Abstract
Impairment of health after overcoming the acute phase of COVID-19 is being observed more and more frequently. Here different symptoms of neurological and/or cardiological origin have been reported. With symptoms, which are very similar to the ones reported but are not caused by SARS-CoV-2, the occurrence of functionally active autoantibodies (fAABs) targeting G-protein coupled receptors (GPCR-fAABs) has been discussed to be involved.
We, therefore investigated, whether GPCR-fAABs are detectable in 31 patients suffering from different Long-COVID-19 symptoms after recovery from the acute phase of the disease.
The spectrum of symptoms was mostly of neurological origin (29/31 patients), including post-COVID-19 fatigue, alopecia, attention deficit, tremor and others. Combined neurological and cardiovascular disorders were reported in 17 of the 31 patients. Two recovered COVID-19 patients were free of follow-up symptoms. All 31 former COVID-19 patients had between 2 and 7 different GPCR-fAABs that acted as receptor agonists. Some of those GPCR-fAABs activate their target receptors which cause a positive chronotropic effect in neonatal rat cardiomyocytes, the read-out in the test system for their detection (bioassay for GPCR-fAAB detection). Other GPCR-fAABs, in opposite, cause a negative chronotropic effect on those cells. The positive chronotropic GPCR-fAABs identified in the blood of Long-COVID patients targeted the β2-adrenoceptor (β2-fAAB), the α1-adrenoceptor (α1-fAAB), the angiotensin II AT1-receptor (AT1-fAAB), and the nociceptin—like opioid receptor (NOC-fAAB). The negative chronotropic GPCR-fAABs identified targeted the muscarinic M2-receptor (M2-fAAB), the MAS-receptor (MAS-fAAB), and the ETA-receptor (ETA-fAAB). It was analysed which of the extracellular receptor loops was targeted by the autoantibodies.
Keywords: Autoantibody, Autoimmunity, COVID-19, Fatigue, Post-covid-19 symptom, Long-COVID
Abbreviations: fAAB, Functional autoantibody; ACE2, Angiotensin-converting enzyme 2 receptors; α1-fAAB, Autoantibody targeting the alpha1-adrenoceptor; AT1-fAAB, Autoantibody targeting the angiotensin II AT1 receptor; β2-fAAB, Autoantibody targeting the beta2-adrenoceptor; CRPS, Complex regional pain syndrome; ETA-fAAB, Autoantibody targeting the endothelin receptor; GPCR, G-protein coupled receptors; MAS-fAAB, Autoantibody targeting the MAS receptor; M2-fAAB, Autoantibody targeting the muscarinic receptor; NOC-fAAB, Functionally active autoantibody against the nociceptin receptor; PoTS, Postural orthostatic tachycardia syndrome; SARS, Severe acute respiratory syndrome; RAS, Renin angiotensin system
Highlights
-
•
Sera from Long-COVID syndrome patients contained functionally active autoantibodies targeting G-protein coupled receptors.
-
•
Autoantibodies target β2- and α1-adrenoceptors, angiotensin II AT1-, muscarinic M2-, MAS-, nociceptin- and ETA-receptors.
-
•
Included syndromes were of neurological and cardiological origin, or a combination of both.
-
•
Such autoantibody patterns have previously been seen in COVID independent neurological deficits and cardiovascular disease.
1. Introduction
The pandemic COVID-19 viral infection is often associated with severe respiratory and neurological complications, cardiovascular problems, microvascular and endothelial disorders, and gastrointestinal diseases. Additionally, these symptoms are often observed in patients who have already recovered from the disease and had negative follow-up coronavirus tests. In their Italian study, Carfi et al. [1] indicated that only 12.6% of investigated patients did not develop any persistent symptoms after recovering from COVID-19. Most of the symptomatic post-infection COVID-19 patients suffered from neurological disorders, such as chronic fatigue syndrome, postural orthostatic tachycardia syndrome (PoTS) and dysautonomia [1]. However, other neurological diseases, such as transverse myelitis, acute necrotising myelitis, Guillain-Barré syndrome and others, have also been reported in several recent case reports on patients following SARS-CoV-2 infection [[2], [3], [4], [5], [6], [7], [8], [9]]. Similar results concerning the extent of post-COVID-19 symptoms were also obtained in a German study that showed only 22% of their investigated COVID-19 patients stayed free of post-disease symptoms [10].
Besides neurological manifestations, patients who recovered from COVID-19 also often developed cardiovascular implications [11]. The most prevalent abnormalities observed included myocardial inflammation, arrhythmia, tachycardia, bradycardia, and atrioventricular (AV) block [10,[12], [13], [14], [15], [16], [17], [18]]. In a large multi-centre study, including the intensive care units of 68 geographically diverse hospitals across the United States, Hayek and co-workers investigated 5019 critically ill COVID-19 patients. They observed that of these 5019 patients, 14% (701/5019) had an in-hospital cardiac arrest which was associated with poor survival, particularly among older patients [19].
Several authors assumed that autoimmune processes, involving the formation of autoantibodies, may be involved in the pathogenesis [20] and development of a post-COVID-19 syndrome [[20], [21], [22]]. In an initial study by Zhou et al. [20], 21 patients critically ill with COVID-19 were investigated for the presence of autoantibodies. It was found that 20% had anti-52 kDa SSA/Ro antibodies (autoantibodies against extractable nuclear antigens), 25% had anti-60 kDa SSA/Ro antibodies and 50% had anti-nuclear antibodies.
