Abstract
Trauma is one of the leading causes of death worldwide. Approximately two-thirds of trauma patients have thoracic injuries. Nonvascular injury to the chest is most common; however, while vascular injuries to the chest make up a small minority of injuries in thoracic trauma, these injuries are most likely to require intervention by interventional radiology (IR). IR plays a vital role, with much to offer, in the evaluation and management of patients with both vascular and nonvascular thoracic trauma; in many cases, IR treatments obviate the need for these patients to go to the operating room. This article reviews the role of IR in the treatment of vascular an nonvascular traumatic thoracic injuries.
Keywords: trauma, hemorrhage, thoracic, pneumothorax, interventional radiology
Trauma is one of the leading causes of death worldwide. Approximately two-thirds of trauma patients have thoracic injuries. 1 Nonvascular injuries to the chest are most common with 49% of thoracic trauma patients presenting with rib fractures, 20% presenting with pneumothorax, and 12% presenting with lung contusions. 2 While vascular injuries to the chest make up only 6% of injuries in thoracic trauma, these injuries are most likely to require intervention by interventional radiology (IR). Iatrogenic injury is another common form of chest trauma. Iatrogenic chest trauma consists of both nonvascular injuries (pneumothorax, barotrauma, tracheal injury, and lymphatic injury) and vascular injuries (intercostal artery (ICA) injury during thoracentesis or chest tube placement or arterial injury during central line placement).
Nonaortic Vascular Trauma
Traumatic and iatrogenic blunt or penetrating injuries can cause damage to vascular structures in the chest. Acute injuries include vessel rupture or dissection, resulting in acute hemorrhage or vessel occlusion. Longer term sequelae of vascular trauma include formation of pseudoaneurysms or arteriovenous malformations. Although rare, injury to these vascular structures can be highly morbid. Depending on the injured vessel's anatomy and location, these vascular injuries can present as a hemothorax, hemoptysis, hematoma formation, cardiac tamponade, or, in the case of coronary artery injury, an acute myocardial infarction. Arterial pseudoaneurysms can rupture long after the initial injury resulting in similar presentations years following the trauma. Arteriovenous malformations can result in paradoxical emboli, high-output heart failure, and other morbid sequelae. Injuries to the nonaortic great vessels are more acutely lethal, typically presenting with significant hemodynamic instability requiring emergent surgical intervention, whereas injuries to smaller vasculature within the chest are more amenable to treatment either endovascularly or percutaneously. In general, IR management of nonaortic vascular injuries is indicated in hemodynamically stable patients with suspicion for vascular injury, typically based on symptoms and imaging findings on contrast-enhanced CT (CECT). Techniques in IR, primarily transarterial embolization (TAE) or stent placement, have been reported as successful treatment options for the management of a variety of nonaortic chest vascular trauma. IR management of vascular injury in the chest generally has high technical and clinical success rates with shorter operative time and hospital stay length than open repair. 3
Intercostal Artery Injury
Blunt or penetrating chest trauma to an ICA can result in extravasation and hemothorax. ICA injury most commonly occurs iatrogenically due to chest tube placement or thoracentesis, although this complication is rare occurring in less than 1% of placements. 4 Hemothorax secondary to iatrogenic ICA injury can have a delayed presentation, with symptoms reported up to 10 days following chest tube placement. 5 The primary management of patients with a stable hemothorax secondary to ICA injury is continued pleural drainage. In patients who are hemodynamically stable and despite pleural drainage have a progressive hemothorax, TAE of the ICA has been shown to be an effective alternative to open surgical thoracotomy and ligation to achieve hemostasis. Suggested indications for TAE include persistent chest tube output of more than 200 mL/hour and observed contrast extravasation on CECT, confirming active bleeding and localization of the injury. 6 In patients with iatrogenic hemorrhage, CECT may not be necessary, as the location of the bleeding is known, and X-ray or ultrasound might be sufficient to confirm persistent hemothorax. 4 IR management using TAE has been shown to successfully treat ICA bleeds and ongoing hemothorax with reported primary technical success rates ranging from 85 to 100% even when embolization of multiple arteries is required. 4 6 7 Successful TAE of the ICA has been reported with Gelfoam pledgets, polyvinyl alcohol (PVA) particles, microcoils, or a combination of embolic agents 6 ( Fig. 1 ). Although TAE of the ICA rarely has complications, branches of the anterior spinal artery have been found to originate from the ICA, particularly in the more caudal arteries (10th–12th thoracic segments), and embolization can result in spinal cord infarction and paralysis. 8 In cases where vascular access is not feasible, Foley balloon compression of the inner chest wall to stop persistent bleeding of an ICA has been reported with success. 9
Fig. 1.

A 66-year-old man with intercostal hemorrhage following bedside chest tube placement by pulmonary medicine. Hemothorax developed requiring large bore (28 Fr) chest tube placement by cardiothoracic surgery. ( a ) Angiography demonstrates active extravasation of contrast from the right T intercostal artery (arrow). ( b ) Resolution of active extravasation of contrast following embolization with 700–900 micron Embosphere particles (Merit Medical Systems, South Jordan, UT). ( c ) Cessation of flow in the distal intercostal artery following coil embolization with Ruby micro coils (black arrow) (Penumbra Medical, Alameda, CA). A pigtail chest tube is also noted (white arrow).
Internal Mammary/Thoracic Artery
The internal mammary/thoracic artery (IMA) is most commonly injured iatrogenically during central venous catheterization (CVC), pericardiocentesis, or sternotomy, although noniatrogenic injuries have been reported. IMA injury can result in hemothorax and/or a mediastinal hematoma with potential for high morbidity due to extracardiac tamponade. 10 The IMA has high blood flow at an average of 150 mL/min which can result in life-threatening hemorrhage within minutes. Injury to the IMA results in lethal shock in 45% of patients. 11 IMA injury can be difficult to diagnose due to the nonspecific imaging findings and the variety of resulting complications. 12 Imaging findings include active contrast extravasation on CECT, the presence of pseudoaneurysm or dissection, or evidence of a hemothorax or hematoma. 12 TAE has been described as a successful treatment option for uncontrolled bleeds of the IMA in hemodynamically stable patients with technical success rates ranging from 91.6 to 100% 10 11 ( Fig. 2 ). TAEs of the IMA were completed with coils, Gelfoam pledgets, and embolic microspheres ( Fig. 3 ). Due to the severity of IMA injury and hemorrhage, around 45% of patients require surgical intervention to control the bleeding. 11
Fig. 2.

