Abstract
Musculoskeletal injuries and associated conditions are the leading cause of physical disability worldwide. The concept of tissue engineering has opened up novel approaches to repair musculoskeletal defects in a fast and/or efficient manner. Biomaterials, cells, and signaling molecules constitute the tissue engineering triad. In the past 40 years, significant progress has been made in developing and optimizing all three components, but only a very limited number of technologies have been successfully translated into clinical applications. A major limiting factor of this barrier to translation is the insufficiency of two-dimensional cell cultures and traditional animal models in informing the safety and efficacy of in-human applications. In recent years, microphysiological systems, often referred to as organ or tissue chips, generated according to tissue engineering principles, have been proposed as the next-generation drug testing models. This chapter aims to first review the current tissue engineering-based approaches that are being applied to fabricate and develop the individual critical elements involved in musculoskeletal organ/tissue chips. We next highlight the general strategy of generating musculoskeletal tissue chips and their potential in future regenerative medicine research. Exemplary microphysiological systems mimicking musculoskeletal tissues are described. With sufficient physiological accuracy and relevance, the human cell-derived, three-dimensional, multi-tissue systems have been used to model a number of orthopedic disorders and to test new treatments. We anticipate that the novel emerging tissue chip technology will continually reshape and improve our understanding of human musculoskeletal pathophysiology, ultimately accelerating the development of advanced pharmaceutics and regenerative therapies.
Keywords: Biological induction, Biomaterial, Disease modeling, Drug testing, Growth factors, Microphysiological system, Organoid, Regenerative medicine, Stem cells, Tissue chip
1. Introduction
The human musculoskeletal system, also known as the locomotor system, provides the human body with structural support and load-bearing capacity and offers protection to the delicate internal organs. The musculoskeletal system enables mechanical functions that subject the tissues to wear and tear as well as injuries over a lifetime of use, leading to debilitating pain and weakening or even loss of functions. In fact, musculoskeletal conditions are the leading cause of physical disability worldwide (James et al. 2018). As musculoskeletal disorders are most prevalent in the elderly, the world’s aging population represent the major contributing factor to the increasing medical and socioeconomic burdens of treating musculoskeletal impairments. Conventional treatments include the use of autografts, allograft, and xenografts, but have several drawbacks such as disease transmission, limited availability and reproducibility, donor scarcity, sterilization-induced alteration in natural matrix properties, and immune rejection. Tissue engineering represents a promising strategy of regenerative medicine to restore musculoskeletal structures and functions.
The field of tissue engineering interfaces engineering, life science, and medicine, with the capability of creating living tissues outside the human body (Langer and Vacanti 1993). Traditionally, these in vitro grown tissues are intended to be implanted into the human body to restore, maintain, augment, or replace diseased, injured, or degenerated tissues. Regenerative medicine, as the term indicates, aims to enable and enhance the body’s natural repair mechanisms to restore the function of otherwise irreparable tissues or organs in situ.
The tissue engineering triad consists of three key elements: scaffolds, cells, and signaling molecules (O’Brien 2011). In the past few decades, considerable progress has been made in the tissue engineering and regenerative medicine (TERM) field, which continues to evolve rapidly. In particular, new biomaterials are being designed, and novel approaches are emerging to fabricate scaffolds with existing biomaterials; the identification of new stem cell sources and functions and the establishment of more biologically accurate organotypic models promise to broaden the applications of TERM technologies; and innovative delivery and administration strategies are being proposed for growth factors and other biologically active molecules. These exciting developments underscore the importance of comprehensive evaluation of the three TERM components to gain an in-depth understanding of their safety, efficacy, and optimal use. In this chapter we focus specifically on the essential TERM elements of relevance to orthopedic applications.
Successful translation of TERM techniques and products requires evidence-based safety and efficacy assessment prior to the clinical trial. Currently, animal models are an essential component in preclinical studies and have been used to assess various biomaterials, cells, and signaling molecules for musculoskeletal regeneration. Small rodents, especially mice and rats, are the most commonly used animal models in musculoskeletal research. The past few decades have witnessed a continuous increase in the use of mice in orthopedic research, while the use of rats remains relatively unchanged due to the difficulties in genetic manipulations (Ericsson et al. 2013). However, in recent years, concerns have risen about the benefit of animal research to humans. A number of studies have shown that most animal experiments are unable to predict the observations in human trials (Mak et al. 2014; Pound and Bracken 2014). In fact, among the 76 highly influential animal studies (each with >500 citations) published on 7 prominent scientific journals, only 37% could be replicated in human randomized trials (Hackam and Redelmeier 2006). Therefore, the benefits of animal models still need to be supported by more systematic evaluations.
In addition to the many commendable achievements in tissue repair, TERM applications have also been successfully extended to disease modeling and drug development over the past decade. Exemplary in vitro models have been established using TERM principles for mimicking musculoskeletal tissues, including bone and cartilage (Lin et al. 2014; Occhetta et al. 2019; Arrigoni et al. 2020), and skeletal muscle (Truskey 2018), and other tissue/organ systems, such as lung (Huh et al. 2010), blood-brain barrier (Phan et al. 2017), gut (Kim et al. 2012), kidney (Jang et al. 2013), liver (Ribeiro et al. 2019), pancreas (Shik Mun et al. 2019), and heart (Nunes et al. 2013). These exciting studies have suggested that in vitro organotypic models and microphysiological systems (MPS), generated with tissue engineering principles and technologies, may serve as convenient and versatile platforms for the comprehensive evaluation of multiple TERM elements. Once the clinical relevance is validated, in vitro models like MPS will be a powerful alternative to current animal models.
We begin this chapter by describing the properties and performances – both in vitro and in vivo – of the scaffolds, cells, and signaling molecules in musculoskeletal tissue engineering. The derivation of organoids from stem cell aggregates and their potential applications are introduced. We then describe the optimal use of the three TERM elements in representative MPS established thus far for modeling musculoskeletal tissues. Finally, we summarize the advantages and disadvantages of different models and envision their utility in the development of drugs/treatments in the future.
2. Key Elements of Musculoskeletal Tissue Models
Scaffolds, cells, and signaling molecules are considered the three key, enabling components of tissue engineering. For musculoskeletal regeneration, the structure and function of native bone, cartilage, skeletal muscle, tendon, and ligament tissues have significant implications on the selection of the three elements mentioned above. Best reparative and regenerative outcomes are achieved when the three elements act synergistically, where the appropriate cell types are seeded into a scaffold that possesses mechanical, structural, and biochemical characteristics akin to those of the native tissue and are given the biochemical and physical signals that induce anabolic and regenerative responses.
2.1. Biomaterials and Scaffolds
Enormous progress in biomaterials research has been made in the past decades. Many different types of natural and synthetic biomaterials have been utilized to fabricate tissue engineering scaffolds, which can be cell-laden or cell-free when implanted. The safety and efficacy of a new biomaterial have to be rigorously assessed prior to its clinical application. This evaluation process routinely includes both in vitro and in vivo tests. We recognize the myriad biomaterial types that have been utilized to regenerate musculoskeletal tissues. This section thus does not intend to provide a thorough description of existing biomaterials, but rather focuses on an analysis of the assessment and performances of the representative, novel biomaterials and scaffolds developed in the past decade.
Bone
Biomaterial preparation for bone regeneration has been facing a persistent challenge to recapitulate the mechanical, structural, and functional characteristics of native bone. Natural biomaterials such as acellular bone matrix and extracellular matrix (ECM) generated by in vitro cultured mesenchymal stem cells (MSCs) have led to robust osteogenesis (Ni et al. 2014; Liu et al. 2019; Rothrauff and Tuan 2020). A myriad of synthetic biomaterials, including metals, ceramics, polymers, and composites, have been developed to address the unmet medical need of bone grafts. Metallic biomaterials still dominate the market of load-bearing bone substitute materials (Chen and Thouas 2015). One of the most commonly used biometals is Ti-6Al-4V alloys. Human MSCs were cultured on additive manufactured Ti-6Al-4V scaffolds, and surface anodization was found to increase the alkaline phosphatase (ALP) production, osteocalcin (OCN) and type I collagen expression, and mineral deposition (Li et al. 2020a; Groessner-Schreiber and Tuan 1992). A major drawback of most metallic implants is the associated “stress shielding effect.” Biodegradable metals such as magnesium alloys have thus been proposed to address this issue. Fracture repair with magnesium-containing orthopedic implants has shown considerable efficacy. Magnesium intramedullary rods were implanted in rat femur, leading to abundant new bone formation through an osteogenic mechanism that involves calcitonin gene-related polypeptide-α (CGRP) (Zhang et al. 2016). It has been noted that there exists a large gap in mechanical properties between existing biodegradable materials and traditional metallic implants. Biodegradable zinc alloys have therefore been developed, tested in vitro with MC3T3-E1 mouse preosteoblast cells and human umbilical vein endothelial cells (HUVECs), and implanted in rat femur to evaluate their degradability (Yang et al. 2020). This group of newly designed zinc alloys was found to show excellent promise in load-bearing orthopedic applications.
Bioceramics mostly possess high chemical stability and biocompatibility; bioactive ceramics are usually osteoconductive and/or osteoinductive. Osteoconductivity is the ability of a surface to support the attachment, proliferation, and migration of bone-forming osteoblasts, while osteoinductivity implies the recruitment and induction of undifferentiated stem cells to become osteoprogenitors (Albrektsson and Johansson 2001). Hydroxyapatite and Bioglass®, as two extensively studies bioactive ceramics, can form direct chemical bonds with surrounding tissues. Because of the close compositional resemblance of calcium phosphates (CaP, minerals containing Ca2+ and phosphate anions) to the inorganic phase of natural bone, CaP ceramics are among the most frequently used bone biomaterials (Bose and Tarafder 2012). The suitability of bioceramics for bone repair is typically assessed by culturing osteoblast-like cells and MSCs on bioceramic surfaces or in bioceramic-conditioned medium (Li et al. 2017a, b, 2020b). As a relatively new bioceramic, 2D nanosilicates have become a topic of increasing interest in bone tissue engineering because of their outstanding osteoconductivity and osteoinductivity. Nanosilicate platelets can be conveniently mixed in three-dimensional (3D) hydrogels to form nanocomposites. A 2D in vitro culture model has been used to assess the osteogenic responses of the MC3T3 E1 subclone 4 cells to nanosilicate-containing scaffolds (Xavier et al. 2015). The cells showed higher quantitative ALP activity and calcium deposition induced by nanosilicate addition. In another study, both in vitro and in vivo models were employed to examine silicate/methacrylated glycol chitosan scaffolds. The in vitro osteoinductivity tests showed markedly increased ALP activity and mineralization as well as upregulated osteogenic gene expression by the encapsulated MSCs in the presence of silicate. In a mouse nonunion calvarial defect model, the silicate-containing, cell-free composites were found to overtly promote native cell infiltration as well as cause remarkably higher bone volume density, bone growth surface area, and trabecular number than either constituent material (Cui et al. 2019).
A main limitation of many bioceramics is their low fracture toughness. To deal with this issue, bioceramics have been combined with biopolymers to obtain composites with improved ductility and toughness (Rezwan et al. 2006). A broader application of biopolymers, especially hydrogels, for musculoskeletal repair is seen in cell- or drug-delivery therapies (Nöth et al. 2010), as will be discussed in Sects. 2.2 and 2.3.
Cartilage
Various natural and synthetic biopolymers have been utilized to maintain or enhance the chondro-phenotype of chondrocytes or chondrogenic differentiation of stem cells, a process where new cartilage ECM is synthesized and deposited. Cartilage scaffolds come in various forms such as porous sponges (Wang et al. 2005), fibers/meshes (Li et al. 2005), and hydrogels (Deng et al. 2019). As a natural biomaterial, MSC-derived ECM was shown in our previous study to be a robust substrate for enhancing the chondrogenesis of articular chondrocytes (Yang et al. 2018). A composite hydrogel, consisting of gelatin, fibrinogen, hyaluronan (HA), and glycerol, was used to 3D print scaffolds containing rabbit articular chondrocytes (Kang et al. 2016). In vitro culture and in vivo implantation in mouse dorsal subcutaneous pockets both showed abundant new cartilage matrix deposition in the 3D printed structure. Sharma et al. (2013) designed a poly(ethylene glycol) diacrylate hydrogel and used it in conjunction with a bioadhesive to repair focal cartilage defects in a caprine model and in human patients (Fig. 1a–c). This soft hydrogel could augment the microfracture treatment and promote cartilage regeneration. Electrospun polymer fiber meshes are usually too dense for cell infiltration. To tackle this issue, cryoelectrospinning, with the mandrel kept at −78 °C, was employed to create an ultraporous nanofiber network that permits the infiltration of cell-laden hydrogels (Formica et al. 2016). The scaffolds were assessed with both bovine and human chondrocytes and led to robust production of type II collagen and sulfated glycosaminoglycans (GAGs), characteristic markers of cartilage, in vitro.
Fig. 1.
