Abstract
The liver is the second most commonly involved solid organ (after spleen) to be injured in blunt abdominal trauma, but liver injury is the most common cause of death in such trauma. In patients with significant blunt abdominal injury, the liver is involved approximately 35 to 45% of the time. Its large size also makes it a vulnerable organ, commonly injured in penetrating trauma. Other than its position and size, the liver is surrounded by fragile parenchyma and its location under the diaphragm makes it vulnerable to shear forces during deceleration injuries. The liver is also a vascular organ made of large, thin-walled vessels with high blood flow. In severe hepatic trauma, hemorrhage is a common complication and uncontrolled bleeding is usually fatal. In fact, in patients with severe abdominal trauma, liver injury is the primary cause of death. This article reviews the clinical presentation of patients with liver injury, the grading system for such injuries that is most frequently used, and management of the patient with liver trauma.
Keywords: liver, trauma, hepatic injury, bleeding, embolization, interventional radiology
Located anteriorly in the right upper quadrant, the liver is the second most commonly involved solid organ (after spleen) to be injured in blunt abdominal trauma. However, liver injury is the most common cause of death in such trauma, due to multiple large vascular systems. Blunt trauma occurs due to motor vehicle accidents, pedestrian accidents, and falls. In patients with significant blunt abdominal injury, the liver is involved approximately 35 to 45% of the time. 1 Its large size also makes it a vulnerable organ, commonly injured in penetrating trauma. Liver injury is found in 40% of patients undergoing laparotomy for stab wounds and 30% of the patients with abdominal gunshot wounds. 1 Other than its position and size, the liver is surrounded by fragile parenchyma and its location under the diaphragm makes it vulnerable to shear forces during deceleration injuries. 2 The liver is also a vascular organ made of large, thin-walled vessels with high blood flow. In severe hepatic trauma, hemorrhage is a common complication and uncontrolled bleeding is usually fatal. In fact, in patients with severe abdominal trauma, liver injury is the primary cause of death with a 10 to 15% mortality rate. 2 The left lobe along the falciform ligament is commonly injured in frontal impact injuries. The right lobe is commonly injured from side impact trauma and is more frequently associated with concurrent injuries to other organ structures. 3
Presentation and Evaluation
Trauma to the anterior or lateral thoracoabdominal wall is highly suggestive of underlying hepatic injury. Patients presenting with blunt or penetrating abdominal injury are quickly assessed for stable airway and circulation. The initial laboratory tests include but are not limited to a comprehensive metabolic panel, complete blood count, coagulation parameters, and lactate levels. Abnormal liver function may not be seen for several hours to days postinjury. 2
Of all the patients with blunt hepatic trauma, approximately 50 to 85% of patients are hemodynamically stable. 1 Right upper quadrant tenderness, abdominal distension, and hypotension suggest the presence of major hepatic injury with hemorrhage. 2 It is important to assess and monitor for signs of hypovolemic shock in such patients. Unstable patients with hypotension are taken for immediate laparotomy, while stable patients undergo spiral computed tomography (CT) scan to assess the abdomen.
The degree of hepatic injury is evaluated radiologically. If available in the trauma bay, the patient will undergo a focused assessment with sonography for trauma (FAST) exam. The FAST exam is used to evaluate the presence of blood in the abdominal and pericardial cavity, although it does not accurately estimate the degree of injury. The sensitivity and specificity of the exam depends on the experience of the operator and can range from 63 to 100%. 2 In hemodynamically stable patients, the degree of injury is assessed with an abdominal and pelvic CT scan with IV contrast to look for areas of active extravasation and nature of liver injury. 2 Using the CT, the physician can also identify concurrent abdominal injuries or the size of hemoperitoneum, if present. Although not used in an acute setting, magnetic resonance cholangiopancreatography (MRCP) is helpful in cases of suspected biliary injury.
Grading of Liver Injuries
Liver injury is graded based on the 2018 AAST (American Association for the Surgery of Trauma) liver injury scale. The criteria for grading are based on imaging findings (CT scan with dual-phase imaging in arterial and portal venous phases), operative criteria, and pathological criteria (which is done postmortem). 4 Although the AAST criteria is universally used to express severity of injury, it is typically used in combination with the hemodynamic status to determine operative or nonoperative management (NOM). 5 Treatment options include NOM, angiographic embolization, and operative management (OM). Grade I and II injuries with stable hemodynamic status typically undergo NOM in the intensive care unit (ICU), whereas hemodynamically unstable and/or high-grade grade V injuries require urgent OM. Stable grade III to IV injuries usually require angiographic evaluation. 2 Table 1 describes the AAST grading system used for liver trauma.
Table 1. AAST grading system used for hepatic trauma.
| AAST grade | Type of injury | Description (CT findings) |
|---|---|---|
| I | Hematoma | Subcapsular, <10% surface area |
| Laceration | Capsular tear, <1 cm parenchymal depth | |
| II | Hematoma | Subcapsular, 10–50% surface area Intraparenchymal <10 cm diameter |
| Laceration | Capsular tear, 1–3 cm parenchymal depth, <10 cm in length | |
| III | Hematoma | Subcapsular, >50% surface area of ruptured subcapsular or parenchymal hematoma Intraparenchymal hematoma >10 cm diameter or expanding |
| Laceration | >3 cm parenchymal depth Vascular injury with active bleeding contained within liver parenchyma |
|
| IV | Laceration | 25–75% hepatic lobe parenchymal disruption; or 1–3 Couinaud's segments |
| Vascular | Active bleeding extending beyond liver parenchyma into peritoneum | |
| V | Laceration | >75% hepatic lobe parenchymal disruption; >3 Couinaud's segments |
| Vascular | Juxtahepatic venous injuries (i.e., retrohepatic vena cava and central major hepatic veins) |
Abbreviations: AAST, American Association for the Surgery of Trauma; CT, computed tomography.