A 55-year-old restrained driver in motor vehicle collision. ( a ) Contrast-enhanced CT demonstrates active hemorrhage in the right breast (arrow) with surrounding hematoma (arrowheads). ( b ) Angiography of a branch of the right internal mammary artery with a vessel cutoff, indicating arterial injury (arrow). ( c ) Angiography after embolization with NBCA glue demonstrates no flow in this successfully treated arterial branch. ( Images courtesy of Patrick Sutphin, MD, PHD—Massachusetts General Hospital .)
Fig. 3.
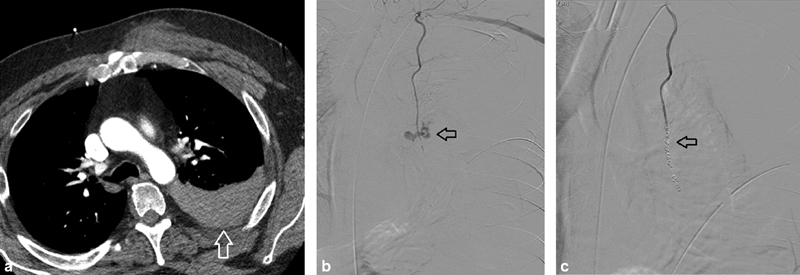
A 43-year-old man with self-inflicted stab wound to the chest in the setting of intentional rivaroxaban overdose. ( a ) CT images demonstrate hemothorax (arrow). ( b ) Selective left internal mammary artery (LIMA) angiography with active extravasation of contrast (arrow). ( c ) Post-coil embolization angiography with successful occlusion of the LIMA (arrow—coil pack). ( Images courtesy of Guy Johnson, MD—University of Washington Medicine .)
Axillosubclavian Artery Injuries
Traumatic injury to the subclavian vessels has high mortality, between 40 and 50%, and high morbidity, particularly due to injury of the brachial plexus, either directly or by associated compression. 13 An estimated 40 to 50% of axillosubclavian injuries can be treated by IR endovascularly, either with TAE or stent graft placement, with the only absolute contraindication being the inability to pass a wire across the injury. 14 A case–control retrospective study of open versus endovascular management of axillosubclavian injuries found similar technical and clinical success between both approaches but significantly reduced in-hospital mortality, rates of sepsis, and surgical-site infection in patients undergoing endovascular treatment. 15 However, many patients with these injuries are emergently treated surgically due to hemodynamic instability. 13 Care must be taken to identify the vertebral artery when placing a stent graft, although occlusion and covering of the origin has been successfully performed in patients in the setting of injury. 16
The most common cause of iatrogenic injury to the axillosubclavian vessels is secondary to CVC placement. 17 Although typically self-resolving, some iatrogenic injuries with large catheters require surgical or endovascular management. Endovascular use of stents, embolic agents, closure devices, and thrombin injections have been shown to be effective in managing iatrogenic axillosubclavian arterial injury secondary to CVC placement in multiple case series. 17
Innominate (Brachiocephalic) Artery Injury
The innominate artery is the second most injured great vessel after the descending aorta. 18 It is highly lethal with an estimated 71% of patients dying before arriving at the hospital. 19 As most patients are hemodynamically unstable with multiple other injuries, they are typically emergently treated surgically; however, endovascular management has been successful. 19 20 Recommended indications for endovascular repair of an innominate artery injury, usually stent graft placement, include patients who are hemodynamically stable with arterial dissection or pseudoaneurysm and not rupture or complete transection. 20 Hybrid procedures, where endovascular balloon tamponade of the hemorrhaging innominate artery as a bridge to surgical repair, have also been successful. 19 Particular care must be taken to clearly identify the origin of the right carotid artery. Patients with injuries near the bifurcation have been successfully managed with kissing covered stents. 21
Lateral Thoracic Artery Injury
Injury to the lateral thoracic artery is rare but can result in hemorrhage, pseudoaneurysm formation, hemoptysis, and hemothorax. Successful and uncomplicated TAE of the lateral thoracic artery and its branches has been reported in cases of injury, both iatrogenic and traumatic. 22
Superior and Inferior Phrenic Artery Injury
Injury to the superior and inferior phrenic arteries is incredibly rare. No reports of superior phrenic artery injury are present in the literature. Six cases of inferior phrenic artery injury due to blunt trauma are reported in the literature which resulted in massive hemothorax ( n = 2), intraperitoneal hemorrhage ( n = 2), and cardiac tamponade ( n = 1). 23 Five of these cases were treated successfully with TAE. The other patient with a diaphragmatic injury required surgical intervention.
