Abstract
The situation of coronavirus disease 2019 (COVID-19) pandemic is rapidly evolving, and medical researchers around the globe are dedicated to finding cures for the disease. Drug repurposing, as an efficient way for drug development, has received a lot of attention. However, the huge amount of studies makes it challenging to keep up to date with the literature on COVID-19 therapeutic development. This review addresses this challenge by grouping the COVID-19 drug repurposing research into three large groups, including clinical trials, computational research, and in vitro protein binding experiments. Particularly, in order to facilitate future drug discovery and the creation of effective drug combinations, drugs are organized by their mechanisms of action and reviewed by their efficacy measured by clinical trials. Providing this subtyping information, we hope this review would serve the scientists, clinicians, and the pharmaceutical industry who are looking at the new therapeutics for COVID-19 treatment.
Keywords: COVID-19, drug repurposing, clinical trial, computational research, in vitro protein interaction assay
Introduction
COVID-19 is an acute respiratory disease caused by the RNA virus SARS-CoV-2. Since its first outbreak in Wuhan, China, the disease has rapidly spread to more than 180 countries around the world. The World Health Organization (WHO) declared it as a public health emergency on Jan 30, 2020, and assessed it as a pandemic on Mar 11, 2020. The situation of the COVID-19 pandemic is continuously evolving. According to WHO COVID-19 situation report No.134 published on Jun 2, 2020, there have been 6.19 million confirmed cases worldwide, and the disease has taken 376,320 lives. Effective treatments are in urgent need, but currently no drug with stable performance has been found for COVID-19.
Medical researchers around the globe are dedicated to understanding and finding cures for the disease. By the time this review is written, there are 3,153 COVID-19 related studies listed on the World Health Organization’s International Clinical Trials Registry Platform (WHO ICTRP), which include studies from countries other than the US, and 1,963 studies listed on ClinicalTrials.gov, which include the US clinical trials only. Among the studies in the US with their study phases documented, most of them are in Phase 2 or Phase 3, and more than 600 drug interventions are included in these trials. Drug repurposing, defined as finding new indications for existing drugs 1, is of particular interest for coping with the COVID-19 urgency. Compared with developing drugs de novo, which was estimated to cost 10–17 years and 800 million USD 1, drug repurposing significantly reduces both the time and money needed as the lengthy and costly ADMET (Absorption, Distribution, Metabolism, Elimination, Toxicity) evaluation can be avoided. If succeeded, this would result in readily available and comparatively affordable medical treatments for COVID-19. Repurposing existing drugs to treat COVID-19 is biologically feasible since SARS-CoV-2 shares some similarities with other coronaviruses such as SARS-CoV and MERS-CoV 2, and there are many successful precedents in repurposing antivirals for new virus targets 3. Actually, most of the drugs currently in clinical trials for COVID-19 are repurposed from approved antiviral drugs. Additionally, with the help of advancing computational methods and mature protein interaction assays, finding potential drug repurposing targets from currently approved drugs or drug candidates.
As a global emergency, the COVID-19 pandemic leads to an explosion of publications, and the research situation is largely unorganized and unstructured: the results of large-scale controlled clinical trials are still on the way, while the results of smaller-scale clinical trials usually contradict with each other. Inevitably, overlapping or similar works exist in computational studies. The amount and complexity of current studies make it hard to keep up to date with the literature on COVID-19 therapeutic development. In the hope to help reduce double efforts, in this review, we grouped the COVID-19 drug repurposing research into three large categories, including clinical trials, computational research, and in vitro experimental studies (Figure 1). In the clinical trial group, drugs are organized and reviewed by their mechanisms of action, which we hope is informative to the discovery of drugs of similar mechanisms and the creation of combinatory treatment. In the computational research group, methods are sorted by their target proteins, and proposed drugs are listed out to prevent duplicated efforts.
Figure 1.
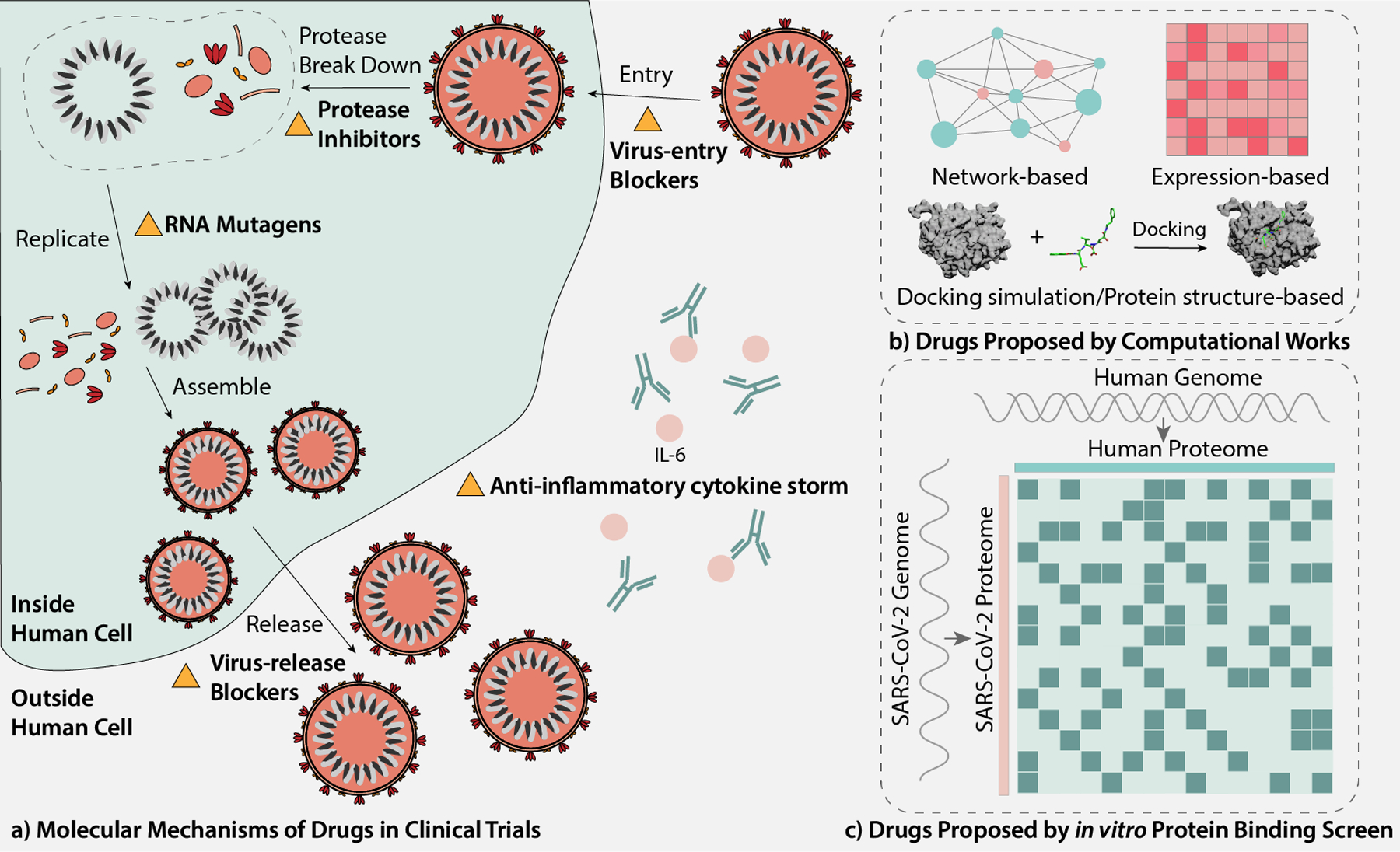
Overview of the review. (a) Molecular mechanisms of drugs in clinical trials. (b) Drugs proposed by computational works. (Molecular docking figure credits to wikipedia Docking (molecular) webpage [https://en.wikipedia.org/wiki/Docking_(molecular)].) (c) Drugs proposed by in vitro protein binding screen.
Drugs in clinical trials for COVID-19.
To facilitate the completeness of this review, we hand-curated the drugs currently on clinical trials by mapping the FDA drug database and PubChem repository. Then, for each identified drug, we screened through literature that reported clinical trial results on PubMed using the drug name plus “COVID-19”. We also searched for ongoing clinical trials for each drug on NIH ClinicalTrials website using the same searching phrases. Of note, trials suspended, withdrawn, terminated, and completed are not included as ongoing trials. For this section, the drugs are organized and reviewed based on their molecular mechanisms. A summary of the type, cohort, drug doses and outcome of the clinical trials mentioned in this section is presented in Table 1.
Table 1.