Nonoperative Management
About 80% of blunt hepatic trauma is treated nonoperatively. 6 NOM is also the treatment of choice for stable patients with stab and gunshot wounds. 7 The most important criteria for NOM are hemodynamic stability and absence of peritoneal irritation. NOM may be used in all hemodynamically stable patients regardless of grade or the volume of hemoperitoneum. 7 NOM requires close patient monitoring, ability for quick access to radiological evaluation, and adequate hospital infrastructure support in case of need for emergent laparotomy. Patients are monitored with serial hematocrits and routine abdominal exams in the ICU. 2 Tarchouli et al showed that NOM in minor liver injuries, grade I to III, had a 89.5% success rate. Furthermore, the study showed that NOM could decrease the risk of short-term and long-term complications from laparotomy including shorter hospital stay, less need for blood transfusion, and lower mortality. 7 Complications of NOM include risk of delayed bleeding, missed bowel injuries, bile leaks, bile peritonitis, hemobilia, abdominal compartment syndrome, and hepatic necrosis/abscess. 7 NOM relies on the coexistence of imaging, hepatic arterial embolization, intensive care surveillance, and delayed surgery. Although NOM could be used in higher grade IV and V injuries, it is more likely to fail. Of the patients who fail NOM, 75% of the time it is due to hemodynamic instability. 2 Failure of NOM does not automatically mean that the patient should undergo surgery, as angiographic therapy may be an effective alternative.
Figs. 1 and 2 demonstrate hepatic traumatic injuries requiring only NOM.
Fig. 1.
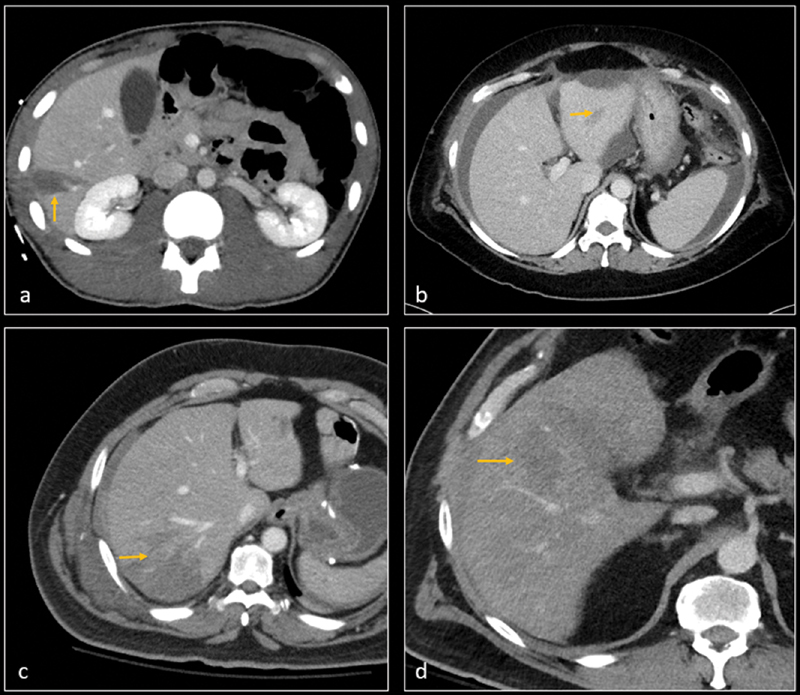
CT angiographies on four different patients show grade 2 liver lacerations (arrows) in segment 5 ( a ), segment 3 ( b ), segment 6 ( c ), and segment 4a ( d ). There was no evidence of active contrast extravasation and patients were hemodynamically stable. All patients remained stable and clinically improved with nonoperative management.
Fig. 2.
