Abstract
Background
A rapidly increasing number of serological surveys for antibodies to SARS-CoV-2 have been reported worldwide. We aimed to synthesise, combine, and assess this large corpus of data.
Methods
In this systematic review and meta-analysis, we searched PubMed, Embase, Web of Science, and five preprint servers for articles published in English between Dec 1, 2019, and Dec 22, 2020. Studies evaluating SARS-CoV-2 seroprevalence in humans after the first identified case in the area were included. Studies that only reported serological responses among patients with COVID-19, those using known infection status samples, or any animal experiments were all excluded. All data used for analysis were extracted from included papers. Study quality was assessed using a standardised scale. We estimated age-specific, sex-specific, and race-specific seroprevalence by WHO regions and subpopulations with different levels of exposures, and the ratio of serology-identified infections to virologically confirmed cases. This study is registered with PROSPERO, CRD42020198253.
Findings
16 506 studies were identified in the initial search, 2523 were assessed for eligibility after removal of duplicates and inappropriate titles and abstracts, and 404 serological studies (representing tests in 5 168 360 individuals) were included in the meta-analysis. In the 82 studies of higher quality, close contacts (18·0%, 95% CI 15·7–20·3) and high-risk health-care workers (17·1%, 9·9–24·4) had higher seroprevalence than did low-risk health-care workers (4·2%, 1·5–6·9) and the general population (8·0%, 6·8–9·2). The heterogeneity between included studies was high, with an overall I2 of 99·9% (p<0·0001). Seroprevalence varied greatly across WHO regions, with the lowest seroprevalence of general populations in the Western Pacific region (1·7%, 95% CI 0·0–5·0). The pooled infection-to-case ratio was similar between the region of the Americas (6·9, 95% CI 2·7–17·3) and the European region (8·4, 6·5–10·7), but higher in India (56·5, 28·5–112·0), the only country in the South-East Asia region with data.
Interpretation
Antibody-mediated herd immunity is far from being reached in most settings. Estimates of the ratio of serologically detected infections per virologically confirmed cases across WHO regions can help provide insights into the true proportion of the population infected from routine confirmation data.
Funding
National Science Fund for Distinguished Young Scholars, Key Emergency Project of Shanghai Science and Technology Committee, Program of Shanghai Academic/Technology Research Leader, National Science and Technology Major project of China, the US National Institutes of Health.
Translation
For the Chinese translation of the abstract see Supplementary Materials section.
Introduction
The COVID-19 pandemic, caused by SARS-CoV-2, was first reported in Wuhan, China, in December, 2019, and quickly spread globally.1 As of Feb 9, 2021, more than 100 million COVID-19 cases, including 2 316 389 deaths, had been reported in 223 countries or regions.2 The true number of SARS-CoV-2 infections is undoubtedly much higher than the officially reported number of cases due to various factors, including the occurrence of asymptomatic infections, variable seeking of health care for clinically mild cases, varied testing strategies in different countries, false-negative virological tests, and incomplete case reporting. Therefore, the reported COVID-19 cases based on clinical identification with virological confirmation only represent a small proportion, with a large number of asymptomatic and mild infections in the general population that might only be identified by seroepidemiological studies.3
Serological studies are a useful tool to estimate the proportion of the population previously infected, to quantify the magnitude of transmission of pathogens, estimate the infection fatality rate,4 assess the effect of interventions,5 and when correlates of protection are available, estimate the degree of population immunity.6, 7 Insights from serological surveillance can be valuable for policy makers and health officials when planning public health decision making.
Several serological investigations across the world have been published during the 12 months of the COVID-19 pandemic, with highly variable estimates of seroprevalence that could largely be due to differences in attack rates, but which also feature heterogeneous sampling strategies and assays used. Several systematic reviews and meta-analyses of SARS-CoV-2 seroprevalence were identified but had limited scope and did not investigate important differences between subpopulations, quantitatively assess study quality, or estimate the infection-to-case ratio.8, 9, 10, 11, 12
Research in context.
Evidence before this study
Serological evidence for SARS-CoV-2 infection is essential to understand the proportion of the population previously infected. Many serological investigations across the world have been done and the data analysed. We searched PubMed, Embase, Web of Science, and five preprint servers for articles published between Dec 1, 2019, and Dec 22, 2020, with the following primary search terms: “SARS-CoV-2”, “COVID-19”, “seroprevalence”, “antibodies”, and “seroepidemiological”. Inclusion criteria were articles published in English that evaluated SARS-CoV-2 seroprevalence in humans after the first identified case in the area, and which reported the assays used. Several narrative reviews only summarised serological data at the early stage of the COVID-19 pandemic without using standard meta-analysis techniques. Another two meta-analyses separately estimated the seroprevalence of general populations and health-care workers, rather than providing a comprehensive assessment of seroprevalence in subpopulations with different levels of exposures. None of these reviews have made pooled estimates of the infection-to-case ratio (serologically detected infections per virologically confirmed cases).
Added value of this study
This systematic review and meta-analysis includes serological data for more than 5 168 360 study participants from 404 serosurveys and provides a comprehensive assessment of the seroprevalence of SARS-CoV-2 human infections. On the basis of study design, laboratory method, and outcome correction, we systematically assessed the overall quality of the existing seroprevalence studies of SARS-CoV-2 and found that it was generally low. A higher prevalence of SARS-CoV-2-specific antibodies was observed in close contacts (18·0%) and high-risk health-care workers (17·1%) than in low-risk health-care workers (4·2%) and the general population (8·0%). Seroprevalence varied hugely across WHO regions, with the highest seroprevalence of general populations in the South-East Asia region (19·6%) and the lowest in the Western Pacific region (1·7%). We also found that young people (<20 years) and older people (≥65 years) were less likely to be seropositive than were individuals aged 20–64 years, and no significant difference was found between men and women. The pooled infection-to-case ratio was similar between the region of the Americas and the European region, but higher in the South-East Asia region.
Implications of all the available evidence
Overall, existing serological evidence shows a higher infection risk among close contacts and health-care workers who do not have access to personal protective equipment. The relatively low prevalence of SARS-CoV-2-specific antibodies among general populations suggests that most populations examined have not been infected, and herd immunity is far from being achieved in most settings. The general low quality of most of the existing seroprevalence studies indicates the effect of differences in study design, laboratory methods, and outcome adjustment on the interpretability of serological studies of human infections with SARS-CoV-2. Therefore, international collaborations to standardise serological survey and laboratory methods are urgently required.
We did a systematic review and meta-analysis to summarise serological surveys for SARS-CoV-2 infections in humans; to comprehensively evaluate the study designs, laboratory methods, and outcome interpretations for each included serological study; and to estimate the risk of infections by populations with different presumed levels of exposure to SARS-CoV-2. We aim for these results to help inform decision makers and researchers as plans are made for the next phases of the global pandemic.
