Abstract
Neural interfaces provide a window into the workings of the nervous system—enabling both biosignal recording and modulation. Traditionally, neural interfaces have been restricted to implanted electrodes to record or modulate electrical activity of the nervous system. Although these electrode systems are both mechanically and operationally robust, they have limited utility due to the resultant macroscale damage from invasive implantation. For this reason, novel nanomaterials are being investigated to enable new strategies to chronically interact with the nervous system at both the cellular and network level. In this feature article, the use of nanomaterials to improve current electrophysiological interfaces, as well as enable new nano-interfaces to modulate neural activity via alternative mechanisms, such as remote transduction of electromagnetic fields are explored. Specifically, this article will review the current use of nanoparticle coatings to enhance electrode function, then an analysis of the cutting-edge, targeted nanoparticle technologies being utilized to interface with both the electrophysiological and biochemical behavior of the nervous system will be provided. Furthermore, an emerging, specialized-use case for neural interfaces will be presented: the modulation of the blood-brain barrier.
Keywords: blood-brain barrier, brain, nanoparticle, neural interface, neurostimulation
1. Introduction
Neural interfaces are engineered systems designed to record or modulate the activity of the central nervous system (CNS) and peripheral nervous system (PNS). Tapping into and recording the activity of the CNS and PNS has been a subject of scientific and medical interest for decades, if not nearly a century,[1–4] as neural stimulation by direct application of electrical current was first reported as early as the late 19th century.[5] Nonetheless, the most viable, widely utilized platforms for interfacing with the CNS and recording signals from excitable cells are still metal or ceramic electrodes placed on the surface of the brain or implanted into the brain.[6,7] By simply implanting a conductive element into brain or peripheral nerve bundle, intra- and extra-cellular potentials of local neurons can be detected, amplified, and recorded; furthermore, electrical signals can be directly applied via the implanted electrode for deliberate stimulation of tissue surrounding the implanted electrode. The applications of this fundamental technology have ranged from the treatment of neurological disorders with electroconvulsive therapy to restoring the ability to hear with cochlear implants.[8–11] Based on these technologies, the future of neural interfaces and brain-computer interfaces hold great promise in diverse biomedical and biotechnological applications, such as prosthesis control, brain-computer interfaces, and regenerative medicine.[12–15]
If the full potential of neural interfaces is to be achieved, myriad limitations of current devices and systems must be overcome. Specifically, the challenges to achieving next-generation neural interfaces fall in the following categories: (i) selectivity, (ii) resolution, (iii) coordination, (iv) stability, and (v) management of host-interface responses. Selectivity is the capability of the interface to record or stimulate select neurons, nerves, or motor units. Resolution is the density, or number of data points taken over time, of the recorded signal. Coordination refers to the alignment of multiple electrophysiological signals in time, or the correlation of electrophysiological signals with biochemical signals. Coordination is a challenge which becomes increasingly more complex as higher levels of signal discrimination and resolution are achieved. Stability requirements mandate that neural interfaces operate without physical or signal degradation for years. These requirements may be tempered by tuning the invasiveness of the delivery technique, which also dictates the host response, whether it be deleterious, such as resultant tissue damage and immune responses to the neural interface. Managing this response of the host to the interface can lead to the reduction of deleterious tissue reactions, such as inflammation and scarring, and enable chronic neural-interfaces to succeed.
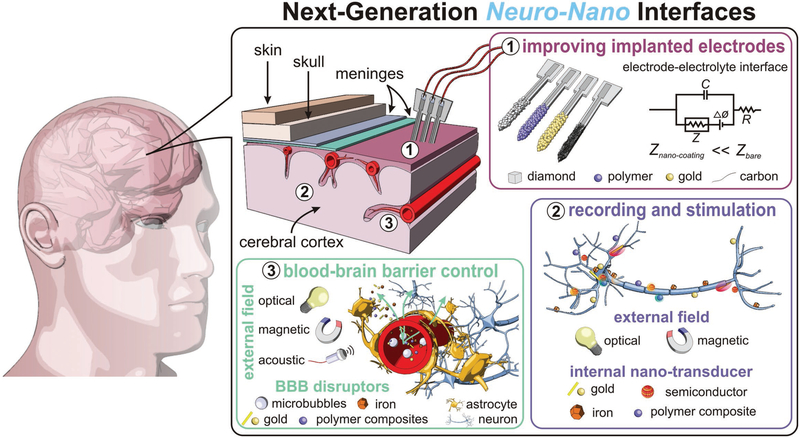
Herein, we explore the recent development of nanoparticles for overcoming the aforementioned challenges and enabling next-generation neuro-nano interfaces. The first decade of the 2000’s witnessed a substantial utility of nanomaterials to fashion novel neural interfaces, including drug delivery, imaging, topographic analysis, and single-cell neuromodulation.t[16] More recently, there has been further exploration of nanoparticles as remote neural interfaces.[17] With increasing capabilities and chemistries to engineer novel nanoparticles, the potential for novel interfaces has continued an upward trend. Briefly, the application of nanoparticle technologies have improved traditional neural interface electrodes;[18,19] furthermore, nanoparticles are small enough to be delivered intravenously, pass through the blood brain barrier (BBB) and interact with individual neurons to increase the capabilities of neuro-nano interfaces.[20–23] Figure 1 illustrates the applications of nanoparticles for next-generation neuro-nano interfaces presented in this Feature Article.
Figure 1.
Neural interfaces provide a window into the workings of the nervous system—enabling both biosignal recording and modulation. Nano-particles are used to improve current electrophysiological interfaces, as well as enable new nano-interfaces to modulate neural activity via alternative mechanisms. (i) Nanoparticle coatings of implanted electrodes reduce interface impedance with dramatically improved recording and stimulation fidelity. Alternatively, nanoparticles can be introduced intravenously and engineered to cross the blood brain barrier and excite neurons. (ii) Targeted-nanoparticles can remotely transduce external fields, e.g., infrared light or alternating magnetic fields, to modulate and stimulate neuronal function. (iii) An emerging, specialized use case for neural interfaces: Using nanoparticles and external fields to control the permeability of the blood-brain barrier for delivery of therapeutic and diagnostic materials into the central nervous system.
2. Nanoparticle Coatings for Neural Electrodes
Microelectrode arrays (MEAs) are the conventional platform for chronic neural recording and stimulation both in vivo and in vitro.[11,24,25] in vivo, these devices are typically implanted in cortical tissue or placed on the dura of the brain. Although physico-chemically robust and clinically proven, MEAs, such as all of the variations of the ubiquitous Utah and Michigan arrays,[26] have many disadvantages including both surgical risk and subsequent foreign body response after implantation. The foreign body response almost certainly leads to an undesirable immune response from surrounding cells, leading to cell death, electrode isolation and failure after long-term implantation on the scale of weeks.[27–29] Recently reported technologies utilize ultrathin dielectric and semiconductor materials to fabricate electronic dura which can be placed on the cortical surface of the brain without the deleterious effects of implantation;[30–32] however, these reported systems only provide superficial recording and stimulation. Lieber et al. have reported injectable MEAs based on conductive mesh. The conductive mesh, composed of polymer threads, were directly injected into the brain and conformed to the mechanical and geometric constraints of brain tissue. The injectable mesh is promising as a nanoscale circuit board for carrying neural recording and stimulation electronics, but this technology is still in its infancy.[33,34] Accordingly, the continued effort to improve implanted neural interfaces by both optimizing the signal quality and modulating host-interface response have turned to the enhancement of conventional MEAs with novel nanomaterials.
The application of nanoparticles to improve implanted electrodes has the potential to overcome the hurdles to next-generation neural interface selectivity and sensitivity by directly modulating the physical contact between the electrodes and neural tissue. Although electrodes provide a reliable, implantable interface to record and stimulate neuronal activity, it is not possible to fabricate electrodes small enough to non-invasively integrate with individual neurons without disrupting surrounding tissue or the cell bodies themselves. Common penetrating electrodes used in clinical settings are the Utah arrays, sieve electrodes, and cuff electrodes.[35–37] These standard smooth surface electrodes have a relatively high impedance resulting in poor signal-to-noise ratios (SNR). Deposition of nanoparticles on electrodes can increase the surface-area to volume ratio of the electrode, facilitating lower impedance to reduce noise.[38] Very simply, nanoparticles provide a higher surface-area-to-volume ratio than conventional materials, and the surface-area of an electrode is inversely proportional to the impedance of the electrode. increasing the surface-area of the neural interface increases the capacitance, which decreases the impedance, Z, defined by the relationship in Equation (1),
| (1) |
Where, i is the imaginary unit, ω is the frequency, C is the capacitance and R is the resistance of the electrode. By reducing the electrode impedance and subsequently reducing the difference in impedance between the tissue and the electrode—improved SNR can be achieved.[39] Furthermore, surface topography of electrodes alters cell adhesion, allowing improved contact with the electrode for better signal quality.[40] This topography can be achieved through a variety of ways, including sandblasting, abrading, etching, lithography, and machining, although nanoparticle deposition allows the integration of new advantageous materials to the electrode surface.[41,42]
Electrodes made entirely of nanoparticles also improve bio-compatibility by matching the mechanical properties of tissue, unlike stiff bulk-material electrodes, and therefore integrating better with the cell environment.[29] The following sections outline the use of carbon, noble metals, and diamond nanoparticles in optimizing implanted neural interfaces.
2.1. Carbon-Based Nanoparticles
Carbon-based nanoparticles offer electrically active and mechanically robust platforms for the enhancement of neural electrode platforms for electrophysiological recording and biochemical sensing. Carbon nanoparticles can be used to lower the impedance of electrodes by increasing the surface-area. In the following section, we will analyze recent developments in carbon-based nanoparticles and relevant nanocomposites used to improve neural interfaces.
2.1.1. Carbon Nanotube (CNT) Functionalized Electrodes
For the purposes of this analysis, we will consider CNTs in the category of nanoparticles when used to augment implanted MEAs. It should be noted that novel neural interfaces are being developed which utilize individual or bundled CNTs as implanted electrodes, specifically to conduct biochemical sensing.[43,44] CNTs offer a variety of desirable characteristics making them suitable for applications in neural interfacing, including high conductivity, high surface-area, and low contact impedance. Functionalization of electrodes with CNTs is an effective method to increase the recording sensitivity of electrodes due to these characteristics.[45] Additionally, CNTs enable unique surface chemistries which modify biological interactions and may promote biocompatibility.[46,47]
The geometry of the individual electrodes of the MEAs, specifically the surface-area, directly impacts the signal-to-noise ratio (SNR) of the recorded neuronal activity. CNTs have been utilized to modify both commercial and custom MEAs to increase surface-area, which improves sensitivity by reducing SNR. Figure 2 presents multiple examples of CNT modified electrodes for neuronal recording. For example, Shin et al. examined CNT-coated tungsten (CNT-W) electrodes. The CNT-W electrodes were tested in vivo by implantation into 76 sequential positions of the brain of a mouse, and neuronal activity was simultaneously recorded.[45] The improved sensitivity and fidelity of the neural recordings was attributed to faster flux of electroactive species across the increased surface-area due to CNT coating.
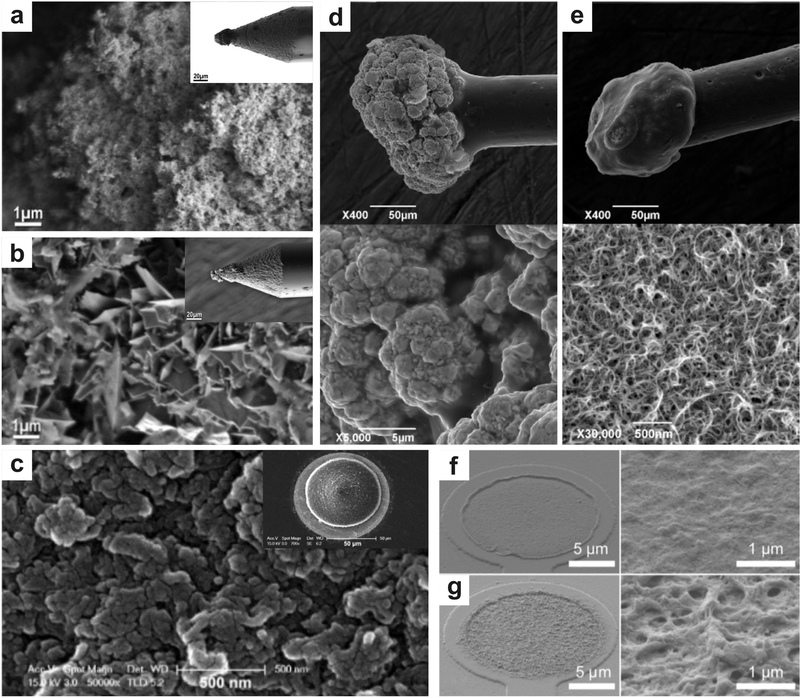
Figure 2.
Micrographs illustrate the myriad nanomaterial functionalization of neural interface electrodes. a-b) CNTs are grown on the tip of a micro-electrode via chemical vapor deposition and a gold-CNT composite is coated on an electrode via a metal plating technique, respectively Reproduced with permission.[49] Copyright 2011, American Chemical Society. CNTs have been deposited on microelectrodes to improve performance in neural recording by increasing the surface-area of the electrodes, which decreases impedance. The CVD electrode displays smaller surface structures and less lamellar structures when compared to the gold-CNT composite, resulting in a greater surface area and mechanical stability, c) Microstructure of PEDOT/MWCNT film deposited in galvanostatic mode on microelectrode (inset) for neuronal recording. Reproduced with permission.[65] Copyright 2013, Elsevier. This deposition technique resulted in longitudinal film growth with lessened electrical cross-talk, resulting in increased spatial resolution of the MEA. d-e) Gold particle and PEDOT:PSS-CNT coated microelectrodes and corresponding microstructures (below), respectively. Reproduced with permission.[51] Copyright 2016, Frontiers Publishing. PEDOT:PSS-CNT coated microelectrodes show a fine nano-structure as compared to the microstructure present on the gold particle coated electrodes. f-g) Morphology of PEDOT and PEDOT-CNT coated electrodes and their microstructures, respectively. Reproduced with permission.[66] Copyright 2012, Frontiers Publishing. The porous structure of the PEDOT-CNT coated electrodes results in a larger surface area, therefore a lower impedance, when compared to the electrodes coated in PEDOT alone.
Electrode facilitated excitation or silencing of neurons is driven by the polarization or depolarization of the cell membrane by exposure to an electrical current.[8] To improve the charge transfer ability at the neural interface, Zhou et al. used amino-functionalized CNTs electrodeposited onto Pt (CNT-Pt) microelectrodes.[48] The CNT-Pt microelectrodes showed a ≈90% decrease in electrode impedance compared to bare Pt microelectrodes. The CNT-Pt electrodes also displayed increased charge storage capacity and an increased safe charge injection limit, 10 × higher than that of bare Pt electrodes. These electrodes displayed high stability when stimulated illustrating a potential use for applications such as neural prostheses. Ansaldo et al. directly grew CNTs directly on the tip of microelectrodes (Figure 2)[49] These Pt-W microelectrodes were fabricated by chemical vapor deposition (CVD) of CNTs. CVD enables the direct growth of CNTs on a microelectrode, rather than deposition of pre-developed CNTs. The CVD-CNT electrodes were reported to have a much wider potential limit, charge injection limit, cathodic currents, and impedance magnitudes when compared to other CNT-functionalized electrodes, which enables better recording and stimulation from the same electrode. The microelectrodes were fabricated from Pt-W wires were coated in CNTs and insulated with quartz glass. Pre-coated electrodes exhibited an impedance ranging from 500 to 700 kΩ at 1 kHz, while CVD-CNT coated electrodes had measured impedances averaging 15 kΩ. More importantly, the high-temperature fabrication of CVD-CNT electrodes result in nanoparticle adhesion that is more stable under mechanical stress when compared to other CNT composite electrodes which utilized dielectric polymers for the insulation layer. Yung-Chan et al. also fabricated flexible CNT MEAs, using commercial polyimide films and deposited chromium and gold layers as the base, with a chemical vapor deposited CNT layer as the contact electrode.[50] They tested this device in vitro by placing the electrode on the caudal photoreceptor on the tail of a crayfish, displaying improved noise and SNR when compared to control MEAs. The MEAs was also used in vivo to record electrocorticography of a rat on the dura surface, also displaying a better SNR and lower impedance per unit area.
Composites of CNTs and conducting or insulating polymers have become popular for acute and chronic neural interfacing (Figure 2).[51–58] CNTs are blended with polymers to produce nanocomposites that limit the leaching of CNTs and improve the electrical characteristics of the polymers. For example, Chen et al. fabricated polypyrrole-CNT nanocomposites via a layer-by-layer (LbL) fabrication method for use in neuronal recording.[59] The surface of neural electrodes was modified with multilayered polypyrrole coated, multi-walled CNTs which improved charge capacity, reduced impedance and increased chemical stability when compared to neural electrodes only coated with multilayered polypyrrole and uncoated Au electrodes. More importantly, preliminary biocompatibility and toxicity tests did not show defects in neurite growth and function, which has been a problem because of CNTs leaching from the MEAs into the neural tissue.[60] This suggests that neurons would maintain function if such a composite electrode was implanted and CNT leaching was at the very least minimized, if not completely avoided, using this material and fabrication technique. Similarly, Baranauskas et al. tested polypyrrole-CNT coating on a Pt-W microelectrode and found significant reduction in the microelectrode impedance at all neuronal signal frequencies (1 to 10000Hz) and up to a 4× increase in SNR.[61] While these polypyrole-CNT electrodes were initially examined in vitro, they outlined a strategy for composing the properties of both conducting polymers and CNTs for implanted MEAs.
Subsequently, CNT-polymer composites have been developed for implanted neural electrodes. individual CNT fibers were insulated with a polystyrene-polybutadiene copolymer, resulting in an electrical impedance 15 to 20 times lower than that of a traditional platinum iridium (Pt-Ir) wire.[29] Furthermore, the flexibility and size of the microelectrodes make them more bio-compatible as they integrate better with neural tissue resulting in less delamination when implanted in the brain.[62] This was tested by implantation into the deep brain of a rat model. Deep brain stimulation using CNT fiber electrodes resulted in alleviating motor symptoms of Parkinson’s disease in the rat model. The brain tissue and electrode were also evaluated six weeks after implantation, displaying reduced inflammatory response when compared to Pt-Ir electrodes. Neuronal activity was also recorded in the primary motor cortex of rats over 4 weeks, showing minimal degradation of signal.
