Abstract
Alzheimer’s disease (AD) is a major public health crisis due to devastating cognitive symptoms, a lack of curative treatments, and increasing prevalence. Most cases are sporadic (>95% of cases) after the age of 65 years, implicating an important role of environmental factors in disease pathogenesis. Environmental neurotoxicants have been implicated in neurodegenerative disorders including Parkinson’s Disease and AD. Animal models of AD and in vitro studies have shed light on potential neuropathological mechanisms, yet the biochemical and molecular underpinnings of AD-relevant environmental neurotoxicity remain poorly understood. Beta-site amyloid precursor protein cleaving enzyme 1 (BACE1) is a potentially critical pathogenic target of environmentally-induced neurotoxicity. BACE1 clearly has a critical role in AD pathophysiology: it is required for amyloid beta production and expression and activity of BACE1 are increased in the AD brain. While the literature on BACE1 in response to environmental insults is limited, current studies, along with extensive AD neurobiology literature suggest that BACE1 deserves attention as an important neurotoxic target. Here, we critically review research on environmental neurotoxicants such as metals, pesticides, herbicides, fungicides, polyfluoroalkyl substances, heterocyclic aromatic amines, advanced glycation end products and acrolein that modulate BACE1 and potential mechanisms of action. While more research is needed to clearly understand whether BACE1 is a critical mediator of AD-relevant neurotoxicity, available reports provide convincing evidence that BACE1 is altered by environmental risk factors associated with AD pathology, implying that BACE1 inhibition and its use as a biomarker should be considered in AD management and research.
Keywords: BACE1, Alzheimer’s disease, oxidative stress, environmental neurotoxicants
1. Introduction
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder characterized by two hallmark pathologies: accumulation of amyloid beta (Aβ) plaques and neurofibrillary tangles (1). Based on genetic predisposition and age of onset, AD is classified into two types: early-onset familial AD and late-onset sporadic AD (2). Sporadic AD accounts for >95% of cases after the age of 65 years (3–5). Although AD was discovered over a century ago, curative and disease-modifying treatments remain elusive. Multifactorial pathology and etiology involving different mechanistic pathways could be the reason for the lack of effective therapies for AD (6). Aging and genetic predisposition play significant roles in the onset of AD (3–5,7). Adjustments to modifiable risk factors, proper management of comorbidities, and maintaining a healthy lifestyle could reduce the risk of dementia (8). Therefore, it is critical to identify and limit exposure to identified risk factors. Environmental toxicants have been extensively linked to neurodegenerative disorders including AD (9). These environmental neurotoxicants have a key role in accelerating disease onset and progression. Metals (10–13), pesticides (14), and dietary toxins (15–17) have been shown to accumulate in the serum and/or the brains of AD patients. However, the pathogenic mechanisms that underlie AD-relevant neurotoxicity resulting from environmental exposures remain understudied. To date, In vivo and in vitro experimental studies have shown that chronic exposure to environmental neurotoxicants such as metals (18–32) pesticides (14,33–36), polyfluoroalkyl substances (37), particulate matter (38,39), and dietary toxins (40–45) induces AD like pathology. It is critical to identify the biochemical and molecular mechanism underlying AD-relevant neurotoxicity because this knowledge can strengthen the understanding of etiological origins and pathology of sporadic AD leading to identification and development of biomarkers and therapies.
In vivo and in vitro studies have shown that beta-site amyloid precursor protein cleaving enzyme 1 (BACE1) might play a key role in environmental neurotoxicant induced AD-like pathology (14,18–49). BACE1 is required for Aβ production (50,51). BACE1 protein and activity are increased in AD patients; BACE1 activity is correlated with Aβ load and markers of oxidative stress in AD patients (52,53). Given the critical role of BACE1, inhibition, or modulation of BACE1, is considered a prime therapeutic goal for reducing Aβ production (54–56).
The role of environmental risk factors in AD has been reviewed numerous times; therefore, in this brief and focused review, we critically discuss data from experimental studies on the environmental neurotoxicants that alter BACE1 and their possible mechanisms of action.
2. BACE1 cell biology
2.1. General overview
BACE1 is a 501 amino acid type 1 transmembrane aspartic protease related to the pepsin family that has the characteristics of β secretase. The catalytic domain of BACE1 contains two signature aspartic protease motifs (Asp-Thr/Ser-Gly-Ser/Thr) that form the active site of the enzyme (50,57–62). Cysteine residues at 216/420, 278/443, and 330/380 form three disulfide bonds and tether the ectodomain of BACE1 in a native tertiary structure (63). BACE1 contains metal binding sites; a copper binding site is present in the cytoplasmic domain (48), and calcium is able to bind to the intracellular domain (64). BACE1 is homologous to BACE2, which is another membrane-bound secretase of the pepsin family, sharing 64% similarity in amino acid sequence. BACE2 is more abundant in peripheral tissues and has low expression in the brain. BACE2 cannot generate Aβ since the preferred BACE2 cleavage site in amyloid precursor protein (APP) is within Aβ (65–68).
BACE1 is synthesized in the endoplasmic reticulum and delivered to the cell surface from the trans-Golgi network. Transcription factors such as nuclear factor κB (NF-κB) (69,70), specificity protein 1 (Sp1) (71), peroxisome proliferator-activated receptor-gamma (PPAR γ) (72), hypoxia inducible factor 1α (HIF-1α) (73,74), Yin Yang 1 (YY1) (75), and nuclear factor of activated T cells (NFAT) (76) amongst others control the transcription of BACE1 (77). Mature BACE1 is internalized from the plasma membrane or directly from the trans-Golgi network and translocated to endosomes (78–80). During its maturation and intracellular trafficking BACE1 undergoes a complex set of post-translational modifications such as glycosylation, acetylation and phosphorylation (63,79,81–85).
BACE1 cleaves membrane-bound substrates only (86). It has maximal activity at an acidic pH (87–90), with the highest activity in the acidic subcellular compartments of the secretory pathway, such as the Golgi apparatus, trans-Golgi network, and endosomes (91,92). BACE is a highly stable protein with a half-life of 12–16 h (81). BACE1 can be degraded by 1) endoproteolysis, 2) the ubiquitin proteasomal pathway (93,94), or 3) the lysosomal pathway (95–99). SUMOylation at K501 residue increases the activity and stability of BACE1 (100). Alterations in BACE1 half-life can affect the level and enzymatic activity of BACE1.
BACE1 also cleaves several other substrates that may be important for processes and functions in the central nervous system. Proteomic studies have identified about 40 novel candidates as BACE1 substrates: including: neuregulin 1 type I and III-β1α, neuregulin 3 (101–104), seizure protein 6 (105,106), sodium gated voltage channel β2 (107–109), Jagged1 and Jagged2 (110,111). Moreover, important binding partners have been identified: Golgi-localized γ-ear-containing ARF-binding proteins (112), phospholipid scramblase 1 (113), SorLAs (114), sortilins (115,116), reticulon/Nogo proteins (117,118), prostate apoptosis response-4 (117), copper chaperone for superoxide dismutase-1 (49), Presenilin (119) among others, bind with BACE1 and modulate its activity and interaction with APP.
2.2. BACE1 distribution
BACE1 is present in most tissues of the body, but the highest activity levels are found in neural tissue (120–123). In the brain, BACE1 is more enriched in the hippocampus (123,124), and hippocampal mossy fiber terminals contain greater BACE1 expression (43,123,125–127). Neurons exhibit the highest BACE1 expression while resting astrocytes in brain do not express BACE1 at detectable levels; however, cultured astrocytes do express active BACE1 protein (128). Chronic activation of astrocytes increases BACE1 (129,130). BACE1-immunoreactive astrocytes are increased in proximity to Aβ plaques in the brains of aged transgenic 2576 mice and AD patients (131,132).
2.3. BACE1 and APP interactions
Interactions between BACE1 and APP have been extensively studied. APP processing is generally divided into two pathways, non-amyloidogenic and amyloidogenic. Non-amyloidogenic α-secretase mediated cleavage of APP releases soluble APP α into the extracellular space, and the C-terminal fragment of APP (CTF83) remains embedded in the plasma membrane. Cleavage of CTF83 by γ-secretase releases a small p3 fragment into the extracellular space and the APP intracellular domain (AICD) into the cytoplasm (133–136). Amyloidogenic BACE1 mediated APP cleavage releases a smaller soluble APP β into the extracellular space, and a larger APP C-terminal fragment (CTF99) remains embedded in the plasma membrane. Cleavage of CTF99 by γ-secretase releases Aβ into the extracellular space and the AICD into the cytoplasm (50,58,137–141).
2.4. BACE1 in AD
BACE1 has a critical role in the pathophysiology of AD. BACE1 expression and activity are increased in AD (142–144). BACE1 activity is correlated with Aβ load and markers of oxidative stress in AD patients (52,53). AD patients have higher CSF BACE1 activity than controls (145). BACE1 accumulates in normal and dystrophic presynaptic terminals surrounding amyloid plaques, causing increase in Aβ production near synapses in the brains of AD patients and AD mouse models (127). Given the critical role of BACE1 in AD pathology, inhibition of BACE1 is considered a prime therapeutic strategy for reducing Aβ production.
In the AD brain, both normal (143,146) and elevated BACE1 mRNA levels have been reported (147), and evidences for the regulation of BACE1 expression at the transcriptional and translational levels has also been presented. Brain extracts from AD patients showed that levels of PPARγ, involved in transcriptional regulation of BACE1 was decreased (148). Ceramide, the lipid second messenger is elevated in the brains of Alzheimer’s disease patients, has been shown to increases the half-life of BACE1 (149).
2.5. BACE1 in aging and MCI
Aging is a major risk factor for AD, where aging is associated with an increase in Aβ levels and accumulation of Αβ in the brain (150,151). BACE1 activity increases significantly in mouse, monkey, and human brains with aging. The increase of BACE1 activity and Αβ accumulation with age potentially predispose to AD (152).
BACE1 activity and protein levels are significantly increased in the brains of patients with mild cognitive impairment (MCI), who have a significant risk of developing AD (153–156). A significant increase in BACE1 activity is also found in cerebrospinal fluid (CSF) and plasma of subjects with MCI (153–155). BACE1 activity could predict progression from prodromal to probable AD stage.
2.6. BACE1 in other neurological diseases
Alteration in BACE1 is also present in other neurological diseases. Elevated levels of Aβ has been reported in human immunodeficiency virus (HIV) patients (157). HIV-associated neurotoxicity is mediated by N-methyl- d-aspartic acid or N-methyl- d-aspartate (NMDA)-dependent elevation of BACE1 and subsequent altered processing of APP (158). BACE1 also has a role in enzymatic cleavage of seizure protein 6, a protein associated with bipolar disorder. BACE1 is significantly upregulated in the plasma of male bipolar disorder patients compared with healthy subjects (159).
3. Environmental neurotoxicants that alter BACE1
Accumulation of Aβ occurs due to overproduction of APP, enhanced amyloidogenic processing of APP by BACE1, and/or a decrease in degradation or clearance of Aβ (33,138,160–165). Here we discuss environmental neurotoxicants that alter amyloidogenic processing of APP by BACE1(Table 1).
Table 1.
Summary of effects of environmental exposures on BACE1.†
| Exposure | Alterations in BACE1 | References |
|---|---|---|
| Metals | ||
| Aluminium | Aluminium exposure increased BACE1 activity in aged New Zealand rabbits and rats, elevated BACE1 levels in rats, and increased BACE1 mRNA in rats and SH-SY5Y cells | (18–22,184–186) |
| Arsenic | Developmental exposure of rats to arsenic increased BACE1 enzymatic activity and BACE1 protein; gene expression of BACE-1 increased in astrocytes exposed to arsenic | (25,203,204) |
| Copper | Copper exposure upregulated BACE1 around Aβ plaques in mice and increased the transcription of BACE1 in PC12 cells BACE1 has a copper-binding site in its cytosolic domain; it competes with SOD1 for the interaction with CCS |
(26,27,48,49) |
| Iron | Ferrous ion exposure increased BACE1 mRNA and protein expression in PC12 cells and neuroblastoma cells. Iron treatment enhanced binding of APP to BACE1, without significant changes in the level of BACE1 in microglia. |
(28,29,201). |
| Lead | Developmental exposure to lead increased expression of BACE1 in monkeys while BACE1 mRNA and protein expression and BACE1 activity were also upregulated in rodents. | (23–25,258–260) |
| Manganese | Manganese exposure increased the transcription of BACE1 in PC12 cells | (27) |
| Silver nanoparticles | Silver nanoparticle exposure increased the protein expression of BACE1 | (32) |
| Zinc | Zinc exposure increased BACE1 in mice and SH-SY5Y cells | (205) |
| Pesticides, fungicides, herbicides | ||
| DDT | DDT exposure increased BACE1 levels in SH-SY5Y cells | (33) |
| Paraquat | Paraquat treatment altered BACE1 subcellular compartmentalization | (36) |
| Rotenone | Rotenone treatment facilitated BACE1 expression and activity | (34) |
| Residues of cyprodinil, mepanipyrim, and pyrimethanil | Cocktail of fungicide residues promoted the expression of BACE1 | (35) |
| Synthetics | ||
| BPA | BPA exposure has been shown to increase the level of BACE1 in SH-SY5Y cells | (227,228) |
| PFOS | Chronic high concentration PFOS exposure in mice increases BACE1 mRNA | (37) |
| Particulates | Chronic exposure of mice to particulate matter increased BACE1 protein levels | (38,39,261) |
| Dieatary toxins/toxicants | ||
| Acrolein | Chronic acrolein exposure increased protein levels of BACE1 in rats and HT22 cells | (44,45,193,255–257) |
| Advanced glycation end products | Pentosidine and GLAP upregulates BACE1 mRNA and protein expression | (43) |
| Heterocyclic aromatic amines | PhIP exposure for acute, sub-acute, and sub-chronic time point increased BACE1 in mice | (43) |
Abbreviations: BPA: Bisphenol A, CCS: Copper chaperone for superoxide dismutase-1 (SOD1), DDT: Dichlorodiphenyltrichloroethane, PFOS: Perfluorooctane sulfonate, PhIP: 2-Amino-1-methyl-6-phenylimidazo (4,5-b)pyridine
3.1. Metals.
Chronic exposure to metals is a health hazard. Exposure to metals can be due to different sources: occupational, dietary (food or water), or in inhaled air (13,166–174). Overall, occupational exposure to metals is higher in terms of dose, but rarer, compared to other sources. Due to the aggressive pace of anthropogenic activities, humans are exposed not only to essential metals such as copper, iron, manganese, and zinc, but also to potentially toxic metals, including aluminium, arsenic, cadmium lead, and mercury (13,168–174). Several clinical studies have shown an increase in metal ions in AD, suggesting that metal ions might be a risk factor associated with the pathogenesis of AD (11–13,160,175–180).
Aluminium:
Epidemiological studies have identified aluminium as a risk factor for AD. Exposure to aluminium may occur through food, drinking water, topically applied cosmetics, and hair, skin, and hygiene products, however inhalation as fine particles is the most relevant way of exposure (169). Here, it is worth noting that the link between AD and aluminium as a causative factor remains highly controversial, with a large amount of negative data (181,182). Aluminium is reported to accumulate in the brain, serum, and CSF of AD patients (11–13,183). In vivo and in vitro studies have demonstrated Aβ accumulation from aluminium exposure is BACE1 mediated. BACE1 activity is increased significantly in the hippocampus of aged New Zealand rabbits treated with aluminium-maltolate (18). Rats treated with aluminium-maltolate had significantly higher levels of BACE1 and γ-secretase enzymes, whereas α-secretase related protein decreased, resulting in increased Aβ production. (19,184,185). Aluminium-maltolate treatment also increased mRNA transcription and enzyme activity of BACE1 in rats (185). AKT/GSK-3β signalling is involved in aluminium-maltolate mediated effects (184). Wang et al. reported that exposure to aluminium-maltolate increased BACE1 expression and reduced the expression of miR29 subtypes; expression of miR29a and miR29b1 was negatively correlated with BACE1 expression (186). AlCl3 exposure also induced severe neurodegeneration, marked with elevated BACE1, Aβ level, and impaired insulin signalling in rats (20). Another study showed upregulation of the gene expression of BACE1 and APP on AlCl3 exposure in rats. This was accompanied by an increase in oxidative stress, neuroinflammation, and activity of acetylcholinesterase; presenilin2 and ER-β expression was downregulated (21). An increase in BACE1 and BACE2 mRNA is seen after co-exposure of SH-SY5Y cells to AlCl3 and Aβ (22).
