Abstract
Gln3 activates Nitrogen Catabolite Repression, NCR-sensitive expression of the genes required for Saccharomyces cerevisiae to scavenge poor nitrogen sources from its environment. The global TorC1 kinase complex negatively regulates nuclear Gln3 localization, interacting with an α-helix in the C-terminal region of Gln3, Gln3656–666. In nitrogen replete conditions, Gln3 is sequestered in the cytoplasm, whereas when TorC1 is down-regulated, in nitrogen restrictive conditions, Gln3 migrates into the nucleus. In this work, we show that the C-terminal Gln3–Tor1 interaction site is required for wild type, rapamycin-elicited, Sit4-dependent nuclear Gln3 localization, but not for its dephosphorylation. In fact, truncated Gln31-384 can enter the nucleus in the absence of Sit4 in both repressive and derepressive growth conditions. However, Gln31-384 can only enter the nucleus if a newly discovered second positively-acting Gln3–Tor1 interaction site remains intact. Importantly, the N- and C-terminal Gln3–Tor1 interaction sites function both autonomously and collaboratively. The N-terminal Gln3–Tor1 interaction site, previously designated Gln3URS contains a predicted α-helix situated within an unstructured coiled-coil region. Eight of the thirteen serine/threonine residues in the Gln3URS are dephosphorylated 3–15-fold with three of them by 10–15-fold. Substituting phosphomimetic aspartate for serine/threonine residues in the Gln3 URS abolishes the N-terminal Gln3–Tor1 interaction, rapamycin-elicited nuclear Gln3 localization, and ½ of the derepressed levels of nuclear Gln3 localization. Cytoplasmic Gln3 sequestration in repressive conditions, however, remains intact. These findings further deconvolve the mechanisms that achieve nitrogen-responsive transcription factor regulation downstream of TorC1.
Keywords: Gln3, Sit4, URS, TOR complex (TorC1), Nitrogen metabolism, Signal transduction, Transcription factors
Introduction
GATA-family Gln3 and Gat1 are the major transcription activators responsible for Nitrogen Catabolite Repression (NCR)-sensitive gene expression. In low-nitrogen conditions, these activators enter the nucleus and activate transcription of genes encoding transporters and enzymes required for Saccharomyces cerevisiae cells to scavenge their environment for any poor/non-preferred nitrogen sources that might be available (e.g. allantoin or proline) (Figure 1A). In luxurious nitrogen conditions (e.g. abundant amino acids, especially glutamine) found in decaying fruits and vegetables, Gln3 and Gat1 exit from the nucleus, thereby repressing NCR-sensitive transcription (Cooper 1982, 2002, 2004; Hofman-Bang 1999; Magasanik and Kaiser 2002; Broach 2012; Ljungdahl and Daignan-Fornier 2012; Conrad et al. 2014; Swinnen et al. 2014; González and Hall 2017; Zhang et al. 2018).
Figure 1.

Regulation of intracellular Gln3 by environmental conditions, regulatory proteins and inhibitors. (A) Green text indicates molecules or growth conditions that elicit or are required for nuclear Gln3 localization. Red text indicates molecules or growth conditions that prevent nuclear Gln3 localization. Green arrows and red bars indicate positive and negative regulation, respectively. (B) Phosphatase requirements for each condition to elicit nuclear Gln3 localization. A more detailed diagram appears in Figure 11 of Tate et al.(2019).
Gln3 responds to growth conditions, loss of global nitrogen regulators, or inhibitors
The above intracellular Gln3 localization has been extensively studied and shown to respond distinctly to multiple growth and experimental nitrogen conditions, including (Figure 1A and Figure 11 in Tate et al. 2019): (i) growth in glutamine vs. proline (Rai et al. 2013), (ii) short and long-term nitrogen starvation (Tate and Cooper 2013), (iii) the loss of its upstream regulators, TorC1, Gcn2 (Cherkasova and Hinnebusch 2003; Hinnebusch 2005; Staschke et al. 2010; Lageix et al. 2015; Rai et al. 2015; Tate et al. 2017; Yuan et al. 2017), the Sit4 and PP2A phosphatases (DiComo and Arndt 1996; Beck and Hall 1999; Jiang and Broach 1999; Bertram et al. 2000; Wang et al. 2003; Tate et al. 2006, 2009, 2010, 2013, 2015, 2019; Yan et al. 2006; Georis et al. 2011), 14-3-3 proteins Bmh1/2 (Tate et al. 2017), the nitrogen network kinase Nnk1 (Breitkreutz et al. 2010), negative regulator Ure2 needed to complex with Gln3 and sequester it in the cytoplasm in nitrogen replete conditions (Lacroute 1971; Drillien and Lacroute 1972; Drillien et al.1973; Cooper 1982, 2004; Courchesne and Magasanik 1988; Coffman et al. 1994; Blinder et al. 1996; Beck and Hall 1999; Cardenas et al. 1999; Cunningham et al. 2000; Kulkarni, et al. 2001; Magasanik and Kaiser 2002; Feller et al. 2013) and (iv) treating cells with the Tor1 kinase and glutamine synthetase inhibitors rapamycin (Rap) and methionine sulfoximine (Msx), respectively (Figure 1A) (Beck and Hall 1999; Bertram et al. 2000; Crespo et al. 2002; Kulkarni et al. 2006; Georis et al. 2011; Rai et al. 2015).
Figure 11.
Two-hybrid assessment of Tor1 association with truncated forms of Gln3. (A) Diagram of Gln3 indicating the end points of sequences present in each of the truncation mutants. (B) Locations of the Tor1 domains. (C) Strain PJ69-4a was transformed with pRR1067 (Gln31-600), pRR1122 (Gln31-476), pRR1120 (Gln31-400), pRR1118 (Gln31-350), pRR1149 (Gln31-240), pRR1147 (Gln31-158), or pRR1145 (Gln31-99). All of these plasmids consisted of the Gal4 activation domain fused to the N-terminus of the respective Gln3 proteins. pJ69-4a transformed with pAS2-1 and pACT2 served as a negative control. pJ69-4a, transformed with both pAV3-1 and pTD1-1, served as a positive control (D). All transformants were tested for Gln3 interaction with full length Tor1 (pASTOR11-2470) or Tor1-1764 (pASTOR11-1764) by streaking cells on the same medium in the presence or absence of 3 mM 3-aminotriazole (3AT). The assays were performed as described in the section “Materials and Methods.”
Gln3 regulation by TorC1, Sit4, and PP2A phosphatases
Gln3 responses to these growth and experimental conditions show specific phosphatase requirements (Tate and Cooper 2013) (Figure 1B). Nuclear Gln3 entry elicited by rapamycin inhibition of TorC1 requires both Sit4 and PP2A (Figure 1B). In contrast, only Sit4 is required for nuclear entry in low nitrogen, i.e. short-term (one to four or five hours depending on the strain) nitrogen starvation or growth in a poorly transported or catabolized nitrogen source. Responses to long-term starvation (six to ten hours, the starvation time needed for cells to G1 arrest) and Msx treatment require neither Sit4 nor PP2A.
Clearly, control of Sit4 and PP2A activities are central to the NCR-sensitive responses. In excess nitrogen TorC1, one of two global nitrogen-responsive regulators, is active and phosphorylates Tor Associated Protein 42 (Tap42) (DiComo and Arndt 1996) (Figure 11 in Tate et al. 2019). Phosphorylated Tap42 binds to TorC1 and both phosphatases. In this configuration, the phosphatases are bound to TorC1 and inactive. Low environmental nitrogen, limited ability to metabolize available nitrogen or rapamycin inhibition of TorC1 releases the Tap42-phosphatase complexes thereby resulting in their activation and ability to dephosphorylate Gln3. Gln3 dissociates from Ure2 and enters the nucleus (Jiang and Broach 1999; Yan et al. 2006).
Complexity of Gln3 regulation
Gln3 phosphorylation/dephosphorylation-mediated regulation, however, is not as straightforward as implied by the abbreviated description above (see Figure 11 in Tate et al. 2019). Three observations exemplify this complexity: (i) Nuclear Gln3 is much more highly phosphorylated than when it is in the cytoplasm under all conditions except when cells are treated with rapamycin. (ii) Gln3 is dephosphorylated in a Sit4- and PP2A-dependent manner when it exits from the nucleus in response to excess nitrogen. (iii) Active Sit4, PP2A, and Ure2 are all required for Gln3 dephosphorylation that occurs when it is sequestered in the cytoplasm of cells cultured in nitrogen replete medium (Figure 11 in Tate et al. 2019). It is important to note that the latter two observations show that Sit4 and PP2A are active in nitrogen-excess as well as nitrogen-limited conditions. The suggested explanation for this apparently paradoxical conclusion is the existence of two phosphatase pools: one associated with Tap42-TorC1 interactions and a second one that is not. Only <5% of Sit4 and <2% of PP2A are associated with and thereby inactivated by TorC1 in excess nitrogen conditions (DiComo and Arndt 1996).
Identification of Gln3 functional and regulatory domains
To gain a greater understanding of Gln3's complex regulation we deconvolved and genetically isolated its various functional domains and branches of its regulation. The approach we used depended on the hypothesis that specific Gln3 residues were associated with each mode of its regulation (Rai et al. 2013). Testing that hypothesis, however, was considered to be improbable given that Gln3 is an exquisitely labile, large protein (730 amino acids), present in very small quantities and consisting of ∼20% (146) serine and threonine residues. The task would likely have been unachievable had it not been for the fact that Gln3 is predicted to be a highly disordered protein containing only a few areas of predicted secondary structures in the absence of other proteins (Tate et al. 2018). This characteristic permitted us and others to genetically identify residues associated with specific Gln3 functions and responses to one experimental perturbation without affecting others (Figure 2A). This included domains for: nuclear export (NES), transcriptional activation, Ure2-binding, an uncharacterized Ure2 Relief Sequence (URS), nuclear entry (two NLSs), DNA-binding, cytoplasmic sequestration in excess nitrogen, rapamycin responsiveness, and Gln3–Tor1 interaction (Carvalho et al. 2001; Kulkarni et al. 2001; Carvalho and Zheng 2003; Rai et al. 2013, 2014, 2015, 2016; Tate et al. 2018).
Figure 2.
Comparison of rapamycin-elicited Gln3-Myc13 mutant protein localization and phosphorylation. (A) Diagram depicting Gln3 control elements, their amino acid positions and the C-terminal regions containing mutations found in plasmids pRR850, pRR1045 and pRR680. The substituted residues in these constructs are shown below the domain diagram. (B) This panel, with exception of the western blot of pRR1045, contains summaries of previously published experiments as noted in the right column of the panel. Electrophoretic mobility and localization of Gln3-Myc13 were determined using transformants of wild type strain JK9-3da. JK9-3da cells were transformed with the pRR536 (Wild type), pRR850, pRR1045 or pRR680. Transformants were grown in YNB-glutamine (Gln) medium to mid-log phase followed by treatment with 200 ng/ml rapamycin (+Rap) for 20 minutes. 5 mls of cells were collected prior to and after rapamycin addition for indirect immunofluorescent microscopy as described in the section “Materials and Methods.” Cultures were also collected for protein extraction and western blot analysis. See the section “Materials and Methods” for protocols.
