding cognitive complaints within patient-relative pairs
An individual patient approach is preferred,27 as analyzing on group-level risks masking differences in individual patients. Therefore, the level of agreement of cognitive complaints was examined within patient-relative pairs.
To examine the level of agreement of cognitive complaints within patient-relative pairs per cognitive domain (ie, total, memory, executive, and attention), we calculated the difference scores of cognitive complaints per patient-relative pair. We subtracted relatives’ scores from patients’ scores, resulting in positive values when patients reported more cognitive complaints than relatives, and negative values when patients reported fewer cognitive complaints. The patient-relative pairs with a difference score of 1 SD above/below the mean of the total cognitive complaints were reported in percentages.
We evaluated the level of agreement with Bland and Altman plots and intraclass correlations (ICC) with a 95% confidence interval. An ICC of <0.5, 0.5-0.75, 0.75-0.9, and >0.9 indicate a poor, moderate, good, and excellent interrater agreement, respectively.28 The Bland and Altman plot of the total cognitive complaints was evaluated to examine the potential relation of the level of agreement (ie, difference scores of total cognitive complaints of patient-relative pairs) and the magnitude of cognitive complaints in patient-relative pairs (ie, means of total cognitive complaints score of patient-relative pairs).
Level of agreement regarding cognitive complaints within patient-relative pairs and its association with patients’ cognitive functioning
A Spearman’s correlation was performed to examine the relationship between the level of agreement (absolute difference score of total cognitive complaints of patient-relative pairs) and patients’ general cognitive functioning (ie, total MoCA score). A statistically significant relation was reported when the level of significance was P < .05. A Spearman’s rho values of 0.1, 0.3, and 0.5 were considered as small, medium, and large effects, respectively.29
Results
In total, 102 brain tumor patients received an invitation between June 2018 and March 2020, of which 48 patients were willing to participate and 47 patients were included in the study. One participant was excluded for analyses since data on the cognitive complaints questionnaire was missing (ie, the MoCA was administered but the questionnaire was never sent by post by the participant). Regarding relatives, 43 were willing to participate, one of which was excluded due to no written informed consent. See Table 1 for the demographic and clinical characteristics of all participants.
Table 1.
Demographic and Clinical Characteristics of All Participants
| n | Patients | n | Relatives | |
|---|---|---|---|---|
| Male (%) | 28 | 59.6 | 12 | 28.6 |
| Age in years (mean [SD]) | 47 | 52 (11.1) | 42 | 53.6 (13.5) |
| Level of education (%) | ||||
| Low | 6 | 13 | ||
| Average | 12 | 26.1 | ||
| High | 28 | 60.9 | ||
| Relationship with the patient (%) | ||||
| Partner | 35 | 83.3 | ||
| Friend | 1 | 2.4 | ||
| Parent | 4 | 9.5 | ||
| Child | 2 | 4.8 | ||
| Tumor type (%) | ||||
| Meningioma | 8 | 17 | ||
| Glioma | 34 | 72.3 | ||
| Other | 5 | 10.6 | ||
| WHO classification (%) | ||||
| WHO grade I | 9 | 19.1 | ||
| WHO grade II | 17 | 36.2 | ||
| WHO grade III | 6 | 12.8 | ||
| WHO grade IV | 9 | 19.1 | ||
| Unknown | 6 | 12.8 | ||
| Time since diagnosis (median, range) | 47 | 1 y and 4 m, 56 d-22 y | ||
| Treatment (%) | ||||
| Resection (Rs) | 18 | 38.3 | ||
| Chemotherapy (Ct) | 1 | 2.1 | ||
| Radiotherapy (Rt) | 3 | 6.4 | ||
| Rs + Ct | 2 | 4.3 | ||
| Rs + Rt | 2 | 4.3 | ||
| Ct + Rt | 2 | 4.3 | ||
| Ct + Rt + Rs | 19 | 40.4 | ||
| Epilepsy (%) | 20 | 42.6 | ||
| MoCA | 45 | |||
| Total (0-30) (mean [SD]) | 25.6 (2.7) | |||
| Below cutoff of 26 (%) | 42.2 | |||
| Working/studying before diagnosis (%) | 43 | 92 | ||
| Self-reported fatigue 0-10 (mean [SD]) | ||||
| Work/education | 35 | 7.0 (2.3) | ||
| Leisure activities | 44 | 4.5 (2.6) | ||
| Travel | 37 | 4.7 (2.7) | ||
| Driving | 28 | 4.2 (3.2) | ||
| Finances | 45 | 4.2 (2.8) | ||
| Use of medication | 42 | 4.3 (2.6) | ||
| Family life | 33 | 1.8 (2.2) | ||
| Social contacts | 43 | 3.0 (3.0) | ||
| Cooking | 46 | 3.2 (2.7) | ||
| Grocery shopping | 41 | 3.5 (2.5) |
Abbreviations: MoCA, Montreal Cognitive Assessment; SD, standard deviation; WHO, World Health Organization.
