Abstract
Cervical carotid and vertebral artery traumatic injuries can have a devastating natural history. This article reviews the epidemiology, mechanisms of injury, clinical presentation, and classification systems pertinent to consideration of endovascular treatment. The growing role of modern endovascular techniques for the treatment of these diseases is presented to equip endovascular surgeons with a framework for critically assessing patients presenting with traumatic cervical cerebrovascular injury.
Keywords: interventional radiology, trauma, carotid artery, vertebral artery, vascular injury
Epidemiology, Mechanisms, and Clinical Presentation
Traumatic injuries of the carotid and vertebral arteries can lead to catastrophic hemorrhagic and ischemic consequences. Understanding of the epidemiology, classification systems, natural history, and emerging evidence for treatment strategies is critical for the appropriate management of these injuries. The prevalence of traumatic cervical carotid and vertebral artery injuries is 20% with penetrating trauma and 1% with blunt trauma. 1 2 These injuries have been historically underappreciated because patients with noncatastrophic injuries may not present until some years later with hemorrhagic and cerebrovascular ischemic insults. 3
Blunt Injury
In the setting of trauma, blunt cervical cerebrovascular injury is seen in 0.1 to 1.6% of all adults, 0.6% of all pediatric patients, and 6 to 12% in the military population. 2 4 The incidence of cerebrovascular injury following blunt trauma with traumatic brain injury is as high as 9.2%. 5 The most common mechanism of injury associated with blunt trauma to the cervical vessels include motor vehicle collision (41–70%), direct cervical trauma (10–20%), automobile versus pedestrian (5–15%), and hanging (5%). 1 6 7 The most common associated injuries include closed-head injuries (50–65%), facial fractures (60%), cervical spine fractures (50%), and thoracic injuries (40–51%). Ninety-two percent of patients with vertebral artery injuries have an associated fracture of the cervical spine involving the transverse foramen or facet subluxation. 8 Multiple cervical vessel involvement is seen in 18 to 38% of blunt cerebrovascular injury patients. 9 10 Blunt carotid and vertebral artery injury has a reported mortality of approximately 30% and severe neurologic morbidity of up to 56% in survivors. 9
The widely adopted Biffl scale (also known as the Denver scale) of blunt carotid injury categorizes the spectrum of cervical arterial vascular injury ( Table 1 ). 6 This should not be confused with the Denver screening criteria which defines the risk for blunt cerebrovascular injury with clinical and historical risk factors.
Table 1. Biffl/Denver grading scale for blunt carotid injury.
| Grade | Definition |
|---|---|
| Grade 1 | Minor intimal irregularity or dissection with less than 25% luminal narrowing |
| Grade 2 | Dissection with intramural hematoma resulting in greater than 25% luminal narrowing |
| Grade 3 | Pseudoaneurysm |
| Grade 4 | Arterial occlusion |
| Grade 5 | Transection with extravasation |
Source: Adapted from Biffl et al. 6
Penetrating Injury
Penetrating injuries in the setting of trauma can involve the carotid sheath of the common (CCA), external (ECA), and internal carotid arteries (ICA); the vertebral artery (VA); the subclavian artery; thyroid vessels; as well as the veins including the vertebral, brachiocephalic, and jugular veins. The incidence of carotid arterial injury in penetrating neck trauma is 11 to 13%. The CCA is the most commonly injured (73%), followed by the ICA (22%) and the ECA (5%). 11 12 VA injuries are far less common in penetrating trauma owing to their course through the vertebral transverse foramen. 13 14 Mechanisms of penetrating trauma are most commonly knife wounds followed by low- and high-velocity projectiles. The most important determinant of the degree of tissue damage is the mechanism and kinetic energy transmitted. Consequences of delayed diagnosis include progression to pseudoaneurysm, arteriovenous fistula, or rupture. There is a 0.3 to 3.4% risk of recurrent stroke from symptomatic cervical arterial dissection in the setting of penetrating trauma. 15 Penetrating injury to the carotid and vertebral arteries has a mortality of 50%. 16 17 18
Penetrating neck injuries are categorized into anatomic zones to help guide management. 19 Zone I is above the angle of the mandible. Zone II is below Zone I to the lower margin of the cricoid cartilage. Zone III is below Zone II to the thoracic outlet.
Iatrogenic Injury
Iatrogenic neck vascular injury can be caused by abrupt neck chiropractic manipulation including rotation or hyperextension. This can result in dissection of the cervical and cranial segments of the carotid or vertebral arteries and can be severe enough to require endovascular stenting and cranial surgery. These injuries can result in permanent disability or death in up to 31% of patients. 20 Iatrogenic needle and catheter injury can be caused by complications of central line placement following cervical nerve root block injections, and head and neck percutaneous biopsies. Cervical arterial dissections can occur during diagnostic cerebral angiography procedures, though these are exceedingly rare with a per procedure incidence of 0.4% with the vertebral artery being the most commonly dissected vessel. 21 More delayed iatrogenic injury from surgical and radiation treatment of head and neck cancer includes carotid blowout syndrome with a prevalence of 3.9% in this population. 22 Arteriovenous fistulas between the carotid artery and adjacent jugular vein can occur as a complication of central line placement. Vertebrovenous fistulas between the vertebral artery and adjacent spinal veins can occur following cervical nerve root injections.
Clinical Presentation
Patients with traumatic carotid or vertebral artery injury most consistently present with ipsilateral headache or pain along the course of the vessel. 23 Ischemia may manifest as a stroke or delayed neurologic deficit. Extracranial vascular injury causes 1% of all strokes and 5% of ischemic strokes in young adults. Clinical signs may include an ipsilateral bruit, Horner's syndrome, anisocoria, shock, or decreased and absent pulses. With rupture or hemorrhage, clinical signs can include meningeal irritation, vocal changes with cervical hemorrhage, and airway compromise resulting in respiratory compromise. A negative physical exam in penetrating neck injury has a 97% negative predictive value for vessel injury. 24 However, including both blunt and penetrating cervical trauma, 20 to 33% of traumatic carotid or vertebral dissections may be asymptomatic. 25 Vertebrovenous arteriovenous fistulas may present with pulsatile tinnitus, as the arterial pressures are increasingly transmitted into the venous system.
Diagnostic Strategies and Differential Considerations
Following an evaluation of the mechanism of injury and visual clinical examination for neck bruising or signs of penetrating trauma deep to the platysma muscle, an assessment of hemodynamic stability determines the diagnostic pathway. Unstable patients with severe penetrating neck injury in nearly all situations require immediate surgical exploration. Penetrating Zone I and Zone III injuries requiring immediate operative intervention should not be delayed by consideration of endovascular treatment options. Early revascularization leads to the best outcomes when managing carotid injuries. Coma portends a poor outcome with these injuries. 26
Radiological imaging is almost universally obtained for suspected vascular injury. Regardless of the imaging modality, common imaging features of vascular injury include identification of an intimal flap, long tapered narrowing or occlusion, vessel dilation with a dissecting aneurysm pattern, or the identification of crescentic intramural hematoma. The choice of imaging modality is guided by availability and inherent diagnostic accuracy based on the mechanism and acuity of injury.
With stroke thrombectomy becoming standard of care for selected large vessel occlusion, multidetector computed tomography angiography (MDCTA) has become more wide available 24/7, is reliably higher in quality, can be rapidly acquired (in minutes), and can detect non–vascular-associated injury. 27 MDCTA sensitivity and specificity for diagnosis of vascular injury approaches 100%. 28 29 30 The rate of clinically significant lesions missed by CTA are low. 31 For these reasons, MDCTA is considered the most appropriate first-line imaging modality in neck trauma.
Catheter-based digital subtraction angiography (DSA) has been the gold standard for the detection of vascular injury for decades before MDCTA. 32 More recently, DSA is performed in conjunction with MDCTA if endovascular procedures such as embolization or stent placement are being considered, or if there is metal causing beam-hardening artifact on MDCTA. With suspected neck vascular injury, DSA should include both carotids and vertebral arteries with imaging of the intracranial carotid segments (Zone I and above) as well as the thoracic outlet and aortic arch (Zone III). DSA has a low rate of significant complication (<1%), though this rate is higher in the emergent or urgent setting of recent trauma. 33 For open surgical planning, DSA has not proven to provide useful information beyond MDCTA. 34 35 36
Duplex ultrasound of the neck is operator dependent. This should be considered only if MDCTA is not available or for follow-up of known injuries in select circumstances. Magnetic resonance angiography (MRA) is technically sophisticated, but in the setting of trauma and an often-unreliable history, it can be contraindicated due to the possible presence of medical implants or metallic foreign bodies. Additional drawbacks of MRA in trauma are the relative lack of emergent availability, longer scan time, and inherent difficulty in monitoring unstable patients. MRA is not considered a first-line study.
