Abstract
Embryo vitrification involves exposure to high concentrations of cryoprotectants and osmotic stress during cooling and warming in the cryopreservation process. Many of these factors can potentially affect gene expression. In this study, in vitro-produced bovine embryos at the blastocyst stage were subjected to vitrification. Four recipients each were used for transferring non-vitrified (n = 80) and vitrified (n = 80) embryos. A total of 12 non-vitrified and 9 vitrified viable day-14 (D14) embryos were recovered by uterine flushing. RNA-seq analysis of the whole embryo or isolated trophectoderm (TE) from vitrified and fresh recovered D14 embryos revealed a total of 927 and 4376 genes with changed expression in embryos and TE isolates, respectively, as a result of vitrification. In addition, we found 671 and 61 genes commonly up- or downregulated in both vitrified whole embryos and TE. Commonly upregulated pathways by vitrification included epithelial adherens junctions, sirtuin signalling, germ cell–sertoli cell junction, ATM signalling, NER and protein ubiquitination pathways. The commonly downregulated pathways included EIF2 signalling, oxidative phosphorylation, mitochondrial dysfunction, regulation of eIF4 and p70S6K signalling and mTOR signalling pathways. Our analysis identified specific pathways and implicated specific gene expression patterns affecting embryo developmental competence that are important to cryopreservation.
Keywords: transcriptome, vitrification, blastocyst, trophectoderm, elongation
Introduction
In vitro-produced (IVP) embryos have become the preferred source of blastocysts for transfer in cattle. According to the International Embryo Technology Society (IETS), in 2018 more than 1 million transferrable embryos were produced in vitro and less than half a million in vivo derived (IVD) (Grothmann 2019). In vitro embryo production serves different purposes depending on the species. In humans, the main objective of in vitro fertilisation (IVF) and in vitro culture (IVC) is to treat infertility. However, in domestic animals, IVP is mostly used to accelerate genetic progress and increase animal production (Sjunnesson 2019). Additionally, in vitro embryo production is used as a tool to enhance other artificial reproductive techniques (ARTs). For example, the use of sex-sorted semen for artificial insemination (AI) in dairy cattle often results in a decline in fertility, mostly due to the low number of sperm used for AI with sex-sorted semen (Frijters et al. 2009). This problem can be easily overcome by performing IVF, for which the number of sperm required is much lower (Xu et al. 2009). Moreover, the production of clones and transgenic animals relies on the in vitro production of embryos. More importantly, in vitro production of embryos has enabled elucidation of many important biochemical and molecular processes that occur during oocyte maturation, fertilisation and at different stages of preimplantation embryo development.
Cryopreservation has become a routine procedure in embryo transfer programs because it not only allows long-term storage, but also the import and export of elite genetics at a relatively low cost. In humans, cryopreservation of embryos has been extremely beneficial because it allows patients to undergo optimal uterus synchronisation, thus increasing the success of IVF cycles (Wong et al. 2014). Cryopreservation refers to the successful preservation of the normal functions of viable cells and tissues at ultra-low temperatures below that at which metabolic and biochemical reactions take place (Chatterjee et al. 2017). Slow freezing and vitrification are the two methods that have been used widely to cryopreserve embryos (Leibo 2008). In cattle, slow freezing is probably the most utilised technique because it allows direct transfer and therefore no laboratory equipment is needed at the moment of embryo transfer (Dochi 2019). However, ice crystals form during slow freezing, which causes structural damage leading to cell lysis. Therefore, vitrification has become an important alternative because the cell damage induced by ice crystals is technically eliminated. In vitrification, embryos are exposed to solutions containing high concentrations of cryoprotectants (penetrating and non-penetrating) that, under ultra-low temperatures (≤ −196°C), allow the transition to a glass-like state, without the formation of intracellular ice-crystals (Rall and Fahy 1985). It has been shown that bovine, mouse, and human blastocysts cryopreserved using vitrification have better survival rates than those preserved by slow freezing (Li et al. 2012; Gupta et al. 2017).
Many studies have attempted to identify the effects of embryo vitrification on gene expression patterns at preimplantation stages. Aksu et al. (2012) reported that vitrification upregulated genes associated with stress responses in IVP bovine blastocysts. When vitrified at the morula stage, IVP bovine embryos that developed into blastocysts showed different expression patterns in genes involved in implantation, lipid oxidation and reactive oxygen species (ROS) generation, apoptosis and cell differentiation (de Oliveira Leme et al. 2016; Gupta et al. 2017). In buffalos, vitrification of IVP blastocysts altered the expression of genes related to cell adhesion (Moussa et al. 2019). In porcine embryos, vitrification, independent of the cryoprotectants, altered the expression of imprinted genes (Bartolac et al. 2018). In mice, vitrification of pronuclear stage embryos led to upregulation of stress-related genes (Boonkusol et al. 2006).
The effects of frozen embryo transfer (FET) on human live births have been reviewed by Blockeel et al. (2019). Their studies provide evidence linking cryopreservation to increased risk of hypertensive disorders of pregnancy and pre-eclampsia. Also, risks for macrosomia and large-for-gestational age are also increased in children born from FET compared with births from fresh embryo transfer.
All the studies cited above focused on the effects of cryopreservation on embryos at preimplantation stages or perinatal neonates. However, there are no reports concerning the effects on embryos beyond the blastocyst stage. Recently developed protocols allow mouse and human embryos to be cultured up to 14 days after IVF in the presence and absence of maternal tissue (Bedzhov et al. 2014; Deglincerti et al. 2016; Shahbazi et al. 2016). In vitro 14-day embryos have helped to reveal key events during implantation and the processes that lead to gastrulation. In humans, implantation occurs before this time point; thus, studies of development beyond the blastocyst stage in vivo are not possible. In the bovine, the day-14 (D14) elongating embryo is a stage of embryo development that can be recovered non-invasively after fertilisation and represents a critical stage of development when many embryos die. Therefore, this study aimed to evaluate the effect of blastocyst vitrification on transcriptome dynamics of D14 bovine embryos by RNA sequencing (RNA-seq). We also provide a comprehensive view of transcriptomes of the bovine D14 elongating embryo and trophectoderm (TE) cells at this critical stage.
Materials and methods
Ethics statement
The experiments were conducted under an animal use protocol (A2017–19) approved by the Louisiana State University Agricultural Center Institutional Animal Care and Use Committee.
Oocyte collection and in vitro maturation
Abattoir-derived cumulus–oocyte complexes (COCs) were purchased from a commercial vendor (DeSoto Bioscience) and matured during shipment overnight in maturation medium. Upon receipt, oocytes undergoing in vitro maturation were removed from the portable incubator (MiniTube product no. 19180/0001) and placed in an incubator at 38.5°C and 6% CO2 to continue maturation to a total of 22 h.
IVF and IVC
All media used were from IVF Bioscience, UK. Media requiring equilibration were placed in an incubator at 38.5°C and 6% CO2 at least 2 h before use.
At 22 h of maturation, the COCs were removed from the in vitro maturation medium and groups of 50 COCs were washed three times in previously equilibrated BO-IVF medium before being transferred, together with no more than 50 μL of media, to a well containing 400 μL of BO-IVF medium. One straw of frozen semen was thawed for 35 s in a 37°C water bath before being washed twice in 4 mL of prewarmed BO-SemenPrep through centrifugation at 400g for 5 min. The pellet from the final wash step was resuspended in 500 μL of BO-SemenPrep. A final concentration of 2 × 106 sperm/mL (~50 μL) was added to each IVF well for oocyte fertilisation.
After 18 h of IVF, cumulus cells of inseminated COCs (presumptive zygotes) were removed by vortexing groups of 50 COCs in a 1.5 mL microcentrifuge tube containing 200μL of BO-Wash, at maximum speed for 2 min. Denuded zygotes were recovered and washed three times in previously equilibrated BO-IVC medium before being placed in groups of 20 into 50 μL drops of BO-IVC overlaid with BO-Oil. In vitro culture was sustained for 7 days in an incubator at 38.5 °C, 6% CO2 and 5% O2.
Vitrification and warming
BO-VitriCool and BO-VitriWarm (IVF Bioscience, UK) were used for embryo vitrification and warming, respectively. Vitrification and thawing protocols were carried out according to manufacturer suggestions.
Embryos at the blastocyst and morula stages were recovered on day 7 of IVC. Transfer medium (IVF Bioscience, UK) was used as a holding medium before beginning vitrification. All vitrification steps were carried out at room temperature. Cohorts of four embryos (blastocysts and/or morula) were placed in BO-Preincubation for 2 min, followed by 2 min in VitriCool 1. Finally, embryos were briefly exposed to VitriCool 2, loaded onto a Cryolock® using, 1 μL of medium and plunged into liquid nitrogen (LN), with a maximum exposure time of 1 min. Embryos remained vitrified in LN for at least 2 weeks.
