Abstract
Outcomes for patients with lower-grade gliomas (LrGGs) continue to improve with advances in molecular characterization and treatment. However, cognitive sequela from the tumor and its treatment leave a significant impact on health-related quality of life for these patients. Several factors affect each patient’s cognition, such as tumor location, treatment, medication, and comorbidities. However, impairments of processing speed, attention, concentration, working memory, and executive function are common across LrGG patients. Cognitive rehabilitation strategies, well established in traumatic brain injury and stroke populations, are based on neural plasticity and functional reorganization. Adapting these strategies for implementation in patients with brain tumors is an active area of research. This article provides an overview of cognitive domains commonly impaired in LrGG patients and evidence for the use of cognitive rehabilitation strategies to address these impairments with the goal of improving health-related quality of life in this patient population.
Keywords: cognitive outcomes, cognitive rehabilitation, low-grade glioma, neurocognitive outcomes, primary brain tumor
Survival for patients with lower-grade diffuse gliomas (LrGGs)–grades II and III—is improving, with a median survival of 5 to 15 years1–5 depending on molecular subtype.6,7 However, these tumors and their related treatments often lead to significant objective and subjective cognitive impairments8–13 that negatively affect patients’ health-related quality of life (HRQOL).14 The reported prevalence of cognitive impairments in LrGG varies widely.15 At presentation, before any treatment (including surgery), 31% to 75% of patients report cognitive impairments11,16 and these are more common with dominant hemispheric lesions.17 Though some cognitive recovery usually occurs within 3 to 6 months of surgery,18–20 19% to 83% of LrGG patients remain impaired or decline further.11,15,21,22
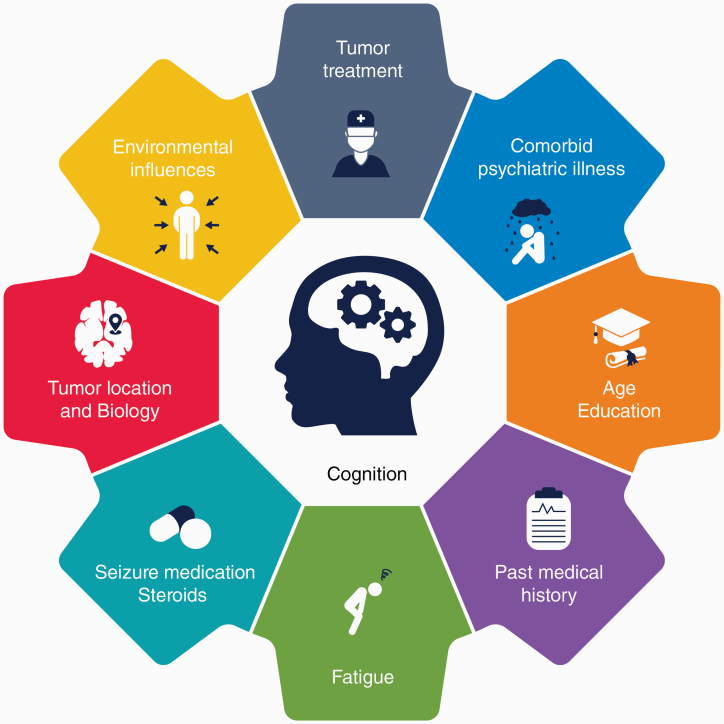
The mechanisms of cognitive impairment in brain tumors, including LrGGs, are multifactorial (Figure 1). The impact of the tumor and its associated treatments can lead to the functional disruption of distributed cognitive networks.10,23–27 Tumor biology, extent of edema, tumor volume, and higher grade have been linked to brain tumor–related cognitive impairment.8,10,11 Isocitrate dehydrogenase mutation, a feature commonly seen in LrGG, correlates with fewer cognitive deficits at presentation and slower lesion momentum over time.10 Standard adjuvant tumor treatments, such as radiotherapy and chemotherapy, are associated with diminished cognition. Radiotherapy affects both the cerebral vasculature and white-matter tracts contributing to demyelination, thickening of vessel walls, focal mineralization, and coagulative necrosis.25 These changes have been linked to cognitive decline that may continue to evolve several years after radiotherapy.28,29 Chemotherapy also has neurotoxic effects on cognition through development of acute and chronic encephalopathy.25,30 In a rodent study of temozolomide, a commonly used alkylating agent in glioma treatment, neurogenesis in the hippocampus was decreased with a negative impact on memory encoding and learning.31 Use of steroid or pain medications, high seizure burden, and several antiepileptic drugs may also exacerbate cognitive inefficiencies.25,32,33
Figure 1.
Factors that influence cognitive impairment in lower-grade glioma (LrGG) patients.
Cognitive rehabilitation is considered a well-established treatment to address cognitive impairments in many neurologic diseases such as traumatic brain injury (TBI), multiple sclerosis, and stroke.34–37 Cognitive rehabilitation “is a systematically applied set of medical and therapeutic services designed to improve cognitive functioning and participation in activities that may be affected by difficulties in one or more cognitive domains.” 38 Further, these services are designed to enhance cognitive abilities, particularly as it relates to improving functional independence and HRQOL.36,38 Cognitive rehabilitation is based on principles of neural plasticity and functional reorganization39,40 with 2 main underlying mechanisms: 1) retraining and 2) functional compensation.35,41–43 Retraining strengthens impaired cognitive skills through repeatedly practicing cognitive tasks,44 whereas functional compensation focuses on honing strategies to modify the environment and/or one’s approach to achieve a goal.41,45 These 2 interventional approaches are often combined,46–49 with compensation being particularly appropriate for treating persistent cognitive impairments.35,36,50
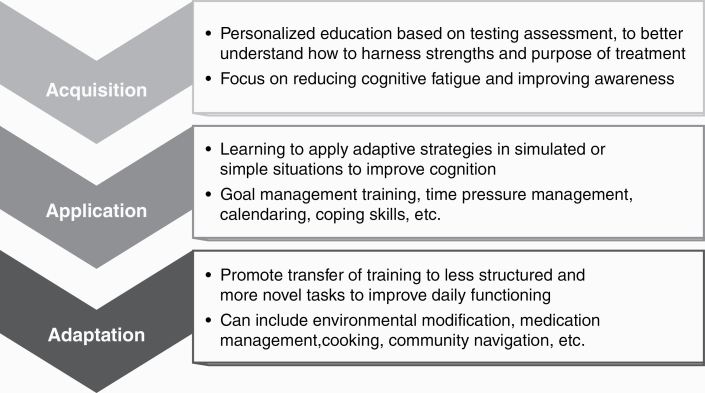
Cognitive rehabilitation typically is informed by neuropsychological assessment and implemented in 3 phases: 1) Acquisition—education about cognitive vulnerabilities and strengths, and beginning to learn possible compensatory strategies; 2) Application—applying learned compensatory strategies in stages toward mastery; and 3) Adaptation—applying prior learned skills with enhanced complexity to aid functional improvement (Figure 2).36,51 Cognitive rehabilitation strategies are increasingly being investigated and applied in brain tumor patients, particularly in LrGG patients with improving survival.