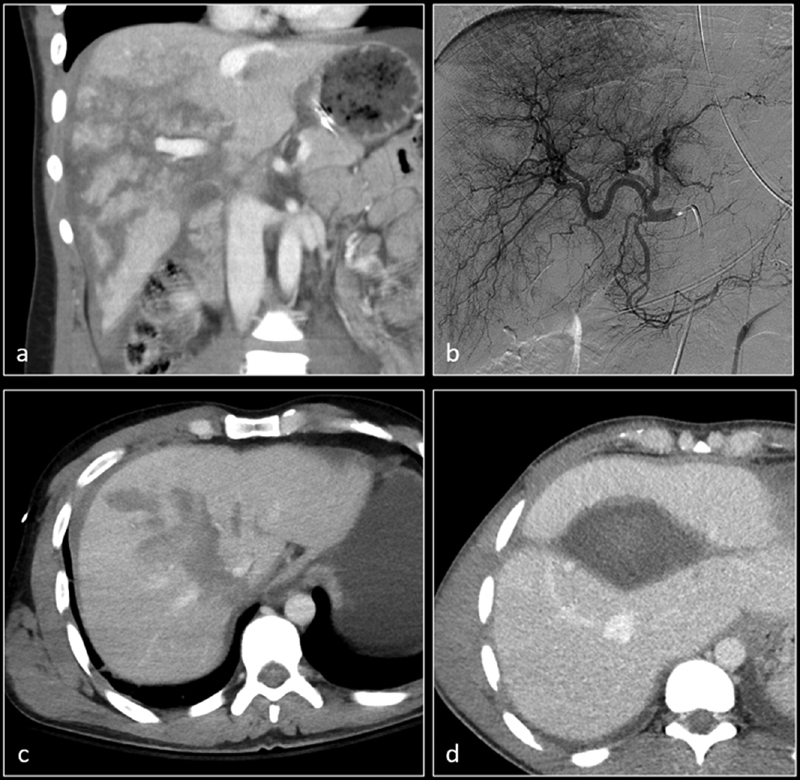
Coronal CT image ( a ) shows grade 4 liver laceration. ( b ) Subsequent angiography demonstrated no active contrast extravasation. A second patient with grade 4 laceration on axial CT image ( c ) and a third patient with large intraparenchymal hemorrhage on axial CT image ( d ) also had negative hepatic angiograms. All patients were managed nonoperatively.
Angiographic Management
Angiographic evaluation with embolization is the treatment of choice in patients who are hemodynamically stable but with imaging evidence of active extravasation, pseudoaneurysm, or arteriovenous/arterioportal fistula. It can also be a treatment option in patients with hemodynamic instability despite operative intervention and a high suspicion for hepatic arterial bleed. Active bleeding can be detected either on CT angiography (detection of a “blush”) or with decreasing serial hematocrit measurements. 3 Grade III and IV injuries are more likely to undergo initial angiographic evaluation, with almost 83% success rate. 2 Arterial embolization protocol uses microcatheter systems and subselective embolization techniques. Gelatin sponge, liquid embolics (such as glue or onyx), and metallic coils are commonly used materials for embolization. 8 Virdis et al reviewed 24 studies on arterial embolization and found a 6% rebleeding rate postembolization, 0.7% mortality, and 28% complication rate. 8 Complications of arterial embolization include biloma (2.8%), hepatic ischemia or necrosis (8.6%), liver abscess (6.8%), gallbladder necrosis (3.6%), abdominal compartment syndrome (2%), peritonitis (1.9%), and septic complications (0.6%). 8 Complications associated with the specific embolic agent have also been reported in literature. 8 Failure or inability to intra-arterially embolize the active hemorrhage may require urgent OM.
Figs. 3 to 11 demonstrate multiple patients with grade II to grade V injuries requiring embolization procedures. Multiple embolic agents, including gelatin sponge, coils, glue, and thrombin, were used as embolic agents.
Fig. 3.
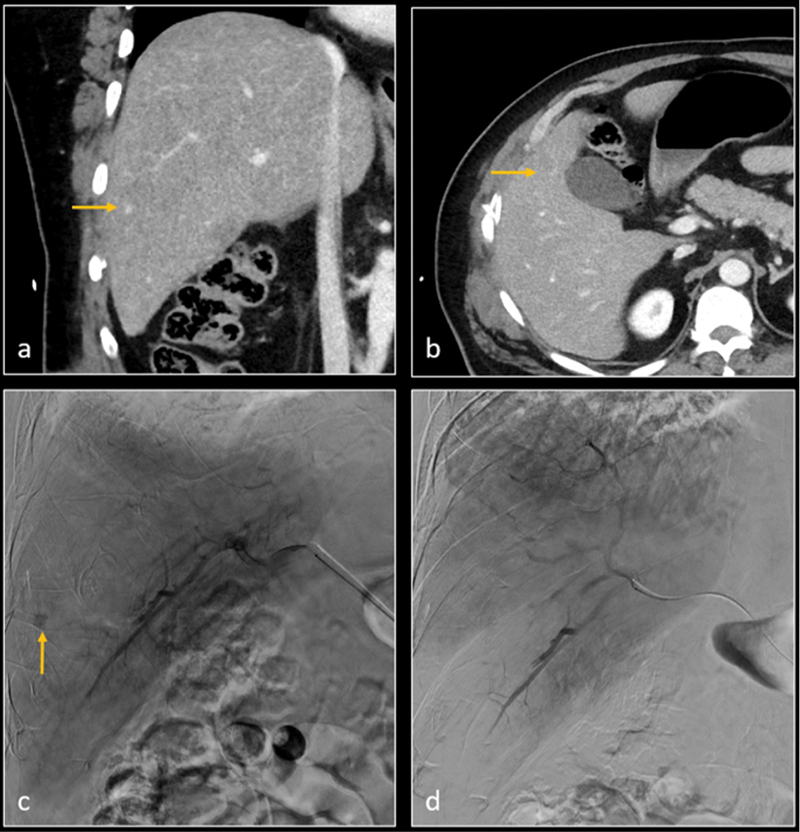
Coronal ( a ) and axial ( b ) CT images demonstrate hepatic segment 5 grade 2 laceration, and a small pseudoaneurysm (arrow). Digital subtraction angiography (DSA—image c ) confirms the pseudoaneurysm (arrow). Gelatin sponge embolization was performed. Subsequent DSA image ( d ) shows resolution of pseudoaneurysm.
