Abstract
Vaccination is the most effective means of controlling infectious disease-related morbidity and mortality. However, due to low immunogenicity of viral antigens, nanomedicine as a new opportunity in new generation of vaccine advancement attracted researcher encouragement. Virosome is a lipidic nanomaterial emerging as FDA approved nanocarriers with promising bioinspiration and biomimetic potency against viral infections. Virosome surface modification with critical viral fusion proteins is the cornerstone of vaccine development. Surface antigens at virosomes innovatively interact with targeted receptors on host cells that evoke humoral or cellular immune responses through antibody-producing B cell and internalization by endocytosis-mediated pathways. To date, several nanovaccine based on virosome formulations have been commercialized against widespread and life-threatening infections. Recently, Great efforts were made to fabricate a virosome-based vaccine platform against a new severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus. Thus, this review provides a novel overview of the virosome based nanovaccine production, properties, and application on the viral disease, especially its importance in SARS-CoV-2 vaccine discovery.
Keywords: Nanovaccine, Virosome, Viral infections, SARS-CoV-2, Bioinspiration
Graphical abstract
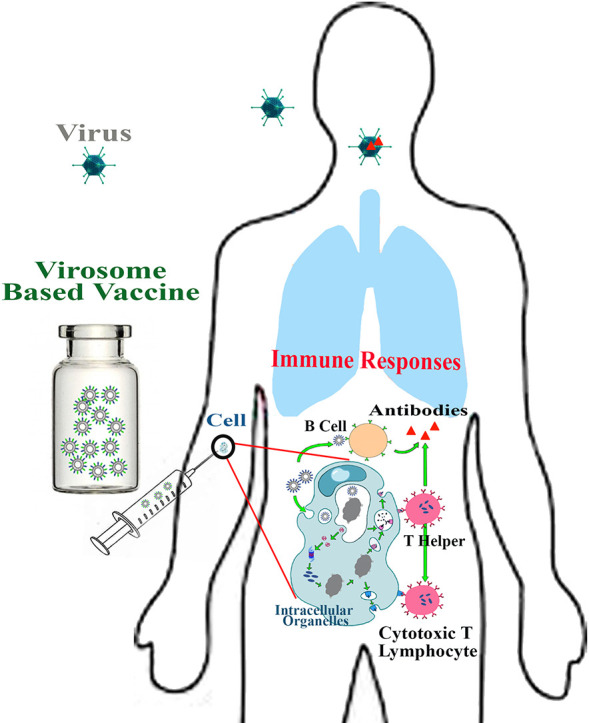
1. Introduction
Viral infections remain a major challenge for global public health, accompanied by a broad range of complications from death and life-threatening disease to mild and asymptomatic diseases. Viral vaccines are the most outstanding public health achievements in medical history. Vaccination is the most effective method of controlling infectious disease-related morbidity and mortality. The World Health Organization (WHO) estimates that vaccination prevents over 2.5 million child deaths each year worldwide [1]. The elevated population density and outgrowth and the excessive population mobility from all areas of the world highly favor the global spreading of pathogens. The climate changes and rising temperatures influence the distribution, abundance, and prevalence of infection-bearing vectors such as mosquitoes or ticks and promote infections with a range of vector-borne diseases. This pattern can be seen in the recent severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic that transmitted infection from bat-borne pathogens to humans [2,3].
Viral vaccines have successfully mitigated the infection transmission and mortality rate of patients. A vaccine is a biological preparation that stimulates antibodies (humoral) or cell-mediated (cellular) immune responses. Besides, vaccination is the critical tool to protect us against malignancies and a wide range of contagious diseases, or even drug dependencies [4].
There are different types of viral vaccines that have been clinically used to date. The conventional types contain either inactivated or live-attenuated viruses or protein subunits of viral particles. Conventional vaccines have some deficiencies include weak cell-mediated immune responses, safety alarm of viral infections in vaccinated individuals, requiring booster doses, and the emergence of immunogenicity [5].
DNA based vaccines encode viral antigens under the control of eukaryotic promoters and induce robust and long-lasting immune responses. Thus, these kinds of vaccines entered large scale clinical trials. However, the safety concerns about integrating DNA-based vaccines into the host cell genome, autoimmune reactions, and antibiotic resistance risk remain a substantial problem [6].
Therefore, highly-focused research in immunology, microbiology, genetics, and structural biology was performed to develop new platforms with considered vaccine-induced host protection. Novel vaccine strategies faced multiple challenges, including the lack of suitable animal models, especially for mucosal delivery, and ambiguous pathways of viral pathogenic mechanisms [7,8].
Nanomedicine has a significant contribution to vaccine production. Some critical characteristics of nanocarriers in biomedical sectors, especially vaccine design, are including cost-effectiveness, improved vaccine stability, encapsulated antigen protection from premature degradation, eliminated booster doses administration necessity, having excellent adjuvant properties, temporal and spatial target delivery, strong innate immune response in humoral and cellular levels, and discovery of desired vaccines for hard to treat viral disease such as acquired immune deficiency syndrome (AIDS) [9,10]. Nanocarriers utilized in vaccine formulations through high-throughput strategies are composed of lipids, proteins, metals, polymers, and other organic elements.
Virosomes are attractive nanocarriers on which many research activities implemented their development [11]. The virosome structure is similar to an enveloped virus whose nucleocapsid was eliminated. Recently, this type of vaccine has attracted much attention, so that various types of virosome-based vaccines have been approved in over 45 countries, and more than 10 million patients have been immunized to date. No age restriction made them applicable in infants and older adults [12,13]. How is this type of vaccine prepared? Why are these vaccines so popular in viral diseases? What are their main features? This study has briefly reviewed the different types of nanovaccines and then focused on the production, formulation, properties, and clinical importance of virosome-based viral nanovaccines. Besides, due to the emergence of virosome based vaccine transvac-2 for coronavirus that causes coronavirus disease 2019 (COVID-19), we especially discuss its importance. However, the potential of virosome in viral vaccine production is our focal point in this review.
2. Nanovaccines
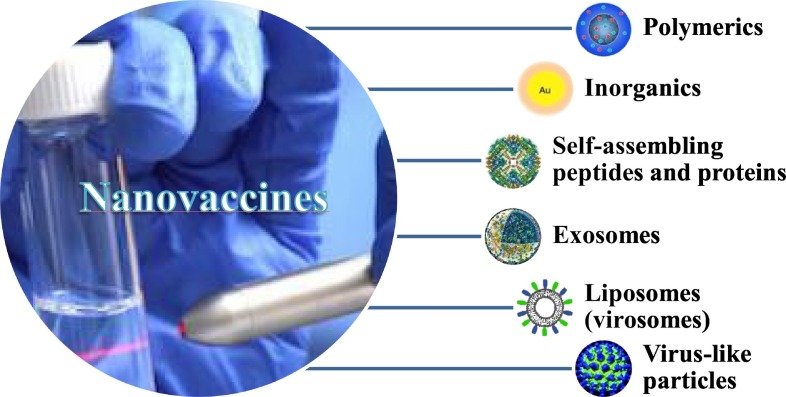
Nanotechnology developed the most crucial approach in the production of new generation vaccines. So far, various types of nanocarriers, including polymers, peptides, virus-like particles (VLPs), and lipid used in vaccine design, have been discussed briefly. However, polymeric and lipid-based nanocarriers are the most extensively studied vehicles [14]. Fig. 1 shows a diagram of the category of nano-based vaccines. This section gathers brief information about the main categories of nanovaccines widely used for viral infections in the clinic.
Fig. 1.
Schematic diagram of nanovaccines classification.
2.1. Polymeric vaccines
Polymeric nanoparticles (NPS) with unique properties are an attractive alternative strategy for vaccine creation. A wide variety of natural and synthetic polymers involves in vaccine decorating, such as gelatin, hyaluronic acid, alginate, polylactic acid (PLA), poly lactic-co-glycolic acid (PLGA), polyethyleneimines (PEI), polyamidoamine (PAMAM), and polyesters [15]. Some useful polymeric NPS are mentioned in Table 1 . For example, Thomas et al. designed an effective mucosal dosage form based on porous PLA and PLGA nanoparticles against hepatitis B viruses administered through pulmonary and inhalation systems. This inhalable nanovaccine is in the preclinical stage [16].
Table 1.
A list of nanocarrier based viral vaccine.
| Virus | Classifications | Components | Target receptors | Status | Ref |
|---|---|---|---|---|---|
| Influenza | O/W Emulsion | IPM1, white oil, soybean oil, and grape-kernel oil | AF032 and AF043 | Preclinical | [28] |
| Hemorrhagic septicemia virus (VHSV) | Polymeric nanoparticles | PLGA | Inactivated VHSV mixed with squalene | Preclinical | [29] |
| Hepatitis B | Polymeric nanoparticles | PLA and PLGA | HBsAg4 | Phase ΙΙ | [16] |
| HPV | VLPs | L1, L2 proteins VLPs | Fusion protein HPV | Market | [30] |
| Merkel Cell Polyomavirus (MCV) | (MCV)-Like Particles | Polyomavirus capsid proteins, VP1 proteins | VP1 proteins | Phase Ι | [31] |
| Melanoma | Dendrimer | Glycan, PAMAM generation 0 or 3 dendrimer, GP100 long synthetic peptide | GP1005, DC-SIGN6, and Langerin receptors | Phase Ι | [32] |
| Herpes simplex virus type 2 (HSV-2) | Calcium phosphate nanoparticles (CAP) | CAP | HSV-2 proteins | Preclinical | [33] |
| Influenza A virus | Gold nanoparticles (AuNPs) | AuNPs, CpG7 | M2e-specific IgG | Preclinical | [34] |
| Viral infectious | Nanogel | Cationic alginate-poly ethylenimine | OVA8 | Preclinical | [35] |
| HIV | Peptide-based nanofibrous hydrogel | DNA, methylamino group, Nap-GFFY9 | DNA encoding the gp 14510 | Preclinical | [36] |
Abbreviations: 1. Isopropyl myristate, 2. Squalene-in-water emulsion, 3. Combination of AF03 with Eisai's synthetic TLR4 agonist, 4. Hepatitis B surface antigen, 5. Glycoprotein 100, 6. Dendritic cell-specific ICAM-3 grabbing non-integrin, 7. The cytosine-guanine-rich oligonucleotide, 8. Ovalbumin antigen, 9. Nap represents naphthalene acetic acid, G represents glycine, F represents phenylalanine, and Y represents tyrosine, 10. HIV-1 glycoprotein 145 envelope.
