Summary sentence for the table of contents:
CRPS patient IgM-mediated C5a complement signaling is pronociceptive in fracture mice.
Keywords: autoimmunity, IgM, C5a complement, cytokines, pain, fracture, complex regional pain syndrome
Summary sentence:
C5a complement and cytokine signaling mediates CRPS patient IgM pronociceptive effects in the skin and spinal cord of fracture mice.
1. Introduction
Complex regional pain syndrome (CRPS) is an enigmatic syndrome that typically develops after limb injury or surgery and presents with distal limb nociceptive, vascular, and bone changes that exceed the expected clinical course of the inciting injury, frequently resulting in significant motor impairment and disability. Autoinflammatory and autoimmune processes may contribute to the development of CRPS.[7,9] Distal limb fracture is the most common cause of CRPS [8,29] and we have investigated the innate and adaptive immune mechanisms supporting nociceptive sensitization in a tibia fracture rodent model closely resembling CRPS.[3] Using anti-CD20 antibodies (rituximab) and muMT mice deficient in B cells and immunoglobulin, we observed that; 1) anti-CD20 antibody treatment of wildtype (WT) fracture mice eliminated B cells and reduced hindpaw allodynia and unweighting, 2) muMT mice had attenuated nociceptive and inflammatory changes at 3 weeks post fracture, 3) IgM immune complexes were deposited in skin and sciatic nerve after fracture in WT mice, but not in muMT mice, and 4) anti-CD20 treatment inhibited post fracture complement membrane attack complex deposition in skin and nerve.[22]
Passive transfer pronociceptive effects were observed when serum or IgM antibodies collected from WT fracture mice were systemically injected into muMT fracture mice, with gradually increasing hindpaw allodynia and unweighting, peaking at 7 days and resolving by 14 days after injection, consistent with the half-life of IgM.[11] These pronociceptive effects were restricted to the fracture hindlimb and not observed in the intact limb. Serum from nonfractured WT mice or IgG from fractured WT mice had no pronociceptive effects. IgM antibody levels gradually increased in the WT mouse fracture limb hind paw skin, sciatic nerve, and corresponding lumbar cord, peaking at 18 weeks after fracture and then declining to baseline levels at 23 weeks post fracture, consistent with the time course of post fracture nociceptive sensitization.[11]
Remarkably, the regionally restricted pronociceptive serum effects observed after injections of WT mouse fracture serum or IgM were replicated when CRPS patient serum or IgM was systemically injected into muMT fracture mice, but normal subject serum and CRPS patient IgG antibodies had no effect.[14] Furthermore, CRPS patient IgM antibodies had unilateral pronociceptive effects when injected intraplantarly or intrathecally in the muMT fracture mice. Early (1–12 months post injury) CRPS patient (n=20) sera were always pronociceptive after systemic injection and chronic (>12 months post injury) CRPS sera were rarely pronociceptive (2/20 patients), while sera from normal subjects (n=20) or from orthopedic trauma patients without CRPS (n=15) were never pronociceptive. Increased CRPS serum IgM binding was observed for keratin 16, histone 3.2, gamma actin, and alpha enolase autoantigens.[14]
Antibody effects are primarily mediated by complement activation. The current study tests the hypothesis that CRPS patient IgM binds to fracture induced autoantigens in the mouse fracture limb skin and corresponding spinal cord, thus initiating an antibody-antigen-complement complex resulting in C5a complement generation and activation of dermal macrophage and spinal microglia C5a receptors, triggering the secretion of inflammatory cytokines capable of sensitizing nociceptive neurons and inducing unilateral hindlimb allodynia and unweighting.
2. Materials and methods
2.1. Human subjects and clinical data collection
After giving informed written consent human subjects were enrolled in the study protocol previously approved by the institutional review board at University Medical Center of Johannes Gutenberg University. All CRPS serum collections were obtained from patients meeting the Budapest scientific criteria for CRPS [15] at the time of blood draw. One experiment utilized IgM derived from the serum of CRPS patients who initially met the Budapest CRPS diagnostic criteria but when reevaluated years later for repeat serum collection later no longer fulfilled the diagnostic criteria for CRPS (recovered CRPS). A commercial company (BioreclamationIVT, Westbury, NY) utilized the study serum processing protocol to prospectively collect normal control subject sera, and these sera tested negative for HIV and HCV antibodies and were non-reactive for HBSAG, HIV-1 RNA, HCV RNA, HBV DNA, and STS.
2.2. Animals
These experiments followed the animal subjects guidelines of the International Association for the Study of Pain and were approved by the Veterans Affairs Palo Alto Health Care System Institutional Animal Care and Use Committee (Palo Alto, CA, USA). These experiments used 3 months old male muMT mice lacking mature B cells and immunoglobulin, on a C57BL/6J congenic background (#002288, Jackson Laboratory, Bar Harbor, ME), with wildtype C57BL/6J controls (#000664, Jackson Laboratory). One experiment used C5a complement receptor deficient mice on a BALB/cJ background (#006845, Jackson Laboratory, Bar Harbor, ME), with wildtype BALB/cJ controls (#000651, Jackson Laboratory). Data collection was conducted blind to group assignment.
2.3. Surgery
Under isoflurane anesthesia a closed right tibia fracture was performed as previously described.[13] The hindlimb was then wrapped in casting tape (Delta-Lite, BSN Medical, Hamburg, Germany) so the hip, knee and ankle were all fixed and at 3 weeks post fracture the cast was removed.
2.4. Hindpaw nociceptive testing
Hindpaw plantar mechanical allodynia was assayed using von Frey filaments according to the “up-down” algorithm as previously described.[4] Hindpaw testing was performed bilaterally and these data were analyzed as the difference between the fracture side and the contralateral untreated side, thus a negative value represents a reduction in the fracture hindpaw withdrawal threshold.
An incapacitance device (IITC Life Science, Woodland Hills, CA) was used to measure bilateral hindlimb weight bearing as previously described.[12] These weight-bearing data were analyzed as a ratio between the right (fracture side) hindlimb weight bearing and the average of right and left hindlimb values ((2R/(R + L)) × 100%), thus a value less than 100% indicates a decrease in weight bearing in the fracture limb.
