Abstract
Background:
While regular exercise exposure is associated with lower risk of cardiovascular disease (CVD) and mortality, mechanisms of exercise-mediated health benefits remain less clear. We used metabolite profiling before and after acute exercise to delineate the metabolic architecture of exercise response patterns in humans.
Methods:
Cardiopulmonary exercise testing (CPET) and metabolite profiling was performed on Framingham Heart Study (FHS) participants (age 53±8 years, 63% women) with blood drawn at rest (n=471) and at peak exercise (n=411).
Results:
We observed changes in circulating levels for 502 of 588 measured metabolites from rest to peak exercise (exercise duration 11.9±2.1 minutes) at a 5% false discovery rate (FDR). Changes included reductions in metabolites implicated in insulin resistance (glutamate −29%, P=1.5x10−55, dimethylguanidinovaleric acid −18%, P=5.8x10−18), and increases in metabolites associated with lipolysis (1-methylnicotinamide, +33%, P=6.1x10−67), nitric oxide bioavailability (arginine/ornithine + citrulline, +29%, P=2.8x10−169), and adipose browning (12,13-dihydroxy-9Z-octadecenoic acid +26%, P=7.4x10−38), among other pathways relevant to cardiometabolic risk. We assayed 177 metabolites in a separate FHS replication sample (n=783, age 54±8 years, 51% women) and observed concordant changes in 164 metabolites (92.6%) at 5% FDR. Exercise-induced metabolite changes were variably related to the amount of exercise performed (peak workload), sex, and body mass index (BMI). There was attenuation of favorable excursions in some metabolites in individuals with higher BMI and greater excursions in select cardioprotective metabolites in women despite less exercise performed. Distinct pre-exercise metabolite levels were associated with different physiologic dimensions of fitness (e.g., ventilatory efficiency, exercise blood pressure, peak VO2). We identified four metabolite signatures of exercise response patterns that were then analyzed in a separate cohort (Framingham Offspring Study; n=2045, age 55±10 years, 51% women), two of which were associated with overall mortality over median follow-up of 23.1 years (P≤0.003 for both).
Conclusions:
In a large sample of community-dwelling individuals, acute exercise elicits widespread changes in the circulating metabolome. Metabolic changes identify pathways central to cardiometabolic health, CVD, and long-term outcome. These findings provide a detailed map of the metabolic response to acute exercise in humans and identify potential mechanisms responsible for the beneficial cardiometabolic effects of exercise for future study.
Keywords: exercise, epidemiology, metabolomics, prevention, cardiopulmonary exercise testing
INTRODUCTION
While pleiotropic beneficial effects of exercise on metabolic, inflammatory, vascular, cardiac, and other systems are well described,1-3 the molecular mechanisms underlying these favorable adaptations are incompletely elucidated. Recent investigations in small samples have suggested the role of circulating molecules elaborated during acute exercise exposure (including exosomes, metabolites, proteins, and non-coding RNAs) in potentially mediating exercise-induced cardiometabolic benefits.4-7 While these studies demonstrate that excursions in functional biomarkers during acute exercise may be a potent probe of human physiology, most studies remain limited by small sample size, incomplete phenotypic characterization of multi-dimensional physiologic response patterns during exercise, and lack of linkage to long-term outcome. A necessary first step toward understanding how exercise improves human health is to link heterogeneous physiological responses to exercise with underlying molecular physiology.
Here, we studied Framingham Heart Study (FHS) participants with simultaneous cardiopulmonary exercise testing (CPET) and assessment of the circulating metabolome at rest and at peak exercise. We hypothesized that a short period of maximum incremental exercise would produce a significant shift in the circulating metabolome, with metabolic alterations reflecting important pathways of exercise-mediated cardiometabolic benefit. Moreover, harnessing multi-dimensional phenotyping of exercise responses during CPET, we evaluated how resting metabolic profiles associated with exercise response patterns that predict future cardiovascular disease (CVD). We also evaluated metabolite signatures of “integrated” responses to exercise (cardiac, systemic and pulmonary vascular, and peripheral) in relation to long-term cardiovascular prognosis. Ultimately, we sought to provide not only a snapshot of metabolic responses to exercise and their relations to multi-dimensional exercise responses in humans, but to also understand how these patterns may explain the mechanisms underlying the widespread cardiovascular benefits of exercise.
METHODS
Data Sharing
The data supporting the study findings will be made available upon reasonable request. FHS data are made publicly available and can be accessed through National Institutes of Health database of genotypes and phenotypes (https://www.ncbi.nlm.nih.gov/gap/).
Study Samples
The FHS is a prospective cohort study of community-dwelling adults in Framingham, Massachusetts that began with the Original Cohort in 1948 (n=5209) and now includes their children and children’s spouses (Second Generation [Gen2], starting in 1971, n=5124),8 and their grandchildren and grandchildren’s spouses (Third Generation [Gen3], enrolled in 2002-2005, n=4095).9 Eligible individuals with at least one parent enrolled in the Gen2 cohort were enrolled in Gen3 from 2002-2005. At the 3rd study visit (2016-2019), CPET was performed as part of the routine exam cycle.10 For the current study, we included the first 482 individuals to undergo CPET with metabolite profiling available. After excluding nine individuals for fasting <8 hours prior to blood sampling and two individuals with multiple extreme outlier metabolite values (>10 SD from mean change), a total of 471 individuals had metabolite profiling performed at rest. Blood sampling at peak exercise was not available for 60 individuals due to participant preference or because the blood draw could not be obtained after two attempts, and therefore 411 individuals had metabolite profiling both at rest and at peak exercise. The next consecutive 783 Gen3 participants with metabolite profiling performed on fasting blood samples were used for replication of exercise-induced changes in metabolite levels. Details of the study flow are shown in Figure I in the Supplement. Assessment of clinical covariates and outcomes are described in the Expanded Methods in the Supplement.
To evaluate the associations of metabolite signatures of exercise and long-term outcomes, 2066 participants from the Gen2 cohort8 with metabolite profiling performed previously on stored blood from the 5th examination cycle (1991-1995)11 were eligible for inclusion. We excluded 21 individuals with inadequate fasting, yielding a sample of 2045 for associating metabolites with death and 1996 individuals without prevalent CVD for associating metabolites with incident CVD. The Boston University Medical Center and Massachusetts General Hospital Institutional Review Boards approved study protocols, and all participants provided written informed consent.
Cardiopulmonary exercise testing (CPET)
Maximal effort CPET was performed on the same cycle ergometer (Lode, the Netherlands), and breath-by-breath gas exchange values were measured by the same metabolic cart (MedGraphics, St. Paul, MN) in all participants. Heart rate was monitored continuously, and blood pressure was measured every two minutes manually via sphygmomanometry. The exercise protocol began with three minutes of unloaded (zero watt) exercise, followed by loaded exercise with an incremental ramp protocol. Two ramp protocols were used (15 and 25 watt/min) and participants were assigned to one of the two protocols based on assessment of estimated peak watts in order to achieve similar exercise time across participants. Recovery measures were taken during three minutes of unloaded cycling and one minute of rest. For this study, our primary measure of exercise capacity was peak oxygen uptake (peak VO2, scaled to body weight, in ml/kg/min), determined as the highest 30-second average during the final 90 seconds of exercise. The maximum predicted VO2 was calculated using the Wasserman equation.12 All measures were adjudicated by two trained exercise physiologists (MT, JB) and reviewed by the study principal investigator (GL) blinded to individual participant metabolite levels.
Metabolite profiling
Blood was sampled at rest (before exercise) and at peak exercise in potassium ethylenediaminetetraacetic (EDTA) tubes. The median time between peak exercise and blood collection was 56 seconds (first and third quartiles 44-72 seconds). EDTA blood collection tubes were centrifuged for 22 minutes at 2500g, 4°C and then stored at −80°C without freeze-thaw cycles until assayed. Metabolite profiling was subsequently performed at the Broad Institute of Harvard and Massachusetts Institute of Technology (Cambridge, MA) using four complementary liquid chromatography tandem mass spectrometry methods (LC-MS) providing broad coverage of metabolite classes: (1) amino acids, amino acid metabolites, acylcarnitines, dipeptides, and other cationic polar metabolites (hydrophobic interaction chromatography in the positive ionization mode; HILIC-positive); (2) sugars, organic acids, purines, pyrimidines, and other anionic polar metabolites (HILIC-negative); (3) lipids (C8-positive); (4) free fatty acids, lipid-derived mediators (eicosanoids), bile acids, and metabolites of intermediate polarity (C18-negative). Detailed metabolite profiling methods are available in the Expanded Methods in the Supplement.
The median inter-assay coefficients of variation (CVs) for the metabolites included in the derivation effort using pooled-plasma samples was 4.94 (25th percentile 2.35, 75th percentile 8.55) and 92% of metabolites had CVs <20% (Table I in the Supplement).
For the replication sample, the single platform with the highest number of metabolites analyzed (HILIC-positive) was run. Metabolite profiling in Gen2 was performed (2009-2011) in the same laboratory (Broad Institute, Cambridge, MA) using previous versions of the HILIC-positive and HILIC-negative platforms, as described.11
Statistical analysis
Data transformations and imputation
For “missing” (below-detection concentrations) metabolite values, we imputed data at 50% of the lowest detected value of that metabolite across participants (separately for resting and peak exercise values).13 Metabolite data were log2-transformed and fold-changes were calculated (log2[peak]/log2[rest]); rest, peak, and fold-change values were then separately rank-normalized (Blom14) to limit skewness and outlier effects.
Metabolic responses to exercise
For each metabolite, we compared the observed mean log2(exercise/rest) versus no change (i.e., log2[exercise/rest]=0) with two-tailed t-tests. To account for multiple testing across metabolites, we used the Benjamini-Hochberg false discovery rate (FDR) method, separately for each metabolite platform and with a 5% FDR significance threshold.
Correlates of metabolic response to exercise
To understand relations of metabolic responses with key demographic and clinical measures, we specified linear models for rank-normalized fold-change (outcome) versus age, sex, body mass index (BMI), workload, and normalized resting metabolite level (predictors). As input to regression analyses, we standardized the distributions of age, log2-BMI and peak watts. Due to large sex differentials in achieved workload, we subtracted the sex-specific mean peak watts from the peak watts value for each participant, pooled men and women, and then standardized (mean 0, variance 1) for analysis. To account for multiple testing, we applied an FDR correction across p-values for estimated regression coefficients separately for each predictor (age, sex, BMI, etc.). Metabolites in each quantified platform (HILIC, C8, C18) were modeled separately, with separate FDR adjustments applied per platform.