Figure 2.
The “Triple A” Model of Cognitive Rehab: 1, Acquisition; 2, Application; and 3, Adaptation.
Common Domains of Cognitive Impairment and Related Cognitive Rehabilitation Strategies in Lower-Grade Glioma Patients
The prevalence of cognitive impairment in LrGG varies across affected domains, particularly those reliant on distributed functional networks and intact white-matter tracts, rather than highly localized cognitive skills.52 LrGG patients commonly demonstrate cognitive impairments in attention and concentration,52,53 processing speed,14,21,52 learning and memory,14,54 and executive functions.14,20,21,53 Recent studies of cognitive rehabilitation strategies to address these impairments have demonstrated feasibility and efficacy in patients with brain tumors (Table 1).35,36,46–50,55–60 However, there are limited studies in LrGG patients alone because most studies include a mixture of low- and high-grade gliomas, as well as meningiomas and metastatic lesions.55,56 The following is an overview of the most common cognitive impairments and related rehabilitation strategies used in brain tumors with an emphasis on data relevant to patients with LrGG. Though we focus on improvement in the targeted cognitive domain, studies support improvements across multiple cognitive domains after rehabilitation, as reflected in Table 1.48 We also discuss practical considerations for implementation.
Table 1.
Cognitive Rehabilitation Studies in Gliomas
| Reference | Study design and intervention | Targeted cognitive domain(s) | Outcome | Sample | |
|---|---|---|---|---|---|
| Combined cognitive retraining and compensation strategies | Gehring et al, 200947 | RCT in lower-grade gliomas | Attention, executive functioning, learning, and memory | At 6 mo: improved attention, verbal memory, and mental fatigue | Total = 140 |
| Intervention: 2 h weekly for 6 sessions including iPad app | Low-grade glioma = 117 | ||||
| Control: wait-list | Anaplastic glioma = 23 | ||||
| van der Linden et al, 201848 | Feasibility RCT in postoperative primary brain tumors | Attention, executive functioning, learning, and memory | Feasibility: 54% met feasibility criteria defined as ≥ 80% completion of retraining and compensation strategies | Total = 13 | |
| Intervention: 3 h weekly for 10 sessions using iPad | Low-grade glioma = 4 | ||||
| Control: wait-list | Meningioma = 7 | ||||
| Other = 2 | |||||
| Zucchella et al, 201349 | RCT in primary brain tumors postoperatively | Orientation, attention, memory, and executive functioning | At 1 mo: improved verbal memory, visual attention | Total = 53 | |
| Intervention: 4 h weekly for 16 sessions | Low-grade glioma = 7 | ||||
| Control: usual care without cognitive training | High-grade glioma = 25 | ||||
| Meningioma = 16 | |||||
| Other = 5 | |||||
| Compensation strategies | Hassler et al, 201059 | Pilot study in glioblastomas and anaplastic gliomas | Perception, concentration, attention, verbal learning and memory, retentiveness, and creativity | At 12 wk: improved verbal learning | Total = 11 |
| Intervention: 10 group sessions of 90 min of compensation (holistic mnemonic training) | Glioblastoma = 7 | ||||
| Anaplastic glioma = 4 | |||||
| Locke et al, 200860 | Primary brain tumors undergoing radiation with caregivers | Executive functioning, learning and memory | At 3 mo: 88% patients using strategies at least once per wk | Total = 13 | |
| Intervention: 50 min daily for 6 sessions of cognitive rehabilitation and problem-solving therapy intervention | Glioma = 11 | ||||
| Control: usual care without cognitive training | Meningioma = 2 | ||||
| Miotto et al, 201361 | Pilot study in untreated primary frontal lobe tumors | Verbal learning and memory, working memory, executive functioning | At 30 min: improved verbal memory | Total = 21 | |
| Intervention: 30 min of strategic semantic organizational training | Glioma = 12 | ||||
| Meningioma = 9 | |||||
| Miotto et al, 201462 | Pilot study in low-grade glioma of left frontal lobe | Verbal learning and memory | At 30 min: improved verbal memory | Total = 9 | |
| Intervention: 30 min of strategic semantic organizational training | Low-grade glioma = 9 | ||||
| Control: matched healthy volunteers | |||||
| Richard et al, 201957 | Pilot RCT in brain tumors | Executive and related attention, memory, and behavioral impairments | At 4 mo: improved HRQOL, executive function, processing speed | Total = 25 | |
| Intervention: 2 h weekly for 8 sessions (GMT or BHP) | Low-grade glioma = 8 | ||||
| Control: wait-list | High-grade glioma = 6 | ||||
| Meningioma = 7 | |||||
| Other = 4 | |||||
| Cognitive retraining | Maschio et al, 201546 | Pilot study in brain tumors | Memory, attention, visuospatial functions, language, and reasoning | Immediately and at 6 mo: improved attention, memory, and verbal fluency | Total = 16 |
| Intervention: 1 h weekly retraining for 10 sessions | Low-grade glioma = 5 | ||||
| Anaplastic glioma = 4 | |||||
| Glioblastoma = 2 | |||||
| Meningioma = 2 | |||||
| Metastases = 3 | |||||
| Yang et al, 201458 | RCT VR for retraining in brain tumors | Attention, learning, and memory | At 1 mo: improved attention, memory, and visual motor coordination, visual learning, and memory | Total = 38 | |
| Intervention: 1.5 h weekly VR retraining and 1 h weekly computer-assisted cognitive rehabilitation for 4 wks | Astrocytoma = 2 | ||||
| Control: 2.5 h weekly of computer-assisted cognitive rehabilitation for 4 wk | Glioblastoma = 5 | ||||
| Meningioma = 10 | |||||
| Metastasis = 6 | |||||
| Other = 15 |
Abbreviations: BHP, Brain Health Program; GMT, Goal Management Training; HRQOL, health related quality of life; RCT, randomized controlled trial; VR, virtual reality.