Coronary Artery Injury
Coronary artery injury is a rare complication of blunt or penetrating trauma to the chest, more commonly seen in iatrogenic injury during cardiac artery catheterization. Mild to moderate trauma can result in arterial dissection and occlusion which will present as an acute myocardial infarction. 24 In blunt trauma, the left anterior descending (LAD) artery is most commonly affected followed by the right coronary artery (RCA) 24 25 26 ( Fig. 4 ), whereas in iatrogenic injury the RCA is more commonly affected. 27 Onset of symptoms and electrocardiographic (ECG) findings of myocardial ischemia secondary to coronary artery occlusion in the setting of trauma are typically delayed with a reported time window of 2 to 56 hours after initial injury, 24 with a review of literature cases finding that in more than 50% of cases, symptoms occurred more than 12 hours after the initial injury. 25 Injury is typically diagnosed by a combination of symptoms, ECG changes, and elevation of cardiac markers. Although standard treatment guidelines for traumatic coronary artery dissections do not exist, results from retrospective analysis suggest that for patients with less than 50% narrowing of a coronary artery, percutaneous transluminal coronary angioplasty (PTCA) is the optimal treatment, whereas patients with more extensive narrowing should undergo immediate coronary bypass. 25 28 Cases with more severe traumatic rupture of the coronary artery can occur which can result in hemopericardium, hemothorax, tamponade, and cardiac ischemia. 24 Coronary artery rupture is more quickly fatal than arterial dissection and is typically managed surgically; however, successful percutaneous treatment of traumatic coronary artery rupture with stent placement has been reported. 29
Fig. 4.

A 37-year-old man involved in head-on motor vehicle collision that developed ST elevations also had grade V liver laceration. ( a ) Cardiac MIP (white arrows) shows abrupt cutoff of the right coronary artery. ( b ) Cardiac curved planar reformat and ( c ) cardiac volume render images—white arrowheads show segment of dissected vessel. Treated by cardiology with two drug-eluting stents. ( Images courtesy of Patrick Sutphin, MD, PhD—Massachusetts General Hospital .)
Bronchial Artery Injury
Injury to the bronchial arteries can present with hemoptysis. Hemoptysis more than 100 mL/hour or total volumes more than 500 mL is considered life-threatening and requires urgent intervention. 30 Although more commonly caused by chronic lung diseases, such as malignancy or bronchiectasis, blunt and penetrating trauma can damage the bronchial arteries and result in acute hemorrhage and massive life-threatening hemoptysis. The etiology of hemoptysis is typically diagnosed with CT or bronchoscopy. The standard diagnostic pathway recommendation is for immediate CT followed by bronchoscopy in cases where the source cannot be identified on imaging. Morbidity from bronchial artery injury, as oppose to other traumatic vascular injuries, is most often due to suffocation from hemorrhage into the bronchial tree rather than exsanguination.
The mainstay of treatment for life-threatening hemoptysis secondary to bronchial artery injury is bronchial artery embolization (BAE). 30 31 BAE has been found to be highly successful for cessation of hemorrhage ranging from 70 to 99%. 30 Although a variety of embolic agents are used, some studies suggest that n -butyl-2-cyanoacrylate (NBCA) is superior to PVA particles and results in higher hemoptysis-free survival. The most common complications of BAE include chest pain and transient dysphagia which is self-limited. 30 Rebleeding occurs in 9.8 to 57.5% of patients; however, patients included in these studies typically have an underlying lung condition rather than bleeding secondary to direct trauma. The most serious complication of BAE is inadvertent embolization of the anterior spinal artery resulting in paralysis. Approximately 5% of patients will have a spinal artery that originates from the bronchial artery. In cases where BAE is not a viable treatment option, such as when the source of bleeding is endobronchial, bronchoscopic or surgical management should be considered. Surgical intervention can be useful in patients where BAE has failed or hemoptysis is recurrent despite multiple BAE treatments. 30
Pulmonary Artery Injuries
Injury to the pulmonary artery (PA) can occur due to blunt chest trauma or more commonly iatrogenically due to PA catheters, also known as Swan-Ganz catheters. 32 Estimated incidence of PA injury due to PA catheterization is low, between 0.01 and 0.47%. Injury can result in dissection or rupture; however, unlike aortic dissections, PA dissections quickly progress and rupture. Manifestations of PA rupture include cardiac arrest or hemodynamic instability secondary to hemorrhage or tamponade, dyspnea secondary to hemothorax, or hemoptysis. Long-term sequelae of PA injury include pseudoaneurysm formation and potential rupture, which can occur up to 60 years following the injury, and arterial fistula formation. 32 PA rupture secondary to PA catheters can be deadly with an average mortality rate of 50% and a mortality rate as high as 75% in anticoagulated patients. Although PA rupture is typically treated surgically, it has been successfully managed by embolization or stent graft placement. Contraindications for IR-directed therapy include hemodynamic instability or massive hemorrhage from the PA. PA pseudoaneurysms that have not ruptured are typically treated by TAE, although CT-guided percutaneous embolization has also been reported. 33 34
Superior Vena Cava Injury
Superior vena cava (SVC) injuries are highly lethal. Noniatrogenic rupture of the SVC is highly lethal and is surgically managed. The most common iatrogenic etiology of SVC injury is rupture secondary to transvenous lead extraction where the mortality rate is greater than 50%. 35 SVC injury can also occur during central catheter placement or during balloon dilation for SVC syndrome. 36 Due to the juxtaposition of the SVC with the thoracic cavity and the pericardium, patients can develop either hemothorax or hemopericardium, and resulting cardiac tamponade. Although iatrogenic SVC ruptures are typically managed surgically, endovascular stent placement has been successful in treating these injuries. Endovascular balloon occlusion of the ruptured SVC as a bridge to surgery has also been reported to improve survival by delaying hemodynamic collapse and improving the surgical field. 35
Nonvascular Injuries
Pneumothorax
Pneumothorax may result from underlying lung disease, trauma, or iatrogenic injury. Penetrating trauma presents with both hemothorax and pneumothorax in 80% of cases due to peripheral lung injury. Blunt trauma may lead to rib fracture or dislocation and subsequent damage to the visceral pleura, with potential to lead to bronchial rupture as recognized by the “fallen lung sign” on chest radiograph. Initial treatment of traumatic pneumothorax involves administration of 100% oxygen, followed by observation, simple aspiration, or tube thoracostomy depending on size and severity of the pneumothorax. 37 Tube thoracostomy is often emergently performed in the trauma bay by surgeons. Patients with a stable traumatic pneumothorax may undergo chest tube placement by IR under CT or a fluoroscopic localization ( Fig. 5 ).
Fig. 5.