Summary of clinical trials
| Author | Type of Study | Cohort | Drugs and Doses | Results |
|---|---|---|---|---|
| remdesivir | ||||
| Grein et al. | Compassionate | 61 patients, oxygen saturation <= 94% or receiving oxygen support | A 10-day course of remdesivir treatment, 200mg intravenously on day 1, 100mg for next 9 days | During a median follow up of 18 days, 36 of 53 (68%) patients improved in terms of the oxygen support class |
| Wang et al. | Randomized, double-blind, placebo-controlled, multicenter | 237 patients, age >= 18, symptom onset <= 12 days, oxygen saturation <= 94%, radiologically confirmed pneumonia | A 10-day course of remdesivir treatment, 200mg intravenously on day 1, 100mg for next 9 days vs. the same amount of placebo | Remdesivir use was not associated with a difference in time to clinical improvement; Patients whose symptom onset <=10 days treated with remdesivir showed a numerically faster time to clinical improvement compared to placebo (not significant) |
| Antinori et al. | Compassionate | 35 patients, 18 in ICU and 17 in the normal ward, age >= 18, oxygen saturation <= 94% or NEWS2 >= 4 or receiving oxygen support | A 10-day course of remdesivir treatment, 200mg intravenously on day 1, 100mg for next 9 days; existing treatments including HCQ was continued, but LPV/r was discontinued | On day 28, 14 patients from the normal ward were discharged, 2 still hospitalized and 1 died, while 6 patients in ICU were discharged, 3 still hospitalized and 8 died. |
| Beigel et al. | Randomized, double-blind, placebo-controlled, multicenter | 1063 patients, age >= 18, oxygen saturation <= 94% or radiologically confirmed pneumonia or receiving oxygen support | A 10-day course of remdesivir treatment, 200mg intravenously on day 1, 100mg for next 9 days vs. the same amount of placebo | Patients with remdesivir had a median to recovery time of 11 days, while patients with placebo had a median to recovery time of 15 days (p<0.001) |
| favipiravir | ||||
| Cai et al. | Open-label, nonrandomized,controlled | 80 patients, age 16–75, disease onset <= 7 days, no severe clinical condition | FPV: oral FPV, 14-day course of treatment, 1600 mg on day 1, twice daily; 600 mg on day 2–14, twice daily; plus interferon (IFN)-a by aerosol inhalation (5 million U twice daily) LPV/r (control): 14-day course of treatment, 400mg/100mg on day 1–14, twice daily; plus interferon (IFN)-a by aerosol inhalation (5 million U twice daily) |
FPV group showed shorter viral clearance time (4(2.5–9) days compared to 11(8–13) days, p < 0.001); FPV group showed improvement in chest imaging (improvement rate of 91.43% compared to 62.22%, p = 0.004) |
| Chen et al. | Prospective, randomized, controlled, open-label, multicenter | 240 patients, age >= 18, initial symptoms <= 12 days | FPV: 1200mg * 2 on day 1 and 600mg * 2 for day 2–10 + conventional therapy Arbidol (control): 200mg * 3/day for 10 days + conventional therapy |
No statistically significant difference in clinical recovery rate on day 7 (71/116 compared to 62/120, p = 0.1396); FPV led to shorter latencies to the relief of pyrexia and cough (p < 0.0001) |
| ribavirin | ||||
| Yuan et al. | Retrospective (analysis of electronic medical reports) | 94 patients | IFN-ɑ, LPV/r, ribavirin, arbidol, FPV, human γ globulin, glucocorticoid | IFN-ɑ + LPV/r and IFN-ɑ + LPV/r + ribavirin treated patients showed a positive correlation between mRNA clearance rate and length of hospital stay |
| Hung et al. | Prospective, open-label, randomized, multicenter, phase 2 | 127 patients, age >= 18, NEWS2 >= 1, symptom onset <= 14 | Combination group: LPV/r 400mg/100mg every 12h + ribavirin 400mg every 12h + IFN-β1b 8 million U every alternate day for 14 days Control group: LPV/r 400mg/100mg every 12h for 14 days |
Combination group had shorter time from the beginning of treatment to negative nasopharyngeal swab (7(5–11) compared to 12(8–15), p = 0.0010) |
| ritonavir-lopinavir | ||||
| Yan et al. | Retrospective | 120 patients | LPV/r: oral, 400mg/100mg, twice daily | Older age and lack of LPV/r treatment lead to prolonged viral shedding independently; Early administration (onset time <= 10 days) of LPV/r can shorten viral shedding (p<0.001) |
| Ye and Luo et al. | Controlled, non-randomized | 47 patients, age 5–68 | Test group: LPV/r 400mg/100mg twice daily or 800mg/200mg once a day with food + adjuvant drugs Control group: Adjuvant drugs including interferon aerosol inhalation and arbidol tablets |
Body temperature decreased faster in the test group (not significant); The abnormal proportion of WBC, lymphocytes, CRP and PLT in the test group was lower than the control group after 3 treatments; The test group had a shorter time before RNA test turns negative (p=0.0219) |
| Cao et al. | Randomized, controlled, open-label | 199 patients, age >= 18, oxygen saturation <= 94% | Test group: LPV/r 400mg/100mg twice daily for 14 days + standard care Control group: standard care |
Time to clinical improvement, mortality rate at day 28, and percentage of patients with positive RNA test at multiple time points were similar in two groups. |
| Wen et al. (full-text not accessible) |
Retrospective | 178 patients | LPV/r group Arbidol group Combination group Control group: conventional treatment |
No significant difference in the rate of negative conversion, clinical improvement, and CT improvement among the 4 groups; Significant difference in the proportion of changing from mild/moderate to severe/critical on day 7 (p=0.017), LPV/r and control group had a smaller proportion of deterioration changing |
| Deng et al. | Retrospective | 33 patients, age>=18, without invasive ventilation | Combination group: arbidol 200mg every 8h, LPV/r 400mg/100mg every 12h; Monotherapy group: LPV/r 400mg/100mg every 12h; Until RNA test negative for 3 times (5–21 days) |
Combination group had higher viral clearance rate on day 7 (p<0.05) and day 14 (p<0.05); Combination group had higher chest image improving rate at day 7 (p<0.05) |
| Zhu et al. | Retrospective | 50 patients, no severe pneumonia or ARDS | LPV/r group: LPV/r 400mg/100mg twice daily for 7 days Arbidol group: 0.2g arbidol, 3 times daily for 7 days All patients received conventional therapy |
No difference in fever duration (p=0.61); arbidol group had less viral load on day 14 (0% compared to 44.1%); arbidol group had shorter RNA test positive duration (p<0.01) |
| Chloroquine | ||||
| Gao et al. | Not declared | More than 100 patients | Not declared | Chloroquine treatment is superior to control treatment in CT improvement and shortening the disease course (No details described) |
| Huang et al. | Controlled, randomized | 22 patients, age >= 18 | Chloroquine: 500mg orally twice daily for 10 days; LPV/r (control): 400mg/100mg orally twice daily for 10 days |
Chloroquine group had shorter time for RNA test to turn negative, faster CT improvement, and shorter time to discharge from hospital |
| Hydroxychloroquine | ||||
| Chen et al. | Randomized, parallel-group | 62 patients, age >= 18, oxygen saturation > 93%, no severe or critical illness | HCQ: 400mg/d for 5 days + standard treatment Control: standard treatment only |
HCQ group showed faster fever and cough remission, a larger proportion of patients with clinical improvement, less progression to severe illness |
| Gautret et al. | observational | 80 patients, mildly infected | HCQ: 200mg orally, 3 times per day for 10 days; Azithromycin: 500mg on day 1 and 250mg on day 2–5; Some patients (with pneumonia, NEWS>=5) also received ceftriaxone |
Clinical improvement and rapid fall of nasopharyngeal viral load were observed |
| Molina et al. | prospective | 11 patients, severe disease, 5 with cancer, 1 with HIV, 2 obesity | HCQ: 600mg per day for 10 days; Azithromycin: 500mg on day 1 and 250mg on day 2–5; |
No evidence for rapid viral clearance and clinical benefits |
| Tang et al. | Randomized, controlled, open-label | 150 patients, 148 mild to moderate disease and 2 severe disease | HCQ group: HCQ 1200mg daily for day 1–3, 800mg daily for the rest of treatment duration (2–3 weeks according to patient condition) + standard care Control group: standard care |
HCQ did not result in a higher proportion of negative conversion |
| Mahevas et al. | Observational | 181 patients, age = 18–80, require oxygen but not intensive care | HCQ group: HCQ 600mg/day, started treatment 48 hours after admission; Control group: no HCQ treatment |
Similar survival rate and clinical improvement in two groups |
| arbidol | ||||
| Chen et al. | Prospective, randomized, controlled, open-label, multicenter | 240 patients, age >= 18, initial symptoms <= 12 days | FPV: 1200mg * 2 on day 1 and 600mg * 2 for day 2–10 + conventional therapy arbidol (control): 200mg * 3/day for 10 days + conventional therapy |
No statistically significant difference in clinical recovery rate on day 7 (71/116 compared to 62/120, p = 0.1396); FPV led to shorter latencies to the relief of pyrexia and cough (p < 0.0001) |
| Wen et al. (full-text not accessible) |
Retrospective | 178 patients | LPV/r group Arbidol group Combination group Control group: conventional treatment |
No significant difference in the rate of negative conversion, clinical improvement, and CT improvement among the 4 groups; Significant difference in the proportion of changing from mild/moderate to severe/critical on day 7 (p=0.017), LPV/r and control group had a smaller proportion of deterioration changing |
| Deng et al. | Retrospective | 33 patients, age>=18, without invasive ventilation | Combination group: arbidol 200mg every 8h, LPV/r 400mg/100mg every 12h; Monotherapy group: LPV/r 400mg/100mg every 12h; Until RNA test negative for 3 times (5–21 days) |
Combination group had higher viral clearance rate on day 7 (p<0.05) and day 14 (p<0.05); Combination group had higher chest image improving rate at day 7 (p<0.05) |
| Zhu et al. | Retrospective | 50 patients, no severe pneumonia or ARDS | LPV/r group: LPV/r 400mg/100mg twice daily for 7 days Arbidol group: 0.2g arbidol, 3 times daily for 7 days All patients received conventional therapy |
No difference in fever duration (p=0.61); arbidol group had less viral load on day 14 (0% compared to 44.1%); arbidol group had shorter RNA test positive duration (p<0.01) |
| Xu et al. | Retrospective, multicenter | 141 patients, age>=18, without ventilation | Combined group: arbidol 200mg, oral, 3 times daily for 7–10 days; IFN-ɑ2β, inhale, twice daily, 5 × 10(5) IU for 10–14 days Monotherapy group: inhale IFN-ɑ2β, twice daily, 5 × 10(5) IU for 10–14 days |
No significant differences between the two groups in terms of viral clearance; Faster CT improvement in the combined therapy group |
| tocilizumab | ||||
| Xu et al. | Observational | 21 patients, severe or critical disease | Tocilizumab: 4–8mg/kg body weight Standard treatment: LPV/r 400mg/100mg twice daily; IFN-ɑ, inhale, twice daily, 5 million U; ribavirin, 500mg 2–3 times daily |
Quick and significant clinical improvements were observed, all patients discharged with a mean of 15.1 days after given tocilizumab |
| Luo et al. | Retrospective | 15 patients, 2 moderately ill, 6 severely ill, 7 critically ill | Different for each patient, see Table 1 in paper | TCZ ameliorated increased CRP rapidly in all patients; treatment failed for 4 patients, 3 dead and 1 aggravated |
| dexamethasone | ||||
| RECOVERY Collaborative Group | Randomized, controlled, open-label | 6425 patients, anyone hospitalized and confirmed with COVID-19 and without risky medical histories, including children < 18 and pregnant or breastfeeding women | dexamethasone 6 mg once daily for 10 days compared with usual care | 28-day mortality reduced one-third in patients need invasive ventilation, one-fifth in patients need oxygen support but not invasive ventilation, no reduction in patients without oxygen support |
RNA mutagens: remdesivir, favipiravir and ribavirin
Since the replication of SARS-CoV-2 depends on the virus protein RNA-dependent RNA polymerase (RdRp), molecules that interfere with the function of RdRp could be potential treatments of COVID-19 by inducing mutations into the virus and blocking virus replication 4. Remdesivir, favipiravir and ribavirin are typical drugs that fall into this category, and the clinical trials about these drugs are reviewed below. Other potential drugs in this category include fluorouracil and acyclovir. However, clinical trials have not yet been conducted to test their efficacy.