Exercise performance relative to resting metabolites
To model associations between exercise performance data (outcomes) with individual resting metabolites (predictors), we used multivariable linear regression models adjusting for sex and age in 471 individuals with resting metabolite data. In sensitivity analyses, we additionally adjusted for resting systolic blood pressure, diabetes, hypertension treatment status, smoking, and prevalent CVD. The list of exercise performance variables was as follows: log2-peak VO2, peak heart rate, aerobic efficiency, peak O2 pulse, log2-recovery half-time, square root VO2 recovery delay, mean arterial pressure (MAP) at 75 watts, and ventilatory efficiency (VE/VCO2) nadir (Table 1). Models relating metabolite levels with peak heart rate and MAP at 75 watts were also adjusted for resting heart rate and MAP, respectively. To account for multiple testing across metabolites, we used a 5% FDR significance threshold.
Table 1.
Cardiopulmonary exercise testing variables
| CPET Variable | Description and Physiologic relevance |
Relationship to Future Outcomes |
Measurement Methodology | Threshold for Abnormal |
Required Exercise Duration |
|---|---|---|---|---|---|
| Exercise Blood Pressure | Integrated measure of arterial compliance,15 autonomic function16 | Predicts future systemic hypertension17, CVD18 | Measurement of DBP + 1/3 (SBP-DBP) at a standardized workload of 75 Watts (% change from resting values) | F>118mmHg18 M>128mmHg18 |
≤ 8 min |
| Peak VO2 | Aerobic capacity, gold standard indicator of cardiorespiratory fitness. Integrates cardiopulmonary, vascular and peripheral skeletal muscle performance | Predicts future metabolic and CVD outcomes including diabetes, heart failure, and death | Highest 30 sec mean VO2 occurring in final 90 seconds of incremental exercise | <80% predicted | Peak |
| Ventilatory efficiency | Minute ventilation required to exchange 1 L/min of CO2. Reflects cardiac output and pulmonary ventilation-perfusion matching during exercise, correlates with pulmonary arterial pressure but not peripheral skeletal muscle performance | Predicts hospitalization and death from heart failure and pulmonary hypertension | Lowest ratio of minute ventilation (VE) to CO2 elimination (VCO2) during exercise. Occurs during early to mid-exercise and is independent of volitional effort. | >34 | <8 min |
| O2 pulse | The product of peripheral oxygen extraction and stroke volume | Predicts CVD outcomes | Slope of VO2 versus heart rate relationship | <80% predicted | Peak |
| Peak heart rate | Reflects ability to augment heart rate in response to exercise, indicative of autonomic function, conduction system integrity | Predicts conduction system disease, CVD outcomes | Highest achieved continuous measurement of heart rate by ECG | <220-Age | Peak |
| Aerobic efficiency | Reflects metabolic cost (in terms of O2 uptake) of performing external work aerobically | Predicts outcomes in established HF | ΔO2 consumption/Δwork (W) during incremental exercise | <8.5 ml/W/min | < 6 min |
| VO2 half-time | Measures of VO2 recovery kinetics that reflect the metabolic consequences of an acute bout of exercise with delays indicative of VO2 deficit accrued during exercise | Predicts outcomes in established CVD | Time for VO2 to decrease to 50% of peak VO2 adjusted for resting VO2 | <65 sec | Peak |
| VO2 recovery delay | Time from the end of loaded exercise until VO2 permanently falls below peak VO2 | <15 sec | Peak |
Canonical correlation analysis
We next sought to understand the joint relationships between the assayed metabolites and integrated cardiopulmonary exercise responses by CPET using canonical correlation analysis (CCA19,20). CCA estimates the joint associations between two sets of multiple variables (in our case, 8 CPET measures and 107 individual circulating metabolites) by creating two separate sets of composite “canonical variates” (a set of CPET variates and a set of metabolite variates) that are “loaded on” (correlated with) each individual variable. The CPET canonical variates are multivariable “scores” that are loaded on different CPET measures; analogously, the metabolite canonical variates are multivariable metabolite-based scores loaded on different individual metabolites. The loadings of each CPET or metabolite measure in each canonical variate are calculated to maximize joint association between each CPET variate and a corresponding metabolite variate, while being uncorrelated with all the other canonical variates. We used CCA to construct maximum correlations between high-dimensional CPET data (8 measures) and circulating metabolite levels (107 metabolites in the HILIC dataset measured in both FHS Gen2 and Gen3). We selected metabolites measured in both Gen2 and Gen3 to allow us to identify metabolite patterns related to multi-dimensional exercise responses in Gen3 and subsequently applied the metabolite weights defined by the CCA in Gen3 to Gen2 data to assess the relationship between CPET-related resting metabolite signatures and long-term outcomes. The CCA was based on partial correlations adjusted for age and sex and the maximum number of variates included in our analysis was selected by an F-test (P<0.05; PROC CANCORR in SAS).
Survival analysis
Cox proportional hazards regression analysis was used to relate the top four CCA-based metabolite scores (standardized to mean 0 and variance 1) to incident CVD and all-cause mortality in Gen2. Models were stratified by sex due to non-proportional hazards between men and women. We fitted a separate model for each CCA-based metabolite score. For both CVD and mortality, we adjusted for age and BMI, and then additionally for systolic blood pressure, hypertension treatment status, diabetes, current smoking, and total/HDL cholesterol.
All analyses were performed in SAS 9.4 (SAS Institute, Cary NC), and/or R (The R Foundation for Statistical Computing, version 4.0.0, http://www.rproject.org).
Pathway analysis
Pathway analysis was performed to identify biological pathways represented by metabolites with statistically significant (5% FDR) changes with exercise. MetaboAnalystR21 was used to map metabolites to genes. Gene enrichment analyses were then performed with clusterProfiler,22 rWikiPathways, and RCy323 against the WikiPathways database of community-curated pathway models.24
RESULTS
Characteristics of the study samples
Gen3 study participants in the derivation sample had a mean age of 53±8 years, 63% were women, 19% had hypertension, 19% were on treatment with lipid-lowering medications, and 8% had diabetes mellitus (Table 2). Gen3 participants in the replication sample displayed similar characteristics with a slightly lower proportion of women. Participants from Gen2 were of similar age (mean age 55±10 years) with 51% women and comparable risk factor burden to Gen3 participants (Table 2).
Table 2.
Characteristics of the study samples
| Characteristic | Generation 3 Derivation (N=471) |
Generation 3 Replication (N=783) |
Generation 2 (N=2045) |
|---|---|---|---|
| Age, years | 53±8 | 54±8 | 55±10 |
| Women, % | 296 (63) | 396 (51) | 1047 (51) |
| Body mass index, kg/m2 | 27.4±5.5 | 27.7±5.1 | 27.6±5.0 |
| Hypertension, % | 88 (19) | 153 (20) | 412 (20) |
| Current smoking, % | 30 (6) | 47 (6) | 384 (19) |
| Diabetes, % | 36 (8) | 42 (5) | 135 (7) |
| Total cholesterol, mg/dL | 188±32 | 192±34 | 207±37 |
| HDL cholesterol, mg/dL | 62±19 | 62±21 | 49±15 |
| Exercise duration, min | 11.9±2.1 | 11.9±2.0 | -- |
| Peak respiratory exchange ratio | 1.2±0.1 | 1.2±0.1 | -- |
| Peak VO2, ml/kg/min | 23.1±7.1 | 23.8±7.3 | -- |
Entries are mean ± SD for continuous variables, n(%) for categorical variables.
The mean exercise duration was 11.9±2.1 minutes with a peak respiratory exchange ratio (RER) of 1.20±0.09, consistent with peak volitional effort during exercise. Average exercise capacity was relatively preserved compared to predicted values (peak oxygen uptake [VO2] 23.1±7.1 ml/kg/min, 92±22% predicted; Table 2). Table 1 specifies the exercise variables measured, their physiologic and prognostic relevance, and previously established abnormal thresholds with sex-specific values for these measures indicated in Table 3.
Table 3.
Exercise characteristics of the Gen3 (derivation) study sample
| Exercise variable | Women (n=296) | Men (n=175) |
|---|---|---|
| Exercise duration, min | 11.5±2.1 | 12.6±2.0 |
| Peak respiratory exchange ratio | 1.2±0.1 | 1.2±0.1 |
| Peak VO2, ml/kg/min | 21.3±6.4 | 26.2±7.2 |
| Peak VO2 % predicted, % | 93.4±23.3 | 89.9±19.5 |
| Peak VO2 absolute, ml/min | 1454.2±390.9 | 2340.1±567.3 |
| Aerobic efficiency, ml/watt* | 9.1±0.9 | 9.6±0.7 |
| VE/VCO2 nadir | 27.7±3 | 26.1±2.7 |
| O2 pulse peak, ml/beat | 9.7±2.2 | 15.4±3.2 |
| VO2 T 1/2 recovery, sec* | 84.4±37.8 | 69.1±24.4 |
| VO2 recovery delay, sec* | 12.5±10.6 | 10.6±17.8 |
| Workload achieved, watts | 132.6±35.9 | 214.2±55.3 |
| Rest heart rate, bpm | 73.9±11.8 | 69.5±11.8 |
| Peak heart rate, bpm | 152.3±18.4 | 154.3±20.9 |
| Rest systolic blood pressure, mm Hg | 123.9±16.4 | 131.6±15.5 |
| Systolic blood pressure at 75W, % change* | 28.5±12.7 | 20.4±10.3 |
| Rest diastolic blood pressure, mm Hg | 79.8±8.1 | 84.7±8.4 |
| Diastolic blood pressure at 75W, % change* | 2.3±8.4 | 0.7±6.4 |
| Mean arterial pressure at 75W, % change* | 13.6±8 | 9.2±6 |
Entries are mean ± SD for continuous variables
N= 470 for aerobic efficiency, 461 for VO2 T 1/2 recovery, 465 for VO2 recovery delay, and 459 for blood pressure at 75W
Widespread changes in the metabolome with acute exercise exposure
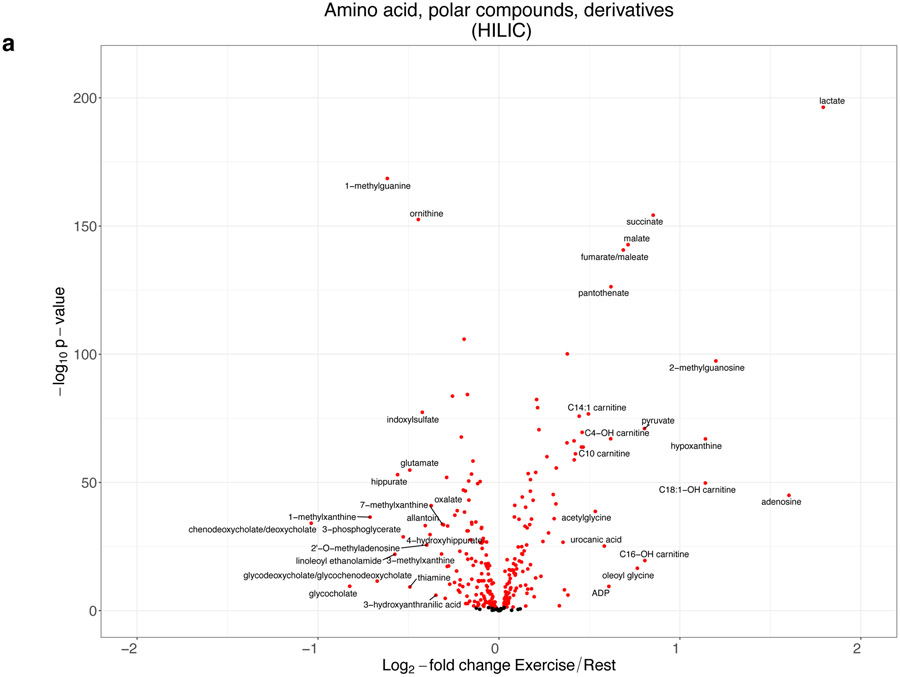
Figure 1 demonstrates the magnitude of change in circulating metabolites with exercise. We observed widespread excursions in the assayed metabolome with 502 of 588 metabolites (85%) demonstrating statistically significant changes with exercise (Table II in the Supplement). In a sensitivity analysis restricted to the 365 individuals with peak RER ≥1.1, we observed consistency of our findings (Figure II in the Supplement).
