Abstract
Traditional classification and prognostic approaches for chronic pain conditions focus primarily on anatomically based clinical characteristics not based on underlying biopsychosocial factors contributing to perception of clinical pain and future pain trajectories. Using a supervised clustering approach in a cohort of temporomandibular disorder (TMD) cases and controls from the Orofacial Pain: Prospective Evaluation and Risk Assessment (OPPERA) study, we recently developed and validated a rapid algorithm (ROPA) to pragmatically classify chronic pain patients into three groups that differed in clinical pain report, biopsychosocial profiles, functional limitations, and comorbid conditions. The present aim was to examine the generalizability of this clustering procedure in two additional cohorts: a cohort of patients with chronic overlapping pain conditions (Complex Persistent Pain Conditions (CPPC) study), and a real-world clinical population of patients seeking treatment at Duke Innovative Pain Therapies (DIPT). In each cohort, we applied ROPA for cluster prediction, which requires only four input variables: pressure pain threshold (PPT) and anxiety, depression, and somatization scales. In both CPPC and DIPT, we distinguished three clusters, including one with more severe clinical characteristics and psychological distress. We observed strong concordance with observed cluster solutions, indicating the ROPA method allows for reliable subtyping of clinical populations with minimal patient burden. The ROPA clustering algorithm represents a rapid and valid stratification tool independent of anatomic diagnosis. ROPA holds promise in classifying patients based on pathophysiological mechanisms rather than structural or anatomical diagnoses. As such, this method of classifying patients will facilitate personalized pain medicine for patients with chronic pain.
1. Introduction
Chronic pain constitutes a significant public health burden, as recent estimates conclude 20% of U.S. adults, or 50 million people, report having a painful condition.[13] The diagnosis and treatment of most chronic pain conditions relies on clinical examination for classification criteria based on anatomically defined pain complaint and associated signs and symptoms. Traditional classification approaches focus primarily on empirical anatomical measurements that are limited in scope and often not based on known pathophysiological mechanisms, frequently leading to sub-optimal treatment outcomes.[7; 32] The multifactorial nature of chronic pain has prompted efforts to classify patients based on psychosocial factors, comorbidities, and functional consequences along with core diagnostic features.[20]
Chronic overlapping pain conditions (COPCs) frequently occur together and share similar biopsychosocial features, symptoms, and risk factors, suggesting they may share underlying etiology.[40] Previous studies using patients with temporomandibular disorders (TMD), a common musculoskeletal pain condition classified as a COPC, have identified etiological features from an array of biopsychosocial measures to classify pain.[4] Stratification procedures may permit the unraveling of the heterogeneity observed in the COPC population, and thus may provide a deeper understanding of etiological mechanisms and facilitate the identification of personalized pain treatments that address pathological processes in specific subpopulations of pain patients.
In previous studies we have identified biopsychosocial profiles that distinguish subgroups of pain patients with TMD. In the Orofacial Pain: Prospective Evaluation and Risk Assessment (OPPERA) study, three clusters were identified using supervised clustering.[4] The clusters differed with respect to experimental pain sensitivity, psychological distress, and clinical features readily assessed in TMD patients. The Adaptive cluster (A) represented 33% of the cohort, and were relatively free of hypersensitivity and psychological distress. The Pain-Sensitive (PS) cluster represented 48% of participants and demonstrated increased muscle pain sensitivity to experimentally applied pressure stimuli. The remaining Global Symptoms (GS) cluster, 18% of participants, reported both increased muscle pain sensitivity and greater psychological disturbance. The three clusters differed in reported pain, functional limitations, and comorbid conditions. TMD-free individuals in the GS cluster had greater risk of developing onset TMD (hazard ratio=2.8) over a four-year follow-up period. We developed a predictive algorithm (Rapid OPPERA Algorithm, or ROPA) using a small set of features (muscle pain sensitivity, somatic symptoms, anxiety, and depression), allowing for assignment to clusters with high accuracy in almost any environment.[4]
Here, we extend the ROPA cluster algorithm to additional cohorts, focusing on the generalizability and stability of the predicted clusters. We consider cohorts of individuals with TMD as well as individuals with other chronic pain conditions. We hypothesize that ROPA will effectively distinguish clusters of chronic pain patients, including those in a multi-specialty clinical environment. The success of such an approach would suggest that there are subtypes of chronic pain patients that are consistent across pain conditions, cohort design, and demographic factors that share common biopsychosocial pathways of vulnerability not fully explained by anatomically or structurally based diagnoses. This will permit the evidence-based implementation of a clustering tool to identify patients that is agnostic to anatomic diagnosis.
2. Methods
Data were analyzed from three observational studies: a TMD cohort study, Orofacial Pain: Prospective Evaluation and Risk Assessment (OPPERA), previously used to identify and characterize the clusters; a case-control study, Complex Persistent Pain Conditions (CPPC): Unique and Shared Pathways of Vulnerability, of five chronic overlapping pain conditions; and a prospective cohort study, Duke Innovative Pain Therapies (DIPT), of chronic pain patients seeking treatment. We first summarize each of the studies and their collected data, and then describe the cluster methodology and validation.
2.1. Description of the OPPERA cohort
The OPPERA study is a prospective cohort study designed to study risk factors of TMD.[39] OPPERA recruited and monitored over 4,000 participants from communities surrounding the University of Maryland, the University at Buffalo (NY), the University of North Carolina, and the University of Florida starting in 2006 as has been previously described.[3] The participants, both male and female and aged 18-44 years, included those with chronic painful TMD as well as healthy controls. Participants were English-speaking, not pregnant or nursing, not receiving orthodontic treatment, and had no significant medical conditions or history of recent facial injury or surgery.
Extensive baseline data were collected from all participants, both in pre-baseline surveys completed at home, and during a 3 hour clinical visit, as described previously.[54] Participants were given the option to complete machine-readable paper and pencil forms mailed to the participant’s home, or e-pdf surveys via online web portal. Participants completed questionnaires on TMD risk factors, psychosocial traits, and demographics. A clinical visit was completed by all participants during which additional questionnaires were collected, a trained examiner assessed the diagnosis or absence of a painful TMD, and sensory testing was performed. TMD was classified based on the Research Diagnostic Criteria for TMD (RDC-TMD).[21] Risk factors and psychosocial features were regularly measured through follow-up questionnaires at three-month intervals. Follow-up questionnaires served to screen for first-onset painful TMD, and clinical examination was performed for participants reporting symptoms. All study procedures were conducted with subjects’ written consent according to protocols approved by the Institutional Review Board (IRB) of the respective enrollment site.
2.2. Description of the CPPC cohort
The CPPC study is an unmatched case-control study of participants with pain designed to identify biological, psychological, and genetic risk factors of frequently overlapping chronic pain conditions.[40] The study recruited 573 chronic pain and 258 healthy volunteers from pain specialty clinics around the University of North Carolina between 2011 and 2014. Participants were English-speaking, not pregnant or nursing, not taking daily opioid medications, and had no significant psychiatric or medical conditions. The study focused on five conditions: fibromyalgia (FM), episodic migraine (EM), vulvar vestibulitis (VVS), irritable bowel syndrome (IBS), and painful temporomandibular disorders (TMD). Participants completed diagnostic questionnaires and a clinical exam for each of the five conditions, including standard diagnostic criteria for EM,[55] FM,[64] and TMD.[21] Women were classified as VVS cases if they reported a history of: excessive pain on contact in the genital region (e.g. insertion of a tampon, or during pelvic examination, during sexual intercourse); or having been told by a gynecologist that they have VVS or vulvodynia; or both. However, women who also reported a history of itching in the genital area for 3 months or more were not classified as VVS cases. IBS was diagnosed using questions assessing Rome II criteria.[38] Subjects also completed a battery of psychosocial questionnaires and sensory testing similar to that given in the OPPERA study[4] and provided a blood sample at their initial visit. Survey data were collected using scannable paper and pencil forms. All subjects gave written informed consent to study procedures. The study was approved by the IRBs of the University of North Carolina and McGill University.
2.3. Description of the DIPT cohort
The DIPT study is a prospective clinic-based study of patients seeking treatment for chronic pain and/or sleep apnea, including 862 consecutive patients of the DIPT clinic between July 2017 and May 2019 who consented to contribute their clinical data to a chronic pain registry. The usage of clinical data for research purposes was explained prior to obtaining informed consent from each participant. All study procedures were approved and monitored by the IRB of Duke University.