Remdesivir:
Remdesivir, a 1′-cyano-substituted adenosine nucleotide analogue prodrug 5, is proposed to have the potential to treat COVID-19 by inducing RNA mutation in SARS-CoV-2. The theoretical evidence of this argument lies in that remdesivir triphosphate can compete with adenosine triphosphate (ATP) for incorporation in Ebola virus, resulting in early termination of the RNA chain 6. It is also found to be able to inhibit the replication of SARS-CoV, MERS-CoV and a wide spectrum of other CoVs in in vitro systems 7. A recent study has also demonstrated its ability to control infection of COVID-19 in vitro 8. A number of clinical trials have been carried out to test remdesivir’s effectiveness on COVID-19. A study treated a cohort of 53 patients with severe COVID-19 with compassionate-use remdesivir for 10 days, 200mg intravenously on day 1 and 100mg for the following 9 days. The results show that during a median follow-up of 18 days after the first dose of remdesivir, 68% of the patients showed clinical improvements in terms of oxygen support 9. However, a randomized, double-blinded, placebo-controlled clinical trial carried out in ten hospitals in Hubei, China looked into remdesivir’s efficacy in a cohort of 237 adults, and the results show that remdesivir is not statistically significantly associated with clinical benefits, while the statistically insignificant reduction in time to clinical improvement in patients within 10 days of symptom onset requires further confirmation in larger cohorts 10. Another smaller-scale study in Italy administered compassionate-use remdesivir for 10 days to a cohort of 35 patients with severe COVID-19 in both ICU and the infectious diseases ward, and the results indicate that remdesivir benefits patients outside ICU 11. By far, the largest-scale study on remdesivir is a recently published double-blind, randomized, controlled study on a cohort of 1059 participants from 10 countries. The results indicate that remdesivir significantly reduced the time to recovery of COVID-19 12. Although the clinical trial results of remdesivir are promising, FDA still has not approved it as a drug against COVID-19 by the time this review is written 13. Besides literature reports, there are 17 ongoing clinical trials on remdesivir’s clinical effect on COVID-19 documented by NIH ClinicalTrials by the time of May 15, 2020 14.
Favipiravir:
Favipiravir-triphosphate can also mimic ATP and GTP for incorporation with RdRp 15, however not as effective as remdesivir 16. In an open-labeled nonrandom controlled study, the effects of favipiravir and ritonavir-lopinavir on SARS-CoV-2 treatment were compared. The favipiravir group exhibited significantly shorter virus clearance time, improved chest imaging and fewer adverse reactions 17. Another retrospective, randomized, controlled study compared the effect of favipiravir and arbidol in a cohort of 240 patients, and found that there are no differences in the recovery rate in the two groups at day 7 18. However, favipiravir led to significantly accelerated relief of symptoms including pyrexia and cough 18. Besides literature reports, there are 16 ongoing clinical trials on favipiravir’s clinical effect on COVID-19 documented by NIH ClinicalTrials by the time of May 20, 2020 14.
Ribavirin:
Ribavirin’s mechanism is similar to that of favipiravir, which also mimics ATP and GTP to incorporate with RdRp 16. A study on a cohort of 94 patients showed that a combination of IFN-α, lopinavir/ritonavir, and ribavirin may be beneficial to patients with SARS-CoV-2 infection 19. Another open-label, randomized, phase-2 trial assessed the efficacy of a combination of IFN-β-1b, lopinavir/ritonavir, and ribavirin on treating SARS-CoV-2 infected patients. The study demonstrated that triple therapy was superior to only using lopinavir/ritonavir in terms of treating patients with mild or moderate SARS-CoV-2 infection 20. However, there aren’t clinical trials that directly assess the efficacy of ribavirin by the time this review is written. Besides literature reports, there is another ongoing clinical trial on the treatment of COVID-19 by a combination of nitazoxanide, ribavirin, and ivermectin. 14.
Protease inhibitors: ritonavir-lopinavir and darunavir
Since the CoVs’ gene expression and replication processes require proteolytic processing of polypeptides into non-structural proteins, it is reasonable to use protease inhibitors to block these processes 21,22. Representative drugs in this category include ritonavir-lopinavir and darunavir.
Ritonavir-lopinavir:
Ritonavir-lopinavir is originally a combination medication for AIDS by inhibiting the protease of HIV 23. A number of clinical trials have been carried out to test whether it is also effective in treating COVID-19. A retrospective study including 120 patients shows that early administration of ritonavir-lopinavir could shorten the time of virus shedding 24. A controlled study involving 47 patients with COVID-19 infection indicated that a combination of ritonavir-lopinavir and adjuvant drugs significantly decreased the number of days for virus clearance compared to adjuvant drugs alone 25. However, a randomized, controlled, open-label trial on 199 patients with SARS-CoV-2 suggested that no additional benefits were observed for the ritonavir-lopinavir treatment 26. But the result of this study is controversial, since there are arguments that it is premature to abandon ritonavir-lopinavir treatment only based on this trial since it is statistically underpowered to show a better improvement, and that the secondary outcomes of the trial suggested that ritonavir-lopinavir has the potential to reduce overall severe-disease and mortality risk 27. Another set of studies investigated ritonavir-lopinavir’s effectiveness compared to or in combination with the antiviral drug arbidol, and the results are not in favor of ritonavir-lopinavir. A retrospective cohort study with 178 patients diagnosed with COVID-19 suggests that no evidence proved that ritonavir-lopinavir or ritonavir-lopinavir combined with arbidol can shorten the disease course 28. A retrospective study with a cohort of 33 patients shows that a combination of arbidol and ritonavir-lopinavir achieved better clinical response compared to using ritonavir-lopinavir only 29. In another retrospective cohort study, 50 patients were divided into ritonavir-lopinavir group and arbidol group, and compared to the ritonavir-lopinavir group, viral clearance is faster in patients in the arbidol group 30. The most common side effects of ritonavir-lopinavir are mild to moderate gastrointestinal adverse effects such as diarrhea, nausea, and vomiting 23. Besides these published trials, there are 31 ongoing clinical trials on ritonavir-lopinavir’s clinical effect on COVID-19 documented by NIH ClinicalTrials at the time of May 20, 2020 14.
Darunavir:
Darunavir is also a protease inhibitor originally used for HIV 31. There are not clinical trials concerning the SARS-CoV-2 treatment effectiveness of darunavir. Based on case studies of three HIV positive patients infected with SARS-CoV-2, Riva et al. suggests that according to these preliminary evidence, darunavir at a dosage of 800mg does not prevent HIV patients from COVID-19 infection, and also may not protect HIV patients from worsening of respiratory function caused by SARS-CoV-2 32. There is one ongoing clinical trial (NCT04252274) that assesses the efficacy and safety of darunavir 14.
Virus-entry blockers: chloroquine, hydroxychloroquine, arbidol, and antibodies against spike (S) protein
SARS-CoV-2 enters the human cell by binding to plasma membrane receptors. Therefore, interfering with this process would block virus entry and thus has the potential to fight virus infection. Drugs in this category include arbidol, and potentially, chloroquine and hydroxychloroquine, and the antibodies against virus spike (S) protein, including LY3819253, JS016 and REGN-COV2.