Fig. 11.
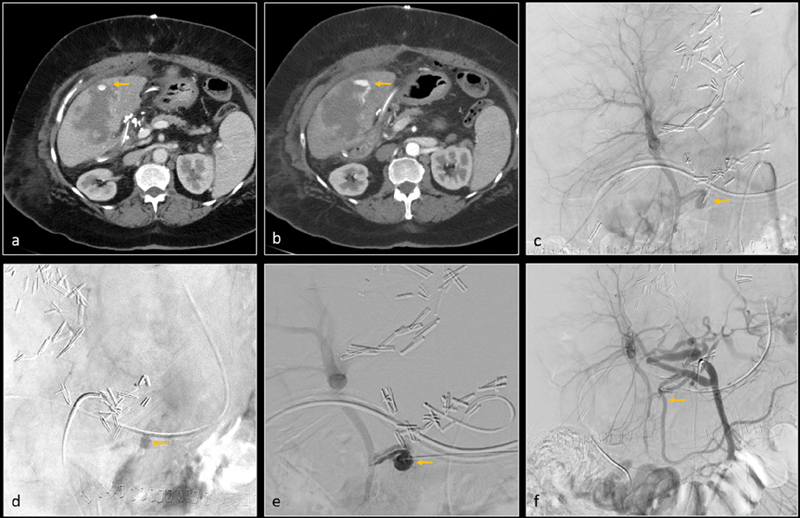
Post–liver transplant, axial CT images ( a and b ) show grade 4 liver laceration and intraparenchymal hemorrhage. There is a small pseudoaneurysm (arrow, image a ) and arterioportal fistula (arrow, image b ) within the hemorrhage. Digital subtraction angiography (DSA) of the replaced right hepatic artery (arising from superior mesenteric artery) was performed. Anteroposterior ( c ) and oblique ( d ) images confirm a pseudoaneurysm (arrows). The pseudoaneurysm was accessed percutaneously under ultrasound guidance. DSA through this percutaneous access ( e ) again confirms the pseudoaneurysm and arterioportal fistula. The pseudoaneurysm was treated with thrombin. Subsequent DSA of the hepatic artery ( f ) shows complete resolution of pseudoaneurysm.
Fig. 4.
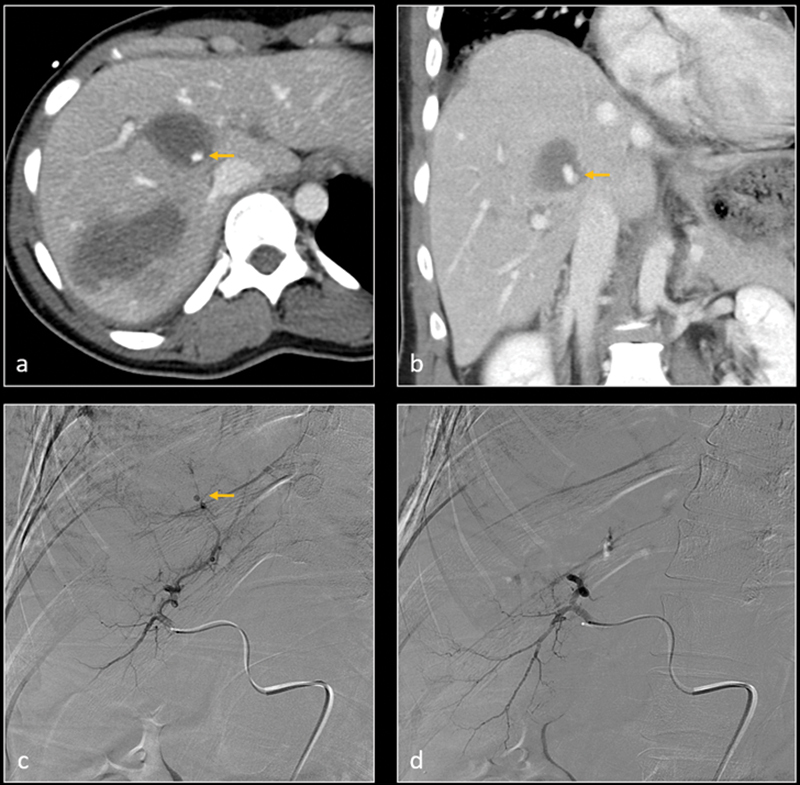
Axial ( a ) and coronal ( b ) CT images demonstrate large intraparenchymal hemorrhage within right hepatic lobe. There is a focus of active contrast extravasation vs. pseudoaneurysm within the hemorrhage (arrow). Subsequent digital subtraction angiography (DSA— c ) identifies the pseudoaneurysm (arrow) and feeding artery. After selective gelatin sponge embolization, DSA ( d ) shows resolution of the pseudoaneurysm.
Fig. 5.