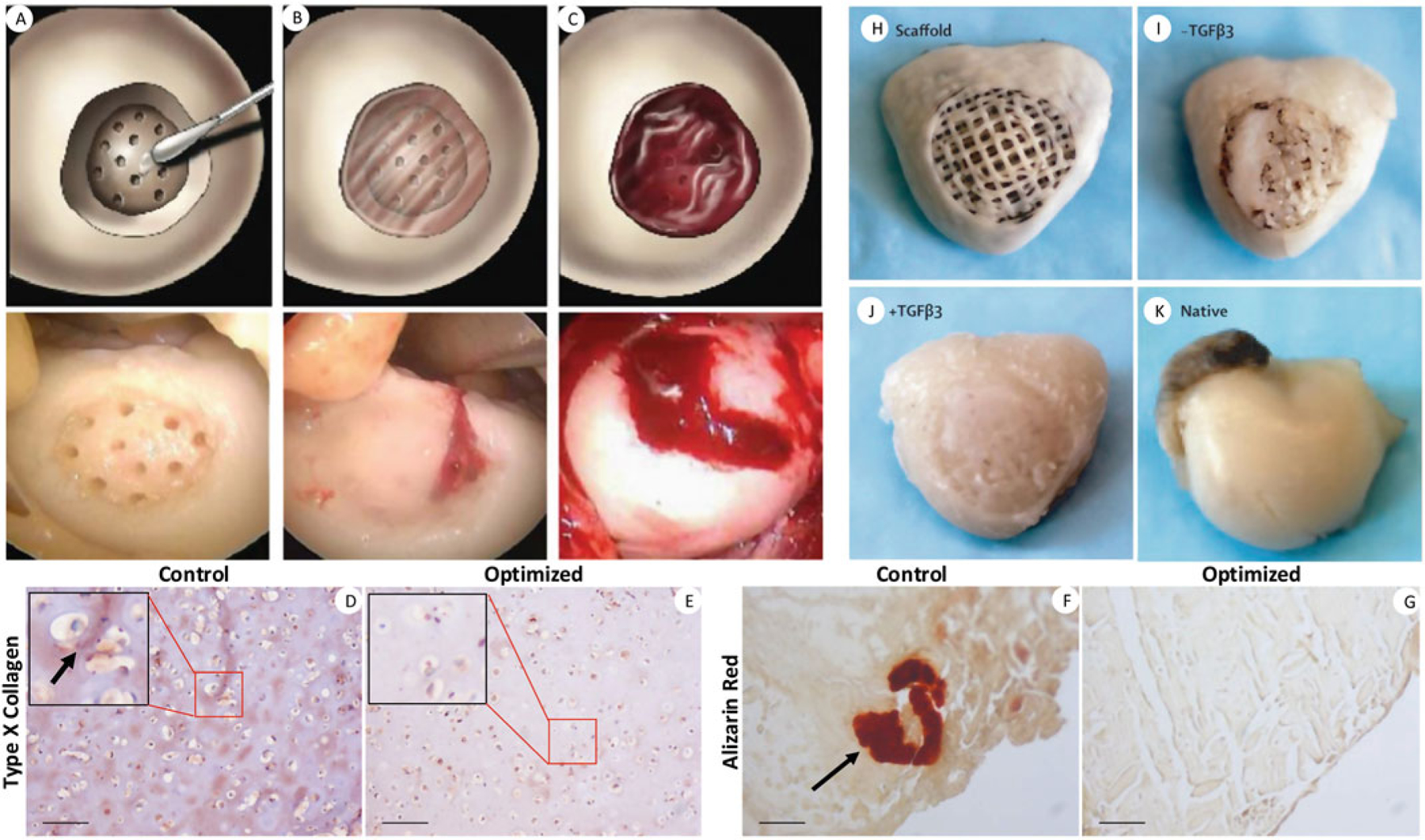
Representative cartilage regeneration strategies harnessing adhesive-hydrogel composites (a–c), MSC chondrogenesis (d–g), and growth factor-induced endogenous cell homing (h–k). In a pilot clinical study, focal cartilage defects in human patients were applied with an adhesive and microfractured (a), filled with injectable, photo-crosslinkable hydrogel (b), which trapped bleeding from the microfracture holes (c). In MSC-based cartilage tissue engineering, an optimized differentiation protocol with exposure to Wnt/β-catenin inhibitor and shorter TGF-β treatment time was found to significantly inhibit chondrocyte hypertrophy both in vitro (d, e) and in a mouse intramuscular implantation model (f, g). Scale bars: 100 μm. A 3D printed scaffold (h) infused with collagen hydrogel and with/without TGF-β3 loading was used to replace a rabbit proximal humeral joint. After 4 months’ implantation, TGFβ3-infused scaffolds showed full articular surface coverage by newly formed cartilage (i) similar to the native tissue (k), while TGFβ3-free scaffolds had only isolated tissue formation (j). Reproduced with permission from Sharma et al. (2013), Deng et al. (2019), and Lee et al. (2010)
Native cartilage undergoes substantial mechanical shear, wear, and compression during a lifetime. The recapitulation of the mechanical and frictional properties has become a key consideration in the design of cartilage biomaterials and scaffolds (Liao et al. 2013). By virtue of its high biocompatibility and safety (and with FDA approval), poly(ethylene glycol) (PEG) has been widely utilized, despite their low mechanical strength, to prepare various hydrogel scaffolds (Benoit et al. 2008; Brandl et al. 2010). An injectable hydrogel derived from 4-arm star PEG showed a local maximum strength of ~20 MPa. This high-strength PEG-based hydrogel supported the proliferation and phenotype maintenance of encapsulated murine chondrocytes, and the injected chondrocyte-laden hydrogels resulted in formation of new hyaline cartilage integrated with the host tissue in a murine osteochondral defect model (Wang et al. 2017). We recently developed a photo-crosslinkable poly-D,L-lactide acid/PEG hydrogel and assessed its chondrogenic potential with human MSCs (Sun et al. 2017). This hydrogel possesses a compressive modulus in the physiological range of native cartilage and supports the differentiation and maintenance of human MSCs and thus holds promise in point-of-care treatment of cartilage defect.
Skeletal Muscle
Generally there are two approaches to skeletal muscle tissue engineering: (1) transplantation of biomaterial scaffolds seeded with muscle cells and other supporting cells and (2) delivery of biomaterial scaffolds, with or without paracrine signaling cells, growth factors, or cytokines, to induce in situ muscle tissue engineering (Kwee and Mooney 2017). In the design of biomaterials for skeletal muscle tissue engineering, it is important to consider the microstructural, physical, and biochemical properties, such as porosity (Hill et al. 2006), degradability (Hong et al. 2011), 2D and 3D patterns (Ku et al. 2012), injectability (Rossi et al. 2011), and native biochemical cues (Perniconi et al. 2011). Electrospinning is suited to a wide variety of natural and synthetic biomaterials, capable of fabricating fibers with varying diameters and orientations and generating scalable, ECM-mimicking scaffolds with desired degree of anisotropy. An alginate-based bioink containing HUVECs has been electrospun onto uniaxially micropatterned PCL/collagen struts, generating scaffolds that provide both topographical and biochemical cues that facilitated the alignment and differentiation of subsequently seeded myocytes (Yeo and Kim 2020). Hydrogels are also commonly used biomaterials for muscle repair. Interestingly, 3D free-standing skeletal muscle fibers engineered from muscle cell-laden Matrigel were found to be able to support the differentiation of neural stem cells into neurons to form neuromuscular junctions (NMJs) (Morimoto et al. 2013).
Cell-free scaffolds used in regenerative medicine eliminate the stringent cell harvest and administration process and obviate the associated regulatory hurdles and possible immune responses. A biologic-free ferrogel, without the incorporation of any bioreagents or cells, could generate externally actuated mechanical suppressions to reduce fibrosis and inflammation and heal myotoxin-induced severe muscle injuries (Cezar et al. 2016). To regenerate volumetric muscle loss (VML), the self-regeneration capability of native muscle does not suffice. Decellularized bladder ECM was used to repair VML in both mice and human patients and resulted in de novo muscle remodeling linked to the recruitment of perivascular stem cells (Sicari et al. 2014).
Tendon and Ligament
Tendons are tough connective tissues that bind muscles to bone, while ligaments connect bones to other bones. A number of design factors, including native tissue anatomy, physical and chemical properties of the materials, and material interactions with native cells, need to be considered to select an optimal biomaterial in tendon and ligament tissue engineering (Kuo et al. 2010).
Our previous study explored the potential application of decellularized tendon-derived, solubilized extracellular matrix (tECM) in adipose stem cell (ASC)-based tendon tissue engineering (Yang et al. 2017). The tECM-supplemented 3D scaffolds not only enhanced the tenogenic differentiation of ASCs and the scaffolds’ mechanical properties but also downregulated the expression of osteogenic markers and matrix metalloproteinases. Decellularized tendon ECM combined with stem cells has also been researched for tendon repair, and the natural, decellularized scaffolds were found to provide an inductive environment for the tenogenic differentiation of MSCs (Youngstrom et al. 2013; Ning et al. 2015). Using the synthetic, degradable biopolymer poly(ε-caprolactone), Wang et al. (2018) fabricated a 3D scaffold with tendon-like mechanical properties and microstructural and hierarchical anisotropy. This scaffold supported tenogenic matrix production by human tenocytes, and the acellular scaffolds showed robust pro-tenogenic properties in a micropig model.
Ligament and tendon have very similar structure in spite of differences in collagen and water content in their ECM. Many biomaterials have, therefore, been designed to engineer both tissues and tested with similar methods (Barber et al. 2013). While tenocytes are the major cellular component of tendons, the cells residing in ligaments are mostly fibroblasts. In in vitro studies, ligament biomaterials and scaffolds are typically evaluated with fibroblasts and MSCs (Correia Pinto et al. 2017; Chang et al. 2020), and in vivo models for ligament tissue engineering are mostly created in small-scale animals.
Tendons and ligaments attach to bone at junctions known as entheses. Biomaterials for enthesis tissue engineering are expected to facilitate integration and smooth load transfer between tendon/ligament and bone. Such biomaterials have been investigated in a number of previous studies and are not detailed here (Font Tellado et al. 2015; Tang et al. 2020).
2.2. Cells
The common cell sources employed to engineer musculoskeletal tissues are shown in Fig. 2a. Because of their multipotency, high proliferative capacity, and relative ease of isolation and expansion, MSCs remain the most promising cell source in musculoskeletal regeneration and in general TERM applications. Although embryonic stem cells (ESCs) have wide differentiation potential and extensive expansion properties, for TERM applications, the hurdles include not only ethical issues related to sourcing but also the risks of tumorigenicity and immune rejection as well as the absence of a standardized protocol for ESC differentiation into musculoskeletal tissues (Jukes et al. 2010). Induced pluripotent stem cells (iPSCs) have received much attention for their pluripotency and virtually unlimited supplies. Since iPSCs can be reprogrammed from somatic cells, they not only obviate the need for embryos but can be made individual-specific, thus avoiding immune rejection issues (Takahashi and Yamanaka 2006). Primary cells from native tissues have also been employed for musculoskeletal regeneration, but they usually have lower availability, are more difficult to culture, and show larger patient-to-patient variability.
Fig. 2.
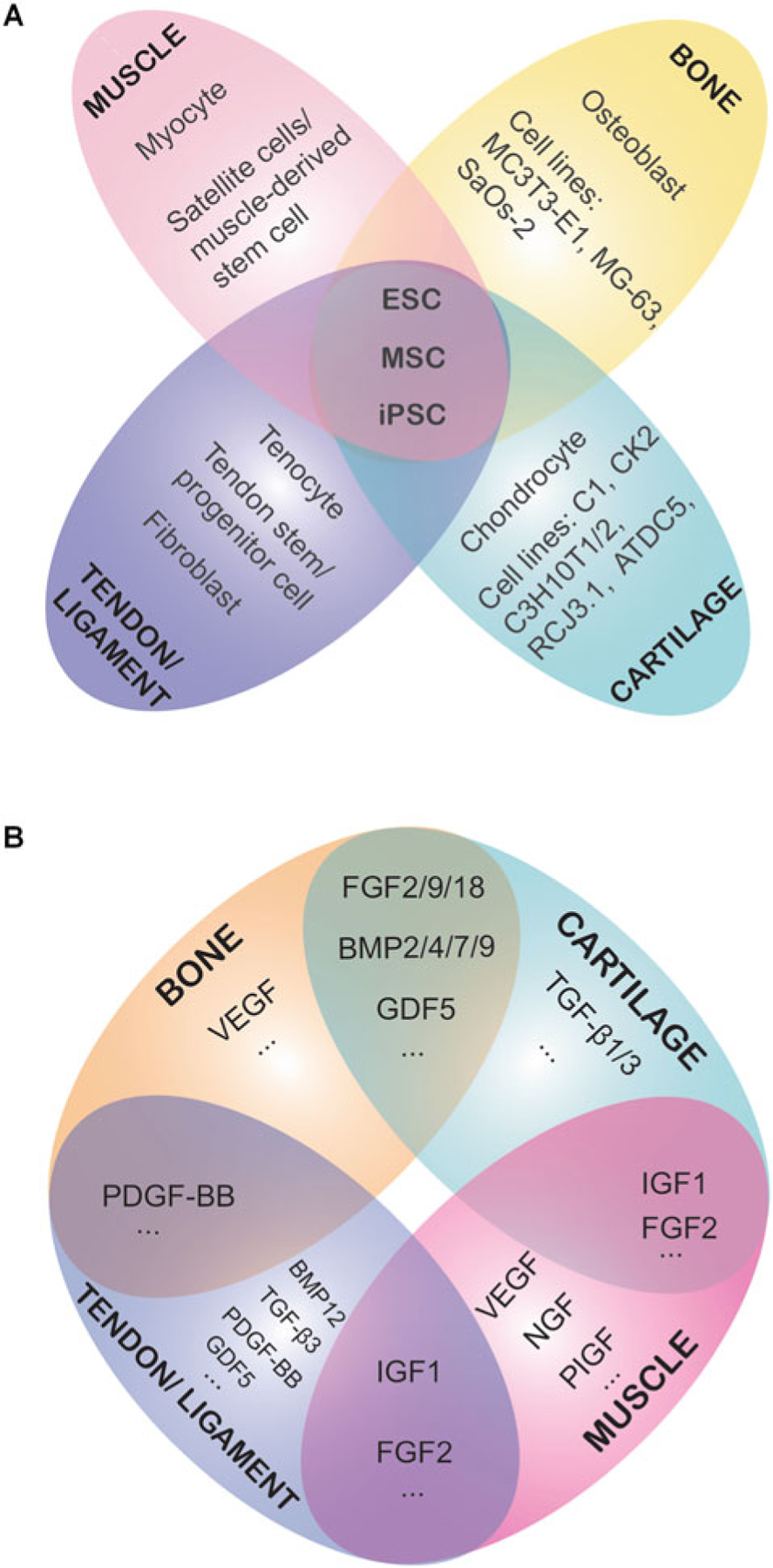
Commonly used cell sources (a) and growth factors (b) for musculoskeletal tissue engineering
Bone
Human/animal primary osteoblastic cells and stabilized osteoblastic cell lines have both been used in in vitro investigations. Osteoblastic cell lines, including SaOs-2, MG-63, and MC3T3-E1, have been compared with primary human osteoblasts (HOBs) in terms of their proliferation ability, mineralization behavior, and gene expression profile. It was found previously that HOBs only share some of the characteristics with each of the cell lines (Czekanska et al. 2014). Although the uses of HOBs are more clinically relevant than cell lines, their applications are limited by the complicated isolation procedures and heterogeneous phenotypes (Czekanska et al. 2012). As a multipotent stem cell, MSCs can be readily differentiated into osteoblasts and have been applied in various scaffolds to induce osteogenesis. Tissue-engineered bone scaffolds are designed to mimic bone ECM and recruit surrounding cells, forming a bone tissue to repair the bone defect (Amini et al. 2012). Recent studies show that MSCs possess robust osteogenic ability when seeded in collagen scaffolds, polymer-mineral scaffolds, fibrous nanocomposite scaffolds, silica-coated scaffolds, as well as their own ECM (Kuttappan et al. 2020; Duan et al. 2018; Gandhimathi et al. 2019; Harvestine et al. 2018; Meinel et al. 2003). However, autologous MSCs have limited availability. Due to patient heterogeneity, allogeneic MSCs show donor age-dependent proliferation rates and raise immunogenic concerns; MSC isolation involves invasive harvesting procedures in the case of bone marrow MSCs. iPSCs have emerged as a novel alternative to MSCs. Osteoblasts derived from iPSC were seeded on a gelatin scaffold and were found to secret calcium, OCN, and bone sialoprotein in vitro and in vivo (Bilousova et al. 2011). Retinoic acid was used to induce human iPSCs to differentiate into osteoblast-like and osteocyte-like cells, and these cells could generate human bone tissues in mouse calvarial defects (Kawai et al. 2019). Nevertheless, with current differentiation protocols, the multi-lineage differentiation capability of iPSCs generally does not match that of MSCs (Diederichs and Tuan 2014), and the optimization of differentiation protocols is warranted in future research.