Lead:
Lead was extensively used in paint, pipes, and gasoline; individuals, and particularly children, living in highly urban areas had the highest exposures to lead in the United States in 1960–1980 (187–189). Accumulating evidence suggests that childhood lead exposures may significantly increase risk for neurodegenerative disease in old age (190). Adults chronically exposed to lead also show abnormalities in brain metabolism (191–193). A positive association was found between an increase in blood lead level and AD mortality after adjustment for competing risks or design effects (194). Animal studies have provided evidence that developmental exposure to lead induces AD-like pathology through BACE1 upregulation. Monkeys exposed to lead as infants had intracellular Aβ and amyloid plaques in the frontal association cortex and expression of APP, BACE1, and Sp1 transcription factor were upregulated in old age (23). DNA methylation appears to have an essential role in these latent effects. Developmental exposure of rats to lead results in upregulation of Aβ, APP and Sp1 transcription factor. BACE1 mRNA, protein expression, and activity were also upregulated later in life (24). Ashok et al. investigated the effects of developmental exposure of rats to individual metals (arsenic, lead, and cadmium) and their combination (at concentrations detected in groundwater of India) on the AD-pathology. They have shown that metals activated the synthesis of Aβ, which was mediated by an increase in APP and APP processing enzymes such as BACE1 and presenilin. Among individual metals, lead triggered maximum induction of Aβ (25).
Copper:
Chronic environmental and occupational exposure to copper causes adverse health effects. The major source of copper is dietary intake from solid food and drinking water; exposure to polluted air is another minor source of copper (171,195). Copper and other essential metals such as iron and zinc are found in Aβ senile plaques and neurofibrillary tangles in AD (175,176). Copper exposure in young 3xTransgenic-AD mice led to an increase in the accumulation of Aβ, which was found to be mediated via the upregulation of BACE1 around Aβ plaques (26). Lin et al. reported that copper increased the transcription of APP and BACE1 in PC12 cells. The increased oxidative stress following copper exposure may be relevant to the expression of BACE1 levels in neurons (27). BACE1 has a copper-binding site in its cytosolic domain (48). Upregulation of BACE1 results in the reduced activity of superoxide dismutase-1 (SOD1), as BACE1 competes with SOD1 for the interaction with CCS (copper chaperone for superoxide dismutase-1 (SOD1)), an important protein that transports copper to SOD1 for its activation (48,49).
Iron:
Brain iron increases with aging, and its accumulation is enhanced in the AD brain in regions such as the parietal cortex, motor cortex, and hippocampus (177–180,196–200). Iron is found in Aβ senile plaques and neurofibrillary tangles (175). The subtoxic concentration of ferrous ions increases APP-α-carboxyl-terminal fragment (APP-α-CTF) associations with A Disintegrin and metalloproteinase domain-containing protein-10 (ADAM10) and APP-β-CTF with BACE1 in PC12 cells. Levels of ADAM10 and BACE1 mRNA and B-cell lymphoma 2 protein expression was also increased (201). In another study, iron treatment impaired APP/Ferroportin 1 complex and enhanced binding of BACE1 to APP without significant changes in the level of BACE1 in microglia (28). FeCl2 is a prooxidant molecule: it can stimulate oxidative stress. In differentiated human neuroblastoma cells, FeCl2 exposure increased Aβ production by up-regulation of BACE1 and gamma-secretase, and down-regulation of alpha-secretase (29).
Arsenic:
Arsenic toxicity is a health concern worldwide as millions of people are exposed to arsenic via drinking water. Epidemiological studies reported that long term arsenic exposure impairs learning and cognitive abilities (170,202). Developmental exposure of rats to arsenic, lead, and cadmium at concentrations detected in groundwater of India activated the synthesis of Aβ, which was mediated by an increase in APP and APP processing enzymes, such as BACE1 (25). Developmental arsenic exposure also induces behavioral deficits accompanied by an increase in BACE1 enzymatic activity, Aβ, and RAGE (203). Gene expression of APP, BACE-1, TNF-α, IL-1β, IL-6, COX-2, and MIF-1 increased significantly in rat cortical astrocytes exposed to a sub-toxic monomethylated metabolite of inorganic arsenic (204).
Zinc:
Zinc exposure could also be a risk factor for AD. Zinc is found in Aβ senile plaques and neurofibrillary tangles in AD (176). APP and presenilin1 double transgenic mice treated with a high dose of zinc in drinking water had a significantly increased amount of zinc levels in the brain. APP expression, Aβ deposition, BACE1, and γ-secretase enzymes increased, whereas α-secretase related protein decreased on Zinc exposure in these mice (205). SH-SY5Y cells overexpressing human APPsw exposed to zinc also confirmed these results (205).
Manganese:
Manganese is a trace element that is essential for brain function. Exposure to toxic concentrations of manganese occurring in occupational settings during mining, ore‐processing, welding, and ferroalloy production has adverse health effects (172,173). Excessive manganese is neurotoxic and has been linked to neurodegenerative disorders (206). Manganese exposure increases the transcription of APP and BACE1 in PC12 cells (27). Guilarte et al. investigated the effect of chronic manganese exposure in non-human primates. Chronic manganese exposure increases a cellular stress response that leads to increased amyloid precursor-like protein1 protein expression and diffuse Aβ plaques in non-human primates (30).
Silver nanoparticles:
Silver nanoparticles (AgNPs) are used as an antimicrobial and antifungal agent in food containers, clothing, pharmaceuticals, and electronics (168). Lin et al. reported that AgNPs accumulates in astrocytes and mouse neuroblastoma neuro-2a cells due to the disruption of tight junction proteins (32). AgNP exposure increases Aβ deposition in a neuronal and triple cell co-culture model of mouse endothelial cells, astrocytes, and neuroblastoma neuro-2a cells (31,32). In another study, AgNP exposure was shown to increase the protein expression of APP, BACE1, presenilin1, and presenilin 2 (32).
3.2. Pesticides, fungicides, and herbicides.
The use of pesticides, fungicides, and herbicides in household and agricultural areas has exponentially increased and polluted the environment, resulting in bioaccumulation of toxicants (207). Exposure to pesticides, fungicides, and herbicide is a risk factor for AD (208). Meta-analysis of data from cohort and case-control studies have shown a positive association between pesticide exposure and AD (209).
Dichlorodiphenyltrichloroethane:
Serum levels of the organochlorine pesticide dichlorodiphenyltrichloroethane (DDT), and its metabolite dichlorodiphenyldichloroethane (DDE), were found to be elevated in AD patients (14). Experimental studies provided the mechanistic link for the association of DDT exposure with AD. Exposure of SH-SY5Y cells to DDE or DDT significantly increased APP levels (14). Another study showed DDT exposure augmented Aβ levels by increasing APP and BACE1 levels, and by reducing the clearance and degradation of Aβ in human neuroglioma H4-AβPPswe cells (33).
Rotenone:
Rotenone is a well-known mitochondrial respiratory inhibitor. Rotenone could produce 1-methyl-4-phenyl pyridine (MPP+) and reproduces the features of Parkinson’s disease (PD) (210,211). Rotenone could also be linked to AD. Rotenone induces mitochondrial dysfunction, mitochondrion-derived ROS formation, and Aβ generation (212). Xiong et al. reported that rotenone treatment elevates Aβ and APP in the retina. Mitochondrial respiratory inhibition and oxidative stress seen on rotenone treatment also facilitate BACE1 expression and activity (34).
Paraquat:
Paraquat is a widely used herbicide around the world. Paraquat exposure has been linked to PD (213). Paraquat exposure induces oxidative stress and mitochondrial damage (214,215). Mild oxidative stress induced on paraquat treatment alters BACE1 subcellular compartmentalization to favor the amyloidogenic processing of APP in primary cortical cells (36).
Cocktails of fungicides residues:
Residues of fungicides cyprodinil, mepanipyrim, and pyrimethanil are detected in many foodstuffs (216). Chronic exposure of transgenic (J20, hAPP Swedish/Indiana) mice to a cocktail of fungicide residues of cyprodinil, mepanipyrim, and pyrimethanil promotes the expression of BACE1 and impairs Aβ clearance. Incubated alone or in a cocktail, these fungicides bind to amyloid plaques ex vivo and promote Aβ peptide fibril formation in vitro (35).
3.3. Synthetic compounds
Perfluorooctane sulfonate:
Perfluorooctane sulfonate (PFOS) is a per- and polyfluoroalkyl substance with extensive applications. PFOS is a major public health concern due to long environmental and biological half-lives (217). Our group has shown that PFOS produces dopaminergic neuropathology in Caenorhabditis elegans (218) and selectively decreases brain dopamine levels in Northern leopard frogs (Rana pipiens) on developmental exposure (219). Developmental PFOS exposure has also been linked to AD pathogenesis. Chronic PFOS exposure in mice increases APP and Aβ1–42 levels; significant up-regulation of BACE1 mRNA was also observed on exposure to a high concentration of PFOS (37).
Bisphenol:
Bisphenol A (BPA) is an environmental endocrine-disrupting chemical. Human exposure to BPA is ubiquitous as it is one of the highest-volume chemicals produced worldwide (220). Epidemiological studies have linked BPA to metabolic disorders (221,222). Accumulating evidence suggests that early-life exposure to BPA impacts neural development in humans (223–226). BPA exposure can also be a risk factor for AD. Prenatal exposure to BPA has been shown to increase the level of NF-κB protein and its target gene BACE1. The upregulation of BACE1 was observed only in males, and there were no significant changes in Aβ levels (227). BPA induced Aβ accumulation and disruption in insulin signalling in SH-SY5Y cells. AD-associated proteins, such as APP, BACE-1, β-CTF, α-CTF, and phosphorylated tau were increased after BPA exposure, and these effects were abrogated by insulin and rosiglitazone treatment (228).
Particulate matter:
Fine and ultrafine particles may be translocated to systemic circulation and the brain through the nasal olfactory pathway (229). The possibility of initial involvement of the olfactory pathway in AD has caused speculation that some inhaled agents might be a risk factor for AD (230). AD-like pathology is seen in individuals residing in cities with high levels of air pollution (231). Chronic exposure of mice to concentrated air particulate matter (PM) (particles measuring 2.5 μm or smaller in diameter and collectively termed PM2.5) increased BACE1 protein levels, APP processing, and Aβ 1–40 levels. This was correlated with an increase in cytokine level and cyclooxygenase-1 and cyclooxygenase-2 protein levels (39). In another study, PM2.5 exposure increased BACE1, deteriorated spatial learning and memory, and synaptic function integrity. These effects were mediated by NF-κB p65-regulated downregulation of miR-574–5p, that targets BACE1 (38).
3.4. Dietary toxins.
The role of the diet in the etiology of AD has received recent attention (232,233). High-fat diets and diets rich in saturated free fatty acids, high glucose, and oxidation products of cholesterol are potential risk factors for AD. They are known to promote amyloidogenic cleavage of APP by BACE1 (234–239). Cooking and processing of food at a high temperature can produce toxic compounds, such as advanced glycation end products (AGEs), heterocyclic aromatic amines (HAAs), acrylamide, and acrolein (240–244). The potential role of dietary toxins is being studied for AD relevance as they are encountered in higher doses and more frequently through one’s life span compared to other environmental contaminants (232,245).
Advanced glycation end products:
AGEs are naturally present in uncooked animal-derived foods; high-temperature cooking accelerates the formation of new AGEs within these foods. AGEs are formed through the Maillard reaction between the aldehyde group of glucose and the amino group of proteins (246,247). AGEs are also formed as a part of normal metabolism within the body, and AGEs accumulate due to certain dietary habits, aging, and in sporadic AD (15). Diets high in AGEs induce AD-like pathology in mice. Transgenic 2576 mice that received a diet high in AGEs had significantly higher levels of oxidative stress, AGEs, the receptor for advanced glycation end products (RAGE), and insoluble Aβ in the hippocampus (41,248). Guglielmotto et al. showed that AGEs such as pentosidine and glyceraldehydes-derived pyridinium (GLAP) upregulate BACE1 mRNA and protein expression through RAGE activation and the consequent activation of NF-κB (41). BACE1 is upregulated in cells overexpressing RAGE and in RAGE-injected brains of Tg2576 mice (40). AGEs/RAGE axis upregulate BACE1 expression via reactive oxygen species (ROS) production (40,41), and activation of nuclear factor of activated T-cells 1 (NFAT1) (40) resulting in Aβ production and deposition in the brain.
Heterocyclic aromatic amines:
Heterocyclic aromatic amines (HAAs) are primarily formed during high-temperature meat cooking (240,241). HAAs are formed through the Maillard reaction between amino acids and sugars, producing pyrimidine, pyridine, or pyrazine, which later reacts with creatine in a heat-dependent reaction (240,241). PhIP (2-Amino-1-methyl-6-phenylimidazo (4,5-b)pyridine) is the most abundant and extensively studied HAA isolated from the crust of cooked meat, and its levels may reach ~15 micrograms/kg uncooked meat (241,249,250). Studies from our lab have reported that PhIP generates reactive free radicals, leading to an increase in the accumulation of ROS (43,251,252). Recently, our group showed that PhIP exposure for acute, sub-acute, and sub-chronic time points produced oxidative damage and alterations in synaptic proteins. Our study demonstrated that sub-chronic PhIP exposure promotes Aβ aggregation by increasing the levels of BACE1, APP, and oxidative damage (43).
Acrolein:
Acrolein is a dietary aldehyde that is present in high concentrations in alcoholic beverages, water, cheese, donuts, coffee, tobacco smoke, industrial waste, and automobile exhaust. It is also formed during deep frying of vegetable and animal fats (253). In vivo, acrolein is formed by the metal-catalyzed oxidation of polyunsaturated fatty acids (254). Acrolein is increased in the amygdala and hippocampus/parahippocampal gyrus in AD patients (16). Chronic acrolein exposure increased protein levels of APP, BACE1, RAGE, and decreased A-disintegrin and ADAM levels in rats (44,45). Acrolein activated MAPK signalling pathways and altered the levels of AD-associated proteins ADAM-10, BACE-1, and RAGE in HT22 murine hippocampal neuronal cells. Inhibitors of MAPK signalling pathways attenuated these effects (255). These effects were associated with oxidative stress, ROS accumulation, glutathione depletion, decreased SOD, and an elevation of MDA (256,257).
4. Exposures and mechanisms that lead to upregulation of BACE1 expression and activity:
Commonly examined detrimental processes of oxidative stress, mitochondrial respiratory inhibition, and direct physical interaction of toxicants with BACE1 are likely important in neurotoxicant induced alteration in BACE1 expression and activity. An increase in BACE1 and subsequent increase in Aβ pathology induced by most of these neurotoxicants was associated with oxidative stress (21,27,34,36,40–43). Oxidative stress has been shown to decrease the activity of α-secretase while promoting the expression and activity of BACE1 (29). Treating primary cortical neurons and HSV-APP cells with hydrogen peroxide (H2O2) increases BACE1 protein and its products (CTFs), in primary neurons, and BACE1 protein and Aβ in HSV-APP cell (262). Inactivating BACE1 with siRNA1 lowers the BACE1, CTF, and Aβ levels following H2O2 treatment. Treating APPwt and APPsw cells with antioxidants reduces superoxide anions and BACE1 activity, indicating that, at least in part, ROS-dependent BACE1 alteration is implicated in Aβ production (212,262). Oxidative stress is also an early event in AD (263); oxidation products, such as 4-hydroxynonenal (HNE) and malondialdehyde (MDA), increases in AD brain tissue. A significant correlation exists between BACE1 activity and markers of oxidative stress in AD, supporting the hypothesis that a direct connection exists between oxidative stress and BACE1 in sporadic AD (53,264,265).