2.5. Hindpaw temperature testing
The temperature of the bilateral hindpaws was measured using a fine wire thermocouple (Omega Engineering, Norwalk, CT) applied to the paw skin, as previously described.[24] These data were analyzed as the difference between the fracture side and the contralateral side, thus a positive value indicates increased temperature in the fracture paw.
2.6. Hind paw thickness testing
A laser sensor technique was used to determine the dorsal-ventral thickness of the bilateral hind paws, as we have previously described.[24] These data were analyzed as the difference between the fracture side and the contralateral side, thus a positive value indicates increased paw thickness on the fracture side.
2.7. C5a complement receptor immunohistochemistry in skin and spinal cord.
Hindpaw plantar skin and lumbar spinal cord tissues were collected, embedded, sectioned (skin: 10-um, cord: 20-um), permeabilized and blocked, exposed to primary and secondary antibodies, mounted on slides, and the sections were imaged using confocal microscopy as previously described.[21,23] Primary antibodies included rabbit anti Iba1, 1:500 (WAKO, Osaka, Japan), mouse anti neuronal nuclei (nenN), 1:1000 (EMD Millipore Corp., Burlington, MA), rat anti C5aR1 monoclonal antibody, 1:500 (Abcam, Cambridge, UK), chicken anti-vimentin polyclonal antibody, 1:4000 (Invitrogen), and monoclonal mouse anti-rat keratin, Pan Ab-1, 1:50 (clone AE1/AE3) (Thermo Fisher Scientific, Waltham, MA), TPSAB1 recombinant rabbit monoclonal antibody, 1:100 (Invitrogen, Carlsbad, CA). Secondary antibodies included Alexa Fluor 594-conjugated affinity pure donkey anti-rabbit IgG (1:1000), Alexa Fluor 647-conjugated affinity pure donkey anti-mouse IgG (1:1000), Alexa Fluor 488-conjugated affinity pure donkey anti-rat IgG (1:1000), Alexa Fluor 594-conjugated affinity pure donkey anti-chicken IgG (1:1000), Alexa Fluor 647-conjugated affinity pure donkey anti-rabbit IgG (1:1000), Alexa Fluor 594-conjugated affinity pure donkey anti-mouse IgG (1:1000) (Jackson ImmunoResearch, West Grove, PA), incubated with respective primary antibodies. Control experiments included incubation of slices in primary and secondary antibody-free solutions and primary antibody pre-absorption control, all of which led to low intensity non-specific staining patterns in preliminary experiments (data not shown). A blinded investigator counted the number of C5aR+Iba1+, C5aR+NeuN+, and C5aR+vimentin+ double positive cells per high-power field (HPF, 400×) in the hindpaw skin and dorsal spinal cord (5 mice per cohort).
2.8. C5a complement signaling experiments in the fracture mouse CRPS model
These experiments examined C5a complement pronociceptive and inflammatory effects in both the tibia fracture mouse CRPS model and in nonfracture controls. The first experiment systemically injected the C5a receptor antagonist PMX53 (4mg/kg, subcutaneously, R&D Systems, Minneapolis, MN) into 3 weeks post tibia fracture wildtype mice the day after cast removal and measured hindpaw von Frey thresholds and weight bearing just prior to injection and at 0.5, 1, 2, and 4 hours after injection. Previously we demonstrated that a similar dosage of PMX53 reduced allodynia and inflammatory cytokine expression in a hindpaw incision mouse trauma model.[5] PMX53 is a small cyclic peptidic molecule specifically targeting the C5aR1 at nanomolar concentrations and acting in a pseudo-irreversible manner, with a serum half-life of 30 minutes.[19] An additional experiment evaluated the 15 weeks time course for the development and resolution of hindpaw allodynia, unweighting, warmth, and edema in C5a receptor deficient tibia fracture mice as compared to wildtype fracture mice.
To localize the sites of C5a pronociceptive activity in the fracture model and to confirm that C5a mediated pronociceptive effects required B cells and immunoglobulin production, we tested the effects of intraplantar (30ug/10ul) and intrathecal (200ng/5ul) injection of the C5a receptor antagonist PMX53 in wildtype fracture mice and in muMT fracture mice lacking B cells and immunoglobulin. Additional experiments tested the effects of intraplantar and intrathecal injection of C5a (recombinant mouse complement component C5a protein, R&D Systems, Minneapolis, MN) or vehicle into muMT fracture and nonfracture mice. The dose of C5a used in these experiments was based on pronociceptive dosages identified in our prior studies in the mouse paw incision model and other investigators work in neuropathic mouse models.[10,18] Mice were tested for hindpaw von Frey allodynia and unweighting at 4 weeks post fracture and then injected either intraplantarly or intrathecally and retested at 0.5, 1, 3, 6, and 24 hours and 7 days after injection.
2.9. Real-time polymerase chain reaction assays for complement and inflammatory cytokines
At 3 weeks after fracture muMT mice were euthanized and the fracture limb hind paw skin or the L4,5 lumber spinal cord tissues were collected, total RNA extracted, and real-time polymerase chain reactions conducted and analyzed as previously described.[12] Tumor necrosis factor (TNF-α), interleukin 1 beta (IL-1β), interleukin 6 (IL-6), nerve growth factor (NGF), hemolytic complement (HC, C5), complement component 1, q subcomponent, alpha polypeptide (C1qa), complement component 1, q subcomponent, beta polypeptide (C1qb), complement component 1, q subcomponent, C chain (C1qc), complement component 5a receptor 1 (C5aR1), complement component 5a receptor 2 (C5aR2) and 18S primer sets (Table 1) were validated on dissociation curves to document single product formation and agarose gel analysis was conducted to confirm the size. All results were confirmed by repeating each experiment three times.
Table 1.
