Abstract
Extensive fibrin deposition in the lungs and altered levels of circulating blood coagulation proteins in COVID-19 patients imply local derangement of pathways that limit fibrin formation and/or promote its clearance. We examined transcriptional profiles of bronchoalveolar lavage fluid (BALF) samples to identify molecular mechanisms underlying these coagulopathies. mRNA levels for regulators of the kallikrein–kinin (C1-inhibitor), coagulation (thrombomodulin, endothelial protein C receptor), and fibrinolytic (urokinase and urokinase receptor) pathways were significantly reduced in COVID-19 patients. While transcripts for several coagulation proteins were increased, those encoding tissue factor, the protein that initiates coagulation and whose expression is frequently increased in inflammatory disorders, were not increased in BALF from COVID-19 patients. Our analysis implicates enhanced propagation of coagulation and decreased fibrinolysis as drivers of the coagulopathy in the lungs of COVID-19 patients.
Research organism: Human
Introduction
The bradykinin storm model for COVID-19 pathogenesis was recently developed from our analyses of gene expression, clinical, autopsy, pathology, and ChIP-Seq data (Garvin et al., 2020). Several clinical studies by other groups have demonstrated positive results from therapeutic interventions predicted by our model (Entrenas Castillo et al., 2020; van de Veerdonk et al., 2020; WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group et al., 2020). Here, we extend this model to include the concurrent dysregulation of the coagulation and fibrinolytic pathways.
SARS-CoV-2 displays considerable tissue tropism (Adachi et al., 2020; Barton et al., 2020; Su et al., 2020; Xu et al., 2020). The lungs are often affected with disease that can range from mild pneumonia, to severe dyspnea and hypoxia, to critical respiratory failure, shock, and multiorgan failure. The time course for the development of severe disease is typically 8–12 days, but some patients rapidly deteriorate about 7 days following development of symptoms. Lung tissue from people who died from COVID-19 has pauci-inflammatory septal capillary injury and luminal and mural fibrin deposition in alveolar septal capillaries (Lax et al., 2020; Magro et al., 2020), thrombi in small- and medium-sized arteries (Lax et al., 2020; Magro et al., 2020), and fibrinous thrombi in small pulmonary arterioles with evidence of tumefaction of the endothelium (Dolhnikoff et al., 2020; Fox et al., 2020; Geerdes-Fenge et al., 2020; Lax et al., 2020). A report that administering tissue plasminogen activator (tPA) to dissolve fibrin transiently alleviates respiratory distress in COVID-19 patients supports the premise that fibrin deposition contributes to the respiratory failure (Wang et al., 2020). Thus, it appears that COVID-19 hypoxemia stems at least partially from fibrin deposits surrounding alveoli that restrict oxygen transfer and microvascular thrombi that cause ventilation–perfusion defects.
Abnormal levels of circulating coagulation proteins are present in patients with COVID-19. Changes include increased levels of coagulation proteins associated with the acute phase response (e.g., fibrinogen and factor VIII) and endothelial activation (e.g., von Willebrand factor), as well as elevated biomarkers of coagulation activation (e.g., D-dimer) (Chen et al., 2020; Gattinoni et al., 2020; Helms et al., 2020; Middeldorp et al., 2020; Panigada et al., 2020; Tang et al., 2020; Zhou et al., 2020a; Helms et al., 2020; Middeldorp et al., 2020; Panigada et al., 2020). Accordingly, thrombotic events have been detected in up to 30% of COVID-19 patients (Helms et al., 2020; Middeldorp et al., 2020; Panigada et al., 2020) including large vessel occlusions such as deep vein thrombosis, pulmonary embolism, and ischemic stroke, as well as microvascular thrombosis and extravascular fibrin deposition in a variety of tissues including lung and skin (Helms et al., 2020; Middeldorp et al., 2020; Panigada et al., 2020). Inappropriate fibrin deposition and thrombosis are thought to stem from the interaction of systemic (blood) changes with tissue-specific dysfunction. However, mechanisms that contribute to prevalence of thrombi and fibrin in the pulmonary vasculature and extravascular space, thought to be a major cause of morbidity and mortality in COVID-19, have remained elusive.
The goal of the present study was to identify changes in the lungs of patients with severe COVID-19 that could contribute to local derangement of hemostatic mechanisms. Bronchoalveolar lavage fluid (BALF) contains lung parenchymal, epithelial, and alveolar cells, as well as immune cells that infiltrate epithelial and luminal spaces. RNA sequencing of BALF provides a snapshot of the transcriptome at the interface where capillary gas exchange occurs and has been used to characterize lung function in many diseases (Gu et al., 2019; Jakieła et al., 2018; Kahn et al., 2015; Sengupta et al., 2019; Sun et al., 2019; Weigt et al., 2019; Zhang et al., 2019). Here, we compared the transcriptional signatures of BALF from patients with COVID-19 and uninfected controls. Our analyses focused on transcripts for proteins that function in coagulation, fibrinolysis, and kinin formation in the lung to identify dysregulated mechanisms that may contribute to COVID-19 pathophysiology.
Results and discussion
Changes in transcripts encoding proteins in the kallikrein–kinin/bradykinin system
Overview of pathways examined
The plasma kallikrein–kinin system is comprised of the protease precursors factor XII (FXII, encoded by F12) and prekallikrein (encoded by KLKB1) and the cofactor high-molecular-weight kininogen (HK, encoded by KNG1) (Schmaier, 2016; Schmaier et al., 2019). In healthy individuals, FXII and prekallikrein undergo reciprocal activation to the proteases FXIIa and kallikrein (Revenko et al., 2011; Schmaier, 2016). Kallikrein cleaves HK to liberate bradykinin, which contributes to setting vascular tone and permeability by interacting with bradykinin receptors (encoded by BDKRB1 and BDKRB2) (Marceau et al., 2020). This process is regulated by C1-Inhibitor (encoded by SERPING1) (Marceau et al., 2020). Congenital C1-Inhibitor deficiency causes hereditary angioedema, which is characterized by bouts of bradykinin-induced soft tissue swelling (Busse and Christiansen, 2020; Cicardi and Zuraw, 2018). C1-Inhibitor in blood is primarily of hepatocyte origin, although it also is produced by other cell types including vascular endothelium (Prada et al., 1998).
Study findings
Transcripts encoding C1-Inhibitor were decreased 80-fold in BALF from COVID-19 patients (Table 1), raising the possibilities that contact activation-initiated thrombin generation is locally dysregulated and control of bradykinin production is compromised. Furthermore, the angiotensin-converting enzyme (ACE) that degrades bradykinin (Cicardi and Zuraw, 2018; Davin et al., 2019) was downregulated eightfold in COVID-19 BALF.