Cartilage
Chondrocytes are the only cell type present in articular cartilage and exist within abundant ECM that is neither vascularized nor innervated. The presence and functional state of chondrocytes are of significant orthopedic clinical importance in the case of degenerative joint diseases, such as osteoarthritis (OA), where the articular cartilage suffers from extensive degeneration, resulting in serious physical debilitation. In order to understand OA progression, it is important to examine the changes in chondrocytes within the cartilage. There has been a relentless pursuit of an optimal chondrocyte culture method to reduce their dedifferentiation during in vitro expansion (Caron et al. 2012), and a number of chondrogenic cell lines from mouse or rat, including C1, C3H10T1/2, ATDC5, CK2, and RCJ3.1, have been generated and widely used (Brown et al. 2014). It is now accepted that abnormal biomechanical and genetic factors target chondrocytes and alter their normal functions (Goldring 2000). Dudek et al. (2016) reported that the chondrocyte clock gene BMAL1 plays a key role in the normal function of articular chondrocytes and hence in cartilage integrity. Besides chondrocytes, MSCs, iPSCs, and ESCs have also been utilized to form cartilage tissues. One of the key challenges in the long-term culture of chondrocytes, either native or derived from chondrogenically differentiated stem or progenitor cells, is the process of hypertrophy, whereby the cells enlarge and enter terminal differentiation, accompanied by apoptosis and calcification. While this differentiation and maturation of chondrocytes is a normal process of long bone development from transitional cartilage as part of the process of endochondral ossification (Saito et al. 2010), the hyaline cartilage of the articular joint surface is a permanent cartilage, and the appearance of hypertrophic chondrocytes is in fact part of the degenerative process in OA (Wang et al. 2004). Researchers have thus explored different methods to generate non-hypertrophic, hyaline cartilage. We found in our previous study that inhibiting the WNT signaling pathway during the differentiation process can promote the MSC chondrogenesis and suppress chondrogenic ossification in vitro and in vivo (Fig. 1d–g) (Deng et al. 2019; Narcisi et al. 2015). Different protocols have been proposed for iPSC chondrogenic differentiation (Dicks et al. 2020; Lach et al. 2019; Hu et al. 2020), and further research is needed for protocol optimization and standardization.
Skeletal Muscle
Regeneration of skeletal muscle is a complex process orchestrated by heterogeneous cell populations. Satellite cells/muscle-derived stem cells (MDSCs) and MSCs are among the most frequently used cell types for repairing defective muscles (Sacco et al. 2008; Montarras et al. 2005; Koponen et al. 2007; Usas and Huard 2007). Human pluripotent stem cells can be successfully differentiated into induced myogenic progenitor cells, which can readily form 3D contractile multinucleated myotubes (Rao et al. 2018). Other less commonly used cell types in muscle regeneration include adipose-derived stem cells and pericytes (Dunn et al. 2019). Satellite cells are positive for PAX7 and PAX3 (Relaix et al. 2005) and generally considered to stay dormant when there is no injury to the muscle. Interestingly, Keefe et al. (2015) demonstrated that satellite cells contribute to myofibers in both injured and healthy adult mouse muscles. Collins et al. (2005) transplanted as few as seven satellite cells in a single intact myofiber into radiation-ablated muscles, where the satellite cells vigorously self-renewed and expanded, generating clusters of new, compact myofibers. Satellite cells have also been delivered in HA hydrogels to a muscle ablation model in mouse, resulting in both structural and functional recovery (Rossi et al. 2011). Multipotent MDSCs have been employed to regenerate muscle and other musculoskeletal tissues (Usas and Huard 2007). A preplate technique was introduced to isolate MDSCs. In this procedure, the minced muscle tissues first undergo enzymatic digestion, and the resultant slurry is plated on collagen-coated flasks. Non-adhering cells in the culture medium are transferred and plated in new collagen-coated flasks, and this process is repeated ~5 times to obtain a slowly adhering cell population that contains MDSCs (Gharaibeh et al. 2008). The maintenance of MDSC potency and quiescence is challenging in in vitro culture. Quarta et al. (2016) created collagen-based artificial muscle fibers that extended the quiescence of mouse and human MDSCs. Bone marrow-derived MSCs, as another promising cell source for muscle repair, have been seeded onto 3D, porous alginate scaffolds loaded with vascular endothelial growth factor (VEGF) and insulin-like growth factor 1 (IGF1), where the local growth factor stimulation could enhance MSC paracrine signaling and support endogenous muscle regeneration in a rat muscle injury (blunt crush) model (Pumberger et al. 2016). The trophic actions of MSCs have been proposed to be a major mechanism underlying the enhanced endogenous muscle repair and regeneration (Sassoli et al. 2012).
Tendon and Ligament
Both terminally differentiated cells and stem cells have been utilized for tendon/ligament regeneration. Tenocytes are characterized by specific markers, such as Scleraxis, Mohawk, and early growth response factor (Asahara et al. 2017). To maintain tenocyte phenotype, many collagen-based scaffolds have been engineered. It was reported that collagen-GAG scaffolds were helpful for the long-term maintenance of tenocyte transcriptomic stability (Caliari et al. 2012). In addition, supplementation of growth/differentiation factor 5 (GDF5) and IGF1 together can rescue the tenocyte phenotype and drive cell proliferation (Caliari and Harley 2013). Tendon stem/progenitor cells (TSPCs) possessing the universal characteristics of stem cells were first identified by Bi et al. (2007). TSPCs reside in a biglycan- and fibromodulin-rich niche and can regenerate tendon-like tissues both in vitro and in vivo. Many studies have explored MSC-based tendon tissue engineering strategies, where MSCs were successfully induced to express tenocyte phenotypes (Kryger et al. 2007; Lee et al. 2011; Li et al. 2015; Tokunaga et al. 2015). It has also been shown that co-culture with stem cells derived from bone marrow or adipose tissue can promote tenocyte proliferation (Chen et al. 2018; Kraus et al. 2013). Recently, Komura et al. (2020) generated a tenocyte induction protocol to differentiate murine iPSCs into tenocyte-like cells, which could significantly reduce scar formation and promote tendon regeneration when implanted in injured mouse tendons. In ligament tissue engineering, ligament-derived fibroblasts (Chang et al. 2020), human dermal fibroblasts (Correia Pinto et al. 2017), and human MSCs have all been employed.
3D Organoids
ESCs, adult stem cells (including MSCs), and iPSCs have been used to engineer almost every musculoskeletal tissue (Fig. 2a), because these cells possess extensive proliferation potential and multi-differentiation potency. Aggregates of stem cells can form 3D structures that recapitulate certain architectural and functional characteristics of some native tissues in vitro, and such 3D cell structures are termed organoids (Takahashi 2019). Various ESC- and iPSC-derived organoids have been derived by employing developmental biology principles, and a number of other organoids were generated by subjecting adult stem cells to conditions that mimic tissue renewal or repair processes in vivo (Clevers 2016). For example, organoids derived from pluripotent stem cells have been studied previously to model the brain (Lancaster et al. 2013), retina (Eiraku et al. 2011), adenohypophysis (Suga et al. 2011), stomach (McCracken et al. 2014), liver (Takebe et al. 2013), lung (Dye et al. 2015), and kidney (Takasato et al. 2014); adult stem cells, which can also undergo extensive proliferation and differentiation to form organoids, have been utilized to mimic stomach (Stange Daniel et al. 2013), prostate (Chua et al. 2014), lung (Desai et al. 2014), salivary gland (Maimets et al. 2016), and esophagus (DeWard et al. 2014), just to name a few. Organoid cultures can often proliferate extensively and thus generate sufficient cells to replace damaged or diseased tissues (Yui et al. 2012). In addition, organoids offer several advantages over animal models and conventional cell culture systems in studying human development, physiology, and pathobiology. Organoids possess multiple cell types as well as 3D structural and morphological characteristics similar to native tissues; shorter duration and lower cost can generally be expected for experimentation with organoid models than with animal models; using patient-derived iPSCs and gene manipulation techniques such as the CRISPR/Cas9 system, patient- and disease-specific organoids with edited genome can greatly advance precision medicine research (Ran et al. 2013; Takebe and Wells 2019). In the history of musculoskeletal research, in 1929, Fell and Robison (1929) cultured skeletal tissue fragments from fowl embryos to model the skeletal development process in vitro. However, a relatively small number of musculoskeletal organoids have been established thus far (Mori et al. 2019). To engineer skeletal muscle tissues, a biomaterial scaffold is usually required to induce myofiber alignment under tension (Maffioletti et al. 2018). Recently, mouse iPSCs were used to fabricate 3D spherical bone/cartilage complex by micro-space culture followed by mechanical shaking (Limraksasin et al. 2020). We recently showed that after brief trypsinization, MSC-impregnated ECM experienced mesenchymal condensation and robust chondrogenesis (Yang et al. 2019).
2.3. Signaling Molecules
Cells residing in native musculoskeletal tissues receive a broad array of signals which can be chemically transmitted via growth factors and other molecules (Fig. 2b) or physically exerted through the immediate ECM. The application of such complex signals in TERM has considerable influences on the outcome. This section focuses on the signaling molecule-conveyed biochemical signals for musculoskeletal regeneration. The effects of some other signals are described in Sect. 3.
Bone
Bone is a highly vascularized tissue that undergoes constant remodeling throughout our lifetime, and signaling molecules play an important role in the complex bone remodeling process. Bone morphogenetic proteins (BMPs), particularly BMP2 and BMP7, show outstanding osteoinductivity and have therefore been widely incorporated in bone scaffolds (Yilgor et al. 2009). BMP2 was previously co-spun with hydroxyapatite-containing silk fibroin/poly(ethylene oxide) solution to fabricate fibrous bone scaffolds (Li et al. 2006; Lee et al. 2013). The presence of BMP2 markedly enhanced mineralization and upregulated osteogenic gene expression. Platelet-derived growth factor-BB (PDGF-BB), one of the five isomeric forms of PDGF, was found to enhance osteogenesis of adipose-derived MSCs and promote bone formation in a distraction osteogenesis rat model (Hung et al. 2015; Moore et al. 2009).
Vascularization plays a critical role in sustaining transplanted cells and/or scaffolds. The use of angiogenic growth factors, especially VEGF, in bone repair has led to encouraging outcomes (Murphy et al. 2000). Poly(lactic-co-glycolic acid) scaffolds containing human bone marrow-derived MSCs and condensed plasmid DNA encoding for BMP-4 and for VEGF were implanted into SCID mice, and the scaffolds containing all three components produced robust bone regeneration (Huang et al. 2005). Besides signaling molecules that act locally, systemic agents such as human growth hormone and parathyroid hormone are also important for bone regeneration (Dimitriou et al. 2011).
Cartilage
Unlike bone, articular cartilage is avascular and recalcitrant to repair and regeneration. Despite the relatively simple tissue composition of cartilage, i.e., chondrocytes embedded in a dense ECM, clinically successful cartilage tissue engineering remains a challenge. The survival and efficacy of transplanted cells in cartilage scaffolds remain controversial. Many approaches utilize biologically active molecules, such as growth factors, to enhance the recruitment of endogenous cells for expedited tissue regeneration. Transforming growth factor beta (TGF-β) plays a critical role in the maintenance of both articular cartilage and subchondral bone homeostasis (Zhen et al. 2013; Zhen and Cao 2014). TGF-β1 promotes MSC chondrogenesis (Miura et al. 1994); TGF-β1 releasing alginate-sulfate scaffold was shown to promote chondrogenesis both in vivo and in vitro (Re’em et al. 2012). Another member in the TGF-β family, TGF-β3, also possesses strong chondrogenic effects (Barry et al. 2001). It was reported that TGF-β3 incorporated within collagen-hyaluronic acid scaffolds could support the chondrogenesis of MSCs and produce cartilage-like ECM (Matsiko et al. 2015). Lee et al. (2010) 3D printed an anatomically correct scaffold and infused it with TGF-β3-loaded collagen hydrogel. It was found that these scaffolds led to regeneration of hyaline cartilage with superior compressive and shear properties and recruited cells significantly more than the TGF-β3-free scaffolds in a rabbit humeral head defect model (Fig. 1h–k). The use of biologically active molecules, therefore, holds promising potential in facilitating cell homing without cell delivery.
BMPs and the growth differentiation factors (GDFs), as members of the TGF-β superfamily, are also able to enhance cartilage regeneration. BMP2, BMP4, and BMP7 have been incorporated in various controlled delivery strategies for cartilage repair (Lam et al. 2015). Sun et al. (2019) found that GDF5 enhanced the migration and chondrogenesis of MSCs in vitro; furthermore, implanting a 3D-bioprinted GDF5-conjugated MSC-laden scaffold led to robust cartilage regeneration in a rabbit knee defect site.
Muscle
Several growth factors, including IGF1, fibroblast growth factor 2 (FGF2), nerve growth factor (NGF), placenta-derived growth factor (PIGF), and VEGF, contribute to muscle repair through different mechanisms (Wei and Huard 2008). IGF is capable of mediating the proliferation and differentiation of muscle stem cells (Adams 2000). Gel delivery of VEGF to ischemic muscle tissue increased the expression of neurotrophic factors and promoted the regrowth and maintenance of damaged axons via NGF/GDNF (nerve growth factor/glial-derived neurotrophic factor) signaling (Shvartsman et al. 2014). The co-delivery of the myogenic factor IGF1 and the angiogenic factor VEGF via an injectable alginate gel has been shown to recover muscle functions from ischemic injuries in a mouse model (Borselli et al. 2010). Wang et al. (2014) engineered a degradable, shape-memory alginate scaffold that delivers myoblasts, IGF, and VEGF through a minimally invasive approach to injured mouse muscle. The implant led to reduced muscle fibrosis, enhanced vascularization, and enhanced functional recovery. A 3D PEG-fibrinogen hydrogel incorporating mouse mesoangioblasts transduced with a PIGF lentivirus was found to attract host vessels and nerves and generate newly formed tissues histologically similar to native muscle in an ablated muscle injury model (Fuoco et al. 2015).
