The past year has seen the rapid global spread of SARS-CoV-2—the virus responsible for the ongoing COVID-19 pandemic. While non-pharmaceutical interventions have been the mainstay of epidemic control to date, vaccination is likely to constitute the definitive, long-term defence strategy against SARS-CoV-2 morbidity, mortality, and transmission, offering the best hope of a return to normal life.
The urgent need for effective vaccines has prompted vaccine developers to pivot towards COVID-19, resulting in rapid growth of preclinical candidates (appendix) and an accelerated vaccine development pipeline.1 In response to the unfolding pandemic and the extraordinary volume and pace of global vaccine research, we developed an online, interactive vaccine tracker hosted by the Vaccine Centre (VaC) at the London School of Hygiene and Tropical Medicine (LSHTM; London, UK). Launched in April 2020, this tracker aims to collate up-to-date information on all COVID-19 vaccine candidates from inception through to deployment, enabling policy makers, researchers, and the public to keep informed of the rapid developments. All code and underlying data for the tracker are freely available and are updated regularly through a Github repository.
The LSHTM VaC tracker is focused on presenting a comprehensive and up-to-date overview of the global vaccine landscape, provided in a user-friendly and engaging format. The landscape feature is updated weekly, collating information on all preclinical and clinical candidates from the WHO COVID-19 vaccine landscape2 and the Milken Institute's tracker,3 as well as information provided directly by developers. An interactive timeline displays clinical trial dates and locations for each candidate, providing users with the option to subset vaccines by platform and development phase.
To summarise key metrics of active and planned vaccine trials, the tracker includes a clinical trials database that captures specifications such as locations, dates, size, study design, masking, randomisation, and recruitment status of registered COVID-19 vaccine trials in humans. Newly registered or updated protocols are identified weekly via the US National Institutes of Health's clinical trials database and the WHO landscape.2 For candidates undergoing phase 3 efficacy testing, clinical trial metrics are fed into an interactive trial map, which displays the global distribution of trials alongside the national case counts in each trial location (derived from the Johns Hopkins University real-time COVID-19 dashboard4), helping place studies within their local epidemiological context.
As data from clinical trials started to become available in peer-reviewed journals and on preprint servers, the need for a single resource presenting results in a standardised and succinct format became increasingly evident. Accordingly, we launched a living review feature on Aug 24, 2020, which allows users to visualise and compare vaccine trial attributes, safety, immunogenicity, and efficacy data from each published report. The living review is updated through weekly searches of medRxiv and PubMed, using the terms “(coronavirus OR COVID OR SARS*) AND vaccin* AND (trial OR phase)”. Descriptive and quantitative data on study design, participant characteristics, safety, immunogenicity, and efficacy are extracted by a single reviewer and then verified by a second. For each study, we present the dosing regimens, randomisation and masking procedures, general inclusion criteria, and the inclusion of pregnant women and HIV-positive individuals. We report any vaccine-related serious adverse events, regardless of prevalence, and any non-serious adverse events with prevalence of 25% or more in one or more study groups. Antibody levels at baseline and 28 days after the final dose (or the nearest available time point) are extracted for each vaccine dosing regimen. Primary outcomes of interest include antigen-specific IgG and neutralising antibodies against wild-type SARS-CoV-2 or pseudovirus, or both. Data on CD4-positive and CD8-positive T-cell responses and any T helper type 1 versus T helper type 2 bias are reported where available. For phase 3 trials, we present efficacy against virologically confirmed symptomatic COVID-19, severe COVID-19, and asymptomatic SARS-CoV-2 infection, stratified by age, ethnicity, or comorbidity status where available.
Finally, with an increasing number of candidates moving from clinical testing to real-world roll-out—albeit not always after publication of phase 3 data—we recently incorporated an implementation feature on the LSHTM VaC tracker. This feature summarises information on vaccine storage requirements, manufacture projections, approval or licensure status, and ONE's Vaccine Access Test scores.5 For each vaccine, we also display the number of countries reporting roll-out based on statistics compiled by Our World in Data.6 As such, the LSHTM VaC tracker summarises all stages of the COVID-19 vaccine development pipeline, from preclinical development through to licensure and implementation.