Attention/Concentration
A majority of everyday functions rely on one’s ability to direct, divide, and sustain attention to aid task completion.63 Attentional abilities interact with many other cognitive domains, and improvements in attention can increase overall cognitive processing.64 Selective attention, or the ability to focus on chosen stimuli while ignoring distractions, is often susceptible to effects of brain disorders, including tumors.63,65 Other aspects of attention, such as sustaining a state of mental concentration over a period of time and/or dividing attention between tasks, are also more challenging following brain disease or injury.63
Attention often declines after glioma surgery,17 and though there is evidence suggesting improvement by 3 to 6 months,9 deficits persist and recovery to presurgical baselines can be tenuous. A variety of treatments and other issues common to brain tumor patients negatively affect attention and concentration, including radiotherapy. A study of low-grade glioma patients treated with radiotherapy vs not found a significant decline in attention with a mean follow-up of 12 years after diagnosis.66 Some antiepileptic drugs are associated with impairments in attention.67 Fatigue and low mood, reported in 42% of low-grade glioma patients following surgery,13 can also contribute to diminished arousal and vigilance, which are needed for attentional focus.68,69
To address attentional issues, emerging studies in brain tumor populations support a combination of cognitive retraining and compensatory strategy training,35,36,70–72 such as fatigue management, relaxation strategies, and pacing education, along with focus on self-awareness and problem solving to reduce attentional lapses.36,73,74 Several studies have demonstrated feasibility with cognitive retraining and compensatory strategy training in LrGG patients with positive cognitive outcomes.47,48 A randomized controlled trial of 6 weekly cognitive rehabilitation sessions (2 hours of combined retraining and compensatory strategy training) vs a waiting-list control group in LrGG demonstrated improvements in brief attention (and verbal memory) at 6 months post intervention.47 Attention-retraining techniques in this study focused on sustained, selective, alternating, and divided attention, whereas compensatory strategy training (didactic and experiential learning) focused on cognitive education, improving awareness, and relaxation strategies.47 An additional study of combined retraining and compensatory strategies in patients with high- and low-grade glioma and meningioma after surgery found significant improvements in visual attention (and verbal memory) at 1 month post intervention compared to a control group.49 The compensatory strategies in the latter study were modeled after work by Cicerone et al35 with metacognitive training (see “Executive Functioning” for more details) and education to improve self-awareness and management of attentional lapses.
In clinical practice, the multidimensional nature of attentional impairments, often identified during neuropsychological testing, necessitates a tailored interventional approach.36,64 Attentional arousal and focus may be addressed by reducing environmental distractors, self-cuing to aid focus, and working in well-lit environments,73,74 whereas difficulty in divided attention is commonly addressed by focusing on sufficient time for response, developing organizational strategies, and doing one task at a time.35,36
Processing Speed
Processing speed usually refers to the speed at which cognitive operations can be performed,75 and is thought of as an interaction between specific cognitive skills, reaction speed, and stimulus transmission.76 Cognitive skills that are more automatic require less processing speed for execution,77 whereas novel and difficult cognitive tasks require higher concerted effort, and thus produce slower processing speed times.76 When processing is slow, completion of tasks in a satisfactory way becomes more challenging.78 Higher-order or more complex tasks, such as abstraction or integration of information, are especially difficult with slower processing as several sources of information are needed simultaneously.78 Processing speed is highly vulnerable to brain injury76 and is seen frequently in LrGG patients.67 Primary brain tumor patients frequently describe mental slowness,79,80 which can lead to frustration when they are unable to keep up with the pace of task demands (eg, conversations and watching television).36,72 Processing speed impairments are particularly associated with damage to cerebral white matter and subcortical systems, as commonly seen in LrGG and from treatment such as radiation.79,80 Certain antiepileptic drugs33 and comorbid psychiatric conditions76,78 may also reduce processing speed.
Rehabilitation strategies to address processing speed impairments focus on compensatory strategy training, including preplanning, problem solving, and improving awareness to more effectively cope with time pressure demands. These strategy training strategies are well established in TBI and stroke, and may be applicable for use with LrGG.36,47,72,81 Clinically, a time pressure management strategy training is commonly used to improve awareness and restructure problem solving before and during a task to reduce time pressure demands.36,72,81 Time pressure management training builds awareness of how slowed thinking negatively affects daily functioning, and emphasizes the importance of performing one task at a time and preplanning to reduce in-the-moment pressure demands. For example, when driving to a new place, patients would be encouraged to study the route in advance, prepare their belongings, and consider setting an alarm to ensure a timely departure. Then during driving, they would focus on keeping ample distance between cars, turning off the radio, and minimizing conversation. If they become overwhelmed they could pull over, use relaxation strategies, and rereview their route when they are calmer. Elements of this approach were integrated into Gehring’s cognitive rehabilitation study with LrGG with an overall positive study benefit.47
Learning and Memory
Memory is a not a single operation, but rather consists of several systems dependent on intact functioning of various cognitive domains.63,82 The impact of neurological insult on memory depends on factors such as anatomical location, treatment, and disease course.69 Patients with left hemispheric gliomas were shown to be more impaired in immediate and delayed verbal recall vs those with intraventricular and posterior fossa tumors prior to surgery.54 Memory impairment is also seen in gliomas involving the thalamus, frontal, and temporal lobes.54 Postoperatively, diminished memory functioning is common in LrGG, with verbal memory impairments noted in 40% to 60% of patients with low-grade gliomas following awake surgery.13 Attention and working memory, which are essential in learning and remembering new information, are vulnerable to impairment following radiotherapy.63,66,83 High fraction dose, in particular, is associated with reduced learning and long-term memory storage capacity.67 Further, chemotherapy may affect hippocampal neurogenesis and learning of new information.31
Rehabilitation interventions focused on memory impairments tend to be broad, given the complexity of memory processes.82,84 Notably, executive impairments also interrupt strategic encoding and memory retrieval85 and are commonly incorporated into memory strategy training.36 A study in brain tumor patients (56% of whom had LrGGs), of 10-week cognitive retraining with exercises in memory, attention, visuospatial functions, language, and reasoning, found immediate improvements in attention, memory, and verbal fluency that were sustained at 6-month follow-up.46 Virtual reality cognitive retraining was also shown in brain tumors (5% of patients with low-grade gliomas) to improve attention, memory, and visual-motor coordination.58
Clinically, it is important to account for the nature and severity of the patient’s memory impairments to guide the rehabilitation approach. Internal memory strategies focus on mentally manipulating information to aid recall (eg, mnemonics, grouping, and semantic categorization), whereas external memory strategies focus on organizational prosthetics.84,86,87 Studies of patients with prefrontal brain tumors after 30 minutes of internal strategy training (semantic categorization) found significantly improved memory with increased contralateral activation seen on functional MRI.61,62 Another study demonstrated improvements in verbal memory in high-grade gliomas after 10 weeks of internal strategy training (mnemonics).59 External memory aids such as written lists, calendaring, labeling, designated places for items, increasing daily structure, notebooks and planners, and alarms show promise for LrGG.47
Executive Functioning
Executive impairments can affect the ability to form and execute goal-directed behavior,88–91 problem solving, and self-monitoring, and can lead to significant negative functional impact.63,89,91,92 Impairments in executive functioning—such as disorganization, cognitive inflexibility, and difficulty planning and multitasking—are particularly common in glioma patients. A meta-analysis of cognitive functioning in 313 glioma patients found postsurgical improvements in complex attention, language, and verbal memory, whereas executive function declined immediately after resection and persisted at 6 months.20
From a rehabilitation perspective, there is emerging evidence for improving executive functioning using metacognitive rehabilitation strategies. Metacognitive training focuses on improving awareness, self-monitoring, and attentional control to more effectively perform daily tasks.93–95 A systematic review in TBI found the greatest improvement when metacognitive approaches were combined with systematic problem-solving interventions,37 and this combination is a promising strategy for managing executive dysfunction in brain tumor populations as well.35,36
A study of patients with primary brain tumors (including 32% low- and 24% high-grade gliomas) found significant improvements in executive functioning using 8-week compensatory metacognitive strategy training combined with brain tumor education, compared to education alone.57 The training and education included exercises for improving awareness of dysexecutive symptoms, monitoring cognitive problems, and applying learned strategies to day-to-day activities. Significant between-group effects were noted at the 4-month posttreatment follow-up, though no immediate posttreatment effects were noted.57 An additional study of patients with primary brain tumors (including LrGG) found that 88% reported problem-solving strategies as helpful and continued to use the intervention 3 months after treatment.60
In clinical practice, compensatory strategy training focused on improving organization and problem solving is often helpful. Routinizing daily tasks,49 linking naturally behaviors together (eg, taking medications with meals), systematic problem solving,57 and optimizing existing organizational strategies47 hold promise for brain tumor patients.36,64,96,97
Language
Patients with gliomas located in the dominant hemisphere often perform worse on cognitive measures of language, verbal learning, and verbal intelligence.98 However, slowed and incoherent speech may also be present in patients with tumors located in the nondominant hemisphere.99 In a study of presumed LrGG patients with dominant-hemisphere tumors, performance was worse on tests of lexical retrieval (object naming and verbal fluency), whereas no impairments in language were found for those with nondominant tumors.100 Although advanced surgical techniques (ie, intraoperative mapping and monitoring) are leading to increased language preservation,22 full recovery from aphasia after resection may not be achieved for all LrGG patients.