Table 1. Differentially expressed coagulation genes.
| Gene | Protein product | Mean COVID-19 | Mean control | Fold change | Log2FC | FDR |
|---|---|---|---|---|---|---|
| A2M | α2-Macroglubulin | 3.9 | 177.6 | −43.5 | −5.4 | 5.2E-15 |
| BDKRB1 | Bradykinin receptor B1 | 3.3 | 0.0 | 258.9 | 8.0 | 4.3E-91 |
| BDKRB2 | Bradykinin receptor B2 | 8.9 | 0.2 | 49.1 | 5.6 | 1.9E-40 |
| F13A1 | Factor XIII-A subunit | 2.5 | 9.2 | −3.6 | −1.8 | 2.6E-05 |
| F13B | Factor XIII-B subunit | 0.6 | 0.0 | 117.8 | 6.9 | 2.7E-31 |
| F12 | Factor XII | 0.5 | 3.2 | −4.4 | −2.1 | 5.7E-10 |
| F11 | Factor XI | 6.6 | 0.1 | 81.2 | 6.3 | 7.9E-79 |
| F10 | Factor X | 3.2 | 0.0 | 169.9 | 7.4 | 7.9E-140 |
| F9 | Factor IX | 0.9 | 0.0 | 189.6 | 7.6 | 2.2E-39 |
| F8 | Factor VIII | 3.9 | 15.8 | −4.6 | −2.2 | 1.6E-40 |
| F7 | Factor VII | 10.1 | 0.0 | 363.5 | 8.5 | 2.9E-73 |
| F5 | Factor V | 9.7 | 6.8 | 1.4 | 0.5 | 0.13 |
| F3 | Tissue Factor | 4.2 | 3.8 | -1.0 | -0.01 | 1 |
| F2 | Prothrombin | 0.9 | 0.0 | 188.8 | 7.6 | 7.8E-67 |
| FGA | Fibrinogen Aα chain | 3.4 | 0.0 | 322.5 | 8.3 | 3.4E-206 |
| FGB | Fibrinogen Bβ chain | 3.7 | 0.0 | 303.9 | 8.2 | 2.4E-151 |
| FGG | Fibrinogen γ chain | 0.7 | 0.0 | 85.4 | 6.4 | 4.8E-17 |
| KLKB1 | Kallikrein B1 | 1.3 | 1.8 | −1.4 | −0.5 | 2.1E-01 |
| KNG1 | Kininogen | 3.3 | 0.0 | 190.7 | 7.6 | 4.2E-163 |
| PLAT | Tissue plasminogen activator | 1.8 | 0.4 | 5.3 | 2.4 | 7.1E-24 |
| PLAU | Urokinase | 3.8 | 158.3 | −37.1 | −5.2 | 8.2E-172 |
| PLAUR | Urokinase receptor | 6.2 | 313.3 | −42.1 | −5.4 | 6.4E-286 |
| PLG | Plasminogen | 2.2 | 0.0 | 75.3 | 6.2 | 7.3E-38 |
| PROC | Protein C | 2.0 | 0.0 | 226.5 | 7.8 | 4.1E-158 |
| PROCR | Endothelial protein C receptor | 1.7 | 57.9 | −33.8 | −5.1 | 1.9E-50 |
| PROS1 | Protein S | 3.2 | 195.3 | −54.2 | −5.8 | 1.1E-174 |
| SERPINA5 | Protein C Inhibitor | 2.7 | 0.0 | 786.8 | 9.6 | 5.4E-145 |
| SERPINB2 | Plasminogen activator inhibitor-2 | 1.0 | 0.5 | 2.3 | 1.2 | 2.1E-01 |
| SERPINC1 | Antithrombin | 1.9 | 0.1 | 13.6 | 3.8 | 3.1E-31 |
| SERPIND1 | Heparin cofactor II | 3.0 | 0.0 | 94.0 | 6.6 | 2.2E-72 |
| SERPINE1 | Plasminogen activator inhibitor-1 | 2.7 | 5.3 | −1.8 | −0.9 | 8.8E-02 |
| SERPINF2 | α2-antiplasmin | 5.4 | 8.2 | −1.4 | −0.5 | 1.1E-02 |
| SERPING1 | C-1 inhibitor | 23.3 | 1923.2 | −80.1 | −6.3 | 5.4E-33 |
| TFPI | Tissue factor pathway inhibitor | 3.4 | 0.4 | 7.7 | 2.9 | 8.7E-19 |
| THBD | Thrombomodulin | 9.6 | 224.0 | −22.2 | −4.5 | 3.2E-48 |
| VWF | Von Willebrand factor | 14.5 | 7.7 | 2.0 | 1.0 | 2.0E-05 |
Changes in transcripts encoding extrinsic pathway proteins that initiate thrombin generation
Overview of pathways examined
The integral cytoplasmic membrane protein tissue factor (TF, encoded by F3) is the primary initiator of thrombin generation in vivo. In many inflammatory conditions, cellular TF expression is upregulated (Edwards et al., 1979), providing a mechanistic explanation for hypercoagulable states associated with a variety of bacterial and viral infections (Grover and Mackman, 2018; Levi and van der Poll, 2017). Tissue factor pathway inhibitor (TFPI, encoded by TFPI) is a Kunitz-type protease inhibitor produced by vascular endothelium (Bajaj et al., 1990) and is the major inhibitor of the factor VIIa (FVIIa)/TF complex (Wood et al., 2014).
Study findings
Surprisingly, transcripts encoding TF were similar in COVID-19 and control BALF samples (Figure 1; Table 1), whereas transcripts for TFPI were increased eightfold in COVID-19 BALF. On balance, these data indicate that pulmonary fibrin deposition does not stem from enhanced local TF production and that counterintuitively, COVID-19 may dampen TF-dependent mechanisms in the lungs.
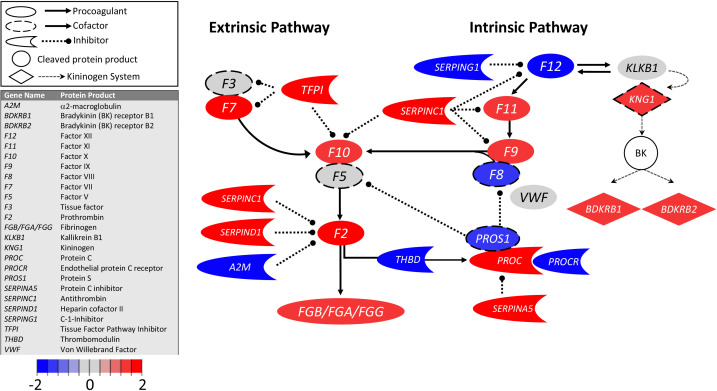
Figure 1. Transcriptional changes in lung induced by COVID-19 infection did not alter F3, but decreased aPC anticoagulant capacity, suggesting decreased inhibition of the propagation phase of coagulation.