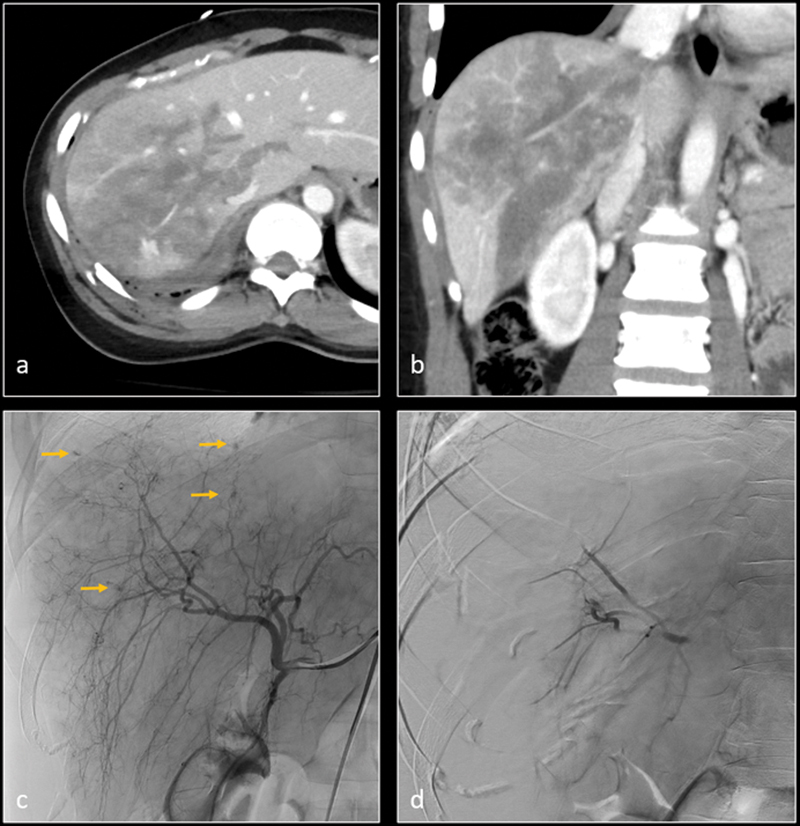
Axial ( a ) and coronal ( b ) CT images demonstrate grade 5 liver laceration. Digital subtraction angiography (DSA) image ( c ) demonstrates multiple micro hemorrhages in the right hepatic lobe (arrows). Nonselective gelatin sponge embolization of the right hepatic artery was performed. Subsequent DSA image ( d ) shows resolution of the micro hemorrhages.
Fig. 6.
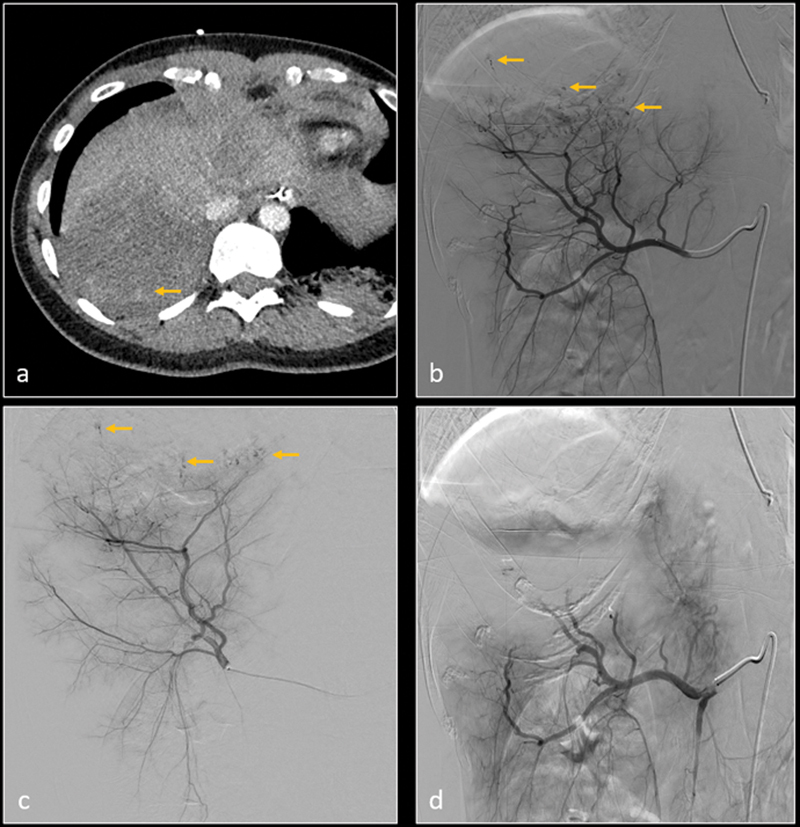
Axial CT image ( a ) shows grade 5 laceration in the right hepatic lobe with poor perfusion and small hemorrhagic foci (arrow). Subsequent digital subtraction angiography (DSA) of right hepatic artery ( b ) and posterior division of right hepatic artery ( c ) confirm multiple micro-hemorrhages (arrows). After gelatin sponge embolization, DSA of right hepatic artery ( d ) shows resolution of micro hemorrhages.
Fig. 7.
