Abstract
Plasmodium falciparum pathogenesis is complex and intimately connected to vascular physiology. This is exemplified by cerebral malaria (CM) a neurovascular complication that accounts for most of the malaria deaths worldwide. P. falciparum sequestration in the brain microvasculature is a hallmark of CM and is not replicated in animal models. Numerous aspects of disease are challenging to fully understand from clinical studies, such as parasite binding tropism or causal pathways in blood-brain barrier breakdown. Recent bioengineering approaches allow for the generation of 3D microvessels and organ-specific vasculature that provide precise control of vessel architecture and flow dynamics and hold great promise for malaria research. Here, we discuss recent and future applications of bioengineered microvessels in malaria pathogenesis research.
Keywords: Cerebral malaria, Plasmodium falciparum, PfEMP1, blood vessels, vascular engineering, 3D microvessels
Plasmodium falciparum interplay with blood vessels
Malaria parasites have a complex and intimate relationship with blood vessels through multiple stages of their life cycle (Figure 1). Sporozoites are introduced into the human body via mosquito bite into the dermis. Sporozoites migrate by gliding motility in the skin and traverse blood vessels to enter the blood circulation [1]. Once in the bloodstream, sporozoites travel to the liver, arrest, and cross the liver sinusoidal barrier to invade hepatocyte cells [2]. After undergoing asexual replication, hepatocyte-derived vesicles containing merozoites, known as merosomes, bud through endothelial cells [3] and are released into the blood circulation [4]. Merosomes become trapped in small blood vessels, such as lung capillaries [5], and are thought to release merozoites, to initiate the blood stage of infection.
Figure 1. P. falciparum interactions with the vasculature along its life cycle.
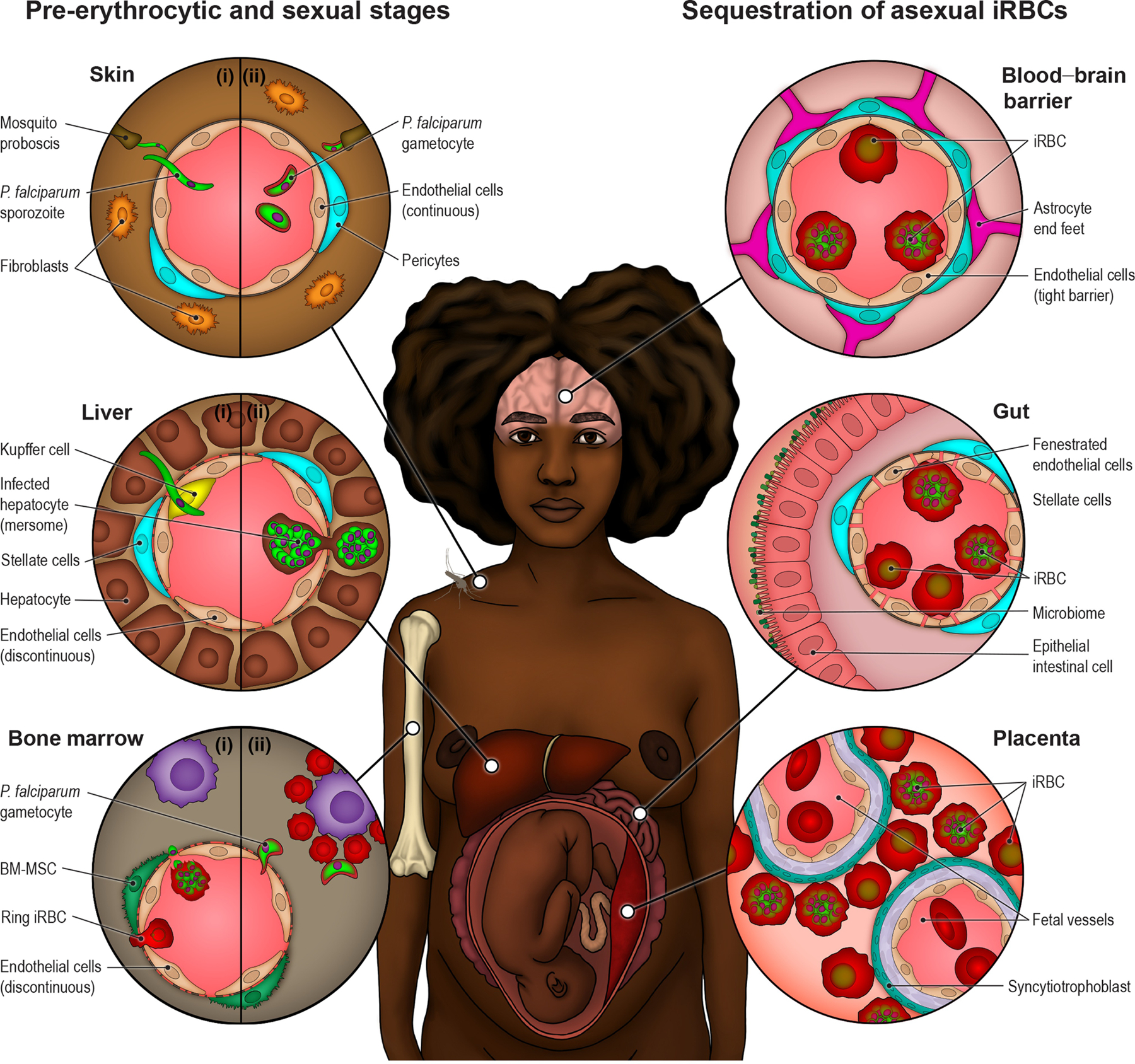
Top left circle (Skin): P. falciparum parasites interact with skin blood vessels when initiating infection (I) and during transmission back to mosquitoes (II). The molecular mechanism of sporozoite crossing across blood vessels or whether mature gametocytes accumulate in skin capillaries remains unknown. Middle left circle (Liver): sporozoites arrest on endothelial cells in the liver and cross the liver sinuosoidal barrier probably through Kupffer cells to infect hepatocytes (I). There, they will asexually multiply into thousands of merozoites that will be released back to bloodstream through budding of membranous structures known as merosomes, containing merozoite parasite forms (II). Left bottom circle (Bone Marrow): P. falciparum sexual development occurs in the bone marrow. Immature ring stage iRBC or merozoites, asexual or sexually committed, have been proposed as candidates to cross the discontinuous bone marrow vasculature (I). Early stage gametocye-iRBCs mature on erythroblastic islands until they re-enter the blood circulation as less-rigid stage V gametocyte-iRBCs (II). Asexual P. falciparum-iRBC sequester in the microvasculature to avoid splenic clearance. There is widespread sequestration in fatal cases. Top right circle (Blood-Brain Barrier): P. falciparum accumulation in the brain is highly pathogenic due to inherent properties of the blood-brain barrier to protect neuronal function. Middle right circle (Gut): One of the main parasite sequestration sites is the gut fenestrated microvasculature, which may contribute to high parasite biomass. Right bottom circle (Placenta): During pregnancy, P. falciparum-iRBCs accumulate in the maternal intervillous space and attached to the syncytiotrophoblast lining in the placenta.
Malaria disease symptoms appear after repeated rounds of parasite replication in red blood cells. Among the human malaria species, P. falciparum is unique for having highly cytoadhesive infected red blood cells (iRBC) that bind to the endothelial lining of blood vessels and cause microvascular obstruction. Sequestration (see Glossary) is thought to have evolved for iRBCs to avoid spleen-dependent clearance mechanisms and is associated with organ-specific complications when parasites reach high burdens. During the blood stage, sexually committed gametocyte-iRBCs develop within the bone marrow sinuses before returning to the blood circulation for uptake within a new mosquito blood meal [6]. These multiple interactions with the vasculature reveal that P. falciparum has evolved a set of complex and sophisticated strategies in synergy with blood vessels and distinct microvascular niches. Although cytoadhesion is central to P. falciparum disease, animals are non-natural hosts for P. falciparum and may not be fully optimal to study all endothelial binding interactions or organ niches. Novel vascular bioengineering approaches could provide new opportunities to study P. falciparum and human vessel interactions in a more physiological way.