Methods
Search strategy and selection criteria
According to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines13 we did a systematic literature review from three peer-reviewed databases (PubMed, Embase, and Web of Science) and five preprint severs (medRxiv, bioRxiv, SSRN, Wellcome Open Research, and Europe PMC) with predefined search terms (appendix 2 pp 10–11). The search terms used for PubMed were as follows: “2019-nCoV” OR “coronavirus disease 2019” OR “COVID-19” OR “severe acute respiratory syndrome coronavirus 2” OR “SARS-CoV-2” AND (seroprevalen* OR seroincidenc* OR seroconversion OR seronegative OR seropositive* OR seroepidemiolog* OR serolog* OR serosurvey* OR antibod* OR infection* AND (“attack rate” OR “cumulative incidence”). Two independent researchers (Xinh C, ZC) screened titles and abstracts of papers published in English from Dec 1, 2019, to Dec 22, 2020, meeting the following criteria: (1) a report of seroprevalence in either the general population or some other well-defined population of non-COVID-19 clinical cases; (2) done after the first reported case in the area; and (3) reporting the specific assays used. We excluded studies that only reported serological responses among patients with COVID-19, those using samples with known infection status (eg, validation studies of assays), and animal experiments. We excluded abstracts of congress meetings or conference proceedings, study protocols, media news, commentaries, reviews, or case reports.
The full texts of included studies after initial screening were scrutinised to assess the overall eligibility based on the inclusion and exclusion criteria by two independent researchers (Xinh C, ZC). A third researcher (HY) was consulted when the two reviewers disagreed on study assessment. For eligible studies, data were extracted by researchers (Xinh C, ZC) on the number of participants who provided specimens and the number of these who were seropositive to calculate the seroprevalence. When data were inconsistent between reviewers, they were asked to discuss and revisit the article until reaching a consensus. If key information, such as the use of personal protective equipment (PPE) of health-care workers, was not reported in the paper, we contacted the corresponding author via email. For each included study, we described its characteristics, laboratory testing method, and primary outcome (appendix 2 pp 12–144).
To assess study quality, we developed a scoring system on the basis of a seroepidemiological protocol from the Consortium for the Standardization of Influenza Seroepidemiology,14 a previously published scoring system for seroprevalence studies of zoonotic influenza viruses,15 and a seroepidemiological protocol developed by WHO.16 We comprehensively assessed study design (representativeness of study participants), laboratory assay (whether internal assay validations or a confirmatory assay was done), and outcome adjustment (correction for demographics or test performance, or both) and an overall score was determined for each included study (appendix 2 pp 145–46). From this score, two researchers (Xinh C, ZC) classified each study's quality into one of four grades: A, B, C, or D. When disagreements arose, a third investigator (HY) was consulted. Grade A, the highest quality category, spanned studies with scores ranging from 10 to 12, grade B from 7 to 9, grade C from 4 to 6, and grade D from 0 to 3. We only included grade A and grade B studies in the main analysis but provide additional results with all studies, irrespective of grade, in appendix 2 (pp 205–06).
Data analysis
Seroprevalence was defined as the prevalence of SARS-CoV-2-specific antibodies at or above a designated antibody titre to define a seropositive result in each original study. For serial cross-sectional studies, we calculated the sum of the total number of participants who provided specimens and total number of seropositive individuals during the whole study period, to avoid repeated inclusion of the same study. Similarly, only data from the first blood collection were analysed for studies with a longitudinal design to limit selection bias associated with retention in the study. For studies that used multiple serological assays, we used the seropositive results from the assay with the highest sensitivity and specificity (calculated by Youden's index). If a study used a confirmatory assay (eg, microneutralisation assay) to validate the positive or equivocal result from the initial screening, we used the results from the confirmatory assay. We also did a sensitivity analysis with results from the other assays (sensitivity analysis 1) and with individuals considered positive if they tested positive in at least one single assay (sensitivity analysis 2). For studies reporting multiple isotypes including IgG, we included only IgG in the main analyses because these isotypes remain elevated for a longer period post-infection than do IgM and IgA.17 If seropositivity based only on IgG was not reported separately or seropositivity reported was based only on total antibodies, these results were also included. Although many studies did adjust for various factors, we decided to use the crude (unadjusted) estimates in our analyses to ease interpretation across different studies and did a sensitivity analysis with seroprevalence adjusted for test performance by using Bayesian measurement error models (sensitivity analysis 3).18
To reduce heterogeneity between individual seroprevalence estimates and to provide more policy-relevant summary statistics, we stratified eligible studies by WHO regions (ie, African region, region of the Americas, Eastern Mediterranean region, European region, South-East Asia region, and Western Pacific region).19 To estimate seroprevalence by different types of exposure, within each WHO region, we categorised all study participants into five groups: (1) close contacts, (2) high-risk health-care workers, (3) low-risk health-care workers, (4) general populations, and (5) poorly defined populations (appendix 2 pp 147–48). The poorly defined population classification represents populations with undefined or unknown exposure to patients with laboratory-confirmed or suspected COVID-19 (eg, blood donors, residual blood samples from laboratories, patients with other diseases), as well as study populations that cannot be categorised into the first four study populations due to limited exposure information (eg, health-care workers without reporting use of PPE or COVID-19-related exposures). On the basis of a random-effect meta-analysis model, we used the inverse variance method to estimate pooled seroprevalence by WHO regions and different subpopulations, combined with the use of the Clopper-Pearson method to calculate 95% CIs.20, 21
For seroprevalence estimates from the general population, we further explored potential determinants affecting the seroprevalence, such as sex, age, race, and the reported cumulative incidence of virologically confirmed SARS-CoV-2 infections (referred to throughout as COVID-19 cases or confirmed COVID-19) for the location. Age-specific, sex-specific, and race-specific seroprevalence and corresponding relative risk (RR) by WHO regions were estimated. Due to evidence that the median time to IgG seroconversion is about 2 weeks,22, 23 we calculated cumulative incidence of confirmed COVID-19 by dividing the number of cases reported in the same target population as the serosurvey 2 weeks before the serosurvey mid-point at the location, by the estimated population size. Spearman's rank correlation was established between the cumulative incidence and the seroprevalence among studies involving the general population and, following this, the corresponding correlation coefficient was calculated. Furthermore, we meta-analysed the number of serologically detected infections (the number of individuals with positive SARS-CoV-2 serology) per confirmed case (the number of reported cases in the target population of the serological study), which we refer to as the infection-to-case ratio, with available epidemiological data included in the articles and other sources.24 Unless reported in the article, we used population size estimates from WorldPop25 or a local statistics bureau.26 Studies were included in the meta-analyses of the ratio of serologically detected infections per confirmed cases if they reported seroprevalence in the representative general population (non-convenience sample) with population data and confirmed case data available for the same population. We estimated the pooled infection-to-case ratio with a random-effect meta-analysis model using inverse variance weighting.