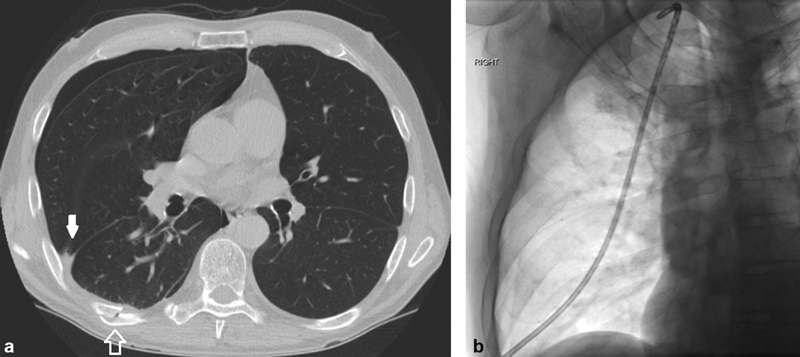
A 57-year-old man with chest pain after fall from a ladder. ( a ) CT chest shows rib fractures (open arrow) and a small right hemopneumothorax (solid arrow). ( b ) The pneumothorax resolved after placement of a 10-Fr pleural drain. ( Images courtesy of Guy Johnson, MD—University of Washington Medicine .)
Iatrogenic pneumothorax is a common complication of thoracic procedures. Stable patients who develop iatrogenic pneumothoraxes can be managed with minimally invasive techniques. The most common cause of pneumothorax is CVC placement, which occurs in up to 1% of cases, with higher rates seen after subclavian line placement as compared with the internal jugular approach. 38 Size of the pneumothorax after CVC placement guides management strategy. Asymptomatic patients with less than 30% pneumothorax size may be managed conservatively. Stable symptomatic patients with larger pneumothorax can be managed with pigtail catheter insertion, while cases of emergent pneumothorax are best managed with large tube thoracostomy. 39 Due to a high risk of tension pneumothorax, patients on mechanical ventilation require initial treatment of any size pneumothorax with tube thoracostomy.
CT-guided lung biopsy is another common cause of iatrogenic pneumothorax. Reported rates after CT-guided needle biopsy vary, with a maximum reported rate above 60%. 40 Occurrence depends on factors including lesion depth, larger needle size, number of pleural punctures, and the presence of preexisting lung disease. 40 41 Choice of approach affects pneumothorax risk, with rates varying between transthoracic (54%) and transbronchial (1–6%) biopsy. 41 42 Typically, the pneumothorax will resolve spontaneously, but 3.3 to 15% of patients require chest tube insertion. Precautionary measures during lung biopsies have been shown to reduce the severity of resultant pneumothoraxes. Embolization of the biopsy tract with gelatin sponge slurry reduces the risk of requiring a chest tube after pneumothorax development, though does not significantly reduce the occurrence of pneumothorax after needle biopsy. 43 Pleural blood patching, which involves sealing of the needle track with 2 to 3 mL of the patient's blood, has also been effective in reducing chest tube requirement. 44 Prone positioning also reduces risk of clinically significant pneumothorax. 45
Similarly, thoracentesis presents a risk of subsequent pneumothorax, with increased risk with larger needles and greater amount of fluid removed. 46 Ultrasound guidance was found to be associated with a lower risk of developing pneumothorax by percentages ranging from 5 to 15% in two studies. 47 48 Other minimally invasive procedures such as pulmonary radiofrequency ablation for pulmonary tumors has been associated with pneumothorax at rates of up to 30%, with 10% requiring chest tube placement. 49
Empyema and Lung Abscesses
Traumatic causes of empyema and/or lung abscess include thoracic surgery, blunt trauma, or other pleural interventions and require immediate attention due to high morbidity and mortality. 50 These are typically diagnosed on CT imaging after clinical suspicion and chest X-ray findings. Ultrasound is the preferred method for procedural guidance given its real-time imaging. CT can provide additional information in select cases such as distinction between empyema and lung abscess. Lung abscesses have been effectively treated with antibiotics alone in 80 to 90% of patients. 51 Tube drainage can be used in refractory cases or hemodynamically unstable patients, though has shown a high complication rate in some studies including development of bronchopleural fistula and pneumothorax. 52 Attempting needle aspiration followed by percutaneous drainage has also been a successful management strategy. 53
Lymphatic Injury
Lymphatic injury has been reported after both iatrogenic and noniatrogenic insults and presents with chylothorax, which develops within 10 days of the initial injury. Postoperative chylothorax is most commonly reported after invasive procedures involving the chest and mediastinum including lobectomy and aortic surgery, the most common being esophagectomy with a reported incidence of 4%. 54 55 Rarer iatrogenic causes such as duct blockage after CVC have also been reported. 56 Noniatrogenic causes of chylothorax secondary to thoracic duct injury (TDI) such as blunt chest trauma, fracture dislocation of thoracic vertebrae, childbirth, and knife wounds have also been reported. 55
Because the thoracic duct above the diaphragm typically begins by following the right side of thoracic vertebrae before crossing to the left at the level of T3/T4, the side of fluid drainage in chylothorax may indicate location of injury along the duct. However, a high level (35%) of embryological differences in thoracic duct course exists in the population, making it especially susceptible to injury during surgery. 57
Conservative initial treatment options involve a combination of dietary modifications such as total parenteral nutrition or a low-fat diet, and chest tube placement, which has been shown to be 84% effective in cure after a median of 8 to 10 days from diagnosis. 58 Thoracic duct embolization (TDE) has rapidly become a preferred treatment method in cases of high-output chylothorax or chylothorax refractory to conservative therapy, with cure and partial response rates of more than 70%. 59 Preprocedural imaging of the thoracic duct includes CT or MR ductography, though these are typically not necessary in traumatic TDI due to high sensitivity of lymphangiography (LAG). Thoracic duct visualization is achieved by either pedal or intranodal LAG using oil-based contrast agents. Pedal LAG requires identification and cannulation of small lymphatics in the foot, after which injection of oil-based dye is followed via fluoroscopy to identify the cisterna chyli (CC) and thoracic duct. Because this procedure is tedious and often technically challenging, ultrasound-guided access of inguinal lymph nodes has become the preferred method for TD visualization, after it was shown to be a suitable alternative to pedal LAG in six patients with faster identification of lymphatic ducts and faster duct cannulation. 60
Once the TD injury is identified on LAG, as determined by leakage of contrast into pleural space, an access approach is determined. While transabdominal access via catheterization of the CC is the most widely used approach, percutaneous transvenous retrograde approach has also been described as an alternative for patients in whom transabdominal access is contraindicated or unsuccessful. This access approach has been reported feasible in case reports via the basilic, brachial, and femoral veins, 61 62 63 but has shown less success in larger studies due to high anatomic variations in the cervical thoracic duct. 64 A third approach via direct access to the cervical thoracic duct, which has proven successful without LAG, has also been reported in patients after unsuccessful transabdominal cannulation or inadequate opacification of the CC on LAG. 65 66
Embolization is achieved via microcoil deployment across the disrupted portion of the thoracic duct followed by injection of N-butyl cyanoacrylate (NBCA) glue in a 1:1 ratio with lipidol for duct polymerization. Therapeutic LAG alone has been shown to occlude lymphatic leaks in up to 70% of low-output (< 500 mL/day) leaks and 35% of normal-to-high output (>500 mL/day) leaks, 67 providing an effective therapeutic option in fluoroscopically unidentifiable chylothorax ( Fig. 6 ).