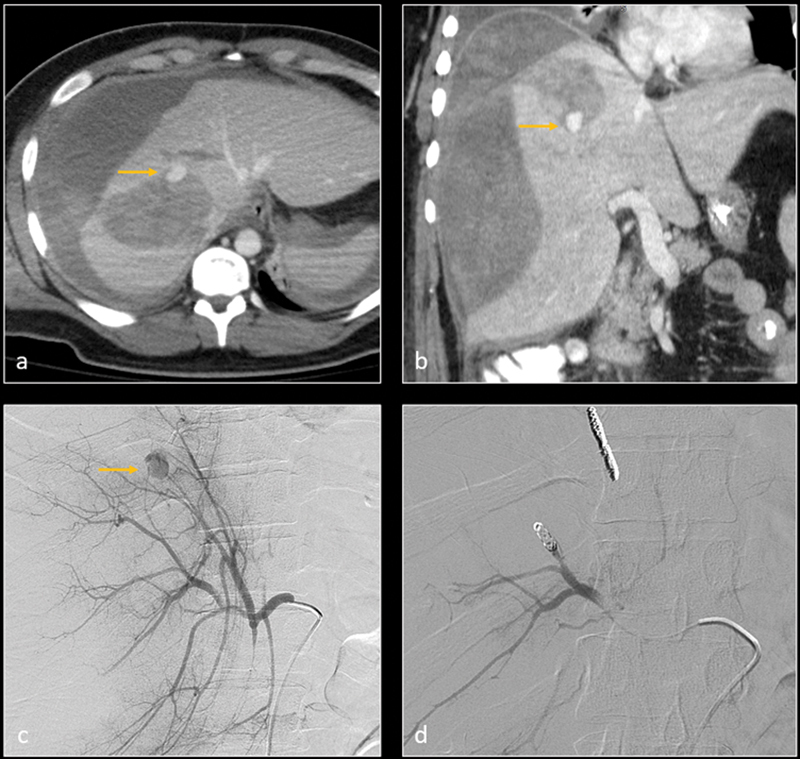
Axial ( a ) and coronal ( b ) CT images in arterial phase demonstrate grade 5 liver laceration with parenchymal hemorrhage, large subcapsular hematoma, and peritoneal hemorrhage. A small pseudoaneurysm vs. active extravasation is visualized within parenchymal hemorrhage (arrow). Digital subtraction angiography (DSA— c ) confirms the pseudoaneurysm (arrow). The feeding artery was embolized with metallic coils. Subsequent DSA image ( d ) shows resolution of the pseudoaneurysm.
Fig. 8.
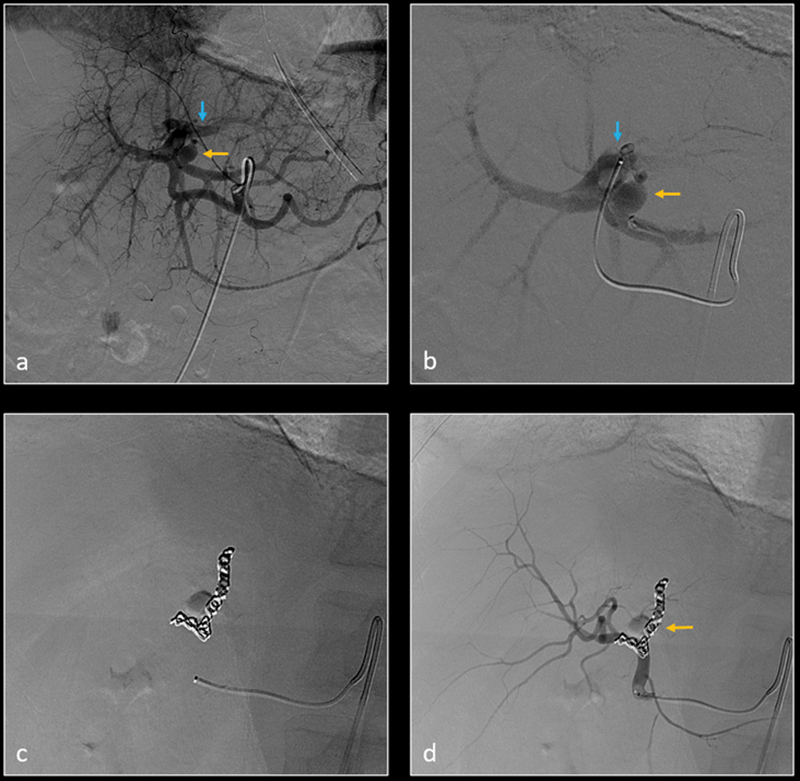
Patient was transferred from outside facility with no imaging studies available at the time of angiography. Digital subtraction angiography (DSA) of left hepatic artery ( a ) and more selective segment 2 hepatic artery ( b ) show a small left hepatic artery pseudoaneurysm (yellow arrow) and left hepatic artery–portal vein fistula (blue arrow). The feeding artery was selectively embolized using metallic coils ( c ). Subsequent DSA of left hepatic artery ( d ) demonstrates resolution of the pseudoaneurysm and arterioportal fistula.
Fig. 9.