Figure 1. The response of the circulating metabolome to acute exercise.
Panels (A-C) display a series of volcano plots of the change in individual metabolites with exercise for different metabolite platforms. Red indicates metabolite levels that are significant at a 5% FDR level. Metabolites are labeled arbitrarily. Of note, inosine is not displayed in the HILIC plot for visualization purposes, as it had an extreme increase with exercise (log2-fold change = 3.2, raw P=5.70x10−111). The full fold-change and significance levels are shown in Table II in the Supplement. Panel (D) displays selected ratios indicative of distinct metabolic phenotypes, including allantoin/urate (related to oxidative stress), kynurenine/tryptophan (related to higher indoleamine 2,3-dioxygenase activity and systemic inflammation), phenylalanine/tyrosine (related to higher inflammation), arginine/[ornithine + citrulline] (related to greater nitric oxide synthesis, improved endothelial function, and lower metabolic syndrome risk), glutamine/glutamate (related to lower cardiometabolic risk). Bars represent the 95% confidence interval for the log2-fold change. P-values are shown in the diagram. Point estimates are colored red for increasing with exercise and blue for decreasing with exercise. Panel (E) shows a plot of the metabolite log2 fold-change with exercise in the replication sample vs. the derivation sample (Table III in the Supplement). The sizes of the points are proportional to the sum of the log2 fold-change in the derivation and replication samples. The dashed line represents equal fold-change in both replication and derivation (y=x). Colors refer to: 1) green, significant (at 5% FDR) with same directionality in both samples; 2) red, significant (at 5% FDR) with opposite directionality in both samples; 3) blue, significant (at 5% FDR) in the derivation sample only; 4) magenta, significant (at 5% FDR) in the replication sample only. 2-methylguanosine was not shown due to a greater proportion of levels below the detection limit in the replication vs. derivation sample limiting interpretation.
Metabolite changes with exercise represented a variety of biological pathways with an approximately equal number of metabolites increasing and decreasing (Table 4). There was an increase in metabolic intermediates reflecting enhanced anaerobic and aerobic respiration, consistent with peak effort exercise: (1) a 246% increase in circulating lactate (P=4.6x10−197, anaerobic respiration); (2) an increase in Krebs cycle span 2 intermediates (succinate, 81% increase, P=5.6x10−155; fumarate, 61% increase, P=2.2x10−141, aerobic respiration); (3) changes in metabolite levels reflecting glycolytic flux (pyruvate, 75% increase, P=9.4x10−72; 3-phosphoglycerate, 31% reduction, P=1.7x10−29); (4) a generalized increase in medium-chain acylcarnitines (mitochondrial fatty acid oxidation); and (5) a 54% increase in pantothenate (P=4.7x10−127), reflecting increased coenzyme A bioavailability and Krebs cycle activity (Table II in the Supplement). A discrete bout of exercise also led to directionally favorable changes in circulating levels of metabolites for which resting levels have been previously implicated in cardiometabolic disease (Table 4). We observed favorable changes with exercise for metabolites associated with insulin resistance and excess adiposity, such as: a 29% decrease in glutamate (P=1.5x10−55) for which higher levels are associated with visceral adiposity, diabetes and hypertension;30,31 2-9% reductions in branched chain amino acids (BCAAs), which are associated with diabetes and CVD;11,60 18% decrease in dimethylguanidinovaleric acid (DMGV; P=5.8x10−18) implicated in hepatic steatosis and diabetes;27 reductions in TAGs with low carbon and double bond numbers implicated in risk for diabetes;36,71 and, decreases in bile acids implicated in adipose tissue metabolism and appetite stimulation.55 Increases in metabolites with inverse associations with cardiometabolic risk were also observed with: a 29% increase in arginine/ornithine + citrulline representing higher levels of nitric oxide bioavailability49 (P=2.8x10−169); 33% increase in 1-methylnicotinamide (P=6.1x10−67), which is associated with lipolysis;26 and a 26% increase in 12,13-dihydroxy-9Z-octadecenoic acid (12,13-diHOME; P=7.4x10−38), a brown-adipose tissue-derived lipokine implicated in skeletal muscle fatty acid metabolism.56
Table 4.
Metabolic response to exercise
| Metabolite class | Metabolites | Δ with exercise |
Adverse Pattern (human/model) |
Clinical correlate and proposed mechanism |
|---|---|---|---|---|
| Insulin sensitivity/vascular risk | 1-methylnicotinamide | ↑ (33%) | ↓ | Anti-thrombotic and anti-inflammatory (via prostacyclin),25 promotes muscle lipolysis26 |
| DMGV | ↓ (−18%) | ↑ | Associated with diabetes, hepatic steatosis and reduced with long-term exercise27-29 | |
| Glutamate Glutamine |
↓ (−29%) ↑ (6%) |
↑ ↓ |
Associated with diabetes and CVD risk,30,31 visceral fat; glutamine metabolism linked to adipose tissue inflammation32 | |
| Branched chain & aromatic amino acids (Ile, Val, Tyr, Phe) | ↓ (−9% to −2%) |
↑ | Greater diabetes and cardiovascular risk;11,33 pro-inflammatory effects (aromatic);34 modulation of branched chain amino acid catabolic pathway35 | |
| Betaine | ↑ (3%) | ↓ | Lower levels associated with diabetes; favorably influenced by lifestyle intervention36 | |
| 2-aminoadipate | ↓ (−3%) | ↑/↓ | Higher 2-aminoadipic acid levels associated with higher incident diabetes; administration of 2-aminoadipic acid may increase beta-cell insulin secretion37 | |
| Dimethylglycine | ↓ (−3%) | ↑ | Higher levels associated with greater risk of diabetes38 | |
| Taurine | ↑ (4%) | ↓ | Pleotropic effects on oxidative stress, angiotensin/NO pathway, and blood pressure39 | |
| Oleoyl glycine | ↑ (70%) | ↓ | Increases insulin sensitivity in adipocytes in vitro40 | |
| Nucleotides and derivatives | Adenosine | ↑ (204%) | ↓ | Lower levels of adenosine in individuals with obstructive coronary disease,41 potentially via modulation of macrophage activation42 |
| Hypoxanthine Inosine |
↑ (121%, 817%) | ↑/↓ | Bioproducts of ischemia-reperfusion in cardiac muscle,43 and may have anti-inflammatory effects44 | |
| Allantoin Urate |
↓ (−25%, −1%) | ↑ | Allantoin is a product of oxidation of uric acid45 (reactive oxygen stress); urate associated with CVD46 | |
| Nitric oxide (NO) pathway | Arginine | ↑ (4%) | ↓ | Arginine (NO precursor) deficiency related to endothelial dysfunction;47 supplementation improves vascular function48 |
| Ornithine Citrulline |
↓ (−27%, −8%) | ↑/↓ | Arginine-citrulline cycle involved in NO generation; ornithine is product of arginase and a precursor to glutamate; higher ornithine/citrulline ratios associated with metabolic syndrome risk49 | |
| Oxidative stress | Cystine | ↓ (−11%) | ↑ | Higher levels associated with greater mortality in patients with coronary artery disease50 |
| 13-HODE | ↑ (12%) | ↑ | See below; marker of increased oxidative stress51 | |
| Cys-gly oxidized | ↑ (12%) | ↑ | Marker of oxidative stress; higher in individuals who develop myocardial infarction52 | |
| Methionine sulfoxide | ↓ (−11%) | ↑ | Oxidative product of methionine; protein methionine oxidation may reflect ischemic oxidative stress in stroke (via NF-kB)53 | |
| Bile acids | Chenodeoxycholate Deoxycholate Glycodeoxycholate Glycoursodeoxycholate Taurodeoxycholate Taurocholate Taurochenodeoxycholate |
↓ (−52% to −16%) |
↑/↓ | Higher plasma levels in obese individuals and type 2 diabetics, inversely related to cognitive restraint of eating. Previously reported to decrease with strenuous exercise;54 complex regulation may depend on type of exercise55 |
| Lipids, lipokines, and lipid precursors | 12,13-diHOME | ↑ (26%) | ↓ | Exercise-induced brown adipose tissue-derived lipokine, increases skeletal muscle fatty acid uptake56 |
| 13-HODE | ↑ (12%) | ↑/↓ | Classically considered a marker of oxidative stress, but may act to decrease pro-inflammatory cytokine release (G-CSF, IL-6)51 | |
| Arachidonate | ↑ (11%) | ↑/↓ | Eicosanoid precursor (regulation of inflammation, vascular dysfunction, platelet reactivity) | |
| Eicosapentaenoate/Docosapentaenoate | ↑ (7%, 15%) | ↑ | Implicated in reduced CVD risk, inflammation | |
| 16-hydroxypalmitate Hexadecanedioate |
↑ (26%, 32%) | NA | Palmitate metabolism | |
| Sphingosine Sphingosine-1 phosphate |
↓ (−13%, −23%) | ↑/↓ | HDL-bound sphingosine-1 phosphate may be anti-atherogenic;57 associated with CVD58,59 | |
| Select sphingomyelins (C16:1 SM, C22:1 SM, C18:1 SM) | ↑ (8% to 12%) | ↓ | Selected sphingomyelins (lower levels) and TAGs (higher levels) associated with higher risk of diabetes38 | |
| Selected triacylglycerols (C48:0 TAG, C50:0 TAG, C46:0 TAG, C42:0 TAG, C44:0 TAG) | ↓ (−23% to −12%) | ↑ | ||
| Medium- and long-chain acylcarnitines (C8:0, C10:0, C12:0, C14:0, C16:0, C18:0) | ↑ (11% to 38%) | ↑ | Related to presence of CAD and future CAD risk60 and are higher in obesity and diabetes61 | |
| Microbial metabolism | Hippurate | ↓ (−32%) | ↑/↓ | Mixed directionality of association for metabolic disease and CVD;62,63 higher levels may be related to higher CVD mortality |
| Trimethylamine-N-oxide | ↓ (−11%) | ↑ | Related to increased CVD risk64 | |
| Tryptophan metabolites | Indoxylsulfate | ↓ (−25%) | ↑ | Metabolite of tryptophan; higher levels related to CVD in chronic kidney disease65 |
| 3-hydroxyanthranilic acid (3-HAA) | ↓ (−21%) | ↑ | Higher level related to greater vascular risk and aneurysm formation, induced by angiotensin II66 | |
| Quinolinate | ↓ (−14%) | ↑ | Associated with increased carotid vascular disease67 and pulmonary vascular dysfunction68 | |
| Tryptophan Kynurenine |
↓ (−10%, −8%) | ↑ | Reflects IDO-tryptophan metabolism; characteristic of pro-inflammatory states | |
| Collagen/fibrosis metabolites | Hydroxyproline | ↓ (16%) | ↑ | Tissue expression of hydroxyproline may reflect increased fibrotic potential69 |
| Steroids | Pregnenolone sulfate | ↑ (10%) | ↑ | Involved in long-term potentiation in the hippocampus70 |
| Glycolysis/anerobic metabolism | Lactate Pyruvate 3-phosphoglycerate |
↑ (246%, (75%) ↓ (−31%) |
NA | Glycolytic metabolism |
| Krebs cycle intermediates and precursors | Succinate Fumarate Aconitate Alpha-ketoglutarate |
↑ (9% to 81%) | NA | Aerobic metabolism; enhanced redox availability to the electron transport chain for aerobic respiration |
| CoA biosynthesis | Pantothenate | ↑ (54%) | NA | Central vitamin cofactor (B5) for fatty acid metabolism and Krebs cycle |
As demonstrated in prior studies, metabolites representing pathways related to oxidative stress and inflammation demonstrated varying directions of activity in response to the physiologic stress of acute exercise. We observed reductions in several metabolites correlated with inflammation with decreases in cystine (oxidized cysteine50), allantoin, and their ratio, and lower kynurenine, which is associated with pro-inflammatory states. Conversely, levels of 13-hydroxyoctadecadienoic acid51 (13-HODE) and arachidonate (both implicated in oxidative stress, inflammation, and its counter-regulation) were increased with exercise.
