Abstract
Age determination from counts of growth layer groups (GLGs) in tooth dentine is a common method for aging marine mammals. Using known-aged animals, we validated this method for acid etched teeth of California sea lions (CSLs), Zalophus californianus. Between 1991 and 2013, the upper left canine (n = 33) was collected opportunistically during necropsy from animals tagged or branded as pups that later died. Overall, 55%–61% of age estimates by GLG counting were within 1 yr of the known-age in the sample of 1–30-yr-old CSLs. Accuracy of age estimates was found to be dependent on age of the CSLs, however. 71%–79% of age estimates were within 1 yr of the known-age in CSLs <10 yr old. These findings support the validity of counting GLGs to estimate age for CSLs <10 yr old to within 1 yr of accuracy.
Keywords: California sea lions, Zalophus californianus, aging, growth layer groups, teeth
The California sea lion (CSLs) (Zalophus californianus) is a widespread species, ranging along the entire West Coast of North America. While the population is thriving at approximately 296,750 (Carretta et al. 2016) they have been impacted by a number of diseases, such as domoic acid toxicity (Scholin et al. 2000, Gulland et al. 2002, Bargu et al. 2012), leptospirosis (Gulland et al. 1996, Gulland 1999), and urogenital carcinoma (Gulland et al. 1996, Lipscomb et al. 2000, Colegrove et al. 2009, Browning et al. 2015). Some of these diseases are associated with specific age groups (e.g., 26% of adults examined postmortem have genital carcinoma; Browning et al. 2015), which highlight the need for accurate age determination to understand disease susceptibility and the role of age in population dynamics. In addition, Carretta et al. (2017) found pinnipeds were the most commonly documented injured or killed by human-related activities averaging a minimum of 389 animals per year. Determining the age of these animals can help assess the impact of this mortality on the population (Laake et al. 2016).
Age determination from counts of growth layer groups (GLGs) in teeth was first described for marine mammals as early as 1840 (Owen 1840) but became widely recognized as a valid aging method around 1950 (Scheffer 1950, Laws 1952). The root of the tooth increases in length over the life of marine mammals while the pulp deposits dentine in the canal until it is completely filled (Scheffer 1950, Benjaminsen 1973, Bowen et al. 1983, Hohn et al. 1989, Laws 1952, Mansfield 1991, Stewart et al. 1996). In instances where teeth can be extracted from either live or dead animals, age can be more accurately estimated by counting incremental growth layer groups (tooth annulation method) that are deposited annually in the cementum or dentine of a tooth (Laws 1952). Accessory layers within the GLGs can represent life history events such as molt, fasting, reproduction, and lactation (Scheffer and Peterson 1967). Biochemical composition analysis of stable isotopes has shown that available prey resources and other environmental parameters can cause fluctuations in the amount of dentine laid making the annual layers sometimes inconsistent in width (Knox et al. 2014).
Estimates of age based on body length, body weight, tooth wear, or reproductive maturity are often used in studies of free-ranging marine mammals due to the logistical challenges of determining the exact age of these long-lived highly mobile animals (Smit 2003, King et al. 2007, Laake et al. 2016). Although there are other methods to age mammals (Klevezal and Kleinenberg 1967, Morris 1972), counting GLGs is commonly used (Scheffer 1950, Thomas 1977), and validated in a number of species of pinnipeds including the harp seal, Pagophilus groenlandicus (Bowen et al. 1983), New Zealand sea lion, Phocarctos hookeri (Childerhouse et al. 2004), Northern fur seal, Callorhinus ursinus (Scheffer 1950, Anas 1970), Cape fur seal, Arctocephalus pusillus (Fletemeyer 1978), New Zealand fur seal, Arctocephalus forsteri (McKenzie et al. 2007), gray seals, Halichoerus grypus (Hewer 1964), and the southern elephant seal, Mirounga leonina (Carrick and Ingham 1962), but not the California sea lion.
The purpose of this study is to validate the tooth annulation method in CSLs using the acid etched method with teeth collected from known-aged animals. For this purpose, we examined the accuracy and precision of age estimation relative to known-ages. Additionally, we examined the accuracy and consistency of age estimates within and between the two readers, then performed post hoc examination of the specimens for insight into the factors important in accurately estimating the age of sea lions by this method.
Methods
Canine teeth (n = 33) were collected from the upper left jaw from known-aged CSLs, ranging from <1 yr to 30 yr of age, during routine necropsies between 1991 and 2013 (Table 1). The sample contained more females (n = 22) than males (n = 11). Most of the study animals (n = 31) were tagged or branded as pups on San Miguel Island, CA, between 1988 and 2010 as part of a long-term monitoring project (DeLong and Melin 2000). At later times, these animals were found dead on the beach, died in rehabilitation, or were euthanized for humane reasons. Two additional sea lion pups were born in captivity and were housed at the U.S. Navy Marine Mammal Program (MMP), San Diego, California, to help with U.S. Navy research (Finneran et al. 2003, 2011; Houser et al. 2010). Their canine teeth were collected during routine necropsies at the time of their natural deaths. All teeth were extracted from the jaw bone, cleaned of remaining tissue, and stored dry until processing.
Table 1.