Incongruency of rapamycin-elicited nuclear Gln3-Myc13 localization and phosphorylation
As the above work evolved, we and others showed four conditions that yield highly nuclear Gln3-Myc13 localization: (i) long-term nitrogen starvation,(ii) loss of Ure2, (iii) expression of a Nuclear Export Sequence (NES) deficient gln3 allele, and (iv) treatment with the glutamine synthetase inhibitor, methionine sulfoximine (Msx) (Beck and Hall 1999; Bertram et al. 2000; Crespo et al. 2002; Tate et al. 2002, 2017, 2019; Cox et al. 2002, 2003, 2004; Tate and Cooper 2013; Rai et al. 2015;). In all these conditions, Gln3-Myc13 (or NES-deficient Gln3-Myc13) is hyper-phosphorylated (Figure 11 in Tate et al. 2019). In contrast, when TorC1 is inhibited with rapamycin, Gln3-Myc13 also relocates to the nucleus, but is hypo-phosphorylated in this condition (Beck and Hall 1999; Bertram et al. 2000). In other words, nuclear localization and phosphorylation levels did not always correlate.
Phenotypes of Gln3 regulatory mutants
The above paradox grew further as we sequentially identified Gln3 domains required for specific responses to environmental conditions. In short, rapamycin dephosphorylated Gln3-Myc13 irrespective of the regulatory element abolished and importantly regardless of the cellular location of Gln3-Myc13 (Figure 2B). Figure 2B, with exception of the northern blot (pRR1045), contains a summary of previously published data as noted in the right-hand column of the figure. In wild type, glutamine-grown cells, rapamycin elicited relocation of Gln3-Myc13 from the cytoplasm to a tripartite distribution in which Gln3 is roughly equally distributed to all three scoring categories, cytoplasmic, nuclear-cytoplasmic, and nuclear (Figure 2B, pRR536, wild type). This treatment also resulted in dephosphorylation of Gln3-Myc13.
Serine to aspartate substitutions in the Gln3–Tor1 interaction site (Gln3656–666) abolished one half of Gln3-Myc13 cytoplasmic sequestration in nitrogen-rich medium, as well as any response to rapamycin addition (Figure 2B, pRR850, Gln3S656D, S659D, S662D-Myc13; Rai et al. 2013). Nuclear Gln3 localization was only half repressed. Nonetheless, rapamycin-treatment led to dephosphorylation of the mutant protein.
Serine to aspartate substitutions in the Gln3 rapamycin response site (Figure 2A, Gln3505–594) also abolished rapamycin-elicited Gln3-Myc13 nuclear localization (Figure 2B, pRR1045, Gln3T526D, T527D, S531D, S535D, S538D, S539D-Myc13; Rai et al. 2014). However, in contrast to the protein expressed from pRR850, cytoplasmic Gln3-Myc13 sequestration was unaffected in repressive YNB-glutamine medium. This was expected as the Gln3–Tor1 interaction remained intact. What was not expected was that rapamycin dephosphorylated the mutant protein (Figure 2B, pRR1045).
Finally, alterations in the cytoplasmic sequestration site (Figure 2A, Gln3477-493) abolished one half of the cytoplasmic Gln3-Myc13 localization resulting in a tripartite distribution (Figure 2B, pRR680, Gln3 S479A, S480A, S483A, S484A, S486A, S487A, S490A, S491A, S492A, S493A, S495A, S496A, S497A-Myc13; Rai et al. 2016). However, in this case, rapamycin addition did result in increased nuclear localization. It is pertinent to note that combining the Gln3 substitutions contained in pRR850 and pRR680 completely abolishes cytoplasmic Gln3-Myc13 sequestration in nitrogen-rich, repressive medium, i.e. their effects were additive (Rai et al. 2016). However, the molecular mechanism through which the combined substitutions achieve this additivity is unknown. Together these experiments further showed that rapamycin-elicited Gln3-Myc13 dephosphorylation was not indicative of its localization. They indicated that the structural requirements for nuclear Gln3-Myc13 localization and hypo-phosphorylation differed and were genetically separable. These incongruencies also correlated with a previous report showing that rapamycin-elicited Gln3-Myc13 hypo-phosphorylation was insufficient to affect its nuclear localization in a YNB-glutamine-grown pph21Δ,pph22Δ mutant (Tate et al. 2009).
Present research objectives
With this foundation of information, we set about to investigate the locus of rapamycin mediated Gln3 dephosphorylation. We focused on the N-terminal region of Gln3 because our previous work centered on its C-terminus and hence little was known about the role of the N-terminal area in Gln3 control. This work supports the conclusion that Gln3 contains two Gln3–Tor1 interaction sites, one N-terminal and the other C-terminal. The N-terminal site is situated in the previously identified Ure2 Relief Sequence (URS). The two sites act both autonomously and collaboratively. Specific serine/threonine residues in the N-terminal site are dephosphorylated in response to rapamycin treatment and substituting phosphomimetic aspartate residues for them eliminates nuclear Gln3-Myc13 localization and Gln3–Tor1 interaction.
Materials and Methods
Strains and culture conditions
The S. cerevisiae strains we used in this work are in Table 1. Transformants, prepared by the lithium acetate method (Ito et al. 1983), were used as soon as possible after transformation (5 or less days).
Table 1.
Strains used in this work
| Strain | Genotype | Reference |
|---|---|---|
| JK9-3da | MATa, leu2-3,112, ura3-52, trp1, his4, rme1, HMLa | Rai et al. (2013) |
| FV029 | MATa, leu2-3,112, ura3-52, trp1, his3, rem1, HMLa, sit4::natMX | Georis et al. (2008) |
| PJ69-4a | Mata, trp1-901, leu2-3,112, ura3-52, his3–200, gal4Δ,gal80Δ, LYS2::GAL1-HIS3, GAL2-ADE2,met2::GAL7-lacZ | Bertram et al. (2000); James et al. (1996) |
Cultures (50 ml) were grown to mid-log phase (A600 nm∼ 0.5) in Yeast Nitrogen Base (YNB, without amino acids or ammonia; VWR Life Science AMRESCO) minimal medium containing the indicated nitrogen source (final concentration, 0.1%). Leucine (120 µg/ml), histidine (20 µg/ml), lysine (40 µg/ml), tryptophan (20 µg/ml), and methionine (20 µg/ml) were added as needed to cover auxotrophic requirements. Cells were treated with 200 ng/ml rapamycin (Sigma) for 15 or 20 minutes or 2 mM methionine sulfoximine (Msx; Sigma) for 30 minutes (Georis et al. 2011).
Plasmid construction and protein–protein interaction assays
Construction and characterization of the Gln3-Myc13 plasmids used in this work may be found as indicated in Table 2. The Gln3 two-hybrid prey plasmids were constructed as described by Rai et al. (2013) using primer sets in Table 3 and interaction vector pACT-2 (Clonetech) which contains the SV-40 T-antigen NLS cloned into the 5’ end of the GAL4AD sequence. Structures of all constructs were verified by restriction mapping and DNA sequence analyses. All plasmids, excluding prey plasmids, contained Gln3 under its wild type native promoter.
Table 2.
Plasmids used in this work
| Plasmid | Residue alterations | Reference |
|---|---|---|
| pRR620 | Gln31-350 | Tate et al. (2018) |
| pRR536 | Gln31-730-Myc13 (full length wild type) | Rai et al. (2014) |
| pRR610 | Gln31-476-Myc13 | Tate et al. (2018) |
| pRR611 | Gln31-384-Myc13 | Tate et al. (2018) |
| pRR612 | Gln31-400-Myc13 | Tate et al. (2018) |
| pRR680 | Gln3 S479A, S480A, S483A, S484A, S486A, S487A, S490A, S491A, S492A, S493A, S495A, S496A, S497A-Myc13 | Rai et al. (2016) |
| pRR754 | Gln3R264D, K265D, S267D | Tate et al. (2018) |
| pRR772 | Gln3K281D, R282D, S285D | Tate et al. (2018) |
| pRR850 | Gln3S656D, S659D, S662D-Myc13 | Rai et al. (2013) |
| pRR1045 | Gln3T526D, T527D, S531D, S535D, S538D, S539D-Myc13 | Rai et al. (2014) |
| pRR1067 | * Gln31-600 | Rai et al. (2013) |
| pRR1118 | * Gln31-350 | This work |
| pRR1120 | * Gln31-400 | This work |
| pRR1122 | * Gln31-476 | This work |
| pRR1124 | * Gln3R264D, K265D, S267D | This work |
| pRR1128 | * Gln3K281D, R282D, S285D | This work |
| pRR1145 | * Gln31-99 | This work |
| pRR1147 | * Gln31-158 | This work |
| pRR1149 | * Gln31-240 | This work |
| pRR1366 | Gln3S249D, S251D, S256D, S257D-Myc13 | Tate et al. (2018) |
| pRR1368 | Gln3S267D, S273F, S274D, T275D, S276D-Myc13 | Tate et al. (2018) |
| pAS2-1 | GAL4 BD fusion vector | Carvalho and Zheng (2003) (Clonetech) |
| pASTOR | ** Tor11-2470 (Full length wild type) | Carvalho and Zheng (2003) |
| pASTOR1 | ** Tor1-1764 | Carvalho and Zheng (2003) |
| pACT2 | GAL4 AD fusion vector | Carvalho and Zheng (2003) (Clonetech) |
| pAV3-1 | (positive control) | X.F.S. Zheng |
| pTD1-1 | SV40 large T antigen (positive control) | Carvalho and Zheng (2003) (Clontech) |
Gal4-activation domain prey fusion protein
Gal4-binding domain bait fusion protein
Table 3.