Vasculature: a heterogenous system
The blood circulatory system provides nutrients and oxygen to all tissues and takes away waste and metabolites for disposal. Blood vessels are organized in a hierarchical branching network, ranging in size from large caliber arteries (from 100 μm to ≤ 1 cm internal diameter) to arterioles (15 – 100 μm) to a dense capillary plexus (4 – 12 μm) where transport interchange takes place. Deoxygenated blood gets drained into venules (10 – 100 μm) and veins (from 100 μm to ≤ 1 cm in diameter). These variations in blood vessel sizes correlate to variations in vascular wall composition, blood flow, and pressure, leading to heterogeneous biophysical forces on the vessel walls. In addition, the microvasculature in different organs has diverse functions to meet the unique demands of the tissue. Endothelial cells line the innermost layer of vessels, and possess heterogeneous phenotypes and functions to regulate the transport between the blood and the surrounding tissue. In dermis, heart, and brain, endothelium forms a continuous layer with low permeability, whereas endothelium in kidneys is fenestrated to allow for filtration and reabsorption of small solutes (see Box 1). Endothelial cells in liver, bone marrow, and spleen are sinusoidal and present large gaps in endothelial-endothelial junctions that facilitate macromolecular and cell diffusion. These heterogeneous organ-specific vessels provide unique interactions with P. falciparum and facilitate different stages of the infection as the parasites enter at the skin, move to the liver, and exploit multiple organ niches throughout the vascular system. To better understand P. falciparum pathogenesis or how it progresses through the human life cycle, future research needs to take vascular heterogeneity into account.
Box 1. Modeling parasite-vessel interaction in response to inflammation and hemodynamic factors.
The endothelium is a dynamic organ. It shifts from an anti-inflammatory and anti-coagulant phenotype in health to a pro-adhesive, procoagulant, and complement-activating phenotype in response to infection and injury. Most malaria infections are asymptomatic with low parasite burdens and limited vascular activation [100]. Only a subset of infections has high parasite burdens with extensive microvascular obstruction, widespread endothelial activation, and endothelial dysfunction. Although PfEMP1s encode diverse binding phenotypes, it is unknown whether the parasite cytoadhesion strategy adjusts to the differing microvascular environments in asymptomatic and symptomatic hosts [100]. Hemodynamic forces are a major driver of endothelial cell phenotypes. Shear stress lowers vascular permeability and the mechano-activated transcriptional programs, such as Kruppel-like factor 2 (KLF2), confer a barrier-strengthening, anti-inflammatory and anti-thrombotic phenotype on endothelial cells [96]. Flow forces have been binned into laminar flow (where vessel geometry is smooth and uniform) and disturbed flow (where vessels bifurcate, high curvature, or from microvascular obstruction). Vessel geometry and microfluidic forces play an important role in thrombosis. For instance, vWF fibers preferentially form near vessel bifurcations and vWF assembly is influenced by 3D vessel architecture, fluid shear stress and flow acceleration [85]. Exposure of 3D human microvessel models to physiological flow forces will enable study of how microvascular endothelial cells respond to inflammatory, pro-coagulant, and hemodynamic forces, as well as to explore the progression to endothelial dysfunction in malaria disease.
Small blood vessels and P. falciparum cytoadhesion
Cytoadherent asexual iRBCs predominantly sequester within small blood vessels with internal diameters smaller than 50 μm. P. falciparum exports proteins into the erythrocyte cytoplasm, many of which are involved in erythrocyte cytoskeletal modifications and inducing knob-like cytoadhesive platforms on the red blood cell surface [7–9]. The main cytoadhesion ligand is P. falciparum erythrocyte membrane protein 1 (PfEMP1), encoded by a family of approximately 60 var genes per parasite genotype. PfEMP1 proteins are expressed in a mutually exclusive fashion and endow different binding properties to iRBCs [10]. The protein family is classified into three main groups (A, B, and C) that encode distinct binding properties for CD36 [11, 12], endothelial protein C receptor (EPCR) [13, 14], and intercellular adhesion molecule 1 (ICAM-1) [15]. CD36 and EPCR are mutually exclusive binding traits [16], and have ancient origins because they are also encoded in the related var-like gene family in the chimpanzee malaria parasite, P. reichenowi [17]. EPCR-binding PfEMP1 variants are enriched in severe malaria infections [18, 19] and linked to brain swelling in cerebral malaria, [16, 20], even though only representing a minor subset of the PfEMP1 repertoire (~11–15%).
Besides controlling blood-tissue exchanges, endothelial cells have a major function in vascular hemostasis by maintaining an anti-thrombotic and anti-inflammatory surface [21]. Endothelial cells respond to inflammatory cytokines by upregulating pro-coagulant and pro-inflammatory pathways [21] (Box 1). The barrier properties of blood vessels are regulated by both barrier disruptive and barrier restorative signaling pathways [22]. Chronic activation or hyperinflammation can result in endothelial dysfunction, an alteration in endothelial state from a resting or “calm” phenotype to a highly activated phenotype where it is unable to perform its normal functions [21]. iRBCs in high parasite biomass are pro-coagulant and interfere with anti-coagulant pathways. Sequestered iRBCs release products that activate endothelial cells and induce barrier disruption in endothelial cell monolayers [23, 24]. They also interfere with EPCR function, which normally counteracts coagulation and induces anti-inflammatory and barrier restorative pathways in endothelial cells [14, 25]. Moreover, in vitro assays provide evidence that products released by schizont-stage P. falciparum-iRBCs interact with thrombin to prolong barrier disruption in endothelial cell monolayers [26, 27]. A better understanding of how endothelial cells integrate inflammatory signals may guide new approaches to treat endothelial dysfunction and vascular leak in severe malaria.
The blood-brain barrier and cerebral malaria pathology
The blood-brain barrier (BBB) presents the lowest permeability coefficient in the human vasculature and is thus essential to preserve brain function. Brain microvascular endothelial cells present a strong barrier phenotype, which is accomplished by elevated expression of tight junction proteins, low levels of transcytosis, and controlled traffic of molecules through specific efflux pumps and solute transporters. The unique properties of the BBB are acquired by its structure and interactions with brain parenchyma cells [28]. Brain pericytes are thought to contribute to the mechanical stability of the capillary wall [29]. In addition, astrocytic end-feet contact and support the vascular bed and secrete factors that enhance the expression of endothelial tight junctional proteins, such as claudin-5 and occludin, and specific transporters, such as glucose transporter-1 (GLUT-1) (Figure 2) [30]. The key role of the BBB in maintaining brain function, makes cerebral microvasculature a highly pathogenic site of sequestration.
Figure 2. Cerebral malaria pathogenesis.
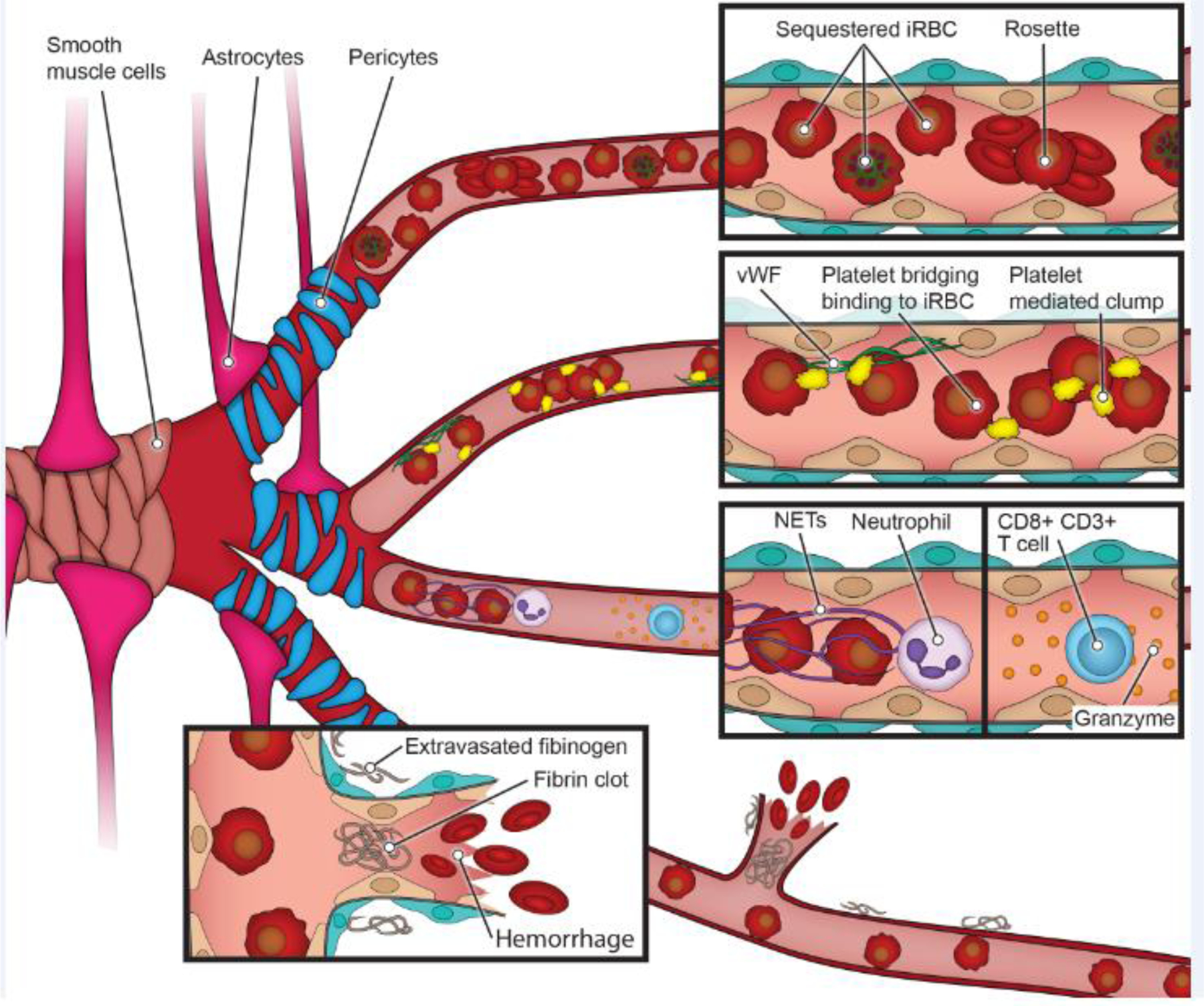
The unique properties of the BBB are achieved through interactions between endothelial cells, perivascular cells (smooth muscle cells in larger vessels and pericytes in the microvasculature) and astrocytes. P. falciparum sequestration in the brain microvasculature is a hallmark of CM and contributes to microvascular obstruction, probably along with rosettes, aggregates of iRBC to uninfected red blood cells. In autopsy studies, BBB disruption is exemplified by ring hemorrhages or fibrinogen deposition in the extravascular space. Platelets might also contribute to CM pathogenesis by bridging iRBC to vWF or endothelium or causing platelet-mediated iRBC clumps. Other cell types implicated in disease are neutrophils and CD8+ T cells. These pathogenic findings are sometimes localized in distinct regions of the brain, such as the white and grey matter [44].