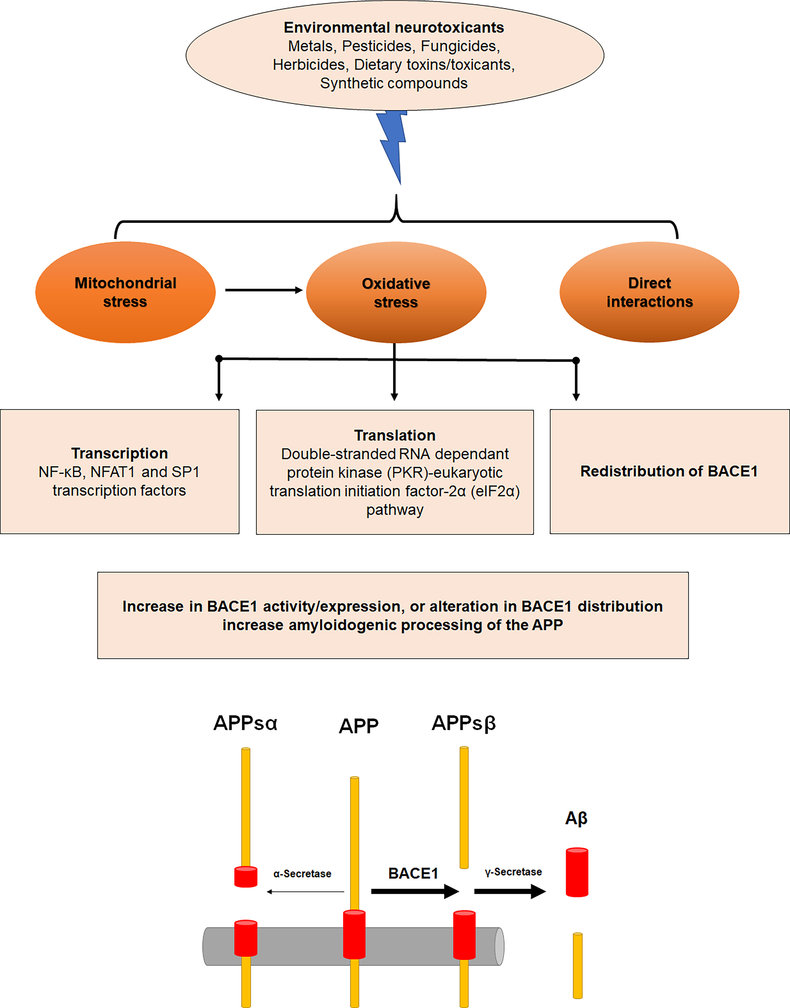
Environmental neurotoxicants upregulate the expression and activity of BACE1, with some increasing BACE1 mRNA level (21,22,24,27,37,41,185,201,204), while others do not affect BACE1 mRNA (35,266). Transcriptional and post-transcriptional control is implicated in BACE1 modulation by oxidative stress (29,266–269). Alterations in BACE1 expression and enzyme activity induced by oxidative stress are mediated through the interplay of multiple signaling pathways (Figure 1).
Figure 1: Neurotoxic targets that lead to upregulation of BACE1 expression and activity:
BACE1 can be an important neurotoxic target in AD-like neurotoxicity induced by environmental neurotoxicants such as metals, pesticides, fungicides, herbicides, dietary toxins/toxicants and synthetic compounds. Environmental neurotoxicants increase BACE1 expression and activity by inducing mitochondrial stress, oxidative stress or by direct interaction. Oxidative stress induced by environmental neurotoxicants can increase transcription and translation of BACE1 or induce redistribution of BACE1 to increase amyloidogenic processing of APP and increase amyloid beta production.
Oxidative stress induced by H2O2 has been shown to increase BACE 1 by potentiating the BACE1 promoter activity and upregulating BACE1 transcription (270). Transcription factors such as NF-κB, NFAT1 and Sp1 have been reported to be activated after exposure to environmental neurtoxicants (23,24,40–42,227). Tamagno et al. reported that the activation of SAPK signaling is involved in oxidant-induced upregulation of BACE1 expression. Exposure of NT2 neurons to HNE upregulates expression of BACE1 and increases intracellular Aβ. HNE-dependent upregulation of BACE1 expression was found to be mediated by activation of c-junN-terminal kinase (JNK) and p38 mitogen-activated protein kinases (p38MAPK) (267). Inducers of JNKs such as hydrogen peroxide increase BACE1 protein level, and inhibition of JNKs abolished this BACE1 induction, confirming that JNK pathways mediate this response (29,267,268). Phosphatidylinositol-3-kinase (PI-3K)/Akt pathway and the extracellular signal-regulated MAP kinase (ERK) pathway are also involved in oxidative stress-mediated BACE1 upregulation. Upregulation of BACE1 protein correlated with increased levels of phosphorylated Akt and ERK1/2 in glutathione peroxidase 4+/− (Gpx4+/−) mice (266). Oxidative stress also alters BACE1 expression at the translational level, and it is mediated by the double-stranded RNA dependant protein kinase (PKR)-eukaryotic translation initiation factor-2α (eIF2α) pathway. A significant correlation was found between BACE1 levels and eIF2α activation in human AD brains. Inhibition of PKR by chemical inhibition or by siRNA significantly attenuates BACE1 protein (269).
Redistribution of BACE1 also facilitates amyloidogenic processing of APP; mild oxidative stress has been reported to promote amyloidogenic processing by redistributing the BACE1 to organelles having optimal environment for BACE1 activity (36). Tan et al. have suggested that depending on the stress intensity inflicted on neurons, oxidative stress may exert a dual effect on BACE1. Mild oxidative stress that does not induce cell death does not increase BACE1 mRNA and protein. Instead, mild oxidative stress promotes amyloidogenic processing by redistributing BACE1 to the trans-Golgi network and early endosomes, which provide an acidic environment an optimal for BACE1 activity. In contrast, in sustained or severe oxidative stress, apoptotic mechanisms would become activated, and BACE1 expression would increase, thereby promoting a more significant Aβ production (36). Hypoxia induction caused a similar biphasic increase of BACE1 (74). However, other studies have also shown the accumulation of BACE1 protein in the absence of cell death (271).
Mitochondrial dysfunction has been suggested to have an early and perhaps a causative role in AD pathogenesis (272–275). AD mice treated with a complex I inhibitor or mice with a genetic defect in complex I, or, showed enhanced Aβ levels in vivo (212). BACE1 expression and its activity appear to be coupled to mitochondrial function. Mitochondrial respiratory inhibition and energy inhibition elevates BACE1 levels and activity (276). Neurotoxicants such as rotenone is a known mitochondrial complex I inhibitors; mitochondrial complex inhibitors and inhibitors of succinate dehydrogenase have been shown to alter BACE1 (34,36). Applications of rotenone, other mitochondrial complex inhibitors, and inhibitors of succinate dehydrogenase increase BACE1 proteins and activity, and Aβ40 levels (34). Paraquat, a known mitochondrial neurotoxicant, induced mild oxidative stress and altered BACE1 subcellular compartmentalization to favor the amyloidogenic processing of APP (36). A strong negative correlation was found between BACE1 and cytochrome c oxidase or succinate dehydrogenase activity at the first synapse on the olfactory pathway (277). Increased BACE1 levels and activity are observed in a variety of experimental conditions likely involving mitochondrial stress and energy disruption (270,276,278). Mitochondrial respiratory inhibitors are generally considered to cause oxidative stress (210,279); it is not possible to differentiate energy inhibition from oxidative stress. Therefore, oxidative stress might be a common element in increasing BACE1. Mitochondrial proteins are also likely important in BACE1 regulation. Over expression Thioredoxin-2 (TXN2), a mitochondrial protein that has a critical role in the scavenging ROS in mitochondria (280) decreases BACE1 transcription; while silencing increases BACE1 transcription. Further, TXN2 knockdown in HEK-APP and SH-SY5Y-APP cells increased the mRNA and protein levels of BACE1 (281). BACE1 also contributes to mitochondrial dysfunction. Accumulation of β-cleaved C-terminal fragment-99 and APP through BACE1 dependent mechanism contributes to mitochondrial dysfunction, and deletion of BACE1 (BACE1+/−) rescued mitochondrial function in 5XFAD mice (282).
Metals can directly interact with BACE1 to modulate APP processing and Aβ release. Biophysical studies have shown an interaction between BACE1, calcium (64), and copper (48,49,283). BACE1 is described as a copper-binding protein; it has a copper-binding site in its cytosolic domain (48,49,283). The cytoplasmic domain of BACE1, a 24-residue peptide corresponding to the C-terminal domain of BACE1, binds a single copper(I) atom with high affinity through cysteine residues. The cytoplasmic domain of BACE1 interacts with CCS that delivers copper to BACE1. Cytosolic CCS forms a stable association with membrane-bound BACE1; CCS protein and BACE1 have been shown to move together in axons of rat primary cortical neurons (48,49). CCS is also essential for the activation of SOD1. CCS binds copper (I) through residues in domains I and III. This enhances the binding of CCS to Zn-SOD1, leading to the formation of a non-covalent heterodimeric CCS–SOD1 complex via domain II of CCS (284). BACE1 interacts with the domain I of CCS, preventing the formation of copper-bound CCS and thereby inhibiting the activation of SOD1. Overexpression of BACE1 results in the reduced activity of SOD1, as BACE1 competes with SOD1 for interaction with CCS (48,49). This interaction provides a link between metal homeostasis and oxidative stress in AD. Calcium also binds to BACE1 and alters its activity. The intracellular domain of BACE1 contains a metal-binding motif Cys-Xaa-Xaa-Cys, one additional cysteine, and potential Calcium-binding residues. Binding studies have provided evidence that calcium binds to BACE1 with high affinity. Low concentrations of calcium can increase the enzymatic activity of BACE1, thereby increasing Aβ production (64). This calcium-BACE1 interaction and generation of the Aβ peptide are supported by the calcium dysregulation hypothesis in aging and AD (285–289).
5. Theoretical BACE1 targets important to neurotoxicity:
Not much is known about the susceptibility of BACE1 catalytic domain and other domains to neurotoxicants. The BACE1 lumenal extension ends are attached by two disulfide bonds connecting directly to the catalytic domain. A span of approximately 11 amino acids attach the catalytic domain of the BACE1 to the lipid membrane, C-terminally of the last disulfide bonded cysteine of the lumenal extension (61). The six Cys residues in these ectodomain form three intramolecular disulfide linkages (Cys(216)-Cys(420), Cys(278)-Cys(443), and Cys(330)-Cys(380)) (62). Fischer et al. reported that the disulfide bonds between Cys(330)-Cys(380) play an important role in the catalytic domain of BACE1 and the other two disulfide bonds are less important for APP processing activity (290). Simulations studies have shown that the absence of disulfide bonds has been shown to weaken/change the interaction strength of BACE1 inhibitors with some residues in the interaction network of BACE1-inhibitors (291). This disulfide bond could be possible a neurotoxic target as disulfide bond formation in other proteins has been reported to be impaired in certain conditions such as hypoxic (292), on exposure to nicotine (293). Koelsch has hypothesized that under hypoxic conditions, two disulfide bonds that attach both ends of the lumenal domain in BACE1 might be incompletely formed, leading to release of the BACE1 catalytic domain away from the lipid bilayer membrane, allowing possibly a greater degree of freedom, increased access to the substrate, and increased activity (294). While plausible, it should be noted that this hypothesis has not yet been tested.
6. BACE1- a potential biomarker and therapeutic target for sporadic AD:
A significant increase in BACE1 level and activity is found in CSF of subjects with MCI and ApoE ε4 genotype carriers with MCI (153,155). Plasma BACE1 activity is also increased in MCI converters and probable AD patients (154). Given the reported 1) alteration of BACE1 expression and/or activity in environmental neurotoxicant induced AD-like pathology in experimental studies and subjects with MCI and APOE ε4 carriers with MCI are at higher risk of developing AD, 2) peripheral BACE1 could be an indicator of MCI and AD risk, and 3) ease of measuring BACE1 in plasma and CSF, and novel PET radioligand [18F]PF-06684511 for in vivo imaging of BACE1; BACE1 has promising potential for use as a clinical diagnostic biomarker for screenings during the presymptomatic stages and monitoring the disease progression in sporadic AD (153–155,295–298).
The important role of BACE1 in the amyloidogenic pathway and modulation of BACE1 by many risk factors associated with sporadic AD has led to the exploration of the potential use of BACE1 as a therapeutic target for AD. BACE1 inhibitors have shown therapeutic effects in AD animal models; however, many BACE1 inhibitors have failed in different phases of clinical trials due to safety concerns (56,299–301). BACE1 null/knockout mice exhibit hypomyelination (302), a significant increase in astrogenesis with a corresponding decrease in neurogenesis in their hippocampi during early development (110), abnormal neuronal clustering in the dentate gyrus (303), and axonal organization defects in the hippocampus (304). Toxicity induced by BACE1 inhibitors and the physiological role of BACE1 makes it difficult to use BACE1 as a treatment for AD.
6. Conclusion and future research:
Environmental neurotoxicants such as metals, pesticides, and dietary toxins have been shown to accumulate in the AD patient; experimental studies in vivo and in vitro have strengthened knowledge about mechanisms associated with it. BACE1 is the initiating and putatively rate-limiting enzyme in Aβ generation. BACE1 expression and/or enzymatic activity are altered in aging, MCI, ApoE ε4 genotype carriers with MCI, and after exposure to various environmental neurotoxicants, indicating that BACE1 is a crucial molecular link between these risk factors and sporadic AD pathogenesis. Although experimental studies have highlighted that oxidative stress, mitochondrial respiratory inhibition, and direct physical interactions might be a common mechanism in environmental neurotoxicant induced BACE1 alterations, the mechanism associated with many environmental neurotoxicants induced BACE1 alteration is still not clearly known. Given the key role that BACE1 has in risk factors associated-AD, future studies are needed to clearly understand these mechanisms. BACE1 undergoes physical interactions with copper and calcium; other metals and reactive environmental neurotoxicants should also be studied to determine if they directly interact with BACE1 to activate it or if they have a neurotoxic target in BACE1. Similar to findings in AD patients, in vivo and in vitro studies also report both normal and elevated BACE1 mRNA along with increased protein expression and/or activity on exposure to environmental neurotoxicants. There is inconsistency in BACE1 measurement; some studies just analyze mRNA or protein expression and/or enzymatic activity. Measuring mRNA, protein expression, activity, and the degradation pathway of BACE1 will help better understand the mechanism associated with BACE1 alteration.
Despite the challenges in BACE1 inhibitor development and failures of BACE1 inhibitor clinical trials, BACE1 inhibitor development should continue by taking into consideration the optimal timing and dose for treatment. There is substantial data indicating the important role of BACE1 in sporadic AD and proving BACE1 inhibitors as disease-modifying agents. Future studies should focus on understanding the physiological functions of other BACE1 substrates and generating inhibitors with an APP selective BACE inhibitory effect. Correlation of BACE1 with disease severity and ease of measuring of BACE1 in plasma and CSF and through in vivo imaging makes BACE1 an ideal candidate for use as a clinical diagnostic biomarker, together with the existing suite of amyloid and tau biomarkers, for screening the presymptomatic stages and monitoring the disease progression in sporadic AD. BACE1 could also be used as a biomarker in drug development.
7. Acknowledgments
J.R.C. was supported by ES025750 from the National Institutes of Health.