In clinical practice, cognitive rehabilitation strategies for general populations with aphasia often focus on improvement in conversational skills, reading comprehension, and language formation.64,101,102 Anomia (a common linguistic complaint among high- and low-grade glioma patients17) is commonly addressed via compensatory strategy training focused on verbal circumlocution, paced speech, associative cuing, and semantic feature analysis (although efficacy is unclear in LrGG).35,103,104 Environmental interventions, such as supportive communication strategy training for caregivers to improve HRQOL by managing expectations and perceptions, have clear support in TBI and stroke, but have yet to be studied in brain tumor populations.105,106
Practical Clinical Considerations
In alignment with the UK National Institutes of Clinical Excellence guidelines for supportive care for adults living with cancer, the rehabilitation needs of patients should be assessed from a multidisciplinary perspective at key points in the disease course.107 This model also has emerging evidence for effectiveness and feasibility in the management of patients with high- and low-grade gliomas.108–110 A prospective, randomized study of 6 to 8 weeks of intensive ambulatory multidisciplinary rehabilitation vs a wait-list control group of brain tumor patients demonstrated improvements in self-care at 3 months.110 Improvements were also noted in psychosocial interactions, communication, and cognitive abilities (problem solving, memory), which were maintained at 6 months.110
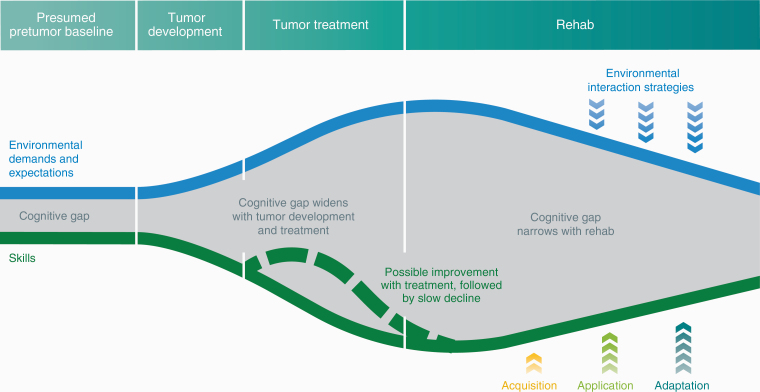
For clinicians and health care professionals working with these patients, considering systematic cognitive screening and actively managing comorbidities that exacerbate cognitive issues are important first steps. By asking patients (and caregivers) early and often about neuropsychological and behavioral changes and related distress, cognitive needs are more likely to be detected early and better managed. A referral to neuropsychology and thorough neuropsychological assessment in patients with suspected cognitive impairment are crucial to identify undertreated neuropsychological symptoms and inform rehabilitation treatment planning. There are no current consensus guidelines about the optimal timing for cognitive intervention in glioma patients. However, given the multiple factors that affect cognition (see Figure 1), cognitive rehabilitation should be considered during periods of psychiatric and medical stability, and after recovery from intensive treatments, such as radiation.56 Cognitive rehabilitation aims to reduce the gap between patients’ demands and abilities by applying the principles and strategies discussed earlier, to learn new ways to achieve their desired goals (Figure 3).
Figure 3.
Schema of Longitudinal Trajectory of Cognitive Impairment Across Disease Trajectory.
The blue line represents environmental demands and expectations such as work, school, and family responsibilities. The green line depicts the patient’s current cognitive skills such as attention/concentration, processing speed, memory, and executive functioning. The gray space between environmental demands and cognitive skills is the cognitive gap. The gap is narrow at “Presumed pretumor baseline” because the patient is well equipped with cognitive skills to manage his or her environmental demands and expectations. During “Tumor development,” the gap widens as skills decline and demands increase. The gap widens further with “Tumor treatment” given that the impact of surgery, radiotherapy, and chemotherapy on cognitive skills and environmental demand increases with factors such as returning to work after treatment. The dotted green line reflects natural recovery of cognitive skills after surgery. With “Rehab,” the gap is narrowed by improving cognitive skills using the Triple A model. Concurrently, environmental demands and expectations are decreased through environmental interaction strategies such as work accommodation, school accommodation, and managing family and patient performance expectations.
Additional factors such as higher seizure burden, certain antiepileptic drugs,33 steroids, and pain medications25,32 may also negatively affect mood and cognitive function. Consideration should be given to alternative antiepileptic drugs and/or referral to psychiatry when appropriate. Sleep issues and fatigue also exacerbate cognitive symptoms, and identifying and treating underlying causes (such as sleep apnea), and discussing sleep hygiene, emotional stressors, exercise, self-pacing education, and pharmacologic management should also be considered.68 Referrals to other care partners such as psychiatry/psychology, speech language pathology, and occupational therapy help to comanage cognitive and emotional symptoms.
Conclusion
As advances in molecular characterization and improvement in treatment extend survival for LrGG patients, increasing attention is being paid to cognitive outcomes and their effect on HRQOL. Impairments in attention, processing speed, learning and memory, executive functioning, and language, commonly experienced by patients with LrGG, often reduce their HRQOL. Results of emerging research of cognitive rehabilitation hold promise for addressing these concerns in LrGG, though there are a number of limitations. Establishing the natural history of cognition over the LrGG disease trajectory is challenged by methodological variability in the literature with a lack of uniform cognitive test batteries, difficulty with study attrition, and smaller sample sizes of heterogeneous histologies captured at varying time points.20 As neuro-oncology moves toward an integrated diagnosis of molecular characterization with histology, there are even fewer data establishing these trajectories among molecular subtypes.