Accuracy and precision of age estimates. Est. = median estimated age, CV = coefficient of variation, D = index of precision.
| Actual age | Reader 1 | Reader 2 | ||||||
|---|---|---|---|---|---|---|---|---|
| yr | mo | Sex | Est. | CV | D | Est. | CV | D |
| 1 | 0 | ♀ | 1 | 43.3 | 25.0 | 1 | 0.0 | 0.0 |
| 1 | 0 | ♀ | 2 | 34.6 | 20.0 | 2 | 24.7 | 14.3 |
| 1 | 0 | ♀ | 0 | 0.0 | 0.0 | 0 | 173 | 100 |
| 1 | 1 | ♀ | 1 | 0.0 | 0.0 | 3 | 21.7 | 12.5 |
| 1 | 2 | ♂ | 1 | 86.6 | 50.0 | 1 | 43.3 | 25.0 |
| 1 | 10 | ♂ | 1 | 86.6 | 50.0 | 1 | 0.0 | 0.0 |
| 2 | 1 | ♂ | 3 | 45.8 | 26.5 | 2 | 0.0 | 0.0 |
| 2 | 8 | ♂ | 4 | 0.0 | 0.0 | 3 | 21.7 | 12.5 |
| 3 | 4 | ♂ | 3 | 21.7 | 12.5 | 4 | 13.3 | 7.7 |
| 4 | 2 | ♀ | 6 | 17.3 | 10.0 | 5 | 12.4 | 7.1 |
| 4 | 4 | ♂ | 6 | 27.0 | 15.6 | 5 | 26.6 | 15.4 |
| 4 | 6 | ♂ | 7 | 8.7 | 5.0 | 6 | 0.0 | 0.0 |
| 4 | 9 | ♂ | 10 | 6.0 | 3.4 | 5 | 12.4 | 7.1 |
| 5 | 2 | ♀ | 6 | 10.2 | 5.9 | 8 | 30.2 | 17.4 |
| 5 | 4 | ♀ | 8 | 12.5 | 7.2 | 6 | 9.1 | 5.3 |
| 5 | 11 | ♂ | 7 | 8.7 | 5.0 | 6 | 10.2 | 5.9 |
| 6 | 0 | ♀ | 6 | 16.7 | 9.6 | 7 | 8.7 | 5.0 |
| 6 | 2 | ♀ | 6 | 16.7 | 9.6 | 6 | 24.7 | 14.3 |
| 7 | 1 | ♀ | 7 | 8.7 | 5.0 | 7 | 8.7 | 5.0 |
| 8 | 1 | ♀ | 8 | 7.5 | 4.3 | 9 | 6.7 | 3.8 |
| 8 | 1 | ♀ | 8 | 6.9 | 4.0 | 9 | 16.4 | 9.4 |
| 8 | 2 | ♀ | 7 | 8.7 | 5.0 | 6 | 0.0 | 0.0 |
| 8 | 10 | ♀ | 7 | 8.7 | 5.0 | 8 | 19.9 | 11.5 |
| 9 | 3 | ♀ | 10 | 6.0 | 3.4 | 11 | 5.1 | 2.9 |
| 11 | 0 | ♀ | 6 | 9.1 | 5.3 | 9 | 22.2 | 12.8 |
| 13 | 2 | ♀ | 10 | 15.8 | 9.1 | 10 | 15.7 | 9.1 |
| 14 | 6 | ♀ | 14 | 4.2 | 2.4 | 15 | 3.9 | 2.3 |
| 16 | 2 | ♀ | 10 | 6.0 | 3.4 | 12 | 8.3 | 4.8 |
| 17 | 0 | ♀ | 11 | 5.4 | 3.1 | 14 | 7.1 | 4.1 |
| 17 | 4 | ♀ | 10 | 40.5 | 23.4 | 12 | 8.3 | 4.8 |
| 18 | 3 | ♀ | 9 | 31.5 | 18.2 | 10 | 0.0 | 0.0 |
| 30 | 1 | ♂ | 17 | 16.5 | 9.5 | 15 | 8.1 | 4.7 |
| 30 | 10 | ♂ | 26 | 30.4 | 17.6 | 12 | 4.9 | 2.9 |
| Mean | 19.6 | 11.3 | 17.2 | 9.9 | ||||
Each tooth was cut longitudinally down the midline, using a slow speed table saw and a diamond edged blade. Teeth with uneven surfaces after cutting were sanded. The cut surface of the clearer half of each tooth was then etched by first soaking in a 15% formic acid solution for 20 min with agitation every few minutes to displace any air bubbles. The teeth were then rinsed in running water for 3 min to remove the formic acid. Next, they were immersed in a 100% acetone bath for 3 min then air dried for 10 min. To remove any white residue, the teeth were rinsed with 70% isopropyl alcohol, rinsed again in running water and blotted with a paper towel until dry. The acid dissolves some of the minerals on the tooth surface more than others, resulting in raised lines and valleys on the tooth surface. The etching process needs to be sufficient to emphasize the raised lines representing the GLGs. If needed, the process was repeated by reducing the formic acid bath to 5 min intervals until fine raised lines could be seen on the dry surface of the tooth. A Number 1 lead pencil was then gently rubbed on the cut surface to enhance the raised lines (Evans and Robertson 2001).
For the first part of the study, age was determined for each tooth without knowledge of the true age. Using a 6.5–10× dissecting microscope, the GLGs were counted on each tooth to estimate age. Counting began at the neonatal line (NNL), the first line laid shortly after birth, and continued to the last distinct dark line closest to the pulp cavity (Fig. 1). From the NNL to the first GLG was considered Year 1. Ages were counted to the last complete GLG.
Figure 1.
A longitudinal section of a CSL tooth prepared for aging. NNL: neonatal line where counting begins. Postnatal dentine continues to fill in with age. GLG: growth layer group. AL: accessory line, or false lines, can be confused as GLGs.