On warming, a single Cryolock® was taken from the LN and placed in VitriWarm 1 in a 35-mm Petri dish. Embryos were recovered from the Cryolock® and subjected to the four sequential media of the VitriWarm protocol, beginning with 3 min in VitriWarm 1. Embryos were then moved to VitriWarm 2 for 2 min followed by VitriWarm 3 for another 2 min. Finally, embryos were washed in VitriWarm 4 for 1 min before being rinsed and held in transfer medium (IVF Bioscience, UK) until being loaded into 0.25-mL straws for transfer. All warming steps were carried out at 38.5°C.
Embryo transfer
Embryos were transferred to non-lactating, 3 year old cross-breed (Bos taurus × Bos indicus) cows. Recipient cows were synchronised with a standard 6-day controlled internal drug release (CIDR) protocol with an ovulation-inducing gonadotrophin releasing hormone–intramuscular (IM) injection 48 h after CIDR removal. A cohort of 20 embryos (blastocysts and morula) were loaded into 0.5 mL straws in transfer medium (IVF Bioscience, UK) and transferred non-surgically to the uterine horn ipsilateral to the ovary with the corpus luteum (CL) of each recipient within 30 min after loading. The presence of a CL was detected through transrectal ultrasonography.
Embryo flushing of D14 embryos and TE isolation
D14 embryos were recovered 7 days after transfer by a standard non-surgical flush with lactated Ringers solution supplemented with 1% calf serum and 0.1% heparin and washed in HEPES-buffered medium before processing for RNA-seq. In this study, pure TE fractions were isolated by placing embryos in a Petri dish with phosphate-buffered saline and performing microsurgery using a microblade under a microscope (Fig. 1). The bovine D14 embryo has an identifiable embryonic disc (Fig. 2a), but isolation of the embryonic disc could not be achieved.
Fig. 1.
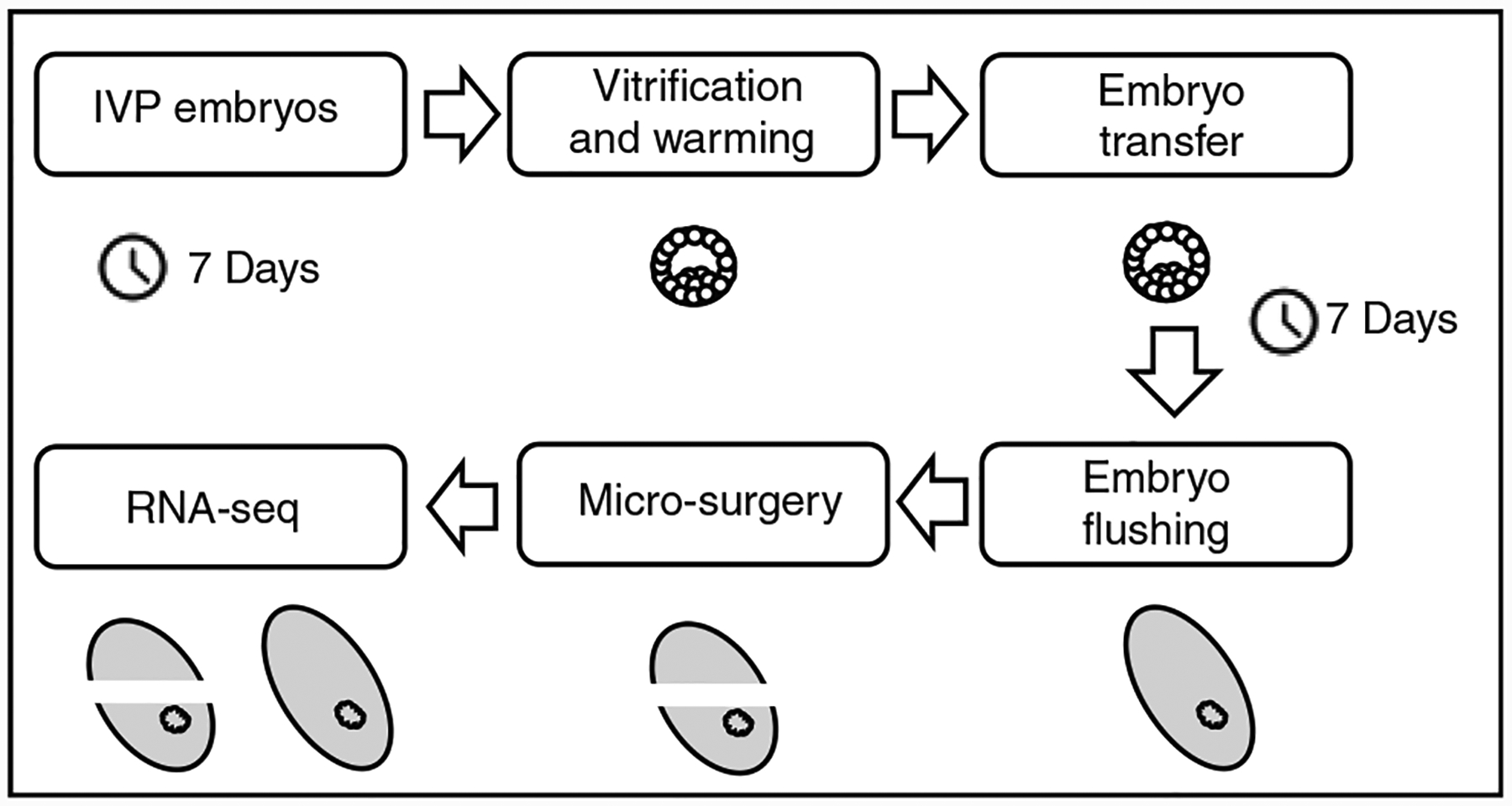
Research design schematic. IVP, in vitro-produced.
Fig. 2.
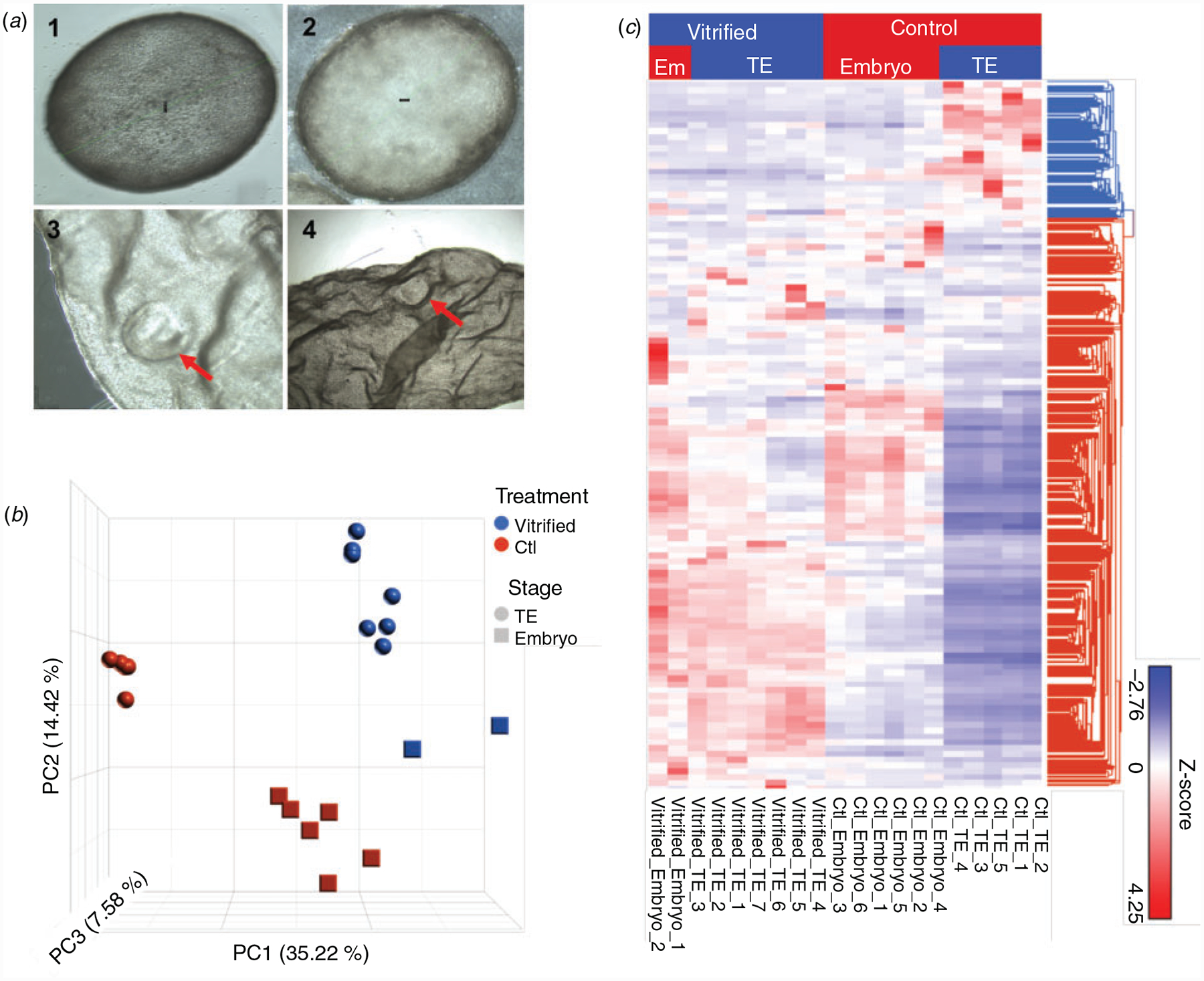
(a) Inset 1, 2: bovine elongating in vivo embryos collected on Day 14 post oestrus with an identifiable embryonic disc. Inset 3, 4: elongated embryo after transfer of non-vitrified blastocyst showing embryonic disc. (b) Principal component (PC) analysis of the transcriptomes for D14 embryos and TE with different treatments demonstrating the reproducibility of the procedures. (c) Hierarchical clustering of differentially expressed genes between fresh and vitrified D14 embryos and TE.