Tendon and Ligament
No consensus has been reached on the optimal tenogenic induction protocol. Previous studies have proposed the use of growth factors such as IGF1, FGF2, PDGF-BB, GDF-5, TGF-β3, connective tissue growth factor (CTGF), and BMP12 to promote tendon cell proliferation and enhance tendon regeneration (Raghavan et al. 2012; Rossi et al. 2011; Wolfman et al. 1997). Many of these growth factors were similarly used for ligament repair (Pauly et al. 2017; Hee et al. 2012). It was previously shown that a 12 h BMP-12 (10 ng/ml) treatment could significantly enhance MSC tenogenic marker expression, and this phenotype could be sustained in vivo in rat tendon defects (Lee et al. 2011). Barsby et al. (2014) found that by loading TGF-β3 into an ESC-seeded 3D collagen scaffold, tendon-associated gene and protein expression by the ESCs could be significantly upregulated.
3. Musculoskeletal Microphysiological Systems
As an in vitro experimental research platform, MPS mimic a tissue or organ by providing living cells with a microenvironment in which the cells can display tissue- or organ-specific phenotypic, structural, and functional characteristics. Successful MPS have been shown to exhibit characteristics that bear high structural and biologic fidelity to tissues that are difficult to achieve in conventional, static 2D, or 3D cultures (Grayson et al. 2010). Namely, MPS-derived musculoskeletal tissues aim to present tissue maturation and complexity as seen in in vivo models, where diverse cellular composition, structurally complex ECM, and interactive signals from biochemical and physical stimuli co-exist. More importantly, the application of MPS not only contributes to achieving musculoskeletal regeneration via the technology of fabricating tissue implants but may also impact the field by establishing disease models that facilitate understanding of musculoskeletal pathogenesis and expediting the development of potential therapies and therapeutic agents. Over the past decade, MPS have been utilized to model various musculoskeletal diseases such as OA (Lin et al. 2019), bone metastasis (Marturano-Kruik et al. 2018), and muscle injury (Agrawal et al. 2017). Examples of pharmacological agents that were tested in musculoskeletal MPS for treating OA include the glucocorticoids dexamethasone and triamcinolone, celecoxib, rapamycin, and HYADD®4 (hyaluronic acid alkylamide) (Lin et al. 2019; Occhetta et al. 2019; Rosser et al. 2019). The physiological relevance and clinical applicability of the MPS depends critically on optimal combination of biomaterial scaffold, appropriate cells, and biochemical and physical signals. Table 1 summarizes the biomaterials, cells, and signals utilized in representative MPS developed to recapitulate musculoskeletal tissues.
Table 1.
Representative MPS that capitulate musculoskeletal tissues/organs, the target tissues/organs, and the biomaterials, cells, and type of stimuli employed
| Target tissues | Biomaterials | Cell types | Signals/stimuli | Physiological/clinical relevance | Ref. |
|---|---|---|---|---|---|
| Cartilage | PEG-based hydrogel | Human chondrocytes | Mechanical compression; dexamethasone, IL-1Ra, rapamycin, celecoxib treatment | Mechanical overload induced OA phenotype in the tissue | Occhetta et al. (2019) |
| Cartilage | Fibrin | Equine chondrocytes | Exposure to TNF-α and IL-1β and steroid (triamcinolone) treatment | The microtissues emulate basic properties of native cartilage and respond to biochemical insults and concurrent steroid treatment | Rosser et al. (2019) |
| Cartilage and synovium | Fibrin | Human articular chondrocytes and synovial fibroblasts | Synovial fluid from OA patients | Synovitis is characterized by the increased infiltrating monocytes and macrophages in synovium (to be verified in model) | Mondadori et al. (2018) |
| Bone | Devitalized bovine trabecular bone | Human MSCs | Interstitial flow of medium | Geometrically complex temporomandibular joint condylar bone structures were created with high structural and biologic fidelity | Grayson et al. (2010) |
| Bone | N.A. (cells directly seeded in PDMS mold) | Murine preosteoblast MC3T3-E1 | Breast cancer cells MDA-MB-231-BRMS1GFP and MDA-MB-231GFP | Formation of mineralized collagen was seen in the chip and hallmarks of breast cancer bone colonization observed | Hao et al. (2018) |
| Bone | Fibrin | Human MSCs, osteodifferentiated MSCs and HUVECs | Breast cancer cell MDA-MB-231 | A vascularized bone-mimic was generated to study breast cancer cell extravasation and test drugs | Jeon et al. (2015) |
| Bone | Decellularized bovine bone | Human MSCs and HUVECs | Breast cancer cell MDA-MB-231 | Drug resistance and progression of breast cancer cells colonizing the bone were observed in the bone perivascular niche-on-a-chip | Marturano-Kruik et al. (2018) |
| Osteochondral tissue | Gelatin | Human MSCs | Interleukin (IL)-1β stimulation | IL-1β treatment of either bone or cartilage induced degenerative responses in the other tissue, suggesting their crosstalk | Lin et al. (2014) |
| Osteochondral tissue | Gelatin | Human iPSCs | IL-1β stimulation on cartilage; celecoxib treatment | The presence of bone aggravated cartilage degeneration | Lin et al. (2019) |
| Skeletal muscle | Collagen type I | Murine skeletal muscle cell line C2C12 | Electrical stimulation | The engineered muscle microtissues contracted when electrically stimulated | Shimizu et al. (2015) |
| Skeletal muscle | Collagen type I with Matrigel™ | Optogenetically encoded myoblasts C2C12 cell expressing Channelrhodopsin-2 | Optical stimulation | The device allows convenient testing of the effects of various factors on muscle maturation, structure, and function | Sakar et al. (2012) |
| Skeletal muscle | Gelatin | C2C12 cell | Cardiotoxin treatment | Cardiotoxin induced structural destruction and reduced passive tension in engineered muscle tissue | Agrawal et al. (2017) |
| Neuromuscular junction | N.A. (scaffold-free culture in a PDMS chip) | Human skeletal muscle myocytes and human iPSC-derived motoneuron | Electrical stimulation and treatment with 3 NMJ toxins | Muscle contraction induced by motoneuron activation declined or ceased with toxin treatment | Santhanam et al. (2018) |
Selection of Biomaterials
Most MPS employ hydrogels as the cell carrier to create a 3D culture environment. In addition to their biocompatibility and high water content, a major advantage of hydrogels in MPS is their injectability, allowing the cell-laden structure to crosslink in situ and conform to a desired geometry. Among the frequently used hydrogels are collagen (Sakar et al. 2012), fibrin (Rosser et al. 2019), and gelatin (Lin et al. 2014). Devitalized bone, as a natural biomaterial, possesses the microstructural, biochemical, and mechanical properties of native bone, provides an inductive niche for mineralization and angiogenesis, and has been successfully used in bone-on-a-chip systems (Marturano-Kruik et al. 2018; Grayson et al. 2010).
Cell Types
As multiple cell types are found in the majority of human tissues, MPS must also replicate this characteristic. Both stem cells and terminally differentiated cells have been utilized to construct MPS. Previous research has shown the feasibility of generating heterogeneous tissues from a single stem cell source through the perfusion of separate induction medium streams (Lin et al. 2014). Cells isolated from diseased tissues can bring with them “memories” of the disorders, thus displaying different phenotypes in vitro compared to those harvested from healthy tissues. For example, chondrocytes from healthy and OA joints were found to possess altered chondrogenic potential (Yang et al. 2006). Many musculoskeletal diseases are patient-specific, with varying stages, genetics, etiology, and drug sensitivity in different individuals. Using individual-specific cells, MPS offer a personalizable approach to understanding disease mechanisms and testing treatment options. Although the use of primary patient cells best recapitulates patient specificity, it is impractical when large cell numbers are needed. iPSCs, with an almost unlimited proliferative capacity, overcome this limitation. Although there is still a long way to go to realize wide clinical applications of iPSCs due to their possible immunogenicity and tumorigenicity and the presence of genetic and epigenetic aberrations, the use of patient-specific iPSCs has contributed significantly to the advancement of precision medicine (Tabar and Studer 2014; Sayed et al. 2016). For example, MPS engineered from iPSCs can serve as an individualized platform for various preclinical tests, thus facilitating the identification of potentially efficacious therapies tailored to individual patients.
Stem cell-derived organoids have been extensively researched to study human physiology and build disease model-in-a-dish (Lancaster et al. 2013). As tissue analogs generated in vitro, organoids are recognized for their higher biological fidelity and have been used in MPS to generate organoid-on-a-chip systems (Skardal et al. 2016). MPS create a dynamic microenvironment mimicking in vivo conditions such as continuous fluidic flow and varying oxygen concentrations, promoting communications between multiple cell/tissue components, and generating potentially more clinically relevant organoid responses to toxins, drugs, and potential medications. In the organoid-on-a-chip research community, increasing attention has been drawn to the use of custom-designed MPS that allow multi-organoid integration to ultimately generate body-on-a-chip systems with sufficient physiological relevance for accurate prediction of native tissue/organ responses to selected treatments (Skardal et al. 2016).
Biochemical and Physical Stimulations
Cells and tissues grown in musculoskeletal MPS can be subjected to various forms of environmental signals, including biochemical (Lin et al. 2014), mechanical (Occhetta et al. 2019), optical (Sakar et al. 2012), and electrical (Santhanam et al. 2018) stimulations, and the elicited responses constitute the MPS readouts. Generally, the application of MPS cultures involves three distinct stages: (1) tissue maturation, (2) disease modeling, and (3) therapeutic drug testing. In the first stage, biochemical signals provided by signaling molecules such as growth factors are usually required if the differentiation of stem cells or progenitors is involved. Other stimulations, including mechanical loading or stretching, interstitial fluid flow, and electromagnetic field, have been shown to be beneficial for enhancing matrix deposition and/or functions of musculoskeletal tissues (Langelaan et al. 2011; Mauck et al. 2000; Grayson et al. 2010). The most common strategy for generating musculoskeletal disease models in MPS is through the introduction of biochemical insults by proinflammatory cytokines or toxins (Lin et al. 2014; Agrawal et al. 2017). Given the high prevalence of mechanical injury-caused musculoskeletal disorders, hyperphysiological mechanical stress-induced pathogenesis is also believed to be of high clinical relevance (Occhetta et al. 2019). Once tissue abnormalities are observed, potential therapeutics targeting the corresponding mode of tissue injury can be introduced to test efficacy in disease modification as well as to evaluate potential toxicity. In some studies disease modeling and drug testing steps occur simultaneously (Rosser et al. 2019). When therapeutics are introduced prior to and/or along with disease causative agents, MPS may provide valuable information on the drugs’ efficacy in not only disease treatment but also disease prevention.
Generally, in the design of the MPS, there exists a tradeoff between device complexity and throughput in the process of balancing the level of biological accuracy and the required technological elements, and it is highly dependent on the needs of the application (Arrigoni et al. 2020). In basic, mechanistic studies, much higher levels of physiological accuracy are desired than in drug screening applications, where throughput and access to resources are important considerations.
As an example, MPS modeling of human synovial joints can serve different purposes. For investigating the pathogenesis and etiology of joint disorders like OA, it is critical to take into account the crosstalk and communication among different joint tissues because OA has long been considered a whole-joint disease (Loeser et al. 2012). The incorporation of multiple tissue components, including cartilage, bone, synovium, and infrapatellar fat pad, in the MPS would be critical to create sufficient physiological relevance. A particular challenge posed by the highly prevalent and debilitating disease of OA is the absence of an effective, FDA-approved disease-modifying medication. A number of candidate disease-modifying OA drugs have been proposed for OA treatment, and there is thus an urgent need of a convenient, efficient, and reliable model for high-throughput drug screening. For this purpose, MPS with low technical sophistication and high cost-effectiveness would be more appropriate. Nevertheless, we believe that investigations using a physiologically relevant and complex MPS, after extensive evaluation and validation, can offer useful guidance, e.g., identification of key readouts, to inform the design of practical and high-throughput systems.
4. Summary and Future Perspectives
It has become clear that intimate crosstalk between different cell types and cell-matrix interactions significantly influence cell proliferation, migration, and differentiation during the regeneration process (Kuraitis et al. 2012; Marsell and Einhorn 2011). Therefore, the traditional 2D culture using one cell type is less informative in predicting future clinical outcomes of potential regenerative treatments. However, 2D cultures are relatively fast and inexpensive and have lower technical requirements compared to animal models and MPS. Thus, conventional 2D dish culture should serve as the first and high-throughput platform to exclude the drugs that display obvious cytotoxicity or inefficacy for further tests. In addition, a 2D dish model allows the easy manipulation of cells, such as gain- or loss-of-function assessment, which enables mechanistic study. The use of animal models is able to partially mimic the local and global physiological changes in humans, recapturing the cell-cell and tissue-tissue crosstalk in tissue injury and repairing processes. In addition, by targeting different mechanisms, animal models are able to simulate different injuries in humans. Therefore, animal models, in particular those using clinically relevant animals, still represent the most powerful models in predicting treatment outcomes in humans. However, as has been recognized for a long time, due to the inherit difference in physiology and anatomic structure, the translation from animal models to human is challenging and not straightforward (Muschler et al. 2010). For example, autologous chondrocyte implantation, a clinically used regenerative method to treat chondral defects, showed robust cartilage repair in the first animal study in rabbits (Grande et al. 1989). However, this technology is mostly only applicable for the treatment of focal defects, and the clinical benefits over older techniques such as microfracture are sometimes questioned (Mollon et al. 2013). Such potential outcome discrepancies between animal models and human patients must be considered when translating regenerative therapies from animal studies to human clinical applications.
As mentioned above, the MPS and organoids have emerged as the next generation of models to assess the utility of regenerative medicine in treating musculoskeletal diseases. The advantages and disadvantages of these technologies, however, are both obvious. With the use of human cells and connective tissues in the manner that is observed in vivo, we may be able to recapitulate the tissue crosstalk and repair processes in humans. The major limitation of current MPS is the insufficient fidelity in replicating native tissues on the anatomical structure, phenotype, or function. In addition, the simulation of some physiological activities, such as mechanical loading, still presents significant technical challenges. Kaarj and Yoon (2019) recently reviewed the methods of delivering mechanical stimuli to MPS. With the progress in scaffold fabrication technologies, in particular 3D printing, as well as the tissue-specific differentiation of iPSCs, the rapid evolution of MPS is expected in the near future. It is noteworthy that immune cells, such as macrophages, which have been shown to play a major role in tissue regeneration (Wynn and Vannella 2016), are often neglected in current MPS. Therefore, successful development and application of MPS requires collaboration among experts from different disciplines and research fields.