Primers Used for Real-time Polymerase Chain Reaction
| Gene | GenBank Accession # | Forward primer | Reverse primer | Product Size(bp) |
|---|---|---|---|---|
| NGF | XM_227525 | acctcttcggacactctgga | gtccgtggctgtggtcttat | 168 |
| IL-1β | NM_031512 | agtctgcacagttccccaac | agacctgacttggcagagga | 230 |
| IL-6 | NM_012589 | cacaagtccggagaggagac | acagtgcatcatcgctgttc | 168 |
| TNF-α | NM_012675 | ctcccagaaaagcaagcaac | cgagcaggaatgagaagagg | 210 |
| C5 | NM_010406 | gagctgtaccaatgccaacc | gaggaatcttcgtgcagcag | 249 |
| C1qa | NM_007572 | taacaccaacaacaaggggc | aggaatccgctgaagatgct | 150 |
| C1qb | NM_009777 | gtgcctggcctctactactt | gcttcaagactaccccacct | 163 |
| C1qc | NM_007574 | gttcaacagcaagcaggtca | tgaggatggggaaagcagag | 213 |
| C5aR1 | NM_001173550 | tggcagcaaacacctttacc | gctgggatataggtttgcgc | 234 |
| C5aR2 | NM_001146005 | gggtttctctgtgtagccct | gtgtgttgttaggcccaagg | 229 |
| 18S | NR_046237 | tcaactttcgatggtagtcgccgt | tccttggatgtggtagccgtttct | 108 |
2.10. CRPS IgM immunoglobulin injection experiments in the fracture mouse CRPS model
This experiment examined the pronociceptive (hindpaw mechanical allodynia and unweighting) effects of early (1–12 months post injury) CRPS patient IgM, chronic (>12 months post injury) CRPS patient IgM or IgG, or normal control subject IgM after intraplantar or intrathecal injection into 4 weeks post fracture muMT mice lacking B cells and immunoglobulin. After clinical evaluation CRPS patient blood was collected in 10ml red top tubes and left undisturbed at room temperature for 60 min to allow clotting, then refrigerated overnight at 4°C and then the blood samples were centrifuged at 2,200g for 20 min at and the serum supernatants were aliquoted and frozen at - 80°C.
IgM was extracted from the pooled serum of early CRPS patients (n = 6), chronic CRPS patients (n = 5), and normal control subjects (n = 2) using a polypropylene column (BioRad, Hercules, CA) which was pre-packed with POROS CaptureSelect™ IgM Affinity Matrix (Thermo fisher Scientific, Leiden, Netherlands). The bound IgM was eluted using 100mM glycine pH 3 with the pH adjusted to 7.4 using 1M Tris pH 8.0, then Slide-A-Lyzer Dialysis Cassettes (10K MWCO, Life Technologies, Carlsbad, CA) were used to remove glycine from protein and the IgM quantified using a NanoDrop ND-1000 UV-Vis spectrophotometer (NanoDrop Technologies, Wilmington, DE) and diluted to 1ug/uL for intrathecal or intraplantar injection.
IgG was extracted from the pooled serum of chronic CRPS patients (n = 5) or from resolved CRPS patients (n=3) using a CaptureSelect IgG-CH1 Affinity Matrix (Thermo Fisher Scientific) with the pH adjusted to 7.4 using 1M Tris pH 8.0, and then, Slide-A-Lyzer Dialysis Cassettes (10K MWCO; Life Technologies) were used to remove glycine from protein, and IgG quantified using a NanoDrop ND-1000 UV-Vis spectrophotometer (NanoDrop Technologies) and diluted in 1 ug/uL for intrathecal or intraplantar injection.
To determine whether the pronociceptive effects of CRPS patient IgM injection required C5a receptor activation in the skin or spinal cord, muMT fracture mice underwent baseline nociceptive behavioral testing (hindpaw von Frey allodynia and unweighting) between 3 and 4 weeks post fracture, and then were injected intraplantarly or intrathecally with early CRPS patient IgM (5ug) with and without co-injection of the C5a receptor antagonist PMX53. Similarly, control subject IgM (5ug/5ul) was also injected intraplantarly or intrathecally into muMT fracture mice with and without the co-injection of PMX53 (30ug). After injection the mice were tested for von Frey allodynia and unweighting at 0.5, 1, 3, 6, and 24 hours post injection. The CRPS patient IgM dosage used in the current study (5ug/5ul) had pronociceptive effects in our prior study after intraplantar or intrathecal injection in muMT fracture mice.[14] The PMX53 dosage used for intraplantar and intrathecal injections in this study (30ug) was derived from our previous observation that an intraplantar injection of PMX53 (30ug) reduced von Frey allodynia in the hindpaw incision model of post operative pain.[18]
To determine whether early CRPS patient IgM could evoke C5a receptor mediated complement and pronociceptive cytokine expression in skin and spinal cord, muMT fracture mice underwent baseline nociceptive behavioral testing (hindpaw von Frey allodynia and unweighting) at 3 weeks post fracture, and then were injected intraplantarly or intrathecally with CRPS patient IgM with and without co-injection of the C5a receptor antagonist PMX53. Approximately 90 minutes after intraplantar or intrathecal injection the hindpaw skin or lumbar spinal cord, respectively, were collected as described above and processed for PCR quantification of TNF, IL-1, IL-6, NGF, C5, C1qa, C1qb, and C1qc mRNA expression.
