Abstract
Tuberculosis (TB) remains an infectious disease of global significance and a leading cause of death in low- and middle-income countries. Significant effort has been directed towards understanding Mycobacterium tuberculosis genomics, virulence, and pathophysiology within the framework of Koch postulates. More recently, the advent of “-omics” approaches has broadened our appreciation of how “commensal” microbes have coevolved with their host and have a central role in shaping health and susceptibility to disease. It is now clear that there is a diverse repertoire of interactions between the microbiota and host immune responses that can either sustain or disrupt homeostasis. In the context of the global efforts to combatting TB, such findings and knowledge have raised important questions: Does microbiome composition indicate or determine susceptibility or resistance to M. tuberculosis infection? Is the development of active disease or latent infection upon M. tuberculosis exposure influenced by the microbiome? Does microbiome composition influence TB therapy outcome and risk of reinfection with M. tuberculosis? Can the microbiome be actively managed to reduce risk of M. tuberculosis infection or recurrence of TB? Here, we explore these questions with a particular focus on microbiome-immune interactions that may affect TB susceptibility, manifestation and progression, the long-term implications of anti-TB therapy, as well as the potential of the host microbiome as target for clinical manipulation.
Tuberculosis is a globally dominant infection with a long-term burden of antibiotic use
Tuberculosis (TB) persists as one of the top 10 causes of death in the world, with currently an estimated 1.4 million deaths annually [1]. Morbidity and mortality are associated with active TB disease, which is believed to develop in 5% to 10% of individuals that are exposed to and infected by Mycobacterium (M.) tuberculosis. In the majority of individuals, M. tuberculosis infection is thought to result in clinically asymptomatic latent tuberculosis infection (LTBI). There is currently no standardized test to confirm the presence of viable M. tuberculosis in individuals with LTBI, and diagnosis is largely based on immunological tests that indicate antigen experience (e.g., skin reactivity to M. tuberculosis purified protein derivatives (PPD); IFNγ release assays (IGRA) detecting reactivity of CD4+ T cells to M. tuberculosis-specific antigens in whole blood). Of note, there are reports of individuals showing no signs of antigen experience or active TB disease in settings of repeated high exposure and transmission of M. tuberculosis. While it is difficult to determine how these “resisters” may be protected from productive infection with M. tuberculosis, a range of innate and adaptive immune mechanisms governed by genetic and epigenetic factors, as well as antigen experience may contribute [2]. It is currently estimated that one quarter of the world’s population is latently infected with M. tuberculosis [3], with a calculated 5% to 10% lifetime risk of developing active TB [1,4]. Nevertheless, a recent review of human cohort studies undertaken before and after antibiotics became available reemphasized that active TB disease most commonly develops within 1 to 2 years of (confirmed or likely) exposure to M. tuberculosis. The review of historic data suggested that the risk for active TB beyond 2 years after exposure declines sharply, arguing that reactivation of LTBI might be a much less common event than currently believed and that active TB later in life might result from re-exposure rather than reactivation [5].
First-line anti-TB antibiotics isoniazid, pyrazinamide, and ethambutol are narrow-spectrum, showing little or no activity outside the mycobacterial genus [6], but are often combined with the broad-spectrum antibiotic rifampin, which affects a wide range of Gram-positive and Gram-negative bacteria [1,7]. Indeed, TB antibiotics are being administered to millions of people every year, with up to 780 narrow- and broad-spectrum antibiotic doses administered over a 9-months period [8,9]. This represents one of the longest duration antibiotic regimens used globally. Given the recognized effects that antibiotics have on the composition and function of the host microbiome [10], it is not surprising that conventional TB therapeutic regimens are associated with long-lasting alterations of the gut microbiota in patients and animal models, with impact noted for up to 8 years in a study following patients that were treated for drug-resistant TB (DR-TB) [11–13]. Moreover, significant risk factors for developing active TB, including HIV infection, malnutrition, smoking, alcohol, and diabetes [1,14–17], are associated with both structural and functional changes in the gut microbiota. How these comorbidities, their clinical management and long-term antibiotic use affect the lung microbiome remains poorly understood [12,18–21]. Yet, profound and long-lasting impact on the microbiota is likely to have deleterious consequences for susceptibility and immune control of infectious diseases, including TB.
The microbiome in health and disease
The colonization of the host by microorganisms begins within minutes of birth or hatching. There is a gradual succession in the diversity and density of these communities, influenced by a myriad of genetic, environmental, and behavioral inputs [22,23]. During those eras of microbiology governed by microscopy and later, culture-based methods, these communities were deemed to be largely comprised of “commensal” microbes: deriving benefits from residing with the host, but with relatively benign and/or unknown impacts on the host itself. The expansion of cultured microbes from different body sites using techniques in anaerobic microbiology helped explain and expand the appreciation of the mutualistic relationships between these communities and their host in terms of structural, metabolic, and immune development [24]. As such, these communities can be considered as the “x-factor” in the genotype x environment x lifestyle interactions governing host response and phenotype. The step advances in nucleic acid sequencing technologies have enabled a phylogenetic and/or gene-based functional assessment of the microbial communities resident at different body sites, and which is commonly referred to as the human microbiome.
By removing the obligatory step of microbial cultivation, a much greater appreciation of the structural and functional dynamics of these communities in the context of health and disease has been developed. In addition to the oral cavity, the microbiota of the large intestine is the most studied compartment of the “human microbiome” [19]. Until recently, microbiome composition was almost exclusively characterized using amplicons produced from the gene encoding 16S rRNA [25]. However, over the last decade, efforts such as the Human Microbiome and integrated Human Microbiome Projects [26] have expanded the scope of investigation to include other regio-specific communities of the human body, the provision of functional as well as taxonomic information via “shotgun metagenomic sequencing” and thereby, a more holistic examination of all 3 domains of life (i.e., Bacteria, Archaea, Eucarya, and their respective viromes) extant (and extinct) in these communities [27–31]. Collectively, these efforts might be summarized into 5 key concepts relevant to our understanding of the roles of the human microbiota in health and disease: First, our microbiota have coevolved with us, drawn from a rather restricted range of the phyla assigned across all 3 domains of life and known to exist in nature. There is a remarkable amount of similarity among the bacterial phyla resident at different body sites, with complexity (and individuality) at different body sites reflected at higher levels of classification [32,33]. Second, this complexity includes a substantial amount of “dark matter” that currently remains biologically uncharacterized at the organismal and genetic level [34]. Third, body sites previously considered to be sterile, such as the healthy lung [35], are now recognized to harbor a variable but nontransient community of microbes considered relevant to sustaining tissue homeostasis with emerging roles in the host defense against pathogenic organisms [36]. Fourth, the advances in food industrialization, medicines, antibiotic use, and hygiene are proposed to impose selective pressures on (at least) the colonic microbiota of Western societies and diminished diversity (“missing microbes”) is linked with the increased incidence of chronic and noncommunicable diseases [37,38]. Indeed, while the definition of a healthy microbiome remains enigmatic, the concept of “dysbiosis” (alterations in measures of microbial diversity and community composition compared to asymptomatic and/or healthy individuals) is now widely considered a hallmark of many chronic and noncommunicable diseases [39,40]. Finally, there are dynamic and bidirectional interactions between the immune system and microbiota with both local and systemic impacts. One example is the multifaceted interplay between the gastrointestinal microbiota and the respiratory tract, coined the gut-lung axis [19]. In this review, we draw on central aspects of these concepts in highlighting the emerging links and implications for TB.
Microbiota in the M. tuberculosis-infected host
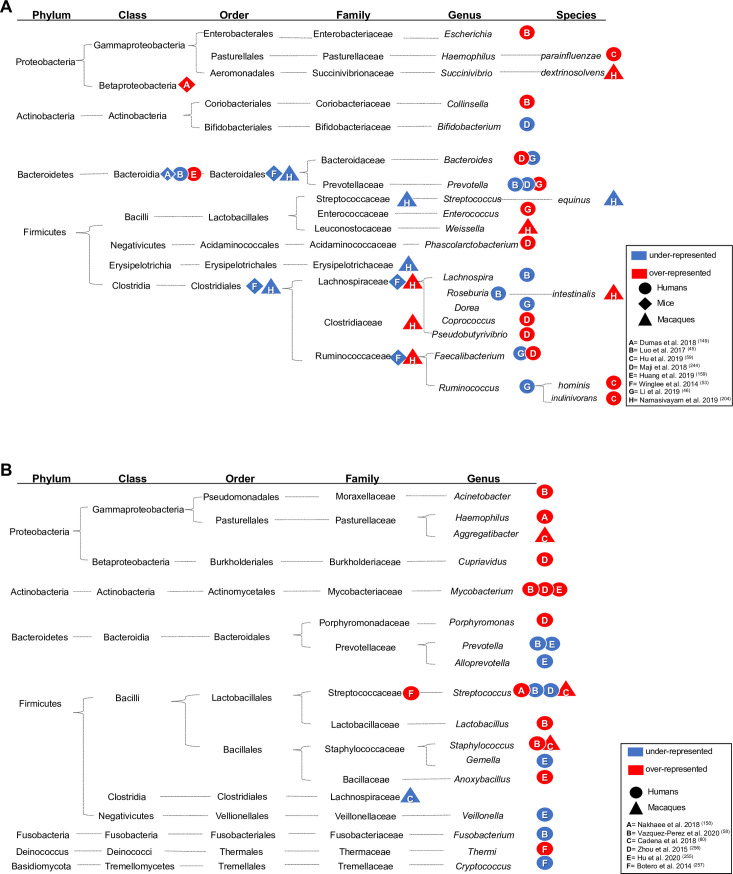
Characterization of the microbiome composition of TB patients and the M. tuberculosis-infected host in animal models has been the subject of significant efforts (Table 1) and has been reviewed in significant detail elsewhere [8,41–44]. Table 1 and Fig 1A and 1B summarize the findings from colonic (fecal) and lung microbiota of humans and animal models of M. tuberculosis infection compared to noninfected “controls”. In general terms, the fecal microbiota profiles of treatment-naïve, new-onset, and recurrent TB patients consistently show a decrease in bacterial diversity compared to control individuals [45,46]. Phylogenetic integration of the data available through these studies reveals changes to the relative abundances of the bacterial lineages affiliated with the families of Ruminococcaceae and/or Lachnospiraceae (Fig 1A). It is important to note that increased and decreased relative abundance, as well as no significant changes have been reported (Table 1 and Fig 1A), highlighting the challenges posed by integrating data obtained across different host organisms, control populations, and study designs. Nevertheless, these 2 bacterial families of the phylum Firmicutes represent the 2 numerically most abundant groups of Gram-positive bacteria in the human colon [47]. Members of both groups are recognized for their capacity to utilize carbohydrates in simple and polymeric forms and govern the schemes of anaerobic fermentation that produce the short-chain fatty acids (SCFAs) acetate and/or butyrate [48]. Butyrate exerts immunomodulatory effects (discussed below), but it is important to emphasize that members of these bacterial lineages also produce other factors that have been ascribed “anti-inflammatory” capacity [49–51], albeit their impact on host responses to M. tuberculosis infection, if any, needs to be explored. Moreover, variable changes in the relative abundances of non spore-forming Gram-negative bacterial lineages assigned to the phylum Bacteroidetes (e.g., Prevotella and Bacteroides) are reported, and relative abundances of Proteobacteria, which, when remarked upon, are increased in M. tuberculosis-infected individuals (Fig 1A). During anaerobic growth, these latter bacterial groups favor the formation of “mixed acids” including succinate, lactate, formate, but also SCFAs such as propionate and acetate [52]. In addition, structural components in particular the Gram-negative bacterial cell wall component lipopolysaccharide (LPS) can trigger substantial pro-inflammatory responses at local and distant sites if epithelial barrier functions are perturbed (discussed below). Taken together, these findings indicate that M. tuberculosis infection is associated with a gut “dysbiosis.” While the cause-and-effect relationship between TB and gut dysbiosis is currently unknown, longitudinal analysis of the fecal microbiota in a mouse model suggest that M. tuberculosis infection causes a significant decrease of the relative abundances of the Lachnospiraceae and Ruminococcaceae families within days of infection [53]. Given that mycobacterial DNA was not detected in fecal samples of infected mice, the selective decrease in bacterial diversity and the dysbiosis observed was unlikely due to the presence of M. tuberculosis within the gut. These findings suggest that the dysbiosis of the colonic microbiota associated with TB may reflect early alterations in the mucosal immune milieu presented in the gut as a consequence of M. tuberculosis infection in the lung, and their translation to selective pressures on the colonic microbiota [53]. Importantly, however, whether (transient) changes in the relative abundance of bacterial taxa affects host responses to M. tuberculosis infection is unknown. In addition, anaerobic growth in the gut is likely to favor metabolic pathways that result in similar classes of metabolites (e.g., SCFAs) across different bacterial taxa. Thus, future studies should aim to combine longitudinal microbiome analyses with transcriptome and metabolome profiling to establish whether changes in the relative abundance of any taxa translate into biologically meaningful changes in the concentrations of immunomodulatory metabolites, and other molecules, at local and distant tissue sites.
Table 1. Summary of microbiome studies performed on animal models of TB and TB patients, investigating the impact of M. tuberculosis infection on the host microbiome.
| Impact of M. tuberculosis infection on the host microbiome | ||||||
|---|---|---|---|---|---|---|
| Location | Specimen | Host and study design | Change in microbiota composition | Effects on the immune system | Sequencing technology and data analysis | Ref |
| Gut | Feces | Newly diagnosed TB patients (NTB, n = 19) and recurrent TB patients (RTB, n = 18); Healthy controls (n = 20)* | Decrease of Prevotella, Lachnospira, Roseburia, and Bacteroidetes in NTB and RTB groups; Enrichment of Escherichia and Collinsella genera in RTB. | Lachnospira and Prevotella directly correlated with CD4+ cell counts in peripheral blood of NTB and inversely correlated with RTB. | 16S rRNA gene amplicon (Illumina) sequencing; Greengenes databaseΔ; Quantitative Insights into Microbial Ecology (QIIME Version 1.7.0°) | [45] |
| Feces | TB patients who did not receive antibiotics 1 month prior to enrollment (n = 18); healthy controls (n = 18) | Decrease of Faecalibacterium, Bacteroides, Ruminococcus, and Dorea; increase of Enterococcus and Prevotella genera. | n.d. | 16S rRNA gene amplicon (Illumina) sequencing; Greengenes databaseΔ; (QIIME v 1.9.1°) | [46] | |
| Feces | TB patients (n = 6) (fecal samples collected before the start of treatment); healthy individuals (n = 6) | Increase of Faecalibacterium, Coprococcus, Phascolarctobacterium, Bacteroides, and Pseudobutyrivibrio; decrease of Prevotella, Bifidobacterium | n.d. | 16S rRNA gene amplicon (Illumina) sequencing; Greengenes databaseΔ; (QIIME v 1.8°) | [244] | |
| Feces | TB patients (n = 46); healthy individuals (n = 31) | Presence of Haemophilus parainfluenzae, Roseburia inulinivorans, and Roseburia hominis in TB patients but not controls | n.d. | Shotgun metagenomic Illumina sequencing; Metaphlan2 (species abundance) | [254] | |
| Feces | TB patients (n = 25); LTBI patients (n = 32); healthy individuals (n = 23) | A higher relative abundance of Bacteroidetes concurrent with low Firmicutes/Bacteroidetes ratio in active TB and LTBI | Positive association of Bacteroidetes and polymorphonuclear neutrophils in TB and LTBI patients; concurrent increase of pro-inflammatory cytokines (IL-6 and IL-1B) and low relative abundance of Bifidobacteriaceae in TB patients | 16S rRNA gene amplicon (Illumina) sequencing; Greengenes databaseΔ; QIIME° | [159] | |
| Feces | Female Balb/c mice (n = 5) infected with Mtb CDC1551 or Mtb H37Rv; preinfection samples from each group as control (n = 3) | Decrease of Clostridiales (Lachnospiraceae, Ruminococcaceae families) and Bacteroidales orders. | n.d. | 16S rRNA gene amplicon (454) pyrosequencing sequencing; Silva databaseΔ; QIIME° | [53] | |
| Feces | Female C57BL/6 mice treated with a cocktail of broad-spectrum antibiotics ceased 2 days before Mtb infection; control group mice w/o Abx treatment; stool samples collected after intranasal Mtb H37Rv infection (n = 4–14 mice/group) | Decrease of Bacteroidetes and Firmicutes; increase of Betaproteobacteria | Decrease in MAIT cells and IL17A in the lungs and increased susceptibility to Mtb | RT-qPCR was performed using phylum-specific primers | [149] | |
| Feces | Female C57BL/6J-CD45a(Ly5a) mice (n = 3–5), 4–8 weeks old, infected with Mtb H37Rv; uninfected age-matched control (n = 3–5), repeated sampling over 20 weeks of infection | Decreased relative abundance of Clostridiales; increased Bacteroidales; although neither significant by 20 weeks | n.d. | 16S rRNA gene amplicon (Illumina) sequencing; custom reference database built from the NCBI 16S rRNA gene sequence and taxonomy database (version May 2016Δ; QIIME v 1.9.1°) | [11] | |
| Feces | Rhesus macaques (n = 4–6) infected with Mtb Erdman | Families Lachnospiraceae, Ruminococcaceae, and
Clostridiaceae significantly increased in animals with severe
disease; members of the family Streptococcaceae,
Erysipelotrichaceae, and the Bacteroidales RF16 and
Clostridiales vadin B660 groups were decreased in the same
group. Roseburia intestinalis, Succinivibrio dextrinosolvens, certain Ruminococcaceae, and Weissella were enriched, and Streptococcus equinus was decreased in some or all animals with severe disease. |
n.d. | 16S rRNA gene amplicon (Illumina) sequencing; Silva databaseΔ; QIIME2/ DADA2°; Shotgun metagenomics with NextSeq 500 platform | [204] | |
| Respiratory tract | BAL | Pulmonary TB patients (TB) (n = 6); healthy controls (n = 10) | Decrease of Streptococcus, Prevotella, Fusobacterium; increase of Lactobacillus, Acinetobacter, Mycobacterium, and Staphylococcus genera. | n.d. | 16S rRNA gene amplicon (Illumina) sequencing; (QIIME v 1.8°) | [58] |
| BAL | Mtb-positive (MTB+, n = 70) and Mtb-negative (MTB−, n = 70) TB patients# | Mycobacterium and Anoxybacillus genera highly abundant in MTB+; MTB− microbiota enriched with Prevotella, Alloprevotella, Veillonella, and Gemella genera. | n.d. | 16S rRNA gene amplicon (Illumina) sequencing; Silva databaseΔ; Mothur (v 1.35.1°) | [255] | |
| BAL | TB patients (n = 10); healthy controls (n = 5) | Presence of the 4 important genus of lung microbiota (Streptococcus, Neisseria, Veillonella, and Haemophilus) | Frequency of Streptococcus directly correlated with TB; frequency of Haemophilus in TB patients is correlated with expression level of T-bet gene (Th1 immune response) | Lung microbiota was detected through culture methods. | [158] | |
| BAL | TB patients (n = 32); healthy controls (n = 24) | Cupriavidus dominance and decrease of Streptococcus in TB patients; wide distribution of Mycobacterium and Porphyromonas in TB patients | n.d. | 16S rRNA gene amplicon (454) pyrosequencing; Ribosomal Database Project (RDP)Δ; Fast UniFrac° | [256] | |
| nasal, oropharynx, sputum samples | TB patients (n = 6); healthy controls (n = 6) | Abundance of Thermi phylum and unclassified sequences belonging to the Streptococcaceae family in TB patients; decrease of the genus Cryptococcus in TB patients | n.d. | 16S rRNA gene and ITS amplicon (454) pyrosequencing; Greengenes databaseΔ; QIIME (v 1.6°) | [257] | |
| OWs, BALs, bronchoscope control samples | Cynomolgus macaques (n = 26) infected with Mtb Erdman | Increase of Aggregibacter, Staphylococcus, Streptococcus, and the unculturable Candidate division SR1 bacteria; decrease of Lachnospiraceae | n.d. | 16S rRNA gene amplicon (Illumina) sequencing; Greengenes databaseΔ; QIIME° | [60] | |
*NTB, no more than 1 week anti-TB treatment; RTB, previously treated and declared as cured prior to recurrence.