The efficacy and generalizability of cognitive rehabilitation strategies are also confounded by variability in test batteries and assessment intervals, lack of control groups, and limited data from randomized trials.111 These limitations complicate our ability to develop a consensus regarding the cognitive needs and related treatments for brain tumor patients, and optimizing timing for intervention.111 Recently published guidelines for standardizing neuropsychological endpoints across cancer,112 including neuro-oncology,113 highlight the importance of addressing these issues and provide foundational work for investigating cognitive rehabilitation approaches in LrGG. Establishing more prospective data to optimize cognitive rehabilitation strategies and the timing of intervention (including strategies for network-based impairments) aligns with the increasing focus on cancer survivorship (including the development of survivorship care plans in neuro-oncology).114 Current challenges to implementation include a scarcity of qualified rehabilitation providers, lack of awareness about the role of cognitive rehabilitation, payer source issues, and underdetection and treatment of compounding mood and cognitive issues. Future considerations include systematic cognitive screening and development of novel telehealth interventions to increase access to care. To this end, we and others are exploring novel cognitive rehabilitation approaches using technology and self-monitoring tools to address some of these barriers (NCT03948490).
Acknowledgments
The authors thank N. Sirivansanti and K. Probst for their artistic support.
Funding
This work was supported by the LoGlio Collective [to all authors]; the Sheri Sobrato Brisson Brain Cancer Fund [to C.W.J., S.C, and S.H.J.]; the Robert Wood Johnson Foundation [grant number 74259 to S.H.J.]; and the National Institute of Neurological Disorders and Stroke [grant number K08 110919-01 to S.H.J.]
Conflict of interest statement. None declared.
References
- 1. Chang SM, Cahill DP, Aldape KD, et al. Treatment of adult lower-grade glioma in the era of genomic medicine. Am Soc Clin Oncol Educ Book. 2016;35:75–81. [DOI] [PubMed] [Google Scholar]
- 2. Buckner JC, Shaw EG, Pugh SL, et al. Radiation plus procarbazine, CCNU, and vincristine in low-grade glioma. N Engl J Med. 2016;374(14):1344–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. van den Bent MJ, Brandes AA, Taphoorn MJ, et al. Adjuvant procarbazine, lomustine, and vincristine chemotherapy in newly diagnosed anaplastic oligodendroglioma: long-term follow-up of EORTC Brain Tumor Group Study 26951. J Clin Oncol. 2013;31(3):344–350. [DOI] [PubMed] [Google Scholar]
- 4. Cairncross G, Wang M, Shaw E, et al. Phase III trial of chemoradiotherapy for anaplastic oligodendroglioma: long-term results of RTOG 9402. J Clin Oncol. 2013;31(3):337–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. van den Bent MJ, Baumert B, Erridge SC, et al. Interim results from the CATNON trial (EORTC study 26053-22054) of treatment with concurrent and adjuvant temozolomide for 1p/19q non-co-deleted anaplastic glioma: a phase 3, randomised, open-label intergroup study. Lancet. 2017;390(10103):1645–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131(6):803–820. [DOI] [PubMed] [Google Scholar]
- 7. Molinaro AM, Taylor JW, Wiencke JK, et al. Genetic and molecular epidemiology of adult diffuse glioma. Nat Rev Neurol. 2019;15(7): 405–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Talacchi A, Santini B, Savazzi S, et al. Cognitive effects of tumour and surgical treatment in glioma patients. J Neurooncol. 2011;103(3):541–549. [DOI] [PubMed] [Google Scholar]
- 9. Satoer D, Visch-Brink E, Smits M, et al. Long-term evaluation of cognition after glioma surgery in eloquent areas. J Neurooncol. 2014;116(1):153–160. [DOI] [PubMed] [Google Scholar]
- 10. Wefel JS, Noll KR, Rao G, et al. Neurocognitive function varies by IDH1 genetic mutation status in patients with malignant glioma prior to surgical resection. Neuro Oncol. 2016;18(12):1656–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. van Kessel E, Baumfalk AE, van Zandvoort MJE, et al. Tumor-related neurocognitive dysfunction in patients with diffuse glioma: a systematic review of neurocognitive functioning prior to anti-tumor treatment. J Neurooncol. 2017;134(1):9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nwachukwu CR, Youland RS, Chioreso C, et al. Health related quality of life (HRQOL) in long-term survivors of pediatric low grade gliomas (LGGs). J Neurooncol. 2015;121(3):599–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Racine CA, Li J, Molinaro AM, Butowski N, et al. Neurocognitive function in newly diagnosed low-grade glioma patients undergoing surgical resection with awake mapping techniques. Neurosurgery. 2015;77(3):371–379. [DOI] [PubMed] [Google Scholar]
- 14. Noll KR, Bradshaw ME, Weinberg JS, et al. Relationships between neurocognitive functioning, mood, and quality of life in patients with temporal lobe glioma. Psychooncology. 2017;26(5):617–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. van Loon EM, Heijenbrok-Kal MH, van Loon WS, et al. Assessment methods and prevalence of cognitive dysfunction in patients with low-grade glioma: a systematic review. J Rehabil Med. 2015;47(6):481–488. [DOI] [PubMed] [Google Scholar]
- 16. Noll KR, Ziu M, Weinberg JS, et al. Neurocognitive functioning in patients with glioma of the left and right temporal lobes. J Neurooncol. 2016;128(2):323–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Santini B, Talacchi A, Squintani G, et al. Cognitive outcome after awake surgery for tumors in language areas. J Neurooncol. 2012;108(2):319–326. [DOI] [PubMed] [Google Scholar]
- 18. Barzilai O, Ben Moshe S, Sitt R, et al. Improvement in cognitive function after surgery for low-grade glioma. J Neurosurg. 2019;130(2):426–434. [DOI] [PubMed] [Google Scholar]
- 19. Hoffermann M, Bruckmann L, Mahdy Ali K, et al. Pre- and postoperative neurocognitive deficits in brain tumor patients assessed by a computer based screening test. J Clin Neurosci. 2017;36:31–36. [DOI] [PubMed] [Google Scholar]
- 20. Ng JCH, See AAQ, Ang TY, et al. Effects of surgery on neurocognitive function in patients with glioma: a meta-analysis of immediate post-operative and long-term follow-up neurocognitive outcomes. J Neurooncol. 2019;141(1):167–182. [DOI] [PubMed] [Google Scholar]