The different etiologies and pathogenesis of musculoskeletal injuries and diseases further amplify the challenges in developing generally accepted regenerative treatments. Additional heterogeneity of musculoskeletal injuries and responses to treatments also arise from the patient’s genetic background; for example, single nucleotide polymorphisms between the major and minor alleles of expressed genes have recently reported to display significantly different regulatory activities (Klein et al. 2019). Therefore, future models must have the capacity to model patient-specific physiology and pathology, in order to allow the development of personalized regenerative treatments. MPS will be the most promising models for such applications. In particular, given that iPSCs have theoretically unlimited expansion capacity as well as potential to generate all human tissues/organs (Shi et al. 2017), MPS derived from patient-specific iPSCs should possess unique advantages over animal models in developing personalized medicine (Fig. 3). However, significant technological advances in stem cell biology to achieve controlled differentiation of iPSCs are clearly needed.
Fig. 3.
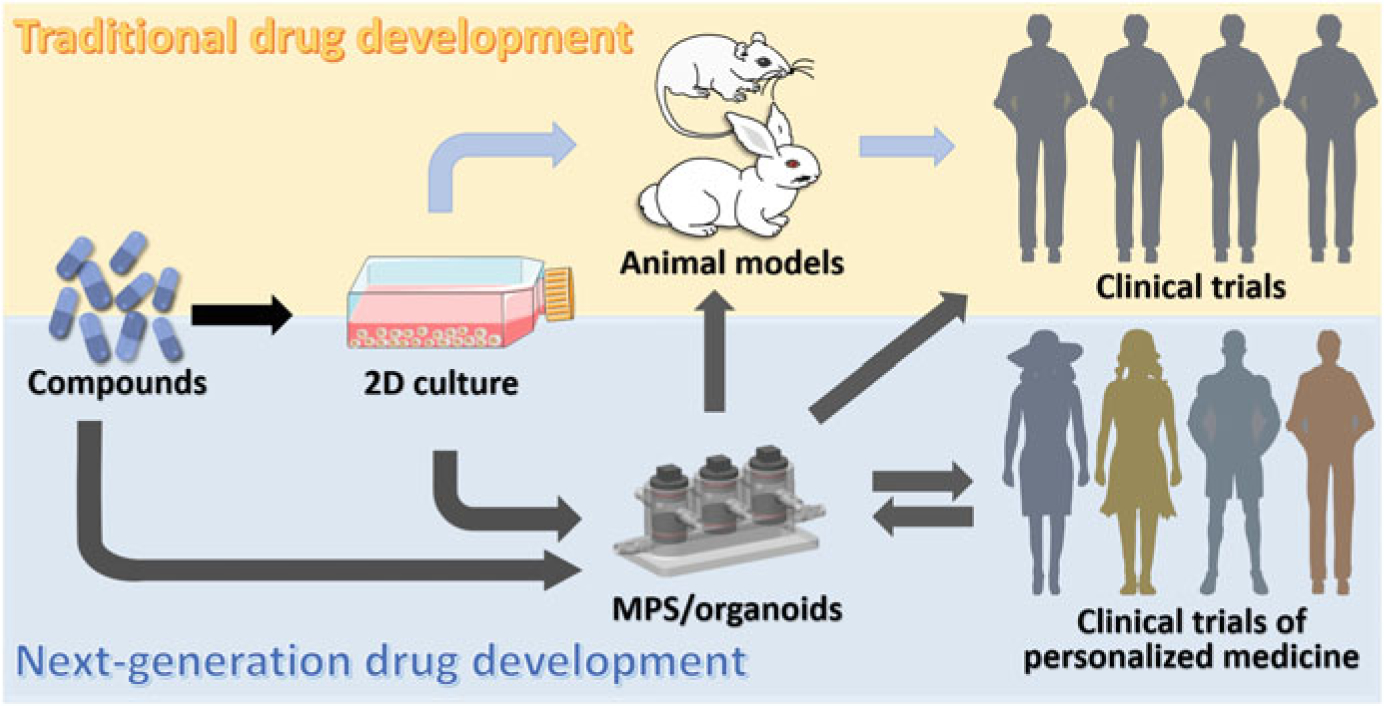
Schematic of traditional and next-generation drug development pipelines
The key requirement for a clinically relevant model is the capability to replicate the human physiology, under both normal and disease conditions, as well as to precisely predict the human response to a treatment. Achieving this relies on extensive validation. In particular, the model should be able to replicate the success or failure of known treatments that have been used clinically. Owing to the limited number and relatively short history of regenerative medicine products that have been used in clinical applications, the validation of new regenerative medicine products with known treatment is often not feasible. Thus, the establishment of a “gold standard” translation pathway is urgently needed, which will require the collaboration among practitioners of life science, pathology, engineering, and clinical medicine.
Acknowledgments
The authors thank Ms. Yuchen He for her assistance with creating Fig. 3. This work was supported by funding from the National Institutes of Health (UG3/UH3TR002136).
Contributor Information
Zhong Li, Center for Cellular and Molecular Engineering, Department of Orthopaedic Surgery, University of Pittsburgh School of Medicine, Pittsburgh, PA, USA.
Shiqi Xiang, Center for Cellular and Molecular Engineering, Department of Orthopaedic Surgery, University of Pittsburgh School of Medicine, Pittsburgh, PA, USA.
Eileen N. Li, Center for Cellular and Molecular Engineering, Department of Orthopaedic Surgery, University of Pittsburgh School of Medicine, Pittsburgh, PA, USA Department of Bioengineering, University of Pittsburgh Swanson School of Engineering, Pittsburgh, PA, USA.
Madalyn R. Fritch, Center for Cellular and Molecular Engineering, Department of Orthopaedic Surgery, University of Pittsburgh School of Medicine, Pittsburgh, PA, USA
Peter G. Alexander, Center for Cellular and Molecular Engineering, Department of Orthopaedic Surgery, University of Pittsburgh School of Medicine, Pittsburgh, PA, USA
Hang Lin, Center for Cellular and Molecular Engineering, Department of Orthopaedic Surgery, University of Pittsburgh School of Medicine, Pittsburgh, PA, USA; Department of Bioengineering, University of Pittsburgh Swanson School of Engineering, Pittsburgh, PA, USA.
Rocky S. Tuan, Center for Cellular and Molecular Engineering, Department of Orthopaedic Surgery, University of Pittsburgh School of Medicine, Pittsburgh, PA, USA Department of Bioengineering, University of Pittsburgh Swanson School of Engineering, Pittsburgh, PA, USA; Institute for Tissue Engineering and Regenerative Medicine, The Chinese University of Hong Kong, Hong Kong SAR, China.
References
- Adams G (2000) Insulin-like growth factor in muscle growth and its potential abuse by athletes. Br J Sports Med 34(6):412–413. 10.1136/bjsm.34.6.412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal G, Aung A, Varghese S (2017) Skeletal muscle-on-a-chip: an in vitro model to evaluate tissue formation and injury. Lab Chip 17(20):3447–3461. 10.1039/C7LC00512A [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrektsson T, Johansson C (2001) Osteoinduction, osteoconduction and osseointegration. Eur Spine J 10(2):S96–S101. 10.1007/s005860100282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amini AR, Laurencin CT, Nukavarapu SP (2012) Bone tissue engineering: recent advances and challenges. Crit Rev Biomed Eng 40(5):363–408. 10.1615/CritRevBiomedEng.v40.i5.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrigoni C, Lopa S, Candrian C, Moretti M (2020) Organs-on-a-chip as model systems for multifactorial musculoskeletal diseases. Curr Opin Biotechnol 63:79–88. 10.1016/j.copbio.2019.12.006 [DOI] [PubMed] [Google Scholar]
- Asahara H, Inui M, Lotz MK (2017) Tendons and ligaments: connecting developmental biology to musculoskeletal disease pathogenesis. J Bone Miner Res 32(9):1773–1782. 10.1002/jbmr.3199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber JG, Handorf AM, Allee TJ, Li W-J (2013) Braided nanofibrous scaffold for tendon and ligament tissue engineering. Tissue Eng Part A 19(11–12):1265–1274. 10.1089/ten.tea.2010.0538 [DOI] [PubMed] [Google Scholar]
- Barry F, Boynton RE, Liu B, Murphy JM (2001) Chondrogenic differentiation of mesenchymal stem cells from bone marrow: differentiation-dependent gene expression of matrix components. Exp Cell Res 268(2):189–200. 10.1006/excr.2001.5278 [DOI] [PubMed] [Google Scholar]
- Barsby T, Bavin EP, Guest DJ (2014) Three-dimensional culture and transforming growth factor beta3 synergistically promote tenogenic differentiation of equine embryo-derived stem cells. Tissue Eng Part A 20(19–20):2604–2613. 10.1089/ten.tea.2013.0457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit DSW, Schwartz MP, Durney AR, Anseth KS (2008) Small functional groups for controlled differentiation of hydrogel-encapsulated human mesenchymal stem cells. Nat Mater 7 (10):816–823. 10.1038/nmat2269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi Y, Ehirchiou D, Kilts TM, Inkson CA, Embree MC, Sonoyama W et al. (2007) Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat Med 13 (10):1219–1227. 10.1038/nm1630 [DOI] [PubMed] [Google Scholar]
- Bilousova G, Jun DH, King KB, De Langhe S, Chick WS, Torchia EC et al. (2011) Osteoblasts derived from induced pluripotent stem cells form calcified structures in scaffolds both in vitro and in vivo. Stem Cells 29(2):206–216. 10.1002/stem.566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borselli C, Storrie H, Benesch-Lee F, Shvartsman D, Cezar C, Lichtman JW et al. (2010) Functional muscle regeneration with combined delivery of angiogenesis and myogenesis factors. Proc Natl Acad Sci U S A 107(8):3287–3292. 10.1073/pnas.0903875106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose S, Tarafder S (2012) Calcium phosphate ceramic systems in growth factor and drug delivery for bone tissue engineering: a review. Acta Biomater 8(4):1401–1421. 10.1016/j.actbio.2011.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandl FP, Seitz AK, Teßmar JKV, Blunk T, Göpferich AM (2010) Enzymatically degradable poly (ethylene glycol) based hydrogels for adipose tissue engineering. Biomaterials 31 (14):3957–3966. 10.1016/j.biomaterials.2010.01.128 [DOI] [PubMed] [Google Scholar]
- Brown PT, Squire MW, Li W-J (2014) Characterization and evaluation of mesenchymal stem cells derived from human embryonic stem cells and bone marrow. Cell Tissue Res 358(1):149–164. 10.1007/s00441-014-1926-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caliari SR, Harley BA (2013) Composite growth factor supplementation strategies to enhance tenocyte bioactivity in aligned collagen-GAG scaffolds. Tissue Eng Part A 19 (9–10):1100–1112. 10.1089/ten.tea.2012.0497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caliari SR, Weisgerber DW, Ramirez MA, Kelkhoff DO, Harley BA (2012) The influence of collagen–glycosaminoglycan scaffold relative density and microstructural anisotropy on tenocyte bioactivity and transcriptomic stability. J Mech Behav Biomed Mater 11:27–40. 10.1016/j.jmbbm.2011.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caron MMJ, Emans PJ, Coolsen MME, Voss L, Surtel DAM, Cremers A et al. (2012) Redifferentiation of dedifferentiated human articular chondrocytes: comparison of 2D and 3D cultures. Osteoarthr Cartil 20(10):1170–1178. 10.1016/j.joca.2012.06.016 [DOI] [PubMed] [Google Scholar]
- Cezar CA, Roche ET, Vandenburgh HH, Duda GN, Walsh CJ, Mooney DJ (2016) Biologic-free mechanically induced muscle regeneration. Proc Natl Acad Sci U S A 113(6):1534–1539. 10.1073/pnas.1517517113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C-W, Lee J-H, Chao P-hG (2020) Chemical optimization for functional ligament tissue engineering. Tissue Eng Part A 26(1–2):102–110. 10.1089/ten.tea.2019.0142 [DOI] [PubMed] [Google Scholar]
- Chen Q, Thouas GA (2015) Metallic implant biomaterials. Mater Sci 87:1–57. 10.1016/j.mser.2014.10.001 [DOI] [Google Scholar]
- Chen Q, Liang Q, Zhuang W, Zhou J, Zhang B, Xu P et al. (2018) Tenocyte proliferation and migration promoted by rat bone marrow mesenchymal stem cell-derived conditioned medium. Biotechnol Lett 40(1):215–224. 10.1007/s10529-017-2446-7 [DOI] [PubMed] [Google Scholar]
- Chua CW, Shibata M, Lei M, Toivanen R, Barlow LJ, Bergren Sarah K et al. (2014) Single luminal epithelial progenitors can generate prostate organoids in culture. Nat Cell Biol 16(10):951–961. 10.1038/ncb3047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H (2016) Modeling development and disease with organoids. Cell 165(7):1586–1597. 10.1016/j.cell.2016.05.082 [DOI] [PubMed] [Google Scholar]
- Collins CA, Olsen I, Zammit PS, Heslop L, Petrie A, Partridge TA, Morgan JE (2005) Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell 122(2):289–301. 10.1016/j.cell.2005.05.010 [DOI] [PubMed] [Google Scholar]
- Correia Pinto V, Costa-Almeida R, Rodrigues I, Guardão L, Soares R, Miranda Guedes R (2017) Exploring the in vitro and in vivo compatibility of PLA, PLA/GNP and PLA/CNT-COOH biodegradable nanocomposites: prospects for tendon and ligament applications. J Biomed Mater Res A 105(8):2182–2190. 10.1002/jbm.a.36075 [DOI] [PubMed] [Google Scholar]
- Cui Z-K, Kim S, Baljon JJ, Wu BM, Aghaloo T, Lee M (2019) Microporous methacrylated glycol chitosan-montmorillonite nanocomposite hydrogel for bone tissue engineering. Nat Commun 10(1):3523. 10.1038/s41467-019-11511-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czekanska E, Stoddart M, Richards R, Hayes J (2012) In search of an osteoblast cell model for in vitro research. Eur Cell Mater 24(4):1–17. 10.22203/ecm.v024a01 [DOI] [PubMed] [Google Scholar]
- Czekanska EM, Stoddart MJ, Ralphs JR, Richards R, Hayes J (2014) A phenotypic comparison of osteoblast cell lines versus human primary osteoblasts for biomaterials testing. J Biomed Mater Res A 102(8):2636–2643. 10.1002/jbm.a.34937 [DOI] [PubMed] [Google Scholar]
- Deng Y, Lei G, Lin Z, Yang Y, Lin H, Tuan RS (2019) Engineering hyaline cartilage from mesenchymal stem cells with low hypertrophy potential via modulation of culture conditions and Wnt/β-catenin pathway. Biomaterials 192:569–578. 10.1016/j.biomaterials.2018.11.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai TJ, Brownfield DG, Krasnow MA (2014) Alveolar progenitor and stem cells in lung development, renewal and cancer. Nature 507(7491):190–194. 10.1038/nature12930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWard AD, Cramer J, Lagasse E (2014) Cellular heterogeneity in the mouse esophagus implicates the presence of a nonquiescent epithelial stem cell population. Cell Rep 9(2):701–711. 10.1016/j.celrep.2014.09.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dicks A, Wu C-L, Steward N, Adkar SS, Gersbach CA, Guilak F (2020) Prospective isolation of chondroprogenitors from human iPSCs based on cell surface markers identified using a CRIS PR-Cas9-generated reporter. Stem Cell Res Ther 11(1):66. 10.1186/s13287-020-01597-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diederichs S, Tuan RS (2014) Functional comparison of human-induced pluripotent stem cell-derived mesenchymal cells and bone marrow-derived mesenchymal stromal cells from the same donor. Stem Cells Dev 23(14):1594–1610. 10.1089/scd.2013.0477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitriou R, Jones E, McGonagle D, Giannoudis PV (2011) Bone regeneration: current concepts and future directions. BMC Med 9(1):66. 10.1186/1741-7015-9-66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan W, Chen C, Haque M, Hayes D, Lopez MJ (2018) Polymer-mineral scaffold augments in vivo equine multipotent stromal cell osteogenesis. Stem Cell Res Ther 9(1):60. 10.1186/s13287-018-0790-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudek M, Gossan N, Yang N, Im H-J, Ruckshanthi JP, Yoshitane H et al. (2016) The chondrocyte clock gene Bmal1 controls cartilage homeostasis and integrity. J Clin Invest 126(1):365–376. 10.1172/JCI82755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn A, Talovic M, Patel K, Patel A, Marcinczyk M, Garg K (2019) Biomaterial and stem cell-based strategies for skeletal muscle regeneration. J Orthop Res 37(6):1246–1262. 10.1002/jor.24212 [DOI] [PubMed] [Google Scholar]