Cognitive Complaints Reported by Patients
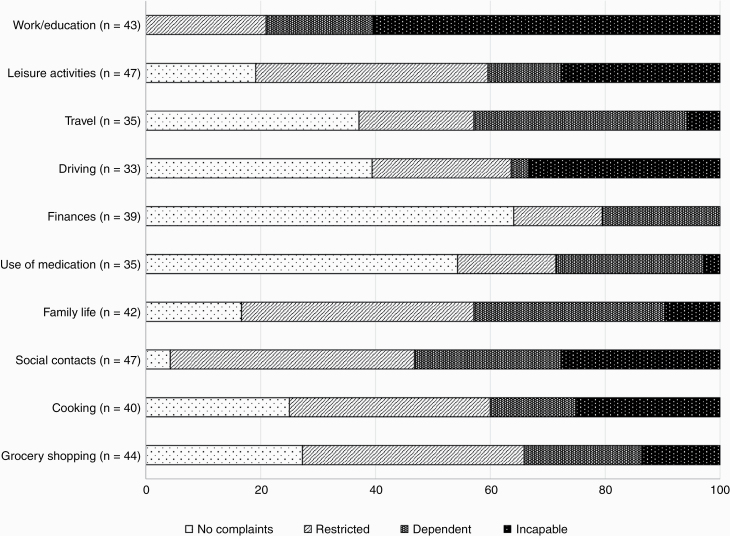
All patients who worked or studied before the brain tumor diagnosis, reported cognitive complaints during work/education activities since the brain tumor (100%), of which 60.5% of the patients reported to feel unable to perform cognitive tasks at work/study. A total of 95.7% reported cognitive complaints in contact with others (social contacts) and 83.3% reported cognitive complaints in family life (see Figure 1). Patients reported most cognitive complaints during activities requiring attentional abilities (mdn = 33, IQR = 35), in comparison to memory complaints (mdn = 22, IQR = 37; z = −2.978, P = .003) and executive complaints (mdn = 23, IQR = 28; z = −4.245, P < .001).
Figure 1.
Patients’ cognitive complaints per daily life activity in frequency (%), distinguished between no complaints, restrictions, dependency, or incapability. The number of patients varied as only participants were included for whom the daily activity was applicable.
Patients’ Cognitive Complaints Related to Clinical Characteristics
Patients with different tumor types (meningioma: mdn = 44.5, mean = 41.9, range = 16-65; glioma: mdn = 24.5, mean = 25.7, range = 7-60; other: mdn = 28, mean = 28.2, range = 5-59), number of treatments (1: mdn = 28; 2: mdn = 28; 3: mdn = 25) or with the absence/presence of epilepsy (absence: mdn = 28; presence: mdn = 27.5), reported comparable total cognitive complaints scores, and also for specific cognitive domains (memory, attention, or executive) (see Table 2).
Table 2.