- 21. Boone M, Roussel M, Chauffert B, et al. Prevalence and profile of cognitive impairment in adult glioma: a sensitivity analysis. J Neurooncol. 2016;129(1):123–130. [DOI] [PubMed] [Google Scholar]
- 22. Duffau H. Diffuse low-grade glioma, oncological outcome and quality of life: a surgical perspective. Curr Opin Oncol. 2018;30(6):383–389. [DOI] [PubMed] [Google Scholar]
- 23. Esposito R, Mattei PA, Briganti C, et al. Modifications of default-mode network connectivity in patients with cerebral glioma. PLoS One. 2012;7(7):e40231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Harris RJ, Bookheimer SY, Cloughesy TF, et al. Altered functional connectivity of the default mode network in diffuse gliomas measured with pseudo-resting state fMRI. J Neurooncol. 2014;116(2):373–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wefel JS, Kayl AE, Meyers CA. Neuropsychological dysfunction associated with cancer and cancer therapies: a conceptual review of an emerging target. Br J Cancer. 2004;90(9):1691–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kesler SR, Noll K, Cahill DP, et al. The effect of IDH1 mutation on the structural connectome in malignant astrocytoma. J Neurooncol. 2017;131(3):565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Stoecklein VM, Stoecklein S, Galiè F, et al. Resting-state fMRI detects alterations in whole brain connectivity related to tumor biology in glioma patients. Neuro-Oncol. 2020;22(9):1388–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Correa DD, Kryza-Lacombe M, Zhou X, et al. A pilot study of neuropsychological functions, APOE and amyloid imaging in patients with gliomas. J Neurooncol. 2018;136(3):613–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pulsifer MB, Duncanson H, Grieco J, et al. Cognitive and adaptive outcomes after proton radiation for pediatric patients with brain tumors. Int J Radiat Oncol Biol Phys. 2018;102(2):391–398. [DOI] [PubMed] [Google Scholar]
- 30. Raisa N, Marhaendraputro EA. The side effects of chemotherapy in glioma. Malang Neurol J. 2019;5(2):92–97. [Google Scholar]
- 31. Nokia MS, Anderson ML, Shors TJ. Chemotherapy disrupts learning, neurogenesis and theta activity in the adult brain. Eur J Neurosci. 2012;36(11):3521–3530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Correa DD, DeAngelis LM, Shi W, et al. Cognitive functions in low-grade gliomas: disease and treatment effects. J Neurooncol. 2007;81(2):175–184. [DOI] [PubMed] [Google Scholar]
- 33. Taphoorn MJB. Neurocognitive sequelae in the treatment of low-grade gliomas. Semin Oncol. 2003;30:45–48. [DOI] [PubMed] [Google Scholar]
- 34. Brissart H, Leroy M, Morele E, et al. Cognitive rehabilitation in multiple sclerosis. Neurocase. 2013;19(6):553–565. [DOI] [PubMed] [Google Scholar]
- 35. Cicerone KD, Langenbahn DM, Braden C, et al. Evidence-based cognitive rehabilitation: updated review of the literature from 2003 through 2008. Arch Phys Med Rehabil. 2011;92(4):519–530. [DOI] [PubMed] [Google Scholar]
- 36. Haskins EC. Cognitive Rehabilitation Manual: Translating Evidence-Based Recommendations Into Practice. In: Trexler LE, ed. Reston, VA:ACRM Publishing; 2014. [Google Scholar]
- 37. Krasny-Pacini A, Chevignard M, Evans J. Goal Management Training for rehabilitation of executive functions: a systematic review of effectiveness in patients with acquired brain injury. Disabil Rehabil. 2014;36(2):105–116. [DOI] [PubMed] [Google Scholar]
- 38. Katz DI, Ashley MJ, O’Shanick GJ, et al. Cognitive rehabilitation: the evidence, funding, and case for advocacy in brain injury. 2006. https://www.biausa.org/downloads/public-affairs/Cognitive_Rehabilitation_Position_Paper.pdf. Accessed August 4, 2020.
- 39. Kleim JA, Jones TA. Principles of experience-dependent neural plasticity: Implications for rehabilitation after brain damage. J Speech Lang Hear Res. 2008;51(1):S225–S239. [DOI] [PubMed] [Google Scholar]
- 40. Prosperini L, Piattella MC, Giannì C, et al. Functional and structural brain plasticity enhanced by motor and cognitive rehabilitation in multiple sclerosis. Neural Plast. 2015;2015. doi: 10.1155/2015/481574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dixon RA, Bäckman L. Principles of compensation in cognitive neurorehabilitation. In: Stuss DT, Winocur G, Robertson IH, eds. Cognitive Neurorehabilitation. Cambridge University Press; 1999:59–72. [Google Scholar]
- 42. Kolb B, Whishaw IQ. Brain plasticity and behavior. Annu Rev Psychol. 1998:49:43–64. [DOI] [PubMed] [Google Scholar]
- 43. Richardson JTE. Clinical and Neuropsychological Aspects of Closed Head Injury. 2 nd ed. Philadelphia, PA:Psychology Press; 2001. [Google Scholar]
- 44. Kurtz MM, Nichols MC. Cognitive rehabilitation for schizophrenia: a review of recent advances. Curr Psychiatry Rev. 2007;3(3):213–221. [Google Scholar]
- 45. Fawcett JW, Rosser AE, Dunnett SB, eds. Neuropsychological rehabilitation. In: Brain Damage, Brain Repair. Oxford: Oxford University Press; 2001:289–297. [Google Scholar]
- 46. Maschio M, Dinapoli L, Fabi A, et al. Cognitive rehabilitation training in patients with brain tumor-related epilepsy and cognitive deficits: a pilot study. J Neurooncol. 2015;125(2):419–426. [DOI] [PubMed] [Google Scholar]
- 47. Gehring K, Sitskoorn MM, Gundy CM, et al. Cognitive rehabilitation in patients with gliomas: a randomized, controlled trial. J Clin Oncol. 2009;27(22):3712–3722. [DOI] [PubMed] [Google Scholar]
- 48. van der Linden SD, Sitskoorn MM, Rutten GM, et al. Feasibility of the evidence-based cognitive telerehabilitation program Remind for patients with primary brain tumors. J Neurooncol. 2018;137(3): 523–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zucchella C, Capone A, Codella V, et al. Cognitive rehabilitation for early post-surgery inpatients affected by primary brain tumor: a randomized, controlled trial. J Neurooncol. 2013;114(1):93–100. [DOI] [PubMed] [Google Scholar]
- 50. Wilson BA, Gracey F, Evans JJ, Bateman A.. Neuropsychological Rehabilitation: Theory, Models, Therapy and Outcome. Cambridge: Cambridge University Press; 2009. doi: 10.1017/CBO9780511581083 [DOI] [Google Scholar]
- 51. Sohlberg MM, Mateer CA.. Introduction to Cognitive Rehabilitation: Theory and Practice. NY: Guilford Press; 1989. [Google Scholar]