Abbreviations:
- MPP+
1-methyl-4-phenyl pyridine
- Aβ
Amyloid beta
- ADAM10
A Disintegrin and metalloproteinase domain-containing protein-10
- AD
Alzheimer’s disease
- APOE4
Apolipoprotein
- APP
Amyloid beta precursor protein
- APP-α-CTF
APP-α-carboxyl-terminal fragment
- AICD
APP intracellular domain
- AGE
Advanced glycation end products
- BACE1
β-Site amyloid precursor protein cleaving enzyme 1
- BPA
Bisphenol A
- CCS
Copper chaperone for superoxide dismutase-1
- CTF83
C-terminal fragment of APP
- CTF99
C-terminal fragment
- CSF
Cerebrospinal fluid
- DDT
Dichlorodiphenyltrichloroethane
- DDE
Dichlorodiphenyldichloroethane
- ERK
extracellular signal-regulated MAP kinase
- eIF2α
Eukaryotic translation initiation factor-2α
- GLAP
Glyceraldehydes-derived pyridinium
- H2O2
Hydrogen peroxide
- HAAs
Heterocyclic aromatic amines
- HIV
human immunodeficiency virus
- HNE
4-hydroxynonenal
- JNK
c-junN-terminal kinase
- MDA
Malondialdehyde
- MCI
Mild cognitive impairment
- NMDA
N-Methyl- d-aspartic acid or N-Methyl- d-aspartate
- NF-κB
Nuclear factor κB
- PFOS
Perfluorooctane sulfonate
- PI-3K
Phosphatidylinositol-3-kinase
- PhIP
2-Amino-1-methyl-6-phenylimidazo(4,5-b)pyridine
- PM
Particulate matter
- ROS
Reactive oxygen species
- RAGE
Receptor for advanced glycation end products
- SOD1
superoxide dismutase-1
- AgNPs
Silver nanoparticles
8. Data availability
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
Reference:
- 1.Ingelsson M, Fukumoto H, Newell KL, Growdon JH, Hedley-Whyte ET, Frosch MP, Albert MS, Hyman BT, Irizarry MC. Early Abeta accumulation and progressive synaptic loss, gliosis, and tangle formation in AD brain. Neurology 2004;62(6):925–31. [DOI] [PubMed] [Google Scholar]
- 2.Van Cauwenberghe C, Van Broeckhoven C, Sleegers K. The genetic landscape of Alzheimer disease: clinical implications and perspectives. Genet Med 2016;18(5):421–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R, Myers RH, Pericak-Vance MA, Risch N, van Duijn CM. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA 1997;278(16):1349–56. [PubMed] [Google Scholar]
- 4.Liu CC, Liu CC, Kanekiyo T, Xu H, Bu G. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat Rev Neurol 2013;9(2):106–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Riedel BC, Thompson PM, Brinton RD. Age, APOE and sex: Triad of risk of Alzheimer’s disease. J Steroid Biochem Mol Biol 2016;160:134–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gong CX, Liu F, Iqbal K. Multifactorial Hypothesis and Multi-Targets for Alzheimer’s Disease. J Alzheimers Dis 2018;64(s1):S107–S117. [DOI] [PubMed] [Google Scholar]
- 7.Saunders AM, Strittmatter WJ, Schmechel D, George-Hyslop PH, Pericak-Vance MA, Joo SH, Rosi BL, Gusella JF, Crapper-MacLachlan DR, Alberts MJ and others. Association of apolipoprotein E allele epsilon 4 with late-onset familial and sporadic Alzheimer’s disease. Neurology 1993;43(8):1467–72. [DOI] [PubMed] [Google Scholar]
- 8.Barnes DE, Yaffe K. The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol 2011;10(9):819–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cannon JR, Greenamyre JT. The role of environmental exposures in neurodegeneration and neurodegenerative diseases. Toxicol Sci 2011;124(2):225–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gerhardsson L, Lundh T, Minthon L, Londos E. Metal concentrations in plasma and cerebrospinal fluid in patients with Alzheimer’s disease. Dement Geriatr Cogn Disord 2008;25(6):508–15. [DOI] [PubMed] [Google Scholar]
- 11.Mirza A, King A, Troakes C, Exley C. Aluminium in brain tissue in familial Alzheimer’s disease. J Trace Elem Med Biol 2017;40:30–36. [DOI] [PubMed] [Google Scholar]
- 12.Perl DP, Brody AR. Alzheimer’s disease: X-ray spectrometric evidence of aluminum accumulation in neurofibrillary tangle-bearing neurons. Science 1980;208(4441):297–9. [DOI] [PubMed] [Google Scholar]
- 13.Exley C, Vickers T. Elevated brain aluminium and early onset Alzheimer’s disease in an individual occupationally exposed to aluminium: a case report. J Med Case Rep 2014;8:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Richardson JR, Roy A, Shalat SL, von Stein RT, Hossain MM, Buckley B, Gearing M, Levey AI, German DC. Elevated serum pesticide levels and risk for Alzheimer disease. JAMA Neurol 2014;71(3):284–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Angeloni C, Zambonin L, Hrelia S. Role of methylglyoxal in Alzheimer’s disease. Biomed Res Int 2014;2014:238485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lovell MA, Xie C, Markesbery WR. Acrolein is increased in Alzheimer’s disease brain and is toxic to primary hippocampal cultures. Neurobiol Aging 2001;22(2):187–94. [DOI] [PubMed] [Google Scholar]
- 17.Luth HJ, Ogunlade V, Kuhla B, Kientsch-Engel R, Stahl P, Webster J, Arendt T, Munch G. Age- and stage-dependent accumulation of advanced glycation end products in intracellular deposits in normal and Alzheimer’s disease brains. Cereb Cortex 2005;15(2):211–20. [DOI] [PubMed] [Google Scholar]
- 18.Panahi N, Mahmoudian M, Mortazavi P, Hashjin GS. Effects of berberine on beta-secretase activity in a rabbit model of Alzheimer’s disease. Arch Med Sci 2013;9(1):146–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang L, Hu J, Zhao Y, Lu X, Zhang Q, Niu Q. Effects of aluminium on beta-amyloid (1–42) and secretases (APP-cleaving enzymes) in rat brain. Neurochem Res 2014;39(7):1338–45. [DOI] [PubMed] [Google Scholar]
- 20.Bazzari FH, Abdallah DM, El-Abhar HS. Chenodeoxycholic Acid Ameliorates AlCl3-Induced Alzheimer’s Disease Neurotoxicity and Cognitive Deterioration via Enhanced Insulin Signaling in Rats. Molecules 2019;24(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wahby MM, Mohammed DS, Newairy AA, Abdou HM, Zaky A. Aluminum-induced molecular neurodegeneration: The protective role of genistein and chickpea extract. Food Chem Toxicol 2017;107(Pt A):57–67. [DOI] [PubMed] [Google Scholar]
- 22.Castorina A, Tiralongo A, Giunta S, Carnazza ML, Scapagnini G, D’Agata V. Early effects of aluminum chloride on beta-secretase mRNA expression in a neuronal model of beta-amyloid toxicity. Cell Biol Toxicol 2010;26(4):367–77. [DOI] [PubMed] [Google Scholar]
- 23.Wu J, Basha MR, Brock B, Cox DP, Cardozo-Pelaez F, McPherson CA, Harry J, Rice DC, Maloney B, Chen D and others. Alzheimer’s disease (AD)-like pathology in aged monkeys after infantile exposure to environmental metal lead (Pb): evidence for a developmental origin and environmental link for AD. J Neurosci 2008;28(1):3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bihaqi SW, Bahmani A, Subaiea GM, Zawia NH. Infantile exposure to lead and late-age cognitive decline: relevance to AD. Alzheimers Dement 2014;10(2):187–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ashok A, Rai NK, Tripathi S, Bandyopadhyay S. Exposure to As-, Cd-, and Pb-mixture induces Abeta, amyloidogenic APP processing and cognitive impairments via oxidative stress-dependent neuroinflammation in young rats. Toxicol Sci 2015;143(1):64–80. [DOI] [PubMed] [Google Scholar]
- 26.Kitazawa M, Cheng D, Laferla FM. Chronic copper exposure exacerbates both amyloid and tau pathology and selectively dysregulates cdk5 in a mouse model of AD. J Neurochem 2009;108(6):1550–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin R, Chen X, Li W, Han Y, Liu P, Pi R. Exposure to metal ions regulates mRNA levels of APP and BACE1 in PC12 cells: blockage by curcumin. Neurosci Lett 2008;440(3):344–7. [DOI] [PubMed] [Google Scholar]
- 28.Gong L, Tian X, Zhou J, Dong Q, Tan Y, Lu Y, Wu J, Zhao Y, Liu X. Iron Dyshomeostasis Induces Binding of APP to BACE1 for Amyloid Pathology, and Impairs APP/Fpn1 Complex in Microglia: Implication in Pathogenesis of Cerebral Microbleeds. Cell Transplant 2019;28(8):1009–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quiroz-Baez R, Rojas E, Arias C. Oxidative stress promotes JNK-dependent amyloidogenic processing of normally expressed human APP by differential modification of alpha-, beta- and gamma-secretase expression. Neurochem Int 2009;55(7):662–70. [DOI] [PubMed] [Google Scholar]
- 30.Guilarte TR, Burton NC, Verina T, Prabhu VV, Becker KG, Syversen T, Schneider JS. Increased APLP1 expression and neurodegeneration in the frontal cortex of manganese-exposed non-human primates. J Neurochem 2008;105(5):1948–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang CL, Hsiao IL, Lin HC, Wang CF, Huang YJ, Chuang CY. Silver nanoparticles affect on gene expression of inflammatory and neurodegenerative responses in mouse brain neural cells. Environ Res 2015;136:253–63. [DOI] [PubMed] [Google Scholar]
- 32.Lin HC, Ho MY, Tsen CM, Huang CC, Wu CC, Huang YJ, Hsiao IL, Chuang CY. From the Cover: Comparative Proteomics Reveals Silver Nanoparticles Alter Fatty Acid Metabolism and Amyloid Beta Clearance for Neuronal Apoptosis in a Triple Cell Coculture Model of the Blood-Brain Barrier. Toxicol Sci 2017;158(1):151–163. [DOI] [PubMed] [Google Scholar]
- 33.Li G, Kim C, Kim J, Yoon H, Zhou H, Kim J. Common Pesticide, Dichlorodiphenyltrichloroethane (DDT), Increases Amyloid-beta Levels by Impairing the Function of ABCA1 and IDE: Implication for Alzheimer’s Disease. J Alzheimers Dis 2015;46(1):109–22. [DOI] [PubMed] [Google Scholar]
- 34.Xiong K, Cai H, Luo XG, Struble RG, Clough RW, Yan XX. Mitochondrial respiratory inhibition and oxidative stress elevate beta-secretase (BACE1) proteins and activity in vivo in the rat retina. Exp Brain Res 2007;181(3):435–46. [DOI] [PubMed] [Google Scholar]
- 35.Lafon PA, Wang Y, Arango-Lievano M, Torrent J, Salvador-Prince L, Mansuy M, Mestre-Frances N, Givalois L, Liu J, Mercader JV and others. Fungicide Residues Exposure and beta-amyloid Aggregation in a Mouse Model of Alzheimer’s Disease. Environ Health Perspect 2020;128(1):17011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tan JL, Li QX, Ciccotosto GD, Crouch PJ, Culvenor JG, White AR, Evin G. Mild oxidative stress induces redistribution of BACE1 in non-apoptotic conditions and promotes the amyloidogenic processing of Alzheimer’s disease amyloid precursor protein. PLoS One 2013;8(4):e61246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Q, Zhao H, Liu W, Zhang Z, Qin H, Luo F, Leng S. Developmental perfluorooctane sulfonate exposure results in tau hyperphosphorylation and beta-amyloid aggregation in adults rats: Incidence for link to Alzheimer’s disease. Toxicology 2016;347–349: 40–6. [DOI] [PubMed] [Google Scholar]
- 38.Ku T, Li B, Gao R, Zhang Y, Yan W, Ji X, Li G, Sang N. NF-kappaB-regulated microRNA-574–5p underlies synaptic and cognitive impairment in response to atmospheric PM2.5 aspiration. Part Fibre Toxicol 2017;14(1):34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bhatt DP, Puig KL, Gorr MW, Wold LE, Combs CK. A pilot study to assess effects of long-term inhalation of airborne particulate matter on early Alzheimer-like changes in the mouse brain. PLoS One 2015;10(5):e0127102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cho HJ, Son SM, Jin SM, Hong HS, Shin DH, Kim SJ, Huh K, Mook-Jung I. RAGE regulates BACE1 and Abeta generation via NFAT1 activation in Alzheimer’s disease animal model. FASEB J 2009;23(8):2639–49. [DOI] [PubMed] [Google Scholar]
- 41.Guglielmotto M, Aragno M, Tamagno E, Vercellinatto I, Visentin S, Medana C, Catalano MG, Smith MA, Perry G, Danni O and others. AGEs/RAGE complex upregulates BACE1 via NF-kappaB pathway activation. Neurobiol Aging 2012;33(1):196 e13–27. [DOI] [PubMed] [Google Scholar]
- 42.Ko SY, Ko HA, Chu KH, Shieh TM, Chi TC, Chen HI, Chang WC, Chang SS. The Possible Mechanism of Advanced Glycation End Products (AGEs) for Alzheimer’s Disease. PLoS One 2015;10(11):e0143345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Syeda T, Foguth RM, Llewellyn E, Cannon JR. PhIP exposure in rodents produces neuropathology potentially relevant to Alzheimer’s disease. Toxicology 2020;437:152436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang YJ, Jin MH, Pi RB, Zhang JJ, Ouyang Y, Chao XJ, Chen MH, Liu PQ, Yu JC, Ramassamy C and others. Acrolein induces Alzheimer’s disease-like pathologies in vitro and in vivo. Toxicol Lett 2013;217(3):184–91. [DOI] [PubMed] [Google Scholar]
- 45.Huang YJ, Zhang L, Shi LY, Wang YY, Yang YB, Ke B, Zhang TY, Qin J. Caloric restriction ameliorates acrolein-induced neurotoxicity in rats. Neurotoxicology 2018;65:44–51. [DOI] [PubMed] [Google Scholar]
- 46.Zawia NH, Basha MR. Environmental risk factors and the developmental basis for Alzheimer’s disease. Rev Neurosci 2005;16(4):325–37. [DOI] [PubMed] [Google Scholar]
- 47.Adwan L, Subaiea GM, Zawia NH. Tolfenamic acid downregulates BACE1 and protects against lead-induced upregulation of Alzheimer’s disease related biomarkers. Neuropharmacology 2014;79:596–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dingwall C A copper-binding site in the cytoplasmic domain of BACE1 identifies a possible link to metal homoeostasis and oxidative stress in Alzheimer’s disease. Biochem Soc Trans 2007;35(Pt 3):571–3. [DOI] [PubMed] [Google Scholar]
- 49.Angeletti B, Waldron KJ, Freeman KB, Bawagan H, Hussain I, Miller CC, Lau KF, Tennant ME, Dennison C, Robinson NJ and others. BACE1 cytoplasmic domain interacts with the copper chaperone for superoxide dismutase-1 and binds copper. J Biol Chem 2005;280(18):17930–7. [DOI] [PubMed] [Google Scholar]
- 50.Vassar R, Bennett BD, Babu-Khan S, Kahn S, Mendiaz EA, Denis P, Teplow DB, Ross S, Amarante P, Loeloff R and others. Beta-secretase cleavage of Alzheimer’s amyloid precursor protein by the transmembrane aspartic protease BACE. Science 1999;286(5440):735–41. [DOI] [PubMed] [Google Scholar]
- 51.Cai H, Wang Y, McCarthy D, Wen H, Borchelt DR, Price DL, Wong PC. BACE1 is the major beta-secretase for generation of Abeta peptides by neurons. Nat Neurosci 2001;4(3):233–4. [DOI] [PubMed] [Google Scholar]
- 52.Li R, Lindholm K, Yang LB, Yue X, Citron M, Yan R, Beach T, Sue L, Sabbagh M, Cai H and others. Amyloid beta peptide load is correlated with increased beta-secretase activity in sporadic Alzheimer’s disease patients. Proc Natl Acad Sci U S A 2004;101(10):3632–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Borghi R, Patriarca S, Traverso N, Piccini A, Storace D, Garuti A, Gabriella C, Patrizio O, Massimo T. The increased activity of BACE1 correlates with oxidative stress in Alzheimer’s disease. Neurobiol Aging 2007;28(7):1009–14. [DOI] [PubMed] [Google Scholar]
- 54.Evin G, Hince C. BACE1 as a therapeutic target in Alzheimer’s disease: rationale and current status. Drugs Aging 2013;30(10):755–64. [DOI] [PubMed] [Google Scholar]
- 55.Das B, Yan R. A Close Look at BACE1 Inhibitors for Alzheimer’s Disease Treatment. CNS Drugs 2019;33(3):251–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hampel H, Vassar R, De Strooper B, Hardy J, Willem M, Singh N, Zhou J, Yan R, Vanmechelen E, De Vos A and others. The beta-Secretase BACE1 in Alzheimer’s Disease. Biol Psychiatry 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hussain I, Powell D, Howlett DR, Tew DG, Meek TD, Chapman C, Gloger IS, Murphy KE, Southan CD, Ryan DM and others. Identification of a novel aspartic protease (Asp 2) as beta-secretase. Mol Cell Neurosci 1999;14(6):419–27. [DOI] [PubMed] [Google Scholar]