The 641 patients with complete clustering data were included in the analytic dataset; these patients were most commonly clinically diagnosed with TMD, FM, trigeminal neuralgia, and headache. Routine standard-of-care (SOC) in this clinic includes psychosocial profiling and a simple trapezius muscle pressure pain threshold measure adapted to the clinical environment. Each patient completed a brief set of web-based pain and psychosocial assessments before their first encounter with a clinician, and then completed follow-up questionnaires before subsequent visits to assess disease progression. The assessments were adapted from the battery of procedures used in OPPERA, but modified for the clinical setting as described in Section 2.4. Patients without English fluency or the cognitive ability to complete the surveys were not asked to participate in the registry; no other exclusion criteria were applied. The full battery of SOC surveys was completed by most DIPT patients in 30-45 minutes; the instrument used for clustering took less than five minutes to complete.
2.4. Study measures
Each of the contributing cohorts collected a variety of sociodemographic and clinical features (Table 1). The OPPERA and CPPC cohorts collected an extensive battery of largely overlapping surveys and clinical measurements. At enrollment, participant sociodemographic characteristics, environmental risk factors (smoking, trauma, injury) and extensive clinical characteristics were recorded.[46; 54] In both studies, measures of autonomic function (heart rate, blood pressure, heart rate variability)[41] and psychophysical pain responsiveness[30] were taken during the clinical exam visit. A battery of psychosocial questionnaires[25] and environmental risk factors (e.g., smoking, regional trauma, injury)[46] were completed by subjects at home, either on paper forms or (in OPPERA only) using a web-based survey platform. This represented a broad variety of measures available, though not necessarily related to painful conditions, for clustering to identify clinically relevant subtypes. A summary of the values of the extensive array of variables for OPPERA has been published.[4] A similar summary for the CPPC cohort appears in the Appendix as Table A3.
Table 1.
Data collection instruments by study.
| Instrument | Abbreviation | OPPERA | CPPC | DIPT | |
|---|---|---|---|---|---|
| Clinical and Diagnostic | Comprehensive Pain and Symptom Questionnaire[46] | CPSQ | X | ||
| Graded Chronic Pain Scale[46] | GCPS | X | |||
| Screening Pain Self Report[46] | SPSR | X | X | X | |
| Short Form Health Survey[62] | SF-12 | X | X | ||
| Jaw Functional Limitation Scale[46] | JFLS | X | |||
| Oral Behaviors Checklist[46] | OBC | X | |||
| Pain, Enjoyment, General Activity[36] | PEG | X | |||
| PainDETECT[27] | PD-Q | X | X | ||
| International Classification of Headache Disorders, 2nd Edition [55] | ICHD-II | X | |||
| American College of Rheumatology Diagnostic Criteria for Fibromyalgia[64] | ACR/FM | X | |||
| Research Diagnostic Criteria for Temporomandibular Disorders [21] | RDC/TMD | X | X | ||
| Short Form McGill Pain Questionnaire[43] | SF-MPQ | X | |||
| Multidimensional Pain Inventory[34] | MPI | X | X | ||
| Pain Response | pressure pain threshold[30] | PPT | X | X | X |
| heat pain threshold and tolerance[30] | HPTT | X | X | ||
| temporal summation[30] | TS | X | X | ||
| pinprick sensitivity[30] | PS | X | |||
| Psychosocial | Coping Strategies Questionnaire-Revised[51] | CSQ-R | X | ||
| Eysenck Personality Questionnaire-Revised (Short Form) [23] | EPQ-R | X | X | ||
| Kohn Reactivity Scale[35] | Kohn | X | X | ||
| Life Experiences Survey[52] | LES | X | X | ||
| Pain Catastrophizing Scale[57] | PCS | X | X | ||
| Lifetime Stressor List/PTSD Checklist-Civilian Version[63] | LSL/PCL-C | X | X | ||
| Pennebaker Inventory of Limbic Languidness[47] | PILL | X | X | ||
| Profile of Mood States Bi-Polar[53] | POMS | X | X | ||
| Pittsburgh Sleep Quality Index[8] | PSQI | X | X | ||
| Symptom Checklist-90 Revised[31] | SCL-90R | X | X | ||
| State–Trait Anxiety Inventory[56] | STAIY | X | X | ||
| Brief Symptom Inventory-18[16] | BSI-18 | X | |||
| Perceived Stress Scale[10] | PSS | X | X | X | |
| Scale of Positive and Negative Experiences[19] | SPANE | X | |||
| Epworth Sleepiness Scale[33] | ESS | X | |||
| Insomnia Severity Index[44] | ISI | X |
The DIPT clinical patient management and research data collection platform also collected a variety of data from all consenting patients as part of their routine care procedures. These included socio-demographics, medical history, and blood sample collection. Clinical pain assessments included the Pain, Enjoyment, General Activity scale (PEG), the Gracely box scale measures of pain intensity and pain unpleasantness, visual analog scale (VAS) pain ratings, and the PainDETECT. A single QST measure was taken: pressure pain threshold measured with an algometer device (Pain Test™ Force Dial FPK/N, Wagner Instruments, Greenwich CT) at the trapezius muscle bilaterally, as was performed in OPPERA.[30] Psychological distress was measured using the Brief Symptom Inventory (BSI-18), which was developed for medical and community settings to measure constructs in the SCL-90R in an 18-item shortened format;[15] the Perceived Stress Scale (PSS); Scale of Positive and Negative Experiences (SPANE); and a brief assessment of coping strategies derived from the Coping Strategies Questionnaire (Revised)[51] and the Chronic Pain Self-Efficacy Scale.[1] Sleep quality was assessed using the Epworth Sleepiness Scale (ESS) and the Insomnia Severity Index (ISI). A retrospective chart review was performed to classify DIPT patients into one or more of 26 groups representing non-overlapping diagnostic pain categories based on examiner-provided ICD-10 codes.
2.5. Clustering measures
Four variables were used to develop ROPA in the OPPERA cluster reliability study[4]: trapezius muscle pressure pain threshold and the anxiety, depression, and somatization subscales from the SCL-90R questionnaire. Pressure pain thresholds (PPT) were acquired using a pressure algometer applied to the center of the trapezius muscle. The algometer’s tip was applied with pressure increasing by 30kPa/s until pain sensation was indicated by the participant. The SCL-90R is a widely used instrument with 90 items that assesses psychological distress. Participants respond as to how bothered they are by particular symptoms, which then defines nine subscales including somatization, depression, and anxiety. The four variables in the algorithm, a subset that promotes generalizability and availability of data across studies, were demonstrated to provide a reliable cluster model in internal and external reliability analysis.[4]
Given the reliability of the four-item clustering approach in OPPERA, as confirmed in an external study, and the availability of these features across our three datasets, we sought to apply ROPA to these cohorts. The four items are available in the CPPC study. However, the DIPT study implemented the BSI-18, a truncated version of the SCL-90R containing only items comprising the anxiety, depression and somatization subscales. The BSI-18 scales are composed of a subset of the items of the respective SCL-90R subscales and are intended to capture the same feature information. This questionnaire was developed with an emphasis on clinical relevance, as it is suitable for quick use in the clinic. We evaluated the correlation between the SCL-90R and BSI-18 items in OPPERA and CPPC, where participants were administered the questions included in the BSI-18 via the SCL-90R (Table A1). Given their significant correlation, we proceed to use the BSI-18 for the DIPT study where the SCL-90R was not observed. The cluster algorithm requires each of the four items to be observed, thus participants with all four clustering measures were used in this analysis.
2.6. Clustering methodology
Using the variables used to identify clusters, we first confirmed the number of clusters in the data. In previous work, the gap statistic was used to identify the number of clusters (k) in order to use a data-driven approach to select a high-quality cluster solution.[58] The gap statistic estimates the difference between the within-cluster sum of squares of a cluster solution and the expected within-cluster sum of squares under a null distribution with clusters. An optimal cluster solution yields small within cluster sum of squares. Thus the cluster solution should have a large gap statistic. We consider the gap statistic for k, the number of clusters, set from 1 to 8. We select the smallest value of k within one standard deviation of the value of k that maximizes the gap statistic. [58]
We applied the ROPA, developed in the previous OPPERA study, to the CPPC and DIPT cohorts. Using the OPPERA dataset, a supervised clustering approach was used to cluster the data to ensure that the clusters were associated with the outcome of interest, namely the presence and severity of TMD.[2] In particular, variable selection was used to identify features most strongly associated with TMD. From this cluster solution, an algorithm was developed to assign new observations to clusters. This algorithm used a nearest centroid classifier to predict cluster assignment based on the validated OPPERA cluster solution. To summarize, data from the OPPERA cohort were normalized and clustered as previously described. Nearest centroid models represent each cluster by a centroid, or the mean of each feature for all subjects within the cluster. The mean values and SDs calculated in the OPPERA cohort were used to normalize the data in the CPPC and DIPT cohorts. Subjects were then assigned by the model to the cluster minimizing the distance between the centroid and given subject. We additionally perform a de novo approach, where standard k-means clustering was applied to identify k clusters. In this approach, the same four items were used in clustering: the somatization, depression, and anxiety scores and the average trapezius PPT. We compare the clusters resulting from ROPA and the novel cluster detection using misclassification rate, calculated as the fraction of individuals placed in the same group across cluster solutions, and the Kappa statistic.