Primers used in this work
| Plasmid | Gln3alterations | Primers |
|---|---|---|
| pRR1118 | * Gln31-350 |
5′-CATGCCATGGAGATGCAAGACGACCCCGAAAATTCGAAGCTG-3′ 5′-CGCGGATCCGATAACGTCCGATTTTAAGGATAATGGCC-3′ pRR620 used as template DNA |
| pRR1120 | * Gln31-400 |
5′-CATGCCATGGAGATGCAAGACGACCCCGAAAATTCGAAGCTG-3′ 5′-CGCGGATCCAGAGTTTTGTTGTAGTGATTTTTTCCTCGA-3′ pRR612 used as template DNA |
| pRR1122 | * Gln31-476 |
5′-CATGCCATGGAGATGCAAGACGACCCCGAAAATTCGAAGCTG-3′ 5′-CGCGGATCCAAAATTAGGAGTAACAGTGTTCGAATTGTG-3′ pR610 used as template DNA |
| pRR1124 | * Gln3R264D, K265D, S267D |
5′-CATGCCATGGAGATGCAAGACGACCCCGAAAATTCGAAGC-3′ pRR754 used as template DNA |
| pRR1128 | * Gln3K281D, R282D, S285D |
5′-CATGCCATGGAGATGCAAGACGACCCCGAAAATTCGAAGCTG-3′ 5′-CGCGGATCCGATAACGTCCGATTTTAAGGATAATGGCC-3′ pRR772 used as template DNA |
| pRR1145 | * Gln31-99 |
5′-CATGCCATGGAGATGCAAGACGACCCCGAAAATTCGAAGCTG-3′ 5′-CGCGGATCCGAGCTGGTGTGGTGTCCTTGACCGTCTTAG-3′ pRR536 used as template DNA |
| pRR1147 | * Gln31-158 |
5′-CATGCCATGGAGATGCAAGACGACCCCGAAAATTCGAAGCTG-3′ 5′-CGCGGATCCGAGGAGCACTAATAGTAGCTGAACG-3′ pRR536 used as template DNA |
| pRR1149 | * Gln31-240 |
5′-CATGCCATGGAGATGCAAGACGACCCCGAAAATTCGAAGCTG-3′ 5′-CGCGGATCCGATTACTATTGTTGCTATTATTATTATTGG-3′ pRR536 used as template DNA |
Plasmids used in 2-hybrid analyses
The protein–protein interaction assay protocols, including strain PJ69-4a and 2-hybrid assay plasmids, with exception of those we constructed, were provided by Bertram et al. (2000) and Carvalho and Zheng (2003). Gal4p-BD and AD fusion proteins were expressed together in yeast strain PJ69-4a. This strain was constructed in the W303 genetic background. Growth of PJ69-4a transformants was measured on plates of synthetic complete medium (SC) lacking leucine, tryptophan, and adenine as the positive control or synthetic complete medium lacking leucine, tryptophan, and histidine plus 3 mM (final concentration) 3- amino-1,2,4-triazole (3AT) to assess whether or not a positive interaction occurred.
Bioinformatic analyses
Saccharomyces cerevisiae Gln3 sequence YER040W, and S. castellii (NRRL-Y12630_AACF01000000), were from the Saccharomyes Genome Data Base (SGD). Sequences for S. paradoxus (AABY01000062.1), S. mikatae (AABZ01000001.1), S. kudriavzevii (AACI02000852.1), S. bayanus (AACG02000070.1), S. pastorianus (ABPO01000005.1), S. (AACF01000032.1), Vanderwaltozyma polyspora (XM001643411.1), and Kluyveromyces waltii (AADM01000165.1) were from the Whole Genome Shotgun (WGS) section of NCBI. The sequences for Kluyveromyces thermotolerans (XM_002555052.1), Kluyveromyces lactis (GCF_000002515.2), Zygosaccharomyces rouxii (GCA_900411385.1), Candida Galbrata (GCA_002219185.1), Naumovozyma castellii (GCA_000237345.1), and Ashbya gossypii (GCA_000091025.4) were from GenBank. These sequences were found by Blast searching these databases, beginning with yeast GLN3 as the query sequence.
Gln3-Myc13 localization and image processing
Cell collection and Gln3-Myc13 visualization by indirect immunofluorescence microscopy were performed as described (Feller et al. 2013; Tate et al. 2019). Microscopic images for presentation were prepared using Adobe Photoshop and Illustrator programs. Level settings (shadow and highlight only) were altered where necessary to avoid any change or loss in cellular detail relative to that observed in the microscope; changes were applied uniformly to the image presented and were similar from one image to another. Mid-tone, gamma settings were never altered. These processed images were used for illustrative presentation only, NOT for scoring Gln3-Myc13 intracellular distributions.
Determination of intracellular Gln3-Myc13 localization
Gln3-Myc13 intracellular localization was manually scored in 200 or more cells for each data point. Unaltered, primary .zvi image files viewed with Zeiss AxioVision 3.0 and 4.8.1 software were exclusively used for scoring purposes. Cells were classified into one of three categories: cytoplasmic (cytoplasmic fluorescent material only, red histogram bars), nuclear-cytoplasmic (fluorescent material appears in both the cytoplasm and co-localizing with DAPI-positive material, DNA, yellow bars), or nuclear (fluorescent material co-localizing only with DAPI-positive material, green bars). Representative “standard” images and detailed descriptions of these categories appear in Figure 2 of Tate et al. 2009. The precision of our scoring has been repeatedly documented (Tate et al. 2006b; 2010; Rai et al. 2013; 2014).
In this work, standard deviations of data from independent experiments appear as error bars. Greatest variation was observed when Gln3-Myc13 was significantly localized to more than one cellular compartment and with the truncated proteins.
Images accompanying the histograms were chosen on the basis that they showed intracellular Gln3-Myc13 distributions as close as possible to those observed by quantitative scoring. However, identifying a field that precisely reflected the more quantitative scoring data was sometimes difficult unless the tagged protein was situated in a single cellular compartment.
Cell collection for western blot analysis
These methods were reproduced from Tate et al. (2019). Cultures were grown to mid-log phase (A600nm = 0.4–0.5) as described above. Once the desired A600nm was reached, or following treatment, the cells were harvested by filtration (using type HA, 0.45-mm Millipore filter), quickly scraped from the filter, placed in a sterile 1.5-ml microcentrifuge tube, and flash-frozen by submerging the microcentrifuge tube and cells in liquid nitrogen for 20–30 sec. The total time for cell harvest to the point of submersion in liquid nitrogen was 25– 35 sec. The tube, still containing liquid nitrogen, was then quickly transferred to –80°C until further processing of the cells was performed.
Protein extraction and western blot analysis
These methods were reproduced from Tate et al. (2019). Extracts for western blots were prepared following the method of Liu et al. 2008. Total protein was extracted by lysing cells in a solution of 0.3 N NaOH, 1.2% β-mercaptoethanol (final concentrations), on ice for 10 min. Protein was then precipitated with trichloroacetic acid (TCA) at a final concentration of 8%, for an additional 10 min on ice. Precipitated protein pellets were then resuspended in 1x sodium dodecyl sulfate (SDS) loading buffer and the extract neutralized with 1 M unbuffered Tris. Crude extracts were then boiled, protein resolved by SDS-PAGE (6 or 7% polyacrylamide) and transferred to nitrocellulose membrane (Bio-Rad) in non-SDS containing buffer. Gels were loaded to optimize comparison of the levels of phosphorylation shown by the proteins, not the levels of proteins produced. The estimated protein concentrations loaded on to the gels differed by less than twofold when a wild type recipient was used, and two- to fourfold when a sit4Δ recipient was used compared to wild type recipient.
Membranes were blocked for 1 hr at room temperature with 5% Carnation milk in 1x TTBS (20 mM Tris-HCL pH 7.5, 0.05% Tween20, 0.5 M NaCl). Membranes were then incubated overnight at room temperature with 9E10 (c-Myc) monoclonal antibody (sc-40; Santa Cruz Biotechnology) at a dilution of 1:1000 in 1x TBS (20 mM Tris-HCL pH 7.5, 0.5 M NaCl) plus 0.25% gelatin. Membranes were washed with 1x TBS and incubated with goat anti-mouse IgG (H + L)-horseradish peroxidase conjugate antibody (Bio-Rad) at a dilution of 1:10,000 for 1 hr in 1x TBS containing 0.005% Tween20 and 0.25% gelatin. Membranes were washed with 1x TBS containing 0.005% Tween20 buffer. Immunoreactive species were detected using the SuperSignal West Pico Chemiluminescent Substrate kit (ThermoScientific) following the manufacturer instructions and results recorded on Classic blue autoradiography Film BX (Midwest Scientific).
Phosphoproteomic analyses
The detailed methods and data set for the phosphoproteomic analyses appear in Hu et al. (2019).
Results
Gln3-Myc13 truncation abolishes its rapamycin-elicited and Sit4-dependent localization
Amino acids substituted in the Gln3 mutant proteins described in Figure 2B were situated in the C-terminal half of Gln3. Therefore, we investigated the effects of completely removing the domains containing them with C-terminal truncations. To this end, we assayed the responses to rapamycin and derepressive nitrogen conditions on the localization of truncated Gln3-Myc13 molecules and the effects of abolishing Sit4 phosphatase on that localization (Figure 3).
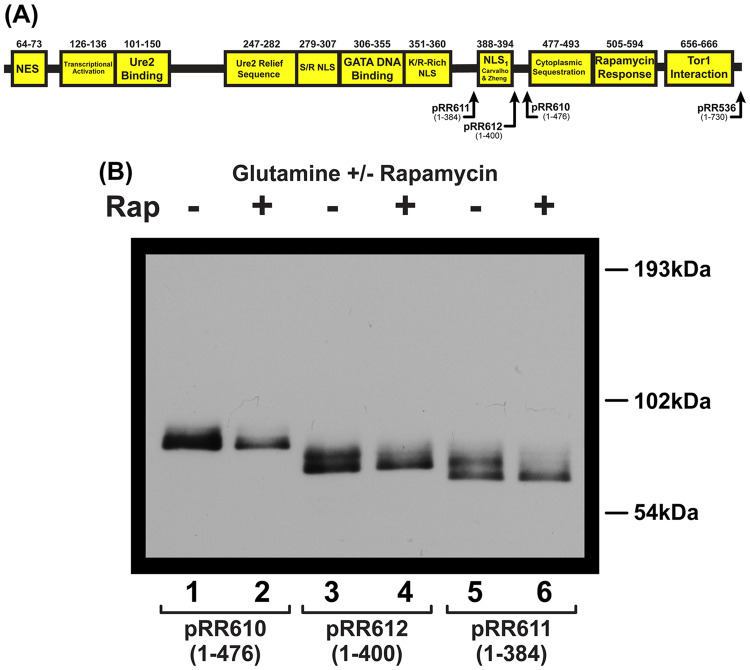
Figure 3.
Effects of C-terminal truncations on Gln3-Myc13 localization in wild type and sit4Δ cells. (A) Diagram depicting the C-terminus of each Gln3-Myc13 C-terminal truncation. (B–E) Wild type (JK9-3da) and sit4Δ (FV029) cells were transformed with plasmids encoding full length, wild type Gln31-730-Myc13 (pRR536) or Gln31-476-Myc13 (pRR610). Cells were grown to mid-log phase in YNB-glutamine (Gln), or -proline (Pro) medium. Rapamycin (200 ng/ml) was added to glutamine-grown cells where indicated (+Rap) for 15 minutes. (B and D) Illustrative images of Gln3-Myc13 localization under the growth conditions scored in the historgrams. Gln3-Myc13 localization was scored as cytoplasmic (red bars), nuclear-cytoplasmic (yellow bars; fluorescent material appearing in both the cytoplasm and colocalizing with DAPI-positive material, DNA) or nuclear (green bars; fluorescent material colocalizing only with DAPI-positive material). (C and E) Histograms depicting the percentage of Gln3 localized to each cellular compartment. Experimental format and scoring criteria were as described in the section “Materials and Methods.” Histogram values represent the averages derived from two biological replicates; error bars indicate standard deviations.