#No healthy individuals recruited as controls, positive M. tuberculosis (Mtb) detection determined by a combination of sputum smear, culture, RT-PCR, and GeneXpert.
ΔTaxonomic assignment.
°Operational Taxonomic Units (OTUs) analysis.
BAL, bronchoalveolar lavage; LTBI, latent tuberculosis infection; n.d., not determined; NTB, newly diagnosed TB patients; OW, oral wash; RTB, recurrent TB patients; RT-qPCR, quantitative reverse transcription PCR; TB, tuberculosis.
Fig 1.
Alterations in microbiome composition (A = gut; B = respiratory tract) in individuals with active TB compared to controls. Significantly over- and underrepresented bacteria in the gut (A) and lungs (B) of TB patients (circle), mice (rhombus), or macaques (triangle) models of TB. Taxonomic details are shown, and over- or underrepresentation of the taxonomic level reported by each study is indicated by a red or blue shape, respectively.
As reflected in Table 1, the studies of the lung microbiota in TB patients and model organisms are fewer and often represent findings obtained from a relatively small number of individuals. Sputum samples have been commonly used to assess the lung microbiome in TB patients [54,55]. However, potential contamination of these samples with bacterial genera typically present in the oropharyngeal microbiota (e.g., Prevotella, Bulleidia, and Atopobium) [18] needs to be considered [56,57]. Alternatively, samples collected via bronchoalveolar lavage (BAL) require more invasive collection methods but are beginning to provide insight into the microbiota of the lower respiratory tract of humans [58]. The largest study to date used BAL to characterize the lung microbiota of human patients with respiratory symptoms and abnormal imaging results, with and without confirmed M. tuberculosis infection [59]. The relatively diverse microbial community (e.g., Streptococcus and Prevotella) in patients without M. tuberculosis [59] contrasted the BAL microbiota of TB patients, which was dominated by M. tuberculosis. This highlights potential challenges for the precise annotation of the TB-associated lung microbiota when using 16S rRNA gene profiling [59]. Nevertheless, longitudinal 16S rRNA-based analyses of oral washes, BAL, and bronchoscopy samples in macaques experimentally infected with M. tuberculosis, revealed increased microbial diversity early after infection (1 month), with the relative abundances of Aggregatibacter, Streptococcus, and Staphylococcus genera elevated by 4 months post infection, and the relative abundances of members of the Lachnospiraceae family being significantly decreased [60]. The magnitude of alterations between individual animals were highly heterogenous, which was discussed to possibly reflect genetic makeup of the individual hosts, previous exposure to infection and treatment, and the heterogenous nature of M. tuberculosis infection in macaques [60]. Indeed, the caveats highlighted by the authors of this study are reflective of shortcomings of most microbiome research to date, which historically has been undertaken as a part of observational and cross-sectional studies. This has led to calls for the utilization of more rigorous study design in both animal models and clinical studies, and the pursuit of multinational and/or multicultural frameworks to enhance demonstration of causality and progress towards clinical outcomes [61–64]. For instance, longitudinal analyses in a defined experimental setting will be vital for better characterizing microbiome dynamics during M. tuberculosis infection, and whether these result from microbial interactions within the niche, or as a consequence of mucosal (and peripheral) immune responses to M. tuberculosis infection. As the importance of microbiome composition of the respiratory tract for susceptibility to infections is emerging [65], constrains imposed by sample type and sequencing approaches will need to be overcome by standardized methods that subtractively enrich microbial DNA from BAL samples, to advance the application of shotgun metagenomic sequencing to provide a more holistic and nonbiased assessment of microbial communities in respiratory health and disease [66,67].
Impact of TB antibiotics treatment on the host microbiome
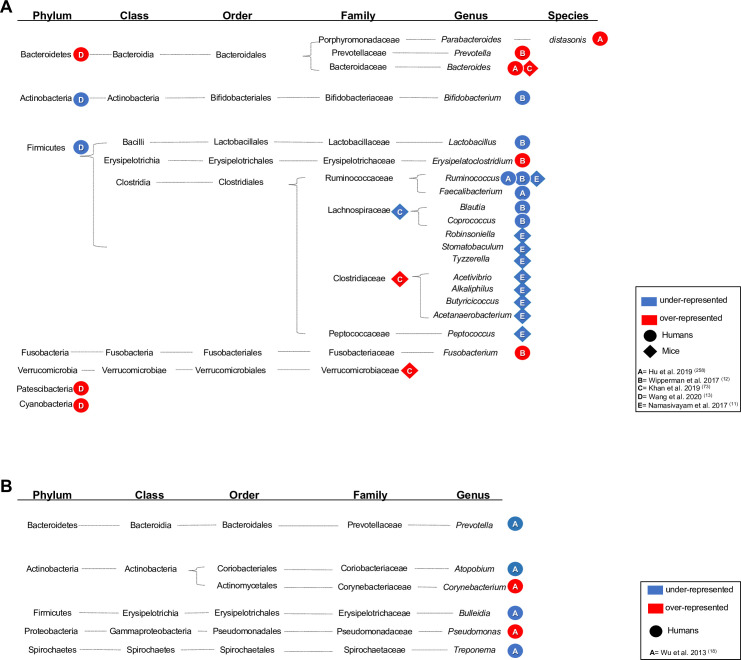
The phenotypic and genetics basis of drug resistance in M. tuberculosis is one of the most significant constraints to improving the clinical management of TB [68]. Treatment regimens for drug-sensitive TB (6 to 9 months) and drug-resistant TB (up to 2 years) are protracted [1]. Antibiotic use disrupts both the composition and total abundance of the microbiota. Whereas there is a limited number of studies addressing this in TB patients and mouse models of M. tuberculosis infection, the results to date indicate that TB antibiotics have a long-lasting impact on the gut microbiome composition [11–13,42–44]. Table 2 summarizes cross-sectional studies in humans and mouse models that have reported effects of TB antibiotics on the microbiota, with Fig 2 providing a phylogenetic integration of the findings to date. A common theme is an antibiotic-induced dysbiosis, with depleted populations of Gram-positive bacteria assigned to the Ruminococcaceae and Lachnospiraceae.
Table 2. Summary of microbiome studies performed on animal models of TB and TB patients, investigating the impact of anti-TB treatment on the host microbiome.
| Effects of anti-TB treatment on the host microbiome composition | |||||||
|---|---|---|---|---|---|---|---|
| Location | Specimen | Host | Treatment | Change in microbiota composition | Effects on the immune system | Sequencing technology and data analyisis | Ref |
| Gut | Feces | LTBI (n = 10), TB (n = 28) TB patients with 1-week anti-TB therapy (TB1, n = 13), TB patients with 2-week anti-TB therapy (T2, n = 10, cured TB patients (TBc, n = 10); healthy individuals (n = 13) | INH, RIF, EMB, and PZA | Decrease of Ruminococcus and Faecalibacterium. Increased abundance of Bacteroides species and Parabacteroides distasonis in all the treatment groups. | n.d. | 16S rRNA gene amplicon (Illumina) sequencing; Ribosomal Database Project (RDP) Δ; Mothur v.1.36.1° | [258] |
| Feces | LTBI (n = 25), TB treatment (n = 19), cured TB patients (n = 19); individuals without Mtb infection (IGRA-) as controls (n = 50) | INH, RIF, EMB, and PZA | Enrichment of Erysipelatoclostridium, Fusobacterium, and Prevotella; decrease of Blautia, Lactobacillus, Coprococcus, Ruminococcus, and Bifidobacterium in the TB treatment group. Depletion of Bacteroides and overabundance of Faecalibacterium, Eubacterium, and Ruminococcus in cured TB group: Enterobacter cloacae, Phascolarctobacterium succinatutens, Methanobrevibacter smithii, Bilophila, and Parabacteroides are biomarkers of cured TB patients. | n.d. | 16S rRNA gene amplicon (Illumina) sequencing; NCBI refseq_rna database with custom scriptsΔ; QIIME°/ Shotgun metagenomic Illumina sequencing; Metaphlan2 (microbial species abundances) and HUMAnN2 (functional pathways) | [12] | |
| Feces | MDR-TB treatment group (n = 6) and untreated controls (n = 26); MDR-TB recovered group (n = 18) and untreated control (n = 28) | MDR-TB treatment | Bacteroidetes, Cyanobacteria, and Patescibacteria are biomarkers for the recovered group: decrease of Actinobacteria and Firmicutes; increase of Bacteroidetes in recovered group. | n.d. | 16S rRNA gene amplicon (Illumina) sequencing; RDP classifier (v 2.2)Δ; Mothur° | [13] | |
| Feces | 6–10 weeks old C57BL/6 mice (n = 5) infected with Mtb H37Rv; fecal samples collected prior to the treatment as baseline (n = 5) | RIF or INH + PYZ | Expansion of Bacteroides, Verrucomicrobiaceae, and decrease in Lachnospiraceae in RIF-treated samples; increase of Clostridiaceae in INH/PYZ-treated mice. | Expression levels of MHCII and production of TNFα and IL-1β significantly reduced after M. tuberculosis infection. Alveolar macrophages more permissive for intracellular M. tuberculosis replication. | 16S rRNA gene amplicon (Illumina) sequencing; Microbiome Analyst web application (community diversity profiling and statistical analysis) | [73] | |
| Feces | 4–8-week-old C57BL/6J-CD45a(Ly5a) female mice (n = 3–5) infected with M. tuberculosis H37Rv; uninfected age-matched control (n = 3–5) | INH, RIF, and PZA + INH and RIF | Decrease of genera Acetivibrio, Robinsoniella, Alkaliphilus, Stomatobaculum, Butyricicoccus, Acetanaerobacterium, Tyzzerella, Ruminococcus, and Peptococcus (all belonging to class Clostridia, phylum Firmicutes). | n.d. | 16S rRNA gene amplicon (Illumina) sequencing; custom reference database built from the NCBI 16S rRNA gene sequence and taxonomy database (version May 2016)Δ; QIIME (v 1.9.1°) | [11] | |
| Respiratory tract | Sputum samples and throat swab samples | New TB group (N-TB, n = 25): patients, cured new TB patients (C-TB, n = 20), recurrent TB group (n = 30), treatment failure group (n = 20); healthy individuals (n = 20) | mix of DS-TB and MDR-TB treatments | Pseudomonas abundance in TB treatment failure patients or recurrent TB than in new or cured TB patients; Prevotella, Bulleidia, Atopobium, and Treponema decrease in recurrent TB patients than new TB group; increased Corynebacterium abundance in recurrent TB than treatment failure TB. | n.d. | 16S rRNA gene amplicon (454) pyrosequencing; Greengenes databaseΔ; QIIME (v 1.5.0°) | [18] |
ΔTaxonomic assignment.
°Operational Taxonomic Units (OTUs) analysis.
DS-TB, drug-susceptible TB; LTBI, latent tuberculosis infection; MDR-TB, multidrug-resistant TB; n.d., not determined; TB, tuberculosis.
Fig 2.
Alterations in microbiome composition (A = gut; B = respiratory tract) of patients upon TB antibiotics treatment. Significantly over- and underrepresented bacteria in the gut (A) and lungs (B) of TB patients (circle), mice (rhombus), or macaques (triangle) models of TB undergoing therapy for drug-sensitive or multidrug-resistant TB. Taxonomic details are shown, and over- or underrepresentation of the taxonomic level reported by each study is indicated by a red or blue shape, respectively.
It is increasingly appreciated that commensal bacteria can confer a form of colonization resistance against nonresident species including pathogens, via competition for metabolic and/or spatial niches, as well as their production of bioactive molecules that can directly inhibit/suppress the growth of susceptible microbes [69]. The sustained use of antibiotics for recalcitrant Clostridioides difficile infection often results in long-term failure of antibiotics to control this infection [69], and this has been used to exemplify how chronic antibiotic use might be a risk factor for reinfection with M. tuberculosis [70,71]. Indeed, long-term impact of TB antibiotics was indicated by a recent study reporting preferential loss of T cell reactivity to M. tuberculosis-derived epitopes that showed similarities with microbiota species [72]. In a mouse model, TB antibiotics altered gut microbiota composition and affected the immune responses to M. tuberculosis infection [73], alluding to the multidimensional complexity of the interplay between resident microbiota at the time of M. tuberculosis infection and the quality of the immune response. Understanding of how prolonged antibiotic use affects predisposition to recurrent TB and/or reinfection is an important area of future investment. Notwithstanding the limits of current studies (e.g., cohort size, mode of sampling), anti-TB antibiotic regimens exert selective pressure and reorganization of the gut and/or lung microbiota with profound and long-lasting effects. Knowledge of the functional implications of these alterations via the gut-lung axis on host immune response are emerging. The following sections examine the physiological and metabolic cues arising from the gut (and lung) microbiota with implications for host susceptibility or resistance to the clinical manifestations of M. tuberculosis infection.
Microbiome-immune crosstalk and host control of M. tuberculosis
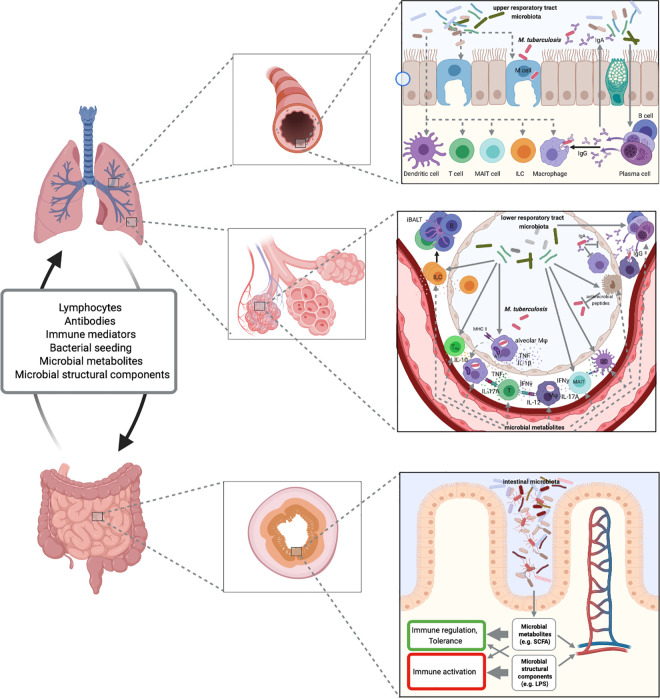
Bioactive metabolites are a key element of the crosstalk between the host and its microbial collective. Such metabolites arise from microbial metabolism (e.g., vitamins) as well as microbe-facilitated modulation of host- or dietary-derived metabolites (e.g., bile acids, SCFAs) [74]. Significant focus to date has been on the metabolic capacity of the gut microbiome, with evidence for impact on immune functions at distant sites, including the lung via the gut-lung-axis [75] (Fig 3). Here, we focus on the emerging concepts of direct and indirect contributions of the host microbiome to host defense mechanisms against M. tuberculosis infection [44].
Fig 3. Proposed microbiome-immune interactions in M. tuberculosis infection.
Microbiota of the upper and lower respiratory tract may define epithelial barrier integrity, M cell frequency, antimicrobial defense, composition, and functionality of innate and adaptive immune mechanisms. Through the gut-lung axis, the microbiota of the intestinal tract influences barrier and immune functions in the periphery and at sites of M. tuberculosis infection. Fig 3 was created with BioRender.
Epithelial barriers and innate immunity
Epithelial cells
The main route of M. tuberculosis entry into the human host is transmission via aerosol droplets. The size of M. tuberculosis-containing droplets allows entry into the alveoli of the lower respiratory tract where the bacteria encounter respiratory epithelium, alveolar macrophages, and resident microbiota. The roles of alveolar epithelial cells in the host defense against M. tuberculosis are incompletely understood. M. tuberculosis has been found in cells of the alveolar epithelium in humans, and infected alveolar epithelial cells in vitro in some but not all studies [76–80]. Transmigration of infected alveolar macrophages from the alveolar space across the epithelium into the interstitium enables engagement of interstitial and recruited inflammatory macrophages, a process important for control of M. tuberculosis [81]. While the importance of the gut microbiota in maintaining gut epithelial integrity and barrier functions is well established [82–85], it is unknown whether microbiota-epithelial interactions shape alveolar macrophage transmigration or macrophage recruitment to sites of M. tuberculosis entry. Pulmonary epithelial cellular defense mechanisms are directly responsive to microbiota-derived SCFAs [86]. Whether production of antimicrobial peptides (AMPs) upon encounter with M. tuberculosis [87,88], is shaped by the lung-resident, or remote, microbiota, will be important to determine as this bears relevance to host defense against M. tuberculosis, and bacterial pathogens more generally. Moreover, microfold (M) cells in the upper respiratory tract have been suggested to act as entry points for M. tuberculosis across the epithelial barrier into mucosa-associated lymphoid tissues, which may result in extrapulmonary manifestation of M. tuberculosis (e.g., cervical lymphadenopathy in the absence of evidence for pulmonary TB) [89,90]. This process has been reported to be facilitated by interactions between the M. tuberculosis virulence factor EsxA and scavenger receptor B1 on M cells in the airway epithelium [91]. With microbiota composition implicated in M cell density and functionality in the gut [92], microbiota contributions to airway M cell functions remain to be elucidated, including implications for M. tuberculosis infection in the antibiotic-naïve or antibiotic-experienced host.