Fig. 6.
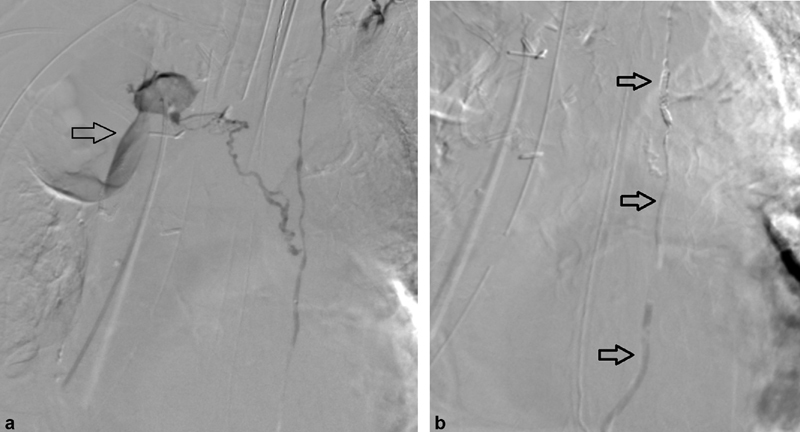
A 78-year-old man with lymphatic leak following right upper lobectomy for squamous cell carcinoma of the lung resulting in high output chylous pleural effusion. ( a ) Contrast injection in the thoracic duct demonstrates leakage of contrast from an accessory duct into the right pleural space (arrow). ( b ) Post embolization contrast injection demonstrates a coil pack and NBCA glue cast in the thoracic duct (arrows) and no extravasation of contrast.
Thoracic duct needle disruption can be attempted in cases of failed TDE. Punctures at the level of the CC via transabdominal approach interrupt lymphatic flow and have achieved cure and partial response in 44% of patients with unsuccessful TD catheterization in a first description. 59 A report of eight patients who underwent TDE or needle disruption found that needle disruption decreased median time to chest tube removal from 7 to 3.5 days, further validating its efficacy. 67
Recently, alternatives involving obliteration and tamponade of interstitium surrounding a TD leak have been demonstrated. Despite prior concerns that NBCA glue polymerizes immediately upon contact with tissues, direct injection of NBCA glue into the interstitial space via transabdominal 68 or translumbar 69 approach has been successful in tamponading lymphatic vessels in select reports. Injection of glue proximal to a lymphatic leak has also been demonstrated in five cases of pelvic lymphocele and may be applicable in TD leaks in which anatomic variation makes the site of leakage inaccessible with more standard methods. 70
Conclusion
Interventional radiology plays a vital role, with much to offer, in the evaluation and management of patients with both vascular and nonvascular thoracic trauma. In a majority of these cases, the minimally invasive nature of IR treatments obviates the need for these patients to go to the operating room. Endovascular options have equal, if not superior, advantages to surgery and play a critical role in the care of these patients. As part of a multidisciplinary approach, an essential understanding of the benefits and risks of the range of IR treatment options available in the care of a patient with thoracic trauma is necessary to provide the best care possible.