Other autoantibodies acting as drivers of the disease have also been reported. It was recently shown by Bastard et al. that over 10% of their investigated COVID-19 patients with a life-threatening pneumonia condition (n = 987) presented with neutralizing autoantibodies against interferon-ω (IFN-ω, n = 13), the 13 types of IFN-α (n = 36), or against both (n = 52). A few of their patients also showed autoantibodies against the other three type I IFNs. In contrast, the authors did not see any of these autoantibodies in 663 patients with asymptomatic or mild SARS-CoV-2 infection and they were only present in 4 of 1227 healthy subjects included for comparison [23]. Bastard et al. concluded from their data that the pre-existence of neutralizing anti-type I IFN autoantibodies was the cause of a critical condition, rather than it being the consequence of the infection [23].
Novelli et al. concluded from their comprehensive systematic review about chronic inflammatory and autoimmune diseases onset during COVID-19 that “it is likely than the autoimmune manifestations described in COVID-19 represent more the results of the inflammatory cascade and the immune activation triggered by the virus rather than a direct effect of the virus per se” [24].
In another study, Lyons-Weiler compared immunogenic peptides of SARS-CoV-2 with sequences of human proteins and found a high number of matching homologous sequences [25]. This would explain the high rate of persisting autoreactivity after SARS-CoV-2 infection. Kreye et al. examined neutralizing anti-SARS antibodies of isolated B-cell clones and observed that some showed “self-reactivity” while others were virus neutralizing only without showing any self-reactivity [26]. The possible impact of autoantibodies on the pathogenesis has most recently been discussed by Khamsi [27].
It is a proven fact that autoimmune processes and the formation of functional autoantibodies (fAABs) directed against G-protein coupled receptors (GPCR) play a role in the development of neurological [[28], [29], [30]] and cardiovascular symptoms [31]. Therefore, in this present study, it was tested if such GPCR-fAABs might also be associated with the development of corresponding post-COVID-19 symptoms. We investigated virus-free sera from 31 recovered COVID-19 patients with respect to the occurrence of GPCR-fAABs.
2. Material and methods
2.1. Patient sera
Sera were obtained from 31 patients, 29 who were still suffering from post-COVID-19 symptoms, after recovery from acute disease and 2 patients who were symptom-free (all positive tested by PCR). All patients signed a written informed consent form which included giving permission to include the anonymised clinical data in a scientific publication, in agreement with the Declaration of Helsinki. Using the RedCap project for data collection and management [32] with the permission from the ethics commission (no: 295-20 B), 6 of the sera were recruited at the University of Erlangen.
2.2. Serum
As a safety-precaution, the COVID-19 patient sera were heat inactivated for 30 min at 56 °C before use. Afterwards, 0.4 mL of the samples were dialysed against 1 L of dialysing buffer (0.15 M NaCl, 10 mM phosphate buffer, pH 7.4; Membra-Cel MD 44, 14 kDa, Serva) for 24 h to remove low-molecular weight bioactive compounds and peptides. Finally, 40 μL of the dialysed samples were added to the bioassay (final dilution of 1:50).
2.3. Bioassay for measurement of GPCR-fAABs
For the identification and characterisation of GPCR-fAABs, a bioassay was used, as described in great detail by Davideit et al. [33] and Wenzel et al. [34] for GPCR-fAABs against the beta1-adrenoceptor, and for other GPCR-fAABs by Wallukat et al. [30,35,36]. After contact with the respective autoantibodies, a change in basal beating rate of spontaneously beating cardiomyocytes expressing GPCR was used as the measuring signal. The receptor specificity was checked by either subsequent addition of specific receptor blockers, resulting in an annulation of this effect, or by addition of corresponding receptor-epitope-competing extracellular loop peptides. In detail: for the specification of the β2-fAABs, the receptor antagonist ICI118.551 (0.1 μM) was used and also neutralizing peptides corresponding to the first (HILMKMWTFGNFWCEFWT) or second (HWYRATHQEAINCYANETCCDFFTNQ) extracellular loop of the human β2-adrenoceptor. The effect of the negative chronotropic muscarinic M2 receptor-autoantibody (M2-fAAB) was blocked by atropine (1 μM). Losartan (1 μM) blocked the effect of the positive chronotropic AT1-fAAB and A779 (1 μM) blocked the effect of the negative chronotropic MAS-fAAB. For the identification of the MAS-fAAB, additional competing peptides corresponding to the first and second extracellular loop of the human MAS receptor of the following sequences: LSIDYALDYELSSGHHYTIVTL and LSGEESHSRSDCRAN, respectively, were exploited. ETA-fAABs were identified by blocking their negative chronotropic effects through the addition of the specific endothelin receptor antagonist BQ123 (0.1 μM) and also competing peptides corresponding to the first or second extracellular loop of the receptor of the following sequences, LPINVFKLLAGRWPFDHNDFGVFLCKL and FEYRGEQHKTCMNATSKFMEFYQDVKD, respectively. The nociceptin receptor antagonist J113397 (0.1 μM) was used to block the effects of the positive chronotropic NOC-fAAB and also competing peptides corresponding to the first (LAVCVGGLLGNCLVMYV) or second (FTLTAMSVDRYVAICHPIRALDVR) extracellular loop. Addition of 1 μM urapidil or prazosin abolished the positive chronotropic effect of α1-fAABs. For the loop analysis the following competing peptides corresponding to the 1st or 2nd extracellular loop of the receptor were used: LGYWAFGRVFCN and GWRQPAPEDETICQINEEPGYVLFSAL, respectively. For all peptides 2 μL of a stock solution of 100 μg/mL was added to 40 μL of the corresponding GPCR-fAAB sample and incubated for 30min before the mixture was transferred to the cells.
