Abstract
Background
Fibroblast growth factor receptor (FGFR) inhibitors are currently used in clinical development. A subset of glioblastomas carries gene fusion of FGFR3 and transforming acidic coiled-coil protein 3. The prevalence of other FGFR3 alterations in glioma is currently unclear.
Methods
We performed RT-PCR in 101 glioblastoma samples to detect FGFR3-TACC3 fusions (“RT-PCR cohort”) and correlated results with FGFR3 immunohistochemistry (IHC). Further, we applied FGFR3 IHC in 552 tissue microarray glioma samples (“TMA cohort”) and validated these results in two external cohorts with 319 patients. Gene panel sequencing was carried out in 88 samples (“NGS cohort”) to identify other possible FGFR3 alterations. Molecular modeling was performed on newly detected mutations.
Results
In the “RT-PCR cohort,” we identified FGFR3-TACC3 fusions in 2/101 glioblastomas. Positive IHC staining was observed in 73/1024 tumor samples of which 10 were strongly positive. In the “NGS cohort,” we identified FGFR3 fusions in 9/88 cases, FGFR3 amplification in 2/88 cases, and FGFR3 gene mutations in 7/88 cases in targeted sequencing. All FGFR3 fusions and amplifications and a novel FGFR3 K649R missense mutation were associated with FGFR3 overexpression (sensitivity and specificity of 93% and 95%, respectively, at cutoff IHC score > 7). Modeling of these data indicated that Tyr647, a residue phosphorylated as a part of FGFR3 activation, is affected by the K649R mutation.
Conclusions
FGFR3 IHC is a useful screening tool for the detection of FGFR3 alterations and could be included in the workflow for isocitrate dehydrogenase (IDH) wild-type glioma diagnostics. Samples with positive FGFR3 staining could then be selected for NGS-based diagnostic tools.
Keywords: FGFR3, glioma, panel sequencing, targeted treatment
Diffusely growing gliomas are aggressive primary brain tumors with a poor prognosis.1 They are further characterized by the absence of prognostically relevant isocitrate dehydrogenase (IDH) 1 and IDH2 hotspot mutations.2 Several molecular subclasses have been described for glioblastoma so far, depending on the clustering method employed.3–6
Fibroblast growth factor receptors (FGFR), which have an important role in cell growth, differentiation, and neovascularization,7 are druggable therapeutic targets. Four receptors (FGFR1-FGFR4) and 18 ligands (FGFR) have been discovered in humans.8 Upon ligand binding, FGFR dimerizes and phosphorylates intracellular kinase domains (eg, Tyr724 and Tyr760 in FGFR3) thus activating several important pathways including Ras/Raf/MEK and PI3K-Akt.8,9 FGFR mutations are most common in urothelial, breast, and endometrial cancer but have been reported in approximately 7% of neoplasms of various histologies.10 The most common FGFR alterations in cancer are mutations and amplifications. Of all FGFRs examined, FGFR3 exhibited the highest rate of gene rearrangements.10 FGFR3 fusions resulting from 4p16.3 rearrangements were detected in bladder cancer and in glioblastomas.11 FGFR3 is most commonly fused to the closely located transforming acidic coiled-coil protein 3 (TACC3) gene. Rarely, FGFR3-BAIAP2L1, FGFR3-CAMK2A, FGFR3-TNIP2, and FGFR3-WHSC1 fusions have been reported.12–14 The fusion protein includes the entire FGFR3 kinase domain, and therefore, the product has ligand-independent constitutive kinase activity, induces mitotic and chromosomal segregation defects, and thus triggering aneuploidy in tumor cells.11 The loss of the 3′-UTR of FGFR3 blocks gene regulation of miR-99a and enhances the expression of the fusion gene.15 Fusion-positive tumors cluster with transcriptional subgroups characterized by mitochondrial activation by PIN4 phosphorylation and subsequent tumor growth.16 Overall, the frequency of FGFR3 fusions in selected brain tumor cohorts has been reported 1.2%-3.5%.3,6,14,17 A very recent study showed that FGFR3 fusion can serve as an independent prognostic factor in glioblastoma.18 FGFR3 protein overexpression in brain tumors is associated with the presence of FGFR3 fusions.13 The prevalence of other FGFR3 alterations in gliomas is currently unclear.
FGFR alterations can be targeted by inhibitors including the multi-tyrosine kinase inhibitors ponatinib, lenvatinib, BGJ398 (Clinical Trial.gov accession number: NCT01975701), and novel oral selective inhibitors with multiple targets among others as outlined in https://www.drugbank.ca/. Some of these FGFR inhibitors are currently under investigation in phase I/II targeted trials for advanced solid tumors.7,19
Molecular diagnostic tools for the identification of suitable patients for these trials currently include whole transcriptome (RNA-Seq) and exome sequencing, targeted DNA sequencing, reverse transcriptase PCR, and immunohistochemistry (IHC).
Glioma cell lines harboring the FGFR3-TACC3 fusion protein are sensitive to FGFR inhibition11 indicating that FGFR3 fusion protein is a therapeutic target in glioma. In fact, two cases of FGFR3-TACC3 fusion-positive glioblastomas were experimentally treated by the pan FGFR inhibitor erdafitinib.17 One patient remained stable for 134 days after the start of the anti-FGFR therapy, the second patient 287 days.
Taken together, FGFR3 fusions represent potential predictive markers for clinical trial inclusions and eventually clinical management. Open questions addressed in this study include (i) the actual frequency of FGFR3-TACC3 and other rare FGFR3 fusions in an unselected collection of glioblastoma sample subset, (ii) the presence and frequency of other FGFR3 alterations beyond FGFR-TACC3, and (iii) the screening role of FGFR3 IHC in a large tissue microarray (TMA) set to detect these alterations in addition to possible FGFR3-TACC3 fusions.