RNA-seq library preparation
The RNA-seq libraries were generated by the Smart-seq2 v4 kit with minor modification of the manufacturer’s instructions. Briefly, whole embryos or isolated TE cells were lysed and mRNA was captured and amplified with the Smart-seq2 v4 kit (Clontech). After AMPure XP beads purification, amplified RNAs were quality checked using the Agilent High Sensitivity D5000 kit (Agilent Technologies). High-quality amplified RNAs were subjected to library preparation (Nextera XT DNA Library Preparation Kit, Illumina) and multiplexed by Nextera XT Indexes (Illumina). The concentration of sequencing libraries was determined by using the Qubit dsDNA HS Assay Kit (Life Technologies) and KAPA Library Quantification kits (KAPA Biosystems). The size of the sequencing libraries was determined by means of High Sensitivity D5000 Assay in a Tapestation 4200 system (Agilent). Pooled indexed libraries were then sequenced on the Illumina HiSeq × platform with 150-bp pair-end reads.
RNA-seq data analysis
The RNA-seq analysis was performed using Partek flow (Partek). Multiplexed sequencing reads that passed quality control filters were trimmed to remove low-quality reads and adaptors. The quality of the reads after filtering was assessed, followed by alignment to the bovine genome UMD3.1.1 by STAR (2.5.3a) with default parameters. Approximately 10 million reads per individual sample were generated. Individual mapped reads were quantified to calculate fragments per kilo-base of exon per million mapped fragments (FPKM) values. Genes with FPKM > 0.1 in at least one sample were kept for downstream analysis, consisting of Pearson correlation, principle component analysis (PCA) and cluster analysis using R (R Foundation for Statistical Computing, version 3.6.0.) Differential gene expression analysis was performed by the Partek Flow GSA algorithm with default parameters. The genes were deemed differentially expressed if they provided a false discovery rate of < 0.05 and fold change > 2. Ingenuity pathway analysis (Qiagen) and KEGG were used to reveal gene ontology and pathways associated with the differentially expressed genes (DEGs) identified.
Results
RNA-seq analysis of D14 embryos and TE isolates
In vitro produced blastocysts (D7) were vitrified–warmed and transferred in groups of 20 into the recipients. Four recipients each were used for transferring non-vitrified (n = 80) and vitrified (n = 80) embryos. In total, 12 (non-vitrified) and 9 (vitrified) D14 embryos were recovered by uterine flushing. To evaluate the effect of vitrification on the transcriptome dynamics of D14 embryos, we profiled the transcriptomes of whole embryos (6 non-vitrified and 2 vitrified embryos with a size of ~2 mm) or isolated TE (4 non-vitrified and 7 vitrified from embryos with a size of ~5 mm) using RNA-seq (Fig. 1). Of note, the attempts to mechanically separate the embryonic disc and TE in this study were not satisfactory. Therefore, smart-seq technology was followed to prepare the RNA-seq libraries from whole embryo and pure TE samples.
Approximately 10 million uniquely mapped reads were obtained on average per sample. The raw FASTQ files and normalised gene expression profiles (FPKM) are available at Gene Expression Omnibus (GEO) (https://www.ncbi.nlm.nih.gov/geo/) under the accession number GSE159891.
Principal component analysis and hierarchical clustering
Principal component analysis of all detected genes was performed to validate the reproducibility of RNA-seq data. All biological replicates showed highly consistent results (Fig. 2b). Principal components 1 (PC1), 2 (PC2), and 3 (PC3) account for 57.22% of the variation in gene expression. To further demonstrate the treatment-dependent nature of the DEGs, these were hierarchically clustered in a heat map (Fig. 2c). As expected, the unbiased hierarchical clustering of all DEGs further showed the separation between treatment groups, which further validated the RNA-seq data.
DEGs
Genes were deemed differentially expressed between treatments if they showed FDR P-value < 0.05 and fold-change > 2. A total of 927 genes were found to be differentially expressed between the vitrified and control embryos. This analysis suggested that vitrification caused the upregulation of 782 genes and the downregulation of 145 (Fig. 3a). In the TE portion, the total DEGs was 4376. A substantial number of genes (4096) were identified as upregulated, but only a small portion (280) were downregulated (Fig. 3b).
Fig. 3.
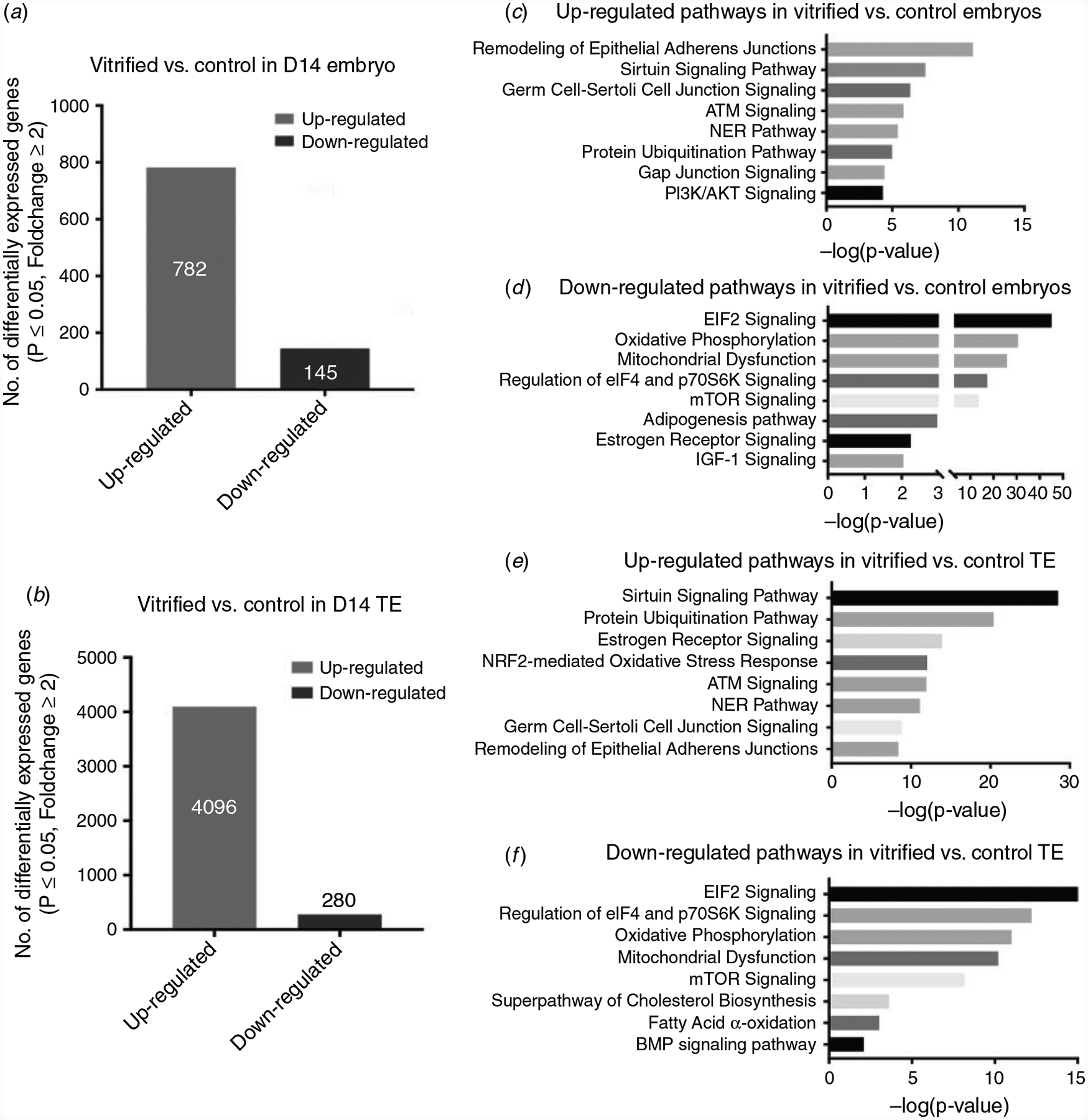
Numbers of differentially expressed genes between fresh and vitrified in D14 embryos (P < 0.05) (a) and TE (b). Ingenuity pathway analysis reveals the main regulated pathways between fresh and vitrified D14 embryos (c, d) and TE (e, f).