Lastly, utilization of poly(3,4-ethylenedioxythiophene) (PEDOT) as a matrix to contain CNTs for neural interfaces provides benefits for neural recording and stimulation. PEDOT, and its close neighbor poly(3,4-ethylenedioxythiophene):poly (styrene sulfonate) (PEDOT:PSS), are highly conductive, bio-compatible and arguably the most broadly utilized conducting polymer.[63-64] In addition, PEDOT has been demonstrated to have better stability when compared to other conductive polymers.[65] Accordingly, many researchers have explored PEDOT CNT composite MEAs.[66–68] In the research presented by Zhou et al., multi-wall CNT-doped PEDOT films (CNT-PEDOT) were electrodeposited onto platinum MEAs.[65] CNT-PEDOT films electrodeposited in a galvanostatic method exhibited greater stability and electrochemical performance compared to CNT-PEDOT films electrodeposited with a potentiostatic method and PEDOT films electrodeposited without CNTs. In vitro tests were performed on rat pheochromocytoma cells (PC12), a cell line with a neural origin commonly used to model neural response, and resulted in increased adhesion and outgrowth of neurites on CNT-PEDOT films when compared to PEDOT films. These results demonstrate that CNT-PEDOT films are non-toxic to neural cells, suggesting that such materials would integrate well with brain tissue. CNT-PEDOT coated platinum wires were also implanted into a rat cortex for in vivo evaluation, and these interfaces elicited a reduced inflammatory response when compared to non-coated platinum wire controls.
Comparable to CNT-polypyrrole composites, CNT-PEDOT composites are typically characterized by increased capacitance and charge injection capacity. Cells displayed high viability and neurite outgrowth on films, further demonstrating that PEDOT-CNT films are a suitable material for interfacing with neurons.[66–68] Kolarcik et al. also used anti-inflammatory doped particles to minimize immune response around implanted electrodes.[69] The conductive polymer PEDOT was used in conjunction with CNTs and the anti-inflammatory drug dexamethasone to coat platinum/iridium dual-shank microelectrodes. While the PEDOT-CNT coated microelectrodes doped with dexamethasone had a higher impedance than the un-doped PEDOT-CNT microelectrodes, they performed comparably to bare electrodes. After examining dose-response in vitro, electrodes were implanted in mice to assess in vivo electrode performance and tissue response. It was found that impedance measurements were decreased in drug-doped electrodes compared to non-doped electrodes over the first few days after in vivo implantation. The anti-inflammatory drug may be reducing foreign body response around the electrode after implantation; therefore, maintaining a low impedance. Doped electrodes also resulted in less neuron death after implantation. Additionally, PEDOT-CNT coated electrodes resulted in a lessened inflammatory response when compared to bare electrodes. This all suggests polymer coatings and anti-inflammatory drug doping may be suitable methods to avoid immune response surrounding an implantable, which will aid in conserving the resolution of the signal recorded by the MEAs.
2.1.2. Nanocrystalline Diamond Used in Neural Interfaces
Diamond is a unique material, growing in popularity, for enhancing the properties of many biomedical applications, including neural interfaces.[70,71] Unlike CNT coatings, or other carbon-based electrodes, boron-doped diamond (BDD) systems are particularly noteworthy because of their tremendous physico-chemical and operational stability under physiological conditions. The nanoscale surface-roughness and topography of nanocrystalline diamond products demonstrate low protein adsorption and non-hemolytic response,[72] which are all beneficial in designing the surface chemistry and activity of implanted electrodes. Piret et al. investigated BDD as a material for electrodes used in neural implants (Figure 3).[73] Two conditions were analyzed: BDD-modified MEAs and BDD-CNT composite MEAs. Both microfabrication methods involved the seeding of diamond nanoparticles on the MEAs, followed by crystal growth to produce nanocrystalline interfaces. The BDD-CNT composite MEAs included vertical CNT growth and an additional diamond encapsulation step to create a 3D-nanocomposite electrode. The BDD-CNT composite MEAs were characterized by reduced impedance and improved SNR compared to the BDD MEAs. After implantation in ex vivo mouse spinal cord sections, BDD-CNT composite MEAs can record low amplitude spiking signals, unlike the BDD MEAs.
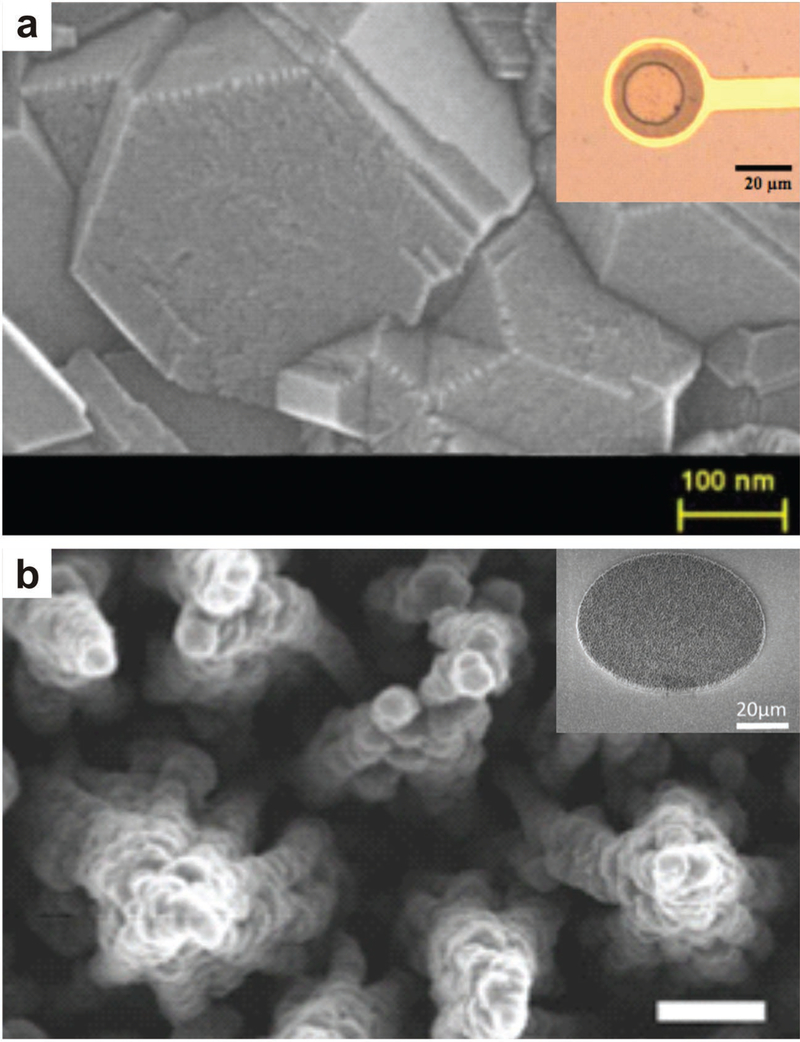
Figure 3.
Electrodes fabricated with boron-doped diamond have improved stability and biocompatibility, a) SEM image of boron-doped diamond microstructure and microelectrode (inset), Reproduced with permission,[74] Copyright 2013, Institute of Electrical and Electronics Engineers. b) 3D-nanostructured boron-doped diamond electrode before addition of insulation layer and microelectrode (inset). Reproduced with permission.[73] Copyright 2015, Elsevier.
Cottance et al. fabricated pristine BDD electrodes from diamond nanoparticles (Figure 3).[74] The diamond array displayed a wider potential window compared to traditional electrodes, ensuring the electrode would not leach ions and degrade over time. Spontaneous activity of an embryonic mouse hindbrain-spinal cord was recorded ex vivo, illustrating that the fabricated BDD microarrays could successfully detect low amplitude signals. Spontaneous activity was also recorded from ex vivo rat retinas. Comparably, Cai et al. reported the use of nanocrystalline diamond pillars to improve the ability of cochlear implants interfacing with auditory neurons.[75] By coating the MEAs with micro-textured nanocrystalline diamond pillars, increased surface-roughness promoted cell growth and improved the physical interface between the electrodes and cells. When compared to bare electrodes, the nanocrystalline diamond pillars exhibited a higher affinity for mouse and human neurons with the axons anchoring around the top of the pillars. This work demonstrates the possible application of nanocrystalline semiconductor coatings to improve cell adhesion to electrodes and better interface MEAs with neural tissue.
Nanocrystalline semiconductors have also found unique utility outside of being the direct recording or stimulation medium. Hermetic coatings are necessary for the biocompatibility of an implantable device, although feedthroughs to allow contact with the surrounding tissue environment are difficult to fabricate and are common points of failure for implanted neural interfaces. Ganeson et al. report the use of hermetic electrical feedthrough arrays fabricated from nitrogen-doped nanocrystalline diamond (NDD).[76] The feedthrough arrays are filled with electrically insulating NDD. The electrodes had low feedthrough and high isolation resistances. The advantageous electrochemical properties enabled by unique properties of nanocrystalline diamond were confirmed; moreover, a higher percentage of rat cortical neurons survived when cultured on the nitrogen-doped nanocrystalline diamond electrodes compared to a polycrystalline diamond substrate and tissue culture plates. Accordingly, we propose that nanocrystalline diamond is a suitable material to make densely packed feedthrough arrays, improving signal and cell interaction when interfacing with neurons.
2.2. Metal and Alloy Nano-Coatings
Noble metal and alloy nanoparticles are useful for improving the conductivity and SNR of implanted electrodes due to their high conductance and surface-area; furthermore, the synthesis and deposition of nanoscale metals on electrode surfaces are a thoroughly explored methodology to offer a well-defined and simplified way to develop biosensors and biotechnologies.[77,78]
Gold nanoparticles (AuNPs) are likely the most popular alternative for the enhancement of implanted MEAs. Castagnola et al. used a PEDOT:PSS composite material to coat a gold microsphere grown on the tip of a thin insulated platinum wire.[51] This electrode was further encapsulated in poly(2-hydroxyethyl methacrylate) (pHEMA) to combat the biocompatibility issues of the nanomaterials, and match the mechanical stiffness of the brain and electrode for better integration. Like many AuNP technologies, the described MEAs were fabricated by LbL methodology. The electrode was tested in vivo by recording neuron signals from the parietal cortex of rats, displaying the pHEMA coating did not affect the electrical performance of the electrode. Additionally, electrodes coated in pHEMA elicited a lower immune response when compared to the uncoated electrodes. Tsai et al. used gold nanoparticles and self-assembled monolayers (SAMs) to modify platinum microelectrodes to detect striatal dopamine, a marker of Parkinson’s disease (Figure 4)[79] An alkanethiol was utilized to form the AuNP SAMs. Alkanethiols are a common group of molecules used to functionalize both AuNPs and the surfaces of metal electrodes. In this case, the AuNPs were used to increase the sensitivity of a platinum electrode. The fast response time, low detection limit, and high SNR facilitated by the AuNP SAMs was attributed to the increase surface-area of the modified electrodes. In vivo studies in the brains of rats confirmed coated electrodes could be used to monitor dopamine release in real time.
Figure 4.
Metallic nanomaterials are incorporated onto neural interface electrodes to improve functionality and stability. a-c) Gold nanopillars are fabricated on MEAs. A cross-section of an HL-1 cell grown on the gold nanopillar array illustrates contact between electrodes and cell membrane, with gaps between the cell membrane and electrode less than 100 nm. Reproduced with permission.[81] Copyright 2011, IOP Publishing. d,e) Schematic for the nanowire geometry and structure for gallium-phosphide nanowire array on MEA. Reproduced with permission.[235] Copyright 2013, Public Library of Science. f) AuNPs are deposited on a Pt electrode and functionalized with a self-assembled monolayer. Reproduced with permission.[79] Copyright 2012, Royal Society of Chemistry. h,k) Silver nanowire electrodes are fabricated using photolithography. Reproduced with permission.[86] Copyright 2014, American Chemical Society. i) Micrographs of nanoporous Au-Pt electrodes, which exhibited high neuron coverage (inset, red) and reduced astrocyte coverage (inset, green). Reproduced with permission.[85] Copyright 2015, American Chemical Society. j) Microelectrodes are coated in AuNP-CNT composite. Reproduced with permission.[84] Copyright 2012, American Chemical Society.
Furthermore, gold nanostructures could be added to the surface of single-cell-sized electrodes, demonstrating significantly lower impedances and higher electrical stimulation capabilities when compared to unmodified gold electrodes.[80] Brüggemann et al. also added gold nanostructures to the surface of microelectrodes for extracellular signal recording (Figure 4)[81] Unlike the aforementioned Au-NP coatings, gold nanopillars were appended to the MEAs to penetrate the neuronal cell membrane and conduct real-time recording. Recorded signal amplitudes were up to 100% higher than those recorded with planar gold electrodes. The recording and stimulation capabilities of the electrodes were tested on rat hippocampal neurons in vitro, displaying low noise levels and a high SNR. Additionally, neurons adjacent to electrodes were easily excited by small current pulses. These results were further verified by Nick et al. when covering microelectrodes with high aspect-ratio gold nanopillars.[82] This platform also exhibited a decrease in impedance, good neuron adhesion, and amplitudes up to ten times higher than conventional gold electrodes. The high amplitudes of this system reduce the necessity for extreme signal amplification, which can introduce further signal distortion and decrease the detection limit.
Beyond the improvement in signal quality, gold nanoparticles can be used to decrease implanted electrode rejection in vivo. Immune response can cause glial capsule formation around an implanted electrode, resulting in an impaired signal and shortened operational lifetime (Figure 4). Zhang et al. demonstrated that AuNPs assembled by LbL).[83] Zhang et al. demonstrated that AuNPs assembled by layer-by-layer (LbL) fabrication techniques on microelectrodes produced more than three-fold reduction in interfacial impedance and an order-of-magnitude increase in charge storage capacity.[84] More importantly, the AuNP-LbL composites displayed minimal bio-fouling after implantation and operation. Chapman et al. proposed using nanoporous gold as an alternative to planar gold coatings to combat scar tissue formation around implanted electrodes.|85| Using an alloy corrosion process, pores were made in coated gold, ranging from 90–150 nm. The nanoporous coating reduces astrocyte coverage while maintaining neuron coverage when tested with co-cultured cells in vitro. The decreased astrocyte coverage and encapsulation intimates the nanoporous gold coatings could improve electrode function in vivo by enabling a larger neuronal interface area by limiting the astrocyte interface area.
Silver and platinum are alternative noble metals which have been explored for neuronal interfaces. Silver nanoparticles have also been explored for use as neural electrode coatings.[86] Ahn et al. fabricated silver nanowire-based microelectrodes (AgNW) on hydrogels with photolithography to attain the precise patterning of small features (Figure 4).[86] They exhibited excellent electrical conductivity, mechanical flexibility, and consistent resistance under hydrated conditions. The demonstration of MEA fabrication on hydrogel substrates is an important step toward neural implants which match the mechanical properties of the brain and other excitable tissue. Similar to silver, platinum has been briefly explored for use in neural interfaces,[87–89] but nano-platinum must be carefully employed due to its potential as a highly reactive catalyst of simple compounds that may be found in physiological media.[90–91] Shah et al. used nano-cluster platinum films to enhance implantable electrode performance.[92] Flexible MEAs were coated with nano-platinum to create electrodes with a decreased impedance that was more stable over a larger range of frequencies and reduced degradation when compared to non-coated MEAs. Angelov et al. used electrophoretic deposition to coat Pt-Ir electrodes with Pt nanoparticles (PtNP).[87] The PtNP-Pt-Ir electrodes were fabricated with different nanoparticle sizes (<10 nm, 50 nm, and composite), and they were compared to bare Pt-Ir electrodes. The PtNP coatings resulted in more stable impedance measurements when compared to non-coated electrodes, and larger PtNPs, i.e., 50 nm, resulted in increased impedance. Electrodes were tested in the subthalamic nucleus of rats, and impedance measurements were evaluated. An increasing impedance for uncoated electrodes was observed over the entire implantation, while the PtNP coatings maintained the electrodes’ impedance over the course of the implantation. It was also reported that cell adhesion and inflammatory response was reduced in PtNP-coated electrodes.
Noble-metal alloy nanoparticles are also an option to take advantage of the positive features offered by multiple metals to reduce the impedance of an electrode.[93–95] Gold and platinum offer synergistic effects, such as improved electron transfer rate, electrocatalytic activity, and stability when compared to AuNPs.[94] Specifically, rough-surface AuPt was deposited on gold microelectrodes by electrodeposition of Au-Pt-Cu nanoparticles and subsequent de-alloying of copper by chemical dissolution. The removal of copper from the nanoparticles created nanoporosity. The AuPt-NP coated gold electrodes exhibited very low impedance, <5% of the impedance of the non-coated electrodes. Neural recordings were compared in vivo by implanting AuNP-coated electrodes and rough-surfaced AuPt-NP-coated electrodes in the brains of rats. Spike amplitude was similar for both conditions, but the rough-surfaced AuPt-NP-coated electrodes exhibited a higher SNR than the AuNP-coated electrodes. Kim et al. presented similar results when AuPt nanoparticles were co-deposited on gold electrodes.[93] The electrode was further modified by covalently binding poly D-lysine onto the AuPt-NP surface. The capacitance of the composite nanoparticle-coated surface was measured to be larger than the capacitance of the AuNP-coated electrodes, and smaller than that of the PtNP-coated electrodes. The composite nanoparticle electrode also has an increased surface-area compared to the AuNP coated electrode. This was tested in vitro by culturing neuronal cells on top of the modified electrodes. A reduction in baseline noise was observed in the AuPt-NP-coated electrodes when compared to AuNP-coated electrodes, suggesting possible applications in long-term neural prostheses due to both mechanical and operational durability.
It should also be noted that noble metals can be partnered with carbon to enhance electrode properties for in vivo neural recording. Zhang et al. electroplated multi-wall carbon nanotube and Au nanoparticle composites onto a silicon neural probe.[96] This group chose to investigate their capability to utilize CNT-Au composites to facilitate improved neuron growth attributed to CNT modification, while also taking advantage of the high conductivity and low toxicity of AuNPs. The impedance was significantly decreased in CNT-Au electrodes when compared to bare electrodes, and an increased charge storage in CNT-Au electrodes was also observed. What will become clear in the following sections is that the common design strategies for neuro-nano interfaces are often rooted in combinatorial approaches, where the integration of multiple nanomaterials contributes to the ultimate, improved performance of the neural interface.
2.3. Polymer and Polymer-Composite Nanoparticle Coatings
An important component for any electrode interfacing with the brain is maintaining contact with surrounding neurons. To do so, neurons must remain viable around the implant, and glial cell accumulation must be minimized because they reduce the ratio of electroactive cells in proximity to the recording or stimulating electrodes. We have previously examined the beneficial incorporation of polymers into nanocomposites for neural interfaces. Polymers enhance the application of nano-coatings due to their tunable mechanical properties, flexibility, biocompatibility, and the broad chemical design space that they offer.