2.2. Self-assembling peptide and protein vaccines
Peptide vaccines are mainly composed of short peptide fragments or proteins, with second or third structures. Self-assembly has a vital role in peptide nanoparticle constitution. Targeted nanovaccines could generate potent immune responses, consequently avoiding allergenic and/or reactogenic sequences [17]. Ferritin is the best example of self-assembled nanoparticles; whose primary function is intracellular iron storage. The ferritin-based vaccine is thermostable with excellent chemical stability. Influenza matrix protein 2(M2e) presented on self-assembling recombinant human heavy chain ferritin (rHF) cage intranasal nanovaccine against homo- and heterosubtypic influenza viruses are in the preclinical stage right now [18].
2.3. VLPs
VLPs are a small fragment of viral structural proteins, i.e., the capsid proteins without nucleic acid-containing epitopes recognized by the host cells. VLPs is a biomimetic nanostructure that can induce strong immunologic responses and high titers of neutralizing antibodies to conformational epitopes. Gardasil is the VLPs based nanovaccine accessible on the market right now, in which VLPs composed of major capsid (L1) proteins of human papillomavirus (HPV) Types 6, 11, 16, 18, 31, 33, 45, 52, and 58 [[19], [20], [21]].
2.4. Inorganic nanovacines
Inorganic nanoparticles have been suggested as a novel vehicle to present virus antigens by intrinsic antimicrobial activities. Gold, silver, and iron oxide nanocarrier are more prevalent inorganic materials used in viral vaccine production [22,23]. Gold nanoparticles (AuNPs) decorated with glycans, HIV (Human Immunodeficiency Virus)-Gag p17 (Peptide1: KKWK-SLYNTVATL), and cytomegalovirus virion protein (CMV pp65) have been used to nanovaccine development against HIV-1 is in preclinical experiments [24].
2.5. Liposomal vaccines
Lipid nanocarriers have emerged as a practical approach in vaccine development. A liposome is constructed of a spherical self-assembled bilayer phospholipid. The possibility of incorporating different antigens (i.e., proteins, peptides, carbohydrates, nucleic acids, and haptens) is the most critical properties of lipid nanocarriers. Liposome formulations for vaccine delivery are commercialized right now. Liposomes have several significant advantages as a vaccine delivery system, including preventing antigens degradation before reaching the target site, carrying hydrophilic and lipophilic compounds, the optimal release profile of antigens, and increasing cellular uptake, and improving antigen-specific immune responses [25,26].
Micelles are another type of self-assembled lipid-based nanoparticles that should be noted at vaccine development, which have been designed by synthetic co-polymers. Therefore, the lipophilic cavity or hydrophilic cavity (reverse micelle) promotes entrapment and delivery of antigens into the target cells [27]. Table 1 mentioned developed nanovaccines and their classifications, components, and mechanism of actions.
Virosome is a kind of lipid nanoparticle that is emerged as a bioinspiration tool confronting viral infections. Now, let see, what are virosomes? What are virosome applications in viral vaccine development?
3. Virosomes
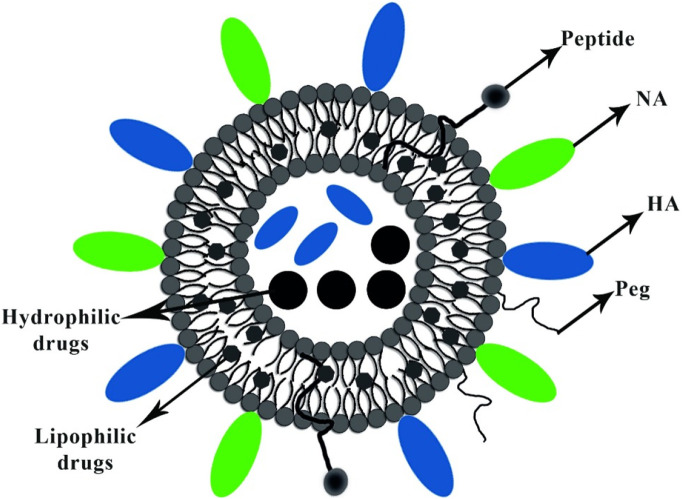
Virosomes are spherical, unilamellar vesicles (60–200 nm) reconstituted of viral envelopes phospholipids with removed nucleocapsid. Fig. 2 shows the schematic picture of the virosome particle. The significant advantage of the virosomes delivery system is adsorbing epitopes of antigens via hydrophobic domains or lipid linker on their surface and inside, furthermore, integrating into phospholipid bilayer. Viral glycoproteins epitopes are incorporated in the virosome surface (peviprotm) or located in the hallow membrane vesicles (pevitertm). Hydrophilic drugs are located in hollow vesicles, while hydrophobic agents mix with bilayer phospholipids. The surface modification of virosomes by hydrophilic polymers such as polyethylene glycol (PEG), polyvinylpyrrolidone (PVP) increased their circulation time and eliminated its significant disadvantages. Incorporation of targeting moieties as the ligand, antibody, and peptide in virosome structure based on active targeting provided opportunities to reach subcellular levels of tumor cells, respiratory and immune system cell components [[37], [38], [39]].
Fig. 2.
Shows the schematic illustration of the virosome particle. Virosome endowed adjuvant properties and functioning as a carrier for delivering several bioactive compounds, including lipophilic and hydrophilic drugs, peptides, and polymers.
Generally, virosomes are preferred to VLPs in vaccine therapy because VLPs have a protein-based structure that confines their movements, mainly when antigens are located near together while locating protein antigens on the surface of fluidic phospholipid substrate in virosomes promoted interactions with host cell receptors [40,41].
Virosomes could be designed by various types of antigen epitope and target different types of host cells. Virosomes are internalized into the host cell at neutral pH; however, virosome fusion with the endosomal membrane is a low pH-dependent route [42].
PH-raising agents such as ammonium chloride (NH4Cl), chloroquine diphosphate (CQ), and bafilomycin A1 increased pH of endosome that would result in virosome efficiency mitigation [43,44].
3.1. Design, preparation, and characterization of virosomes
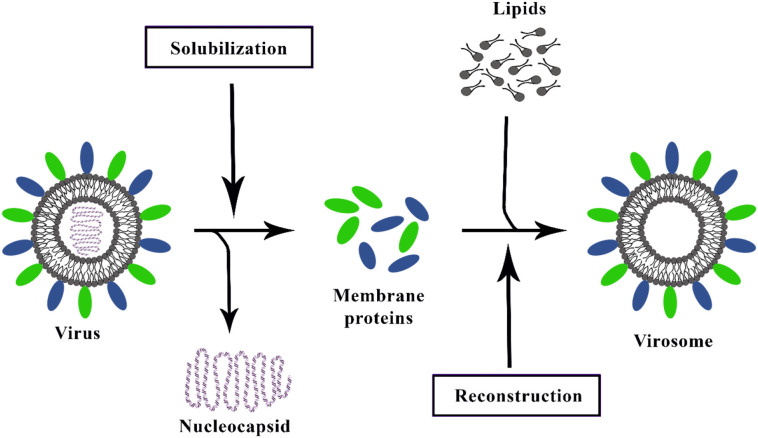
Virosomes closely mimic the native morphology of the virus, especially size and shape. Their preparation techniques are simple, cost-effective, and follows similar main steps. Virosome is produced in three main steps, as shown in Fig. 3 :
-
A.
At the initial step, an enveloped virus is solubilized by detergent, and then the nucleocapsid structure is removed after ultracentrifugation.
-
B.
Detergent is eliminated, then the sedimented viral proteins from the supernatants were extracted.
-
C.
At the end, viral antigens epitopes and viral fusion peptide, which are relatively hydrophobic proteins, and lipid, reconstitute hollow membrane vesicles [40,45,46]. Moreover, virosome virulence could be promoted by virus components (RNA, DNA, or proteins) and antiviral drug addition.
Fig. 3.
The schematic representation of the current virosome preparation method.
In some virosome formulations, viruses have been inactivated upon exposure to UV radiation, and the viral genome breaks into many fragments. Then, detergent is added to the solution. The hemagglutinating virus of Japan (HVJ; Sendai virus) has been prepared in this way [47].
The efficacy of fusogenic virosome dramatically depends on the detergents used. After solubilization, it is also challenging to remove low-CMC detergents like octaethylene glycol mono (n-dodecyl) ether (C12E8), Triton X-100, nonidert p-40, and others. High-CMC detergents like nonionic detergent octylglucoside (OG) lead to nonactive virosome, and after that, are replaced by another detergent. However, OG removal is easily achieved from a solution that facilitates viral protein incorporation into the bilayer membrane. Viral antigens are deposited, and detergent is omitted by either dialysis or hydrophobic resin (like Bio-Beads SM-2).
Virosome is fabricated by a massive expansion of phospholipids and biomaterial. However, the purified viral lipid is preferred in most commercialized vaccines product. Virosomal pool (VP) is the final result of preparations which must be characterized. Virosome size and structure are determined by electron microscope-based and dynamic light scattering (DLS) analysis. The presence of viral proteins such as HA and NA content was determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) test.
Fluorescent resonance energy transfer assay (FRET) by adding a fluorescent probe, pyrene-phosphatidylcholine (pyrene-PC), was used to determine virosomes fusion activity and other cell-binding assay procedures. However, the fusion activity is indirectly evaluated by monitoring hemolytic activity, which correlates nearly to fusion activity and indicates a pH dependence similar to fusion. Lamellarity can be determined by small edge x-beam dissipating, 13p-NMR, freeze-fracture electron microscopy. Pyrogenicity can be determined by rabbit fever test, Limulus amoebocyte lysate (LAL) test. Surface chemical analysis is evaluated by static auxiliary particle mass spectrometry. Sucrose gradient ultracentrifugation techniques are most frequently used for virosomes purification.