We evaluated the cluster assignments by the rapid cluster algorithm in multiple ways. First, the demographic characteristics of individuals in each cluster, including testing for differences in means of each feature across the three clusters, were compared. We considered the distributions of clinical pain ratings, pain measures, and psychosocial measures by cluster. We tested for differences in means across all three clusters, and for pairwise differences between the clusters. We also investigated the distribution of cluster assignments by condition for the CPPC study. All analyses were performed in R version 3.6.3.
3. Results
Data were analyzed from three observational studies. We first summarize each of the studies and their collected data. We then evaluate the reliability and consistency of clustering methods in the three cohorts.
3.1. Cohort Demographics
The sociodemographic characteristics of the participants in the three cohorts are described in Table 2. In the present analysis, the OPPERA cohort contains 1,031 chronic TMD cases and 3,247 TMD-free controls; the CPPC cohort contains 426 cases with any of the five index pain conditions and 240 controls; and the DIPT cohort contains 641 individuals seeking pain treatment. These cohorts were studied under different protocols and, as such, differ with respect to demographic characteristics. In each study, the majority of participants were female. The CPPC cohort was predominantly female (87.8%), with the majority of the DIPT (70.4%) and OPPERA (61.7%) participants female as well. Each of the studies enrolled mostly non-Hispanic white individuals followed by African American individuals; the OPPERA, DIPT, and CPPC studies were 55.2%, 80.8%, and 70.0% non-Hispanic white participants, respectively. The OPPERA study considered individuals 18-44 years old. The DIPT study included all ages, with the majority at least 45 years old and ranging from 14-91. The CPPC cohort included ages 18-64, with the majority aged 25-34.
Table 2:
Demographic characteristics and case status by cohort and cluster assignment by ROPA.
| OPPERA, n (%) | n=4278 | A (n=1426) |
PS (n=2062) |
GS (n=790) |
P† |
|---|---|---|---|---|---|
| Male | 1637 | 839 (0.51) | 558 (0.34) | 240 (0.15) | <0.0001 |
| Female | 2641 | 587 (0.22) | 1504 (0.57) | 550 (0.21) | |
| Non-Hispanic white | 2361 | 780 (0.33) | 1126 (0.48) | 455 (0.19) | 0.08 |
| African American | 1171 | 381 (0.33) | 596 (0.51) | 194 (0.17) | |
| Asian | 340 | 111 (0.33) | 160 (0.47) | 69 (0.2) | |
| Hispanic | 273 | 102 (0.37) | 129 (0.47) | 42 (0.15) | |
| Other | 133 | 52 (0.39) | 51 (0.38) | 30 (0.23) | |
| Age <18 | 0 | 0 (0) | 0 (0) | 0 (0) | <0.0001 |
| Age 18-24 | 2067 | 730 (0.35) | 1027 (0.5) | 310 (0.15) | |
| Age 25-34 | 1228 | 379 (0.31) | 593 (0.48) | 256 (0.21) | |
| Age 35-44 | 983 | 317 (0.32) | 442 (0.45) | 224 (0.23) | |
| Age >44 | 0 | 0 (0) | 0 (0) | 0 (0) | |
| Cases | 1031 | 88 (0.09) | 540 (0.52) | 403 (0.39) | <0.0001 |
| Controls | 3247 | 1338 (0.41) | 1522 (0.47) | 387 (0.12) | |
| DIPT, n (%) | n=641 | A (n=230) | PS (n=259) | GS (n=152) |
P† |
| Male | 190 | 100 (0.53) | 49 (0.26) | 41 (0.22) | <0.0001 |
| Female | 451 | 130 (0.29) | 210 (0.47) | 111 (0.25) | |
| White | 518 | 177 (0.34) | 215 (0.42) | 126 (0.24) | 0.06 |
| African American | 64 | 20 (0.31) | 29 (0.45) | 15 (0.23) | |
| Asian | 20 | 12 (0.6) | 5 (0.25) | 3 (0.15) | |
| Other | 39 | 21 (0.54) | 10 (0.26) | 8 (0.21) | |
| Age <18 | 2 | 1 (0.5) | 0 (0) | 1 (0.5) | 0.37 |
| Age 18-24 | 30 | 6 (0.2) | 13 (0.43) | 11 (0.37) | |
| Age 25-34 | 88 | 36 (0.41) | 33 (0.38) | 19 (0.22) | |
| Age 35-44 | 120 | 49 (0.41) | 47 (0.39) | 24 (0.2) | |
| Age >44 | 401 | 138 (0.34) | 166 (0.41) | 97 (0.24) | |
| CPPC, n (%) | n=666 | A (n=388) | PS (n=127) | GS (n=151) |
P† |
| Male | 81 | 67 (0.83) | 6 (0.07) | 8 (0.1) | <0.0001 |
| Female | 585 | 321 (0.55) | 121 (0.21) | 143 (0.24) | |
| White | 466 | 274 (0.59) | 89 (0.19) | 103 (0.22) | 0.47 |
| African American | 148 | 82 (0.55) | 25 (0.17) | 41 (0.28) | |
| Asian | 18 | 12 (0.67) | 4 (0.22) | 2 (0.11) | |
| Other | 34 | 20 (0.59) | 9 (0.26) | 5 (0.15) | |
| Age <18 | 0 | 0 (0) | 0 (0) | 0 (0) | 0.0002 |
| Age 18-24 | 113 | 74 (0.65) | 27 (0.24) | 12 (0.11) | |
| Age 25-34 | 231 | 132 (0.57) | 52 (0.23) | 47 (0.2) | |
| Age 35-44 | 168 | 84 (0.5) | 31 (0.18) | 53 (0.32) | |
| Age >44 | 154 | 98 (0.64) | 17 (0.11) | 39 (0.25) | |
| Healthy control | 240 | 211 (0.88) | 17 (0.07) | 12 (0.05) | |
| Fibromyalgia | 104 | 5 (0.05) | 32 (0.31) | 67 (0.64) | |
| Episodic migraine | 273 | 96 (0.35) | 74 (0.27) | 103 (0.38) | |
| Irritable bowel syndrome | 228 | 82 (0.36) | 56 (0.25) | 90 (0.39) | |
| Temporomandibular disorder | 176 | 32 (0.18) | 54 (0.31) | 90 (0.51) | |
| Vulvar vestibulitis (n=585) | 146 | 53 (0.36) | 39 (0.27) | 54 (0.37) |
Indicates that the sample is only among female patients, where n=585.
Indicates that the p-value tests the null hypothesis that the percentage in each cluster does not differ across levels of the demographic feature.
3.2. Cluster discovery
In this study, we performed clustering via a rapid cluster algorithm (ROPA) based on four features of interest: trapezius PPT, and the depression, somatization, and anxiety subscales of the SCL-90R in the CPPC cohort, and BSI-18 in the DIPT cohort based on availability. The relationship between the SCL-90R and BSI-18 scales was evaluated using Pearson correlation on pairwise complete observations. Consistent with previous observations of internal consistency and convergent validity between the two instruments,[48] the subscales derived from SCL-90R and BSI-18 are strongly correlated in our datasets. In particular, the correlations range from 0.92 to 0.98 for the three constructs (anxiety, depression, and somatization) in each of the OPPERA and CPPC cohorts where both scales were measured. The correlations are provided in Appendix Table 1. The BSI-18 subscales were calculated for each of the studies, and only observations with complete information on the four cluster features were retained. The cluster features differed in distribution across study, with means and standard deviations given in Appendix Table 2. In particular, the CPPC cohort had a higher average PPT trapezius score. The psychosocial measures were similar across the cohorts.
We evaluated the number of clusters in the data, k, using the gap statistic. We evaluated the cluster solution at each value of k for each study as reported in Appendix Figure 1. We observe that the gap statistic is maximized for k=3 clusters for the CPPC and OPPERA data. The gap statistic for k=3 in the DIPT setting is also the smallest value of k within one standard deviation of the value of k that maximizes the gap statistic. Thus, the following de novo clustering steps proceeded with k-means clustering setting k=3 for all cohorts.
3.3. Cluster Reliability
We evaluated the reliability of ROPA by comparing the cluster labels to de novo cluster assignments. In each comparison, the rapid algorithm and de novo clustering assigned over 87% of subjects to the same cluster (Table 3). Using centroids derived from OPPERA, 89.6% of CPPC subjects and 87.5% of DIPT patients were assigned to the same clusters as the de novo solution.
