Abstract
Traumatic injuries to the kidney and collecting system can range widely from small lacerations to significant bleeding and its sequelae. Urinary obstruction can occur in the renal pelvis, ureters, or urethra. Interventional radiology plays a significant role in treatment and management, in many cases requiring emergent action. Endovascular embolization is frequently the first-line approach to treating hemorrhage. Percutaneous interventions for urinary obstruction include nephrostomy and suprapubic catheter placement. In this article, we outline the clinical approach and interventional methods used in the evaluation and treatment of renal trauma. Several case presentations demonstrate the role of interventional radiology in renal trauma.
Keywords: interventional radiology, renal trauma, embolization, page kidney, renal hemorrhage
Trauma is the leading cause of death in the United States for individuals up to 45 years of age, and it is the fourth leading cause of death for all ages. 1 Renal injury is involved in less than 4% of trauma cases—with estimates ranging between 1.4 and 3.25%. 2 In trauma patients with intra-abdominal injuries, however, approximately 24% had renal involvement. 3 Given the relatively low prevalence of clinically significant renal injury, the evidence for the management of renal trauma has historically relied heavily on expert opinion and single-institution retrospective studies. In 2004, for example, the World Health Organization convened an international committee to codify the evidence for renal trauma clinical management. 2 At that time, the only level 1 quality data available was a single randomized control trial in 56 patients with renal trauma undergoing surgical management. 4 Over time, there has been a gradual movement toward nonsurgical management and greater involvement of interventional radiology (IR) in the clinical management of these patients.
Epidemiology
The vast majority of renal injuries result from blunt trauma, though there is significant geographic variation. In the developing world, this typically results from motor vehicle crashes or falls. In a review of the epidemiology of renal trauma, Voelzke and Leddy found that 84% of renal trauma was blunt force, while 14% was from penetrating trauma. 5 In adults, 63% of causes of blunt trauma cases came from motor vehicle collisions (MVCs), with falls making up to 14% and sports injuries up to 11%. In the pediatric population, MVC accounted for 30%, fall accounted for 27%, pedestrian struck by car accounted for 13%, and sports accounted for 12%. Penetrating trauma was exclusively from gunshot wounds (65%) and stabbing (35%).
The patient population affected by renal trauma is disproportionately young (mean age: 20–30) and male. 2 The role of IR in trauma management has been well-established, particularly as the overwhelming majority of renal trauma patients are nowadays managed nonoperatively. 6 Voelzke and Leddy's review estimated the median percent of nonoperative care for adult blunt renal injury at 94.8%, with an estimated 5.4% nephrectomy rate. 5 In a different study of 46 blunt trauma patients, the use of surgical exploration was limited to patients with major vessel injury, ureteral disruption, pancreatic ductal injury, or gastrointestinal perforation. 7
A significant proportion of renal trauma is iatrogenic, though the specific etiology will likely vary depending on institutional practice patterns. In one case series of 21 patients, the specific injury etiology was nearly evenly distributed between renal biopsy, percutaneous nephrolithotomy (PCNL), nephron-sparing surgery, guide-wire–induced arterial perforation during coronary angiography or renal stenting, percutaneous nephrostomy, renal pyeloplasty, and surgical nephrectomy. 8
As with other aspects of IR clinical practice, a key branch point in the clinical management of intervenable causes of hemorrhage is whether the patient is hemodynamically stable; hemodynamically unstable patients with signs of active hemorrhage may go directly to the operating room for surgical exploration. Blunt trauma affecting the kidneys typically requires significant force, and coexisting abdominal injuries are common. 2 IR inclusion in clinical management for these patients is key, particularly in high-energy trauma, as IR's minimally invasive endovascular and percutaneous approaches can save many patients from unnecessary surgery and morbidity.
Pathophysiology
The kidneys are protected by their retroperitoneal location, surrounded by fat and Gerota's fascia, as well as the abdominal organs anteriorly and the back muscles and spine posteriorly. Acceleration forces may result in blunt impact to the ribs or abdominal wall directly leading to renal parenchymal or vascular injury. The main attachment points for the kidneys are the renal pedicle and the ureteropelvic junction. Significant deceleration secondary to blunt trauma can therefore lead to renal pedicle injury, including thrombosis or rupture. 2 9 Penetrating injuries are more likely to cause severe renal injury, though they are less common. 2 Penetrating injuries through the anterior abdomen can injure key renal structures that are directed toward the midline of the body, such as the pelvis, hilum, and pedicle. Parenchymal injuries, which tend to be less severe, are more common with penetrating injuries through the flanks. 2
The underlying kidney may be abnormal predisposing it to injury with lower force impact. Of the abnormalities seen in renal trauma cases, hydronephrosis is the most frequent accounting for 38% with cysts accounting for another 17%. These scenarios may make the kidney more incompressible and therefore vulnerable to rupture.