References
- 1.Ludwig C, Koryllos A. Management of chest trauma. J Thorac Dis. 2017;9 03:S172–S177. doi: 10.21037/jtd.2017.03.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kulshrestha P, Munshi I, Wait R. Profile of chest trauma in a level I trauma center. J Trauma. 2004;57(03):576–581. doi: 10.1097/01.ta.0000091107.00699.c7. [DOI] [PubMed] [Google Scholar]
- 3.Carrillo E H, Heniford B T, Senler S O, Dykes J R, Maniscalco S P, Richardson J D. Embolization therapy as an alternative to thoracotomy in vascular injuries of the chest wall. Am Surg. 1998;64(12):1142–1148. [PubMed] [Google Scholar]
- 4.Chemelli A P, Thauerer M, Wiedermann F, Strasak A, Klocker J, Chemelli-Steingruber I E. Transcatheter arterial embolization for the management of iatrogenic and blunt traumatic intercostal artery injuries. J Vasc Surg. 2009;49(06):1505–1513. doi: 10.1016/j.jvs.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 5.Chen R B, Hu H J, Matro E. Transcatheter arterial embolization for intercostal arterial bleeding in a patient after chest tube insertion. J Thorac Dis. 2015;7(07):E194–E197. doi: 10.3978/j.issn.2072-1439.2015.07.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tamburini N, Carriel N, Cavallesco G. Technical results, clinical efficacy and predictors of outcome of intercostal arteries embolization for hemothorax: a two-institutions' experience. J Thorac Dis. 2019;11(11):4693–4699. doi: 10.21037/jtd.2019.10.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stampfl U, Sommer C-M, Bellemann N. Emergency embolization for the treatment of acute hemorrhage from intercostal arteries. Emerg Radiol. 2014;21(06):565–570. doi: 10.1007/s10140-014-1231-3. [DOI] [PubMed] [Google Scholar]
- 8.Ozoilo K, Stein M. Paraplegia complicating embolization for bleeding intercostal artery in penetrating trauma. Inj Extra. 2013;44(08):70–73. [Google Scholar]
- 9.Chao B F, Jian Y J, Hao H Z. Balloon Foley catheter compression as a treatment for intercostal vessel bleeding. Injury. 2011;42(09):958–959. doi: 10.1016/j.injury.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 10.Corvino F, Giurazza F, Cangiano G. Safety and effectiveness of transcatheter embolization in the treatment of internal mammary artery injuries. Radiol Med (Torino) 2018;123(05):369–377. doi: 10.1007/s11547-017-0844-5. [DOI] [PubMed] [Google Scholar]
- 11.Chen J M, Lv J, Ma K, Yan J. Assessment of internal mammary artery injury after blunt chest trauma: a literature review. J Zhejiang Univ Sci B. 2014;15(10):864–869. doi: 10.1631/jzus.B1400098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Braatz T, Mirvis S E, Killeen K, Lightman N I. CT diagnosis of internal mammary artery injury caused by blunt trauma. Clin Radiol. 2001;56(02):120–123. doi: 10.1053/crad.2000.0572. [DOI] [PubMed] [Google Scholar]
- 13.Johnson C A. Endovascular management of peripheral vascular trauma. Semin Intervent Radiol. 2010;27(01):38–43. doi: 10.1055/s-0030-1247887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hourmozdi J J, Markin A, Johnson B, Fleming P R, Miller J B. Routine chest radiography is not necessary after ultrasound-guided right internal jugular vein catheterization. Crit Care Med. 2016;44(09):e804–e808. doi: 10.1097/CCM.0000000000001737. [DOI] [PubMed] [Google Scholar]
- 15.Branco B C, Boutrous M L, DuBose J J. Outcome comparison between open and endovascular management of axillosubclavian arterial injuries. J Vasc Surg. 2016;63(03):702–709. doi: 10.1016/j.jvs.2015.08.117. [DOI] [PubMed] [Google Scholar]
- 16.Mwipatayi B P, Jeffery P, Beningfield S J, Motale P, Tunnicliffe J, Navsaria P H. Management of extra-cranial vertebral artery injuries. Eur J Vasc Endovasc Surg. 2004;27(02):157–162. doi: 10.1016/j.ejvs.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 17.Abi-Jaoudeh N, Turba U C, Arslan B. Management of subclavian arterial injuries following inadvertent arterial puncture during central venous catheter placement. J Vasc Interv Radiol. 2009;20(03):396–402. doi: 10.1016/j.jvir.2008.12.409. [DOI] [PubMed] [Google Scholar]
- 18.O'Connor J V, Byrne C, Scalea T M, Griffith B P, Neschis D G. Vascular injuries after blunt chest trauma: diagnosis and management. Scand J Trauma Resusc Emerg Med. 2009;17:42. doi: 10.1186/1757-7241-17-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Graham J M, Feliciano D V, Mattox K L, Beall A C., Jr Innominate vascular injury. J Trauma. 1982;22(08):647–655. doi: 10.1097/00005373-198208000-00001. [DOI] [PubMed] [Google Scholar]
- 20.du Toit D F, Odendaal W, Lambrechts A, Warren B L. Surgical and endovascular management of penetrating innominate artery injuries. Eur J Vasc Endovasc Surg. 2008;36(01):56–62. doi: 10.1016/j.ejvs.2008.01.024. [DOI] [PubMed] [Google Scholar]
- 21.Hu S-L, Wang C-X, Lu H-J, Yuan Y. Management of injuries near the innominate artery bifurcation using an accurate kissing Viabahn stent technique. J Int Med Res. 2020;48(05):3.00060520912104E14. doi: 10.1177/0300060520912104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tingerides C, Annamalai G, Kaduri S, Dey C, Pugash R, David E. Transcatheter arterial embolization for chest wall trauma—10-year experience from a single trauma center. J Vasc Interv Radiol. 2015;26(01):151–152. [Google Scholar]
- 23.Aoki M, Shibuya K, Kaneko M. Massive hemothorax due to inferior phrenic artery injury after blunt trauma. World J Emerg Surg. 2015;10:58. doi: 10.1186/s13017-015-0052-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abu-Hmeidan J H, Arrowaili A I, Yousef R S. Coronary artery rupture in blunt thoracic trauma: a case report and review of literature. J Cardiothorac Surg. 2016;11(01):119. doi: 10.1186/s13019-016-0528-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keresztesi A A, Asofie G, Jung H. Traumatic coronary dissection: case presentation and literature review. J Interdisciplin Med. 2016;1(03):282–286. [Google Scholar]