Gln3-Myc13 localization was rapamycin-responsive, NCR-sensitive and completely Sit4-dependent in transformants containing full length, wild type Gln31-730-Myc13 pRR536 (Figure 3, A–C). Analogous experiments were then performed with glutamine-grown, wild type and sit4Δ transformation recipients containing the individual truncation plasmids: Gln31-476-Myc13 (pRR610), Gln31-400-Myc13 (pRR612) and Gln31-384-Myc13 (pRR611) (Figure 3D and E; Figure 4).
Figure 4.

Effects of C-terminal truncations on Gln3-Myc13 localization in wild type and sit4Δ cells. Wild type (JK9-3da) and sit4Δ (FV029) cells were transformed with pRR612 (Gln31-400-Myc13), or pRR611 (Gln31-384-Myc13). Experimental design and data presentation were as described in Figure 3.
All of the gln3 mutant transformants lost about half of their cytoplasmic localization in repressive glutamine medium as previously documented when the C-terminal Gln3–Tor1 interaction site (Gln3656–666) was abolished (Rai et al. 2013). None of the mutant gln3 transformants responded to rapamycin (Figure 3, B–E; Figure 4). A tripartite distribution, with Gln3-Myc13 more-or-less uniformly distributed among the three scoring categories, was observed with untreated and treated, wild type and sit4Δ recipients. As noted earlier, such a tripartite intra-cellular Gln3 distribution is characteristic of cells in which one of two major Gln3 regulatory pathways is abolished (Rai et al. 2013, 2014, 2016; Tate et al. 2017). These experiments yielded two important observations: (i) nuclear Gln3-Myc13 localization that occurred in these gln3 truncation mutants was no longer Sit4-dependent and (ii) rapamycin responsiveness and the absolute Sit4 requirement for nuclear Gln3-Myc13 entry were coordinately lost. Neither active Sit4 nor rapamycin-inactivated TorC1 were required for nuclear Gln3-Myc13 entry when the C-terminal regulatory domains were eliminated in cells provided with excess nitrogen (glutamine) (Figure 3, C and E; Figure 4).
The truncation experiments were then performed in wild type cells using a derepressive nitrogen source (proline) whose uptake and metabolism require NCR-sensitive gene products. Wild type Gln3-Myc13 and the three gln3 mutants were significantly more nuclear in the proline-grown transformants when compared to cells grown with repressive glutamine as the nitrogen source (Figures 3 and 4). Derepressed nuclear Gln3-Myc13 localization per se remained intact in the absence of the C-terminal regulatory region.
As noted above, the loss of Sit4 had no effect on localization of the mutant proteins compared to wild type when glutamine was provided as sole nitrogen source (Figures 3 and 4). However, with proline as nitrogen source, there was reduced nuclear Gln3-Myc13 localization when compared to wild type recipient cells. These results, however, contrasted sharply with those observed with wild type, full length Gln3-Myc13 in the sit4Δ recipient and argued in favor of a partial Sit4 requirement for fully derepressed levels of nuclear Gln3-Myc13 even though there was no rapamycin response. We also noted that nuclear truncated Gln3-Myc13 localization was somewhat less reproducible than when the full-length protein was used.
Together these experiments indicated that: (i) Significant nuclear localization of C-terminally truncated Gln3-Myc13 could be achieved in the absence of Sit4 and presence of active TorC1, i.e. in glutamine-grown transformants. (ii) Rapamycin-responsive intracellular Gln3-Myc13 localization, and its Sit4-dependence correlated with the presence of the C-terminal but not the N-terminal portion of Gln3-Myc13. (iii) The C-terminal region of Gln3 was not required to observe derepression of nuclear Gln3-Myc13 localization, but the loss of Sit4 reduced it.
gln3 truncation proteins are dephosphorylated by rapamycin-treatment
As nuclear localization of truncated Gln3-Myc13 proteins no longer responded to rapamycin, we queried whether rapamycin elicited their dephosphorylation. It did! Addition of rapamycin to glutamine-grown wild type recipient cells containing the individual truncation plasmids increased Gln3-Myc13 mobilities characteristic of its dephosphorylation (Figure 5).
Figure 5.
Rapamycin-elicited dephosphorylation is retained in C-terminally truncated Gln3-Myc13 proteins. (A) Gln3 domain diagram showing the locations of the truncations analyzed in this experiment. (B). Wild type (JK9-3da) cells were transformed with pRR610 (Gln31-476-Myc13), pRR612 (Gln31-400-Myc13) or pRR611 (Gln31-384-Myc13). Transformants were grown to mid-log phase in YNB-glutamine (Gln) medium. Rapamycin (200 ng/ml) was added where indicated (+Rap) for 15 minutes. Cultures were collected for protein extraction and western blot analysis as described (see the section “Materials and Methods” for protocols).
Sit4 dephosphorylates truncated Gln3-Myc13 proteins when TorC1 is up- and downregulated
Rapamycin-elicited nuclear Gln3-Myc13 localization and dephosphorylation has been previously taken as evidence for down-regulation of TorC1 and concomitant up-regulation of Tap42-Sit4-mediated Gln3 dephosphorylation (Beck and Hall 1999; Bertram et al. 2000; Yan et al. 2006). Having genetically separated Gln3-Myc13 localization from its dephosphorylation (Figures 2, 3 and 5), we asked whether Sit4 continued to participate in the dephosphorylation of truncated Gln3-Myc13 proteins. The mobility of each truncated Gln3-Myc13 protein decreased in glutamine-grown sit4Δ transformants relative to that observed in wild type recipient cells (Figure 6A). Remarkably, Sit4 actively dephosphorylated all three truncated Gln3-Myc13 proteins irrespective of whether TorC1 was activated by the excess nitrogen in YNB-glutamine medium or increasingly inactive as occurs with ammonia or proline as sole nitrogen sources (Figure 6, B and C).
Figure 6.
Dephosphorylation of C-terminally truncated Gln3-Myc13 proteins is Sit4-dependent. Wild type (JK9-3da) and sit4Δ (FV029) cells were transformed with plasmids encoding Gln31-476-Myc13 (pRR610), Gln31-400-Myc13 (pRR612), or Gln31-384-Myc13 (pRR611). Transformants were grown to mid-log phase in YNB-glutamine (A), ammonia (B) or proline (C) as the sole nitrogen source. Cell collection and processing for western blot analysis were performed as described in the section “Materials and Methods.”
These data showed rapamycin-responsive, Sit4-dependent, Gln3 dephosphorylation in the N-terminal portion of the protein. It occurred despite rapamycin addition having little if any effect on nuclear localization of the C-terminal-truncated Gln3-Myc13 molecules and the absence of the C-terminal Gln3–Tor1 interaction site (Gln3656–666) (Rai et al. 2013). These observations raised three important questions: (i) What N-terminal Gln3 residues were required for rapamycin-elicited dephosphorylation? (ii) Was rapamycin-elicited dephosphorylation associated with a Tor-rapamycin response site as occurs at Gln3505–594 or alternatively a Tor1-dependent site as occurs at Gln3656–666? (iii) Was the site of rapamycin-elicited dephosphorylation associated with a predicted α-helix as occurs at both Gln3505–594 and Gln3656–666?
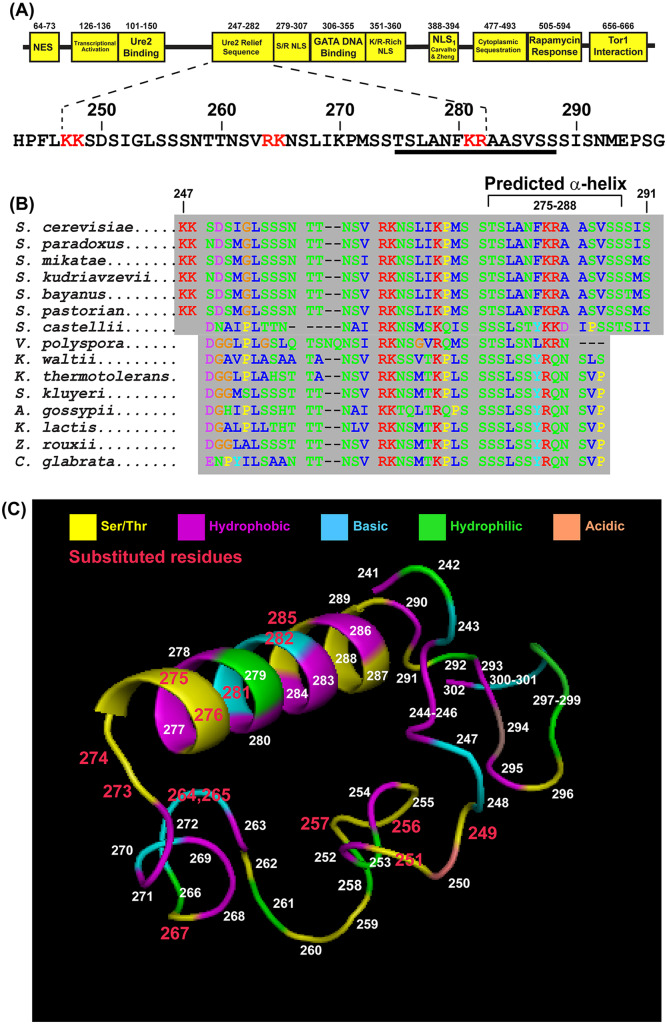
Predicted structure of the Ure2 relief sequence (URS)
To begin addressing these questions, we first inspected the N-terminal Gln3 domain structure. One region we had previously identified, but knew little about, was the Ure2 Relief Sequence (URS, Gln3247-282) which contained a highly conserved sequence, Gln3247-282 (Figure 7, A and B) (Tate et al. 2018). This sequence must be functional if Gln3-Myc13 is to enter the nuclei of Ure2 plus cells, but not those when the URE2 gene is deleted (Tate et al. 2018). It consists of three segments defined by pairs of basic amino acids followed by serine and hydrophobic-rich residues (Figure 7A).
Figure 7.
Predicted secondary structure of the highly conserved Gln3URS. (A)The Gln3URS sequence and its position in Gln3. (B) Highly conserved Gln3 sequences containing the Gln3URS. Amino acid types are color coded: hydrophobic (blue); hydrophilic (green); basic (red); acidic (purple); and proline, though hydrophobic, (yellow). The sequence predicted to possess an α-helical secondary structure is indicated. (C) PyMol modeling showing the predicted secondary structure of Gln3241–302. Colored regions represent amino acid characteristics: basic (cyan) serine/threonine (yellow), hydrophobic (magenta), hydrophilic (green) and acidic (peach). Numbers indicate the Gln3 residue locations. Red numbers indicate the residues that were substituted in this work.
Given this somewhat strange sequence, we modeled Gln3 residues 241 to 302 using PEP-FOLD and PHYRE2 and visualized the most likely three-dimensional (3 D) structure with PyMol legacy version 0.99 and found it to contain a predicted 14-residue amphipathic α-helix (Gln3275-288 inclusive) embedded in a disordered coiled-coil region (Figure 7C). This was an engaging finding because the URS contained a predicted α-helix similar in size to those at the C-terminal Tor1-Gln3 interacting Gln3656–666 and the rapamycin-responsive site, Gln3583-594. It is, however, important to emphasize that our 3 D peptide model was constructed in the absence of a complete Gln3 structure or any other protein(s) with which especially the Gln3241–302 peptide might interact. Despite these caveats, the URS merited further attention as participating in N-terminal Gln3 phosphorylation.