Two readers aged each tooth three times, with at least 2 wk between each reading. The median of the three readings was used as the estimated age for each reader. Reader 1 and Reader 2 were an inexperienced and experienced reader, respectively, as defined by Lawson et al. (1992). GLG estimates were made without reference to specimen information, such as total body length, reproductive status, or previous GLG counts. The known-age for each animal was determined by subtracting death date from the mean pupping date of 15 June as recorded by DeLong and Melin (2000) for CSLs. Once the readings were complete, each reader’s estimated age was compared to the actual age of each animal to evaluate the accuracy of using counts of dentinal GLGs in acid etched canines to determine age for CSLs. Ages of known animals were rounded down to the nearest year for comparison.
For the second part of the study, multiple images were taken of each tooth using a high definition digital microscope camera (Leica M170-HD), at 10× magnification and stitched into one complete image using Image-Pro Premier software version: 9.3 64-bit (Media Cybernetics, Inc., Rockville, MD). These high-resolution images were examined by both readers to identify the GLGs with knowledge of the known-age and to identify if patterns could be associated between GLG width, tooth size or sex. The best images are discussed below.
Statistical Analysis
To measure the accuracy of the GLG counting method, we calculated the percentage of estimated ages that were to within 1 yr of the known-age. We measured the effects of CSL age, CSL sex, and reader on the age estimates by ANCOVA (type III sum of squares) in R Studio v 1.1.456. Coefficient of variation and index of precision were calculated for each reader’s age estimates.
Results
Accuracy of Age Estimates by Counting GLGs
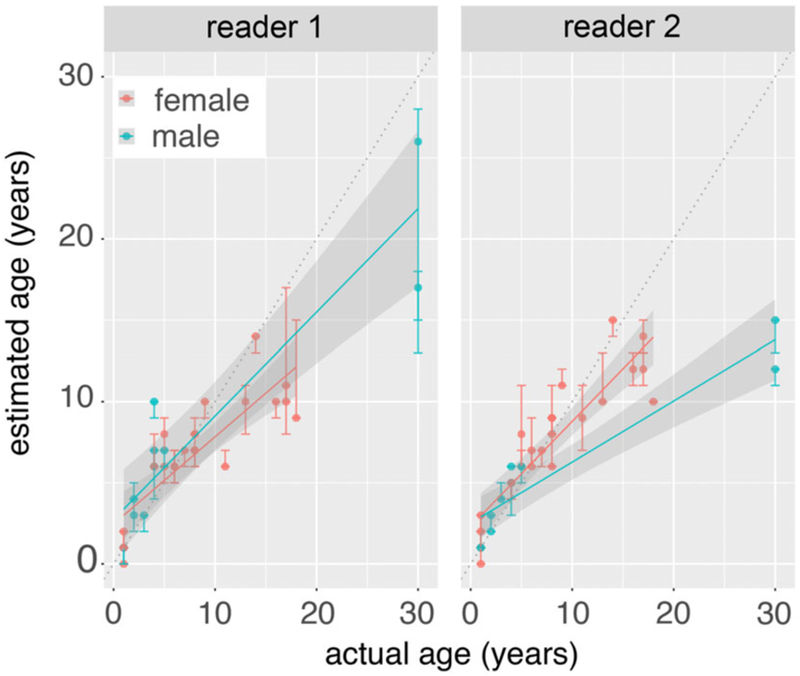
In a sample of 33 CSLs, approximately 55%-61% of age estimates by counting GLGs were within 1 yr of the known-age (Table 2). Accuracy of age estimates was dependent on age of the CSLs (P < 2E−16; Fig. 2, Table 3). Both readers underestimated the ages of CSLs >10 yr old in most cases (Fig. 2). In the subset of 24 CSLs that were <10 yr old, 71%–79% of age estimates were within 1 yr of the known-age, and 88%-96% were within 2 yr of the known-age (Table 2). Furthermore, in CSLs <10 yr old, Reader 1 estimated the known-age exactly in 10 CSLs (42%), and Reader 2 estimated known-age exactly in 8 CSLs (33%). While we failed to find that sex affected the accuracy of age estimates directly (P = 0.4727), an interaction detected using ANCOVA showed that the accuracy of age estimates varied between readers in the oldest male CSLs (P = 0.00198; Table 3).
Table 2.
Accuracy of age estimates relative to known ages.
| Reader 1 | Reader 2 | |||||
|---|---|---|---|---|---|---|
| Age ranges | 1–30 | <10 | >10 | 1–30 | <10 | >10 |
| Number of teeth | 33 | 24 | 9 | 33 | 24 | 9 |
| Accurate within 1 yr (%) | 55 | 71 | 11 | 61 | 79 | 11 |
| Accurate within 2 yr (%) | 67 | 88 | 11 | 76 | 96 | 22 |
| Accurate within 5 yr (%) | 82 | 96 | 44 | 91 | 100 | 67 |
| Accurate within 10 yr (%) | 100 | 100 | 89 | 94 | 100 | 78 |
| Accurate within 15 yr (%) | 100 | 100 | 100 | 97 | 100 | 89 |
| Accurate within 20 yr (%) | 100 | 100 | 100 | 100 | 100 | 100 |
Figure 2.
Accuracy of age estimates by counting GLGs in acid etched CSL canines. Plots show the relationship of the known-ages in 33 CSLs to the median + range of age estimates determined independently, in triplicate, by two blinded readers. Lines denote the linear regression lines + 95% confidence interval, separated by sea lion sex and dotted black lines denote the ideal (i.e., 1 to 1) relationship of known and estimated age.