Important differential imaging considerations that can mimic traumatic vessel injury include atherosclerotic plaque causing short-segment stenosis with diffuse distal adaptive narrowing, reversible vasospasm caused by catheter-induced irritation during diagnostic angiography, and Takayasu arteritis with smooth symmetric narrowing of the arch vessels.
Classification, Natural History, and Treatment Considerations
The pathophysiologic classification system of cervical cerebrovascular injury provides a framework for understanding the injury scale used for triage and treatment consideration.
Dissections are defined as an intimal defect with delamination of the vessel wall allowing blood to enter a false lumen with a blind pouch. 37 38 Presence of an intimal flap is pathognomonic. A long, tapered segment of vascular narrowing or occlusion described as flame-shaped can be present with variable enlargement of the lumen caliber. Stenosis of the true lumen may be caused by mass effect from the false lumen.
Pseudoaneurysms are defined as vessel wall disruption beyond the intimal layer with involvement of the media and occasionally the adventitia. This is most commonly seen in the upper neck and skull base presumably due to the transition between the mobile and more fixed segments of the cervical vessels as they enter the skull. 39
Transections are seen as eccentric vascular protrusions with variable extravasation. Arteriovenous fistulas are absent from the Biffl blunt carotid injury scale but are appropriate considerations for inclusion in a larger discussion of traumatic cervical cerebrovascular injury.
General Endovascular Treatment Considerations
In the modern endovascular era, these minimally invasive techniques are becoming safer with both advances in device technology and growing knowledge of the technical risks. 40 While there has been a trend toward decreased open surgical management of blunt carotid injury with a steady increase in endovascular treatment, the overall rate of intervention versus nonoperative medical management has remained unchanged. 41 Penetrating trauma in Zones I and III that have historically been primarily managed with open surgery is increasingly being managed endovascularly as adjunct techniques and sometimes as primary methods for treatment. 42 43
Broadening the application of the Biffl blunt carotid injury scale to both blunt and penetrating trauma is useful in the discussion of the natural history and endovascular treatment of these traumatic vascular injuries. While we will use the Biffl scale as a framework for discussing the management of blunt and penetrating trauma, it should be noted that references to this scale in the literature are usually focused on blunt cerebrovascular traumatic injury.
Grade 1: Intimal Irregularity and Minor Dissection
Grade 1 vascular injuries ( Fig. 1 ) are characterized as angiographically apparent small intimal irregularities and dissections with less than 25% luminal stenosis. Evaluation of these injuries weeks to months later indicates that 57 to 70% of these heal on follow-up, 4 to 14% progress to a higher grade, and 1 to 3% have a risk of ischemic complications. 15 44 Short-term follow-up imaging is prudent for low-grade lesions managed conservatively. One series demonstrated that angiographic evaluation after 7 to 10 days changed management in 61% of initially low-grade blunt cerebrovascular injuries. 45
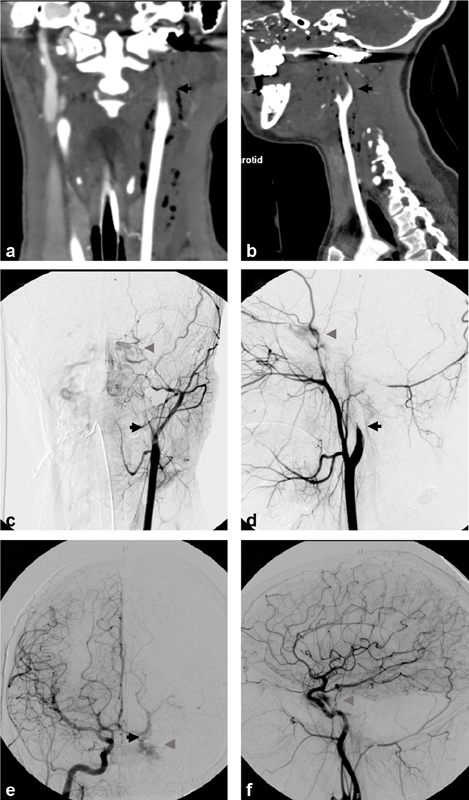
Fig. 1.
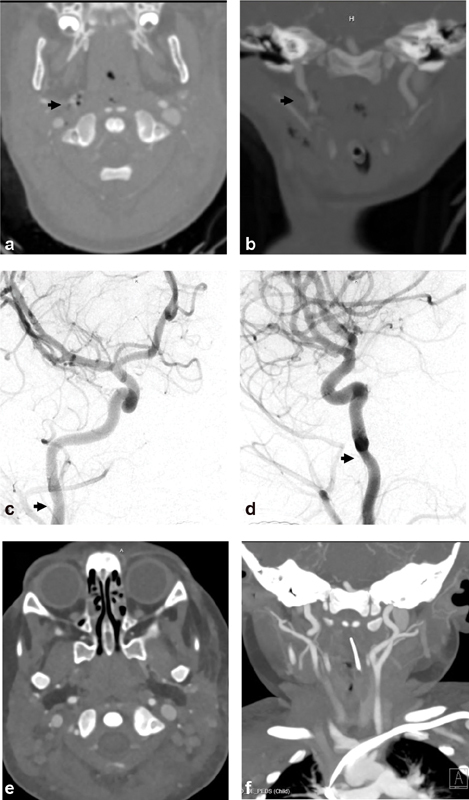
Biffl grade 1: A 22-month-old girl presented after multiple dog bites resulting in head, neck, and body injuries including skull and spine fractures. Axial ( a ) and coronal ( b ) computed tomography angiographic (CTA) images demonstrate small focal irregularity (arrows) of the distal right cervical internal carotid artery (ICA). Digital subtraction angiographic (DSA) images ( c —anteroposterior projection, d —lateral projection) demonstrate a Biffl grade 1 injury with focal narrowing (arrows) of the distal cervical right ICA near the skull base (arrows). Follow-up CTA ( e —axial, f —coronal images) 1 week later demonstrates resolution of the mild focal right ICA stenosis.
Grade 1 injuries are treated medically with antithrombotics to prevent ischemic complications during the healing phase while prothrombotic subintimal collagen is exposed to the lumen. 32 There has been no reported difference in clinical or imaging outcomes with heparin, single-antiplatelet, or dual-antiplatelet treatment. 32 45 46 47 Heparin with transition to antiplatelet agents is a reasonable approach with treatment continuation until there is imaging resolution of the injury which corresponds to the period of thromboembolic ischemic risk. Small grade 1 dissections caused during catheter angiography can be managed conservatively with antithrombotic medication or no treatment with close monitoring for neurologic events. 21
Grade 2: Dissection with More than 25% Stenosis
Grade 2 vascular injuries ( Fig. 2 ) are dissections with large intimal flaps that result in more than 25% luminal stenosis. The spontaneous healing of these injuries is much less frequently than grade 1 injuries, with 29 to 70% progressing to pseudoaneurysms or occlusions. 6 45 The mortality from posttraumatic cervical carotid grade 2 dissection ranges from 20 to 40% with significant neurologic sequelae ranging from 12 to 80%. Mortality from vertebral artery grade 2 posttraumatic dissection ranges from 4 to 8% with significant neurologic sequelae ranging from 14 to 24%. 2
Fig. 2.