- 58.Lin X, Koelsch G, Wu S, Downs D, Dashti A, Tang J. Human aspartic protease memapsin 2 cleaves the beta-secretase site of beta-amyloid precursor protein. Proc Natl Acad Sci U S A 2000;97(4):1456–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sinha S, Anderson JP, Barbour R, Basi GS, Caccavello R, Davis D, Doan M, Dovey HF, Frigon N, Hong J and others. Purification and cloning of amyloid precursor protein beta-secretase from human brain. Nature 1999;402(6761):537–40. [DOI] [PubMed] [Google Scholar]
- 60.Yan R, Bienkowski MJ, Shuck ME, Miao H, Tory MC, Pauley AM, Brashier JR, Stratman NC, Mathews WR, Buhl AE and others. Membrane-anchored aspartyl protease with Alzheimer’s disease beta-secretase activity. Nature 1999;402(6761):533–7. [DOI] [PubMed] [Google Scholar]
- 61.Hong L, Koelsch G, Lin X, Wu S, Terzyan S, Ghosh AK, Zhang XC, Tang J. Structure of the protease domain of memapsin 2 (beta-secretase) complexed with inhibitor. Science 2000;290(5489):150–3. [DOI] [PubMed] [Google Scholar]
- 62.Haniu M, Denis P, Young Y, Mendiaz EA, Fuller J, Hui JO, Bennett BD, Kahn S, Ross S, Burgess T and others. Characterization of Alzheimer’s beta - secretase protein BACE. A pepsin family member with unusual properties. J Biol Chem 2000;275(28):21099–106. [DOI] [PubMed] [Google Scholar]
- 63.Wang H, Li R, Shen Y. beta-Secretase: its biology as a therapeutic target in diseases. Trends Pharmacol Sci 2013;34(4):215–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hayley M, Perspicace S, Schulthess T, Seelig J. Calcium enhances the proteolytic activity of BACE1: An in vitro biophysical and biochemical characterization of the BACE1-calcium interaction. Biochim Biophys Acta 2009;1788(9):1933–8. [DOI] [PubMed] [Google Scholar]
- 65.Yan R, Munzner JB, Shuck ME, Bienkowski MJ. BACE2 functions as an alternative alpha-secretase in cells. J Biol Chem 2001;276(36):34019–27. [DOI] [PubMed] [Google Scholar]
- 66.Kandalepas PC, Vassar R. Identification and biology of beta-secretase. J Neurochem 2012;120 Suppl 1:55–61. [DOI] [PubMed] [Google Scholar]
- 67.Bennett BD, Babu-Khan S, Loeloff R, Louis JC, Curran E, Citron M, Vassar R. Expression analysis of BACE2 in brain and peripheral tissues. J Biol Chem 2000;275(27):20647–51. [DOI] [PubMed] [Google Scholar]
- 68.Farzan M, Schnitzler CE, Vasilieva N, Leung D, Choe H. BACE2, a beta - secretase homolog, cleaves at the beta site and within the amyloid-beta region of the amyloid-beta precursor protein. Proc Natl Acad Sci U S A 2000;97(17):9712–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chami L, Buggia-Prevot V, Duplan E, Del Prete D, Chami M, Peyron JF, Checler F. Nuclear factor-kappaB regulates betaAPP and beta- and gamma-secretases differently at physiological and supraphysiological Abeta concentrations. J Biol Chem 2012;287(29):24573–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bourne KZ, Ferrari DC, Lange-Dohna C, Rossner S, Wood TG, Perez-Polo JR. Differential regulation of BACE1 promoter activity by nuclear factor-kappaB in neurons and glia upon exposure to beta-amyloid peptides. J Neurosci Res 2007;85(6):1194–204. [DOI] [PubMed] [Google Scholar]
- 71.Christensen MA, Zhou W, Qing H, Lehman A, Philipsen S, Song W. Transcriptional regulation of BACE1, the beta-amyloid precursor protein beta-secretase, by Sp1. Mol Cell Biol 2004;24(2):865–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sastre M, Dewachter I, Landreth GE, Willson TM, Klockgether T, van Leuven F, Heneka MT. Nonsteroidal anti-inflammatory drugs and peroxisome proliferator-activated receptor-gamma agonists modulate immunostimulated processing of amyloid precursor protein through regulation of beta-secretase. J Neurosci 2003;23(30):9796–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang X, Zhou K, Wang R, Cui J, Lipton SA, Liao FF, Xu H, Zhang YW. Hypoxia-inducible factor 1alpha (HIF-1alpha)-mediated hypoxia increases BACE1 expression and beta-amyloid generation. J Biol Chem 2007;282(15):10873–80. [DOI] [PubMed] [Google Scholar]
- 74.Guglielmotto M, Aragno M, Autelli R, Giliberto L, Novo E, Colombatto S, Danni O, Parola M, Smith MA, Perry G and others. The up-regulation of BACE1 mediated by hypoxia and ischemic injury: role of oxidative stress and HIF1alpha. J Neurochem 2009;108(4):1045–56. [DOI] [PubMed] [Google Scholar]
- 75.Nowak K, Lange-Dohna C, Zeitschel U, Gunther A, Luscher B, Robitzki A, Perez-Polo R, Rossner S. The transcription factor Yin Yang 1 is an activator of BACE1 expression. J Neurochem 2006;96(6):1696–707. [DOI] [PubMed] [Google Scholar]
- 76.Cho HJ, Jin SM, Youn HD, Huh K, Mook-Jung I. Disrupted intracellular calcium regulates BACE1 gene expression via nuclear factor of activated T cells 1 (NFAT 1) signaling. Aging Cell 2008;7(2):137–47. [DOI] [PubMed] [Google Scholar]
- 77.Chen XF, Zhang YW, Xu H, Bu G. Transcriptional regulation and its misregulation in Alzheimer’s disease. Mol Brain 2013;6:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tan JZA, Fourriere L, Wang J, Perez F, Boncompain G, Gleeson PA. Distinct anterograde trafficking pathways of BACE1 and amyloid precursor protein from the TGN and the regulation of amyloid-beta production. Mol Biol Cell 2020;31(1):27–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tan J, Evin G. Beta-site APP-cleaving enzyme 1 trafficking and Alzheimer’s disease pathogenesis. J Neurochem 2012;120(6):869–80. [DOI] [PubMed] [Google Scholar]
- 80.Chia PZ, Toh WH, Sharples R, Gasnereau I, Hill AF, Gleeson PA. Intracellular itinerary of internalised beta-secretase, BACE1, and its potential impact on beta-amyloid peptide biogenesis. Traffic 2013;14(9):997–1013. [DOI] [PubMed] [Google Scholar]
- 81.Huse JT, Pijak DS, Leslie GJ, Lee VM, Doms RW. Maturation and endosomal targeting of beta-site amyloid precursor protein-cleaving enzyme. The Alzheimer’s disease beta-secretase. J Biol Chem 2000;275(43):33729–37. [DOI] [PubMed] [Google Scholar]
- 82.Creemers JW, Ines Dominguez D, Plets E, Serneels L, Taylor NA, Multhaup G, Craessaerts K, Annaert W, De Strooper B. Processing of beta-secretase by furin and other members of the proprotein convertase family. J Biol Chem 2001;276(6):4211–7. [DOI] [PubMed] [Google Scholar]
- 83.Capell A, Steiner H, Willem M, Kaiser H, Meyer C, Walter J, Lammich S, Multhaup G, Haass C. Maturation and pro-peptide cleavage of beta-secretase. J Biol Chem 2000;275(40):30849–54. [DOI] [PubMed] [Google Scholar]
- 84.Bennett BD, Denis P, Haniu M, Teplow DB, Kahn S, Louis JC, Citron M, Vassar R. A furin-like convertase mediates propeptide cleavage of BACE, the Alzheimer’s beta -secretase. J Biol Chem 2000;275(48):37712–7. [DOI] [PubMed] [Google Scholar]
- 85.Benjannet S, Elagoz A, Wickham L, Mamarbachi M, Munzer JS, Basak A, Lazure C, Cromlish JA, Sisodia S, Checler F and others. Post-translational processing of beta-secretase (beta-amyloid-converting enzyme) and its ectodomain shedding. The pro- and transmembrane/cytosolic domains affect its cellular activity and amyloid-beta production. J Biol Chem 2001;276(14):10879–87. [DOI] [PubMed] [Google Scholar]
- 86.Citron M, Teplow DB, Selkoe DJ. Generation of amyloid beta protein from its precursor is sequence specific. Neuron 1995;14(3):661–70. [DOI] [PubMed] [Google Scholar]
- 87.Knops J, Suomensaari S, Lee M, McConlogue L, Seubert P, Sinha S. Cell-type and amyloid precursor protein-type specific inhibition of A beta release by bafilomycin A1, a selective inhibitor of vacuolar ATPases. J Biol Chem 1995;270(6):2419–22. [DOI] [PubMed] [Google Scholar]
- 88.Haass C, Hung AY, Schlossmacher MG, Teplow DB, Selkoe DJ. beta-Amyloid peptide and a 3-kDa fragment are derived by distinct cellular mechanisms. J Biol Chem 1993;268(5):3021–4. [PubMed] [Google Scholar]
- 89.Haass C, Capell A, Citron M, Teplow DB, Selkoe DJ. The vacuolar H(+)-ATPase inhibitor bafilomycin A1 differentially affects proteolytic processing of mutant and wild-type beta-amyloid precursor protein. J Biol Chem 1995;270(11):6186–92. [DOI] [PubMed] [Google Scholar]
- 90.Shimizu H, Tosaki A, Kaneko K, Hisano T, Sakurai T, Nukina N. Crystal structure of an active form of BACE1, an enzyme responsible for amyloid beta protein production. Mol Cell Biol 2008;28(11):3663–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Koo EH, Squazzo SL. Evidence that production and release of amyloid beta-protein involves the endocytic pathway. J Biol Chem 1994;269(26):17386–9. [PubMed] [Google Scholar]
- 92.Haass C, Lemere CA, Capell A, Citron M, Seubert P, Schenk D, Lannfelt L, Selkoe DJ. The Swedish mutation causes early-onset Alzheimer’s disease by beta-secretase cleavage within the secretory pathway. Nat Med 1995;1(12):1291–6. [DOI] [PubMed] [Google Scholar]
- 93.Qing H, Zhou W, Christensen MA, Sun X, Tong Y, Song W. Degradation of BACE by the ubiquitin-proteasome pathway. FASEB J 2004;18(13):1571–3. [DOI] [PubMed] [Google Scholar]
- 94.Zhang M, Deng Y, Luo Y, Zhang S, Zou H, Cai F, Wada K, Song W. Control of BACE1 degradation and APP processing by ubiquitin carboxyl-terminal hydrolase L1. J Neurochem 2012;120(6):1129–38. [DOI] [PubMed] [Google Scholar]
- 95.Ye X, Cai Q. Snapin-mediated BACE1 retrograde transport is essential for its degradation in lysosomes and regulation of APP processing in neurons. Cell Rep 2014;6(1):24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang R, Ying Z, Zhao J, Zhang Y, Wang R, Lu H, Deng Y, Song W, Qing H. Lys(203) and Lys(382) are essential for the proteasomal degradation of BACE1. Curr Alzheimer Res 2012;9(5):606–15. [DOI] [PubMed] [Google Scholar]
- 97.Schnoder L, Hao W, Qin Y, Liu S, Tomic I, Liu X, Fassbender K, Liu Y. Deficiency of Neuronal p38alpha MAPK Attenuates Amyloid Pathology in Alzheimer Disease Mouse and Cell Models through Facilitating Lysosomal Degradation of BACE1. J Biol Chem 2016;291(5):2067–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Feng T, Tammineni P, Agrawal C, Jeong YY, Cai Q. Autophagy-mediated Regulation of BACE1 Protein Trafficking and Degradation. J Biol Chem 2017;292(5):1679–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bera S, Camblor-Perujo S, Calleja Barca E, Negrete-Hurtado A, Racho J, De Bruyckere E, Wittich C, Ellrich N, Martins S, Adjaye J and others. AP-2 reduces amyloidogenesis by promoting BACE1 trafficking and degradation in neurons. EMBO Rep 2020;21(6):e47954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bao J, Qin M, Mahaman YAR, Zhang B, Huang F, Zeng K, Xia Y, Ke D, Wang Q, Liu R and others. BACE1 SUMOylation increases its stability and escalates the protease activity in Alzheimer’s disease. Proc Natl Acad Sci U S A 2018;115(15):3954–3959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Willem M, Garratt AN, Novak B, Citron M, Kaufmann S, Rittger A, DeStrooper B, Saftig P, Birchmeier C, Haass C. Control of peripheral nerve myelination by the beta-secretase BACE1. Science 2006;314(5799):664–6. [DOI] [PubMed] [Google Scholar]
- 102.Luo X, Prior M, He W, Hu X, Tang X, Shen W, Yadav S, Kiryu-Seo S, Miller R, Trapp BD and others. Cleavage of neuregulin-1 by BACE1 or ADAM10 protein produces differential effects on myelination. J Biol Chem 2011;286(27):23967–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hu X, He W, Diaconu C, Tang X, Kidd GJ, Macklin WB, Trapp BD, Yan R. Genetic deletion of BACE1 in mice affects remyelination of sciatic nerves. FASEB J 2008;22(8):2970–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fleck D, van Bebber F, Colombo A, Galante C, Schwenk BM, Rabe L, Hampel H, Novak B, Kremmer E, Tahirovic S and others. Dual cleavage of neuregulin 1 type III by BACE1 and ADAM17 liberates its EGF-like domain and allows paracrine signaling. J Neurosci 2013;33(18):7856–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhu K, Xiang X, Filser S, Marinkovic P, Dorostkar MM, Crux S, Neumann U, Shimshek DR, Rammes G, Haass C and others. Beta-Site Amyloid Precursor Protein Cleaving Enzyme 1 Inhibition Impairs Synaptic Plasticity via Seizure Protein 6. Biol Psychiatry 2018;83(5):428–437. [DOI] [PubMed] [Google Scholar]
- 106.Causevic M, Dominko K, Malnar M, Vidatic L, Cermak S, Pigoni M, Kuhn PH, Colombo A, Havas D, Flunkert S and others. BACE1-cleavage of Sez6 and Sez6L is elevated in Niemann-Pick type C disease mouse brains. PLoS One 2018;13(7):e0200344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wong HK, Sakurai T, Oyama F, Kaneko K, Wada K, Miyazaki H, Kurosawa M, De Strooper B, Saftig P, Nukina N. beta Subunits of voltage-gated sodium channels are novel substrates of beta-site amyloid precursor protein-cleaving enzyme (BACE1) and gamma-secretase. J Biol Chem 2005;280(24):23009–17. [DOI] [PubMed] [Google Scholar]
- 108.Kim DY, Gersbacher MT, Inquimbert P, Kovacs DM. Reduced sodium channel Na(v)1.1 levels in BACE1-null mice. J Biol Chem 2011;286(10):8106–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kim DY, Carey BW, Wang H, Ingano LA, Binshtok AM, Wertz MH, Pettingell WH, He P, Lee VM, Woolf CJ and others. BACE1 regulates voltage-gated sodium channels and neuronal activity. Nat Cell Biol 2007;9(7):755–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hu X, He W, Luo X, Tsubota KE, Yan R. BACE1 regulates hippocampal astrogenesis via the Jagged1-Notch pathway. Cell Rep 2013;4(1):40–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.He W, Hu J, Xia Y, Yan R. beta-site amyloid precursor protein cleaving enzyme 1(BACE1) regulates Notch signaling by controlling the cleavage of Jagged 1 (Jag1) and Jagged 2 (Jag2) proteins. J Biol Chem 2014;289(30):20630–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tesco G, Koh YH, Kang EL, Cameron AN, Das S, Sena-Esteves M, Hiltunen M, Yang SH, Zhong Z, Shen Y and others. Depletion of GGA3 stabilizes BACE and enhances beta-secretase activity. Neuron 2007;54(5):721–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kametaka S, Shibata M, Moroe K, Kanamori S, Ohsawa Y, Waguri S, Sims PJ, Emoto K, Umeda M, Uchiyama Y. Identification of phospholipid scramblase 1 as a novel interacting molecule with beta -secretase (beta -site amyloid precursor protein (APP) cleaving enzyme (BACE)). J Biol Chem 2003;278(17):15239–45. [DOI] [PubMed] [Google Scholar]
- 114.Spoelgen R, von Arnim CA, Thomas AV, Peltan ID, Koker M, Deng A, Irizarry MC, Andersen OM, Willnow TE, Hyman BT. Interaction of the cytosolic domains of sorLA/LR11 with the amyloid precursor protein (APP) and beta-secretase beta-site APP-cleaving enzyme. J Neurosci 2006;26(2):418–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Marks N, Berg MJ. BACE and gamma-secretase characterization and their sorting as therapeutic targets to reduce amyloidogenesis. Neurochem Res 2010;35(2):181–210. [DOI] [PubMed] [Google Scholar]
- 116.Finan GM, Okada H, Kim TW. BACE1 retrograde trafficking is uniquely regulated by the cytoplasmic domain of sortilin. J Biol Chem 2011;286(14):12602–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.He W, Lu Y, Qahwash I, Hu XY, Chang A, Yan R. Reticulon family members modulate BACE1 activity and amyloid-beta peptide generation. Nat Med 2004;10(9):959–65. [DOI] [PubMed] [Google Scholar]
- 118.Murayama KS, Kametani F, Saito S, Kume H, Akiyama H, Araki W. Reticulons RTN3 and RTN4-B/C interact with BACE1 and inhibit its ability to produce amyloid beta-protein. Eur J Neurosci 2006;24(5):1237–44. [DOI] [PubMed] [Google Scholar]