Our knowledge of cerebral malaria has been acquired through examination of autopsy samples and more recently through neuroimaging studies. Magnetic resonance imaging (MRI) has indicated that severe brain swelling is associated with fatality in pediatric cerebral malaria [31]. Although the pathophysiological changes leading to brain swelling in cerebral malaria are incompletely understood, venous congestion and vascular engorgement are common findings, and MRI has found strong evidence for vasogenic edema in children [32, 33]. Brain swelling also occurs in adult CM, although at milder levels [34, 35]. Fatality in adults has been recently associated to severe hypoxia, which might be triggered by P. falciparum microvascular obstruction [35]. In both children and adults, the neurovascular pathology on MRI is compatible with Posterior Reversible Encephalopathy Syndrome (PRES), a neurological disorder that is characterized by vasogenic edema and endothelial dysfunction including breakdown of the BBB. PRES is reversible and patient survivors recover from brain swelling in 48–72h [32, 33].
Histological examination of pediatric brain autopsies has confirmed evidence of BBB breakdown. Fatal pediatric CM cases are divided into two groups: dense sequestration only (CM1) and dense sequestration accompanied by extra-erythrocytic pigment, ring hemorrhages, and evidence of systemic activation of coagulation (CM2) [36, 37]. Both groups have fibrinogen leakage to brain parenchyma, indicating BBB breakdown (Figure 2) [36, 38]. In addition, high levels of extracellular histones and plasma cell-free DNA are associated with brain swelling on MRI [39, 40]. Accumulation of iRBCs is common in both white and grey matter of the brain. Sequestration is highest in capillary beds or marginalized in post-capillary venules [38, 41]. The same pattern is evident in retinal imaging with higher accumulation in smaller vessels and on the venular side of circulation (small venules > post-capillary venules > large venules > pre-capillary arterioles > small arterioles > large arterioles) [42]. This pattern may arise in part because cells experience deacceleration and lower shear stress as they exit the capillaries into the larger post-capillary venule spaces. Collectively, these findings suggest interactions between sequestration, coagulation, and BBB dysregulation in cerebral malaria, albeit the extent of brain coagulopathy varies in fatal cases.
Both CM1 and CM2 cases show perivascular brain activation and pathology including myelin damage in cortex, subcortex, white matter and brain stem, and reactive astrocytes in all brain regions except the cortex [36]. Ring hemorrhages are concentrated in the white matter and watershed areas where blood supply is decreased. It has been hypothesized that biophysical and biological components may account for these brain differences [43, 44]. White matter vasculature presents longer and less ramified small blood vessels and is more prone to upstream occlusive damage. By comparison, grey matter small vessels are organized in a dense and sponge-like network with multiple anastomosis and collateral pathways, which might prevent local increase in flow resistance after occlusion and hemorrhages [38]. In addition, it has been hypothesized that differences in perivascular cell composition might account for different responses between white and grey matter that could confer diverse barrier properties or influence the localized response to sequestered iRBCs (reviewed in [44]).
Host response and cerebral malaria pathogenesis
A pro-coagulant and hyperinflammatory host response might additionally contribute to vascular dysfunction in CM patients. For example, fibrin deposits are common in pediatric brain autopsies upstream of ring hemorrhages (Figure 2). Moreover, low circulating platelet levels has been linked to fatal brain swelling in pediatric CM [20]. Whereas thrombocytopenia might have multiple systemic causes, platelet accumulation in the brain vasculature might have pathogenic consequences [45, 46]. Platelets have both immunogenic and pro-coagulant functions, which might lead to a dual protective or damaging effect on blood vessels. Activated platelets secrete pro-inflammatory cytokines, including tumor necrosis factor alpha (TNFα) [47], transforming growth factor TGF-β1, and IL-1β [48], that enhance the local inflammatory milieu. They also release, microparticles that potentially inhibit parasite growth [49]. Platelets can bridge binding of iRBC without tropism to the brain endothelium [50], and may promote microvascular obstruction by platelet-mediated clumping of iRBC [51] or iRBC binding to platelet-von Willebrand Factor (vWF) multimers secreted upon endothelial activation or injury [52] (Figure 2).
Recent studies have revealed increased transcriptional signatures of neutrophil activation and the presence of neutrophil granular proteins in plasma of severe malaria patients [53, 54]. In addition, a recent study has shown that neutrophil extracellular traps (NETs) are found at sites of iRBC sequestration [55] (Figure 2). Like platelets, neutrophils and NETs might have a dual protective-harmful role ( reviewed [56]). An additional cell type that has recently been associated to CM are CD8+ T cells. Autopsy studies have shown their enrichment in the choroid plexus [57] or the lumen of veins colocalizing with granzyme B staining [58]. In vitro vascular engineered models may provide a means to dissect and disentangle the individual and collective contribution of P. falciparum iRBC, platelets, neutrophils and CD8+ T cells in CM pathogenesis.
Organ-specific P. falciparum-iRBC cytoadhesion
Children who die from cerebral malaria have massive sequestration of iRBC in the brain, but also in the vasculature of the gastrointestinal tract and/or subcutaneous adipose tissue of the skin. Other sites of sequestration include the heart, lung, spleen, and to a lesser extent the kidney [59]. Despite evidence of a broad sequestration [59], parasite tropism for most organ/vascular sites remains terra incognito.
Although the gut is considered a relatively non-pathogenic sequestration site, malaria patients frequently have gastrointestinal symptoms, and the gastrointestinal vasculature (from the stomach to the large colon) is an intense site of sequestration in fatal pediatric cerebral malaria cases (Figure 1) [41, 59]. Gut sequestration has also been described in an adult CM autopsy series [38]. The gastrointestinal tract represents an enormous surface area and has voluminous fenestrated capillary beds for iRBC sequestration, and therefore makes a major contribution to the high parasite burdens in fatal cases [59]. In agreement with autopsy findings, the microcirculatory blood flow of the rectal mucosa is markedly disturbed in adult severe malaria patients [60]. The extent of mucosal microvascular obstruction and blocked capillaries by in vivo imaging is proportional to disease severity and correlates with base deficit in plasma and the concentration of lactate [60]. Moreover, acidic microbial products contribute to metabolic acidosis in malaria, indicating that intestinal barrier function may be compromised [61]. Metabolic acidosis is associated with high mortality and significantly increases the risk of cerebral malaria mortality [62, 63]. Collectively, these findings implicate gut sequestration in high parasite burdens and raise the possibility of a gut-brain axis in CM. It remains to be determined if the same or different parasite binding variants sequester in gut and brain.