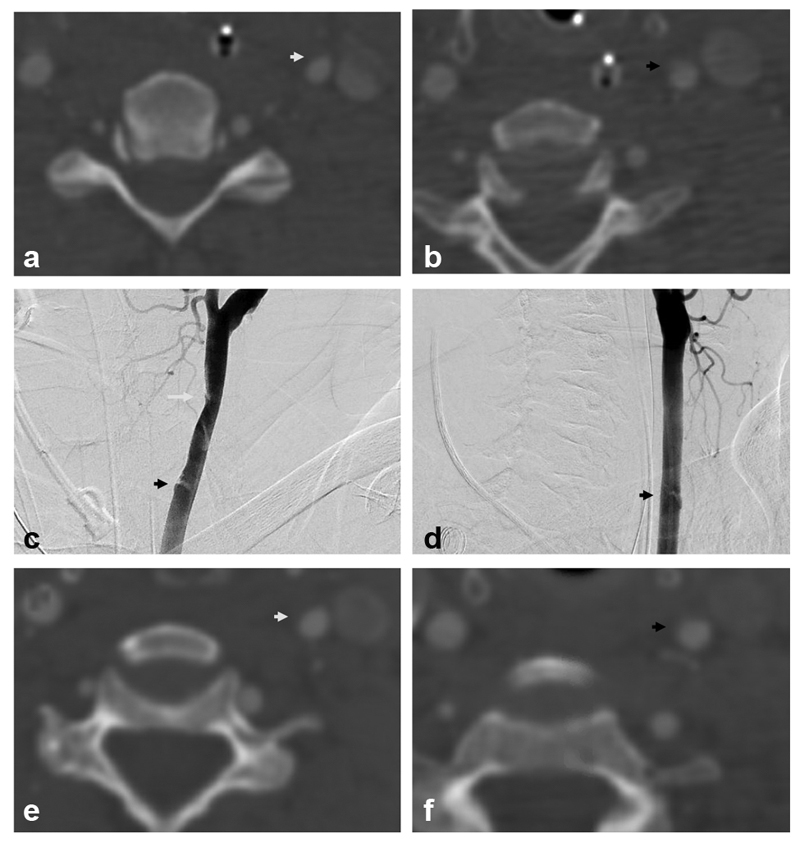
Biffl grade 2: A 55-year old presented after motor vehicle collision with cervical spine fracture and mild traumatic brain injury (TBI). Axial computed tomography angiographic (CTA) images demonstrate mild narrowing of the left common carotid artery (CCA) at C5 ( a , white arrow) and a dissection flap at C6/7 ( b , black arrow). Digital subtraction angiographic (DSA) images in oblique projections ( c, d ) demonstrate a Biffl grade 2 injury with focal narrowing of the left CCA at C5 (white arrow) and dissection flap at C6/7 (black arrows). Follow-up CTA axial images ( e, f ) 6 weeks later demonstrate improvement in vessel caliber at C5 (white arrow) and C6/7 (black arrow).
The goal of treatment in grade 2 injuries is to prevent false lumen expansion, increase the true lumen diameter, thrombolysis in the setting of acute stroke, and fenestration or stenting with larger dissections. Antithrombotics are essential for the prevention of ischemic sequelae and may be used as primary treatment in asymptomatic patients or as an adjunct with intervention. In cases that are symptomatic despite medical management or if the dissection becomes flow-limiting, endovascular intervention is becoming standard, accepted management. 48 49 However, candidates for endovascular intervention should be selected with caution since a large retrospective review in a level I trauma center comparing outcomes demonstrated a 45% rate of vessel occlusion with carotid stenting versus 5% with antithrombotics in patients with grade 2 carotid artery. 50 Vertebral artery grade 2 dissection treatment is more controversial with no level I and limited level II evidence for treatment. If asymptomatic, the vast majority of vertebral artery dissections are treated with anticoagulation or antiplatelet therapy. 51
Endovascular treatment options for grade 2 injuries include the use of various categories of stents. A meta-analyses of 64 cases demonstrated a 99% technical success rate, 1.3% postprocedural complication rate, and 1.4% neurological event rate with subsequent meta-analysis confirming these findings. 52 53 54 55 The most useful schema for categorizing stents is into balloon-expandable versus self-expanding, bare metal versus covered, and flow-diverting stents.
Self-expanding stents are more common and more widely studied with grade 2 injuries. While they are more challenging to precisely position than balloon-expandable stents, they are preferred in the cervical vessels for their ability to tolerate external compression. The Wallstent (Boston Scientific Corporation) was the most commonly reported endovascular stent in this setting, but is no longer available. The SMART stent (Cordis Corporation) has been reported as used in 18% of cases in one series with a very high rate of technical success. 53 Balloon-expandable stents can be placed more precisely, but are not recommended due to their susceptibility to external compression and resulting stenosis.
Bare metal stents can help with apposition of the dissection flap against the adventitial wall, and in grade 2 injury bare metal stents are preferred over covered stents.
Flow-diverting devices are stents with a low porosity woven design. The most widely used internal carotid flow-diverting device is the Pipeline Embolization Device (Medtronic, Minneapolis, MN). Flow diverters lack the self-expanding properties of more porous stents but offer advantages in tortuous segments in the high cervical and skull base regions. This approach has been demonstrated in a few case series with good results in the cervical and vertebral arteries, but represents an off-label use of this device in the United States. 56 57 58 Newer hybrid flow-diverting stents with less porosity but more intermediate self-expanding properties are emerging and may represent a more favorable compromise for future device selection.
Grade 3: Pseudoaneurysms
Grade 3 pseudoaneurysms ( Fig. 3 ) represent vascular injuries involving the media and adventitial layers of the vessel wall. Pseudoaneurysms of the cervical carotid or vertebral artery can result in cerebral ischemia via thromboembolism or flow-limiting mass effect. Pseudoaneurysms have only an 8% rate of angiographic improvement without treatment. 6 45
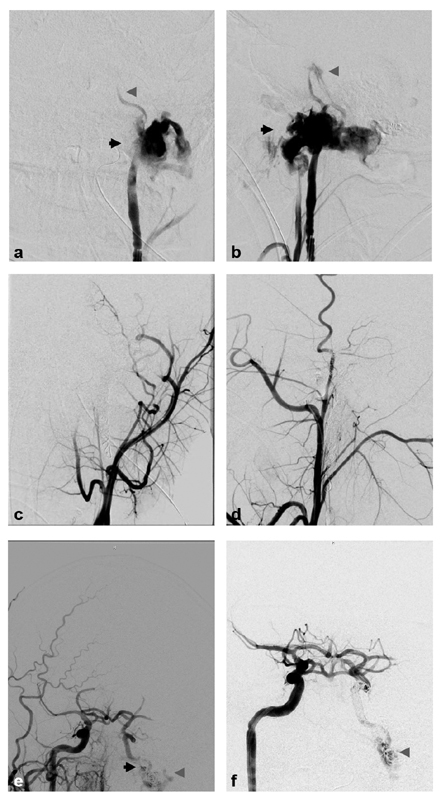
Fig. 3.
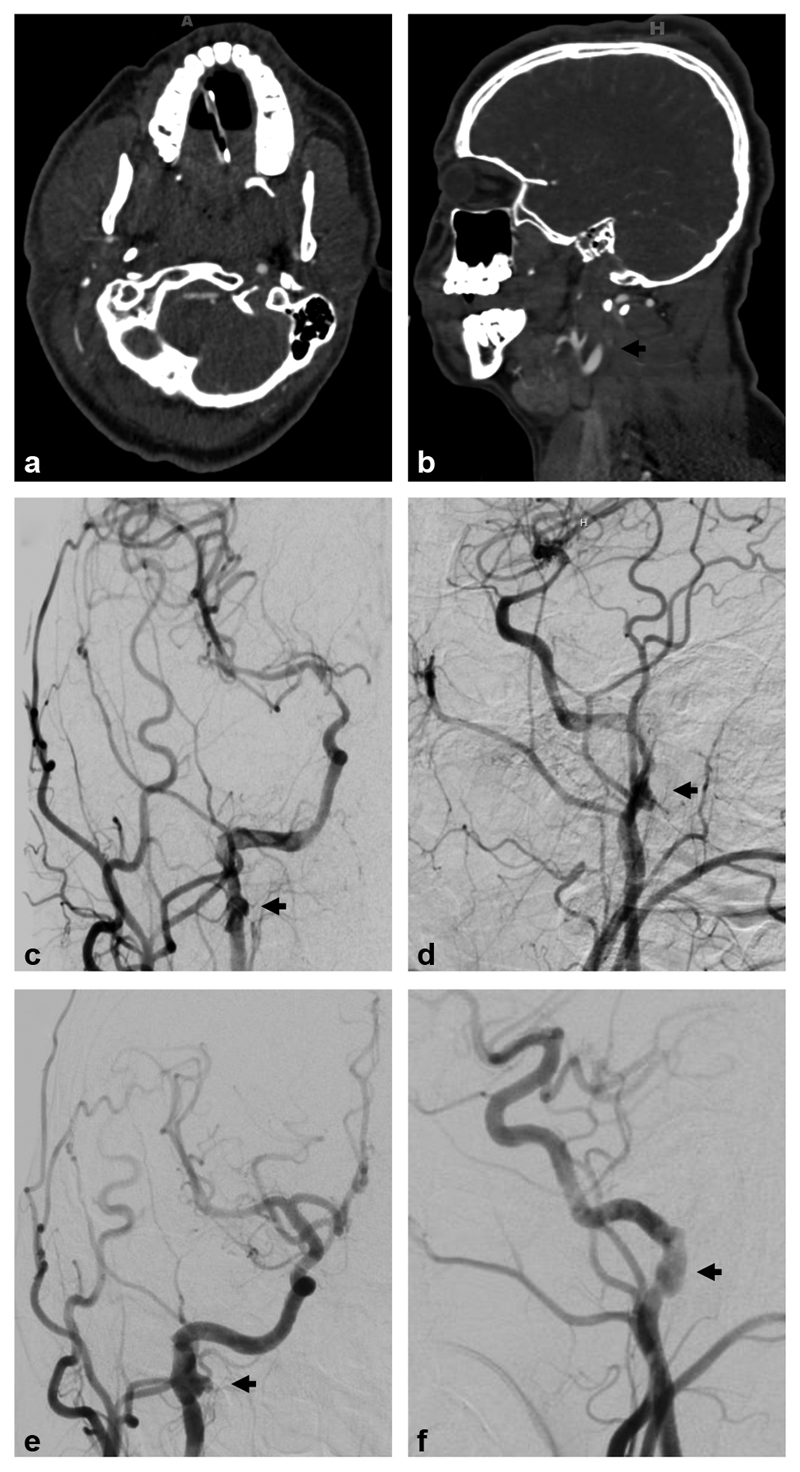
Biffl grade 3: A 16-year-old male presented after a motor vehicle collision with multiple skull and facial fractures and severe TBI. Axial ( a ) and sagittal ( b ) computed tomography angiographic images demonstrate nonopacification (black arrow) of the distal right cervical internal carotid artery (ICA). Digital subtraction angiographic (DSA) images ( c —anteroposterior projection, d —lateral projection) demonstrate a Biffl grade 3 injury with dissection flap beginning in the cervical right ICA and extending into the cavernous ICA with a pseudoaneurysm in the distal cervical ICA (black arrows), and resultant flow limitation. Subsequent follow-up DSA ( e —anteroposterior projection, f —lateral projection) 3 months later demonstrates partial healing of the right ICA with persistent pseudoaneurysm (black arrows).