Host-microbiota interactions are critical in governing tissue homeostasis at sites of close contact as well as distant sites. Yet, microbial dysbiosis and compromised but barrier functions, e.g., in the context of chronic inflammation and antibiotics treatment, have been implicated in inflammation and metabolic dysfunction at distant sites. This is driven at least in part through innate immune activation of macrophages and other innate immune cells by microbiota-derived bacterial products such as LPS [83,93–96] (Fig 3). Some studies have questioned whether epithelial functions in the gut are altered in TB patients and how this might affect pharmacodynamics of TB antibiotics and have returned varying results [97–101]. With long-term antibiotics regimens and sustained alterations of the gut microbiota, it is relevant to query if and how the integrity of epithelial barriers (e.g., gastrointestinal and respiratory tract) is affected in TB patients during and after treatment, and whether this has long-term consequences for tissue and organ homeostasis, immune functions, metabolism, cognition, and behavior [83].
Macrophages
Macrophages are major host cells for intracellular M. tuberculosis, and bacterial interference with macrophage antimicrobial defense mechanisms enable intracellular persistence and replication [102]. The immune-regulatory and metabolic phenotype of alveolar macrophages, as well as ready availability of nutrients key to intracellular M. tuberculosis survival have been implicated in facilitating the exponential intracellular replication of M. tuberculosis within alveolar macrophages for several days post infection [81]. The airway microbiota has been implicated in defining alveolar macrophage functions [75,103], including during M. tuberculosis infection [73]. Infection of mice with M. tuberculosis after a course of treatment with the TB antibiotics isoniazid and pyrazinamide for 8 weeks resulted in slightly higher lung bacterial burden. This was accompanied by an altered phenotype of alveolar macrophages, including diminished MHCII expression, TNF and IL-1β production, as well as cellular respiration and ATP production [73]. Alveolar macrophages derived from such antibiotic-treated mice were diminished in their ability to control intracellular M. tuberculosis replication in ex vivo cultures. The authors linked functional dysbiosis to these outcomes, which were reversed by fecal microbiota transfer (FMT). It is interesting to note that the antibiotic-driven phenotypic alteration of alveolar macrophages was not inducible in in vitro culture in the presence of isoniazid and pyrazinamide but required the in vivo tissue context [73], suggesting that alveolar macrophage phenotypic imprinting required tissue- and/or microbiome-derived factors. In this context, it is noteworthy that in in vitro cultures of PBMC, the presence of lactic acid bacteria has been reported to enhance M. tuberculosis-induced IFNγ production and promoted IFNγ-driven macrophage antimicrobial defense mechanisms [104]. Thus, positioning the microbiota as one of the likely sources of cues that define alveolar macrophage functions related to antimicrobial defense, inflammation, and engagement of adaptive immunity is important for our understanding of early host control of M. tuberculosis infection with implications for developing active disease or LTBI.
Innate and innate-like lymphoid cells
Microbial products, including metabolites, distinctly guide development and functions of innate and innate-like lymphocytes. Conversely, the localization of innate and innate-like lymphoid cells to mucosal sites directs the composition and spatial distribution of the microbiota [105]. SCFAs such as acetate, propionate, and butyrate are the most abundant bacterial products derived from commensal bacterial fermentation of dietary fibers in the intestine and have been found to regulate cellular metabolism and exert potent immune-regulatory functions [106,107]. SCFAs instruct the proliferation and function of group 3 innate lymphoid cells (ILC3) [108], which play central roles in immune responses at mucosal and epithelial sites, including the lung [109]. Control of M. tuberculosis infection is critically dependent on intact IL-12 and IFNγ signaling, and IFNγ-mediated protection is largely attributed to adaptive T cell responses [110]. However, more recently, contributions of innate and innate-like lymphoid cells have been unveiled.
Based on their cytokine expression profiles, ILCs are categorized into group 1, including natural killer (NK) cells and noncytotoxic type 1 ILCs (IFNγ, TNF), group 2 (IL-4/5/13), and group 3 (IL-17/22) [111].
IFNγ-expressing NK cells have been described to accumulate in the pleural fluid of patients with TB pleurisy [112]. Individuals with LTBI exhibited increased numbers of circulating NK cells in peripheral blood and these cells exhibited increased cytotoxic capacity associated with high expression of granzyme B and perforin [113], and accumulation of CD27+ NK cells in the lung has also been associated with LTBI in nonhuman primates [114]. In contrast, circulating NK cells were markedly decreased in peripheral blood of patients with active TB [113]. NK cells have been reported to contribute to CD8+ T cell responses, and lyse mycobacteria-infected monocytes, macrophages, as well as regulatory T cells expanded in the presence of mycobacterial antigens [115–117]. Patients with active TB exhibit diminished proportions of type 1, 2, and 3 ILCs, but not NK cells, in peripheral blood [118], which is thought to be a result of ILC accumulation in infected lungs, as has been shown for mice infected with M. tuberculosis or Mycobacterium bovis bacille Calmette-Guérin (BCG) [118,119]. Transcriptome analyses of ILC2s and ILC3s isolated from lungs of TB patients revealed distinct immune signatures [118], suggesting specific functional contributions. Early studies in mice indicated that deficiency in T and B lymphocytes as well as ILCs (RAG2−/− γc−/−) resulted in higher susceptibility to M. tuberculosis infection compared to T and B cell deficiency (RAG2−/−), which was attributed to IL-12-driven IFNγ production by innate lymphocytes [120]. More recently, specific contributions of group 3 ILCs to host control of M. tuberculosis early during infection have emerged, specifically in the formation of inducible bronchus-associated lymphoid tissue (iBALT) [118], which is associated with a degree of host protection early during M. tuberculosis infection [121].
Due to the intimate connection between microbiota and ILCs, many questions arise from these recent observations, including: Are ILC3 contributions to immune control of M. tuberculosis shaped by the metabolic capacity of the microbiome (e.g., dynamics and relative abundance of SCFA at mucosal sites and in the periphery [108]? Do (myco)bacteria-derived components or TB antibiotics direct ILC3 functions, e.g., through engagement of arylhydrocarbon receptor (AhR) [122,123], a ligand-dependent transcription factor that governs ILC3 functions [124]? Are microbiota-derived metabolites that drive IL-22 production at mucosal sites (e.g., tryptophan derivatives) [125] linked to the host control of M. tuberculosis attributed to type 3 ILC and IL-22 [118,126,127]? Does plasticity within type 1 ILC (i.e., conversion of NK cells to type I ILCs) occur during M. tuberculosis infection, similar to what has been described recently in the context of Toxoplasma gondii infection [128] and tumor immune evasion [129]? Is ILC functionality at the sites of M. tuberculosis infection reflective of the ILC composition detectable in peripheral blood and do alterations in the periphery indicate relevance to host control, e.g., as discussed for NK cell dynamics in active TB versus LTBI and healthy controls [113,130,131]?
MAIT cells
Innate-like lymphocytes, including mucosa-associated invariant T cells (MAIT), natural killer T cells (NKT), and γδ T cells recognize microbially derived nonpeptide antigens via semi-invariant T cell receptors (TCRs) resulting in cytokine production and/or cytotoxic activity. Among these, MAIT cell development has been closely linked to the presence of the microbiota driven by thymic presentation of bacteria-derived antigen [132–135], although microbiota-independent MAIT cell development during embryogenesis has also been reported [136]. MAIT cells are abundant in barrier tissues and at mucosal sites, including the lung, apart from representing up to 10% of circulating human T cells [137]. The evolutionary conserved MAIT cell TCRs have been shown to recognize the vitamin B2 precursor metabolite, 5-(2-oxopropylideneamino)-6-D-ribitylaminouracil (5-OP-RU), presented by the MHC-1-like molecule MR1 [138,139]. In addition, IL-18 and IL-12 can drive antigen-independent activation of MAIT cells [140]. TCR-mediated MAIT cell effector functions include cytokine production (predominantly IL-17A by MAIT cells in mice and human tissues; IFNγ, TNF in human blood), cytotoxicity against cells that present antigen via MR1, and bystander activation of dendritic cells [137].
Peripheral blood MAIT cell numbers are significantly diminished in TB patients [141–146] and have been noted to negatively correlate with TB disease severity [143]. A TB household contact study reported that MAIT cells in peripheral blood show signatures of activation [147]. Whereas MAIT cell accumulation in infected lungs has been reported for some bacterial pathogens, studies in M. tuberculosis-infected nonhuman primates have shown only limited accumulation in infected lung tissue [148]. Observations in mice appear to suggest a more nuanced picture of MAIT cell contributions to the host control of mycobacterial infection in this model organism. Initial studies indicated contributions of MAIT cells to early host control of mycobacterial infection in the lung upon aerosol or intranasal challenge, as well as in spleen after intravenous delivery of bacteria, albeit with relatively small and transient effects [141,149,150]. In contrast, a recent study using MR1-deficient mice reported no difference in the ability to control M. tuberculosis infection compared to wild-type mice [151]. Exogenous administration of 5-OP-RU (in conjunction with Toll-like receptor (TLR) agonists) prior to M. tuberculosis infection resulted in expansion of MAIT cells but did not affect M. tuberculosis burden in the lung [151,152], despite delayed CD4+ T cell priming in mesenteric lymph nodes [151]. On the other hand, therapeutic administration of 5-OP-RU well into the chronic phase of M. tuberculosis infection conferred some protection in the lung dependent on IL-17A, but not TNF or IFNγ. A possible interpretation of these observations is that the microenvironment and/or activation status of MAIT cells at the time of stimulation skews their cytokine profile towards regulatory or inflammatory functions [151]. Whether exogenous application of MAIT cell antigen would have similar effects in humans will be important to establish, especially considering the relative higher abundance of a MAIT cells in humans when compared to laboratory mice [137]. Such insights will be critical especially if targeted engagement of MAIT cells is to be explored for host-directed interventions in TB [151]. Thus, experimental evidence to date suggests that MAIT cells contribute to host responses against M. tuberculosis infection, and that it appears to be important to determine whether the timing of their engagement in the context of infection is beneficial or detrimental to immune responses that control mycobacterial infections. Of note, a genetic polymorphism in MR1 has been associated with TB susceptibility and manifestation in humans [153], and household contact studies have led to the hypothesis that MAIT cells in early stages of M. tuberculosis exposure are associated with protection from productive infection [147,154]. Findings that abundance or depletion of distinct bacterial species correlates with distinct MAIT cell functionality (e.g., IFNγ, granzyme B expression) in a TB household contact study [147] might be reflective of the impact of phylogenetic diversity, relative demand for riboflavin, and/or carbon source utilization within microbial ecosystems as indicated in in vitro studies on MAIT cell activation [155,156]. Whether these observations translate into in vivo settings with diverse microbial ecosystems at different anatomical sites requires further investment into more detailed analyses on how the microbiome shapes innate immune cell responses at mucosal barriers (Fig 3).
Adaptive immunity
T cells
CD4+ T cells are critical in the host control of M. tuberculosis infection, with contributions of CD8+ T and B lymphocytes increasingly appreciated. Inflammatory circuits, e.g., driven by IL-12/IFNγ, TNF, and IL-17, are central to controlling M. tuberculosis, yet tight regulation of these immune effector mechanisms, e.g., by regulatory T (Treg) cells and IL-10, is essential for preventing severe pathology and poor pathogen control [110]. With the growing understanding of how dynamic interactions between microbiota and the host immune system define the development and functions of lymphocytes [157], there is a growing interest in how the microbiota shapes adaptive immune responses that are critical for the host control of M. tuberculosis infection [44,158].
There is evidence suggesting that microbiota composition licenses T cell functions critical to controlling M. tuberculosis infection. A recent study in a small cohort of patients with active TB (prior to treatment commencement), LTBI and healthy controls reported a positive correlation between the abundance of Coriobacteriaceae in fecal samples of LTBI individuals with M. tuberculosis antigen-specific IFNγ responses in peripheral blood [159]. Observations in mice indicate the extent and qualitative impact of antibiotic-induced dysbiosis might differentially impact on immune mechanisms that control M. tuberculosis. Specifically, impaired host control of M. tuberculosis in mice exposed to broad-spectrum antibiotics exposure was associated with decreased proportions of IFNγ+ and TNF+ CD4+ T cells alongside an increased percentage FoxP3-positive Treg cells in the spleen [160]. In contrast, mice treated with the narrow-spectrum TB antibiotics isoniazid and pyrazinamide displayed a comparatively slight increase in M. tuberculosis lung burden at the onset of the chronic phase of infection, which was associated with impaired antimicrobial defense by alveolar macrophages, without impact on the percentages of TB antigen-specific T cells [73]. In both settings, FMT experiments in mice rescued antibiotic-induced impairment of M. tuberculosis control by the host [73,160]. The impact of broad-spectrum antibiotics on mycobacteria-specific T cell responses has been extended to a vaccine setting in mice with impaired CD4 and CD8 activation, as well as impaired generation of lung-resident and effector memory T cells [161].
There are examples of microbiota species that have been suggested to poise the host towards Th1 responses, including Klebsiella aeromobilis, Klebsiella pneumoniae, and Bilophila wadsworthia [162,163]. Defining if and how specific bacterial groups or species within the microbiota gear M. tuberculosis-specific T cell responses towards increased effector functions (e.g., IFNγ, TNF) and whether this translates into benefits for the host in controlling M. tuberculosis might offer opportunities for targeted intervention. This might encompass promotion of a specific microbiota composition but could equally be explored for metabolic capacities of the microbiota that define host immune functions. Microbial products and metabolites, in particular SCFAs, have been established as key mediators of immune-modulatory functions of the microbiota [164]. In this context, the potential contributions of SCFAs such as butyrate have become of particular interest (Fig 3).
Butyrate reduced M. tuberculosis antigen-specific IFNγ and IL-17A production and elevated IL-10 production of in vitro cultured human peripheral blood mononuclear cells (PBMCs) [165,166]. This is consistent with the immune-modulatory functions of butyrate, which are driven by suppression of histone deacetylase (HDAC) activity that enhances FOXP3 expression and Treg differentiation [167,168]. Additional effects of SCFA on immune functions include reprogramming of Th1 cells towards IL-10 production [169], inhibition of HDAC-dependent epigenetic regulation of inflammatory gene expression (e.g., IL12b, Nos2) by macrophages and dendritic cells [170,171], as well as limiting neutrophil activation [172]. Thus, the SCFA profile arising from a particular microbiome composition may impair immune effector mechanisms that are central to effective host control of M. tuberculosis. If present at the time of M. tuberculosis encounter, this may represent a risk factor for successful infection and progression to active TB. Support for this hypothesis may be drawn from a recent study in a cohort of HIV+ healthy individuals undergoing antiretroviral therapy (ART) in a high-TB incidence environment. Individuals undergoing ART are characterized by SCFA-producing microbiota in their lower airways, and in this cohort, SCFA serum concentrations positively correlated with elevated risk of subsequently developing TB, as well as induction of FoxP3+ Tregs in PPD-stimulated cultures of BAL lymphocytes [165]. Elevated serum SCFA concentrations were associated with increased presence of Prevotella in the lower airways [165]. These correlations encourage investigation of how SCFA production locally in the lung, or systemically, might hamper mucosal immune defense mechanisms against M. tuberculosis infection. This might seem counterintuitive when considering the decrease in the relative abundance of Ruminococcaceae and/or Lachnospiraceae described in some studies (Table 1 and Fig 1A). However, if altered microbiota composition in the context of active TB disease was accompanied by diminished SCFA concentrations at peripheral sites, one might speculate that microbiota changes upon M. tuberculosis infection could be reflective of a directly or indirectly driven host adaptation to enable effective Th1 immune responses that control M. tuberculosis. Carefully designed longitudinal studies, integrating taxonomic, metagenomic, metabolomic, and immunological analyses in a prospective setting will be necessary to establish whether a microbiome composition functionally geared towards a specific metabolic output governs establishment and host control of M. tuberculosis infection.
B cells and antibodies
Mucosal and systemic antibody responses are directly shaped by the microbiome. Exploration of these microbiota-immune interactions has largely focused on the gut microbiota, a critical regulator of gut immunoglobulin A (IgA) production [173,174]. Microbiota-derived SCFAs gear B cell metabolism and gene expression towards antibody production [175]. TLR-mediated sensing of the microbiota by epithelial and dendritic cells drives expression of a proliferation-inducing ligand (APRIL) and B cell-activating factor (BAFF), which promote B cell survival and IgA production by plasma cells [176–180]. There is emerging evidence that microbial cues at oral and respiratory epithelial sites similarly shape B cell functions and antibody responses [180–182]. Despite these well-established links between microbiota and antibody responses, it remains largely unknown how these contribute to host responses during M. tuberculosis infection and TB disease.
The B cell compartment in peripheral blood undergoes dynamic changes during M. tuberculosis infection, and relative abundance of memory B cells, plasma blasts, and plasma cells has been correlated with TB disease state (reviewed in [183]). M. tuberculosis infection induces robust antibody responses, yet the contributions of B cells to the immune control of the infection are incompletely understood and have remained controversial. Different mouse models of B cell deficiency indicated protective contributions of B cells during M. tuberculosis infection, through regulation of tissue pathology and local inflammatory cytokine responses [184–186]. B cell depletion (anti-CD20, rituximab) in M. tuberculosis-infected nonhuman primates did not affect overall lung pathology, bacterial burden, and clinical outcome in an early disease setting. Nevertheless, at the level of individual granulomas, B cell contributions to bacterial control, production of IL-6 and IL-10, as well as diminishing the frequency of T cells expressing IL-2, IL-10, or IL-17 have been reported [187].