Variability between studies was determined by the heterogeneity tests with Higgins' I 2 statistic. We explored the reasons for variations among eligible studies and examined whether prevalence of SARS-CoV-2-specific antibodies varied by study location, study quality, level of exposure, and test performance by multivariable meta-regression models. For all statistical tests, a two tailed p value of less than 0·05 was considered statistically significant. All statistical analyses were done with R (version 3.6.3), with the meta package to do the meta-analysis. This study is registered with PROSPERO, CRD42020198253.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
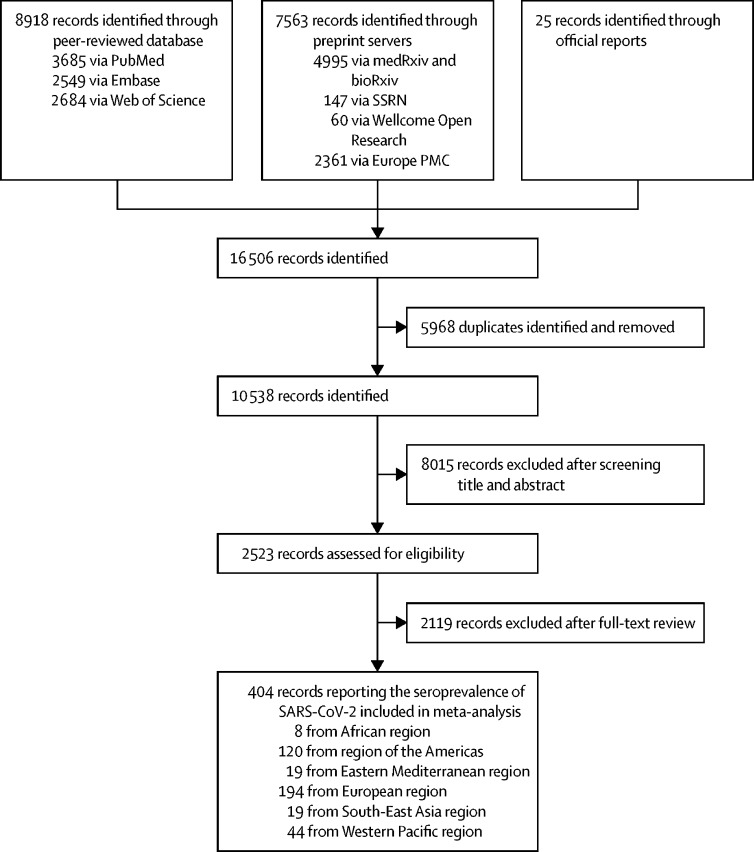
We identified a total of 16 506 studies after systematically searching multiple data sources, with 8918 identified from peer-reviewed databases, 7563 from preprint severs, and 25 reports from governments or health authorities. After excluding 5968 duplicates and a further 8015 following screening of titles and abstracts, 2523 studies reporting serological evidence of SARS-CoV-2 infections were assessed for eligibility. 2119 studies were considered ineligible for inclusion, resulting in a total of 404 studies involving 5 168 360 participants included in the meta-analysis after full-text scrutiny (figure 1 ). Most studies were done in the European region (n=194), the region of the Americas (n=120), and the Western Pacific region (n=44; figure 1, figure 2 ; appendix 2 p 218). Among 388 studies reporting the exact starting sampling date, 18 (5%) were done after more than 75% of the total cases (in that country, state, or province) had been reported as of Dec 22, 2020, most of which (17 of 18, 94%) were done in China (appendix 2 pp 208–17).
Figure 1.
Study selection
Flowchart of the selection of serological studies of SARS-CoV-2 infection from Dec 1, 2019, to Dec 22, 2020.
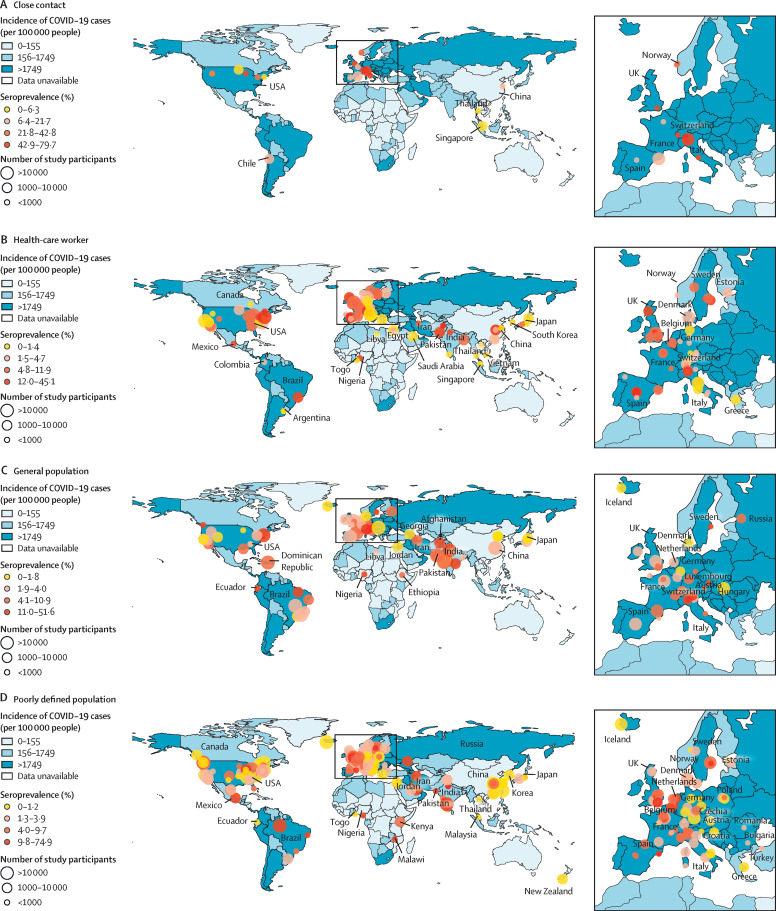
Figure 2.
Geographical distribution of SARS-CoV-2 serosurveys by study populations from Dec 1, 2019, to Dec 22, 2020
The colours on the maps indicate the cumulative incidence of reported cases, with darker colours representing higher values. Cumulative incidence data are reproduced from the WHO COVID-19 Dashboard.
The overall quality of studies was low based on our grading system, with only 20% (82 of 404) classified as grade A or grade B studies included in the main analysis (appendix 2 pp 182–84). Most studies were categorised as grade C or grade D, including all but two studies of high-risk health-care workers and close contacts. Of the 84 general population-based studies, ten were classified as grade A and 28 were classified as grade B (appendix 2 p 207).
About two thirds of studies (259 of 404, 64%) described serological results from convenience samples, while 45 studies (11%) used multistage or stratified random sampling to select study participants. Most studies measured IgG antibodies using chemiluminescence immunoassays (182 of 404, 45%), followed by ELISA, (162 of 404, 40%), and lateral flow immunoassays (97 of 404, 24%), with 20% (82 of 404) of studies using more than one serological assay. Additionally, 42 studies used a neutralisation assay to detect neutralising antibodies. Among 323 studies that reported the target protein for serological assays, 219 (68%) studies used tests targeting the S protein, and 191 (59%) used tests targeting the N protein. More than half of the studies (243 of 404, 60%) reported age-specific or sex-specific seroprevalence or corrected their findings for age or sex or both. 60 studies (15%) adjusted for sensitivity and specificity of the serological assays.