Endovascular treatment options ( Fig. 4 ) include stent-assisted coiling of the pseudoaneurysm component with bare metal stents employed as scaffolding. Coiling microcatheters may be intentionally “trapped” prior to stent deployment or the microcatheter may be navigated through the struts of a deployed stent. Flow-diverting stents may also be used for more effective scaffolding of a coil mass within a pseudoaneurysm with the additional benefit of flow diversion, but a microcatheter cannot subsequently be navigated through the wall of a flow-diverting stent. Balloon-assisted coiling is possible in the appropriate anatomic configuration. Stenting with covered stent grafts is increasingly being used to seal pseudoaneurysms of the cervical carotid and vertebral arteries to help prevent type IV endoleaks. 59 60 61 62 63 PTFE-covered self-expanding nitinol stents such as Viabahn (Gore and Associates) are favorable due to the lack of PTFE porosity. 64 The relatively greater amount of foreign material on the stent requires long-term antiplatelet therapy. Long-term outcomes with covered stents in this setting are still being established.
Fig. 4.
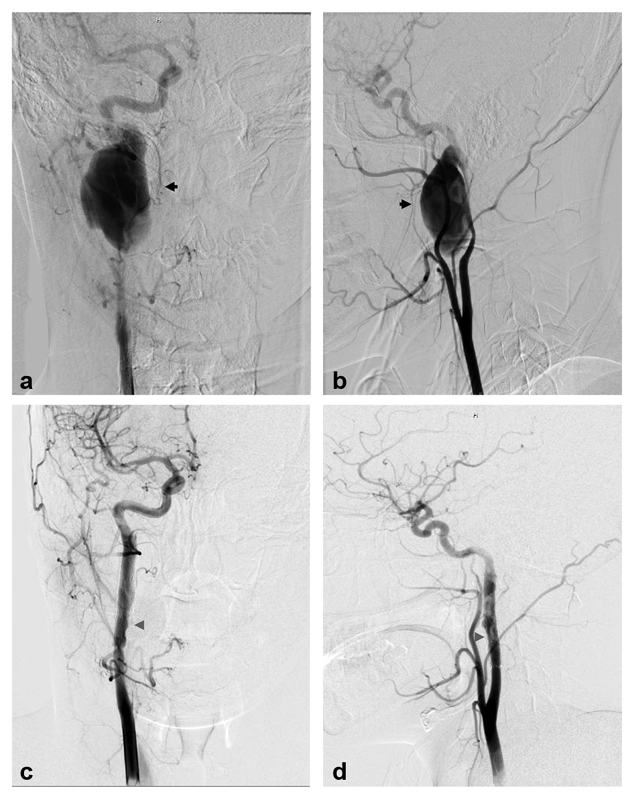
Pseudoaneurysm stenting: A 21-year-old male presented after a motor vehicle collision with multiple facial fractures and Horner's syndrome. Digital subtraction angiographic (DSA) images ( a, c —anteroposterior projection; b, d —lateral projection) demonstrate a Biffl grade 3 injury with dissection of the cervical right ICA and a large pseudoaneurysm (black arrows) in the distal cervical ICA, with resultant flow limitation. He underwent endovascular treatment with placement of two EV3 Protégé Rx (ev3, Inc., Plymouth, MN) self-expanding stents across the pseudoaneurysm. Subsequent follow-up DSA ( c, d ) 15 months later demonstrates healing of the right ICA pseudoaneurysm with mild in-stent stenosis (gray arrowheads).
Endovascular parent vessel embolization therapy is a consideration in appropriately selected cases of traumatic pseudoaneurysms. The goal of vessel sacrifice (also known as deconstruction) is to control active hemorrhage and secure the injured segment of the vessel to prevent future hemorrhage. 65 This may be necessary in patients with an absolute contraindication to antiplatelet medication precluding placement of a stent or if stent placement is technically not feasible. 66 If the patient is hemodynamically stable, a balloon occlusion test to assess for adequate collateral circulation and prognosis estimation is appropriate prior to permanent occlusion. 64 Acute emergent occlusion of a carotid artery can result in a stroke rate of 30 to 50%. 67 Permanent occlusion in the carotid or vertebral artery is achievable with coil embolization. This occlusion is achieved by both the mechanical effect of the coil mass and the formation of clot within the coil mass. Coils with Dacron or nylon fibers used in the peripheral circulation may be less effective in the carotid or vertebral artery because they often fail to achieve the packing density necessary for flow stasis and thrombosis. Numerous coils are often necessary for occlusion of a cervical carotid or vertebral artery with a coil mass density of 35% often regarded as minimal for permanent occlusion. Neurovascular coils measuring 0.018 inches in diameter are often better suited to achieve this packing density owing to their softness. In the setting of trauma, clot formation in the coil mass may be delayed due to clotting factor consumption elsewhere. Self-expanding occlusion devices such as the Amplatzer vascular plug (AGA Medical Corp., Plymouth, MN) may be used as a primary occlusion device or in conjunction with coils. Often multiple vascular plugs may be necessary for successful occlusion and the length of available vessel for device deployment should be considered in the planning. Liquid embolic agents such as n-butyl cyanoacrylate glue (NBCA) which rapidly polymerizes in contact with ionic solutions such as blood or saline may be used to form an occlusive cast that is not dependent on the presence of clotting factors and may be considered as an adjunct for vessel sacrifice with coils. NBCA use requires prior experience as the delivery catheter can become permanently adhered to the glue cast if not appropriately administered. Vessel embolization with NBCA represents an off-label use in the United States.
Any attempt at stent grafting or deconstructive sacrifice should take into account potential collaterals from the ECA and reversed distal ICA flow. ECA coil embolization and ICA occlusion distal to the pseudoaneurysm are usually appropriate to consider in these circumstances.
Vertebral artery pseudoaneurysms and dissecting aneurysms are characterized by an irregular and increased luminal diameter. These are especially important to identify as they carry an increased risk of rupture and distal embolism. They are often treated aggressively with coil embolization with distal and proximal occlusion or with covered stents.