Complaints Scores (Total, Memory, Attention, Executive) Compared Between Patient Groups With Different Tumor Etiology or Treatment Characteristics
| Complaints scores | Type of tumor (median [IQR]) | |||
|---|---|---|---|---|
| Meningioma (n = 8) | Glioma (n = 34) | Other* (n = 5) | Statistics | |
| Total | 44.5 (30.3) | 24.5 (30) | 28 (38.5) | χ 2(2) = 5.052, P = .080 |
| Memory | 46 (32.8) | 16.5 (45.8) | 20 (36) | χ 2(2) = 5.552, P = .062 |
| Attention | 54 (35.8) | 31.5 (33.8) | 43 (32) | χ 2(2) = 6.472, P = .039 |
| Executive | 35 (34.5) | 22.5 (25.3) | 17 (46) | χ 2(2) = 2.709, P = .258 |
| Complaints scores | Number of treatments (median [IQR]) | |||
| 1 (n = 21) | 2 (n = 7) | 3 (n = 19) | Statistics | |
| Total | 28 (40) | 28 (24) | 25 (30) | χ 2(2) = .832, P = .660 |
| Memory | 22 (36.5) | 20 (39) | 22 (34) | χ 2(2) = 1.194, P = .550 |
| Attention | 43 (42.5) | 33 (22) | 30 (20) | χ 2(2) = 4.812, P = .090 |
| Executive | 23 (39.5) | 17 (25) | 22 (28) | χ 2(2) = .129, P = .938 |
| Complaints scores | Epilepsy (median [IQR]) | |||
| Absence (n = 20) | Presence (n = 27) | Statistics | ||
| Total | 28 (29) | 27.5 (33.3) | U = 268.5, z = −.032, P = .974 | |
| Memory | 20 (37) | 22 (39.8) | U = 254, z = −.035, P = .730 | |
| Attention | 37 (35) | 33 (25) | U = 242.5, z = −.592, P = .554 | |
| Executive | 22 (26) | 23.5 (28.8) | U = 261.5, z = −.183, P = .855 | |
Abbreviations: IQR, interquartile range; n, number of patients.
*Note: These patients were diagnosed with: hemangioblastoma, subependymoma, medulloblastoma, lymphoma, or cerebral metastases.
Estimated Cognitive Complaints of Patients Reported by relatives
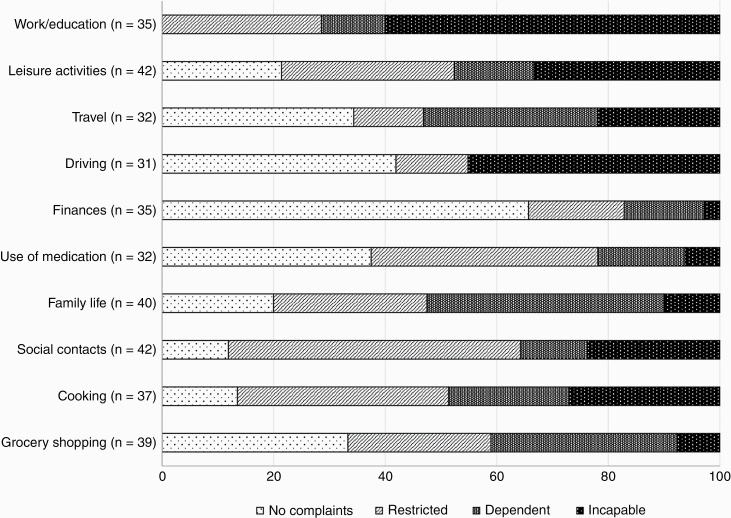
All relatives estimated patients having cognitive complaints during work/education (100%), of which 60% of the relatives estimated patients being unable to perform cognitive tasks at work/study since the brain tumor (see Figure 2). A total of 88.1% of relatives estimated cognitive complaints regarding maintaining contact with others (social contacts) and 86.5% during cooking. Relatives also estimated most cognitive complaints during activities that required attentional abilities (mdn = 36, IQR = 42.5), compared to memory complaints (mdn = 20.5, IQR = 33.8; z = −3.946, P < .001) and executive complaints (mdn = 26, IQR = 28.8; z = −4.038, P < .001).
Figure 2.
Relatives’ estimated cognitive complaints of patients per daily life activity in frequency (%), distinguished between no complaints, restrictions, dependency, or incapability. The number of relatives varied as only participants were included for whom the daily activity was applicable.