Figure shows differential gene expression (log2 fold change) of coagulation pathway transcripts in BALF of COVID-19 patients; the image illustrates mechanistic relationships of the protein products of the identified transcripts during coagulation. Shading indicates relative expression in COVID-19 patients compared to controls: increased (red) or decreased (blue). There was minimal change in F3 (encoding tissue factor ) and increased TFPI (encoding the major inhibitor of tissue factor activity). There was decreased THBD, PROCR, and PROS1 (encoding proteins that enhance anticoagulant activity) and increased SERPINA5 (encoding protein C inhibitor). There was also decreased SERPING1 (encoding C1-Inhibitor). Other transcripts showing changes (e.g., F11, F10, F7, F2) encode proteins typically produced in the liver; local expression of these proteins is unclear.
Changes in transcripts encoding intrinsic pathway proteins that initiate thrombin generation
Overview of pathways examined
Increased thrombin generation can also be initiated by the plasma protease FXIIa (Maas and Renné, 2018; Stavrou, 2018). While FXIIa does not contribute to hemostasis, it is implicated in thrombo-inflammatory conditions. FXIIa converts factor XI (FXI, encoded by F11) to the protease FXIa (Ivanov et al., 2017; Long et al., 2016; Schmaier, 2016; Wang et al., 2019), a potent activator of the coagulation protease factor IX (FIX, encoded by F9) (Mohammed et al., 2018).
Study findings
Transcripts encoding FXII were slightly decreased (−4-fold) in COVID-19 and control BALF samples (Figure 1; Table 1), but the expression of the C1 inhibitor (encoded by SERPING1), which normally inhibits the FXII protein activity, is downregulated 80-fold. While it is not known if pulmonary tissues normally produce FXI or FIX, transcripts for FXI (81-fold) and FIX (190-fold) were both increased in BALF from COVID-19 patients. Numerous studies have reported correlations between plasma FIX and FXI levels and risk for venous thromboembolism and ischemic stroke (Ammollo et al., 2014; Folsom et al., 2015; Mohammed et al., 2018; Preis et al., 2017; Salomon et al., 2011, Salomon et al., 2008; Siegerink et al., 2010; Suri et al., 2010). Perhaps local increases in FIX or FXI expression enhance thrombin generation and promote thrombus formation.
Changes in transcripts encoding anticoagulant proteins
Overview of pathways examined
Healthy lung endothelium has membrane-associated anticoagulant proteins that inhibit different points in the thrombin generation pathway. These include TFPI, which inhibits the initiation of coagulation as described above, and thrombomodulin (encoded by THBD) and endothelial protein C receptor (EPCR, encoded by PROCR), which coordinate to inhibit the propagation of coagulation. Thrombomodulin captures thrombin and converts it from a procoagulant enzyme to an activator of protein C (encoded by PROC) in a process enhanced by binding of PC to EPCR. Activated PC with its cofactor protein S (encoded by PROS1) downregulates thrombin generation by inactivating factors VIIIa and Va and produces a cytoprotective/anti-inflammatory effect when bound to EPCR through cleavage of protease activated receptor-1 on endothelial cells (Ito et al., 2019; Riewald et al., 2002).
Study findings
Transcripts encoding thrombomodulin (−22-fold) and EPCR (−33-fold) were each reduced in BALF from COVID-19 patients compared with controls (Figure 1; Table 1), suggesting SARS-CoV-2 is associated with reduced expression of these proteins on vascular endothelium. We also observed a marked increase in protein C transcripts in BALF from COVID-19 patients (227-fold), but a significant reduction in PS transcripts (−54-fold). Since protein C and protein S in blood are primarily synthesized by hepatocytes, the importance of local synthesis of these proteins is not known. However, expression patterns in BALF are consistent with the observation that the plasma concentration of protein C is moderately elevated, and protein S moderately reduced, in COVID-19 patients (Panigada et al., 2020). In COVID-19 BALF, there was also a substantial increase (786-fold) in mRNA for protein C inhibitor (encoded by SERPINA5), a serpin regulator of activated protein C. Taken as a whole, the transcript pattern in COVID-19 BALF suggests a diminished capacity of the endothelial anticoagulant system to downregulate local thrombin generation.
Changes in transcripts that encode fibrinogen and proteins in the fibrinolytic pathway
Overview of pathways examined
The fibrinolytic system is responsible for enzymatic degradation of fibrin. Plasminogen activators convert plasminogen to plasmin, which cleaves fibrin and generates fibrin degradation products, including the circulating biomarker D-dimer. Cells within the lung express plasminogen activators (e.g., [tPA, encoded by PLAT], urokinase plasminogen activator [uPA, encoded by PLAU], and the uPA receptor [uPAR, encoded by PLAUR]) that prevent fibrin accumulation in small airways and the alveolar compartment and that maintain blood vessel patency (Shetty et al., 2008). uPA and uPAR are expressed by lung epithelial cells, alveolar macrophages, and fibroblasts, and reduced expression of these proteins contributes to acute lung injury (Shetty et al., 2008). The plasminogen activators are inhibited by plasminogen activator inhibitor-1 (PAI-1, encoded by SERPINE1). Plasma fibrinogen, a dimer of trimers (Aα2Bβ2γ2) encoded by three genes, FGA, FGB, and FGG, is synthesized primarily by hepatocytes (Pieters and Wolberg, 2019); however, synthesis has been reported in stimulated cultured lung alveolar epithelial cells and in alveolar epithelium in an animal model of bacterial pneumonia (Guadiz et al., 1997; Simpson-Haidaris et al., 1998).
Study findings
Transcripts encoding the fibrinogen chains were increased in COVID-19 BALF (Table 1, Figure 2; ). Our findings suggest that local fibrinogen expression in the lungs of COVID-19 patients, like fibrinogen expression by hepatocytes, is upregulated during the acute phase response and may provide additional substrate for local thrombin-mediated fibrin production. Our analysis uncovered evidence of reduced expression of transcripts encoding uPA (−37-fold) and uPAR (−42-fold) (Table 1, Figure 2) in BALF from COVID-19 patients. Expression of transcripts encoding tPA was very low in BALF from healthy individuals. Transcripts encoding tPA were elevated (fivefold) in COVID-19 BALF, but absolute expression was low compared to uPA and uPAR transcripts in uninfected controls (Table 1, Figure 2). Similarly, expression of transcript for plasminogen (encoded by PLG) was elevated in COVID-19 BALF but remained low compared to other transcripts. The combination of increased fibrinogen expression and reduced production of uPA and uPAR indicates a loss of local fibrinolytic capacity, consistent with a lung environment permissive of the accumulation of fibrin deposits within pulmonary vessels and the alveolar space (Lax et al., 2020; Magro et al., 2020).