Additional experiments evaluated whether the pronociceptive effects of early CRPS patient IgM injection required cytokine signaling in the skin or spinal cord. The muMT fracture mice underwent baseline nociceptive behavioral testing (hindpaw von Frey allodynia and unweighting) between 3 and 4 weeks post fracture, and then the global cytokine inhibitor pentoxifylline (Sigma Aldrich, St. Louis, MO) was administered by gavage for 2 consecutive days (200gm/kg, daily), then the mice were injected intraplantarly or intrathecally with early CRPS patient IgM. An additional cohort of muMT fracture mice were treated with pentoxifylline but were not injected with IgM. Another group of muMT fracture mice were injected intraplantarly or intrathecally with CRPS patient IgM without pentoxifylline treatment. After IgM intraplantar injection the mice were tested for von Frey allodynia and unweighting at 1, 3, 6, and 24 hours post injection. After IgM intrathecal injection the mice were tested for von Frey allodynia and unweighting at 0.5, 1, 3, 6, and 24 hours post injection. Previously we demonstrated that the pentoxifylline dosage used in the current study (200mg/kg, PO daily) was analgesic in fracture rats and reduced post fracture increases in mRNA and protein levels of TNF-α, IL-1β, and IL-6 in the skin of the injured limb.[35]
Lastly, the pronociceptive effects of intraplantar or intrathecal injections of chronic (> 12 month duration) CRPS patient IgM or IgG were evaluated in muMT fracture mice. After baseline testing at 3 weeks post fracture, muMT mice were injected intraplantarly or intrathecally with 5ug of either chronic CRPS patient IgM, chronic CRPS IgG, resolved CRPS IgM, or control subject IgM, and then all mice were tested for von Frey allodynia and unweighting at 0.5, 1, 3, 6, and 24 hours post injection. In our prior study the early CRPS patient IgM dosage used in the current study (5 ug) had pronociceptive effects after intraplantar or intrathecal injection in muMT fracture mice.[14]
2.11. C5b-9 complement immunostaining in skin after CRPS IgM intraplantar injection
This experiment examined the effect of CRPS IgM treatment on C5b-9 complement deposition in hindpaw skin of muMT fracture mice. Three weeks post fracture muMT mice underwent unilateral intraplantar injection in the fracture limb paw with either control or CRPS IgM (5ug/5ul). At 1.5 – 2 hours after the injection, mice were euthanized and hindpaw plantar skin tissues were collected, embedded, sectioned (10-um), permeabilized and blocked, exposed to primary and secondary antibodies, mounted on slides, and the sections were imaged using confocal microscopy as previously described.[21,23] Primary antibodies were polyclonal rabbit anti-C5b9, 1:250 (Abcam) and monoclonal mouse anti-rat keratin, Pan Ab-1, 1:50 (clone AE1/AE3, Thermo Fisher Scientific) and secondary antibodies were donkey anti-rabbit immunoglobulin G conjugated with Alexa Fluor 594, 1:500 and donkey anti-mouse immunoglobulin G conjugated with Alexa Fluor 488, 1:500 (Jackson ImmunoResearch Laboratories, West Grove, PA, USA). The immunohistochemistry data was quantified by a blinded investigator and is presented in the figures as mean intensity per high-powered field. Control experiments included incubation of slices in primary and secondary antibody-free solutions, both of which led to low-intensity nonspecific staining patterns in preliminary experiments (data not shown).