M. tuberculosis infection in the immune-competent host elicits robust antibody responses against diverse mycobacterial protein and oligosaccharide antigens [188]. Recent insights into potential roles of antibody-mediated modulation of M. tuberculosis control by host cells [189,190] have reinvigorated the interest in B cell functions in TB. Antibody-mediated opsonization (serum or purified IgG) has been implicated in M. tuberculosis restriction by infected human and mouse macrophages associated with enhanced phagocytosis and delivery to phagolysosomal compartments [189–194]. More detailed insights into patient-specific patterns and functional contributions of IgG subtypes in this context will be of great value, especially in light of earlier observations implicating distinct outcomes of activating versus inhibitory Fcγ receptors for the host control of M. tuberculosis infection [195]. Antibiotics-mediated depletion of resident microbiota has been associated with decreased pulmonary IgA production, which has been associated with increased susceptibility to pulmonary bacterial infections in humans and mice [180]. This observation likely bears relevance for M. tuberculosis infection in light of reports that passive transfer of purified, mycobacteria-specific IgA diminished bacterial burden in infected lungs [196–198]. The molecular and cellular mechanisms underlying this protection are incompletely understood but may include IgA-mediated inhibition of infection of macrophages and lung epithelial cells with contributions of the human FcαRI IgA receptor [190,198]. Humoral immune responses in individuals infected with M. tuberculosis are highly heterogenous and influenced by complex interactions of a number of factors, including age, state of infection (active TB disease or LTBI), and immune competency (e.g., HIV, diabetes). With the fundamental contributions of the microbiota to shaping local airway mucosal as well as systemic antibody responses [173,199], it is imperative to define how microbiota-defined local and systemic antibody responses affect host susceptibility and manifestation (active disease versus LTBI) during M. tuberculosis infection. The design of future studies needs to include considerations on the impact of systemic and mucosal antigen exposure on antibody repertoire [199]. Isotype- and/or target cell-specific functional differences of M. tuberculosis-specific antibodies may be further defined by distinct glycosylation profiles characteristic to disease state, i.e., active versus latent TB [189]. It will be important to determine whether treatment with TB antibiotics causes secondary IgA deficiency [180] and whether this poses risks for (re)infection with M. tuberculosis. A comprehensive view of B cell functionality, beyond antibody responses, in this context will further enhance understanding of cellular drivers of local inflammatory responses [185,187], macrophage polarization [200], neutrophilia [185,201], and immune regulation [202,203].
Are there opportunities for microbiota-focused preventative and adjunct-therapeutic strategies?
With the notion that the larger collective of “commensal microorganisms” may, directly and indirectly, shape host susceptibility to M. tuberculosis (re)infection, protective immune responses, and disease manifestation, the questions arising now center on how this knowledge might translate into therapeutic or preventative measures. Areas of focus include opportunities at the gene product (e.g., metabolites and bioactives), organismal (e.g., probiotics, genetically modified organisms (GMO), FMTs), and dietary level of interventions to correct microbial dysbiosis or specifically deliver functional capabilities that reshape host immune responses and resilience to M. tuberculosis infection and/or recurrence.
Strategies that promote the introduction and/or restoration of a “beneficial” microbiota, such as dietary interventions or defined probiotic formulations may prove to be an effective strategy to complement TB treatment, in particular in correcting the long-lasting dysbiosis that occurs as consequence of prolonged TB antibiotics regimens. Moreover, gut microbiota composition prior to infection has been found to correlate with disease manifestation in nonhuman primates experimentally infected with M. tuberculosis, which raises the possibility of defining a gut microbiota that reduces host susceptibility to M. tuberculosis infection and TB disease manifestation [204]. Gut microbiota diversity, abundance, and host immune response are strongly impacted by diet and nutrition and much still needs to be learned about these interrelationships in the context of disease susceptibility and prevalence associated with under-, mal-, and overnutrition [120]. Protein–calorie undernutrition, type 2 diabetes associated with overnutrition, and micronutrient deficiencies (e.g., vitamin D) are risk factors for developing active TB [205–208].
Probiotics such as Bifidobacterium spp. as an adjunct therapy with conventional TB antibiotics are reported to restore and maintain what is considered a “healthy microbiome” [209–211]. A longitudinal study in TB patients reported that a multi-strain probiotic formulation (Lactobacillus acidophilus, Lactobacillus casei, Lactobacillus rhamnosus, Lactobacillus bulgaricus, Bifidobacterium breve, Bifidobacterium longum, and Streptococcus thermophilus) combined with supplementation of vitamins B1, B6, and B12 increased serum concentrations of IFNγ and IL-12, compared to the control group receiving only anti-TB antibiotics and vitamin B6 [212]. Whether rational design of safe-for-human-use probiotics can include the design of strains that withstand TB antibiotic therapy as proposed recently [213] remains to be carefully evaluated.
Immune cross-reactivity between mycobacterial species as well as direct impact on the microbiota are associated with beneficial effects of orally administered heat-killed Mycobacterium manresensis. Indeed, formulations using this environmental bacterium that is commonly found in drinking water are being explored for potential benefits in the treatment of TB. In a susceptible mouse model of M. tuberculosis infection, orally administered heat-killed M. manresensis reduced lung pathology, bacterial burden, and inflammatory responses, and in combination with TB antibiotics, expanded the life span of infected mice when compared to mice treated only with antibiotics [214]. Following on from early clinical safety profiling [215,216], a placebo-controlled randomized interventional trial in HIV–negative and HIV–positive individuals undergoing treatment for TB is currently analyzing the impact of a M. manresensis-based food supplement on gut microbiota composition, antigen-specific CD4+ T cell responses, as well as time to sputum conversion and reduction in bacterial burden (NCT03851159).
Perhaps the most dramatic approach to “probiotic therapy” is the integration of FMT into clinical practice. Although practiced by some cultural groups for centuries [217], FMT has recently become a mainstream intervention for the treatment of recurrent Clostridioides difficile infection, offering high therapeutic efficacy and with limited prevalence of adverse events, at least in the short term [218]. These findings have catalyzed global interest in both research and clinical settings for the evaluation of FMT as induction therapy for a variety of medical conditions where gut “dysbiosis” is implicated [219–221]. In the context of TB, the findings that FMT reversed the increased susceptibility of antibiotic-treated mice to M. tuberculosis infection [73,160] warrants further investigation into microbiota compositions that confer benefits to the host. In summary, probiotics as an adjunct and/or therapeutic option for the restoration of gut homeostasis has long been investigated and continues to hold promise, and this extends to their potential as adjunct therapeutics alongside TB antibiotics [222–224].
With current limitations of probiotics and FMT, dietary interventions, defined microbial metabolites, and actively secreted bioactives might offer a pragmatic alternative. For example, indolepropionic acid (IPA), which is produced by bacteria taxonomically affiliated with the Clostridiales, including Peptostreptococcus anaerobius, has been shown to inhibit growth of M. tuberculosis, both in vitro and in vivo. This has been attributed to antagonistic effects of IPA on M. tuberculosis tryptophan biosynthesis, leading to suggestions that IPA per se and/or targeting the M. tuberculosis tryptophan pathway may be avenues for the discovery of novel antimycobacterials [225–228]. Additional positive effects of IPA on epithelial barrier function as well as activation of innate and adaptive immune responses [229–232] might be worth exploring for dually acting compounds. A second example are bacteria-derived AMPs, which directly affect microbial ecology, including specific inhibition of bacterial pathogens [233,234]. The in vitro antimycobacterial activity of bacteriocins isolated from Lactobacillus salivarius, Streptococcus cricetus, and Enterococcus faecalis exceeds that of the TB antibiotic rifampicin [235], with nisin and lacticin being effective towards M. tuberculosis, Mycobacterium kansasii, Mycobacterium smegmatis, and Mycobacterium avium subspecies paratuberculosis [236,237]. Synergism with TB antimicrobials, such as those reported for bacteriocin AS-48 from E. faecalis and ethambutol [238] may offer avenues for exploration, e.g., whether combinations allow for shortening of current antibiotics regimens or reducing antibiotic dosing to limit toxic side effects.
Notwithstanding the notion that SCFAs poise host immune mechanisms towards a permissive environment for M. tuberculosis infection, whether modulation of SCFA production might be a target for intervention in TB requires careful consideration. With SCFA the primary microbial metabolites released within the gastrointestinal tract, host evolution has favored the development of sensor-regulatory pathways linked with immune and/or metabolic pathways that can monitor and respond to alterations in these primary microbial metabolites. In chronic diseases with characteristic gut dysbiosis (e.g., inflammatory bowel disease), the presumptive reduction in butyrate-producing bacteria is widely considered to compromise barrier integrity, mucin production, and FoxP3+ Treg cell production [239,240]. While the link between SCFAs and host immune responses is relatively well characterized, the minimal effective concentrations of SCFA needed for the maintenance of barrier integrity and regulatory immune responses are less well understood. In that context, the therapeutic efficacy of specifically modulating colonic butyrate and/or other SCFA concentrations via oral or colonic routes of administration are, at best, mixed [241]. Such findings suggest that reaching threshold concentrations of colonic SCFA are necessary but not sufficient to bias mucosal integrity and immune responses. Indeed, additional metabolic capabilities being defined in “beneficial” bacteria such as Faecalibacterium prausnitzii [242,243] highlight the complexity of microbial metabolites and secreted products that define the sustainability of gut homeostasis and poise (mucosal) immune responses.
Conclusions
Confidence in whether the microbiome composition is associated with host susceptibility M. tuberculosis infection or can indeed skew effector mechanisms towards improved or diminished pathogen control requires carefully designed prospective and longitudinal studies in large cohorts. The integration of microbiome, metagenome, and metabolome analyses, ideally in the lung as well as the gut and potentially other distant sites, alongside immunological characterization will be essential. Additionally, important confounding factors such as nutritional status, coinfection(s), and other comorbidities [165,244] will need to be integrated into study and cohort design. Careful considerations will need to be given to sampling techniques, as well as appropriate control samples and cohorts [8].
Candidate microbiota/microbe/metabolite approaches and functional studies in animal models of TB will be invaluable to further elucidate causality between microbiota composition, metabolic capacity, and the immune control of M. tuberculosis infection. It will be particularly important to determine the interplay between microbiota and immune components at distinct stages of infection and disease. Our discussions above highlight the importance of acknowledging potential composite effects of innate and adaptive immune cell functions, and the multidimensional interplay between microbiota and host defense mechanisms. For example, butyrate enhanced antimicrobial defense in macrophages (e.g., AMP expression and autophagy), thereby increasing control of extracellular and intracellular bacterial pathogens, including mycobacteria [245]. Yet, SCFAs are emerging to create a permissive immune milieu for M. tuberculosis infection in the host at least in part through their immune-modulatory effects on adaptive immune responses. Moreover, detailed studies are required to fill current knowledge gaps on the host interactions with viruses, fungi, and protozoa in the human microbiome, which likely has profound implications for shaping host responses to infections [246,247].
Restoration of TB antibiotic-induced dysbiosis is an attractive and seemingly achievable target. Nevertheless, the transition of probiotics from being dietary supplements to an evidence-based predictive intervention in clinical settings remains elusive [248,249]. Similarly, the potential that FMT might serve to augment the treatment and immune control of M. tuberculosis infection, as indicated in mouse studies [73,160], is attractive. With the accelerating increase in reports associating microbiota composition with human pathologies, some level of caution is warranted, e.g., in relation to invariably positive outcomes from studies using human microbiota-associated or humanized gnotobiotic animal models [61]. Additional critical considerations need to be given to the ethical, cultural, and safety implications of selecting and using stool samples for FMT, which continue to be reviewed and assessed for other conditions where gut dysbiosis is diagnostic [250]. Similarly, interest in using diet as a first-line intervention for the correction of microbiota-immune interactions and promoting gut homeostasis in digestive health and disease have gained considerable momentum in recent years [251,252]. Translation of these findings to the context of TB may offer insights over and above gains made by promoting a more protein–calorie-rich diet in societies afflicted by mal- and/or undernutrition. But not unlike the constraints associated with the advancement of probiotics, FMT, and next-generation versions of both, the translation of such observations into evidence-based interventions is contingent on further refinement of the approaches used to produce such evidence [253].
In summary, notwithstanding the increasing body of literature focused on establishing links between the microbiome and the immune control of TB, as with most microbiome-focused research, the challenge at hand will be to establish causality, which would deliver solid foundations for the pursuit of targeted interventions in TB.
Acknowledgments
The authors acknowledge support by The University of Queensland Diamantina Institute, The University of Queensland Faculty of Medicine, as well as the infrastructure provided by the Translational Research Institute.
Funding Statement
The authors received no specific funding for this work.
References
- 1.World Health Organization. Global tuberculosis report 2020.