Level of Agreement Regarding Cognitive Complaints Within Patient-Relative Pairs
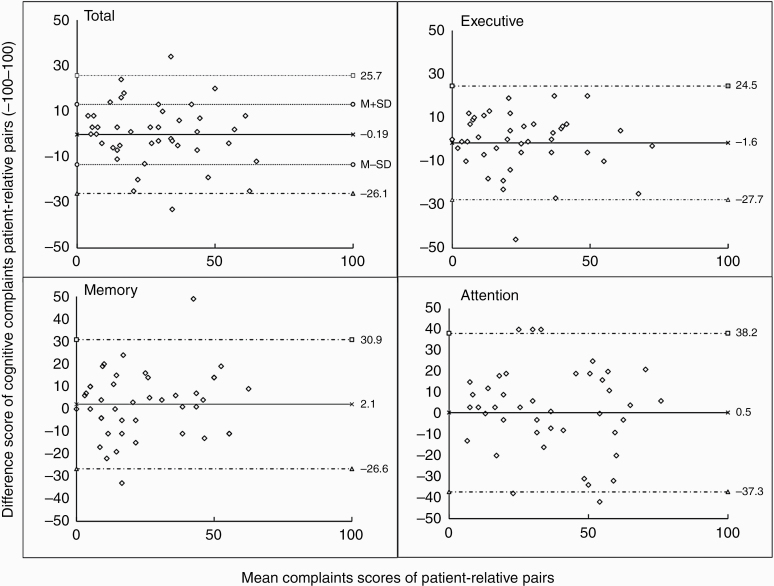
Of all patient-relative pairs, 76% had a maximum difference complaints score within 1 SD from the mean (mean + 1 SD; M = −0.19, SD = 13.2) (see Figure 3). For the remaining 24% patient-relative pairs, a difference complaints score above or below 1 SD from the mean was found, indicating a substantial disagreement. From these pairs, 12% reported patients more complaints than relatives (range difference score: 14-34 points). The other 12% included pairs in which patients reported fewer complaints (range difference score: 19-33 points).
Figure 3.
Bland and Altman plots of cognitive complaints difference score of patient-relative pairs. The dotted lines represent the upper/lower 95% confidence interval. The straight lines represent the mean. Note: Positive values describe patients reporting more cognitive complaints than their relatives, and negative values fewer cognitive complaints compared to their relatives.
The interrater agreement (ICC) was good for total cognitive complaints (0.84, 0.7-0.91), memory complaints (0.82, 0.67-0.91), and executive complaints (0.86, 0.74-0.92). A moderate interrater agreement was found for attention complaints (0.73, ranging 0.51-0.86), indicating less agreement in patient-relative pairs for this domain. The level of agreement was not associated with the magnitude of total cognitive complaints in patient-relative pairs (see Figure 3).
Level of Agreement Regarding Cognitive Complaints Within Patient-Relative Pairs and Its Association With Patients’ Cognitive Functioning
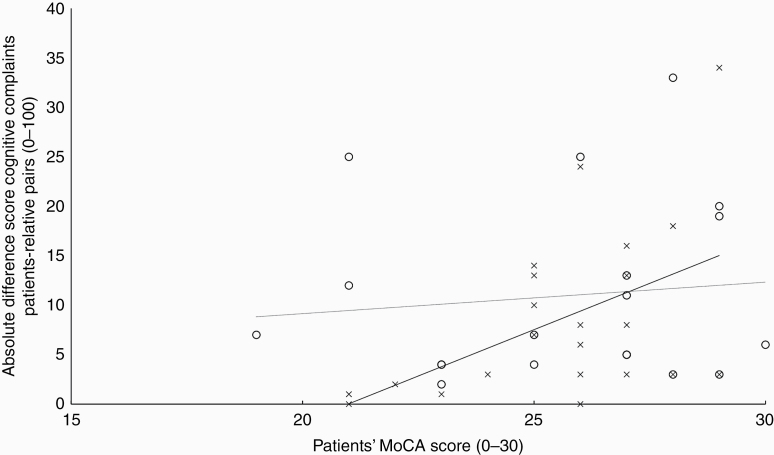
The patients’ cognitive functioning, as assessed by the MoCA, was not associated with the level of agreement regarding cognitive complaints between patients’ experiences and their relatives’ estimations, rs = .270, P = .092 (see Figure 4). This finding indicates that discordance in patient-relative pairs was not related to the degree of patients’ cognitive deficits.
Figure 4.
Scatterplot visualizing the association between the absolute difference score of total cognitive complaints of patient-relative pairs and patients’ general cognitive functioning (MoCA). Note: Crosses represent patients reporting more cognitive complaints than their relatives’ estimations (black linear trendline), and circles represent patients reporting fewer cognitive complaints compared to their relatives (dotted linear trendline).