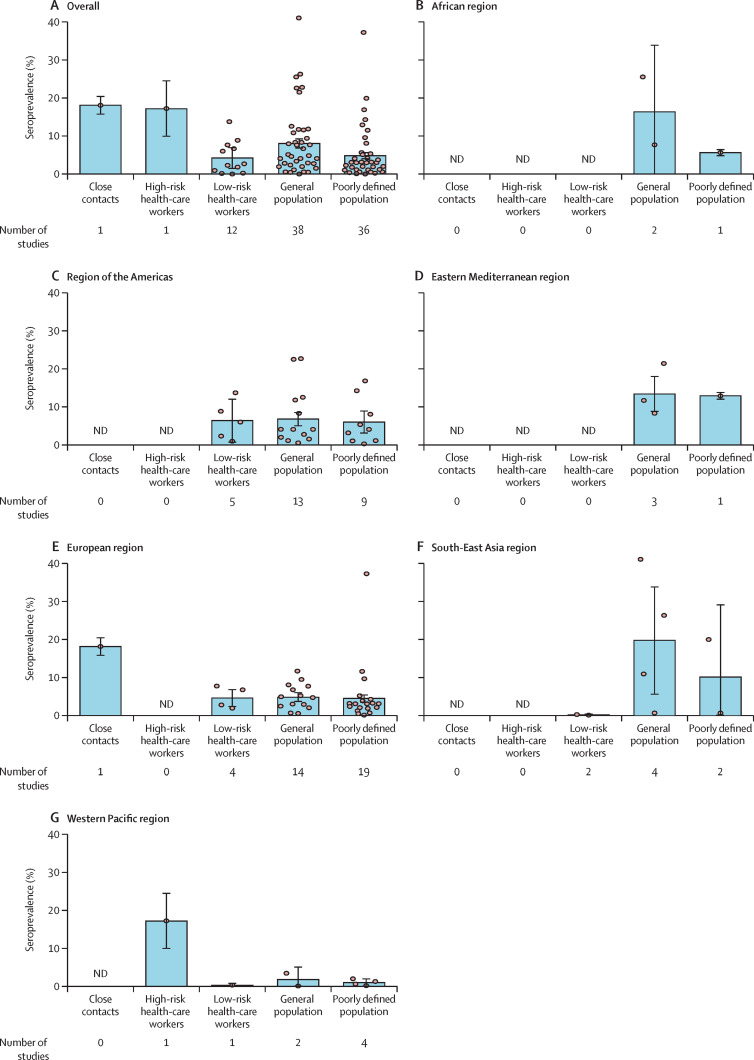
Among 82 grade A and grade B studies, seroprevalence varied across WHO regions and study populations (figure 3 ). Generally, close contacts (18·0%, 95% CI 15·7–20·3) and high-risk health-care workers (17·1%, 9·9–24·4) had a higher seroprevalence than did low-risk health-care workers (4·2%, 1·5–6·9) and general populations (8·0%, 6·8–9·2; figure 3A, appendix 2 pp 185–87). The seroprevalence of the populations in studies that did not specify exposure was 4·8% (95% CI 4·0–5·6; figure 3A, appendix 2 pp 185–87). Pooled estimates of seroprevalence in the general population was highest from four studies done in the South-East Asia region (19·6%, 95% CI 5·5–33·6, all in India), followed by two studies done in the African region (16·3%, 0·0–33·7), Eastern Mediterranean region (three studies, 13·4%, 8·8–18·0), region of the Americas (13 studies, 6·8%, 5·0–8·5), and European region (14 studies, 4·7%, 3·6–5·9), with the lowest seroprevalence in studies done in the Western Pacific region (two studies, 1·7%, 0·0–5·0; figure 3B–G, appendix 2 pp 185–87). Sensitivity analyses using different definitions of positivity and accounting for serological test performance showed no qualitative differences from the primary results (appendix 2 pp 188–92).
Figure 3.
Estimated seroprevalence by WHO regions and study populations
The bar represents the pooled estimates and the error bars represent the 95% CI. Each dot represents the result of one single study. ND=no data.
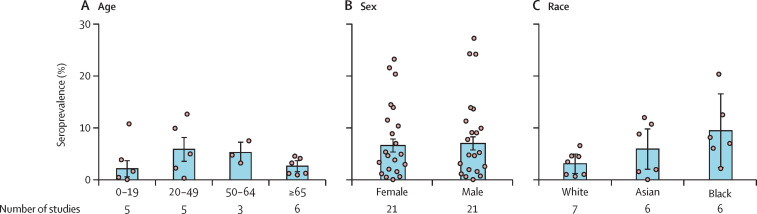
Within grade A and grade B studies of the general population, seroprevalence of those younger than 20 years was 2·1% (95% CI 0·5–3·6), 5·8% (3·5–8·1) for those aged 20–49, 5·2% (3·2–7·2) for those aged 50–64, and 2·6% (1·5–3·6) for those aged 65 years and older (figure 4A ). After further merging the middle two age groups (20–49 years and 50–64 years), the RR of seropositivity in the young (<20 years) was approximately 20% lower than that of working age adults (20–64 years; RR 0·77, 95% CI 0·72–0·84; appendix 2 pp 195–96). The risk of seropositivity in older people (≥65 years) was also lower than for working age adults (RR 0·76, 0·59–0·96; appendix 2 pp 195–96).
Figure 4.
Estimated seroprevalence by age groups, sex, and race
The pooled seroprevalence for men (7·0%, 5·7–8·2) and women (6·6%, 5·3–7·8) was similar, with 52% (11 of 21) of sex-specific seroprevalence point estimates being higher in men than in women (figure 4B). Similarly, pooling sex-specific relative risks across studies to adjust for the differences in risk across settings revealed no significant increase in risk of seropositivity in men (RR 1·02, 95% CI 0·95–1·09), with similar estimates across WHO regions (appendix 2 pp 195–96). Furthermore, across the seven studies that compared different races, Black (RR 2·70, 95% CI 2·30–3·18) and Asian (RR 1·91, 1·82–2·03) individuals showed a significantly higher risk of infection than did White individuals (figure 4C, appendix 2 pp 195–96).
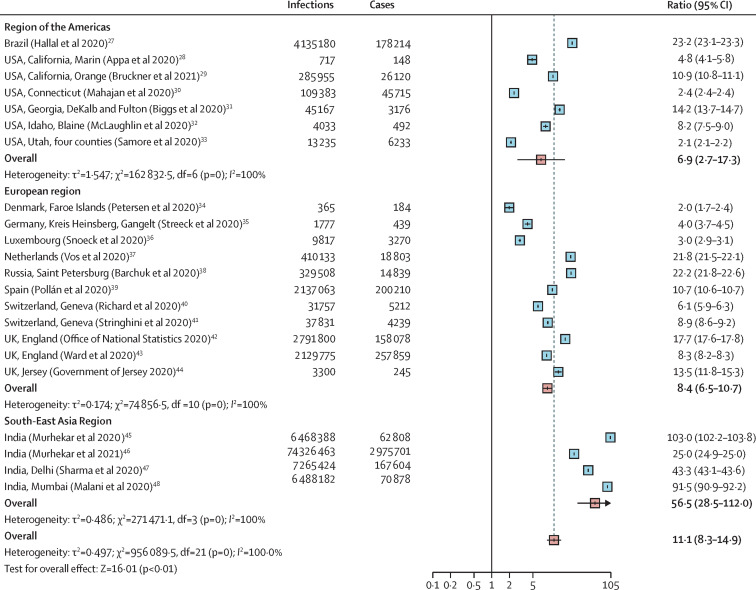
The relationship between reported COVID-19 incidence (confirmed cases reported from public sources) and the number of infections identified through serological surveys can be useful for understanding the evolution of the pandemic without serological surveillance in each and every locale. For studies of the general population, the cumulative reported incidence of COVID-19 correlated with seroprevalence across locations (Spearman's rank correlation coefficient, 0·59; appendix 2 pp 223–24). For studies including individuals from general populations, the ratio of serologically detected infections to virologically confirmed cases varied across locations, with a pooled ratio of 11·1 (95% CI 8·3–14·9), suggesting that for each virologically confirmed SARS-CoV-2 infection, at least ten infections remained undetected by surveillance systems. This ratio was similar in the region of the Americas (6·9, 2·7–17·3) and the European region (8·4, 6·5–10·7), but higher in the South-East Asia region (56·5, 28·5–112·0); although this final estimate is only based on studies done in India (figure 5 ).