Virosomal sterility is evaluated by conventional liposomal sterilization techniques, including filtration, γ-irradiation, saturated steam, dry heat, ethylene oxide, UV sterilization, and dense gas technique. However, each technique showed its advantages and disadvantages—moreover, much effort was required to develop a widely applicable sterilization technique [48].
3.2. Immunologic features of virosome based nanovaccines
Virosomes have been emerging as FDA approved nanocarriers for human use due to their excellent tolerance and safety profile (8–10). Subunit vaccines presented poor performance against viral invasions, while virosomes create strong humoral and cellular immune responses similar to a potent adjuvant. Potency and mechanisms of virosome based vaccines actions is affected by several factors, such as chemical compounds, particle size distribution and homogeneity, charge, morphology, and location of antigens and/or adjuvants in a carrier structure and, finally, the route of administration.
Location of antigens epitopes in virosome structure is the critical factor in initiating rapid innate and adaptive immune responses, immunoglobulin receptors of the B cells quickly recognizing antigens on the surface of virosomes and secrete virus-specific antibodies. However, T cell-independent responses are non-durable and short-lived and usually induced IgM antibodies secreting. T cells are a critical component of adaptive immunity and generating protective immune responses against viral infections. T cell receptor (TCR) binds to broken-down peptides of the antigen presented on major histocompatibility complexes (MHC) molecules. MHC class I and MHC class II reside on antigen-presenting cell (APCs) surface. MHC-I presentation pathway simulated the CD8+ cytotoxic T lymphocytes (CTLs) and MHC-II antigen leading to activated CD4+ helper T cells pathway. APCs uptake antigens and transport them into draining lymph nodes, crucial for induction of a long-lived memory and robust IgG response [44,49,50].
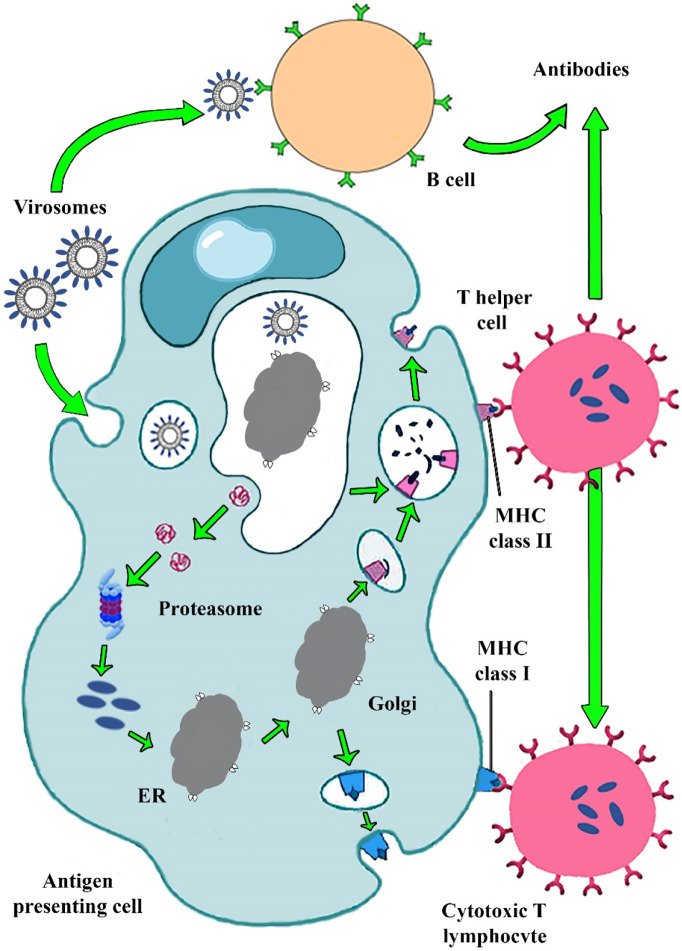
Fig. 4 shows that the reconstituted virosome is mostly similar to the enveloped virus structure that induced strong humoral and cellular immune responses.
Fig. 4.
Immunogenic pathway of virosome based vaccine presented in Fig. 2. Virosome binding to pathogen recognition receptors on APCs cell caused IgG, IgA, IgM antibody releases. Virosome entered the cell by endocytic pathway. A viral peptide derived from virosome degradation in endosomal low pH binding to MHC-І and MHC-IІ molecules stimulated T helper and CTL cells. CTLs removed viral infections and produced cellular immunity.
Immunopotentiating reconstituted influenza virosomes (IRIVs) are the desired example of versatile and potent nanocarriers for vaccine development. They harbor hemagglutinin (HA) and neuraminidase (NA) antigens from the influenza virus. Salic acid presented on the APCs membranes is a main target of IRIVs, activated endocytosis pathway and, followed by intracellular delivery of virosome payload. B cell lymphocyte is drawn in humoral immune response when immunoglobulin receptors can recognize influenza antigens such as HA incorporated in virosome on a B cell as a foreign pathogen. The cell membrane fusion mechanism of the virosome occurs following the endocytosis mediated pathway. HA binds to sialic acid receptors on the host cell, which causes virosome fusion to the membrane and endosomal formation. HA is composed of 2 epitopes: HA1 and HA2. Virosome–endosome membrane fusion due to HA2 epitopes conformational changes occur in endosomal low PH ~ 5. Virosome content releases in cytosol and bonds with MHC-І molecules that finally activate CTLs lymphocytes. Also, spike proteins and other viral peptides result from the virosome degradation bond with MHC-IІ molecules. MHC-IІ interaction with CD4+ activates T helper lymphocyte responses, which creates CTLs responses. CTLs immune response is essential for counteracting viral attacks. However, T helper lymphocyte stimulates B cells to secreted IgG, IgA, and IgM antibodies [40,[51], [52], [53]].
3.3. Applications of virosome technology in biomedical sciences
3.3.1. Virosome immunogens
Virosome mimics the enveloped virus fusion pathway to evoke potent humoral and cellular immune responses. Host immune system recognizes virosomes as a pathogen that interact with immunoglobulin (IG) receptors on B cells. Repetitive exposure of antigens and IG receptors activates APCs and antigen-specific T helper cells. Also, influenza derived virosomes directly can produce intense stimulation in B cells without T helper cell participation [44].
Virosome surface modification with critical viral fusion proteins is the cornerstone of vaccine development. Surface antigens of Virosomes interact with targeted receptors on host cells that engaged in virus-host cell membrane fusion. Virosome evokes cellular immune responses through internalization by endocytosis-mediated pathways. Several triggers, including receptors and low PH (5–6) of endosomes, induced conformational changes in antigens epitopes, which leads to virosome-endosomes fusion. Activation of CTLs depends on signals generation by dendritic cells, T helper cells, and other cells. Finally, T-helper lymphocyte cells activate CTLs releasing cytotoxic granules against viral invading [54].
3.3.2. Epitope-displaying virosome nanocarriers
Antigen incorporation in the virosomes structure followed by standard virosome procedure, as represented in Fig. 2, can be employed. The optimized concentration of antigens epitopes was put into the solubilized viral membrane suspension before detergent removal. Proteins or peptides are often available in lyophilized form on the market that could be appended in prepared suspension.
For example, antigens extracted from at least two strains of enveloped virus 0.5 mg of P1 peptide 10–50 mg of OVA or 1–5 mg of HPV type 16 L1 proteins to 1 Amol of an endogenous lipid belong to virus enveloped and 1.5 Amol of exogenous lipids. Fusion properties concerns of the reconstituted virosomes are eliminated by optimizing the condition of the solubilizing step.
For example, the influenza virosome has been generated by reconstitution of the influenza HA and NA antigens epitopes added to viral membrane suspension. Sendai fusion protein (F-protein), with or without the HA and NA proteins, rejoined in viral lipids to reassemble Sendai virus virosome structures. Vesicular stomatitis virus (VSV) G-protein put in viral lipids to the preformed VSV virosome.
3.3.3. Virosome delivery platform
Overall, standard buffered saline (135–150 Mm NaCl) were equipped in virosome formulations, but other suitable solution such as phosphate buffer saline (PBS) also exists. These compositions should be sterilized using techniques like membrane filtration. Additive substances append to the prepared formulation is essential to simulate isotonic physiological conditions, including buffering agents, pH adjusting, osmolality adjustment, and wetting agents (sodium acetate, sodium lactate, sodium chloride, potassium chloride, calcium chloride, sorbitan monolaurate, triethanolamine oleate, and the like) [37].
The final concentration of virosomes is presented in the vaccine formulations, currently is varied from 20 to 200 mg/mL. Virosome concentrations are optimized depending on administration routes and treatment approaches. Auxiliary components can be added to the vaccine formulation to target the specific cell types [55].
Virosome is a versatile antigen delivery system administered through various routes, including injection, mucosal (oral, respiratory, vaginal, rectal), and intradermal delivery. The virosomes can also be encapsulated into implantable devices for long-term release. Non-invasive mucosal routes are preferred because of their needle-free delivery system and eliminated require of trained health workers and sterile injection equipment. Mucosal tissue and lymph nodes comprise 90% of the immune cell component [56]. Vaccine loses its potency due to heat exposure; hence, heat independent vaccine development is essential. Thermostable delivery platform development by MACIVIVA project is the acronym for the “manufacturing process for cold chain independent virosome-based vaccines”. This technology is of great importance in developing countries where adequate cooling equipment could not be provided for long time vaccine storage [57].
4. Different types of virosome-based viral nanovaccines
To date, several vaccine adjuvants based on virosome formulations have been commercialized against widespread and life-threatening infections, including influenza (Inflexal® V) and hepatitis A (Epaxal®). However, many efforts have been made to develop a potent vaccine against HIV-1, HPV, and cancer mentioned below. Also, SARS-CoV-2 is an enveloped virus that vaccine project as transvac 2 is going on. In Table 2 , we described some virosome vaccine briefly.