Polymer nanoparticles are most apparently useful for their ability to facilitate neural adhesion to an electrode surface by both chemical and physical engineering. Ho et al. used polymer nanoparticles to create neural guidance conduits with surface roughness to induce cell adhesion.[97] The nanoparticles consisted of a poly(glycidal methacrylate) (PMMA) and Fe3O4 composite core, coated in the conductive polymer PEDOT:PSS. A cationic polyethylenimine layer was used to electrostatically conjugate the PEDOT:PSS coating to the PMMA-Fe3O4 nanoparticle core. Capillary force lithography was used to linearly organize the nanoparticles, creating parallel channels 800 nm long and 130 nm wide, with about 1.5 μm between lines. Neurites displayed preferential attachment to the nanoparticle channels compared to non-coated gaps, when cultured on top of the patterned arrays. Cultured neurons were electrically stimulated through the conductive microarrays, displaying greater cell area, as well as dendritic patterning along the linear channels. This cellular response in adhesion and excitation suggested the PEDOT:PSS coated conductive polymers patterned on a substrate as an innovative new method to locally excite neurons in vivo.
Not only can polymers be used the promote cell adhesion by physical patterning, but polymer nanocomposite electrodes can be engineered to match the mechanical properties of brain tissue to decrease immune response around an implantable.[98–99] Capadona et al. have reported a polymer nanocomposite composed of a poly(vinyl acetate) solution with “nanowhiskers” made from cellulose nanocrystals. The tensile storage modulus of the material decreases upon implantation and has minimal swelling compared to other hydrogel materials. The electrodes were implanted into mice, with histological analysis confirming decreased microglia density around the polymer nanocomposite electrodes. Additionally, more neurons were present around the polymer nanocomposite electrodes, suggesting the polymer electrodes would have better signal integrity than that of smooth electrodes due to more exposure to surrounding neurons. Analysis of long-term implantation confirmed that the neuroinflammatory response of the implantable was dramatically decreased for the nanocomposite electrodes when compared to silicon implants.[100] Also, the BBB integrity was conserved around the nanocomposite electrodes, weeks after implantation, unlike the poly(vinyl acetate)-coated silicone implants. By utilizing soft nanomaterials to match the mechanical properties of an implanted electrode with the brain, these early reports illustrate a path towards reducing glial scarring and promoting neuron viability around implants, indicating improved signal integrity for extended-use applications.
More pro-active routes to reducing deleterious foreign body responses to implanted electrodes are also being developed. Because of the broad chemical design space offered by polymer nanoparticles and polymer nanoparticle composites, neural implants can be engineered to directly deliver anti-inflammatory drugs. Huang et al. explored the use of controlled-release nanoparticles to combat the immune response of cells surrounding implanted electrodes.[101] Rather than coating electrodes in mechanically compliant materials to try to avoid an immune response, these researchers decided to actively treat inflammation using oligo-proanthocyanidin (OPC) released from chitosan nanoparticles coating the implanted electrodes. The coated electrode had a lower impedance and improved SNR compared to uncoated electrodes, like other nano-coatings, but these incorporate active anti-inflammatory agents. Inflammatory activity was reduced with cultured neurons, and glial cell recruitment to the electrode surface was decreased.
Kolarcik et al. also used anti-inflammatory doped particles to minimize foreign body response around implanted electrodes.[69] PEDOT, CNTs, and the anti-inflammatory drug dexa-methasone was compounded and coated onto Pt-Ir dual-shank microelectrodes. Electrodes were implanted in mice to assess in vivo electrode performance and tissue response. It was found that impedance measurements were decreased in drug-doped electrodes compared to non-doped electrodes over the first few days after in vivo implantation, suggesting the anti-inflammatory drug may be reducing inflammatory response around the electrode. The use of the drug-doped electrodes also resulted in less neuron death after implantation.
The continued exploration of drug-releasing nanoparticles will continue to creep into the next-generation of neural interfaces as a promising route to overcome foreign body response and enable chronic implantation.
3. Nanoparticles as Systemically Delivered Neural Interfaces
Invasive and bulky, electrodes are often not a feasible method to stimulate and modulate the CNS or PNS, specifically when the area of interest is in the deep brain, chronic utility is necessary, or the risk of surgery is either too great or impractical for the final outcome. Of the aforementioned nanotechnologies that utilize nanoparticles to enable improved implanted electrodes, none employ the capabilities of nanoparticles to remotely access and interface with the individual neurons at the deepest regions of the CNS via systemic or targeted delivery. Accordingly, a recent body of literature has reported the utility of circulating nanoparticles to access the CNS and PNS to be remotely activated for neural modulation or stimulation.
Neurons respond to a variety of stimuli. Traditionally this constitutes electrical stimulation through implanted electrodes, as discussed heretofore; however, neurons also respond to thermal, physical and chemical stimuli. For example, nanoparticles can be engineered to transduce an external stimulus to activate ion channels, leading to resultant action potentials; furthermore, the stimulation or modulation effects can be restricted to certain cell types by targeting surface receptors unique to the cell type of interest.[102]
The following section will analyze recent reports on the utility of nanoparticles to freely interface with the CNS and PNS, transduce external fields and provide a non-invasive system to record, stimulate or modulate the function of neurons.
3.1. Light-Activated Nanoparticles as Neural Interfaces
Nanoparticles in contact with neurons can transduce the radiation of external or implanted light sources, offering single-cell or wide field neuronal stimulation, modulation or silencing. The general illumination of neurons can affect their activity either by light-induced temperature rise, exploiting the intrinsic physical, and chemical dynamics of neuronal membranes,[103–106] or by photovoltaic effects that transduce light into an electrical stimulation.[107–108] Moreover, the addition of optically active nanoparticles can improve the efficiency of transducing optical signals into neuronal stimulation or modulation. In addition, the field of optogenetics is supporting novel ventures into modulating neurons with unprecedented precision and specificity,[109] and even treat several diseases such as hereditary blindness, epilepsy, or Parkinson’s disease with the possibility of wireless implants for chronic light stimulation[110–112] With the development of better genetic engineering techniques and understanding of neurobiology, optically active nanoparticle transducers are a promising class of materials for neural interfaces.
The future application of optically excited nanoparticles for interfacing with the brain depends on the development of systems that can be activated non-invasively with a high-energy conversion that do not damage tissue. This will require significant improvements in both nanomaterials and hardware used for the optical stimulation. Most importantly, the sheer quantity of novel optically active nanoparticles reported is increasing, and most recently, a few select subsets of these nanotechnologies have been explored as minimally invasive ways to interface with the brain.[102] In the following sections, optically controlled neural interfacing and nanoparticle transducers will be outlined, discussing the advantages and disadvantages of the methodologies, as well as possible applications.
3.1.1. Photonic Polymer Nanoparticles
Nanoparticles are fabricated in various geometries from polymers or metals and conjugated with antibodies or active groups to specifically target areas of the CNS. Additionally, nanoparticles can be engineered for activity across select regions of the electromagnetic spectrum, which enables more deliberate excitation. As such, many nanoparticle designs are optically active in the near-infrared band,[113–116] and this is of particular note because NIR radiation has the best transmission into biological tissue. So, by deploying NIR-active nanoparticles into the CNS or PNS, NIR radiation can be used to target nanoparticles in the deep brain and areas that are outside of the limits of most implantable electrodes.[117] This includes through the skull and into tissues that are greater than 500 μm and even up to multiple cm in depth.[118–119] Additionally, NIR can be applied at higher than 15 J cm−2 without causing any tissue heating or damage, which is more than enough for the applications discussed.[117–120]
Localized heating can be achieved to excite cells with near-infrared (NIR) light stimulation to thermally actuate cells with nanoparticles.[102,121] Additionally, NIR light can be used to target deep-brain tissue with high levels of specificity and reduced noise. Although thermal tissue damage is a concern for such methods, many groups display successful in vivo cellular control without detectable tissue damage. Polymeric nanoparticles can also be used to polarize neurons in the brain, triggering action potentials. Rather than electrical excitation, like those induced by implanted electrodes, thermal activation of nanoparticles by external stimulus can cause the release of molecules from the polymeric substrate, affecting the surrounding cells. Li et al. studied neural activation using NIR release of biomolecules (Figure 5).[122] They take advantage of polypyrrole microgels to release thermal energy when triggered by NIR irradiation. Additionally, the same material can be used for light-controlled release of neurotransmitters. This is especially useful because NIR can penetrate deep into tissues. Specifically, they used chemotactic assays to look at neurons growing toward and away from microgels. They observed that neuronal dendrites turn toward a chemoattractant (Netrin) releasing microgel and collapse away from a chemo-repellant (Sema3A) releasing microgel, when the microgels are exposed to NIR. The response is absent when the chemical agent is trapped within the microgel when not exposed to NIR. In vivo, the experimenters used NIR to control the release of neuro-transmitters in the mouse brain, measuring spiking activities of neurons when activated by NIR. The microgels that released glutamate upon NIR resulted in a significantly higher number of spikes when brain electrophysiology was recorded with an implanted electrode. The microgels releasing a receptor antagonist (DNQX) displayed a lower level of spikes when exposed to NIR. Radiation was emitted cyclically, resulting in almost full recovery of spike number when the brain was not irradiated. This method is discussed as a possible tool for controlled drug delivery.
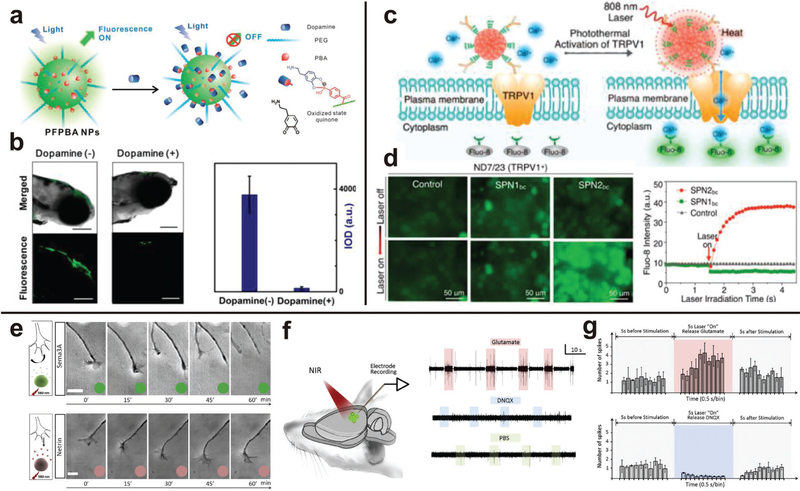
Figure 5.
Polymer-derived nanoparticles can be used to interface with the brain without implanted electrodes. a-b) Zebrafish microinjected with PFPBA nanoparticles were used to optically detect dopamine by fluorescence. Reprinted with permission.[125] Copyright 2015, American Chemical Society, c-d) Neurons were thermally activated when nanoparticles were excited by IR radiation, resulting in remotely controlled depolarization of neurons. Reproduced with permission.[123] Copyright 2016, American Chemical Society. e-g) Polymer nanoparticles functionalized with neuro-active molecules directed the growth of axons, and neural activity was regulated by remote NIR irradiation. Reproduced with permission.[122] Copyright 2015, Elsevier.
Rather than using microgels to release a chemical agent, polymers can emit thermal energy to activate surrounding cells. Also using a NIR-excited polymer, Lyu et al. used semi-conducting nanoparticles to control thermosensitive ion channels in neurons (Figure 5).[123] A semiconducting copolymer, poly(cyclopentadithiophene-alt-diketopyrrolopyrrole), was fashioned into nanoparticles and conjugated with anti-TRPVl antibodies to activate thermosensitive ion channels which can be activated to allow an influx of calcium ions. These particles were cultured with mouse neuroblastoma/rat DRG neuron hybrid ND7/23 cells and after optical excitation, calcium influx into cells was increased leading to depolarization. The strategy of utilizing semiconducting polymers has also been explored with alternative materials. Although not utilizing nanoparticles, Feyen et al. similarly uses polymers for light-mediated inhibition of electrical activity in the form of conjugated films.[124] Illuminated poly(3-hexylthiophene-2,5-diyl) (P3HT) causes hyper-polarization of the neuronal membrane, reducing the action potential firing. P3HT has a large excitation spectrum in the visible range. Effects of the excited polymer were tested by measuring the electrical activity of primary hippocampal neurons, explanted retinas, and brain slices when cultured on P3HT. It was found that the spike frequency of hippocampal neurons decreased significantly when P3HT was excited. Additionally, hyperpolarization was greatly decreased upon light stimulation of P3HT. Brain slices and explanted retinas displayed similar trends when on top of an excited P3HT phenyl-C61-butyric-acid-methyl ester (PCBM) blend. In juxtaposition, these two studies suggest that there is considerable opportunity in utilizing semi-conducting polymers to instigate neuron depolarization and stimulation, and a better understanding of the fundamental mechanism for these properties can better inform the design of semiconducting nanoparticles for neural interfaces.
Polymeric nanoparticles can respond to circulating molecules to record biological mechanisms for ex vivo analysis, or respond to an external source to create a cellular response in the brain. Unique in that they are flexible, biocompatible, and tunable, polymeric materials can be engineered to release thermal energy to actuate surrounding cells or change material properties to release chemicals in the brain. Although advancements are being made to expand the applications of polymeric nanoparticles, specifically to thermally excite them via NIR, these technologies have yet to be developed and tested in vivo as a nanoparticle platform for intravenous use. For this reason, semiconducting composite nanoparticles are currently a more realistic platform for possible clinical use.
Analyte detection in the brain can be achieved with circulating polymer nanoparticles. If an intravenously introduced particle fluoresces when in contact with the molecule of interest, intensity measurements can be obtained with non-invasive imaging techniques. These intensity measurements can be correlated with concentration curves to determine how much analyte is in the brain. In a clinical setting, this method would be useful to assess real-time neurotransmitter concentration in the brain to determine course of treatment without invasive electrode implantation or sampling of cerebrospinal fluid. Qian et al. use a polymer-based fluorescence emissive nanoparticle with phenylboronic acid (PBA) tags on the surface to sense the neurotransmitter dopamine (Figure 5).[125] The nanoparticles, referred to as PFPBA-NPs, were cultured with PC12 cells to sense dopamine using fluorescence imaging. PC12 cells are known to release dopamine in hypoxic situations; therefore cells were cultured in anaerobic phosphate buffered saline and observed. Cells were shown to endocytose PFPBA-NPs, therefore when endogenous dopamine was released due to hypoxia the intracellular dopamine levels decreased and a significantly higher emission mean density was observed when compared to controls. Additionally, the nanoparticles were used to sense dopamine in a zebrafish brain. PFPBA-NPs were microinjected into zebrafish brain ventricles, and fluorescently imaged in real-time. Dopamine molecules were introduced to the zebrafish brain, displaying much higher integrated optical density without dopamine when compared to that with the exogenous dopamine. The nanoparticles displayed high sensitivity and selectivity, making them candidates for detecting neurotransmitters to diagnose and treat neurotransmitter-related diseases.
3.1.2. Plasmonic Gold Nanoparticles
Similar to polymer nanoparticles, AuNPs can be used to activate thermally responsive modulation of ion-gated-channels in cells. As previously stated, gold is an especially popular element for synthesizing nanoparticles due to the ability to fabricate various geometries and low toxicity; moreover, gold nanostructures have tunable photonic behavior directly dependent on their geometry.[126,127] Upon exposure to NIR radiation, localized surface plasmon resonance causes thermal fluctuation that affects surrounding cells.[127–129] As a neural interface, AuNPs can transduce light energy to thermal energy, selectively exciting heat-activated ion channels in neurons (Figure 6)[130] Neurons are polarized by the activated ion channels, eliciting a response to remotely control cells in vivo. For example, Paviolo et al. investigated the excitation of poly (4-styrenesulfonic acid)-coated and silica-coated gold nanorods (AuNRs) and their effects on neurite growth when exposed to continuous NIR radiation (Figure 6).[131] Cytotoxicity was reduced by coating the AuNRs; moreover, the cultures exposed to coated-AuNRs produced a significantly higher percentage of neurons with neurites when compared to uncoated-AuNRs. Neurons did exhibit lower viability when exposed to a 780 nm laser at irradiances higher than 250 mW-cm-2. The cells recovered after one day and reduced irradiance resulted in more outgrowth of neurites, conserving the possibility of clinical use. These effects of AuNRs on cell differentiation and growth have potential applications in peripheral nerve regeneration and treatment after spinal cord injury.
Figure 6.
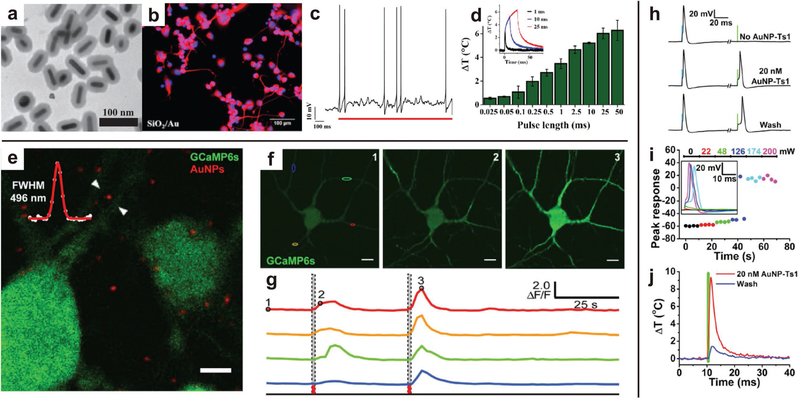
Semiconductor nanoparticles can be excited by external light sources to stimulate growth and activity of neurons. a-d) Polymer-coated AuNRs excited by NIR resulted in neurite outgrowth. Current-clamp recording of a neuron showed action potentials fired in response to a single laser pulse, which is attributed to hyperthermal response of the AuNRs. Increased laser pulse produced an increased mean peak temperature change. Representative temperature profiles for various pulse lengths (inset). Reproduced with permission.[131] Copyright 2013, John Wiley & Sons. e-g) Fluorescent micrographs of Ca2+ concentration in neurons (green) and AuNPs (red). Representative images of varying Ca2+ concentration (green) in response to NIR excitation and corresponding spectral intensity response, illustrating the activation of neurons with NIR excitation at time points indicated by dashed lines. Reproduced with permission.[130] Copyright 2016, Nature Publishing Group. h-j) Functionalized AuNPs are robustly localized to neurons, and continue to stimulate neuronal activity after multiple washings. Increasing irradiance induces greater cell depolarization, triggering action potentials. The NIR-activated depolarization is attributed to the recorded, localized hyperthermia induced by excitation of the functionalized AuNPs. Reproduced with permission.[133] Copyright 2015, Cell Press.