Figure 5.
Estimated ratio of serologically detected infections to confirmed cases of COVID-19
The size of boxes represents the weight for each study. The whisker represents the 95% CI. Values higher than 1 suggest greater under-reporting of infections (due to both mild or asymptomatic infections, care-seeking behaviours, and testing practices).
Discussion
With the increasing availability of serological assays for SARS-CoV-2, a large body of literature describing seroprevalence studies in different populations has emerged. In this study, we examine the quality and results of 404 reports of seroprevalence studies from around the globe, both published and in preprint form. In general, the quality of existing serological studies was low, involving less rigorous sampling strategies, poorly validated and non-standardised laboratory methods, and scarcity of statistical correction for demographics and test performance in analyses. As expected, we found that close contacts and high-risk health-care workers had a higher prevalence of SARS-CoV-2-specific antibodies than did low-risk health-care workers and the general population. Young individuals (<20 years) and older people (≥65 years) were less likely to be seropositive than those aged 20–64 years, and there was no significant difference in seroprevalence between men and women. Additionally, we found that the ratios of infections per confirmed case were similar in the European region and the region of the Americas on average, but higher in the South-East Asia region (in which all estimates were from India).
Representative serosurveys can provide useful snapshots of the infection history of a population. However, very few studies provided representative estimates for their target population. An optimal study design for estimating seroprevalence includes a detailed sampling framework, rigorous sampling methods (ie, multistage, stratified sampling), and adjustments for selection bias and assay performance.14
Various detection assays were used for determination of seropositivity.49 We found large variations in test performance, targeted antigens and immunoglobulin isotypes, and threshold used. Additionally, more than half of the studies lacked independent validations of the sensitivity and specificity of the diagnostic kits before assessment of serosurvey samples to verify their initial results.50, 51, 52 Furthermore, few independent validations were done in the target population of serosurvey, which might lead to mis-specification of assay performance.53 Notably, although WHO has established a generic population-based serological study protocol, standardised guidelines and procedures for laboratory testing are scarce, which might contribute towards such heterogeneity in performance and reporting of results. We call on national and international governance bodies to develop standardised antibody testing protocols and reporting practices and create biobanks of reference standards (eg, monoclonal antibodies), to reduce laboratory-to-laboratory variations, thus facilitating the comparability and interpretability among seroprevalence studies. Despite the WHO recommendations, the estimates described by many of the population-based serosurveys did not adjust for the demographic structure of the target population,16 nor for the testing performance (sensitivity and specificity) of the assay, which made the comparison among studies difficult.
Most of the high-quality serological surveys identified were done in the region of the Americas and the European region, predominantly in general populations.31, 33, 36, 41, 54, 55, 56, 57, 58 The number of high-quality studies of exposed populations were few, especially for health-care workers and close contacts, and studies to address this knowledge gap are needed.59, 60, 61, 62 For the other four WHO regions examined, there was a paucity of high-quality studies across all populations examined, suggesting that attention should be paid to optimise the design of future seroepidemiological studies to include good representativeness of samples, standardised laboratory methods, and reasonable adjustments. Higher-quality studies provide more accurate measures of disease burden and transmission to better inform public health efforts against COVID-19.
We found high seroprevalence among high-risk health-care workers, defined as those who provided routine medical care to patients with COVID-19, who did not have access to PPE. On the contrary, low-risk health-care workers, defined as those wearing adequate PPE or those who provided care for patients who did not have COVID-19, had significantly lower seroprevalence than their high-risk counterparts, indicating the necessity of proper use of PPE for front-line health-care workers.63 We found a pooled seroprevalence of 8·0% in the general population, suggesting that globally, the number of people infected by the end of 2020 was unlikely to satisfy estimates of what it would take to achieve antibody-mediated herd immunity.39, 41, 64 The seroprevalence in the general population also varied across WHO regions, with a higher prevalence of SARS-CoV-2-specific antibodies in the South-East Asia region (eg, India, range: 10·8–40·8%)46, 47, 48 contrasting with a relatively lower seroprevalence in the Western Pacific region (eg, China, range: 0·8–3·3%),65, 66 indicating different levels of community transmission of SARS-CoV-2 in different locales, potentially due to differences in non-pharmaceutical interventions.67 Additionally, the very limited number of high-quality studies in all but two of the WHO regions underlines our incomplete understanding of SARS-CoV-2 transmission in much of the world.39, 43, 68, 69, 70, 71 We also found notable differences in seroprevalence between age groups, with the seroprevalence increasing with age among participants younger than 65 years. We found that the young (<20 years) were less likely to be seropositive than were individuals aged 20–64 years, consistent with reports of lower numbers of virally confirmed cases in children compared with other age groups.72 In some areas, this might have been due to the effects of lockdowns limiting exposures of school-aged populations, in contrast to adult-aged essential workers who had continued community exposure.73, 74 The prevalence of SARS-CoV-2-specific antibodies and the relative risk of being seropositive among older people (≥65 years) was also low, which could be explained by poorer serological responses after infection,75 lower rates of infection as a result of biological differences or, perhaps more likely, due to behaviour changes leading to reduced frequency of infectious contacts.
The seroprevalence is a reflection of the duration and intensity of community transmission. By use of a regression analysis, we showed that higher cumulative incidence of reported cases is associated with higher seroprevalence. For locations where data on the number of confirmed cases were available, we found that the number of infections per confirmed case varied greatly, although estimates in Europe and the USA were similar on average. Such evidence indicates the existence of many undetected cases in the community and provides a range of scaling factors for translating the observed confirmed cases into unobserved infections in the community.27
Our study has several limitations. First, although we did meta-regression and subgroup analysis to explore heterogeneity of varied seroprevalence for different populations, there are still some factors that we have not taken into account, so that some heterogeneity cannot be well explained quantitatively. Second, misclassification bias can occur due to the limited information on exposures for the study populations, especially for the so-called poorly defined populations. For certain variables (eg, the use of PPE for health-care workers) for which data cannot be extracted from the original articles, we tried to contact the authors, but the response rate was low. Third, we pooled the estimates of studies at different stages of the pandemic, which makes interpretation of the pooled estimates more nuanced. However, we did summarise the relationship between starting timepoint and local epidemic to demonstrate temporal distributions for each included study. Fourth, current seroprevalence estimates are limited by the lack of knowledge of the time-varying sensitivities of the immunoassays used, which might have led to underestimation of seroprevalence, especially those of asymptomatic or mild cases. Additional longitudinal studies are needed to examine the long-term kinetics of antibody responses to inform appropriate correction for immunoassay sensitivity. Finally, we have not included studies examining the use of T-cell responses for estimates of prevalence. Although there is evidence that SARS-CoV-2-specific T-cell responses might be detectable in those that lack antibody response, measuring T-cell responses at a population level is not feasible.76
In conclusion, the overall quality of the existing seroprevalence studies of SARS-CoV-2 is low and international efforts to standardise study design and assays are urgently required.16 Pooled estimates of SARS-CoV-2 seroprevalence based on currently available data show a higher infection risk among close contacts and health-care workers who did not use PPE, while the relatively low prevalence of SARS-CoV-2-specific antibodies among general populations suggests that the majority of examined populations have not been infected. Therefore, antibody-mediated herd immunity is probably far from being reached in most settings, and continuous serological monitoring is necessary to inform public health decision making.