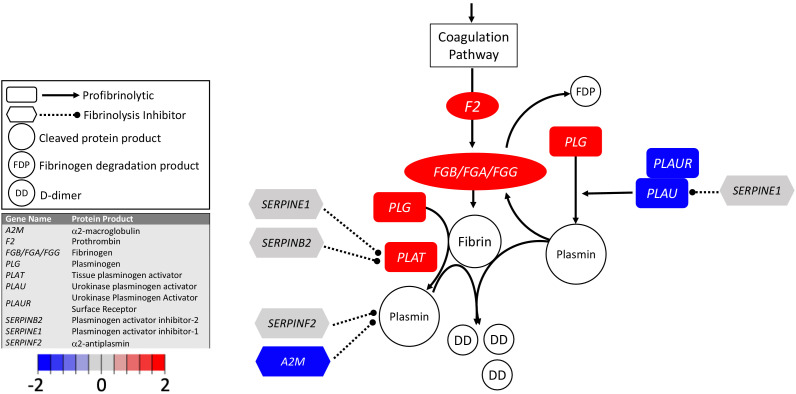
Figure 2. Transcriptional changes in lung induced by COVID-19 infection decreased PLAU and PLAUR, suggesting diminished fibrinolytic activity.
Figure shows differential gene expression (log2 fold change) of fibrinolytic pathway transcripts in BALF of COVID-19 patients; the image illustrates mechanistic relationships of the protein products of the identified transcripts during fibrinolysis. Shading indicates relative expression in COVID-19 patients compared to controls: increased (red) or decreased (blue). There was a moderate increase in PLAT (encoding tPA). There was also enhanced expression of FGB, FGA, and FGG (encoding fibrinogen chains) and decreased expression of PLAU and PLAUR (encoding uPA and uPAR, respectively). Other transcripts showing changes (e.g., F2, PLG) encode proteins typically produced in the liver; local expression of these proteins is unclear.
Conclusions
We detected pronounced changes in mRNA levels encoding proteins involved in regulation of coagulation, fibrinolysis, and inflammation in the lungs of COVID-19 patients. These changes include reductions in transcripts for proteins known to be expressed in normal pulmonary tissue (thrombomodulin, EPCR, uPA, uPAR) that would compromise the functions of anticoagulant and fibrinolytic pathways. In concert with enhanced production of bradykinin and vascular permeability, these changes are likely to create an environment in which plasma proteins are exposed to the extravascular space, resulting in increased fibrin production and reduced fibrin degradation, enabling the fibrin deposits in pulmonary vessels and alveolar spaces observed in autopsies of COVID-19 patients (Dolhnikoff et al., 2020; Fox et al., 2020; Geerdes-Fenge et al., 2020; Lax et al., 2020), and contributing to the virulence of the virus.
An unexpected finding was the lack of change in transcripts for F3, which encodes TF, the integral membrane protein responsible for triggering thrombin generation during hemostasis. TF expression is enhanced in a wide range of thrombo-inflammatory disorders, and its over-expression is thought to be important for driving consumptive coagulation. The apparent lack of increase in F3, coupled with an apparent increase in TFPI transcripts, raises the possibility that pathways unrelated to the FVIIa/TF complex may play a significant role in COVID-19-associated thrombosis. In this regard, thrombomodulin and EPCR transcripts were substantially decreased, suggesting reduced capacity of the lung endothelium to downregulate the propagation of thrombin generation. In addition, changes in regulation or activation of the plasma kallikrein–kinin system and contact activation may be important. This is consistent with our hypothesis that COVID-19 triggers a bradykinin storm (Garvin et al., 2020), the net effect of increased bradykinin production, reduced BK degradation, and increased local expression of bradykinin receptors, is expected to enhance vasodilation and vascular permeability, exposing blood components to extravascular proteins that promote fibrin formation and contribute to tissue damage.
Fibrin deposits and thrombi have been observed in organs other than the lungs in COVID-19 patients, including kidney (Su et al., 2020) and spleen (Mestres et al., 2020). It is tempting to speculate that transcriptional changes observed in BALF may occur in these other tissues. Currently, it is not clear whether fibrin deposition in specific organs indicates localized infection of those organs, perhaps stemming from differential distribution of the SARS-CoV-2 receptor ACE2, or reflects organ susceptibility to systemic changes induced by the infection. Analysis of transcriptional and proteomic changes in different organs in concert with information on virus entry into parenchymal cells may help explain the pattern of organ-specific thrombosis during SARS-CoV-2 infection.
Our study has limitations. Although we detected changes in multiple gene transcripts, we did not identify specific cell types for each transcript, which may include lung parenchymal cells, leukocytes, megakaryocytes (Fox et al., 2020), and other cells present in native and inflammatory states that undergo transcriptional changes in response to infection. Single-cell analyses will be required to better understand these changes during SARS-CoV-2 infection. As with all transcriptional data, we could not determine whether changes in mRNA manifest as changes in protein expression. While histologic analyses clearly demonstrate fibrin deposition in the lung, further studies using agnostic and targeted proteomics, as well as biochemical analyses will be needed to confirm causation. We focused on changes in coagulation and fibrinolytic pathways in the present study; however, the pathologic processes observed in the lungs of COVID-19 patients are likely to be influenced by changes in other pathways, such as those involving complement. Crosstalk between coagulation, fibrinolytic, and complement pathways is well documented (Ewald and Eisenberg, 1995; Foley et al., 2016; Fox et al., 2020; Kleniewski and Donaldson, 1987). Despite these limitations, it is instructive to consider how the changes in coagulation and fibrinolytic gene expression documented in our study of BALF may represent common events occurring in multiple vascular beds in COVID-19, as identification of a common dysfunction may reveal a targetable mechanism to prevent thrombosis in these patients.
Materials and methods
Study participants
The nine BALF samples from 5 patients used in this study were collected from patients in Wuhan, China. All patients displayed pneumonia and other severe symptoms upon admission to the hospital in Wuhan, China, in late December 2019 and were therefore admitted to the ICU (Table 2). They were originally used for RNA sequencing to identify the etiological agent for COVID-19 and to determine the genomic sequence of SARS-CoV-2. Human mRNA sequences from these samples were used to develop our bradykinin storm model (Garvin et al., 2020), but have not previously been analyzed to understand localized coagulopathy in the lungs of patients with COVID-19 (Zhou et al., 2020b). We compared mRNA transcripts for proteins involved in coagulation, fibrinolysis, and kinin formation in BALF from these samples with those from 40 controls from a study on the contribution of obesity to disease severity in asthmatics (Michalovich et al., 2019). The 40 control individuals included smokers and non-smokers, asthmatics and non-asthmatics, as well as obese and non-obese individuals. Given this diverse set of morbidities and the resulting variance in gene expression, they represent an informative control for patients with COVID-19 as statistically significant changes detected due to COVID-19 are not just representing subtle shifts in variance (Table 1). Expression datasets are available from the NCBI Sequence Read Archive under the accession numbers PRJNA605983 (severe acute respiratory syndrome coronavirus 2 raw sequence reads) and PRJNA434133 (Microbiome and Inflammatory Interactions in Obese and Severe Asthmatic Adults).