Conversely, the kidney is not a major sequestration site, but acute kidney injury and renal failure is frequent in children [64] an adult severe malaria [63], respectively. The kidney contains two highly specialized microvasculature, the glomerular capillaries and the tubular capillaries, with distinct cell structures and specialized functions. The glomerular endothelial cells are fenestrated and covered by a thick glycocalyx that facilitates the sieving properties of glomerular filtration. The peritubular capillary endothelial cells have a thin fenestrated diaphragm, which facilitates reabsorption and secretion of products between blood and adjacent tubular epithelial cells. The red average blood cell velocity is higher in the glomerular capillaries (~16.7 mm/s) compared to the peritubular capillaries (4.7 mm/s) [65]. Quantitative ultrastructure studies show that parasite sequestration is higher in the peritubular capillaries than glomerular capillaries, and that malaria-associated renal failure was associated with renal tubular injury rather than glomerulonephritis [66]. The parasite binding variants of the kidney remain unknown.
In pregnant women, P. falciparum-iRBCs sequester within the maternal intervillous spaces and bind to the syncytiotrophoblast lining causing placental malaria (Figure 1). The placenta architecture is highly complex and remodels during pregnancy. Blood flow is much slower in the placenta than other organs which may facilitate iRBC sequestration [67]. Whereas the mean flow velocity in a third trimester placenta is of 0.4 mm/s, other microvascular beds present a flow velocity gradient between 1 and 16 mm/s. Likewise, iRBCs are exposed to a much lower wall shear stress (0.5–2.3 dyn/cm2) in the placenta than in the systemic microvasculature (1–6 dyn/cm2) [68]. Placental binding parasites adhere to a unique low sulfated chondroitin sulfate A in the placenta [69, 70]. This causes placental malaria-derived complications, such as premature birth or miscarriage. The heterogeneity and complexity of organ microvasculature highlights the need for better in vitro models that recapitulate functional organ-specific microvessel beds and their varied flow dynamics to study parasite-vascular tropism in CM and severe malaria (Box 2).
Box 2: Engineered 3D microvessels: a versatile system and applications in malaria pathogenesis research.
Controlled vessel size and geometry: The fabrication of 3D microvessel networks is not limited to previously published 13 x 13 grids or parallel channels connected by capillary-size vessels (Figure 3). The combination of soft lithography, injection molding and laser photoablation allows the design of custom-made geometries. This provides control of vessel length and diameter, number of branches, branching angle, and vessel curvature. The 3D microvessel network will determine microfluidic properties which 1) regulate endothelial cell phenotype, including gene expression and transcriptional profile or 2) how blood cells interact with the vessel wall. These systems can be used to study parasite sequestration in engineered blood vessels that precisely mimic the shape and curve of natural organ-specific microvessels, as well as to explore how vessel geometry might determine parasite-mediated vascular damage.
Fabrication of organ-specific cell types and vasculature: These fabrication technologies support the growth and development of new vascular-specific microvasculature models, including human kidney fenestrated peritubular microvascular endothelial cells [98] or even models that combine two microfluidic networks, an endothelial and an epithelial, that recapitulate the vascular-tubular renal interface in the kidney [99]. Additionally, patient specific cells can be used, either primary cells or iPSC- differentiated cells. This offers controlled experimental conditions to study parasite-vessel tropism and investigate disease mechanisms leading to specific endothelial damage in different regions of the brain or in other organs.
Controlled perfusion of blood components: Attaching the 3D microvessel network into a flow pump allows for precise control of biological and biomechanical parameters in a step-wise fashion. For example, different blood cells or molecules (e.g. cytokines, clotting factors, patient blood samples) can be perfused independently, sequentially or in combination at controlled microfluidic conditions to dissect how human or P. falciparum factors contribute to severe disease, individually or in synergy.
Modeling P. falciparum cytoadhesion in vitro
The factors that determine iRBC-organ sequestration patterns are incompletely understood. The simplest assay and workhorse for investigating parasite binding is the static cytoadhesion assay [12, 15]. In this assay, iRBCs settle on cells or spotted proteins and then are gently washed. This technique has the significant advantage in that it is easy to implement, even in a low-tech field research setting, and therefore has seen abundant usage for characterizing receptor and endothelial-specific interactions [71–75]. For instance, this approach has shown that the same parasite variants can bind to primary human brain, lung, and heart microvascular endothelial cells [76]. However, a major disadvantage is that this assay mostly measures the strength of iRBC attachment during washing steps and provides limited insights into iRBC capture from flow and other flow-based considerations that may be important for vascular tropism. To account for this limitation, flow-based microfluidic systems have been applied [77, 78]. While much more difficult to implement in a field setting, a recent study using commercial linear flow chambers found that increased number of iRBC from cerebral malaria patients could bind to primary brain endothelial cell monolayer under flow than uncomplicated malaria cases [79], suggesting expansion of brain-tropic parasites in cerebral malaria patients. While significant mechanistic insights have been gained from static and flow-based binding assays, 2D monolayer formats lack important microvascular parameters, such as lumen dimensions, branching architecture, and vessel curvature that influence microfluidic parameters and endothelial transcription and behavior. New in vitro vascular engineering models pave the way for more physiological studies for malaria pathogenesis research.
New avenues in vascular in vitro engineering
Recent advances in tissue engineering have allowed for the development of various modeling strategies to study vascular diseases and brings their potential to push the frontier of malaria research. Different vascular models have been generated with varying complexities. While vascular networks can be generated via self-assembly either as cells in a gel [80, 81] or vascularized organoids [82] from stem cells, the lack of control on vascular structure and perfusion has limited their usage in studies that require refined flow control. Recent advances have been focused on the creation of 3D perfusable microvascular models through a variety of manufacturing techniques. These methods offer different levels of cellular, structural and physiological complexity to investigate the interaction of molecular and cellular processes in different organ environments.
PDMS-based microfluidic channels and networks
Twenty years ago, soft lithography technology was developed to transfer the silicon-based microfabrication technology into silicone-based materials, such as polydimethylsiloxane (PDMS). This technology has been exploited to fabricate microchannels and transparent networks that can be studied under precise microfluidic flow when connected to a flow pump. PDMS microchannel networks extended the horizon of malaria research by providing tools to study rheology of P. falciparum-iRBC through geometries that mimicked capillary constrictions or splenic endothelial slits in PDMS models (reviewed in [83]). However, limitations exist in the use of PDMS and similar materials, due to the high chemical absorbability and its rigidness, which is far from recapitulating the biomechanical properties of the vessel wall or the lubricating properties of endothelial cells. Additionally, this platform imposes difficulties in studying the effect of the perivascular cell’s extracellular matrix on endothelial cell phenotype and heterogeneity.
Hydrogel-based microvascular networks
To better mimic the microvascular environment, soft lithography techniques were later extended into hydrogels platforms to build microfluidic networks in cell-compatible biomimetic extracellular matrices, such as collagen and fibrin. The resultant channels can be seeded with organ-specific endothelial cells in channels with different branching architecture, and perfused with defined flow conditions [84] (Figure 3). Conventional soft lithography techniques can generate robust vessels at diameters of 50 to 500 μm in collagen but they tend to collapse at smaller sizes [85]. An advantage of the hydrogel format is that perivascular cells, like pericytes, can be seeded into the biomatrix and directly interact with endothelial cells [84]. Other advantages of hydrogel-based vascular engineering platforms are described in Figure 3 and Box 2.
Figure 3. Bioengineered in vitro vascular models.