Pairwise comparisons to reveal common genes up- and downregulated between the treatments evaluated were assessed through Venn analysis (Fig. 4). There was a substantial overlap of DEGs (671) between upregulated genes in vitrified embryos and TE, which represented 85.8% of the total upregulated genes identified in the whole embryos. Conversely, 61 DEGs were found to be commonly downregulated in the embryos and TE. On the other hand, 21 DEGs were found to be downregulated in embryos and upregulated in TE isolates and only 6 DEGs were found to be upregulated in embryos and downregulated in isolated TE.
Fig. 4.
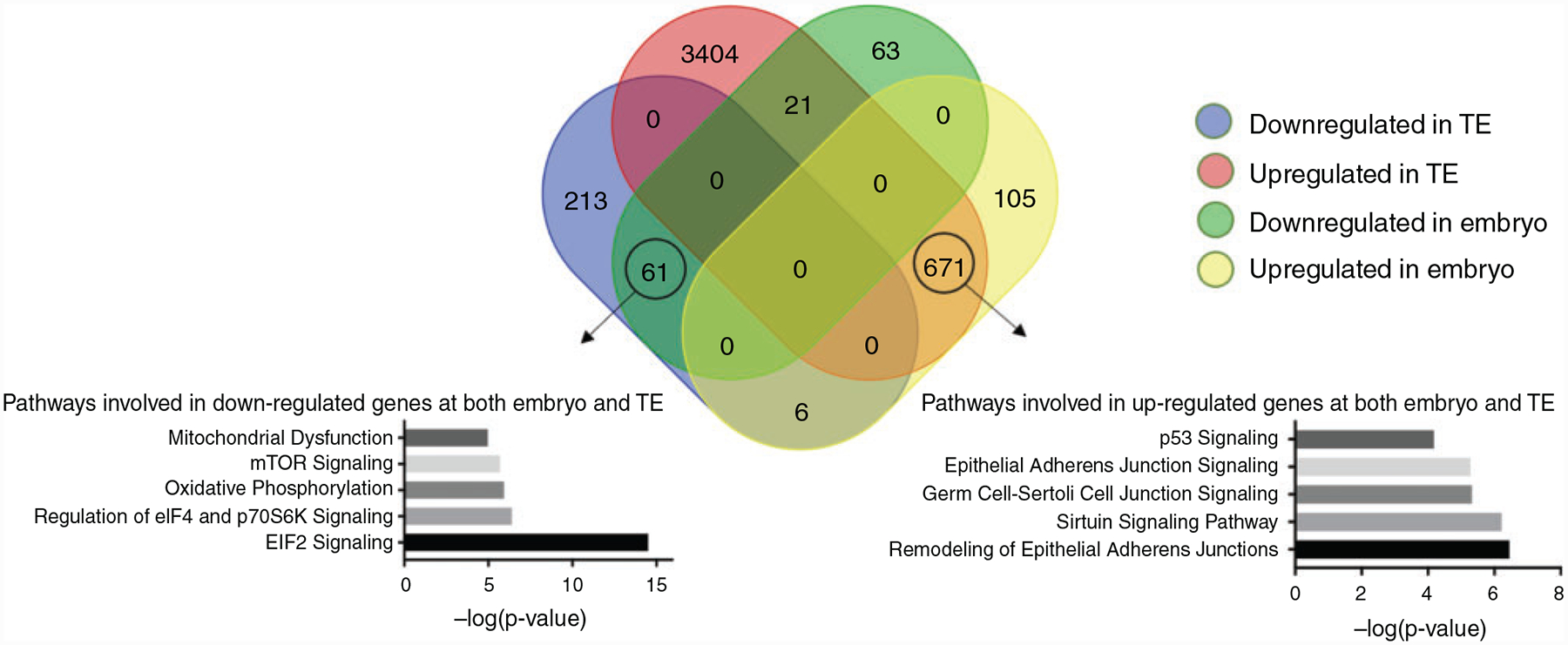
Venn diagram showing the numbers of differentially expressed genes between fresh and vitrified D14 embryos and TE. Pathway analysis based on commonly up- and downregulated genes between fresh and vitrified D14 embryos and TE reveals the main regulated pathways.
GO and pathway analysis of DEGs
A series of pathways were determined to be upregulated as result of vitrification in both TE and whole embryos, including epithelial adherens junctions, sirtuin signalling, germ cell–Sertoli cell junction, ataxia telangiectasia mutated (ATM) signalling, nucleotide excision repair (NER) and protein ubiquitination pathways (Fig. 3c, e).
Other pathways identified as upregulated by vitrification were those associated with cell proliferation, such as cell proliferation, positive regulation of stem cell proliferation, cell cycle, mitotic cell cycle, DNA replication initiation and DNA biosynthesis (Tables 1, 2). In addition, DNA repair mechanisms appeared upregulated, including cellular response to DNA damage stimulus, DNA repair and base excision repair (Tables 1, 2). Also, pathways related to mRNA surveillance and RNA degradation were upregulated in TE isolates of embryos that underwent vitrification. In addition, the p53 signalling pathway and other pathways controlling apoptosis, normally activated under severe DNA damage, also appeared upregulated as a result of vitrification in whole embryos and TE isolates (Tables 1, 2).
Table 1.
Enriched GO terms found upregulated in D14 embryos as a result of vitrification
| GO ID | G0 term | Source | P value | Fold enrichment |
|---|---|---|---|---|
| G0:0006974 | Cellular response to DNA damage stimulus | Embryo | 3.31E-04 | 3.093 |
| TE | 3.81E-06 | 2.072 | ||
| G0:0006281 | DNA repair | Embryo | 9.17E-04 | 2.939 |
| TE | 2.83E-07 | 2.211 | ||
| G0:0006284 | Base-excision repair | Embryo | 3.68E-02 | 3.918 |
| TE | 9.10E-03 | 2.304 | ||
| G0:0006457 | Protein folding | Embryo | 5.70E-03 | 2.637 |
| TE | 1.27E-05 | 2.046 | ||
| G0:0090002 | Establishment of protein localisation to plasma membrane | Embryo | 1.53E-02 | 4.030 |
| TE | 1.19E-02 | 2.437 | ||
| GO:0090004 | Positive regulation of establishment of protein localisation to plasma membrane | Embryo | 2.29E-02 | 4.521 |
| TE | 8.72E-03 | 2.437 | ||
| G0:0031648 | Protein destabilisation | Embryo | 3.68E-02 | 3.918 |
| TE | 1.94E-04 | 2.880 | ||
| G0:0008283 | Cell proliferation | Embryo | 2.06E-02 | 2.108 |
| G0:0007049 | Cell cycle | Embryo | 1.82E-02 | 2.501 |
| G0:2000648 | Positive regulation of stem cell proliferation | Embryo | 4.25E-02 | 8.816 |
| G0:0030388 | fructose 1,6-bisphosphate metabolic process | Embryo | 3.28E-02 | 10.076 |
| G0:0006468 | Protein phosphorylation | Embryo | 3.16E-02 | 2.059 |
| G0:0008637 | Apoptotic mitochondrial changes | Embryo | 1.95E-02 | 6.717 |
| G0:0000278 | Mitotic cell cycle | TE | 1.51E-02 | 2.400 |
| G0:0006270 | DNA replication initiation | TE | 1.33E-03 | 3.017 |
| G0:0071897 | DNA biosynthetic process | TE | 2.57E-04 | 3.840 |
| G0:0061014 | Positive regulation of mRNA catabolic process | TE | 3.51E-02 | 3.600 |
| G0:0006099 | Tricarboxylic acid cycle | TE | 2.58E-05 | 3.323 |
GO, gene ontology; TE, trophectoderm
Table 2.