Discussion
In the current study, we first reflected on the severity of cognitive complaints (ie, regarding both daily life activities and cognitive domains) in brain tumor patients. Next, the association between patients’ cognitive complaints and clinical characteristics regarding tumor etiology and treatment characteristics was examined. Finally, the level of agreement of patients’ reported cognitive complaints and relatives’ estimations was examined, and its potential association with the magnitude of cognitive complaints in patient-relative pairs and patients’ cognitive functioning.
Our study showed that cognitive complaints commonly occur in brain tumor patients. The majority of the patients (60.5%) reported not being able to perform work-/education-related activities due to cognitive complaints since the brain tumor. Previous research showed that brain tumor patients often do not return to work or only return to work with an adjusted schedule because of a variation of symptoms (eg, fatigue) and disabilities (eg, neurological impairment, little motivation, lack of self-awareness).20,30 Patients’ cognitive complaints during social activities (ie, social contacts and family life) were also frequently present. They were mainly described as, despite having complaints, still being able to do it independently or with help from others. While patients are still able to fulfill a social role, maintaining certain family and social roles may be challenging for brain tumor patients.31 Cognitive complaints in daily life situations could be linked to Instrumental Activities of Daily Living (IADL). One might therefore argue that IADL is susceptible to more than just cognition and the level of cognitive functions. This is obviously the case; physical impairments and mood disorders can have a serious negative impact on IADL as well.14,32 The CoCo-P, however, clearly aims at the cognitive complaints, as it stresses per item 1 cognitive domain per daily life situation, such as work, family life, or traveling. In addition, patients and caregivers can indicate how tiring those situations of daily life are to them. So, both fatigue and cognitive complaints can be estimated or assessed with the CoCo-P. When one needs a complete overview of complaints in daily life situations, one should add other questionnaires and/or inventories to the protocol.
Furthermore, most frequently reported cognitive complaints occurred during daily life activities involving attentional processes. Problems in attentional skills complicate the ability to perform daily life activities, such as working or preparing dinner.1,31 However, daily life activities (eg, working) usually require multiple skills such as planning, mental flexibility, and problem-solving simultaneously and therefore rely on multiple cognitive processes.14 Fatigue, reported by 25%-90% of brain tumor patients,33 may have contributed to the cognitive complaints involving attentional processes (to give an example, 1 attention item on the current inventory was “I can carry out my tasks and activities in busy surroundings”).
Patients with different tumor etiology and treatment characteristics reported a comparable amount of cognitive complaints. Even though differences were found between the groups, this was statistically not confirmed. Caution should be taken due to relatively small sample sizes (n ranging from 5 to 37 per subgroup), which may have sketched a different picture. In contrast, cognitive deficits, as assessed with conventional neuropsychological tests, have been associated with treatment characteristics before.3 For instance, the use of antiepileptic drugs was found to be a risk factor for cognitive deficits in the study on low-grade glioma patients.3 However, we know from earlier research that cognitive complaints are not necessarily an indication of cognitive deficits. In other words, cognitive complaints and deficits are not always related.3,5–7 Cognitive abilities are affected by multiple factors other than treatment characteristics, such as psychological distress and fatigue.6,33
Relatives perceived cognitive complaints of patients were mostly reported during work/education (100%), social contacts (88.1%), and cooking (86.5%). Similar to patients, most cognitive complaints were related to attentional processes. Overall, a moderate-good agreement on cognitive complaints was found between patients and relatives. Patients and their relatives usually agree more on more easily observable behavior of patients (eg, fatigue), rather than invisible symptoms (eg, mood).34 Although in a quarter of the patient-relative pairs, a substantial disagreement was found. Disagreements on cognitive complaints within patient-relative pairs were described by either patients reporting more or less cognitive complaints than their relatives’ estimations. The level of agreement of patient-relative pairs seemed not to be associated with the magnitude of cognitive complaints in patient-relative pairs nor patients’ cognitive functioning as assessed by a cognitive screener. However, multiple other factors are likely involved in developing cognitive complaints. For instance, affective disturbances (eg, psychological distress, depression, anxiety) and fatigue have strongly been associated with cognitive complaints,6,33,35,36 and have become very important in the management of the disease.37,38 Affective disturbances and fatigue probably have affected the magnitude of cognitive complaints and consequently the patient-relative agreement. To give an example, a patient suffering from psychological distress may have reported significantly more cognitive restrictions than his/her relative estimated. Future research is necessary to elaborate more on the impact of these factors on