- 2.Simmons JD, Stein CM, Seshadri C, Campo M, Alter G, Fortune S, et al. Immunological mechanisms of human resistance to persistent Mycobacterium tuberculosis infection. Nat Rev Immunol. 2018;18(9):575–89. Epub 2018/06/14. 10.1038/s41577-018-0025-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen A, Mathiasen VD, Schon T, Wejse C. The global prevalence of latent tuberculosis: a systematic review and meta-analysis. Eur Respir J. 2019;54(3). Artn 1900655 10.1183/13993003.00655-2019 WOS:000489163100035. [DOI] [PubMed] [Google Scholar]
- 4.Ahmad S. Pathogenesis, immunology, and diagnosis of latent Mycobacterium tuberculosis infection. Clin Dev Immunol. 2011;2011:814943. Epub 2011/01/15. 10.1155/2011/814943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Behr MA, Edelstein PH, Ramakrishnan L. Revisiting the timetable of tuberculosis. BMJ. 2018;362:k2738. Epub 2018/08/25. 10.1136/bmj.k2738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Melander RJ, Zurawski DV, Melander C. Narrow-spectrum antibacterial agents. Medchemcomm 2018;9(1):12–21. 10.1039/C7MD00528H WOS:000423465800002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Forrest GN, Tamura K. Rifampin combination therapy for nonmycobacterial infections. Clin Microbiol Rev. 2010;23(1):14–34. Epub 2010/01/13. 10.1128/CMR.00034-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Naidoo CC, Nyawo GR, Wu BG, Walzl G, Warren RM, Segal LN, et al. The microbiome and tuberculosis: state of the art, potential applications, and defining the clinical research agenda. Lancet Respir Med. 2019;7(10):892–906. Epub 2019/03/27. 10.1016/S2213-2600(18)30501-0 . [DOI] [PubMed] [Google Scholar]
- 9.Hawn TR, Matheson AI, Maley SN, Vandal O. Host-Directed Therapeutics for Tuberculosis: Can We Harness the Host? Microbiol Mol Biol R. 2013;77(4):608–27. 10.1128/MMBR.00032-13 WOS:000327945100005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Langdon A, Crook N, Dantas G. The effects of antibiotics on the microbiome throughout development and alternative approaches for therapeutic modulation. Genome Med. 2016;8(1):39. Epub 2016/04/15. 10.1186/s13073-016-0294-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Namasivayam S, Maiga M, Yuan W, Thovarai V, Costa DL, Mittereder LR, et al. Longitudinal profiling reveals a persistent intestinal dysbiosis triggered by conventional anti-tuberculosis therapy. Microbiome. 2017;5(1):71. Epub 2017/07/08. 10.1186/s40168-017-0286-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wipperman MF, Fitzgerald DW, Juste MAJ, Taur Y, Namasivayam S, Sher A, et al. Antibiotic treatment for Tuberculosis induces a profound dysbiosis of the microbiome that persists long after therapy is completed. Sci Rep. 2017;7(1):10767. Epub 2017/09/09. 10.1038/s41598-017-10346-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang J, Xiong K, Zhao S, Zhang C, Zhang J, Xu L, et al. Long-Term Effects of Multi-Drug-Resistant Tuberculosis Treatment on Gut Microbiota and Its Health Consequences. Front Microbiol. 2020;11:53. Epub 2020/02/23. 10.3389/fmicb.2020.00053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.du Preez K, Mandalakas AM, Kirchner HL, Grewal HM, Schaaf HS, van Wyk SS, et al. Environmental tobacco smoke exposure increases Mycobacterium tuberculosis infection risk in children. Int J Tuberc Lung Dis. 2011;15(11):1490–6, i. Epub 2011/10/20. 10.5588/ijtld.10.0759 . [DOI] [PubMed] [Google Scholar]
- 15.Narasimhan P, Wood J, MacIntyre CR, Mathai D. Risk Factors for Tuberculosis. Pulm Med. 2013;2013. Artn 82893910.1155/2013/828939. WOS:000216516500029. 10.1155/2013/828939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rehm J, Samokhvalov AV, Neuman MG, Room R, Parry C, Lonnroth K, et al. The association between alcohol use, alcohol use disorders and tuberculosis (TB). A systematic review. BMC Public Health. 2009;9. Artn 45010. 1186/1471-2458-9-450. WOS:000273039500001. 10.1186/1471-2458-9-450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Havlir DV, Getahun H, Sanne I, Nunn P. Opportunities and challenges for HIV care in overlapping HIV and TB epidemics. JAMA. 2008;300(4):423–30. Epub 2008/07/24. 10.1001/jama.300.4.423 . [DOI] [PubMed] [Google Scholar]
- 18.Wu J, Liu W, He L, Huang F, Chen J, Cui P, et al. Sputum microbiota associated with new, recurrent and treatment failure tuberculosis. PLoS ONE. 2013;8(12):e83445. Epub 2013/12/19. 10.1371/journal.pone.0083445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Budden KF, Gellatly SL, Wood DLA, Cooper MA, Morrison M, Hugenholtz P, et al. Emerging pathogenic links between microbiota and the gut-lung axis. Nat Rev Microbiol. 2017;15 (1):55–63. 10.1038/nrmicro.2016.142 WOS:000391345200008. [DOI] [PubMed] [Google Scholar]
- 20.Becattini S, Taur Y, Pamer EG. Antibiotic-Induced Changes in the Intestinal Microbiota and Disease. Trends Mol Med. 2016;22 (6):458–78. 10.1016/j.molmed.2016.04.003 WOS:000377728100005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jernberg C, Lofmark S, Edlund C, Jansson JK. Long-term ecological impacts of antibiotic administration on the human intestinal microbiota. ISME J. 2007;1 (1):56–66. 10.1038/ismej.2007.3 WOS:000249215800009. [DOI] [PubMed] [Google Scholar]
- 22.Org E, Parks BW, Joo JW, Emert B, Schwartzman W, Kang EY, et al. Genetic and environmental control of host-gut microbiota interactions. Genome Res. 2015;25(10):1558–69. Epub 2015/08/12. 10.1101/gr.194118.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ottman N, Smidt H, de Vos WM, Belzer C. The function of our microbiota: who is out there and what do they do? Front Cell Infect Microbiol. 2012;2:104. Epub 2012/08/25. 10.3389/fcimb.2012.00104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grenham S, Clarke G, Cryan JF, Dinan TG. Brain-gut-microbe communication in health and disease. Front Physiol. 2011;2:94. Epub 2011/12/14. 10.3389/fphys.2011.00094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim H, Kim S, Jung S. Instruction of microbiome taxonomic profiling based on 16S rRNA sequencing. J Microbiol. 2020;58 (3):193–205. 10.1007/s12275-020-9556-y WOS:000519389300003. [DOI] [PubMed] [Google Scholar]
- 26.NIH Human Microbiome Portfolio Analysis Team. A review of 10 years of human microbiome research activities at the US National Institutes of Health, Fiscal Years 2007–2016. Microbiome. 2019;7(1):31. Epub 2019/02/28. 10.1186/s40168-019-0620-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morgan XC, Huttenhower C. Chapter 12: Human microbiome analysis. PLoS Comput Biol. 2012;8(12):e1002808. Epub 2013/01/10. 10.1371/journal.pcbi.1002808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Borrel G, Brugere JF, Gribaldo S, Schmitz RA, Moissl-Eichinger C. The host-associated archaeome. Nat Rev Microbiol 2020;18(11):622–36. Epub 2020/07/22. 10.1038/s41579-020-0407-y . [DOI] [PubMed] [Google Scholar]
- 29.Chin VK, Yong VC, Chong PP, Amin Nordin S, Basir R, Abdullah M. Mycobiome in the Gut: A Multiperspective Review. Mediat Inflamm. 2020;2020:9560684. Epub 2020/04/24. 10.1155/2020/9560684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Malla MA, Dubey A, Kumar A, Yadav S, Hashem A, Abd Allah EF. Exploring the Human Microbiome: The Potential Future Role of Next-Generation Sequencing in Disease Diagnosis and Treatment. Front Immunol. 2018;9:2868. Epub 2019/01/23. 10.3389/fimmu.2018.02868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Santiago-Rodriguez TM, Hollister EB. Human Virome and Disease: High-Throughput Sequencing for Virus Discovery, Identification of Phage-Bacteria Dysbiosis and Development of Therapeutic Approaches with Emphasis on the Human Gut. Viruses. 2019;11(7). Epub 2019/07/22. 10.3390/v11070656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Foster KR, Schluter J, Coyte KZ, Rakoff-Nahoum S. The evolution of the host microbiome as an ecosystem on a leash. Nature. 2017;548(7665):43–51. Epub 2017/08/05. 10.1038/nature23292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spor A, Koren O, Ley R. Unravelling the effects of the environment and host genotype on the gut microbiome. Nat Rev Microbiol 2011;9(4):279–90. Epub 2011/03/17. 10.1038/nrmicro2540 . [DOI] [PubMed] [Google Scholar]
- 34.Bernard G, Pathmanathan JS, Lannes R, Lopez P, Bapteste E. Microbial Dark Matter Investigations: How Microbial Studies Transform Biological Knowledge and Empirically Sketch a Logic of Scientific Discovery. Genome Biol Evol. 2018;10 (3):707–15. 10.1093/gbe/evy031 WOS:000429483700001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Faner R, Sibila O, Agusti A, Bernasconi E, Chalmers JD, Huffnagle GB, et al. The microbiome in respiratory medicine: current challenges and future perspectives. Eur Respir J. 2017;49(4). Artn 1602086 10.1183/13993003.02086-2016 WOS:000400342500020. [DOI] [PubMed] [Google Scholar]
- 36.Pickard JM, Zeng MY, Caruso R, Nunez G. Gut microbiota: Role in pathogen colonization, immune responses, and inflammatory disease. Immunol Rev. 2017;279(1):70–89. Epub 2017/09/01. 10.1111/imr.12567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blaser MJ. The theory of disappearing microbiota and the epidemics of chronic diseases. Nat Rev Immunol. 2017;17(8):461–3. Epub 2017/07/28. 10.1038/nri.2017.77 . [DOI] [PubMed] [Google Scholar]
- 38.Wu GD, Compher C, Chen EZ, Smith SA, Shah RD, Bittinger K, et al. Comparative metabolomics in vegans and omnivores reveal constraints on diet-dependent gut microbiota metabolite production. Gut. 2016;65(1):63–72. Epub 2014/11/29. 10.1136/gutjnl-2014-308209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang R, Ju Z, Zhou PK. A gut dysbiotic microbiota-based hypothesis of human-to-human transmission of non-communicable diseases. Sci Total Environ. 2020;745:141030. Epub 2020/07/30. 10.1016/j.scitotenv.2020.141030 . [DOI] [PubMed] [Google Scholar]
- 40.Wilkins LJ, Monga M, Miller AW. Defining Dysbiosis for a Cluster of Chronic Diseases. Sci Rep. 2019;9(1):12918. Epub 2019/09/11. 10.1038/s41598-019-49452-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wood MR, Yu EA, Mehta S. The Human Microbiome in the Fight Against Tuberculosis. Am J Trop Med Hyg. 2017;96(6):1274–84. Epub 2017/07/19. 10.4269/ajtmh.16-0581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Namasivayam S, Sher A, Glickman MS, Wipperman MF. The Microbiome and Tuberculosis: Early Evidence for Cross Talk. MBio. 2018;9(5). 10.1128/mBio.01420-18 WOS:000449472200013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hong BY, Maulen NP, Adami AJ, Granados H, Balcells ME, Cervantes J. Microbiome Changes during Tuberculosis and Antituberculous Therapy. Clin Microbiol Rev. 2016;29(4):915–26. Epub 2016/09/10. 10.1128/CMR.00096-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eribo OA, du Plessis N, Ozturk M, Guler R, Walzl G, Chegou NN. The gut microbiome in tuberculosis susceptibility and treatment response: guilty or not guilty? Cell Mol Life Sci. 2020;77(8):1497–509. Epub 2019/11/16. 10.1007/s00018-019-03370-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Luo M, Liu Y, Wu P, Luo DX, Sun Q, Zheng H, et al. Alternation of Gut Microbiota in Patients with Pulmonary Tuberculosis. Front Physiol. 2017;8:822. Epub 2017/12/06. 10.3389/fphys.2017.00822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li W, Zhu Y, Liao Q, Wang Z, Wan C. Characterization of gut microbiota in children with pulmonary tuberculosis. BMC Pediatr. 2019;19(1):445. Epub 2019/11/19. 10.1186/s12887-019-1782-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rinninella E, Raoul P, Cintoni M, Franceschi F, Miggiano GAD, Gasbarrini A, et al. What is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms. 2019;7(1). ARTN 14 10.3390/microorganisms7010014. WOS:000457122800004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Louis P, Flint HJ. Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiol Lett. 2009;294(1):1–8. Epub 2009/02/19. 10.1111/j.1574-6968.2009.01514.x . [DOI] [PubMed] [Google Scholar]
- 49.Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science. 2011;331(6015):337–41. Epub 2011/01/06. 10.1126/science.1198469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Atarashi K, Tanoue T, Oshima K, Suda W, Nagano Y, Nishikawa H, et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature. 2013;500(7461):232–6. Epub 2013/07/12. 10.1038/nature12331 . [DOI] [PubMed] [Google Scholar]
- 51.Sokol H, Pigneur B, Watterlot L, Lakhdari O, Bermudez-Humaran LG, Gratadoux JJ, et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. P Natl Acad Sci USA. 2008;105(43):16731–6. 10.1073/pnas.0804812105 WOS:000260913500059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ward B. Chapter 11—Bacterial Energy Metabolism. In: Tang Y-W SM, Liu D, Poxton I, Schwartzman J, editor. Molecular Medical Microbiology (Second Edition): Boston: Academic Press,; 2015. p. 201–33. [Google Scholar]
- 53.Winglee K, Eloe-Fadrosh E, Gupta S, Guo H, Fraser C, Bishai W. Aerosol Mycobacterium tuberculosis infection causes rapid loss of diversity in gut microbiota. PLoS ONE. 2014;9(5):e97048. Epub 2014/05/14. 10.1371/journal.pone.0097048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cui Z, Zhou Y, Li H, Zhang Y, Zhang S, Tang S, et al. Complex sputum microbial composition in patients with pulmonary tuberculosis. BMC Microbiol. 2012;12:276. Epub 2012/11/28. 10.1186/1471-2180-12-276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cheung MK, Lam WY, Fung WYW, Law PTW, Au CH, Nong WY, et al. Sputum Microbiota in Tuberculosis as Revealed by 16S rRNA Pyrosequencing. PLoS ONE. 2013;8(1). ARTN e54574 10.1371/journal.pone.0054574 WOS:000314023600075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Goddard AF, Staudinger BJ, Dowd SE, Joshi-Datar A, Wolcott RD, Aitken ML, et al. Direct sampling of cystic fibrosis lungs indicates that DNA-based analyses of upper-airway specimens can misrepresent lung microbiota. P Natl Acad Sci USA. 2012;109(34):13769–74. 10.1073/pnas.1107435109 WOS:000308085200065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hogan DA, Willger SD, Dolben EL, Hampton TH, Stanton BA, Morrison HG, et al. Analysis of Lung Microbiota in Bronchoalveolar Lavage, Protected Brush and Sputum Samples from Subjects with Mild-To-Moderate Cystic Fibrosis Lung Disease. PLoS ONE. 2016;11(3):e0149998. Epub 2016/03/05. 10.1371/journal.pone.0149998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vazquez-Perez JA, Carrillo CO, Iniguez-Garcia MA, Romero-Espinoza I, Marquez-Garcia JE, Falcon LI, et al. Alveolar microbiota profile in patients with human pulmonary tuberculosis and interstitial pneumonia. Microb Pathog. 2020;139:103851. Epub 2019/11/13. 10.1016/j.micpath.2019.103851 . [DOI] [PubMed] [Google Scholar]
- 59.Hu Y, Feng Y, Wu J, Liu F, Zhang Z, Hao Y, et al. The Gut Microbiome Signatures Discriminate Healthy From Pulmonary Tuberculosis Patients. Front Cell Infect Microbiol. 2019;9:90. Epub 2019/04/20. 10.3389/fcimb.2019.00090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cadena AM, Ma Y, Ding T, Bryant M, Maiello P, Geber A, et al. Profiling the airway in the macaque model of tuberculosis reveals variable microbial dysbiosis and alteration of community structure. Microbiome. 2018;6(1):180. Epub 2018/10/12. 10.1186/s40168-018-0560-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Walter J, Armet AM, Finlay BB, Shanahan F. Establishing or Exaggerating Causality for the Gut Microbiome: Lessons from Human Microbiota-Associated Rodents. Cell. 2020;180 (2):221–32. 10.1016/j.cell.2019.12.025 WOS:000508908200006. [DOI] [PubMed] [Google Scholar]
- 62.Cani PD. Human gut microbiome: hopes, threats and promises. Gut. 2018;67(9):1716–25. Epub 2018/06/24. 10.1136/gutjnl-2018-316723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Quigley EMM, Gajula P. Recent advances in modulating the microbiome. F1000Res. 2020;9. Epub 2020/02/13. 10.12688/f1000research.20204.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sperber AD, Gwee KA, Hungin AP, Corazziari E, Fukudo S, Gerson C, et al. Conducting multinational, cross-cultural research in the functional gastrointestinal disorders: issues and recommendations. A Rome Foundation working team report. Aliment Pharmacol Ther. 2014;40(9):1094–102. Epub 2014/09/02. 10.1111/apt.12942 . [DOI] [PubMed] [Google Scholar]
- 65.Clark SE. Commensal bacteria in the upper respiratory tract regulate susceptibility to infection. Curr Opin Immunol. 2020;66:42–9. Epub 2020/05/18. 10.1016/j.coi.2020.03.010 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Carney SM, Clemente JC, Cox MJ, Dickson RP, Huang YJ, Kitsios GD, et al. Methods in Lung Microbiome Research. Am J Resp Cell Mol. 2020;62(3):283–99. 10.1165/rcmb.2019-0273TR WOS:000518195100008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bachmann NL, Rockett RJ, Timms VJ, Sintchenko V. Advances in Clinical Sample Preparation for Identification and Characterization of Bacterial Pathogens Using Metagenomics. Front Public Health. 2018;6. ARTN 3633389/fpubh.2018.00363. WOS:000453243000001. 10.3389/fpubh.2018.00363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lange C, Dheda K, Chesov D, Mandalakas AM, Udwadia Z, Horsburgh CR. Management of drug-resistant tuberculosis. Lancet. 2019;394(10202):953–66. 10.1016/S0140-6736(19)31882-3 WOS:000485840600027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Buffie CG, Pamer EG. Microbiota-mediated colonization resistance against intestinal pathogens. Nat Rev Immunol. 2013;13(11):790–801. Epub 2013/10/08. 10.1038/nri3535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Van Rie A, Warren R, Richardson M, Victor TC, Gie RP, Enarson DA, et al. Exogenous reinfection as a cause of recurrent tuberculosis after curative treatment. New Engl J Med. 1999;341(16):1174–9. 10.1056/NEJM199910143411602 WOS:000083087400002. [DOI] [PubMed] [Google Scholar]