2.12. Statistical analysis
Statistical analysis was performed using a two-way repeated measures ANOVA or a one-way ANOVA with Sidak multiple comparisons test for post-hoc contrasts. Data are presented as the mean ± standard error of the mean, and differences are considered significant at a P value less than 0.05 (Prism 5, GraphPad Software, San Diego, CA).
3. Results
3.1. C5a receptor signaling caused post fracture hindpaw nociceptive sensitization and warmth
Subcutaneous injection of the C5a receptor antagonist PMX53 (4mg/kg) into 3 weeks post fracture wildtype mice reduced hindpaw von Frey allodynia and unweighting at 1 hour post injection (Fig. 1 A,B) and this analgesic effect resolved within 2 hours after injection. Fracture mice deficient for the C5a receptor developed attenuated hindpaw allodynia and unweighting at 3 weeks post fracture and exhibited an accelerated resolution of hindpaw nociceptive sensitization, compared to wildtype fracture mice (Fig. 1 C,D). The C5a receptor deficient fracture mice also failed to develop hindpaw warmth, but did develop hindpaw edema similar to wildtype fracture mice (Fig. 1 E,F).
Figure 1. Complement 5a receptor (C5aR) signaling contributed to post fracture (FX) nociceptive sensitization.
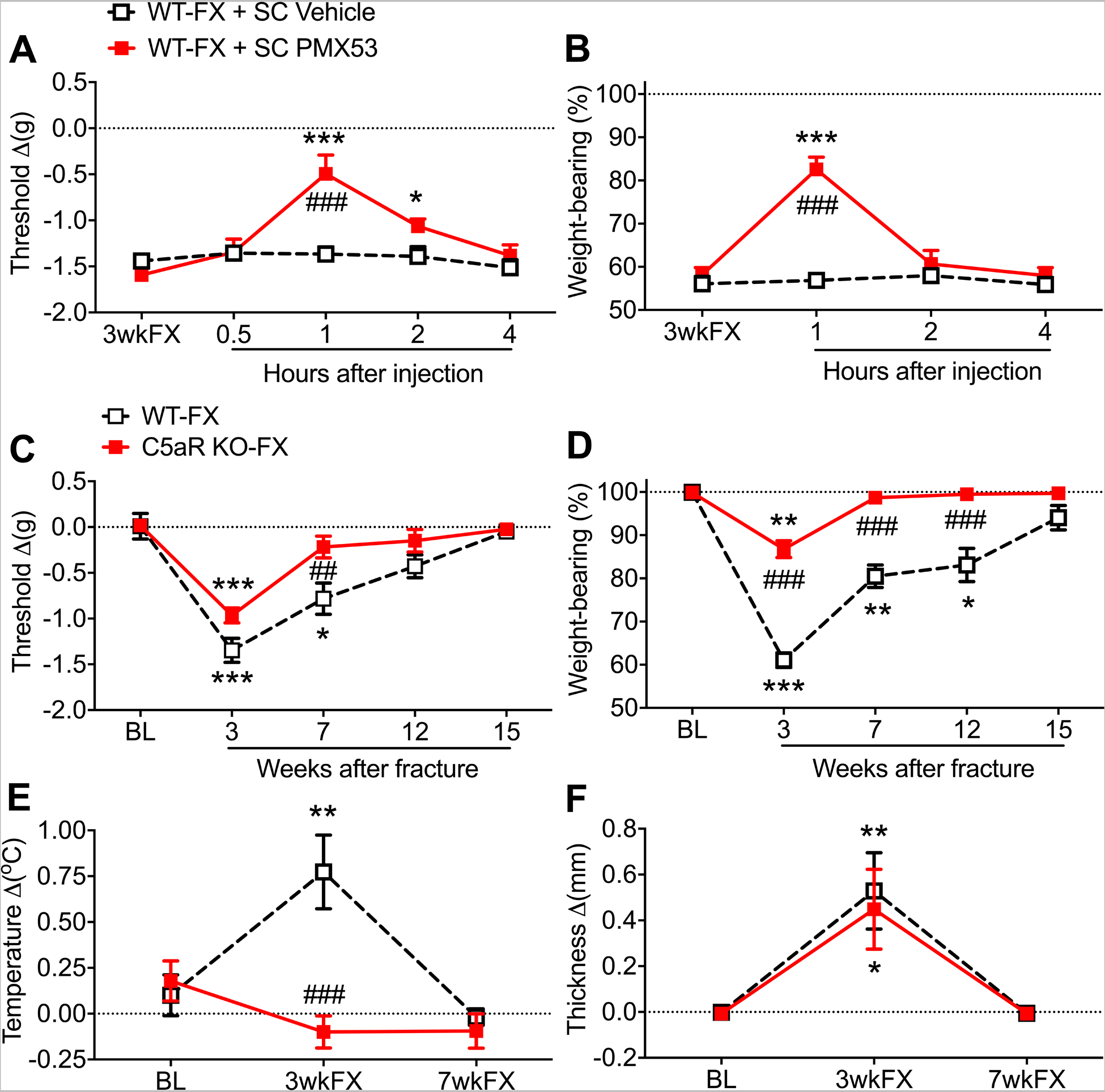
At 3 weeks after tibia FX and casting wildtype (WT) C57BL6 mice exhibited unilateral hindpaw von Frey allodynia (A) and unweighting (B). The subcutaneous injection of a C5aR antagonist (PMX53, 4mg/kg, SC) transiently reversed allodynia and unweighting in the FX mice. When C5aR deficient mice on a BALB/cJ background underwent tibia FX/casting they developed attenuated hindpaw allodynia (C), unweighting (D), and warmth (E) compared to WT BALB/cJ FX mice, but hindpaw edema (F) in the C5aR deficient FX mice was similar to that observed in WT FX mice. Measurements for A, C, E, and F represent the difference between the FX side and contralateral paw, thus a negative von Frey threshold value represents a decrease in mechanical withdrawal thresholds on the affected side and positive temperature and thickness values represent increased hindpaw warmth and edema on the FX side. Measurements for B and D represent weight-bearing on the FX hind limb as a ratio to half of the total bilateral hind limb loading, thus, a percentage lower than 100% represents hindpaw unweighting. A 2-way repeated measures analysis of variance was used to test the effects of each treatment group on the dependent variables over time, using a Sidak correction test for post hoc contrasts. Data are expressed as mean values ± SEM, n = 7 per cohort. #P < 0.05, ## P < 0.01, and ### P < 0.001 for differences between the treatment groups, *P < 0.05, ** P < 0.01, and *** P < 0.001 for differences over time from 3wkFX (A,B) or BL (C-F) values. WT: wildtype mice, FX: fracture, SC: subcutaneous injection, PMX53: C5aR antagonist, BL: baseline, 3wkFX: 3 weeks after fracture, 7wkFX: 7 weeks after fracture
3.2. Fracture increased C5a receptor expressing macrophages in the ipsilateral hindpaw