Materials and Methods
Biological Specimen
In total, the study consisted of 1047 analyzed samples. First, 728 patients with brain tumor samples were enrolled in this retrospective study (Figure 1) and were assigned into three cohorts. The “RT-PCR cohort” included 101 subsequently operated fresh-frozen glioblastoma samples that were analyzed by RT-PCR for frequency of FGFR3-TACC3 fusion. Our TMA cohort (“TMA cohort”) consisted of 552 formalin-fixed, paraffin-embedded (FFPE) glioma samples (for detailed epidemiological data and molecular data, see Table 1). The third cohort (“NGS [next-generation sequencing] cohort”) consisted of 75 additional unselected glioblastoma samples with tumor progression after standard treatment. The NGS cohort was enriched for specific cases from the RT-PCR (n = 3) and TMA (n = 10) cohorts due to their FGFR3 IHC expression and included two IDH-mutant cases (total n = 88 tumor FFPE samples for targeted sequencing). The FFPE samples were obtained from patients undergoing surgery for astrocytic and oligodendroglial brain tumors between 2000 and 2018 at the University Hospital Tuebingen. All three cohorts were evaluated for FGFR3 staining in IHC. Staining result frequencies were validated in two external TMA cohorts. Validation TMA dataset #1 was provided from the Neurological Institute/Edinger Institute, University of Frankfurt, Germany and consisted of 232 IDH wild-type primary glioblastoma samples.20 Validation TMA dataset #2 with 87 glioblastoma samples was provided by the Neuropathology Heidelberg. The study was authorized by the respective ethics board (number 788/2016BO2). Histological diagnosis, molecular typing, and grading for each tumor sample were performed according to the current WHO classification of CNS (central nervous system) tumors.21 Tumor location, gender, survival, tumor status (primary/progression), and patient age were retrieved from the clinical records.
Figure 1.
CONSORT diagram depicting samples used in the study. A immunoreactive (IR) score > 7 corresponds to overexpression in FGFR3 immunohistochemistry evaluation results. Abbreviations: NGS = next-generation sequencing; TMA = tissue microarray; RT-PCR = reverse transcriptase PCR; IHC = immunohistochemistry; FGFR3 = fibroblast growth factor receptor 3.
Table 1.
Epidemiology of Tissue Microarray Cohort
| Diagnosis | WHO grade | Cases | Gender (F/M) | Mean age (range) | Primary/recurrent | IDH mutant/IDH wild type | MGMT methylated/unmethylated | ATRX loss/retention | LOH1p19q / retention |
|---|---|---|---|---|---|---|---|---|---|
| Oligodendroglioma | II | 61 | 28/33 | 45.1 (17-74) | 56/5 | 61/0 | 9/0 | 1/60 | 61/0 |
| Anaplastic oligodendroglioma | III | 32 | 14/18 | 46.7 (26-79) | 19/13 | 32/0 | 21/1 | 1/31 | 32/0 |
| Astrocytoma | II | 53 | 19/34 | 42.8 (5-70) | 45/8 | 36/17 | 4/7 | 33/20 | 0/9 |
| Anaplastic astrocytoma | III | 61 | 20/41 | 43.9 (10-76) | 38/23 | 35/26 | 2/8 | 34/27 | 0/5 |
| Glioblastoma | IV | 215 | 81/134 | 59.5 (10-88) | 179/36 | 20/195 | 57/84 | 25/190 | 0/2 |
| Gliosarcoma | IV | 48 | 18/30 | 59.8 (26-83) | 30/18 | 0/48 | 15/26 | 0/48 | ND |
| Pilocytic astrocytoma | I | 82 | 43/39 | 23.3 (2-78) | 67/15 | 0/82 | ND | 0/82 | ND |
| All tumors | 552 | 223/329 | 48.5 (2-88) | 434/118 | 184/368 | 108/126 | 94/376 | 93/16 |
Abbreviations: M, male; F, female; IDH, isocitrate dehydrogenase; ND, not determined; LOH, loss of heterozygosity; MGMT, O6-methylguanine DNA methyltransferase; ATRX, alpha thalassemia/mental retardation syndrome X-linked; WHO, World Health Organization.
Molecular Diagnostics
IDH1/2, H3F3A, ATRX (alpha thalassemia/mental retardation syndrome X-linked), and LOH1p/19q (loss of heterozygosity) analysis in the Tuebingen cohort was performed as described previously.22 Briefly, the IDH1 R132H and H3F3A K27M mutational status was first determined in IHC. Samples lacking the R132H mutation and from patients less than 55 years old were further examined by direct pyrosequencing of the relevant exons for IDH1 and 2 hotspot mutations. Cases with ATRX loss and IDH1/2 wild-type status or cases with midline location were sequenced for H3F3A K27 and G34 mutations. LOH1p/19q was examined in IDH1/2 mutant tumors with ATRX retention using 5 tetranucleotide markers for each chromosomal region. Allele signal intensity of each tumor sample was always compared with the corresponding allele band of the blood control sample taken from the same patient. O6-methylguanine DNA methyltransferase (MGMT) methylation status was determined by pyrosequencing of CpG islands 74-48. Methylation classification analysis was performed by applying EPIC analysis in two cases (Illumina, Carlsbad, CA, USA) as previously described.5 Tumors were then classified with an established brain tumor classifier V11b4 (www.molecularneuropathology.org) using the obtained IDAT files. Classifier scores with a probability greater than 0.9 were taken as indicative for the respective methylation class. Cases with inconsistent molecular data were not included. Tumors with identification of FGFR3-TACC3 fusion or other FGFR3 alteration were additionally stained for CD34 (Clone QBEnd-10, Dako, Glostrup, DK) on full slides.
FGFR3 RT-PCR
In brief, cDNA was mixed with 7 μl nuclease-free water and 12 μl master mix. The master mix contained 10 μl MyTaq HS Mix (BIOLINE, Luckenwalde, Germany), 1 μl of a forward primer, and 1 μl of a reverse primer. All primers were selected using NCBI/Primer-BLAST blast sequence data of FGFR3 (NM_000142.4) and TACC3 (NM_006342.2) obtained at http://www.ncbi.nlm.nih.gov/tools/primer-blast/. (For primer details and cycling conditions see Supplementary Table 1). The expected product length for the wild type was 404 bp, and for the FGFR3-TACC3 fusion product, it depended on the type of fusion, but could not exceed 4690 bp for the primer combination 3 and 4, 3504 bp for the primer combination 5 and 6, and 3854 bp for the primer combination 5 and 7. In all cases, the estimated sizes of the amplification products detected corresponded to the predicted sizes.