- 52. Hendriks EJ, Habets EJJ, Taphoorn MJB, et al. Linking late cognitive outcome with glioma surgery location using resection cavity maps. Hum Brain Mapp. 2018;39(5):2064–2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Cochereau J, Herbet G, Duffau H. Patients with incidental WHO grade II glioma frequently suffer from neuropsychological disturbances. Acta Neurochir (Wien). 2016;158(2):305–312. [DOI] [PubMed] [Google Scholar]
- 54. Miotto EC, Silva Junior A, Corrêa Silva C, et al. Cognitive impairments in patients with low grade gliomas and high grade gliomas. Arq Neuropsiquiatr. 2011;69(4):596–601. [DOI] [PubMed] [Google Scholar]
- 55. van Lonkhuizen PJC, Klaver KM, Wefel JS, et al. Interventions for cognitive problems in adults with brain cancer: a narrative review. Eur J Cancer Care (Engl). 2019;28(3):e13088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Coomans MB, van der Linden SD, Gehring K, et al. Treatment of cognitive deficits in brain tumour patients: current status and future directions. Curr Opin Oncol. 2019;31(6):540–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Richard NM, Bernstein LJ, Mason WP, et al. Cognitive rehabilitation for executive dysfunction in brain tumor patients: a pilot randomized controlled trial. J Neurooncol. 2019;142(3):565–575. [DOI] [PubMed] [Google Scholar]
- 58. Yang S, Chun MH, Son YR. Effect of virtual reality on cognitive dysfunction in patients with brain tumor. Ann Rehabil Med. 2014;38(6):726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hassler MR, Elandt K, Preusser M, et al. Neurocognitive training in patients with high-grade glioma: a pilot study. J Neurooncol. 2010;97(1):109–115. [DOI] [PubMed] [Google Scholar]
- 60. Locke DE, Cerhan JH, Wu W, et al. Cognitive rehabilitation and problem-solving to improve quality of life of patients with primary brain tumors: a pilot study. J Support Oncol. 2008;6(8):383–391. [PubMed] [Google Scholar]
- 61. Miotto EC, Savage CR, Evans JJ, et al. Semantic strategy training increases memory performance and brain activity in patients with prefrontal cortex lesions. Clin Neurol Neurosurg. 2013;115(3): 309–316. [DOI] [PubMed] [Google Scholar]
- 62. Miotto EC, Balardin JB, Vieira G, et al. Right inferior frontal gyrus activation is associated with memory improvement in patients with left frontal low-grade glioma resection. PLoS One. 2014;9(8):e105987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lezak MD, Howieson DB, Bigler ED, Tranel D.. Neuropsychological Assessment. 5th ed. Oxford:Oxford University Press Inc; 2012. [Google Scholar]
- 64. Johnstone B, Stonnington HH, eds. Rehabilitation of Neuropsychological Disorders: A Practical Guide for Rehabilitation Professionals. 2nd ed. NY:Psychology Press; 2009. [Google Scholar]
- 65. Scott JG. Attention/concentration: the distractible patient. In: Schoenberg MR, Scott JG, eds. The Little Black Book of Neuropsychology: A Syndrome-Based Approach. NY:Springer US; 2011:149–158. [Google Scholar]
- 66. Douw L, Klein M, Fagel SS, et al. Cognitive and radiological effects of radiotherapy in patients with low-grade glioma: long-term follow-up. Lancet Neurol. 2009;8(9):810–818. [DOI] [PubMed] [Google Scholar]
- 67. Klein M, Heimans JJ, Aaronson NK, et al. Effect of radiotherapy and other treatment-related factors on mid-term to long-term cognitive sequelae in low-grade gliomas: a comparative study. Lancet. 2002;360(9343):1361–1368. [DOI] [PubMed] [Google Scholar]
- 68. Armstrong TS, Gilbert MR. Practical strategies for management of fatigue and sleep disorders in people with brain tumors. Neuro Oncol. 2012;14(Suppl 4):iv65–iv72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Skeel RL, Edwards S. The assessment and rehabilitation of memory impairments. In: Schoenberg MR, Scott JG, eds. Rehabilitation of Neuropsychological Disorders: A Practical Guide for Rehabilitation Professionals. 2nd ed. NY:Psychology Press; 2009:47–74. [Google Scholar]
- 70. Bogdanova Y, Yee MK, Ho VT, et al. Computerized cognitive rehabilitation of attention and executive function in acquired brain injury: a systematic review. J Head Trauma Rehabil. 2016;31(6):419–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Sohlberg MM, Avery J, Kennedy M, et al. Practice guidelines for direct attention training. J Med Speech-Lang Pathol. 2003;11(3): xix–xxxix. [Google Scholar]
- 72. Winkens I, Van Heugten CM, Wade DT, et al. Efficacy of time pressure management in stroke patients with slowed information processing: a randomized controlled trial. Arch Phys Med Rehabil. 2009;90(10):1672–1679. [DOI] [PubMed] [Google Scholar]
- 73. Sohlberg MM, Mateer CA. Improving attention and managing attentional problems. Adapting rehabilitation techniques to adults with ADD. Ann N Y Acad Sci. 2001;931:359–375. [PubMed] [Google Scholar]
- 74. Whyte J. Neurologic disorders of attention and arousal: assessment and treatment. Arch Phys Med Rehabil. 1992;73(11):1094–1103. [PubMed] [Google Scholar]
- 75. Reichenberg A, Harvey PD. Neuropsychological impairments in schizophrenia: Integration of performance-based and brain imaging findings. Psychol Bull. 2007;133(5):833–858. [DOI] [PubMed] [Google Scholar]
- 76. Koziol LF, Budding DE.. Subcortical Structures and Cognition: Implications for Neuropsychological Assessment. NY: Springer Science + Business Media LLC; 2009. [Google Scholar]
- 77. Luu P, Tucker DM, Stripling R. Neural mechanisms for learning actions in context. Brain Res. 2007;1179:89–105. [DOI] [PubMed] [Google Scholar]
- 78. Kail R, Salthouse TA. Processing speed as a mental capacity. Acta Psychol (Amst). 1994;86(2–3):199–225. [DOI] [PubMed] [Google Scholar]
- 79. Cohen RA. The Neuropsychology of Attention. 2nd ed. NY:Springer US; 2014. [Google Scholar]
- 80. Schatz J, Kramer JH, Ablin A, et al. Processing speed, working memory, and IQ: a developmental model of cognitive deficits following cranial radiation therapy. Neuropsychology. 2000;14(2):189–200. [DOI] [PubMed] [Google Scholar]
- 81. Fasotti L, Kovacs F, Eling PATM, Brouwer WH.. Time pressure management as a compensatory strategy training after closed head injury. Neuropsychol Rehabil. 2000;10(1):47–65. [Google Scholar]
- 82. Squire LR, Zola SM. Structure and function of declarative and nondeclarative memory systems. Proc Natl Acad Sci U S A. 1996;93(24):13515–13522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Baddeley A. Is working memory still working? Eur Psychol. 2002;7(2):85–97. [Google Scholar]