Additional upregulated pathways identified by KEGG in D14 embryos as a result of vitrification
| KEGG ID | KEGG term | Source | P value | Fold enrichment |
|---|---|---|---|---|
| bta04110 | Cell cycle | Embryo | 1.32E-03 | 2.974 |
| TE | 7.07E-10 | 2.506 | ||
| bta03410 | Base-excision repair | Embryo | 5.27E-03 | 5.200 |
| TE | 1.97E-07 | 3.757 | ||
| bta01200 | Carbon metabolism | Embryo | 1.39E-03 | 3.148 |
| TE | 9.31E-07 | 2.275 | ||
| bta00051 | Fructose and mannose metabolism | Embryo | 2.16E-02 | 4.613 |
| TE | 1.40E-02 | 2.315 | ||
| bta01230 | Biosynthesis of amino acids | Embryo | 3.10E-03 | 3.625 |
| TE | 8.13E-06 | 2.482 | ||
| bta04115 | p53 signalling pathway | Embryo | 3.66E-02 | 2.820 |
| bta05230 | Central carbon metabolism in cancer | Embryo | 1.29E-03 | 4.151 |
| bta00520 | Amino sugar and nucleotide sugar metabolism | Embryo | 6.06E-03 | 4.171 |
| bta04141 | Protein processing in endoplasmic reticulum | Embryo | 2.53E-04 | 2.877 |
| bta03008 | Ribosome biogenesis in eukaryotes | Embryo | 1.21E-08 | 2.808 |
| bta03030 | DNA replication | TE | 1.26E-04 | 2.900 |
| bta03015 | Messenger RNA surveillance pathway | TE | 1.20E-06 | 2.420 |
| bta03018 | RNA degradation | TE | 7.01E-04 | 2.061 |
| bta00020 | Citrate cycle (TCA) | TE | 8.69E-06 | 3.480 |
| bta00240 | Pyrimidine metabolism | TE | 4.38E-06 | 2.264 |
| bta00970 | Aminoacyl-tRNA biosynthesis | TE | 2.90E-06 | 3.045 |
TE, trophectoderm
Moreover, vitrification appeared to upregulate metabolic pathways that are important in cell proliferation and biomass synthesis. The mechanisms involved were fructose and mannose metabolism, carbon metabolism, central carbon metabolism in cancer, pyrimidine and purine metabolism, biosynthesis of amino acids, and protein synthesis, processing, transport/localisation, and destabilisation/degradation (Tables 1, 2). Rapid cell division and biomass synthesis are also characteristic of preimplantation embryos beyond the blastocyst stage.
The commonly downregulated pathways in TE and embryos included EIF2 signalling, oxidative phosphorylation, mitochondrial dysfunction, regulation of eIF4 and P70S6K signalling, and mTOR signalling (Fig. 3d, f).
Discussion
The ability to cryopreserve gametes and embryos has been a valuable tool for reproductive management in all mammalian species, especially farm animals. Vitrification is a commonly used cryopreservation method, which involves exposure of the embryos to high concentrations of cryoprotectants and ultra-low temperatures to generate very rapid cooling rates. These conditions allow the transition of the cryoprotective solution to a glass-like state without formation of intracellular ice crystals. It is widely accepted that IVP embryos show lower cryotolerance than IVD embryos, which has been attributed to the elevated accumulation of lipid droplets during IVC (Abe et al. 2002). Regardless, vitrification has proven to be a better alternative for cryopreserving IVP embryos in many species of farm animals, including cattle (Mahmoudzadeh et al. 1994; Nedambale et al. 2004), sheep (Dos Santos-Neto et al. 2017), pig (reviewed in Berthelot et al. 2003) and horse (Moussa et al. 2005; Choi and Hinrichs 2017).
This study aimed to reveal the transcriptomic dynamics of IVP blastocysts that were vitrified–warmed before transfer and then non-surgically recovered 7 days later (day 14 of development). In this study, we transferred embryos in groups of 20. Although there is evidence that multiple embryo transfer (MET) can intensify uterine signals at day 13 after ovulation (Spencer et al. 2013), Gómez and Muñoz (2015) suggest that MET is a suitable model for revealing aspects of in vivo maternal–embryo interactions because each embryo creates its own microenvironment in the endometrium. Therefore, we assumed that the changes in gene expression of D14 embryos reported in this study were due to the effects of vitrification. Once recovered, D14 embryos were subjected to microsurgery to create isolates of the embryonic disc and TE. However, although TE isolation was successful, the attempts to isolate the embryonic disc were imprecise. Thus, whole embryo and TE isolates were used for library preparation, not embryonic disc isolates.
RNA-seq revealed that a total of 782 and 4096 genes were upregulated in whole embryos and TE isolates, respectively, as an effect of vitrification. Although it is not appropriate to directly compare the number of DGEs between TE and whole embryos given that the TE isolates were actually derived from elongated embryos with a size of ~5 mm compared with the whole embryo with ~2 mm, thus we expected much more effects by vitrification in TE groups as many more genes are dynamically activated while the embryos develop and become more elongated. Of these, 671 were commonly upregulated in the both whole embryos and TE isolates. One of the upregulated pathways was involved in remodelling of epithelial adherens junctions, which are essential during early embryo development for the establishment of cell–cell adhesion in developing sheets of epithelial cells (Pollard et al. 2017). The asymmetric proliferation of stem cells is also regulated by adherens cell junctions, which determine the plane of division by controlling the orientation of the mitotic spindle (Pollard et al. 2017). The sirtuin signalling pathway was also upregulated as an effect of vitrification. Sirtuins are proteins that are involved in several biological functions, including the regulation of chromatin structure, DNA repair, gene expression, cell differentiation, metabolic homeostasis, senescence and apoptosis (reviewed in Rodriguez et al. 2013). SIRT1 is a sirtuin involved in the cellular stress response under metabolic, genotoxic and oxidative stress (Raynes et al. 2013). Interestingly, the SIRT1 gene appeared to be upregulated in TE isolates, indicating that the stress caused by vitrification at the blastocyst stage persisted through the preimplantation embryo development. These results are consistent with those reported by Burkuš et al. (2015), which indicated that exposure of pregnant mice to stress during preimplantation caused impaired development of the conceptus and offspring. In addition, in an experiment carried out in rabbits, it was reported that vitrification of morula-stage embryos affected conceptus development at preimplantation stages, during implantation and after implantation (Vicente et al. 2013).
Preimplantation embryos undergo massive growth in the uterus after hatching (Senger 2012). However, our results indicated that vitrification induced even more rapid cellular proliferation. This observation was supported by pathway analysis revealing several upregulated pathways involved with proliferation, including cell proliferation, cell cycle, mitotic cell cycle, DNA replication initiation and DNA biosynthesis (Tables 1, 2). Moreover, pathways involved in the negative regulation of cell growth appeared to be downregulated in whole embryos after vitrification. Although cells undergoing rapid proliferation have a high risk of DNA damage (Miermont et al. 2019), vitrification has also been shown to directly cause DNA damage. Therefore, it is not surprising that DNA repair mechanisms were upregulated in the D14 embryos and TE isolates from previously vitrified blastocysts. These pathways include cellular responses to DNA damage stimulus, DNA repair and base excision repair (Tables 1, 2). In addition, vitrification upregulated mRNA surveillance pathways and RNA degradation (Table 2). Rapid cell proliferation also involves the synthesis of biomass, including nucleic acids, fatty acids and proteins. In the vitrification groups we found upregulation of multiple pathways involved in synthesis of biomass, including one-carbon metabolism, central carbon metabolism in cancer, pyrimidine and purine metabolism, biosynthesis of amino acids and protein processing in the endoplasmic reticulum (Tables 1, 2). The protein ubiquitination pathway was upregulated in both whole embryos and TE isolates. Ubiquitination serves several functions, including proteolytic protein degradation, gene expression through histone mono-ubiquitination and apoptosis (Orlowski 1999; Callis 2014). This suggests that embryos subjected to vitrification are undergoing high rates of proliferation, which results in DNA damage, triggering many downstream pathways that function to amend genotoxic effects and/or programed cell death.