FGFR3 and TACC3 Immunohistochemistry
Staining was performed on the Benchmark IHC/ISH (Ventana Medical Systems) after several optimization rounds for tissue pretreatment, antigen demasking, and antibody dilution. FGFR3 protein expression was detected by IHC using a mouse monoclonal antibody raised against amino acids 25-124 of FGFR3 of human origin (sc-13121; Santa Cruz Biotechnology). Tumors with confirmed FGFR3-TACC3 fusion served as positive control.13 For TACC3 protein expression, a mouse monoclonal antibody raised against a partial recombinant TACC3 (Clone 6C4, H00010460-M02; Abnova) was used. FFPE fixed human tonsils served as positive control. Staining conditions were as follows: FGFR3: OptiView CC1 pretreatment for 32 minutes, 1:50 dilution, incubated at 37°C for 40 minutes. TACC3: IView CC1 pretreatment for 32 minutes, 1:100 dilution incubated at 37°C for 32 minutes, Amplifier A and B (Dako, Glostrup, DK) for 8 minutes. All slides were then counterstained with hematoxylin for 2 minutes. FGFR3 and TACC3 staining intensities were scored as published previously in 0 (no staining), 1 (weak staining), 2 (moderate staining), and 3 (strong staining), for exemplary images, see Supplementary Figure 1.13 In addition, positive staining in tumors was quantified as follows: 1 (up to 24% tumor cells positive), 2 (25%-49% tumor cells positive), 3 (50%-74% tumor cells positive), and 4 (more than 75% tumor cells positive). Staining intensities were multiplied with staining quantification into a combined immunoreactivity score (IRS) ranging from 0 to 12. IRS scores > 7 were determined in this study as FGFR3 overexpression, IRS scores 9-12 were considered strongly positive.
Gene Panel Sequencing and Variant Calling
Gene panel sequencing and target enrichment were performed according to standard procedures (CeGaT GmbH, Tuebingen, Germany) as described previously.23 The panel includes 710 genes (TUM.4, full list available in the Supplementary file). Sequencing was carried out on Illumina NovaSeq 6000 platform as 100 bp paired-end runs. Reads were demultiplexed using Illumina bcl2fastq (2.19) (Illumina, San Diego, CA, USA). Adapter sequences were removed with Skewer 0.2.2 and the trimmed reads mapped to the human reference genome (hg19) using the Burrows-Wheeler Aligner (BWA-MEM 0.7.17). Variants were called using SAMtools and VarScan (2.3.3) and annotated after artifacts, and duplicates had been removed with in-house tools (CeGaT GmbH, Tuebingen, Germany). Somatic variants were reported when tumor samples had a minimum of 30 reads and at least 5 reads reported the same changes. The absolute detection limit was set to 5% of null allele frequency. Structural variants were called using Manta-SV (version 1.4) with tumor-only analysis parameters, and a minimum of 5 independent reads were required for fusion detection.
Molecular Modeling
Protein structure database 4K3324 was used as a template for modeling FGFR3 variants. Protein structure was modeled using Maestro Suite 2019-2 (Schrödinger, LLC, New York, NY, USA) with Prime. The protein structure was prepared using the default options of Protein Preparation Wizard. After K649R mutation, side-chain and main-chain positions were re-modeled by using Prime side-chain optimization with an option to allow also main-chain movements. Resulting protein structures were analyzed with Maestro and PyMOL, and the image was created with PyMOL (The PyMOL Molecular Graphics System, Version 2.0, Schrödinger, LLC, New York, NY, USA).
Statistical Analysis
Quantitative and statistical analyses were performed using JMP 7.0 (SAS Institute, Cary, NJ, USA). For correlation analyses, we performed unpaired, two-tailed Student’s t-test and the Fisher’s exact test to identify possible significant associations or differences between two pairs. Kaplan-Meier test was performed for survival analysis. Univariate analyses of the different variables were obtained with 95% confidence intervals (CIs). A P-value <.05 was considered as significant.
Results
Consecutive Screening of Glioblastoma by RT-PCR Confirms Low Frequency of FGFR3 Fusions
We analyzed 101 consecutive glioblastoma cases with available fresh-frozen tissue for the frequency of FGFR3-TACC3 fusions by RT-PCR. Epidemiological details of this “RT-PCR cohort” are listed in Supplementary Table 2.
We identified two cases with FGFR3 primers for exon 17-exon 10 fusion (Cases #1 and #3, Figures 2A and 4). Sanger sequencing of the RT-PCR products in both cases confirmed breakpoints at FGFR3 exon 17 joining TACC3 exon 10 sequences (Figure 2B). By using specific primers located in exon 8, 6, and 5, no further common FGFR3 fusions cases were identified. Thus, the frequency of known FGFR3 fusions in consecutively sampled glioblastoma identified by RT-PCR was 1.9%.
Figure 2.
FGFR3 fusions in glioblastoma: A, RT-PCR product for FGFR3 exon 17-TACC3 exon 10 primer pairs. B, Confirmatory Sanger sequence of FGFR3-TACC breakpoint in Case #1. C, FGFR3 (left column) and TACC3 (right column) immunohistochemistry in a FGFR-TACC3 fusion-positive (Case #1) and fusion-negative (Case #4) tumor. D, Full mounted slide staining confirming strong FGFR3 immunoreactivity score (IRS: 12, insets: full photo of stained TMA punch, scale bars: 200 µm).
Figure 4.
Summary of genetic profiles found in FGFR3-altered tumors (yellow box: protein-affecting mutation, violet amp: amplification, brown del: deletion, MGMT promotor M: methylated, U: unmethylated, blue loss: nuclear expression absent, *: IHC data from primary tumor). The FGFR3-TACC3 fusions in Cases #1 and #3 were also identified by RT-PCR.
Both FGFR3 fusion-positive cases (Cases #1 and #3) were IDH wild-type glioblastomas located in the right temporal lobe. Interestingly in histology, besides standard glioblastoma features, an extensive vascularization pattern was observed in Case #1 (representative images are shown in Supplementary Figure 2) as previously described in FGFR3 mutant tumors.25,26
We subjected the RT-PCR cohort cases to FGFR3 and TACC3 IHC. Sufficient corresponding FFPE tissue for FGFR3 and TACC3 staining evaluation with sufficient tumor content (=>70%) was available in 78/101 frozen samples. Positive staining in FGFR3 IHC positive cases was generally diffusely cytoplasmic with very occasional additional nuclear staining (Supplementary Figure 2). Endothelial cells remained negative. Adjacent normal human brain was mostly negative except some weak staining of the cerebral molecular layer. Sixty-seven cases (85%) were FGFR3 negative (IRS score 2 or lower), 7 cases (9%) showed focal and weak FGFR3 expression (IRS 3-5), and one case moderate FGFR3 expression (IRS 6-8). Three cases (4%) showed strong FGFR3 IHC expression in the majority of tumor cells (all IRS score 12, Figure 2 C+D). These 3 strongly positive cases included the two samples in which the FGFR3-TACC3 fusion was also identified by RT-PCR. The same RT-PCR cohort samples with FGFR3 IHC were also immunostained for the prototypical fusion partner TACC3. Sixty-two cases including the two detected fusion-positive cases (79%) were considered strongly TACC3 positive (IRS score 8 or higher), 1 case was moderately positive (IRS: 6, 1%), 11 cases weakly positive (IRS 4, 14%), and 4 cases were considered negative (IRS 0-2, 5%). TACC3 staining was observed in the cytoplasm of tumor cells with increased expression in multinucleated tumor cells. Nuclear staining was observed in apoptotic bodies. In contrast to FGFR3 IHC, the TACC3 staining IRS was not associated with FGFR3-TACC3 fusion (Pearson’s chi-square: P = .989) and did not correlate to FGFR3 staining IRS (P = 1.0).