Chloroquine:
Chloroquine has been used as an anti-malaria drug for many years. It’s antiviral mechanism is not completely clear, while there are studies suggesting that it disrupts virus-receptor binding by interfering with glycosylation of the human cell membrane receptor angiotensin-converting enzyme 2 (ACE2) 33. A recent study proposed that the virus entrance process not only involves spike protein binding to ACE2 but also host gangliosides, and chloroquine interferes with this process by competing with the virus’s spike protein to bind to gangliosides 34. Gao et al. reported in a letter that there are clinical trials that demonstrated that chloroquine performed better than control treatment in improving clinical outcomes of COVID-19 infected patients 35. However, the letter didn’t give any details of the clinical trials. A controlled study in a cohort of 22 patients showed that compared to ritonavir-lopinavir treatment, chloroquine phosphate significantly reduced the disease duration 36. However, large-scale studies are still in urgent need to determine the effectiveness of chloroquine.
Hydroxychloroquine:
Hydroxychloroquine is the hydroxylated form of chloroquine, and thus they share similar antiviral mechanisms. Some early small-scale trials found hydroxychloroquine effective for mild COVID-19 treatment. For example, a randomized controlled trial with 62 patients demonstrated that the use of hydroxychloroquine significantly shortened the disease course 37. Another pilot observational study in a cohort of 80 mildly infected patients also shows that combined therapy using hydroxychloroquine and Azithromycin may improve the situation of infected patients 38. However, another study mentioned that they failed to observe strong clinical improvement when using the same drugs and doses to treat 11 patients severely infected with COVID-19 39. The results of a series of larger-scale studies also cast doubt on the effectiveness of hydroxychloroquine. A recent randomized controlled study involving 150 mild to moderate patients concludes that no evidence suggests that hydroxychloroquine treatment performs better than standard patient care, and the adverse effect of hydroxychloroquine is higher 40. Another recent observational study in 181 patients with SARS-CoV-2 who required oxygen but not intensive care also does not support the effectiveness of hydroxychloroquine 41. Besides, it is reported that hydroxychloroquine add-on therapy to ritonavir-lopinavir may have many potential adverse effects including cardiac, metabolic and neurological symptoms etc., and should be used with caution 42. FDA recently established a summary of safety issues brought by chloroquine and hydroxychloroquine, including severe heart rhythm problems, blood and lymph system disorders, kidney injuries, and liver problems and failure, and cautioned against use of these drugs outside hospital settings 43.
Arbidol:
Arbidol is a broad-spectrum antiviral. Previous studies on viruses such as HCV, influenza virus etc. demonstrated that it interferes with various steps of the virus life-cycle, including virus entry, endocytosis, endosomal trafficking etc. 44. There is a lack of clinical trials that directly measure the efficacy of arbidol in treating COVID-19. Most of the clinical trials related to arbidol use it as a control group or in combination with other drugs. As mentioned in the ritonavir-lopinavir section, there are clinical studies suggesting that arbidol monotherapy 30 and arbidol combined with ritonavir-lopinavir 29 perform better at shortening the duration of the disease compared to ritonavir-lopinavir only. However, there is another clinical trial stating that no evidence suggests that arbidol combined with ritonavir-lopinavir would shorten the disease course 28. A randomized controlled study that compares the efficacy of arbidol and favipiravir in a cohort of 240 patients showed no significant difference in 7-day recovery rate between the two groups, while favipiravir led to earlier improvement of symptoms including pyrexia and cough 18. Recently, a retrospective cohort study involving 141 adult patients without ventilation suggests that there is almost no difference in clinical outcomes between arbidol monotherapy and arbidol combined with IFN-2b, and the study infers that combined therapy may be used to improve the situation of mild patients while it may not be able to accelerate virus clearance 45. There are three ongoing clinical trials that evaluate the efficacy and safety of arbidol for COVID-19 infection treatment 14.
LY3819253:
LY3819253, developed by the pharmaceutical company Eli Lilly, is the world’s first neutralizing antibody that goes into clinical trials. It is a potent monoclonal antibody against the SARS-CoV-2 spike (S) protein. The current Phase 1 (NCT04411628, 40 participants) and Phase 2 (NCT04427501, 400 participants) are randomized, double-blind, placebo-controlled studies with mild or moderate infected participants, and both are anticipated to end in mid to late August, 2020 14.
JS016:
JS016 is also a neutralizing antibody against SARS-CoV-2 spike (S) protein. It is developed by the pharmaceutical Shanghai Junshi Bioscience and entered Phase 1 clinical trial in early June (NCT04441918). The randomized, double-blind and placebo-controlled clinical trial aims at evaluating the safety of the product based on the experience of 40 healthy participants 14. Studies in vitro and in rhesus monkeys show that JS016 (CB6) has the ability to inhibit SARS-CoV-2 infection 46. The completion date of the trial is expected to be in mid December, 2020.
REGN-COV2:
REGN-COV2 is a combination therapy containing the antibodies REGN10933 and REGN10987, and is currently under Phase 1 clinical trial (NCT04426695) 14. The antibodies are generated from humanized mice and convalescent humans, both proved to be efficiently targeting the receptor-binding domain of the spike protein 47. There are expected to be 1860 participants in the Phase 1 trail, and the study completion date is going to be in June 2021.
Virus-release blockers: oseltamivir
This category of medication inhibits the release of virus from the infected cell, thus blocks virus transmission. A typical drug in this category is oseltamivir. Studies in influenza viruses show that it binds to and inhibits the virus neuraminidase enzyme, which facilitates virus release from the infected cell 48. There are currently no completed clinical trials for oseltamivir’s efficacy in treating COVID-19. Four ongoing clinical trials are dedicated to assess the efficacy and safety of oseltamivir 14.
Non-virus-targeting treatments: tocilizumab, dexamethasone, CD24Fc and dapagliflozin
Cytokine storm is a crucial factor that leads to the acute respiratory distress syndrome (ARDS) and multiple organ failure, which would suddenly exacerbate the disease and finally lead to death. Therefore, inhibition of the cytokine storm is an important step in COVID-19 treatment 49. Drugs in this category include IL-6 inhibitors (tocilizumab) and CD24Fc. Besides these treatments that directly target cytokines, metabolic modulators can also reduce adverse events brought by SARS-CoV-2 infection. These drugs include the corticosteroid drug dexamethasone and SGLT2 inhibitor dapagliflozin.
Tocilizumab:
IL-6 level is highly positively related to COVID-19 disease severity. The monoclonal antibody tocilizumab, an IL-6 receptor antagonist, is used in most cases of COVID-19 treatment where IL-6 is targeted. An observational study on 20 patients with severe or critical COVID-19 infection showed that the use of tocilizumab immediately improved clinical outcomes 50. Another observational study in 15 patients, 13 of which are severely or critically ill, also demonstrated that tocilizumab may be a useful therapy, and repeated dose is recommended for patients with elevated IL-6 level 51. However, two cases with adverse effects are reported, and the author advised clinicians to be cautious about hypertriglyceridemia when using tocilizumab 52.
CD24Fc:
CD24Fc, composed of the non-polymorphic regions of CD24 attached to the Fc region of human IgG1, is an immunomodulator that can suppress the expression of multiple cytokines 14. It is currently in Phase 2/Phase 3 clinical trial stage, and the current randomized, double-blind, placebo-controlled Phase 3 trial (NCT04317040) evaluates the safety efficacy of CD24Fc in treating COVID-19 in the cohort of 230 patients 14. The study completion date is expected to be in December 2020 14.
Dexamethasone:
Dexamethasone is an FDA approved synthetic corticosteroid that suppresses the immune system by inhibiting naive T cell proliferation and differentiation 53, and is the first-line treatment for immune-related complications. A large-scale randomized, controlled, open-label trial involving 6425 patients observed that dexamethasone reduced 28-day mortality rate by one-third in patients receiving invasive ventilation, and by one-fifth in patients receiving oxygen but not invasive ventilation 54. It does not reduce mortality rate in patients not requiring oxygen support 54. Metabolic side effects of dexamethasone include a mild increase of blood glucose level 55, ocular hypertension and cataract 56, neuropsychological side effects such as mood and behavior change 57, and osteoporosis 58. However, these adverse effects are mostly associated with long-term high dose dexamethasone treatments, while its benefit-risk profile is favorable for short-term treatments 59. WHO is in the process of adding dexamethasone into COVID-19 treatment guidelines 59.
Dapagliflozin:
Dapagliflozin is a sodium-glucose cotransporter-2 (SGLT2) inhibitor, and is hypothesised to be able to prevent serious side effects caused by SARS-CoV-2 infection by preventing low PH in cells 60. However, it is suggested to be carefully used together with insulin to prevent the side effect of euglycemic diabetic ketoacidosis 60. A randomized, double-blind, placebo controlled Phase 3 study (NCT04350593) is being carried out to evaluate the safety and efficacy of dapagliflozin in preventing adverse events in a cohort of 900 COVID-19 patients 14. The completion date is expected to be in December 2020 14.
Finally, it is worth noting that there are no drugs passed clinical trials and approved by FDA for COVID-19 by the time this review is written. Phase 3 trials compare a new drug to the standard-of-care drug, and Phase 4 trials test new drugs approved by the FDA for short-lived and long-lasting side effects and safety 61. For the COVID-19 situation, drugs passed Phase 3 or Phase 4 may be considered as passed clinical trials. There are currently 9 completed Phase 3 trials and 2 completed Phase 4 trials concerning COVID-19 on the clinicaltrials.gov webpage, involving drugs such as remdesivir (positive), favipiravir (result not posted), hydroxychloroquine (unclear, larger dataset needed; negative), baricitinib (result not posted), methylprednisolone therapy (result not posted), liposomal lactoferrin (result not posted), and danoprevir (result not posted) 14.