3. Results
Several different GPCR-fAABs were identified in the 31 sera of recovered COVID-19 patients. All 31 investigated patients had between 2 and 7 different GPCR-fAAB (Table 1). This was a surprising unexpected effect. In healthy controls, which are included in many studies, these autoantibodies are only found in a small percentage [37,38]”.
Table 1.
Overview of post-COVID-19 symptoms and accompanying GPCR-fAABs.
| Patient no. |
Gender |
Age (years) |
Running no. |
Symptom class |
Symptoms |
Neuro-active fAABs |
Vasoactive fAABs |
Neuro- and vasocative fAABs |
RAS-specific fAABs |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neuro∗ | Cardiovasc∗∗ | Neuro∗ | Cardiovasc∗∗ | Noc-fAAB§ | β2-fAAB$ | α1-fAAB& | ETA-fAAB+ | M2-fAAB% | AT1-fAAB? | MAS-fAAB# | ||||
| 1 | F | 48 | 1 | x | x | Fatigue, Alopecia, Anomic aphasia | Tachycardia | x | x | x | x | x | x | |
| 7 | F | 55 | 2 | x | x | Fatigue, Alopecia | Tachycardia | x | x | x | x | x | x | x |
| 11 | F | 39 | 3 | x | x | Fatigue, Alopecia | Tachycardia | x | x | x | x | x | ||
| 19 | F | 34 | 4 | x | x | Fatigue, PoTS, Tremor | Tachycardia | x | x | x | x | x | ||
| 22 | F | 34 | 5 | x | x | Fatigue, Alopecia | Tachycardia | x | x | x | x | x | ||
| 29 | F | 49 | 6 | x | x | PoTS | Tachycardia | x | x | x | x | x | ||
| 26 | M | 28 | 7 | x | x | PoTS | Tachycardia, Hypertension | x | x | x | x | x | ||
| 30 | M | 55 | 8 | x | x | PoTS | Bradycardia | x | x | x | x | |||
| 27 | M | 69 | 9 | x | x | PoTS, Attention deficit | Tachycardia | x | x | x | x | x | ||
| 31 | M | 44 | 10 | x | x | Attention deficit | Bradycardia | x | x | x | x | |||
| 3 | F | 56 | 11 | x | x | Fatigue, Attention deficit | Tachycardia, | x | x | x | x | x | x | |
| 21 | F | 28 | 12 | x | x | Attention deficit, Tremor, Dysautonomia | Arrhythmia | x | x | x | x | |||
| 18 | F | 53 | 13 | x | x | Tremor, Attention deficit | Tachycardia | x | x | |||||
| 20 | M | 54 | 14 | x | x | Attention deficit | Tachycardia, Hypertension | x | x | |||||
| 14 | F | 57 | 15 | x | x | Fatigue, Anomic aphasia | Arrhythmia, Hypertension | x | x | x | x | x | ||
| 23 | F | 50 | 16 | x | x | Eczema, Alopecia | Myocarditis | x | x | x | x | x | ||
| 28 | M | 65 | 17 | x | x | Smell/Taste disorder. | Tachycardia, Myocarditis | x | x | x | x | x | ||
| 24 | F | 33 | 18 | x | x | Fatigue, PoTS | n.a. | x | x | x | x | x | x | |
| 2 | M | 42 | 19 | x | – | Fatigue, Alopecia | n.a. | x | x | x | x | |||
| 4 | M | 50 | 20 | x | – | Fatigue | n.a. | x | x | x | x | |||
| 5 | F | 45 | 21 | x | – | Fatigue | n.a. | x | x | x | x | |||
| 6 | F | 36 | 22 | x | – | Tremor, Alopecia, Dysautonomia | n.a. | x | x | x | x | x | x | x |
| 9 | F | 50 | 23 | x | – | Fatigue | n.a. | x | x | x | x | x | ||
| 10 | F | 48 | 24 | x | – | Fatigue | n.a. | x | x | x | x | x | ||
| 12 | F | 53 | 25 | x | – | Fatigue, Attention deficit | n.a. | x | x | x | x | x | ||
| 15 | F | 46 | 26 | x | – | Fatigue, Alopecia, Polyneuropathy | n.a. | x | x | x | x | x | ||
| 17 | F | 49 | 27 | x | – | Fatigue, PoTS, Tremor | n.a. | x | x | |||||
| 25 | F | 58 | 28 | x | – | Attention deficit, Neuropathy | n.a. | x | x | x | x | x | ||
| 13 | F | 26 | 29 | x | – | Fatigue | n.a. | x | x | x | x | |||
| 8 | M | 71 | 30 | – | – | Symptom free | Symptom free | x | x | x | x | x | x | |
| 16 | M | 54 | 31 | – | – | Symptom free | Symptom free | x | x | x | x | x | ||
Neuro∗ = neurological symptoms; Cardiovasc∗∗ = cardiovascular symptoms, n.a. = not applicable, PoTS = postural orthostatic tachycardia syndrome; NOC-fAAB§ = functionally active autoantibody against the nociceptin receptor, β2-fAAB$ = autoantibody targeting the beta2-adrenoceptor, α1-fAAB& = autoantibody targeting the alpha1-adrenoceptor, ETA-fAAB+ = autoantibody targeting the endothelin receptor, M2-fAAB% = autoantibody targeting the muscarinic receptor, AT1-fAAB? = autoantibody targeting the angiotensin II AT1 receptor, MAS-fAAB# = autoantibody targeting the MAS receptor.