- 71.Verver S, Warren RM, Beyers N, Richardson M, van der Spuy GD, Borgdorff MW, et al. Rate of reinfection tuberculosis after successful treatment is higher than rate of new tuberculosis. Am J Resp Crit Care. 2005;171(12):1430–5. 10.1164/rccm.200409-1200OC WOS:000229711200017. [DOI] [PubMed] [Google Scholar]
- 72.Scriba TJ, Carpenter C, Pro SC, Sidney J, Musvosvi M, Rozot V, et al. Differential Recognition of Mycobacterium tuberculosis-Specific Epitopes as a Function of Tuberculosis Disease History. Am J Respir Crit Care Med. 2017;196(6):772–81. Epub 2017/08/02. 10.1164/rccm.201706-1208OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Khan N, Mendonca L, Dhariwal A, Fontes G, Menzies D, Xia J, et al. Intestinal dysbiosis compromises alveolar macrophage immunity to Mycobacterium tuberculosis. Mucosal Immunol. 2019;12(3):772–83. Epub 2019/02/21. 10.1038/s41385-019-0147-3 . [DOI] [PubMed] [Google Scholar]
- 74.Blacher E, Levy M, Tatirovsky E, Elinav E. Microbiome-Modulated Metabolites at the Interface of Host Immunity. J Immunol. 2017;198 (2):572–80. 10.4049/jimmunol.1601247 WOS:000392405000004. [DOI] [PubMed] [Google Scholar]
- 75.Brown RL, Sequeira RP, Clarke TB. The microbiota protects against respiratory infection via GM-CSF signaling. Nat Commun. 2017;8(1):1512. Epub 2017/11/17. 10.1038/s41467-017-01803-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cohen SB, Gern BH, Delahaye JL, Adams KN, Plumlee CR, Winkler JK, et al. Alveolar Macrophages Provide an Early Mycobacterium tuberculosis Niche and Initiate Dissemination. Cell Host Microbe. 2018;24(3):439–+. 10.1016/j.chom.2018.08.001 WOS:000446887200015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ryndak MB, Leal S. Mycobacterium tuberculosis Primary Infection and Dissemination: A Critical Role for Alveolar Epithelial Cells. Front Cell Infect Mi. 2019;9. ARTN 299 10.3389/fcimb.2019.00299 WOS:000482048000001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Harriff MJ, Karamooz E, Burr A, Grant WF, Canfield ET, Sorensen ML, et al. Endosomal MR1 Trafficking Plays a Key Role in Presentation of Mycobacterium tuberculosis Ligands to MAIT Cells. PLoS Pathog. 2016;12(3). ARTN e 10.1371/journal.ppat.1005524 WOS:000378154800039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Reuschl AK, Edwards MR, Parker R, Connell DW, Hoang L, Halliday A, et al. Innate activation of human primary epithelial cells broadens the host response to Mycobacterium tuberculosis in the airways. PLoS Pathog. 2017;13(9). ARTN e 10.1371/journal.ppat.1006577 WOS:000411968300021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fine KL, Metcalfe MG, White E, Virji M, Karls RK, Quinn FD. Involvement of the autophagy pathway in trafficking of Mycobacterium tuberculosis bacilli through cultured human type II epithelial cells. Cell Microbiol. 2012;14(9):1402–14. 10.1111/j.1462-5822.2012.01804.x WOS:000307844700008. [DOI] [PubMed] [Google Scholar]
- 81.Corleis B, Dorhoi A. Early dynamics of innate immunity during pulmonary tuberculosis. Immunol Lett. 2020;221:56–60. 10.1016/j.imlet.2020.02.010 WOS:000527910600008. [DOI] [PubMed] [Google Scholar]
- 82.Ostaff MJ, Stange EF, Wehkamp J. Antimicrobial peptides and gut microbiota in homeostasis and pathology. EMBO Mol Med. 2013;5(10):1465–83. Epub 2013/09/17. 10.1002/emmm.201201773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sommer F, Backhed F. The gut microbiota—masters of host development and physiology. Nat Rev Microbiol. 2013;11(4):227–38. Epub 2013/02/26. 10.1038/nrmicro2974 . [DOI] [PubMed] [Google Scholar]
- 84.Burgueno JF, Abreu MT. Epithelial Toll-like receptors and their role in gut homeostasis and disease. Nat Rev Gastroenterol Hepatol. 2020;17(5):263–78. Epub 2020/02/28. 10.1038/s41575-019-0261-4 . [DOI] [PubMed] [Google Scholar]
- 85.Kayama H, Takeda K. Manipulation of epithelial integrity and mucosal immunity by host and microbiota-derived metabolites. Eur J Immunol. 2020;50(7):921–31. Epub 2020/06/09. 10.1002/eji.201948478 . [DOI] [PubMed] [Google Scholar]
- 86.Antunes KH, Fachi JL, de Paula R, da Silva EF, Pral LP, dos Santos AA, et al. Microbiota-derived acetate protects against respiratory syncytial virus infection through a GPR43-type 1 interferon response. Nat Commun. 2019;10. ARTN 10.1038/s41467-019-11152-6 WOS:000476721900021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rivas-Santiago B, Serrano CJ, Enciso-Moreno JA. Susceptibility to Infectious Diseases Based on Antimicrobial Peptide Production. Infect Immun. 2009;77(11):4690–5. 10.1128/IAI.01515-08 WOS:000270885800001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sow FB, Nandakumar S, Velu V, Kellar KL, Schlesinger LS, Amara RR, et al. Mycobacterium tuberculosis components stimulate production of the antimicrobial peptide hepcidin. Tuberculosis (Edinb). 2011;91(4):314–21. Epub 2011/04/13. 10.1016/j.tube.2011.03.003 . [DOI] [PubMed] [Google Scholar]
- 89.Teitelbaum R, Schubert W, Gunther L, Kress Y, Macaluso F, Pollard JW, et al. The M cell as a portal of entry to the lung for the bacterial pathogen Mycobacterium tuberculosis. Immunity. 1999;10(6):641–50. 10.1016/s1074-7613(00)80063-1 WOS:000081209600002. [DOI] [PubMed] [Google Scholar]
- 90.Nair VR, Franco LH, Zacharia VM, Khan HS, Stamm CE, You W, et al. Microfold Cells Actively Translocate Mycobacterium tuberculosis to Initiate Infection. Cell Rep. 2016;16(5):1253–8. Epub 2016/07/28. 10.1016/j.celrep.2016.06.080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Khan HS, Nair VR, Ruhl CR, Alvarez-Arguedas S, Rendiz JLG, Franco LH, et al. Identification of scavenger receptor B1 as the airway microfold cell receptor for Mycobacterium tuberculosis. elife. 2020;9. ARTN e52551 10.7554/eLife.52551 WOS:000519908300001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Donaldson DS, Pollock J, Vohra P, Stevens MP, Mabbott NA. Microbial Stimulation Reverses the Age-Related Decline in M Cells in Aged Mice. Iscience. 2020;23(6). ARTN 10.1016/j.isci.2020.101147 WOS:000548210600005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Caesar R, Reigstad CS, Backhed HK, Reinhardt C, Ketonen M, Lunden GO, et al. Gut-derived lipopolysaccharide augments adipose macrophage accumulation but is not essential for impaired glucose or insulin tolerance in mice. Gut. 2012;61(12):1701–7. Epub 2012/04/27. 10.1136/gutjnl-2011-301689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57(6):1470–81. Epub 2008/02/29. 10.2337/db07-1403 . [DOI] [PubMed] [Google Scholar]
- 95.Cani PD, Possemiers S, Van de Wiele T, Guiot Y, Everard A, Rottier O, et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut. 2009;58(8):1091–103. Epub 2009/02/26. 10.1136/gut.2008.165886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang J, Chen WD, Wang YD. The Relationship Between Gut Microbiota and Inflammatory Diseases: The Role of Macrophages. Front Microbiol. 2020;11:1065. Epub 2020/06/26. 10.3389/fmicb.2020.01065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Barroso EC, Pinheiro VG, Facanha MC, Carvalho MR, Moura ME, Campelo CL, et al. Serum concentrations of rifampin, isoniazid, and intestinal absorption, permeability in patients with multidrug resistant tuberculosis. Am J Trop Med Hyg. 2009;81(2):322–9. Epub 2009/07/29. . [PubMed] [Google Scholar]
- 98.Facanha MC, Gondim AM, Pinheiro VG, Barroso EC, Peloquin CA, Guerrant RL, et al. Intestinal barrier function and serum concentrations of rifampin, isoniazid and pyrazinamide in patients with pulmonary tuberculosis. Braz J Infect Dis. 2009;13(3):210–7. Epub 2010/03/02. 10.1590/s1413-86702009000300011 . [DOI] [PubMed] [Google Scholar]
- 99.Goovaerts O, Jennes W, Massinga-Loembe M, Ceulemans A, Worodria W, Mayanja-Kizza H, et al. LPS-binding protein and IL-6 mark paradoxical tuberculosis immune reconstitution inflammatory syndrome in HIV patients. PLoS ONE. 2013;8(11):e81856. Epub 2013/12/07. 10.1371/journal.pone.0081856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pinheiro VG, Ramos LM, Monteiro HS, Barroso EC, Bushen OY, Facanha MC, et al. Intestinal permeability and malabsorption of rifampin and isoniazid in active pulmonary tuberculosis. Braz J Infect Dis. 2006;10(6):374–9. Epub 2007/04/11. 10.1590/s1413-86702006000600003 . [DOI] [PubMed] [Google Scholar]
- 101.Vinnard C, Ravimohan S, Tamuhla N, Pasipanodya J, Srivastava S, Modongo C, et al. Markers of gut dysfunction do not explain low rifampicin bioavailability in HIV-associated TB. J Antimicrob Chemother. 2017;72(7):2020–7. Epub 2017/05/05. 10.1093/jac/dkx111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Upadhyay S, Mittal E, Philips JA. Tuberculosis and the art of macrophage manipulation. Pathog Dis. 2018;76(4). Epub 2018/05/16. 10.1093/femspd/fty037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Siegel SJ, Tamashiro E, Weiser JN. Clearance of Pneumococcal Colonization in Infants Is Delayed through Altered Macrophage Trafficking. PLoS Pathog. 2015;11(6). ARTN e1005004 10.1371/journal.ppat.1005004 WOS:000357400900060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ghadimi D, de Vrese M, Heller KJ, Schrezenmeir J. Lactic acid bacteria enhance autophagic ability of mononuclear phagocytes by increasing Th1 autophagy-promoting cytokine (IFN-gamma) and nitric oxide (NO) levels and reducing Th2 autophagy-restraining cytokines (IL-4 and IL-13) in response to Mycobacterium tuberculosis antigen. Int Immunopharmacol. 2010;10(6):694–706. Epub 2010/04/13. 10.1016/j.intimp.2010.03.014 . [DOI] [PubMed] [Google Scholar]
- 105.Constantinides MG. Interactions between the microbiota and innate and innate-like lymphocytes. J Leukoc Biol. 2018;103 (3):409–19. 10.1002/JLB.3RI0917-378R WOS:000426256900005. [DOI] [PubMed] [Google Scholar]
- 106.den Besten G, van Eunen K, Groen AK, Venema K, Reijngoud DJ, Bakker BM. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res. 2013;54(9):2325–40. Epub 2013/07/04. 10.1194/jlr.R036012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Correa RO, Fachi JL, Vieira A, Sato FT, Vinolo MAR. Regulation of immune cell function by short-chain fatty acids. Clin Transl Immunol. 2016;5. UNSP e73 10.1038/cti.2016.17 WOS:000379340300007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chun E, Lavoie S, Fonseca-Pereira D, Bae S, Michaud M, Hoveyda HR, et al. Metabolite-Sensing Receptor Ffar2 Regulates Colonic Group 3 Innate Lymphoid Cells and Gut Immunity. Immunity. 2019;51(5):871–+. 10.1016/j.immuni.2019.09.014 WOS:000497811300011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ardain A, Porterfield JZ, Kloverpris HN, Leslie A. Type 3 ILCs in Lung Disease. Front Immunol. 2019;10. ARTN 92 10.3389/fimmu.2019.00092 WOS:000457116600001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.O’Garra A, Redford PS, McNab FW, Bloom CI, Wilkinson RJ, Berry MPR. The Immune Response in Tuberculosis. Annu Rev Immunol. 2013;31:475–527. 10.1146/annurev-immunol-032712-095939 WOS:000321861600017. [DOI] [PubMed] [Google Scholar]
- 111.Vivier E, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, et al. Innate Lymphoid Cells: 10 Years On. Cell. 2018;174(5):1054–66. Epub 2018/08/25. 10.1016/j.cell.2018.07.017 . [DOI] [PubMed] [Google Scholar]
- 112.Schierloh P, Yokobori N, Alemán M, Musella RM, Beigier-Bompadre M, Saab MA, et al. Increased susceptibility to apoptosis of CD56dimCD16+ NK cells induces the enrichment of IFN-gamma-producing CD56bright cells in tuberculous pleurisy. J Immunol. 2005. November 15;175(10):6852–60. 10.4049/jimmunol.175.10.6852 . [DOI] [PubMed] [Google Scholar]
- 113.Chowdhury RR, Vallania F, Yang QT, Angel CJL, Darboe F, Penn-Nicholson A, et al. A multi-cohort study of the immune factors associated with M. tuberculosis infection outcomes. Nature. 2018;560(7720):644–+. 10.1038/s41586-018-0439-x WOS:000443218600050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Esaulova E, Das S, Singh DK, Choreno-Parra JA, Swain A, Arthur L, et al. The immune landscape in tuberculosis reveals populations linked to disease and latency. Cell Host Microbe. 2020. Epub 2020/12/20. 10.1016/j.chom.2020.11.013 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Roy S, Barnes PF, Garg A, Wu S, Cosman D, Vankayalapati R. NK cells lyse T regulatory cells that expand in response to an intracellular pathogen. J Immunol. 2008;180(3):1729–36. Epub 2008/01/23. 10.4049/jimmunol.180.3.1729 . [DOI] [PubMed] [Google Scholar]
- 116.Vankayalapati R, Garg A, Porgador A, Griffith DE, Klucar P, Safi H, et al. Role of NK cell-activating receptors and their ligands in the lysis of mononuclear phagocytes infected with an intracellular bacterium. J Immunol. 2005;175(7):4611–7. Epub 2005/09/24. 10.4049/jimmunol.175.7.4611 . [DOI] [PubMed] [Google Scholar]
- 117.Vankayalapati R, Wizel B, Weis SE, Safi H, Lakey DL, Mandelboim O, et al. The NKp46 receptor contributes to NK cell lysis of mononuclear phagocytes infected with an intracellular bacterium. J Immunol. 2002;168(7):3451–7. Epub 2002/03/22. 10.4049/jimmunol.168.7.3451 . [DOI] [PubMed] [Google Scholar]
- 118.Ardain A, Domingo-Gonzalez R, Das S, Kazer SW, Howard NC, Singh A, et al. Group 3 innate lymphoid cells mediate early protective immunity against tuberculosis. Nature. 2019;570(7762):528–32. Epub 2019/06/07. 10.1038/s41586-019-1276-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Steigler P, Daniels NJ, McCulloch TR, Ryder BM, Sandford SK, Kirman JR. BCG vaccination drives accumulation and effector function of innate lymphoid cells in murine lungs. Immunol Cell Biol. 2018;96(4):379–89. Epub 2018/01/25. 10.1111/imcb.12007 . [DOI] [PubMed] [Google Scholar]
- 120.Feng CG, Kaviratne M, Rothfuchs AG, Cheever A, Hieny S, Young HA, et al. NK cell-derived IFN-gamma differentially regulates innate resistance and neutrophil response in T cell-deficient hosts infected with Mycobacterium tuberculosis. J Immunol. 2006;177(10):7086–93. Epub 2006/11/04. 10.4049/jimmunol.177.10.7086 . [DOI] [PubMed] [Google Scholar]
- 121.Marin ND, Dunlap MD, Kaushal D, Khader SA. Friend or Foe: The Protective and Pathological Roles of Inducible Bronchus-Associated Lymphoid Tissue in Pulmonary Diseases. J Immunol. 2019;202(9):2519–26. Epub 2019/04/24. 10.4049/jimmunol.1801135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Moura-Alves P, Fae K, Houthuys E, Dorhoi A, Kreuchwig A, Furkert J, et al. AhR sensing of bacterial pigments regulates antibacterial defence. Nature. 2014;512(7515):387–92. Epub 2014/08/15. 10.1038/nature13684 . [DOI] [PubMed] [Google Scholar]
- 123.Puyskens A, Stinn A, van der Vaart M, Kreuchwig A, Protze J, Pei G, et al. Aryl Hydrocarbon Receptor Modulation by Tuberculosis Drugs Impairs Host Defense and Treatment Outcomes. Cell Host Microbe. 2020;27(2):238–+. 10.1016/j.chom.2019.12.005 WOS:000514177500014. [DOI] [PubMed] [Google Scholar]
- 124.Qiu J, Heller JJ, Guo XH, Chen ZME, Fish K, Fu YX, et al. The Aryl Hydrocarbon Receptor Regulates Gut Immunity through Modulation of Innate Lymphoid Cells. Immunity. 2012;36 (1):92–104. 10.1016/j.immuni.2011.11.011 WOS:000299766000013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zelante T, Iannitti RG, Cunha C, De Luca A, Giovannini G, Pieraccini G, et al. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity. 2013;39(2):372–85. Epub 2013/08/27. 10.1016/j.immuni.2013.08.003 . [DOI] [PubMed] [Google Scholar]
- 126.Tripathi D, Radhakrishnan RK, Sivangala Thandi R, Paidipally P, Devalraju KP, Neela VSK, et al. IL-22 produced by type 3 innate lymphoid cells (ILC3s) reduces the mortality of type 2 diabetes mellitus (T2DM) mice infected with Mycobacterium tuberculosis. PLoS Pathog. 2019;15(12):e1008140. Epub 2019/12/07. 10.1371/journal.ppat.1008140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Treerat P, Prince O, Cruz-Lagunas A, Munoz-Torrico M, Salazar-Lezama MA, Selman M, et al. Novel role for IL-22 in protection during chronic Mycobacterium tuberculosis HN878 infection. Mucosal Immunol. 2017;10(4):1069–81. Epub 2017/03/02. 10.1038/mi.2017.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Park E, Patel S, Wang Q, Andhey P, Zaitsev K, Porter S, et al. Toxoplasma gondii infection drives conversion of NK cells into ILC1-like cells. elife. 2019;8. Epub 2019/08/09. 10.7554/eLife.47605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gao Y, Souza-Fonseca-Guimaraes F, Bald T, Ng SS, Young A, Ngiow SF, et al. Tumor immunoevasion by the conversion of effector NK cells into type 1 innate lymphoid cells. Nat Immunol. 2017;18(9):1004–15. Epub 2017/08/02. 10.1038/ni.3800 . [DOI] [PubMed] [Google Scholar]
- 130.Cai Y, Dai YC, Wang YJ, Yang QQ, Guo JB, Wei CL, et al. Single-cell transcriptomics of blood reveals a natural killer cell subset depletion in tuberculosis. EBioMedicine. 2020;53. 10.1016/j.ebiom.2020.102686 WOS:000523581700031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Harris LD, Khayumbi J, Ongalo J, Sasser LE, Tonui J, Campbell A, et al. Distinct Human NK Cell Phenotypes and Functional Responses to Mycobacterium tuberculosis in Adults From TB Endemic and Non-endemic Regions. Front Cell Infect Microbiol. 2020;10:120. Epub 2020/04/09. 10.3389/fcimb.2020.00120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Treiner E, Duban L, Bahram S, Radosavljevic M, Wanner V, Tilloy F, et al. Selection of evolutionarily conserved mucosal-associated invariant T cells by MR1. Nature. 2003;422 (6928):164–9. 10.1038/nature01433 WOS:000181488900045. [DOI] [PubMed] [Google Scholar]
- 133.Koay HF, Gherardin NA, Enders A, Loh L, Mackay LK, Almeida CF, et al. A three-stage intrathymic development pathway for the mucosal-associated invariant T cell lineage. Nat Immunol. 2016;17(11):1300–11. 10.1038/ni.3565 WOS:000386193200010. [DOI] [PubMed] [Google Scholar]