Table 3:
Cluster reliability.
| DIPT cohort cluster assignments (% Agreement: 89.6%, Kappa: 0.84) |
CPPC cohort cluster assignments (% Agreement: 87.5% , Kappa: 0.78) |
||||||
|---|---|---|---|---|---|---|---|
| Predicted by OPPERA algorithm | Predicted by OPPERA algorithm | ||||||
| De novo | A | PS | GS | De novo | A | PS | GS |
| A | 227 | 8 | 14 | A | 368 | 0 | 17 |
| PS | 3 | 251 | 42 | PS | 20 | 127 | 46 |
| GS | 0 | 0 | 96 | GS | 0 | 0 | 88 |
Across-cohort validation of cluster reliability was assessed by comparing the cluster assigned using “de novo” k-means clustering with that predicted using the OPPERA algorithm based on the “nearest centroid” method. The misclassification error rate is the proportion of misclassified assignments out of the total number of observations.
3.4. Clinical Presentation of Clusters
As previously observed in OPPERA,[4] the three clusters identified in the CPPC and DIPT cohorts using the four measures (anxiety, depression, somatization, and trapezius PPT) can be characterized as “Adaptive,” “Pain Sensitive,” and “Global Symptoms”. As shown in Fig. 1, the Adaptive cluster rated low in both pain sensitivity and affective distress. The Pain Sensitive cluster was resilient to emotional distress, but had a low PPT, indicating high muscle pain sensitivity. The Global Symptoms cluster exhibited high levels of both pain sensitivity and high scores on the measures of anxiety, depression, and somatization.
Figure 1.
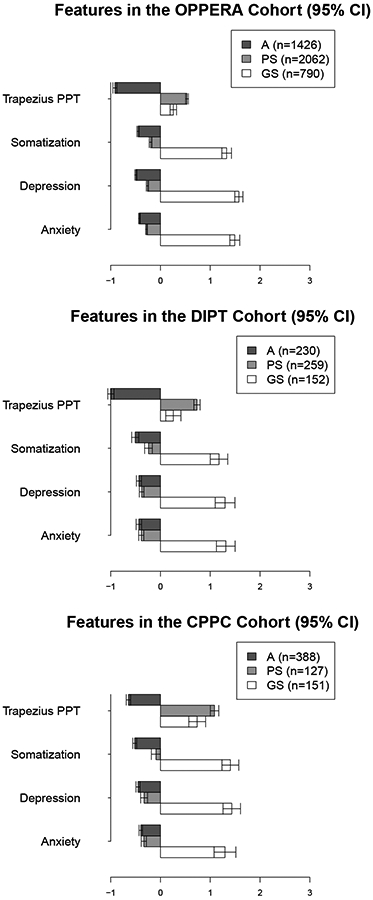
Mean values (95% CI) for the cluster features in each of the three cohorts. Cluster labels were obtained by assigning individuals using ROPA; variables in the figures have been standardized.
Note: The features presented are for clusters based on ROPA.
Demographic characteristics of each cohort are described by cluster in Table 2. In each cohort, significant differences between clusters were observed for sex, and in the OPPERA and CPPC cohorts, by age. As only a small number of DIPT participants were not being treated for pain (having sleep apnea as the primary complaint), no analysis based on case status was made. In all cohorts, sex was significantly associated with cluster membership, with increased frequency of males in the A cluster and more females in the PS cluster. In both research cohorts (OPPERA and CPPC) and the clinical cohort (DIPT), females were more prevalent in the GS cluster than males. Race was not significantly associated with cluster membership in any cohort. Age was significantly associated in each cohort, with greater age often associated with a higher frequency in the GS group.
Clinical pain was assessed by self-report using a 0-100 scale in all three cohorts. As previously reported, we observed between-cluster differences in clinical pain ratings in OPPERA (data not shown; see [4] for analysis). In CPPC, GS subjects reported higher levels of pain and a larger percentage of the day in pain (Table 4). For the PS cluster subjects, pain ratings were intermediate in value, and A cluster subjects reported the least pain and time spent in pain. In DIPT, clinical pain ratings and time spent in pain were greater in GS (Table 4). The GS cluster also reported higher pain intensity and unpleasantness obtained using the Gracely Box Scale[29] in both CPPC and DIPT cohorts; PS and A clusters did not differ in these ratings.
Table 4:
Clinical pain ratings within clusters predicted by ROPA.
| Feature | A (n=230) | PS (n=259) | GS (n=152) | P-value† | A v. PS‡ | PS v. GS‡ | A v. GS‡ | |
|---|---|---|---|---|---|---|---|---|
| DIPT (n=641) | Rating of average pain (0-100) | 55.84 (1.59) | 55.22 (1.35) | 66.9 (1.48) | 7.97E−07 | 7.85E−01 | 5.86E−08 | 2.88E−06 |
| % of waking day with pain | 64.17 (2.16) | 70.33 (1.79) | 83.51 (1.65) | 9.98E−09 | 4.30E−02 | 4.69E−07 | 1.52E−10 | |
| Rating of pain unpleasantness (0-20) | 12.08 (0.32) | 11.72 (0.26) | 14.16 (0.33) | 8.90E−07 | 4.18E−01 | 7.70E−08 | 3.21E−05 | |
| Rating of pain intensity (0-20) | 12.69 (0.32) | 12.73 (0.26) | 14.25 (0.32) | 1.64E−03 | 9.23E−01 | 4.94E−04 | 1.38E−03 | |
| Pain density (Average pain % of waking day in pain) | 3985.43 (185.66) | 4144.21 (151.82) | 5747.41 (183.75) | 2.22E−10 | 5.41E−01 | 5.86E−10 | 8.56E−10 | |
| Feature | A (n=388) | PS (n=127) | GS (n=151) | P-value† | A v. PS‡ | PS v. GS‡ | A v. GS‡ | |
| CPPC (n=666) | Rating of average facial pain (0-100) | 24.72 (1.21) | 34.03 (1.95) | 47.8 (2.17) | 3.71E−12 | 2.77E−03 | 7.57E−05 | 7.49E−12 |
| % of waking day with facial pain | 20.9 (2.8) | 33.12 (3.1) | 53.61 (2.77) | 5.17E−08 | 4.29E−02 | 4.17E−05 | 2.48E−08 | |
| Rating of facial pain unpleasantness (0-20) | 4.33 (0.24) | 5.08 (0.3) | 8.33 (0.39) | 4.10E−10 | 2.28E−01 | 2.13E−07 | 7.05E−09 | |
| Rating of facial pain intensity (0-20) | 4.7 (0.26) | 5.68 (0.38) | 9.66 (0.44) | 5.56E−12 | 1.66E−01 | 9.25E−08 | 7.73E−11 | |
| Facial pain density | 8.82 (0.66) | 14.28 (1.7) | 31.57 (2.18) | 4.63E−14 | 3.29E−02 | 7.61E−07 | 4.50E−13 | |
| Rating of average bodily pain (0-100) | 30.57 (1.15) | 50.72 (1.94) | 64.53 (1.87) | 2.84E−12 | 1.11E−04 | 4.49E−04 | 2.22E−10 | |
| % of waking day with bodily pain | 37.5 (1.78) | 72.7 (8.39) | 75.62 (2.27) | 1.36E−03 | 1.86E−02 | 8.31E−01 | 2.88E−07 | |
| Rating of bodily pain unpleasantness (0-20) | 6.82 (0.24) | 8.31 (0.39) | 11.29 (0.31) | 3.09E−07 | 1.82E−01 | 2.02E−04 | 4.29E−05 | |
| Rating of bodily pain intensity (0-20) | 7.39 (0.28) | 9.76 (0.48) | 13 (0.32) | 4.32E−08 | 7.81E−02 | 5.87E−04 | 1.46E−05 | |
| Bodily pain density | 18.82 (0.91) | 42.44 (4.67) | 53.81 (2.08) | 3.16E−05 | 7.79E−03 | 1.76E−01 | 3.73E−11 |
Indicates that the p-value tests the null hypothesis that the mean value of the pain rating does not differ between the three clusters.
Indicates that the p-value tests the null hypothesis that the mean value of the pain rating does not differ between the two compared clusters.