Positive FGFR3 Immunohistochemistry Identifies Gliomas with Distinct Histological Features Associated with FGFR3 Fusion
FGFR3 staining in the “RT-PCR cohort” was strongly expressed in the cases with FGFR3-TACC3 fusion; therefore, we next screened additional 552 glioma samples (“TMA cohort,” Figure 3, for sample details, see Table 1). Five hundred and seventeen samples were negative (93%, immunoreactive score 0: 475 cases, IRS 1: 3 cases, IRS 2: 39 cases). Weak (and mostly focal) staining was observed in 19 cases (3.6%, IRS 4: all cases), moderate staining was observed in 11 cases (2.1%, IRS score 6: 6 samples, IRS score 8: 5 samples), and strong staining was seen in 5 cases (0.9%, IRS score 9: 1 case, score 12: 4 cases). All 16 TMA samples with moderate to strong FGFR3 positivity were re-stained as whole-mount sections for confirmation and overall slide assessment did not result in score change. Tumors with strong FGFR3 IHC displayed distinct morphological growth patterns (illustrated and described in Figure 5).
Figure 3.
FGFR3 immunohistochemistry distribution among the glioma tumor cohorts analyzed in this study. Positive FGFR3 staining was observed in 73 out of 1024 samples total. FGFR3 overexpression (scores > 7, Figure 1) was detected in 26 samples. Strong FGFR3 staining was observed in 1% of all tumors analyzed (10/1024) and was significantly associated with the presence of a FGFR3-TACC3 fusion.
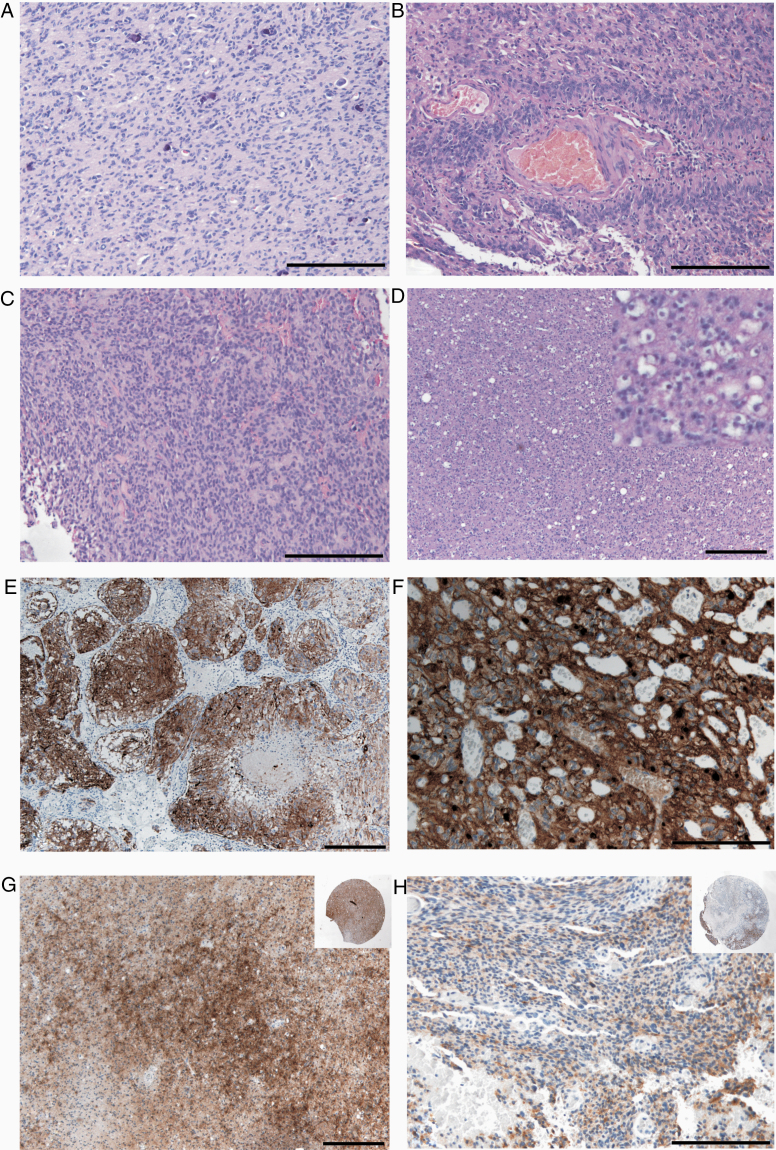
Figure 5.
Histological patterns observed in FGFR3-positive tumors as reported by Bielle et al.26A, Monomorphous ovoid nuclei with focal microcalcifications (HE). B, Attachment of tumor cells to vessels by thin cytoplasmic processes resembling vague pseudorosettes. C, Organization of tumor cells in pseudo-ependymal pseudorosettes (HE). D, Isolated tumor cells surrounded by pale ovoid cytoplasm (HE, inset higher magnification). E, Organoid nesting of tumor cells (FGFR3 immunohistochemistry) with surrounding vascularization. F, Endocrinoid network of thin capillaries and tumor cells with pale cytoplasm and membranous enrichment of FGFR3 immunohistochemistry. G, Primary tumor with moderate FGFR3 immunohistochemistry (IRS score 6) and reduced expression in tumor recurrence one year later (IRS score 2, insets show corresponding stained tissue microarray punches). Scale bars: 200 µm.
FGFR3 immunoreactive scores of the TMA cohort were not associated with tumor WHO grade (Pearson’s chi-square, P = .14), gender (P = .78), tumor location (P = .99), tumor progression (P = .29), IDH mutation status (P = .08), ATRX status (P = .36), MGMT promoter methylation (P = .10), and patient age (logistic fit, P = .31). All 48 gliosarcomas, 80/82 pilocytic astrocytomas, and 89/93 oligodendroglial tumors were classified as FGFR3 negative. Strong FGFR3 expression significantly correlated with astrocytic phenotype (P = .04).