- 134.Constantinides MG, Link VM, Tamoutounour S, Wong AC, Perez-Chaparro PJ, Han SJ, et al. MAIT cells are imprinted by the microbiota in early life and promote tissue repair. Science. 2019;366(6464):445–+. ARTN eaax6624 10.1126/science.aax6624 WOS:000493177900038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Legoux F, Bellet D, Daviaud C, El Morr Y, Darbois A, Niort K, et al. Microbial metabolites control the thymic development of mucosal-associated invariant T cells. Science. 2019;366(6464):494–+. 10.1126/science.aaw2719 WOS:000493177900048. [DOI] [PubMed] [Google Scholar]
- 136.Leeansyah E, Loh L, Nixon DF, Sandberg JK. Acquisition of innate-like microbial reactivity in mucosal tissues during human fetal MAIT-cell development. Nat Commun. 2014;5. ARTN 3143 10.1038/ncomms4143 WOS:000331097100007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Godfrey DI, Koay HF, McCluskey J, Gherardin NA. The biology and functional importance of MAIT cells. Nat Immunol. 2019;20(9):1110–28. 10.1038/s41590-019-0444-8 WOS:000482212600012. [DOI] [PubMed] [Google Scholar]
- 138.Kjer-Nielsen L, Patel O, Corbett AJ, Le Nours J, Meehan B, Liu LG, et al. MR1 presents microbial vitamin B metabolites to MAIT cells. Nature. 2012;491(7426):717–+. 10.1038/nature11605 WOS:000311606000036. [DOI] [PubMed] [Google Scholar]
- 139.Corbett AJ, Eckle SBG, Birkinshaw RW, Liu LG, Patel O, Mahony J, et al. T-cell activation by transitory neo-antigens derived from distinct microbial pathways. Nature. 2014;509(7500):361–+. 10.1038/nature13160 WOS:000336121200039. [DOI] [PubMed] [Google Scholar]
- 140.Ussher JE, Bilton M, Attwod E, Shadwell J, Richardson R, de Lara C, et al. CD161(++)CD8(+) T cells, including the MAIT cell subset, are specifically activated by IL-12+IL-18 in a TCR-independent manner. Eur J Immunol. 2014;44(1):195–203. 10.1002/eji.201343509 WOS:000330803400020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Le Bourhis L, Martin E, Peguillet I, Guihot A, Froux N, Core M, et al. Antimicrobial activity of mucosal-associated invariant T cells. Nat Immunol. 2010;11 (8):701–U66. 10.1038/ni.1890 WOS:000280149400011. [DOI] [PubMed] [Google Scholar]
- 142.Gold MC, Eid T, Smyk-Pearson S, Eberling Y, Swarbrick GM, Langley SM, et al. Human thymic MR1-restricted MAIT cells are innate pathogen-reactive effectors that adapt following thymic egress. Mucosal Immunol. 2013;6(1):35–44. Epub 2012/06/14. 10.1038/mi.2012.45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kwon YS, Cho YN, Kim MJ, Jin HM, Jung HJ, Kang JH, et al. Mucosal-associated invariant T cells are numerically and functionally deficient in patients with mycobacterial infection and reflect disease activity. Tuberculosis. 2015;95 (3):267–74. 10.1016/j.tube.2015.03.004 WOS:000354126100006. [DOI] [PubMed] [Google Scholar]
- 144.Yang QT, Xu Q, Chen Q, Li J, Zhang MX, Cai Y, et al. Discriminating Active Tuberculosis from Latent Tuberculosis Infection by flow cytometric measurement of CD161-expressing T cells. Sci Rep-Uk. 2015;5. ARTN 17918 10.1038/srep17918 WOS:000365964700001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sharma PK, Wong EB, Napier RJ, Bishai WR, Ndung’u T, Kasprowicz VO, et al. High expression of CD26 accurately identifies human bacteria-reactive MR1-restricted MAIT cells. Immunology. 2015;145 (3):443–53. 10.1111/imm.12461 WOS:000356699200012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Jiang J, Wang XJ, An HJ, Yang BF, Cao ZH, Liu YH, et al. Mucosal-associated Invariant T-Cell Function Is Modulated by Programmed Death-1 Signaling in Patients with Active Tuberculosis. Am J Resp Crit Care. 2014;190 (3):329–39. 10.1164/rccm.201401-0106OC WOS:000340021900015. [DOI] [PubMed] [Google Scholar]
- 147.Vorkas CK, Wipperman MF, Li K, Bean J, Bhattarai SK, Adamow M, et al. Mucosal-associated invariant and gammadelta T cell subsets respond to initial Mycobacterium tuberculosis infection. JCI Insight. 2018;3(19). Epub 2018/10/05. 10.1172/jci.insight.121899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kauffman KD, Sallin MA, Hoft SG, Sakai S, Moore R, Wilder-Kofie T, et al. Limited Pulmonary Mucosal-Associated Invariant T Cell Accumulation and Activation during Mycobacterium tuberculosis Infection in Rhesus Macaques. Infect Immun. 2018;86(12). ARTN e00431-18 10.1128/IAI.00431-18 WOS:000450630200004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Dumas A, Corral D, Colom A, Levillain F, Peixoto A, Hudrisier D, et al. The Host Microbiota Contributes to Early Protection Against Lung Colonization by Mycobacterium tuberculosis. Front Immunol. 2018;9:2656. Epub 2018/11/30. 10.3389/fimmu.2018.02656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Chua WJ, Truscott SM, Eickhoff CS, Blazevic A, Hoft DF, Hansen TH. Polyclonal Mucosa-Associated Invariant T Cells Have Unique Innate Functions in Bacterial Infection. Infect Immun. 2012;80(9):3256–67. 10.1128/IAI.00279-12 WOS:000307869100027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Sakai S, Kauffman KD, Oh S, Nelson CE, Barry CE, 3rd, Barber DL. MAIT cell-directed therapy of Mycobacterium tuberculosis infection. Mucosal Immunol. 2021;14(1):199–208. Epub 2020/08/20. 10.1038/s41385-020-0332-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Vorkas CK, Levy O, Skular M, Li K, Aube J, Glickman MS. Efficient 5-OP-RU-Induced Enrichment of Mucosa-Associated Invariant T Cells in the Murine Lung Does Not Enhance Control of Aerosol Mycobacterium tuberculosis Infection. Infect Immun. 2020;89(1). Epub 2020/10/21. 10.1128/IAI.00524-20 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Seshadri C, Thuong NTT, Mai NTH, Bang ND, Chau TTH, Lewinsohn DM, et al. A polymorphism in human MR1 is associated with mRNA expression and susceptibility to tuberculosis. Genes Immun. 2017;18 (1):8–14. 10.1038/gene.2016.41 WOS:000393734600002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Coulter F, Parrish A, Manning D, Kampmann B, Mendy J, Garand M, et al. IL-17 Production from T Helper 17, Mucosal-Associated Invariant T, and gammadelta Cells in Tuberculosis Infection and Disease. Front Immunol. 2017;8:1252. Epub 2017/10/28. 10.3389/fimmu.2017.01252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Krause JL, Schape SS, Schattenberg F, Muller S, Ackermann G, Rolle-Kampczyk UE, et al. The Activation of Mucosal-Associated Invariant T (MAIT) Cells Is Affected by Microbial Diversity and Riboflavin Utilization in vitro. Front Microbiol. 2020;11:755. Epub 2020/05/12. 10.3389/fmicb.2020.00755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Schmaler M, Colone A, Spagnuolo J, Zimmermann M, Lepore M, Kalinichenko A, et al. Modulation of bacterial metabolism by the microenvironment controls MAIT cell stimulation. Mucosal Immunol. 2018;11(4):1060–70. Epub 2018/05/11. 10.1038/s41385-018-0020-9 . [DOI] [PubMed] [Google Scholar]
- 157.Skelly AN, Sato Y, Kearney S, Honda K. Mining the microbiota for microbial and metabolite-based immunotherapies. Nat Rev Immunol. 2019;19(5):305–23. Epub 2019/03/13. 10.1038/s41577-019-0144-5 . [DOI] [PubMed] [Google Scholar]
- 158.Nakhaee M, Rezaee A, Basiri R, Soleimanpour S, Ghazvini K. Relation between lower respiratory tract microbiota and type of immune response against tuberculosis. Microb Pathog. 2018;120:161–5. 10.1016/j.micpath.2018.04.054 WOS:000452578300022. [DOI] [PubMed] [Google Scholar]
- 159.Huang SF, Yang YY, Chou KT, Fung CP, Wang FD, Su WJ. Systemic proinflammation after Mycobacterium tuberculosis infection was correlated to the gut microbiome in HIV-uninfected humans. Eur J Clin Investig. 2019;49(5):e13068. Epub 2019/01/09. 10.1111/eci.13068 . [DOI] [PubMed] [Google Scholar]
- 160.Khan N, Vidyarthi A, Nadeem S, Negi S, Nair G, Agrewala JN. Alteration in the Gut Microbiota Provokes Susceptibility to Tuberculosis. Front Immunol. 2016;7:529. Epub 2016/12/15. 10.3389/fimmu.2016.00529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Nadeem S, Maurya SK, Das DK, Khan N, Agrewala JN. Gut Dysbiosis Thwarts the Efficacy of Vaccine Against Mycobacterium tuberculosis. Front Immunol. 2020;11. ARTN 726 10.3389/fimmu.2020.00726 WOS:000538887300001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Atarashi K, Suda W, Luo CW, Kawaguchi T, Motoo I, Narushima S, et al. Ectopic colonization of oral bacteria in the intestine drives T(H)1 cell induction and inflammation. Science. 2017;358(6361):359–+. 10.1126/science.aan4526 WOS:000413251000041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Devkota S, Wang Y, Musch MW, Leone V, Fehlner-Peach H, Nadimpalli A, et al. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10-/- mice. Nature. 2012;487(7405):104–8. Epub 2012/06/23. 10.1038/nature11225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Skelly AN, Sato Y, Kearney S, Honda K. Mining the microbiota for microbial and metabolite-based immunotherapies. Nat Rev Immunol. 2019;19 (5):305–23. 10.1038/s41577-019-0144-5 WOS:000466381300011. [DOI] [PubMed] [Google Scholar]
- 165.Segal LN, Clemente JC, Li Y, Ruan C, Cao J, Danckers M, et al. Anaerobic Bacterial Fermentation Products Increase Tuberculosis Risk in Antiretroviral-Drug-Treated HIV Patients. Cell Host Microbe. 2017;21(4):530–7 e4. Epub 2017/04/04. 10.1016/j.chom.2017.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Lachmandas E, van den Heuvel CN, Damen MS, Cleophas MC, Netea MG, van Crevel R. Diabetes Mellitus and Increased Tuberculosis Susceptibility: The Role of Short-Chain Fatty Acids. J Diabetes Res. 2016;2016:6014631. Epub 2016/04/09. 10.1155/2016/6014631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly YM, et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341(6145):569–73. Epub 2013/07/06. 10.1126/science.1241165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504(7480):446–50. Epub 2013/11/15. 10.1038/nature12721 . [DOI] [PubMed] [Google Scholar]
- 169.Sun MM, Wu W, Chen L, Yang WJ, Huang XS, Ma CY, et al. Microbiota-derived short-chain fatty acids promote Th1 cell IL-10 production to maintain intestinal homeostasis. Nat Commun. 2018;9. ARTN 3555 10.1038/s41467-018-05901-2 WOS:000443465100007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P, et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504(7480):451–5. Epub 2013/11/15. 10.1038/nature12726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Chang PV, Hao LM, Offermanns S, Medzhitov R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. P Natl Acad Sci USA. 2014;111(6):2247–52. 10.1073/pnas.1322269111 WOS:000330999600048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Vinolo MAR, Rodrigues HG, Nachbar RT, Curi R. Regulation of Inflammation by Short Chain Fatty Acids. Nutrients. 2011;3 (10):858–76. 10.3390/nu3100858 WOS:000298248600002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.McCoy KD, Ronchi F, Geuking MB. Host-microbiota interactions and adaptive immunity. Immunol Rev. 2017;279(1):63–9. 10.1111/imr.12575 WOS:000408672700006. [DOI] [PubMed] [Google Scholar]
- 174.Pabst O. New concepts in the generation and functions of IgA. Nat Rev Immunol. 2012;12(12):821–32. 10.1038/nri3322 WOS:000311479500011. [DOI] [PubMed] [Google Scholar]
- 175.Kim M, Qie YQ, Park J, Kim CH. Gut Microbial Metabolites Fuel Host Antibody Responses. Cell Host Microbe. 2016;20(2):202–14. 10.1016/j.chom.2016.07.001 WOS:000381587500012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Tezuka H, Abe Y, Asano J, Sato T, Liu JJ, Iwata M, et al. Prominent Role for Plasmacytoid Dendritic Cells in Mucosal T Cell-Independent IgA Induction. Immunity. 2011;34 (2):247–57. 10.1016/j.immuni.2011.02.002 WOS:000287989500014. [DOI] [PubMed] [Google Scholar]
- 177.McCarthy DD, Kujawa J, Wilson C, Papandile A, Poreci U, Porfilio EA, et al. Mice overexpressing BAFF develop a commensal flora-dependent, IgA-associated nephropathy. J Clin Invest. 2011;121(10):3991–4002. 10.1172/JCI45563 WOS:000295601000027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Litinskiy MB, Nardelli B, Hilbert DM, He B, Schaffer A, Casali P, et al. DCs induce CD40-independent immunoglobulin class switching through BLyS and APRIL. Nat Immunol. 2002;3(9):822–9. 10.1038/ni829 WOS:000177694900010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.He B, Xu W, Santini PA, Polydorides AD, Chiu A, Estrella J, et al. Intestinal bacteria trigger T cell-independent immunoglobulin A(2) class switching by inducing epithelial-cell secretion of the cytokine APRIL. Immunity. 2007;26 (6):812–26. 10.1016/j.immuni.2007.04.014 WOS:000247646900016. [DOI] [PubMed] [Google Scholar]
- 180.Robak OH, Heimesaat MM, Kruglov AA, Prepens S, Ninnemann J, Gutbier B, et al. Antibiotic treatment-induced secondary IgA deficiency enhances susceptibility to Pseudomonas aeruginosa pneumonia. J Clin Invest. 2018;128(8):3535–45. 10.1172/JCI97065 WOS:000440461500034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Ruane D, Chorny A, Lee H, Faith J, Pandey G, Shan M, et al. Microbiota regulate the ability of lung dendritic cells to induce IgA class-switch recombination and generate protective gastrointestinal immune responses. J Exp Med. 2016;213(1):53–73. 10.1084/jem.20150567 WOS:000368337400007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Pasquali C, Salami O, Taneja M, Gollwitzer ES, Trompette A, Pattaroni C, et al. Enhanced mucosal antibody production and protection against respiratory infections following an orally administered bacterial extract. Front Med. 2014;1. ARTN 41 10.3389/fmed.2014.00056 WOS:000219242600036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Choreno-Parra JA, Weinstein LI, Yunis EJ, Zuniga J, Hernandez-Pando R. Thinking Outside the Box: Innate- and B Cell-Memory Responses as Novel Protective Mechanisms Against Tuberculosis. Front Immunol. 2020;11:226. Epub 2020/03/03. 10.3389/fimmu.2020.00226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Vordermeier HM, Venkatprasad N, Harris DP, Ivanyi J. Increase of tuberculous infection in the organs of B cell-deficient mice. Clin Exp Immunol. 1996;106(2):312–6. 10.1046/j.1365-2249.1996.d01-845.x WOS:A1996VQ33800018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Maglione PJ, Xu JY, Chan J. B cells moderate inflammatory progression and enhance bacterial containment upon pulmonary challenge with Mycobacterium tuberculosis. J Immunol. 2007;178(11):7222–34. 10.4049/jimmunol.178.11.7222 WOS:000246896300063. [DOI] [PubMed] [Google Scholar]
- 186.Bosio CM, Gardner D, Elkins KL. Infection of B cell-deficient mice with CDC 1551, a clinical isolate of Mycobacterium tuberculosis: Delay in dissemination and development of lung pathology. J Immunol. 2000;164(12):6417–25. 10.4049/jimmunol.164.12.6417 WOS:000087508500042. [DOI] [PubMed] [Google Scholar]
- 187.Phuah J, Wong EA, Gideon HP, Maiello P, Coleman MT, Hendricks MR, et al. Effects of B Cell Depletion on Early Mycobacterium tuberculosis Infection in Cynomolgus Macaques. Infect Immun. 2016;84(5):1301–11. 10.1128/IAI.00083-16 WOS:000377106600006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Achkar JM, Prados-Rosales R. Updates on antibody functions in Mycobacterium tuberculosis infection and their relevance for developing a vaccine against tuberculosis. Curr Opin Immunol. 2018;53:30–7. 10.1016/j.coi.2018.04.004 WOS:000448096600007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Lu LL, Chung AW, Rosebrock TR, Ghebremichael M, Yu WH, Grace PS, et al. A Functional Role for Antibodies in Tuberculosis. Cell. 2016;167(2):433–+. 10.1016/j.cell.2016.08.072 WOS:000386343100019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Zimmermann N, Thormann V, Hu B, Kohler AB, Imai-Matsushima A, Locht C, et al. Human isotype-dependent inhibitory antibody responses against Mycobacterium tuberculosis. EMBO Mol Med. 2016;8(11):1325–39. 10.15252/emmm.201606330 WOS:000387111900007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Chen TT, Blanc C, Liu YY, Ishida E, Singer S, Xu JY, et al. Capsular glycan recognition provides antibody-mediated immunity against tuberculosis. J Clin Invest. 2020;130(4):1808–22. 10.1172/JCI128459 WOS:000530101000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Chen TT, Blanc C, Eder AZ, Prados-Rosales R, Souza ACO, Kim RS, et al. Association of Human Antibodies to Arabinomannan With Enhanced Mycobacterial Opsonophagocytosis and Intracellular Growth Reduction. J Infect Dis. 2016;214(2):300–10. 10.1093/infdis/jiw141 WOS:000379822900018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Kumar SK, Singh P, Sinha S. Naturally produced opsonizing antibodies restrict the survival of Mycobacterium tuberculosis in human macrophages by augmenting phagosome maturation. Open Biol. 2015;5(12). ARTN 150171 10.1098/rsob.150171 WOS:000367482100005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Teitelbaum R, Glatman-Freedman A, Chen B, Robbins JB, Unanue E, Casadevall A, et al. A mAb recognizing a surface antigen of Mycobacterium tuberculosis enhances host survival. P Natl Acad Sci USA. 1998;95(26):15688–93. 10.1073/pnas.95.26.15688 WOS:000077697200098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Maglione PJ, Xu J, Casadevall A, Chan J. Fc gamma receptors regulate immune activation and susceptibility during Mycobacterium tuberculosis infection. J Immunol. 2008;180(5):3329–38. Epub 2008/02/23. 10.4049/jimmunol.180.5.3329 . [DOI] [PubMed] [Google Scholar]