Transduction of IR radiation by AuNRs has also been used for electrophysiological stimulation. To avoid the deleterious effects of continuous NIR radiation, Eom et al. used pulsed infrared neural stimulation, rather than continuous NIR, with plasmonic AuNRs to assess the effects on neuron excitation in the sciatic nerve of a rat.[132] The AuNRs had a maximum absorption peak at a 997 nm wavelength, and during pulsed IR stimulation, the compound nerve action potentials of sciatic nerves was increased. Additionally, nerve tissue showed no evidence of damage from laser exposure at low levels (≈1 J-cm−2) and extensive damage with higher laser exposure (≈2 J-cm−2). Carvalho-de-Souza et al. similarly uses gold nanoparticles to optically stimulate nanoparticles, but they took advantage of modified surface chemistry to target specific cells (Figure 6).[133] Ts1, a neurotoxin binding voltage-gated sodium channels, and anti-TRPVl and anti-P2X3 antibodies, targeting surface proteins, were used to selectively bind AuNPs to neurons and increase operational lifetime. Heat transduction by the gold nanoparticles affects the capacitance of the cell membrane, depolarizing the cell and creating action potentials, and this can be a strategy for neural stimulation as many photonic and plasmonic nanoparticles display localized hyperthermia in an applied field.
3.1.3. Quantum Dots
Quantum dots (QDs) are semiconductor nanoparticles, typically composed of III-V or II-VI elements that have been designed and synthesized with myriad surface functionality for intracellular targeting, protein labelling and other applications for neuronal interfacing.[134–138]
QDs exhibit photocurrent generation from visible or NIR radiation which may be used as a neural interface. As such, QDs have recently been explored as transducers to both monitor and excite neurons. Marshall et al. use semiconductor nanoparticles, such as ultra bright QDs, to monitor spiking activity across large populations of individual neurons.[139] This is due to the much greater emission intensity under one and two photon imaging. Additionally, the nanocrystals are demonstrated to not elicit a negative response in surrounding neurons. Realtime imaging of the action potential of a single neuron was also conceptualized using QDs.[140] Rowland et al. created an electronic system mimicking QDs trapped in a cell membrane and quantified applied voltage by measuring QD fluorescence. This system illustrates a potential use of QDs to non-invasively measure the polarization of electrically active cells without patch-clamps or implanted electrodes. QDs can also be used to monitor temperature fluctuation, enabling the quantification of heat dissipation in neuronal cells.[141] With increasing temperatures the fluorescence spectrum of quantum dots shift to longer wavelengths. This technique was used to determine that the temperature of the cell body was higher than that of neurites in cultured human neurons. Expansion of this technology to in vivo use would be a diagnostic tool to correlate localized temperature fluctuations, hyperthermia or hypothermia, with neural activity. Lugo et al. suggest a way to optically modulate the membrane potential of cells, which would lead to possible methods for neural stimulation. CdTe and CdSe quantum dots can be introduced near the membrane of a cell, excited, and then neuron activation may be observed.[134] After excitation of the QDs, the cells were likely hyperpolarized due to effect on the potassium or sodium channels.
Narrow band absorption, narrow band emission and high quantum efficiency, are inherent to QDs; therefore, designer surface chemistries can be applied without effecting these optoelectronic characteristics. As such, surface modifications can be made to direct QDs toward the membrane of neurons. Targeting and tracking of specific cells with QDs was demonstrated by Agarwal et al. by observing CdSe/ZnS QDs in the developing chick embryo brain.[142] The QDs were functionalized with both a zwitterionic ligand that targets neurons and a peptide known to facilitate endosomal escape. These modified QDs were found to distribute throughout the brain without cell toxicity, and neural stem cells were tracked throughout brain development. The synergy of both optical cell tracking and optoelectronic stimulation with QDs can be used in a clinical setting to treat neurological disorders and aid conjugated species to cross the blood-brain barrier. Although QDs did not need to pass the BBB in this previous study, as they were injected straight into the spinal cord, one of the most favorable features of QD systems is the ability to fluorescently image QD as they pass through the BBB to better understand the possibility for intravenous delivery.[143]
QDs can also be used to target other cell types, such as microglia which operate as macrophages in the CNS, to selectively deliver nanoparticles throughout the CNS. Interestingly, unmodified QDs were found to be selectively taken up by microglia cells.[144] This was demonstrated by Minami et al., who cultured QDs with mixed cortical cultures and found that microglia sequestered QDs via receptor biding and endocytosis, rather than previously postulated phagocytosis. These observations confirm the necessity of surface-modification to specifically target neurons, but open a potential alternate route into the CNS, if escape from microglia can be achieved.
Although useful for the applications described previously, the toxicity of QDs is a topic of concern.[145,146] Chen et al. demonstrated CdSe and ZnS QDs impaired the function of cultured HeLa and hippocampal cells, causing autophagy.[147] This damages the synaptic transmission and plasticity of neurons, insinuating that QDs may not be a safe route for the clinical setting. Toxicity of CdTe QDs was also tested in the motor neurons of nematodes, with the animals presenting abnormal behavior when exposed to QDs.[148] Furthermore, Corazzari et al. investigate the uptake of QDs into neurons.[149] They found that QDs were internalized into the cytosolic and lysosomal compartments in cells, and when sequestered at low pH, such as the lysosomal compartment, the engineered coatings of QDs destabilize resulting in the leaching of metal ions from the core which results in cytotoxicity. This concern has been addressed in a study suggesting the use of InP/ZnS QDs as an alternative to CdSe/ZnS QDs.[150] InP QD cores displayed significantly less toxicity than that of CdSe QD cores when cultured with human lung carcinoma and human neuroblastoma cells. This was further demonstrated by delivering QDs to a Drosophila in vivo model, although InP QDs did have an increased apoptotic rate compared to non-QD exposed controls when cultured with Drosophila larval hemocytes. Consequently, QDs may need further investigation to better understand how they chronically interact with cells and animal models before clinical use.[151] The risk of QD toxicity could be preemptively combatted, if the mechanism of cell toxicity is known. Then combinatorial approaches, such as incorporation of the antioxidant N-acetylcysteine, could be introduced into the QD system to prevent deleterious effects.[152]
3.1.4. Photonic Upconversion with Nanoparticles
Upconversion is the absorption of two or more photons resulting in the emission of a photon of a shorter wavelength, i.e., higher energy, than the original impinging photons. Upconversion can be utilized to convert NIR radiation, which is transmitted by most biological tissue, into higher energy photons to induce biochemical or electrophysiological reactions. UCNPs are typically composed of lanthanide-doped nanoparticles.[153] UCNP activation involves the excitation of the lanthanide dopant with NIR radiation, which then excites erbium to emit light in the visible range. This upconverted light then activates neurons surrounding the particle. This system is useful because it enables the exposure of cells to visible light without any implanted optical device; furthermore, the optoelectronic properties of UCNPs are chemically robust and photo-stable, making them suitable for long-term application in the CNS and other biomedical applications.[154–160] Specifically, UCNPs are gaining momentum as alternative neuro-nano interfaces and optogenetic transducers because early reports suggest negligible toxicity at low dosage;[161] however, there is significant research that needs to be carried out to elucidate the mechanisms of the long-term biodistribution and potential toxicity of UCNPs.
Portioli et al. demonstrated evidence to support a claim of biocompatibility of UCNPs by assessing citrate-stabilized lanthanide-doped nanoparticles ((Er, Yb)-doped CaF2 and SrF2 nanoparticles) interaction with brain cells.[162] In vitro analysis involved culturing UCNPs with human dendritic cells and neurons. Significant cell death was not observed. Additionally, UCNPs did not affect cell viability, even at the highest concentration tested (5000 μg-mL−1). Nanoparticles were also found endocytosed in dendritic cells. Biodistribution was also assessed using an in vivo model, analyzing mouse tissue after infiltration with nanoparticles. Neurons internalized nanoparticles without exhibition of decreased viability, and nanoparticles were found in the mouse brain parenchyma without exhibition of inflammation. These results suggested the benign transversal of the BBB. When assessed using histology, brain tissue with nanoparticles showed no changes in microglia morphology.
With the suggestion that UCNP are non-toxic and can cross the BBB to interact with the CNS, actual neural response must be determined. One response analyzed was differentiation of cells when co-cultured with UCNPs. Guan et al. investigated UCNP effects on neurite outgrowth (Figure 7).[163] NaYF4:Yb/Er UCNP were coated with poly(ethylene imine), PEG, and poly(acrylic acid) to assess cytotoxicity and change the surface charge as compared to the traditional oleic acid coated UCNPs. Neurite outgrowth increased for all UCNP type when exposed to NIR. Additionally, it was determined that the UCNPs were internalized into brain cells, albeit in low percentages, 1.7% in vivo and 5.4% in vitro. Therefore, these nanoparticles may be able to reach the CNS to induce neurite outgrowth if introduced intravenously, but may require substantial dosing to achieve significant distribution in the CNS. Such substantial dosing could result in toxicity; therefore, delivery would need to be improved to expand application to clinical settings.
Figure 7.
Upconversion nanoparticles can be used to excite genetically modified neurons. a,b) A schematic of the energy transfer mechanism of YbEr UCNPs, and results from their application in the NIR activation of channelrhodopsin illustrate NIR activated neuronal firing and neuronal depolarization. Reproduced with permission.[164] Copyright 2016, American Chemical Society. c,d) Representative electron micrograph of polymer-coated UNCPs which were utilized to induce neurite outgrowth when incubated with cells and excited with NIR. Reproduced with permission.[163] Copyright 2014, John Wiley & Sons.
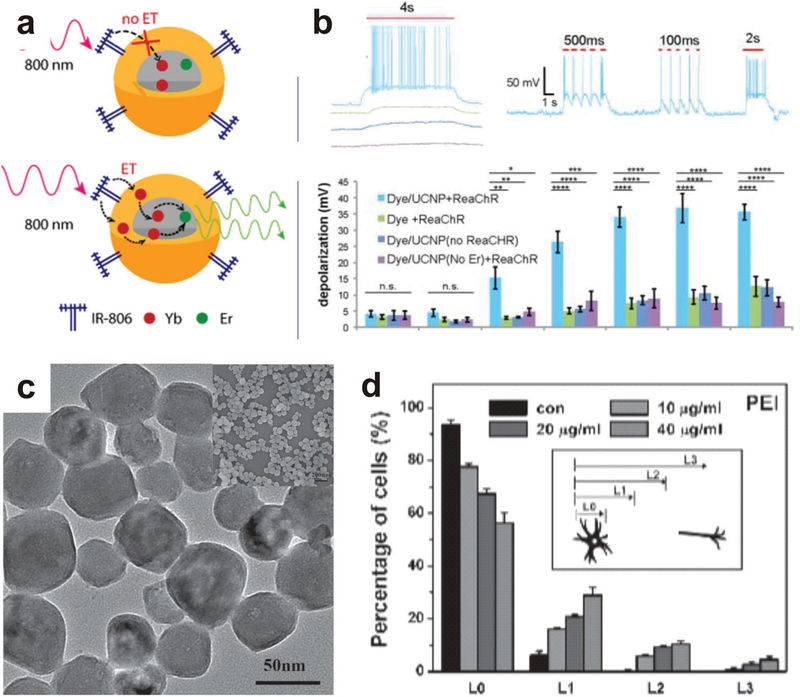
Another neural response assessed was neuronal stimulation. Wu et al. discuss the use of NIR dye-sensitized UCNPs made from PMMA to enhance the upconversion efficiency (Figure 7).[164] Nanoparticles were doped with Yb3+ ions in two conditions: the core only and the shell and core together. It was found that doping in the shell resulted in a much higher up-conversion enhancement intensity when irradiated at 800 nm, such that Yb3+ shell-doped particles can transfer energy into the 2% Er3+ core and emit upconverted light. UCNPs were also made water-soluble by encapsulating them in Pluronic F127, creating a hydrophilic micelle and making them suitable for many pharmaceutical compositions. When cultured with these UCNPs, hippocampal neurons exhibited a much higher level of depolarization and firing when exposed to NIR radiation. By further modifying the surface and doping location of UCNPs, the upconversion efficiency of UCNPs can be improved to create a more suitable platform for in vivo experimentation and clinical application.
UCNPs are unique and creative methods to excite cells without harmful materials or invasive implantable devices. Similar to QDs, the inherent properties of UCNPs can be exploited to create a multiplexed neural stimulating and visualization system. This can be achieved through the addition of MRI of the UCNPs during IR radiation. To this end, Smith et al. used a NaGdF4:Yb,Er core particle with a poly(glycidyl methacrylate) (PGMA) coating[154] Using NIR radiation, these UCNPs were heated to excite neurons, and then tracked via MRI. Future research directions can include in vivo testing of the multiplexed stimulation and visualization to remotely control CNS or PNS function. As optogenetic systems progress, UCNPs can be deployed for remote activation of genes. One drawback is that the optical response is limited to the cells in the direct vicinity of the nanoparticles, which can be a disadvantage if greater tissue excitation is of interest. This system can be further improved by modifying nanoparticles to target cell types of interest, as well as continuing to surface modify the nanoparticles to avoid an immune response and facilitate transport through the blood-brain-barrier with better biodistribution.
3.2. Magnetic and Acoustic Activation of Nanoparticles
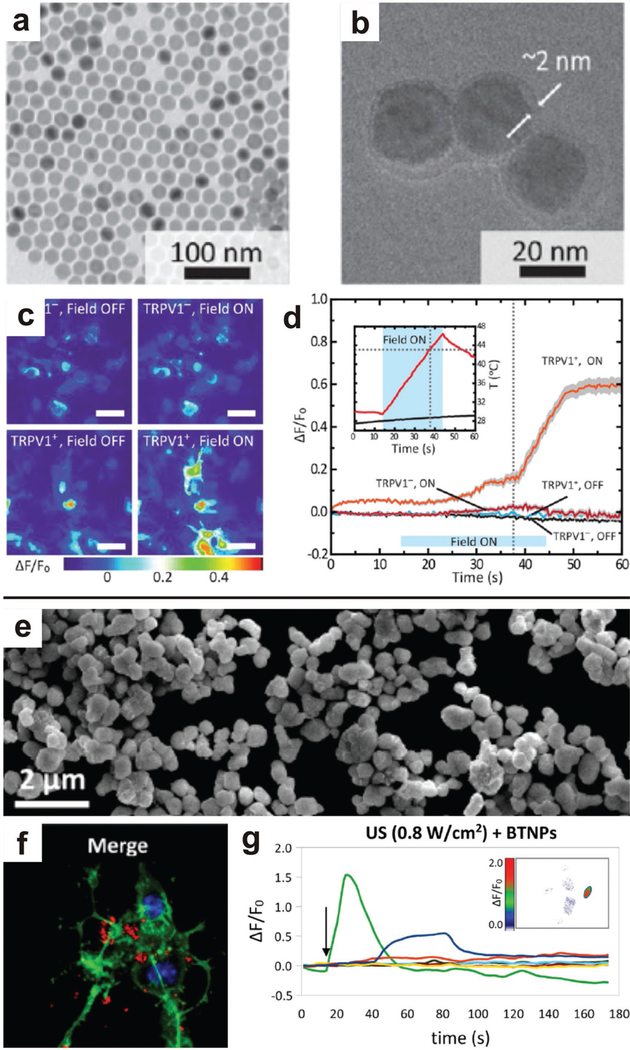
Optically activated hyperthermia, magnetic nanoparticles (MNPs) can thermally induce a cellular response by application of an external magnetic field; furthermore, MNPs can be used as contrast agents during magnetic resonance imaging (MRI) to confirm the bio-distribution of the particles.[165–167] For example, Chen et al. remotely activated heat-sensitive capsaicin receptor TRPV1 using MNPs and low-radiofrequency alternating magnetic fields (0.1–1 MHz) (Figure 8).[168] Biochemical activity was stimulated in deep brain regions with minimal noise or non-specific activity. A similar strategy, which used localized hyperthermia, was applied to degrade amyloid plaques characteristic of Alzheimer’s disease.[169] While these reports illustrate the potential for MNPs to remotely modulate CNS function, continued efforts will surely develop new methods for targeting and stimulating broad swaths of neural circuity.
Figure 8.
External fields can be applied to remotely stimulated neurons via nanoparticle transducers. a-d) Magnetic nanoparticles generate localized hyperthermia when exposed to an alternating magnetic field. This effect was employed to stimulate the heat-sensitive receptor TRPV1, and heat maps show the activation of TRPV1 when magnetic nanoparticles and an external field are introduced into the neuronal culture. Upon application of the magnetic field, both TRPV1 was activated and there was significant temperature increase, illustrating the operation of the magnetic nanoparticles to stimulate the TRPV1 activation via remote controlled hyperthermia. Reproduced with permission.[168] Copyright 2015, American Association for the Advancement of Science. e-g) Piezoelectric barium titanate nanoparticles associate with neuronal cell membranes and calcium flux can be modulated by applied ultrasound. Arrow indicated application of ultrasound pulse. Reproduced with permission.[172] Copyright 2015, American Chemical Society.
To this end, MNPs can also be partnered with implanted devices to target select areas of the brain. Accoto et al. investigate interfacing with the peripheral nervous system with electromagnetic stimulation and electrical recording, rather than the traditional use of electric means for both excitation and signal collection.[170] Electromagnetically exciting neurons improves the SNR due to lessened physiological response around the stimulus. The MNPs intensified the field signal and locally excited neurons of interest due to surface-functionalized targeted antibodies. The MNPs are trapped within a polymeric coating on the electrode, to be released around the electrode upon implantation. A feasibility analysis revealed that stimulation thresholds could be reached with the proposed technology, with next steps being fabrication and in vitro and ex vivo tests. More recently, the fabrication of implantable micro-coils has been successful, displayed to excite in vitro brain slices and not elicit an immune response in vivo.[171] The electromagnetic implantable does not degrade and display reduced performance over time. By partnering this tested technology with the nanoparticle method suggested by Accoto et al., the signal can be better targeted to individual neurons or further amplified.
In addition to localized hyperthermia induced by an alternating magnetic field, some MNPs also exhibit piezoelectric properties which can be activated by external ultrasound. Marino et al. exploited the properties of barium titanate nanoparticles to generate remote stimulation of neurons (Figure 8).[172] After association of the barium titanate nanoparticles with the cell membrane, applied ultrasound resulted in stimulation of Na+ and Ca2+ ion channels.
4. A Special Case: Interfacing with the Blood-Brain Barrier
While many neural interfaces utilize brute force to physically connect with the CNS or PNS, there is a secondary interface at play in the brain that performs a particularly important role in neural function. The blood-brain barrier (BBB) is the highly selective, cellular barricade that separates the circulatory system and CNS; moreover, the BBB is the gatekeeper between noninvasive neuro-nano interfaces and current neural interface technologies. First, the BBB directly effects the performance of electrodes implanted in the brain. BBB degradation and the resultant diffusion of macromolecules eliciting an immune response has been determined as part of the foreign body response surrounding an electrode.[173]
In relation to novel nanoparticles and our endeavor to systemically deliver them to the CNS, the BBB is a highly selective interface, due to tight junctions between the constituent endothelium and astrocytes, preventing passive transport into the brain.[174–175] Cell-surface receptors facilitate transcellular transport, but paracellular transport is extremely limited in the brain. Intranasal administration is one avenue to bypass the BBB to deliver materials to the CNS.[176] The olfactory and trigeminal nerves in the nasal cavity allow direct access to the CNS via extracellular transport along the nasal nerve pathways or intracellular diffusion and endocytosis.[177] By circumventing the BBB, nanoparticles and other materials do not have to be specially designed to control bioactivity to reach the brain; however, intranasal delivery is one of the least common delivery methods for neural interfaces. Disadvantages of intranasal treatment include unknown and limiting effects of nasal diseases, small absorption area, and membrane permeability variability.[178] Accordingly, nanoparticles intended for use as neural interfaces must be particularly designed to cross or modulate the BBB if delivered intravenously or orally.