Vascular Anatomy
Interventionalists should be aware of classical and variant renal vascular anatomy for preprocedural planning. The renal arteries classically arise from the abdominal aorta at the L1–L2 level, below the branching point of the superior mesenteric artery. 10 In a study of 133 cadavers, Sampaio and Passos demonstrated the existence of eight different anatomical variants and their respective prevalence ( Fig. 1 ). 11
Fig. 1.
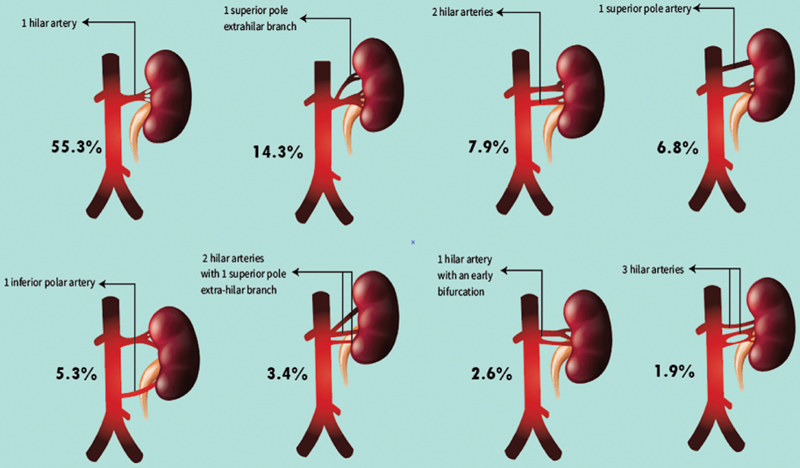
Graphic illustration of variant renal anatomy (adapted from Sampaio et al). 11
Interventional Techniques
The role of IR in the clinical management of renal trauma depends on the type and grade of renal injury, as well as the hemodynamic stability of the patient. Hemodynamically unstable patients would typically undergo intraoperative evaluation, though some institutions have published successful endovascular treatment of even grade V renal injuries in hemodynamically unstable patients. 12 Similarly, some interventionalists have proposed a paradigm to accelerate the IR workflow and allow for the treatment of hemodynamically unstable patients: among other things, by including arterial sheath placement as part of the initial trauma survey and creating a third “conductor” role in which a proceduralist coordinates with the trauma team in the control room and directs the interventionalist performing the procedure in the angiography suite. 13 For hemodynamically stable patients, abdominal CT with intravenous contrast is typically the initial study of choice. 2 CT accurately demonstrates most perirenal, vascular, parenchymal, and collecting system injuries. CT imaging for the evaluation of renal trauma should include unenhanced, postcontrast corticomedullary (to identify renal parenchymal and vascular injuries), and postcontrast delayed phases (to identify injuries to the urinary collecting system) to maximize its diagnostic yield. 14 MRI may be considered for patients with contraindication to contrast-enhanced CT imaging (e.g., pregnancy, renal insufficiency, or contrast allergy).
The American Association for the Surgery of Trauma (AAST) has proposed a grading system of renal injury that is divided into five separate categories determined primarily by CT imaging findings ( Table 1 ). 15 Separate grading scales have been proposed for the classification of ureteral and bladder injuries. 14
Table 1. American Association for Surgery in Trauma (AAST) Kidney Injury scale—2018 revision 15 .
| AAST grade | Imaging criteria (CT findings) |
|---|---|
| I | Subcapsular hematoma and/or parenchymal contusion without laceration |
| II | Perirenal hematoma confined to Gerota's fascia Parenchymal laceration ≤1 cm without urinary extravasation |
| III | Parenchymal laceration >1 cm without urinary extravasation or collecting system rupture Any injury in the presence of a kidney vascular injury or active bleeding contained within Gerota's fascia |
| IV | Parenchymal laceration extending into urinary collecting system with urinary extravasation Renal pelvis laceration and/or complete ureteropelvic disruption Segmental renal vein or artery injury Active bleeding beyond Gerota's fascia into the retroperitoneum or peritoneum Segmental or complete kidney infarction(s) due to vessel thrombosis without active bleeding |
| V | Main renal artery or vein laceration or avulsion of hilum Devascularized kidney with active bleeding Shattered kidney with loss of identifiable parenchymal renal anatomy |
Vascular interventions are performed in cases of main or branch renal artery injuries. These can be treated with balloon occlusion (which can achieve hemostasis as a temporizing measure until definitive treatment), stent grafts (particularly to treat large vessel injury), and transarterial embolization, which has high rates of success. 16 17 The contemporary availability of a myriad of coaxial microcatheters allows for selective and superselective catheterization of virtually the entire arterial circulation. In one study, 94% of embolizations achieved control of hemorrhage and 82% of patients required no further intervention. 18 19 Similarly, in cases of renal laceration/hemorrhage, delayed renal bleeding in patients with extensive renal injury can be managed successfully with angiographic embolization. Renal arteriovenous fistulas can lead to rapid blood loss and may have delayed presentation. These can be treated with selective embolization of the involved artery. Similarly, traumatic and iatrogenic renal artery pseudoaneurysms can cause delayed or recurrent hemorrhage. 20 Iatrogenic trauma includes postbiopsy complications and can even result in Page kidney or renal vein thrombosis.