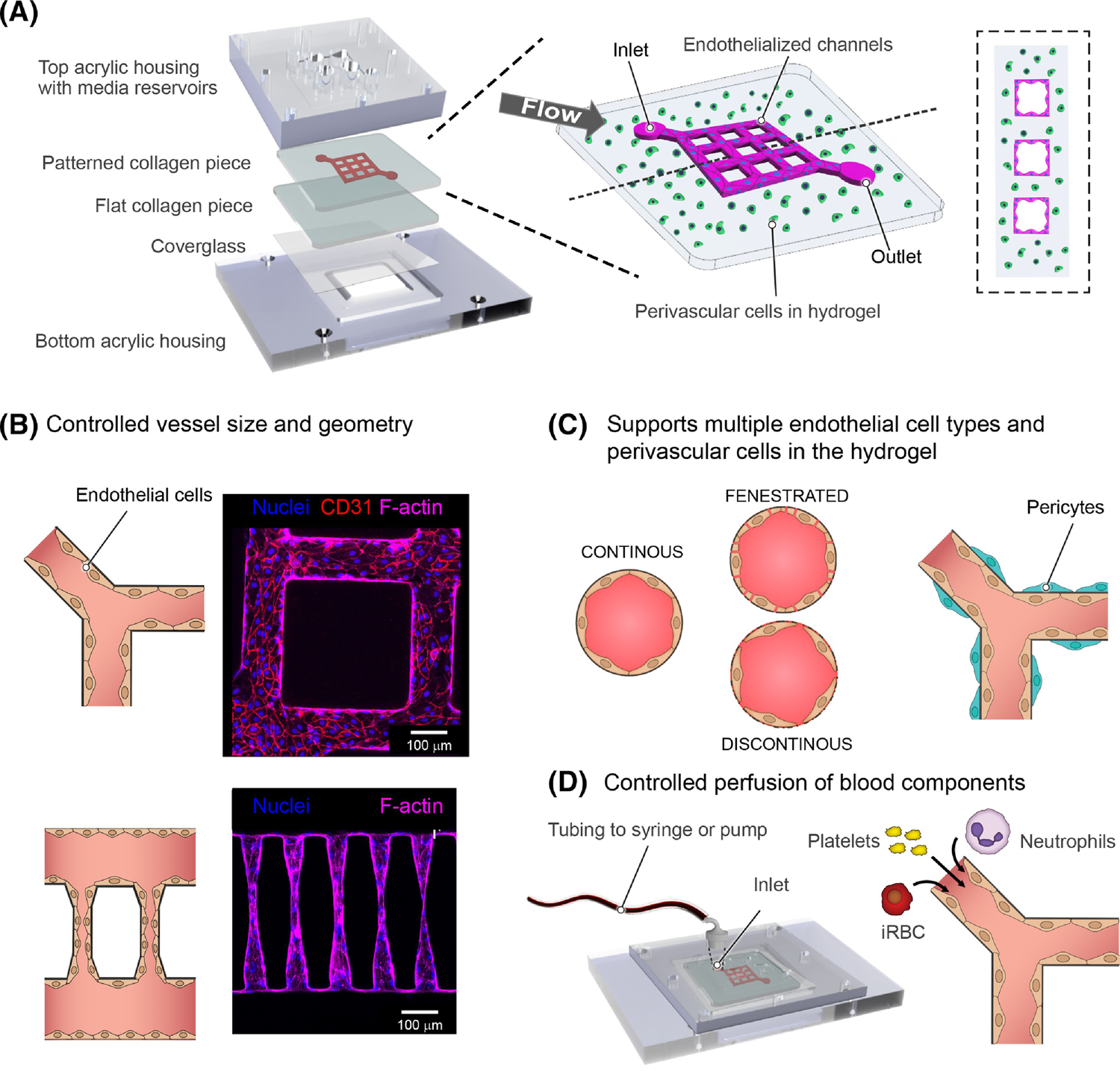
a. Rendering of device components (left), and schematic representation of 3D microvessels with a 3x3 grid microfluidic network (right). Bioengineered 3D microvessels are a versatile model. b. By combining fabrication methods like soft-lithography and photoablation control can be achieved over vessel size, diameter and network geometry. This is exemplified by immunofluorescences showing a portion of a 13X13 grid composed of a network of 100 μm diameter vessels (top), and vessels mimicking arteriole-capillary-venule transition with a diameter of 5–15 μm in the narrowest region (bottom). c. Engineered 3D microvessels are compatible with multiple endothelial cell types including continuous, fenestrated and sinusoidal endothelium, and support the growth of perivascular and parenchyma cells within the hydrogel. d. The use of a predetermined network provides refined control over microfluidic properties when connected to a flow pump. Perfusion of different blood components through the device can be independent, sequential or combined to understand the independent or synergistic contribution of parasite and host components in CM.
The microvessel format has been used in several recent studies for investigating vessel wall interactions that are relevant for malaria research. For example, a 13x13 grid network design based on human umbilical vein endothelial cells was used to create a large range of physiological and pathological flow velocities that have been used to study flow driven interactions of vWF and platelets [85]. This analysis showed that the ability of vWF to assemble into thicker fibers and more complex meshworks was influenced by vessel geometry and microfluidic properties (Box 1) The same grid-based 3D microvessel format was adapted to study P. falciparum-iRBC interactions with primary brain endothelial cells [86]. This study found that iRBC binding to 3D brain microvessels is strongly influenced by physiological differences in flow shear that exists within the brain microcirculation, and that parasite adhesion strength is highly sensitive to changes in EPCR and ICAM-1 expression levels on the endothelial cell surface caused by TNFα activation. Some PfEMP1-clonal parasite lines presented increased sequestration, while no difference was found in others, depending on their combinatorial binding properties [86]. These findings suggest a highly tuned strategy for parasite sequestration under different conditions experienced during human infection.
Endothelial-only 3D microvessel models have also been used to understand changes in endothelial permeability during malaria infections. For instance, the endothelial-cell only 3D microvessel 13x13 model has been used to study barrier function in response to human CM sera [87]. In a different endothelial cell-only model built from a crosslinked agarose–gelatin interpenetrating polymer-network (IPN) hydrogel, iRBCs caused occlusion of 20 μm microvessels followed by an immediate increase of permeability. Two days after occlusion, the microvessels recovered baseline permeability levels and endothelial cells were found to have engulfed the malaria pigment, hemozoin [88]. To better mimic brain pathogenic events in CM patients, future models might incorporate hypoxia.
Alternatives approaches to build perfusable tubes and networks in hydrogels, include subtractive molding [89] and bioprinting [90–92]. Success was demonstrated via subtractive molding to remove a needle from a hydrogel after gelation and form a hollow lumen for endothelial cell seeding and culture. Bioprinting has been demonstrated in either the direct printing of highly viscous biomaterials or sacrificial materials [90, 91] that can be removed after the surrounding hydrogel or scaffolds are cross-linked, followed by endothelial cell seeding and culture. This highly automated process has shown success in generating thick, complex 3D channels for endothelial cell seeding and culture to form defined networks. These approaches allow the integration of perivascular and extravascular cells, and controlled perfusion but are restricted to relatively large diameter vessels (100 μm or larger), which are larger than the capillary and post-capillary venules where parasite sequestration usually occurs [36, 42].
More recently, multiphoton ablation technology has been used to create capillary networks (down to 5 μm) between two wider parallel microvessels [93]. Multiphoton ablation allows precise control of vessel diameter and exploits angiogenesis for endothelial cell in-growth to connect the two wider microvessels. We recently generated an arteriole-capillary-venule unit by combining soft-lithography based approach and multiphoton ablation, and used it to study the biomechanics of iRBC sequestration and microvascular occlusion. Whereas normal red blood cells readily traversed the capillary-sized constriction (5–10 μm) with negligible vessel wall interactions, iRBC displayed increased tumbling motions and accumulated. Sequestration was influenced by changes in red blood cell deformability, parasite adhesion properties, and changes in velocity experienced by cells as they move through different-sized vessels. Similar to human infections, iRBC predominantly sequester within the capillary and postcapillary-sized vessels of the arteriole-capillary-venule units [93]. Future models could include the presence of other host blood cells, including platelets, neutrophils or rosettes.
Blood brain barrier models
These new bioengineered microvessel models have shown promise in revealing new vascular biology phenomena and have provided molecular insights into molecular, microfluidic, and geometric factors that promote microvascular obstruction in malaria. Future CM models need to better recapitulate the unique cellular environment and barrier properties of the BBB. Many efforts have been focused to the development of 3D-BBB models with different degrees of success. Two promising models that combine PDMS and hydrogel based microfluidic fabrication methods have achieved physiological low permeability rates similar to human BBB [94, 95]. Both models incorporate endothelial cells differentiated from induced pluripotent stem cells and primary astrocytes and pericytes that increase the expression and strength of tight junctions and BBB specific transporters. These models have outstanding potential for studying barrier function. However, they present some limitations as either they do not reproduce the dimensions and branching architecture of the brain microvasculature [94], or do not offer a reproducible flow control which makes difficult the generation of biological replicates [95]. As flow has an important role to tune endothelial cell transcriptional networks [96] or BBB properties [97], future CM in vitro vascular models need to achieve low BBB permeability rates and consistently reproduce brain branching networks and flow properties.