Two functionally active autoantibodies, that were seen in almost all investigated former COVID-19 patients, were directed against the β2-adrenoceptor (β2-fAAB) and the muscarinic M2 receptor (M2-fAAB). These fAABs induced a positive and a negative chronotropic response on their targeted receptors, respectively.
Two other fAABs that were also present in 29 (90%) of the 31 investigated post-COVID-19 patients were directed against the angiotensin II AT1 receptor (fAT1-AAB) and the angiotensin 1-7 MAS receptor (MAS-AAB). These receptors belong to the renin angiotensin system (RAS) and cause a positive and negative chronotropic effect, respectively, when targeted by the respective fAABs.
Post-infection hair loss (alopecia) was experienced by 8 of the recovered patients. In sera of these patients, three additional GPCR-fAABs were discovered: the negative chronotropic ETA-fAAB (4/8), the positive chronotropic NOC-fAAB (5/8), and the positive chronotropic α1-AAB (3/8). Not every alopecia patient showed all three of these GPCR-fAABs. Instead, their occurrence varied, and a pattern is not yet detectable. As shown in (Table 1), 2 of the 31 investigated post COVID-19 patients developed fAABs without showing any symptoms.
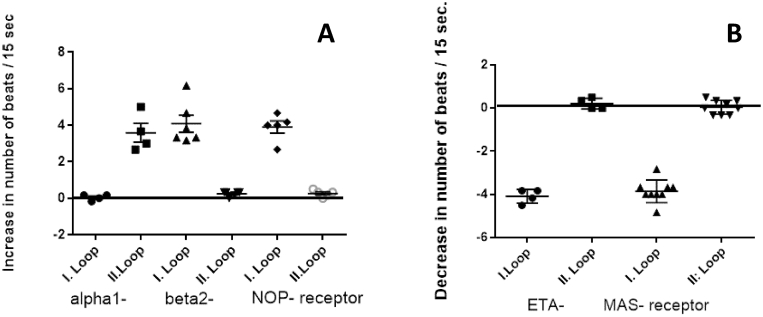
In a next step it was analysed at selected ETA-1-fAAB, MAS-fAAB and α1-fAAB, β2-fAAB and NOC-fAAB positive samples which of the extracellular loops of the receptors were targeted by the respective autoantibodies by addition of competing loop peptides (Fig. 1). Here it was clearly to see that with four of the tested autoantibodies (ETA-1-fAAB, MAS-fAAB, β2-fAAB, and NOC-fAAB) in each of the investigated cases the second extracellular loop of the receptor was targeted by the autoantibody and consequently neutralized by addition of its competing loop peptide. α1-fAAB containing samples targeted the 1st extracellular loop of the receptor. This analysis provided additional evidence that no nonspecific effects had influenced the outcome.
Fig. 1.
Loop analysis of selected GPCR-fAAB positive samples. A: positive chronotropic GPCR-fABBs: α1-fAAB, β2-fAAB and NOC-fAAB and B: negative chronotropic GPCR-fABBs: ETA-1-fAAB, MAS-fAAB samples were preincubated with 0.2 μg of the corresponding competing loop peptides as indicated under Material and methods for 30 min before the mixture was added to the cells for the recording of the corresponding GPCR-fAAB effect. In the case of competition, the chronotropic response was abolished, which was achieved in four cases by the loop peptides specific to the 2nd extracellular loops. Only the α1-fAAB targeted the 1st extracellular loop.
4. Discussion
The astonishing finding of this investigation is the fact that an unusually high number of GPCR-fAABs were detected in the serum of recovered COVID-19 patients who mostly suffered from a variety of different post-COVID-19 symptoms. Due to the functionality of such GPCR-fAABs, the question of whether these GPCR-fAABs may play a role in the development of post-COVID-19 symptoms is raised.
A continuing fatigue-like symptom, persisting long after virus follow-up tests are negative, was a frequently reported impairment in patients of this study (17/31), and other studies [1]. For patients suffering from a classical coronavirus-independent fatigue syndrome, the occurrence of β2-fAABs, M2-fAABs and, in some cases, also ETA-fAABs has already been reported before [39]. Here, with this post-COVID-19 study, almost all investigated sera contained β2-fAABs and M2-fAABs. The combination of β2-fAABs and M2-fAABs have also been identified in sera of patients suffering from PoTS and dysautonomia [40], both of which are conditions now observed in post-COVID-19 patients (7/31 and 2/31, respectively, not overlapping). Furthermore, this combination of β2-fAABs with M2-fAABs had also been identified before by our group, in patients with complex regional pain syndrome (CRPS) [41], in patients suffering from narcolepsy type 1, here additionally with the NOC-fAAB in 9 of 10 cases [36] and in patients with small fibre diseases.