The segmental branches of the renal artery are terminal arteries. This means that occlusion of an injured vessel will inevitably cause devascularization of the renal parenchyma fed by that artery and its branches. The advent of microcatheters with inner diameters ranging from 0.018″ down to 0.010″ allows for the selection of the distal-most artery, closest to the site of bleeding. This minimizes the amount of renal parenchyma damaged during intentional occlusion of the injured artery. That being said, main renal artery embolization is still occasionally performed. 5
The most commonly used embolic agents in the trauma setting include Gelfoam, particles, and coils; the specific choice of agent will depend on the location of injury and operator preference. Ethanol embolization of a bleeding renal arterial stump after open nephrectomy has also been reported. 21
Nonvascular interventions may be performed when there is injury to the collecting system, ureter, bladder, or urethra. A possible complication of ureteral or collecting system rupture is urinary extravasation, which can lead to urinomas or perinephric abscesses that may require percutaneous intervention. Procedures such as nephrostomy, PCNU placement, suprapubic catheter placement, and ureteroplasty may be performed. Persistence of urinomas despite appropriate drainage catheter placement indicates persistent leakage from the collecting system, which may require placement of nephroureteral catheters to maximize urinary diversion. 22 Routine cultures of urinoma fluid and empiric antibiotic therapy until cultures speciate have been recommended to minimize the risk posttraumatic perinephric abscesses.
As with all interventions, procedural complications may arise. In a review of 10 published series on endovascular treatment for acute renal injury, complications were rare but included renal abscess, dislodged microcoils, parenchymal loss, renal branch intimal dissection, ectopic coil placement, and nephrectomy for hypertension after nontarget main renal artery embolization, among others. 23 Repeat or follow-up procedures are often required. Failure of initial renal artery embolization have been reported to be as high as 16.5%. 24 In one retrospective review of over 117 renal arterial embolizations for the treatment of hemorrhage after PCNL, risk factors for failure of initial embolization included multiple percutaneous access sites, more than two bleeding sites seen on angiography, and use of gelatin sponge alone as the embolic material. 25
Case Presentations
Case 1: Grade IV Injury after Fall
A 66-year-old man was brought to the emergency department after a fall from a height of 15 feet. He was initially hemodynamically stable and a CT was obtained ( Fig. 2a, b ) showing grade IV renal trauma with hyperdense material within the laceration, consistent with active bleeding (red arrow, Fig. 2a ). The left renal artery and vein are seen surrounded by the retroperitoneal hematoma (white arrow), concerning for the possibility of laceration. He was admitted to the ICU for observation.
Fig. 2.

Grade 4 left renal laceration after fall. Axial ( a ) and coronal ( b ) CT images showing laceration of the left kidney with hyperdense material (red arrow) showing active hemorrhage. Angiography ( c ) shows area of bleeding in the inferior pole (white arrow). After coil embolization ( d ) the bleeding is controlled. The lack of parenchymal contrast peripheral to the coils in the medial inferior pole represents devascularized tissue.
Although he remained hemodynamically stable, the patient's hemoglobin decreased from 11.3 g/dL on admission to 7.7 g/dL. IR was consulted. Angiography revealed multiple foci of active extravasation in the inferior left renal pole in conjunction with a large, brisk left interpolar renal pseudoaneurysm (white arrow, Fig. 2c ). This was treated with coil embolization (white arrows, Fig. 2d ), with resolution of active extravasation and decreased flow to the left renal pseudoaneurysm (black arrow, Fig. 2d —coil pack from unrelated embolization procedure).
Case 2: Grade IV Renal Artery Injury after Percutaneous Nephrostomy
A 74-year-old man with nephrolithiasis and chronic kidney disease (Cr 1.5) presented to an outside hospital with bilateral obstructive nephrolithiasis. He underwent right percutaneous nephrostomy tube placement complicated by renal subcapsular hematoma leading to hemorrhagic shock requiring vasopressors and blood transfusions. Fig. 3 (a, b) demonstrates the retroperitoneal hematoma (white arrows) and percutaneous nephrostomy tube (red arrow). He was then transferred to the authors' institution for IR consultation. Right renal angiography ( Fig. 3c – e ) revealed active extravasation of contrast from two right renal artery inferior pole branch vessels. A microcatheter was used to select each bleeding area and microcoils used to embolize ( Fig. 3c–e : arrows—percutaneous nephrostomy tube and ureteral stent; arrowheads—postembolization coil pack).
Fig. 3.