Data sharing
All datasets generated and analysed are available in the Article and appendix 2. Some tables of appendix 2 in Excel format are available at Github, and are more informative than the PDF supplement provided with the Article.
Acknowledgments
Acknowledgments
We thank Qianli Wang, Wei Wang, Qianhui Wu, Yongli Zhang, Junbo Chen, and Yuxia Liang from the Fudan University (Shanghai, China). This study was funded by the National Science Fund for Distinguished Young Scholars (grant number 81525023), Key Emergency Project of Shanghai Science and Technology Committee (grant number 20411950100), Program of Shanghai Academic/Technology Research Leader (grant number 18XD1400300), National Science and Technology Major project of China (grant numbers 2018ZX10713001–007, 2017ZX10103009–005, 2018ZX10201001–010), and the US National Institutes of Health (R01 AI135115 to DTL and ASA). The views expressed are those of the authors and do not necessarily represent the institutions with which the authors are affiliated.
Editorial note: the Lancet Group takes a neutral position with respect to territorial claims in published maps and institutional affiliations.
Contributors
HY designed and supervised the study. Xinh C and ZC did the literature search, set up the database, and did all statistical analyses. Xinh C, ZC, ASA, and DTL co-drafted the first version of the article. Xinh C, ZC, XD, WL, ZZ, Xing C, RS, TZ, and NZ helped with checking data and constructed the figures. Xinh C, ZC, and HY have verified the underlying data used in the analysis. DTL, ASA, JY, MA, CV, and HY commented on the data and its interpretation and critically revised the content. All authors contributed to review and revision, approved the final manuscript as submitted, and agree to be accountable for all aspects of the work. All authors had full access to all the data in the study and accept responsibility for the decision to submit for publication.
Declaration of interests
HY reports research funding from Sanofi Pasteur and Shanghai Roche Pharmaceutical Company. MA reports research funding from Seqirus. DTL and ASA report research funding from the US National Institutes of Health. None of the research funding is related to COVID-19. All other authors declare no competing interests.
Supplementary Materials
References
- 1.Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO Coronavirus disease (COVID-19) pandemic. 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019
- 3.Munster VJ, Koopmans M, van Doremalen N, van Riel D, de Wit E. A novel coronavirus emerging in China—key questions for impact assessment. N Engl J Med. 2020;382:692–694. doi: 10.1056/NEJMp2000929. [DOI] [PubMed] [Google Scholar]
- 4.Perez-Saez J, Lauer SA, Kaiser L, et al. Serology-informed estimates of SARS-CoV-2 infection fatality risk in Geneva, Switzerland. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30584-3. published online July 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sughayer MA, Mansour A, Al Nuirat A, Souan L, Ghanem M, Siag M. Covid-19 seroprevalence rate in healthy blood donors from a community under strict lockdown measures. medRxiv. 2020 doi: 10.1101/2020.06.06.20123919. published online June 7, 2020. (preprint). [DOI] [Google Scholar]
- 6.Griffin S. Covid-19: Herd immunity is “unethical and unachievable,” say experts after report of 5% seroprevalence in Spain. BMJ. 2020;370 doi: 10.1136/bmj.m2728. [DOI] [PubMed] [Google Scholar]
- 7.Verity R, Okell LC, Dorigatti I, et al. Estimates of the severity of coronavirus disease 2019: a model-based analysis. Lancet Infect Dis. 2020;20:669–677. doi: 10.1016/S1473-3099(20)30243-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bobrovitz N, Arora RK, Yan T, et al. Lessons from a rapid systematic review of early SARS-CoV-2 serosurveys. medRxiv. 2020 doi: 10.1101/2020.05.10.20097451. published online May 14, 2020. (preprint). [DOI] [Google Scholar]
- 9.Arora RK, Joseph A, Van Wyk J, et al. SeroTracker: a global SARS-CoV-2 seroprevalence dashboard. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30631-9. published online Aug 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Franceschi VB, Santos AS, Glaeser AB, et al. Population-based prevalence surveys during the COVID-19 pandemic: a systematic review. medRxiv. 2020 doi: 10.1101/2020.10.20.20216259. published online Oct 22, 2020. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galanis P, Vraka I, Fragkou D, Bilali A, Kaitelidou D. Seroprevalence of SARS-CoV-2 antibodies and associated factors in health care workers: a systematic review and meta-analysis. J Hosp Infect. 2020;108:120–134. doi: 10.1016/j.jhin.2020.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rostami A, Sepidarkish M, Leeflang MMG, et al. SARS-CoV-2 seroprevalence worldwide: a systematic review and meta-analysis. Clin Microbiol Infect. 2020 doi: 10.1016/j.cmi.2020.10.020. published online Oct 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6 [PMC free article] [PubMed] [Google Scholar]
- 14.Horby PW, Laurie KL, Cowling BJ, et al. CONSISE statement on the Reporting of Seroepidemiologic Studies for influenza (ROSES-I statement): an extension of the STROBE statement. Influenza Other Respir Viruses. 2017;11:2–14. doi: 10.1111/irv.12411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sikkema RS, Freidl GS, de Bruin E, Koopmans M. Weighing serological evidence of human exposure to animal influenza viruses—a literature review. Euro Surveill. 2016;21 doi: 10.2807/1560-7917.ES.2016.21.44.30388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.WHO Population-based age-stratified seroepidemiological investigation protocol for coronavirus 2019 (COVID-19) infection. 2020. https://apps.who.int/iris/rest/bitstreams/1278667/retrieve
- 17.Ma H, Zeng W, He H, et al. Serum IgA, IgM, and IgG responses in COVID-19. Cell Mol Immunol. 2020;17:773–775. doi: 10.1038/s41423-020-0474-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gelman A, Carpenter B. Bayesian analysis of tests with unknown specificity and sensitivity. J R Stat Soc Ser C Appl Stat. 2020;69:1269–1283. doi: 10.1111/rssc.12435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rostami A, Sepidarkish M, Leeflang M, et al. First snap-shot meta-analysis to estimate the prevalence of serum antibodies to SARS-CoV-2 in humans. medRxiv. 2020 doi: 10.1101/2020.08.31.20185017. published online Sept 2. (preprint). [DOI] [Google Scholar]
- 20.Clopper CJ, Pearson ES. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika. 1934;26:404–413. [Google Scholar]