Another of the most highly upregulated pathways following vitrification and common in both the TE isolates and whole embryos was the ATM signalling pathway. The protein ATM is a kinase that is crucial in maintaining genome integrity. When DNA damage occurs, ATM is activated and induces upregulation of cell cycle checkpoints, causing arrest in order for DNA repair to occur (Stracker et al. 2013). The ATM kinase also participates in senescence and apoptosis. Programmed cell death in response to severe DNA damage is under p53 regulation, the stability of which relies on phosphorylation by ATM and Chk2 (serine/threonine kinase) (Awasthi et al. 2015). One of the most important effects of the high concentration of cryoprotectants used in vitrification is osmotic shock. As a participant in the regulation of cellular homeostasis, the ATM signalling pathway is also activated under osmotic stress, which causes activation of downstream targets, including p53 (Bakkenist and Kastan 2003). In our experiment, both the TP53 gene, which encodes the p53 protein, and the p53 signalling pathway were upregulated (Table 2). In addition, the pathway apoptotic mitochondrial changes were upregulated in the embryos (Table 1). This finding suggested that the effects of vitrification on DNA damage and osmotic stress were inducing apoptosis via the ATM and p53 pathways. Our analysis concords with the results obtained by Sandeep et al. (2018) who vitrified IVP morula-stage buffalo embryos and reported higher apoptosis rates in morula that developed into blastocysts after warming compared with IVP fresh blastocysts. Similarly, Desai et al. (2008) reported a high incidence of apoptosis in vitrified murine blastocysts. In their study they suggested artificial collapse (AC) as a technique to reduce DNA damage and subsequent cell death. In addition, Li et al. (2012) also found less DNA-induced apoptosis using AC, leading to the conclusion that blastocoele collapse before vitrification is a safe approach for improving blastocyst survival in mice and humans. Likewise, AC is probably the best approach for cryopreserving equine D7 and D8 embryos, because the cryoprotectants do not easily diffuse through the embryonic capsule when performing conventional vitrification (Diaz et al. 2016; Diaz et al. 2018). Further research is necessary to elucidate the benefits of AC in bovine embryos undergoing vitrification.
Vitrification resulted in downregulation of 145 and 280 genes in whole embryos and TE isolates, respectively. Pathway analysis revealed that among the common pathways exhibiting the greatest downregulation were those associated with protein translation initiation, stress response and mitochondrial dysfunction. In eukaryotes, translation initiation is probably the most regulated step in protein synthesis. In our study, we found pathways involved in the regulation of translation initiation, including the eIF2, eIF4, P70S6K, and mTOR signalling pathways, to be downregulated as an effect of vitrification.
The eukaryotic initiation factor 4 (eIF4) family is involved in the binding of mRNA to the small ribosomal subunit during the initiation of translation. This is why the eIF4 family is associated with cell growth and proliferation, cellular transformation and inhibition of apoptosis (reviewed in Hernández and Vazquez-Pianzola 2005). Under stress conditions, translation is inactivated through the phosphorylation of eIF4E by p38, a stress-activated mitogen-activated protein kinase (MAPK) (Sheikh and Fornace 1999). The eIF2 functions to bring the Met–tRNAi, which is the first to be integrated into every peptide chain and forms a preinitiation complex with the 40S ribosomal subunit and other initiation factors (1, 1A, 5 and 3) (Schmitt et al. 2010). Similar to eIF4, eIF2 plays a role in translational regulation under stress conditions. Amino acid deficiency and an increase in misfolded proteins are some of the stressors that can cause the phosphorylation of the α subunit of eIF2, leading to inhibition of translation initiation (Wek et al. 2006; Stolboushkina and Garber 2011). p53 is also involved in the control of eIF2α and eIF4E phosphorylation. Activation of p53 by p38 leads to the phosphorylation of eIF2α and dephosphorylation of eIF4E, causing a change that leads to the inhibition of autophagy and activation of apoptotic pathways (Jiang et al. 2014). In addition, it has been shown that tumour necrosis factor and TRAIL (apoptosis-inducing THF-like ligand), two apoptotic inducers, stimulate phosphorylation of eIF2α (Jeffrey et al. 2002). The mTOR (serine/threonine kinase) signalling pathway is also involved in translation initiation through mTORC1 whose major downstream targets are eIF4E-binding proteins and S6 kinase (Saxton and Sabatini 2017). P70S6K is associated with cellular growth by promoting protein synthesis and cell survival through inactivation of BAD, a proapoptotic molecule (Harada et al. 2001). Thus, downregulation of the mTOR pathway and P70S6K is most likely to result in activation of apoptosis. In this experiment, we found in TE isolates that vitrification caused upregulation of the gene MAPK11, which codes for p38β, one of the four p38 MAPK described to date. These findings suggest that at the translational level, the stress response as result of vitrification is being mediated by p38 activity, which might result in higher apoptotic rates.
The effects of vitrification on mitochondria have been long studied. In oocytes, vitrification causes changes in mitochondrial distribution (Rho et al. 2002; Nazmara et al. 2014), mitochondrial DNA copy number (Amoushahi et al. 2017), ATP production (Zhao et al. 2011; Dai et al. 2015) and mitochondrial membrane potential (Lei et al. 2014; Amoushahi et al. 2017). In mouse zygotes (2 pronuclei), Zhao et al. (2009) reported that vitrification had an effect on mitochondrial distribution and mitochondrial membrane potential that was directly associated with ATP production. In rabbit embryos, vitrification caused the differential expression of proteins involved in impaired oxidative metabolism related to ATP synthesis (Garcia-Dominguez et al. 2018). In our study, mitochondrial dysfunction and oxidative phosphorylation pathways were downregulated in the vitrification groups. However, the tricarboxylic acid (TCA) cycle was upregulated (Table 2), suggesting that embryos that underwent vitrification at the blastocyst stage are in an energy imbalance and that the products resulting from the TCA cycle are being used for other purposes such as biomass generation, a requirement in rapid proliferating cells. This metabolic phenotype has been previously proposed to occur in preimplantation embryos (Krisher and Prather 2012).
Conclusion
Embryo cryopreservation is a widely used technique in both human fertility programs and for the propagation of genetics in farm animals. In this study we vitrified in vitro-produced bovine blastocysts, aiming to determine the lasting effects, at the transcriptomic level, of the high concentrations of cryoprotectants and very rapid cooling rates at D14 of development. As part of the study, we wanted to describe the effects on the two most marked cell populations at this stage, the embryonic disc and TE; however, our attempts to separate these two cell types were not satisfactory. Thus, whole embryos and TE isolates were used to create libraries for RNA-seq analysis. Results indicated that a substantial number of genes were differentially expressed between the vitrified and control groups. Among the identified pathways were those involved in the cellular stress response, cell proliferation, DNA repair, control of translation initiation and mitochondrial dysfunction. We conclude that blastocyst vitrification induced rapid cell proliferation in D14 embryos with a high risk of DNA damage leading to the activation of DNA repair mechanisms or apoptosis. Also, D14 embryos that had previously undergone vitrification seem to be in a negative energy balance, redirecting most of the energy to the synthesis of cellular components. Finally, although viable offspring are born from vitrified embryos, we do not know the lasting genetic effects. This study indicates metabolic irregularities and increased risk of genetic errors that persist for at least the first 7 days following vitrification. These may lead to increased risk of spontaneous abortion or lifelong issues if they are not resolved during fetal development. As such, further investigation on the lasting genetic expression effects of vitrification and how we can mitigate these are warranted.
Acknowledgement
This work was supported by the USDA-NIFA grant (2019-67016-29863), the LSU-ACRES (1920R0447), NICHD (1R01HD102533), and USDA-NIFA W4171.