Two of the identified GPCR-fAABs, observed in over 90% of the investigated COVID-19 patient sera (29/31), were directed against receptors of RAS, namely the angiotensin II AT1 receptor and the angiotensin (1–7) MAS receptor. These vasoactive AT1-fAABs had been identified before in patients with malignant hypertension, therapy-resistant hypertension, preeclampsia, and kidney diseases [[42], [43], [44]]. Moreover, Dragun et al. [44] showed that AT1-fAABs induced the rejection of kidneys in a subgroup of patients that underwent kidney transplantation. The authors also showed that the transfer of human AT1-fAABs to kidney transplanted rats caused the occlusion of the kidney arteries in the recipients.
Given the evidence described above, it may be that vasoactive processes, caused by the occurrence of AT1-fAABs and MAS-fAABs, might also be involved in the pathogenesis of post-COVID-19 symptoms. However, it is highly unlikely that the pathophysiological effects are caused by the fAABs alone. For example, Lukitsch and co-workers [45] already showed that the addition of the human AT1-fAABs to isolated kidney arteries induced a contraction of these arteries, but only in ischemic arteries and in arteries that were taken from kidney transplanted rats. Arteries obtained from healthy rats did not respond to AT1-fAABs, even though they reacted to their natural agonist, angiotensin II (which confirmed that the receptors were intact). Taken together, these data demonstrated that the AT1-fAABs did not act alone but needed ischaemic or inflammatory cofactors to have full effect. With respect to the COVID-19 situation, this is of course an absolutely obvious situation. Thrombo-inflammatory factors which even may become predictive markers for COVID-19 complications have been described by Cremer et al. [46]. Other immune biomarkers, as taken together by Fouladseresht et al. [47] have also been reported.
To date, the evidence suggests that a combination of ischaemic or inflammatory cofactors and autoantibodies can act to maintain a cardiac inflammatory process. Specifically, it has been shown that AT1-fAABs and α1-fAABs can influence the maturation and degranulation of cardiac mast cells [48], suggesting that they can contribute to inflammation.
It has also already been reported that COVID-19 induces an imbalance of RAS through viral-occupation of the angiotensin-converting enzyme 2 (ACE2) receptors, which reduces the generation of protective-peptides angiotensin-(1–7) and (1–9). This subsequently decreases the stimulation the MAS- and angiotensin II ATR2-receptors, and is accompanied by an overstimulation of the AT1-receptors due to reduced degradation of angiotensin II by ACE2 [49]. Therefore, Steckelings and Sumners [49] recently suggested that ATR2 receptor agonists could be used to treat COVID-19-induced disorders of various organ systems.
This is in good agreement with the identification of MAS-fAABs in over 90% of the symptomatic patients examined. Autoantibodies against this receptor have been observed before in a cancer patient after chemotherapy [50] and in patients with multiple sclerosis (MS) (unpublished results). In MS patients we observed the combination of MAS-fAABs with α1-fAABs. In this context, it is interesting to see that in several case reports it was shown that COVID-19 patients can develop neurological complications like transverse myelitis [2] and Guillain-Barré syndrome [5,7]. Whether the MAS-fAABs observed in this COVID-19 study is involved in the development of the reported neurological symptoms should be clarified in further investigations.
Furthermore, our data showed that 2 of the patients with a mild COVID-19 infection developed fAABs but not the symptoms as seen in the other recovered COVID-19 patients. We assume that in both of these patients adaption processes might have prevented the binding of fAABs to the receptor or the receptors are not available for the fAABs as described by Lukitsch and co-worker for angiotensin II AT1 fAAB before [45].
We strongly assume that the GPCR-fAABs play an important role in the development and maintenance of post-COVID symptoms. These GPCR-fAABs persistently stimulate their corresponding receptors and the normal, physiological, cell-protective desensitisation of the receptors is inhibited by the fAABs themselves [51].
It has already been shown in other diseases, such as idiopathic dilated cardiomyopathy (here it is the autoantibody targeting the beta1-adrenoceotor), that GPCR-fAABs play a significant role in the pathogenesis of the disease [31]. Additionally, the removal of these fAABs by immunoadsorption led to an improvement in cardiac function and to a significant increase in survival rate [52,53]. A similar beneficial therapeutic effect of the removal of GPCR-fAABs has also been observed in β2-fAAB positive, therapy-refractory, open-angle glaucoma patients. Here, the removal of the fAABs by immunoadsorption resulted in a reduction of the ocular pressure [54].
With respect to post-COVID-19 symptoms, Masuccio et al. reported a patient who was suffering from post-infection acute motor axonal neuropathy and myelitis. The patient tested positive for the ganglioside anti-GD1b IgM autoantibody, and a partial recovery was achieved through plasma exchange combined with subsequent immunoglobulin substitution [7].
The Sars-CoV-2 spike protein is a potential epitopic target for biomimicry-induced autoimmunological processes [25]. Therefore, we feel it will be extremely important to investigate whether GPCR-fAABs will also become detectable after immunisation by vaccination against the virus.