Table 2. Clinical data for patients from which BALF was extracted and analyzed for this study.
| GISAID accession | Isolate | NCBI accession | SRA accessions | Swab date | Age | Sex | Patient no | Date onset | Symptoms_admission | Status 1/13/20 | Diagnosis history |
|---|---|---|---|---|---|---|---|---|---|---|---|
| EPI_ISL_402127 | WIV02 | MN996527 | SRR11092058, SRR11092063 | 12/30/19 | 32 | Male | ICU-04 | 12/19/19 | Fever, cough, dyspnea | Fever, intermitttent cough | Negative |
| EPI_ISL_402124 | WIV04 | MN996528 | SRR11092057, SRR11092062 | 12/30/19 | 49 | Female | ICU-06 | 12/27/19 | Fever (37.9C), palpitation | Fever, malaise, cough | Coronavirus (nt) |
| EPI_ISL_402128 | WIV05 | MN996529 | SRR11092061 | 12/30/19 | 52 | Female | ICU-08 | 12/22/19 | Fever (38C), expectoration, malaise, dyspnea | Recovered, disharged | Streptococcus pneumoniae (nt) |
| EPI_ISL_402129 | WIV06 | MN996530 | SRR11092056, SRR11092060 | 12/30/19 | 40 | Male | ICU-09 | 12/28/20 | Fever (38C), expectoration | Fever (38C), expectoration, dizziness | Negative |
| EPI_ISL_402130 | WIV07 | MN996531 | SRR11092059, SRR11092064 | 12/30/19 | 56 | Male | ICU-10 | 12/20/19 | Fever, dyspnea, chest tightness | Fever, malaise, cough, dyspnea | Negative |
Gene expression analysis
The CLC Genomics Workbench (20.0.3) was used to trim FASTQ files downloaded from the NCBI Sequence Read Archive to remove any adapter sequences or other artifacts from processing. The RNA reads were mapped to the GRCh38_latest_rna.fna version of the human transcriptome. Mapping parameters included a cost penalty of two for mismatches and three for small insertions or deletions (INDEL), and similarity and length fraction were both set to 0.95. The resulting transcript profiles were manually inspected to account for expression artifacts, such as reads mapping solely to repetitive elements such as the Alu transposable element. Transcripts whose counts came solely from (or were dominated by) reads at repetitive elements were removed from the analysis. Where transcripts per million (TPM) values were zero (transcripts were not detected), we used the lowest TPM across samples to two decimal places. If all samples in a group had no detected transcripts, we used the lowest TPM to two decimal places from the entire set of genes queried. The edgeR package (McCarthy et al., 2012; Robinson et al., 2010) was used to identify genes that were differentially expressed in COVID-19 patient samples compared to controls. Count data were scaled to normalize for library size and normalization factors determined as instructed in edgeR documentation. Dispersion was estimated, and the read counts for each gene were fit with a negative binomial model. Each gene was tested for differential expression. The Benjamini–Hochberg method was used to determine the false discovery rate.
Acknowledgements
We would like to acknowledge funding from the Oak Ridge National Laboratory, Laboratory Directed Research and Development Fund, LOIS:10074 (which supported the systems biology work), and the National Institutes of Health, U24 HL148865: the LungMap Consortium (which supported tissue and cell-based expression conceptualization). Funding was also provided by the National Institutes of Health grants HL068835 (AM), HL143403 (AW), HL126974 (AW), 3RF1AG053303-01S2 (DJ), and HL140025 (DG) to support analyses and interpretation of the coagulation and fibrinolytic pathways. This research used resources of the Oak Ridge Leadership Computing Facility, which is a DOE Office of Science User Facility supported under Contract DE-AC05-00OR22725. This research used resources of the Compute and Data Environment for Science (CADES) at the Oak Ridge National Laboratory.
Funding Statement
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Contributor Information
Daniel Jacobson, Email: jacobsonda@ornl.gov.
Noriaki Emoto, Kobe Pharmaceutical University, Japan.
Jos WM van der Meer, Radboud University Medical Centre, Netherlands.
Funding Information
This paper was supported by the following grants:
Oak Ridge National Laboratory LOIS:10074 to Michael R Garvin, J Izaak Miller, Daniel Jacobson.
National Institutes of Health U24 HL148865 to Bruce Aronow.
National Institutes of Health HL068835 to Alan E Mast.
National Institutes of Health HL143403 to Alisa S Wolberg.
National Institutes of Health HL126974 to Alisa S Wolberg.
National Institutes of Health HL140025 to David Gailani.
National Institute on Aging 3RF1AG053303-01S2 to Daniel Jacobson, Michael R Garvin.
Additional information
Competing interests
receives research funding from Novo Nordisk and has received honoraria for serving on Novo Nordisk advisory boards.
receives research funding from Takeda and Bristol Myers Squibb.
receives research funding from Bayer and has received honoraria for serving on Anthos, Bristol-Myers Squibb, Ionis and Janssen advisory boards.
No competing interests declared.
Author contributions
Conceptualization, Formal analysis, Investigation, Visualization, Writing - original draft, Writing - review and editing.
Conceptualization, Formal analysis, Investigation, Visualization, Writing - original draft, Writing - review and editing.
Conceptualization, Formal analysis, Investigation, Visualization, Writing - original draft, Writing - review and editing.
Conceptualization, Data curation, Formal analysis, Visualization, Writing - original draft, Writing - review and editing.
Visualization.
Formal analysis, Investigation, Methodology.
Conceptualization, Formal analysis, Investigation, Visualization, Writing - original draft, Writing - review and editing.
Conceptualization, Supervision, Funding acquisition, Investigation, Visualization, Methodology, Writing - original draft, Project administration, Writing - review and editing.
Additional files
Data availability
All data generated or analysed during this study are included in the manuscript and supporting files. Data for control and COVID-19 bronchoalveolar lavage samples are available in the Sequence Read Archive at NCBI.