Pain sensitivity and psychosocial measures are described by cluster in Table 5, with additional measures obtained from the CPPC cohort presented in Table A5. All features in Table 5 differ across clusters. In particular, we observe that the GS cluster has higher psychological measures. The PS and A clusters tended to be more similar across factors when compared to the GS cluster. In the CPPC cohort, the GS group differed significantly from the A group for all measures as shown in Table 5, and as shown also differed from the PS cluster on all measures except heat pain tolerance and threshold. The A cluster also differed from PS on all measures except for the POMS overall negative subscale. In the DIPT cohort, the GS cluster exhibited significantly higher clinical pain ratings and psychosocial distress than the A and PS clusters (Table 5). Compared to the A cluster, the PS cluster had higher PEG scores for average clinical pain and interference with activity, but not for interference with enjoyment. There were also no differences between A and PS on measures related to perceived stress, negative and positive affect, catastrophizing and effectiveness of pain decrease, or on the sleep-related measures (Table 5). These findings are consistent with those previously reported in the OPPERA cohort[4] and a more extensive listing of variables for the CPPC cohort is provided in Table A5.
Table 5:
Average values of representative pain and psychosocial measures within clusters predicted by ROPA.
| Feature | A (n=230) | PS (n=259) | GS (n=152) | P-value† | A v. PS‡ | PS v. GS‡ | A v. GS‡ | |
|---|---|---|---|---|---|---|---|---|
| DIPT | PEG Average Pain (0-10) | 5.11 (0.18) | 5.49 (0.14) | 6.64 (0.15) | 9.88E−09 | 1.00E−01 | 1.16E−07 | 5.23E−10 |
| PEG Interference Activity (0-10) | 4.75 (0.22) | 5.41 (0.19) | 6.95 (0.21) | 4.29E−11 | 1.96E−02 | 1.10E−07 | 2.53E−12 | |
| PEG Interference Enjoyment (0-10) | 5.51 (0.22) | 5.88 (0.17) | 7.51 (0.18) | 9.43E−11 | 1.78E−01 | 2.65E−10 | 7.91E−12 | |
| PainDETECT score (0-38) | 8.05 (0.46) | 11.38 (0.51) | 17.31 (0.69) | 0.00E+00 | 1.69E−06 | 3.40E−11 | 4.43E−24 | |
| PSS score (0-40) | 17.51 (0.24) | 17.44 (0.22) | 21.28 (0.3) | 0.00E+00 | 8.31E−01 | 2.09E−21 | 9.91E−20 | |
| Rating of catastrophic thinking (0-100) | 36.78 (1.91) | 37.78 (1.72) | 53.19 (2.14) | 9.87E−09 | 6.96E−01 | 4.27E−08 | 2.29E−08 | |
| Rating of effectiveness to decrease pain (0-100) | 49.78 (2.07) | 48.26 (1.69) | 40.26 (2.26) | 4.58E−03 | 5.69E−01 | 4.91E−03 | 2.05E−03 | |
| SPANE Positive score (6-30) | 23.43 (0.26) | 22.89 (0.26) | 17.45 (0.33) | 0.00E+00 | 1.42E−01 | 7.27E−32 | 2.05E−36 | |
| SPANE Negative score (6-30) | 12.48 (0.23) | 12.66 (0.24) | 18.25 (0.34) | 0.00E+00 | 5.97E−01 | 1.33E−32 | 2.17E−34 | |
| SPANE Affect balance (−24-24) | 10.94 (0.45) | 10.23 (0.44) | −0.8 (0.6) | 0.00E+00 | 2.58E−01 | 2.03E−37 | 9.13E−41 | |
| ISI sum score (0-28) | 7.78 (0.37) | 8.47 (0.38) | 14.33 (0.6) | 0.00E+00 | 1.89E−01 | 8.71E−15 | 1.01E−17 | |
| ESE sum score (0-24) | 5.83 (0.3) | 5.64 (0.28) | 8.12 (0.45) | 6.33E−07 | 6.36E−01 | 4.85E−06 | 3.15E−05 | |
| Feature | A (n=1426) | PS (n=2062) | GS (n=790) | P-value† | A v. PS‡ | PS v. GS‡ | A v. GS‡ | |
| OPPERA | PSS score (0-40) | 11.84 (0.14) | 15.23 (0.12) | 22.89 (0.19) | 0.00E+00 | 4.63E−68 | 9.45E−189 | 4.37E−304 |
| PSQI global score (0-21) | 4.08 (0.07) | 5.04 (0.06) | 8.69 (0.14) | 0.00E+00 | 2.54E−25 | 2.60E−104 | 4.81E−148 | |
| PILL score (0-270) | 28.27 (0.47) | 39.11 (0.48) | 70.81 (1.1) | 0.00E+00 | 3.28E−57 | 2.39E−119 | 8.30E−184 | |
| KOHN global score (0-120) | 67.29 (0.31) | 73.98 (0.24) | 77.73 (0.42) | 0.00E+00 | 2.26E−63 | 1.66E−14 | 2.24E−79 | |
| Feature | A (n=388) | PS (n=127) | GS (n=151) | P-value† | A v. PS‡ | PS v. GS‡ | A v. GS‡ | |
| CPPC | PSS score (0-40) | 12.91 (0.3) | 14.43 (0.48) | 22.61 (0.5) | 0.00E+00 | 8.20E−03 | 9.44E−26 | 6.36E−42 |
| Heat pain threshold | 43.96 (0.16) | 41.77 (0.3) | 42.05 (0.3) | 9.71E−14 | 9.02E−10 | 5.09E−01 | 5.99E−08 | |
| Heat pain tolerance | 47.02 (0.11) | 45.07 (0.26) | 45.32 (0.26) | 0.00E+00 | 5.50E−11 | 4.83E−01 | 1.02E−08 | |
| No. neck sites painful to palpation (0-14) | 0.8 (0.11) | 4.39 (0.4) | 6.6 (0.42) | 0.00E+00 | 6.02E−15 | 1.58E−04 | 2.38E−28 | |
| No. body sites painful to palpation (0-14) | 1.73 (0.16) | 6.85 (0.4) | 8.33 (0.37) | 0.00E+00 | 7.44E−24 | 7.49E−03 | 1.24E−38 | |
| KOHN global score (0-120) | 74.81 (0.54) | 79.02 (1.03) | 83.39 (1.01) | 8.37E−14 | 4.83E−04 | 3.21E−03 | 3.52E−12 | |
| PCS global score (0-52) | 8.68 (0.42) | 11.95 (0.78) | 22.99 (0.98) | 0.00E+00 | 3.24E−04 | 4.00E−16 | 6.65E−29 | |
| PILL score (0-270) | 36.1 (1.09) | 56.26 (2.15) | 91.58 (2.81) | 0.00E+00 | 3.10E−14 | 1.38E−19 | 2.61E−43 | |
| POMS overall negative subscale | 52.65 (0.82) | 54.94 (1.25) | 79.83 (1.47) | 0.00E+00 | 1.32E−01 | 8.32E−29 | 2.21E−38 | |
| PSQI global score (0-21) | 4.85 (0.16) | 7.27 (0.36) | 10.67 (0.31) | 0.00E+00 | 1.34E−08 | 8.14E−11 | 2.79E−36 |
Note: These variables were not used in developing the cluster solutions. Additional measures obtained in the CPPC cohort are presented in Appendix Table 5. See [4] for additional measures obtained in the OPPERA cohort.
Indicates that the p-value tests the null hypothesis that the mean value of the measure does not differ between the three clusters.
Indicates that the p-value tests the null hypothesis that the mean value of the measure does not differ between the two compared clusters.
The CPPC cohort assessed individuals with multiple index conditions. Figure 2 demonstrates the distribution of cluster assignments for each of the index conditions. Note that individuals were evaluated for each condition and could record multiple index conditions. Because VVS was only evaluated in women, the cluster distribution is only evaluated among women. The majority of healthy controls were in the A cluster. As the count of conditions increases, we observe an increase in the proportion of individuals in the GS cluster and decrease in the proportion of individuals in the A cluster.
Figure 2.
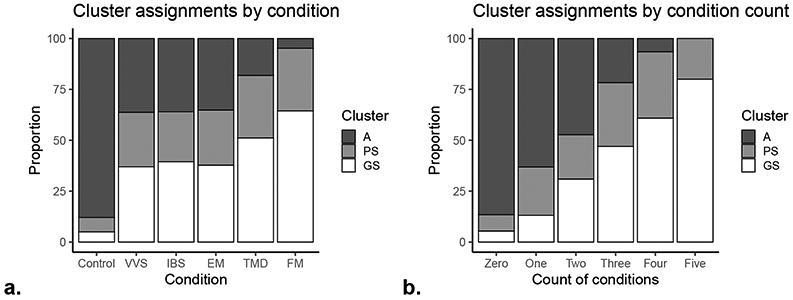
Cluster distribution for index conditions and count of index conditions in the CPPC study. Individuals in CPPC were evaluated for each condition; only women were evaluated for VVS. The clusters are defined by ROPA.
Note: The features presented are for clusters based on ROPA.