5. Conclusion
Our results indicated that all 29 investigated symptomatic post-COVID-19 patients developed fAABs directed against different GPCRs, known to be able to disturb the balance of neuronal and vascular processes. Most of these patients developed an antibody pattern consisting of β2-fAABs, M2-fAABs, AT1-fAABs, and MAS-fAABs. These agonistic fAABs activate their corresponding receptors like classical agonists. The observed specific GPCR-fAAB pattern has been observed before in several neurological and cardiac disorders and might also support the development of neurological and/or cardiovascular symptoms after COVID-19 recovery. These results provide valuable clues that are worth pursuing and investigating further.
6. Limitations of the study
A major limitation of this study is that it is only a snapshot. Therefore, the causal relationship between the presence of GPCR-fAAB and the disease cannot be shown. In order to be able to identify causal relationships, samples from former COVID patients must be systematically collected over a longer period of time for GPCR-fAAB detection. Afterwards the data have to be retrospectively assigned to recovering and non-recovering Long-COVID-19 patients.
Data availability
All available data are in included in this study.
Ethical standard
Informed consent and permission to publish this information was obtained from every patient included in this study.
Informed consent
Written informed consent was collected from the patients for the inclusion of anonymised clinical data in a scientific publication, in agreement with the Declaration of Helsinki.
Compliance with ethical standards
.
Funding
Part of this work was funded by the Berlin Cures GmbH, Germany. The funder provided support in the form of salaries but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Authors contributions
All authors contributed to the study conception and design. Material preparation and data collection and analysis were performed by Gerd Wallukat, Bettina Hohberger, Katrin Wenzel, Julia Fürst, Sarah Schulze-Rothe, Anne Wallukat, Anne-Sophie Hönicke, and Johannes Müller. The first draft of the manuscript was written by Annekathrin Haberland and all authors commented on previous versions of the manuscript. All authors read, revised, and approved the final manuscript.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests. G. Wallukat, K. Wenzel, S. Schulze-Rothe, A. Wallukat, A.S. Hönicke, and J. Müller are employed by the Berlin Cures GmbH. G. Wallukat and J. Müller are shareholders of the Berlin Cures Holding AG, the holding company of Berlin Cures. The authors declare no competing interests. All other authors have nothing to declare. The authors have no other relevant affiliations or financial involvement with any organisation or entity with a financial interest in or financial conflict with the subject or materials discussed in the manuscript apart from those which are disclosed.
Acknowledgements
The authors thank all the patients and health care workers for their support in this study. Annekathrin Haberland helped to write the manuscript. We thank Sabine Bartel for the supply of the rat cardiomyocytes. This manuscript was proofread by Proof-Reading-Service.com.
References
- 1.Carfì A., Bernabei R., Landi F. Persistent symptoms in patients after acute COVID-19. J. Am. Med. Assoc. 2020;324:603–605. doi: 10.1001/jama.2020.12603. Gemelli Against COVID-19 Post-Acute Care Study Group. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Munz M., Wessendorf S., Koretsis G. Acute transverse myelitis after COVID-19 pneumonia. J. Neurol. 2020;267:2196–2197. doi: 10.1007/s00415-020-09934-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chow C.C.N., Magnussen J., Ip J., Su Y. Acute transverse myelitis in COVID-19 infection. BMJ Case Rep. 2020;13(8) doi: 10.1136/bcr-2020-236720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maideniuc C., Memon A.B. Acute necrotizing myelitis and acute motor axonal neuropathy in a COVID-19 patient. J. Neurol. 2020;9:1–3. doi: 10.1007/s00415-020-10145-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Toscano G., Palmerini F., Ravaglia S. Guillain–barré syndrome associated with SARS-CoV-2. N. Engl. J. Med. 2020;382(26):2574–2576.. doi: 10.1056/NEJMc2009191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zachariadis A., Tulbu A., Strambo D. Transverse myelitis related to COVID-19 infection. J. Neurol. Dec 2020;267(12):3459–3461. doi: 10.1007/s00415-020-09997-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Masuccio F.G., Barra M., Claudio G., Claudio S. A rare case of acute motor axonal neuropathy and myelitis related to SARS-CoV-2 infection. J. Neurol. 2020;17:1–4. doi: 10.1007/s00415-020-10219-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chakraborty U., Chandra A., Ray A.K., Biswas P. COVID-19-associated acute transverse myelitis: a rare entity. BMJ Case Rep. 2020;13(8) doi: 10.1136/bcr-2020-238668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaur H., Mason J.A., Bajracharya M. Transverse myelitis in a child with COVID-19. Pediatr. Neurol. 2020;112:5–6. doi: 10.1016/j.pediatrneurol.2020.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Puntmann V.O., Carerj M.L., Wieters I. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5(11):1265–1273. doi: 10.1001/jamacardio.2020.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo T., Fan Y., Chen M. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:811–818. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lindner D., Fitzek A., Bräuninger H. Association of cardiac infection with SARS-CoV-2 in confirmed COVID-19 autopsy cases. JAMA Cardiol. 2020;5(11):1281–1285. doi: 10.1001/jamacardio.2020.3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Driggin E., Madhavan M.V., Bikdeli B. Cardiovascular considerations for patients, health care workers, and health systems during the COVID-19 pandemic. J. Am. Coll. Cardiol. 2020;75:2352–2371. doi: 10.1016/j.jacc.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nishiga M., Wang D.W., Han Y. COVID-19 and cardiovascular disease: from basic mechanisms to clinical perspectives. Nat. Rev. Cardiol. 2020;17:543–558. doi: 10.1038/s41569-020-0413-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amaratunga E.A., Corwin D.S., Moran L., Snyder R. Bradycardia in patients with COVID-19: a calm before the storm? Cureus. 2020;12 doi: 10.7759/cureus.8599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gupta M.D., Qamar A., Mp G. Bradyarrhythmias in patients with COVID-19: a case series. Indian Pacing Electrophysiol. J. 2020;20:211–212. doi: 10.1016/j.ipej.2020.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ashok V., Loke W.I. Case report: high-grade atrioventricular block in suspected COVID-19 myocarditis. Eur. Heart J. Case Rep. 2020;4:1–6. doi: 10.1093/ehjcr/ytaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kir D., Mohan C., Sancassani R. Heart brake: an unusual cardiac manifestation of COVID-19. JACC Case Rep. 2020;2:1252–1255. doi: 10.1016/j.jaccas.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hayek S.S., Brenner S.K., Azam T.U. In-hospital cardiac arrest in critically ill patients with covid-19: multicenter cohort study. BMJ. 2020;371:m3513. doi: 10.1136/bmj.m3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou Y., Han T., Chen J. Clinical and autoimmune characteristics of severe and critical cases of COVID-19. Clin. Transl. Sci. 2020;13(6):1077–1086. doi: 10.1111/cts.12805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ehrenfeld M., Tincani A., Andreoli L. Covid-19 and autoimmunity. Autoimmun. Rev. 2020;19:102597. doi: 10.1016/j.autrev.2020.102597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang E.Y., Mao T., Klein J. vol. 12. medRxiv 2020; 2020. Diverse Functional Autoantibodies in Patients with COVID-19. 10.20247205. [DOI] [PubMed] [Google Scholar]
- 23.Bastard P., Rosen L.B., Zhang Q. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science. 2020;370(6515) doi: 10.1126/science.abd4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Novelli L., Motta F., De Santis M. The JANUS of chronic inflammatory and autoimmune diseases onset during COVID-19 - a systematic review of the literature. J. Autoimmun. 2020;117 doi: 10.1016/j.jaut.2020.102592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lyons-Weiler J. Pathogenic priming likely contributes to serious and critical illness and mortality in COVID-19 via autoimmunity. J. Transl. Autoimmun. 2020;3 doi: 10.1016/j.jtauto.2020.100051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kreye J., Reincke S.M., Kornau H.-C. A therapeutic non-self-reactive SARS-CoV-2 antibody protects from lung pathology in a COVID-19 hamster model. Cell. 2020;183:1058–1069. doi: 10.1016/j.cell.2020.09.049. e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khamsi R. Rogue antibodies could be driving severe COVID-19. Nature. 2021;590(7844):29–31. doi: 10.1038/d41586-021-00149-1. https://www.nature.com/articles/d41586-021-00149-1 [DOI] [PubMed] [Google Scholar]
- 28.Shoenfeld Y., Ryabkova V.A., Scheibenbogen C. Complex syndromes of chronic pain, fatigue and cognitive impairment linked to autoimmune dysautonomia and small fiber neuropathy. Clin. Immunol. 2020;214:108384. doi: 10.1016/j.clim.2020.108384. [DOI] [PubMed] [Google Scholar]
- 29.Scheibenbogen C., Loebel M., Freitag H. Immunoadsorption to remove ß2 adrenergic receptor antibodies in Chronic Fatigue Syndrome CFS/ME. PLoS One. 2018;13 doi: 10.1371/journal.pone.0193672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wallukat G., Prüss H., Müller J., Schimke I. Functional autoantibodies in patients with different forms of dementia. PLoS One. 2018;13 doi: 10.1371/journal.pone.0192778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Düngen H.-D., Dordevic A., Felix S.B. β1-Adrenoreceptor autoantibodies in heart failure: physiology and therapeutic implications. Circ. Heart Fail. 2020;13 doi: 10.1161/CIRCHEARTFAILURE.119.006155. [DOI] [PubMed] [Google Scholar]
- 32.Harris P.A., Taylor R., Thielke R. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inf. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davideit H., Haberland A., Bartel S. Determination of agonistically acting autoantibodies to the adrenergic beta-1 receptor by cellular bioassay. Methods Mol. Biol. 2019;1901:95–102. doi: 10.1007/978-1-4939-8949-2_8. [DOI] [PubMed] [Google Scholar]