Subcapsular hematoma after nephrostomy. Axial ( a ) and coronal ( b ) CT images showing large subcapsular hematoma after placement of a right percutaneous nephrostomy catheter (PCN). The PCN is partially imaged (red arrow). Angiogram shows two areas of active extravasation ( c ). Oblique angiogram shows microcatheter placing coils in the more central focus ( d ) and AP angiogram shows placement of more distal micro-coils ( e ) to treat the more peripheral focus of hemorrhage.
Case 3: Persistent Hematuria after Percutaneous Access for Lithotripsy
A 73-year-old woman with history of nephrolithiasis underwent right percutaneous nephrostomy tube placement for the purposes of lithotripsy. This was complicated by persistent hematuria and falling hemoglobin count. IR was consulted and took the patient directly to angiography ( Fig. 4 ). This revealed a 2-cm renal artery pseudoaneurysm (arrow, Fig. 4a, b ). A microcatheter was used to navigate peripherally, closer to the site of arterial injury where coil embolization was performed. Subsequent to coil placement, angiography shows an area of devascularization peripheral to the coils (black arrow, Fig. 4c ). The use of microcatheters allows minimizing the amount of renal parenchyma sacrificed when performing coil embolization (white arrow, Fig. 4c —postembolization coil pack).
Fig. 4.

Persistent hematuria after percutaneous access for lithotripsy. Panel a shows angiography of the right renal artery with formation of a 2 cm pseudoaneurysm. Superselective angiography was performed with catheterization of a distal branch near the site of injury ( b ). Post-coil embolization angiogram shows a small area of devascularization distal to the coils ( c ).
Case 4: Renal Artery Hemorrhage after Partial Nephrectomy
A 56-year-old man presented with gross hematuria after a partial left nephrectomy. Angiography ( Fig. 5 ) demonstrated a focal area of irregularity consistent with pseudoaneurysm formation involving a major branch of the superior left renal artery (arrow, Fig. 5a ). This was coil embolized (arrow, Fig. 5b ). The inferior left renal artery was unaffected (arrow, Fig. 5c ).
Fig. 5.

Pseudoaneurysm after partial nephrectomy. Angiogram ( a ) showing pseudoaneurysm (white arrow) in a branch of the left renal artery. The inferior pole of the kidney was not visualized suggesting the presence of a second renal artery arising from the aorta. Post coil embolization ( b ) shows occlusion of the injured vessel. Angiography of the inferior left renal artery was then performed showing it was unaffected ( c ).
Case 5: Renal Laceration Due to Stabbing
A 30-year-old man with stab wounds to the bilateral upper extremities, right chest and left flank, presented to the emergency department. CT showed a resulting grade III renal laceration in the interpolar region of the left kidney, perirenal hematoma, and acute arterial hemorrhage (arrow, Fig. 6a, b ). He was taken for angiography which revealed active left mid pole renal extravasation (arrow, Fig. 6c ). The main subsegmental renal arterial branch supplying the area of active extravasation was selected and embolized with Gelfoam pledget (also known as a Gelfoam “torpedo”). While Gelfoam is not considered a permanent embolic, it occludes the target vessel for a variable period on the order of weeks. This is frequently enough time for the vessel wall injury to close and may allow for future reperfusion of the vessel after the Gelfoam is resorbed. Fig. 6d demonstrates no further contrast extravasation postembolization.
Fig. 6.

Renal laceration from stabbing. Axial ( a ) and coronal ( b ) CT showed a grade III renal laceration in the interpolar region of the left kidney, perirenal hematoma and acute arterial hemorrhage. Angiography ( c ) revealed active left mid pole renal extravasation (red arrow). The main subsegmental renal arterial branch supplying the area of active extravasation was selected and embolized with Gelfoam pledget (aka torpedo). Post embolization angiogram shows cessation of hemorrhage ( d ).
Case 6: Surgical Trauma, PCNL Dilator through Renal Pelvis
A 58-year-old woman underwent attempted PCNL at an outside hospital ( Fig. 7 ) in which a 24-Fr dilator was passed through the renal pelvis into the lesser sac. Initially, a Foley catheter was placed through the defect, with the balloon inflated next to posterior gastric lumen. A CT demonstrated left anterior perinephric hematoma, with evidence of contrast extravasation, from the left renal vessels, tracking into the lower pelvis. There was also left renal collecting system contrast extravasation (arrows, Fig. 7a ), in keeping with a perforated renal collecting system, surrounding the left kidney, posterior to the gastric lumen, around the liver, and interspersed in the right upper quadrant small bowel mesentery.
Fig. 7.