- 84. Elliott M, Parente F. Efficacy of memory rehabilitation therapy: a meta-analysis of TBI and stroke cognitive rehabilitation literature. Brain Inj. 2014;28(12):1610–1616. [DOI] [PubMed] [Google Scholar]
- 85. Strangman GE, O’Neil-Pirozzi TM, Goldstein R, et al. Prediction of memory rehabilitation outcomes in traumatic brain injury by using functional magnetic resonance imaging. Arch Phys Med Rehabil. 2008;89(5):974–981. [DOI] [PubMed] [Google Scholar]
- 86. OʼNeil-Pirozzi TM, Strangman GE, Goldstein R, et al. A controlled treatment study of internal memory strategies (I-MEMS) following traumatic brain injury . J Head Trauma Rehabil. 2010;25(1): 43–51. [DOI] [PubMed] [Google Scholar]
- 87. OʼNeil-Pirozzi TM, Kennedy MRT, Sohlberg MM. Evidence-based practice for the use of internal strategies as a memory compensation technique after brain injury: a systematic review. J Head Trauma Rehabil. 2016;31(4):E1–E11. [DOI] [PubMed] [Google Scholar]
- 88. Lezak MD. The problem of assessing executive functions. Int J Psychol. 1982;17:281–297. [Google Scholar]
- 89. Goldberg E. The New Executive Brain: Frontal Lobes in a Complex World. Oxford: Oxford University Press Inc; 2009. [Google Scholar]
- 90. Scott JG, Schoenberg MR. Frontal lobe/executive functioning. In: The Little Black Book of Neuropsychology: A Syndrome-Based Approach. NY:Springer US; 2011:219–248. [Google Scholar]
- 91. Tranel D, Hathaway-Nepple J, Anderson SW. Impaired behavior on real-world tasks following damage to the ventromedial prefrontal cortex. J Clin Exp Neuropsychol. 2007;29(3):319–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Anderson V, Levin HS, Jacobs R. Executive functions after frontal lobe injury: a developmental perspective. In: Stuss DT, Knight RT, eds. Principles of Frontal Lobe Function. Oxford:Oxford University Press Inc; 2002:504–527. [Google Scholar]
- 93. Stamenova V, Levine B. Effectiveness of goal management training in improving executive functions: a meta-analysis. Neuropsychol Rehabil. 2019;29(10):1569–1599. [DOI] [PubMed] [Google Scholar]
- 94. Levine B, Schweizer TA, O’Connor C, et al. Rehabilitation of executive functioning in patients with frontal lobe brain damage with goal management training. Front Hum Neurosci. 2011;5:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Levine B, Robertson IH, Clare L, et al. Rehabilitation of executive functioning: an experimental-clinical validation of goal management training. J Int Neuropsychol Soc. 2000;6(3):299–312. [DOI] [PubMed] [Google Scholar]
- 96. Perna R, Loughan AR, Talka K. Executive functioning and adaptive living skills after acquired brain injury. Appl Neuropsychol Adult. 2012;19(4):263–271. [DOI] [PubMed] [Google Scholar]
- 97. Stuss DT, Knight RT, eds. Principles of Frontal Lobe Function. Oxford:Oxford University Press Inc; 2002. [Google Scholar]
- 98. Scheibel RS, Meyers CA, Levin VA. Cognitive dysfunction following surgery for intracerebral glioma: influence of histopathology, lesion location, and treatment. J Neurooncol. 1996;30(1):61–69. [DOI] [PubMed] [Google Scholar]
- 99. Lilja A, Brun A, Salford LG, et al. Neuropsychological indexes of a partial frontal syndrome in patients with nonfrontal gliomas. Neuropsychology. 1992;6(4):315–326. [Google Scholar]
- 100. Antonsson M, Jakola A, Longoni F, et al. Post-surgical effects on language in patients with presumed low-grade glioma. Acta Neurol Scand. 2018;137(5):469–480. [DOI] [PubMed] [Google Scholar]
- 101. Winans-Mitrik RL, Hula WD, Dickey MW, Schumacher JG, Swoyer B, Doyle PJ. Description of an intensive residential aphasia treatment program: rationale, clinical processes, and outcomes. Am J Speech Lang Pathol. 2014;23(2):S330–S342. [DOI] [PubMed] [Google Scholar]
- 102. Yu ZZ, Jiang SJ, Jia ZS, et al. Study on language rehabilitation for aphasia. Chin Med J (Engl). 2017;130(12):1491–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Francis DR, Clark N, Humphreys GW. Circumlocution-induced naming (CIN): a treatment for effecting generalisation in anomia? Aphasiology. 2002;16(3):243–259. [Google Scholar]
- 104. Maddy KM, Capilouto GJ, McComas KL. The effectiveness of semantic feature analysis: an evidence-based systematic review. Ann Phys Rehabil Med. 2014;57(4):254–267. [DOI] [PubMed] [Google Scholar]
- 105. Turner S, Whitworth A. Conversational partner training programmes in aphasia: a review of key themes and participants’ roles. Aphasiology. 2006;20(6):483–510. [Google Scholar]
- 106. Simmons-Mackie N, Raymer A, Armstrong E, et al. Communication partner training in aphasia: a systematic review. Arch Phys Med Rehabil. 2010;91(12):1814–1837. [DOI] [PubMed] [Google Scholar]
- 107. National Institute for Clinical Excellence, Great Britain, National Health Service. Improving Supportive and Palliative Care for Adults With Cancer: the Manual. London, UK: National Institute for Clinical Excellence; 2004. [Google Scholar]
- 108. Bartolo M, Zucchella C, Pace A, et al. Early rehabilitation after surgery improves functional outcome in inpatients with brain tumours. J Neurooncol. 2012;107(3):537–544. [DOI] [PubMed] [Google Scholar]
- 109. Sherer M, Meyers CA, Bergloff P. Efficacy of postacute brain injury rehabilitation for patients with primary malignant brain tumors. Cancer. 1997;80(2):250–257. [PubMed] [Google Scholar]
- 110. Khan F, Amatya B, Drummond K, et al. Effectiveness of integrated multidisciplinary rehabilitation in primary brain cancer survivors in an Australian community cohort: a controlled clinical trial. J Rehabil Med. 2014;46(8):754–760. [DOI] [PubMed] [Google Scholar]
- 111. Habets EJJ, Taphoorn MJB, Klein M, et al. The level of reporting of neurocognitive outcomes in randomised controlled trials of brain tumour patients: A systematic review. Eur J Cancer. 2018;100:104–125. [DOI] [PubMed] [Google Scholar]
- 112. Wefel JS, Vardy J, Ahles T, et al. International Cognition and Cancer Task Force recommendations to harmonise studies of cognitive function in patients with cancer. Lancet Oncol. 2011;12(7):703–708. [DOI] [PubMed] [Google Scholar]
- 113. van den Bent MJ, Wefel JS, Schiff D, et al. Response assessment in neuro-oncology (a report of the RANO group): assessment of outcome in trials of diffuse low-grade gliomas. Lancet Oncol. 2011;12(6):583–593. [DOI] [PubMed] [Google Scholar]
- 114. Leeper HE, Acquaye AA, Bell S, et al. Survivorship care planning in neuro-oncology. Neurooncol Pract. 2018;5(1):3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]