4. Discussion
Previous subgroup identification of healthy patients and those experiencing pain symptoms identified three clusters with distinct pain and biopsychosocial profiles.[4] This initial exercise in the OPPERA study[4] relied on a comprehensive battery of questionnaires and experimental quantitative sensory testing (QST) consisting of over 200 phenotypic features. The 25 features most significantly associated with TMD, including measures of psychological distress and pain sensitivity, were used to calculate the clusters. Because comprehensive phenotyping and clustering is impractical for clinical use, we sought to evaluate the performance of a cluster prediction algorithm in a heterogeneous sample using a truncated assessment protocol that can be used in the clinical setting that is accurate and produces little disruption in clinical workflow.
This study demonstrates the utility and validity of a novel clustering approach applicable to both healthy and clinical pain populations that share similar phenotypic profiles regardless of the anatomical source of pain. This work extends the three-subtype solution previously identified to different clinical settings and provides evidence of its reliability as a stand-alone algorithm for assigning new individuals to phenotype-driven clusters. We used data from three different cohorts that varied with respect to recruitment method, inclusion and exclusion criteria, sociodemographic characteristics, and more. In each setting, we further validated ROPA by applying cluster solutions across studies and replicating de novo cluster findings.
We further demonstrate that this cluster solution is not exclusively applicable to painful TMD, but more broadly relevant to other specific chronic pain conditions as well as a mixed group of patients seeking treatment for chronic pain. As with other efforts to identify subgroups of chronic pain patients, including osteoarthritis[11; 12; 14; 45], fibromyalgia[28; 61; 65], and neuropathic pain[6; 26], initial development of this algorithm focused on a defined pain condition, limiting its generalizability. In contrast with many of these previous studies, the phenotypes the algorithm uses for assignment to clusters may be measured in almost anyone, regardless of pain status, as our overarching goal has been to identify individuals carrying elevated risk of developing a pain disorder, whether in the presence or absence of instigating etiology. In addition to participants from the OPPERA study, which focused on painful TMD, the present analysis included the CPPC cohort, which examined individuals with five chronic overlapping pain conditions (COPCs) that vary with respect to anatomical classification, including fibromyalgia (FM), episodic migraine (EM), vulvar vestibulitis (VVS), irritable bowel syndrome (IBS), and painful temporomandibular disorders (TMD). Also, the DIPT study included patients seeking treatment in a specialized pain clinic presenting with painful symptoms, regardless of anatomical location. Differences in the populations from which these cohorts draw from may contribute to some observed differences in the gap statistic and de novo cluster solutions. Nonetheless, phenotypic cluster profile may be a more meaningful functional classification strategy than current anatomically based diagnostic criteria, illuminating underlying mechanisms related to pain processing and psychological distress with the goal of informing treatment approaches.[60] The fact that the distinguishing features of each cluster are consistent across cohorts supports the underlying concepts of pain amplification and psychosocial distress as important contributors to chronic pain in the conditions examined in this study.[17] Molecular biomarkers characteristic of pathological changes in these domains would therefore be associated more strongly with clusters than with anatomical diagnosis. Likewise, treatment modalities matched to cluster assignment are much more likely to address the specific pathophysiology of the patient rather than a treatment based primarily on a patient’s anatomical diagnosis (e.g., lower back pain, TMD, headache, etc.).
Reliance on extensive questionnaires limits clinical utility; it is impractical to implement detailed data collection in a typical clinical setting, as extensive questionnaires have a prohibitively high patient and clinical burden. Other classification schemes intended to distinguish mechanistically distinct subpopulations within a defined disorder or a symptom-free population typically rely on extensive QST procedures[5; 26; 49; 59], psychological assessment[2; 50], or most frequently a combination[24; 37; 61; 65]. The rapid OPPERA clustering procedure validated a reduced set of measures, feasibly and efficiently administered in a typical clinical practice, which can be used to assign individual patients to a specific diagnostic and prognostic cluster. Of note, our theoretical approach to clustering is based on the concept of shared disease mechanisms (i.e., a biopsychosocial model) and is not based on the anatomical site where pain is reported.[17] Previously studied cohorts were limited to TMD cases and healthy controls; other pain conditions were not incorporated and therefore the generalization of the clustering process to other pain conditions has not yet been evaluated. We used additional cohorts, with a broader spectrum of pain conditions, to show that this cluster solution is not specific to TMD patients, but instead is more broadly relevant to patients with chronic overlapping pain conditions (COPCs).[40] We propose that the clustering solution will enable treatment decisions based on etiologically relevant cluster membership, complementing treatment specific to the anatomically based diagnosis.
This clustering method has the potential to significantly impact clinical practice. Previous work designed to identify patient subtypes required extensive questionnaires and physical examination. The present approach provides considerable advantages over existing examination-based risk stratification schemes, as obtaining the four input variables is viable in routine clinical practice and applicable to non-pain populations such as those undergoing pre-operative optimization.[18; 66] Further validation for applicability for risk stratification purposes would be important. We have validated a brief protocol for classification that may be performed in essentially any clinical setting, and are now using the procedure routinely in an EPIC software implementation to acquire data for both clinical patient management and research purposes with very little impact on clinic work flow.
Furthermore, we have confirmed that the information provided by ROPA has clinical relevance. In particular, the GS cluster, which shows characteristics of CNS dysregulation of somatosensory processing and affective regulation, is associated with increased sensitivity to painful stimuli, decreased heart rate power and variability, severity of stress, disturbed sleep, negative affect, and catastrophizing, factors that are known to contribute to pain chronicity[9; 22; 42] and which can be targeted therapeutically in a holistic approach to pain management. The PS and GS clusters are also associated with increased comorbidity of COPCs, which suggests that central and/or peripheral sensitization processes modify pain processing beyond the site of the primary complaint resulting in multiple pain complaints across the body. Assignment to PS or GS clusters should prompt a comprehensive screen for comorbid pain conditions. GS cluster patients should be additionally evaluated for referral for psychological services.
Although this work extends the original cluster development to a more diverse, real-world clinical population, it still requires further validation beyond DIPT’s population of largely white and female patients who are able to afford treatment. This study is also limited by the pain conditions available for study in the included cohorts. Future research should examine the performance of the clustering algorithm in patients with pain due to neuropathic or rheumatic mechanisms, cancer pain, and acute post-operative pain in order to determine if these other pain disorders, not part of the traditional COPC rubric, exhibit the same characteristics as markers for cluster assignment. The DIPT patients were not subjected to comprehensive diagnostic screening to identify all indications of pain, so currently we were unable to address comorbidity in this clinical population. Likewise, this cohort lacks complete longitudinal data on treatments and outcomes, which will be necessary to evaluate trajectories of individuals undergoing specific therapeutic modalities. Ongoing studies will examine the stability of the clusters over time and whether a change in cluster severity (e.g., GS to PS or A, PS to A) corresponds with clinically relevant improvement in symptoms and the patient’s perception of change in their clinical condition. If so, cluster assignment and changes in cluster assignment may represent a new way of consolidating multiple univariate features into a global index that can be used to assess “value-based” pain care. Future algorithm refinement should consider additional approaches such as mixed models in order to leverage large datasets where missingness in cluster features is observed. Future research is also needed to evaluate the success of treatments according to cluster assignment in order to translate these findings into a clinical decision-making tool for a personalized medicine approach in the clinical setting.
In summary, in this study we present evidence of the validity and reliability of a unique patient clustering method (ROPA) that can be implemented in various clinical settings to rapidly identify patients with greater pain sensitivity, psychosocial distress, and likelihood of high impact pain. We have phenotypically characterized the clusters in three independent cohorts. We have shown that the clustering procedure is generalizable to COPCs regardless of anatomical diagnosis, and that a greater number of painful conditions is associated with belonging to a more severe cluster. This patient classification method may assist clinicians in determining treatment strategies that target the biopsychosocial factors contributing to pain chronicity.
Supplementary Material
Acknowledgments
The OPPERA study was supported by National Institutes of Health/National Institute for Dental and Craniofacial Research grant (NIDCR) U01DE017018. The OPPERA program also acknowledges resources specifically provided for this project by the participating institutions: Battelle Memorial Institute; University at Buffalo; University of Florida; University of Maryland; and University of North Carolina at Chapel Hill. The CPPC study was supported by NIH/National Institute of Neurological Disorders and Stroke (NINDS) grant NS045685 to the University of North Carolina at Chapel Hill. Funding and support for the DIPT study was generously provided by the Department of Anesthesiology of Duke University Medical Center.
Footnotes
Conflict of Interest Statement:
Thomas Buchheit is a consultant for Mainstay Medical, Summus, and Best Doctors. He declares no conflicts of interest. William Maixner is a consultant to Orthogen Inc and a member of the Supervisory Board. He declares no conflicts of interest. Other authors report no conflicts of interest related to this work.