Of the 177 metabolites significant at a 5% FDR level from the HILIC-positive platform in our derivation cohort and measured in the replication cohort, we observed consistent signals in the direction of exercise-induced metabolite change in 164 (92.6%, at 5% FDR; Figure 1e and Table III in the Supplement).
In silico pathway analysis
Filtering for metabolites that changed significantly with exercise (at a 5% FDR) and with a specified HMDB identifier produced sets of 251 metabolites in the HILIC platforms, 173 metabolites in the C8 platform, and 58 metabolites in the C18 platform. Using MetaboAnalystR21 to perform compound-gene mapping, 106 of 251 HILIC metabolites were mapped to 2141 genes, 4 of 173 C8 metabolites mapped to 7 genes, and 16 of 58 C18 metabolites mapped to 291 genes. The genes from these mappings were then used to perform enrichment analysis. The top pathway analysis results are displayed as dot plots (Figure III in the Supplement). We observed enrichment of pathways related to amino acid metabolism, peroxisome proliferator-activated receptor signaling, eicosanoid and prostaglandin metabolism, insulin signaling, angiopoietin-like protein 8 regulation, and brain-derived neurotrophic factor signaling, all of which have been implicated in cardiovascular and metabolic diseases.
Determinants of metabolite changes with exercise
To evaluate the relations of metabolite changes with exercise to clinical variables (age, sex, and BMI) and to the amount of exercise performed (workload achieved), we specified multivariable-adjusted linear regression models (Figure IV in the Supplement, Tables IV-VI in the Supplement). As expected, we observed increases in metabolites involved in glycolysis and anaerobic metabolism (e.g., lactate, pyruvate, fumarate) to be related to higher amounts of exercise (Figure V in the Supplement). By contrast, exercise-induced changes in many metabolites related with increased insulin sensitivity, greater nitric oxide bioavailability, and favorable shifts in the circulating lipidome, were not associated with the amount of exercise performed.
We observed significant heterogeneity in the exercise-induced change in several metabolites by BMI and sex (Figure 2 and Figure IV in the Supplement, Tables IV-VI in the Supplement). For example, DMGV (higher levels associated with hepatic steatosis) decreased with exercise, with a magnitude of decrease associated with BMI, such that individuals with higher BMI had a less marked reduction in DMGV with exercise (Figure 2a). These findings suggest that there may be less metabolic “plasticity” with exercise in individuals who have a higher BMI for certain pathways important to cardiometabolic health. We also observed distinct metabolic responses to exercise by sex, with higher excursions in metabolites linked to anaerobic metabolism (e.g., pyruvate, lactate) with greater muscle mass in men, yet greater favorable changes in select metabolites involved in cardiometabolic health (e.g., reductions in DMGV and pro-inflammatory tryptophan-kynurenine metabolites, and increases in hexadecanedioate and oleoyl glycine) in women (Figure 2b). After accounting for the other variables in a multivariable model, excursions of only a small number (n=3: sorbitol, C10:2 acylcarnitine, C14:2 acylcarnitine) of metabolites were significantly related to age (Figure IV in the Supplement).
Figure 2. Metabolic architecture of acute exercise.
Panel (A) shows the mean (dot) and 95% confidence interval (bar) for log2 fold-changes across three different BMI strata: lean (BMI <25 kg/m2), overweight (BMI 25 to <30 kg/m2), obese (BMI ≥30 kg/m2). These are crude (unadjusted) fold changes for each metabolite across BMI strata. Metabolites were selected for display based on significance for fold-change with exercise (Table II in the Supplement), significance for BMI in fully adjusted regressions (Tables IV-VI in the Supplement), and curation into pathways of cardiometabolic health (Table 4). Similarly, panel (B) shows the mean (dot) and 95% confidence interval (bar) for log2 fold-changes for men and women. With exercise, women displayed greater reductions in metabolites associated with impaired insulin sensitivity28 and increased vascular risk58 and greater increases in cardioprotective free fatty acids. By contrast, men demonstrated higher excursions in metabolites involved in cellular metabolism, likely due to greater muscle mass.
Abbreviations: FAs, fatty acids; skel, skeletal
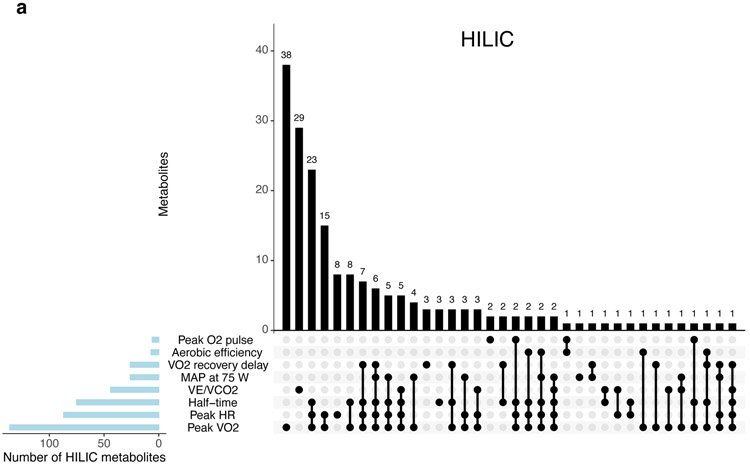
Exercise responses in relation to the resting metabolome
In age- and sex-adjusted models, resting blood metabolite levels were distinctly associated with one or more exercise response pattern measured by CPET (Figure 3 and Tables VII-IX in the Supplement). These findings were largely consistent in models additionally adjusted for clinical factors including resting systolic blood pressure, diabetes, hypertension treatment status, smoking, and prevalent CVD (Figure VI in the Supplement, Tables X-XII in the Supplement). We focused on patterns of unique metabolite associations with three complementary exercise measures: global aerobic capacity (peak VO2), systemic vascular function (mean arterial pressure at 75 watts), and right heart-pulmonary vascular performance during exercise (VE/VCO2) as illustrated in Figure 3d. As expected, given its dependence on both central cardiac and peripheral factors, peak VO2 was associated with the largest number of metabolites (Figure 3a-c). We observed associations between lower peak VO2 and higher levels of metabolites reflecting impaired peripheral muscle and fat metabolism (DMGV, 1-methylnicotinomide, 2-aminoadipate), insulin resistance (BCAAs), oxidative stress-related purine degradation (hypoxanthine, urate) and greater reliance on anaerobic metabolism in a state of rest (lactate).
Figure 3. Resting metabolite profiles are differentially associated with multi-dimensional physiologic measurements during exercise.
Panels (A-C) display the number of resting metabolite levels (per platform, in columns) that are significantly associated (at a 5% FDR) with combinations of CPET measures (in rows) in age- and sex-adjusted linear regression models. Panel (D) displays the estimated regression coefficients for select metabolites in regressions for three key physiological exercise responses: peak VO2, VE/VCO2 nadir, and MAP at 75 watts. Each CPET variable demonstrates a pattern of associations with metabolites representing distinct physiologic processes as noted. Details of metabolite functions and associations with cardiometabolic traits are shown in Table 4. Asterisks are used to denote statistical significance as follows: * = FDR >0.01 to ≤0.05; ** = FDR >0.001 to ≤0.01; *** = FDR ≤0.001. Raw data for these plots are shown in Table VII-IX in the Supplement.
Abbreviations: NO, nitric oxide
Submaximum exercise blood pressure response is associated with future development of overt resting hypertension17 and CVD,18 but the metabolic pathways underlying these associations have not been established. Plasma levels of several plasmalogens were inversely related to exercise blood pressure in models adjusted for resting blood pressure (Figure 3d). Plasmalogens, which serve as endogenous anti-oxidants and generate vasoactive mediators,72 are lower in overt hypertension and vascular disease73 but have not been previously related to exercise blood pressure responses. We also observed associations of MAP at 75 watts with several amino acids and amino acid derivatives previously associated with resting blood pressure (glutamate – associated with higher exercise MAP, and asparagine and glycine associated with lower MAP).74 A large number of triacylglycerols (TAGs) were associated with exercise MAP with direct associations noted for TAGs with lower carbon number and double bond content and inverse associations noted with TAGs with higher carbon number and double bond content.