Drugs that have been proposed by computational works.
Significant efforts have been put into the computational works for prioritizing previous FDA-approved drugs for repurposing to treat COVID-19. In this section, we summarized the general categories of computational drug repurposing methods to help reduce duplicated works (Table 2).
Table 2.
Drugs proposed by computational methods
(bold words indicate drugs in clinical trials reviewed above)
| Author | Protein in focus | Proposed drugs/molecules (bold words indicate drugs in clinical trials reviewed above) |
|---|---|---|
| Category 1. Network-based algorithms | ||
| Zhou et al. | 119 proteins in the HCoV-host interactome network | irbesartan, toremifene, camphor, equilin, mesalazine, mercaptopurine, paroxetine, sirolimus, carvedilol, colchicine, dactinomycin, melatonin, quinacrine, eplerenone, emodin, oxymetholone |
| Cava et al. | Angiotensin converting enzyme 2 (ACE2) | LMB-2, L-778123, didanosine, lomustine, fumarate, vatiquinone, lentinan, flutamide, photofrin, medroxyprogesterone acetate, dihydrokainate, letrozole, mesalamine, cerulenin, thiabendazole, trichostatin, nimesulide, fluticasone propionate, semapimod, iratumumab, ivacaftor, SGN-30, retinol, QBW251, lumacaftor, apigenin, NS-398, tezacaftor, naproxen, esflurbiprofen, mefenamic acid, VK-19911, alglucosidase alfa, ibutilide, fumarate, amiodarone, hydrochloride, venetoclax |
| Category 2. Expression-based algorithms | ||
| He and Garmire | Angiotensin converting enzyme 2 (ACE2) | COL-3, CGP-60474 |
| Category 3. Docking simulation or protein structure based algorithms | ||
| Wu et al. | 18 SARS-CoV-2 proteins and 2 human proteins: Nsp1, Nsp3, Nsp7-Nsp8, Nsp9-Nsp10, Nsp14-Nsp16, 3CLpro, E-channel (E protein), ORF7a, Spike, ACE2, C-terminal RNA binding domain (CRBD), N-terminal RNA binding domain (NRBD), helicase, RdRp, TMPRSSS2 |
ribavirin, valganciclovir, β-Thymidine, aspartame, oxprenolol, lymecycline, chlorhexidine, alfuzosin, cilastatin, famotidine, valganciclovir, ceftibuten, fenoterol, fludarabine, etc. (only listed part of the results) |
| AI-Khafaji et al. | SARS-CoV-2 main protease (Mpro or 3CLpro) | saquinavir, ritonavir, remdesivir, delavirdine, cefuroxime axetil, oseltamivir, prevacid |
| Shah et al. | SARS-CoV-2 main protease (Mpro or 3CLpro) | lopinavir, asunaprevir, remdesivir, CGP42112A, indinavir, ritonavir, ABT450, marboran (methisazone), galidesivir |
| Kandeel and AI-Nazawi | SARS-CoV-2 main protease (Mpro or 3CLpro) | chromocarb, ribavirin, telbivudine, vitamin B12, aminophylline, nicotinamide, triflusal etc. (only listed part of the results) |
| Mahanta et al. | SARS-CoV-2 main protease (Mpro or 3CLpro) | viomycin |
| Pant et al. | SARS-CoV-2 main protease (Mpro or 3CLpro) | cobicistat, ritonavir, lopinavir, darunavir |
| Junmei Wang | SARS-CoV-2 main protease (Mpro or 3CLpro) | carfilzomib, eravacycline, valrubicin, lopinavir, elbasvir |
| Odhar et al. | SARS-CoV-2 main protease (Mpro or 3CLpro) | conivaptan, olaparib, loxapine, sonidegib, azelastine, idelalisib, tolvaptan, perampanel, suvorexant, ponatinib |
| Mittal et al. | SARS-CoV-2 main protease (Mpro or 3CLpro) | leupeptin, hemisulphate, pepstatin A, nelfinavir, birinapant, lypression, octreotide |
| Das et al. | SARS-CoV-2 main protease (Mpro or 3CLpro) |
ritonavir, emetine, lopinavir, indinavir (only listed part of the results) |
| Farag et al. | SARS-CoV-2 main protease (Mpro or 3CLpro) | darunavir, mitoxantrone, nelfinavir, moexpril, daunorubicin, rosuvastatin, saquinavir, metamizole, bepotastine, benzonatate, atovaqoune |
| Gimeno et al. | SARS-CoV-2 main protease (Mpro or 3CLpro) | perampanel, carprofen, celecoxib, alprazolam, trovafloxacin, sarafloxacin, ethyl biscoumacetate |
| Elfiky | RNA-dependent RNA polymerase (RdRp) | ribavirin, remdesivir, sofosbuvir, galidesivir, tenofovir, hydroxychloroquine, cefuroxime, favipiravir, setrobuvir, YAK, IDX-184 |
| Gupta et al. | SARS-CoV-2 envelope (E) protein | belachinal, macaflavanone E, vibsanol B |
| Beck et al. | SARS-CoV-2 main protease (Mpro or 3CLpro), RdRp, Helicase, 3’−5’ exonuclease, endoRNAse, 2’-O-ribose methyltransferase | atazanavir, ganciclovir, lopinavir, ritonavir, darunavir, etc. (only listed part of the results) |
| Elmezayen et al. | SARS-CoV-2 main protease (Mpro or 3CLpro), human transmembrane protease serine 2 (TMPRSS2) | talampicillin, lurasidone, rubitecan, loprazolam (only listed part of the results) |
| Hall and Ji | SARS-CoV-2 main protease (Mpro or 3CLpro), Spike (S) protein | cangrelor, NADH, flavin adenine dinucleotide (FAD) adeflavin, comeprol, Coenzyme A, tiludronate, zanamivir, bortezomib, saquinavir, cangrelor, carfilzomib, indinavir, remdesivir |
| Batra et al. | Spike (S) protein or Spike (S) protein-ACE2 interface complex | pemirolast, sulfamethoxazole, valaciclovir, sulfamerazine, tazobactam, nitrofurantoin |
| Oliveira et al. | Spike (S) protein | suramin sodium, 5-hydroxytrytophan, dihydroergocristine mesylate, quinupristin, nilotinib, dexamethasone-21-sulfobenzoate, tirilazad, selamectin, acetyldigitoxin, doramectin |
| Park et al. | Spike (S) protein | CR3022 human antibody, F26G19 mouse antibody, D12 mouse antibody |
Network-based algorithms
Zhou et al. integrated HCoV-host interactions, drug-target network and human protein interactome together and proposed 16 drugs and 3 drug combinations for SARS-CoV-2 infection treatment 62. In this study, CoV-associated host proteins were collected, and based on these proteins, HCoV-host interactome was generated. Then, potential drugs are identified by measuring network proximity between HCoV-specific network and drug-target network in the human interactome 62. Another study focused on the main cell receptor of SARS-CoV-2, the Angiotensin converting enzyme 2 (ACE2) 63. A protein-protein interaction network containing genes co-expressed with ACE2 was constructed, and focus was placed on genes that were already associated with drugs. A total of 36 potential drugs were proposed by this method 63.
Expression-based algorithms
In an expression-based drug repurposing study, based on the statement that inhibition of the Angiotensin converting enzyme 2 (ACE2) may be the mechanism of lung injury induced by SARS-CoV-2, two potential repurposed drugs were proposed for COVID-19 treatment since they reversed the change of gene expression patterns caused by ACE2 inhibitor 64.
Docking simulation or protein structure-based drug design
There are a comparatively large number of studies under this category, which can be further divided into two sub-categories:
Docking simulation for small molecule treatment predictions: The general steps for this kind of drug design method are 1) predict target protein structures using homology modeling or retrieve established crystal structures from databases; 2) screen for molecules that can bind to the target proteins using virtual docking simulation; 3) validation of the most promising molecules using methods such as molecular dynamic simulation etc. (Figure 2). Differences between studies mainly lie in the choice of protein targets, the docking sites on the protein targets, the drug/molecule databases and the virtual screening algorithms. Several virus or host proteins that are crucial for virus invasion or replication are in focus for drug design. SARS-CoV-2 3C-like main protease (3CLpro or Mpro), as the first SARS-CoV-2 protein whose crystal structure has been discovered 65, becomes the target of most molecular docking drug screening studies 66–77. To highlight a few, Gimeno et al. integrated the predictions of 3 molecular docking softwares (Glide, FRED and AutoDock Vina), only selecting the drugs that are predicted to have high binding affinity to Mpro by all the three softwares 76. Wang 71 and Mittal et al. 74 both used molecular dynamic simulation followed by binding free energy calculations to validate the top docking molecules. Other popular targets include RNA-dependent RNA polymerase (RdRp) 78, spike (S) protein 79,77, and spike (S) protein-human ACE2 interface 80. Besides, several studies investigated relatively novel targets, such as cellular transmembrane protease serine 2 (TMPRSS2) 72 and SARS-CoV-2 envelope (E) protein 81. Instead of focusing on only one or two proteins, there are some large scale studies that focused on more than two protein targets. An early study carried out by Wu et al. modeled and screened for drugs against 18 SARS-CoV-2 proteins and 2 host proteins 82. And another study by Beck et al. screened for drugs against 5 virus proteins using their own pre-trained deep learning-based drug-target interaction model 83. Finally, Shi et al. developed a new molecular docking-based web server that facilitates protein structure-based drug screening 84. As the structure of RNA dependent RNA-polymerase (RdRp) has been established very recently 4, we forecast that more inhibitors may be proposed for this protein target.