Table 3.
ANCOVA of estimated age.
| Variable | Sum sq. | df | F | P |
|---|---|---|---|---|
| Intercept | 94.09 | 1 | 19.45 | 1.73E–05 |
| actual age | 630.47 | 1 | 130.33 | 2.54E–23 |
| Gender | 2.50 | 1 | 0.52 | 0.4728 |
| Reader | 0.60 | 1 | 0.12 | 0.7246 |
| actual age:gender | 0.02 | 1 | 0.00 | 0.9475 |
| actual age:reader | 3.92 | 1 | 0.81 | 0.3689 |
| gender:reader | 1.29 | 1 | 0.27 | 0.6068 |
| actual age:gender:reader | 47.60 | 1 | 9.84 | 0.0020 |
The mean coefficient of variation for age estimates by counting GLGs were 19.6% and 17.2% for Reader 1 and Reader 2, respectively (Table 1). In 20 of 24 (83%) CSLs <10 yr old, age estimates by the two readers were within 1 year (Table 1). Similarly, ANCOVA detected no significant main effects of the reader on the age estimates (P = 0.72459: Table 3).
GLG Patterns and Guidance
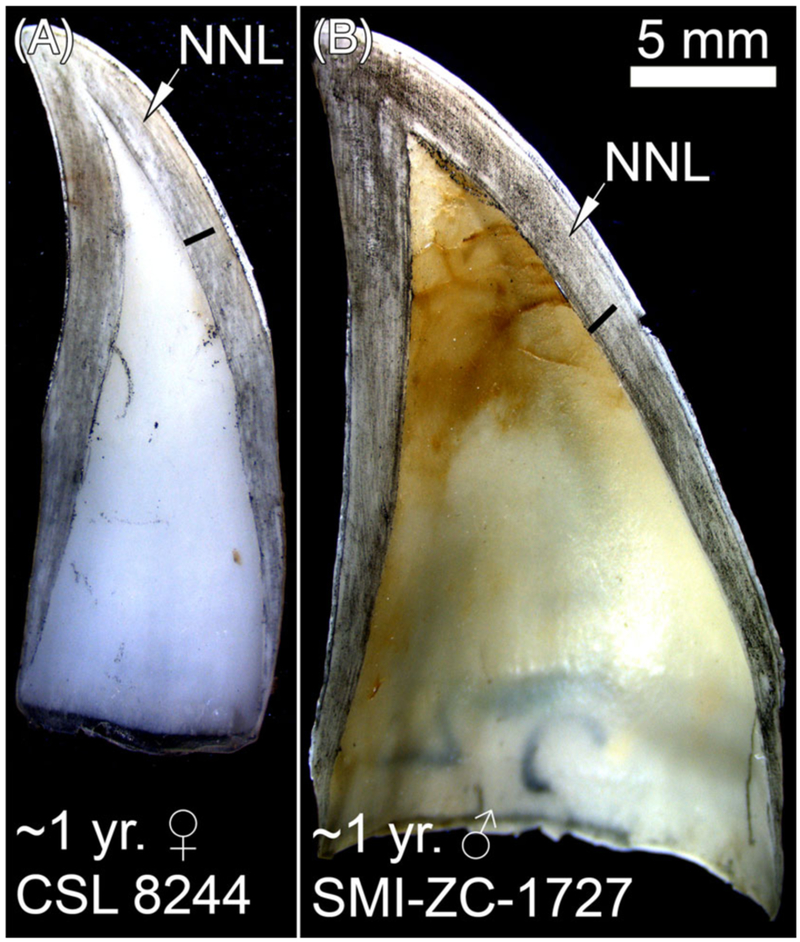
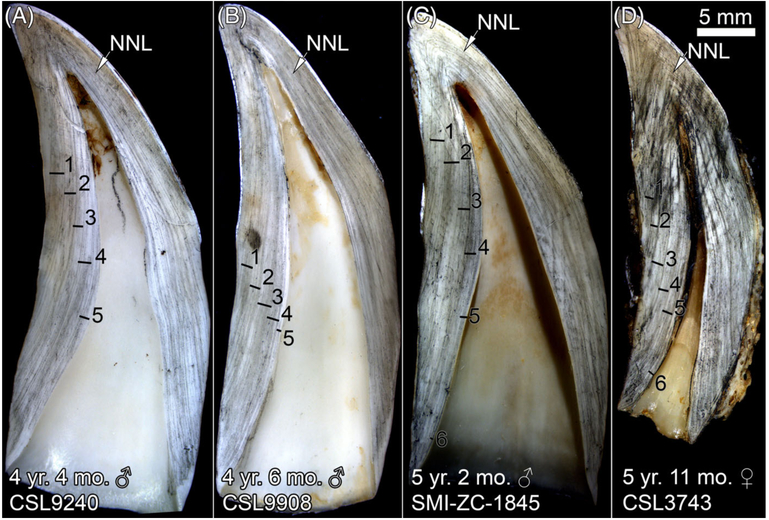
GLG boundaries are narrow, dark parallel lines counted from the NNL to the pulp cavity (Fig. 1). In young CSLs, the NNL was often difficult to discern. For example, the NNL in CSL 8244 is faint but can be followed almost to the end of the crown-root junction (Fig. 3A). The lack of a distinct GLG before the edge of the pulp cavity suggests that CSL 8244 is under 1 yr old. Likewise, the NNL is difficult to discern in SMI-ZC-1727 (Fig. 3B). Notice that both the tooth and pulp cavity are wider in the male than in the female (Fig. 3). In adult CSLs, the NNL was difficult to discern in some specimens (Fig. 4A, B, D), but easily visible in others (Fig. 4C).