References
- [1].Anderson KO, Dowds BN, Pelletz RE, Edwards WT, Peeters-Asdourian C. Development and initial validation of a scale to measure self-efficacy beliefs in patients with chronic pain. Pain 1995;63(1):77–84. [DOI] [PubMed] [Google Scholar]
- [2].Backryd E, Persson EB, Larsson AI, Fischer MR, Gerdle B. Chronic pain patients can be classified into four groups: Clustering-based discriminant analysis of psychometric data from 4665 patients referred to a multidisciplinary pain centre (a SQRP study). PLoS ONE 2018;13(2):e0192623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bair E, Brownstein NC, Ohrbach R, Greenspan JD, Dubner R, Fillingim RB, Maixner W, Smith SB, Diatchenko L, Gonzalez Y. Study protocol, sample characteristics, and loss to follow-up: the OPPERA prospective cohort study. The Journal of Pain 2013;14(12):T2–T19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Bair E, Gaynor S, Slade GD, Ohrbach R, Fillingim RB, Greenspan JD, Dubner R, Smith SB, Diatchenko L, Maixner W. Identification of clusters of individuals relevant to temporomandibular disorders and other chronic pain conditions: the OPPERA study. Pain 2016;157(6):1266–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Baron R, Maier C, Attal N, Binder A, Bouhassira D, Cruccu G, Finnerup NB, Haanpaa M, Hansson P, Hullemann P, Jensen TS, Freynhagen R, Kennedy JD, Magerl W, Mainka T, Reimer M, Rice AS, Segerdahl M, Serra J, Sindrup S, Sommer C, Tolle T, Vollert J, Treede RD. Peripheral neuropathic pain: a mechanism-related organizing principle based on sensory profiles. Pain 2017;158(2):261–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bouhassira D, Wilhelm S, Schacht A, Perrot S, Kosek E, Cruccu G, Freynhagen R, Tesfaye S, Lledó A, Choy E, Marchettini P, Micó JA, Spaeth M, Skljarevski V, Tölle T. Neuropathic pain phenotyping as a predictor of treatment response in painful diabetic neuropathy: data from the randomized, double-blind, COMBO-DN study. Pain 2014;155(10):2171–2179. [DOI] [PubMed] [Google Scholar]
- [7].Brox JI, Reikeras O, Nygaard O, Sorensen R, Indahl A, Holm I, Keller A, Ingebrigtsen T, Grundnes O, Lange JE, Friis A. Lumbar instrumented fusion compared with cognitive intervention and exercises in patients with chronic back pain after previous surgery for disc herniation: a prospective randomized controlled study. Pain 2006;122(1-2):145–155. [DOI] [PubMed] [Google Scholar]
- [8].Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 1989;28(2):193–213. [DOI] [PubMed] [Google Scholar]
- [9].Choy EH. The role of sleep in pain and fibromyalgia. Nat Rev Rheumatol 2015;11(9):513–520. [DOI] [PubMed] [Google Scholar]
- [10].Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav 1983;24:385–396. [PubMed] [Google Scholar]
- [11].Cruz-Almeida Y, Cardoso J, Riley JL 3rd, Goodin B, King CD, Petrov M, Bartley EJ, Sibille KT, Glover TL, Herbert MS, Bulls HW, Addison A, Staud R, Redden D, Bradley LA, Fillingim RB. Physical performance and movement-evoked pain profiles in community-dwelling individuals at risk for knee osteoarthritis. Exp Gerontol 2017;98:186–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Cruz-Almeida Y, King CD, Goodin BR, Sibille KT, Glover TL, Riley JL, Sotolongo A, Herbert MS, Schmidt J, Fessler BJ, Redden DT, Staud R, Bradley LA, Fillingim RB. Psychological profiles and pain characteristics of older adults with knee osteoarthritis. Arthritis Care Res (Hoboken) 2013;65(11):1786–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Dahlhamer J, Lucas J, Zelaya C, Nahin R, Mackey S, DeBar L, Kerns R, Von Korff M, Porter L, Helmick C. Prevalence of chronic pain and high-impact chronic pain among adults - United States, 2016. MMWR Morbidity and mortality weekly report 2018;67(36):1001–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].de Luca K, Parkinson L, Downie A, Blyth F, Byles J. Three subgroups of pain profiles identified in 227 women with arthritis: a latent class analysis. Clin Rheumatol 2016. [DOI] [PubMed] [Google Scholar]
- [15].Derogatis LR. The Brief Symptom Inventory–18 (BSI-18): Administration, Scoring and Procedures Manual. Minneapolis, MN: National Computer Systems, 2000. [Google Scholar]
- [16].Derogatis LR, Melisaratos N. The Brief Symptom Inventory: An introductory report. Psychol Med 1983;13:595–605. [PubMed] [Google Scholar]
- [17].Diatchenko L, Nackley A, Slade G, Fillingim R, Maixner W. Idiopathic pain disorders--pathways of vulnerability. Pain 2006;123(3):226–230. [DOI] [PubMed] [Google Scholar]
- [18].Diebo BG, Tishelman JC, Horn S, Poorman GW, Jalai C, Segreto FA, Bortz CA, Gerling MC, Lafage V, White AP, Mok JM, Cha TD, Eastlack RK, Radcliff KE, Paulino CB, Passias PG. The impact of mental health on patient-reported outcomes in cervical radiculopathy or myelopathy surgery. Journal of clinical neuroscience : official journal of the Neurosurgical Society of Australasia 2018;54:102–108. [DOI] [PubMed] [Google Scholar]
- [19].Diener E, Wirtz D, Tov W, Kim-Prieto C, Choi D-w, Oishi S, Biswas-Diener R. New well-being measures: Short scales to assess flourishing and positive and negative feelings. Social Indicators Research 2010;97(2):143–156. [Google Scholar]
- [20].Dworkin RH, Bruehl S, Fillingim RB, Loeser JD, Terman GW, Turk DC. Multidimensional Diagnostic Criteria for Chronic Pain: Introduction to the ACTTION-American Pain Society Pain Taxonomy (AAPT). J Pain 2016;17(9 Suppl):T1–9. [DOI] [PubMed] [Google Scholar]
- [21].Dworkin S, LeResche L. Research diagnostic criteria for temporomandibular disorders: review, criteria, examinations and specifications, critique. J Craniomandib Disord 1992;6(4):301–355. [PubMed] [Google Scholar]
- [22].Edwards RR, Dworkin RH, Sullivan MD, Turk DC, Wasan AD. The role of psychosocial processes in the development and maintenance of chronic pain. J Pain 2016;17(9 Suppl):T70–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Eysenck SBG, Eysenck HJ, Barrett P. A revised version of the psychoticism scale. Pers Individ Dif 1985;6:21–29. [Google Scholar]
- [24].Farmer AD, Coen SJ, Kano M, Paine PA, Shwahdi M, Jafari J, Kishor J, Worthen SF, Rossiter HE, Kumari V, Williams SCR, Brammer M, Giampietro VP, Droney J, Riley J, Furlong PL, Knowles CH, Lightman SL, Azizl Q. Psychophysiological responses to pain identify reproducible human clusters. Pain 2013;In press(0). [DOI] [PubMed] [Google Scholar]
- [25].Fillingim RB, Ohrbach R, Greenspan JD, Knott C, Dubner R, Bair E, Baraian C, Slade GD, Maixner W. Potential psychosocial risk factors for chronic TMD: descriptive data and empirically identified domains from the OPPERA Case-Control Study. J Pain 2011;12(11, Supplement):T46–T60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Freeman R, Baron R, Bouhassira D, Cabrera J, Emir B. Sensory profiles of patients with neuropathic pain based on the neuropathic pain symptoms and signs. Pain 2014;155(2):367–376. [DOI] [PubMed] [Google Scholar]
- [27].Freynhagen R, Baron R, Gockel U, Tolle TR. painDETECT: a new screening questionnaire to identify neuropathic components in patients with back pain. Current medical research and opinion 2006;22(10):1911–1920. [DOI] [PubMed] [Google Scholar]
- [28].Giesecke T, Williams DA, Harris RE, Cupps TR, Tian X, Tian TX, Gracely RH, Clauw DJ. Subgrouping of fibromyalgia patients on the basis of pressure-pain thresholds and psychological factors. Arthritis Rheum 2003;48(10):2916–2922. [DOI] [PubMed] [Google Scholar]
- [29].Gracely RH, McGrath F, Dubner R. Ratio scales of sensory and affective verbal pain descriptors. Pain 1978;5(1):5–18. [DOI] [PubMed] [Google Scholar]
- [30].Greenspan JD, Slade GD, Bair E, Dubner R, Fillingim RB, Ohrbach R, Knott C, Mulkey F, Rothwell R, Maixner W. Pain sensitivity risk factors for chronic TMD: descriptive data and empirically identified domains from the OPPERA Case Control Study. J Pain 2011;12(11, Supplement):T61–T74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Hardt J, Gerbershagen HU, Franke P. The symptom check-list, SCL-90-R: its use and characteristics in chronic pain patients. Eur J Pain 2000;4(2):137–148. [DOI] [PubMed] [Google Scholar]