The following sections outline nanoparticle surface modifications that can aid transport into the brain, BBB modulation to permit transport of nanoparticles, and we comment on the correlation of BBB integrity to neural interface performance.
4.1. Nanoparticles to Infiltrate the BBB
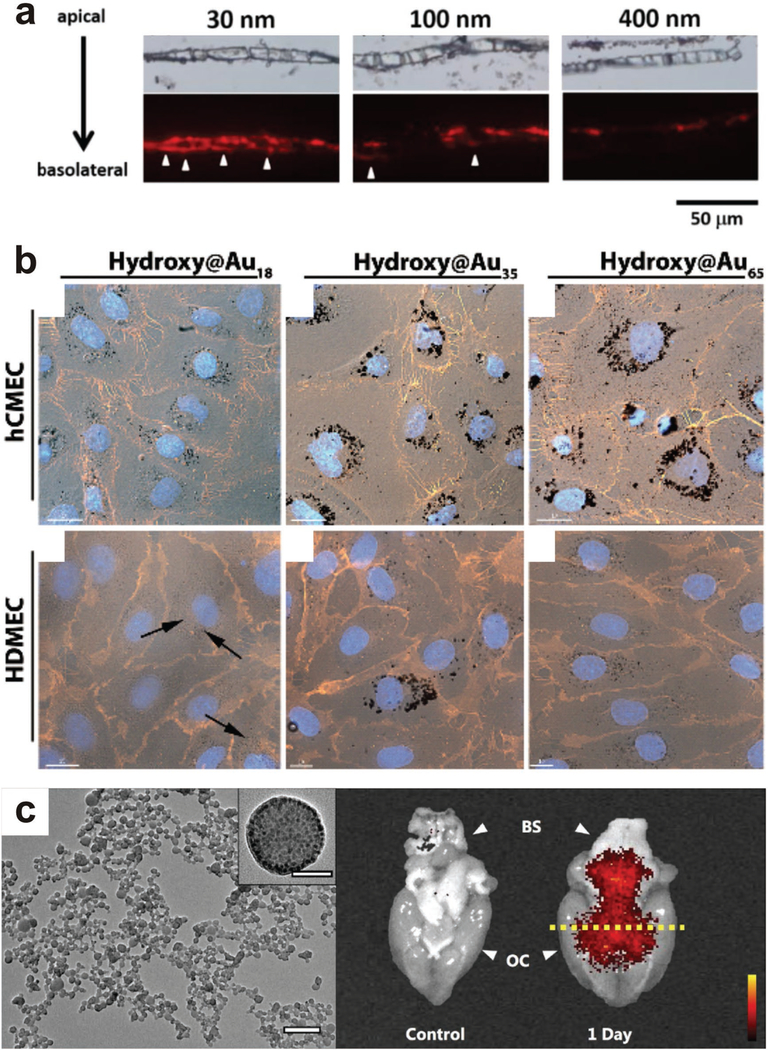
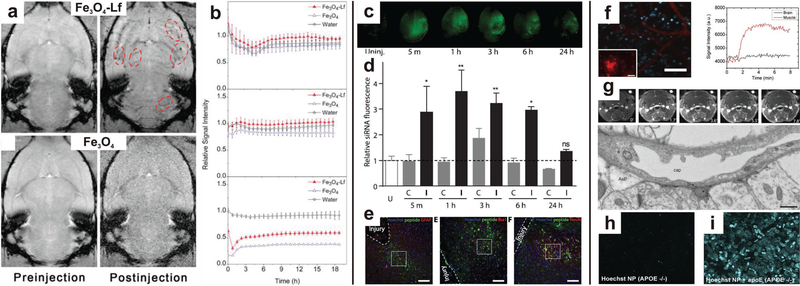
Circulation time and biodistribution are two of the most important considerations when intravenously injecting nanoparticles into the circulatory system. Due to the high selectivity of the BBB, this is a specifically relevant consideration when attempting to deliver nanoparticles to the CNS. Unmodified nanoparticles absorb plasma components, are phagocytized by macrophages, and cleared from the circulatory system in a relatively short period of time.[179] This prevents delivery to the desired tissue. Additionally, nanoparticles tend to accumulate in the liver and spleen rather than other tissues of interest.[180] Much of this transport is dependent on particle size, and smaller particles have a greater tendency to successfully cross the BBB compared to large (>100 nm) particles.[181] Hanada et al. assessed the effects of particle size on transport through the BBB using a simple in vitro model (Figure 9).|182] Primary rat brain microvascular endothelial cells were co-cultured with rat brain pericytes in a transwell. Silica nanoparticles of varying sizes were introduced to the transwell and transport through the cell layer was assessed. It was determined that only the smaller 30 nm silica nanoparticles could be transported through the BBB. Exposure to the 30 nm nanoparticles caused thinning of the endothelial cell layer and “loosened” tight junctions connecting neighboring cells, suggesting even small uncoated nanoparticles could cause barrier damage. QDs were also used to assess the effect of surface charge on travel through the BBB, with significantly more cationic quantum dots travelling through the BBB compared to anionic and neutral QDs, demonstrating that surface charge also has a significant effect on the ability of a nanoparticle to travel across the BBB.
Figure 9.
Physiochemical properties of nanoparticles determine how they cross the BBB. a) Silica nanoparticles traverse a BBB model based on particle size. Reproduced with permission.[182] Copyright 2014, MDPI. b) More polymer-coated nanoparticles accumulated in brain endothelial cells when compared to dermal endothelial cells, showing potential preference for the BBB. Reproduced with permission.[187] Copyright 2014, Royal Society of Chemistry. c) Albumin-coated magnetite nanoparticles were localized in the brain after a transient breakdown of the BBB, illustrating the potential for delivery of large nanoparticles into neural tissue by temporal modulation of BBB properties. Reproduced with permission.[238] Copyright 2016, Nature Publishing Group.
Many groups consider the effects of nanoparticle size and surface-charge when engineering nanoparticles to target tissues without corollary clearance.[181–185] A common technique to reduce nanoparticle clearance, as well as increase endothelial permeability, is to PEG-ylate nanoparticles. This involves coating nanoparticles with PEG, a technique that has been shown to increase selective uptake into the brain and increase circulation time due to increased hydrophilicity, which reduces protein adsorpiton, and an exhibited preference for interaction with the endothelium of the brain.[186] Liu et al. evaluated PEG-ylated silica nanoparticles for selective transport across the BBB.[184] Three different sizes of nanoparticles were compared, and the transport efficiency increased as particle size decreased, with a maximum efficiency of ≈25% for PSiNPs with a diameter of 25 nm. Nance et al. systematically compared the effects of nanoparticle coating and travel through the BBB. The effects of size and surface-coating on nanoparticle transport through the BBB was assessed by comparing PEG-ylated particles of varying size treated with carboxylic acid (COOH) to produce an anionic surface-charge[181] Polystyrene nanoparticles were coated with PEG or COOH and introduced to ex vivo tissue samples. PEG-NPs had increasing mean square displacement over time at 40 and 100 nm diameters, while COOH-NPs had very little displacement in tissue. Nanoparticles could diffuse through the ex vivo models when densely coated with low molecular weight PEG with diameters >100 nm, and in vivo, PEG-coated nanoparticles with diameters of <100 nm could travel into brain tissue, while PEG-coated nanoparticles with diameters of 200 nm were unable to cross the BBB.
Other polymers have also been demonstrated to successfully target the brain when coated onto nanoparticles. Freese et al. coated AuNPs with poly(2-hydroxypropylmethacrylamide) (pH PM A) to target uptake into the brain, targeted at select cells (Figure 9)[187] It was reported that pHPMA-AuNPs were internalized into the perinuclear region of the cells, and stored in vesicle-like structures. Additionally, the brain cells had a higher uptake of pHPMA-AuNPs than the skin cells, suggesting pHPMA is an effective molecule for targeting and internalization. Also, more pHPMA-AuNP internalization occurred for smaller pHPMA nanoparticles than larger hPMA-NPs, concluding that 35 nm particles were optimal for cellular uptake. However, transport across primary porcine brain endothelial cells on transwells displayed a very low quantity of pHPMA-NPs crossed the BBB because pHPMA-AuNPs were sequestered within vesicles within cells. Although the nanoparticles may deliver increased circulation time and localization at the brain tissue, they may not completely cross the BBB.
Besides circumventing the BBB by employing “stealth” coatings, researchers have attempted to directly challenge the performance of astrocytes. Astrocytes are key in maintaining brain homeostasis, therefore the ability to modulate ion channels is useful for the treatment of a variety of pathophysiologies, including ischemia, stroke, and Alzheimer disease. Posati et al. used hydrotalcite-like compounds (HTlc) to manipulate astrocyte ion channels and aquaporins in vitro.[188] Specifically, the HTlc nanoparticles arrange in a crystalline manner that creates a film with nanoroughness and rod-like structures, and they were used to affect to influx of potassium ions mediated by the Kir4.1 channel in conjunction with aquaporin 4 (AQP4). Rat neocortical astroglial cells differentiated when cultured on the HTlc nanoparticles; furthermore, AQP4 and Kir4.1 were upregulated when cells were cultured on HTlc films. From these results, HTlc nanoparticles are an improved platform to study molecular modulation of astrocytes without the addition of chemical elements, which may lead to methods for modulating the function of astrocytes in the BBB.
Accordingly, the ability to accurately identify both the region of nanoparticle localization and residence time is critically important to the design of a neuro-nano interface. In vitro models such as transwells typically cannot be used to quantify nanoparticle transport efficiency through the BBB due to the insufficient barrier properties. Essentially, a significant number of nanoparticles could have travelled through areas of low confluency that do not truly represent an accurate model of the BBB. This concern was also expressed by Bramini et al., and it was evaluated by utilizing 3D-imaging to accurately assess particle location on cell barriers.[189] As a solution, they suggest that the use of proper imaging could allow selective study of areas with full cell confluency and well established cell interactions to understand nanoparticle endocytosis without in vivo models. A model endothelium was stained to determine areas with barrier defects, and confocal microscopy was used to follow nanoparticle movement through the barrier. Functionalizing particle surfaces with stealth materials facilitates the transport of nanoparticles through the BBB into the nervous system at larger sizes and with lessened immune response; however, differentiating uptake into endothelial cells and delivery across the BBB to neuronal tissue is still a critical characterization hurdle. More importantly, the ability to target or ensure predominantly localization at the BBB may help achieve better transport and delivery to the CNS.
4.2. Receptor-Mediated Transport Across the BBB
Rather than modifying nanoparticles to infiltrate and localize in the endothelium of the BBB, there is substantial benefit modifying the surface of nanoparticles with specific biomacromolecules, i.e., proteins, antibodies, or peptides, to interact with receptors on the surface of the endothelial cell. This method has been demonstrated to very selectively target endothelial cells, and even activate biochemical pathways (Figures 10 and Figure 9). For example, a number of techniques have been reported that utilize nanoparticles to apply forces to specific receptors on the surface of cells, modulating mechano-responsive ion channels.[190,191] Such a technique can be easily extrapolated as an exciting route for stimulation of neurons or modulation of the BBB.
Figure 10.
Receptor-mediated transport across the BBB can be facilitated by protein, antibody, peptide, or hormone conjugation to the surface of nanoparticles. a,b) Lactoferrin-functionalized nanoparticles are localized in the brain; whereas, bare nanoparticles do not cross the BBB. TEER values are reduced during the initial exposure to lactoferrin-functionalized nanoparticles but quickly recover and are attributed to measurement fluctuation, suggesting the receptor-mediated crossing of the BBB does not permanently disrupt the barrier function. Reproduced with permission.[166] Copyright 2012, American Chemical Society. c-e) RVG-targeted nanoparticles accumulate in the hemisphere of the brain with a disrupted BBB. Quantification of nanoparticles in the contralateral (C) and injured hemisphere (I) illustrate significant accumulation when the BBB is disrupted. Astrocytes (GFAP+), microglia (Iba1), and neurons (NeuN) localized at the injury site, where there were reduced astrocytes and microglia after application of the neuron-targeted nanoparticles. Reproduced with permission.[236] Copyright 2016, American Chemical Society. f-i) PBCA nanoparticles functionalized with APOE across the BBB, but increase DTPA signal intensity is not measured in the brain, suggesting the nanoparticles do not disrupt the BBB. This is further illustrated by an intact BBB, and APOE knockout mice did not display significant uptake of the nanoparticles without the APOE functionalization. Reproduced with permission.[237] Copyright 2011, National Academy of Science.
Guarnieri et al. investigated the effect of viral fusion peptide gH625 conjugation on polystyrene nanoparticle transport across the BBB.[192] The peptide gH625 was previously observed to be key in facilitating the transport of the Herpes virus through a cell membrane; therefore, it was chosen to force particle endocytosis into endothelial cells. Bare nanoparticles were transported into vesicles facilitated by motor proteins. The gH625-NPs displayed a more diffusive transport pattern through the cellular membrane. Additionally, significantly more conjugated gH625-NPs were internalized in the endothelial cells when compared to bare nanoparticles. Endocytosis can also be induced by conjugating specific antibodies to nanoparticles. Yemisci et al. induced particle transcytosis with a transferrin-targeted antibody.[193] Streptavidin-ylated anti-transferrin receptor-1 monoclonal antibodies were conjugated to chitosan-PEG-biotin polymer nanoparticles. Transferrin is a receptor found on the cellular membrane responsible for transporting iron into the cell via receptor-mediated endocytosis.[194] Using in vivo mouse models, it was observed that significantly more modified nanoparticles were transported into the brain compared to controls, confirming the activation of the transferrin receptor as an effective way to induce transcytosis and transport across the BBB. Biodistribution would need to be assessed to expand applications to clinical settings, as transferrin receptors are present in other organ systems. Other proteins in the transferrin family have also been investigated as agents to facilitate traversal of the BBB. Lactoferrin promotes receptor-mediated transcytosis on cerebral endothelial cells with a higher uptake efficiency than transferrin receptors activated by anti-transferrin antibodies. Qiao et al. conjugated lactoferrin to PEG-ylated MNPs (Figure 10).[166] TEER was used to quantify the BBB integrity of an in vitro model before and after introducing the lactoferrin-modified nanoparticles and controls. It was reported that lactoferrin-modified nanoparticles had a significantly higher transport efficiency and reduced the TEER, i.e., damaged the BBB model, when compared to controls. Using MRI-enhanced visualization in vivo, it was shown that the lactoferrin-modified nanoparticles crossed the BBB and were not trapped in the endothelium during transcytosis. Also using proteins to mediate transport, Gregori et al. functionalized particles with a modified fragment of human Apolipoprotein E (mApoE)[183] This protein targets a low-density lipoprotein-receptor that is upregulated on brain endothelium when compared to other endothelial cell types. This protein was conjugated onto a self-assembled nanoparticle fabricated from poly(N,N-dimethylacrylamide)-block-polystyrene (PDMA-b-PS). Block co-polymer nanoparticles are notable for being highly stable and suitable carrier and release systems for bioactive molecules. Functionalized nanoparticles were internalized more than nanoparticles without mApoE, demonstrating that lipoprotein-receptor targeting is an effective way to delivery nanoparticles to the brain. The multitude of biomacromolecules being used for targeting nanoparticles around the body provide a very broad design space to develop nanoparticle with can both target the BBB and cross it without significant disruption. Considering the receptors discussed are not solely found in the brain endothelium, the identification of receptors exclusive to the BBB would prove valuable for developing strategies for precision delivery of nanoparticles to the brain while avoiding broad distribution and activity.
Hormone receptor-mediated transcytosis may provide an alternative class of homing molecules to ferry nanoparticles across the BBB[195–197] Shilo et al. fabricated insulin-modified gold nanoparticles to assess transport across the BBB via such a pathway.[198] The authors’ strategy aimed to exploit the neuro-vascular insulin receptors to initiate transcytosis across the BBB,[199,200] while organs without insulin receptors would possess an endothelium too occlusive to allow passive transport of insulin-modified nanoparticles. This was tested in vivo by injecting mice with nanoparticles and quantifying the distribution of the insulin-modified nanoparticles across multiple organs. The quantity of insulin-modified nanoparticles resident in the brain was five times that of the control nanoparticles. By targeting receptors only found on the brain’s endothelium, nanoparticles can be deliberately accumulated in proximity to the BBB. This strategy may reduce the concentration of nanoparticles required for treatment and potentially avoid accumulation and clearance via the liver and kidney.
While insulin receptor-mediated transcytosis is a promising, preliminary strategy to ferry nanoparticles across the BBB, systemic delivery of hormones may raise serious hazards, such as the use of insulin causing an acute fluctuation in blood glucose concentrations. Continued evaluation of these materials and identification of unique neurovascular receptors, i.e., receptors not found or found in significantly reduced quantity in other organs, will ideally assuage such concerns, and enable new neuro-nano interfaces and non-invasive delivery strategies.
4.3. Physical Alteration of the BBB to Enable Neuro-Nano Interfaces
As an alternative to modifying particles to induce endocytosis into, and finally across, the endothelium of the BBB, novel techniques aim to transiently compromise the BBB to permit the transport of nanoparticles through gaps between cells into the CNS (Figure 11). Remote modulation methods to induce and control the permeability of the BBB are under investigation for myriad applications, including the optimization of delivering chemotherapeutics and other small-molecule drugs[21]
Figure 11.
Nanoparticle delivery across the BBB can be facilitated by temporarily disrupting the BBB using external stimuli. a) Applying a magnetic force externally allows the transport of magnetic nanoparticles into the brain. Reproduced with permission.[223] Copyright 2012, Elsevier. b) MRI-assisted ultrasound facilitates the delivery of gadolinium nanoparticles to the brain, and more gadolinium entered the brain at higher pressures. Reproduced with permission.[204] Copyright 2014, Elsevier. (c) Temporary disruption in the BBB due to injected magnetic nanoparticles and an applied external field which induces localized hyperthermia and is illustrated by the transport of Evans blue dye into the treated hemisphere of the brain. Reproduced with permission.[165] Copyright 2015, Elsevier.
Popular non-invasive methods to alter the permeability of the BBB rely on guided ultrasound.[201–203] This technique involves the delivery of focused ultrasound energy to stimulate precise regions of the brain, which result in the degradation of the barrier properties of the BBB. Such a modulation of the barrier properties may allow the transport of circulating nanoparticles across the BBB. For example, Nance et al. uses MRI-guided focused ultrasound to temporarily permeabilize the BBB, allowing the transport of polystyrene-PEG nanoparticles.[204] It was also determined that 60 nm PS-PEGNPs could be delivered to the brain with MRI-guided focused ultrasound without uptake into other non-targeted regions.