We subjected an independent TMA validation set (Figure 3, first validation set #1) consisting of 232 IDH wild-type glioblastoma samples at the time of initial diagnosis to FGFR3 IHC. Two hundred and eleven cases were scored negative (91%, IRS score 0-2), 17 cases showed weak immunoreactivity (7%, IRS score 3-5), three cases were moderately positive (1.2%, IRS score 6-8), and one case was strongly positive (0.4%, IRS score 12). Repeated staining on full slides was carried out for all four moderately to strongly stained TMA punches and confirmed positive staining in full slides, although in two cases IRS scores then were changed (IRS score from 12 to 9 and from 8 to 6, Figure 5G and H) due to more heterogenous staining observed on full slides, indicating potential subclonal expression of FGFR3 in some cases. Here, too, the strongly FGFR3-positive cases showed distinct morphological patterns (Figure 5B). A second independent validation set (Figure 3, TMA validation set #2) consisting of 87 glioblastoma samples was stained for FGFR3 with the same conditions as in the previous cohorts. Eighty-three cases scored negative (96%, IRS 0-2), 3 cases were weakly positive (3%, IRS 4), and one case moderately positive (1%, IRS 6). No strongly positive cases were found. Confirmatory staining on full slides led to the reclassification of one weakly positive case to negative (from IRS 4 to 2). Both validation sets showed a similar low rate of FGFR3-positive samples as the initial TMA cohort (Figure 3). After combining data from all cohorts, FGFR3 overexpression (using the determined cutoff IHC score > 7) was found in 2.5% (26/1024) glioma samples, and strong FGFR3 overexpression was observed in 1% (10/1024) samples.
The Detection of FGFR3 Overexpression by Immunohistochemistry Is Associated with FGFR3 Sequence Variants, Amplifications, or Fusion
Gene panel sequencing was carried out in the “NGS cohort” (unselected cases n = 75 Figure 1) and in 13 preselected cases with FGFR3 overexpression. FGFR3 IHC data were available in all 88 cases (Figure 3). Genetic alteration of FGFR3 was detected in 15/88 cases analyzed (n = 5/75 (7%) unselected cases and n = 9/13 (69%) preselected cases). Molecular overview of all findings is shown in Figure 4. Calling of structural variants identified two cases with FGFR3 (exon 17)-TACC3 (exon 10) fusion, one case each with FGFR3-TACC3 17-11, 16-8, 18-13, and 18-8 fusion. One case exhibited FGFR3 amplification and additional FGFR3 rearrangement in the last exon to chromosome 6 without currently known gene at this location (Case #3, contig positions: chr4:1808908-chr6:3506534). Case #2 additionally harbored an FGFR3 copy number gain and another FGFR3 rearrangement that joined the WHSC1 gene (also known as NSD2 nuclear receptor-binding SET domain protein), located downstream of FGFR3. The frequency of FGFR3 fusions in the unselected NGS cohort was 1.3% (n = 1/75) and in the RT-PCR cohort including NGS data was 3% (n = 3/101). As the FGFR3 fusion in Case #2 was not detected in RT-PCR, the sensitivity and specificity to detect a FGFR3 fusion in RT-PCR compared to NGS was 67% and 100%, respectively (positive predictive value: 100%, negative predictive value: 99%, n = 101). One case with strong FGFR3 expression and three cases with moderate FGFR3 expression showed no detectable FGFR3 fusion, amplification, or sequence variants. The sensitivity and specificity to detect a FGFR3 fusion in IHC compared to NGS was 95% and 100%, respectively, at optimal cutoff for IRS score > 7 (positive predictive value: 76%, negative predictive value: 100%, n = 88). Case #1 with FGFR3-TACC3 fusion previously detected in RT-PCR revealed an additional novel hemizygous FGFR3 p.Lys649Arg missense mutation (c.1946A > G) located in the intracellular tyrosine kinase domain. The subsequent amino acid position p.Lys650 is frequently mutated in bladder cancer, where a constitutively activated kinase with impaired internalization and degradation results in prolonged FGFR3 signaling (COSMIC database accession number: COSM719). It is also remarkable that Tyr647, a residue phosphorylated as a part of FGFR3 activation, is directly affected by the p.Lys649Arg mutation as side chains of residue 649 and 647 are in direct interaction (PDB ID: 4K33, Supplementary Figure 4).27
The IDH1 (Arg132His)-mutated case with low FGFR3 expression showed a subclonal p.Leu363Alafs*32 frameshift alteration (Case #8). This frameshift is located just ahead the transmembrane domain of FGFR3 spanning from amino acids 376-396 (Uniprot accession number: P22607). Both fusion-positive cases with low-grade glioma histology showed strong cytoplasmic CD34 immunoreactivity in the tumor cells as previously reported for polymorphous low-grade neuroepithelial tumor of the young with FGFR3 or FGFR1 fusions.28 Methylation analysis using the brain tumor classifier displayed the highest score for methylation class family low-grade glioma, subtype ganglioglioma in one case, and methylation class family glioblastoma, IDH wild type, subclass RTK II in the other case. Among the cases with high-grade histology, CD34 immunoreactivity was observed in 4/9 cases with FGFR3 fusion cases and was absent in all other FGFR3 altered cases (Figure 4).
Novel FGFR3 Sequence Variants in Glioma Without FGFR3 IHC Overexpression
Only 1 of 75 unselected NGS cohort cases displayed strong FGFR3 expression associated with FGFR3 fusion. One case showed weak expression and the remaining 73 cases were negative in IHC (97%, Figure 3). Among the FGFR3 IHC-negative cases in the NGS cohort, we identified five FGFR3 variants previously not reported in glioma. Three were FGFR3 missense (c.148C > T, p.Pro62Ser; c.1630G > A, p.Ala544Thr and c.2080C > T, p.Pro694Ser), one frameshift (c.938dupT, p.Ser314Glnfs*2), and one stop-gain mutation (c.1059G > A). The frameshift and the stop-gain mutation are located ahead of the transmembrane domain. The Pro62Ser variant is located within the Ig-like C2-type 1 domain, which is considered dispensable for FGF binding.29 The Leiden Open Variation Database (V3.0) classifies the Pro62Ser variant as likely benign. The Ala544Thr variant is located in the intracellular tyrosine kinase domain and has not yet been described in gliomas. Its clinical significance is unclear. The Pro694Ser variant is located in the C-lobe part of protein kinase domain and is considered pathogenic (COSMIC accession number: COSM4166489). So far, this variant has been only reported in gastrointestinal cancer. None of these five glioma samples with FGFR3 variants displayed positive staining in repeated FGFR3 IHC on whole-mount slides. The Pro62Ser mutated case was an IDH1 Arg132His-mutant glioblastoma and one of the other cases showed a H3F3A Gly34 mutation, which is often seen in pediatric glioblastomas. The sensitivity and specificity of IHC to detect any type of activating FGFR3 alteration was 93% and 95%, respectively, at optimal cutoff for IHC score > 7 (positive predictive value: 76%, negative predictive value: 99%, n = 88).