Alteration of the Gln3 URS basic residues abolishes rapamycin elicited nuclear Gln3-Myc13 localization
What first drew our attention to the URS were the three pairs of basic residues each separated by fifteen amino acids (Figure 7A and Tate et al. 2018). Additionally, 1/3 of those amino acids (13 of 36) were serine/threonine residues. Our previous work had shown that substitution of aspartate for these basic residues and a nearby serine in the URS (pRR754 Gln3R264D, K265D, S267D and pRR772 Gln3K281D, R282D, S285D) completely abolished the ability of rapamycin to elicit nuclear Gln3-Myc13 localization (Figure 8C, a summary of data from Tate et al. 2018). The first of these substitutions were situated in the disordered coiled-coil region of the protein (Figure 8A, pRR754 and Figure 8B, images 1–3), whereas the second set was within the predicted α-helical region (Figure 8A, pRR772 and Figure 8B, images 4–6). It is important to note that these substitutions, as drastic as they were, did not prevent nuclear entry of the mutant proteins in ure2Δ recipient cells (Tate et al. 2018).
Figure 8.
Rapamycin responsiveness of Gln3-Myc13 localization is abolished when aspartate is substituted for basic and serine residues in the predicted disordered loop and amphipathic α-helical regions of the Gln3URS. (A) Gln3URS with aspartate-substituted residues indicated by asterisks; the predicted 14 residue amphipathic α-helix is underlined. (B) Ribbon and space-filling models of the Gln3URS showing the locations of the substituted residues. Residue color scheme: basic (cyan), hydrophobic (magenta), hydrophilic (green), substituted serines (yellow), remaining serine/threonines that were not substituted (orange). Structures in image 3 was derived by rotating the structure in image 2 horizontally ∼180°. Structures in image 6 was derived by rotating the structure in images 5 vertically ∼180°. (C) Contains summaries of previously published experiments as noted in the right column of the panel. Effects of rapamycin addition on Gln3-Myc13 localization. Experimental format and data presentation were as described in Figures 2–4. Transformants contained pRR536 (wild type Gln3), pRR754 (Gln3R264D, K265D, S267D) or pRR772 (Gln3K281D, R282D, S285D).
That earlier work also showed the same result if aspartates were substituted for only serine/threonine residues (pRR1366 Gln3S249D, S251D, S256D, S257D and pRR1368 Gln3S267D, S273D, S274D, T275D, S276D) in the URS (Tate et al. 2018). Gln3-Myc13 remained staunchly sequestered in the cytoplasm irrespective of whether or not the glutamine-grown mutants were treated with rapamycin (Figure 9C, a summary of data from Tate et al. 2018). These serine/threonine residues in pRR1366 were situated in the coiled-coil region (Figure 9A substituted residues denoted with asterisk’s and in 9B images 1–3), whereas those in pRR1368 were in the N-terminal end of the putative α-helix (Figure 9, A and 9B, images 4–6). It is pertinent to note that the mutants were otherwise full length Gln3-Myc13 proteins. The substituted serine/threonine residues in the mutant Gln3-Myc13 peptides are exposed in the predicted space-filling structures (colored yellow in the models, 8B and 9B, images 2, 3, 5, and 6). However, we again emphasize that residues apparently exposed in the models shown in Figures 8B and 9B of the isolated Gln3241–302 peptide may not be in the same confirmation/location in an intact Gln3 molecule or a Gln3 molecule associated with other protein(s).
Figure 9.
Rapamycin responsiveness of Gln3-Myc13 localization is abolished when aspartate is substituted for serine/threonine residues in the predicted disordered loop and amphipathic α-helical regions of the Gln3URS. (A) URS sequence containing amino acid substitutions in loop and helical regions of Gln3. (B) Pymol modeling showing position of mutated amino acids in the Gln3URS sequence. (C) This panel contains summaries of previously published experiments as noted in the right column of the panel. Wild type strain JK9-3da was transformed with either pRR536 (Gln31-730-Myc13), pRR1366 (Gln3S249D, S251D, S256D, S257D), or pRR1368 (Gln3S267D, S273F, S274D, T275D, S276D). Transformants were grown in YNB-glutamine (Gln) medium to mid-log phase followed by treatment with 200 ng/ml rapamycin (+Rap) for 15 minutes. Experimental format and data presentation were as described in Figures 3, 4, 8 and the section “Materials and Methods.”
Alteration of the Gln3 URS serine/threonine residues abolishes ½ of derepressed nuclear Gln3 localization
In contrast to the above data, cytoplasmic sequestration of wild type Gln3-Myc13 (pRR536) decreased by one half, when ammonia replaced glutamine as the nitrogen source (compare Figures 9C and 10; Figure 10 contains data similar to that in Tate et al. 2018). The tripartite Gln3-Myc13 distribution observed in the ammonia-grown cells correlated with that of truncated Gln3-Myc13 proteins produced in glutamine-grown cells lacking Sit4 (Compare Figure 10B, pRR536 with Figure3, D and E; Figure 4) or in cells where the Gln3656–666-Tor1 interaction site was abolished (Figure 2B, pRR850).
Figure 10.
Nitrogen catabolite repression of Gln3-Myc13 localization is altered when aspartate is substituted for serine/threonine residues in the predicted disordered loop and α-helical regions of the Gln3URS. Wild type strain JK9-3da was transformed with either pRR536 (Gln31-730-Myc13), pRR1366 (Gln3S249D, S251D, S256D, S257D), or pRR1368 (Gln3S267D, S273F, S274D, T275D, S276D). Transformants were grown in YNB-ammonia (Am.) or -proline (Pro) medium to mid-log phase. Experimental format and data presentation are as described in Figures 3 and 4. Error bars are standard deviations of two biological replicates. Similar conditions to those used in this experiment may also be found by comparing data obtained comparing Gln vs. Pro as nitrogen source and ammonia vs. ammonia + Msx presented in Tate et al.(2018).
An analogous response was observed with proline vs. ammonia as nitrogen source. In proline-grown, wild-type transformants (pRR536), Gln3-Myc13 was highly nuclear. When ammonia replaced proline, about one half of the nuclear localization was lost again yielding a typical tripartite Gln3-Myc13 distribution. The remaining one half of nuclear Gln3-Myc13 localization present in ammonia-grown wild type cells was additionally lost in the similarly cultured URS mutants (Figure 10B, compare pRR536 vs. pRR1366 and pRR1368).
Similarly, the highly nuclear-Gln3-Myc13 observed in proline-grown wild type cells was reduced by one half upon the loss of URS function in the pRR1366 and pRR1368 transformants. Further, that remaining one half of nuclear Gln3-Myc13 localization observed in proline grown pRR1366 and pRR1368 transformants was itself lost when more repressive glutamine or ammonia, replaced proline (Figure 10, pRR1366 and pRR1368 vs. Figure 9 pRR1366 and pRR1368). Note that Gln3-Myc13 distributions in the mutant transformants behaved in parallel. In each of the above cases, the tripartite distributions are those observed when one of two regulatory pathways controlling Gln3 localization is lost.
Gln3–Tor1 interaction requires a functional URS
We speculated that the above results pointed to the loss of one mode of Gln3 regulation and a potential involvement of TorC1 in results obtained with the truncation and URS substitution mutants. Additionally, earlier work raised the possibility of an unidentified Gln3–Tor1 interaction site existing N-terminal to Gln3600. Recall that the previously identified Gln3–Tor1 interaction site was located at Gln3656–666 (Rai et al. 2013; Figure 11C, Gln31-600, pRR1067).
Therefore, we searched for the location of a Tor1 interaction site to assess whether its location correlated with any of the above data. To that end, we performed 2-hybrid analyses with a set of C-terminal truncation plasmids using Tor11-2470 and Tor11-1764 as baits (Methods and Rai et al. 2013). Tor11-2470 is a full-length molecule. Tor11-1764 is a C-terminal truncation containing the HEAT repeats and ∼3/4 of the FAT domain, but lacks the remainder of the FAT domain, the rapamycin binding FRB, PI3K-like kinase and short C-terminal FATC domains (Figure 11B) (https://www.uniprot.org/uniprot/P35169). The prey constructs were Gln3 C-terminal truncations terminating at residues 476, 400, 350, 240, 158, and 99 (Figure 11C, pRR1122, pRR1120, pRR1118, pRR1149, pRR1147, and pRR1145, respectively).
C-terminal truncations to Gln31-400 interacted positively with both baits (Figure 11C, columns 2 and 4). However, elimination of the next 50 residues to Gln3350, abolished Gln3 interaction with Tor11-1764 but not Tor11-2470. Further truncation to Gln3240 and beyond abolished the interaction with Tor11-2470 as well (Figure 11, columns 2 and 4).
Three functional domains exist within the 110 Gln3 residues eliminated between Gln3350 and Gln3240: (i) the GATA DNA binding domain, (ii) a Ser/basic amino acid-rich nuclear localization sequence (S/R NLS) and (iii) the URS. Abolishment of rapamycin-responsive nuclear Gln3-Myc13localization in the URS substitution mutants (Figures 8–10) motivated us to determine the effect of these substitutions on the Gln3–Tor1 interaction.
Aspartate substitutions in either the coiled-coil portion (Figure 12B, images 1 and 2, pRR1124) or predicted α-helix (images 3 and 4, pRR1128) of the URS eliminated the interaction of these Gln3 mutant proteins with Tor11-1764 (Figure 12C, columns, 3 and 4). Note that these are the same substitutions that abolished rapamycin-elicited nuclear Gln3-Myc13 localization in otherwise full-length, wild type Gln3-Myc13 proteins (Figures 8, pRR754 and pRR772 and Figure 9, pRR1366 and pRR1368). In contrast, Tor11-2470 continued to interact with proteins encoded by these gln3 prey plasmids (Figure 12C, columns 1 and 2, pRR1124 and pRR1128). This was not too surprising because the C-terminal Gln3–Tor1 interaction site at Gln3656–666 remained intact in these full-length mutant proteins.
Figure 12.
Aspartate substitutions in the Gln3URS abolish Gln3–Tor1 interaction. (A and B) Locations of aspartate substitutions in Gln3 primary and predicted secondary structures of proteins encoded by pRR1124 (Gln3R264D, K265K, S267D) and pRR1128 (Gln3K281D, R282D, S285D). The substitutions in pRR1124 and pRR1128 were the same as those analyzed in pRR754 and pRR772, respectively (Figure 7). Color coding of the Gln3 secondary structure images are the same as in Figures 8 and 9. (C) The two-hybrid experiments were performed and presented as described in Figure 11.
Rapamycin elicited dephosphorylation of URS Ser/Thr residues
The above observations identified multiple serine/threonine residues in the URS that were required for nuclear Gln3-Myc13 localization and interaction with Tor1. Moreover, these residues were contained in Gln3-Myc13 truncation proteins that were dephosphorylated in a Sit4-dependent manner in rapamycin-treated cells. Together, these data raised the possibility that URS serine/threonine residues were phosphorylated in vivo and dephosphorylated upon inhibiting TorC1 with rapamycin.