Figure 3.
Sectioned teeth from CSLs approximately 1 yr old. Arrow, NNL = neonatal line, Black bar = GLG.
Figure 4.
Sectioned teeth from CSLs ranging from 4 to 5 yr old. Arrow, NNL = neonatal line, Black bar = GLG.
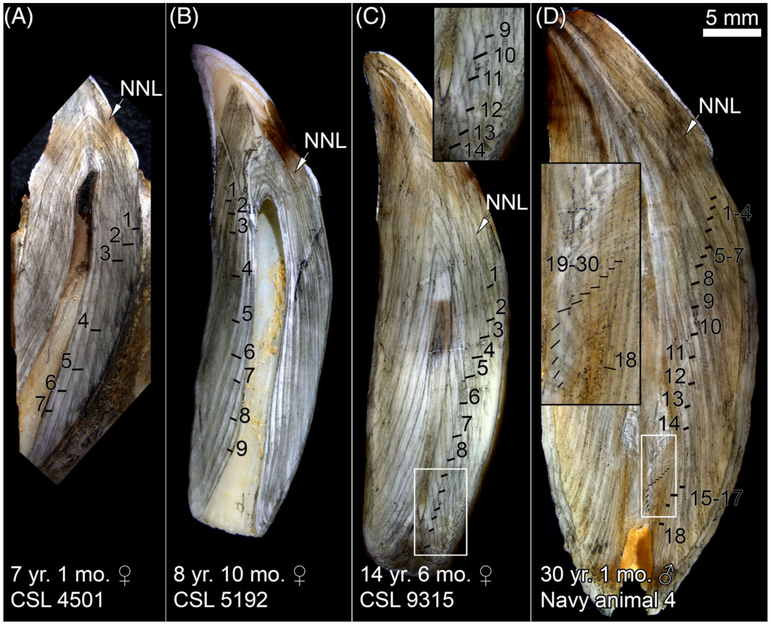
The GLGs were faint in some adult specimens but distinct in others (compare those in Fig. 4 to those in Fig. 5A–C). The fuzzy appearance of GLGs 4 and 5 in CSL 5192 (Fig. 5B) are likely due to the off-center cut of the tooth and the interrupted GLGs are an artifact of the cutting process. The brown staining in the top right enamel is due to uneven wear.
Figure 5.
Sectioned teeth from CSLs ranging from 7 yr to 30 yr old. Arrow, NNL = neonatal line, Black bar = GLG.
Accessory lines are dark lines that could be mistaken for GLG boundaries, but do not typically mirror the length or width of the adjacent GLG. An example of accessory lines can be seen in CSL 9240 (Fig. 4A), where narrowly spaced lines are present in GLGs 2 and 3. Accessory lines are also evident in GLG 1 of SMI-ZC-1845 (Fig. 4C).
Partial-year GLGs (towards the pulp cavity) were narrower than the preceding GLG and did not have a distinct dark edge (Fig. 4, 5). Because of the subjectivity of determining the months of a partial GLG, we rounded ages down to the last complete GLG.
As CSLs age, the pulp cavity narrows (compare pulp cavities in Fig. 5 to those in Fig. 4A–C) and narrowing of GLGs increases near the pulp cavity. Narrowing of the pulp cavity is accentuated in females as they grow (compare the female in Fig. 4D to males in Fig. 4A–C). In older CSLs, the recently laid GLGs close to the pulp cavity were only visible near the base of the tooth and became increasingly narrow near the pulp cavity and were more variable in orientation (Fig. 5). For example, in Navy animal 4 (Fig. 5D), GLGs are clear until GLG 17, then narrow. In the inset of Figure 5D, the remaining GLGs are tightly packed with some extending across the tooth rather than the length of the tooth. Both readers underestimated the age of this CSL due to the difficulty of discerning the small late GLGs with less distinct dark boundaries. The pulp cavity is not completely closed yet in this 30-yr-old male.
Discussion
Our findings support the accuracy of counting GLGs for age estimation of CSLs <10 yr old. The accuracy of age estimates using the GLG method in CSLs was dependent on CSL age and readers underestimated ages of CSLs >10 yr of age in almost every case. Accuracy in the present study was lower than other pinniped studies that aged animals <10 yr of age (Bowen et al. 1983, Mansfield 1991), but similar to studies of other otariids and polar bears, Ursus maritimus, that aged animals up to 15 yr (Childerhouse et al. 2004, Christensen-Dalsgaard et al. 2010) (Table 4). Comparing precision to that in other pinniped studies was difficult since CV and D values were not reported in these studies. Accuracy and precision could be improved in future CSL studies by using this known-age data set to practice identifying and counting GLGs across a range of ages. In addition, for imprecise ages, adding additional readings beyond the standard of three may help to improve accuracy (McCann 1993, Childerhouse et al. 2004).
Table 4.