In conjunction with direct application of ultrasound to disrupt the BBB and permit nanoparticle transport, the utilization of microbubbles to transduce ultrasound application has been recently explored. Microbubble contrast agents, injected intravenously, are activated by ultrasound, producing mechanical, chemical, and thermal effects that lead to temporary disruption of the BBB.[205–213] By taking a small liberty, we may consider these microbubble contrast agents as nanoparticle technologies,[214] and Fan et al. have even utilized microbubbles as carriers for gene delivery across the BBB.[215] Chu et al. have characterized the mechanisms by which focused-ultrasound (FUS) attenuation of microbubbles disrupts the BBB by comparing the mechanical index (MI) to the cavitation index (CI).[216] These indices are derived from backscattered acoustic emissions of FUS, and these indices are used to quantify the microbubble contraction/expansion (subharmonic/ultraharmonic emissions) and microbubble disruption (wideband emissions). This is referred to as stable cavitation quantified by the CI and inertial cavitation quantified by the MI, respectively. Inertial cavitation has been traditionally measured to gauge FUS-microbubble mediated BBB opening, though stable cavitation is being reported to have a more substantial effect.[217–218] FUS was used at varying frequencies to non-invasively and locally induce BBB opening in mouse models, with MI and CI determined through the use of dynamic-contrast-enhanced MRI and passive cavitation detection. By quantifying these indexes, the dynamic effects of BBB disruption with FUS attenuated microbubbles can be gauged to precisely control degree and duration of BBB opening, which is especially important for determining both dose and duration of treatment in the clinical settings. Such techniques are potential tools for delivery of nanoparticle interfaces without inducing a chronic disruption of the BBB. However, while focused ultrasound has been illustrated to permit nanoparticles to travel into the brain, there are many factors—including ultrasound doses, attenuation factors and variability in timing the duration of the BBB opening which will require significant studies to further define the proper operational parameters.[219,220]
Rather than creating barrier-disrupting effects with ultrasound, the localized heating and mechanical stressors achieved with ultrasound may also be achieved via the application of external magnetic fields. It has been shown that mild hyperthermia can increase BBB permeability.[221,222] Kong et al. demonstrated that MNPs can cross the BBB of a mouse model when an external magnetic field is applied (Figure 11).[223] Composite MNPs were fabricated by trapping MNPs and fluorophores within polystyrene nanospheres, resulting in nanocomposites with diameters of ≈100 nm. Accumulation of MNPs in the brain without an external field was not observed. An external magnetic field was applied locally by implanting a magnet in the right hemisphere of a mouse cerebral cortex and intravenously introducing MNPs, which accumulated where the implant was located. Significantly more MNPs were retained in the brain when an external magnetic field was applied compared to no magnetic field. Additionally, when a magnetic field was applied MNPs were observed to cross the BBB into the perivascular space and parenchyma without any histological changes which would be present in tissue or vessel damage. Large perivascular aggregates of MNPs (>800 nm) were observed in stained brain sections previously exposed to the magnet, suggesting that MNPs may have travelled through the vessel while clustered, although the study rationalizes that the MNPs are endocytosed into endothelial cells through confocal microscopy. In vitro cell culture with MNPs produced no cell death or immunotoxicity and displayed MNP cellular internalization meaning uptake is membrane mediated. This was further demonstrated by Tabatabaei et al., where magnetic heating of nanoparticles can increase permeability of the BBB.[165] Rather than fluorescent nanoparticles, fluorescent Evans Blue dye was used to determine the integrity of the BBB. Significantly more dye was detected in the brain with magnetically heated nanoparticles. The brain was almost completely cleared of dye after two hours of recovery, indicating that heating MNPs in the brain does not cause permanent damage of the BBB. These results are promising for use of localized hyperthermia in targeted delivery of nanoparticles across the BBB for neural interfaces.
Regardless of the modality of BBB permeabilization, what remains to be characterized is the optimal timeline for maximal BBB disruption and penetrations of nanoparticles. In addition, alternative physical modalities, such as electric field modulation of the BBB have not been systematically studied. Frequency-tuned electric fields have been studied as a cancer therapy, and electrical current has been used to open the BBB.[218–220] These techniques achieve irreversible electroporation and possible tissue ablation, which while viable for cancer treatment, is not ideal for delivery of nanoparticles for neuro-nano interfaces. When using methods that modify the permeability of the BBB, unwanted molecules or pathogens may be able to enter the brain, just as the desired nanoparticles do. For this reason, it would need to be heavily assured that there is no systemic infection before treatment, or treatment would need to be partnered with antibiotics or antivirals as a preventative measure. Unfortunately, the imaging and targeting techniques required to selectively permeate barriers next to particles of interest are not yet realized, limiting the possibility of this technique for nanoparticle delivery into the brain.
4.4. Electrodes and the BBB
As previously discussed, the short-lived stability of electrodes implanted in the brain is an issue that can be addressed with nanoparticle deposition on the electrode surface, which reduces both fouling and fibrotic encapsulation.[29] Cell death and immune response in close vicinity to the electrode causes surface degradation, change of material properties, and reduced signal integrity. In addition, glial capsule formation around a foreign object can isolate the electrode from the tissue of interest, eliminating the signal (Figure 12).[173] Glial scarring can also reduce contact between the electrode and neurons, reducing signal integrity.[224] It has recently been discovered that BBB leakage may influence brain tissue reaction to implanted bodies too.[224,225] Loss of barrier function can lead to antigens and serum albumin leakage from vessels, which can induce apoptosis in surrounding cells and instigate an inflammatory response.
Figure 12.
Neural interface stability can be compromised due to BBB gap formation and release of macromolecules causing an exacerbated immune response. a) Chronic neural implant failure cause chronic BBB failure, which induces a cascade of deleterious immune responses including glial scarring and neurodegeneration which results in recording failures. In general, the SNR increases over the course of the implantation, and soon after implantation the presence of reactive astrocytes is high (GFAP+, red). After 16 weeks of implantation, reactive astrocytes are still localized around the implanted electrode interface. Reproduced with permission.[173] Copyright 2013, Elsevier. b) By employing mechanically compliant nanocomposites, the neuroinflammatory response is mitigated and recruitment of reactive astrocytes and subsequent glial scarring is reduced. Reproduced with permission.[100] Copyright 2014, Institute of Physics.
Unlike acute wounds to the BBB which exhibit resolution, implantation of an electrode or other interface results in a foreign body response and persistent leakage of the BBB[226,227] Sawyer et al. investigated the foreign body response due to implanted electrodes, and its effects on BBB integrity and leakage. Fluorescent polystyrene nanoparticles were injected in mice with a previously implanted foreign body to assess tissue integrity around the implant.[224] Within 50 and 100 μm of the implant, BBB gaps were reported. Gold nanoparticle tracers were also used to identify the location of the nanoparticles outside the neuro-vasculature. Particles with diameters ranging from 20–1000 nm were used to quantify the size of the gaps in the BBB around the implant. It was found that particles as large as 500 nm could travel into the brain parenchyma. An acute inflammatory response was also observed, suggesting that nanoparticles were phagocytosed and could be transported through the BBB via monocytes. The cause of the persistent gaps in the BBB, i.e., mechanical insertion versus expansion of the gaps after the foreign body and inflammatory response, was not delineated; yet, in the reported results, the gaps persisted for weeks within the vicinity of the implant without significant spreading. These results lend to the contention between the common hypothesis that the foreign-body response and the BBB stabilize after approximately four weeks post-implantation[228] and the recent reports of a much lengthier, almost persistent, foreign-body response and BBB dysfunction due to implanted devices.[173,227,229,230]
Saxena et al. also explored the relationship between BBB function and electrode degradation.[173,224] Electrodes were implanted into rat brains and barrier integrity was correlated with electrode performance. It was found that the electrode SNR steadily declined over a 16-week period. Additionally, there was a relative increase in reactive astrocytes surrounding the implant site 16 weeks after implantation. Areas where the BBB was compromised accumulated neurotoxic factors, particularly near electrodes collecting signals with poor quality.
As a better understanding of the BBB’s function and modes of dysfunction are achieved, such information will dictate the design parameters for next-generation neuronal interfaces. Most importantly, the discussed results highlight the need for alternative methods to interface with the brain, as electrodes do not seem practically reliable for long-term use without the application of complex particle coatings and anti-inflammatory drugs in the acute setting. Consequently, many of the circulating particle techniques described previously suggest a promising platform for long-term neural interfacing that avoid acute mechanical damage to neural tissue and minimize any chronic foreign-body response. In addition, the capability to circumvent the damage to the BBB by modulating, i.e., opening then closing, the BBB to deliver nanoparticles to the CNS will be tremendously important for the development of new neuro-nano interfaces.
5. Future Outlook and Perspective
Prior to drawing any conclusions, it is necessary to briefly comment on the toxicological interactions of nanoparticles with the CNS and PNS. Although numerous in vivo and in vitro studies have provided evidence of the toxic effects of various types of nanoparticles,[231–234] our understanding of the potential health and safety issues regarding nanoparticles lags behind the daily plethora of reports touting the next-greatest nanotechnology and their rapid run towards commercialization. While many reports are emerging on the potential deleterious impact of nanoparticles, the advances in understanding the complexities of nanotoxicology seem to trail that of biomedical nanotechnologies. This may be due to the relative popularity and news-worthiness of a newly designed, promising nano-therapeutic or nanotechnology versus a systematic, seminal study of the neuro-toxicological effect of a narrowly defined population of nanomaterials. In any case, it can be expected that with further application of nanoparticles, both systematic studies and post facto analysis of nanoparticles and neurotoxicity will be formulated and become of critical importance to next-generation neuro-nano interfaces.
With that said, the horizon is lined with researchers exploring nanomaterial technologies to enable single-cell electronic connections, silicon-neuron hybrid circuits, and remote modulation of entire neural circuits via genetic engineering. As the neuro-nano interfaces become more capable, we will also have to evaluate the effects of any altered function due to their chronic application. There are many challenges faced, both in terms of biological and hardware complexity, and significant breakthroughs must be made in a wide range of scientific disciplines, from synthetic biology to neuroscience and low-power electronics.
In this feature, we presented the state-of-the-art nanoparticle technologies utilized for neural stimulation, recording and modulation which will lead the way toward next-generation neural interfaces. We have described three main classes of these technologies: (i) nano-coatings of implanted electrodes; (ii) nanoparticle transducers; and (iii) modulation of the BBB. Although, many aspects need further investigation, it has been demonstrated that the physico-chemical properties like particle geometry, surface-charge and chemical composition are vital to performance. In general, smaller nanoparticles may undergo passive transport across the BBB. The surface-charge can also be tuned to limit protein adsorption and mitigate immune responses to lengthen circulation times. Nanoparticle coatings to reduce the impedance of implanted electrodes have also become a standard step in the optimization of neural recording hardware. Independent nanoparticles are also emerging as neural interface materials. Optically responsive nanoparticles targeted or delivered to neurons can transduce applied fields and instigate neural stimulation. Similarly, MNPs can be used to elicit thermal reactions from cells with simultaneous imaging with MRI. Finally, and perhaps the most important for the future success of neuro-nano interfaces, is the development of non-invasive and non-destructive methods to modulate the barrier properties of the BBB and permit transport of systemically delivered nanoparticles. Better understanding and control of the BBB will enable future experiments to question the efficacy of neuro-nano interfaces for chronic applications.
Ultimately, the use of nanoparticles as neural interfaces is progressing down a bright path, leading toward fascinating tools to understand, modify, control and heal the human nervous system, especially the brain. This promise also leads to one conclusion, in which the capability of nanoparticles to accurately quantify changes in the neural environment with cellular resolution suggests the possibility of developing feedback-loops for immediate in vivo response, i.e., non-invasive neural recording with real-time biochemical or electrophysiological augmentation.
Acknowledgements
This work was sponsored by the National Science Foundation (EEC1160483) through a NSF Nanosystems Engineering Research Center (NERC) for Advanced Self-Powered Systems of Integrated Sensors and Technologies (ASSIST). ATY is supported through the NIH Integrative Vascular Biology Traineeship (NIH T32HL069768).
Biographies

Ashlyn T. Young is a doctoral student in Dr. Daniele’s laboratory. Her current research focuses on strategies for engineering organ-on-chip models of the human blood-brain barrier. She received her bachelor’s degree in Applied Science from UNC-Chapel Hill in 2015.

Neil Cornwell is an undergraduate student in Dr. Daniele’s laboratory. His current research focuses on the growth of human endothelium in microfluidic devices. He is pursuing a bachelor’s degree in Biomedical Engineering from NC State University with an expected graduation date in 2019.

Michael A. Daniele joined the Department of Electrical & Computer Engineering at NC State University and the Joint Department of Biomedical Engineering at UNC-Chapel Hill and NC State University in 2015. He earned a B.S. from Rutgers University in 2009 and a Ph.D. in Materials Science & Engineering at Clemson University in 2012. His laboratory’s primary area of interest is the broad application of soft materials to engineer systems which monitor, mimic or augment biological function. Specific topics of research include wearable and implantable microdevices and organ-on-chip models.
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
Contributor Information
Ashlyn T. Young, Joint Department of Biomedical Engineering, University of North Carolina at Chapel Hill, and North Carolina State University, 911 Oval Dr., Raleigh, NC 27695, USA
Neil Cornwell, Joint Department of Biomedical Engineering, University of North Carolina at Chapel Hill, and North Carolina State University, 911 Oval Dr., Raleigh, NC 27695, USA.
Michael A. Daniele, Joint Department of Biomedical Engineering, University of North Carolina at Chapel Hill, and North Carolina State University, 911 Oval Dr., Raleigh, NC 27695, USA Department of Electrical & Computer Engineering, North Carolina State University, 890 Oval Dr., Raleigh, NC 27695, USA.