Ventilatory efficiency (VE/VCO2) reflects pulmonary ventilation-perfusion matching during exercise, is elevated proportionate to pulmonary vascular resistance, and decreases in response to pulmonary vasodilatory therapy in patients with overt pulmonary hypertension.10,75 VE/VCO2 was uniquely associated with long-chain acylcarnitines, which are elevated in pulmonary arterial hypertension,68,76 as well as 16-hydroxypalmitate, which is known to become elevated in the setting of impaired fatty acid beta-oxidation characteristic of pulmonary hypertension.77 Beta-hydroxybutyrate was also related to VE/VCO2 and has recently been shown to be elevated in overt pulmonary arterial hypertension.78 Other free fatty acids (e.g., dodecanoate, eicosatrienoate, hexadecanedioate) related to VE/VCO2 in this study have not been previously related to pulmonary vascular function.
Metabolite signatures of integrated exercise responses are associated with long-term survival
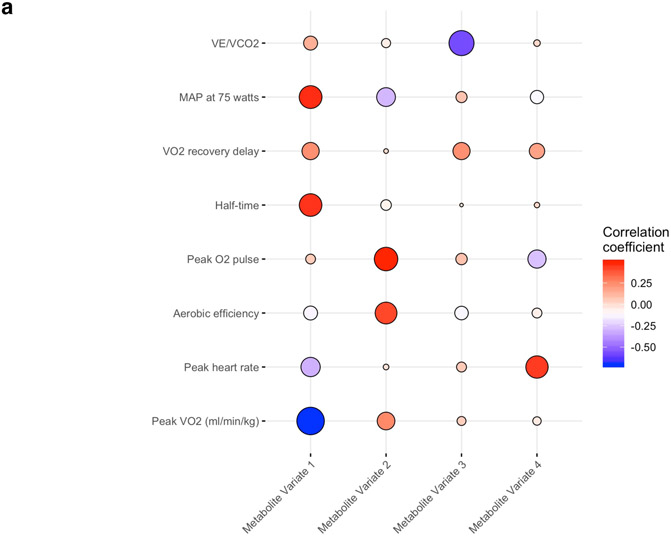
We next sought to derive resting metabolite signatures of integrated exercise responses, leveraging data across the eight CPET measures described in Table 1. CCA was used to create metabolite and CPET ‘scores’ that can be used to define metabolite signatures of multi-dimensional exercise data (Tables XIII-XIV in the Supplement). Figure 4a shows the correlations of the first four metabolite variates with each CPET variable. Metabolite variate 1 was most strongly correlated with lower peak VO2, higher submaximal exercise blood pressure, and delayed O2 recovery kinetics following exercise (Table XV in the Supplement). Metabolite variate 2 was correlated with favorable O2 uptake kinetics (higher peak VO2, aerobic efficiency, O2 pulse) and lower 75 watt mean arterial pressure. Metabolite variate 3 was correlated with lower VE/VCO2, and metabolite variate 4 was correlated with higher peak heart rate. The metabolites most highly correlated with each canonical variate are shown in Figure 4b (and Table XVI in the Supplement).
Figure 4. Correlations of metabolites with integrated exercise responses.
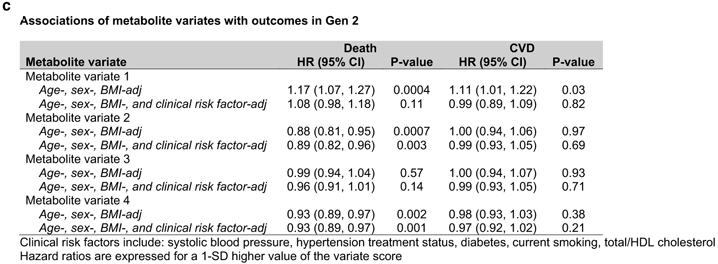
Panel (A) shows the age- and sex-adjusted partial correlations of each exercise measure with metabolite variates determined by canonical correlation analysis. The size of each circle is proportional to the absolute value of the correlation coefficient, and its color represents the directionality of correlation. Panel (B) represents a heatmap of the age- and sex-adjusted partial correlations of metabolites with metabolite variates. The metabolites shown here have a correlation coefficient ≥0.25 with any of the metabolite variates. Panel (C) displays the multivariable-adjusted associations of the four metabolite variates with death and incident CVD.
We then evaluated the associations of metabolite ‘variate scores’ with long-term outcomes (Figure 4c). During median follow-up of 23.1 (limits 0.1-27.3) years, 702 deaths occurred in 2,045 individuals. In age-, sex-, and BMI-adjusted analyses, metabolite variates 1, 2, and 4 were associated with mortality; variates 2 and 4 remained statistically significant after further adjustment for CVD risk factors (Figure 4c). In 1996 individuals without CVD at baseline, 489 CVD events occurred during median follow-up of 22.7 (limits 0.1-27.3) years. Variate 1 was associated with higher CVD risk in age-, sex-, and BMI-adjusted models, with attenuation of the association after further multivariable adjustment.
DISCUSSION
Exercise is associated with broad benefits on cardiovascular, metabolic, and general health, though precise mechanisms by which these ends are met remain unclear. We provide a comprehensive, quantitative assessment of global human metabolism before and after an acute bout of exercise in a large sample of well-phenotyped, community-dwelling individuals. We observed a dramatic shift in >80% of annotated metabolites in the circulating metabolome in response to ≈12 minutes of incremental exercise, with beneficial alterations in levels of metabolites representing key metabolic pathways central to obesity, insulin resistance, oxidative stress, inflammation, vascular reactivity, and longevity, including a variety of novel metabolic mediators and pathways not extensively described. Dynamic changes in metabolite levels varied by BMI and sex but notably were not associated with age. Importantly, we observed distinct resting metabolite signatures of abnormal systemic and pulmonary vascular responses to exercise as well as global fitness as captured by peak VO2. Finally, we linked metabolite signatures of eight CPET measures to mortality and CVD in nearly 2000 individuals with ≈23 years’ follow-up. Collectively, these findings underscore the complex, heterogeneous metabolic response to acute exercise and its relation to physiologic mechanisms and long-term prognosis.
In addition to workload-related increases in metabolites reflecting aerobic and anaerobic cellular metabolism (e.g., lactate, Krebs cycle intermediates,4 acylcarnitines,7,68,79 and purine catabolites indicating heightened ATP turnover80,81), we detected favorable shifts in a number of metabolites for which resting levels were previously shown to be associated with cardiometabolic disease. These include reductions in several metabolites associated with future diabetes, including BCAAs,11,60 glutamate,30,31 DMGV (associated with hepatic fat27-29), as well as select TAGs (lower carbon number and double bond content71), and pro-atherogenic ceramide derivatives (sphingosine and shingosine-1-phosphate58,59). We also observed favorable increases in metabolites associated with lower cardiometabolic risk, such as 1-methylnicotinamide (promotes lipolysis26), glutamine, and 12,13-diHOME (promotes skeletal muscle fatty acid uptake56). Shifts in many of these metabolites were not related to workload achieved, suggesting that a “salutary switch” may be turned on by brief exercise that results in favorable changes in circulating levels of metabolites reflecting a healthier metabolic state.
Favorable metabolite shifts may be “blunted” in those with a higher BMI, even after adjustment for workload, suggesting obesity or its related metabolic perturbations (e.g., insulin resistance) may confer resistance to the benefits of exercise. We also observed distinct metabolic responses to exercise between men and women consistent with known sex heterogeneity in cardiorespiratory fitness. Men appeared to experience greater excursions in metabolites linked to cellular metabolism, while changes in several metabolites with putative beneficial implications were more prominent in females. Whether these are the result of sexual dimorphism in body composition (e.g., greater lean muscle mass in men), underlying mechanisms of exercise response, or residual confounding remains a source of future study in large samples specifically designed to address this hypothesis.82 Notably, we observed consistency of metabolite changes with exercise across a broad age spectrum, which corresponds to previous findings of similar clinical benefits of exercise among different age groups.83
By combining detailed multi-dimensional exercise measures obtained via CPET with metabolite profiling, we were able to evaluate metabolite signatures of discrete physiologic exercise responses. We observed distinct metabolite signatures for peak VO2 (reflecting whole-body metabolic responses), submaximum blood pressure response (reflecting systemic vascular function), and VE/VCO2 (reflecting pulmonary vascular dysfunction and impaired cardiac performance). Peak VO2 was associated with resting levels of a large number of metabolites, including those related to fat metabolism, insulin resistance, oxidative stress, and anaerobic metabolism, reflecting a combination of central cardiovascular function and peripheral oxygen utilization. Adverse submaximum blood pressure responses were directly associated with higher levels of TAG species related to CVD risk,71 higher levels of amino acid derivates linked to resting systemic hypertension,74 and lower levels of vaso-protective plasmalogens.72,73 VE/VCO2, which reflects ventilation-perfusion matching in the pulmonary circulation and is elevated in proportion to pulmonary vascular resistance10,75 demonstrated associations with various metabolites related to overt resting pulmonary arterial hypertension including acylcarnitines,68,76 16-hydroxypalmitate,77 and beta-hydroxybutyrate.78 These findings offer mechanistic insight into the metabolic pathways distinctly involved in organ-specific physiologic responses to exercise that lie upstream of overt forms of disease states evident at rest (i.e. pulmonary arterial hypertension). Indeed, composite metabolite signatures of integrated exercise responses were associated with mortality after adjustment for traditional CVD risk factors, consistent with strong associations between cardiorespiratory fitness and both cardiovascular84 and non-cardiovascular mortality.85
Our findings are highly consistent with and extend those from a recent study by Contrepois et al. which reported multi-omic changes with acute exercise in 36 individuals.7 Similar to this published report, we observed increases in metabolites involved in anaerobic and aerobic respiration, complex lipids such as sphingomyelins, nucleotide derivatives, and reductions in BCAAs, among others.7 While these consistencies suggest the external validity of our findings, our report fundamentally extends the published literature in this space in several important ways. First, the larger sample size in the present study allowed detection of exercise changes in metabolites relevant to cardiometabolic disease not previously reported likely due to enhanced statistical power. These include exercise-induced decreases in bile acids and modest changes in glutamine74 (linked with longevity and CVD), furthering the understanding of biological pathways related to exercise responses. In our study, exercise-induced changes in metabolites were replicated in a separate sample, providing strong support of the validity of our key findings. The greater sample size across a broader, heterogeneous population also affords insights into potential modification of observed exercise responses by obesity, age, and sex. Moreover, the comprehensive CPET testing here allowed us to link resting metabolite levels to specific exercise phenotypes reflecting distinct physiologies. These CPET phenotypes may offer insight into metabolic dysfunction related to the early, subclinical stages of CVD development: for example, association of acylcarnitines with VE/VCO2 slope in the FHS (a population without overt clinical disease) were similar to those in animal models and patients with symptomatic pulmonary arterial hypertension.76 Similarly, we related metabolite classes such as plasmalogens to exercise blood pressure with adjustment for resting blood pressure, thereby creating signatures that are specific to an exercise response pattern that antedates future hypertension.17 Lastly, by exporting a metabolomic score representing multi-dimensional exercise responses to a separate cohort, we demonstrated prognostically significant associations of metabolic exercise signatures with long-term health outcomes. These contributions highlight the importance of broad phenotyping across a large sample to clarify potential disease mechanisms for perturbation, mechanistic evaluation, and targeting.