Immunohistochemistry in hindpaw plantar skin sections demonstrated a 6-fold increase in C5aR expressing Iba-1 positive activated macrophages in the dermis of 3-week fracture mice, vs intact controls (Fig. 2). Dermal fibroblasts also expressed C5aRs, but fracture did not significantly up-regulate the number of double-labeled vimentin positive fibroblasts. Hindpaw skin sections were also immunostained with C5aR, keratin (a keratinocyte marker), and tryptase (a mast cell marker), but no double-labeling for C5aR was observed in keratinocytes or mast cells (data not shown).
Figure 2. Fracture caused an increase in C5aR expressing macrophages in hindpaw skin.
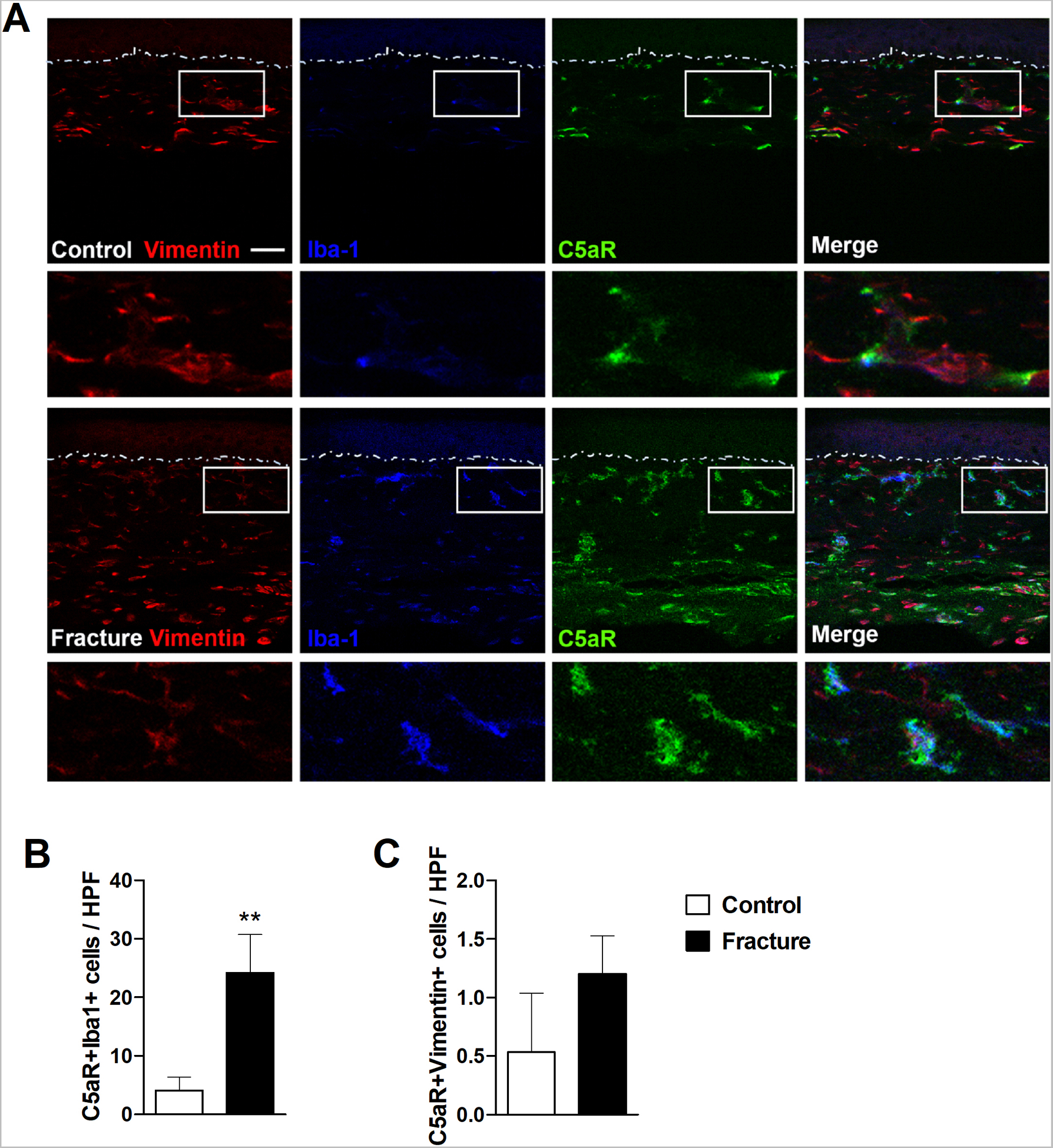
At 3 weeks post fracture immunohistochemistry was used to identify C5aR cellular expression in the fracture hindpaw skin. (A) Representative confocal fluorescent images of vimentin (red, a fibroblast marker), Iba1 (blue, a macrophage marker), and C5aR1 (green). The top row of images are from an intact control mouse, the second row are the enlargements of the box regions of the first row, the third row of images are from a fracture mouse, and the bottom row panels are the enlargements of the box regions of the third row. Triple labeling demonstrated a 6-fold increase in C5aR-positive macrophages in the hindpaw skin after fracture. Dotted lines indicate the epidermal-dermal boundary. Scale bar = 25 μm. Bar graphs present the average number of C5aR+Iba1+ (B) and C5aR+vimentin+(C) double-labeled cells per high-power field (HPF) in the hindpaw skin of fracture and intact control mice. An unpaired student’s t-test was used to test differences between treatment groups. Data are expressed as mean values ± SEM, n = 5 per cohort. **p<0.01 for fracture vs controls.
3.3. Pronociceptive C5a signaling in the fracture mouse hindpaw skin requires immunoglobulin
Intraplantar injection of PMX53 (30ug/5ul) into the injured limb hindpaw of 4 weeks post fracture wildtype mice partially reversed allodynia and unweighting between 0.5 and 3 hours post injection, but had no effect in muMT fracture mice, indicating that immunoglobulin is required for the post fracture development of C5a pronociceptive effects (Fig. 3 A,B). Intraplantar injection of C5a (200ng/5ul) into the injured hindpaw of 4 weeks post fracture muMT mice caused increased allodynia and unweighting lasting for 3 hours, but had no effect in muMT nonfracture mice, indicating that the pronociceptive effects of activating C5a receptors in the hindpaw skin are dependent on the tibia fracture 4 weeks prior to testing (Fig. 3 C,D). Interestingly, at 4 weeks post fracture there was an increase in the mRNA expression of C5aR1 and C5aR2 receptors in the hindpaw skin of muMT fracture mice (Fig. 3E,F), indicating up-regulated C5a signaling after fracture. Furthermore, C5a protein levels increased in the hindpaw skin of wildtype mice after fracture (Fig. 3G), a possible explanation for the analgesic effectiveness of PMX53 intraplantar injections in wildtype fracture mice (Fig. 3 A,B).
Figure 3. C5aR cutaneous signaling contributed to post fracture nociceptive sensitization in wildtype mice, but not in muMT mice lacking IgM.