cognitive complaints and the patient-relative agreement, for example, by using multiple regression analyses. Even though the patient-relative interrater agreements turned out to be sufficient, several patient-relative pairs disagreed substantially, emphasizing the importance of an individual patient-relative approach. Taken these findings into consideration and the possibility of patients’ or relatives’ over- or underestimation of cognitive abilities,3,15 clinicians should pay attention to both patients’ as their relatives’ perspectives on cognitive complaints, regardless of the visibility and severity of the brain tumor consequences. Clinicians could encourage patient-relative pairs to communicate about cognitive complaints and their potential different perspectives on these to reach mutual understanding. By informing brain tumor patients about the effects of having a brain tumor and undergoing treatments (eg, cognitive) and addressing any (future) concerns, psychological distress may be reduced or prevented.31
Strengths and Limitations
A strength of this study was the detailed inventory of cognitive complaints during daily life activities in brain tumor patients who were receiving outpatient rehabilitation care. All outpatients were included regardless of the tumor type, received treatment(s), or the absence/presence of epilepsy, resulting in an actual reflection of the cognitive complaints in this patient group. On the other hand, the variation of tumor etiology and treatment characteristics in these patients can be considered a limitation, as this resulted in some small group sizes when comparing cognitive complaints between patient groups with different tumor etiology and treatment characteristics (n ranging from 5 to 37 per subgroup). Future research with larger patient groups could reflect more on potential predictors of cognitive complaints in brain tumor patients and interrelationships between tumor etiology and treatment characteristics by performing multiple linear regression analyses. Another strength concerns the large group of relatives reporting on their estimations on patients’ complaints. This way, also relatives’ perspectives on situations are considered. Clinicians can therefore pay attention to both perspectives, and improve communication and mutual understanding. Improving mutual communication and understanding might prevent stressful situations in which patients and relatives do not understand each other.
A limitation we want to address regards the MoCA, which we used as a cognitive screener in the current study. The MoCA has a poor sensitivity for cognitive deficits in brain tumor patients.26 Especially mild cognitive deficits are hard to detect, and therefore the severity of cognitive deficits may have been underestimated in the current study. However, as the MoCA is a brief (ie, we wanted to burden patients at short as possible as they had other clinical appointments the same day), easy to administer, and well-tolerated cognitive screener by brain tumor patients25 assessing patient’s global cognitive function, we decided to use it. The current finding indicating that discordance of cognitive complaints in patient-relative pairs is not related to patients’ cognitive deficits should be interpreted with caution and be further examined in future studies with more extensive neuropsychological assessments. An extensive neuropsychological assessment could be helpful to provide more precise cognitive data and could also elaborate on specific cognitive domains. Furthermore, the use of the novel CoCo-P inventory could be addressed as a limitation, as this inventory has not yet been evaluated in terms of reliability and validity.16 For that reason, results in terms of specific cognitive constructs (attention, memory, and executive functions) should be interpreted with caution. Face validity was considered adequate, since patients with acquired brain injury considered the daily life activities representative for the difficulties they encounter in daily life.16 However, as it is yet unclear which daily life activities are relevant especially for brain tumor patients specifically,14 the current study might have failed to include relevant daily activities. Nevertheless, no relevant missing activities were reported by patients in the additional remarks.
Conclusion
Cognitive complaints are common in brain tumor patients and should therefore be assessed systematically. Both patients and their relatives reported most cognitive complaints during patient’s work/study and social contacts. Most cognitive complaints occurred during daily life activities that required attentional abilities. No specific clinical characteristics (ie, tumor type, number of treatments, epilepsy) were associated with the degree of patients’ cognitive complaints. Overall, a reasonable level of agreement within patient-relative pairs was determined for all cognitive domains (ie, total, memory, attention, and executive). A quarter of the patient-relative pairs (24%) disagreed substantially on patients’ cognitive complaints and relatives’ reports, which were described by patients reporting either more or fewer complaints than their relatives estimated. Neither the magnitude of cognitive complaints in patient-relative pairs or patients’ cognitive functioning on a cognitive screener were found to be indicators for significantly more patient-relative discordance in cognitive complaints.
The clinical message is to systematically assess cognitive complaints considering their importance for patient participation. Thereby attention must be paid to both patients’ and relatives’ perspectives on cognitive complaints. Clinicians could encourage communication to reach mutual understanding in patient-relative pairs.