- 21.Newcombe RG. Two-sided confidence intervals for the single proportion: comparison of seven methods. Stat Med. 1998;17:857–872. doi: 10.1002/(sici)1097-0258(19980430)17:8<857::aid-sim777>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 22.Borremans B, Gamble A, Prager KC, et al. Quantifying antibody kinetics and RNA detection during early-phase SARS-CoV-2 infection by time since symptom onset. eLife. 2020;9 doi: 10.7554/eLife.60122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Post N, Eddy D, Huntley C, et al. Antibody response to SARS-CoV-2 infection in humans: a systematic review. PLoS One. 2020;15 doi: 10.1371/journal.pone.0244126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Federal Office of Public Health New coronavirus: situation in Switzerland. 2020. https://www.bag.admin.ch/bag/en/home/krankheiten/ausbrueche-epidemien-pandemien/aktuelle-ausbrueche-epidemien/novel-cov/situation-schweiz-und-international.html#-1680104524
- 25.WorldPop WorldPop gridded population estimate datasets and tools. 2020. https://www.worldpop.org/geodata/listing?id=79
- 26.Federal Statistical Office STAT-TAB - Interactive tables. 2020. https://www.pxweb.bfs.admin.ch/pxweb/en/px-x-0103010000_101/-/px-x-0103010000_101.px/?rxid=34873e36-d320-4c20-b931-8f0596e0e667
- 27.Hallal PC, Hartwig FP, Horta BL, et al. SARS-CoV-2 antibody prevalence in Brazil: results from two successive nationwide serological household surveys. Lancet Glob Health. 2020;8:e1390–e1398. doi: 10.1016/S2214-109X(20)30387-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Biggs HM, Harris JB, Breakwell L, et al. Estimated community seroprevalence of SARS-CoV-2 antibodies—two Georgia counties, April 28–May 3, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:965–970. doi: 10.15585/mmwr.mm6929e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Samore MH, Looney A, Orleans B, et al. SARS-CoV-2 seroprevalence and detection fraction in Utah urban populations from a probability-based sample. medRxiv. 2020 doi: 10.1101/2020.10.26.20219907. published online Oct 27. (preprint). [DOI] [Google Scholar]
- 36.Snoeck CJ, Vaillant M, Abdelrahman T, et al. Prevalence of SARS-CoV-2 infection in the Luxembourgish population: the CON-VINCE study. medRxiv. 2020 doi: 10.1101/2020.05.11.20092916. published online May 18, 2020. (preprint). [DOI] [Google Scholar]
- 39.Pollán M, Pérez-Gómez B, Pastor-Barriuso R, et al. Prevalence of SARS-CoV-2 in Spain (ENE-COVID): a nationwide, population-based seroepidemiological study. Lancet. 2020;396:535–544. doi: 10.1016/S0140-6736(20)31483-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stringhini S, Wisniak A, Piumatti G, et al. Seroprevalence of anti-SARS-CoV-2 IgG antibodies in Geneva, Switzerland (SEROCoV-POP): a population-based study. Lancet. 2020;396:313–319. doi: 10.1016/S0140-6736(20)31304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ward H, Cooke G, Atchison C, et al. Declining prevalence of antibody positivity to SARS-CoV-2: a community study of 365,000 adults. medRxiv. 2020 doi: 10.1101/2020.10.26.20219725. published online Oct 27. (preprint). [DOI] [Google Scholar]
- 46.Murhekar MV, Bhatnagar T, Selvaraju S, et al. SARS-CoV-2 antibody prevalence in India: findings from the second nationwide household serosurvey, August–September 2020. Lancet Glob Health. 2021;9:e257–e266. doi: 10.1016/S2214-109X(20)30544-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sharma N, Sharma P, Basu S, et al. The seroprevalence and trends of SARS-CoV-2 in Delhi, India: a repeated population-based seroepidemiological study. medRxiv. 2020 doi: 10.1101/2020.12.13.20248123. published online Dec 14. (preprint). [DOI] [PubMed] [Google Scholar]
- 48.Malani A, Shah D, Kang G, et al. Seroprevalence of SARS-CoV-2 in slums versus non-slums in Mumbai, India. Lancet Glob Health. 2020;9:e110–e111. doi: 10.1016/S2214-109X(20)30467-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lisboa Bastos M, Tavaziva G, Abidi SK, et al. Diagnostic accuracy of serological tests for covid-19: systematic review and meta-analysis. BMJ. 2020;370 doi: 10.1136/bmj.m2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen Y, Tong X, Wang J, et al. High SARS-CoV-2 antibody prevalence among healthcare workers exposed to COVID-19 patients. J Infect. 2020;81:420–426. doi: 10.1016/j.jinf.2020.05.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Payne DC, Smith-Jeffcoat SE, Nowak G, et al. SARS-CoV-2 infections and serologic responses from a sample of US navy service members—USS Theodore Roosevelt, April 2020. MMWR Morb Mortal Wkly Rep. 2020;69:714–721. doi: 10.15585/mmwr.mm6923e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kai-Wang To K, Chi-Chung Cheng V, Cai J-P, et al. Seroprevalence of SARS-CoV-2 in Hong Kong and in residents evacuated from Hubei province, China: a multicohort study. Lancet Microbe. 2020;1:e111–e118. doi: 10.1016/S2666-5247(20)30053-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yadouleton A, Sander A-L, Moreira-Soto A, et al. Limited specificity of serologic tests for SARS-CoV-2 antibody detection, Benin. 2021. https://wwwnc.cdc.gov/eid/article/27/1/20-3281_article [DOI] [PMC free article] [PubMed]
- 54.Gudbjartsson DF, Norddahl GL, Melsted P, et al. Humoral immune response to SARS-CoV-2 in Iceland. N Engl J Med. 2020;383:1724–1734. doi: 10.1056/NEJMoa2026116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sood N, Simon P, Ebner P, et al. Seroprevalence of SARS-CoV-2-specific antibodies among adults in Los Angeles County, California, on April 10–11, 2020. JAMA. 2020;323:2425–2427. doi: 10.1001/jama.2020.8279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rosenberg ES, Tesoriero JM, Rosenthal EM, et al. Cumulative incidence and diagnosis of SARS-CoV-2 infection in New York. Ann Epidemiol. 2020;48:23–29.e4. doi: 10.1016/j.annepidem.2020.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Naranbhai V, Chang CC, Beltran WFG, et al. High seroprevalence of anti-SARS-CoV-2 antibodies in Chelsea, Massachusetts. J Infect Dis. 2020;222:1955–1959. doi: 10.1093/infdis/jiaa579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bendavid E, Mulaney B, Sood N, et al. COVID-19 antibody seroprevalence in Santa Clara County, California. medRxiv. 2020 doi: 10.1101/2020.04.14.20062463. published online April 30. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Iversen K, Bundgaard H, Hasselbalch RB, et al. Risk of COVID-19 in health-care workers in Denmark: an observational cohort study. Lancet Infect Dis. 2020;20:1401–1408. doi: 10.1016/S1473-3099(20)30589-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moscola J, Sembajwe G, Jarrett M, et al. Prevalence of SARS-CoV-2 antibodies in health care personnel in the New York City area. JAMA. 2020;324:893–895. doi: 10.1001/jama.2020.14765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Garcia-Basteiro AL, Moncunill G, Tortajada M, et al. Seroprevalence of antibodies against SARS-CoV-2 among health care workers in a large Spanish reference hospital. Nat Commun. 2020;11 doi: 10.1038/s41467-020-17318-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Self WH, Tenforde MW, Stubblefield WB, et al. Seroprevalence of SARS-CoV-2 among frontline health care personnel in a multistate hospital network—13 academic medical centers, April–June 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1221–1226. doi: 10.15585/mmwr.mm6935e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu M, Cheng SZ, Xu KW, et al. Use of personal protective equipment against coronavirus disease 2019 by healthcare professionals in Wuhan, China: cross sectional study. BMJ. 2020;369 doi: 10.1136/bmj.m2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Guo L, Ren L, Yang S, et al. Profiling early humoral response to diagnose novel coronavirus disease (COVID-19) Clin Infect Dis. 2020;71:778–785. doi: 10.1093/cid/ciaa310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang X, Gao W, Cui S, et al. A population-based seroprevalence survey of severe acute respiratory syndrome coronavirus 2 infection in Beijing, China. medRxiv. 2020 doi: 10.1101/2020.09.23.20197756. published Sept 23. (preprint). [DOI] [Google Scholar]