Table 2.
A list of virosome based formulation.
| Target | Vaccine composition | Status | Dosage form | Ref |
|---|---|---|---|---|
| Respiratory Syncytial Virus (RSV) | Virosomes, HA, NA, RSV fusion proteins | Under GMP8 | IM | [58] |
| HIV-1 | Virosomes, HA, NA, P1, gp41, TLR7/81 | New GMP | Mucose | [57] |
| Hepatitis A | Virosomes, HA, NA, HAV2 | Marketed | Injection | [59] |
| Influenza | Virosomes HA, NA | Marketed | Injection | [53] |
| Plasmodium falciparum | Virosomes, AMA-13, CSP4, PfCyRPA5 | Phase I/II | Injection | [60,61] |
| Melanoma | Virosomes, Recombinant vaccinia virus encoding five melanoma epitopes, octo-mel-rVV6 | Phase I/II | Intradermal | [62] |
| SARS-CoV-2 | Virosomes HA, NA, s protein8 | Phase I/II | Injection |
Abbreviations: 1. Tool like receptor 7/8, 2. Inactivated hepatitis A virus, 3. Apical membrane antigen 1, 4. Circumsporozoite protein, 5. Plasmodium falciparum cysteine-rich protective antigens, 6. Three co-stimulatory molecules, 7. Spike protein, 8. Good manufacturing practice.
4.1. Influenza
Conventional influenza vaccine platforms protect the population against some highly pathogenic strains; however, adequate information elucidates that these products do not have enough protection against the future pandemic. Therefore, new vaccine technology development is essential to produce community immunity. Virosome based influenza vaccines are a novel FDA approved technology that mitigates viral morbidity and mortality rate [63].
Inflexal®V is the trivalent influenza virus virosome vaccine in which the formulation contains an inactivated form of two virus strains and one B virus strain with the influenza virus's specific antigen HA and NA subunits [52].
Like other virosomes preparation techniques, in influenza virosome development, the optimized amount of detergents has solubilized influenza viruses, their nucleocapsid was removed; consequently, IRIV naturally formed in the presence of viral lipids and glycoproteins. Phospholipids (PL), especially phosphatidylcholines (PC), attain in virosome reconstitutions. PC assigned 70% of virus lipid content that 30% achieved by envelope phospholipids originating from the influenza virus to provide NA and HA glycoproteins [64]. In Fig. 5 , we showed the schematic illustration of influenza reconstituted virosome.
Fig. 5.
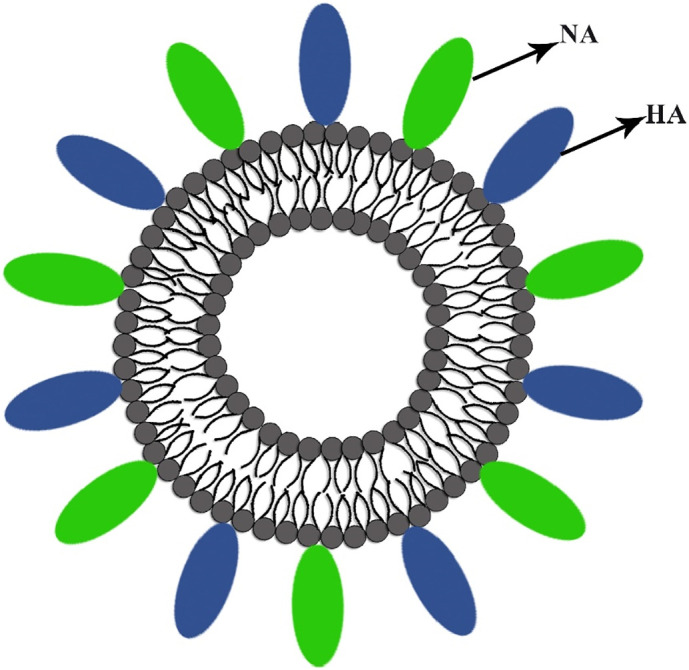
Schematic design of influenza virosome.
The NA has a tetramer structure consisting of four similar monomers, spherical subunits hydrophobically anchored into IRIV phospholipid membrane by a central stalk. NA function significantly affects the viral virulence and improves the action of virosomes. N-acetylneuraminic acid (sialic acid) from bound sugar residues reduces the host's mucus viscosity and facilitates the release of progeny viruses at the budding stage and access to epithelial cells. The HA epitopes comprised of HA1 and HA2 subunit played a crucial role in the virus-endosomal fusion and provoked cellular immunity. Besides, HA incorporation into the virosome membrane is confined to virosome-virosome fusion [65]. First, Inflexal®V has been produced by the Swiss Serum and Vaccine Institute, Berne, Switzerland, since 1997, which is now commercialized in over 20 countries worldwide by different trade names as Isiflu® V in Italy and as Viroflu® in the United Kingdom. Up to date, 18 clinical trials have been done on more than 2500 healthy volunteers reporting the safety and efficacy of Inflexal®V. This vaccine presented outstanding tolerability in all age groups and exceeded 10 million vaccine doses have been administered [66]. Inflexal®V eliminated the conventional influenza vaccine limitations, contained no thiomersal or formaldehyde in formulations, and reduced allergic reactions by the lowest OVA content. Producing stabilized vaccine formulation under varying storage conditions significantly improved vaccination safety and decreased preservation cost. Inflexal®V has been shown to maintain high levels of HA content for 24 months [67]. As WHO recommended, and due to the high mutation rate of influenza viruses that changes the amino acid composition of the HA and NA antigens and the mean diameter of virosome particle size, they will notify each season's new influenza strains [46,[68], [69], [70]].
4.2. Hepatitis A
Hepatitis A is a life-threatening disease caused by Hepatovirus A (HAV). HAV is an RNA virus surrounded by cubic or icosahedral capsid proteins of 27–32 nm diameter. This virus is stable at low pH, temperature and inactivated in heat above 85 °c. Vaccination in the high-risk population against HAV could provide universal immunization. Several types of vaccines developed for HAV as Havrix®, Vaqta®, Avaxim®, and Epaxel® that Epaxel® are the only aluminum-free product. Fig. 6 presented a schematic feature of the virosome used in Epaxel formulation. Epaxel® developed by virosome is decorated with inactivated HAV, while aluminum hydroxide adjuvant was used as the substrate for attachment of HAV inactivated virus in other vaccines. Aluminum hydroxide adjuvant declined local inflammatory responses. This potent viral vaccine provides more rapid immune responses with a higher seroconversion rate at day 14, accompanied by a higher geometric mean titer (GMT) [71]. Epaxel® has excellent tolerability and high immunogenicity, conferring the protection of at least 9–11 years in vaccinated individuals.
Fig. 6.
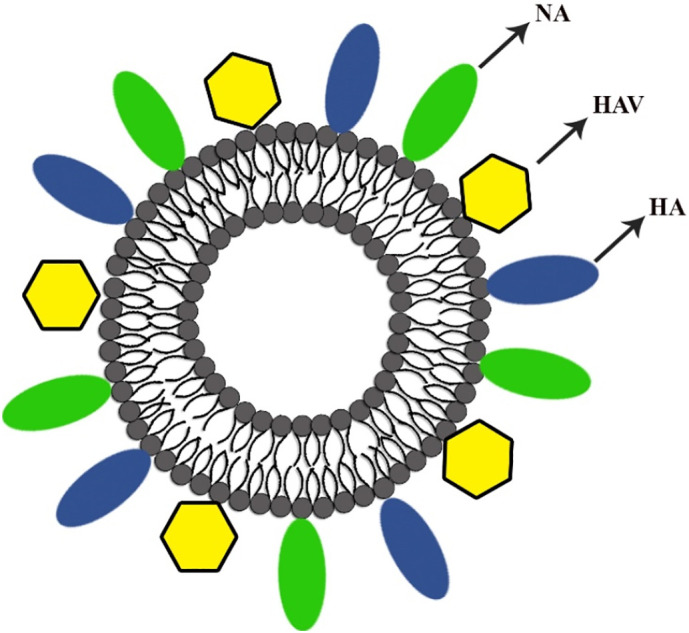
Schematic picture of virosomal vaccine for HAV.
Epaxel® preparation technique is similar to the influenza vaccine. The inactivated influenza virus is used for Epaxel® production. The reconstituted virosome consists of approximately 150 nm bilayer phospholipids, lecithin (phosphatidylcholine), and cephalin (phosphatidylethanolamine) with viral envelope antigens, including fusion proteins, HA, NA. Besides, the final structure surface decorated with formalin-inactivated HAV caused intrinsic adjuvant properties. Initially, inactivated HAV triggers B cell to proliferate and its progeny to differentiate into antibody-secreting cells. HA and NA facilitated the uptake of Epaxel® virosome to the immunocompetent cells, and sialic acid residues were abundantly expressed on dendritic cells (DCs) macrophages caused rapid cell uptake by endosomal-mediated pathway. It finally activated MHC-I, MHC-II, and lymphocytes (B, T helper, CTLs). Therefore, Epaxel® evoked both humoral and cell-mediated immunity [72].
4.3. HIV
Human Immunodeficiency Virus type 1 (HIV-1) is a lentiviral retrovirus causing AIDS. AIDS is a life-threatening and rapid contagious disease in which over 35 million deaths occur in the world from 70 million infected people [73,74].
HIV virosomal vaccines have acceptable results in the clinical phase Ι and are expected to be available in the market as soon as possible. This virosomal vaccine platform could be delivered by intramuscular, intradermal, or subcutaneous injections. Also, the mucosal delivery dosage form of the HIV virosome vaccine could provide potent immune responses. The high HIV virulence route is through mucosal tissue; therefore, the initial mucosal antibody production is the substantial defense against HIV pathogens [[75], [76], [77]]. In the study, sublingual dosage form vaccine confronting HIV developed physical robustness, particles, and antigen integrity and stability. Influenza enveloped viruses are used for virosome preparation, decorated with HIV-1 virulence antigens such as gp41, p1 peptides, and 3 m-052 adjuvants. Gp41 antigen facilitates host cell infection via interaction CD4 receptors on the immune component cell-like helper T cells and macrophages. Also, the P1 peptide improves the function and movement of protein epitopes. 3 m-052 adjuvants help develop long-lasting immunity confronting HIV-1 viruses, incorporated via 18 carbon fatty-acid chain into virosome phospholipid bilayer. 3 m-052 is a thermostable adjuvant that increased virosome membrane rigidity [78,79].