- 26.Ginzburg E, Dygert J, Parra-Davila E, Lynn M, Almeida J, Mayor M. Coronary artery stenting for occlusive dissection after blunt chest trauma. J Trauma. 1998;45(01):157–161. doi: 10.1097/00005373-199807000-00034. [DOI] [PubMed] [Google Scholar]
- 27.Iannaccone S F, Sopková D, Dinič M, Farkaš D. Fatal coronary artery dissection caused by a diagnostic coronary catheterization: a case report and review of literature. Rev Med Leg. 2018;(26):369–373. [Google Scholar]
- 28.Vanzetto G, Berger-Coz E, Barone-Rochette G. Prevalence, therapeutic management and medium-term prognosis of spontaneous coronary artery dissection: results from a database of 11,605 patients. Eur J Cardiothorac Surg. 2009;35(02):250–254. doi: 10.1016/j.ejcts.2008.10.023. [DOI] [PubMed] [Google Scholar]
- 29.Ekici B, Erkan A F, Kütük U, Töre H F. Successful management of coronary artery rupture with stent-graft: a case report. Case Rep Med. 2014;2014:391843. doi: 10.1155/2014/391843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kathuria H, Hollingsworth H M, Vilvendhan R, Reardon C. Management of life-threatening hemoptysis. J Intensive Care. 2020;8(01):23. doi: 10.1186/s40560-020-00441-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sopko D R, Smith T P. Bronchial artery embolization for hemoptysis. Semin Intervent Radiol. 2011;28(01):48–62. doi: 10.1055/s-0031-1273940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yanagawa Y, Ishikawa K, Nagasawa H. Traumatic pulmonary artery injury: a review of the recent literature. Vessel Plus. 2018;2(01):1. [Google Scholar]
- 33.David A, Liberge R, Perret C, Frampas E, Douane F. CT-guided direct percutaneous treatment of a ruptured pulmonary artery pseudoaneurysm using N-butyl cyanoacrylate. Diagn Interv Imaging. 2017;98(12):903–904. doi: 10.1016/j.diii.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 34.Lafita V, Borge M A, Demos T C. Pulmonary artery pseudoaneurysm: etiology, presentation, diagnosis, and treatment. Semin Intervent Radiol. 2007;24(01):119–123. doi: 10.1055/s-2007-971202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Azarrafiy R, Tsang D C, Boyle T A, Wilkoff B L, Carrillo R G. Compliant endovascular balloon reduces the lethality of superior vena cava tears during transvenous lead extractions. Heart Rhythm. 2017;14(09):1400–1404. doi: 10.1016/j.hrthm.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 36.Lou X, Brunner M P, Wilkoff B L, Martin D O, Clair D G, Soltesz E G. Successful stent implantation for superior vena cava injury during transvenous lead extraction. HeartRhythm Case Rep. 2015;1(06):394–396. doi: 10.1016/j.hrcr.2014.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sharma A, Jindal P. Principles of diagnosis and management of traumatic pneumothorax. J Emerg Trauma Shock. 2008;1(01):34–41. doi: 10.4103/0974-2700.41789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barnes T W, Morgenthaler T I, Olson E J, Hesley G K, Decker P A, Ryu J H. Sonographically guided thoracentesis and rate of pneumothorax. J Clin Ultrasound. 2005;33(09):442–446. doi: 10.1002/jcu.20163. [DOI] [PubMed] [Google Scholar]
- 39.Steinke K, Sewell P E, Dupuy D. Pulmonary radiofrequency ablation--an international study survey. Anticancer Res. 2004;24(01):339–343. [PubMed] [Google Scholar]
- 40.Duncan M, Wijesekera N, Padley S. Interventional radiology of the thorax. Respirology. 2010;15(03):401–412. doi: 10.1111/j.1440-1843.2010.01729.x. [DOI] [PubMed] [Google Scholar]
- 41.Boskovic T, Stanic J, Pena-Karan S. Pneumothorax after transthoracic needle biopsy of lung lesions under CT guidance. J Thorac Dis. 2014;6(01) 01:S99–S107. doi: 10.3978/j.issn.2072-1439.2013.12.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boskovic T, Stojanovic M, Stanic J. Pneumothorax after transbronchial needle biopsy. J Thorac Dis. 2014;6 04:S427–S434. doi: 10.3978/j.issn.2072-1439.2014.08.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tran A A, Brown S B, Rosenberg J, Hovsepian D M. Tract embolization with gelatin sponge slurry for prevention of pneumothorax after percutaneous computed tomography-guided lung biopsy. Cardiovasc Intervent Radiol. 2014;37(06):1546–1553. doi: 10.1007/s00270-013-0823-8. [DOI] [PubMed] [Google Scholar]
- 44.Wagner J M, Hinshaw J L, Lubner M G. CT-guided lung biopsies: pleural blood patching reduces the rate of chest tube placement for postbiopsy pneumothorax. AJR Am J Roentgenol. 2011;197(04):783–788. doi: 10.2214/AJR.10.6324. [DOI] [PubMed] [Google Scholar]
- 45.Covey A M, Gandhi R, Brody L A, Getrajdman G, Thaler H T, Brown K T. Factors associated with pneumothorax and pneumothorax requiring treatment after percutaneous lung biopsy in 443 consecutive patients. J Vasc Interv Radiol. 2004;15(05):479–483. doi: 10.1097/01.rvi.0000124951.24134.50. [DOI] [PubMed] [Google Scholar]
- 46.Josephson T, Nordenskjold C A, Larsson J, Rosenberg L U, Kaijser M. Amount drained at ultrasound-guided thoracentesis and risk of pneumothorax. Acta Radiol. 2009;50(01):42–47. doi: 10.1080/02841850802590460. [DOI] [PubMed] [Google Scholar]
- 47.Raptopoulos V, Davis L M, Lee G, Umali C, Lew R, Irwin R S. Factors affecting the development of pneumothorax associated with thoracentesis. AJR Am J Roentgenol. 1991;156(05):917–920. doi: 10.2214/ajr.156.5.2017951. [DOI] [PubMed] [Google Scholar]