A comparison table of aging studies using teeth, CV = coefficient of variation, D = index of precision.
| Study | Common name | Age ranges | Aging method | Age determination | CV (Reader 1/2) | D (Reader 1/2) | Validation |
|---|---|---|---|---|---|---|---|
| This study | California sea lion | <1–30 yr old | Acid etched upper canine | Median of 3 readings | 19.6/17.2 | 11.3/9.9 | 55/61% aged within 1 yr of actual age |
| Bowen et al. 1983 | Harp seal | 3 mo–10 yr old | Thin-sectioned lower canine | Mean of 5 readings | n/a | n/a | 83.4% aged exactly (mostly young animals) |
| Mansfield 1991 | Gray seal | 1–10 yr old | Thin-sectioned lower canine | 2/3 repeated readings used or 3+/5 repeated readings used | n/a | n/a | 93% aged exactly for 3 readings and 94% for 5 readings |
| Bernt 1996 | Gray seal | 2–20 yr old | Thin-sectioned incisor | 2/3 repeated readings used or mean of 3 readings | n/a | n/a | 83%, 63%, 93% aged exactly by readers 1, 2 and 3 |
| Childerhouse et al. 2004 | New Zealand sea lion | 4–15 yr old | Thin-sectioned postcanine | 2/3 repeated readings used or mean of 3 readings | n/a | n/a | 55% aged within 1 yr of actual age |
| Christensen-Dalsgaard et al. 2010 | Polar bear | 1–15 yr old | Thin-sectioned premolar | 3 readings | 18.2/15.2 | n/a (used a linear regression model) | 50/53% aged within 1 yr of actual age |
The lower accuracy in CSLs >10 yr is likely accentuated in this study since the majority of the teeth were from females (Table 1), which have narrower teeth than males of the same age class (Lowry and Folk 1990), and therefore have narrower GLGs (Fig. 3, 4). Mansfield (1991) reported that GLGs are better defined in male teeth.
Age estimation by counting GLGs provides a greater range of age estimation with reasonable accuracy than age estimation based on length or weight, which are only predictive for CSLs up to 3 yr and 3.5–8 mo, respectively (Laake et al. 2016). The GLG counting method should be useful for studies using specimens from deceased CSLs collected at rehabilitation centers as the majority of these animals are <10 yr of age (80% of intakes at The Marine Mammal Center). Male CSLs become sexually mature at 4–5 yr and females become reproductive between 5 yr and 7 yr of age (Melin et al. 2012). Thus, the GLG counting method provides sufficient accuracy to examine fishery impacts on juveniles and adults, two age classes of importance for population management (Laake et al. 2016). Estimation of reproductive parameters or survival rates using ages estimated via acid etched teeth will likely produce reasonable estimates for parameters associated with CSLs <10 yr but should be used with caution for older animals. Older animals were consistently underestimated in the present study; estimates could therefore reasonably be considered minimum estimates of age. Dendrochronology studies (Hanson et al. 2009, Knox et al. 2014, Hamilton and Evans 2018) that sample individual GLGs to look at temporal changes in the environment or other parameters should use younger teeth to reduce the chance of error in CSL studies.
Accuracy of future CSL age estimates by counting GLGs in acid etched CSL canines could be increased with the knowledge of GLG variability. For example, in old CSLs, we found that GLGs are densely compacted and do not always extend to the root of the tooth and may need to be counted near the center of the long axis (Fig. 5D). Also, estimating age of very young CSLs (1–2 yr) was challenging due to indistinct NNLs and GLGs. Also, narrower pulp cavities and greater compaction of GLGs should be expected in female CSLs due to the narrower width of their teeth (Lowry and Folk 1990). Thus, familiarity with the factors affecting accuracy of age estimation found using this known-age data set may increase the accuracy of age estimates in future studies. Whether decalcified and stained thin-sections or varying etching times improves the clarity of GLGs should also be explored in future studies. When possible, both canines should be collected to allow for a second preparation attempt when either curved teeth or human error results in a poor section. Awareness of tooth preparation artifacts, relative age, sex, accessory lines, and reader experience should improve the accuracy of age estimation.
Conclusion
This study demonstrates that counting annual GLGs in the dentine of acid etched canine teeth of CSLs is a reliable method of age estimation, particularly in CSLs <10 yr of age. Post hoc examination of the old teeth using the known-age as a guide revealed that diminished accuracy of age estimates in older animals was associated with increasing difficulty in identifying clear GLGs due to narrowing or closure of the pulp cavity and compaction of the GLGs with aging. Findings also suggest that reader experience and CSL sex are potential factors in the accuracy of age estimation by counting GLGs.
Acknowledgments
The authors would like to thank the U.S. Navy Marine Mammal Program, the National Marine Mammal Foundation, The Marine Mammal Center, California Academy of Sciences, and the Alaska Fisheries Science Center for the use of extracted teeth and correlated data. We would also like to thank the staff and volunteers at The Marine Mammal Center for extraction, cleaning, and preparation of the majority of the teeth in the study. Thank you to Kelly Robertson and Susan Chivers for reviewing and editing this manuscript. This project was conducted under permit MMPA 18786 to Frances Gulland. BAW was supported by NIH T32 DC005361.
Contributor Information
Lauren B. Rust, The Marine Mammal Center, 2000 Bunker Road, Sausalito, California 94965, U.S.A.;.
Kerri Danil, Southwest Fisheries Science Center, NMFS, NOAA, 8901 La Jolla Shores Drive, La Jolla, California 92037, U.S.A.;.
Sharon R. Melin, Alaska Fisheries Science Center, NMFS, NOAA, 7600 Sand Point Way N.E., Building 4, Seattle, Washington 98115, U.S.A.;
Brent Wilkerson, Department of Biological Sciences, University of Washington, Seattle, Washington 98195, U.S.A..