In another study, the thermostable HIV-1 virosomal vaccine produced by thermostable HIV-1 vaccine is composed of influenza enveloped virosome with HA, NA antigen epitopes, lecithin, cephalin, and other phospholipids that are reconstituted with gp41 virosome type1 and p1 virosome type2, both type of virosome engineered by addition of 3 m-052 adjuvants and toll-like receptor (TLR7/8) and trehalose sugar [80]. TLR7/8 function is similar to adjuvants and stimulated TNF, IFN, IL12 cytokine secretion, which induces dendritic cell maturation and expression of co-stimulatory molecules such as CD80, CD86. Finally, TLR7/8 reinforces T cell and B cell immune responses. Vaccination with the stable forms of the virosomal HIV-1 candidate vaccine should elicit relevant protective antibodies, even if the product has been stored accidentally for a few days or weeks at 40 °C or frozen during shipment [57,81,82].
4.4. HPV
The HPV is a sexually transmitted family virus with over 170 types that 40 types could transmit. HPV infection was assigned maximum prevalence among cervical cancer cases, which prevailed in cervical intraepithelial neoplasia (CIN) [83]. The studies in recent years were sustained on the efficacy of virosome based prophylactic vaccines for HPV.
E6 and E7 key viral oncogenes in HPV independently provided perpetuate infections in host cells via the effect on cell cycle checkpoints. Also, both of them have a synergistic effect on each other function. The presence of E6 and E7 in the cytosol of APCs evoke cellular responses. Virosome contains E6 and E7 protein fusing with the host cell membrane via endocytosis mediated pathway. Virosomes depleted their content into cell cytoplasm through endosomal fusion (pH 5). Studies showed that recombinant HPV16 E7 incorporated in fusion-active influenza virosome induced strong CTLs responses and prevented the outgrowth of an HPV16- transformed tumor. Additionally, immunization with E7-virosomes induced IgG antibody responses against E7 [84].
4.5. Viral virosome vaccines against cancer
Virosome therapeutic agents were developed more significantly in multimodal vehicles against advanced-stage cancer. Virosome is the desired carrier for immunogenic substances and chemotropic agent delivery in oncologic fields. Oncolytic viruses comprise natural and recombinant mutants that replicate in tumor cells and reinforce anti-tumor functions [85]. Virosome could make delivery tumor antigens, including Tumor-Specific Antigens (TSA) and Tumor-Associated Antigens (TAA). Virosome compression to the viral vector has much safety and is persuaded to be used. The HVJ; Sendai virus can serve as multimodal tools against cancer applications [86].
Several studies were mentioned on the virosome application in cancer therapy. Reconstituted influenza virus envelopes (virosomes) were used in several preclinical studies and clinical trials for cancer treatment, such as ovarian carcinoma (OVCAR-3). Viral HA membrane fusion activity was inhibited by PEG-derivatized lipids incorporation into the virosome membrane, and then FabP fragments of mAb 323/A3 (anti-epithelial glycoprotein-2) were conjugated to the distal end of PEG on the virosomes. This study suggested that influenza virosomes had desirable properties in cytosolic delivery [87]. Waelti et al. showed that the virosome densely covered with HA spikes, conjugated with HER-2/neu (p185her2) oncogene produced a new selective drug delivery system inhibiting tumor progression [88].
5. Recent progress on virosome-based vaccines for SARS-CoV-2
The COVID-19 pandemic caused the SARS-CoV-2 originated from Wuhan, China, in December 2019; however, several studies warned the first sign of disease in February 2019 [88]. Since the last report on 5 December 2020, more than 62 million cases have been reported in 210 countries, of which 1,480,000 have died.
SARS-CoV-2 is an enveloped spherical particle with a diameter of approximately 60–150 nm and a club-shaped spikes glycoprotein coating about 20 nm long. It belongs to the genus Betacoronavirus within the family Coronaviridae. SARS-CoV-2 has an extensive positive-strand RNA genome, comprised of ~3 k nucleotides [89]. Weak, reduced proofreading activity of viral RNA polymerases leads to a high mutation rate. Consequently, RNA viruses are prone to evolve resistance to drugs and to escape from immune surveillance. Up to date, a variant strain of SARS-CoV-2 has been identified, and recent studies reported more than 40 types of them that transmitted pattern, and clinical symptoms have been localized to each region. All the above mentioned, and the development of an appropriate COVID 19 vaccine remains a critical challenge [90].
The coronaviral genome encodes structural proteins that playing a vital role in producing infectious virions and the severity of the disease. CoV S protein is one of the critical components in receptor binding and virus-host cell fusion, and it is also the primary target for neutralizing antibodies and vaccine design [91,92]. Recent studies have shown that SARS-CoV E protein is another virulence factor that can contribute to therapeutic targets. Some coronaviruses also contain a hem agglutinin-esterase protein (HE). The lectin domain of HE protein helps the attachment of virus-host cells. Therefore, specific surface antigens and phospholipids of SARS-CoV2 were purified, and they can be used in virosome vaccine production. Virosome particles are a promising vehicle in SARS-CoV2 vaccine development. Transvac 2 project is a virosomal- based vaccine in progress by European MI Matrix Company.
Coronaviruses infected host cells via binding to Angiotensin-converting enzyme 2 (ACE2) and activated the endocytosis pathway [89]. Besides, SARS-CoV chiefly contains basic amino acid residue that is intensified virus membrane fusion by electrostatic attraction with the heparan sulfate proteoglycans present on the surface of target cells. SARS-CoV entry into the target cell via endosome and the low pH-dependent mechanism is essential for cytoplasm infectious.
Coronaviruses replicate in the cytoplasm, where viral RNA is synthesized in a specific, flask-shaped compartment surrounded by a double membrane. These changes include the formation of double-membrane vesicles, the presence of nucleocapsid inclusions, and granulations in the cytoplasm [93,94].
6. Regulations for virosome-based nano vaccines
Virosome based vaccine development with long-term stability at room temperature is highly desirable and is the main purpose of the MACIVIVA project. Characteristics of virosomes based vaccines include Quality by Design (QbD) ultimately must be established based on regulatory agencies such as European Medicines Agency (EMA), US Food and Drug Administration (FDA), International Council for Harmonization (ICH), and the other valid regulatory organization step by step. In the pharmaceutical manufacturing context, QbD represents a quality vaccine formulation containing active substances, excipients, and other components. Predominantly, QbD is determined by principles of critical quality attributes (CQAs) and quality target product profile (QTPP).
CQAs mainly include optimization process of size, polydispersity index (PDI), shape, stability, Z-potential, the surface to volume ratio, morphology, surface decoration, structural organization, stimuli responsiveness, thermodynamic properties, which are critical to guarantee the safety and efficacy of the nanoformulation. For example, virosome size and concentration must be evaluated by standard laboratory equipment. Besides, virosome-based vaccine formulation must be reproducible, cost-effective, and high output. Then, the interaction of the CQAs parameter with patients' needs must be evaluated. The QTPP was determined by the vaccine performance, indication, and the modality of administration. Moreover, clinical evaluation of vaccines should be adopted by the international regulatory of WHO guidelines [95].
Manufacturing aspects of nanovaccine must exactly be evaluated. For example, sterilization is not required in nasal spray vaccine dosage form production, and the control of microbial content is sufficient [96]. Vaccine storage conditions should be accurately regulated based on vaccine components. For example, freeze exposure destroys vaccines contain aluminum salt. Temperature labeling, shipping standards, and IATA time should be inserted on vaccine packages.
At the end, nanovaccine production should be classified and packaged based on World Health Organization (WHO) Guidelines on the international packaging and shipping of vaccines [97].
7. Perspectives
Virosome is biomimetic, and FDA approved nanocarrier for pharmaceutical approaches. This biodegradable, biocompatible, nontoxic biomolecule entailed high safety profile in vaccine development. It can be applied with almost all administration routes and combined with other adjuvants comfortably. According to Table 2, two successful vaccines based on virosome formulation were launched into the market, and many products are in the preclinical and clinical stage. The virosome-based vaccine induces potent humoral and cellular immune responses critical in confronting life-threatening viral diseases. In many instances, RNA viruses are accompanied by high mutation rates. Virosomes are versatile carriers that could develop multi antigens epitopes against all the strains of RNA viruses. Virosome cannot replicate and incorporate into the host genome. Thus, this nanovaccine with an excellent safety profile provides a new horizon in vaccine development and eliminates medical complications and concerns about next-generation products.
However, the rapid disintegration of virosome formulations is a major drawback for pharmaceutical usage ignored by stability optimization. Thermostable virosome-based vaccine by mucosal route administration is a bioinspiration compound that could revolutionize vaccination patterns in developing countries.
8. Conclusion
Virosome is an auspicious vehicle to develop vigorous nanovaccines. The virosome preparation method is relatively simple and possesses versatile delivery routes. A key feature of virosomes is the surface organization similarity to the native virions that could evoke strong immune responses. To date, many virosome products are approved by administration authorities in different countries and commercialized. Especial efforts should be put on virosome-based viral vaccine improvements and further tests to accelerate the approval process. Transvac 2 is a new virosomal vaccine designed against sarscov2 viruses that are in phase ΙΙ clinical trials. The transvac 2 vaccine is an innovative compound expected to be helpful in the recent viral pandemic.
Acknowledgment
The authors would like to offer their special thanks to Ms.sedigheh babaei for editing the figures of the manuscript.