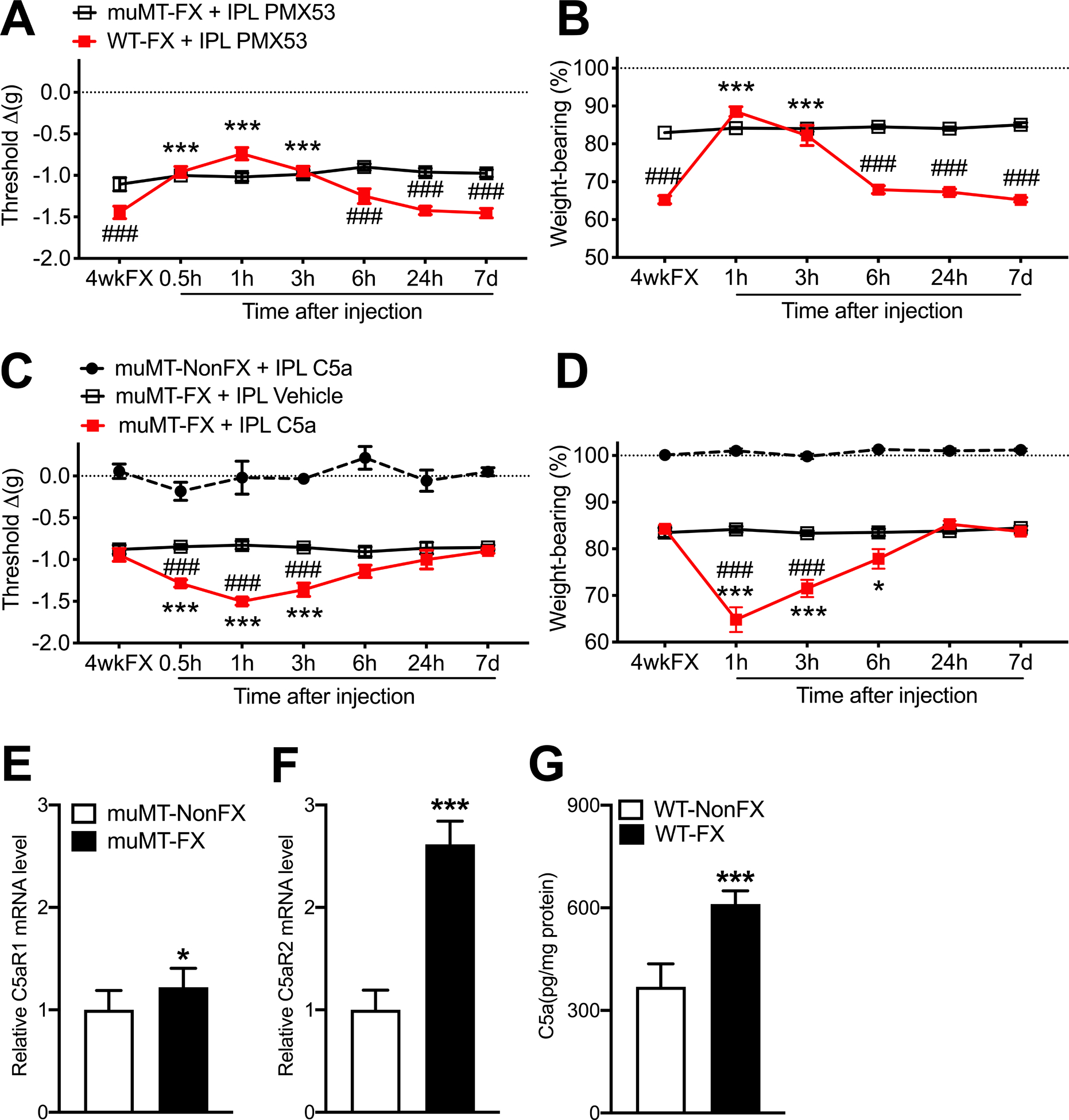
By 4 weeks post tibia fracture and casting (4wkFX) the wildtype (WT) mice had developed robust hindpaw von Frey allodynia (A) and unweighting (B), but muMT mice lacking IgM exhibited attenuated post FX hindpaw allodynia and unweighting. Intradermal plantar injection of a C5aR antagonist (PMX53, 30ug/5ul, IPL) transiently reversed allodynia (A) and unweighting (B) in WT FX mice, but not in muMT FX mice lacking IgM. Intradermal plantar injection of C5a (200ng/5ul, IPL) in muMT FX mice caused increased von Frey allodynia (C) and unweighting (D), and these pronociceptive effects of intradermal C5a were restricted to the FX limb (data not shown) and were not observed when C5a was intraplantarly injected into nonfracture (nonFX) muMT mice. MuMT FX mice exhibited increased C5aR1 and C5aR2 mRNA expression in the hindpaw skin, relative to nonFX muMT mice (E,F), a possible explanation for the lack of pronociceptive effects observed after C5a IPL injection in nonFX muMT mice (C). WT FX mice exhibited increased C5a protein levels in the hindpaw skin, related to WT nonFX mice (G). A 2-way repeated measures analysis of variance was used to test the effects of each treatment group on the dependent variables over time, using a Sidak correction test for post hoc contrasts. Data are expressed as mean values ± SEM, n = 7–8 per cohort. #P < 0.05, ## P < 0.01, and ### P < 0.001 for differences between the treatment groups, *P < 0.05, ** P < 0.01, and *** P < 0.001 for differences over time from 4wkFX values. muMT: mice lacking B cells and immunoglobulin, WT: wildtype C57BL/6 mice, FX: fracture, IPL: intradermal plantar injection, PMX53: C5aR antagonist, 4wkFX: 4 weeks after fracture, C5a: complement 5a
3.4. Intraplantar injection of CRPS patient IgM induced C5a and cytokine mediated inflammatory pronociceptive effects in fracture mouse skin
Intraplantar injection of control subject IgM (5ug/5ul) into the injured hindpaw of 4 weeks post fracture muMT mice, with or without the co-injection of the C5aR antagonist PMX53 (30ug/5ul), had no effect on hindpaw allodynia and unweighting (Fig. 4 A,B). Intraplantar injection of CRPS patient IgM (5ug/5ul) in muMT fracture mice caused increased hindpaw allodynia and unweighting, and this pronociceptive antibody effect was completely blocked by PMX53 co-injection (Fig. 4 C,D), indicating that CRPS IgM pronociceptive effects in fracture mice are dependent on C5a signaling. Similarly, pretreatment with the global cytokine inhibitor pentoxifylline (200mg/k, PO daily) for 2 days prior also blocked CRPS IgM pronociceptive effects in the injured hindpaw skin (Fig. 4 E,F). Another experiment observed that intraplantar injection of CRPS patient IgM (5ug/5ul) into the hindpaw of muMT fracture mice evoked increased hindpaw skin mRNA expression of TNF, IL-1, and IL-6 inflammatory cytokines and increased C5, C1qa, C1qb, and C1qc complement components, relative to the effects of intraplantar injection of control subject IgM (Fig.5). Co-injection of CRPS IgM with PMX53 (30ug/5ul) completely blocked CRPS IgM evoked cytokine expression in the skin, indicating that the cutaneous inflammatory effects of CRPS IgM require C5a signaling (Fig. 5). Intraplantar injection of CRPS IgM also caused increased C5b-9 complement immunostaining in the epidermal keratinocytes of muMT mouse fracture hindpaw skin, relative to intraplantar injection of control IgM in muMT fracture mice (Fig. 6). Collectively, these results support the hypothesis that CRPS IgM binds to antigens in the fracture hindpaw skin, activating the classical complement cascade generating C5a that activates C5aR receptors on cutaneous immune cells, triggering inflammatory cytokine up-regulation and subsequent nociceptive sensitization.