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
References
- Abe H, Yamashita S, Satoh T, and Hoshi H (2002). Accumulation of cytoplasmic lipid droplets in bovine embryos and cryotolerance of embryos developed in different culture systems using serum-free or serum-containing media. Mol. Reprod. Dev 61(1), 57–66. doi: 10.1002/MRD.1131 [DOI] [PubMed] [Google Scholar]
- Aksu DA, Agca C, Aksu S, Bagis H, Akkoc T, Caputcu AT, Arat S, Taskin AC, Kizil SH, Karasahin T, Akyol N, Satilmis M, Sagirkaya H, Ustuner B, Nur Z, and Agca Y (2012). Gene expression profiles of vitrified in vitro- and in vivo-derived bovine blastocysts. Mol. Reprod. Dev 79(9), 613–625. doi: 10.1002/MRD.22068 [DOI] [PubMed] [Google Scholar]
- Amoushahi M, Salehnia M, and Mowla SJ (2017). Vitrification of Mouse MII Oocyte Decreases the Mitochondrial DNA Copy Number, TFAM Gene Expression and Mitochondrial Enzyme Activity. J. Reprod. Infertil 18(4), 343–351. [PMC free article] [PubMed] [Google Scholar]
- Awasthi P, Foiani M, and Kumar A (2015). ATM and ATR signaling at a glance. J. Cell Sci 128(23), 4255–4262. doi: 10.1242/JCS.169730 [DOI] [PubMed] [Google Scholar]
- Bakkenist CJ, and Kastan MB (2003). DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature 421(6922), 499–506. doi: 10.1038/NATURE01368 [DOI] [PubMed] [Google Scholar]
- Bartolac LK, Lowe JL, Koustas G, Grupen CG, and Sjöblom C (2018). Vitrification, not cryoprotectant exposure, alters the expression of developmentally important genes in in vitro produced porcine blastocysts. Cryobiology 80, 70–76. doi: 10.1016/J.CRYOBIOL.2017.12.001 [DOI] [PubMed] [Google Scholar]
- Bedzhov I, Leung CY, Bialecka M, and Zernicka-Goetz M (2014). In vitro culture of mouse blastocysts beyond the implantation stages. Nat. Protoc 9(12), 2732–2739. doi: 10.1038/NPROT.2014.186 [DOI] [PubMed] [Google Scholar]
- Berthelot F, Martinat-Botté F, Vajta G, and Terqui M (2003). Cryopreservation of porcine embryos: state of the art. Livest. Prod. Sci 83(1), 73–83. doi: 10.1016/S0301-6226(03)00038-1 [DOI] [Google Scholar]
- Blockeel C, Campbell A, Coticchio G, Esler J, Garcia-Velasco JA, Santulli P, and Pinborg A (2019). Should we still perform fresh embryo transfers in ART? Hum. Reprod 34(12), 2319–2329. doi: 10.1093/HUMREP/DEZ233 [DOI] [PubMed] [Google Scholar]
- Boonkusol D, Gal AB, Bodo S, Gorhony B, Kitiyanant Y, and Dinnyes A (2006). Gene expression profiles and in vitro development following vitrification of pronuclear and 8-cell stage mouse embryos. Mol. Reprod. Dev 73(6), 700–708. doi: 10.1002/MRD.20450 [DOI] [PubMed] [Google Scholar]
- Burkuš J, Kačmarová M, Kubandová J, Kokošová N, Fabianová K, Fabian D, Koppel J, and Čikoš Š (2015). Stress exposure during the preimplantation period affects blastocyst lineages and offspring development. J. Reprod. Dev 61(4), 325–331. doi: 10.1262/JRD.2015-012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callis J (2014). The ubiquitination machinery of the ubiquitin system. Arabidopsis Book 12, e0174. doi: 10.1199/TAB.0174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee A, Saha D, Niemann H, Gryshkov O, Glasmacher B, and Hofmann N (2017). Effects of cryopreservation on the epigenetic profile of cells. Cryobiology 74, 1–7. doi: 10.1016/J.CRYOBIOL.2016.12.002 [DOI] [PubMed] [Google Scholar]
- Choi Y-H, and Hinrichs K (2017). Vitrification of in vitro-produced and in vivo-recovered equine blastocysts in a clinical program. Theriogenology 87, 48–54. doi: 10.1016/J.THERIOGENOLOGY.2016.08.005 [DOI] [PubMed] [Google Scholar]
- Dai J, Wu C, Muneri CW, Niu Y, Zhang S, Rui R, and Zhang D (2015). Changes in mitochondrial function in porcine vitrified MII-stage oocytes and their impacts on apoptosis and developmental ability. Cryobiology 71(2), 291–298. doi: 10.1016/J.CRYOBIOL.2015.08.002 [DOI] [PubMed] [Google Scholar]
- de Oliveira Leme L, Dufort I, Spricigo JFW, Braga TF, Sirard M-A, Franco MM, and Dode MAN (2016). Effect of vitrification using the Cryotop method on the gene expression profile of in vitro-produced bovine embryos. Theriogenology 85(4), 724733.e1. doi: 10.1016/J.THERIOGENOLOGY.2015.10.016 [DOI] [PubMed] [Google Scholar]
- Deglincerti A, Croft GF, Pietila LN, Zernicka-Goetz M, Siggia ED, and Brivanlou AH (2016). Self-organization of the in vitro attached human embryo. Nature 533(7602), 251–254. doi: 10.1038/NATURE17948 [DOI] [PubMed] [Google Scholar]
- Desai N, Szeptycki J, Scott M, AbdelHafez FF, and Goldfarb J (2008). Artificial Collapse of Blastocysts Before Vitrification: Mechanical vs. Laser Technique and Effect on Survival, Cell Number, and Cell Death in Early and Expanded Blastocysts. Cell Preserv. Technol 6(3), 181–190. doi: 10.1089/CPT.2008.0007 [DOI] [Google Scholar]
- Diaz F, Bondiolli K, Paccamonti D, and Gentry GT (2016). Cryopreservation of Day 8 equine embryos after blastocyst micromanipulation and vitrification. Theriogenology 85(5), 894–903. doi: 10.1016/J.THERIOGENOLOGY.2015.10.039 [DOI] [PubMed] [Google Scholar]
- Diaz FA, Gutierrez EJ, Cramer E, Paccamonti DL, Gentry GT, and Bondioli KR (2018). Pregnancy Rates Following Low-Temperature Storage of Large Equine Embryos Before Vitrification. J. Equine Vet. Sci 64, 12–16. doi: 10.1016/J.JEVS.2018.01.009 [DOI] [PubMed] [Google Scholar]
- Dochi O (2019). Direct transfer of frozen-thawed bovine embryos and its application in cattle reproduction management. J. Reprod. Dev 65(5), 389–396. doi: 10.1262/JRD.2019-025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dos Santos-Neto PC, Cuadro F, Barrera N, Crispo M, and Menchaca A (2017). Embryo survival and birth rate after minimum volume vitrification or slow freezing of in vivo and in vitro produced ovine embryos. Cryobiology 78, 8–14. doi: 10.1016/J.CRYOBIOL.2017.08.002 [DOI] [PubMed] [Google Scholar]
- Frijters ACJ, Mullaart E, Roelofs RMG, van Hoorne RP, Moreno JF, Moreno O, and Merton JS (2009). What affects fertility of sexed bull semen more, low sperm dosage or the sorting process? Theriogenology 71(1), 64–67. doi: 10.1016/J.THERIOGENOLOGY.2008.09.025 [DOI] [PubMed] [Google Scholar]
- Garcia-Dominguez X, Peñaranda DS, Estruch G, Blanca J, García-Carpintero V, Cañizares J, Marco-Jiménez F, and Vicente JS (2018). Long-term phenotypic effects following vitrified-thawed embryo transfer in a rabbit model. bioRxiv 410514. doi: 10.1101/410514 [DOI] [Google Scholar]
- Gómez E, and Muñoz M (2015). Multiple-embryo transfer for studying very early maternal–embryo interactions in cattle. Reproduction 150(2), R35–R43. doi: 10.1530/REP-14-0465 [DOI] [PubMed] [Google Scholar]
- Grothmann H (2019) 46th Annual Conference of the IETS. In ‘Embryo Technology Newsletter. Vol. 37.’ (International Embryo Technology Society; Champaign, IL: ) [Google Scholar]
- Gupta A, Singh J, Dufort I, Robert C, Dias FCF, and Anzar M (2017). Transcriptomic difference in bovine blastocysts following vitrification and slow freezing at morula stage. PLoS One 12(11), e0187268. doi: 10.1371/JOURNAL.PONE.0187268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada H, Andersen JS, Mann M, Terada N, and Korsmeyer SJ (2001). p70S6 kinase signals cell survival as well as growth, inactivating the pro-apoptotic molecule BAD. Proc. Natl. Acad. Sci. USA 98(17), 9666–9670. doi: 10.1073/PNAS.171301998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández G, and Vazquez-Pianzola P (2005). Functional diversity of the eukaryotic translation initiation factors belonging to eIF4 families. Mech. Dev 122(7–8), 865–876. doi: 10.1016/J.MOD.2005.04.002 [DOI] [PubMed] [Google Scholar]
- Jeffrey IW, Bushell M, Tilleray VJ, Morley S, and Clemens MJ (2002). Inhibition of protein synthesis in apoptosis: differential requirements by the tumor necrosis factor alpha family and a DNA-damaging agent for caspases and the double-stranded RNA-dependent protein kinase. Cancer Res. 62(8), 2272–2280. [PubMed] [Google Scholar]