- [32].Jarvik JG, Hollingworth W, Martin B, Emerson SS, Gray DT, Overman S, Robinson D, Staiger T, Wessbecher F, Sullivan SD, Kreuter W, Deyo RA. Rapid magnetic resonance imaging vs radiographs for patients with low back pain: a randomized controlled trial. Jama 2003;289(21):2810–2818. [DOI] [PubMed] [Google Scholar]
- [33].Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 1991;14(6):540–545. [DOI] [PubMed] [Google Scholar]
- [34].Kerns RD, Turk DC, Rudy TE. The West Haven-Yale Multidimensional Pain Inventory (WHYMPI). Pain 1985;23(4):345–356. [DOI] [PubMed] [Google Scholar]
- [35].Kohn PM. Sensation-seeking, augment-reducing, and strength of the nervous system. In: Spence J, Izard D, editors. Motivation, Emotion, and Personality. Amsterdam, NL: Elsevier, 1985. pp. 167–173. [Google Scholar]
- [36].Krebs EE, Lorenz KA, Bair MJ, Damush TM, Wu J, Sutherland JM, Asch SM, Kroenke K. Development and initial validation of the PEG, a three-item scale assessing pain intensity and interference. J Gen Intern Med 2009;24(6):733–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Larsson B, Gerdle B, Bernfort L, Levin LA, Dragioti E. Distinctive subgroups derived by cluster analysis based on pain and psychological symptoms in Swedish older adults with chronic pain - a population study (PainS65+). BMC geriatrics 2017;17(1):200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology 2006;130(5):1480–1491. [DOI] [PubMed] [Google Scholar]
- [39].Maixner W, Diatchenko L, Dubner R, Fillingim RB, Greenspan JD, Knott C, Ohrbach R, Weir B, Slade GD. Orofacial Pain Prospective Evaluation and Risk Assessment Study: The OPPERA Study. J Pain 2011;12(11, Supplement):T4–T11.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Maixner W, Fillingim RB, Williams DA, Smith SB, Slade GD. Overlapping chronic pain conditions: Implications for diagnosis and classification. J Pain 2016;17(9 Suppl):T93–t107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Maixner W, Greenspan JD, Dubner R, Bair E, Mulkey F, Miller V, Knott C, Slade GD, Ohrbach R, Diatchenko L, Fillingim RB. Potential autonomic risk factors for chronic TMD: descriptive data and empirically identified domains from the OPPERA Case-Control Study. J Pain 2011;12(11, Supplement):T75–T91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Meints SM, Edwards RR. Evaluating psychosocial contributions to chronic pain outcomes. Prog Neuropsychopharmacol Biol Psychiatry 2018;87(Pt B):168–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Melzack R The short-form McGill pain questionnaire. Pain 1987;30(2):191–197. [DOI] [PubMed] [Google Scholar]
- [44].Morin CM, Belleville G, Belanger L, Ivers H. The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep 2011;34(5):601–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Murphy SL, Lyden AK, Phillips K, Clauw DJ, Williams DA. Subgroups of older adults with osteoarthritis based upon differing comorbid symptom presentations and potential underlying pain mechanisms. Arthritis Res Ther 2011;13(4):R135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Ohrbach R, Fillingim RB, Mulkey F, Gonzalez Y, Gordon S, Gremillion H, Lim P-F, Ribeiro-Dasilva M, Greenspan JD, Knott C, Maixner W, Slade G. Clinical findings and pain symptoms as potential risk factors for chronic TMD: descriptive data and empirically identified domains from the OPPERA Case-Control Study. J Pain 2011;12(11, Supplement):T27–T45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Pennebaker JW. The psychology of physical symptoms (Appendix B). New York: Raven Press, 1982. [Google Scholar]
- [48].Prinz U, Nutzinger DO, Schulz H, Petermann F, Braukhaus C, Andreas S. Comparative psychometric analyses of the SCL-90-R and its short versions in patients with affective disorders. BMC Psychiatry 2013;13:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Rabey M, Slater H, O’Sullivan P, Beales D, Smith A. Somatosensory nociceptive characteristics differentiate subgroups in people with chronic low back pain: a cluster analysis. Pain 2015;156(10):1874–1884. [DOI] [PubMed] [Google Scholar]
- [50].Rabey M, Smith A, Beales D, Slater H, O’Sullivan P. Differing psychologically derived clusters in people with chronic low back pain are associated with different multidimensional profiles. Clin J Pain 2016;32(12):1015–1027. [DOI] [PubMed] [Google Scholar]
- [51].Rosenstiel AK, Keefe FJ. The use of coping strategies in chronic low back pain patients: Relationship to patient characteristics and current adjustment. Pain 1983;17:33–44. [DOI] [PubMed] [Google Scholar]
- [52].Sarason IG, Johnson JH, Siegel JM. Assessing the impact of life changes: development of the Life Experiences Survey. J Consult Clin Psychol 1978;46:932–946. [DOI] [PubMed] [Google Scholar]
- [53].Shacham S A shortened version of the Profile of Mood States. Journal of personality assessment 1983;47(3):305–306. [DOI] [PubMed] [Google Scholar]
- [54].Slade GD, Bair E, By K, Mulkey F, Baraian C, Rothwell R, Reynolds M, Miller V, Gonzalez Y, Gordon S, Ribeiro-Dasilva M, Lim PF, Greenspan JD, Dubner R, Fillingim RB, Diatchenko L, Maixner W, Dampier D, Knott C, Ohrbach R. Study methods, recruitment, sociodemographic findings, and demographic representativeness in the OPPERA Study. J Pain 2011;12(11, Supplement):T12–T26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Society IH. The International Classification of Headache Disorders: 2nd edition. Cephalalgia 2004;24 Suppl 1:9–160. [DOI] [PubMed] [Google Scholar]
- [56].Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory (Form Y1). Palo Alto, CA: Consulting Psychologists Press, 1983. [Google Scholar]
- [57].Sullivan MJL, Bishop SR, Pivik J. The Pain Catastrophizing Scale: Development and validation. Psychological Assessment 1995;7(4):524–532. [Google Scholar]
- [58].Tibshirani R, Walther G, Hastie T. Estimating the number of clusters in a data set via the gap statistic. Journal of the Royal Statistical Society: Series B (Statistical Methodology) 2001;63(2):411–423. [Google Scholar]
- [59].Vaegter HB, Graven-Nielsen T. Pain modulatory phenotypes differentiate subgroups with different clinical and experimental pain sensitivity. Pain 2016;157(7):1480–1488. [DOI] [PubMed] [Google Scholar]
- [60].Vardeh D, Mannion RJ, Woolf CJ. Toward a mechanism-based approach to pain diagnosis. J Pain 2016;17(9 Suppl):T50–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Vincent A, Hoskin TL, Whipple MO, Clauw DJ, Barton DL, Benzo RP, Williams DA. OMERACT-based fibromyalgia symptom subgroups: an exploratory cluster analysis. Arthritis Res Ther 2014;16(5):463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Ware J Jr., Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Medical care 1996;34(3):220–233. [DOI] [PubMed] [Google Scholar]
- [63].Weathers F, Litz B, Herman D, Huska J, Keane T. The PTSD Checklist (PCL): Reliability, Validity, and Diagnostic Utility. The Annual Convention of the International Society for Traumatic Stress Studies. San Antonio, TX, 1993. [Google Scholar]
- [64].Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, Tugwell P, Campbell SM, Abeles M, Clark P, et al. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum 1990;33(2):160–172. [DOI] [PubMed] [Google Scholar]
- [65].Yim YR, Lee KE, Park DJ, Kim SH, Nah SS, Lee JH, Kim SK, Lee YA, Hong SJ, Kim HS, Lee HS, Kim HA, Joung CI, Kim SH, Lee SS. Identifying fibromyalgia subgroups using cluster analysis: Relationships with clinical variables. Eur J Pain 2017;21(2):374–384. [DOI] [PubMed] [Google Scholar]
- [66].Zeppieri KE, Butera KA, Iams D, Parvataneni HK, George SZ. The role of social support and psychological distress in predicting discharge: A pilot study for hip and knee arthroplasty patients. J Arthroplasty 2019;34(11):2555–2560. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