Literature Cited
- Anas RE 1970. Accuracy in assigning ages to fur seals. Journal of WildlifeManagement 34:844–852. [Google Scholar]
- Bargu S, Goldstein T, Roberts K, Li C and Gulland F. 2012. Pseudo-nitzschia blooms, domoic acid, and related California sea lion strandings in Monterey Bay, California. Marine Mammal Science 28:237–253. [Google Scholar]
- Benjaminsen T 1973. Age determination and the growth and age distribution from cementum growth layers of bearded seals at Svalbard. Fiskeridirektoratets skrifter, Serie Havundersøkelser 16:159–170. [Google Scholar]
- Bernt KE 1996. Age estimation of grey seals (Halichoerus grypus) using incisors. Marine Mammal Science 12:476–482. [Google Scholar]
- Bowen WD, Sergeant DE and Øritsland T. 1983. Validation of age estimation in the harp seal, Pagophilus groenlandicus, using dentinal annuli. Canadian Journal of Fisheries and Aquatic Sciences 40:1430–1441. [Google Scholar]
- Browning HM, Gulland FMD, Hammond JA, Colegrove KM, and Hall AJ. 2015. Common cancer in a wild animal: The California sea lion (Zalophus californianus) as an emerging model for carcinogenesis. Philosophical Transactions Royal Society B: Biological Sciences 370(1673): 20140228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carretta JV, Oleson EM, Baker J, et al. 2016. U.S. Pacific marine mammal stock assessments: 2015. U.S. Department of Commerce, NOAA Technical Memorandum NMFS-SWFSC-561. 419 pp. [Google Scholar]
- Carretta JV, Forney KA, Oleson EM, et al. 2017. U.S. Pacific marine mammal stock assessments: 2016. U.S. Department of Commerce, NOAA Technical Memorandum NMFS-SWFSC-577. 414 pp. [Google Scholar]
- Carrick R, and Ingham SE. 1962. Studies on the southern elephant seal, Mirounga leonina (L.). II. Canine tooth structure in relation to function and age determination. CSIRO Wildlife Research 7: 102–118. [Google Scholar]
- Childerhouse S, Dickie G and Hessel G. 2004. Ageing live New Zealand sea lions (Phocartos hookeri) using the first post-canine. Wildlife Research 31:177–181. [Google Scholar]
- Christensen-Dalsgaard SN, Aars J, Andersen M, Lockyer C and Yoccoz NG. 2010. Accuracy and precision in estimation of age of Norwegian Arctic polar bears (Ursus maritimus) using dental cementum layers from known-age individuals. Polar Biology 33:589–597. [Google Scholar]
- Colegrove KM, Gulland FMD, Naydan DK and Lowenstine LJ. 2009. The normal genital tract of the female California sea lion (Zalophus californianus): Cyclic changes in histomorphology and hormone receptor distribution. The Anatomical Record 292:1801–1817. [DOI] [PubMed] [Google Scholar]
- DeLong RL, and Melin SR. 2000. Thirty years of pinniped research at San Miguel Island. Pages 401–406 in Browne DR, Mitchell KL and Chaney HW, eds. Proceedings of the Fifth California Channel Islands Symposium. Santa Barbara Museum of Natural History, Santa Barbara, CA. [Google Scholar]
- DeLong RL, Melin SR, Laake JL, Morris P, Orr AJ and Harris JD. 2017. Age-and sex-specific survival of California sea lions (Zalophus californianus) on San Miguel Island, California. Marine Mammal Science 33:1097–1125. [Google Scholar]
- Evans K, and Robertson K. 2001. A note on the preparation of sperm whale (Physeter macrocephalus) teeth for age determination. Journal of Cetacean Research and Management 3:101–107. [Google Scholar]
- Finneran JJ, Dear R, Carder DA and Ridgway SH. 2003. Auditory and behavioral responses of California sea lions (Zalophus californianus) to single underwater impulses from an arc-gap transducer. Journal of the Acoustical Society of America 114:1667–1677. [DOI] [PubMed] [Google Scholar]
- Finneran JJ, Mulsow JL, E Schlundt C and Houser DS. 2011. Dolphin and sea lion auditory evoked potentials in response to single and multiple swept amplitude tones. Journal of the Acoustical Society of America 130:1038–1048. [DOI] [PubMed] [Google Scholar]
- Fletemeyer JR 1978. Laminate in the teeth of the Cape fur seal used for age determination. Life Science 22:695–698. [DOI] [PubMed] [Google Scholar]
- Gulland FMD, Koski M, Lowenstine LJ, Colagrass A, Morgan L and Spraker T. 1996a. Leptospirosis in California sea lions (Zalophus californianus) stranded along the central California coast, 1981–1994. Journal of Wildlife Diseases 32:572–580. [DOI] [PubMed] [Google Scholar]
- Gulland FMD 1999. Leptospirosis in marine mammals. Pages 469–471 in Fowler ME and Miller RE, eds. Zoo and wild animal medicine: Current therapy 4. W. B. Saunders Company, Philadelphia, PA. [Google Scholar]
- Gulland FMD, Trupkiewicz JG, Spraker TR and Lowenstine LJ. 1996b. Metastatic carcinoma of probable transitional cell origin in 66 free-living California sea lions (Zalophus californianus), 1979–1994. Journal of Wildlife Diseases 32:250–258. [DOI] [PubMed] [Google Scholar]
- Gulland FMD, Haulena M, Fauquier D, et al. 2002. Domoic acid toxicity in Californian sea lions (Zalophus californianus): Clinical signs, treatment and survival. Veterinary Record 150:475–480. [DOI] [PubMed] [Google Scholar]