Our study has several important limitations. Though our sample size was more than an order of magnitude higher than previously published data in this space, we recruited primarily white, middle-aged adults and focused on acute exercise on a cycle. Certainly, this limits generalizability by age, race, disability, and chronicity and type of exercise, though metabolite excursions observed here are largely consistent with signals from previous studies.4,7,29 Cycle exercise results in lower maximal CPET measures and is less influenced by body mass compared with treadmill exercise; differences in the activity of metabolic pathways based on exercise modality should be examined in future studies. While we did observe sex- and BMI-related heterogeneity in the metabolic response to exercise, true evidence of modification of effect by sex, BMI, and other important clinical features will require larger studies with more comprehensive phenotyping of other confounders known to display differences by sex (e.g., muscle and fat distribution) and perturbational studies (i.e., weight loss). We observed an approximately equal proportion of metabolites that increased with exercise (that may be affected by exercise-associated hemoconcentration) as metabolites that decreased with exercise (the effect of which would be attenuated by exercise-related hemoconcentration), suggesting that hemoconcentration during this brief period of exercise may not substantially confound the observed changes. The duration of acute, favorable exercise-induced changes in metabolite levels was also not captured due to limitations in feasibility of serial post-exercise sampling in a population study. Lastly, we observed metabolite signatures of exercise responses to be more closely associated with future mortality than with incident CVD, highlighting that exercise-related metabolite pathways associate with prognosis outside of their role in CVD.
In conclusion, acute exercise exerts specific, widespread effects on the circulating metabolome, many of which are related to favorable mechanisms of health. Metabolic responses to exercise are not necessarily related only to the work performed, but may be modulated by key clinical features, including sex and obesity. These results not only provide a detailed resource regarding the metabolic architecture of acute exercise in humans, but also pinpoint specific pathways of prognostically-relevant, exercise-mediated metabolic adaptation, suggesting novel avenues for future mechanistic investigation and intervention.
Supplementary Material
CLINICAL PERSPECTIVE.
What is new?
To better understand how acute exercise influences the human metabolome, we measured the levels of 588 circulating metabolites before and immediately following ≈12 minutes of exercise in 411 community-dwelling individuals who underwent cardiopulmonary exercise testing (CPET) and replicated key findings in a separate sample (n=783).
Significant changes occurred in >80% of circulating metabolites, including favorable shifts in many metabolites previously linked to cardiometabolic outcomes.
We observed distinct associations of resting metabolites with physiologic exercise responses captured by CPET and metabolite signatures of multi-dimensional exercise responses were associated with the risk of death in a separate sample (n ≈2000).
What are the clinical implications?
In this study, we observed favorable exercise-induced changes in circulating levels of metabolites related to insulin resistance, obesity, lipolysis, oxidative stress, inflammation, vascular reactivity, and longevity, with blunting of advantageous changes observed in individuals with higher body mass index and different metabolite excursions in men and women.
These findings provide a detailed description of the metabolic effects of acute exercise, link specific metabolic pathways with exercise response variables and long-term health outcomes, and spotlight metabolic pathways involved in exercise responses for future study.
Acknowledgments:
We acknowledge the dedication of the FHS study participants without whom this research would not be possible.
Funding:
This work was supported by the National Heart, Lung and Blood Institute’s Framingham Heart Study (Contracts N01-HC-25195, HHSN268201500001I, and 75N92019D00031) and by NIH grants K23-HL138260 (MN), 1R01HL131029 (RSV and GDL), R01HL134893 (JEH), R01HL140224 (JEH), R01HL142809 (RM), and American Heart Association grant 15GPSGC24800006 (GDL). RSV is supported in part by the Evans Medical Foundation and the Jay and Louis Coffman Endowment from the Department of Medicine, Boston University School of Medicine. RVS and VLM are supported by the National Institutes of Health and the American Heart Association.
NON-STANDARD ABBREVIATIONS AND ACRONYMS
- FHS
Framingham Heart Study
- CPET
cardiopulmonary exercise testing
- CVD
cardiovascular disease
- Gen2
Framingham Heart Study Second Generation
- Gen3
Framingham Heart Study Third Generation
- Peak VO2
peak oxygen uptake
- EDTA
ethylenediaminetetraacetic
- LC-MS
liquid chromatography tandem mass spectrometry
- HILIC-positive
hydrophobic interaction chromatography in the positive ionization mode
- HILIC-negative
hydrophobic interaction chromatography in the negative ionization mode
- CVs
coefficients of variation
- FDR
false discovery rate
- BMI
body mass index
- MAP
mean arterial pressure
- VE/VCO2
ventilatory efficiency
- CCA
canonical correlation analysis
- BCAAs
branched chain amino acids
- DMGV
dimethylguanidinovaleric acid
- 12,13-diHOME
12,13-dihydroxy-9Z-octadecenoic acid
- 13-HODE
13-hydroxyoctadecadienoic acid
- TAGs
triacylglycerols
Footnotes
Disclosures: Dr. Shah has funding from the National Institutes of Health and American Heart Association. In the past 12 months, he has received consulting funds from Best Doctors and MyoKardia, neither of which is relevant to the current report. In addition, he is co-inventor on a patent related to ex-RNA signatures of cardiac remodeling. Dr. Murthy owns stock in Amgen, General Electric and Cardinal Health. He has received speaking honoraria from, serves as a scientific advisor for, and owns stock options in Ionetix. He has received research funding and speaking honoraria from Siemens Medical Imaging. He has served as a scientific advisor for Curium and has received expert witness fees from Jubilant Draximage. He has received a speaking honorarium from 2Quart Medical. He has received non-financial research support from INVIA Medical Imaging Solutions. Dr. Lewis has research funding from the National Institutes of Health and the American Heart Association as well as Amgen, Cytokinetics, Applied Therapeutics, AstraZeneca, Sonivie in relation to projects and clinical trials investigating exercise capacity that are distinct from this work. He has served as a scientific advisor for Pfizer, Merck, Boehringer-Ingelheim, Novartis, American Regent, Relypsa, Cyclerion, Cytokinetics, and Amgen and receives royalties from UpToDate for scientific content authorship related to exercise physiology.
References
- 1.Zaccardi F, O'Donovan G, Webb DR, Yates T, Kurl S, Khunti K, Davies MJ and Laukkanen JA. Cardiorespiratory fitness and risk of type 2 diabetes mellitus: A 23-year cohort study and a meta-analysis of prospective studies. Atherosclerosis. 2015;243:131–137. [DOI] [PubMed] [Google Scholar]
- 2.Kokkinos P Cardiorespiratory Fitness, Exercise, and Blood Pressure. Hypertension. 2014;64:1160–1164. [DOI] [PubMed] [Google Scholar]
- 3.Green DJ, Walsh JH, Maiorana A, Best MJ, Taylor RR and O'Driscoll JG. Exercise-induced improvement in endothelial dysfunction is not mediated by changes in CV risk factors: pooled analysis of diverse patient populations. Am J Physiol Heart Circ Physiol. 2003;285:H2679–H2687. [DOI] [PubMed] [Google Scholar]
- 4.Lewis GD, Farrell L, Wood MJ, Martinovic M, Arany Z, Rowe GC, Souza A, Cheng S, McCabe EL, Yang E, et al. Metabolic signatures of exercise in human plasma. Sci Transl Med. 2010;2:33ra37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shah R, Yeri A, Das A, Courtright-Lim A, Ziegler O, Gervino E, Ocel J, Quintero-Pinzon P, Wooster L, Bailey CS, et al. Small RNA-seq during acute maximal exercise reveal RNAs involved in vascular inflammation and cardiometabolic health: brief report. Am J Physiol Heart Circ Physiol. 2017;313:H1162–H1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rigamonti AE, Bollati V, Pergoli L, Iodice S, De Col A, Tamini S, Cicolini S, Tringali G, De Micheli R, Cella SG, et al. Effects of an acute bout of exercise on circulating extracellular vesicles: tissue-, sex-, and BMI-related differences. Int J Obes (Lond). 2020;44:1108–1118. [DOI] [PubMed] [Google Scholar]
- 7.Contrepois K, Wu S, Moneghetti KJ, Hornburg D, Ahadi S, Tsai MS, Metwally AA, Wei E, Lee-McMullen B, Quijada JV, et al. Molecular Choreography of Acute Exercise. Cell. 2020;181:1112–1130 e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kannel WB, Feinleib M, McNamara PM, Garrison RJ and Castelli WP. An investigation of coronary heart disease in families. The Framingham offspring study. Am J Epidemiol. 1979;110:281–290. [DOI] [PubMed] [Google Scholar]
- 9.Splansky GL, Corey D, Yang Q, Atwood LD, Cupples LA, Benjamin EJ, D'Agostino RB Sr., Fox CS, Larson MG, Murabito JM, et al. The Third Generation Cohort of the National Heart, Lung, and Blood Institute's Framingham Heart Study: design, recruitment, and initial examination. Am J Epidemiol. 2007;165:1328–1335. [DOI] [PubMed] [Google Scholar]
- 10.Nayor M, Xanthakis V, Tanguay M, Blodgett JB, Shah RV, Schoenike M, Sbarbaro J, Farrell R, Malhotra R, Houstis NE, et al. Clinical and Hemodynamic Associations and Prognostic Implications of Ventilatory Efficiency in Patients With Preserved Left Ventricular Systolic Function. Circ Heart Fail. 2020;13:e006729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang TJ, Larson MG, Vasan RS, Cheng S, Rhee EP, McCabe E, Lewis GD, Fox CS, Jacques PF, Fernandez C, et al. Metabolite profiles and the risk of developing diabetes. Nat Med. 2011;17:448–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hansen JE, Sue DY and Wasserman K. Predicted values for clinical exercise testing. Am Rev Respir Dis. 1984;129:S49–55. [DOI] [PubMed] [Google Scholar]