Concluding remarks
P. falciparum sequestration is broadly distributed in diverse microvasculature beds in fatal CM cases [59]. Vascular dysfunction contributes to the complex array of multi-system organ complications that malaria patients can suffer. Along the circulatory system, the endothelial lining of blood vessels presents differences in receptor expression, and organ-specific microvessels present distinctive branching architectures and flow properties that may influence parasite sequestration burdens. Likewise, the unique functions of each microvascular bed, given by distinct perivascular cell composition and endothelial cell phenotype, might influence how blood vessels react to parasite, inflammatory stimuli and immune cells. To better understand organ-specific disease mechanisms in malaria and evaluate therapeutic interventions, in vitro models need to mimic disease mechanisms found in patients and recapitulate functional and structural heterogeneity of organ-microvessel beds (see Outstanding questions).
Outstanding questions:
Malaria pathogenesis
Do factors released by P. falciparum-iRBCs sequestration cause BBB breakdown?
How do endothelial cells integrate inflammatory stimuli from malaria parasites, systemic factors and infiltrating host cells?
How do host cells, including platelets, neutrophils, and CD8+ T cells contribute to brain endothelial activation and BBB breakdown?
Is there cross-talk between endothelial cells, perivascular cells, and parenchymal cells in CM pathophysiology?
Can we design adjunctive therapies that prevent vascular disfunction in severe and cerebral malaria?
Vascular engineering for malaria research:
Can we use in vitro vascular engineering to develop BBB or neurovascular unit models that recapitulate brain microvascular structure and flow-induced properties to understand microvascular obstruction in the brain?
Can these models (or simplified versions) be used to understand CM malaria pathogenesis in the BBB and brain parenchyma or to evaluate new therapeutic interventions?
Can vascular tissue-specific models, including placenta, kidney and gut, be developed to understand other organ-complications in severe malaria?
Can vascular engineered models be used to investigate other stages of the malaria life-cycle including sporozoite interactions with dermal or liver sinusoidal barrier or gametocyte-bone marrow interactions?
Altogether, engineered in vitro 3D microvessels offer a versatile opportunity to consistently reproduce and understand the heterogenous pathology of a complex disease such as malaria. Hydrogel-based methods provide an extracellular matrix that supports the growth of multiple cell types. Some of these methodologies allow control over network branching, microfluidic properties, and internal lumen dimensions with regulated perfusion of different parasite and blood components that, independently or collectively, might cause CM pathogenesis. In the last decade multiple organ-specific vascular models have been developed by using endothelial from primary origin or differentiated from iPSC, including kidney [98, 99], brain [86] or BBB models [94, 95]. These models hold great promise to gain a better understanding of the organ-specific disease mechanisms in CM patients. Likewise, although less explored, future models of the skin, liver, or bone marrow microvasculature might reveal new findings in the biology of pre-erythrocytic and transmission malaria stages.
Highlights.
Research into cytoadhesive complications of Plasmodium falciparum infection has been hampered by the lack of suitable animal models and limitations of endothelial cell monolayer models.
Bioengineered microvessels offer precise control over vascular cell types, branching architecture, lumen diameter, and flow dynamics. They provide new opportunities for investigating Plasmodium-vessel interactions.
3D microvessels models have been used to explore how flow dynamics, vessel diameter, and endothelial activation influence sequestration. They can also model the endothelial cells response to host and parasite inflammatory products.
Future bioengineered models could include tissue-specific vasculature to investigate organ-specific injury in severe malaria, evaluate novel therapeutic interactions, or improve our understanding of other key parasite-blood vessel interactions in the malaria life cycle.
Acknowledgements
We thank Adriana Lippy for drawing the illustrations in Figures 1 and 2. This work was supported by the core program funding of the European Molecular Biology Laboratory (MB), R01 HL130488, R01 AI141602 and R01 AI148802 (JDS and YZ). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We apologize to colleagues whose work was not cited due to space constraints.
Glossary:
- Bioprinting
use of 3D printing technology with materials that sustain viable living cells. The main limitation for vascular engineering is that current technologies do not allow the fabrication of small diameter vessels
- Blood-brain barrier
Highly impermeable blood vessels that separate the blood from brain tissue. It is composed by endothelial cells, and surrounding pericytes, astrocytes and extracellular matrix
- Cerebral malaria
Severe malaria complication characterized by patient coma and neurovascular pathology. Sequestration of P. falciparum-iRBC is a hallmark of cerebral malaria
- Fenestrated (endothelium)
Fenestrations are round or oval 60–80 nm diameter transcellular pores in endothelial cells. Flow through the fenestrae is controlled by a diaphragm and allows for small protein and molecule diffusion
- Flow chambers
Microfluidic devices that can be perfused through a flow pump. Endothelial cells grow in a 2D monolayer on plastic and glass. They can be commercial or self-fabricated and generally have a channel width of 1mm.
- Flow resistance
The force opposing the flow of blood through a vessel which is dependent on vessel length and branching structure, vessel diameter, and viscosity of the blood
- Hydrogel
biomaterial that sustains cellular growth in 3D. Examples of hydrogels are collagen, fibrin or Matrigel
- In vitro vascular engineered models
In vitro models that mimic the three-dimensional structure of blood vessels and that are perfusable with media and blood components
- Induced Pluripotent Stem Cells (iPSC)
Pluripotent stem cells that can be generated from any somatic cell, for example white blood cells or dermal fibroblasts. They can propagate indefinitely and differentiate in any cell of the human body
- Perfusable microvascular models
Engineered 3D microvessels that can be perfused with media and flow components in a controlled way
- Placental malaria
Malaria complication associated with the adhesion of infected erythrocytes within the intervillous space of the placenta
- Ring hemorrhages
Brain microvascular hemorrhages with a round shape that are usually found following a capillary blocked by fibrin depositions
- Self-assembly/ Self-assembled models
Models where cells are seeded in hydrogels and self-organize organized replicas of tissue and organs. Organoids are examples of self-assembled models
- Sequestration
Accumulation of P. falciparum-iRBC in the microvasculature caused by iRBC binding/ cytoadhesion to endothelial cells
- Shear stress
Force that causes deformation of materials and cells by slippage along a plane, for example the endothelium. It can vary depending on fluid viscosity, flow rate, vessel diameter or length and branching network
- Sinusoid
Small blood vessels of 30–40 um diameter with wide gaps in endothelial junctions, that take the place of capillaries and venules in the liver, bone marrow and spleen
- Soft lithography
Fabrication technique to build microfluidic networks or devices that uses silicon-based materials and photomasks
- Subtractive molding
Manufacturing technique used to create channels by the gelation of a material around a removable object, such as a needle, which is then removed from the material leaving an open lumen
- Tight junction
Multiprotein complex at endothelial cell junctions that controls the passage of ions, fluids and small molecules through paracellular transport (in between cells)
- Vascular engorgement
Local congestion and enlargement of blood vessels. In CM it is likely to be caused by sequestered P. falciparum-iRBC
- Vascular permeability
Capacity of blood vessels to allow exchange of small molecules, or even cells, between blood and the surrounding tissue
- Vascular wall
Blood vessel structure composed of endothelial cells, perivascular cells (pericytes or smooth muscle cells) and extracellular matrix
- Vascularized organoids
Organoids are simplified version of organs grown in vitro with realistic cell types and tissue organization. Although different organoid types containing endothelial cells have been generated, they are generally not perfusable in vitro, which poses limitations for malaria research
- Vasogenic (brain) edema
Brain swelling cause by blood-brain barrier breakdown
- Von Willebrand Factor
glycoprotein released after endothelial activation. It forms long strings and has an important role in coagulation because it binds to platelets and coagulation factors
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Vanderberg JP and Frevert U (2004) Intravital microscopy demonstrating antibody-mediated immobilisation of Plasmodium berghei sporozoites injected into skin by mosquitoes. Int. J. Parasitol 34 (9), 991–996. [DOI] [PubMed] [Google Scholar]