The following previously published datasets were used:
Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, Chen HD, Chen J, Luo Y, Guo H, Jiang RD, Liu MQ, Chen Y, Shen XR, Wang X, Zheng XS, Zhao K, Chen QJ, Deng F, Liu LL, Yan B, Zhan FX, Wang YY, Xiao GF, Shi ZL. 2020. Severe acute respiratory syndrome coronavirus 2 Raw sequence reads. NCBI BioProject. PRJNA605983
GlaxoSmithKline 2018. Microbiome and Inflammatory Interactions in Obese and Severe Asthmatic Adults. NCBI BioProject. PRJNA434133
References
- Adachi T, Chong JM, Nakajima N, Sano M, Yamazaki J, Miyamoto I, Nishioka H, Akita H, Sato Y, Kataoka M, Katano H, Tobiume M, Sekizuka T, Itokawa K, Kuroda M, Suzuki T. Clinicopathologic and immunohistochemical findings from autopsy of patient with COVID-19, japan. Emerging Infectious Diseases. 2020;26:2157–2161. doi: 10.3201/eid2609.201353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammollo CT, Semeraro F, Colucci M, Simioni P. Factor IX-Padua enhances the fibrinolytic resistance of plasma clots. Thrombosis and Haemostasis. 2014;111:226–232. doi: 10.1160/TH13-06-0489. [DOI] [PubMed] [Google Scholar]
- Bajaj MS, Kuppuswamy MN, Saito H, Spitzer SG, Bajaj SP. Cultured normal human hepatocytes do not synthesize lipoprotein-associated coagulation inhibitor: evidence that endothelium is the principal site of its synthesis. PNAS. 1990;87:8869–8873. doi: 10.1073/pnas.87.22.8869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton LM, Duval EJ, Stroberg E, Ghosh S, Mukhopadhyay S. COVID-19 autopsies, Oklahoma, USA. American Journal of Clinical Pathology. 2020;153:725–733. doi: 10.1093/ajcp/aqaa062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busse PJ, Christiansen SC. Hereditary angioedema. New England Journal of Medicine. 2020;382:1136–1148. doi: 10.1056/NEJMra1808012. [DOI] [PubMed] [Google Scholar]
- Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. The Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicardi M, Zuraw BL. Angioedema due to bradykinin dysregulation. The Journal of Allergy and Clinical Immunology: In Practice. 2018;6:1132–1141. doi: 10.1016/j.jaip.2018.04.022. [DOI] [PubMed] [Google Scholar]
- Davin L, Marechal P, Lancellotti P, Martinez C, Pierard L, Radermecker R. Angioedema: a rare and sometimes delayed side effect of angiotensin-converting enzyme inhibitors. Acta Cardiologica. 2019;74:277–281. doi: 10.1080/00015385.2018.1507477. [DOI] [PubMed] [Google Scholar]
- Dolhnikoff M, Duarte-Neto AN, de Almeida Monteiro RA, da Silva LFF, de Oliveira EP, Saldiva PHN, Mauad T, Negri EM. Pathological evidence of pulmonary thrombotic phenomena in severe COVID-19. Journal of Thrombosis and Haemostasis. 2020;18:1517–1519. doi: 10.1111/jth.14844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards RL, Rickles FR, Bobrove AM. Mononuclear cell tissue factor: cell of origin and requirements for activation. Blood. 1979;54:359–370. doi: 10.1182/blood.V54.2.359.359. [DOI] [PubMed] [Google Scholar]
- Entrenas Castillo M, Entrenas Costa LM, Vaquero Barrios JM, Alcalá Díaz JF, López Miranda J, Bouillon R, Quesada Gomez JM. "Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: A pilot randomized clinical study". The Journal of Steroid Biochemistry and Molecular Biology. 2020;203:105751. doi: 10.1016/j.jsbmb.2020.105751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewald GA, Eisenberg PR. Plasmin-mediated activation of contact system in response to pharmacological thrombolysis. Circulation. 1995;91:28–36. doi: 10.1161/01.CIR.91.1.28. [DOI] [PubMed] [Google Scholar]
- Foley JH, Walton BL, Aleman MM, O'Byrne AM, Lei V, Harrasser M, Foley KA, Wolberg AS, Conway EM. Complement activation in arterial and venous thrombosis is mediated by plasmin. EBioMedicine. 2016;5:175–182. doi: 10.1016/j.ebiom.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folsom AR, Tang W, Roetker NS, Heckbert SR, Cushman M, Pankow JS. Prospective study of circulating factor XI and incident venous thromboembolism: the longitudinal investigation of thromboembolism etiology (LITE) American Journal of Hematology. 2015;90:1047–1051. doi: 10.1002/ajh.24168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox SE, Akmatbekov A, Harbert JL, Li G, Quincy Brown J, Vander Heide RS. Pulmonary and cardiac pathology in Covid-19: the first autopsy series from new orleans. medRxiv. 2020 doi: 10.1101/2020.04.06.20050575. [DOI] [PMC free article] [PubMed]
- Garvin MR, Alvarez C, Miller JI, Prates ET, Walker AM, Amos BK, Mast AE, Justice A, Aronow B, Jacobson D. A mechanistic model and therapeutic interventions for COVID-19 involving a RAS-mediated bradykinin storm. eLife. 2020;9:e59177. doi: 10.7554/eLife.59177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattinoni L, Coppola S, Cressoni M, Busana M, Rossi S, Chiumello D. COVID-19 does not lead to a “Typical” Acute Respiratory Distress Syndrome. American Journal of Respiratory and Critical Care Medicine. 2020;201:1299–1300. doi: 10.1164/rccm.202003-0817LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geerdes-Fenge HF, Reisinger EC, Arndt H. Pulmonary artery embolism in COVID-19 despite thrombosis prophylaxis. Deutsches Aerzteblatt Online. 2020;117:297. doi: 10.3238/arztebl.2020.0297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grover SP, Mackman N. Tissue factor: an essential mediator of hemostasis and trigger of thrombosis. Arteriosclerosis, Thrombosis, and Vascular Biology. 2018;38:709–725. doi: 10.1161/ATVBAHA.117.309846. [DOI] [PubMed] [Google Scholar]
- Gu L, Deng H, Ren Z, Zhao Y, Yu S, Guo Y, Dai J, Chen X, Li K, Li R, Wang G. Dynamic changes in the microbiome and mucosal immune microenvironment of the lower respiratory tract by influenza virus infection. Frontiers in Microbiology. 2019;10:2491. doi: 10.3389/fmicb.2019.02491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guadiz G, Sporn LA, Goss RA, Lawrence SO, Marder VJ, Simpson-Haidaris PJ. Polarized secretion of fibrinogen by lung epithelial cells. American Journal of Respiratory Cell and Molecular Biology. 1997;17:60–69. doi: 10.1165/ajrcmb.17.1.2730. [DOI] [PubMed] [Google Scholar]