- 13.Yeri A, Murphy RA, Marron MM, Clish C, Harris TB, Lewis GD, Newman AB, Murthy VL and Shah RV. Metabolite Profiles of Healthy Aging Index Are Associated With Cardiovascular Disease in African Americans: The Health, Aging, and Body Composition Study. J Gerontol A Biol Sci Med Sci. 2019;74:68–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blom G Statistical estimates and transformed beta-variables. Stockholm, Sweden: Almqvist & Wiksell; 1958. [Google Scholar]
- 15.Tanaka H, Dinenno FA, Monahan KD, Clevenger CM, DeSouza CA and Seals DR. Aging, habitual exercise, and dynamic arterial compliance. Circulation. 2000;102:1270–1275. [DOI] [PubMed] [Google Scholar]
- 16.Julius S Abnormalities of autonomic nervous control in human hypertension. Cardiovasc Drugs Ther. 1994;8 Suppl 1:11–20. [DOI] [PubMed] [Google Scholar]
- 17.Singh JP, Larson MG, Manolio TA, O'Donnell CJ, Lauer M, Evans JC and Levy D. Blood pressure response during treadmill testing as a risk factor for new-onset hypertension. The Framingham heart study. Circulation. 1999;99:1831–1836. [DOI] [PubMed] [Google Scholar]
- 18.Lewis GD, Gona P, Larson MG, Plehn JF, Benjamin EJ, O'Donnell CJ, Levy D, Vasan RS and Wang TJ. Exercise blood pressure and the risk of incident cardiovascular disease (from the Framingham Heart Study). Am J Cardiol. 2008;101:1614–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cooley WW and Lohnes PR. Multivariate Data Analysis. New York: John Wiley & Sons, Inc; 1971. [Google Scholar]
- 20.Sherry A and Henson RK. Conducting and interpreting canonical correlation analysis in personality research: a user-friendly primer. J Pers Assess. 2005;84:37–48. [DOI] [PubMed] [Google Scholar]
- 21.Chong J, Yamamoto M and Xia J. MetaboAnalystR 2.0: From Raw Spectra to Biological Insights. Metabolites. 2019;9:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu G, Wang LG, Han Y and He QY. clusterProfiler: an R package for comparing biological themes among gene clusters. Omics. 2012;16:284–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gustavsen JA, Pai S, Isserlin R, Demchak B and Pico AR. RCy3: Network biology using Cytoscape from within R. F1000Res. 2019;8:1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Slenter DN, Kutmon M, Hanspers K, Riutta A, Windsor J, Nunes N, Melius J, Cirillo E, Coort SL, Digles D, et al. WikiPathways: a multifaceted pathway database bridging metabolomics to other omics research. Nucleic Acids Res. 2018;46:D661–D667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chlopicki S, Swies J, Mogielnicki A, Buczko W, Bartus M, Lomnicka M, Adamus J and Gebicki J. 1-Methylnicotinamide (MNA), a primary metabolite of nicotinamide, exerts anti-thrombotic activity mediated by a cyclooxygenase-2/prostacyclin pathway. Br J Pharmacol. 2007;152:230–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Strom K, Morales-Alamo D, Ottosson F, Edlund A, Hjort L, Jorgensen SW, Almgren P, Zhou Y, Martin-Rincon M, Ekman C, et al. N(1)-methylnicotinamide is a signalling molecule produced in skeletal muscle coordinating energy metabolism. Sci Rep. 2018;8:3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O'Sullivan JF, Morningstar JE, Yang Q, Zheng B, Gao Y, Jeanfavre S, Scott J, Fernandez C, Zheng H, O'Connor S, et al. Dimethylguanidino valeric acid is a marker of liver fat and predicts diabetes. J Clin Invest. 2017;127:4394–4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ottosson F, Ericson U, Almgren P, Smith E, Brunkwall L, Hellstrand S, Nilsson PM, Orho-Melander M, Fernandez C and Melander O. Dimethylguanidino Valerate: A Lifestyle-Related Metabolite Associated With Future Coronary Artery Disease and Cardiovascular Mortality. J Am Heart Assoc. 2019;8:e012846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robbins JM, Herzig M, Morningstar J, Sarzynski MA, Cruz DE, Wang TJ, Gao Y, Wilson JG, Bouchard C, Rankinen T, et al. Association of Dimethylguanidino Valeric Acid With Partial Resistance to Metabolic Health Benefits of Regular Exercise. JAMA Cardiol. 2019;4:636–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zheng Y, Hu FB, Ruiz-Canela M, Clish CB, Dennis C, Salas-Salvado J, Hruby A, Liang L, Toledo E, Corella D, et al. Metabolites of Glutamate Metabolism Are Associated With Incident Cardiovascular Events in the PREDIMED PREvencion con DIeta MEDiterranea (PREDIMED) Trial. J Am Heart Assoc. 2016;5:e003755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu X, Zheng Y, Guasch-Ferre M, Ruiz-Canela M, Toledo E, Clish C, Liang L, Razquin C, Corella D, Estruch R, et al. High plasma glutamate and low glutamine-to-glutamate ratio are associated with type 2 diabetes: Case-cohort study within the PREDIMED trial. Nutr Metab Cardiovasc Dis. 2019;29:1040–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Petrus P, Lecoutre S, Dollet L, Wiel C, Sulen A, Gao H, Tavira B, Laurencikiene J, Rooyackers O, Checa A, et al. Glutamine Links Obesity to Inflammation in Human White Adipose Tissue. Cell Metab. 2020;31:375–390. [DOI] [PubMed] [Google Scholar]
- 33.Wurtz P, Havulinna AS, Soininen P, Tynkkynen T, Prieto-Merino D, Tillin T, Ghorbani A, Artati A, Wang Q, Tiainen M, et al. Metabolite profiling and cardiovascular event risk: a prospective study of 3 population-based cohorts. Circulation. 2015;131:774–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murr C, Grammer TB, Meinitzer A, Kleber ME, Marz W and Fuchs D. Immune activation and inflammation in patients with cardiovascular disease are associated with higher phenylalanine to tyrosine ratios: the ludwigshafen risk and cardiovascular health study. J Amino Acids. 2014;2014:783730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou M, Shao J, Wu CY, Shu L, Dong W, Liu Y, Chen M, Wynn RM, Wang J, Wang J, et al. Targeting BCAA Catabolism to Treat Obesity-Associated Insulin Resistance. Diabetes. 2019;68:1730–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Walford GA, Ma Y, Clish C, Florez JC, Wang TJ, Gerszten RE and Diabetes Prevention Program Research G. Metabolite profiles of diabetes incidence and intervention response in the Diabetes Prevention Program. Diabetes. 2016;65:1424–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang TJ, Ngo D, Psychogios N, Dejam A, Larson MG, Vasan RS, Ghorbani A, O'Sullivan J, Cheng S, Rhee EP, et al. 2-Aminoadipic acid is a biomarker for diabetes risk. J Clin Invest. 2013;123:4309–4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen ZZ, Liu J, Morningstar J, Heckman-Stoddard BM, Lee CG, Dagogo-Jack S, Ferguson JF, Hamman RF, Knowler WC, Mather KJ, et al. Metabolite Profiles of Incident Diabetes and Heterogeneity of Treatment Effect in the Diabetes Prevention Program. Diabetes. 2019;68:2337–2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sun Q, Wang B, Li Y, Sun F, Li P, Xia W, Zhou X, Li Q, Wang X, Chen J, et al. Taurine Supplementation Lowers Blood Pressure and Improves Vascular Function in Prehypertension: Randomized, Double-Blind, Placebo-Controlled Study. Hypertension. 2016;67:541–549. [DOI] [PubMed] [Google Scholar]
- 40.Wang S, Xu Q, Shu G, Wang L, Gao P, Xi Q, Zhang Y, Jiang Q and Zhu X. N-Oleoyl glycine, a lipoamino acid, stimulates adipogenesis associated with activation of CB1 receptor and Akt signaling pathway in 3T3-L1 adipocyte. Biochem Biophys Res Commun. 2015;466:438–443. [DOI] [PubMed] [Google Scholar]
- 41.Simard T, Jung R, Labinaz A, Faraz MA, Ramirez FD, Di Santo P, Perry-Nguyen D, Pitcher I, Motazedian P, Gaudet C, et al. Evaluation of Plasma Adenosine as a Marker of Cardiovascular Risk: Analytical and Biological Considerations. J Am Heart Assoc. 2019;8:e012228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gessi S, Fogli E, Sacchetto V, Merighi S, Varani K, Preti D, Leung E, Maclennan S and Borea PA. Adenosine modulates HIF-1{alpha}, VEGF, IL-8, and foam cell formation in a human model of hypoxic foam cells. Arterioscler Thromb Vasc Biol. 2010;30:90–97. [DOI] [PubMed] [Google Scholar]
- 43.Farthing DE, Farthing CA and Xi L. Inosine and hypoxanthine as novel biomarkers for cardiac ischemia: from bench to point-of-care. Exp Biol Med (Maywood). 2015;240:821–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hasko G, Kuhel DG, Nemeth ZH, Mabley JG, Stachlewitz RF, Virag L, Lohinai Z, Southan GJ, Salzman AL and Szabo C. Inosine inhibits inflammatory cytokine production by a posttranscriptional mechanism and protects against endotoxin-induced shock. J Immunol. 2000;164:1013–1019. [DOI] [PubMed] [Google Scholar]
- 45.Grootveld M and Halliwell B. Measurement of allantoin and uric acid in human body fluids. A potential index of free-radical reactions in vivo? Biochem J. 1987;243:803–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Feig DI, Kang DH and Johnson RJ. Uric acid and cardiovascular risk. N Engl J Med. 2008;359:1811–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kamada Y, Nagaretani H, Tamura S, Ohama T, Maruyama T, Hiraoka H, Yamashita S, Yamada A, Kiso S, Inui Y, et al. Vascular endothelial dysfunction resulting from L-arginine deficiency in a patient with lysinuric protein intolerance. J Clin Invest. 2001;108:717–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Creager MA, Gallagher SJ, Girerd XJ, Coleman SM, Dzau VJ and Cooke JP. L-arginine improves endothelium-dependent vasodilation in hypercholesterolemic humans. J Clin Invest. 1992;90:1248–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moon J, Kim OY, Jo G and Shin MJ. Alterations in Circulating Amino Acid Metabolite Ratio Associated with Arginase Activity Are Potential Indicators of Metabolic Syndrome: The Korean Genome and Epidemiology Study. Nutrients. 2017;9:740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Patel RS, Ghasemzadeh N, Eapen DJ, Sher S, Arshad S, Ko YA, Veledar E, Samady H, Zafari AM, Sperling L, et al. Novel Biomarker of Oxidative Stress Is Associated With Risk of Death in Patients With Coronary Artery Disease. Circulation. 2016;133:361–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nieman DC, Meaney MP, John CS, Knagge KJ and Chen H. 9- and 13-Hydroxy-octadecadienoic acids (9+13 HODE) are inversely related to granulocyte colony stimulating factor and IL-6 in runners after 2h running. Brain Behav Immun. 2016;56:246–252.. [DOI] [PubMed] [Google Scholar]