The Molecular Profile of the FGFR3 Fusion-Positive Cases Is Similar to Other Glioblastoma, IDH Wild Type
None of the cases with FGFR3 fusion showed ATRX loss or pathogenic EGFR/IDH mutations. EGFR (epidermal growth factor receptor) amplification was seen in 1/9, CDKN2A/B deletion was seen in 3/9, CDK4 amplification in 2/9, and telomerase reverse transcriptase (TERT) hotspot mutations (C228T and C250T) were present in 4/9 tumors with FGFR3 fusion (for full mutation profile of FGFR3 mutant cases, see Figure 4). Kaplan-Meier survival analysis showed no significant differences between FGFR3 wild-type and FGFR3-mutant tumors (n = 302, log-rank: 0.18, median: 799 days).
Discussion
The FGFR3-TACC3 fusion as potential therapeutic target results in subsequent FGFR3 protein overexpression in glioblastoma.17 FGFR3 IHC (especially in the TMA cohort) allowed us the rapid identification of cases with FGFR3-TACC3 fusions in a large cohort of total 1024 gliomas. We observed strong expression of FGFR3 protein in 10 (1%) of tumors. Further, 16 (1.4%) of the tumors had a moderate FGFR3 expression (Figure 3). The strong expression of FGFR3 was clearly associated with molecular detection of FGFR3 fusions in IDH wild-type, non-EGFR amplified glioblastomas (Figures 1 and 2). Two FGFR3 fusion-positive glioblastomas showed additional FGFR3 amplification, and we detected in one fusion-positive case a novel missense mutation linked to constitutive kinase activation associated with prolonged FGFR3 signaling (Supplementary Figure 4). Unbiased consecutive workup of glioblastoma samples indicates that 2.3% (combined data from RT-PCR and unselected NGS cohort) of all tumors carry a FGFR3 fusion. In contrast, only 2 out of 169 tumors diagnosed as low-grade gliomas in histology had FGFR3 fusions (Figure 4). Previously reported incidence of FGFR3-TACC3 fusion in selected glioblastoma cohorts vary between 1.3% and 8.3%.15,17,18,30,31
Due to the close proximity of FGFR3 and TACC3, fusion detection by fluorescence in situ hybridization is difficult32 and most studies have employed RNA-sequencing strategies.17,18,26,30 Using whole transcriptome sequencing, Parker et al. identified four FGFR3 fusions in 48 glioblastomas.15 Granberg et al. demonstrated the ability of pulldown-based targeted sequencing in FFPE tissue to identify FGFR3 fusions.13 Here, we demonstrate that gene panel sequencing of FFPE tissue and variant calling with the Manta algorithm33 enable the detection of FGFR3 fusions in addition to the identification of amplifications and pathogenic variants, all of them associated with FGFR3 protein overexpression in these tumors. Furthermore, we detected, in three tumors, multiple molecular alterations affecting the FGFR3 gene in the same FGFR3 overexpressing tumor (Figure 4), and we also identified FGFR3 fusions not detectable in RT-PCR. One of our cases exhibited simultaneously a FGFR3-TACC3 fusion, a FGFR3-WHSC1 fusion and FGFR3 amplification. A similar case was previously reported by Granberg et al.13 Another case showed the presence of a novel FGFR3 Lys649Arg mutation in addition to a FGFR3-TACC3 fusion. Interestingly, molecular modeling indicated that this mutation directly affects Tyr647, a residue phosphorylated as a part of FGFR3 activation and thus likely contributes to FGFR3 overexpression in the tumor. It is also possible that Lys649Arg actually may mimic to some extent the effects of the 647 phosphorylation. Long-term modeling might provide further insights. FGFR3 alterations that do not show statistically significant patterns of co-occurrence may still have important interactions biologically. From an evolutionary standpoint, coselection of independent oncogenic alterations may improve tumor growth and survival as seen frequently in non–small-cell lung cancer. Recently, two FGFR fusion-positive cases with coexisting Lys650Thr mutation were reported in two glioblastomas25 and the publicly available TCGA cohort contains a glioblastoma case with Lys650Glu mutation.34 Mutation of FGFR3 position 650 is frequently seen in bladder cancer and is associated with FGFR3 overexpression.10 Of interest is the pathogenic Pro694Ser mutation we detected in one glioblastoma without FGFR3 overexpression. This variant has been reported previously in a metastasizing colorectal carcinoma.35 The wild-type sequence has three rigid proline residues (694, 696, and 699) near each other. As serine has much higher flexibility, this mutation may affect protein dynamics and protein interactions implicating another potential target. Bielle et al. reported constant immunoreactivity of FGFR3 fusion-positive tumors with a sensitivity of 100% and specificity of 92% (they reported only one fusion-positive case with weak FGFR3 staining), suggesting that FGFR IHC can be used as a screening marker.26 We identified FGFR3 fusions in 90% gliomas with strong and in 25% of gliomas with moderate FGFR3 expression associated with distinct histological patterns (Figure 5). We therefore recommend a cost-effective approach to sequence all tumors with FGFR3 IHC overexpression (ie, IRS score > 7) for targetable FGFR3 fusions and activating mutations (sensitivity and specificity of 93% and 95%, respectively). Based on our data, this means that only 2.4% of all glioblastomas need to be selected for molecular analysis to achieve a FGFR3 alteration detection rate of approximately 67%. In line with our observations, a recent study identified FGFR3 fusions in 10 out of 15 FGFR3 preselected, positively stained cases.13 Another large screening study identified FGFR3 fusions in partly preselected, 84% immunopositive glioblastomas.18 Our cohort included four FGFR3-positive IHC cases without molecular FGFR3 correlates that were recognizable with our methodology. It remains to be determined whether such cases will also be responsive for FGFR inhibitor treatment. Interestingly, the only fusion-negative case with strong FGFR3 IHC (score 12) was a pediatric glioblastoma. In this regard, FGFR3 expression without associated FGFR3 fusion has been reported in other pediatric tumor entities, especially in pilocytic astrocytomas.36 For this reason, we included 82 pilocytic astrocytomas in our TMA cohort but observed only 2 cases with weak FGFR3 expression. So far, none of the previous studies have detected FGFR3 fusions in pediatric astrocytomas and ependymomas, in contrast to frequently observed FGFR1 gene alterations in up to 5% of pilocytic astrocytoma.10,37 Two histologically low-grade CNS tumors in our cohort also carried out the FGFR3 fusion. One tumor had been classified initially as diffuse astrocytoma, IDH wild-type grade II, and the other case was considered to be a ganglioglioma grade I without BRAF gene mutation. Strong CD34 overexpression, therefore, prompted the differential diagnosis of a recently described entity called “polymorphous low-grade neuroepithelial tumor of the young (PLNTY)” and FGFR3-TACC3 fusions have been described in this tumor entity.28,37,38 Diffuse astrocytomas with FGFR3 fusion usually exhibit a molecular profile that molecularly corresponds to glioblastoma, that is, the presence of 7p/10q-signature or TERT hotspot mutations.26 This molecular profile was found in one of our low-grade tumors and indeed methylation profiling classified this tumor as glioblastoma.