- Dye BR, Hill DR, Ferguson MA, Tsai Y-H, Nagy MS, Dyal R et al. (2015) In vitro generation of human pluripotent stem cell derived lung organoids. Elife 4:e05098. 10.7554/eLife.05098.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiraku M, Takata N, Ishibashi H, Kawada M, Sakakura E, Okuda S et al. (2011) Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature 472(7341):51–56. 10.1038/nature09941 [DOI] [PubMed] [Google Scholar]
- Ericsson AC, Crim MJ, Franklin CL (2013) A brief history of animal modeling. Mo Med 110 (3):201–205 [PMC free article] [PubMed] [Google Scholar]
- Fell HB, Robison R (1929) The growth, development and phosphatase activity of embryonic avian femora and limb-buds cultivated in vitro. Biochem J 23(4):767–784. 10.1042/bj0230767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Font Tellado S, Balmayor ER, Van Griensven M (2015) Strategies to engineer tendon/ligament-to-bone interface: biomaterials, cells and growth factors. Adv Drug Deliv Rev 94:126–140. 10.1016/j.addr.2015.03.004 [DOI] [PubMed] [Google Scholar]
- Formica FA, Öztürk E, Hess SC, Stark WJ, Maniura-Weber K, Rottmar M, Zenobi-Wong M (2016) A bioinspired ultraporous nanofiber-hydrogel mimic of the cartilage extracellular matrix. Adv Healthc Mater 5(24):3129–3138. 10.1002/adhm.201600867 [DOI] [PubMed] [Google Scholar]
- Fuoco C, Rizzi R, Biondo A, Longa E, Mascaro A, Shapira-Schweitzer K et al. (2015) In vivo generation of a mature and functional artificial skeletal muscle. EMBO Mol Med 7(4):411–422. 10.15252/emmm.201404062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhimathi C, Quek YJ, Ezhilarasu H, Ramakrishna S, Bay B-H, Srinivasan DK (2019) Osteogenic differentiation of mesenchymal stem cells with silica-coated gold nanoparticles for bone tissue engineering. Int J Mol Sci 20(20):5135. 10.3390/ijms20205135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gharaibeh B, Lu A, Tebbets J, Zheng B, Feduska J, Crisan M et al. (2008) Isolation of a slowly adhering cell fraction containing stem cells from murine skeletal muscle by the preplate technique. Nat Protoc 3(9):1501–1509. 10.1038/nprot.2008.142 [DOI] [PubMed] [Google Scholar]
- Goldring MB (2000) The role of the chondrocyte in osteoarthritis. Arthritis Rheumatol 43 (9):1916–1926. [DOI] [PubMed] [Google Scholar]
- Grande DA, Pitman MI, Peterson L, Menche D, Klein M (1989) The repair of experimentally produced defects in rabbit articular cartilage by autologous chondrocyte transplantation. J Orthop Res 7(2):208–218. 10.1002/jor.1100070208 [DOI] [PubMed] [Google Scholar]
- Grayson WL, Fröhlich M, Yeager K, Bhumiratana S, Chan ME, Cannizzaro C et al. (2010) Engineering anatomically shaped human bone grafts. Proc Natl Acad Sci U S A 107 (8):3299–3304. 10.1073/pnas.0905439106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groessner-Schreiber B, Tuan RS (1992) Enhanced extracellular matrix production and mineralization by osteoblasts cultured on titanium surfaces in vitro. J Cell Sci 101(1):209–217 [DOI] [PubMed] [Google Scholar]
- Hackam DG, Redelmeier DA (2006) Translation of research evidence from animals to humans. JAMA 296(14):1727–1732. 10.1001/jama.296.14.1731 [DOI] [PubMed] [Google Scholar]
- Hao S, Ha L, Cheng G, Wan Y, Xia Y, Sosnoski DM et al. (2018) A spontaneous 3D bone-on-a-chip for bone metastasis study of breast cancer cells. Small 14(12):1702787. 10.1002/smll.201702787 [DOI] [PubMed] [Google Scholar]
- Harvestine JN, Orbay H, Chen JY, Sahar DE, Leach JK (2018) Cell-secreted extracellular matrix, independent of cell source, promotes the osteogenic differentiation of human stromal vascular fraction. J Mater Chem B 6(24):4104–4115. 10.1039/C7TB02787G [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hee CK, Dines JS, Solchaga LA, Shah VR, Hollinger JO (2012) Regenerative tendon and ligament healing: opportunities with recombinant human platelet-derived growth factor BB-homodimer. Tissue Eng Part B Rev 18(3):225–234. 10.1089/ten.teb.2011.0603 [DOI] [PubMed] [Google Scholar]
- Hill E, Boontheekul T, Mooney DJ (2006) Regulating activation of transplanted cells controls tissue regeneration. Proc Natl Acad Sci U S A 103(8):2494–2499. 10.1073/pnas.0506004103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong Y, Takanari K, Amoroso NJ, Hashizume R, Brennan-Pierce EP, Freund JM et al. (2011) An elastomeric patch electrospun from a blended solution of dermal extracellular matrix and biodegradable polyurethane for rat abdominal wall repair. Tissue Eng Part C Methods 18 (2):122–132. 10.1089/ten.tec.2011.0295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Chen L, Gao Y, Cheng P, Yang L, Wu C, Jie Q (2020) A lithium-containing biomaterial promotes chondrogenic differentiation of induced pluripotent stem cells with reducing hypertrophy. Stem Cell Res Ther 11(1):77. 10.1186/s13287-020-01606-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YC, Kaigler D, Rice KG, Krebsbach PH, Mooney DJ (2005) Combined angiogenic and osteogenic factor delivery enhances bone marrow stromal cell-driven bone regeneration. J Bone Miner Res 20(5):848–857. 10.1359/JBMR.041226 [DOI] [PubMed] [Google Scholar]
- Huh D, Matthews BD, Mammoto A, Montoya-Zavala M, Hsin HY, Ingber DE (2010) Reconstituting organ-level lung functions on a chip. Science 328(5986):1662–1668. 10.1126/science.1188302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung BP, Hutton DL, Kozielski KL, Bishop CJ, Naved B, Green JJ et al. (2015) Platelet-derived growth factor BB enhances osteogenesis of adipose-derived but not bone marrow-derived mesenchymal stromal/stem cells. Stem Cells 33(9):2773–2784. 10.1002/stem.2060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- James SL, Abate D, Abate KH, Abay SM, Abbafati C, Abbasi N et al. (2018) Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 392(10159):1789–1858. 10.1016/S0140-6736(18)32279-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang K-J, Mehr AP, Hamilton GA, McPartlin LA, Chung S, Suh K-Y, Ingber DE (2013) Human kidney proximal tubule-on-a-chip for drug transport and nephrotoxicity assessment. Integr Biol 5(9):1119–1129. 10.1039/c3ib40049b [DOI] [PubMed] [Google Scholar]
- Jeon JS, Bersini S, Gilardi M, Dubini G, Charest JL, Moretti M, Kamm RD (2015) Human 3D vascularized organotypic microfluidic assays to study breast cancer cell extravasation. Proc Natl Acad Sci U S A 112(1):214–219. 10.1073/pnas.1417115112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jukes JM, van Blitterswijk CA, de Boer J (2010) Skeletal tissue engineering using embryonic stem cells. J Tissue Eng Regen Med 4(3):165–180. 10.1002/term.234 [DOI] [PubMed] [Google Scholar]
- Kaarj K, Yoon J-Y (2019) Methods of delivering mechanical stimuli to organ-on-a-chip. Micromachines 10(10):700. 10.3390/mi10100700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang H-W, Lee SJ, Ko IK, Kengla C, Yoo JJ, Atala A (2016) A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat Biotechnol 34(3):312. 10.1038/nbt.3413 [DOI] [PubMed] [Google Scholar]
- Kawai S, Yoshitomi H, Sunaga J, Alev C, Nagata S, Nishio M et al. (2019) In vitro bone-like nodules generated from patient-derived iPSCs recapitulate pathological bone phenotypes. Nat Biomed Eng 3(7):558–570. 10.1038/s41551-019-0410-7 [DOI] [PubMed] [Google Scholar]
- Keefe AC, Lawson JA, Flygare SD, Fox ZD, Colasanto MP, Mathew SJ et al. (2015) Muscle stem cells contribute to myofibres in sedentary adult mice. Nat Commun 6(1):1–11. 10.1038/ncomms8087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Huh D, Hamilton G, Ingber DE (2012) Human gut-on-a-chip inhabited by microbial flora that experiences intestinal peristalsis-like motions and flow. Lab Chip 12(12):2165–2174. 10.1039/C2LC40074J [DOI] [PubMed] [Google Scholar]
- Klein JC, Keith A, Rice SJ, Shepherd C, Agarwal V, Loughlin J, Shendure J (2019) Functional testing of thousands of osteoarthritis-associated variants for regulatory activity. Nat Commun 10 (1):2434. 10.1038/s41467-019-10439-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komura S, Satake T, Goto A, Aoki H, Shibata H, Ito K et al. (2020) Induced pluripotent stem cell-derived tenocyte-like cells promote the regeneration of injured tendons in mice. Sci Rep 10 (1):1–12. 10.1038/s41598-020-61063-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koponen JK, Kekarainen T, Heinonen SE, Laitinen A, Nystedt J, Laine J, Ylä-Herttuala S (2007) Umbilical cord blood-derived progenitor cells enhance muscle regeneration in mouse hindlimb ischemia model. Mol Ther 15(12):2172–2177. 10.1038/sj.mt.6300302 [DOI] [PubMed] [Google Scholar]
- Kraus A, Woon C, Raghavan S, Megerle K, Pham H, Chang J (2013) Co-culture of human adipose-derived stem cells with tenocytes increases proliferation and induces differentiation into a tenogenic lineage. Plast Reconstr Surg 132(5):754e–766e. 10.1097/PRS.0b013e3182a48b46 [DOI] [PubMed] [Google Scholar]
- Kryger GS, Chong AK, Costa M, Pham H, Bates SJ, Chang J (2007) A comparison of tenocytes and mesenchymal stem cells for use in flexor tendon tissue engineering. J Hand Surg Am 32 (5):597–605. 10.1016/j.jhsa.2007.02.018 [DOI] [PubMed] [Google Scholar]
- Ku SH, Lee SH, Park CB (2012) Synergic effects of nanofiber alignment and electroactivity on myoblast differentiation. Biomaterials 33(26):6098–6104. 10.1016/j.biomaterials.2012.05.018 [DOI] [PubMed] [Google Scholar]
- Kuo CK, Marturano JE, Tuan RS (2010) Novel strategies in tendon and ligament tissue engineering: advanced biomaterials and regeneration motifs. BMC Sports Sci Med Rehabil 2(1):20. 10.1186/1758-2555-2-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuraitis D, Giordano C, Ruel M, Musarò A, Suuronen EJ (2012) Exploiting extracellular matrix-stem cell interactions: a review of natural materials for therapeutic muscle regeneration. Biomaterials 33(2):428–443. 10.1016/j.biomaterials.2011.09.078 [DOI] [PubMed] [Google Scholar]
- Kuttappan S, J-i J, Sabu CK, Menon D, Tabata Y, Nair MB (2020) Bioinspired nanocomposite fibrous scaffold mediated delivery of ONO-1301 and BMP2 enhance bone regeneration in critical sized defect. Mater Sci Eng C 110:110591. 10.1016/j.msec.2019.110591 [DOI] [PubMed] [Google Scholar]
- Kwee BJ, Mooney DJ (2017) Biomaterials for skeletal muscle tissue engineering. Curr Opin Biotechnol 47:16–22. 10.1016/j.copbio.2017.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lach MS, Wroblewska J, Kulcenty K, Richter M, Trzeciak T, Suchorska WM (2019) Chondrogenic differentiation of pluripotent stem cells under controllable serum-free conditions. Int J Mol Sci 20(11):2711. 10.3390/ijms20112711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam J, Lu S, Kasper FK, Mikos AG (2015) Strategies for controlled delivery of biologics for cartilage repair. Adv Drug Deliv Rev 84:123–134. 10.1016/j.addr.2014.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster MA, Renner M, Martin C-A, Wenzel D, Bicknell LS, Hurles ME et al. (2013) Cerebral organoids model human brain development and microcephaly. Nature 501(7467):373–379. 10.1038/nature12517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langelaan MLP, Boonen KJM, Rosaria-Chak KY, van der Schaft DWJ, Post MJ, Baaijens FPT (2011) Advanced maturation by electrical stimulation: differences in response between C2C12 and primary muscle progenitor cells. J Tissue Eng Regen Med 5(7):529–539. 10.1002/term.345 [DOI] [PubMed] [Google Scholar]
- Langer R, Vacanti JP (1993) Tissue engineering. Science 260(5110):920–926. 10.1126/science.8493529 [DOI] [PubMed] [Google Scholar]
- Lee CH, Cook JL, Mendelson A, Moioli EK, Yao H, Mao JJ (2010) Regeneration of the articular surface of the rabbit synovial joint by cell homing: a proof of concept study. Lancet 376 (9739):440–448. 10.1016/S0140-6736(10)60668-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JY, Zhou Z, Taub PJ, Ramcharan M, Li Y, Akinbiyi T et al. (2011) BMP-12 treatment of adult mesenchymal stem cells in vitro augments tendon-like tissue formation and defect repair in vivo. PLoS One 6(3):e17531–e17531. 10.1371/journal.pone.0017531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SS, Huang BJ, Kaltz SR, Sur S, Newcomb CJ, Stock SR et al. (2013) Bone regeneration with low dose BMP-2 amplified by biomimetic supramolecular nanofibers within collagen scaffolds. Biomaterials 34(2):452–459. 10.1016/j.biomaterials.2012.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W-J, Tuli R, Okafor C, Derfoul A, Danielson KG, Hall DJ, Tuan RS (2005) A three-dimensional nanofibrous scaffold for cartilage tissue engineering using human mesenchymal stem cells. Biomaterials 26(6):599–609. 10.1016/j.biomaterials.2004.03.005 [DOI] [PubMed] [Google Scholar]