- 119.Kuzuya A, Uemura K, Kitagawa N, Aoyagi N, Kihara T, Ninomiya H, Ishiura S, Takahashi R, Shimohama S. Presenilin 1 is involved in the maturation of beta-site amyloid precursor protein-cleaving enzyme 1 (BACE1). J Neurosci Res 2007;85(1):153–65. [DOI] [PubMed] [Google Scholar]
- 120.Yan R Physiological Functions of the beta-Site Amyloid Precursor Protein Cleaving Enzyme 1 and 2. Front Mol Neurosci 2017;10:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Vassar R The beta-secretase, BACE: a prime drug target for Alzheimer’s disease. J Mol Neurosci 2001;17(2):157–70. [DOI] [PubMed] [Google Scholar]
- 122.Cole SL, Vassar R. The Alzheimer’s disease beta-secretase enzyme, BACE1. Mol Neurodegener 2007;2:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Laird FM, Cai H, Savonenko AV, Farah MH, He K, Melnikova T, Wen H, Chiang HC, Xu G, Koliatsos VE and others. BACE1, a major determinant of selective vulnerability of the brain to amyloid-beta amyloidogenesis, is essential for cognitive, emotional, and synaptic functions. J Neurosci 2005;25(50):11693–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Irizarry MC, Locascio JJ, Hyman BT. beta-site APP cleaving enzyme mRNA expression in APP transgenic mice: anatomical overlap with transgene expression and static levels with aging. Am J Pathol 2001;158(1):173–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhao J, Fu Y, Yasvoina M, Shao P, Hitt B, O’Connor T, Logan S, Maus E, Citron M, Berry R and others. Beta-site amyloid precursor protein cleaving enzyme 1 levels become elevated in neurons around amyloid plaques: implications for Alzheimer’s disease pathogenesis. J Neurosci 2007;27(14):3639–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hitt B, Riordan SM, Kukreja L, Eimer WA, Rajapaksha TW, Vassar R. beta-Site amyloid precursor protein (APP)-cleaving enzyme 1 (BACE1)-deficient mice exhibit a close homolog of L1 (CHL1) loss-of-function phenotype involving axon guidance defects. J Biol Chem 2012;287(46):38408–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kandalepas PC, Sadleir KR, Eimer WA, Zhao J, Nicholson DA, Vassar R. The Alzheimer’s beta-secretase BACE1 localizes to normal presynaptic terminals and to dystrophic presynaptic terminals surrounding amyloid plaques. Acta Neuropathol 2013;126(3):329–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhao J, Paganini L, Mucke L, Gordon M, Refolo L, Carman M, Sinha S, Oltersdorf T, Lieberburg I, McConlogue L. Beta-secretase processing of the beta-amyloid precursor protein in transgenic mice is efficient in neurons but inefficient in astrocytes. J Biol Chem 1996;271(49):31407–11. [DOI] [PubMed] [Google Scholar]
- 129.Rossner S, Lange-Dohna C, Zeitschel U, Perez-Polo JR. Alzheimer’s disease beta-secretase BACE1 is not a neuron-specific enzyme. J Neurochem 2005;92(2):226–34. [DOI] [PubMed] [Google Scholar]
- 130.Rossner S, Apelt J, Schliebs R, Perez-Polo JR, Bigl V. Neuronal and glial beta-secretase (BACE) protein expression in transgenic Tg2576 mice with amyloid plaque pathology. J Neurosci Res 2001;64(5):437–46. [DOI] [PubMed] [Google Scholar]
- 131.Hong HS, Hwang EM, Sim HJ, Cho HJ, Boo JH, Oh SS, Kim SU, Mook-Jung I. Interferon gamma stimulates beta-secretase expression and sAPPbeta production in astrocytes. Biochem Biophys Res Commun 2003;307(4):922–7. [DOI] [PubMed] [Google Scholar]
- 132.Hartlage-Rubsamen M, Zeitschel U, Apelt J, Gartner U, Franke H, Stahl T, Gunther A, Schliebs R, Penkowa M, Bigl V and others. Astrocytic expression of the Alzheimer’s disease beta-secretase (BACE1) is stimulus-dependent. Glia 2003;41(2):169–79. [DOI] [PubMed] [Google Scholar]
- 133.Sisodia SS. Beta-amyloid precursor protein cleavage by a membrane-bound protease. Proc Natl Acad Sci U S A 1992;89(13):6075–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lammich S, Kojro E, Postina R, Gilbert S, Pfeiffer R, Jasionowski M, Haass C, Fahrenholz F. Constitutive and regulated alpha-secretase cleavage of Alzheimer’s amyloid precursor protein by a disintegrin metalloprotease. Proc Natl Acad Sci U S A 1999;96(7):3922–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kojro E, Fahrenholz F. The non-amyloidogenic pathway: structure and function of alpha-secretases. Subcell Biochem 2005;38:105–27. [DOI] [PubMed] [Google Scholar]
- 136.Hiraoka Y, Ohno M, Yoshida K, Okawa K, Tomimoto H, Kita T, Nishi E. Enhancement of alpha-secretase cleavage of amyloid precursor protein by a metalloendopeptidase nardilysin. J Neurochem 2007;102(5):1595–1605. [DOI] [PubMed] [Google Scholar]
- 137.Zheng H, Koo EH. Biology and pathophysiology of the amyloid precursor protein. Mol Neurodegener 2011;6(1):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zhang YW, Thompson R, Zhang H, Xu H. APP processing in Alzheimer’s disease. Mol Brain 2011;4:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wilkins HM, Swerdlow RH. Amyloid precursor protein processing and bioenergetics. Brain Res Bull 2017;133:71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.O’Brien RJ, Wong PC. Amyloid precursor protein processing and Alzheimer’s disease. Annu Rev Neurosci 2011;34:185–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Nathalie P, Jean-Noel O. Processing of amyloid precursor protein and amyloid peptide neurotoxicity. Curr Alzheimer Res 2008;5(2):92–9. [DOI] [PubMed] [Google Scholar]
- 142.Yang LB, Lindholm K, Yan R, Citron M, Xia W, Yang XL, Beach T, Sue L, Wong P, Price D and others. Elevated beta-secretase expression and enzymatic activity detected in sporadic Alzheimer disease. Nat Med 2003;9(1):3–4. [DOI] [PubMed] [Google Scholar]
- 143.Holsinger RM, McLean CA, Beyreuther K, Masters CL, Evin G. Increased expression of the amyloid precursor beta-secretase in Alzheimer’s disease. Ann Neurol 2002;51(6):783–6. [DOI] [PubMed] [Google Scholar]
- 144.Fukumoto H, Cheung BS, Hyman BT, Irizarry MC. Beta-secretase protein and activity are increased in the neocortex in Alzheimer disease. Arch Neurol 2002;59(9):1381–9. [DOI] [PubMed] [Google Scholar]
- 145.Zetterberg H, Andreasson U, Hansson O, Wu G, Sankaranarayanan S, Andersson ME, Buchhave P, Londos E, Umek RM, Minthon L and others. Elevated cerebrospinal fluid BACE1 activity in incipient Alzheimer disease. Arch Neurol 2008;65(8):1102–7. [DOI] [PubMed] [Google Scholar]
- 146.Preece P, Virley DJ, Costandi M, Coombes R, Moss SJ, Mudge AW, Jazin E, Cairns NJ. Beta-secretase (BACE) and GSK-3 mRNA levels in Alzheimer’s disease. Brain Res Mol Brain Res 2003;116(1–2):155–8. [DOI] [PubMed] [Google Scholar]
- 147.Li Q, Sudhof TC. Cleavage of amyloid-beta precursor protein and amyloid-beta precursor-like protein by BACE 1. J Biol Chem 2004;279(11):10542–50. [DOI] [PubMed] [Google Scholar]
- 148.Sastre M, Dewachter I, Rossner S, Bogdanovic N, Rosen E, Borghgraef P, Evert BO, Dumitrescu-Ozimek L, Thal DR, Landreth G and others. Nonsteroidal anti-inflammatory drugs repress beta-secretase gene promoter activity by the activation of PPARgamma. Proc Natl Acad Sci U S A 2006;103(2):443–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Puglielli L, Ellis BC, Saunders AJ, Kovacs DM. Ceramide stabilizes beta-site amyloid precursor protein-cleaving enzyme 1 and promotes amyloid beta-peptide biogenesis. J Biol Chem 2003;278(22):19777–83. [DOI] [PubMed] [Google Scholar]
- 150.Price JL, Davis PB, Morris JC, White DL. The distribution of tangles, plaques and related immunohistochemical markers in healthy aging and Alzheimer’s disease. Neurobiol Aging 1991;12(4):295–312. [DOI] [PubMed] [Google Scholar]
- 151.Fukumoto H, Asami-Odaka A, Suzuki N, Shimada H, Ihara Y, Iwatsubo T. Amyloid beta protein deposition in normal aging has the same characteristics as that in Alzheimer’s disease. Predominance of A beta 42(43) and association of A beta 40 with cored plaques. Am J Pathol 1996;148(1):259–65. [PMC free article] [PubMed] [Google Scholar]
- 152.Fukumoto H, Rosene DL, Moss MB, Raju S, Hyman BT, Irizarry MC. Beta-secretase activity increases with aging in human, monkey, and mouse brain. Am J Pathol 2004;164(2):719–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zhong Z, Ewers M, Teipel S, Burger K, Wallin A, Blennow K, He P, McAllister C, Hampel H, Shen Y. Levels of beta-secretase (BACE1) in cerebrospinal fluid as a predictor of risk in mild cognitive impairment. Arch Gen Psychiatry 2007;64(6):718–26. [DOI] [PubMed] [Google Scholar]
- 154.Shen Y, Wang H, Sun Q, Yao H, Keegan AP, Mullan M, Wilson J, Lista S, Leyhe T, Laske C and others. Increased Plasma Beta-Secretase 1 May Predict Conversion to Alzheimer’s Disease Dementia in Individuals With Mild Cognitive Impairment. Biol Psychiatry 2018;83(5):447–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ewers M, Zhong Z, Burger K, Wallin A, Blennow K, Teipel SJ, Shen Y, Hampel H. Increased CSF-BACE 1 activity is associated with ApoE-epsilon 4 genotype in subjects with mild cognitive impairment and Alzheimer’s disease. Brain 2008;131(Pt 5):1252–8. [DOI] [PubMed] [Google Scholar]
- 156.Cheng X, He P, Lee T, Yao H, Li R, Shen Y. High activities of BACE1 in brains with mild cognitive impairment. Am J Pathol 2014;184(1):141–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Achim CL, Adame A, Dumaop W, Everall IP, Masliah E, Neurobehavioral Research C. Increased accumulation of intraneuronal amyloid beta in HIV-infected patients. J Neuroimmune Pharmacol 2009;4(2):190–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Stern AL, Ghura S, Gannon PJ, Akay-Espinoza C, Phan JM, Yee AC, Vassar R, Gelman BB, Kolson DL, Jordan-Sciutto KL. BACE1 Mediates HIV-Associated and Excitotoxic Neuronal Damage Through an APP-Dependent Mechanism. J Neurosci 2018;38(18):4288–4300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ghafouri-Fard S, Taheri M, Arsang-Jang S, Kholghi Oskooei V, Omrani MD. Sex-based dimorphisms in expression of BDNF and BACE1 in bipolar patients. Compr Psychiatry 2019;91:29–33. [DOI] [PubMed] [Google Scholar]
- 160.Singh I, Sagare AP, Coma M, Perlmutter D, Gelein R, Bell RD, Deane RJ, Zhong E, Parisi M, Ciszewski J and others. Low levels of copper disrupt brain amyloid-beta homeostasis by altering its production and clearance. Proc Natl Acad Sci U S A 2013;110(36):14771–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Selkoe DJ. Clearing the brain’s amyloid cobwebs. Neuron 2001;32(2):177–80. [DOI] [PubMed] [Google Scholar]
- 162.Mukherjee A, Hersh LB. Regulation of amyloid beta-peptide levels by enzymatic degradation. J Alzheimers Dis 2002;4(5):341–8. [DOI] [PubMed] [Google Scholar]
- 163.Morelli L, Bulloj A, Leal MC, Castano EM. Amyloid beta degradation: a challenging task for brain peptidases. Subcell Biochem 2005;38:129–45. [DOI] [PubMed] [Google Scholar]
- 164.Kitazawa M, Hsu HW, Medeiros R. Copper Exposure Perturbs Brain Inflammatory Responses and Impairs Clearance of Amyloid-Beta. Toxicol Sci 2016;152(1):194–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Chow VW, Mattson MP, Wong PC, Gleichmann M. An overview of APP processing enzymes and products. Neuromolecular Med 2010;12(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Oliveira A, Cacodcar J, Motghare DD. Morbidity among iron ore mine workers in Goa. Indian J Public Health 2014;58(1):57–60. [DOI] [PubMed] [Google Scholar]
- 167.Mortimer JA, Borenstein AR, Nelson LM. Associations of welding and manganese exposure with Parkinson disease: review and meta-analysis. Neurology 2012;79(11):1174–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Burdusel AC, Gherasim O, Grumezescu AM, Mogoanta L, Ficai A, Andronescu E. Biomedical Applications of Silver Nanoparticles: An Up-to-Date Overview. Nanomaterials (Basel) 2018;8(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Exley C Human exposure to aluminium. Environ Sci Process Impacts 2013;15(10):1807–1816. [DOI] [PubMed] [Google Scholar]
- 170.Calderon J, Navarro ME, Jimenez-Capdeville ME, Santos-Diaz MA, Golden A, Rodriguez-Leyva I, Borja-Aburto V, Diaz-Barriga F. Exposure to arsenic and lead and neuropsychological development in Mexican children. Environ Res 2001;85(2):69–76. [DOI] [PubMed] [Google Scholar]
- 171.Brewer GJ. Copper-2 Ingestion, Plus Increased Meat Eating Leading to Increased Copper Absorption, Are Major Factors Behind the Current Epidemic of Alzheimer’s Disease. Nutrients 2015;7(12):10053–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Bowler RM, Roels HA, Nakagawa S, Drezgic M, Diamond E, Park R, Koller W, Bowler RP, Mergler D, Bouchard M and others. Dose-effect relationships between manganese exposure and neurological, neuropsychological and pulmonary function in confined space bridge welders. Occup Environ Med 2007;64(3):167–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Bast-Pettersen R, Ellingsen DG, Hetland SM, Thomassen Y. Neuropsychological function in manganese alloy plant workers. Int Arch Occup Environ Health 2004;77(4):277–87. [DOI] [PubMed] [Google Scholar]
- 174.Huat TJ, Camats-Perna J, Newcombe EA, Valmas N, Kitazawa M, Medeiros R. Metal Toxicity Links to Alzheimer’s Disease and Neuroinflammation. J Mol Biol 2019;431(9):1843–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lovell MA, Robertson JD, Teesdale WJ, Campbell JL, Markesbery WR. Copper, iron and zinc in Alzheimer’s disease senile plaques. J Neurol Sci 1998;158(1):47–52. [DOI] [PubMed] [Google Scholar]
- 176.Miller LM, Wang Q, Telivala TP, Smith RJ, Lanzirotti A, Miklossy J. Synchrotron-based infrared and X-ray imaging shows focalized accumulation of Cu and Zn co-localized with beta-amyloid deposits in Alzheimer’s disease. J Struct Biol 2006;155(1):30–7. [DOI] [PubMed] [Google Scholar]
- 177.Tao Y, Wang Y, Rogers JT, Wang F. Perturbed iron distribution in Alzheimer’s disease serum, cerebrospinal fluid, and selected brain regions: a systematic review and meta-analysis. J Alzheimers Dis 2014;42(2):679–90. [DOI] [PubMed] [Google Scholar]
- 178.Pfefferbaum A, Adalsteinsson E, Rohlfing T, Sullivan EV. MRI estimates of brain iron concentration in normal aging: comparison of field-dependent (FDRI) and phase (SWI) methods. Neuroimage 2009;47(2):493–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Langkammer C, Ropele S, Pirpamer L, Fazekas F, Schmidt R. MRI for iron mapping in Alzheimer’s disease. Neurodegener Dis 2014;13(2–3):189–91. [DOI] [PubMed] [Google Scholar]
- 180.Ghadery C, Pirpamer L, Hofer E, Langkammer C, Petrovic K, Loitfelder M, Schwingenschuh P, Seiler S, Duering M, Jouvent E and others. R2* mapping for brain iron: associations with cognition in normal aging. Neurobiol Aging 2015;36(2):925–32. [DOI] [PubMed] [Google Scholar]
- 181.Lidsky TI. Is the Aluminum Hypothesis dead? J Occup Environ Med 2014;56(5 Suppl):S73–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Aluminum Tomljenovic L. and Alzheimer’s disease: after a century of controversy, is there a plausible link? J Alzheimers Dis 2011;23(4):567–98. [DOI] [PubMed] [Google Scholar]
- 183.Virk SA, Eslick GD. Aluminum Levels in Brain, Serum, and Cerebrospinal Fluid are Higher in Alzheimer’s Disease Cases than in Controls: A Series of Meta-Analyses. J Alzheimers Dis 2015;47(3):629–38. [DOI] [PubMed] [Google Scholar]