- 2.Tavares J et al. (2013) Role of host cell traversal by the malaria sporozoite during liver infection. J Exp Med 210 (5), 905–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burda PC et al. (2017) Manipulation of the Host Cell Membrane during Plasmodium Liver Stage Egress. mBio 8 (2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sturm A et al. (2006) Manipulation of host hepatocytes by the malaria parasite for delivery into liver sinusoids. Science 313 (5791), 1287–90. [DOI] [PubMed] [Google Scholar]
- 5.Baer K et al. (2007) Release of hepatic Plasmodium yoelii merozoites into the pulmonary microvasculature. PLoS Pathog 3 (11), e171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Venugopal K et al. (2020) Plasmodium asexual growth and sexual development in the haematopoietic niche of the host. Nat Rev Microbiol 18 (3), 177–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marti M et al. (2004) Targeting malaria virulence and remodeling proteins to the host erythrocyte. Science 306 (5703), 1930–3. [DOI] [PubMed] [Google Scholar]
- 8.Hiller NL et al. (2004) A host-targeting signal in virulence proteins reveals a secretome in malarial infection. Science 306 (5703), 1934–1937. [DOI] [PubMed] [Google Scholar]
- 9.Maier AG et al. (2008) Exported proteins required for virulence and rigidity of Plasmodium falciparum-infected human erythrocytes. Cell 134 (1), 48–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith JD (2014) The role of PfEMP1 adhesion domain classification in Plasmodium falciparum pathogenesis research. Mol Biochem Parasitol 195 (2), 82–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hsieh FL et al. (2016) The structural basis for CD36 binding by the malaria parasite. Nat Commun 7, 12837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barnwell JW et al. (1989) A human 88-kD membrane glycoprotein (CD36) functions in vitro as a receptor for a cytoadherence ligand on Plasmodium falciparum-infected erythrocytes. J Clin Invest 84 (3), 765–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lau CK et al. (2015) Structural conservation despite huge sequence diversity allows EPCR binding by the PfEMP1 family implicated in severe childhood malaria. Cell host & microbe 17 (1), 118–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Turner L et al. (2013) Severe malaria is associated with parasite binding to endothelial protein C receptor. Nature 498 (7455), 502–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ockenhouse CF et al. (1992) Plasmodium falciparum-infected erythrocytes bind ICAM-1 at a site distinct from LFA-1, Mac-1, and human rhinovirus. Cell 68 (1), 63–9. [DOI] [PubMed] [Google Scholar]
- 16.Lennartz F et al. (2017) Structure-Guided Identification of a Family of Dual Receptor-Binding PfEMP1 that Is Associated with Cerebral Malaria. Cell Host Microbe 21 (3), 403–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brazier AJ et al. (2017) Pathogenicity Determinants of the Human Malaria Parasite Plasmodium falciparum Have Ancient Origins. mSphere 2 (1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lavstsen T et al. (2012) Plasmodium falciparum erythrocyte membrane protein 1 domain cassettes 8 and 13 are associated with severe malaria in children. Proc Natl Acad Sci U S A 109 (26), E1791–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bernabeu M et al. (2016) Severe adult malaria is associated with specific PfEMP1 adhesion types and high parasite biomass. Proc Natl Acad Sci U S A 113 (23), E3270–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kessler A et al. (2017) Linking EPCR-Binding PfEMP1 to Brain Swelling in Pediatric Cerebral Malaria. Cell Host Microbe 22 (5), 601–614 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pober JS and Sessa WC (2014) Inflammation and the blood microvascular system. Cold Spring Harb Perspect Biol 7 (1), a016345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mehta D and Malik AB (2006) Signaling mechanisms regulating endothelial permeability. Physiol Rev 86 (1), 279–367. [DOI] [PubMed] [Google Scholar]
- 23.Tripathi AK et al. (2007) Plasmodium falciparum-infected erythrocytes decrease the integrity of human blood-brain barrier endothelial cell monolayers. J. Infect. Dis 195 (7), 942–950. [DOI] [PubMed] [Google Scholar]
- 24.Gillrie MR and Ho M (2017) Dynamic interactions of Plasmodium spp. with vascular endothelium. Tissue Barriers 5 (1), e1268667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bernabeu M and Smith JD (2017) EPCR and Malaria Severity: The Center of a Perfect Storm. Trends Parasitol 33 (4), 295–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Avril M et al. (2019) Interplay of Plasmodium falciparum and thrombin in brain endothelial barrier disruption. Sci Rep 9 (1), 13142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Storm J et al. (2020) Testing the effect of PAR1 inhibitors on Plasmodium falciparum-induced loss of endothelial cell barrier function. Wellcome Open Res 5, 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao Z et al. (2015) Establishment and Dysfunction of the Blood-Brain Barrier. Cell 163 (5), 1064–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lindahl P et al. (1997) Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science 277 (5323), 242–5. [DOI] [PubMed] [Google Scholar]
- 30.Abbott NJ et al. (2006) Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci 7 (1), 41–53. [DOI] [PubMed] [Google Scholar]
- 31.Seydel KB et al. (2015) Brain swelling and death in children with cerebral malaria. N Engl J Med 372 (12), 1126–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mohanty S et al. (2017) Magnetic Resonance Imaging of Cerebral Malaria Patients Reveals Distinct Pathogenetic Processes in Different Parts of the Brain. mSphere 2 (3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Potchen MJ et al. (2018) 1.5 Tesla Magnetic Resonance Imaging to Investigate Potential Etiologies of Brain Swelling in Pediatric Cerebral Malaria. Am J Trop Med Hyg 98 (2), 497–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maude RJ et al. (2014) Randomized controlled trial of levamisole hydrochloride as adjunctive therapy in severe falciparum malaria with high parasitemia. J Infect Dis 209 (1), 120–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sahu PK et al. (2020) Brain Magnetic Resonance Imaging Reveals Different Courses of Disease in Pediatric and Adult Cerebral Malaria. Clin Infect Dis 10.1093/cid/ciaa1647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dorovini-Zis K et al. (2011) The neuropathology of fatal cerebral malaria in malawian children. Am J Pathol 178 (5), 2146–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taylor TE et al. (2004) Differentiating the pathologies of cerebral malaria by postmortem parasite counts. Nat Med 10 (2), 143–5. [DOI] [PubMed] [Google Scholar]
- 38.Spitz S (1946) The pathology of acute falciparum malaria. Mil Surg 99 (5), 555–72. [PubMed] [Google Scholar]
- 39.Moxon CA et al. (2020) Parasite histones are toxic to brain endothelium and link blood barrier breakdown and thrombosis in cerebral malaria. Blood Adv 4 (13), 2851–2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vera IM et al. (2020) Plasma cell-free DNA predicts pediatric cerebral malaria severity. JCI Insight 5 (12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Milner DA Jr. et al. (2015) Quantitative Assessment of Multiorgan Sequestration of Parasites in Fatal Pediatric Cerebral Malaria. J Infect Dis 212 (8), 1317–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barrera V et al. (2018) Neurovascular sequestration in paediatric P. falciparum malaria is visible clinically in the retina. Elife 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maccormick IJ et al. (2014) Cerebral malaria in children: using the retina to study the brain. Brain. [DOI] [PMC free article] [PubMed]
- 44.Noumbissi ME et al. (2018) Brain vascular heterogeneity: implications for disease pathogenesis and design of in vitro blood-brain barrier models. Fluids Barriers CNS 15 (1), 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grau GE et al. (2003) Platelet accumulation in brain microvessels in fatal pediatric cerebral malaria. J Infect Dis 187 (3), 461–6. [DOI] [PubMed] [Google Scholar]
- 46.Hochman SE et al. (2015) Fatal Pediatric Cerebral Malaria Is Associated with Intravascular Monocytes and Platelets That Are Increased with HIV Coinfection. MBio 6 (5), e01390–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grau GE et al. (1989) Tumor necrosis factor and disease severity in children with falciparum malaria. N Engl J Med 320 (24), 1586–1591. [DOI] [PubMed] [Google Scholar]
- 48.Garraud O et al. (2012) Platelets and cytokines: How and why? Transfus Clin Biol 19 (3), 104–8. [DOI] [PubMed] [Google Scholar]
- 49.McMorran BJ et al. (2012) Platelet factor 4 and Duffy antigen required for platelet killing of Plasmodium falciparum. Science 338 (6112), 1348–51. [DOI] [PubMed] [Google Scholar]
- 50.Wassmer SC et al. (2004) Platelets reorient Plasmodium falciparum-infected erythrocyte cytoadhesion to activated endothelial cells. J. Infect. Dis 189 (2), 180–189. [DOI] [PubMed] [Google Scholar]
- 51.Chotivanich K et al. (2004) Platelet-induced autoagglutination of Plasmodium falciparum-infected red blood cells and disease severity in Thailand. J Infect Dis 189 (6), 1052–5. [DOI] [PubMed] [Google Scholar]