Surgical trauma after attempted PCNL. During a PCNL procedure, a 24 Fr dilator perforated the left renal collecting system. Initially a Foley catheter was placed through the defect, with the balloon inflated next to posterior gastric lumen ( a ). The CT Abdomen demonstrated a left anterior perinephric hematoma, with evidence of contrast extravasation, from the left renal vessels, tracking into the lower pelvis. There was also left renal collecting system contrast extravasation, in keeping with a perforated renal collecting system, surrounding the left kidney, posterior to the gastric lumen, around the liver and interspersed in the right upper quadrant small bowel mesentery. ( b ) In the OR, urology placed an internal double-J ureteral stent. A guidewire was placed, percutaneously entering the left kidney, traversing to the lesser sac, looping back into the left renal collecting system/ureter, subsequently terminating in the bladder. Angiography was then performed. The mesenteric vessels were uninjured ( c ). Left renal artery angiography ( d ) demonstrated irregularity and luminal narrowing consistent with arterial injury involving a left lower pole segmental artery (red arrow). This was embolized with 3 mm and 4 mm coils. Final injection of contrast into the Foley catheter demonstrated filling of the collecting system with extravasation into the retroperitoneum ( e ). This access was subsequently used to place a percutaneous nephroureteral stent ( f ). With prolonged decompression of the collecting system, the hemorrhage into the collecting system ultimately dissipated.
The patient was taken to a hybrid operating room/angiography suite. Urology placed internal double-J ureteral stent (black arrow, Fig. 7b ). A guidewire was placed, percutaneously entering the left kidney, traversing to the lesser sac, looping back into the left renal collecting system/ureter, subsequently terminating in the bladder (white arrow, Fig. 7b ). Angiography was then performed. The mesenteric vessels were uninjured ( Fig. 7c ). Repeat angiography as the catheter was withdrawn into the collecting system confirmed the mesenteric vessels were not a source of hemorrhage (not shown). Left renal artery angiography demonstrated irregularity and luminal narrowing consistent with arterial injury involving a left lower pole segmental artery (arrow, Fig. 7d ). This was embolized with 3- and 4-mm coils (arrow, Fig. 7e ). Final injection of contrast into the Foley catheter demonstrated filling of the collecting system with extravasation into the retroperitoneum. This access was subsequently used to place a percutaneous nephroureteral stent (white arrow, Fig. 7f ). With prolonged decompression of the collecting system, the hemorrhage into the collecting system ultimately dissipated (black arrow, Fig. 7f —ureteral stent).
Case 7: Page Kidney with Active Arterial Extravasation
A 20-year-old man presented with a stab wound to the right flank ( Fig. 8 ). On CT, there was an evident left-sided renal laceration involving the posterior superior pole with evidence of active arterial extravasation (arrow, Fig. 8a ) and a large perinephric hematoma (star, Fig. 8a, b ) resulting in distortion of the posterior kidney. The patient became hypertensive raising concern for “Page phenomenon” or “Page kidney.” In 1939, Irvine Page published an experiment in which he wrapped one or both kidneys in a dog model with cellophane resulting in hypertension. 26 Hypertension in the context of a perinephric hematoma or any externally compressing process should raise concern for decreased blood flow to the kidney and compromised renal function.
Fig. 8.

Page kidney with active arterial extravasation. A 20-year-old male presented with a stab wound to the right flank. Axial ( a ) and sagittal ( b ) CT images show right-sided renal laceration involving the posterior superior pole with evidence of active arterial extravasation and a large perinephric hematoma resulting in distortion of the posterior kidney. The patient became hypertensive raising concern Page kidney. A selective right renal arteriogram was performed showing contrast extravasation ( c , white arrow). This was coiled embolized ( d ).
A selective right renal arteriogram was performed. It showed normal patency and caliber of the right renal artery. The right renal artery divided into superior and inferior segmental branches and then into tertiary branches. In the suspected area of injury, two small peripheral arteries were seen that had irregular caliber, one of which was associated with active contrast extravasation beyond the renal capsule into the retroperitoneum (arrow, Fig. 8c ). The irregular artery was embolized with coils (white arrow, Fig. 8d ), with resultant resolution of the contrast extravasation and hypoperfusion beyond the coil pack (black arrow, Fig. 8d ).
Case 8: Hematoma after Renal Biopsy
A 61-year-old man underwent a renal biopsy 2 weeks prior to presentation with hypotension and hemoglobin drop ( Fig. 9a ). A CT showed subcapsular hematoma (white arrows, Fig. 9a, b ) and active extravasation (red arrow, Fig. 9b ). Angiography revealed an area of active extravasation in the right lower pole (white arrow, Fig. 9c ). The space between the peripheral renal parenchyma and the capsular arteries represents the subcapsular hematoma (double black arrow, Fig. 9c ). The injured arterial branch was selectively catheterized and embolized with microcoils (arrow, Fig. 9e —postembolization coil pack.
Fig. 9.