- 66.Ling R, Yu Y, He J, et al. Seroprevalence and epidemiological characteristics of immunoglobulin M and G antibodies against SARS-CoV-2 in asymptomatic people in Wuhan, China. medRxiv. 2020 doi: 10.1101/2020.06.16.20132423. published online Aug 25. (preprint). [DOI] [Google Scholar]
- 67.Lai S, Ruktanonchai NW, Zhou L, et al. Effect of non-pharmaceutical interventions to contain COVID-19 in China. Nature. 2020;585:410–413. doi: 10.1038/s41586-020-2293-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Majiya H, Aliyu-Paiko M, Balogu VT, et al. Seroprevalence of COVID-19 in Niger State. medRxiv. 2020 doi: 10.1101/2020.08.04.20168112. published online Aug 5. (preprint). [DOI] [Google Scholar]
- 69.Stadlbauer D, Tan J, Jiang K, et al. Repeated cross-sectional sero-monitoring of SARS-CoV-2 in New York City. Nature. 2020 doi: 10.1038/s41586-020-2912-6. published online Nov 3. [DOI] [PubMed] [Google Scholar]
- 70.Dodd RY, Xu M, Stramer SL. Change in donor characteristics and antibodies to SARS-CoV-2 in donated blood in the US, June–August 2020. JAMA. 2020;324:1677–1679. doi: 10.1001/jama.2020.18598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Uyoga S, Adetifa IMO, Karanja HK, et al. Seroprevalence of anti-SARS-CoV-2 IgG antibodies in Kenyan blood donors. Science. 2021;371:79–82. doi: 10.1126/science.abe1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gudbjartsson DF, Helgason A, Jonsson H, et al. Spread of SARS-CoV-2 in the Icelandic population. N Engl J Med. 2020;382:2302–2315. doi: 10.1056/NEJMoa2006100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Del Fava E, Cimentada J, Perrotta D, et al. The differential impact of physical distancing strategies on social contacts relevant for the spread of COVID-19: evidence from a multi-country survey. medRxiv. 2020 doi: 10.1101/2020.05.15.20102657. published online Dec 18. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang J, Litvinova M, Liang Y, et al. Changes in contact patterns shape the dynamics of the COVID-19 outbreak in China. Science. 2020;368:1481–1486. doi: 10.1126/science.abb8001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pawelec G, Weng NP. Can an effective SARS-CoV-2 vaccine be developed for the older population? Immun Ageing. 2020;17:8. doi: 10.1186/s12979-020-00180-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sekine T, Perez-Potti A, Rivera-Ballesteros O, et al. Robust T cell immunity in convalescent individuals with asymptomatic or mild COVID-19. Cell. 2020;183:158. doi: 10.1016/j.cell.2020.08.017. 68.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Uncited References
- 28.Appa A, Takahashi S, Rodriguez-Barraquer I, et al. Universal PCR and antibody testing demonstrate little to no transmission of SARS-CoV-2 in a rural community. medRxiv. 2020 doi: 10.1101/2020.08.15.20175786. published online Aug 17. (preprint). [DOI] [Google Scholar]
- 29.Bruckner TA, Parker DM, Bartell SM, et al. Estimated seroprevalence of SARS-CoV-2 antibodies among adults in Orange County, California. Sci Rep. 2021;11 doi: 10.1038/s41598-021-82662-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mahajan S, Srinivasan R, Redlich CA, et al. Seroprevalence of SARS-CoV-2-specific IgG antibodies among adults living in Connecticut: post-infection prevalence (PIP) study. Am J Med. 2020 doi: 10.1016/j.amjmed.2020.09.024. published online Oct 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McLaughlin C, Doll MK, Morrison KT, et al. High community SARS-CoV-2 antibody seroprevalence in a ski resort community, Blaine County, Idaho, US. Preliminary results. medRxiv. 2020 doi: 10.1101/2020.07.19.20157198. published online July 21. (preprint). [DOI] [Google Scholar]
- 34.Petersen MS, Strøm M, Christiansen DH, et al. Seroprevalence of SARS-CoV-2-specific antibodies, Faroe Islands. Emerg Infect Dis. 2020;26:2761–2763. doi: 10.3201/eid2611.202736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Streeck H, Schulte B, Kümmerer BM, et al. Infection fatality rate of SARS-CoV-2 in a super-spreading event in Germany. Nat Commun. 2020;11 doi: 10.1038/s41467-020-19509-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vos ERA, den Hartog G, Schepp RM, et al. Nationwide seroprevalence of SARS-CoV-2 and identification of risk factors in the general population of the Netherlands during the first epidemic wave. J Epidemiol Community Health. 2020 doi: 10.1136/jech-2020-215678. published online Nov 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barchuk A, Skougarevskiy D, Titaev K, et al. Seroprevalence of SARS-CoV-2 antibodies in Saint Petersburg, Russia: a population-based study. medRxiv. 2020 doi: 10.1101/2020.11.02.20221309. published online Nov 4. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Richard A, Wisniak A, Perez-Saez J, et al. Seroprevalence of anti-SARS-CoV-2 IgG antibodies, risk factors for infection and associated symptoms in Geneva, Switzerland: a population-based study. medRxiv. 2020 doi: 10.1101/2020.12.16.20248180. published online Dec 18. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Office of National Statistics Coronavirus (COVID-19) infection survey. 2020. https://www.ons.gov.uk/file?uri=/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/datasets/coronaviruscovid19infectionsurveydata/2020/previous/v26/covid19infectionsurveydatasets20201002.xlsx
- 44.Government of Jersey SARS-CoV-2: prevalence of antibodies in Jersey. Preliminary analysis. 2020. https://www.gov.je/SiteCollectionDocuments/Government%20and%20administration/R%20Prevalence%20of%20antibodies%202020508%20SJ.pdf
- 45.Murhekar MV, Bhatnagar T, Selvaraju S, et al. Prevalence of SARS-CoV-2 infection in India: findings from the national serosurvey, May-June 2020. Indian J Med Res. 2020;152:48–60. doi: 10.4103/ijmr.IJMR_3290_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All datasets generated and analysed are available in the Article and appendix 2. Some tables of appendix 2 in Excel format are available at Github, and are more informative than the PDF supplement provided with the Article.