We were able to evaluate this possibility experimentally due to the generosity of Claudio De Virgilio and his colleagues (Hu et al. 2019). Mass spectrometry analyses were performed with SD medium-grown cells in the presence vs. absence of rapamycin treatment (200 ng/ml for 30 minutes). These data showed that 11 of 17 Gln3 URS serine/threonine residues showed a high probability of being dephosphorylated in rapamycin-treated cells (Table 4). All of the identified residues showed very low posterior error probabilities (PEP) and high Andromeda scores (Kället al. 2008; Cox et al. 2011). Phosphorylation levels in eight of the eleven residues decreased more than twofold in the rapamycin-treated cells: Gln3 residues 251, 256, 257, 262, 273, 275, 276, and 289 (Figure 13). Importantly, rapamycin decreased phosphorylation of residues 256, 257 and 262 by 10- to 15-fold. Decreased phosphorylation observed with the other residues was a more moderate three to sixfold.
Table 4.
Identification of phosphorylated serine/threonine residues in the Gln3URS
| PEP | Score | Position | Sequence window | Phospho-Ser/Thr probabilities |
|---|---|---|---|---|
| 2.84E–34 | 238 | 251 | SNNSNIQHPFLKKSDSIGLSSSNTTNSVRKN | KSDS(1)IGLSSSNTTNSVR |
| 5.35E–69 | 285 | 256 | IQHPFLKKSDSIGLSSSNTTNSVRKNSLIKP | KSDSIGLSS(0.833)S(0.163)NT(0.003)(0.001)NSVR |
| 3.73E–8 | 335 | 257 | QHPFLKKSDSIGLSSSNTTNSVRKNSLIKPM |
SDSIGLSS(0.002)S(0.993)NT(0.005) T (0.001)NSVRK |
| 1.06E–36 | 262 | 262 | KKSDSIGLSSSNTTNSVRKNSLIKPMSSTSL |
KSDSIGLS(0.024)S(0.018)S(0.877)N T(0.064)T(0.022)NS(0.996)VRK |
| 1.04E–37 | 253 | 267 | IGLSSSNTTNSVRKNSLIKPMSSTSLANFKR | KNS(1)LIKPMS(0.844)S(0.132)T(0.021) |
| S(0.003)LANFK | ||||
| 1.45E–80 | 313 | 273 | NTTNSVRKNSLIKPMSSTSLANFKRAASVSS | PMS(0.999)S(0.001)TSLANFK |
| 2.44E–05 | 152 | 275 | TNSVRKNSLIKPMSSTSLANFKRAASVSSSI | PMS(0.924)S(0.082)T(0.942) |
| 1.25E–42 | 266 | 276 | NSVRKNSLIKPMSSTSLANFKRAASVSSSIS |
NSLIKPMS(0.958)S(0.042) T(0.003)S(0.997)LANFKR |
| 4.47E–57 | 286 | 285 | KPMSSTSLANFKRAASVSSSISNMEPSGQNK | AAS(1)VSSSISNMEPSGQNK |
| 4.34E–28 | 232 | 288 | SSTSLANFKRAASVSSSISNMEPSGQNKKPL |
AASVS(0.002)S(0.798)S(0.2) ISNMEPSGQNK |
| 1.93E–28 | 240 | 289 | STSLANFKRAASVSSSISNMEPSGQNKKPLI |
RAAS(0.994)VS(0.006)S(0.007)S(0.992) IS(0.001)NMEPSGQNK |
The data were obtained using the methods cited in the section “Materials and Methods.”
Figure 13.

Rapamycin decreases the phosphorylation of serine and threonine residues in the Gln3URS. Phosphorylation levels in untreated cells and those treated with 200 ng/mL rapamycin for 30 minutes. The histograms indicate the average of the assay results, whereas the filled circles indicate the actual values of biological replicates from which the histograms were generated. These are the quantitative data associated with the analysis described in Table 4.
Dephosphorylated residues 275, 276, and 289 are situated at both ends of the predicted amphipathic α-helix (Figure 14, A and B). Residues 251, 256, 257, 262 and 273 are in the unstructured regions of the URS (Figure 14C). Correlating with the dephosphorylation data, residues 251, 256 and 257 in pR1366 and residues 273, 275, and 276 in pRR1368 were those that abolished rapamycin-elicited nuclear Gln3-Myc13 localization when modified. With space filling models of the whole URS sequence, serine/threonine residues identified as being dephosphorylated in rapamycin treated cells were largely exposed in the predicted structure (Figure 14C, yellow).
Figure 14.
Locations of Gln3 serine and threonine residues in the predicted α-helix (A and B) and complete Gln3URS (C) that were dephosphorylated by treating cells with 200 ng/mL rapamycin. The phosphorylated/dephosphorylated residues are designated by their primary sequence locations and were derived from the data in Table 4. Color coding as in Figures 2, 8, 9, and 12.
Discussion
We have investigated the mechanisms by which nitrogen-responsive TorC1 kinase regulates the NCR-sensitive transcriptional activator, Gln3. The experiments were predicated on the idea that the individual regulatory pathways that cumulatively achieve NCR-sensitive Gln3 regulation each have a target on the Gln3 molecule itself (Rai et al. 2013). Present experiments have identified a new Gln3 target and characterized its functions and interactions with others in the molecule.
With one paradoxical exception (Tate et al. 2015), TorC1-Gln3 interaction has been uniformly concluded to negatively regulate Gln3 intracellular localization and thereby NCR-sensitive transcription (Beck and Hall 1999; Bertram et al. 2000). However, this work expands our understanding of the TorC1-Gln3 interactions, showing the existence of two TorC1 interacting sites situated at the C-terminal and N-terminal regions of Gln3, one acting negatively and the other positively. Moreover, our evidence supports the contention that the two Gln3 sites can function separately as well as together. The C-terminal site is required for full repression (cytoplasmic sequestration) and rapamycin-dependent nuclear Gln3 localization, whereas the N-terminal site is required for fully derepressed nuclear Gln3 localization and rapamycin elicited nuclear Gln3 localization.
C-terminal Gln3–Tor1 interaction site functions negatively
Eliminating or inactivating the C-terminal eleven residue peptide predicted to be an α-helix (Gln3656–666), which interacts directly or indirectly with Tor1, reduces the level of cytoplasmic Gln3 sequestration to ½ of that observed when the wild type (Gln31-730) is cultured in excess nitrogen (Rai et al. 2013). This loss of cytoplasmic sequestration, i.e. down-regulation, results in the characteristic tripartite intracellular Gln3 distribution. Not surprisingly, elimination of the C-terminal Tor1 interaction site also abolishes rapamycin elicited nuclear Gln3 localization. Consistent with this negative regulation, TorC1 kinase phosphorylates Gln3 in vitro (Bertram et al. 2000).
Just N-terminal of the Gln3–Tor1 interaction helix is a Gln3 domain specifically required for rapamycin-elicited nuclear Gln3 localization (Rai et al. 2014). However, Tor1-mediated cytoplasmic Gln3 sequestration and Gln3–Tor1 interaction are unaffected by alteration of this rapamycin response site. Together, the C-terminal Gln3–Tor1 interaction site down-regulates nuclear Gln3 localization in excess nitrogen conditions. Then rapamycin, in concert with the Gln3 rapamycin response site, relieves this down-regulation. Both characteristics are consistent with TorC1 acting as a negative regulator of nuclear Gln3 localization.
Strikingly, however, eliminating the C-terminal Gln3–Tor1 interaction and/or the rapamycin response site has no effect on: (i) rapamycin-elicited Gln3-Myc13 dephosphorylation or (ii) derepression of nuclear Gln3-Myc13 localization in a poor nitrogen source (proline). In fact, none of the sequences C-terminal of Gln31-384 influence rapamycin elicited Gln3 dephosphorylation in nitrogen-rich glutamine or its fully derepressed nuclear localization in proline medium.
N-terminal Gln3–Tor1 interaction site functions positively
Our work shows a second, positively acting Gln3–Tor1 interacting site situated in an N-terminal, disordered, coiled-coil region of Gln3 containing a predicted 14 residue α-helix (Figure 7). We previously identified this region as the Gln3 URS (Ure2 Relief Sequence, Gln3247-282), because it was required for nuclear Gln3 localization when Ure2 was present but not when it was absent (Tate et al. 2019). Here we have shown that wild type URS residues are positively required for: (i) nuclear Gln3-Myc13 localization in glutamine-grown, rapamycin-treated cells, (ii) ½ of derepressed nuclear Gln3-Myc13 localization that occurs in derepressive proline medium, and (iii) Gln3–Tor1 interaction; all in the absence of the C-terminal Gln3–Tor1 interaction site.
How might TorC1 function positively in the regulation of nuclear Gln3 localization? There are at least two conceptual ways to speculatively interpret the three observations listed in the previous paragraph. (i) The fact that loss of Tor1-Gln3-Myc13 interaction correlates with the first two phenotypes could lead to the conclusion that a cause-effect relationship exists with TorC1 acting in a positive manner when interacting with the N-terminal Gln3 site. (ii) Alternatively, one may reason that the three observations correlate but do not derive from a direct cause-effect relationship. The Gln3–Tor1 interaction site is demonstrably acting positively, i.e. it is required for all three phenotypes. However, TorC1 itself could still be acting negatively. One way that this might occur is that TorC1, interacting with and phosphorylating the N-terminal Gln3 site inhibits its ability to mediate: (i) rapamycin induced nuclear Gln3-Myc13 localization in glutamine medium and (ii) full nuclear Gln3-Myc13 localization in derepressive proline medium. This is consistent with the observations that phosphomimetic substitutions of URS residues abolish these functions. Here, loss of the Tor1-Gln3 interaction would be speculated to result from TorC1 having much greater affinity for the hypo-phosphorylated Gln3 interacting site than when the URS is hyper-phosphorylated. In other words, two negative events causing a positive outcome.
The two Gln3–Tor1 interaction sites function autonomously and collaboratively
The two Gln3–Tor1 interaction sites act both autonomously and collaboratively. Functioning autonomously, the C-terminal Gln3–Tor1 interaction site is a primary determinant for regulating intracellular Gln3 localization in repressive conditions accounting for one half of Gln3’s cytoplasmic sequestration (Figure 2). We reason that it functions autonomously because loss of the N-terminal site has no effect on maintaining full cytoplasmic Gln3-Myc13 sequestration in repressive glutamine medium (Figure 9). Further, Gln3-Myc13 dephosphorylation occurs normally in the absence of the C-terminal Gln3–Tor1 interaction site (Figure 5). On the other hand, the two Gln3–Tor1 interaction sites also function collaboratively. Both sites must be functional for rapamycin to elicit nuclear Gln3 localization. The loss of either site eliminates nuclear Gln3 entry (Figures 2, 8 and 9).