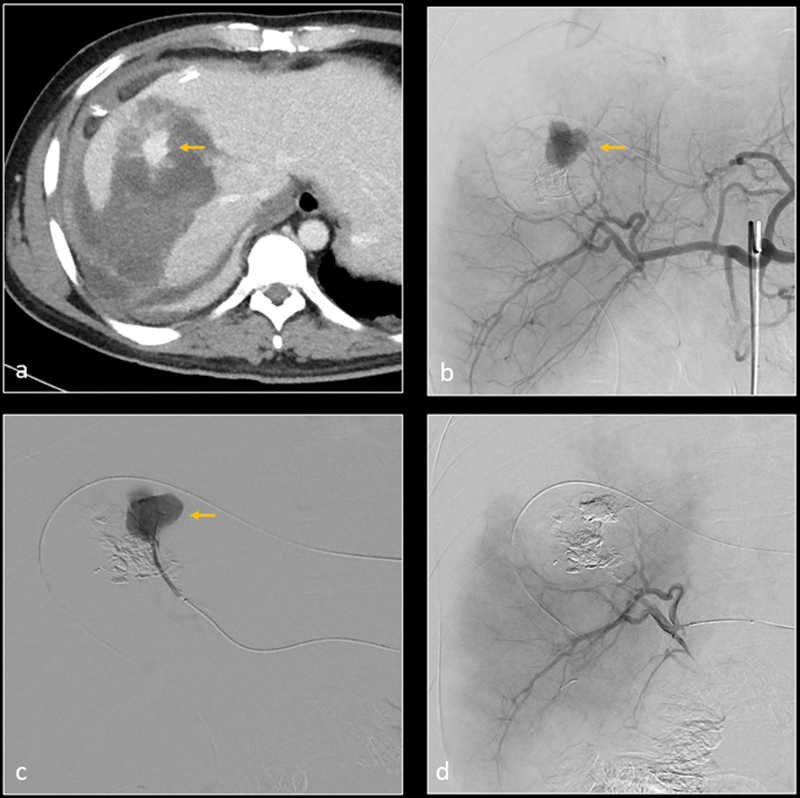
Axial ( a ) and coronal ( b ) CT images demonstrate grade 5 liver laceration. Selective digital subtraction angiography (DSA) of the right hepatic artery (RHA) anterior division demonstrates a small pseudoaneurysm (yellow arrow) and parenchymal blush (blue arrow). Selective embolization of the anterior division of RHA was performed with N-butyl cyanoacrylate (n-BCA) glue. Subsequent DSA image ( d ) shows resolution of the pseudoaneurysm and the suspected parenchymal hemorrhage.
Fig. 10.
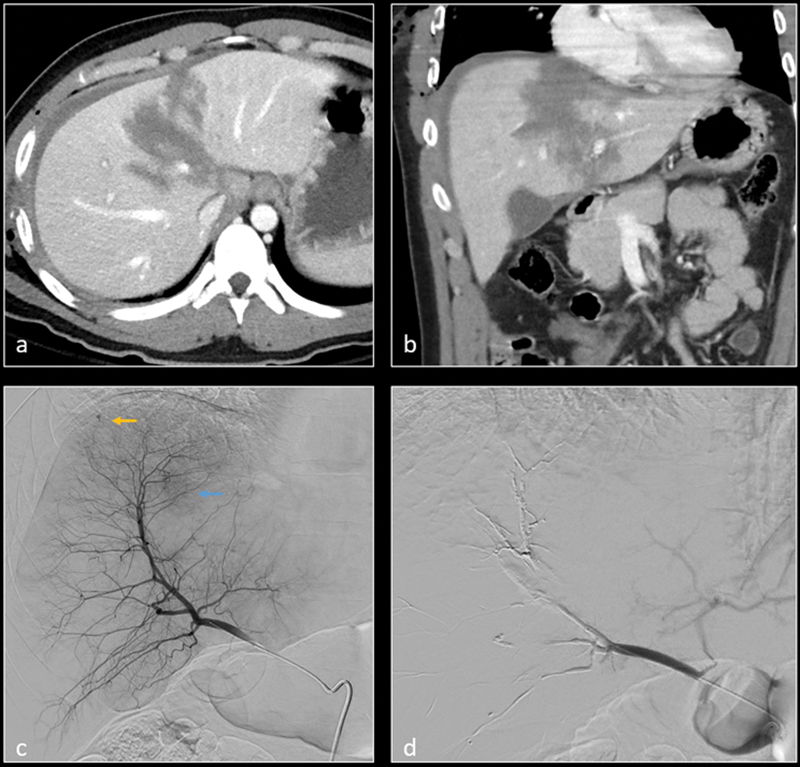
Patient had emergent hepatic arterial embolization at outside facility (imaging studies not available) and was then transferred for higher level care. Axial CT ( a ) image shows grade 5 liver laceration with focus of active contrast extravasation (arrow). Digital subtraction angiography (DSA) of the common hepatic artery ( b ) and anterior division of right hepatic artery ( c ) confirm active contrast extravasation. Selective embolization was performed using n-BCA glue. Postembolization DSA ( d ) image of right hepatic artery shows no further hemorrhage. Large wedge-shaped area of hypoperfusion in the right lobe corresponds to the lacerated and subsequently embolized segments.
Fig. 12 demonstrates a late complication of NOM of hepatic traumatic injury, resulting in liver abscess formation. These late complications may also be amenable to image-guided interventions, as was performed in this instance.
Fig. 12.