Acknowledgments
We thank all participants for their contribution to this study. Also, we would like to thank Neeltje Op ’t Hoog for her help in collecting the data.
Funding
This work was supported by Handicap.nl [grant numbers R2015010 and R201705758].
Conflict of interest statement. The authors report no conflict of interest.
References
- 1. Rijnen SJM, Meskal I, Bakker M, et al. Cognitive outcomes in meningioma patients undergoing surgery: individual changes over time and predictors of late cognitive functioning. Neuro Oncol. 2019;21(7):911–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. van Kessel E, Baumfalk AE, van Zandvoort MJE, et al. Tumor-related neurocognitive dysfunction in patients with diffuse glioma: a systematic review of neurocognitive functioning prior to anti-tumor treatment. J Neurooncol. 2017;134(1):9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Taphoorn MJ, Klein M. Cognitive deficits in adult patients with brain tumours. Lancet Neurol. 2004;3(3):159–168. [DOI] [PubMed] [Google Scholar]
- 4. Talacchi A, Santini B, Savazzi S, et al. Cognitive effects of tumour and surgical treatment in glioma patients. J Neurooncol. 2011;103(3):541–549. [DOI] [PubMed] [Google Scholar]
- 5. Nijsse B, van Heugten CM, van Mierlo ML, et al. Psychological factors are associated with subjective cognitive complaints 2 months post-stroke. Neuropsychol Rehabil. 2017;27(1):99–115. [DOI] [PubMed] [Google Scholar]
- 6. Pranckeviciene A, Deltuva VP, Tamasauskas A, et al. Association between psychological distress, subjective cognitive complaints and objective neuropsychological functioning in brain tumor patients. Clin Neurol Neurosurg. 2017;163:18–23. [DOI] [PubMed] [Google Scholar]
- 7. Van Rijsbergen M. Subjective Cognitive Complaints after Stroke: Prevalence, Determinants and Course over Time. s.l.: Ridderprint BV.; 2017. https://research.tilburguniversity.edu/en/publications/subjective-cognitive-complaints-after-stroke-prevalence-determina [Google Scholar]
- 8. van Rijsbergen MW, Mark RE, de Kort PL, et al. Subjective cognitive complaints after stroke: a systematic review. J Stroke Cerebrovasc Dis. 2014;23(3):408–420. [DOI] [PubMed] [Google Scholar]
- 9. Meadows KA. Patient-reported outcome measures: an overview. Br J Community Nurs. 2011;16(3):146–151. [DOI] [PubMed] [Google Scholar]
- 10. Nugent BD, Weimer J, Choi CJ, et al. Work productivity and neuropsychological function in persons with skull base tumors. Neurooncol Pract. 2014;1(3):106–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Feuerstein M, Hansen JA, Calvio LC, et al. Work productivity in brain tumor survivors. J Occup Environ Med. 2007;49(7):803–811. [DOI] [PubMed] [Google Scholar]
- 12. Schepers VPM, van der Vossen S, Berkelbach van der Sprenkel JW, et al. Participation restrictions in patients after surgery for cerebral meningioma. J Rehabil Med. 2018;50(10):879–885. [DOI] [PubMed] [Google Scholar]
- 13. Efficace F, Taphoorn M. Methodological issues in designing and reporting health-related quality of life in cancer clinical trials: the challenge of brain cancer studies. J Neurooncol. 2012;108(2):221–226. [DOI] [PubMed] [Google Scholar]
- 14. Oort Q, Taphoorn MJB, Sikkes SAM, et al. Evaluation of the content coverage of questionnaires containing basic and instrumental activities of daily living (ADL) used in adult patients with brain tumors. J Neurooncol. 2019;143(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vallat-Azouvi C, Paillat C, Bercovici S, et al. Subjective complaints after acquired brain injury: presentation of the Brain Injury Complaint Questionnaire (BICoQ). J Neurosci Res. 2018;96(4):601–611. [DOI] [PubMed] [Google Scholar]
- 16. Spreij LA, Sluiter D, Gosselt IK, et al. CoCo - participation: the development and clinical use of a novel inventory measuring cognitive complaints in daily life. Neuropsychol. Rehabil. 2019;2:1–23. doi: 10.1080/09602011.2019.1691017 [DOI] [PubMed] [Google Scholar]