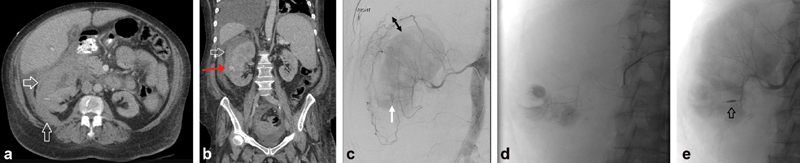
Hematoma after renal biopsy. A 61-year-old male presented with hypotension and hemoglobin drop 2 weeks after a renal biopsy. CT abdomen and pelvis with contrast showed subcapsular hematoma ( a, b ) and active extravasation ( b , red arrow). Angiography revealed an area of active extravasation in the right lower pole ( c , white arrow). The space between the peripheral renal parenchyma and the capsular arteries represents the subcapsular hematoma. The injured arterial branch was selectively catheterized ( d ) and embolized with micro-coils ( e ).
Case 9: Pseudoaneurysm after Partial Nephrectomy
An 80-year-old man underwent partial nephrectomy and subsequently developed hematuria. Given the relationship in time to the surgery, cross-sectional imaging was deferred and the patient was taken directly to the angiography suite. A selective left renal angiography demonstrated a 1.5-cm left lower pole pseudoaneurysm (arrow, Fig. 10a ) and arteriovenous fistula as evidenced by an early draining renal vein (arrowhead, Fig. 10a ). The feeding vessel was successfully catheterized and embolized with microcoils.
Fig. 10.

Pseudoaneurysm after left partial nephrectomy. An 80-year-old male presented with hematuria after partial nephrectomy. Angiogram ( a ) demonstrated a 1.5 cm pseudoaneurysm which was treated with micro-coils ( b ).
Case 10: Pseudoaneurysm and Retroperitoneal Hematoma after Renal Biopsy
A 75-year-old man presented to the emergency department 2 days after a renal biopsy. CT showed a 4-mm left renal artery pseudoaneurysm in the lower pole (red arrow, Fig. 11a ) with formation of a retroperitoneal hematoma (white arrow, Fig. 11a ). Adjacent to the pseudoaneurysm, there is active extravasation posteriorly (yellow arrow, Fig. 11b ), likely along the biopsy tract, into a left retroperitoneal hematoma measuring approximately 8.3 × 10.9 × 9.6 cm. Angiography demonstrated three small pseudoaneurysms arising from small arterial branches within the lower pole of the left kidney (circled, Fig. 11c ), and an arteriovenous fistula involving a branch arising from the superior aspect of the inferior branch of the left renal artery within the interpolar region of the left kidney, as evidenced by an early draining vein (arrow, Fig. 11c ). The injured vessel was embolized with coils (arrow, Fig. 11d ).
Fig. 11.

Pseudoaneurysm and retroperitoneal hematoma after renal biopsy. A 75-year-old male presented to the Emergency Department 2 days after a renal biopsy. A CT abdomen and pelvis with IV contrast showed a 4-mm left renal artery pseudoaneurysm in the lower pole ( a , red arrow) with formation of a retroperitoneal hematoma ( a , white arrow). Adjacent to the pseudoaneurysm there is active extravasation posteriorly ( b , yellow arrow), likely along the biopsy tract, into a left retroperitoneal hematoma. Angiography demonstrated 3 small pseudoaneurysms arising from small arterial branches within the lower pole of the left kidney, and an arteriovenous fistula involving a branch arising from the superior aspect of the inferior branch of the left renal artery within the interpolar region of the left kidney ( c ). The injured vessel was embolized with coils ( d ).
Case 11: Renal Biopsy Complicated by Intercostal Artery Injury
An 18-year-old man presented to IR for a left renal mass biopsy ( Fig. 12 ). A hematoma formed immediately during the procedure (star, Fig. 11a ) and the existing needle guide (arrow, Fig. 11a ) was injected with Gelfoam (noted as gas in the biopsy tract, Fig. 12b ). A follow-up CT scan showed the development of a large left pleural effusion (not shown). The patient was transfused 2 units of packed red blood cells and was immediately taken for angiography. Selective angiography of the left eleventh intercostal artery (arrow, Fig. 12c ) showed active hemorrhage (arrowhead, Fig. 12c ). The microcatheter was placed distal to the point of bleeding and coil embolized back to the proximal artery (arrows, Fig. 12d ). A chest tube was also placed at that time yielding output of 2.3 L of blood.
Fig. 12.

Renal biopsy complicated by intercostal artery injury. An 18-year-old male presented for a IR left renal mass biopsy ( a ). A hematoma formed immediately and needle guide was injected with gelfoam ( b ). The patient subsequently developed a large left pleural effusion and was immediately taken for angiography. Selective angiography of the left eleventh intercostal artery ( c ) showed active hemorrhage. The microcatheter was placed distal to the point of bleeding and coiled embolized back to the proximal artery ( d ).