Docking simulation for antibodies treatment: The binding of SARS-CoV-2 spike (S) protein with human ACE2 protein is believed to facilitate SARS-CoV-2 to enter human cells 85,86, making this process a good target. Using antibody-antigen docking simulation, Park et al. proposed that the human antibody CR3022 may have high affinity to SARS-CoV-2 spike protein and thus it may be a potential treatment of COVID-19 87.
Figure 2.
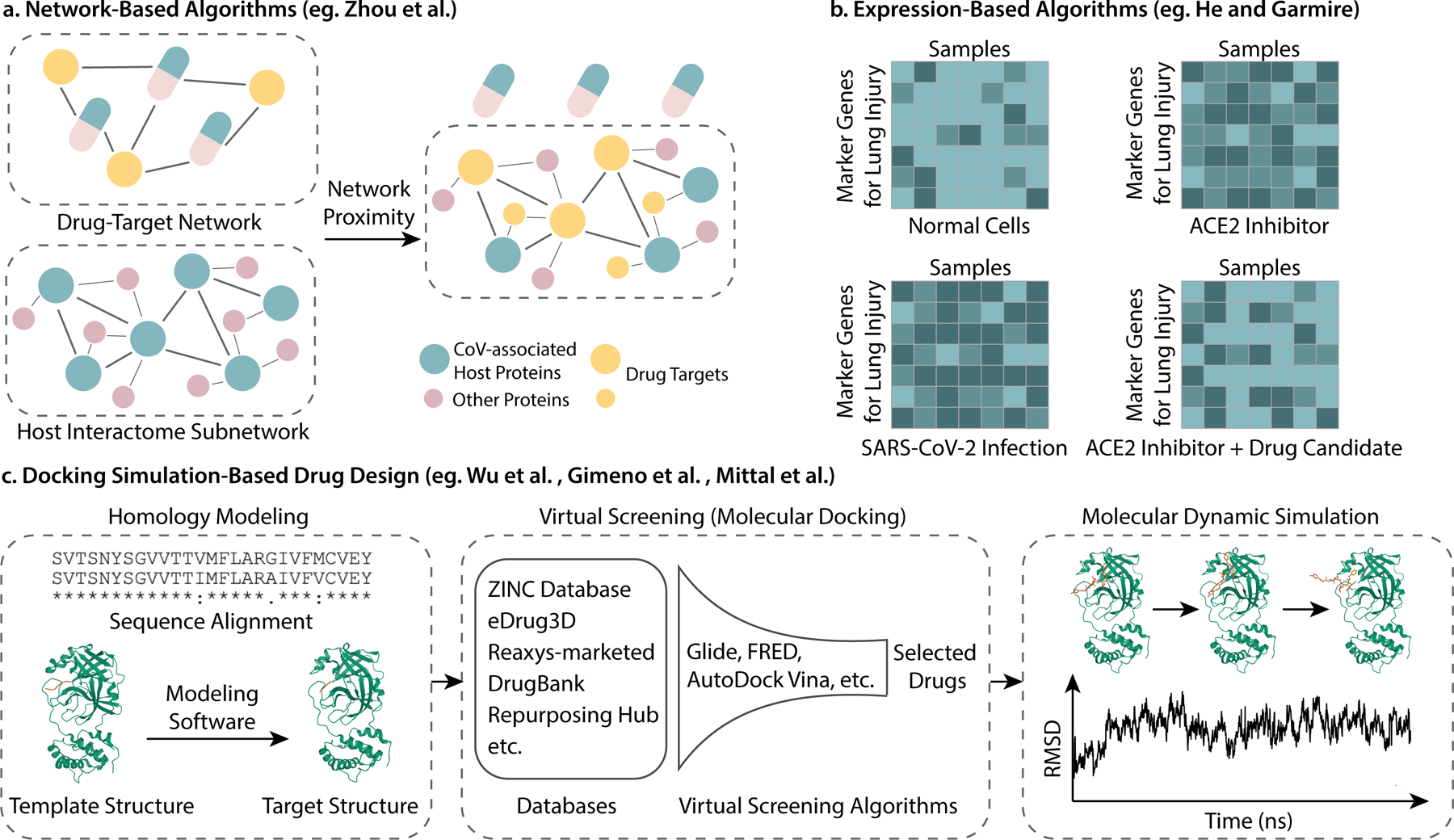
Illustration of computational drug repurposing methods. (a) Network-based algorithms, using the work of Zhou et al. as an example; (b) Expression-based algorithms, using the work of He and Garmire as an example; (c) General pipeline of docking simulation-based drug design (Protein structures credit to RCSB PDB 3AW0 [https://www.rcsb.org/structure/3AW0] and 6LU7 [https://www.rcsb.org/structure/6LU7]. Sequence alignment performed by UniProt [https://www.uniprot.org/align/]). Note that the RMSD plot in the figure is only for illustration.)
Besides drug repurposing, computational methods are also used for vaccine design. Multiple in-silico studies have been carried out to design multi-epitope vaccines against SARS-CoV-2 88–90. General workflow of vaccine design includes the retrieval of antigenic protein sequences, predicting potential epitopes, construction of the vaccine, and validating the binding ability of the designed vaccine with TLR3 immune receptor using docking simulation. Antigenic proteins used in these studies include SARS-CoV-2 spike glycoprotein88, nucleocapsid89, ORF3a89, and non-structural proteins 90.
It is worth mentioning that for this part of the review, we mostly focused on the repurposing of currently approved drugs or drug candidates under clinical trial. There are a number of enlightening studies that focus on finding new drugs from plants or other natural products or designing new molecules 91 that are not included here since they are outside the scope of this review.
Drugs that have been proposed by in vitro protein binding assays.
Studies have been carried out for genome-wide in vitro binding screening of the virus proteins and human proteins, and drugs that directly target these proteins can thus be proposed 92,93,65. In this section, we will review the methods and progress in this area (Table 3).
Table 3.
Drugs proposed by in vitro protein binding assays
(bold words indicate drugs in clinical trials reviewed above)
| Author | Main Method | Proposed Drugs/Molecules (bold words indicate drugs in clinical trials reviewed above) |
|---|---|---|
| Gordon et al. | AP-MS | silmitasertib, valproic acid, haloperidol, entacapone, indomethacin, metformin, ponatinib, ribavirin, migalastat, etc. (only listed part of the results) |
| Li et al. | Y2H and co-IP, AP-LC-MS | Does not contain screening for drugs, only identified protein-protein interaction network |
| Jin et al. | FRET | ebselen, shikonin, tideglusib, PX-12, disulfiram, carmofur |
Gordon et al. cloned, tagged and expressed 26 of the SARS-CoV-2 proteins in human cells, and then identified 332 SARS-CoV-2-human protein interactions using affinity-purification mass spectrometry, among which 67 druggable proteins and 69 potential drugs are identified 92. Li et al. first used SARS-CoV-2 genome-wide yeast-two hybrid (Y2H) and co-immunoprecipitations (co-IP) to identify the intra-viral protein-protein interactions. Then they cloned and overexpressed each of the virus genes and determined host-virus interactome using affinity-purification, liquid chromatography and mass spectrometry (AP-LC-MS) 93. Jin et al. purified Mpro, and then used fluorescence resonance energy transfer (FRET) assay to screen through the Mpro binding ability of more than 10,000 compounds including approved drugs or drug candidates 65.
Outlook
In this review, we summarized drugs against COVID-19 proposed by clinical trials, computational approaches and in vitro protein binding assays. From the clinical trials session, we conclude that there is not a single drug for which consistent positive response has been reported yet, and large-scale controlled trials are in urgent need. Additionally, the clinical trials reviewed in this work reveal that there are differences in drug efficacy between mild or moderate infected patients and severe or critical patients. Thus, analysis and reports taking into account these factors may be informative. From the computational study summary, we learned that some of the drugs proposed by computational methods have already been put into clinical use, which validates the methods in some way.
Current small-scale pilot trials point to the necessity for future large-scale, well-controlled trials to resolve certain inconsistency in results, as disagreements in the reported drug response can root from differences in dosage, baseline biometrics and population groups. With more clinical trial results coming in, they will also enable meta-analysis to stratify these variables across centers and trials. Besides, an in-depth reflection on the causes and solutions of challenges faced by clinical trials, such as small sample sizes, result consistency, and efficiency of result delivery would be very helpful for future clinical trials.
With the effort of researchers around the world, a variety of unconventional drugs and treatments are explored. Synthetic peptide against COVID-19, for example, is one of the novel treatment options that deserve attention due to its relatively fast and inexpensive synthesis process and better safety. There are currently a handful of peptide treatments against COVID-19 under clinical trials, such as Angiotensin peptide (1–7) (NCT04375124) and LSALT peptide (NCT04402957), and several suggested by studies and clinical trials, such as modified α-ketoamide inhibitors 94 and Solnatide 95. Future reviews may consider providing a more detailed summary of the development of peptide treatments.
Additionally, antibodies and vaccines play crucial roles in the battle against COVID-19. Future work may provide more complete and in-depth reviews focusing on the development of antibodies and vaccines against COVID-19.
Acknowledgment
This work is supported by NIH R35-GM133346.
Biographies
Biosketches
Xueqing Wang is a Master’s student at the Department of Computational Medicine and Bioinformatics, the University of Michigan Medical School, Ann Arbor, MI.