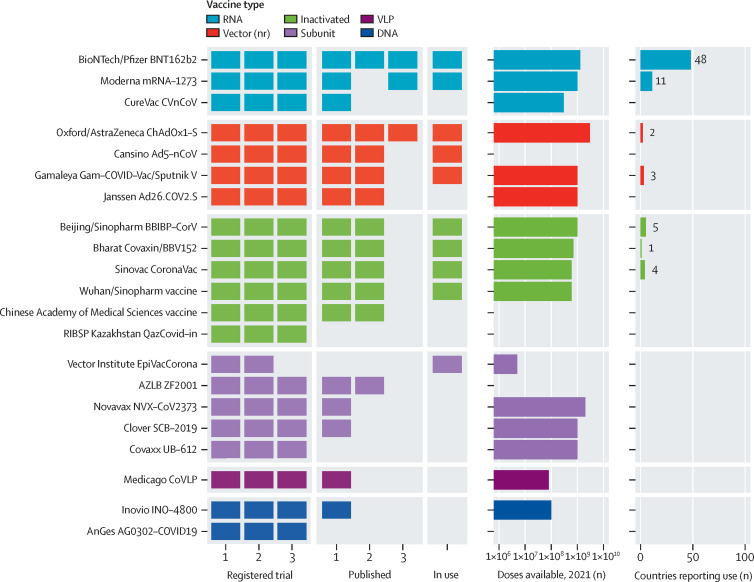
The COVID-19 pandemic has spurred advances in vaccine development at an extraordinary scale, and the LSHTM VaC tracker provides a clear testimony of this progress. As of Jan 26, 2021, the COVID-19 vaccine landscape featured 291 candidates (appendix). Our clinical trials database includes 156 registered studies spanning 70 separate candidates, of which 20 are currently undergoing phase 3 efficacy testing (figure ). Innovations in platforms, manufacturing, delivery, and trial design have been notable, for example RNA-based, plant-derived, and intranasal vaccines, and combined phase 1/2 or 2/3 trials. 31 published reports spanning 18 separate candidates have shown encouraging safety and immunogenicity profiles for inactivated (n=5), non-replicating viral vector (n=4), RNA (n=3), protein subunit (n=4), DNA (n=1), and virus-like particle (n=1) vaccines; and three published reports have confirmed the potential of vaccines to yield robust protective efficacy against COVID-19. We can only hope that further candidates follow—as the scale of the pandemic escalates, the urgent need for multiple effective vaccines and their equitable global distribution is clear.
Figure.
Status of the COVID-19 vaccine landscape
Includes vaccine candidates in phase 3 testing, use, or both as of Jan 26, 2021. AZLB=Anhui Zhifei Longcom Biopharmaceutical. nr=non-replicating. RIBSP=Research Institute for Biological Safety Problems. VLP=virus-like particle.
The rapid progress in COVID-19 vaccine development is a testament not only to the dedication and innovation of research teams, but also to the public who have volunteered for clinical testing. Active or planned trials include more than 690 000 individuals across the globe, and this number will grow as further studies commence. This altruism must be matched with clear and transparent information regarding COVID-19 vaccine safety, efficacy, and implementation policy decisions in the national and global context. The implications of the ongoing vaccine roll-out extend beyond COVID-19 and will set the tone for public confidence and enthusiasm regarding vaccination for decades to come.
Acknowledgments
We declare no competing interests.
Supplementary Material
References
- 1.Krammer F. SARS-CoV-2 vaccines in development. Nature. 2020;586:516–527. doi: 10.1038/s41586-020-2798-3. [DOI] [PubMed] [Google Scholar]
- 2.WHO Draft landscape of COVID-19 candidate vaccines. 2021. https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines
- 3.Milken Institute COVID-19 treatment and vaccine tracker. https://covid-19tracker.milkeninstitute.org
- 4.Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;20:533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.ONE Vaccine access test. 2021. https://www.one.org/international/vaccine-access-test/
- 6.Our World in Data Coronavirus (COVID-19) vaccinations. 2021. https://ourworldindata.org/covid-vaccinations
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.