Case 12: Urethral Injury after Motorcycle Accident
A 57-year-old man presented to the emergency department after a motorcycle accident. Initial plain radiographs ( Fig. 13a ) revealed bilateral inferior pubic rami, bilateral acetabulum, and left sacral fractures. CT also revealed evidence of prostatic hemorrhage (arrow, Fig. 13b ) suggesting genitourinary laceration. A retrograde urethrogram demonstrated injury of the membranous and prostatic urethra (arrow, Fig. 13c ). Initial attempt at Foley catheter placement in the emergency room was unsuccessful, with return of bloody output. A suprapubic catheter was then placed by IR under direct ultrasound visualization at the bedside, with return of 300 mL of bloody urine ( Fig. 13d ; arrow denotes introducer). Plain radiography confirmed good placement of the suprapubic catheter (arrow, Fig. 13e ). This allowed stabilization of the patient who then underwent orthopaedic surgery for multiple pelvic fractures. Flexible cystoscopy in the operating room later revealed a lateral posterior defect in the prostatomembranous urethra that was bypassed under cystoscopy to allow Foley placement and urethral realignment.
Fig. 13.

Suprapubic catheter placement for traumatic urethral transection. A 57-year-old male who presented after a motorcycle accident. Initial imaging revealed bilateral inferior pubic rami, bilateral acetabulum and left sacral fractures ( a ). CT also revealed evidence of prostatic hemorrhage ( b , black arrow). A retrograde urethrogram demonstrated injury of the membranous and prostatic urethra ( c , white arrow). An SPT was placed by IR under direct ultrasound visualization at the bedside, with return of 300cc of bloody urine ( d ). Pelvis radiograph after surgical intervention shows the SPT in place ( e ).
Conclusion
Historically, the management of renal trauma has gradually shifted toward an increasing emphasis on expectant management and minimally invasive interventions. 27 Nearly a third of patients with high-grade renal injury after trauma have evidence of extravasation from renal arterial branches. 7 The clear majority of these can be managed with transarterial embolization alone. The main endovascular interventions offered by IR are temporizing balloon occlusion, embolization of bleeding arterial branches, and stenting of renal arterial injuries. 14
Iatrogenic injury as seen in the earlier case presentations can result in similar findings to penetrating trauma. Consideration should also be given to any intervening structures that may be injured such as an intercostal artery. Expeditious drainage of hematoma or urinary diversion may also be necessary in some patients. With the use of these minimally invasive techniques, many patients with even severe renal trauma can be managed nonsurgically offering the ability to preserve the kidney and long-term renal function. Recent advancements in the optimization of IR workflow in trauma have potential to further expand the role of IR for the management of hemodynamically unstable trauma patients.
Footnotes
Conflict of Interest The authors have no conflicts of interest.
References
- 1.WISQARS Leading Causes of Death Reports . Accessed August 4, 2020 at:https://webappa.cdc.gov/sasweb/ncipc/leadcause.html
- 2.Santucci R A, Wessells H, Bartsch G. Evaluation and management of renal injuries: consensus statement of the renal trauma subcommittee. BJU Int. 2004;93(07):937–954. doi: 10.1111/j.1464-4096.2004.04820.x. [DOI] [PubMed] [Google Scholar]
- 3.Smith J, Caldwell E, D'Amours S, Jalaludin B, Sugrue M. Abdominal trauma: a disease in evolution. ANZ J Surg. 2005;75(09):790–794. doi: 10.1111/j.1445-2197.2005.03524.x. [DOI] [PubMed] [Google Scholar]
- 4.Gonzalez R P, Falimirski M, Holevar M R, Evankovich C.Surgical management of renal trauma: is vascular control necessary? J Trauma 199947061039–1042., discussion 1042–1044 [DOI] [PubMed] [Google Scholar]
- 5.Voelzke B B, Leddy L. The epidemiology of renal trauma. Transl Androl Urol. 2014;3(02):143–149. doi: 10.3978/j.issn.2223-4683.2014.04.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zealley I A, Chakraverty S. The role of interventional radiology in trauma. BMJ. 2010;340:c497. doi: 10.1136/bmj.c497. [DOI] [PubMed] [Google Scholar]
- 7.Hagiwara A, Sakaki S, Goto H. The role of interventional radiology in the management of blunt renal injury: a practical protocol. J Trauma. 2001;51(03):526–531. doi: 10.1097/00005373-200109000-00017. [DOI] [PubMed] [Google Scholar]