- Helms J, Tacquard C, Severac F, Leonard-Lorant I, Ohana M, Delabranche X, Merdji H, Clere-Jehl R, Schenck M, Fagot Gandet F, Fafi-Kremer S, Castelain V, Schneider F, Grunebaum L, Anglés-Cano E, Sattler L, Mertes P-M, Meziani F. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Medicine. 2020;46:1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T, Thachil J, Asakura H, Levy JH, Iba T. Thrombomodulin in disseminated intravascular coagulation and other critical conditions-a multi-faceted anticoagulant protein with therapeutic potential. Critical Care. 2019;23:280. doi: 10.1186/s13054-019-2552-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov I, Shakhawat R, Sun MF, Dickeson SK, Puy C, McCarty OJ, Gruber A, Matafonov A, Gailani D. Nucleic acids as cofactors for factor XI and prekallikrein activation: different roles for high-molecular-weight kininogen. Thrombosis and Haemostasis. 2017;117:671–681. doi: 10.1160/TH16-09-0691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakieła B, Gielicz A, Plutecka H, Przybyszowski M, Soja J, Sładek K, Bochenek G. Bronchoalveolar lavage transcriptome and eicosanoid profiles reveal heterogeneity of lower airway inflammation in aspirin-exacerbated respiratory disease (AERD) Allergy and Immunology. 2018;52:PA5010. doi: 10.1183/13993003.congress-2018.pa5010. [DOI] [PubMed] [Google Scholar]
- Kahn N, Granzow M, Meister M, Muley T, Herth FJF, Kreuter M. Transcriptome analysis in endobronchial epithelial lining fluid compared to bronchoalveolar lavage in idiopathic pulmonary fibrosis. Pneumologie. 2015;69:1556653. doi: 10.1055/s-0035-1556653. [DOI] [Google Scholar]
- Kleniewski J, Donaldson VH. Comparison of human high molecular weight kininogen digestion by plasma kallikrein and by plasmin A revised method of purification of high molecular weight kininogen. The Journal of Laboratory and Clinical Medicine. 1987;109:469–479. [PubMed] [Google Scholar]
- Lax SF, Skok K, Zechner P, Kessler HH, Kaufmann N, Koelblinger C, Vander K, Bargfrieder U, Trauner M. Pulmonary arterial thrombosis in COVID-19 with fatal outcome : results from a prospective, Single-Center, clinicopathologic case series. Annals of Internal Medicine. 2020;173:350–361. doi: 10.7326/M20-2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi M, van der Poll T. Coagulation and sepsis. Thrombosis Research. 2017;149:38–44. doi: 10.1016/j.thromres.2016.11.007. [DOI] [PubMed] [Google Scholar]
- Long AT, Kenne E, Jung R, Fuchs TA, Renné T. Contact system revisited: an interface between inflammation, coagulation, and innate immunity. Journal of Thrombosis and Haemostasis. 2016;14:427–437. doi: 10.1111/jth.13235. [DOI] [PubMed] [Google Scholar]
- Maas C, Renné T. Coagulation factor XII in thrombosis and inflammation. Blood. 2018;131:1903–1909. doi: 10.1182/blood-2017-04-569111. [DOI] [PubMed] [Google Scholar]
- Magro C, Mulvey JJ, Berlin D, Nuovo G, Salvatore S, Harp J, Baxter-Stoltzfus A, Laurence J. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Translational Research. 2020;220:1–13. doi: 10.1016/j.trsl.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marceau F, Bachelard H, Bouthillier J, Fortin JP, Morissette G, Bawolak MT, Charest-Morin X, Gera L. Bradykinin receptors: agonists, antagonists, expression, signaling, and adaptation to sustained stimulation. International Immunopharmacology. 2020;82:106305. doi: 10.1016/j.intimp.2020.106305. [DOI] [PubMed] [Google Scholar]
- McCarthy DJ, Chen Y, Smyth GK. Differential expression analysis of multifactor RNA-Seq experiments with respect to biological variation. Nucleic Acids Research. 2012;40:4288–4297. doi: 10.1093/nar/gks042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mestres G, Puigmacià R, Blanco C, Yugueros X, Esturrica M, Riambau V. Risk of peripheral arterial thrombosis in COVID-19. Journal of Vascular Surgery. 2020;72:756–757. doi: 10.1016/j.jvs.2020.04.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalovich D, Rodriguez-Perez N, Smolinska S, Pirozynski M, Mayhew D, Uddin S, Van Horn S, Sokolowska M, Altunbulakli C, Eljaszewicz A, Pugin B, Barcik W, Kurnik-Lucka M, Saunders KA, Simpson KD, Schmid-Grendelmeier P, Ferstl R, Frei R, Sievi N, Kohler M, Gajdanowicz P, Graversen KB, Lindholm Bøgh K, Jutel M, Brown JR, Akdis CA, Hessel EM, O'Mahony L. Obesity and disease severity magnify disturbed microbiome-immune interactions in asthma patients. Nature Communications. 2019;10:5711. doi: 10.1038/s41467-019-13751-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middeldorp S, Coppens M, van Haaps TF, Foppen M, Vlaar AP, Müller MCA, Bouman CCS, Beenen LFM, Kootte RS, Heijmans J, Smits LP, Bonta PI, van Es N. Incidence of venous thromboembolism in hospitalized patients with COVID-19. Journal of Thrombosis and Haemostasis. 2020;18:1995–2002. doi: 10.1111/jth.14888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammed BM, Matafonov A, Ivanov I, Sun MF, Cheng Q, Dickeson SK, Li C, Sun D, Verhamme IM, Emsley J, Gailani D. An update on factor XI structure and function. Thrombosis Research. 2018;161:94–105. doi: 10.1016/j.thromres.2017.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panigada M, Bottino N, Tagliabue P, Grasselli G, Novembrino C, Chantarangkul V, Pesenti A, Peyvandi F, Tripodi A. Hypercoagulability of COVID-19 patients in intensive care unit: a report of thromboelastography findings and other parameters of hemostasis. Journal of Thrombosis and Haemostasis. 2020;18:1738–1742. doi: 10.1111/jth.14850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieters M, Wolberg AS. Fibrinogen and fibrin: an illustrated review. Research and Practice in Thrombosis and Haemostasis. 2019;3:161–172. doi: 10.1002/rth2.12191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prada AE, Zahedi K, Davis AE. Regulation of C1 inhibitor synthesis. Immunobiology. 1998;199:377–388. doi: 10.1016/S0171-2985(98)80042-9. [DOI] [PubMed] [Google Scholar]
- Preis M, Hirsch J, Kotler A, Zoabi A, Stein N, Rennert G, Saliba W. Factor XI deficiency is associated with lower risk for cardiovascular and venous thromboembolism events. Blood. 2017;129:1210–1215. doi: 10.1182/blood-2016-09-742262. [DOI] [PubMed] [Google Scholar]
- Revenko AS, Gao D, Crosby JR, Bhattacharjee G, Zhao C, May C, Gailani D, Monia BP, MacLeod AR. Selective depletion of plasma prekallikrein or coagulation factor XII inhibits thrombosis in mice without increased risk of bleeding. Blood. 2011;118:5302–5311. doi: 10.1182/blood-2011-05-355248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riewald M, Petrovan RJ, Donner A, Mueller BM, Ruf W. Activation of endothelial cell protease activated receptor 1 by the protein C pathway. Science. 2002;296:1880–1882. doi: 10.1126/science.1071699. [DOI] [PubMed] [Google Scholar]
- Robinson MD, McCarthy DJ, Smyth GK. edgeR: a bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon O, Steinberg DM, Koren-Morag N, Tanne D, Seligsohn U. Reduced incidence of ischemic stroke in patients with severe factor XI deficiency. Blood. 2008;111:4113–4117. doi: 10.1182/blood-2007-10-120139. [DOI] [PubMed] [Google Scholar]