Yuanfang Guan is an associate professor at the Department of Computational Medicine and Bioinformatics, the University of Michigan Medical School, Ann Arbor, MI. She obtained her Ph.D in molecular biology from Princeton University. Her research focuses on drug response prediction and she has led the development of the winning algorithms of multiple community-based drug response challenges including the AstraZenaca Sanger Drug Combination Prediction Challenge, Rheumatoid Arthritis DREAM Challenge, Malaria DREAM Challenge, Prostate Cancer Challenge. Link to lab website: https://guanlab.ccmb.med.umich.edu/
References
- 1.Tobinick EL The value of drug repositioning in the current pharmaceutical market. Drug News Perspect. 22, 119–125 (2009). [DOI] [PubMed] [Google Scholar]
- 2.Chen B et al. Overview of lethal human coronaviruses. Signal Transduct Target Ther 5, 89 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mercorelli B, Palù G & Loregian A Drug Repurposing for Viral Infectious Diseases: How Far Are We? Trends Microbiol. 26, 865–876 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gao Y et al. Structure of the RNA-dependent RNA polymerase from COVID-19 virus. Science 368, 779–782 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tchesnokov EP, Feng JY, Porter DP & Götte M Mechanism of Inhibition of Ebola Virus RNA-Dependent RNA Polymerase by Remdesivir. Viruses 11, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saha A et al. Probable Molecular Mechanism of Remdesivir for the Treatment of COVID-19: Need to Know More. Arch. Med. Res (2020) 10.1016/j.arcmed.2020.05.001. [DOI] [PMC free article] [PubMed]
- 7.Sheahan TP et al. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Science Translational Medicine vol. 9 eaal3653 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang M et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res 30, 269–271 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grein J et al. Compassionate Use of Remdesivir for Patients with Severe Covid-19. N. Engl. J. Med. (2020) 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed]
- 10.Wang Y et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet 395, 1569–1578 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Antinori S et al. Compassionate remdesivir treatment of severe Covid-19 pneumonia in intensive care unit (ICU) and Non-ICU patients: Clinical outcome and differences in post_treatment hospitalisation status. Pharmacol. Res. 104899 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beigel JH et al. Remdesivir for the treatment of Covid-19—preliminary report. N. Engl. J. Med (2020). [DOI] [PubMed]
- 13.Office of the Commissioner. Coronavirus Disease 2019 (COVID-19). U.S. Food and Drug Administration https://www.fda.gov/emergency-preparedness-and-response/counterterrorism-and-emerging-threats/coronavirus-disease-2019-covid-19 (2020). [PubMed]
- 14.Home - ClinicalTrials.gov. https://ClinicalTrials.gov.
- 15.Furuta Y et al. Favipiravir (T-705), a novel viral RNA polymerase inhibitor. Antiviral Res 100, 446–454 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gordon CJ et al. Remdesivir is a direct-acting antiviral that inhibits RNA-dependent RNA polymerase from severe acute respiratory syndrome coronavirus 2 with high potency. J. Biol. Chem 295, 6785–6797 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cai Q et al. Experimental Treatment with Favipiravir for COVID-19: An Open-Label Control Study. Engineering (2020) 10.1016/j.eng.2020.03.007. [DOI] [PMC free article] [PubMed]
- 18.Chen C et al. Favipiravir versus arbidol for COVID-19: a randomized clinical trial. medRxiv 2020. DOI 10, 17–20037432. [Google Scholar]
- 19.Yuan J et al. The correlation between viral clearance and biochemical outcomes of 94 COVID-19 infected discharged patients. Inflammation Research vol. 69 599–606 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hung IF-N et al. Triple combination of interferon beta-1b, lopinavir--ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet (2020). [DOI] [PMC free article] [PubMed]
- 21.Xue X et al. Structures of two coronavirus main proteases: implications for substrate binding and antiviral drug design. J. Virol 82, 2515–2527 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muralidharan N, Sakthivel R, Velmurugan D & Gromiha MM Computational studies of drug repurposing and synergism of lopinavir, oseltamivir and ritonavir binding with SARS-CoV-2 protease against COVID-19. J. Biomol. Struct. Dyn. 1–6 (2020). [DOI] [PubMed]
- 23.Chandwani A & Shuter J Lopinavir/ritonavir in the treatment of HIV-1 infection: a review. Ther. Clin. Risk Manag. 4, 1023 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yan D et al. Factors associated with prolonged viral shedding and impact of Lopinavir/Ritonavir treatment in hospitalised non-critically ill patients with SARS-CoV-2 infection. European Respiratory Journal 2000799 (2020) 10.1183/13993003.00799-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ye X-T et al. Clinical efficacy of lopinavir/ritonavir in the treatment of Coronavirus disease 2019. Eur. Rev. Med. Pharmacol. Sci 24, 3390–3396 (2020). [DOI] [PubMed] [Google Scholar]
- 26.Cao B et al. A Trial of Lopinavir–Ritonavir in Adults Hospitalized with Severe Covid-19. N. Engl. J. Med. (2020) 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed]
- 27.Carmona-Bayonas A, Jimenez-Fonseca P & Castañón E A Trial of Lopinavir-Ritonavir in Covid-19. The New England journal of medicine vol. 382 e68 (2020). [DOI] [PubMed] [Google Scholar]
- 28.Wen CY et al. [Real-world efficacy and safety of lopinavir/ritonavir and arbidol in treating with COVID-19 : an observational cohort study]. Zhonghua Nei Ke Za Zhi 59, E012 (2020).32388937 [Google Scholar]
- 29.Deng L et al. Arbidol combined with LPV/r versus LPV/r alone against Corona Virus Disease 2019: A retrospective cohort study. J. Infect. (2020) 10.1016/j.jinf.2020.03.002. [DOI] [PMC free article] [PubMed]
- 30.Zhu Z et al. Arbidol monotherapy is superior to lopinavir/ritonavir in treating COVID-19. J. Infect (2020) 10.1016/j.jinf.2020.03.060. [DOI] [PMC free article] [PubMed]
- 31.Spagnuolo V, Castagna A & Lazzarin A Darunavir for the treatment of HIV infection. Expert Opinion on Pharmacotherapy vol. 19 1149–1163 (2018). [DOI] [PubMed] [Google Scholar]
- 32.Riva A et al. Darunavir does not prevent SARS-CoV-2 infection in HIV patients. Pharmacol. Res. 157, 104826 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vincent MJ et al. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol. J 2, 69 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fantini J, Di Scala C, Chahinian H & Yahi N Structural and molecular modelling studies reveal a new mechanism of action of chloroquine and hydroxychloroquine against SARS-CoV-2 infection. Int. J. Antimicrob. Agents 55, 105960 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gao J, Tian Z & Yang X Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci. Trends 14, 72–73 (2020). [DOI] [PubMed] [Google Scholar]
- 36.Huang M et al. Treating COVID-19 with Chloroquine. J. Mol. Cell Biol 12, 322–325 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen Z et al. Efficacy of hydroxychloroquine in patients with COVID-19: results of a randomized clinical trial. 10.1101/2020.03.22.20040758. [DOI]
- 38.Gautret P et al. Clinical and microbiological effect of a combination of hydroxychloroquine and azithromycin in 80 COVID-19 patients with at least a six-day follow up: A pilot observational study. Travel Med. Infect. Dis 101663 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Molina JM et al. No evidence of rapid antiviral clearance or clinical benefit with the combination of hydroxychloroquine and azithromycin in patients with severe COVID-19 infection. Med. Mal. Infect. 50, 384 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tang W et al. Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: open label, randomised controlled trial. BMJ 369, m1849 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mahévas M et al. Clinical efficacy of hydroxychloroquine in patients with covid-19 pneumonia who require oxygen: observational comparative study using routine care data. BMJ 369, m1844 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chong VH et al. Conduction abnormalities in hydroxychloroquine add on therapy to lopinavir/ritonavir in COVID‐19. J. Med. Virol (2020) 10.1002/jmv.26004. [DOI] [PMC free article] [PubMed]
- 43.Center for Drug Evaluation & Research. FDA cautions use of hydroxychloroquine/chloroquine for COVID-19. U.S. Food and Drug Administration https://www.fda.gov/drugs/drug-safety-and-availability/fda-cautions-against-use-hydroxychloroquine-or-chloroquine-covid-19-outside-hospital-setting-or (2020).
- 44.Blaising J, Polyak SJ & Pécheur E-I Arbidol as a broad-spectrum antiviral: an update. Antiviral Res. 107, 84–94 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu P et al. Arbidol/IFN-α2b Therapy for Patients With Corona Virus Disease 2019: A Retrospective Multicenter Cohort Study. Microbes Infect. (2020) 10.1016/j.micinf.2020.05.012. [DOI] [PMC free article] [PubMed]
- 46.Shi R et al. A human neutralizing antibody targets the receptor-binding site of SARS-CoV-2. Nature (2020) 10.1038/s41586-020-2381-y. [DOI] [PubMed]
- 47.Hansen J et al. Studies in humanized mice and convalescent humans yield a SARS-CoV-2 antibody cocktail. Science (2020) 10.1126/science.abd0827. [DOI] [PMC free article] [PubMed]
- 48.Whitley RJ The role of oseltamivir in the treatment and prevention of influenza in children. Expert Opin. Drug Metab. Toxicol. 3, 755–767 (2007). [DOI] [PubMed] [Google Scholar]
- 49.Ye Q, Wang B & Mao J The pathogenesis and treatment of the `Cytokine Storm’ in COVID-19. J. Infect 80, 607–613 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xu X et al. Effective treatment of severe COVID-19 patients with tocilizumab. ChinaXiv 202003, V1 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Luo P et al. Tocilizumab treatment in COVID-19: a single center experience. J. Med. Virol. (2020). [DOI] [PMC free article] [PubMed]
- 52.Morrison AR et al. Letter to the Editor: Acute hypertriglyceridemia in patients with COVID‐19 receiving tocilizumab. J. Med. Virol. (2020) 10.1002/jmv.25907. [DOI] [PMC free article] [PubMed]
- 53.Giles AJ et al. Dexamethasone-induced immunosuppression: mechanisms and implications for immunotherapy. J Immunother Cancer 6, 51 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Horby P et al. Effect of dexamethasone in hospitalized patients with COVID-19: preliminary report. MedRxiv (2020).