- 8.Ierardi A M, Floridi C, Fontana F. Transcatheter embolisation of iatrogenic renal vascular injuries. Radiol Med (Torino) 2014;119(04):261–268. doi: 10.1007/s11547-013-0343-2. [DOI] [PubMed] [Google Scholar]
- 9.Schmidlin F, Farshad M, Bidaut L. Biomechanical analysis and clinical treatment of blunt renal trauma. Swiss Surg. 1998;5(05):237–243. [PubMed] [Google Scholar]
- 10.Ramaswamy R S, Darcy M D. Arterial embolization for the treatment of renal masses and traumatic renal injuries. Tech Vasc Interv Radiol. 2016;19(03):203–210. doi: 10.1053/j.tvir.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 11.Sampaio F JB, Passos M ARF. Renal arteries: anatomic study for surgical and radiological practice. Surg Radiol Anat. 1992;14(02):113–117. doi: 10.1007/BF01794885. [DOI] [PubMed] [Google Scholar]
- 12.Brewer M E, Jr, Strnad B T, Daley B J. Percutaneous embolization for the management of grade 5 renal trauma in hemodynamically unstable patients: initial experience. J Urol. 2009;181(04):1737–1741. doi: 10.1016/j.juro.2008.11.100. [DOI] [PubMed] [Google Scholar]
- 13.Matsumoto J, Lohman B D, Morimoto K, Ichinose Y, Hattori T, Taira Y.Damage control interventional radiology (DCIR) in prompt and rapid endovascular strategies in trauma occasions (PRESTO): a new paradigm Diagn Interv Imaging 201596(7-8):687–691. [DOI] [PubMed] [Google Scholar]
- 14.Mani N BS, Kim L. The role of interventional radiology in urologic tract trauma. Semin Intervent Radiol. 2011;28(04):415–423. doi: 10.1055/s-0031-1296084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.AAST Patient Assessment Committee . Kozar R A, Crandall M, Shanmuganathan K. Organ injury scaling 2018 update: spleen, liver, and kidney. J Trauma Acute Care Surg. 2018;85(06):1119–1122. doi: 10.1097/TA.0000000000002058. [DOI] [PubMed] [Google Scholar]
- 16.Gould J E, Vedantham S. The role of interventional radiology in trauma. Semin Intervent Radiol. 2006;23(03):270–278. doi: 10.1055/s-2006-948766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vignali C, Lonzi S, Bargellini I. Vascular injuries after percutaneous renal procedures: treatment by transcatheter embolization. Eur Radiol. 2004;14(04):723–729. doi: 10.1007/s00330-003-2009-2. [DOI] [PubMed] [Google Scholar]
- 18.Fisher R G, Ben-Menachem Y, Whigham C. Stab wounds of the renal artery branches: angiographic diagnosis and treatment by embolization. AJR Am J Roentgenol. 1989;152(06):1231–1235. doi: 10.2214/ajr.152.6.1231. [DOI] [PubMed] [Google Scholar]
- 19.Uflacker R, Paolini R M, Lima S. Management of traumatic hematuria by selective renal artery embolization. J Urol. 1984;132(04):662–667. doi: 10.1016/s0022-5347(17)49810-7. [DOI] [PubMed] [Google Scholar]
- 20.Lee R S, Porter J R. Traumatic renal artery pseudoaneurysm: diagnosis and management techniques. J Trauma. 2003;55(05):972–978. doi: 10.1097/01.TA.0000032251.70194.65. [DOI] [PubMed] [Google Scholar]
- 21.Markovic B, Markovic Z, Pejcic T. Embolization of insufficiently ligated renal artery after open nephrectomy with absolute ethanol injection. Diagn Interv Radiol. 2011;17(01):88–91. doi: 10.4261/1305-3825.DIR.2309-08.2. [DOI] [PubMed] [Google Scholar]
- 22.Titton R L, Gervais D A, Boland G W, Mueller P R. Renal trauma: radiologic evaluation and percutaneous treatment of nonvascular injuries. AJR Am J Roentgenol. 2002;178(06):1507–1511. doi: 10.2214/ajr.178.6.1781507. [DOI] [PubMed] [Google Scholar]
- 23.Breyer B N, McAninch J W, Elliott S P, Master V A.Minimally invasive endovascular techniques to treat acute renal hemorrhage J Urol 2008179062248–2252., discussion 2253 [DOI] [PubMed] [Google Scholar]
- 24.Menaker J, Joseph B, Stein D M, Scalea T M. Angiointervention: high rates of failure following blunt renal injuries. World J Surg. 2011;35(03):520–527. doi: 10.1007/s00268-010-0927-0. [DOI] [PubMed] [Google Scholar]
- 25.Zeng G, Zhao Z, Wan S. Failure of initial renal arterial embolization for severe post-percutaneous nephrolithotomy hemorrhage: a multicenter study of risk factors. J Urol. 2013;190(06):2133–2138. doi: 10.1016/j.juro.2013.06.085. [DOI] [PubMed] [Google Scholar]
- 26.Page I H. The production of persistent arterial hypertension by cellophane perinephritis. J Am Med Assoc. 1939;113(23):2046–2048. [Google Scholar]
- 27.Colaco M, Navarrete R A, MacDonald S M, Stitzel J D, Terlecki R P. Nationwide procedural trends for renal trauma management. Ann Surg. 2019;269(02):367–369. doi: 10.1097/SLA.0000000000002475. [DOI] [PubMed] [Google Scholar]