- Salomon O, Steinberg DM, Zucker M, Varon D, Zivelin A, Seligsohn U. Patients with severe factor XI deficiency have a reduced incidence of deep-vein thrombosis. Thrombosis and Haemostasis. 2011;105:269–273. doi: 10.1160/TH10-05-0307. [DOI] [PubMed] [Google Scholar]
- Schmaier AH. The contact activation and kallikrein/kinin systems: pathophysiologic and physiologic activities. Journal of Thrombosis and Haemostasis. 2016;14:28–39. doi: 10.1111/jth.13194. [DOI] [PubMed] [Google Scholar]
- Schmaier AH, Emsley J, Feener EP, Gailani D, Govers-Riemslag JWP, Kaplan AP, Maas C, Morrissey JH, Renné T, Sidelmann JJ, Meijers JCM. Nomenclature of factor XI and the contact system. Journal of Thrombosis and Haemostasis : JTH. 2019;17:2216–2219. doi: 10.1111/jth.14595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta S, Tang SY, Devine JC, Anderson ST, Nayak S, Zhang SL, Valenzuela A, Fisher DG, Grant GR, López CB, FitzGerald GA. Circadian control of lung inflammation in influenza infection. Nature Communications. 2019;10:4107. doi: 10.1038/s41467-019-11400-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetty S, Padijnayayveetil J, Tucker T, Stankowska D, Idell S. The fibrinolytic system and the regulation of lung epithelial cell proteolysis, signaling, and cellular viability. American Journal of Physiology-Lung Cellular and Molecular Physiology. 2008;295:L967–L975. doi: 10.1152/ajplung.90349.2008. [DOI] [PubMed] [Google Scholar]
- Siegerink B, Govers-Riemslag JW, Rosendaal FR, Ten Cate H, Algra A. Intrinsic coagulation activation and the risk of arterial thrombosis in young women: results from the risk of arterial thrombosis in relation to oral contraceptives (RATIO) case-control study. Circulation. 2010;122:1854–1861. doi: 10.1161/CIRCULATIONAHA.110.943738. [DOI] [PubMed] [Google Scholar]
- Simpson-Haidaris PJ, Courtney MA, Wright TW, Goss R, Harmsen A, Gigliotti F. Induction of fibrinogen expression in the lung epithelium during Pneumocystis carinii pneumonia. Infection and Immunity. 1998;66:4431–4439. doi: 10.1128/IAI.66.9.4431-4439.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavrou EX. Factor XII in inflammation and wound healing. Current Opinion in Hematology. 2018;25:403–409. doi: 10.1097/MOH.0000000000000450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su H, Yang M, Wan C, Yi LX, Tang F, Zhu HY, Yi F, Yang HC, Fogo AB, Nie X, Zhang C. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney International. 2020;98:219–227. doi: 10.1016/j.kint.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q, Fang L, Roth M, Tang X, Papakonstantinou E, Zhai W, Louis R, Heinen V, Schleich FN, Lu S, Savic S, Tamm M, Stolz D. Bronchial thermoplasty decreases airway remodelling by blocking epithelium-derived heat shock protein-60 secretion and protein arginine methyltransferase-1 in fibroblasts. European Respiratory Journal. 2019;54:1900300. doi: 10.1183/13993003.00300-2019. [DOI] [PubMed] [Google Scholar]
- Suri MFK, Yamagishi K, Aleksic N, Hannan PJ, Folsom AR. Novel hemostatic factor levels and risk of ischemic stroke: the atherosclerosis risk in communities (ARIC) Study. Cerebrovascular Diseases. 2010;29:497–502. doi: 10.1159/000297966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. Journal of Thrombosis and Haemostasis. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Veerdonk FL, Kouijzer IJE, de Nooijer AH, van der Hoeven HG, Maas C, Netea MG, Brüggemann RJM. Outcomes associated with use of a kinin B2 receptor antagonist among patients with COVID-19. JAMA Network Open. 2020;3:e2017708. doi: 10.1001/jamanetworkopen.2020.17708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Ivanov I, Smith SA, Gailani D, Morrissey JH. Polyphosphate, Zn2+ and high molecular weight kininogen modulate individual reactions of the contact pathway of blood clotting. Journal of Thrombosis and Haemostasis. 2019;17:2131–2140. doi: 10.1111/jth.14612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Hajizadeh N, Moore EE, McIntyre RC, Moore PK, Veress LA, Yaffe MB, Moore HB, Barrett CD. Tissue plasminogen activator (tPA) treatment for COVID-19 associated acute respiratory distress syndrome (ARDS): A case series. Journal of Thrombosis and Haemostasis. 2020;18:1752–1755. doi: 10.1111/jth.14828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigt SS, Wang X, Palchevskiy V, Li X, Patel N, Ross DJ, Reynolds J, Shah PD, Danziger-Isakov LA, Sweet SC, Singer LG, Budev M, Palmer S, Belperio JA. Usefulness of gene expression profiling of bronchoalveolar lavage cells in acute lung allograft rejection. The Journal of Heart and Lung Transplantation. 2019;38:845–855. doi: 10.1016/j.healun.2019.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group. Sterne JAC, Murthy S, Diaz JV, Slutsky AS, Villar J, Angus DC, Annane D, Azevedo LCP, Berwanger O, Cavalcanti AB, Dequin PF, Du B, Emberson J, Fisher D, Giraudeau B, Gordon AC, Granholm A, Green C, Haynes R, Heming N, Higgins JPT, Horby P, Jüni P, Landray MJ, Le Gouge A, Leclerc M, Lim WS, Machado FR, McArthur C, Meziani F, Møller MH, Perner A, Petersen MW, Savovic J, Tomazini B, Veiga VC, Webb S, Marshall JC. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a Meta-analysis. Jama. 2020;324:1330–1341. doi: 10.1001/jama.2020.17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood JP, Ellery PE, Maroney SA, Mast AE. Biology of tissue factor pathway inhibitor. Blood. 2014;123:2934–2943. doi: 10.1182/blood-2013-11-512764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L, Tai Y, Bai C, Gao T, Song J, Xia P, Dong J, Zhao J, Wang FS. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. The Lancet Respiratory Medicine. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HH, Li CX, Tang LF. The differential expression profiles of miRNA-let 7a, 7b, and 7c in bronchoalveolar lavage fluid from infants with asthma and airway foreign bodies. Journal of Evidence-Based Integrative Medicine. 2019;24:2515690X18821906. doi: 10.1177/2515690X18821906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. The Lancet. 2020a;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, Chen HD, Chen J, Luo Y, Guo H, Jiang RD, Liu MQ, Chen Y, Shen XR, Wang X, Zheng XS, Zhao K, Chen QJ, Deng F, Liu LL, Yan B, Zhan FX, Wang YY, Xiao GF, Shi ZL. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020b;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]