- 52.Bridges DJ et al. (2010) Rapid activation of endothelial cells enables Plasmodium falciparum adhesion to platelet-decorated von Willebrand factor strings. Blood 115 (7), 1472–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Feintuch CM et al. (2016) Activated Neutrophils Are Associated with Pediatric Cerebral Malaria Vasculopathy in Malawian Children. MBio 7 (1), e01300–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee HJ et al. (2018) Integrated pathogen load and dual transcriptome analysis of systemic host-pathogen interactions in severe malaria. Sci Transl Med 10 (447). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Knackstedt SL et al. (2019) Neutrophil extracellular traps drive inflammatory pathogenesis in malaria. Sci Immunol 4 (40). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Amulic B et al. (2020) A More Granular View of Neutrophils in Malaria. Trends Parasitol 36 (6), 501–503. [DOI] [PubMed] [Google Scholar]
- 57.Barrera V et al. (2019) Comparison of CD8(+) T Cell Accumulation in the Brain During Human and Murine Cerebral Malaria. Front Immunol 10, 1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Riggle BA et al. (2020) CD8+ T cells target cerebrovasculature in children with cerebral malaria. J Clin Invest 130 (3), 1128–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Milner DA Jr. et al. (2014) The systemic pathology of cerebral malaria in African children. Front Cell Infect Microbiol 4, 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dondorp AM et al. (2008) Direct in vivo assessment of microcirculatory dysfunction in severe falciparum malaria. J Infect Dis 197 (1), 79–84. [DOI] [PubMed] [Google Scholar]
- 61.Leopold SJ et al. (2019) Identifying the Components of Acidosis in Patients With Severe Plasmodium falciparum Malaria Using Metabolomics. J Infect Dis 219 (11), 1766–1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Marsh K et al. (1995) Indicators of life-threatening malaria in African children. N Engl J Med 332 (21), 1399–404. [DOI] [PubMed] [Google Scholar]
- 63.Dondorp AM et al. (2008) The relationship between age and the manifestations of and mortality associated with severe malaria. Clin Infect Dis 47 (2), 151–7. [DOI] [PubMed] [Google Scholar]
- 64.Conroy AL et al. (2019) Acute kidney injury is associated with impaired cognition and chronic kidney disease in a prospective cohort of children with severe malaria. BMC Med 17 (1), 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kang JJ et al. (2006) Quantitative imaging of basic functions in renal (patho)physiology. Am J Physiol Renal Physiol 291 (2), F495–502. [DOI] [PubMed] [Google Scholar]
- 66.Nguansangiam S et al. (2007) A quantitative ultrastructural study of renal pathology in fatal Plasmodium falciparum malaria. Trop Med Int Health 12 (9), 1037–50. [DOI] [PubMed] [Google Scholar]
- 67.Brabin BJ et al. (2004) The sick placenta-the role of malaria. Placenta 25 (5), 359–78. [DOI] [PubMed] [Google Scholar]
- 68.Lecarpentier E et al. (2016) Computational Fluid Dynamic Simulations of Maternal Circulation: Wall Shear Stress in the Human Placenta and Its Biological Implications. PLoS One 11 (1), e0147262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Achur RN et al. (2008) Structural interactions in chondroitin 4-sulfate mediated adherence of Plasmodium falciparum infected erythrocytes in human placenta during pregnancy-associated malaria. Biochemistry 47 (47), 12635–12643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fried M and Duffy PE (1996) Adherence of Plasmodium falciparum to chondroitin sulfate A in the human placenta. Science 272 (5267), 1502–4. [DOI] [PubMed] [Google Scholar]
- 71.Avril M et al. (2012) A restricted subset of var genes mediates adherence of Plasmodium falciparum-infected erythrocytes to brain endothelial cells. Proc Natl Acad Sci U S A 109 (26), E1782–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Claessens A et al. (2012) A subset of group A-like var genes encodes the malaria parasite ligands for binding to human brain endothelial cells. Proc Natl Acad Sci U S A 109 (26), E1772–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Udeinya IJ et al. (1981) Falciparum malaria-infected erythrocytes specifically bind to cultured human endothelial cells. Science 213 (4507), 555–557. [DOI] [PubMed] [Google Scholar]
- 74.Berendt AR et al. (1989) Intercellular adhesion molecule-1 is an endothelial cell adhesion receptor for Plasmodium falciparum. Nature 341 (6237), 57–59. [DOI] [PubMed] [Google Scholar]
- 75.Barnwell JW et al. (1985) Monoclonal antibody OKM5 inhibits the in vitro binding of Plasmodium falciparum-infected erythrocytes to monocytes, endothelial, and C32 melanoma cells. J Immunol 135 (5), 3494–7. [PubMed] [Google Scholar]
- 76.Avril M et al. (2013) DC8 and DC13 var genes associated with severe malaria bind avidly to diverse endothelial cells. PLoS Pathog 9 (6), e1003430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cooke BM et al. (1994) Rolling and stationary cytoadhesion of red blood cells parasitized by Plasmodium falciparum: separate roles for ICAM-1, CD36 and thrombospondin. Br J Haematol 87 (1), 162–70. [DOI] [PubMed] [Google Scholar]
- 78.Wick TM and Louis V (1991) Cytoadherence of Plasmodium falciparum-infected erythrocytes to human umbilical vein and human dermal microvascular endothelial cells under shear conditions. Am J Trop Med Hyg 45 (5), 578–86. [DOI] [PubMed] [Google Scholar]
- 79.Storm J et al. (2019) Cerebral malaria is associated with differential cytoadherence to brain endothelial cells. EMBO Mol Med 11 (2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen X et al. (2009) Prevascularization of a fibrin-based tissue construct accelerates the formation of functional anastomosis with host vasculature. Tissue Eng Part A 15 (6), 1363–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Whisler JA et al. (2014) Control of perfusable microvascular network morphology using a multiculture microfluidic system. Tissue Eng Part C Methods 20 (7), 543–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Collen A et al. (2003) Membrane-type matrix metalloproteinase-mediated angiogenesis in a fibrin-collagen matrix. Blood 101 (5), 1810–7. [DOI] [PubMed] [Google Scholar]
- 83.Depond M et al. (2019) Methods to Investigate the Deformability of RBC During Malaria. Front Physiol 10, 1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zheng Y et al. (2012) In vitro microvessels for the study of angiogenesis and thrombosis. Proc Natl Acad Sci U S A 109 (24), 9342–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zheng Y et al. (2015) Flow-driven assembly of VWF fibres and webs in in vitro microvessels. Nat Commun 6, 7858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bernabeu M et al. (2019) Binding Heterogeneity of Plasmodium falciparum to Engineered 3D Brain Microvessels Is Mediated by EPCR and ICAM-1. MBio 10 (3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Barker KR et al. (2017) miR-155 Modifies Inflammation, Endothelial Activation and Blood-Brain Barrier Dysfunction in Cerebral Malaria. Mol Med 23, 24–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Qiu Y et al. (2018) Microvasculature-on-a-chip for the long-term study of endothelial barrier dysfunction and microvascular obstruction in disease. Nat Biomed Eng 2, 453–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Raghavan S et al. (2010) Geometrically controlled endothelial tubulogenesis in micropatterned gels. Tissue Eng Part A 16 (7), 2255–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Miller JS et al. (2012) Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues. Nat Mater 11 (9), 768–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kinstlinger IS et al. (2020) Generation of model tissues with dendritic vascular networks via sacrificial laser-sintered carbohydrate templates. Nat Biomed Eng 4 (9), 916–932. [DOI] [PubMed] [Google Scholar]
- 92.Kolesky DB et al. (2014) 3D bioprinting of vascularized, heterogeneous cell-laden tissue constructs. Adv Mater 26 (19), 3124–30. [DOI] [PubMed] [Google Scholar]
- 93.Arakawa C et al. (2020) Biophysical and biomolecular interactions of malaria-infected erythrocytes in engineered human capillaries. Sci Adv 6 (3), eaay7243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Park TE et al. (2019) Hypoxia-enhanced Blood-Brain Barrier Chip recapitulates human barrier function and shuttling of drugs and antibodies. Nat Commun 10 (1), 2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Campisi M et al. (2018) 3D self-organized microvascular model of the human blood-brain barrier with endothelial cells, pericytes and astrocytes. Biomaterials 180, 117–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Topper JN and Gimbrone MA Jr. (1999) Blood flow and vascular gene expression: fluid shear stress as a modulator of endothelial phenotype. Mol Med Today 5 (1), 40–6. [DOI] [PubMed] [Google Scholar]
- 97.Bouhrira N et al. (2020) Disturbed flow disrupts the blood-brain barrier in a 3D bifurcation model. Biofabrication 12 (2), 025020. [DOI] [PubMed] [Google Scholar]
- 98.Ligresti G et al. (2016) A Novel Three-Dimensional Human Peritubular Microvascular System. J Am Soc Nephrol 27 (8), 2370–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rayner SG et al. (2018) Reconstructing the Human Renal Vascular-Tubular Unit In Vitro. Adv Healthc Mater 7 (23), e1801120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Andrade CM et al. (2020) Increased circulation time of Plasmodium falciparum underlies persistent asymptomatic infection in the dry season. Nat Med 26,1929–1940. [DOI] [PubMed] [Google Scholar]