References
- [1].Kovacs GTA, Science 1990, 248, 1280. [PubMed] [Google Scholar]
- [2].Hodgkin AL, Huxley AF, Nature 1939, 744, 710. [Google Scholar]
- [3].Vidal JJ, Annu. Rev. Biophys. Bioeng 1973, 2, 157. [DOI] [PubMed] [Google Scholar]
- [4].Vidal JJ, Proc. IEEE 1977, 65, 633. [Google Scholar]
- [5].Bartholow R, Am. J. Med. Sci 1874, 66, 305. [Google Scholar]
- [6].Grill WM, Am. Sci 2010, 98, 48. [Google Scholar]
- [7].Grill WM, Norman SE, Bellamkonda RV, Annu. Rev. Biomed. Eng 2009, 11, 1. [DOI] [PubMed] [Google Scholar]
- [8].Rajasethupathy P, Ferenczi E, Deisseroth K, Cell 2016, 165, 524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Zeng FG, Rebscher S, Harrison W, Sun X, Feng H, IEEE Rev Biomed. Eng 2008, 1, 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Merrill DR, Bikson M, Jefferys JGR, J. Neurosci. Meth 2005, 141, 171. [DOI] [PubMed] [Google Scholar]
- [11].Cogan SF, Annu. Rev. Biomed. Eng 2008, 10, 275. [DOI] [PubMed] [Google Scholar]
- [12].Wolpaw JR, McFarland DJ, Proc. Natl. Acad. Sci. USA 2004, 101, 17849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hochberg LR, Bacher D, Jarosiewicz B, Masse NY, Simeral JD, Vogel J, Haddadin S, Liu J, Cash SS, van der Smagt P, Donoghue JP, Nature 2012, 485, 372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Deer TR, Krames E, Mekhail N, Pope J, Leong M, Stanton-Hicks M, Golovac S, Kapural L, Alo K, Anderson J, Foreman RD, Caraway D, Narouze S, Linderoth B, Buvanendran A, Feler C, Poree L, Lynch P, McJunkin T, Swing T, Staats P, Liem L, Williams K, Neuromodulation 2014, 17, 599. [DOI] [PubMed] [Google Scholar]
- [15].Minev IR, Musienko P, Hirsch A, Barraud Q, Wenger N, Moraud EM, Gandar J, Capogrosso M, Milekovic T, Asboth L, Torres RF, Vachicouras N, Liu QH, Pavlova N, Duis S, Larmagnac A, Voros J, Micera S, Suo ZG, Courtine G, Lacour SP, Science 2015, 347, 159. [DOI] [PubMed] [Google Scholar]
- [16].Kotov NA, Winter JO, Clements IP, Jan E, Timko BP, Campidelli S, Pathak S, Mazzatenta A, Lieber CM, Prato M, Bellamkonda RV, Silva GA, Kam NWS, Patolsky F, Ballerini L, Adv. Mater 2009, 21, 3970. [Google Scholar]
- [17].Colombo E, Feyen P, Antognazza MR, Lanzani G, Benfenati F, Front. Neurosci 2016, 10, 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Thompson CH, Zoratti MJ, Langhals NB, Purcell EK, Tissue Eng. Part B 2016, 22, 125. [DOI] [PubMed] [Google Scholar]
- [19].Zhang HN, Shih J, Zhu J, Kotov NA, Nano Lett. 2012, 12, 3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Wang YC, Guo L, Front. Neurosci 2016, 10.26858590 [Google Scholar]
- [21].Appelboom G, Detappe A, LoPresti M, Kunjachan S, Mitrasinovic S, Goldman S, Chang SD, Tillement O, Neuro-Oncology 2016, 18, 1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Das S, Carnicer-Lombarte A, Fawcett JW, Bora U, Prog. Neuro-biol 2016, 142, 1. [DOI] [PubMed] [Google Scholar]
- [23].Pisanello F, Sileo L, De Vittorio M, Frontiers Neurosci. 2016, 10, 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Schwartz AB, Annu. Rev Neurosci 2004, 27, 487. [DOI] [PubMed] [Google Scholar]
- [25].Maynard EM, Nordhausen CT, Normann RA, Electroencephalogr. Clin. Neurophysiol 1997, 102, 228. [DOI] [PubMed] [Google Scholar]
- [26].Hoogerwerf AC, Wise KD, IEEE T. Bio-Med Eng 1994, 41, 1136. [DOI] [PubMed] [Google Scholar]
- [27].Seymour JP, Kipke DR, Biomaterials 2007, 28, 3594. [DOI] [PubMed] [Google Scholar]
- [28].Groothuis J, Ramsey NF, Ramakers GMJ, van der Plasse G, Brain Stimul. 2014, 7, 1. [DOI] [PubMed] [Google Scholar]
- [29].Vitale F, Summerson SR, Aazhang B, Kemere C, Pasquali M, ACS Nano 2015, 9, 4465. [DOI] [PubMed] [Google Scholar]
- [30].Jeong JW, Shin G, Il Park S, Yu KJ, Xu LZ, Rogers JA, Neuron 2015, 86, 175. [DOI] [PubMed] [Google Scholar]
- [31].Norton JJS, Lee DS, Lee JW, Lee W, Kwon O, Won P, Jung SY, Cheng HY, Jeong JW, Akce A, Umunna S, Na I, Kwon YH, Wang XQ, Liu ZJ, Paik U, Huang YG, Bretl T, Yeo WH, Rogers JA, Proc. Natl. Acad. Sci. USA 2015, 112, 3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Viventi J, Kim DH, Vigeland L, Frechette ES, Blanco JA, Kim YS, Avrin AE, Tiruvadi VR, Hwang SW, Vanleer AC, Wulsin DF, Davis K, Gelber CE, Palmer L, Van der Spiegel J, Wu J, Xiao JL, Huang YG, Contreras D, Rogers JA, Litt B, Nat. Neurosci 2011, 14, 1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Xie C, Liu J, Fu TM, Dai XC, Zhou W, Lieber CM, Nat. Mater 2015, 14, 1286. [DOI] [PubMed] [Google Scholar]
- [34].Hong GS, Fu TM, Zhou T, Schuhmann TG, Huang JL, Lieber CM, Nano Lett. 2015, 15, 6979. [DOI] [PubMed] [Google Scholar]
- [35].Ortiz-Catalan M, Branemark R, Hakansson B, Delbeke J, Biomed. Eng. Online 2012, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Seifert JL, Desai V, Watson RC, Musa T, Kim YT, Keefer EW, Romero MI, IEEE T Neur. Sys. Reh 2012, 20, 220. [DOI] [PubMed] [Google Scholar]
- [37].Normann RA, Fernandez E, J. Neural Eng 2016, 13, 061003. [DOI] [PubMed] [Google Scholar]
- [38].Zhao Z, Gong R, Huang H, Wang J, Sensors-Basel 2016, 16, 880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Kappenman ES, Luck SJ, Psychophysiology 2010, 47, 888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Hoffman-Kim D, Mitchel JA, Bellamkonda RV, Annu. Rev. Biomed. Eng 2010, 12, 203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Nikkhah M, Edalat F, Manoucheri S, Khademhosseini A, Biomaterials 2012, 33, 5230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Nguyen AT, Sathe SR, Yim EKF, J. Phys. Condens. Mat 2016, 28, 183001. [DOI] [PubMed] [Google Scholar]
- [43].Lugo-Morales LZ, Loziuk PL, Corder AK, Toups JV, Roberts JG, McCaffrey KA, Sombers LA, Anal. Chem 2013, 85, 8780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Schmidt AC, Wang X, Zhu YT, Sombers LA, Acs Nano 2013, 7, 7864. [DOI] [PubMed] [Google Scholar]
- [45].Shin JH, Kim GB, Lee EJ, An T, Shin K, Lee SE, Choi W, Lee S, Latchoumane C, Shin H-S, Lim G, Adv. Healthcare Mater 2014, 3, 245. [DOI] [PubMed] [Google Scholar]
- [46].Heister E, Brunner EW, Dieckmann GR, Jurewicz I, Dalton AB, Acs Appl. Mater. Interfaces 2013, 5, 1870. [DOI] [PubMed] [Google Scholar]
- [47].Amenta V, Aschberger K, Wiley Interdiscip Rev. -Nanomed. Nano-biol 2015, 7, 371. [DOI] [PubMed] [Google Scholar]
- [48].Zhou H, Wang T, Duan YY, J. Electroanal. Chem 2013, 688, 69. [Google Scholar]
- [49].Ansaldo A, Castagnola E, Maggiolini E, Fadiga L, Ricci D, Acs Nano 2011, 5, 2206. [DOI] [PubMed] [Google Scholar]
- [50].Yung-Chan C, Hui-Lin H, Yu-Tao L, Huan-Chieh S, Shiang-Jie Y, Chang-Hsiao C, Wei-Lun H, Tri-Rung Y, Shih-Rung Y, Da-Jeng Y, Yen-Chung C, Hsin C,J. Neural. Eng 2011, 8, 034001.21474876 [Google Scholar]
- [51].Castagnola E, Maggiolini E, Ceseracciu L, Ciarpella F, Zucchini E, De Faveri S, Fadiga L, Ricci D, Front. Neurosci 2016, 10, 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Kozai TDY, Catt K, Du ZH, Na K, Srivannavit O, Haque RUM, Seymour J, Wise KD, Yoon E, Cui XT, IEEE T. Bio-Med. Eng 2016, 63, 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Castagnola E, Maiolo L, Maggiolini E, Minotti A, Marrani M, Maita F, Pecora A, Angotzi GN, Ansaldo A, Boffini M, Fadiga L, Fortunato G, Ricci D, IEEE T. Neurol. Sys. Reh 2015, 23, 342. [DOI] [PubMed] [Google Scholar]
- [54].Samba R, Herrmann T, Zeck G, J. Neural Eng 2015, 12, 016014. [DOI] [PubMed] [Google Scholar]
- [55].Kolarcik CL, Catt K, Rost E, Albrecht IN, Bourbeau D, Du ZH, Kozai TDY, Luo XL, Weber DJ, Cui XT,J. Neural. Eng 2015, 12, 016008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Luo XL, Weaver CL, Zhou DD, Greenberg R, Cui XYT, Biomaterials 2011, 32, 5551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Chen HL, Guo LH, Ferhan AR, Kim DH, J. Phys. Chem. C 2011. 115, 5492. [Google Scholar]
- [58].David-Pur M, Bareket-Keren L, Beit-Yaakov G, Raz-Prag D, Hanein Y, Biomed. Microdevices 2014, 16, 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Chen H, Guo L, Ferhan AR, Kim D-H, The Journal of Physical Chemistry C 2011, 115, 5492. [Google Scholar]
- [60].Nunes A, Al-Jamal K, Nakajima T, Hariz M, Kostarelos K, Arch. Toxicol 2012, 86, 1009. [DOI] [PubMed] [Google Scholar]
- [61].Baranauskas G, Maggiolini E, Castagnola E, Ansaldo A, Mazzoni A, Angotzi GN, Vato A, Ricci D, Panzeri S, Fadiga L, J. Neural. Eng 2011, 8, 066013. [DOI] [PubMed] [Google Scholar]
- [62].Chen R, Canales A, Anikeeva P, Nat. Rev. Mater 2017, 2, 16093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Kirchmeyer S, Reuter K,J. Mater. Chem 2005, 15, 2077. [Google Scholar]
- [64].Huang J, Miller PF, de Mello JC, de Mello AJ, Bradley DDC, Synth. Met 2003, 139, 569. [Google Scholar]
- [65].Zhou H, Cheng X, Rao L, Li T, Duan YY, Acta Biomater. 2013, 9, 6439. [DOI] [PubMed] [Google Scholar]
- [66].Gerwig R, Fuchsberger K, Schroeppel B, Link G, Heusel G, Kraushaar U, Schuhmann W, Stett A, Stelzle M, Front. Neuroeng 2012, 5, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Chen S, Pei W, Gui Q, Tang R, Chen Y, Zhao S, Wang H, Chen H, Sensors Act. A: Physical 2013, 193, 141. [Google Scholar]
- [68].Luo X, Weaver CL, Zhou DD, Greenberg R, Cui XT, Biomaterials 2011, 32, 5551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Kolarcik CL, Catt K, Rost E, Albrecht IN, Bourbeau D, Du Z, Kozai TD, Luo X, Weber DJ, Cui XT, J. Neural. Eng 2015, 72, 016008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Skoog SA, Kumar G, Zheng JW, Sumant AV, Goering PL, Narayan RJ, J. Mater. Sci. -Mater. Med 2016, 27, 187. [DOI] [PubMed] [Google Scholar]
- [71].Garrett DJ, Tong W, Simpson DA, Meffin H, Carbon 2016, 102, 437. [Google Scholar]
- [72].Skoog SA, Lu QJ, Malinauskas RA, Sumant AV, Zheng JW, Goering PL, Narayan RJ, Casey BJ, J. Biomed. Mater. Res. A 2017, 105, 253. [DOI] [PubMed] [Google Scholar]
- [73].Piret G, Hébert C, Mazellier J-P, Rousseau L, Scorsone E, Cottance M, Lissorgues G, Heuschkel MO, Picaud S, Bergonzo P, Yvert B, Biomaterials 2015, 53, 173. [DOI] [PubMed] [Google Scholar]
- [74].Cottance M, Nazeer S, Rousseau L, Lissorgues G, Bongrain A, Kiran R, Scorsone E, Bergonzo P, Bendali A, Picaud S, Joucla S, Yvert B, presented at 2013 Symposium on Design, Test, Integration and Packaging of MEMS/MOEMS (DTIP), 16–18 April 2013, 2013. [Google Scholar]
- [75].Cai Y, Edin F, Jin Z, Alexsson A, Gudjonsson O, Liu W, Rask-Andersen H, Karlsson M, Li H, Acta Biomater. 2016, 31, 211. [DOI] [PubMed] [Google Scholar]
- [76].Ganesan K, Garrett DJ, Ahnood A, Shivdasani MN, Tong W, Turnley AM, Fox K, Meffin H, Prawer S, Biomaterials 2014, 35, 908. [DOI] [PubMed] [Google Scholar]
- [77].Doria G, Conde J, Veigas B, Giestas L, Almeida C, Assuncao M, Rosa J, Baptista PV, Sensors-Basel 2012, 12, 1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Daniel MC, Astruc D, Chem. Rev 2004, 104, 293. [DOI] [PubMed] [Google Scholar]
- [79].Tsai T-C, Guo C-X, Han H-Z, Li Y-T, Huang Y-Z, Li C-M, Chen J-JJ, Analyst 2012, 137, 2813. [DOI] [PubMed] [Google Scholar]
- [80].Kim R, Hong N, Nam Y, Biotechnol. J 2013, 8, 206. [DOI] [PubMed] [Google Scholar]
- [81].Brüggemann D, Wolfrum B, Maybeck V, Mourzina Y, Jansen M, Offenhäusser A, Nanotechnology 2011, 22, 265104. [DOI] [PubMed] [Google Scholar]
- [82].Nick C, Quednau S, Sarwar R, Schlaak HF, Thielemann C, Microsystem Technol. 2014, 20, 1849. [Google Scholar]
- [83].Xie Y, Martini N, Hassler C, Kirch RD, Stieglitz T, Seifert A, Hofmann UG, Front. Neuroeng 2014, 7, 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Zhang H, Shih J, Zhu J, Kotov NA, Nano Lett. 2012, 12, 3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Chapman CA, Chen H, Stamou M, Biener J, Biener MM, Lein PJ, Seker E, ACS Appl. Mater. Interfaces 2015, 7, 7093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Ahn Y, Lee H, Lee D, Lee Y, Acs Appl. Mater. Inter 2014, 6, 18401. [DOI] [PubMed] [Google Scholar]
- [87].Angelov SD, Koenen S, Jakobi J, Heissler HE, Alam M, Schwabe K, Barcikowski S, Krauss JK, J. Nanobiotechnol 2016, 14, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Rui Y, Liu J, Wang Y, Yang C, Microsystem Technol. 2011, 17, 437. [Google Scholar]
- [89].Zhang Y, Li KY, Jin C, Wang YT, Geng L, Sun YJ, Tian HC, Liu JQ, Jin XJ,J. Laryngol. Otol 2014, 128, 679. [DOI] [PubMed] [Google Scholar]
- [90].Chen B, Garland NT, Geder J, Pruessner M, Mootz E, Cargill A, Leners A, Vokshi G, Davis J, Burns W, Daniele MA, Kogot J, Medintz IL, Claussen JC, ACS Appl. Mater. Interfaces 2016, 8, 30941. [DOI] [PubMed] [Google Scholar]
- [91].Claussen JC, Daniele MA, Geder J, Pruessner M, Makinen AJ, Melde BJ, Twigg M, Verbarg JM, Medintz IL, Acs Appl. Mater. Interfaces 2014, 6, 17837. [DOI] [PubMed] [Google Scholar]
- [92].Shah KG, Tolosa VM, Tooker AC, Felix SH, Pannu SS, Proc. IEEE Eng. Med. Biol. Soc 2013, 2013, 1546. [DOI] [PubMed] [Google Scholar]
- [93].miKim YH, Kim AY, Kim GH, Han YH, Chung M-A, Jung S-D, Biomed. Microdevices 2016, 18, 14. [DOI] [PubMed] [Google Scholar]
- [94].Zhao Z, Gong R, Zheng L, Wang J, Sensors 2016, 16, 1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Kim YH, Kim GH, Kim AY, Jung SD, Proc. IEEE Nano-technol 2015, 238. [Google Scholar]
- [96].Zhang SS, Tsang WM, Srinivas M, Sun T, Singh N, Kwong DL, Lee C, Appl. Phys. Lett 2014, 104, 193105. [Google Scholar]
- [97].Ho D, Zou J, Chen X, Munshi A, Smith NM, Agarwal V, Hodgetts SI, Plant GW, Bakker AJ, Harvey AR, Luzinov I, Iyer KS, ACS Nano 2015, 9, 1767. [DOI] [PubMed] [Google Scholar]
- [98].Harris JP, Capadona JR, Miller RH, Healy BC, Shanmuganathan K, Rowan SJ, Weder C, Tyler DJ, J. Neural. Eng 2011, 8, 066011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Harris JP, Hess AE, Rowan SJ, Weder C, Zorman CA, Tyler DJ, Capadona JR, J. Neural. Eng 2011, 8, 046010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Nguyen JK, Park DJ, Skousen JL, Hess-Dunning AE, Tyler DJ, Rowan SJ, Weder C, Capadona JR, J. Neural. Eng 2014, 11, 056014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Huang WC, Lai HY, Kuo LW, Liao CH, Chang PH, Liu TC, Chen SY, Chen YY, Adv. Mater 2015, 27, 4186. [DOI] [PubMed] [Google Scholar]
- [102].Pisanello F, Sileo L, De Vittorio M, Front. Neurosci 2016, 10, 468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Duke AR, Jenkins MW, Lu H, McManus JM, Chiel HJ, Jansen ED, Sci. Rep 2013, 3, 2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Farah N, Zoubi A, Matar S, Golan L, Marom A, Butson CR, Brosh I, Shoham S, J. Neural. Eng 2013, 10, 056004. [DOI] [PubMed] [Google Scholar]
- [105].Martino N, Feyen P, Porro M, Bossio C, Zucchetti E, Ghezzi D, Benfenati F, Lanzani G, Antognazza MR, Sci. Rep 2015, 5, 8911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Shapiro MG, Homma K, Villarreal S, Richter CP, Bezanilla F, Nat. Commun 2012, 3, 736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Ghezzi D, Antognazza MR, Dal Maschio M, Lanzarini E, Benfenati F, Lanzani G, Nat. Commun 2011, 2, 166. [DOI] [PubMed] [Google Scholar]
- [108].Mandel Y, Goetz G, Lavinsky D, Huie P, Mathieson K, Wang LL, Kamins T, Galambos L, Manivanh R, Harris J, Palanker D, Nat. Commun 2013, 4, 1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Packer AM, Roska B, Hausser M, Nat. Neurosci 2013, 16, 805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Montgomery KL, Yeh AJ, Ho JS, Tsao V, Iyer SM, Grosenick L, Ferenczi EA, Tanabe Y, Deisseroth K, Delp SL, Poon ASY, Nat. Methods 2015, 12, 969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Deisseroth K, Nat. Methods 2011, 8, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Busskamp V, Roska B, Curr. Opin. Neurobiol 2011, 21, 942. [DOI] [PubMed] [Google Scholar]
- [113].Liang Y, Gao W, Peng X, Deng X, Sun C, Wu H, He B, Biomaterials 2016, 100, 76. [DOI] [PubMed] [Google Scholar]
- [114].Dou QQ, Guo HC, Ye E, Mater. Sci. Eng. C Mater. Biol. Appl 2014, 45, 635. [DOI] [PubMed] [Google Scholar]
- [115].Daniele MA, Shaughnessy ML, Roeder R, Childress A, Bandera YP, Foulger S, Acs Nano 2013, 7, 203. [DOI] [PubMed] [Google Scholar]
- [116].Jetty R, Bandera YP, Daniele MA, Hanor D, Hung HI, Ramshesh V, Duperreault MF, Nieminen AL, Lemasters JJ, Foulger SH, J. Mater. Chem. B 2013, 1, 4542. [DOI] [PubMed] [Google Scholar]
- [117].Hososhima S, Yuasa H, Ishizuka T, Hoque MR, Yamashita T, Yamanaka A, Sugano E, Tomita H, Yawo H, Sci. Rep 2015, 5, 16533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Weissleder R, Nat. Biotechnol 2001, 19, 316. [DOI] [PubMed] [Google Scholar]