Grade 4: Arterial Occlusion
Grade 4 traumatic cervical arterial occlusion ( Fig. 5 ) has a high risk of cerebral infarction without medical therapy and rarely recanalize spontaneously. 6 45 With medical therapy, the long-term annual stroke risk is 0.7% which is double the 0.3% annual ipsilateral stroke risk in occlusions that recanalize. 15 68
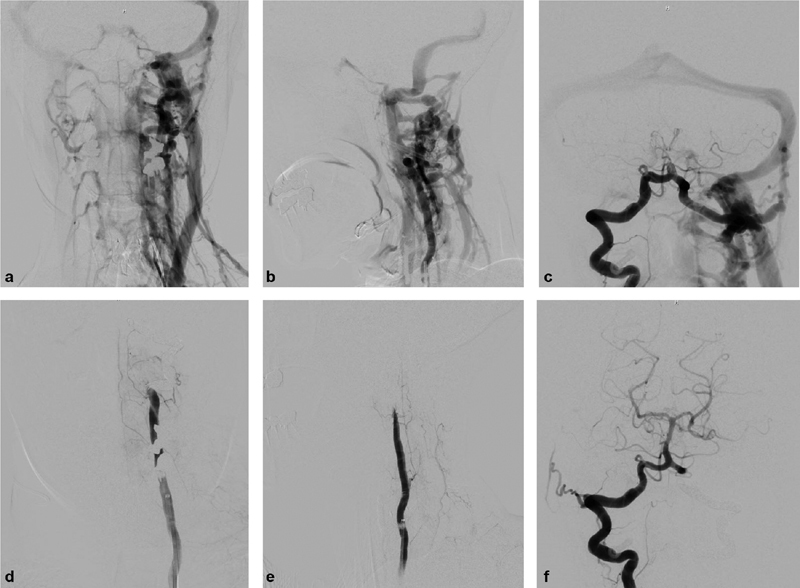
Fig. 5.
Biffl grade 4: An 18-year-old female sustained a gunshot wound to the face with multiple skull and facial fractures and C1 fracture. Coronal ( a ) and sagittal ( b ) computed tomography angiographic images demonstrate occlusion of the distal left cervical internal carotid artery (ICA) and reconstitution of the supraclinoid left ICA (images not shown). Digital subtraction angiographic (DSA) images ( c, e —anteroposterior projections; d, f —lateral projections) demonstrate a Biffl grade 4 injury with occlusion of the distal cervical left ICA (arrows; c, d ) and reconstitution of the supraclinoid left ICA ( e, f ) via right to left collateral flow. There is also early venous drainage (gray arrowheads) indicative of a direct carotid cavernous fistula ( e, f ).
Treatment considerations are limited because traversing the occlusion with a microwire may be impossible or carry a high risk of exacerbating existing intimal vessel damage. Stump occlusions can, however, be a source of emboli. Each case should be considered individually as there is equipoise on the risks versus benefit, and limited case series have demonstrated the safety of crossing severe stenosis (90%) or occlusion with a microcatheter and microwire. 69
Grade 5: Arterial Transection
Grade 5 traumatic cervical carotid or vertebral transections ( Fig. 6 ) understandably have a high rate of morbidity and mortality rates as high as 50%. 45 Treatment consideration begins emergently with surgical management when presenting with shock in accordance with Advanced Trauma Life Support guidelines. 70 Open surgery has been the most common treatment especially in shock trauma presentations.
Fig. 6.
Biffl grade 5: A 23-year-old male sustained a gunshot wound to the head and neck with multiple skull and facial fractures. Computed tomography angiographic demonstrated occlusion of the left cervical internal carotid artery (ICA) and reconstitution of the supraclinoid left ICA (images not shown). Digital subtraction angiographic (DSA) images ( a, c, e —anteroposterior projections; b, d, f —lateral projections) from a left injection demonstrated a Biffl grade 5 injury with extravasation (black arrows) from a transected left cervical ICA ( a, b ). He underwent emergent endovascular coil and glue embolization for sacrifice of the proximal left ICA stump ( c, d ). DSA images from a right injection demonstrated continued extravasation due to retrograde filling of the distal end of the transected left ICA via collateral flow ( e ). This was treated with coil occlusion of the left ICA distal to the transection ( f ) via retrograde access from the right ICA across the anterior communicating artery. There is also early venous drainage (gray arrowheads) indicative of an arteriovenous fistula ( a, b, e, f ).
Emergent endovascular treatment of cervical arterial transection is reserved as adjunctive measures or when lesions are not accessible surgically. Endovascular interventions include emergent permanent occlusion using the previously mentioned devices or temporary balloon occlusion to aid with surgical exposure. 62 63 64 67 69 71 72 Reviews of stent grafting indicate a 95% efficacy rate for endovascular covered stents in controlling acute hemorrhage. There is a reported 30% rebleed rate in these series suggesting this may be considered a temporizing measure in these injuries. 73 74 Small series have reported various levels of limited success in controlling bleeding in patients with grade 5 transections with stent-assisted coiling, percutaneous thrombin injection, and glue embolization which may be considered in extreme circumstances. While extremely limited by selection bias of more stable patients, studies of acute vessel sacrifice with modern endovascular techniques have reported 8 to 10% mortality and 2.3 to 3.4% ischemic stroke rate which compares favorably to surgical ligation with a reported 40% mortality and 60% major neurologic morbidity. 64
Arteriovenous Fistulas in the Neck
The natural history of arteriovenous fistulas in the neck is limited to case series and case reports. Treatment is largely guided by the progression of symptoms related to the shunting and arterial pressure present in the venous vasculature. Treatment of the fistula point with vessel sacrifice may be considered if there is appropriate collateral flow while taking measures to minimize the risk of thromboembolic complications.
Iatrogenic ICA to jugular vein fistula formation as a result of a central line placement should prompt immediate consultation with a surgical service without any attempt at removal of the catheter if it is still in place. These are best managed in the operating room where the catheter can be removed with control of flow in the CCA and ICA.
Vertebrovenous fistulas ( Fig. 7 ) can result from neck trauma or iatrogenic cervical nerve root injections. These can rarely thrombose spontaneously, but there are too few reported cases to postulate on their natural history. Vertebral artery injuries in the V2 and V3 segments are more difficult to access surgically than endovascularly. The majority are asymptomatic. Auscultation may reveal a bruit. If the fistula progresses and develops venous congestion, symptoms can include neck pain, cervical radiculopathy, tinnitus, spinal cord symptoms, vertigo, and diplopia. The majority of reported cases have been treated successfully with vessel sacrifice to exclude the fistula point from circulation. If the vertebral artery has a dominant cervical spinal artery or terminates in the ipsilateral posterior inferior cerebellar artery without collateral supply from the contralateral vertebral artery, the use of covered stents may be necessary in an attempt to maintain blood supply to the cervical spinal cord or medulla and inferior cerebellum. A balloon-occlusion test may be warranted to guide treatment planning. 75 76 77 78 79 80
Fig. 7.
Vertebrovenous fistula: A 59-year-old female presented with pulsatile tinnitus several months after a cervical nerve root injection was found to have a high flow left vertebrovenous fistula and was treated with left vertebral artery endovascular coil deconstruction. Digital subtraction angiographic (DSA) images ( a, c —anteroposterior projection; b —lateral projection) demonstrate a high flow vertebrovenous fistula arising from the V3 segment of the left vertebral artery with rapid filling of engorged neck veins, intraspinal venous plexus, and retrograde flow through the transverse-sigmoid sinus. Post treatment images ( d, f —anteroposterior projection; e —lateral projection) demonstrate complete occlusion of the left vertebral artery V3 segment at the fistulous point, and normal filling of the remaining vertebrobasilar circulation from the right vertebral artery ( f ).
Additional Endovascular Treatment Considerations
With regard to the timing of endovascular interventions for the treatment of blunt cervical arterial injuries, there is evidence that delaying endovascular treatment for at least 24 hours after presentation if possible may have a significant mortality benefit independent of clinical presentation. 41 Of course, this needs to be weighed against the patient's clinical acuity of presentation.
In the subset of patients presenting with acute stroke with intracranial large vessel occlusions and tandem cervical carotid artery dissection, there is supporting evidence for endovascular mechanical thrombectomy with good outcomes having been described in 70% of cases. 81 82
Postprocedural Management
Antithrombotic treatment is crucial following cerebrovascular arterial stenting to prevent stent occlusion. There is a higher rate of thrombosis with vessel injury than with stenting for atheromatous stenosis. 50 Dual-antiplatelet therapy with 325 mg aspirin and 75 mg clopidogrel daily for at least 6 weeks to 6 months followed by indefinite aspirin monotherapy is generally accepted based on treatment strategies for atheromatous stenosis. 1 42 Alternatively, aspirin 81 mg once daily and ticagrelor 45 to 90 mg twice daily may be considered to offset the 5 to 30% clopidogrel nonresponsiveness rate in patients. 83 However, cost-associated patient noncompliance with ticagrelor should be considered.