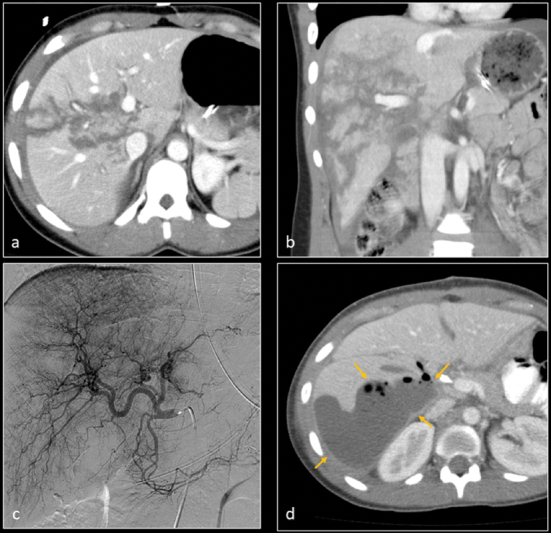
Axial ( a ) and coronal ( b ) CT images demonstrate grade 5 laceration of right hepatic lobe. Digital subtraction angiography ( c ) of hepatic arteries did not show active hemorrhage. Patient was managed nonoperatively. About 2 weeks later, he developed septic shock. Contrast-enhanced axial CT image ( d ) shows a well-formed abscess in the right hepatic lobe. A drainage catheter was subsequently inserted in the hepatic abscess collection.
Operative Management
Hemodynamic instability is an important indicator for urgent OM. Patients may go to the operating room either directly from the trauma bay, or after the failure of NOM, or after angiographic stabilization or failure for definitive surgical repair. Emergency laparotomy is rare, with an incidence of 20%, and is indicated when the patient experiences the “lethal triad” 6 of coagulopathy, acidosis, and hypothermia. Emergent laparotomy management includes first controlling hemorrhage, and then controlling the gastrointestinal contamination by packing the abdomen and localizing the injury. 2 To minimize hemorrhage, the liver may be manually compressed, the porta hepatis may be clamped using the Pringle maneuver, or laparotomy pads may be used for perihepatic packing. Patients experiencing uncontrolled hemorrhage usually have high-grade V injuries and experience high mortality. 2 More destructive injuries may require selective ligation of the feeding hepatic artery or haptic lobectomy. Angiography with embolization may be used subsequently for selective patients and is an adjunct treatment for patients with initial damage control laparotomy. In patients not undergoing damage control, other hemostatic techniques can also be applied based on the injury type. Parenchymal injuries can be reapproximated using suture hepatorrhaphy. Hemostatic agents can also be placed on the damaged parenchyma to maintain hemostasis. 2 Common postoperative complications are infectious and include pneumonia, peritonitis, and intraabdominal abscess. In a retrospective study looking at OM of hepatic trauma, hepatorrhaphy was the most commonly performed surgical management, and damage control was applied only on 6.5% of patients. The patients undergoing OM had a survival rate of only 87.5%. 9 This study showed that despite advances in OM, mortality rates from liver trauma still remain high.
Conclusion
Interventional radiologist has significant role in the evaluation and management of hepatic trauma. Despite advances in OM of trauma patients, current literature supports primary angiographic evaluation in patients not suitable for NOM, as well as supportive role in patients after failed NOM or prior/adjunct to OM. A good understanding of the hepatic vascular anatomy and excellent microcatheter skills is essential for best patient outcome.
Footnotes
Conflict of Interest None declared.
References
- 1.Feliciano D V, Rozycki G S. Hepatic trauma. Scand J Surg. 2002;91(01):72–79. doi: 10.1177/145749690209100112. [DOI] [PubMed] [Google Scholar]
- 2.Taghavi S, Askari R. Treasure Island, FL: StatPearls; 2020. Liver Trauma. [Google Scholar]
- 3.Padia S A, Ingraham C R, Moriarty J M.Society of Interventional Radiology Position Statement on endovascular intervention for trauma J Vasc Interv Radiol 20203103363–36900., e2 [DOI] [PubMed] [Google Scholar]
- 4.AAST Patient Assessment Committee . Kozar R A, Crandall M, Shanmuganathan K. Organ injury scaling 2018 update: spleen, liver, and kidney. J Trauma Acute Care Surg. 2018;85(06):1119–1122. doi: 10.1097/TA.0000000000002058. [DOI] [PubMed] [Google Scholar]
- 5.Ruscelli P, Gemini A, Rimini M. The role of grade of injury in non-operative management of blunt hepatic and splenic trauma: case series from a multicenter experience. Medicine (Baltimore) 2019;98(35):e16746. doi: 10.1097/MD.0000000000016746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Letoublon C, Amariutei A, Taton N.Management of blunt hepatic trauma J Visc Surg 2016153(4, Suppl):33–43. [DOI] [PubMed] [Google Scholar]
- 7.Tarchouli M, Elabsi M, Njoumi N, Essarghini M, Echarrab M, Chkoff M R. Liver trauma: What current management? Hepatobiliary Pancreat Dis Int. 2018;17(01):39–44. doi: 10.1016/j.hbpd.2018.01.013. [DOI] [PubMed] [Google Scholar]
- 8.Virdis F, Reccia I, Di Saverio S. Clinical outcomes of primary arterial embolization in severe hepatic trauma: a systematic review. Diagn Interv Imaging. 2019;100(02):65–75. doi: 10.1016/j.diii.2018.10.004. [DOI] [PubMed] [Google Scholar]
- 9.Kalil M, Amaral I M. Epidemiological evaluation of hepatic trauma victims undergoing surgery. Rev Col Bras Cir. 2016;43(01):22–27. doi: 10.1590/0100-69912016001006. [DOI] [PubMed] [Google Scholar]