References
- 1.Rauch S., Jasny E., Schmidt K.E., Petsch B. Frontiers in Immunology 9(1963) 2018. New vaccine technologies to combat outbreak situations. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tu Y.-F., Chien C.-S., Yarmishyn A.A., Lin Y.-Y., Luo Y.-H., Lin Y.-T., Lai W.-Y., Yang D.-M., Chou S.-J., Yang Y.-P. A review of SARS-CoV-2 and the ongoing clinical trials. Int. J. Mol. Sci. 2020;21(7):2657. doi: 10.3390/ijms21072657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H., Wu Y., Zhang L., Yu Z., Fang M., Yu T., Wang Y., Pan S., Zou X., Yuan S., Shang Y. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir. Med. 2020;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Delany I., Rappuoli R., De Gregorio E. Vaccines for the 21st century. EMBO molecular medicine. 2014;6(6):708–720. doi: 10.1002/emmm.201403876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pati R., Shevtsov M., Sonawane A. Nanoparticle vaccines against infectious diseases. Front. Immunol. 2018;9(2224) doi: 10.3389/fimmu.2018.02224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Medjitna T., Stadler C., Bruckner L., Griot C., Ottiger H. DNA vaccines: safety aspect assessment and regulation. Dev. Biol. 2006;126:261–270. [PubMed] [Google Scholar]
- 7.Lee N.-H., Lee J.-A., Park S.-Y., Song C.-S., Choi I.-S., Lee J.-B. A review of vaccine development and research for industry animals in Korea. Clinical and experimental vaccine research. 2012;1(1):18–34. doi: 10.7774/cevr.2012.1.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Criscuolo E., Caputo V., Diotti R.A., Sautto G.A., Kirchenbaum G.A., Clementi N. Alternative methods of vaccine delivery: an overview of edible and intradermal vaccines. J Immunol Res. 2019;8:303–648. doi: 10.1155/2019/8303648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Emadi F., Emadi A., Gholami A. A comprehensive insight towards pharmaceutical aspects of graphene nanosheets. Curr. Pharm. Biotechnol. 2020;21(11):1016–1027. doi: 10.2174/1389201021666200318131422. [DOI] [PubMed] [Google Scholar]
- 10.Borzouyan Dastjerdi M., Amini A., Nazari M., Cheng C., Benson V., Gholami A., Ghasemi Y. Novel versatile 3D bio-scaffold made of natural biocompatible hagfish exudate for tissue growth and organoid modeling. Int. J. Biol. Macromol. 2020;158:894–902. doi: 10.1016/j.ijbiomac.2020.05.024. [DOI] [PubMed] [Google Scholar]
- 11.Kennedy R.B., Ovsyannikova I.G., Palese P., Poland G.A. Current challenges in vaccinology. Front. Immunol. 2020;11(1181) doi: 10.3389/fimmu.2020.01181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vijayan V., Mohapatra A., Uthaman S., Park I.-K. Recent advances in nanovaccines using biomimetic immunomodulatory materials. Pharmaceutics. 2019;11(10):534. doi: 10.3390/pharmaceutics11100534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olive C., Sun H.K., Ho M.F., Dyer J., Horváth A., Toth I., Good M.F. Intranasal administration is an effective mucosal vaccine delivery route for self-adjuvanting lipid core peptides targeting the group A streptococcal M protein. J. Infect. Dis. 2006;194(3):316–324. doi: 10.1086/505580. [DOI] [PubMed] [Google Scholar]
- 14.Hajizade A., Ebrahimi F., Salmanian A.-H., Arpanaei A., Amani J. Nanoparticles in Vaccine Development. Journal of Applied Biotechnology Reports. 2014;1(4):125–134. [Google Scholar]
- 15.Sulczewski F.B., Liszbinski R.B., Romão P.R., Junior L.C.R. Nanoparticle vaccines against viral infections. Arch. Virol. 2018;163(9):2313–2325. doi: 10.1007/s00705-018-3856-0. [DOI] [PubMed] [Google Scholar]
- 16.Thomas C., Rawat A., Hope-Weeks L., Ahsan F. Aerosolized PLA and PLGA nanoparticles enhance humoral, mucosal and cytokine responses to hepatitis B vaccine. Mol. Pharm. 2011;8(2):405–415. doi: 10.1021/mp100255c. [DOI] [PubMed] [Google Scholar]
- 17.López-Sagaseta J., Malito E., Rappuoli R., Bottomley M.J. Self-assembling protein nanoparticles in the design of vaccines. Computational and Structural Biotechnology Journal. 2016;14:58–68. doi: 10.1016/j.csbj.2015.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qi M., Zhang X.-E., Sun X., Zhang X., Yao Y., Liu S., Chen Z., Li W., Zhang Z., Chen J., Cui Z. 14(13) 2018. Influenza Nanovaccines: Intranasal nanovaccine Confers Homo- and Hetero-subtypic Influenza Protection (Small 13/2018), Small (Weinheim an der Bergstrasse, Germany) pp. 1856–1870. [DOI] [PubMed] [Google Scholar]
- 19.Gomes A.C., Mohsen M., Bachmann M.F. Harnessing nanoparticles for immunomodulation and vaccines. Vaccines. 2017;5(1):6. doi: 10.3390/vaccines5010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garg H., Mehmetoglu-Gurbuz T., Joshi A. Virus Like Particles (VLP) as multivalent vaccine candidate against Chikungunya, Japanese Encephalitis, Yellow Fever and Zika Virus. Sci. Rep. 2020;10(1):17–40. doi: 10.1038/s41598-020-61103-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Urakami A., Sakurai A., Ishikawa M., Yap M.L., Flores-Garcia Y., Haseda Y., Aoshi T., Zavala F.P., Rossmann M.G., Kuno S., Ueno R., Akahata W. Development of a novel virus-like particle vaccine platform that mimics the immature form of Alphavirus. Clin. Vaccine Immunol. 2017;24(7):17–90. doi: 10.1128/CVI.00090-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mousavi S.M., Zarei M., Hashemi S.A., Ramakrishna S., Chiang W.-H., Lai C.W., Gholami A. Gold nanostars-diagnosis, bioimaging and biomedical applications. Drug Metab. Rev. 2020;52(2):299–318. doi: 10.1080/03602532.2020.1734021. [DOI] [PubMed] [Google Scholar]
- 23.Gholami A., Mousavi S.M., Hashemi S.A., Ghasemi Y., Chiang W.-H., Parvin N. Current trends in chemical modifications of magnetic nanoparticles for targeted drug delivery in cancer chemotherapy. Drug Metab. Rev. 2020;52(1):205–224. doi: 10.1080/03602532.2020.1726943. [DOI] [PubMed] [Google Scholar]
- 24.Climent N., García I., Marradi M., Chiodo F., Miralles L., Maleno M.J., Gatell J.M., García F., Penadés S., Plana M. Loading dendritic cells with gold nanoparticles (GNPs) bearing HIV-peptides and mannosides enhance HIV-specific T cell responses, Nanomedicine. Nanotechnology, Biology and Medicine. 2018;14(2):339–351. doi: 10.1016/j.nano.2017.11.009. [DOI] [PubMed] [Google Scholar]
- 25.Wang N., Chen M., Wang T. Liposomes used as a vaccine adjuvant-delivery system: from basics to clinical immunization. J. Control. Release. 2019;303:130–150. doi: 10.1016/j.jconrel.2019.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.He H., Yuan D., Wu Y., Cao Y. Pharmacokinetics and pharmacodynamics modeling and simulation systems to support the development and regulation of liposomal drugs. Pharmaceutics. 2019;11(3):110. doi: 10.3390/pharmaceutics11030110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Facciolà A., Visalli G., Laganà P., La Fauci V., Squeri R., Pellicanò G.F., Nunnari G., Trovato M., Di Pietro A. The new era of vaccines: the “nanovaccinology”. Eur. Rev. Med. Pharmacol. Sci. 2019;23(16):7163–7182. doi: 10.26355/eurrev_201908_18763. [DOI] [PubMed] [Google Scholar]
- 28.Zhao L., Zhu Z., Ma L., Li Y. O/W nanoemulsion as an adjuvant for an inactivated H3N2 influenza vaccine: based on particle properties and mode of carrying. Int. J. Nanomedicine. 2020;15:2071–2083. doi: 10.2147/IJN.S232677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kole S., Qadiri S.S.N., Shin S.-M., Kim W.-S., Lee J., Jung S.-J. PLGA encapsulated inactivated-viral vaccine: formulation and evaluation of its protective efficacy against viral haemorrhagic septicaemia virus (VHSV) infection in olive flounder (Paralichthys olivaceus) vaccinated by mucosal delivery routes. Vaccine. 2019;37(7):973–983. doi: 10.1016/j.vaccine.2018.12.063. [DOI] [PubMed] [Google Scholar]
- 30.Olczak P., Roden R.B.S. Progress in L2-based prophylactic vaccine development for protection against diverse human papillomavirus genotypes and associated diseases. Vaccines. 2020;8(4):568. doi: 10.3390/vaccines8040568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Touzé A., Gaitan J., Arnold F., Cazal R., Fleury M.J., Combelas N., Sizaret P.-Y., Guyetant S., Maruani A., Baay M., Tognon M., Coursaget P. Generation of Merkel Cell Polyomavirus (MCV)-like Particles and their application to detection of MCV antibodies. J. Clin. Microbiol. 2010;48(5):1767–1770. doi: 10.1128/JCM.01691-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duinkerken S., Horrevorts S.K., Kalay H., Ambrosini M., Rutte L., de Gruijl T.D., Garcia-Vallejo J.J., van Kooyk Y. Glyco-dendrimers as intradermal anti-tumor vaccine targeting multiple skin DC subsets. Theranostics. 2019;9(20):5797–5809. doi: 10.7150/thno.35059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.He Q., Mitchell A., Morcol T., Bell S.J. Calcium phosphate nanoparticles induce mucosal immunity and protection against herpes simplex virus type 2. Clin. Diagn. Lab. Immunol. 2002;9(5):1021–1024. doi: 10.1128/CDLI.9.5.1021-1024.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tao W., Ziemer K.S., Gill H.S. Gold nanoparticle–M2e conjugate coformulated with CpG induces protective immunity against influenza A virus. Nanomedicine. 2014;9(2):237–251. doi: 10.2217/nnm.13.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li P., Luo Z., Liu P., Gao N., Zhang Y., Pan H., Liu L., Wang C., Cai L., Ma Y. Bioreducible alginate-poly(ethylenimine) nanogels as an antigen-delivery system robustly enhance vaccine-elicited humoral and cellular immune responses. J. Control. Release. 2013;168(3):271–279. doi: 10.1016/j.jconrel.2013.03.025. [DOI] [PubMed] [Google Scholar]