- Li C, Vepari C, Jin H-J, Kim HJ, Kaplan DL (2006) Electrospun silk-BMP-2 scaffolds for bone tissue engineering. Biomaterials 27(16):3115–3124. 10.1016/j.biomaterials.2006.01.022 [DOI] [PubMed] [Google Scholar]
- Li Y, Ramcharan M, Zhou Z, Leong DJ, Akinbiyi T, Majeska RJ, Sun HB (2015) The role of scleraxis in fate determination of mesenchymal stem cells for tenocyte differentiation. Sci Rep 5:13149. 10.1038/srep13149 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Li Z, Bi S, Thompson BC, Li R, Khor KA (2017a) Multifunctional bioceramic-based composites reinforced with silica-coated carbon nanotube core-shell structures. Ceram Int 43:16084–16093. 10.1016/j.ceramint.2017.08.125 [DOI] [Google Scholar]
- Li Z, Khun NW, Tang X-Z, Liu E, Khor KA (2017b) Mechanical, tribological and biological properties of novel 45S5 Bioglass® composites reinforced with in situ reduced graphene oxide. J Mech Behav Biomed Mater 65:77–89. 10.1016/j.jmbbm.2016.08.007 [DOI] [PubMed] [Google Scholar]
- Li J, Mutreja I, Hooper GJ, Clinch K, Lim K, Evans G, Woodfield TF (2020a) Combined infection control and enhanced osteogenic differentiation capacity on additive manufactured Ti-6Al-4V are mediated via titania nanotube delivery of novel biofilm inhibitors. Adv Mater Interfaces 7:1901963. 10.1002/admi.201901963 [DOI] [Google Scholar]
- Li Z, Zhu W, Bi S, Li R, Hu H, Lin H et al. (2020b) Incorporating silica-coated graphene in bioceramic nanocomposites to simultaneously enhance mechanical and biological performance. J Biomed Mater Res A 108(4):1016–1027. 10.1002/jbm.a.36880 [DOI] [PubMed] [Google Scholar]
- Liao I, Moutos FT, Estes BT, Zhao X, Guilak F (2013) Composite three-dimensional woven scaffolds with interpenetrating network hydrogels to create functional synthetic articular cartilage. Adv Funct Mater 23(47):5833–5839. 10.1002/adfm.201300483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limraksasin P, Kondo T, Zhang M, Okawa H, Osathanon T, Pavasant P, Egusa H (2020) In vitro fabrication of hybrid bone/cartilage complex using mouse induced pluripotent stem cells. Int J Mol Sci 21(2):581. 10.3390/ijms21020581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H, Lozito TP, Alexander PG, Gottardi R, Tuan RS (2014) Stem cell-based microphysiological osteochondral system to model tissue response to interleukin-1β. Mol Pharm 11(7):2203–2212. 10.1021/mp500136b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Z, Li Z, Li EN, Li X, Del Duke CJ, Shen H et al. (2019) Osteochondral tissue chip derived from iPSCs: modeling OA pathologies and testing drugs. Front Bioeng Biotechnol 7:411. 10.3389/fbioe.2019.00411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Kuang B, Rothrauff BB, Tuan RS, Lin H (2019) Robust bone regeneration through endochondral ossification of human mesenchymal stem cells within their own extracellular matrix. Biomaterials 218:119336. 10.1016/j.biomaterials.2019.119336 [DOI] [PubMed] [Google Scholar]
- Loeser RF, Goldring SR, Scanzello CR, Goldring MB (2012) Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum 64(6):1697–1707. 10.1002/art.34453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffioletti SM, Sarcar S, Henderson ABH, Mannhardt I, Pinton L, Moyle LA et al. (2018) Three-dimensional human iPSC-derived artificial skeletal muscles model muscular dystrophies and enable multilineage tissue engineering. Cell Rep 23(3):899–908. 10.1016/j.celrep.2018.03.091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maimets M, Rocchi C, Bron R, Pringle S, Kuipers J, Giepmans BN et al. (2016) Long-term in vitro expansion of salivary gland stem cells driven by Wnt signals. Stem Cell Rep 6(1):150–162. 10.1016/j.stemcr.2015.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak IW, Evaniew N, Ghert M (2014) Lost in translation: animal models and clinical trials in cancer treatment. Am J Transl Res 6(2):114–118 [PMC free article] [PubMed] [Google Scholar]
- Marsell R, Einhorn TA (2011) The biology of fracture healing. Injury 42(6):551–555. 10.1016/j.injury.2011.03.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marturano-Kruik A, Nava MM, Yeager K, Chramiec A, Hao L, Robinson S et al. (2018) Human bone perivascular niche-on-a-chip for studying metastatic colonization. Proc Natl Acad Sci U S A 115(6):1256–1261. 10.1073/pnas.1714282115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsiko A, Levingstone TJ, Gleeson JP, O’Brien FJ (2015) Incorporation of TGF-Beta 3 within collagen–hyaluronic acid scaffolds improves their chondrogenic potential. Adv Healthc Mater 4 (8):1175–1179. 10.1002/adhm.201500053 [DOI] [PubMed] [Google Scholar]
- Mauck RL, Soltz MA, Wang CCB, Wong DD, Chao P-HG, Valhmu WB et al. (2000) Functional tissue engineering of articular cartilage through dynamic loading of chondrocyte-seeded agarose gels. J Biomech Eng 122(3):252–260. 10.1115/1.429656 [DOI] [PubMed] [Google Scholar]
- McCracken KW, Catá EM, Crawford CM, Sinagoga KL, Schumacher M, Rockich BE et al. (2014)Modelling human development and disease in pluripotent stem-cell-derived gastric organoids. Nature 516(7531):400–404. 10.1038/nature13863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinel L, Zoidis E, Zapf J, Hassa P, Hottiger MO, Auer JA et al. (2003) Localized insulin-like growth factor I delivery to enhance new bone formation. Bone 33(4):660–672. 10.1016/S8756-3282(03)00207-2 [DOI] [PubMed] [Google Scholar]
- Miura Y, Fitzsimmons JS, Commisso CN, Gallay SH, O’Driscoll SW (1994) Enhancement of periosteal chondrogenesis in vitro. Dose-response for transforming growth factor-beta 1 (TGF-beta 1). Clin Orthop Relat Res 301:271–280 [PubMed] [Google Scholar]
- Mollon B, Kandel R, Chahal J, Theodoropoulos J (2013) The clinical status of cartilage tissue regeneration in humans. Osteoarthr Cartil 21(12):1824–1833. 10.1016/j.joca.2013.08.024 [DOI] [PubMed] [Google Scholar]
- Mondadori C, Visone R, Rasponi M, Redaelli A, Moretti M, Lopa S (2018) Development of an organotypic microfluidic model to reproduce monocyte extravasation process in the osteoarthritic joint. Osteoarthr Cartil 26:S122. 10.1016/j.joca.2018.02.267 [DOI] [Google Scholar]
- Montarras D, Morgan J, Collins C, Relaix F, Zaffran S, Cumano A et al. (2005) Direct isolation of satellite cells for skeletal muscle regeneration. Science 309(5743):2064–2067. 10.1126/science.1114758 [DOI] [PubMed] [Google Scholar]
- Moore DC, Ehrlich MG, McAllister SC, Machan JT, Hart CE, Voigt C et al. (2009) Recombinant human platelet-derived growth factor-BB augmentation of new-bone formation in a rat model of distraction osteogenesis. J Bone Joint Surg Am 91(8):1973–1984. 10.2106/JBJS.H.00540 [DOI] [PubMed] [Google Scholar]
- Mori S, Sakakura E, Tsunekawa Y, Hagiwara M, Suzuki T, Eiraku M (2019) Self-organized formation of developing appendages from murine pluripotent stem cells. Nat Commun 10 (1):1–13. 10.1038/s41467-019-11702-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto Y, Kato-Negishi M, Onoe H, Takeuchi S (2013) Three-dimensional neuron–muscle constructs with neuromuscular junctions. Biomaterials 34(37):9413–9419. 10.1016/j.biomaterials.2013.08.062 [DOI] [PubMed] [Google Scholar]
- Murphy WL, Peters MC, Kohn DH, Mooney DJ (2000) Sustained release of vascular endothelial growth factor from mineralized poly(lactide-co-glycolide) scaffolds for tissue engineering. Biomaterials 21(24):2521–2527. 10.1016/S0142-9612(00)00120-4 [DOI] [PubMed] [Google Scholar]
- Muschler GF, Raut VP, Patterson TE, Wenke JC, Hollinger JO (2010) The design and use of animal models for translational research in bone tissue engineering and regenerative medicine. Tissue Eng Part B Rev 16(1):123–145. 10.1089/ten.teb.2009.0658 [DOI] [PubMed] [Google Scholar]
- Narcisi R, Cleary MA, Brama PA, Hoogduijn MJ, Tüysüz N, ten Berge D, van Osch GJ (2015) Long-term expansion, enhanced chondrogenic potential, and suppression of endochondral ossification of adult human MSCs via WNT signaling modulation. Stem Cell Rep 4 (3):459–472. 10.1016/j.stemcr.2015.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni P, Ding Q, Fan M, Liao J, Qian Z, Luo J et al. (2014) Injectable thermosensitive PEG–PCL–PEG hydrogel/acellular bone matrix composite for bone regeneration in cranial defects. Biomaterials 35(1):236–248. 10.1016/j.biomaterials.2013.10.016 [DOI] [PubMed] [Google Scholar]
- Ning L-J, Zhang Y-J, Zhang Y, Qing Q, Jiang Y-L, Yang J-L et al. (2015) The utilization of decellularized tendon slices to provide an inductive microenvironment for the proliferation and tenogenic differentiation of stem cells. Biomaterials 52:539–550. 10.1016/j.biomaterials.2015.02.061 [DOI] [PubMed] [Google Scholar]
- Nöth U, Rackwitz L, Steinert AF, Tuan RS (2010) Cell delivery therapeutics for musculoskeletal regeneration. Adv Drug Deliv Rev 62(7–8):765–783. 10.1016/j.addr.2010.04.004 [DOI] [PubMed] [Google Scholar]
- Nunes SS, Miklas JW, Liu J, Aschar-Sobbi R, Xiao Y, Zhang B et al. (2013) Biowire: a platform for maturation of human pluripotent stem cell–derived cardiomyocytes. Nat Methods 10 (8):781–787. 10.1038/nmeth.2524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien FJ (2011) Biomaterials & scaffolds for tissue engineering. Mater Today 14(3):88–95. 10.1016/S1369-7021(11)70058-X [DOI] [Google Scholar]
- Occhetta P, Mainardi A, Votta E, Vallmajo-Martin Q, Ehrbar M, Martin I et al. (2019) Hyperphysiological compression of articular cartilage induces an osteoarthritic phenotype in a cartilage-on-a-chip model. Nat Biomed Eng 3(7):545–557. 10.1038/s41551-019-0406-3 [DOI] [PubMed] [Google Scholar]
- Pauly HM, Sathy BN, Olvera D, McCarthy HO, Kelly DJ, Popat KC et al. (2017) Hierarchically structured electrospun scaffolds with chemically conjugated growth factor for ligament tissue engineering. Tissue Eng Part A 23(15–16):823–836. 10.1089/ten.tea.2016.0480 [DOI] [PubMed] [Google Scholar]
- Perniconi B, Costa A, Aulino P, Teodori L, Adamo S, Coletti D (2011) The pro-myogenic environment provided by whole organ scale acellular scaffolds from skeletal muscle. Biomaterials 32(31):7870–7882. 10.1016/j.biomaterials.2011.07.016 [DOI] [PubMed] [Google Scholar]
- Phan DT, Bender RHF, Andrejecsk JW, Sobrino A, Hachey SJ, George SC, Hughes CC (2017) Blood-brain barrier-on-a-chip: microphysiological systems that capture the complexity of the blood-central nervous system interface. Exp Biol Med 242(17):1669–1678. 10.1177/1535370217694100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pound P, Bracken MB (2014) Is animal research sufficiently evidence based to be a cornerstone of biomedical research? BMJ 348:g3387. 10.1136/bmj.g3387 [DOI] [PubMed] [Google Scholar]
- Pumberger M, Qazi TH, Ehrentraut MC, Textor M, Kueper J, Stoltenburg-Didinger G et al. (2016) Synthetic niche to modulate regenerative potential of MSCs and enhance skeletal muscle regeneration. Biomaterials 99:95–108. 10.1016/j.biomaterials.2016.05.009 [DOI] [PubMed] [Google Scholar]
- Quarta M, Brett JO, DiMarco R, De Morree A, Boutet SC, Chacon R et al. (2016) An artificial niche preserves the quiescence of muscle stem cells and enhances their therapeutic efficacy. Nat Biotechnol 34(7):752–759. 10.1038/nbt.3576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavan SS, Woon CYL, Kraus A, Megerle K, Pham H, Chang J (2012) Optimization of human tendon tissue engineering: synergistic effects of growth factors for use in tendon scaffold repopulation. Plast Reconstr Surg 129(2):479–489. 10.1097/PRS.0b013e31823aeb94 [DOI] [PubMed] [Google Scholar]
- Ran FA, Hsu PD, Wright J, Agarwala V, Scott DA, Zhang F (2013) Genome engineering using the CRISPR-Cas9 system. Nat Protoc 8:2281. https://www.nature.com/articles/nprot.2013.143#supplementary-information [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao L, Qian Y, Khodabukus A, Ribar T, Bursac N (2018) Engineering human pluripotent stem cells into a functional skeletal muscle tissue. Nat Commun 9(1):126. 10.1038/s41467-017-02636-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Re’em T, Kaminer-Israeli Y, Ruvinov E, Cohen S (2012) Chondrogenesis of hMSC in affinity-bound TGF-beta scaffolds. Biomaterials 33(3):751–761. 10.1016/j.biomaterials.2011.10.007 [DOI] [PubMed] [Google Scholar]
- Relaix F, Rocancourt D, Mansouri A, Buckingham M (2005) A Pax3/Pax7-dependent population of skeletal muscle progenitor cells. Nature 435(7044):948–953. 10.1038/nature03594 [DOI] [PubMed] [Google Scholar]
- Rezwan K, Chen QZ, Blaker JJ, Boccaccini AR (2006) Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials 27 (18):3413–3431. 10.1016/j.biomaterials.2006.01.039 [DOI] [PubMed] [Google Scholar]