- [119].Chu LL, Wang SW, Li KH, Xi W, Zhao XY, Qian J, Biomed. Opt. Express 2014, 5, 4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Henderson TA, Morries LD, Neuropsych. Dis. Treat 2015, 11, 2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Zhang YW, Huang L, Li ZJ, Ma GL, Zhou YB, Han G, Acs Nano 2016, 10, 3881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Li W, Luo R, Lin X, Jadhav AD, Zhang Z, Yan L, Chan C-Y, Chen X, He J, Chen C-H, Shi P, Biomaterials 2015, 65, 76. [DOI] [PubMed] [Google Scholar]
- [123].Lyu Y, Xie C, Chechetka SA, Miyako E, Pu KY, J. Am. Chem. Soc 2016, 138, 9049. [DOI] [PubMed] [Google Scholar]
- [124].Feyen P, Colombo E, Endeman D, Nova M, Laudato L, Martino N, Antognazza MR, Lanzani G, Benfenati F, Ghezzi D, Sci. Rep 2016, 6, 22718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Qian CG, Zhu S, Feng PJ, Chen YL, Yu JC, Tang X, Liu Y, Shen QD, Acs Appl. Mater. Interfaces 2015, 7, 18581. [DOI] [PubMed] [Google Scholar]
- [126].Yeh Y-C, Creran B, Rotello VM, Nanoscale 2012, 4, 1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Paviolo C, Thompson AC, Yong JW, Brown WGA, Stoddart PR, J. Neural Eng 2014, 11, 065002. [DOI] [PubMed] [Google Scholar]
- [128].Yoo S, Hong S, Choi Y, Park JH, Nam Y, Acs Nano 2014, 8, 8040. [DOI] [PubMed] [Google Scholar]
- [129].Yoo S, Kim R, Park JH, Nam Y, Acs Nano 2016, 10, 4274. [DOI] [PubMed] [Google Scholar]
- [130].Lavoie-Cardinal F, Salesse C, Bergeron E, Meunier M, De Koninck P, Sci. Rep 2016, 6, 20619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Paviolo C, Haycock JW, Yong J, Yu A, Stoddart PR, McArthur SL, Biotechnol. Bioeng 2013, 110, 2277. [DOI] [PubMed] [Google Scholar]
- [132].Eom K, Kim J, Choi JM, Kang T, Chang JW, Byun KM, Jun SB, Kim SJ, Small 2014, 10, 3853. [DOI] [PubMed] [Google Scholar]
- [133].Carvalho-de-Souza JL, Treger JS, Dang B, Kent SBH, Pepperberg DR, Bezanilla F, Neuron 2015, 86, 207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Lugo K, Miao XY, Rieke F, Lin LY, Biomed. Opt. Express 2012, 3, 447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Getz T, Qin J, Medintz IL, Delehanty JB, Susumu K, Dawson PE, Dawson G, J Neurochemistry 2016, 139, 872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Walters R, Medintz IL, Delehanty JB, Stewart MH, Susumu K, Huston AL, Dawson PE, Dawson G, ASN Neuro 2015, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Breger J, Delehanty JB, Medintz IL, Wiley Interdiscip Rev. Nanomed. Nanobiotechnol 2015, 7, 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Delehanty JB, Medintz IL, Ther. Delivery 2013, 4, 127. [DOI] [PubMed] [Google Scholar]
- [139].Marshall JD, Schnitzer MJ, Acs Nano 2013, 7, 4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Rowland CE, Susumu K, Stewart MH, Oh E, Makinen AJ, O’Shaughnessy TJ, Kushto G, Wolak MA, Erickson JS, Efros AL, Huston AL, Delehanty JB, Nano Lett. 2015, 15, 6848. [DOI] [PubMed] [Google Scholar]
- [141].Tanimoto R, Hiraiwa T, Nakai Y, Shindo Y, Oka K, Hiroi N, Funahashi A, Sci. Rep 2016, 6, 22071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Agarwal R, Domowicz MS, Schwartz NB, Henry J, Medintz I, Delehanty JB, Stewart MH, Susumu K, Huston AL, Deschamps JR, Dawson PE, Palomo V, Dawson G, Acs Chem. Neurosci 2015, 6, 494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Xu G, Mahajan S, Roy I, Yong KT, Front. Pharmacol 2013, 4, 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Minami SS, Sun B, Popat K, Kauppinen T, Pleiss M, Zhou Y, Ward ME, Floreancig P, Mucke L, Desai T, Gan L, J. Neuroinflammation 2012, 9, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Hoshino A, Hanada S, Yamamoto K, Arch. Toxicol 2011, 85, 707. [DOI] [PubMed] [Google Scholar]
- [146].Wu T, Zhang T, Chen Y, Tang M, J. Appl. Toxicol 2016, 36, 345. [DOI] [PubMed] [Google Scholar]
- [147].Chen L, Miao Y, Chen L, Jin P, Zha Y, Chai Y, Zheng F, Zhang Y, Zhou W, Zhang J, Wen L, Wang M, Biomaterials 2013, 34, 10172. [DOI] [PubMed] [Google Scholar]
- [148].Zhao Y, Wang X, Wu Q, Li Y, Wang D, J. Hazard. Mater 2015, 283, 480. [DOI] [PubMed] [Google Scholar]
- [149].Corazzari I, Gilardino A, Dalmazzo S, Fubini B, Lovisolo D, Toxicol. in vitro 2013, 27, 752. [DOI] [PubMed] [Google Scholar]
- [150].Brunetti V, Chibli H, Fiammengo R, Galeone A, Malvindi MA, Vecchio G, Cingolani R, Nadeau JL, Pompa PP, Nanoscale 2013, 5, 307. [DOI] [PubMed] [Google Scholar]
- [151].Tsoi KM, Dai Q, Alman BA, Chan WC, Acc. Chem. Res 2013, 46, 662. [DOI] [PubMed] [Google Scholar]
- [152].Wu T, He K, Zhan Q, Ang S, Ying J, Zhang S, Zhang T, Xue Y, Chen Y, Tang M, Toxicol. Res 2015, 4, 1613. [Google Scholar]
- [153].Wu X, Chen G, Shen J, Li Z, Zhang Y, Han G, Bioconjug. Chem 2015, 26, 166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Smith NM, Ho DW, Munshi AM, House MJ, Dunlop SA, Fitzgerald M, Iyer KS, N. J. Chem 2016, 40, 6692. [Google Scholar]
- [155].Bansal A, Liu H, Jayakumar MK, Andersson-Engels S, Zhang Y, Small 2016, 12, 1732. [DOI] [PubMed] [Google Scholar]
- [156].Shah S, Liu JJ, Pasquale N, Lai J, McGowan H, Pang ZP, Lee KB, Nanoscale 2015, 7, 16571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Sedlmeier A, Gorris HH, Chem. Soc. Rev 2015, 44, 1526. [DOI] [PubMed] [Google Scholar]
- [158].Guo H, Sun S, Nanoscale 2012, 4, 6692. [DOI] [PubMed] [Google Scholar]
- [159].Bogdan N, Rodriguez EM, Sanz-Rodriguez F, de la Cruz MC, Juarranz A, Jaque D, Sole JG, Capobianco JA, Nanoscale 2012, 4, 3647. [DOI] [PubMed] [Google Scholar]
- [160].Morgan CG, Mitchell AC, Biosens. Bioelectron 2007, 22, 1769. [DOI] [PubMed] [Google Scholar]
- [161].Gnach A, Lipinski T, Bednarkiewicz A, Rybka J, Capobianco JA, Chem. Soc. Rev 2015, 44, 1561. [DOI] [PubMed] [Google Scholar]
- [162].Portioli C, Pedroni M, Benati D, Donini M, Bonafede R, Mariotti R, Perbellini L, Cerpelloni M, Dusi S, Speghini A, Bentivoglio M, Nanomedicine 2016, DOI: 10.2217/nnm-2016-0297. [DOI] [PubMed] [Google Scholar]
- [163].Guan YJ, Li M, Dong K, Ren JS, Qu XG, Small 2014, 10, 3655. [DOI] [PubMed] [Google Scholar]
- [164].Wu X, Zhang Y, Takle K, Bilsel O, Li Z, Lee H, Zhang Z, Li D, Fan W, Duan C, Chan EM, Lois C, Xiang Y, Han G, Acs Nano 2016, 10, 1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Tabatabaei SN, Girouard H, Carret AS, Martel S, J. Controlled Release 2015, 206, 49. [DOI] [PubMed] [Google Scholar]
- [166].Qiao R, Jia Q, Huwel S, Xia R, Liu T, Gao F, Galla HJ, Gao M, Acs Nano 2012, 6, 3304. [DOI] [PubMed] [Google Scholar]
- [167].Huang H, Delikanli S, Zeng H, Ferkey DM, Pralle A, Nat. Nanotechnol 2010, 5, 602. [DOI] [PubMed] [Google Scholar]
- [168].Chen R, Romero G, Christiansen MG, Mohr A, Anikeeva P, Science 2015, 347, 1477. [DOI] [PubMed] [Google Scholar]
- [169].Loynachan CN, Romero G, Christiansen MG, Chen R, Ellison R, O’Malley TT, Froriep UP, Walsh DM, Anikeeva P, Adv. Healthcare Mater 2015, 4, 2100. [DOI] [PubMed] [Google Scholar]
- [170].Accoto D, Francomano MT, Rainer A, Trombetta M, Rossini PM, Guglielmelli E, Med. Hypotheses 2013, 81, 322. [DOI] [PubMed] [Google Scholar]
- [171].Lee SW, Fallegger F, Casse BD, Fried SI, Sci. Adv 2016, 2, e1600889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Marino A, Arai S, Hou YY, Sinibaldi E, Pellegrino M, Chang YT, Mazzolai B, Mattoli V, Suzuki M, Ciofani G, Acs Nano 2015, 9, 7678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Saxena T, Karumbaiah L, Gaupp EA, Patkar R, Patil K, Betancur M, Stanley GB, Bellamkonda RV, Biomaterials 2013, 34, 4703. [DOI] [PubMed] [Google Scholar]
- [174].Keaney J, Campbell M, FEBSJ. 2015, 282, 4067. [DOI] [PubMed] [Google Scholar]
- [175].Janzer RC, Raff MC, Nature 1987, 325, 253. [DOI] [PubMed] [Google Scholar]
- [176].Hanson LR, Frey WH 2nd, Bmc Neurosci. 2008, 9, S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Chapman CD, Frey WH 2nd, Craft S, Danielyan L, Hallschmid M, Schioth HB, Benedict C, Pharm. Res 2013, 30, 2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Jain KK, in Drug Delivery Systems, DOI: 10.1007/978-1-59745-210-6_1 (Ed: Jain KK), Humana Press, Totowa, NJ: 2008, p. 1. [DOI] [Google Scholar]
- [179].Wohlfart S, Gelperina S, Kreuter J, J. Controlled Release 2012, 161, 264. [DOI] [PubMed] [Google Scholar]
- [180].Tosi G, Bortot B, Ruozi B, Dolcetta D, Vandelli MA, Forni F, Severini GM, Curr. Med. Chem 2013, 20, 2212. [DOI] [PubMed] [Google Scholar]
- [181].Nance EA, Woodworth GF, Sailor KA, Shih TY, Xu QG, Swaminathan G, Xiang D, Eberhart C, Hanes J, Sci. Transl. Med 2012, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Hanada S, Fujioka K, Inoue Y, Kanaya F, Manome Y, Yamamoto K, Int. J. Mol. Sci 2014, 15, 1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Gregori M, Bertani D, Cazzaniga E, Orlando A, Mauri M, Bianchi A, Re F, Sesana S, Minniti S, Francolini M, Cagnotto A, Salmona M, Nardo L, Salerno D, Mantegazza F, Masserini M, Simonutti R, Macromol. Biosci 2015, 15, 1687. [DOI] [PubMed] [Google Scholar]
- [184].Liu D, Lin BQ, Shao W, Zhu Z, Ji TH, Yang CY, Acs Appl. Mater. Interfaces 2014, 6, 2131. [DOI] [PubMed] [Google Scholar]
- [185].Sun ZZ, Yathindranath V, Worden M, Hegmann T, Miller D, FasebJ. 2013, 27. [Google Scholar]
- [186].Calvo P, Gouritin B, Chacun H, Desmaele D, D’Angelo J, Noel J-P, Georgin D, Fattal E, Andreux JP, Couvreur P, Pharm. Res 2001, 78, 1157. [DOI] [PubMed] [Google Scholar]
- [187].Freese C, Unger RE, Deller RC, Gibson MI, Brochhausen C, Klok HA, Kirkpatrick CJ, Biomater. Sci 2013, 7, 824. [DOI] [PubMed] [Google Scholar]
- [188].Posati T, Pistone A, Saracino E, Formaggio F, Mola MG, Troni E, Sagnella A, Nocchetti M, Barbalinardo M, Valle F, Bonetti S, Caprini M, Nicchia GP, Zamboni R, Muccini M, Benfenati V, Sci. Rep 2016, 6, 31226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Bramini M, Ye D, Hallerbach A, Raghnaill MN, Salvati A, Aberg C, Dawson KA, Acs Nano 2014, 8, 4304. [DOI] [PubMed] [Google Scholar]
- [190].Hughes S, El Haj AJ, Dobson J, Med. Eng. Phys 2005, 27, 754. [DOI] [PubMed] [Google Scholar]
- [191].Kunze A, Tseng P, Godzich C, Murray C, Caputo A, Schweizer FE, Di Carlo D, Acs Nano 2015, 9, 3664. [DOI] [PubMed] [Google Scholar]
- [192].Guarnieri D, Falanga A, Muscetti O, Tarallo R, Fusco S, Galdiero M, Galdiero S, Netti PA, Small 2013, 9, 853. [DOI] [PubMed] [Google Scholar]
- [193].Yemisci M, Caban S, Gursoy-Ozdemir Y, Lule S, Novoa-Carballal R, Riguera R, Fernandez-Megia E, Andrieux K, Couvreur P, Capan Y, Dalkara T, Cerebr J. Blood F Met. 2015, 35, 469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Herbison CE, Thorstensen K, Chua ACG, Graham RM, Leedman P, Olynyk JK, Trinder D, Am. J. Physi. - Cell Physiol 2009, 297, C1567. [DOI] [PubMed] [Google Scholar]
- [195].Zhang TT, Li W, Meng GM, Wang P, Liao WZ, Biomater. Sci 2016, 4, 219. [DOI] [PubMed] [Google Scholar]
- [196].Wang YY, Lui PCW, Li JY, Immunotherapy 2009, 7, 983. [DOI] [PubMed] [Google Scholar]
- [197].Ulbrich K, Knobloch T, Kreuter J,Drug Target J. 2011, 79, 125. [DOI] [PubMed] [Google Scholar]
- [198].Shilo M, Motiei M, Hana P, Popovtzer R, Nanoscale 2014, 6, 2146. [DOI] [PubMed] [Google Scholar]
- [199].Pardridge WM, Eisenberg J, Yang J, J. Neurochem 1985, 44, 1771. [DOI] [PubMed] [Google Scholar]
- [200].Georgieva JV, Hoekstra D, Zuhorn IS, Pharmaceutics 2014, 6, 557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Torrice M, Chem. Eng. News 2016, 94, 6. [Google Scholar]
- [202].Mooney SJ, Shah K, Yeung S, Burgess A, Aubert I, Hynynen K, Plos One 2016, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Mitrasinovic S, Appelboom G, Detappe A, Connolly ES, J. Clin. Neurosci 2016, 28, 187. [DOI] [PubMed] [Google Scholar]
- [204].Nance E, Timbie K, Miller GW, Song J, Louttit C, Klibanov AL, Shih TY, Swaminathan G, Tamargo RJ, Woodworth GF, Hanes J, Price RJ, J. Controlled Release 2014, 789, 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Wang ST, Samiotaki G, Olumolade O, Feshitan JA, Konofagou EE, Ultrasound Med. Biol 2014, 40, 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Samiotaki G, Vlachos F, Tung YS, Konofagou EE, Magn. Reson. Med 2012, 67, 769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Wiedemair W, Tukovic Z, Jasak H, Poulikakos D, Kurtcuoglu V, Phys. Med. Biol 2012, 57, 1019. [DOI] [PubMed] [Google Scholar]
- [208].Vlachos F, Tung YS, Konofagou E, Magn. Reson. Med 2011, 66, 821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Choi JJ, Feshitan JA, Baseri B, Wang SG, Tung YS, Borden MA, Konofagou EE, IEEE T. Bio-Med. Eng 2010, 57, 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [210].Wang F, Cheng Y, Mei J, Song Y, Yang YQ, Liu YJ, Wang ZB, J. Ultrasound Med 2009, 28, 1501. [DOI] [PubMed] [Google Scholar]
- [211].Xie F, Lof J, Matsunaga T, Zutshi R, Unger E, Porter TR, J. Am. Coll. Cardiol 2009, 53, A252. [Google Scholar]
- [212].Liu P, Gao YH, Tan K, Zhu XH, Liu Z, Li P, Narula J, Vannan M, Circulation 2005, 112, U602. [Google Scholar]
- [213].Chu PC, Liu HL, Lai HY, Lin CY, Tsai HC, Pei YC, Sci Rep-Uk 2015, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [214].Meairs S, Pharmaceutics 2015, 7, 275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [215].Fan CH, Chang EL, Ting CY, Lin YC, Liao EC, Huang CY, Chang YC, Chan HL, Wei KC, Yeh CK, Biomaterials 2016, 106, 46. [DOI] [PubMed] [Google Scholar]
- [216].Chu PC, Chai WY, Tsai CH, Kang ST, Yeh CK, Liu HL, Sci. Rep 2016, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Chen H, Konofagou EE, Cereb J. Flow and Metab. 2014, 34, 1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [218].Sun T, Samiotaki G, Wang S, Acosta C, Chen CC, Konofagou EE, Phys. Med. Biol 2015, 60, 9079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [219].Downs ME, Buch A, Sierra C, Karakatsani ME, Chen SS, Konofagou EE, Ferrera VP, Plos One 2015, 10. [Google Scholar]
- [220].Yang FY, Liu SH, Ho FM, Chang CH, J. Acoust. Soc. Am 2009, 126, 3344. [DOI] [PubMed] [Google Scholar]
- [221].Dan M, Bae Y, Pittman TA, Yokel RA, Pharm. Res 2015, 32, 1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [222].Gong W, Wang ZY, Liu N, Lin W, Wang XP, Xu D, Liu HY, Zeng CY, Xie XY, Mei XG, Lu WL, Biol. Pharm. Bull 2011, 34, 1058. [DOI] [PubMed] [Google Scholar]
- [223].Kong SD, Lee J, Ramachandran S, Eliceiri BP, Shubayev VI, Lal R, Jin S, J. Controlled Release 2012, 164, 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [224].Sawyer AJ, Kyriakides TR, J. Neural Eng 2013, 10, 016013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [225].Winslow BD, Tresco PA, Biomaterials 2010, 31, 1558. [DOI] [PubMed] [Google Scholar]
- [226].Winslow BD, Christensen MB, Yang WK, Solzbacher F, Tresco PA, Biomaterials 2010, 31, 9163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [227].Potter KA, Buck AC, Self WK, Capadona JR, J. Neural. Eng 2012, 9, 046020. [DOI] [PubMed] [Google Scholar]
- [228].Tresco PA, Winslow BD, Crit. Rev. Biomed. Eng 2011, 39, 29. [DOI] [PubMed] [Google Scholar]
- [229].Berces Z, Toth K, Marton G, Pal I, Kovats-Megyesi B, Fekete Z, Ulbert I, Pongracz A, Sci. Rep 2016, 6, 35944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [230].Prodanov D, Delbeke J, Front. Neurosci 2016, 10, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [231].Shin SW, Song IH, Um SH, Nanomaterials 2015, 5, 1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [232].Win-Shwe TT, Fujimaki H, Int. J. Mol. Sci 2011, 12, 6267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [233].Baalousha M, Lead JR, Nat. Nanotechnol 2013, 8, 308. [DOI] [PubMed] [Google Scholar]
- [234].Ngobili TA, Daniele MA, Exp. Biol. Med 2016, 241, 1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [235].Suyatin DB, Wallman L, Thelin J, Prinz CN, Jorntell H, Samuelson L, Montelius L, Schouenborg J, PLOS One 2013, 8, e56673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [236].Kwon EJ, Skalak M, Lo Bu R, Bhatia SN, ACS Nano 2016, 10, 7926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [237].Koffie RM, Farrar CT, Saidi LJ, William CM, Hyman BT, Spires-Jones TL, Proc. Natl. Acad. Sci. USA 2011, 108, 18837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [238].Smith NM, Gachulincova I, Ho D, Bailey C, Bartlett CA, Norret M, Murphy J, Buckley A, Rigby PJ, House MJ, St Pierre T, Fitzgerald M, Iyer KS, Dunlop SA, Sci. Rep 2016, 6, 22595. [DOI] [PMC free article] [PubMed] [Google Scholar]