Previous studies reported that FGFR3-TACC3 fusion-positive gliomas are IDH1/IDH2 wild type and frequently have TERT promoter mutations (74%) or CDKN2A loss (60%).13,26 Further molecular characterization indicated that FGFR3 fusions are mutually exclusive with EGFR or PDGFRα amplification and that fusion-positive tumors are more frequently associated with CDK4 and MDM amplification.13,17,18,26 In agreement, our FGFR3 fusion-positive cohort consisted of IDH wild-type tumors with CDK4 amplification or PTEN deletion in 22%, CDKN2A/B loss in 33%, and TERT mutation in 44% (Figure 4). However, we observed a single fusion-positive case21 with low-level EGFR amplification (3-4 copies), suggesting that there are rare exceptions with concomitant FGFR3 and EGFR alterations. Interestingly, the pediatric tumor with strong FGFR3 staining but without detectable fusion39 exhibited a pathogenic EGFR R222C mutation occasionally observed in CNS tumors with a molecular profile similar to prototypical EGFR amplified glioblastoma. This highlights possible differences between adult and pediatric FGFR3-expressing tumors.
Morphological patterns such as microcalcifications, cell arrangements in vague pseudorosettes, and increased vascularization in IDH wild-type glioblastomas (Figure 5) should prompt FGFR3 immunostaining as first and cost-effective screening step followed by further molecular analysis of FGFR3 when moderate to strong cytoplasmic expression in tumor cells is present.26 Di Stefano et al. reported FGFR3 to be homogenously expressed in their cohorts,17 whereas Granberg et al. observed spatial heterogenous distribution intensities indicative of subclonal FGFR3 distribution.13 When we compared staining intensities and distribution scores in our TMA punches with full slide stains of the same cases, only one case with weak staining had to be reclassified. We therefore recommend performing FGFR3 IHC staining in routine on full slides. In very small glioma samples, FGFR3-expressing cases may remain undetected. Limitations of the study are that the preselected cases in the sequencing cohort were moderately to strongly stained tumors and thus represent a selected subset of the TMA cohort. However, in all 68 FGFR3 IHC-negative tumors we sequenced, we did not detect significant IHC expression in full slides, so that the risk of missing cases with FGFR3 fusion has to be regarded as low.
Our findings indicate the low frequency and a high variability in FGFR3-TACC3 and other FGFR3 fusions. FGFR3 IHC in conjunction with histology patterns is a reliable method to identify many tumors with oncogenic FGFR3 alterations (sensitivity and specificity of 93% and 95%, respectively) and therefore could be included in the routine diagnostic workflow. FGFR3 IHC recognizes the common FGFR3-TACC3 fusions as well as FGFR3 amplifications and other novel actionable FGFR3 sequence variations variant. The use of gene panels may be best suited to identify the underlying alteration associated with FGFR3 overexpression and may also detect novel FGFR3 sequence variants without FGFR3 overexpression.
Supplementary material
Supplementary material is available online at Neuro-Oncology (http://neuro-oncology.oxfordjournals.org/).
Funding
G.T. acknowledges intramural funding of the Medical Faculty Tübingen (Demonstratorprojekt Personalisierte Medizin). M.M. would like to thank the Luxembourg National Research Fond (FNR) for the support (FNR PEARL P16/BM/11192868 grant).
Author contributions
All authors made substantial contributions to the study, that is, conception, design, sample acquisition, clinical data acquisition, data analysis, data interpretation, and coordination of experiments and collaborations. J.S. and G.T. designed and coordinated the study, evaluated, analyzed, and interpreted the molecular results. J.S. wrote the first draft of the manuscript, created figures, and data table. L.Z. performed the RT-PCR experiments, J.Sp. performed sequencing of RT-PCR products, S.B. provided panel sequencing data, M.M. and D.C. provided curated TMA samples; I.B. and M.S. provided clinical data. A.P. carried out molecular modeling studies concerning the mutations. All authors read, modified the manuscript and approved the final version.
Conflict of interest statement. G.T. served on Advisory Boards of BMS, MSD, and AbbVie; received research and/or travel grants from Roche Diagnostics, Novocure, and Medac, received speakers’ fees from Medac and Novocure. L.Z., J.Sp., M.M., D.C., I.B., M.S., S.B., A.P., and J.S. have no conflict of interest to declare.