Rapamycin elicits dephosphorylation of serine/threonine residues in the N-terminal Gln3–Tor1 interaction site
Existence of a Gln3–Tor1 interaction associated with the Gln3 URS and the loss of nuclear Gln3-Myc13 localization when phosphomimetic aspartate residues were substituted for serines and threonine in that site suggested that the positive function of the Gln3–Tor1 interaction site might be regulated by phosphorylation. Supporting that suggestion, eight of the thirteen serine/threonine residues in the Gln3 URS are dephosphorylated in rapamycin-treated cells by 3–15-fold with 3 of them by 10–15-fold. These rapamycin-sensitive phosphorylated residues are situated at both ends of the predicted α-helix and are exposed in space filling models of the entire Gln3 URS (Figure 14). Although we do not know the nature of potential interactions of the Gln3 URS with other proteins, the positions of its dephosphorylated residues in the present models are consistent with them being available to do so.
N-terminal Gln3–Tor1 interaction may inform an unresolved paradox
Earlier, we reported the somewhat puzzling observation that components of the Gtr-Ego complex are required for cell growth and normal nuclear Gln3-Myc13 localization in derepressive proline medium, following a down-shift from glutamine to proline medium or following nitrogen starvation (Tate et al. 2015). It was hard at the time to envision how this complex might function in such a positive manner. A priori, one would have expected its presence to negatively regulate nuclear Gln3 localization, because the Gtr-Ego complex was known to be clearly required for TorC1 kinase activation in nitrogen replete conditions (Binda et al. 2009, 2010; Bonfils et al. 2012). Activated TorC1 kinase, in turn, was accepted to down-regulate or repress nuclear Gln3-Myc13 localization (Beck and Hall 1999). Therefore, by that reasoning, elimination of the Gtr-Ego complex should have facilitated rather than prohibited nuclear Gln3 localization. Here, by one line of reasoning, we have extended those earlier observations by showing that a Tor1-Gln3 interaction itself correlates with achieving full nuclear Gln3 localization in derepressive growth conditions or in response to rapamycin treatment. Note the N-terminal Gln3–Tor1 interaction site remains required even though both TorC1 and the C-terminal Gln3–Tor1 interaction sites are intact. What remains unknown is how Gtr-Ego and TorC1 together actually achieve the apparent positive regulation of rapamycin-responsive nuclear Gln3 localization.
Distinct Sit4 functions for nuclear Gln3 dephosphorylation and nuclear localization
Another prescient finding of this work is the multi-conditional participation of Sit4 phosphatase. When both Gln3–Tor1 interaction sites are intact, Sit4 is absolutely required for nuclear Gln3 localization whether in response to rapamycin addition or growth in derepressive proline medium. However, elimination of the C-terminal site results in the coordinate loss of both rapamycin-elicited nuclear Gln3 localization and the absolute requirement for Sit4 participation in nuclear Gln3 localization in derepressive conditions (Figures 3 and 4). Yet Gln3 dephosphorylation remains normally rapamycin-stimulated and Sit4-dependent under these same nitrogen replete conditions. This is not the first time gross Gln3 dephosphorylation and nuclear localization have been separated from one another. Recall that rapamycin elicited Gln3 dephosphorylation alone is insufficient for nuclear entry of Gln3 in the absence of PP2A phosphatase activity (Tate et al. 2009).
Unanswered questions—Does Gln3 reside in two cytoplasmic locations?
Why is it that the two Gln3–Tor1 interaction sites function additively for imposition/relief of cytoplasmic Gln3 sequestration, whereas they are both required in an all or none manner for rapamycin-elicited nuclear Gln3 localization? Why is Sit4 absolutely required for nuclear Gln3 localization when both sites are present, but not when only the N-terminal site is present? Yet, Sit4 remains required for Gln3 dephosphorylation in the absence of the C-terminal site.
In the above context, two previously reported observations are likely to be important. First, Sit4 and Sit4-Tap42 both are required for wild type intracellular Gln3 regulation (Tate et al. 2019). Second, De Virgilio's laboratory has shown that TorC1 resides in two pools within the cell, one associated with the vacuole and the other with perivacuolar endosomes (Hatakeyama et al. 2019; Hatakeyama and De Virgilio 2019b). We and others have shown that genes encoding components of protein trafficking pathways are required for wild type NCR-sensitive Gln3 localization and transcriptional activation (Cox et al. 2004; Zurita-Martinez et al. 2007; Puria et al. 2008;Rohde et al. 2008; Fayyadkazan et al. 2014; Kingsbury and Cardenas 2016; Hatakeyama and De Virgilio 2019). The above correlations raise the possibilities that Gln3 may interact with TorC1 in two spatially separated cellular locations as also might the Sit4 and Sit4-Tap42 phosphatases. They also lead us to speculate that determining the relationships that bind these observations together and order them in time will further expand our knowledge of NCR-sensitive transcriptional regulation and explain how TorC1 positively and negatively regulates Gln3 and thereby NCR-sensitive transcription.
Data availability
Following publication, strains and plasmids will be provided upon request, but only for non-commercial purposes. Commercial and commercial-development uses are prohibited. Materials provided may not be transferred to a third party without written consent. This will be done in accordance with NIH guidelines.
Acknowledgements
The authors express their sincere gratitude to Professor Jörn Dengjel, and Drs. Serena Raucci and Michael Stumpe, and Zehan Hufor generously providing the data used in this work prior to publication.
Funding
This work was supported by NIH grant GM35642-27, the Harriet S. Van Vleet Chair of Excellence, USA-LTAUSA18 grant LTAUSA18162, and Swiss National Science Foundation 310030-184671.
Conflicts of interest
None declared.
References
- Beck T, Hall MN.. 1999. The TOR signaling pathway controls nuclear localization of nutrient-regulated transcription factors. Nature. 402:689–692. [DOI] [PubMed] [Google Scholar]
- Bertram PG, Choi JH, Carvalho J, Ai W, Zeng C, et al. 2000. Tripartite regulation of Gln3p by TOR, Ure2p, and phosphatases. J Biol Chem. 275:35727–35733. [DOI] [PubMed] [Google Scholar]
- Binda M, Bonfils G, Panchaud N, Péli-Gulli MP, De Virgilio C.. 2010. An EGOcentric view of TORC1 signaling. Cell Cycle. 9:221–222. [DOI] [PubMed] [Google Scholar]
- Binda M, Péli-Gulli MP, Bonfils G, Panchaud N, Urban J, et al. 2009. The Vam6 GEF controls TORC1 by activating the EGO complex. Mol Cell. 35:563–573. [DOI] [PubMed] [Google Scholar]
- Blinder D, Coschigano PW, Magasanik B.. 1996. Interaction of the GATA factor Gln3p with the nitrogen regulator Ure2p in Saccharomyces cerevisiae. J Bacteriol. 178:4734–4736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonfils G, Jaquenoud M, Bontron S, Ostrowicz C, Ungermann C, et al. 2012. Leucyl-tRNA synthetase controls TORC1 via the EGO complex. Mol Cell. 46:105–110. [DOI] [PubMed] [Google Scholar]
- Breitkreutz A, Choi H, Sharom JR, Boucher L, Neduva V, et al. 2010. A global protein kinase and phosphatase interaction network in yeast. Science. 328:1043–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broach JR. 2012. Nutritional control of growth and development in yeast. Genetics. 192:73–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas ME, Cutler NS, Lorenz MC, Di Como CJ, Heitman J.. 1999. The TOR signaling cascade regulates gene expression in response to nutrients. Genes Dev. 13:3271–3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho J, , Bertram P G, , Wente S R, , Zheng X F. 2001. Phosphorylation regulates the interaction between Gln3p and the nuclear import factor Srp1p. J Biol Chem. 276:25359–25365. [DOI] [PubMed] [Google Scholar]
- Carvalho J, Zheng XF.. 2003. Domains of Gln3p interacting with karyopherins, Ure2p, and the target of rapamycin protein. J Biol Chem. 278:16878–16886. [DOI] [PubMed] [Google Scholar]
- Cherkasova VA, Hinnebusch A.. 2003. Translational control by TOR and TAP42 through dephosphorylation of eIF2alpha kinase GCN2. Genes Dev. 17:859–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffman JA, el Berry HM, Cooper TG.. 1994. The URE2 protein regulates nitrogen catabolic gene expression through the GATAA-containing UASNTR element in Saccharomyces cerevisiae. J Bacteriol. 176:7476–7483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad M, Schothorst J, Kankipati HN, Van Zeebroeck G, Rubio-Texeira M, et al. 2014. Nutrient sensing and signaling in the yeast Saccharomyces cerevisiae. FEMS Microbiol Rev. 38:254–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper TG. 1982. Nitrogen metabolism in Saccharomyces cerevisiae. In:Strathern JN, Jones EW, Broach JR, editors. Molecular Biology of the Yeast Saccharomyces: metabolism and Gene Expression. New York: Cold Spring Harbor Laboratory, Cold Spring Harbor. p.39–99. [Google Scholar]
- Cooper TG. 2002. Transmitting the signal of excess nitrogen in Saccharomyces cerevisiae from the Tor proteins to the GATA factors: connecting the dots. FEMS Microbiol Rev. 26:223–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper TG. 2004. Integrated regulation of the nitrogen-carbon interface in nutrient-induced responses in eukaryotic cells. In: Winderickx Jand Taylor PM, editors. Topics in Current Genetics, Vol. 7, Chapter 9. Berlin-Heidelberg: Springer-Verlag. p. 225–257. [Google Scholar]
- Courchesne WE, Magasanik B.. 1988. Regulation of nitrogen assimilation in Saccharomyces cerevisiae: roles of the URE2 and GLN3 genes. J Bacteriol. 170:708–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox KH, Kulkarni A, Tate JJ, Cooper TG.. 2004. Gln3 phosphorylation and intracellular localization in nutrient limitation and starvation differ from those generated by rapamycin inhibition of Tor1/2 in Saccharomyces cerevisiae. J Biol Chem. 279:10270–10278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox J, Neuhauser N, Michalski A, Scheltema RA, Olsen JV, et al. 2011. Andromeda: a peptide search engine integrated into the Max Quant environment. J Proteome Res. 10:1794–1805. [DOI] [PubMed] [Google Scholar]
- Cox KH, Tate JJ, Cooper TG.. 2002. Cytoplasmic compartmentation of Gln3 during nitrogen catabolite repression and the mechanism of its nuclear localization during carbon starvation in Saccharomyces cerevisiae. J Biol Chem. 277:37559–37566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox KH, Tate JJ, Cooper TG.. 2004. Actin cytoskeleton is required for nuclear accumulation of Gln3 in response to nitrogen limitation but not rapamycin treatment in Saccharomyces cerevisiae. J Biol Chem. 279:19294–19301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespo JL, Powers T, Fowler B, Hall MN.. 2002. The TOR-controlled transcription activators GLN3, RTG1, and RTG3 are regulated in response to intracellular levels of glutamine. Proc Natl Acad Sci USA. 99:6784–6789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham TS, Andhare R, Cooper TG.. 2000. Nitrogen catabolite repression of DAL80 expression depends on the relative levels of Gat1p and Ure2p production in Saccharomyces cerevisiae. J Biol Chem. 275:14408–14414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Como CJ, Arndt KT.. 1996. Nutrients, via the Tor proteins, stimulate the association of Tap42 with type 2A phosphatases. Genes Dev. 10:1904–1916. [DOI] [PubMed] [Google Scholar]