- 17. Rueda AD, Lau KM, Saito N, et al. ; Alzheimer’s Disease Neuroimaging Initiative. Self-rated and informant-rated everyday function in comparison to objective markers of Alzheimer’s disease. Alzheimers Dement. 2015;11(9):1080–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hochstenbach J, Prigatano G, Mulder T. Patients’ and relatives’ reports of disturbances 9 months after stroke: subjective changes in physical functioning, cognition, emotion, and behavior. Arch Phys Med Rehabil. 2005;86(8):1587–1593. [DOI] [PubMed] [Google Scholar]
- 19. Randazzo D, Peters KB. Psychosocial distress and its effects on the health-related quality of life of primary brain tumor patients. CNS Oncol. 2016;5(4):241–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liaset IF, Kvam L. Experiences of returning to work after brain tumor treatment. Work. 2018;60(4):603–612. [DOI] [PubMed] [Google Scholar]
- 21. Verhage F. Intelligence and age in a Dutch sample. Hum. Dev. 1965;8:238–245. [Google Scholar]
- 22. Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(2):97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–699. [DOI] [PubMed] [Google Scholar]
- 24. Dautzenberg PLJ, de Jonghe JFM.. Montreal Cognitive Assessment: afname- en scoringinstructies. 2004. https://tvgg.nl/wp-content/uploads/sites/2/2019/10/12439_2010_Article_218.pdf [Google Scholar]
- 25. Olson RA, Chhanabhai T, McKenzie M. Feasibility study of the Montreal Cognitive Assessment (MoCA) in patients with brain metastases. Support Care Cancer. 2008;16(11):1273–1278. [DOI] [PubMed] [Google Scholar]
- 26. Robinson GA, Biggs V, Walker DG. Cognitive screening in brain tumors: short but sensitive enough? Front Oncol. 2015;5:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. van Loenen IS, Rijnen SJM, Bruijn J, et al. Group changes in cognitive performance after surgery mask changes in individual patients with glioblastoma. World Neurosurg. 2018;117:e172–e179. [DOI] [PubMed] [Google Scholar]
- 28. Koo TK, Li MY. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med. 2016;15(2):155–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Field A. Discovering Statistics Using SPSS Third Edition. London: Sage Publications Ltd.; 2009. [Google Scholar]
- 30. Thurin E, Corell A, Gulati S, et al. Return to work following meningioma surgery: a Swedish nationwide registry-based matched cohort study. Neurooncol Pract. 2020;7(3):320–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bartolo M, Soffietti R, Klein M.. Neurorehabilitation in Neuro-Oncology. Cham: Springer Nature Switzerland; 2019. [Google Scholar]
- 32. Kiosses DN, Alexopoulos GS. IADL functions, cognitive deficits, and severity of depression: a preliminary study. Am J Geriatr Psychiatry. 2005;13(3):244–249. [DOI] [PubMed] [Google Scholar]
- 33. Day J, Gillespie DC, Rooney AG, et al. Neurocognitive deficits and neurocognitive rehabilitation in adult brain tumors. Curr Treat Options Neurol. 2016;18(5):22. [DOI] [PubMed] [Google Scholar]
- 34. Rooney AG, McNamara S, Mackinnon M, et al. Screening for major depressive disorder in adults with glioma using the PHQ-9: a comparison of patient versus proxy reports. J Neurooncol. 2013;113(1):49–55. [DOI] [PubMed] [Google Scholar]
- 35. van der Linden SD, Gehring K, Rutten GM, et al. Prevalence and correlates of fatigue in patients with meningioma before and after surgery. Neurooncol Pract. 2020;7(1):77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gehring K, Taphoorn MJ, Sitskoorn MM, et al. Predictors of subjective versus objective cognitive functioning in patients with stable grades II and III glioma. Neurooncol Pract. 2015;2(1):20–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Van der Linden SD. Research into Neuropsychological Assessment and Cognitive Rehabilitation in Brain Tumor Patients after Surgery. Ridderprint; 2019. https://research.tilburguniversity.edu/en/publications/research-into-neuropsychological-assessment-and-cognitive-rehabil [Google Scholar]
- 38. Amidei C. Symptom-based interventions to promote quality survivorship. Neuro Oncol. 2018;20(suppl_7):vii27–vii39. [DOI] [PMC free article] [PubMed] [Google Scholar]