References
- 1. Claes A, Idema AJ, Wesseling P. Diffuse glioma growth: a guerilla war. Acta Neuropathol. 2007;114(5):443–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Watanabe T, Nobusawa S, Kleihues P, et al. IDH1 mutations are early events in the development of astrocytomas and oligodendrogliomas. Am J Pathol. 2009;174(4):1149–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brennan CW, Verhaak RG, McKenna A, et al. The somatic genomic landscape of glioblastoma. Cell. 2013;155(2):462–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Phillips HS, Kharbanda S, Chen R, et al. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell. 2006;9(3):157–173. [DOI] [PubMed] [Google Scholar]
- 5. Capper D, Jones DTW, Sill M, et al. DNA methylation-based classification of central nervous system tumours. Nature. 2018;555(7697):469–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. TCGA. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455(7216):1061–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Touat M, Ileana E, Postel-Vinay S, et al. Targeting FGFR signaling in cancer. Clin Cancer Res. 2015;21(12):2684–2694. [DOI] [PubMed] [Google Scholar]
- 8. Katoh M, Nakagama H. FGF receptors: cancer biology and therapeutics. Med Res Rev. 2014;34(2):280–300. [DOI] [PubMed] [Google Scholar]
- 9. Nelson KN, Meyer AN, Wang CG, et al. Oncogenic driver FGFR3-TACC3 is dependent on membrane trafficking and ERK signaling. Oncotarget. 2018;9(76):34306–34319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Helsten T, Elkin S, Arthur E, et al. The FGFR landscape in cancer: analysis of 4853 tumors by next-generation sequencing. Clin Cancer Res. 2016;22(1):259–267. [DOI] [PubMed] [Google Scholar]
- 11. Singh D, Chan JM, Zoppoli P, et al. Transforming fusions of FGFR and TACC genes in human glioblastoma. Science. 2012;337(6099):1231–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Williams SV, Hurst CD, Knowles MA. Oncogenic FGFR3 gene fusions in bladder cancer. Hum Mol Genet. 2013;22(4):795–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Granberg KJ, Annala M, Lehtinen B, et al. Strong FGFR3 staining is a marker for FGFR3 fusions in diffuse gliomas. Neuro Oncol. 2017;19(9):1206–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Costa R, Carneiro BA, Taxter T, et al. FGFR3-TACC3 fusion in solid tumors: mini review. Oncotarget. 2016;7(34):55924–55938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Parker BC, Annala MJ, Cogdell DE, et al. The tumorigenic FGFR3-TACC3 gene fusion escapes miR-99a regulation in glioblastoma. J Clin Invest. 2013;123(2):855–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Frattini V, Pagnotta SM, Tala, et al. A metabolic function of FGFR3-TACC3 gene fusions in cancer. Nature. 2018;553(7687):222–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Di Stefano AL, Fucci A, Frattini V, et al. Detection, characterization, and inhibition of FGFR-TACC fusions in IDH wild-type glioma. Clin Cancer Res. 2015;21(14):3307–3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Di Stefano AL, Picca A, Saragoussi E, et al. Clinical, molecular and radiomic profile of gliomas with FGFR3-TACC3 fusions. Neuro Oncol. 2020;22(11):1614–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Voss MH, Hierro C, Heist RS, et al. A phase I, open-label, multicenter, dose-escalation study of the oral selective FGFR inhibitor Debio 1347 in patients with advanced solid tumors harboring FGFR gene alterations. Clin Cancer Res. 2019;25(9):2699–2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jennewein L, Ronellenfitsch MW, Antonietti P, et al. Diagnostic and clinical relevance of the autophago-lysosomal network in human gliomas. Oncotarget. 2016;7(15):20016–20032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–820. [DOI] [PubMed] [Google Scholar]
- 22. Ebrahimi A, Skardelly M, Bonzheim I, et al. ATRX immunostaining predicts IDH and H3F3A status in gliomas. Acta Neuropathol Commun. 2016;4(1):60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dohrn MF, Glöckle N, Mulahasanovic L, et al. Frequent genes in rare diseases: panel-based next generation sequencing to disclose causal mutations in hereditary neuropathies. J Neurochem. 2017;143(5):507–522. [DOI] [PubMed] [Google Scholar]
- 24. Huang Z, Chen H, Blais S, et al. Structural mimicry of a-loop tyrosine phosphorylation by a pathogenic FGF receptor 3 mutation. Structure. 2013;21(10):1889–1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ballester LY, Moghadamtousi SZ, Leeds NE, et al. Coexisting FGFR3 p.K650T mutation in two FGFR3-TACC3 fusion glioma cases. Acta Neuropathol Commun. 2019;7(1):63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bielle F, Di Stefano AL, Meyronet D, et al. Diffuse gliomas with FGFR3-TACC3 fusion have characteristic histopathological and molecular features. Brain Pathol. 2018;28(5):674–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Webster MK, D’Avis PY, Robertson SC, et al. Profound ligand-independent kinase activation of fibroblast growth factor receptor 3 by the activation loop mutation responsible for a lethal skeletal dysplasia, thanatophoric dysplasia type II. Mol Cell Biol. 1996;16(8):4081–4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Huse JT, Snuderl M, Jones DT, et al. Polymorphous low-grade neuroepithelial tumor of the young (PLNTY): an epileptogenic neoplasm with oligodendroglioma-like components, aberrant CD34 expression, and genetic alterations involving the MAP kinase pathway. Acta Neuropathol. 2017;133(3):417–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Olsen SK, Ibrahimi OA, Raucci A, et al. Insights into the molecular basis for fibroblast growth factor receptor autoinhibition and ligand-binding promiscuity. Proc Natl Acad Sci U S A. 2004;101(4):935–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Stransky N, Cerami E, Schalm S, et al. The landscape of kinase fusions in cancer. Nat Commun. 2014;5:4846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bao ZS, Chen HM, Yang MY, et al. RNA-seq of 272 gliomas revealed a novel, recurrent PTPRZ1-MET fusion transcript in secondary glioblastomas. Genome Res. 2014;24(11):1765–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kurobe M, Kojima T, Nishimura K, et al. Development of RNA-FISH Assay for Detection of Oncogenic FGFR3-TACC3 fusion genes in FFPE samples. PLoS One. 2016;11(12):e0165109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chen X, Schulz-Trieglaff O, Shaw R, et al. Manta: rapid detection of structural variants and indels for germline and cancer sequencing applications. Bioinformatics. 2016;32(8):1220–1222. [DOI] [PubMed] [Google Scholar]
- 34. Frattini V, Trifonov V, Chan JM, et al. The integrated landscape of driver genomic alterations in glioblastoma. Nat Genet. 2013;45(10):1141–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kovaleva V, Geissler AL, Lutz L, et al. Spatio-temporal mutation profiles of case-matched colorectal carcinomas and their metastases reveal unique de novo mutations in metachronous lung metastases by targeted next generation sequencing. Mol Cancer. 2016;15(1):63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lehtinen B, Raita A, Kesseli J, et al. Clinical association analysis of ependymomas and pilocytic astrocytomas reveals elevated FGFR3 and FGFR1 expression in aggressive ependymomas. BMC Cancer. 2017;17(1):310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Johnson A, Severson E, Gay L, et al. Comprehensive genomic profiling of 282 pediatric low- and high-grade gliomas reveals genomic drivers, tumor mutational burden, and hypermutation signatures. Oncologist. 2017;22(12):1478–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Riva G, Cima L, Villanova M, et al. Low-grade neuroepithelial tumor: unusual presentation in an adult without history of seizures. Neuropathology. 2018;38(5):557–560. [DOI] [PubMed] [Google Scholar]
- 39. Schittenhelm J, Mittelbronn M, Meyermann R, et al. Confirmation of R132H mutation of isocitrate dehydrogenase 1 as an independent prognostic factor in anaplastic astrocytoma. Acta Neuropathol. 2011;122(5):651–652. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.