- Drillien R, Aigle M, Lacroute F.. 1973. Yeast mutants pleiotropically impaired in the regulation of the two glutamate dehydrogenases. Biochem Biophys Res. Commun. 53:367–372. [DOI] [PubMed] [Google Scholar]
- Drillien R, Lacroute F.. 1972. Ureidosuccinic acid uptake in yeast and some aspects of its regulation. J Bacteriol. 109:203–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fayyadkazan M, Tate JJ, Vierendeels F, Cooper TG, Dubois E, et al. 2014. Components of Golgi-to-vacuole trafficking are required for nitrogen- and TORC1-responsive regulation of the yeast GATA factors. Microbiologyopen. 3:271–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feller A, Georis I, Tate JJ, Cooper TG, Dubois E.. 2013. Alterations in the Ure2 αCap domain elicit different GATA factor responses to rapamycin treatment and nitrogen limitation. J Biol Chem. 288:1841–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georis I, Tate JJ, Cooper TG, Dubois E.. 2008. Tor pathway control of the nitrogen-responsive DAL5 gene bifurcates at the level of Gln3 and Gat1 regulation in Saccharomyces cerevisiae. J Biol Chem. 283:8919–8929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georis I, Tate JJ, Cooper TG, Dubois E.. 2011a. Nitrogen-responsive regulation of GATA protein family activators Gln3 and Gat1 occurs by two distinct pathways, one inhibited by rapamycin and the other by methionine sulfoximine. J Biol Chem. 286:44897–44912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georis I, Tate JJ, Feller A, Cooper TG, Dubois E.. 2011b. Intranuclear function for protein phosphatase 2A: Pph21 and Pph22 are required for rapamycin-induced GATA factor binding to the DAL5 promoter in yeast. Mol Cell Biol. 31:92–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González A, Hall MN.. 2017. Nutrient sensing and TOR signaling in yeast and mammals. Embo J. 36:397–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatakeyama R, De Virgilio C.. 2019. TORC1 specifically inhibits microautophagy through ESCRT-0. Curr Genet. 65:1243–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatakeyama R, De Virgilio C.. 2019b. A spatially and functionally distinct pool of TORC1 defines signaling endosomes in yeast. Autophagy. 15:915–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatakeyama R, Péli-Gulli MP, Hu Z, Jaquenoud M, Garcia Osuna GM, et al. 2019. Spatially distinct pools of TORC1 balance protein homeostasis. Mol Cell. 73:325–338.e8. [DOI] [PubMed] [Google Scholar]
- Hinnebusch AG. 2005. Translational regulation of GCN4 and the general amino acid control of yeast. Annu Rev Microbiol. 59:407–450. [DOI] [PubMed] [Google Scholar]
- Hofman-Bang J. 1999. Nitrogen catabolite repression in Saccharomyces cerevisiae. MB. 12:35–71. [DOI] [PubMed] [Google Scholar]
- Hu Z, Raucci S, Jaquenoud M, Hatakeyama R, Stumpe M, et al. 2019. Multilayered control of protein turnover by TORC1 and Atg1.Cell Reports. 28:3486–3496.e6. [DOI] [PubMed] [Google Scholar]
- Ito H, Fukuda Y, Murata K, Kimura A.. 1983. Transfomation of intact yeast cells treated with alkali ions. J Bacteriol. 153:163–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James P, Halladay J, Craig EA.. 1996. Genomic libraries and a host strain designed for highly efficient two-hybrid selection in yeast. Genetics. 144:1425–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Broach JR.. 1999. Tor proteins and protein phosphatase 2A reciprocally regulate Tap42 in controlling cell growth in yeast. Embo J. 18:2782–2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Käll L, Storey JD, MacCoss MJ, Noble WS.. 2008. posterior error probabilities and false discovery rates: two sides of the same coin. J Proteome Res. 7:40–44. [DOI] [PubMed] [Google Scholar]
- Kingsbury JM, Cardenas ME.. 2016. Vesicular trafficking systems impact TORC1-controlled transcriptional programs in Saccharomyces cerevisiae. G3 (Bethesda). 6:641–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni AA, Abul-Hamd AT, Rai R, Berry HE, Cooper TG.. 2001. Gln3p nuclear localization and interaction with Ure2p in Saccharomyces cerevisiae. J Biol Chem. 276:32136–32144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni AA, Buford TD, Rai R, Cooper TG.. 2006. Differing responses of Gat1 and Gln3 phosphorylation and localization to rapamycin and methionine sulfoximine treatment in Saccharomyces cerevisiae. FEMS Yeast Res. 6:218–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacroute F. 1971. Non-Mendelian mutation allowing ureidosuccinic acid uptake in yeast. J Bacteriol. 106:519–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lageix S, Zhang J, Rothenburg S, Hinnebusch AG.. 2015. Interaction between the tRNA-binding and C-terminal domains of Yeast Gcn2 regulates kinase activity in vivo. PLoS Genet. 11:e1004991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, , Thornton J, , Spírek M, , Butow R A. 2008. Activation of the SPS amino acid-sensing pathway in Saccharomyces cerevisiae correlates with the phosphorylation state of a sensor component, Ptr3. Mol Cell Biol. 28:551–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljungdahl PO, Daignan-Fornier B.. 2012. Regulation of amino acid, nucleotide, and phosphate metabolism in Saccharomyces cerevisiae. Genetics. 190:885–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magasanik B, Kaiser CA.. 2002. Nitrogen regulation in Saccharomyces cerevisiae. Gene. 290:1–18. [DOI] [PubMed] [Google Scholar]
- Puria R, Zurita-Martinez SA, Cardenas ME.. 2008. Nuclear translocation of Gln3 in response to nutrient signals requires Golgi-to-endosome trafficking in Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 105:7194–7199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai R, Tate JJ, Nelson DR, Cooper TG.. 2013. gln3 mutations dissociate responses to nitrogen limitation (nitrogen catabolite repression) and rapamycin inhibition of TorC1. J Biol Chem. 288:2789–2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai R, Tate JJ, Shanmuganatham K, Howe MM, Cooper TG.. 2014. A domain in the transcription activator Gln3 specifically required for rapamycin responsiveness. J Biol Chem. 289:18999–19018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai R, Tate JJ, Shanmuganatham K, Howe MM, Nelson D, et al. 2015. Nuclear Gln3 import is regulated by nitrogen catabolite repression whereas export is specifically regulated by glutamine. Genetics. 201:989–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai R, Tate JJ, Cooper TG.. 2016. Multiple targets on the Gln3 transcription activator are cumulatively required for control of its cytoplasmic sequestration. G3. 6:1391–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohde JR, Bastidas R, Puria R, Cardenas M.. 2008. Nutritional control via Tor signaling in Saccharomyces cerevisiae. Curr Opin Microbiol. 11:153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staschke KA, Dey S, Zaborske JM, Palam LR, McClintick JN, et al. 2010. Integration of general amino acid control and target of rapamycin (TOR) regulatory pathways in nitrogen assimilation in yeast. J Biol Chem. 285:16893–16911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swinnen E, Ghillebert R, Wilms T, Winderickx J.. 2014. Molecular mechanisms linking the evolutionary conserved TORC1-Sch9 nutrient signaling branch to lifespan regulation in Saccharomyces cerevisiae. FEMS Yeast Res. 14:17–32. [DOI] [PubMed] [Google Scholar]
- Tate JJ, Buford D, Rai R, Cooper TG.. 2017. General amino acid control and 14-3-3 proteins Bmh1/2 are required for nitrogen catabolite repression-sensitive regulation of Gln3 and Gat1 localization. Genetics. 205:633–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate JJ, Cooper TG.. 2013. Five conditions commonly used to down-regulate tor complex 1 generate different physiological situations exhibiting distinct requirements and outcomes. J Biol Chem. 288:27243–27262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate JJ, Cox KH, Rai R, Cooper TG.. 2002. Mks1p is required for negative regulation of retrograde gene expression in Saccharomyces cerevisiae but does not affect nitrogen catabolite repression-sensitive gene expression. JBiol Chem. 277:20477–20482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate JJ, Feller A, Dubois E, Cooper TG.. 2006. Saccharomyces cerevisiae Sit4 phosphatase is active irrespective of the nitrogen source provided, and Gln3 phosphorylation levels become nitrogen source-responsive in a sit4-deleted strain. J Biol Chem. 281:37980–37992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate JJ, Georis I, Dubois E, Cooper TG.. 2010. Distinct phosphatase requirements and GATA factor responses to nitrogen catabolite repression and rapamycin treatment in Saccharomyces cerevisiae. J Biol Chem. 285:17880–17895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate JJ, Georis I, Feller A, Dubois E, Cooper TG.. 2009. Rapamycin-induced Gln3 dephosphorylation is insufficient for nuclear localization: Sit4 and PP2A phosphatases are regulated and function differently. J Biol Chem. 284:2522–2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate JJ, Georis I, Rai R, Vierendeels F, Dubois E, et al. 2015. GATA factor regulation in excess nitrogen occurs independently of Gtr-Ego complex-dependent TorC1 activation. G3 (Bethesda). 5:1625–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate JJ, Rai R, Cooper R,TG.. 2018. More than one way in: three Gln3 sequences required to relieve negative Ure2 regulation and support nuclear Gln3 import in Saccharomyces cerevisiae. Genetics. 208:207–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate JJ, Tolley EA, Cooper TG.. 2019. Sit4 and PP2A dephosphorylate nitrogen catabolite repression-sensitive Gln3 when TorC1 is up- as well as downregulated. Genetics. 212:1205–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Wang X, Jiang Y.. 2003. Interaction with Tap42 is required for the essential function of Sit4 and type 2A phosphatases. MBoC. 14:4342–4351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Du G, Zhou J, Chen J.. 2018. Regulation of sensing, transportation, and catabolism of nitrogen sources in Saccharomyces cerevisiae. Microbiol Mol Biol Rev. 82:e00040-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan G, Shen X, Jiang Y.. 2006. Rapamycin activates Tap42-associated phosphatases by abrogating their association with Tor complex 1. Embo J. 25:3546–3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan W, Guo S, Gao J, Zhong M, Yan G, et al. 2017. General control nonderepressible2 (GCN2) kinase inhibits target of rapamycin complex 1 in response to amino acid starvation in Saccharomyces cerevisiae. J Biol Chem. 292:2660–2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurita-Martinez SA, Puria R, Pan X, Boeke JD, Cardenas ME.. 2007. Efficient Tor signaling requires a functional class C Vps protein complex in Saccharomyces cerevisiae. Genetics. 176:2139–2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Following publication, strains and plasmids will be provided upon request, but only for non-commercial purposes. Commercial and commercial-development uses are prohibited. Materials provided may not be transferred to a third party without written consent. This will be done in accordance with NIH guidelines.