- 36.Tian Y., Wang H., Liu Y., Mao L., Chen W., Zhu Z., Liu W., Zheng W., Zhao Y., Kong D., Yang Z., Zhang W., Shao Y., Jiang X. A peptide-based nanofibrous hydrogel as a promising DNA nanovector for optimizing the efficacy of HIV vaccine. Nano Lett. 2014;14(3):1439–1445. doi: 10.1021/nl404560v. [DOI] [PubMed] [Google Scholar]
- 37.Kalra N., Dhanya V., Saini V., Jeyabalan G. Virosomes: as a drug delivery carrier. American Journal of Advanced Drug Delivery. 2013;1(1):29–35. [Google Scholar]
- 38.Amacker M., Engler O., Kammer A.R., Vadrucci S., Oberholzer D., Cerny A., Zurbriggen R. Peptide-loaded chimeric influenza virosomes for efficient in vivo induction of cytotoxic T cells. Int. Immunol. 2005;17(6):695–704. doi: 10.1093/intimm/dxh249. [DOI] [PubMed] [Google Scholar]
- 39.Wang Z.B., Xu J. Better adjuvants for better vaccines: progress in adjuvant delivery systems, modifications, and adjuvant-antigen codelivery. Vaccines. 2020;8(1) doi: 10.3390/vaccines8010128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huckriede A., Bungener L., Stegmann T., Daemen T., Medema J., Palache A.M., Wilschut J. The virosome concept for influenza vaccines. Vaccine. 2005;23:S26–S38. doi: 10.1016/j.vaccine.2005.04.026. [DOI] [PubMed] [Google Scholar]
- 41.Qian C., Liu X., Xu Q., Wang Z., Chen J., Li T., Zheng Q., Yu H., Gu Y., Li S., Xia N. Recent progress on the versatility of virus-like particles. Vaccines. 2020;8(1) doi: 10.3390/vaccines8010139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cech P.G., Aebi T., Abdallah M.S., Mpina M., Machunda E.B., Westerfeld N., Stoffel S.A., Zurbriggen R., Pluschke G., Tanner M., Daubenberger C., Genton B., Abdulla S. Virosome-formulated plasmodium falciparum AMA-1 & CSP derived peptides as malaria vaccine: randomized phase 1b trial in semi-immune adults & children. PLoS One. 2011;6(7):e22273. doi: 10.1371/journal.pone.0022273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scheule R.K. Novel preparation of functional Sindbis virosomes. Biochemistry. 1986;25(15):4223–4232. doi: 10.1021/bi00363a009. [DOI] [PubMed] [Google Scholar]
- 44.Bungener L., Huckriede A., de Mare A., de Vries-Idema J., Wilschut J., Daemen T. Virosome-mediated delivery of protein antigens in vivo: efficient induction of class I MHC-restricted cytotoxic T lymphocyte activity. Vaccine. 2005;23(10):1232–1241. doi: 10.1016/j.vaccine.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 45.Kaneda Y. Virosomes: evolution of the liposome as a targeted drug delivery system. Adv. Drug Deliv. Rev. 2000;43(2):197–205. doi: 10.1016/s0169-409x(00)00069-7. [DOI] [PubMed] [Google Scholar]
- 46.P. Schoen, R. Bron, J. Wilschut, Delivery of foreign substances to cells mediated by fusion-active reconstituted influenza virus envelopes (Virosomes), Journal of Liposome Research 3 (2008) 767–792.
- 47.Kaneda Y., Nakajima T., Nishikawa T., Yamamoto S., Ikegami H., Suzuki N., Nakamura H., Morishita R., Kotani H. Hemagglutinating virus of Japan (HVJ) envelope vector as a versatile gene delivery system. Mol. Ther. 2002;6(2):219–226. doi: 10.1006/mthe.2002.0647. [DOI] [PubMed] [Google Scholar]
- 48.Pöltl-Frank F., Zurbriggen R., Helg A., Stuart F., Robinson J., Glück R., Pluschke G. Use of reconstituted influenza virus virosomes as an immunopotentiating delivery system for a peptide-based vaccine. Clin. Exp. Immunol. 1999;117(3):496–503. doi: 10.1046/j.1365-2249.1999.00989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stegmann T., Kamphuis T., Meijerhof T., Goud E., de Haan A., Wilschut J. Lipopeptide-adjuvanted respiratory syncytial virus virosomes: a safe and immunogenic non-replicating vaccine formulation. Vaccine. 2010;28(34):5543–5550. doi: 10.1016/j.vaccine.2010.06.041. [DOI] [PubMed] [Google Scholar]
- 50.Kammer A.R., Amacker M., Rasi S., Westerfeld N., Gremion C., Neuhaus D., Zurbriggen R. A new and versatile virosomal antigen delivery system to induce cellular and humoral immune responses. Vaccine. 2007;25(41):7065–7074. doi: 10.1016/j.vaccine.2007.07.052. [DOI] [PubMed] [Google Scholar]
- 51.Jazayeri S.D., Poh C.L. Development of universal influenza vaccines targeting conserved viral proteins. Vaccines. 2019;7(4):169. doi: 10.3390/vaccines7040169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Otten G., Matassa V., Ciarlet M., Leav B. A phase 1, randomized, observer blind, antigen and adjuvant dosage finding clinical trial to evaluate the safety and immunogenicity of an adjuvanted, trivalent subunit influenza vaccine in adults ≥65 years of age. Vaccine. 2020;38(3):578–587. doi: 10.1016/j.vaccine.2019.10.058. [DOI] [PubMed] [Google Scholar]
- 53.Summerfield A., McCullough K.C. Dendritic cells in innate and adaptive immune responses against influenza virus. Viruses. 2009;1(3):1022–1034. doi: 10.3390/v1031022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bhardwaj P., Bhatia E., Sharma S., Ahamad N., Banerjee R. Advancements in prophylactic and therapeutic nanovaccines. Acta Biomater. 2020;108:1–21. doi: 10.1016/j.actbio.2020.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu H., Tu Z., Feng F., Shi H., Chen K., Xu X. Virosome, a hybrid vehicle for efficient and safe drug delivery and its emerging application in cancer treatment. Acta Pharma. 2015;65(2):105–116. doi: 10.1515/acph-2015-0019. [DOI] [PubMed] [Google Scholar]
- 56.Bernasconi V., Norling K., Bally M., Höök F., Lycke N.Y. Mucosal vaccine development based on liposome technology. J Immunol Res. 2016;(2016):5482087. doi: 10.1155/2016/5482087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Amacker M., Smardon C., Mason L., Sorrell J., Jeffery K., Adler M., Bhoelan F., Belova O., Spengler M., Punnamoottil B., Schwaller M., Bonduelle O., Combadière B., Stegmann T., Naylor A., Johnson R., Wong D., Fleury S. New GMP manufacturing processes to obtain thermostable HIV-1 gp41 virosomes under solid forms for various mucosal vaccination routes. npj Vaccines. 2020;5(1):41. doi: 10.1038/s41541-020-0190-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lederhofer J., van Lent J., Bhoelan F., Karneva Z., de Haan A., Wilschut J.C., Stegmann T. Development of a Virosomal RSV vaccine containing 3D-PHAD® adjuvant: formulation, composition, and long-term stability. Pharm. Res. 2018;35(9) doi: 10.1007/s11095-018-2453-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hatz C., van der Ploeg R., Beck B.R., Frösner G., Hunt M., Herzog C. Successful memory response following a booster dose with a virosome-formulated hepatitis a vaccine delayed up to 11 years. Clinical and vaccine immunology : CVI. 2011;18(5):885–887. doi: 10.1128/CVI.00358-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thompson F.M., Porter D.W., Okitsu S.L., Westerfeld N., Vogel D., Todryk S., Poulton I., Correa S., Hutchings C., Berthoud T., Dunachie S., Andrews L., Williams J.L., Sinden R., Gilbert S.C., Pluschke G., Zurbriggen R., Hill A.V.S. Evidence of blood stage efficacy with a virosomal malaria vaccine in a phase IIa clinical trial. PLoS One. 2008;3(1):1493. doi: 10.1371/journal.pone.0001493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pluschke G., Tamborrini M. Development of a virosomal malaria vaccine candidate: from synthetic peptide design to clinical concept validation. Futur. Virol. 2012;7(8):779–790. [Google Scholar]
- 62.Adamina M., Weber W.P., Rosenthal R., Schumacher R., Zajac P., Guller U., Frey D.M., Oertli D., Zuber M., Heberer M., Spagnoli G.C. Heterologous prime-boost immunotherapy of melanoma patients with Influenza virosomes, and recombinant Vaccinia virus encoding 5 melanoma epitopes and 3 co-stimulatory molecules. A multi-centre phase I/II open labeled clinical trial. Contemporary Clinical Trials. 2008;29(2):165–181. doi: 10.1016/j.cct.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 63.Khalaj-Hedayati A., Chua C.L.L., Smooker P., Lee K.W. Nanoparticles in influenza subunit vaccine development: Immunogenicity enhancement. Influenza Other Respir. Viruses. 2019;14(1):92–101. doi: 10.1111/irv.12697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Abdoli A., Soleimanjahi H., Tavassoti Kheiri M., Jamali A., Mazaheri V., Abdollahpour Alitappeh M. An H1-H3 chimeric influenza virosome confers complete protection against lethal challenge with PR8 (H1N1) and X47 (H3N2) viruses in mice. Pathogens and Disease. 2014;72(3):197–207. doi: 10.1111/2049-632X.12206. [DOI] [PubMed] [Google Scholar]