- Jiang Q, Li F, Shi K, Wu P, An J, Yang Y, and Xu C (2014). Involvement of p38 in signal switching from autophagy to apoptosis via the PERK/eIF2α/ATF4 axis in selenite-treated NB4 cells. Cell Death Dis. 5(5), e1270. doi: 10.1038/CDDIS.2014.200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krisher RL, and Prather RS (2012). A role for the Warburg effect in preimplantation embryo development: metabolic modification to support rapid cell proliferation. Mol. Reprod. Dev 79(5), 311–320. doi: 10.1002/MRD.22037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei T, Guo N, Tan M. h., and Li Y. f. (2014). Effect of mouse oocyte vitrification on mitochondrial membrane potential and distribution. Journal of Huazhong University of Science and Technology [Medical Sciences] 34(1), 99–102. doi: 10.1007/S11596-014-1238-8 [DOI] [PubMed] [Google Scholar]
- Leibo SP (2008). Cryopreservation of oocytes and embryos: Optimization by theoretical versus empirical analysis. Theriogenology 69(1), 37–47. doi: 10.1016/J.THERIOGENOLOGY.2007.10.006 [DOI] [PubMed] [Google Scholar]
- Li L, Zhang X, Zhao L, Xia X, and Wang W (2012). Comparison of DNA apoptosis in mouse and human blastocysts after vitrification and slow freezing. Mol. Reprod. Dev 79(3), 229–236. doi: 10.1002/MRD.22018 [DOI] [PubMed] [Google Scholar]
- Mahmoudzadeh AR, Van Soom A, Ysebaert MT, and de Kruif A (1994). Comparison of two-step vitrification versus controlled freezing on survival of in vitro produced cattle embryos. Theriogenology 42(8), 1389–1397. doi: 10.1016/0093-691X(94)90259-L [DOI] [Google Scholar]
- Miermont A, Antolović V, Lenn T, Nichols JME, Millward LJ, and Chubb JR (2019). The fate of cells undergoing spontaneous DNA damage during development. Development 146(12), dev174268. doi: 10.1242/DEV.174268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussa M, Bersinger I, Doligez P, Guignot F, Duchamp G, Vidament M, Mermillod P, and Bruyas JF (2005). In vitro comparisons of two cryopreservation techniques for equine embryos: Slow-cooling and open pulled straw (OPS) vitrification. Theriogenology 64(7), 1619–1632. doi: 10.1016/J.THERIOGENOLOGY.2005.04.001 [DOI] [PubMed] [Google Scholar]
- Moussa M, Yang C-Y, Zheng H-Y, Li M-Q, Yu N-Q, Yan S-F, Huang J-X, and Shang J-H (2019). Vitrification alters cell adhesion related genes in pre-implantation buffalo embryos: Protective role of β-mercaptoethanol. Theriogenology 125, 317–323. doi: 10.1016/J.THERIOGENOLOGY.2018.11.013 [DOI] [PubMed] [Google Scholar]
- Nazmara Z, Salehnia M, and HosseinKhani S (2014). Mitochondrial distribution and ATP content of vitrified, in vitro matured mouse oocytes. Avicenna J. Med. Biotechnol 6(4), 210–217. [PMC free article] [PubMed] [Google Scholar]
- Nedambale TL, Dinnyés A, Groen W, Dobrinsky JR, Tian XC, and Yang X (2004). Comparison on in vitro fertilized bovine embryos cultured in KSOM or SOF and cryopreserved by slow freezing or vitrification. Theriogenology 62(3–4), 437–449. doi: 10.1016/J.THERIOGENOLOGY.2003.10.020 [DOI] [PubMed] [Google Scholar]
- Orlowski RZ (1999). The role of the ubiquitin-proteasome pathway in apoptosis. Cell Death Differ. 6(4), 303–313. doi: 10.1038/SJ.CDD.4400505 [DOI] [PubMed] [Google Scholar]
- Pollard TD, Earnshaw WC, Lippincott-Schwartz J, and Johnson GT (2017) Chapter 31 - Intercellular Junctions. In ‘Cell Biology (Third Edition).’ (Eds Pollard TD, Earnshaw WC, Lippincott-Schwartz J and Johnson GT) pp. 543–553. (Elsevier; ) [Google Scholar]
- Rall WF, and Fahy GM (1985). Ice-free cryopreservation of mouse embryos at −196 [deg]C by vitrification. Nature 313(6003), 573–575. doi: 10.1038/313573A0 [DOI] [PubMed] [Google Scholar]
- Raynes R, Brunquell J, and Westerheide SD (2013). Stress Inducibility of SIRT1 and Its Role in Cytoprotection and Cancer. Genes Cancer 4(3–4), 172–182. doi: 10.1177/1947601913484497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rho G-J, Kim S, Yoo J-G, Balasubramanian S, Lee H-J, and Choe S-Y (2002). Microtubulin configuration and mitochondrial distribution after ultra-rapid cooling of bovine oocytes. Mol. Reprod. Dev 63(4), 464–470. doi: 10.1002/MRD.10196 [DOI] [PubMed] [Google Scholar]
- Rodriguez RM, Fernandez AF, and Fraga MF (2013). Role of sirtuins in stem cell differentiation. Genes Cancer 4(3–4), 105–111. doi: 10.1177/1947601913479798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandeep RKD, Pratap SA, Mohan JL, and Chandra BC (2018). Developmental Potential and Apoptosis Incidence of In Vitro Produced Buffalo Embryos Vitrified by Solid Surface Technique. Journal of Animal Research 8(4), 571–577. doi: 10.30954/2277-940X.08.2018.4 [DOI] [Google Scholar]
- Saxton RA, and Sabatini DM (2017). mTOR Signaling in Growth, Metabolism, and Disease. Cell 168(6), 960–976. doi: 10.1016/J.CELL.2017.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt E, Naveau M, and Mechulam Y (2010). Eukaryotic and archaeal translation initiation factor 2: a heterotrimeric tRNA carrier. FEBS Lett. 584(2), 405–412. doi: 10.1016/J.FEBSLET.2009.11.002 [DOI] [PubMed] [Google Scholar]
- Senger PL (2012) ‘Pathways to pregnancy & parturition (Third Edition).’ (Current Conceptions, Inc.) [Google Scholar]
- Shahbazi MN, Jedrusik A, Vuoristo S, Recher G, Hupalowska A, Bolton V, Fogarty NNM, Campbell A, Devito L, Ilic D, Khalaf Y, Niakan KK, Fishel S, and Zernicka-Goetz M (2016). Self-organization of the human embryo in the absence of maternal tissues. Nat. Cell Biol 18(6), 700–708. doi: 10.1038/NCB3347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheikh MS, and Fornace AJ (1999). Regulation of translation initiation following stress. Oncogene 18(45), 6121–6128. doi: 10.1038/SJ.ONC.1203131 [DOI] [PubMed] [Google Scholar]
- Sjunnesson Y (2019). In vitro fertilisation in domestic mammals: a brief overview. Ups. J. Med. Sci 125, 68–76. doi: 10.1080/03009734.2019.1697911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer TE, Forde N, Dorniak P, Hansen TR, Romero JJ, and Lonergan P (2013). Conceptus-derived prostaglandins regulate gene expression in the endometrium prior to pregnancy recognition in ruminants. Reproduction 146(4), 377–387. doi: 10.1530/REP-13-0165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolboushkina EA, and Garber MB (2011). Eukaryotic type translation initiation factor 2: Structure–functional aspects. Biochemistry (Mosc.) 76(3), 283–294. doi: 10.1134/S0006297911030011 [DOI] [PubMed] [Google Scholar]
- Stracker TH, Roig I, Knobel PA, and Marjanović M (2013). The ATM signaling network in development and disease. Front. Genet 4, 37. doi: 10.3389/FGENE.2013.00037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicente JS, Saenz-de-Juano MD, Jiménez-Trigos E, Viudes-de-Castro MP, Peñaranda DS, and Marco-Jiménez F (2013). Rabbit morula vitrification reduces early foetal growth and increases losses throughout gestation. Cryobiology 67(3), 321–326. doi: 10.1016/J.CRYOBIOL.2013.09.165 [DOI] [PubMed] [Google Scholar]
- Wek RC, Jiang HY, and Anthony TG (2006). Coping with stress: eIF2 kinases and translational control. Biochem. Soc. Trans 34(1), 7–11. doi: 10.1042/BST0340007 [DOI] [PubMed] [Google Scholar]
- Wong KM, Mastenbroek S, and Repping S (2014). Cryopreservation of human embryos and its contribution to in vitro fertilization success rates. Fertil. Steril 102(1), 19–26. doi: 10.1016/J.FERTNSTERT.2014.05.027 [DOI] [PubMed] [Google Scholar]
- Xu J, Chaubal SA, and Du F (2009). Optimizing IVF with sexed sperm in cattle. Theriogenology 71(1), 39–47. doi: 10.1016/J.THERIOGENOLOGY.2008.09.012 [DOI] [PubMed] [Google Scholar]
- Zhao XM, Fu XW, Hou YP, Yan CL, Suo L, Wang YP, Zhu HB, Dinnyés A, and Zhu SE (2009). Effect of vitrification on mitochondrial distribution and membrane potential in mouse two pronuclear (2-PN) embryos. Mol. Reprod. Dev 76(11), 1056–1063. doi: 10.1002/MRD.21064 [DOI] [PubMed] [Google Scholar]
- Zhao X-M, Du W-H, Wang D, Hao H-S, Liu Y, Qin T, and Zhu H-B (2011). Recovery of mitochondrial function and endogenous antioxidant systems in vitrified bovine oocytes during extended in vitro culture. Mol. Reprod. Dev 78(12), 942–950. doi: 10.1002/MRD.21389 [DOI] [PubMed] [Google Scholar]