- 48.Kornbau C, Lee K C, Hughes G D, Firstenberg M S. Central line complications. Int J Crit Illn Inj Sci. 2015;5(03):170–178. doi: 10.4103/2229-5151.164940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tsotsolis N, Tsirgogianni K, Kioumis I. Pneumothorax as a complication of central venous catheter insertion. Ann Transl Med. 2015;3(03):40. doi: 10.3978/j.issn.2305-5839.2015.02.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.O'Connor J V, Chi A, Joshi M, DuBose J, Scalea T M. Post-traumatic empyema: aetiology, surgery and outcome in 125 consecutive patients. Injury. 2013;44(09):1153–1158. doi: 10.1016/j.injury.2012.03.025. [DOI] [PubMed] [Google Scholar]
- 51.Wali S O. An update on the drainage of pyogenic lung abscesses. Ann Thorac Med. 2012;7(01):3–7. doi: 10.4103/1817-1737.91552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yunus M. CT guided transthoracic catheter drainage of intrapulmonary abscess. J Pak Med Assoc. 2009;59(10):703–709. [PubMed] [Google Scholar]
- 53.Hoffer F A, Bloom D A, Colin A A, Fishman S J. Lung abscess versus necrotizing pneumonia: implications for interventional therapy. Pediatr Radiol. 1999;29(02):87–91. doi: 10.1007/s002470050547. [DOI] [PubMed] [Google Scholar]
- 54.Dougenis D, Walker W S, Cameron E W, Walbaum P R. Management of chylothorax complicating extensive esophageal resection. Surg Gynecol Obstet. 1992;174(06):501–506. [PubMed] [Google Scholar]
- 55.Bryant A S, Minnich D J, Wei B, Cerfolio R J.The incidence and management of postoperative chylothorax after pulmonary resection and thoracic mediastinal lymph node dissection Ann Thorac Surg 20149801232–235., discussion 235–237 [DOI] [PubMed] [Google Scholar]
- 56.Kurekci E, Kaye R, Koehler M. Chylothorax and chylopericardium: a complication of a central venous catheter. J Pediatr. 1998;132(06):1064–1066. doi: 10.1016/s0022-3476(98)70414-7. [DOI] [PubMed] [Google Scholar]
- 57.McGrath E E, Blades Z, Anderson P B. Chylothorax: aetiology, diagnosis and therapeutic options. Respir Med. 2010;104(01):1–8. doi: 10.1016/j.rmed.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 58.Takuwa T, Yoshida J, Ono S. Low-fat diet management strategy for chylothorax after pulmonary resection and lymph node dissection for primary lung cancer. J Thorac Cardiovasc Surg. 2013;146(03):571–574. doi: 10.1016/j.jtcvs.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 59.Cope C, Kaiser L R. Management of unremitting chylothorax by percutaneous embolization and blockage of retroperitoneal lymphatic vessels in 42 patients. J Vasc Interv Radiol. 2002;13(11):1139–1148. doi: 10.1016/s1051-0443(07)61956-3. [DOI] [PubMed] [Google Scholar]
- 60.Nadolski G J, Itkin M. Feasibility of ultrasound-guided intranodal lymphangiogram for thoracic duct embolization. J Vasc Interv Radiol. 2012;23(05):613–616. doi: 10.1016/j.jvir.2012.01.078. [DOI] [PubMed] [Google Scholar]
- 61.Mittleider D, Dykes T A, Cicuto K P, Amberson S M, Leusner C R.Retrograde cannulation of the thoracic duct and embolization of the cisterna chyli in the treatment of chylous ascites J Vasc Interv Radiol 200819(2, Pt 1):285–290. [DOI] [PubMed] [Google Scholar]
- 62.Koike Y, Hirai C, Nishimura J, Moriya N, Katsumata Y. Percutaneous transvenous embolization of the thoracic duct in the treatment of chylothorax in two patients. J Vasc Interv Radiol. 2013;24(01):135–137. doi: 10.1016/j.jvir.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 63.Arslan B, Masrani A, Tasse J C, Stenson K, Turba ÜC. Superselective retrograde lymphatic duct embolization for management of postoperative lymphatic leak. Diagn Interv Radiol. 2017;23(05):379–380. doi: 10.5152/dir.2017.16514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kariya S, Nakatani M, Ueno Y. Transvenous retrograde thoracic ductography: initial experience with 13 consecutive cases. Cardiovasc Intervent Radiol. 2018;41(03):406–414. doi: 10.1007/s00270-017-1814-y. [DOI] [PubMed] [Google Scholar]
- 65.Pieper C C, Schild H H. Direct cervical puncture for retrograde thoracic duct embolization in a postoperative cervical lymphatic fistula. J Vasc Interv Radiol. 2015;26(09):1405–1408. doi: 10.1016/j.jvir.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 66.Reis S P, MacFarlane J, Anene A, Pillai A K. Direct percutaneous access to the cervical portion of the thoracic duct, an alternative to traditional access through the cisterna chyli. J Vasc Interv Radiol. 2015;26(12):1902–1904. doi: 10.1016/j.jvir.2015.08.021. [DOI] [PubMed] [Google Scholar]
- 67.Binkert C A, Yucel E K, Davison B D, Sugarbaker D J, Baum R A. Percutaneous treatment of high-output chylothorax with embolization or needle disruption technique. J Vasc Interv Radiol. 2005;16(09):1257–1262. doi: 10.1097/01.rvi.0000167869.36093.43. [DOI] [PubMed] [Google Scholar]
- 68.Hur S, Shin J H, Lee I J. Early experience in the management of postoperative lymphatic leakage using lipiodol lymphangiography and adjunctive glue embolization. J Vasc Interv Radiol. 2016;27(08):1177–11860. doi: 10.1016/j.jvir.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 69.Itou C, Koizumi J, Myojin K, Yamashita T, Mori N, Imai Y. A case of refractory chylous ascites after nephrectomy successfully treated with percutaneous obliteration using adhesive glue. Jpn J Radiol. 2013;31(01):71–74. doi: 10.1007/s11604-012-0146-8. [DOI] [PubMed] [Google Scholar]
- 70.Baek Y, Won J H, Chang S-J. lymphatic embolization for the treatment of pelvic lymphoceles: preliminary experience in five patients. J Vasc Interv Radiol. 2016;27(08):1170–1176. doi: 10.1016/j.jvir.2016.04.011. [DOI] [PubMed] [Google Scholar]