- 65.Huckriede A., Bungener L., ter Veer W., Holtrop M., Daemen T., Palache A.M., Wilschut J. Influenza virosomes: combining optimal presentation of hemagglutinin with immunopotentiating activity. Vaccine. 2003;21(9):925–931. doi: 10.1016/s0264-410x(02)00542-x. [DOI] [PubMed] [Google Scholar]
- 66.Soema P.C., Kompier R., Amorij J.-P., Kersten G.F.A. Current and next generation influenza vaccines: formulation and production strategies. Eur. J. Pharm. Biopharm. 2015;94:251–263. doi: 10.1016/j.ejpb.2015.05.023. [DOI] [PubMed] [Google Scholar]
- 67.Waelti E.R., Glück R. Delivery to cancer cells of antisense L- myc oligonucleotides incorporated in fusogenic, cationic-lipid-reconstituted influenza-virus envelopes (cationic virosomes) Int. J. Cancer. 1998;77(5):728–733. doi: 10.1002/(sici)1097-0215(19980831)77:5<728::aid-ijc11>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 68.Calzas C., Chevalier C. Innovative mucosal vaccine formulations against influenza a virus infections. Front. Immunol. 2019;10(1605) doi: 10.3389/fimmu.2019.01605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Glück R., Mischler R., Finkel B., Que J.U., Cryz S.J., Scarpa B. Immunogenicity of new virosome influenza vaccine in elderly people. Lancet. 1994;344(8916):160–163. doi: 10.1016/s0140-6736(94)92758-8. [DOI] [PubMed] [Google Scholar]
- 70.Mischler R., Metcalfe I.C. Inflexal®V a trivalent virosome subunit influenza vaccine: production. Vaccine. 2002;20:B17–B23. doi: 10.1016/s0264-410x(02)00512-1. [DOI] [PubMed] [Google Scholar]
- 71.Hatz C., van der Ploeg R., Beck B.R., Frösner G., Hunt M., Herzog C. Successful memory response following a booster dose with a virosome-formulated hepatitis a vaccine delayed up to 11 years. Clin. Vaccine Immunol. 2011;18(5):885–887. doi: 10.1128/CVI.00358-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bovier P.A. Epaxal: a virosomal vaccine to prevent hepatitis a infection. Expert review of vaccines. 2008;7(8):1141–1150. doi: 10.1586/14760584.7.8.1141. [DOI] [PubMed] [Google Scholar]
- 73.Brouwer P.J.M., Sanders R.W. Presentation of HIV-1 envelope glycoprotein trimers on diverse nanoparticle platforms. Curr. Opin. HIV AIDS. 2019;14(4):302–308. doi: 10.1097/COH.0000000000000549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gao Y., McKay P.F., Mann J.F.S. Advances in HIV-1 vaccine development. Viruses. 2018;10(4):167. doi: 10.3390/v10040167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ebensen T., Debarry J., Pedersen G.K., Blazejewska P., Weissmann S., Schulze K., McCullough K.C., Cox R.J., Guzmán C.A. Mucosal administration of Cycle-Di-nucleotide-adjuvanted virosomes efficiently induces protection against Influenza H5N1 in mice. Front. Immunol. 2017;8(1223) doi: 10.3389/fimmu.2017.01223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Duchemin M., Tudor D., Cottignies-Calamarte A., Bomsel M. Antibody-dependent cellular phagocytosis of HIV-1-infected cells is efficiently triggered by IgA targeting HIV-1 envelope subunit gp41. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.01141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bomsel M., Tudor D., Drillet A.-S., Alfsen A., Ganor Y., Roger M.-G., Mouz N., Amacker M., Chalifour A., Diomede L., Devillier G., Cong Z., Wei Q., Gao H., Qin C., Yang G.-B., Zurbriggen R., Lopalco L., Fleury S. Immunization with HIV-1 gp41 subunit virosomes induces mucosal antibodies protecting nonhuman primates against vaginal SHIV challenges. Immunity. 2011;34(2):269–280. doi: 10.1016/j.immuni.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 78.Mayorga O., Bühler S., Jaeger V.K., Bally S., Hatz C., Frösner G., Protzer U., Van Damme P., Egger M., Herzog C. Single-dose hepatitis a immunization: 7.5-year observational pilot study in Nicaraguan children to assess protective effectiveness and humoral immune memory response. J. Infect. Dis. 2016;214(10):1498–1506. doi: 10.1093/infdis/jiw411. [DOI] [PubMed] [Google Scholar]
- 79.Wong Y.T., Smardon C., Shirkhani K., Amacker M., Fleury S., Stegmann A.J.H. Oral dispersible vaccine comprising virosomes. US Patent App. 2020;16(/696):752. [Google Scholar]
- 80.Pastorino B., Baronti C., Gould E.A., Charrel R.N., de Lamballerie X. Effect of chemical stabilizers on the thermostability and infectivity of a representative panel of freeze dried viruses. PLoS One. 2015;10(4) doi: 10.1371/journal.pone.0118963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ruprecht R.M., Lakhashe S.K. Antibody-mediated immune exclusion of HIV. Curr. Opin. HIV AIDS. 2017;12(3):222–228. doi: 10.1097/COH.0000000000000369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kozlowski P.A., Aldovini A. Mucosal vaccine approaches for prevention of HIV and SIV transmission. Curr. Immunol. Rev. 2019;15(1):102–122. doi: 10.2174/1573395514666180605092054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.C.K. Chan, G. Aimagambetova, T. Ukybassova, K. Kongrtay, A. Azizan, Human papillomavirus infection and cervical cancer: epidemiology, screening, and vaccination—review of current perspectives, Journal of Oncology 2019(3257939) (2019). [DOI] [PMC free article] [PubMed]
- 84.Bungener L., de Mare A., de Vries-Idema J., Sehr P., van der Zee A., Wilschut J., Daemen T. A virosomal immunization strategy against cervical cancer and pre-malignant cervical disease. Antivir. Ther. 2006;11(6):717–727. [PubMed] [Google Scholar]
- 85.Angel J., Chaperot L., Molens J.-P., Mezin P., Amacker M., Zurbriggen R., Grichine A., Plumas J. Virosome-mediated delivery of tumor antigen to plasmacytoid dendritic cells. Vaccine. 2007;25(19):3913–3921. doi: 10.1016/j.vaccine.2007.01.101. [DOI] [PubMed] [Google Scholar]
- 86.Saga K., Kaneda Y. Virosome presents multimodel cancer therapy without viral replication. Biomed. Res. Int. 2013;(2013):764706. doi: 10.1155/2013/764706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mastrobattista E., Schoen P., Wilschut J., Crommelin D.J.A., Storm G. Targeting influenza virosomes to ovarian carcinoma cells. FEBS Lett. 2001;509(1):71–76. doi: 10.1016/s0014-5793(01)03112-x. [DOI] [PubMed] [Google Scholar]
- 88.Waelti E., Wegmann N., Schwaninger R., Wetterwald A., Wingenfeld C., Rothen-Rutishauser B., Gimmi C.D. Targeting HER-2/neu with Antirat Neu virosomes for cancer therapy. Cancer Res. 2002;62(2):437–444. [PubMed] [Google Scholar]
- 89.Hashemi S.A., Golab Behbahan N.G., Bahrani S., Mousavi S.M., Gholami A., Ramakrishna S., Firoozsani M., Moghadami M., Lankarani K.B., Omidifar N. Ultra-sensitive viral glycoprotein detection NanoSystem toward accurate tracing SARS-CoV-2 in biological/non-biological media. Biosens. Bioelectron. 2021;171:112731. doi: 10.1016/j.bios.2020.112731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mousavi S.M., Hashemi S.A., Parvin N., Gholami A., Ramakrishna S., Omidifar N., Moghadami M., Chiang W.-H., Mazraedoost S. Recent biotechnological approaches for treatment of novel COVID-19: from bench to clinical trial. Drug Metab. Rev. 2020:1–30. doi: 10.1080/03602532.2020.1845201. [DOI] [PubMed] [Google Scholar]
- 91.Kuate S., Cinatl J., Doerr H.W., Überla K. Exosomal vaccines containing the S protein of the SARS coronavirus induce high levels of neutralizing antibodies. Virology. 2007;362(1):26–37. doi: 10.1016/j.virol.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Coleman C.M., Liu Y.V., Mu H., Taylor J.K., Massare M., Flyer D.C., Glenn G.M., Smith G.E., Frieman M.B. Purified coronavirus spike protein nanoparticles induce coronavirus neutralizing antibodies in mice. Vaccine. 2014;32(26):3169–3174. doi: 10.1016/j.vaccine.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.White J.M., Whittaker G.R. Fusion of enveloped viruses in endosomes. Traffic. 2016;17(6):593–614. doi: 10.1111/tra.12389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gupta T., Gupta S.K. Potential adjuvants for the development of a SARS-CoV-2 vaccine based on experimental results from similar coronaviruses. Int. Immunopharmacol. 2020;86 doi: 10.1016/j.intimp.2020.106717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.W.H. Organization . World Health Organization; Geneva: 2004. Guidelines on Clinical Evaluation of vaccines: Regulatory Expectations, WHO Expert Committee on Biological Standardization. Fifty-second Report; pp. 035–101. [Google Scholar]
- 96.Dacoba T.G., Ruiz-Gatón L., Benito A., Klein M., Dupin D., Luo M., Menta M., Teijeiro-Osorio D., Loinaz I., Alonso M.J., Crecente-Campo J. Technological challenges in the preclinical development of an HIV nanovaccine candidate. Drug Delivery and Translational Research. 2020;10(3):621–634. doi: 10.1007/s13346-020-00721-8. [DOI] [PubMed] [Google Scholar]
- 97.W.H. Organization . World Health Organization; 2005. Guidelines on the International Packaging and Shipping of Vaccines. [Google Scholar]