- Ribeiro AJS, Yang X, Patel V, Madabushi R, Strauss DG (2019) Liver microphysiological systems for predicting and evaluating drug effects. Clin Pharmacol Ther 106(1):139–147. 10.1002/cpt.1458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosser J, Bachmann B, Jordan C, Ribitsch I, Haltmayer E, Gueltekin S et al. (2019) Microfluidic nutrient gradient–based three-dimensional chondrocyte culture-on-a-chip as an in vitro equine arthritis model. Mater Today 4:100023. 10.1016/j.mtbio.2019.100023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi CA, Flaibani M, Blaauw B, Pozzobon M, Figallo E, Reggiani C et al. (2011) In vivo tissue engineering of functional skeletal muscle by freshly isolated satellite cells embedded in a photopolymerizable hydrogel. FASEB J 25(7):2296–2304. 10.1096/fj.10-174755 [DOI] [PubMed] [Google Scholar]
- Rothrauff B, Tuan R (2020) Decellularized bone extracellular matrix in skeletal tissue engineering. Biochem Soc Trans. (in press). 10.1042/BST20190079 [DOI] [PubMed] [Google Scholar]
- Sacco A, Doyonnas R, Kraft P, Vitorovic S, Blau HM (2008) Self-renewal and expansion of single transplanted muscle stem cells. Nature 456(7221):502–506. 10.1038/nature07384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T, Fukai A, Mabuchi A, Ikeda T, Yano F, Ohba S et al. (2010) Transcriptional regulation of endochondral ossification by HIF-2α during skeletal growth and osteoarthritis development. Nat Med 16(6):678–686. 10.1038/nm.2146 [DOI] [PubMed] [Google Scholar]
- Sakar MS, Neal D, Boudou T, Borochin MA, Li Y, Weiss R et al. (2012) Formation and optogenetic control of engineered 3D skeletal muscle bioactuators. Lab Chip 12(23):4976–4985. 10.1039/C2LC40338B [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santhanam N, Kumanchik L, Guo X, Sommerhage F, Cai Y, Jackson M et al. (2018) Stem cell derived phenotypic human neuromuscular junction model for dose response evaluation of therapeutics. Biomaterials 166:64–78. 10.1016/j.biomaterials.2018.02.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassoli C, Zecchi-Orlandini S, Formigli L (2012) Trophic actions of bone marrow-derived mesenchymal stromal cells for muscle repair/regeneration. Cell 1(4):832–850. 10.3390/cells1040832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayed N, Liu C, Wu JC (2016) Translation of human-induced pluripotent stem cells: from clinical trial in a dish to precision medicine. J Am Coll Cardiol 67(18):2161–2176. 10.1016/j.jacc.2016.01.083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma B, Fermanian S, Gibson M, Unterman S, Herzka DA, Cascio B et al. (2013) Human cartilage repair with a photoreactive adhesive-hydrogel composite. Sci Transl Med 5 (167):167ra166. 10.1126/scitranslmed.3004838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Inoue H, Wu JC, Yamanaka S (2017) Induced pluripotent stem cell technology: a decade of progress. Nat Rev Drug Discov 16(2):115–130. 10.1038/nrd.2016.245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shik Mun K, Arora K, Huang Y, Yang F, Yarlagadda S, Ramananda Y et al. (2019) Patient-derived pancreas-on-a-chip to model cystic fibrosis-related disorders. Nat Commun 10(1):3124. 10.1038/s41467-019-11178-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu K, Araki H, Sakata K, Tonomura W, Hashida M, Konishi S (2015) Microfluidic devices for construction of contractile skeletal muscle microtissues. J Biosci Bioeng 119(2):212–216. 10.1016/j.jbiosc.2014.07.003 [DOI] [PubMed] [Google Scholar]
- Shvartsman D, Storrie-White H, Lee K, Kearney C, Brudno Y, Ho N et al. (2014) Sustained delivery of VEGF maintains innervation and promotes reperfusion in ischemic skeletal muscles via NGF/GDNF signaling. Mol Ther 22(7):1243–1253. 10.1038/mt.2014.76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sicari BM, Rubin JP, Dearth CL, Wolf MT, Ambrosio F, Boninger M et al. (2014) An acellular biologic scaffold promotes skeletal muscle formation in mice and humans with volumetric muscle loss. Sci Transl Med 6(234):234ra258. 10.1126/scitranslmed.3008085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skardal A, Shupe T, Atala A (2016) Organoid-on-a-chip and body-on-a-chip systems for drug screening and disease modeling. Drug Discov Today 21(9):1399–1411. 10.1016/j.drudis.2016.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stange Daniel E, Koo B-K, Huch M, Sibbel G, Basak O, Lyubimova A et al. (2013) Differentiated Troy+ chief cells act as reserve stem cells to generate all lineages of the stomach epithelium. Cell 155(2):357–368. 10.1016/j.cell.2013.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suga H, Kadoshima T, Minaguchi M, Ohgushi M, Soen M, Nakano T et al. (2011) Self-formation of functional adenohypophysis in three-dimensional culture. Nature 480(7375):57–62. 10.1038/nature10637 [DOI] [PubMed] [Google Scholar]
- Sun AX, Lin H, Fritch MR, Shen H, Alexander PG, DeHart M, Tuan RS (2017) Chondrogenesis of human bone marrow mesenchymal stem cells in 3-dimensional, photocrosslinked hydrogel constructs: effect of cell seeding density and material stiffness. Acta Biomater 58:302–311. 10.1016/j.actbio.2017.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, You Y, Jiang W, Zhai Z, Dai K (2019) 3D-bioprinting a genetically inspired cartilage scaffold with GDF5-conjugated BMSC-laden hydrogel and polymer for cartilage repair. Theranostics 9(23):6949. 10.7150/thno.38061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabar V, Studer L (2014) Pluripotent stem cells in regenerative medicine: challenges and recent progress. Nat Rev Genet 15:82. 10.1038/nrg3563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T (2019) Organoids for drug discovery and personalized medicine. Annu Rev Pharmacol Toxicol 59(1):447–462. 10.1146/annurev-pharmtox-010818-021108 [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126(4):663–676. 10.1016/j.cell.2006.07.024 [DOI] [PubMed] [Google Scholar]
- Takasato M, Er PX, Becroft M, Vanslambrouck JM, Stanley EG, Elefanty AG, Little MH (2014) Directing human embryonic stem cell differentiation towards a renal lineage generates a self-organizing kidney. Nat Cell Biol 16(1):118–126. 10.1038/ncb2894 [DOI] [PubMed] [Google Scholar]
- Takebe T, Wells JM (2019) Organoids by design. Science 364(6444):956–959. 10.1126/science.aaw7567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takebe T, Sekine K, Enomura M, Koike H, Kimura M, Ogaeri T et al. (2013) Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature 499(7459):481–484. 10.1038/nature12271 [DOI] [PubMed] [Google Scholar]
- Tang Y, Chen C, Liu F, Xie S, Qu J, Li M et al. (2020) Structure and ingredient-based biomimetic scaffolds combining with autologous bone marrow-derived mesenchymal stem cell sheets for bone-tendon healing. Biomaterials 241:119837. 10.1016/j.biomaterials.2020.119837 [DOI] [PubMed] [Google Scholar]
- Tokunaga T, Shukunami C, Okamoto N, Taniwaki T, Oka K, Sakamoto H et al. (2015) FGF-2 stimulates the growth of tenogenic progenitor cells to facilitate the generation of tenomodulin-positive tenocytes in a rat rotator cuff healing model. Am J Sports Med 43(10):2411–2422. 10.1177/0363546515597488 [DOI] [PubMed] [Google Scholar]
- Truskey GA (2018) Development and application of human skeletal muscle microphysiological systems. Lab Chip 18(20):3061–3073. 10.1039/C8LC00553B [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usas A, Huard J (2007) Muscle-derived stem cells for tissue engineering and regenerative therapy. Biomaterials 28(36):5401–5406. 10.1016/j.biomaterials.2007.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Manner PA, Horner A, Shum L, Tuan RS, Nuckolls GH (2004) Regulation of MMP-13 expression by RUNX2 and FGF2 in osteoarthritic cartilage. Osteoarthr Cartil 12(12):963–973. 10.1016/j.joca.2004.08.008 [DOI] [PubMed] [Google Scholar]
- Wang Y, Kim U-J, Blasioli DJ, Kim H-J, Kaplan DL (2005) In vitro cartilage tissue engineering with 3D porous aqueous-derived silk scaffolds and mesenchymal stem cells. Biomaterials 26 (34):7082–7094. 10.1016/j.biomaterials.2005.05.022 [DOI] [PubMed] [Google Scholar]
- Wang L, Cao L, Shansky J, Wang Z, Mooney D, Vandenburgh H (2014) Minimally invasive approach to the repair of injured skeletal muscle with a shape-memory scaffold. Mol Ther 22 (8):1441–1449. 10.1038/mt.2014.78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Zhang F, Tsang WP, Wan C, Wu C (2017) Fabrication of injectable high strength hydrogel based on 4-arm star PEG for cartilage tissue engineering. Biomaterials 120:11–21. 10.1016/j.biomaterials.2016.12.015 [DOI] [PubMed] [Google Scholar]
- Wang Z, Lee WJ, Koh BTH, Hong M, Wang W, Lim PN et al. (2018) Functional regeneration of tendons using scaffolds with physical anisotropy engineered via microarchitectural manipulation. Sci Adv 4(10):eaat4537. 10.1126/sciadv.aat4537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei S, Huard J (2008) Tissue therapy: implications of regenerative medicine for skeletal muscle. In: Atala A, Lanza R, Thomson JA, Nerem RM (eds) Principles of regenerative medicine. Academic Press, San Diego, pp 1232–1247. 10.1016/B978-012369410-2.50074-7 [DOI] [Google Scholar]
- Wolfman NM, Hattersley G, Cox K, Celeste AJ, Nelson R, Yamaji N et al. (1997) Ectopic induction of tendon and ligament in rats by growth and differentiation factors 5, 6, and 7, members of the TGF-beta gene family. J Clin Invest 100(2):321–330. 10.1172/JCI119537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn TA, Vannella KM (2016) Macrophages in tissue repair, regeneration, and fibrosis. Immunity 44(3):450–462. 10.1016/j.immuni.2016.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xavier JR, Thakur T, Desai P, Jaiswal MK, Sears N, Cosgriff-Hernandez E et al. (2015) Bioactive nanoengineered hydrogels for bone tissue engineering: a growth-factor-free approach. ACS Nano 9(3):3109–3118. 10.1021/nn507488s [DOI] [PubMed] [Google Scholar]
- Yang KGA, Saris DBF, Geuze RE, van Rijen MHP, van der Helm YJM, Verbout AJ et al. (2006) Altered in vitro chondrogenic properties of chondrocytes harvested from unaffected cartilage in osteoarthritic joints. Osteoarthr Cartil 14(6):561–570. 10.1016/j.joca.2005.12.002 [DOI] [PubMed] [Google Scholar]
- Yang G, Rothrauff BB, Lin H, Yu S, Tuan RS (2017) Tendon-derived extracellular matrix enhances transforming growth factor-β3-induced tenogenic differentiation of human adipose-derived stem cells. Tissue Eng Part A 23(3–4):166–176. 10.1089/ten.tea.2015.0498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Lin H, Shen H, Wang B, Lei G, Tuan RS (2018) Mesenchymal stem cell-derived extracellular matrix enhances chondrogenic phenotype of and cartilage formation by encapsulated chondrocytes in vitro and in vivo. Acta Biomater 69:71–82. 10.1016/j.actbio.2017.12.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Liu Y, Lin Z, Shen H, Lucas C, Kuang B et al. (2019) Condensation-driven chondrogenesis of human mesenchymal stem cells within their own extracellular matrix: formation of cartilage with low hypertrophy and physiologically relevant mechanical properties. Adv Biosyst 3 (12):1900229. 10.1002/adbi.201900229 [DOI] [PubMed] [Google Scholar]
- Yang H, Jia B, Zhang Z, Qu X, Li G, Lin W et al. (2020) Alloying design of biodegradable zinc as promising bone implants for load-bearing applications. Nat Commun 11(1):401. 10.1038/s41467-019-14153-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo M, Kim G (2020) Micro/nano-hierarchical scaffold fabricated using a cell electrospinning/3D printing process for co-culturing myoblasts and HUVECs to induce myoblast alignment and differentiation. Acta Biomater 107:102–114. 10.1016/j.actbio.2020.02.042 [DOI] [PubMed] [Google Scholar]
- Yilgor P, Tuzlakoglu K, Reis RL, Hasirci N, Hasirci V (2009) Incorporation of a sequential BMP-2/BMP-7 delivery system into chitosan-based scaffolds for bone tissue engineering. Biomaterials 30(21):3551–3559. 10.1016/j.biomaterials.2009.03.024 [DOI] [PubMed] [Google Scholar]
- Youngstrom DW, Barrett JG, Jose RR, Kaplan DL (2013) Functional characterization of detergent-decellularized equine tendon extracellular matrix for tissue engineering applications. PLoS One 8(5):e64151–e64151. 10.1371/journal.pone.0064151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yui S, Nakamura T, Sato T, Nemoto Y, Mizutani T, Zheng X et al. (2012) Functional engraftment of colon epithelium expanded in vitro from a single adult Lgr5+ stem cell. Nat Med 18 (4):618–623. 10.1038/nm.2695 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Xu J, Ruan YC, Yu MK, O’Laughlin M, Wise H et al. (2016) Implant-derived magnesium induces local neuronal production of CGRP to improve bone-fracture healing in rats. Nat Med 22(10):1160–1169. 10.1038/nm.4162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen G, Cao X (2014) Targeting TGFβ signaling in subchondral bone and articular cartilage homeostasis. Trends Pharmacol Sci 35(5):227–236. 10.1016/j.tips.2014.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen G, Wen C, Jia X, Li Y, Crane JL, Mears SC et al. (2013) Inhibition of TGF-β signaling in mesenchymal stem cells of subchondral bone attenuates osteoarthritis. Nat Med 19(6):704–712. 10.1038/nm.3143 [DOI] [PMC free article] [PubMed] [Google Scholar]