- 184.Huang W, Cheng P, Yu K, Han Y, Song M, Li Y. Hyperforin attenuates aluminum-induced Abeta production and Tau phosphorylation via regulating Akt/GSK-3beta signaling pathway in PC12 cells. Biomed Pharmacother 2017;96:1–6. [DOI] [PubMed] [Google Scholar]
- 185.Liang RF, Li WQ, Wang H, Wang JX, Niu Q. Impact of sub-chronic aluminium-maltolate exposure on catabolism of amyloid precursor protein in rats. Biomed Environ Sci 2013;26(6):445–52. [DOI] [PubMed] [Google Scholar]
- 186.Wang LP, Hu JL, Zhao Y, Zhang L, Niu Q. [Influence of aluminum on microRNA29 and beta-site amyloid precursor protein cleaving enzyme 1 in the brain of rats]. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi 2017;35(2):81–84. [DOI] [PubMed] [Google Scholar]
- 187.Robbins N, Zhang ZF, Sun J, Ketterer ME, Lalumandier JA, Shulze RA. Childhood lead exposure and uptake in teeth in the Cleveland area during the era of leaded gasoline. Sci Total Environ 2010;408(19):4118–27. [DOI] [PubMed] [Google Scholar]
- 188.Pirkle JL, Brody DJ, Gunter EW, Kramer RA, Paschal DC, Flegal KM, Matte TD. The decline in blood lead levels in the United States. The National Health and Nutrition Examination Surveys (NHANES). JAMA 1994;272(4):284–91. [PubMed] [Google Scholar]
- 189.Annest JL, Mahaffey KR, Cox DH, Roberts J. BLood lead levels for persons 6 months-74 years of age: United States, 1976–80. Adv Data 1982(79):1–23. [PubMed] [Google Scholar]
- 190.Reuben A Childhood Lead Exposure and Adult Neurodegenerative Disease. J Alzheimers Dis 2018;64(1):17–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Weisskopf MG, Hu H, Sparrow D, Lenkinski RE, Wright RO. Proton magnetic resonance spectroscopic evidence of glial effects of cumulative lead exposure in the adult human hippocampus. Environ Health Perspect 2007;115(4):519–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Jiang YM, Long LL, Zhu XY, Zheng H, Fu X, Ou SY, Wei DL, Zhou HL, Zheng W. Evidence for altered hippocampal volume and brain metabolites in workers occupationally exposed to lead: a study by magnetic resonance imaging and (1)H magnetic resonance spectroscopy. Toxicol Lett 2008;181(2):118–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Hsieh TJ, Chen YC, Li CW, Liu GC, Chiu YW, Chuang HY. A proton magnetic resonance spectroscopy study of the chronic lead effect on the Basal ganglion and frontal and occipital lobes in middle-age adults. Environ Health Perspect 2009;117(6):941–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Horton CJ, Weng HY, Wells EM. Association between blood lead level and subsequent Alzheimer’s disease mortality. Environ Epidemiol 2019;3(3):e045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Hsu HW, Bondy SC, Kitazawa M. Environmental and Dietary Exposure to Copper and Its Cellular Mechanisms Linking to Alzheimer’s Disease. Toxicol Sci 2018;163(2):338–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Ding B, Chen KM, Ling HW, Sun F, Li X, Wan T, Chai WM, Zhang H, Zhan Y, Guan YJ. Correlation of iron in the hippocampus with MMSE in patients with Alzheimer’s disease. J Magn Reson Imaging 2009;29(4):793–8. [DOI] [PubMed] [Google Scholar]
- 197.Bilgic B, Pfefferbaum A, Rohlfing T, Sullivan EV, Adalsteinsson E. MRI estimates of brain iron concentration in normal aging using quantitative susceptibility mapping. Neuroimage 2012;59(3):2625–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Bartzokis G, Tishler TA. MRI evaluation of basal ganglia ferritin iron and neurotoxicity in Alzheimer’s and Huntingon’s disease. Cell Mol Biol (Noisy-le-grand) 2000;46(4):821–33. [PubMed] [Google Scholar]
- 199.Bartzokis G, Sultzer D, Mintz J, Holt LE, Marx P, Phelan CK, Marder SR. In vivo evaluation of brain iron in Alzheimer’s disease and normal subjects using MRI. Biol Psychiatry 1994;35(7):480–7. [DOI] [PubMed] [Google Scholar]
- 200.Smith MA, Zhu X, Tabaton M, Liu G, McKeel DW Jr., Cohen ML, Wang X, Siedlak SL, Dwyer BE, Hayashi T and others. Increased iron and free radical generation in preclinical Alzheimer disease and mild cognitive impairment. J Alzheimers Dis 2010;19(1):363–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Kim CH, Yoo YM. Altered APP Carboxyl-Terminal Processing Under Ferrous Iron Treatment in PC12 Cells. Korean J Physiol Pharmacol 2013;17(3):189–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Hamadani JD, Tofail F, Nermell B, Gardner R, Shiraji S, Bottai M, Arifeen SE, Huda SN, Vahter M. Critical windows of exposure for arsenic-associated impairment of cognitive function in pre-school girls and boys: a population-based cohort study. Int J Epidemiol 2011;40(6):1593–604. [DOI] [PubMed] [Google Scholar]
- 203.Nino SA, Martel-Gallegos G, Castro-Zavala A, Ortega-Berlanga B, Delgado JM, Hernandez-Mendoza H, Romero-Guzman E, Rios-Lugo J, Rosales-Mendoza S, Jimenez-Capdeville ME and others. Chronic Arsenic Exposure Increases Abeta(1–42) Production and Receptor for Advanced Glycation End Products Expression in Rat Brain. Chem Res Toxicol 2018;31(1):13–21. [DOI] [PubMed] [Google Scholar]
- 204.Escudero-Lourdes C, Uresti-Rivera EE, Oliva-Gonzalez C, Torres-Ramos MA, Aguirre-Banuelos P, Gandolfi AJ. Cortical Astrocytes Acutely Exposed to the Monomethylarsonous Acid (MMA(III)) Show Increased Pro-inflammatory Cytokines Gene Expression that is Consistent with APP and BACE-1: Overexpression. Neurochem Res 2016;41(10):2559–2572. [DOI] [PubMed] [Google Scholar]
- 205.Wang CY, Wang T, Zheng W, Zhao BL, Danscher G, Chen YH, Wang ZY. Zinc overload enhances APP cleavage and Abeta deposition in the Alzheimer mouse brain. PLoS One 2010;5(12):e15349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Lucchini RG, Albini E, Benedetti L, Borghesi S, Coccaglio R, Malara EC, Parrinello G, Garattini S, Resola S, Alessio L. High prevalence of Parkinsonian disorders associated to manganese exposure in the vicinities of ferroalloy industries. Am J Ind Med 2007;50(11):788–800. [DOI] [PubMed] [Google Scholar]
- 207.Nicolopoulou-Stamati P, Maipas S, Kotampasi C, Stamatis P, Hens L. Chemical Pesticides and Human Health: The Urgent Need for a New Concept in Agriculture. Front Public Health 2016;4:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Tang BL. Neuropathological Mechanisms Associated with Pesticides in Alzheimer’s Disease. Toxics 2020;8(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Yan D, Zhang Y, Liu L, Yan H. Pesticide exposure and risk of Alzheimer’s disease: a systematic review and meta-analysis. Sci Rep 2016;6:32222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Sherer TB, Betarbet R, Testa CM, Seo BB, Richardson JR, Kim JH, Miller GW, Yagi T, Matsuno-Yagi A, Greenamyre JT. Mechanism of toxicity in rotenone models of Parkinson’s disease. J Neurosci 2003;23(34):10756–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Cannon JR, Tapias V, Na HM, Honick AS, Drolet RE, Greenamyre JT. A highly reproducible rotenone model of Parkinson’s disease. Neurobiol Dis 2009;34(2):279–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Leuner K, Schutt T, Kurz C, Eckert SH, Schiller C, Occhipinti A, Mai S, Jendrach M, Eckert GP, Kruse SE and others. Mitochondrion-derived reactive oxygen species lead to enhanced amyloid beta formation. Antioxid Redox Signal 2012;16(12):1421–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Berry C, La Vecchia C, Nicotera P. Paraquat and Parkinson’s disease. Cell Death Differ 2010;17(7):1115–25. [DOI] [PubMed] [Google Scholar]
- 214.Franco R, Li S, Rodriguez-Rocha H, Burns M, Panayiotidis MI. Molecular mechanisms of pesticide-induced neurotoxicity: Relevance to Parkinson’s disease. Chem Biol Interact 2010;188(2):289–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Chen L, Na R, Boldt E, Ran Q. NLRP3 inflammasome activation by mitochondrial reactive oxygen species plays a key role in long-term cognitive impairment induced by paraquat exposure. Neurobiol Aging 2015;36(9):2533–43. [DOI] [PubMed] [Google Scholar]
- 216.European Food Safety A. The 2017 European Union report on pesticide residues in food. EFSA J 2019;17(6):e05743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Jian JM, Chen D, Han FJ, Guo Y, Zeng L, Lu X, Wang F. A short review on human exposure to and tissue distribution of per- and polyfluoroalkyl substances (PFASs). Sci Total Environ 2018;636:1058–1069. [DOI] [PubMed] [Google Scholar]
- 218.Sammi SR, Foguth RM, Nieves CS, De Perre C, Wipf P, McMurray CT, Lee LS, Cannon JR. Perfluorooctane Sulfonate (PFOS) Produces Dopaminergic Neuropathology in Caenorhabditis elegans. Toxicol Sci 2019;172(2):417–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Foguth RM, Flynn RW, de Perre C, Iacchetta M, Lee LS, Sepulveda MS, Cannon JR. Developmental exposure to perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA) selectively decreases brain dopamine levels in Northern leopard frogs. Toxicol Appl Pharmacol 2019;377:114623. [DOI] [PubMed] [Google Scholar]
- 220.Vandenberg LN, Chahoud I, Heindel JJ, Padmanabhan V, Paumgartten FJ, Schoenfelder G. Urinary, circulating, and tissue biomonitoring studies indicate widespread exposure to bisphenol A. Environ Health Perspect 2010;118(8):1055–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Shankar A, Teppala S. Relationship between urinary bisphenol A levels and diabetes mellitus. J Clin Endocrinol Metab 2011;96(12):3822–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Wang T, Li M, Chen B, Xu M, Xu Y, Huang Y, Lu J, Chen Y, Wang W, Li X and others. Urinary bisphenol A (BPA) concentration associates with obesity and insulin resistance. J Clin Endocrinol Metab 2012;97(2):E223–7. [DOI] [PubMed] [Google Scholar]
- 223.Braun JM, Kalkbrenner AE, Calafat AM, Yolton K, Ye X, Dietrich KN, Lanphear BP. Impact of early-life bisphenol A exposure on behavior and executive function in children. Pediatrics 2011;128(5):873–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Braun JM, Yolton K, Dietrich KN, Hornung R, Ye X, Calafat AM, Lanphear BP. Prenatal bisphenol A exposure and early childhood behavior. Environ Health Perspect 2009;117(12):1945–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Harley KG, Gunier RB, Kogut K, Johnson C, Bradman A, Calafat AM, Eskenazi B. Prenatal and early childhood bisphenol A concentrations and behavior in school-aged children. Environ Res 2013;126:43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Miodovnik A, Engel SM, Zhu C, Ye X, Soorya LV, Silva MJ, Calafat AM, Wolff MS. Endocrine disruptors and childhood social impairment. Neurotoxicology 2011;32(2):261–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Sukjamnong S, Thongkorn S, Kanlayaprasit S, Saeliw T, Hussem K, Warayanon W, Hu VW, Tencomnao T, Sarachana T. Prenatal exposure to bisphenol A alters the transcriptome-interactome profiles of genes associated with Alzheimer’s disease in the offspring hippocampus. Sci Rep 2020;10(1):9487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Wang T, Xie C, Yu P, Fang F, Zhu J, Cheng J, Gu A, Wang J, Xiao H. Involvement of Insulin Signaling Disturbances in Bisphenol A-Induced Alzheimer’s Disease-like Neurotoxicity. Sci Rep 2017;7(1):7497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Oberdorster G, Sharp Z, Atudorei V, Elder A, Gelein R, Kreyling W, Cox C. Translocation of inhaled ultrafine particles to the brain. Inhal Toxicol 2004;16(6–7):437–45. [DOI] [PubMed] [Google Scholar]
- 230.Pearson RC, Esiri MM, Hiorns RW, Wilcock GK, Powell TP. Anatomical correlates of the distribution of the pathological changes in the neocortex in Alzheimer disease. Proc Natl Acad Sci U S A 1985;82(13):4531–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Calderon-Garciduenas L, Reed W, Maronpot RR, Henriquez-Roldan C, Delgado-Chavez R, Calderon-Garciduenas A, Dragustinovis I, Franco-Lira M, Aragon-Flores M, Solt AC and others. Brain inflammation and Alzheimer’s-like pathology in individuals exposed to severe air pollution. Toxicol Pathol 2004;32(6):650–8. [DOI] [PubMed] [Google Scholar]
- 232.Grant WB. Using Multicountry Ecological and Observational Studies to Determine Dietary Risk Factors for Alzheimer’s Disease. J Am Coll Nutr 2016;35(5):476–89. [DOI] [PubMed] [Google Scholar]
- 233.Perrone L, Grant WB. Observational and ecological studies of dietary advanced glycation end products in national diets and Alzheimer’s disease incidence and prevalence. J Alzheimers Dis 2015;45(3):965–79. [DOI] [PubMed] [Google Scholar]
- 234.Marwarha G, Schommer J, Lund J, Schommer T, Ghribi O. Palmitate-induced C/EBP homologous protein activation leads to NF-kappaB-mediated increase in BACE1 activity and amyloid beta genesis. J Neurochem 2018;144(6):761–779. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 235.Marwarha G, Rostad S, Lilek J, Kleinjan M, Schommer J, Ghribi O. Palmitate Increases beta-site AbetaPP-Cleavage Enzyme 1 Activity and Amyloid-beta Genesis by Evoking Endoplasmic Reticulum Stress and Subsequent C/EBP Homologous Protein Activation. J Alzheimers Dis 2017;57(3):907–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Marwarha G, Raza S, Prasanthi JR, Ghribi O. Gadd153 and NF-kappaB crosstalk regulates 27-hydroxycholesterol-induced increase in BACE1 and beta-amyloid production in human neuroblastoma SH-SY5Y cells. PLoS One 2013;8(8):e70773. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 237.Marwarha G, Claycombe-Larson K, Lund J, Ghribi O. Palmitate-Induced SREBP1 Expression and Activation Underlies the Increased BACE 1 Activity and Amyloid Beta Genesis. Mol Neurobiol 2019;56(7):5256–5269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Maesako M, Uemura M, Tashiro Y, Sasaki K, Watanabe K, Noda Y, Ueda K, Asada-Utsugi M, Kubota M, Okawa K and others. High Fat Diet Enhances beta-Site Cleavage of Amyloid Precursor Protein (APP) via Promoting beta-Site APP Cleaving Enzyme 1/Adaptor Protein 2/Clathrin Complex Formation. PLoS One 2015;10(9):e0131199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Lee HJ, Ryu JM, Jung YH, Lee SJ, Kim JY, Lee SH, Hwang IK, Seong JK, Han HJ. High glucose upregulates BACE1-mediated Abeta production through ROS-dependent HIF-1alpha and LXRalpha/ABCA1-regulated lipid raft reorganization in SK-N-MC cells. Sci Rep 2016;6:36746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Skog KI, Johansson MA, Jagerstad MI. Carcinogenic heterocyclic amines in model systems and cooked foods: a review on formation, occurrence and intake. Food Chem Toxicol 1998;36(9–10):879–96. [DOI] [PubMed] [Google Scholar]
- 241.Matsumoto T, Yoshida D, Tomita H. Determination of mutagens, amino-alpha-carbolines in grilled foods and cigarette smoke condensate. Cancer Lett 1981;12(1–2):105–10. [DOI] [PubMed] [Google Scholar]
- 242.Liu ZM, Tse LA, Chen B, Wu S, Chan D, Kowk T, Woo J, Xiang YT, Wong SY. Dietary acrylamide exposure was associated with mild cognition decline among non-smoking Chinese elderly men. Sci Rep 2017;7(1):6395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Gray JI, Morton ID. Some toxic compounds produced in food by cooking and processing. J Hum Nutr 1981;35(1):5–23. [DOI] [PubMed] [Google Scholar]
- 244.Abraham K, Andres S, Palavinskas R, Berg K, Appel KE, Lampen A. Toxicology and risk assessment of acrolein in food. Mol Nutr Food Res 2011;55(9):1277–90. [DOI] [PubMed] [Google Scholar]