Emergent carotid or vertebral stenting requires immediate initiation of an antiplatelet regimen which can complicate hemorrhage control in multisystem trauma. If deemed appropriate, a bolus dosing of aspirin 300 to 600 mg per rectum or crushed and administered via nasogastric or orogastric tube can be considered in addition to a second antiplatelet agent. Individual institutional pharmacy guidelines should be consulted if a short-acting antiplatelet agent such as IV cangrelor is initiated during stent placement which can be transitioned to clopidogrel or ticagrelor. Alternatively, some practitioners administer crushed clopidogrel via a gastric tube.
There are no well-established recommendations for imaging follow-up after endovascular treatment of a traumatic cervical cerebrovascular injury. General recommendations include 4- to 6-week follow-up angiography (CTA or DSA) in lesions that may progress to a higher grade. Long-term evaluation of stented vessels can be performed by CTA or ultrasound at an interval of 3, 6, or 12 months with no established firm guidelines. 40
Conclusion
Endovascular treatment options for traumatic cervical cerebrovascular injuries continue to rapidly improve with advances in diagnostic imaging techniques, adjunctive medical therapy, device technology, and growing evidence for their appropriate use. Though cervical carotid and vertebral artery injury can have a devastating natural history, endovascularly trained physicians with appropriate knowledge in the use of these technologies have the potential to have a tremendous impact on outcomes for these patients.
Footnotes
Conflict of Interest None declared.
References
- 1.Fabian T C, Patton J H, Jr, Croce M A, Minard G, Kudsk K A, Pritchard F E.Blunt carotid injury. Importance of early diagnosis and anticoagulant therapy Ann Surg 199622305513–522., discussion 522–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harrigan M R, Hadley M N, Dhall S S. Management of vertebral artery injuries following non-penetrating cervical trauma. Neurosurgery. 2013;72 02:234–243. doi: 10.1227/NEU.0b013e31827765f5. [DOI] [PubMed] [Google Scholar]
- 3.Cogbill T H, Moore E E, Meissner M. The spectrum of blunt injury to the carotid artery: a multicenter perspective. J Trauma. 1994;37(03):473–479. doi: 10.1097/00005373-199409000-00024. [DOI] [PubMed] [Google Scholar]
- 4.White J M, Stannard A, Burkhardt G E, Eastridge B J, Blackbourne L H, Rasmussen T E. The epidemiology of vascular injury in the wars in Iraq and Afghanistan. Ann Surg. 2011;253(06):1184–1189. doi: 10.1097/SLA.0b013e31820752e3. [DOI] [PubMed] [Google Scholar]
- 5.Esnault P, Cardinale M, Boret H. Blunt cerebrovascular injuries in severe traumatic brain injury: incidence, risk factors, and evolution. J Neurosurg. 2017;127(01):16–22. doi: 10.3171/2016.4.JNS152600. [DOI] [PubMed] [Google Scholar]
- 6.Biffl W L, Moore E E, Offner P J, Brega K E, Franciose R J, Burch J M. Blunt carotid arterial injuries: implications of a new grading scale. J Trauma. 1999;47(05):845–853. doi: 10.1097/00005373-199911000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Berne J D, Norwood S H, McAuley C E, Vallina V L, Creath R G, McLarty J. The high morbidity of blunt cerebrovascular injury in an unscreened population: more evidence of the need for mandatory screening protocols. J Am Coll Surg. 2001;192(03):314–321. doi: 10.1016/s1072-7515(01)00772-4. [DOI] [PubMed] [Google Scholar]
- 8.Miller P R, Fabian T C, Croce M A.Prospective screening for blunt cerebrovascular injuries: analysis of diagnostic modalities and outcomes Ann Surg 200223603386–393., discussion 393–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kerwin A J, Bynoe R P, Murray J. Liberalized screening for blunt carotid and vertebral artery injuries is justified. J Trauma. 2001;51(02):308–314. doi: 10.1097/00005373-200108000-00013. [DOI] [PubMed] [Google Scholar]
- 10.Berne J D, Norwood S H, McAuley C E, Villareal D H.Helical computed tomographic angiography: an excellent screening test for blunt cerebrovascular injury J Trauma 2004570111–17., discussion 17–19 [DOI] [PubMed] [Google Scholar]
- 11.Asensio J A, Vu T, Mazzini F N. Penetrating carotid artery: uncommon complex and lethal injuries. Eur J Trauma Emerg Surg. 2011;37(05):429. doi: 10.1007/s00068-011-0132-3. [DOI] [PubMed] [Google Scholar]
- 12.Tisherman S A, Bokhari F, Collier B. Clinical practice guideline: penetrating zone II neck trauma. J Trauma. 2008;64(05):1392–1405. doi: 10.1097/TA.0b013e3181692116. [DOI] [PubMed] [Google Scholar]
- 13.Lee V H, Brown R D, Jr, Mandrekar J N, Mokri B. Incidence and outcome of cervical artery dissection: a population-based study. Neurology. 2006;67(10):1809–1812. doi: 10.1212/01.wnl.0000244486.30455.71. [DOI] [PubMed] [Google Scholar]
- 14.Béjot Y, Daubail B, Debette S, Durier J, Giroud M. Incidence and outcome of cerebrovascular events related to cervical artery dissection: the Dijon Stroke Registry. Int J Stroke. 2014;9(07):879–882. doi: 10.1111/ijs.12154. [DOI] [PubMed] [Google Scholar]
- 15.Touzé E, Gauvrit J, Meder J, Mas J. Prognosis of cervical artery dissection. Front Neurol Neurosci. 2005;20:129–139. doi: 10.1159/000088157. [DOI] [PubMed] [Google Scholar]
- 16.Landreneau R J, Weigelt J A, Megison S M, Meier D E, Fry W J. Combined carotid-vertebral arterial trauma. Arch Surg. 1992;127(03):301–304. doi: 10.1001/archsurg.1992.01420030067012. [DOI] [PubMed] [Google Scholar]
- 17.Herrera F A, Mareno J A, Easter D. Management of penetrating neck injuries: zone II. J Surg Educ. 2007;64(02):75–78. doi: 10.1016/j.jsurg.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 18.Bladergroen M, Brockman R, Luna G, Kohler T, Johansen K. A twelve-year survey of cervicothoracic vascular injuries. Am J Surg. 1989;157(05):483–486. doi: 10.1016/0002-9610(89)90640-5. [DOI] [PubMed] [Google Scholar]
- 19.Roon A J, Christensen N. Evaluation and treatment of penetrating cervical injuries. J Trauma. 1979;19(06):391–397. doi: 10.1097/00005373-197906000-00001. [DOI] [PubMed] [Google Scholar]
- 20.Albuquerque F C, Hu Y C, Dashti S R. Craniocervical arterial dissections as sequelae of chiropractic manipulation: patterns of injury and management. J Neurosurg. 2011;115(06):1197–1205. doi: 10.3171/2011.8.JNS111212. [DOI] [PubMed] [Google Scholar]
- 21.Groves A P, Kansagra A P, Cross D T, III, Moran C J, Derdeyn C P. Acute management and outcomes of iatrogenic dissections during cerebral angiography. J Neurointerv Surg. 2017;9(05):499–501. doi: 10.1136/neurintsurg-2016-012285. [DOI] [PubMed] [Google Scholar]
- 22.Chang F C, Lirng J F, Luo C B. Patients with head and neck cancers and associated postirradiated carotid blowout syndrome: endovascular therapeutic methods and outcomes. J Vasc Surg. 2008;47(05):936–945. doi: 10.1016/j.jvs.2007.12.030. [DOI] [PubMed] [Google Scholar]
- 23.Yang S T, Huang Y C, Chuang C C, Hsu P W. Traumatic internal carotid artery dissection. J Clin Neurosci. 2006;13(01):123–128. doi: 10.1016/j.jocn.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 24.Azuaje R E, Jacobson L E, Glover J. Reliability of physical examination as a predictor of vascular injury after penetrating neck trauma. Am Surg. 2003;69(09):804–807. [PubMed] [Google Scholar]