- 196.Williams A, Reljic R, Naylor I, Clark SO, Falero-Diaz G, Singh M, et al. Passive protection with immunoglobulin A antibodies against tuberculous early infection of the lungs. Immunology. 2004;111(3):328–33. Epub 2004/03/11. 10.1111/j.1365-2567.2004.01809.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Lopez Y, Yero D, Falero-Diaz G, Olivares N, Sarmiento ME, Sifontes S, et al. Induction of a protective response with an IgA monoclonal antibody against Mycobacterium tuberculosis 16kDa protein in a model of progressive pulmonary infection. Int J Med Microbiol. 2009;299(6):447–52. Epub 2009/01/23. 10.1016/j.ijmm.2008.10.007 . [DOI] [PubMed] [Google Scholar]
- 198.Balu S, Reljic R, Lewis MJ, Pleass RJ, McIntosh R, van Kooten C, et al. A novel human IgA monoclonal antibody protects against tuberculosis. J Immunol. 2011;186(5):3113–9. Epub 2011/01/25. 10.4049/jimmunol.1003189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Li H, Limenitakis JP, Greiff V, Yilmaz B, Scharen O, Urbaniak C, et al. Mucosal or systemic microbiota exposures shape the B cell repertoire. Nature. 2020;584(7820):274–8. Epub 2020/08/08. 10.1038/s41586-020-2564-6 . [DOI] [PubMed] [Google Scholar]
- 200.Benard A, Sakwa I, Schierloh P, Colom A, Mercier I, Tailleux L, et al. B Cells Producing Type I IFN Modulate Macrophage Polarization in Tuberculosis. Am J Respir Crit Care Med. 2018;197(6):801–13. Epub 2017/11/22. 10.1164/rccm.201707-1475OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Kozakiewicz L, Chen Y, Xu J, Wang Y, Dunussi-Joannopoulos K, Ou Q, et al. B cells regulate neutrophilia during Mycobacterium tuberculosis infection and BCG vaccination by modulating the interleukin-17 response. PLoS Pathog. 2013;9(7):e1003472. Epub 2013/07/16. 10.1371/journal.ppat.1003472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Rosser EC, Piper CJM, Matei DE, Blair PA, Rendeiro AF, Orford M, et al. Microbiota-Derived Metabolites Suppress Arthritis by Amplifying Aryl-Hydrocarbon Receptor Activation in Regulatory B Cells. Cell Metab. 2020;31(4):837–51 e10. Epub 2020/03/28. 10.1016/j.cmet.2020.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Schierloh P, Landoni V, Balboa L, Musella RM, Castagnino J, Morana E, et al. Human pleural B-cells regulate IFN-gamma production by local T-cells and NK cells in a Mycobacterium tuberculosis-induced delayed hypersensitivity reaction. Clin Sci (Lond). 2014;127(6):391–403. Epub 2014/04/03. 10.1042/CS20130769 . [DOI] [PubMed] [Google Scholar]
- 204.Namasivayam S, Kauffman KD, McCulloch JA, Yuan WX, Thovarai V, Mittereder LR, et al. Correlation between Disease Severity and the Intestinal Microbiome in Mycobacterium tuberculosis-Infected Rhesus Macaques. Mbio. 2019;10(3). ARTN e01018-19 10.1128/mBio.01018-19 WOS:000473596500017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Dooley KE, Chaisson RE. Tuberculosis and diabetes mellitus: convergence of two epidemics. Lancet Infect Dis. 2009;9(12):737–46. 10.1016/S1473-3099(09)70282-8 WOS:000272292000016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Talat N, Perry S, Parsonnet J, Dawood G, Hussain R. Vitamin D Deficiency and Tuberculosis Progression. Emerg Infect Dis. 2010;16(5):853–5. 10.3201/eid1605.091693 WOS:000277209900020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Sita-Lumsden A, Lapthorn G, Swaminathan R, Milburn HJ. Reactivation of tuberculosis and vitamin D deficiency: the contribution of diet and exposure to sunlight. Thorax. 2007;62(11):1003–7. Epub 2007/05/29. 10.1136/thx.2006.070060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Koethe JR, von Reyn CF. Protein-calorie malnutrition, macronutrient supplements, and tuberculosis. Int J Tuberc Lung Dis. 2016;20(7):857–63. Epub 2016/06/12. 10.5588/ijtld.15.0936 . [DOI] [PubMed] [Google Scholar]
- 209.Gueimonde M, Sanchez B, de los Reyes-Gavilán CG, Margolles A. Antibiotic resistance in probiotic bacteria. Front Microbiol. 2013;4:202. Epub 2013/07/25. 10.3389/fmicb.2013.00202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Fouhy F, O’Connell Motherway M, Fitzgerald GF, Ross RP, Stanton C, van Sinderen D, et al. In silico assigned resistance genes confer Bifidobacterium with partial resistance to aminoglycosides but not to beta-lactams. PLoS ONE. 2013;8(12):e82653. Epub 2013/12/11. 10.1371/journal.pone.0082653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Duranti S, Lugli GA, Mancabelli L, Turroni F, Milani C, Mangifesta M, et al. Prevalence of Antibiotic Resistance Genes among Human Gut-Derived Bifidobacteria. Appl Environ Microbiol. 2017;83(3). Epub 2016/11/20. 10.1128/AEM.02894-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Suprapti B, Suharjono S, Raising R, Yulistiani Y, Izzah Z, Nilamsari WP, et al. Effects of Probiotics and Vitamin B Supplementation on IFN-gamma and IL-12 Levels During Intensive Phase Treatment of Tuberculosis. Indones J Pharm. 2018;29(2):80–5. 10.14499/indonesianjpharm29iss2pp80 WOS:000442168100005. [DOI] [Google Scholar]
- 213.Lokesh D, Parkesh R, Kammara R. Bifidobacterium adolescentis is intrinsically resistant to antitubercular drugs. Sci Rep. 2018;8(1):11897. Epub 2018/08/11. 10.1038/s41598-018-30429-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Cardona P, Marzo-Escartin E, Tapia G, Diaz J, Garcia V, Varela I, et al. Oral Administration of Heat-Killed Mycobacterium manresensis Delays Progression toward Active Tuberculosis in C3HeB/FeJ Mice. Front Microbiol. 2015;6:1482. Epub 2016/01/19. 10.3389/fmicb.2015.01482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Tukvadze N, Cardona P, Vashakidze S, Shubladze N, Avaliani Z, Vilaplana C, et al. Development of the food supplement Nyaditum resae as a new tool to reduce the risk of tuberculosis development. Int J Mycobacteriol. 2016;5 Suppl 1:S101–S2. Epub 2017/01/04. 10.1016/j.ijmyco.2016.09.073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Montane E, Barriocanal AM, Arellano AL, Valderrama A, Sanz Y, Perez-Alvarez N, et al. Pilot, double-blind, randomized, placebo-controlled clinical trial of the supplement food Nyaditum resae (R) in adults with or without latent TB infection: Safety and immunogenicity. PLoS ONE. 2017;12(2). ARTN e0171294 10.1371/journal.pone.0171294 WOS:000394231800052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.de Groot PF, Frissen MN, de Clercq NC, Nieuwdorp M. Fecal microbiota transplantation in metabolic syndrome: History, present and future. Gut Microbes. 2017;8(3):253–67. Epub 2017/06/14. 10.1080/19490976.2017.1293224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Baxter M, Colville A. Adverse events in faecal microbiota transplant: a review of the literature. J Hosp Infect. 2016;92(2):117–27. Epub 2016/01/25. 10.1016/j.jhin.2015.10.024 . [DOI] [PubMed] [Google Scholar]
- 219.Allegretti JR, Kearney S, Li N, Bogart E, Bullock K, Gerber GK, et al. Recurrent Clostridium difficile infection associates with distinct bile acid and microbiome profiles. Aliment Pharmacol Ther. 2016;43(11):1142–53. Epub 2016/04/19. 10.1111/apt.13616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Borody T, Fischer M, Mitchell S, Campbell J. Fecal microbiota transplantation in gastrointestinal disease: 2015 update and the road ahead. Expert Rev Gastroenterol Hepatol. 2015;9(11):1379–91. Epub 2015/09/29. 10.1586/17474124.2015.1086267 . [DOI] [PubMed] [Google Scholar]
- 221.Paramsothy S, Walsh AJ, Borody T, Samuel D, van den Bogaerde J, Leong RW, et al. Gastroenterologist perceptions of faecal microbiota transplantation. World J Gastroenterol. 2015;21(38):10907–14. Epub 2015/10/20. 10.3748/wjg.v21.i38.10907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Spinas E, Saggini A, Kritas SK, Cerulli G, Caraffa A, Antinolfi P, et al. Crosstalk between Vitamin B and Immunity. J Biol Reg Homeos Ag. 2015;29(2):283–8. WOS:000356992900003. [PubMed] [Google Scholar]
- 223.Forsythe P. Probiotics and lung immune responses. Ann Am Thorac Soc. 2014;11 Suppl 1:S33–7. Epub 2014/01/21. 10.1513/AnnalsATS.201306-156MG . [DOI] [PubMed] [Google Scholar]
- 224.Mortaz E, Adcock IM, Folkerts G, Barnes PJ, Paul Vos A, Garssen J. Probiotics in the management of lung diseases. Mediat Inflamm. 2013;2013:751068. Epub 2013/06/06. 10.1155/2013/751068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Negatu DA, Yamada Y, Xi Y, Go ML, Zimmerman M, Ganapathy U, et al. Gut Microbiota Metabolite Indole Propionic Acid Targets Tryptophan Biosynthesis in Mycobacterium tuberculosis. MBio. 2019;10(2). ARTN e02781-18 10.1128/mBio.02781-18 WOS:000465077600067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Abrahams KA, Cox JAG, Futterer K, Rullas J, Ortega-Muro F, Loman NJ, et al. Inhibiting mycobacterial tryptophan synthase by targeting the inter-subunit interface. Sci Rep-Uk. 2017;7. ARTN 9430 10.1038/s41598-017-09642-y WOS:000408448100023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Zhang YJJ, Reddy MC, Ioerger TR, Rothchild AC, Dartois V, Schuster BM, et al. Tryptophan Biosynthesis Protects Mycobacteria from CD4 T-Cell-Mediated Killing. Cell. 2013;155(6):1296–308. 10.1016/j.cell.2013.10.045 WOS:000328271100014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Wellington S, Nag PP, Michalska K, Johnston SE, Jedrzejczak RP, Kaushik VK, et al. A small-molecule allosteric inhibitor of Mycobacterium tuberculosis tryptophan synthase. Nat Chem Biol. 2017;13(9):943–+. 10.1038/nchembio.2420 WOS:000407929800009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Dodd D, Spitzer MH, Van Treuren W, Merrill BD, Hryckowian AJ, Higginbottom SK, et al. A gut bacterial pathway metabolizes aromatic amino acids into nine circulating metabolites. Nature. 2017;551(7682):648–52. Epub 2017/11/24. 10.1038/nature24661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Venkatesh M, Mukherjee S, Wang H, Li H, Sun K, Benechet AP, et al. Symbiotic bacterial metabolites regulate gastrointestinal barrier function via the xenobiotic sensor PXR and Toll-like receptor 4. Immunity. 2014;41(2):296–310. Epub 2014/07/30. 10.1016/j.immuni.2014.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Fung TC, Olson CA, Hsiao EY. Interactions between the microbiota, immune and nervous systems in health and disease. Nat Neurosci. 2017;20(2):145–55. Epub 2017/01/17. 10.1038/nn.4476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Negatu DA, Liu JJJ, Zimmerman M, Kaya F, Dartois V, Aldrich CC, et al. Whole-Cell Screen of Fragment Library Identifies Gut Microbiota Metabolite Indole Propionic Acid as Antitubercular. Antimicrob Agents Chemother. 2018;62(3). Epub 2017/12/13. 10.1128/AAC.01571-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Milshteyn A, Schneider JS, Brady SF. Mining the metabiome: identifying novel natural products from microbial communities. Chem Biol. 2014;21(9):1211–23. Epub 2014/09/23. 10.1016/j.chembiol.2014.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Kommineni S, Bretl DJ, Lam V, Chakraborty R, Hayward M, Simpson P, et al. Bacteriocin production augments niche competition by enterococci in the mammalian gastrointestinal tract. Nature. 2015;526(7575):719–22. Epub 2015/10/20. 10.1038/nature15524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Sosunov V, Mischenko V, Eruslanov B, Svetoch E, Shakina Y, Stern N, et al. Antimycobacterial activity of bacteriocins and their complexes with liposomes. J Antimicrob Chemother. 2007;59(5):919–25. Epub 2007/03/10. 10.1093/jac/dkm053 . [DOI] [PubMed] [Google Scholar]
- 236.Mota-Meira M, LaPointe G, Lacroix C, Lavoie MC. MICs of mutacin B-Ny266, nisin A, vancomycin, and oxacillin against bacterial pathogens. Antimicrob Agents Chemother. 2000;44(1):24–9. Epub 1999/12/22. 10.1128/aac.44.1.24-29.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Carroll J, Draper LA, O’Connor PM, Coffey A, Hill C, Ross RP, et al. Comparison of the activities of the lantibiotics nisin and lacticin 3147 against clinically significant mycobacteria. Int J Antimicrob Agents. 2010;36(2):132–6. Epub 2010/06/16. 10.1016/j.ijantimicag.2010.03.029 . [DOI] [PubMed] [Google Scholar]
- 238.Aguilar-Perez C, Gracia B, Rodrigues L, Vitoria A, Cebrian R, Deboosere N, et al. Synergy between Circular Bacteriocin AS-48 and Ethambutol against Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2018;62(9). Epub 2018/07/11. 10.1128/AAC.00359-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Hamer HM, Jonkers D, Venema K, Vanhoutvin S, Troost FJ, Brummer RJ. Review article: the role of butyrate on colonic function. Aliment Pharmacol Ther. 2008;27(2):104–19. Epub 2007/11/02. 10.1111/j.1365-2036.2007.03562.x . [DOI] [PubMed] [Google Scholar]
- 240.Bach Knudsen KE, Laerke HN, Hedemann MS, Nielsen TS, Ingerslev AK, Gundelund Nielsen DS, et al. Impact of Diet-Modulated Butyrate Production on Intestinal Barrier Function and Inflammation. Nutrients. 2018;10(10). Epub 2018/10/17. 10.3390/nu10101499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Parada Venegas D, De la Fuente MK, Landskron G, Gonzalez MJ, Quera R, Dijkstra G, et al. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front Immunol. 2019;10:277. Epub 2019/03/28. 10.3389/fimmu.2019.00277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Miquel S, Leclerc M, Martin R, Chain F, Lenoir M, Raguideau S, et al. Identification of metabolic signatures linked to anti-inflammatory effects of Faecalibacterium prausnitzii. MBio. 2015;6(2). Epub 2015/04/23. 10.1128/mBio.00300-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Quevrain E, Maubert MA, Michon C, Chain F, Marquant R, Tailhades J, et al. Identification of an anti-inflammatory protein from Faecalibacterium prausnitzii, a commensal bacterium deficient in Crohn’s disease. Gut. 2016;65(3):415–25. Epub 2015/06/06. 10.1136/gutjnl-2014-307649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Maji A, Misra R, Dhakan DB, Gupta V, Mahato NK, Saxena R, et al. Gut microbiome contributes to impairment of immunity in pulmonary tuberculosis patients by alteration of butyrate and propionate producers. Environ Microbiol. 2018;20(1):402–19. Epub 2018/01/13. 10.1111/1462-2920.14015 . [DOI] [PubMed] [Google Scholar]
- 245.Schulthess J, Pandey S, Capitani M, Rue-Albrecht KC, Arnold I, Franchini F, et al. The Short Chain Fatty Acid Butyrate Imprints an Antimicrobial Program in Macrophages. Immunity. 2019;50(2):432–+. 10.1016/j.immuni.2018.12.018 WOS:000459006700017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Nguyen LD, Viscogliosi E, Delhaes L. The lung mycobiome: an emerging field of the human respiratory microbiome. Front Microbiol. 2015;6:89. Epub 2015/03/13. 10.3389/fmicb.2015.00089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Weaver D, Gago S, Bromley M, Bowyer P. The Human Lung Mycobiome in Chronic Respiratory Disease: Limitations of Methods and Our Current Understanding. Curr Fungal Infect R. 2019;13(3):109–19. 10.1007/s12281-019-00347-5 WOS:000482387300005. [DOI] [Google Scholar]
- 248.Khoruts A, Staley C, Sadowsky MJ. Faecal microbiota transplantation for Clostridioides difficile: mechanisms and pharmacology. Nat Rev Gastroenterol Hepatol. 2020. Epub 2020/08/28. 10.1038/s41575-020-0350-4 . [DOI] [PubMed] [Google Scholar]
- 249.Preidis GA, Weizman AV, Kashyap PC, Morgan RL. AGA Technical Review on the Role of Probiotics in the Management of Gastrointestinal Disorders. Gastroenterology. 2020;159(2):708–38 e4. Epub 2020/06/13. 10.1053/j.gastro.2020.05.060 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Mullish BH, Quraishi MN, Segal JP, McCune VL, Baxter M, Marsden GL, et al. The use of faecal microbiota transplant as treatment for recurrent or refractory Clostridium difficile infection and other potential indications: joint British Society of Gastroenterology (BSG) and Healthcare Infection Society (HIS) guidelines. Gut. 2018;67(11):1920–41. Epub 2018/08/30. 10.1136/gutjnl-2018-316818 . [DOI] [PubMed] [Google Scholar]
- 251.Levine A, Boneh RS, Wine E. Evolving role of diet in the pathogenesis and treatment of inflammatory bowel diseases. Gut. 2018;67(9):1726–+. 10.1136/gutjnl-2017-315866 WOS:000445080300019. [DOI] [PubMed] [Google Scholar]
- 252.Camilleri M, Lyle BJ, Madsen KL, Sonnenburg J, Verbeke K, Wu GD. Role for diet in normal gut barrier function: developing guidance within the framework of food-labeling regulations. Am J Physiol-Gastr L. 2019;317(1):G17–G39. 10.1152/ajpgi.00063.2019 WOS:000474866500003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Daniel H. Diet and the gut microbiome: from hype to hypothesis. Br J Nutr. 2020;124(6):521–30. Epub 2020/04/03. 10.1017/S0007114520001142 . [DOI] [PubMed] [Google Scholar]
- 254.Hu YF, Feng YQ, Wu JN, Liu F, Zhang ZG, Hao YN, et al. The Gut Microbiome Signatures Discriminate Healthy From Pulmonary Tuberculosis Patients. Front Cell Infect Mi. 2019;9. ARTN 90 10.3389/fcimb.2019.00090 WOS:000463586600001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Hu Y, Kang Y, Liu X, Cheng M, Dong J, Sun L, et al. Distinct lung microbial community states in patients with pulmonary tuberculosis. Sci China Life Sci. 2020. Epub 2020/04/19. 10.1007/s11427-019-1614-0 . [DOI] [PubMed] [Google Scholar]
- 256.Zhou YH, Lin FS, Cui ZL, Zhang XR, Hu CM, Shen T, et al. Correlation between Either Cupriavidus or Porphyromonas and Primary Pulmonary Tuberculosis Found by Analysing the Microbiota in Patients’ Bronchoalveolar Lavage Fluid. PLoS ONE. 2015;10(5). ARTN e0124194 10.1371/journal.pone.0124194 WOS:000354931700004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Botero LE, Delgado-Serrano L, Cepeda ML, Bustos JR, Anzola JM, Del Portillo P, et al. Respiratory tract clinical sample selection for microbiota analysis in patients with pulmonary tuberculosis. Microbiome. 2014;2. Artn 29 10.1186/2049-2618-2-29 WOS:000363195000001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Hu Y, Yang Q, Liu B, Dong J, Sun L, Zhu Y, et al. Gut microbiota associated with pulmonary tuberculosis and dysbiosis caused by anti-tuberculosis drugs. J Infect. 2019;78(4):317–22. Epub 2018/08/15. 10.1016/j.jinf.2018.08.006 . [DOI] [PubMed] [Google Scholar]