- 34.Wenzel K., Schulze-Rothe S., Haberland A. Performance and in-house validation of a bioassay for the determination of beta1-autoantibodies found in patients with cardiomyopathy. Heliyon. 2017;3 doi: 10.1016/j.heliyon.2017.e00362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wallukat G., Wenzel K., Schimke I. Analytics of functional autoantibodies in patients with chagas disease. Methods Mol. Biol. 2019;1955:247–261. doi: 10.1007/978-1-4939-9148-8_19. [DOI] [PubMed] [Google Scholar]
- 36.Orjatsalo M., Partinen E., Wallukat G. Activating autoantibodies against G protein-coupled receptors in narcolepsy type 1. Sleep Med. 2020;77:82–87. doi: 10.1016/j.sleep.2020.11.038. [DOI] [PubMed] [Google Scholar]
- 37.Wallukat G., Jandrig B., Becker N.-P. Autoantibodies directed against α1-adrenergic receptor and endothelin receptor A in patients with prostate cancer. Auto Immun. Highlights. 2020;11:13. doi: 10.1186/s13317-020-00136-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hohberger B., Kunze R., Wallukat G. Autoantibodies activating the β2-adrenergic receptor characterize patients with primary and secondary glaucoma. Front. Immunol. 2019;10:2112. doi: 10.3389/fimmu.2019.02112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Loebel M., Grabowski P., Heidecke H. Antibodies to β adrenergic and muscarinic cholinergic receptors in patients with Chronic Fatigue Syndrome. Brain Behav. Immun. 2016;52:32–39. doi: 10.1016/j.bbi.2015.09.013. [DOI] [PubMed] [Google Scholar]
- 40.Kharraziha I., Axelsson J., Ricci F. Serum activity against G protein-coupled receptors and severity of orthostatic symptoms in postural orthostatic tachycardia syndrome. J. Am. Heart Assoc. 2020;9 doi: 10.1161/JAHA.120.015989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kohr D., Singh P., Tschernatsch M. Autoimmunity against the β2 adrenergic receptor and muscarinic-2 receptor in complex regional pain syndrome. Pain. 2011;152:2690–2700. doi: 10.1016/j.pain.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 42.Fu M.L., Herlitz H., Schulze W. Autoantibodies against the angiotensin receptor (AT1) in patients with hypertension. J. Hypertens. 2000;18:945–953. doi: 10.1097/00004872-200018070-00017. [DOI] [PubMed] [Google Scholar]
- 43.Wallukat G., Neichel D., Nissen E. Agonistic autoantibodies directed against the angiotensin II AT1 receptor in patients with preeclampsia. Can. J. Physiol. Pharmacol. 2003;81:79–83. doi: 10.1139/y02-160. [DOI] [PubMed] [Google Scholar]
- 44.Dragun D., Müller D.N., Bräsen J.H. Angiotensin II type 1-receptor activating antibodies in renal-allograft rejection. N. Engl. J. Med. 2005;352:558–569. doi: 10.1056/NEJMoa035717. [DOI] [PubMed] [Google Scholar]
- 45.Lukitsch I., Kehr J., Chaykovska L. Renal ischemia and transplantation predispose to vascular constriction mediated by angiotensin II type 1 receptor-activating antibodies. Transplantation. 2012;94:8–13. doi: 10.1097/TP.0b013e3182529bb7. [DOI] [PubMed] [Google Scholar]
- 46.Cremer S., Jakob C., Berkowitsch A. Elevated markers of thrombo-inflammatory activation predict outcome in patients with cardiovascular comorbidities and COVID-19 disease: insights from the LEOSS registry. Clin. Res. Cardiol. 2020;19:1–12. doi: 10.1007/s00392-020-01769-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fouladseresht H., Doroudchi M., Rokhtabnak N. Predictive monitoring and therapeutic immune biomarkers in the management of clinical complications of COVID-19. Cytokine Growth Factor Rev. 2020;58:32–48. doi: 10.1016/j.cytogfr.2020.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Okruhlicova L., Morwinski R., Schulze W. Autoantibodies against G-protein-coupled receptors modulate heart mast cells. Cell. Mol. Immunol. 2007;4:127–133. [PubMed] [Google Scholar]
- 49.Steckelings U.M., Sumners C. Correcting the imbalanced protective RAS in COVID-19 with angiotensin AT2-receptor agonists. Clin. Sci. (Lond.) 2020;134:2987–3006. doi: 10.1042/CS20200922. [DOI] [PubMed] [Google Scholar]
- 50.Haberland A., Santos R.A.S., Schimke I., Wallukat G. Are agonistic autoantibodies against G-protein coupled receptors involved in the development of long-term side effects of tumor chemotherapy? Case Rep. Oncol. 2013;6:104–108. doi: 10.1159/000348425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wallukat G., Morwinski M., Kowal K. Autoantibodies against the beta-adrenergic receptor in human myocarditis and dilated cardiomyopathy: beta-adrenergic agonism without desensitization. Eur. Heart J. 1991;12 doi: 10.1093/eurheartj/12.suppl_d.178. Suppl D:178–181. [DOI] [PubMed] [Google Scholar]
- 52.Dandel M., Wallukat G., Englert A. Long-term benefits of immunoadsorption in β(1)-adrenoceptor autoantibody-positive transplant candidates with dilated cardiomyopathy. Eur. J. Heart Fail. 2012;14:1374–1388. doi: 10.1093/eurjhf/hfs123. [DOI] [PubMed] [Google Scholar]
- 53.Müller J., Wallukat G., Dandel M. Immunoglobulin adsorption in patients with idiopathic dilated cardiomyopathy. Circulation. 2000;101:385–391. doi: 10.1161/01.cir.101.4.385. [DOI] [PubMed] [Google Scholar]
- 54.Jünemann A., Hohberger B., Rech J. Agonistic autoantibodies to the β2-adrenergic receptor involved in the pathogenesis of open-angle glaucoma. Front. Immunol. 2018;9:145. doi: 10.3389/fimmu.2018.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All available data are in included in this study.