- 25.Scheid R, Zimmer C, Schroeter M L, Ballaschke O, von Cramon D Y. The clinical spectrum of blunt cerebrovascular injury. Neurologist. 2006;12(05):255–262. doi: 10.1097/01.nrl.0000243977.17242.ab. [DOI] [PubMed] [Google Scholar]
- 26.Ledgerwood A M, Mullins R J, Lucas C E. Primary repair vs ligation for carotid artery injuries. Arch Surg. 1980;115(04):488–493. doi: 10.1001/archsurg.1980.01380040110019. [DOI] [PubMed] [Google Scholar]
- 27.Uyeda J W, Anderson S W, Sakai O, Soto J A.CT angiography in trauma Radiol Clin North Am 20104802423–438., ix–x [DOI] [PubMed] [Google Scholar]
- 28.Bodanapally U K, Dreizin D, Sliker C W, Boscak A R, Reddy R P. Vascular injuries to the neck after penetrating trauma: diagnostic performance of 40- and 64-MDCT angiography. AJR Am J Roentgenol. 2015;205(04):866–872. doi: 10.2214/AJR.14.14161. [DOI] [PubMed] [Google Scholar]
- 29.Schroeder J W, Baskaran V, Aygun N. Imaging of traumatic arterial injuries in the neck with an emphasis on CTA. Emerg Radiol. 2010;17(02):109–122. doi: 10.1007/s10140-009-0835-5. [DOI] [PubMed] [Google Scholar]
- 30.Inaba K, Branco B C, Menaker J.Evaluation of multidetector computed tomography for penetrating neck injury: a prospective multicenter study J Trauma Acute Care Surg 20127203576–583., discussion 583–584, quiz 803–804 [DOI] [PubMed] [Google Scholar]
- 31.Shahan C P, Magnotti L J, Stickley S M. A safe and effective management strategy for blunt cerebrovascular injury: avoiding unnecessary anticoagulation and eliminating stroke. J Trauma Acute Care Surg. 2016;80(06):915–922. doi: 10.1097/TA.0000000000001041. [DOI] [PubMed] [Google Scholar]
- 32.Bromberg W J, Collier B C, Diebel L N. Blunt cerebrovascular injury practice management guidelines: the Eastern Association for the Surgery of Trauma. J Trauma. 2010;68(02):471–477. doi: 10.1097/TA.0b013e3181cb43da. [DOI] [PubMed] [Google Scholar]
- 33.Kaufmann T J, Huston J, III, Mandrekar J N, Schleck C D, Thielen K R, Kallmes D F. Complications of diagnostic cerebral angiography: evaluation of 19,826 consecutive patients. Radiology. 2007;243(03):812–819. doi: 10.1148/radiol.2433060536. [DOI] [PubMed] [Google Scholar]
- 34.Múnera F, Cohn S, Rivas L A. Penetrating injuries of the neck: use of helical computed tomographic angiography. J Trauma. 2005;58(02):413–418. doi: 10.1097/01.ta.0000141892.07192.55. [DOI] [PubMed] [Google Scholar]
- 35.Múnera F, Soto J A, Palacio D, Velez S M, Medina E. Diagnosis of arterial injuries caused by penetrating trauma to the neck: comparison of helical CT angiography and conventional angiography. Radiology. 2000;216(02):356–362. doi: 10.1148/radiology.216.2.r00jl25356. [DOI] [PubMed] [Google Scholar]
- 36.Demetriades D, Skalkides J, Sofianos C, Melissas J, Franklin J. Carotid artery injuries: experience with 124 cases. J Trauma. 1989;29(01):91–94. [PubMed] [Google Scholar]
- 37.Fusco M R, Harrigan M R.Cerebrovascular dissections--a review part I: spontaneous dissections Neurosurgery 20116801242–257., discussion 257 [DOI] [PubMed] [Google Scholar]
- 38.Fusco M R, Harrigan M R.Cerebrovascular dissections: a review. Part II: Blunt cerebrovascular injury Neurosurgery 20116802517–530., discussion 530 [DOI] [PubMed] [Google Scholar]
- 39.Moon T H, Kim S H, Lee J W, Huh S K. Clinical analysis of traumatic cerebral pseudoaneurysms. Korean J Neurotrauma. 2015;11(02):124–130. doi: 10.13004/kjnt.2015.11.2.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yamada R, Miller C, Guimaraes M, Schönholz C. Overview of endovascular management of traumatic carotid lesions Determining appropriate intervention based on lesion severity and patient characteristics. Endov Today. 2016;15(01):79–82. [Google Scholar]
- 41.Blitzer D N, Ottochian M, O'Connor J V.Timing of intervention may influence outcomes in blunt injury to the carotid artery J Vasc Surg 202071041323–1.332E8., e1325 [DOI] [PubMed] [Google Scholar]
- 42.Starnes B W, Arthurs Z M. Endovascular management of vascular trauma. Perspect Vasc Surg Endovasc Ther. 2006;18(02):114–129. doi: 10.1177/1531003506293418. [DOI] [PubMed] [Google Scholar]
- 43.Ditmars M L, Klein S R, Bongard F S. Diagnosis and management of zone III carotid injuries. Injury. 1997;28(08):515–520. doi: 10.1016/s0020-1383(97)00058-2. [DOI] [PubMed] [Google Scholar]
- 44.Scott W W, Sharp S, Figueroa S A. Clinical and radiographic outcomes following traumatic Grade 1 and 2 carotid artery injuries: a 10-year retrospective analysis from a Level I trauma center. The Parkland Carotid and Vertebral Artery Injury Survey. J Neurosurg. 2015;122(05):1196–1201. doi: 10.3171/2015.1.JNS14642. [DOI] [PubMed] [Google Scholar]
- 45.Biffl W L, Ray C E, Jr, Moore E E.Treatment-related outcomes from blunt cerebrovascular injuries: importance of routine follow-up arteriography Ann Surg 200223505699–706., discussion 706–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wahl W L, Brandt M M, Thompson B G, Taheri P A, Greenfield L J. Antiplatelet therapy: an alternative to heparin for blunt carotid injury. J Trauma. 2002;52(05):896–901. doi: 10.1097/00005373-200205000-00012. [DOI] [PubMed] [Google Scholar]
- 47.Cothren C C, Biffl W L, Moore E E, Kashuk J L, Johnson J L. Treatment for blunt cerebrovascular injuries: equivalence of anticoagulation and antiplatelet agents. Arch Surg. 2009;144(07):685–690. doi: 10.1001/archsurg.2009.111. [DOI] [PubMed] [Google Scholar]
- 48.DuBose J, Recinos G, Teixeira P G, Inaba K, Demetriades D. Endovascular stenting for the treatment of traumatic internal carotid injuries: expanding experience. J Trauma. 2008;65(06):1561–1566. doi: 10.1097/TA.0b013e31817fd954. [DOI] [PubMed] [Google Scholar]
- 49.Lee T S, Ducic Y, Gordin E, Stroman D. Management of carotid artery trauma. Craniomaxillofac Trauma Reconstr. 2014;7(03):175–189. doi: 10.1055/s-0034-1372521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cothren C C, Moore E E, Ray C E., JrCarotid artery stents for blunt cerebrovascular injury: risks exceed benefits Arch Surg 200514005480–485., discussion 485–486 [DOI] [PubMed] [Google Scholar]
- 51.CADISS Trial Investigators . Markus H S, Hayter E, Levi C, Feldman A, Venables G, Norris J. Antiplatelet treatment compared with anticoagulation treatment for cervical artery dissection (CADISS): a randomised trial. Lancet Neurol. 2015;14(04):361–367. doi: 10.1016/S1474-4422(15)70018-9. [DOI] [PubMed] [Google Scholar]
- 52.Duke B J, Ryu R K, Coldwell D M, Brega K E. Treatment of blunt injury to the carotid artery by using endovascular stents: an early experience. J Neurosurg. 1997;87(06):825–829. doi: 10.3171/jns.1997.87.6.0825. [DOI] [PubMed] [Google Scholar]
- 53.Pham M H, Rahme R J, Arnaout O.Endovascular stenting of extracranial carotid and vertebral artery dissections: a systematic review of the literature Neurosurgery 20116804856–866., discussion 866 [DOI] [PubMed] [Google Scholar]
- 54.Xianjun H, Zhiming Z.A systematic review of endovascular management of internal carotid artery dissections Intervent Neurol 20131(3-4):164–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kansagra A P, Cooke D L, English J D. Current trends in endovascular management of traumatic cerebrovascular injury. J Neurointerv Surg. 2014;6(01):47–50. doi: 10.1136/neurintsurg-2012-010605. [DOI] [PubMed] [Google Scholar]