- Hanson NN, Wurster CM, Bird MI, Reid K and Boyd IL. 2009. Intrinsic and extrinsic forcing in life histories: Patterns of growth and stable isotopes in male Antarctic fur seal teeth. Marine Ecology Progress Series 388:263–272. [Google Scholar]
- Hamilton V, and Evans K. 2018. Establishing growth chronologies from marine mammal teeth: A method applicable across species. Journal of Experimental Marine Biology and Ecology 505:24–34. [Google Scholar]
- Hewer HR 1964. The determination of age, sexual maturity, longevity and a life table in the grey seal (Halichoerus grypus). Proceedings of the Zoological Society of London 142:593–634. [Google Scholar]
- Hohn AA, Scott MD, Wells RS, Sweeney JC and Irvine AB. 1989. Growth layers in teeth from known-age, free-ranging bottlenose dolphins. Marine Mammal Science 5:315–342. [Google Scholar]
- Houser DS, Finneran JJ and Ridgway SH. 2010. Research with Navy marine mammals benefits animal care, conservation and biology. International Journal of Comparative Psychology 23:249–268. [Google Scholar]
- King JC, Gelatt TS, Pitcher KW and Pendelton GW. 2007. A field based method for estimating age in free-ranging Steller sea lion (Eumetopias jubatus) less than twenty-four months of age. Marine Mammal Science 23:262–271. [Google Scholar]
- Klevezal GA, and Kleinenberg SE. 1967. Age determination of mammals from annual layers in teeth and bones. Fisheries Research Board of Canada Translation Series 1024. 142 pp. Available at http://www.dfo-mpo.gc.ca/Library/137851.pdf. [Google Scholar]
- Knox TC, Stuart-Williams H, Warneke RM, Hoskins AJ and Arnould JPY. 2014. Analysis of growth and stable isotopes in teeth of male Australian fur seals reveals interannual variability in prey resources. Marine Mammal Science 30:763–781. [Google Scholar]
- Laake JL, Melin SR, Orr AJ, Greig DJ, Prager KC, DeLong RL and Harris JD. 2016. California sea lion sex- and age-specific morphometry. U.S. Department of Commerce, NOAA Technical Memorandum NMFSAFSC-312. 21 pp. [Google Scholar]
- Laws RM 1952. A new method of age determination for mammals. Nature 169:972–973. [DOI] [PubMed] [Google Scholar]
- Lawson J, Hamilton G and Bowen W. 1992. Factors affecting accuracy of age determination in the harp seal, Phoca groenlandica. Marine Mammal Science 8:169–171. [Google Scholar]
- Lipscomb TP, Scott DP, Garber RL, et al. 2000. Common metastatic carcinoma of California sea lions (Zalophus californianus): Evidence of genital origin and association with novel gammaherpesvirus. Veterinary Pathology 37:609–617. [DOI] [PubMed] [Google Scholar]
- Lowry MS, and Folk RL. 1990. Sex determination of the California sea lion (Zalophus californianus) from canine teeth. Marine Mammal Science 6: 25–31. [Google Scholar]
- Mansfield AW 1991. Accuracy of age determination in the grey seal Halichoerus grypus of Eastern Canada. Marine Mammal Science 7:44–49. [Google Scholar]
- McCann TS 1993. Age determination. Pages 199–227 in Laws RM, ed. Antarctic seals: Research, methods and techniques. Cambridge University Press, Cambridge, U.K. [Google Scholar]
- McKenzie J, Page B, Shaughnessy PD and Hindell MA. 2007. Age and reproductive maturity of New Zealand fur seals (Arctocephalus forsteri) in southern Australia. Journal of Mammalogy 88:639–648. [Google Scholar]
- Melin SR, Laake JL, DeLong RL and Siniff DB. 2012. Age-specific recruitment and natality of California sea lions at San Miguel Island, California. Marine Mammal Science 28:751–776. [Google Scholar]
- Morris PA 1972. A review of mammalian age determination methods. Mammal Review 2:69–104. [Google Scholar]
- Owen R 1840. Treatise on the comparative anatomy of the teeth, their physiological relations, mode of development, and microscopic structures, in the vertebrate animals. Hippolyte Bailliere, London, U.K. [PMC free article] [PubMed] [Google Scholar]
- Scheffer VB 1950. Growth layers on the teeth of Pinnipedia as an indication of age. Science 112:309–311. [DOI] [PubMed] [Google Scholar]
- Scheffer VB, and Peterson RS. 1967. Growth layers in teeth of suckling fur seals. Growth 31:35–38. [PubMed] [Google Scholar]
- Scholin CA, Gulland F, Doucette GJ, et al. 2000. Mortality of sea lions along the central California coast linked to a toxic diatom bloom. Nature 403: 80–84. [DOI] [PubMed] [Google Scholar]
- Smit A-M. 2003. Age determination and establishment of reproductive condition from marine mammal biopsy sampling. DOC Science Internal Series 133. 13 pp. [Google Scholar]
- Stewart REA, Stewart BE, Stirling I and Street E. 1996. Counts of growth layer groups in cementum and dentine in ringed seals (Phoca hispida). Marine Mammal Science 12:383–401. [Google Scholar]
- Thomas DC 1977. Metachromatic staining of dental cementum for mammalian age determination. Journal of Wildlife Management 41:207–210. [Google Scholar]