- 245.Francis H, Stevenson R. The longer-term impacts of Western diet on human cognition and the brain. Appetite 2013;63:119–28. [DOI] [PubMed] [Google Scholar]
- 246.Uribarri J, Woodruff S, Goodman S, Cai W, Chen X, Pyzik R, Yong A, Striker GE, Vlassara H. Advanced glycation end products in foods and a practical guide to their reduction in the diet. J Am Diet Assoc 2010;110(6):911–16 e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Goldberg T, Cai W, Peppa M, Dardaine V, Baliga BS, Uribarri J, Vlassara H. Advanced glycoxidation end products in commonly consumed foods. J Am Diet Assoc 2004;104(8):1287–91. [DOI] [PubMed] [Google Scholar]
- 248.Lubitz I, Ricny J, Atrakchi-Baranes D, Shemesh C, Kravitz E, Liraz-Zaltsman S, Maksin-Matveev A, Cooper I, Leibowitz A, Uribarri J and others. High dietary advanced glycation end products are associated with poorer spatial learning and accelerated Abeta deposition in an Alzheimer mouse model. Aging Cell 2016;15(2):309–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Felton JS, Knize MG, Shen NH, Lewis PR, Andresen BD, Happe J, Hatch FT. The isolation and identification of a new mutagen from fried ground beef: 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP). Carcinogenesis 1986;7(7):1081–6. [DOI] [PubMed] [Google Scholar]
- 250.Felton JS, Knize MG, Shen NH, Andresen BD, Bjeldanes LF, Hatch FT. Identification of the mutagens in cooked beef. Environ Health Perspect 1986;67:17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Lawana V, Um SY, Rochet JC, Turesky RJ, Shannahan JH, Cannon JR. Neuromelanin Modulates Heterocyclic Aromatic Amine-Induced Dopaminergic Neurotoxicity. Toxicol Sci 2020;173(1):171–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Griggs AM, Agim ZS, Mishra VR, Tambe MA, Director-Myska AE, Turteltaub KW, McCabe GP, Rochet JC, Cannon JR. 2-Amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) is selectively toxic to primary dopaminergic neurons in vitro. Toxicol Sci 2014;140(1):179–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Stevens JF, Maier CS. Acrolein: sources, metabolism, and biomolecular interactions relevant to human health and disease. Mol Nutr Food Res 2008;52(1):7–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Uchida K, Kanematsu M, Morimitsu Y, Osawa T, Noguchi N, Niki E. Acrolein is a product of lipid peroxidation reaction. Formation of free acrolein and its conjugate with lysine residues in oxidized low density lipoproteins. J Biol Chem 1998;273(26):16058–66. [DOI] [PubMed] [Google Scholar]
- 255.Liu M, Huang Y, Qin J, Wang Y, Ke B, Yang Y. Inhibition of MAPKs Signaling Pathways Prevents Acrolein-Induced Neurotoxicity in HT22 Mouse Hippocampal Cells. Biol Pharm Bull 2019;42(4):617–622. [DOI] [PubMed] [Google Scholar]
- 256.Huang Y, Jin M, Pi R, Zhang J, Chen M, Ouyang Y, Liu A, Chao X, Liu P, Liu J and others. Protective effects of caffeic acid and caffeic acid phenethyl ester against acrolein-induced neurotoxicity in HT22 mouse hippocampal cells. Neurosci Lett 2013;535:146–51. [DOI] [PubMed] [Google Scholar]
- 257.Shi LY, Zhang L, Li H, Liu TL, Lai JC, Wu ZB, Qin J. Protective effects of curcumin on acrolein-induced neurotoxicity in HT22 mouse hippocampal cells. Pharmacol Rep 2018;70(5):1040–1046. [DOI] [PubMed] [Google Scholar]
- 258.Wu Z, Bai L, Tu R, Zhang L, Ba Y, Zhang H, Li X, Cheng X, Li W, Huang H. Disruption of synaptic expression pattern and age-related DNA oxidation in a neuronal model of lead-induced toxicity. Environ Toxicol Pharmacol 2020;76:103350. [DOI] [PubMed] [Google Scholar]
- 259.Wu S, Liu H, Zhao H, Wang X, Chen J, Xia D, Xiao C, Cheng J, Zhao Z, He Y. Environmental lead exposure aggravates the progression of Alzheimer’s disease in mice by targeting on blood brain barrier. Toxicol Lett 2020;319:138–147. [DOI] [PubMed] [Google Scholar]
- 260.Ayyalasomayajula N, Bandaru M, Dixit PK, Ajumeera R, Chetty CS, Challa S. Inactivation of GAP-43 due to the depletion of cellular calcium by the Pb and amyloid peptide induced toxicity: An in vitro approach. Chem Biol Interact 2020;316:108927. [DOI] [PubMed] [Google Scholar]
- 261.Milani C, Farina F, Botto L, Massimino L, Lonati E, Donzelli E, Ballarini E, Crippa L, Marmiroli P, Bulbarelli A and others. Systemic Exposure to Air Pollution Induces Oxidative Stress and Inflammation in Mouse Brain, Contributing to Neurodegeneration Onset. Int J Mol Sci 2020;21(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Kao SC, Krichevsky AM, Kosik KS, Tsai LH. BACE1 suppression by RNA interference in primary cortical neurons. J Biol Chem 2004;279(3):1942–9. [DOI] [PubMed] [Google Scholar]
- 263.Nunomura A, Perry G, Aliev G, Hirai K, Takeda A, Balraj EK, Jones PK, Ghanbari H, Wataya T, Shimohama S and others. Oxidative damage is the earliest event in Alzheimer disease. J Neuropathol Exp Neurol 2001;60(8):759–67. [DOI] [PubMed] [Google Scholar]
- 264.Guglielmotto M, Giliberto L, Tamagno E, Tabaton M. Oxidative stress mediates the pathogenic effect of different Alzheimer’s disease risk factors. Front Aging Neurosci 2010;2:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Apelt J, Bigl M, Wunderlich P, Schliebs R. Aging-related increase in oxidative stress correlates with developmental pattern of beta-secretase activity and beta-amyloid plaque formation in transgenic Tg2576 mice with Alzheimer-like pathology. Int J Dev Neurosci 2004;22(7):475–84. [DOI] [PubMed] [Google Scholar]
- 266.Chen L, Na R, Gu M, Richardson A, Ran Q. Lipid peroxidation up-regulates BACE1 expression in vivo: a possible early event of amyloidogenesis in Alzheimer’s disease. J Neurochem 2008;107(1):197–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Tamagno E, Parola M, Bardini P, Piccini A, Borghi R, Guglielmotto M, Santoro G, Davit A, Danni O, Smith MA and others. Beta-site APP cleaving enzyme up-regulation induced by 4-hydroxynonenal is mediated by stress-activated protein kinases pathways. J Neurochem 2005;92(3):628–36. [DOI] [PubMed] [Google Scholar]
- 268.Tamagno E, Guglielmotto M, Aragno M, Borghi R, Autelli R, Giliberto L, Muraca G, Danni O, Zhu X, Smith MA and others. Oxidative stress activates a positive feedback between the gamma- and beta-secretase cleavages of the beta-amyloid precursor protein. J Neurochem 2008;104(3):683–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Mouton-Liger F, Paquet C, Dumurgier J, Bouras C, Pradier L, Gray F, Hugon J. Oxidative stress increases BACE1 protein levels through activation of the PKR-eIF2alpha pathway. Biochim Biophys Acta 2012;1822(6):885–96. [DOI] [PubMed] [Google Scholar]
- 270.Tong Y, Zhou W, Fung V, Christensen MA, Qing H, Sun X, Song W. Oxidative stress potentiates BACE1 gene expression and Abeta generation. J Neural Transm (Vienna) 2005;112(3):455–69. [DOI] [PubMed] [Google Scholar]
- 271.Tamagno E, Bardini P, Obbili A, Vitali A, Borghi R, Zaccheo D, Pronzato MA, Danni O, Smith MA, Perry G and others. Oxidative stress increases expression and activity of BACE in NT2 neurons. Neurobiol Dis 2002;10(3):279–88. [DOI] [PubMed] [Google Scholar]
- 272.Moreira PI, Carvalho C, Zhu X, Smith MA, Perry G. Mitochondrial dysfunction is a trigger of Alzheimer’s disease pathophysiology. Biochim Biophys Acta 2010;1802(1):2–10. [DOI] [PubMed] [Google Scholar]
- 273.Eckert A, Schmitt K, Gotz J. Mitochondrial dysfunction - the beginning of the end in Alzheimer’s disease? Separate and synergistic modes of tau and amyloid-beta toxicity. Alzheimers Res Ther 2011;3(2):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Reddy PH, Tripathi R, Troung Q, Tirumala K, Reddy TP, Anekonda V, Shirendeb UP, Calkins MJ, Reddy AP, Mao P and others. Abnormal mitochondrial dynamics and synaptic degeneration as early events in Alzheimer’s disease: implications to mitochondria-targeted antioxidant therapeutics. Biochim Biophys Acta 2012;1822(5):639–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Hauptmann S, Scherping I, Drose S, Brandt U, Schulz KL, Jendrach M, Leuner K, Eckert A, Muller WE. Mitochondrial dysfunction: an early event in Alzheimer pathology accumulates with age in AD transgenic mice. Neurobiol Aging 2009;30(10):1574–86. [DOI] [PubMed] [Google Scholar]
- 276.Velliquette RA, O’Connor T, Vassar R. Energy inhibition elevates beta-secretase levels and activity and is potentially amyloidogenic in APP transgenic mice: possible early events in Alzheimer’s disease pathogenesis. J Neurosci 2005;25(47):10874–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Yan XX, Xiong K, Luo XG, Struble RG, Clough RW. beta-Secretase expression in normal and functionally deprived rat olfactory bulbs: inverse correlation with oxidative metabolic activity. J Comp Neurol 2007;501(1):52–69. [DOI] [PubMed] [Google Scholar]
- 278.Blasko I, Beer R, Bigl M, Apelt J, Franz G, Rudzki D, Ransmayr G, Kampfl A, Schliebs R. Experimental traumatic brain injury in rats stimulates the expression, production and activity of Alzheimer’s disease beta-secretase (BACE-1). J Neural Transm (Vienna) 2004;111(4):523–36. [DOI] [PubMed] [Google Scholar]
- 279.Wang L, Duan Q, Wang T, Ahmed M, Zhang N, Li Y, Li L, Yao X. Mitochondrial Respiratory Chain Inhibitors Involved in ROS Production Induced by Acute High Concentrations of Iodide and the Effects of SOD as a Protective Factor. Oxid Med Cell Longev 2015;2015:217670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Tanaka T, Hosoi F, Yamaguchi-Iwai Y, Nakamura H, Masutani H, Ueda S, Nishiyama A, Takeda S, Wada H, Spyrou G and others. Thioredoxin-2 (TRX-2) is an essential gene regulating mitochondria-dependent apoptosis. EMBO J 2002;21(7):1695–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Li KY, Xiang XJ, Song L, Chen J, Luo B, Wen QX, Zhong BR, Zhou GF, Deng XJ, Ma YL and others. Mitochondrial TXN2 attenuates amyloidogenesis via selective inhibition of BACE1 expression. J Neurochem 2020. [DOI] [PubMed] [Google Scholar]
- 282.Devi L, Ohno M. Mitochondrial dysfunction and accumulation of the beta-secretase-cleaved C-terminal fragment of APP in Alzheimer’s disease transgenic mice. Neurobiol Dis 2012;45(1):417–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Bittner HJ, Guixa-Gonzalez R, Hildebrand PW. Structural basis for the interaction of the beta-secretase with copper. Biochim Biophys Acta Biomembr 2018;1860(5):1105–1113. [DOI] [PubMed] [Google Scholar]
- 284.Furukawa Y, Torres AS, O’Halloran TV. Oxygen-induced maturation of SOD1: a key role for disulfide formation by the copper chaperone CCS. EMBO J 2004;23(14):2872–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Disterhoft JF, Moyer JR Jr., Thompson LT. The calcium rationale in aging and Alzheimer’s disease. Evidence from an animal model of normal aging. Ann N Y Acad Sci 1994;747:382–406. [DOI] [PubMed] [Google Scholar]
- 286.Gibson GE, Peterson C. Calcium and the aging nervous system. Neurobiol Aging 1987;8(4):329–43. [DOI] [PubMed] [Google Scholar]
- 287.Khachaturian ZS. The role of calcium regulation in brain aging: reexamination of a hypothesis. Aging (Milano) 1989;1(1):17–34. [DOI] [PubMed] [Google Scholar]
- 288.Landfield PW. ‘Increased calcium-current’ hypothesis of brain aging. Neurobiol Aging 1987;8(4):346–7. [DOI] [PubMed] [Google Scholar]
- 289.Thibault O, Gant JC, Landfield PW. Expansion of the calcium hypothesis of brain aging and Alzheimer’s disease: minding the store. Aging Cell 2007;6(3):307–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Fischer F, Molinari M, Bodendorf U, Paganetti P. The disulphide bonds in the catalytic domain of BACE are critical but not essential for amyloid precursor protein processing activity. J Neurochem 2002;80(6):1079–88. [DOI] [PubMed] [Google Scholar]
- 291.Chen J, Yin B, Wang W, Sun H. Effects of Disulfide Bonds on Binding of Inhibitors to beta-Amyloid Cleaving Enzyme 1 Decoded by Multiple Replica Accelerated Molecular Dynamics Simulations. ACS Chem Neurosci 2020;11(12):1811–1826. [DOI] [PubMed] [Google Scholar]
- 292.Koritzinsky M, Levitin F, van den Beucken T, Rumantir RA, Harding NJ, Chu KC, Boutros PC, Braakman I, Wouters BG. Two phases of disulfide bond formation have differing requirements for oxygen. J Cell Biol 2013;203(4):615–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Wong MK, Nicholson CJ, Holloway AC, Hardy DB. Maternal nicotine exposure leads to impaired disulfide bond formation and augmented endoplasmic reticulum stress in the rat placenta. PLoS One 2015;10(3):e0122295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Koelsch G BACE1 Function and Inhibition: Implications of Intervention in the Amyloid Pathway of Alzheimer’s Disease Pathology. Molecules 2017;22(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Zhang L, Chen L, Dutra JK, Beck EM, Nag S, Takano A, Amini N, Arakawa R, Brodney MA, Buzon LM and others. Identification of a Novel Positron Emission Tomography (PET) Ligand for Imaging beta-Site Amyloid Precursor Protein Cleaving Enzyme 1 (BACE-1) in Brain. J Med Chem 2018;61(8):3296–3308. [DOI] [PubMed] [Google Scholar]
- 296.Takano A, Chen L, Nag S, Brodney MA, Arakawa R, Chang C, Amini N, Doran SD, Dutra JK, McCarthy TJ and others. Quantitative Analysis of (18)F-PF-06684511, a Novel PET Radioligand for Selective beta-Secretase 1 Imaging, in Nonhuman Primate Brain. J Nucl Med 2019;60(7):992–997. [DOI] [PubMed] [Google Scholar]
- 297.Cervellati C, Trentini A, Rosta V, Passaro A, Bosi C, Sanz JM, Bonazzi S, Pacifico S, Seripa D, Valacchi G and others. Serum beta-secretase 1 (BACE1) activity as candidate biomarker for late-onset Alzheimer’s disease. Geroscience 2020;42(1):159–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Arakawa R, Takano A, Stenkrona P, Stepanov V, Nag S, Jahan M, Gryback P, Bolin M, Chen L, Zhang L and others. PET imaging of beta-secretase 1 in the human brain: radiation dosimetry, quantification, and test-retest examination of [(18)F]PF-06684511. Eur J Nucl Med Mol Imaging 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Moussa-Pacha NM, Abdin SM, Omar HA, Alniss H, Al-Tel TH. BACE1 inhibitors: Current status and future directions in treating Alzheimer’s disease. Med Res Rev 2020;40(1):339–384. [DOI] [PubMed] [Google Scholar]
- 300.Imbimbo BP, Watling M. Investigational BACE inhibitors for the treatment of Alzheimer’s disease. Expert Opin Investig Drugs 2019;28(11):967–975. [DOI] [PubMed] [Google Scholar]
- 301.Coimbra JRM, Marques DFF, Baptista SJ, Pereira CMF, Moreira PI, Dinis TCP, Santos AE, Salvador JAR. Highlights in BACE1 Inhibitors for Alzheimer’s Disease Treatment. Front Chem 2018;6:178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Hu X, Hou H, Bastian C, He W, Qiu S, Ge Y, Yin X, Kidd GJ, Brunet S, Trapp BD and others. BACE1 regulates the proliferation and cellular functions of Schwann cells. Glia 2017;65(5):712–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Hou H, Fan Q, He W, Suh H, Hu X, Yan R. BACE1 Deficiency Causes Abnormal Neuronal Clustering in the Dentate Gyrus. Stem Cell Reports 2017;9(1):217–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Vassar R Editorial: Implications for BACE1 Inhibitor Clinical Trials: Adult Conditional BACE1 Knockout Mice Exhibit Axonal Organization Defects in the Hippocampus. J Prev Alzheimers Dis 2019;6(2):78–84. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.