- 56.Brzezicki G, Rivet D J, Reavey-Cantwell J. Pipeline embolization device for treatment of high cervical and skull base carotid artery dissections: clinical case series. J Neurointerv Surg. 2016;8(07):722–728. doi: 10.1136/neurintsurg-2015-011653. [DOI] [PubMed] [Google Scholar]
- 57.Fischer S, Perez M A, Kurre W, Albes G, Bäzner H, Henkes H.Pipeline embolization device for the treatment of intra- and extracranial fusiform and dissecting aneurysms: initial experience and long-term follow-up Neurosurgery 20147504364–374., discussion 374 [DOI] [PubMed] [Google Scholar]
- 58.Zeleňák K, Zeleňáková J, DeRiggo J, Kurča E, Kantorová E, Poláček H. Treatment of cervical internal carotid artery spontaneous dissection with pseudoaneurysm and unilateral lower cranial nerves palsy by two silk flow diverters. Cardiovasc Intervent Radiol. 2013;36(04):1147–1150. doi: 10.1007/s00270-012-0472-3. [DOI] [PubMed] [Google Scholar]
- 59.Ahn J Y, Chung Y S, Lee B H, Choi S W, Kim O J. Stent-graft placement in a traumatic internal carotid-internal jugular fistula and pseudoaneurysm. J Clin Neurosci. 2004;11(06):636–639. doi: 10.1016/j.jocn.2003.10.025. [DOI] [PubMed] [Google Scholar]
- 60.Kooraki S, Grohmann J, Elshikh S, Urbach H, Meckel S. Covered stents for exclusion of iatrogenic common carotid artery-internal jugular vein fistula and brachiocephalic artery pseudoaneurysm. J Neurointerv Surg. 2016;8(08):e31. doi: 10.1136/neurintsurg-2015-011760.rep. [DOI] [PubMed] [Google Scholar]
- 61.Maras D, Lioupis C, Magoufis G, Tsamopoulos N, Moulakakis K, Andrikopoulos V. Covered stent-graft treatment of traumatic internal carotid artery pseudoaneurysms: a review. Cardiovasc Intervent Radiol. 2006;29(06):958–968. doi: 10.1007/s00270-005-0367-7. [DOI] [PubMed] [Google Scholar]
- 62.Schönholz C, Krajcer Z, Carlos Parodi J. Stent-graft treatment of pseudoaneurysms and arteriovenous fistulae in the carotid artery. Vascular. 2006;14(03):123–129. doi: 10.2310/6670.2006.00034. [DOI] [PubMed] [Google Scholar]
- 63.Schönholz C J, Uflacker R, De Gregorio M A, Parodi J C. Stent-graft treatment of trauma to the supra-aortic arteries. A review. J Cardiovasc Surg (Torino) 2007;48(05):537–549. [PubMed] [Google Scholar]
- 64.Manzoor N F, Rezaee R P, Ray A. Contemporary management of carotid blowout syndrome utilizing endovascular techniques. Laryngoscope. 2017;127(02):383–390. doi: 10.1002/lary.26144. [DOI] [PubMed] [Google Scholar]
- 65.Radvany M G, Gailloud P. Endovascular management of neurovascular arterial injuries in the face and neck. Semin Intervent Radiol. 2010;27(01):44–54. doi: 10.1055/s-0030-1247888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Eckard D A, Purdy P D, Bonte F J. Temporary balloon occlusion of the carotid artery combined with brain blood flow imaging as a test to predict tolerance prior to permanent carotid sacrifice. AJNR Am J Neuroradiol. 1992;13(06):1565–1569. [PMC free article] [PubMed] [Google Scholar]
- 67.Mazumdar A, Derdeyn C P, Holloway W, Moran C J, Cross D T., III Update on endovascular management of the carotid blowout syndrome. Neuroimaging Clin N Am. 2009;19(02):271–281. doi: 10.1016/j.nic.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 68.Kremer C, Mosso M, Georgiadis D. Carotid dissection with permanent and transient occlusion or severe stenosis: long-term outcome. Neurology. 2003;60(02):271–275. doi: 10.1212/01.wnl.0000043580.70857.92. [DOI] [PubMed] [Google Scholar]
- 69.Cohen J E, Gomori J M, Itshayek E. Single-center experience on endovascular reconstruction of traumatic internal carotid artery dissections. J Trauma Acute Care Surg. 2012;72(01):216–221. doi: 10.1097/TA.0b013e31823f630a. [DOI] [PubMed] [Google Scholar]
- 70.Kortbeek J B, Al Turki S A, Ali J. Advanced trauma life support, 8th edition, the evidence for change. J Trauma. 2008;64(06):1638–1650. doi: 10.1097/TA.0b013e3181744b03. [DOI] [PubMed] [Google Scholar]
- 71.Klein G E, Szolar D H, Raith J, Frühwirth H, Pascher O, Hausegger K A. Posttraumatic extracranial aneurysm of the internal carotid artery: combined endovascular treatment with coils and stents. AJNR Am J Neuroradiol. 1997;18(07):1261–1264. [PMC free article] [PubMed] [Google Scholar]
- 72.Lesley W S. Endosurgical repair of an iatrogenic facial arteriovenous fistula due to percutaneous trigeminal balloon rhizotomy. J Neurosurg Sci. 2007;51(04):177–180. [PubMed] [Google Scholar]
- 73.Gaba R C, West D L, Bui J T, Owens C A, Marden F A. Covered stent treatment of carotid blowout syndrome. Semin Intervent Radiol. 2007;24(01):47–52. doi: 10.1055/s-2007-971189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shah H, Gemmete J J, Chaudhary N, Pandey A S, Ansari S A. Acute life-threatening hemorrhage in patients with head and neck cancer presenting with carotid blowout syndrome: follow-up results after initial hemostasis with covered-stent placement. AJNR Am J Neuroradiol. 2011;32(04):743–747. doi: 10.3174/ajnr.A2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Aljobeh A, Sorenson T J, Bortolotti C, Cloft H, Lanzino G. Vertebral arteriovenous fistula: a review article. World Neurosurg. 2019;122:e1388–e1397. doi: 10.1016/j.wneu.2018.11.063. [DOI] [PubMed] [Google Scholar]
- 76.Herrera D A, Vargas S A, Dublin A B. Endovascular treatment of traumatic injuries of the vertebral artery. AJNR Am J Neuroradiol. 2008;29(08):1585–1589. doi: 10.3174/ajnr.A1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Higashida R T, Halbach V V, Tsai F Y. Interventional neurovascular treatment of traumatic carotid and vertebral artery lesions: results in 234 cases. AJR Am J Roentgenol. 1989;153(03):577–582. doi: 10.2214/ajr.153.3.577. [DOI] [PubMed] [Google Scholar]
- 78.Miller R E, Hieshima G B, Giannotta S L, Grinnell V S, Mehringer C M, Kerin D S. Acute traumatic vertebral arteriovenous fistula: balloon occlusion with the use of a contralateral approach. Neurosurgery. 1984;14(02):225–229. doi: 10.1227/00006123-198402000-00020. [DOI] [PubMed] [Google Scholar]
- 79.Santos-Franco J A, Zenteno M, Lee A.Dissecting aneurysms of the vertebrobasilar system. A comprehensive review on natural history and treatment options Neurosurg Rev 20083102131–140., discussion 140 [DOI] [PubMed] [Google Scholar]
- 80.Yi A C, Palmer E, Luh G Y, Jacobson J P, Smith D C. Endovascular treatment of carotid and vertebral pseudoaneurysms with covered stents. AJNR Am J Neuroradiol. 2008;29(05):983–987. doi: 10.3174/ajnr.A0946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Delgado F, Bravo I, Jiménez E. Endovascular treatment in the acute and non-acute phases of carotid dissection: a therapeutic approach. J Neurointerv Surg. 2017;9(01):11–16. doi: 10.1136/neurintsurg-2016-012475. [DOI] [PubMed] [Google Scholar]
- 82.Marnat G, Mourand I, Eker O. Endovascular management of tandem occlusion stroke related to internal carotid artery dissection using a distal to proximal approach: insight from the RECOST study. AJNR Am J Neuroradiol. 2016;37(07):1281–1288. doi: 10.3174/ajnr.A4752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Müller-Schunk S, Linn J, Peters N. Monitoring of clopidogrel-related platelet inhibition: correlation of nonresponse with clinical outcome in supra-aortic stenting. AJNR Am J Neuroradiol. 2008;29(04):786–791. doi: 10.3174/ajnr.A0917. [DOI] [PMC free article] [PubMed] [Google Scholar]