- 55.Blanchard D & van Wissen K Adverse Effects of Dexamethasone in Surgical Patients. The American journal of nursing vol. 119 19 (2019). [DOI] [PubMed] [Google Scholar]
- 56.Fassbender Adeniran JM, Jusufbegovic D & Schaal S Common and Rare Ocular Side-effects of the Dexamethasone Implant. Ocul. Immunol. Inflamm. 25, 834–840 (2017). [DOI] [PubMed] [Google Scholar]
- 57.Warris LT et al. Does dexamethasone induce more neuropsychological side effects than prednisone in pediatric acute lymphoblastic leukemia? A systematic review. Pediatr. Blood Cancer 61, 1313–1318 (2014). [DOI] [PubMed] [Google Scholar]
- 58.Black R & Grodzinsky AJ Dexamethasone: chondroprotective corticosteroid or catabolic killer? Eur. Cell. Mater. 38, 246–263 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Q&A: Dexamethasone and COVID-19. https://www.who.int/news-room/q-a-detail/q-a-dexamethasone-and-covid-19.
- 60.Cure E & Cure MC Can dapagliflozin have a protective effect against COVID-19 infection? A hypothesis. Diabetes Metab. Syndr. 14, 405 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nguyen V, Sweet BV & Macek T Defining the phases of clinical trials. Am. J. Health. Syst. Pharm. 63, 710–711 (2006). [DOI] [PubMed] [Google Scholar]
- 62.Zhou Y et al. Network-based drug repurposing for novel coronavirus 2019-nCoV/SARS-CoV-2. Cell Discovery vol. 6 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cava C, Bertoli G & Castiglioni I In Silico Discovery of Candidate Drugs against Covid-19. Viruses 12, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.He B & Garmire L Prediction of repurposed drugs for treating lung injury in COVID-19. arXiv [q-bio.TO] (2020). [DOI] [PMC free article] [PubMed]
- 65.Jin Z et al. Structure of M pro from SARS-CoV-2 and discovery of its inhibitors. Nature 1–5 (2020). [DOI] [PubMed]
- 66.Al-Khafaji K, Al-Duhaidahawi D & Taskin Tok T Using integrated computational approaches to identify safe and rapid treatment for SARS-CoV-2. J. Biomol. Struct. Dyn. 1–9 (2020). [DOI] [PMC free article] [PubMed]
- 67.Shah B, Modi P & Sagar SR In silico studies on therapeutic agents for COVID-19: Drug repurposing approach. Life Sci. 252, 117652 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mahanta S et al. Potential anti-viral activity of approved repurposed drug against main protease of SARS-CoV-2: an in silico based approach. J. Biomol. Struct. Dyn. 1–10 (2020). [DOI] [PubMed]
- 69.Kandeel M & Al-Nazawi M Virtual screening and repurposing of FDA approved drugs against COVID-19 main protease. Life Sci 251, 117627 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pant S, Singh M, Ravichandiran V, Murty USN & Srivastava HK Peptide-like and small-molecule inhibitors against Covid-19. J. Biomol. Struct. Dyn. 1–10 (2020). [DOI] [PMC free article] [PubMed]
- 71.Wang J Fast Identification of Possible Drug Treatment of Coronavirus Disease −19 (COVID-19) Through Computational Drug Repurposing Study. ChemRxiv (2020) 10.26434/chemrxiv.11875446. [DOI] [PMC free article] [PubMed]
- 72.Elmezayen AD, Al-Obaidi A, Şahin AT & Yelekçi K Drug repurposing for coronavirus (COVID-19): in silico screening of known drugs against coronavirus 3CL hydrolase and protease enzymes. J. Biomol. Struct. Dyn. 1–13 (2020). [DOI] [PMC free article] [PubMed]
- 73.Odhar HA et al. Molecular docking and dynamics simulation of FDA approved drugs with the main protease from 2019 novel coronavirus. Bioinformation 16, 236–244 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mittal L, Kumari A, Srivastava M, Singh M & Asthana S Identification of potential molecules against COVID-19 main protease through structure-guided virtual screening approach. J. Biomol. Struct. Dyn. 1–19 (2020). [DOI] [PMC free article] [PubMed]
- 75.Das S, Sarmah S, Lyndem S & Singha Roy A An investigation into the identification of potential inhibitors of SARS-CoV-2 main protease using molecular docking study. J. Biomol. Struct. Dyn. 1–11 (2020). [DOI] [PMC free article] [PubMed]
- 76.Gimeno A et al. Prediction of Novel Inhibitors of the Main Protease (M-pro) of SARS-CoV-2 through Consensus Docking and Drug Reposition. Int. J. Mol. Sci 21, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hall DC & Ji H-F A search for medications to treat COVID-19 via in silico molecular docking models of the SARS-CoV-2 spike glycoprotein and 3CL protease. Travel Med. Infect. Dis 101646 (2020). [DOI] [PMC free article] [PubMed]
- 78.Elfiky AA Ribavirin, Remdesivir, Sofosbuvir, Galidesivir, and Tenofovir against SARS-CoV-2 RNA dependent RNA polymerase (RdRp): A molecular docking study. Life Sci. 253, 117592 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.de Oliveira OV, Rocha GB, Paluch AS & Costa LT Repurposing approved drugs as inhibitors of SARS-CoV-2 S-protein from molecular modeling and virtual screening. J. Biomol. Struct. Dyn. 1–10 (2020). [DOI] [PMC free article] [PubMed]
- 80.Batra R et al. Screening of Therapeutic Agents for COVID-19 using Machine Learning and Ensemble Docking Simulations. arXiv [q-bio.BM] (2020). [DOI] [PubMed]
- 81.Gupta MK et al. In-silico approaches to detect inhibitors of the human severe acute respiratory syndrome coronavirus envelope protein ion channel. J. Biomol. Struct. Dyn. 1–11 (2020). [DOI] [PMC free article] [PubMed]
- 82.Wu C et al. Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharm Sin B (2020) 10.1016/j.apsb.2020.02.008. [DOI] [PMC free article] [PubMed]
- 83.Beck BR, Shin B, Choi Y, Park S & Kang K Predicting commercially available antiviral drugs that may act on the novel coronavirus (SARS-CoV-2) through a drug-target interaction deep learning model. Comput. Struct. Biotechnol. J. 18, 784–790 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shi Y et al. D3Targets-2019-nCoV: a webserver for predicting drug targets and for multi-target and multi-site based virtual screening against COVID-19. Acta Pharm Sin B (2020) 10.1016/j.apsb.2020.04.006. [DOI] [PMC free article] [PubMed]
- 85.Wan Y, Shang J, Graham R, Baric RS & Li F Receptor Recognition by the Novel Coronavirus from Wuhan: an Analysis Based on Decade-Long Structural Studies of SARS Coronavirus. J. Virol. 94, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang H, Penninger JM, Li Y, Zhong N & Slutsky AS Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med 46, 586–590 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Park T et al. Spike protein binding prediction with neutralizing antibodies of SARS-CoV-2. bioRxiv (2020).
- 88.Abraham Peele K, Srihansa T, Krupanidhi S, Vijaya Sai A & Venkateswarulu TC Design of multi-epitope vaccine candidate against SARS-CoV-2: a in-silico study. J. Biomol. Struct. Dyn. 1–9 (2020). [DOI] [PMC free article] [PubMed]
- 89.Enayatkhani M et al. Reverse vaccinology approach to design a novel multi-epitope vaccine candidate against COVID-19: an in silico study. J. Biomol. Struct. Dyn. 1–16 (2020). [DOI] [PMC free article] [PubMed]
- 90.Ojha R et al. High throughput and comprehensive approach to develop multiepitope vaccine against minacious COVID-19. Eur. J. Pharm. Sci 151, 105375 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tang B et al. AI-aided design of novel targeted covalent inhibitors against SARS-CoV-2. bioRxiv 2020.03.03.972133 (2020) 10.1101/2020.03.03.972133. [DOI] [PMC free article] [PubMed]
- 92.Gordon DE, Jang GM, Bouhaddou M, Xu J & Obernier K A SARS-CoV-2-human protein-protein interaction map reveals drug targets and potential drug-repurposing. BioRxiv (2020). [DOI] [PMC free article] [PubMed]
- 93.Liang Q et al. Virus-host interactome and proteomic survey of PMBCs from COVID-19 patients reveal potential virulence factors influencing SARS-CoV-2 pathogenesis. bioRxiv (2020). [DOI] [PMC free article] [PubMed]
- 94.Zhang L et al. Crystal structure of SARS-CoV-2 main protease provides a basis for design of improved α-ketoamide inhibitors. Science 368, 409–412 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Krenn K et al. Inhaled AP301 for treatment of pulmonary edema in mechanically ventilated patients with acute respiratory distress syndrome: a phase IIa randomized placebo-controlled trial. Crit. Care 21, 194 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]