- 52.Drogan D, Weikert C, Dierkes J, Klipstein-Grobusch K, Buijsse B, Mohlig M, Pfeiffer AF, Pischon T, Spranger J and Boeing H. Plasma gamma-glutamyltransferase, cysteinyl-glycine, and oxidized low-density lipoprotein: a pathway associated with myocardial infarction risk? Arterioscler Thromb Vasc Biol. 2010;30:2053–2058. [DOI] [PubMed] [Google Scholar]
- 53.Gu SX, Blokhin IO, Wilson KM, Dhanesha N, Doddapattar P, Grumbach IM, Chauhan AK and Lentz SR. Protein methionine oxidation augments reperfusion injury in acute ischemic stroke. JCI Insight. 2016;1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Danese E, Salvagno GL, Tarperi C, Negrini D, Montagnana M, Festa L, Sanchis-Gomar F, Schena F and Lippi G. Middle-distance running acutely influences the concentration and composition of serum bile acids: Potential implications for cancer risk? Oncotarget. 2017;8:52775–52782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Morville T, Sahl RE, Trammell SA, Svenningsen JS, Gillum MP, Helge JW and Clemmensen C. Divergent effects of resistance and endurance exercise on plasma bile acids, FGF19, and FGF21 in humans. JCI Insight. 2018;3:e122737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stanford KI, Lynes MD, Takahashi H, Baer LA, Arts PJ, May FJ, Lehnig AC, Middelbeek RJW, Richard JJ, So K, et al. 12,13-diHOME: An Exercise-Induced Lipokine that Increases Skeletal Muscle Fatty Acid Uptake. Cell Metab. 2018;27:1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kimura T, Sato K, Malchinkhuu E, Tomura H, Tamama K, Kuwabara A, Murakami M and Okajima F. High-density lipoprotein stimulates endothelial cell migration and survival through sphingosine 1-phosphate and its receptors. Arterioscler Thromb Vasc Biol. 2003;23:1283–1288. [DOI] [PubMed] [Google Scholar]
- 58.Deutschman DH, Carstens JS, Klepper RL, Smith WS, Page MT, Young TR, Gleason LA, Nakajima N and Sabbadini RA. Predicting obstructive coronary artery disease with serum sphingosine-1-phosphate. Am Heart J. 2003;146:62–68. [DOI] [PubMed] [Google Scholar]
- 59.Sattler KJ, Elbasan S, Keul P, Elter-Schulz M, Bode C, Graler MH, Brocker-Preuss M, Budde T, Erbel R, Heusch G, et al. Sphingosine 1-phosphate levels in plasma and HDL are altered in coronary artery disease. Basic Res Cardiol. 2010;105:821–832. [DOI] [PubMed] [Google Scholar]
- 60.Shah SH, Bain JR, Muehlbauer MJ, Stevens RD, Crosslin DR, Haynes C, Dungan J, Newby LK, Hauser ER, Ginsburg GS, et al. Association of a peripheral blood metabolic profile with coronary artery disease and risk of subsequent cardiovascular events. Circ Cardiovasc Genet. 2010;3:207–214. [DOI] [PubMed] [Google Scholar]
- 61.Mihalik SJ, Goodpaster BH, Kelley DE, Chace DH, Vockley J, Toledo FG and DeLany JP. Increased levels of plasma acylcarnitines in obesity and type 2 diabetes and identification of a marker of glucolipotoxicity. Obesity (Silver Spring). 2010;18:1695–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pallister T, Jackson MA, Martin TC, Zierer J, Jennings A, Mohney RP, MacGregor A, Steves CJ, Cassidy A, Spector TD, et al. Hippurate as a metabolomic marker of gut microbiome diversity: Modulation by diet and relationship to metabolic syndrome. Sci Rep. 2017;7:13670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shafi T, Meyer TW, Hostetter TH, Melamed ML, Parekh RS, Hwang S, Banerjee T, Coresh J and Powe NR. Free Levels of Selected Organic Solutes and Cardiovascular Morbidity and Mortality in Hemodialysis Patients: Results from the Retained Organic Solutes and Clinical Outcomes (ROSCO) Investigators. PLoS One. 2015;10:e0126048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, Feldstein AE, Britt EB, Fu X, Chung YM, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hung SC, Kuo KL, Wu CC and Tarng DC. Indoxyl Sulfate: A Novel Cardiovascular Risk Factor in Chronic Kidney Disease. J Am Heart Assoc. 2017;6:e005022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang Q, Ding Y, Song P, Zhu H, Okon I, Ding YN, Chen HZ, Liu DP and Zou MH. Tryptophan-Derived 3-Hydroxyanthranilic Acid Contributes to Angiotensin II-Induced Abdominal Aortic Aneurysm Formation in Mice In Vivo. Circulation. 2017;136:2271–2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pawlak K, Brzosko S, Mysliwiec M and Pawlak D. Kynurenine, quinolinic acid--the new factors linked to carotid atherosclerosis in patients with end-stage renal disease. Atherosclerosis. 2009;204:561–566. [DOI] [PubMed] [Google Scholar]
- 68.Lewis GD, Ngo D, Hemnes AR, Farrell L, Domos C, Pappagianopoulos PP, Dhakal BP, Souza A, Shi X, Pugh ME, et al. Metabolic Profiling of Right Ventricular-Pulmonary Vascular Function Reveals Circulating Biomarkers of Pulmonary Hypertension. J Am Coll Cardiol. 2016;67:174–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lavall D, Selzer C, Schuster P, Lenski M, Adam O, Schafers HJ, Bohm M and Laufs U. The mineralocorticoid receptor promotes fibrotic remodeling in atrial fibrillation. J Biol Chem. 2014;289:6656–6668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sabeti J, Nelson TE, Purdy RH and Gruol DL. Steroid pregnenolone sulfate enhances NMDA-receptor-independent long-term potentiation at hippocampal CA1 synapses: role for L-type calcium channels and sigma-receptors. Hippocampus. 2007;17:349–369. [DOI] [PubMed] [Google Scholar]
- 71.Rhee EP, Cheng S, Larson MG, Walford GA, Lewis GD, McCabe E, Yang E, Farrell L, Fox CS, O'Donnell CJ, et al. Lipid profiling identifies a triacylglycerol signature of insulin resistance and improves diabetes prediction in humans. J Clin Invest. 2011;121:1402–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nishimukai M, Maeba R, Yamazaki Y, Nezu T, Sakurai T, Takahashi Y, Hui SP, Chiba H, Okazaki T and Hara H. Serum choline plasmalogens, particularly those with oleic acid in sn-2, are associated with proatherogenic state. J Lipid Res. 2014;55:956–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Graessler J, Schwudke D, Schwarz PE, Herzog R, Shevchenko A and Bornstein SR. Top-down lipidomics reveals ether lipid deficiency in blood plasma of hypertensive patients. PloS one. 2009;4:e6261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cheng S, Rhee EP, Larson MG, Lewis GD, McCabe EL, Shen D, Palma MJ, Roberts LD, Dejam A, Souza AL, et al. Metabolite profiling identifies pathways associated with metabolic risk in humans. Circulation. 2012;125:2222–2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lewis GD, Shah RV, Pappagianopolas PP, Systrom DM and Semigran MJ. Determinants of ventilatory efficiency in heart failure: the role of right ventricular performance and pulmonary vascular tone. Circ Heart Fail. 2008;1:227–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Brittain EL, Talati M, Fessel JP, Zhu H, Penner N, Calcutt MW, West JD, Funke M, Lewis GD, Gerszten RE, et al. Fatty Acid Metabolic Defects and Right Ventricular Lipotoxicity in Human Pulmonary Arterial Hypertension. Circulation. 2016;133:1936–1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wanders RJ, Komen J and Kemp S. Fatty acid omega-oxidation as a rescue pathway for fatty acid oxidation disorders in humans. FEBS J. 2011;278:182–194. [DOI] [PubMed] [Google Scholar]
- 78.Heresi GA, Mey JT, Bartholomew JR, Haddadin IS, Tonelli AR, Dweik RA, Kirwan JP and Kalhan SC. Plasma metabolomic profile in chronic thromboembolic pulmonary hypertension. Pulm Circ. 2020;10:2045894019890553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang J, Light AR, Hoppel CL, Campbell C, Chandler CJ, Burnett DJ, Souza EC, Casazza GA, Hughen RW, Keim NL, et al. Acylcarnitines as markers of exercise-associated fuel partitioning, xenometabolism, and potential signals to muscle afferent neurons. Exp Physiol. 2017;102:48–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Muhsen Ali A, Burleigh M, Daskalaki E, Zhang T, Easton C and Watson DG. Metabolomic Profiling of Submaximal Exercise at a Standardised Relative Intensity in Healthy Adults. Metabolites. 2016;6:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Enea C, Seguin F, Petitpas-Mulliez J, Boildieu N, Boisseau N, Delpech N, Diaz V, Eugene M and Dugue B. (1)H NMR-based metabolomics approach for exploring urinary metabolome modifications after acute and chronic physical exercise. Anal Bioanal Chem. 2010;396:1167–1176. [DOI] [PubMed] [Google Scholar]
- 82.Sanford JA, Nogiec CD, Lindholm ME, Adkins JN, Amar D, Dasari S, Drugan JK, Fernandez FM, Radom-Aizik S, Schenk S, et al. Molecular Transducers of Physical Activity Consortium (MoTrPAC): Mapping the Dynamic Responses to Exercise. Cell. 2020;181:1464–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lira FS, Pimentel GD, Santos RV, Oyama LM, Damaso AR, Oller do Nascimento CM, Viana VA, Boscolo RA, Grassmann V, Santana MG, et al. Exercise training improves sleep pattern and metabolic profile in elderly people in a time-dependent manner. Lipids Health Dis. 2011;10:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kodama S, Saito K, Tanaka S, Maki M, Yachi Y, Asumi M, Sugawara A, Totsuka K, Shimano H, Ohashi Y, et al. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: a meta-analysis. JAMA. 2009;301:2024–2035. [DOI] [PubMed] [Google Scholar]
- 85.Marshall CH, Al-Mallah MH, Dardari Z, Brawner CA, Lamerato LE, Keteyian SJ, Ehrman JK, Visvanathan K and Blaha MJ. Cardiorespiratory fitness and incident lung and colorectal cancer in men and women: Results from the Henry Ford Exercise Testing (FIT) cohort. Cancer. 2019;125:2594–2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Paynter NP, Balasubramanian R, Giulianini F, Wang DD, Tinker LF, Gopal S, Deik AA, Bullock K, Pierce KA, Scott J, Martinez-Gonzalez MA, Estruch R, Manson JE, Cook NR, Albert CM, Clish CB and Rexrode KM. Metabolic Predictors of Incident Coronary Heart Disease in Women. Circulation. 2018;137:841–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.