Figure 4. The pronociceptive effects of CRPS patient IgM antibodies in the fracture hindpaw of muMT mice were mediated by C5aR and cytokine signaling.
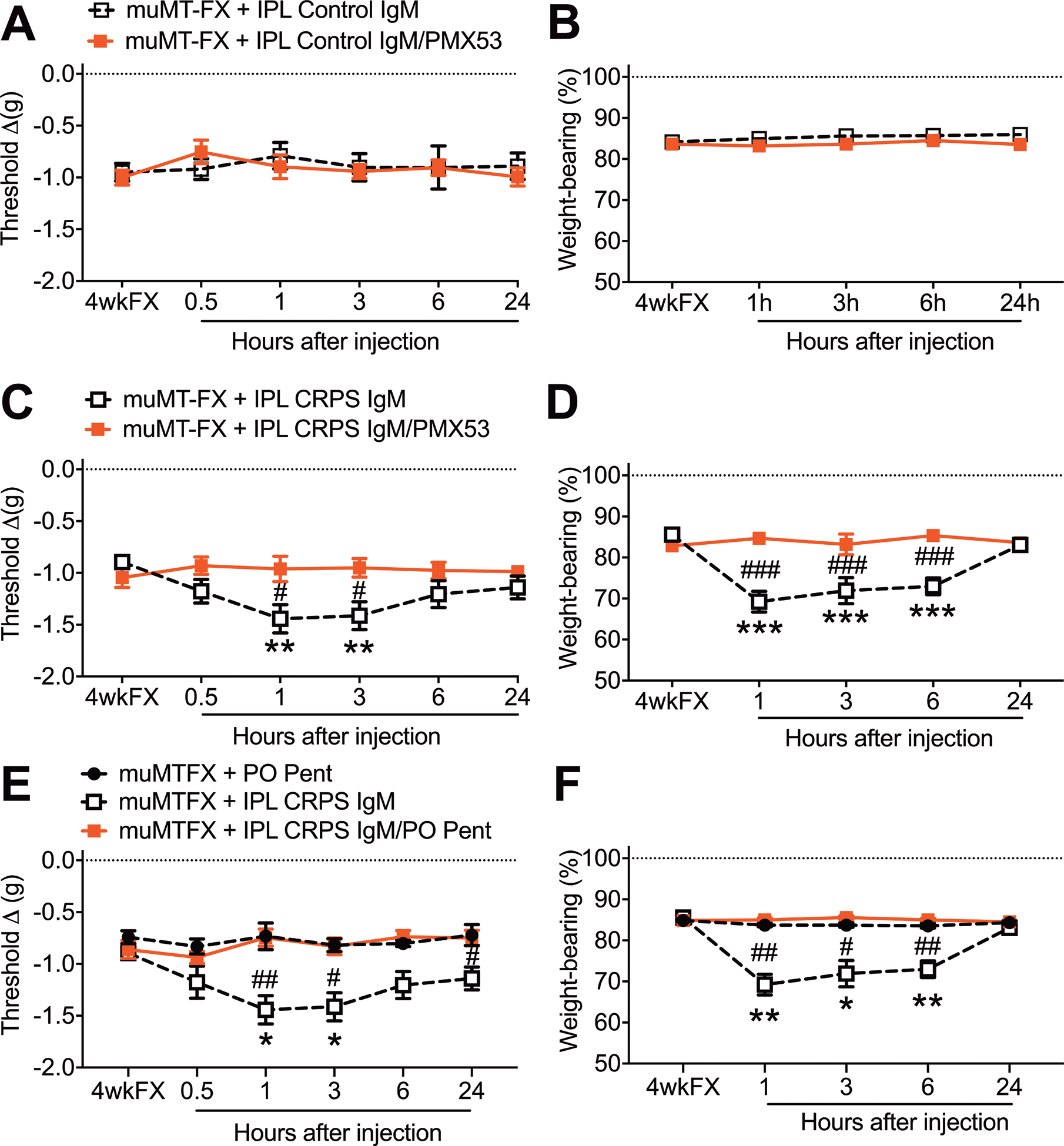
Intraplantar injection of normal subject Control IgM (5ug/5ul, IPL), with or without a C5aR antagonist (PMX53, 30ug/5ul, IPL), into 4 weeks post fracture (4wkFX) muMT mice lacking IgM had no effect on hindpaw von Frey allodynia (A) and unweighting (B). Intraplantar injection of early (< 12 months duration) CRPS patient IgM (5ug/5ul, IPL) had pronociceptive effects in muMT-FX mice, evoking increased von Frey allodynia (C) and unweighting (D) over a 3–6 hour post injection period, and these pronociceptive effects were restricted to the FX limb (data not shown). Intradermal plantar injection of CRPS patient IgM with co-injection of a C5aR antagonist (PMX53, 30ug/5ul, IPL) blocked IgM pronociceptive effects on hindpaw allodynia (C) and unweighting (D) in muMT FX mice. Similarly, pretreatment with the global cytokine inhibitor pentoxifylline (200mg/kg, PO daily) for 2 days also blocked IgM pronociceptive effects on allodynia (E) and unweighting (F) in muMT FX mice. A 2-way repeated measures analysis of variance was used to test the effects of each treatment group on the dependent variables over time, using a Sidak correction test for post hoc contrasts. Data are expressed as mean values ± SEM, n = 7–8 per cohort. #P < 0.05, ## P < 0.01, and ### P < 0.001 for differences between the treatment groups, *P < 0.05, ** P < 0.01, and *** P < 0.001 for differences over time from the pre-injection 4wkFX values. muMT: mice lacking B cells and immunoglobulin, FX: fracture, IPL: intradermal plantar injection, PMX53: C5aR antagonist, PO Pent: oral pentoxifylline, C5a, 4wkFX: 4 weeks after fracture
Figure 5. Intraplantar injection of CRPS patient IgM induced the cutaneous expression of TNF, IL-1, IL-6, C1qa, C1qb, C1qc in muMT fracture mice.
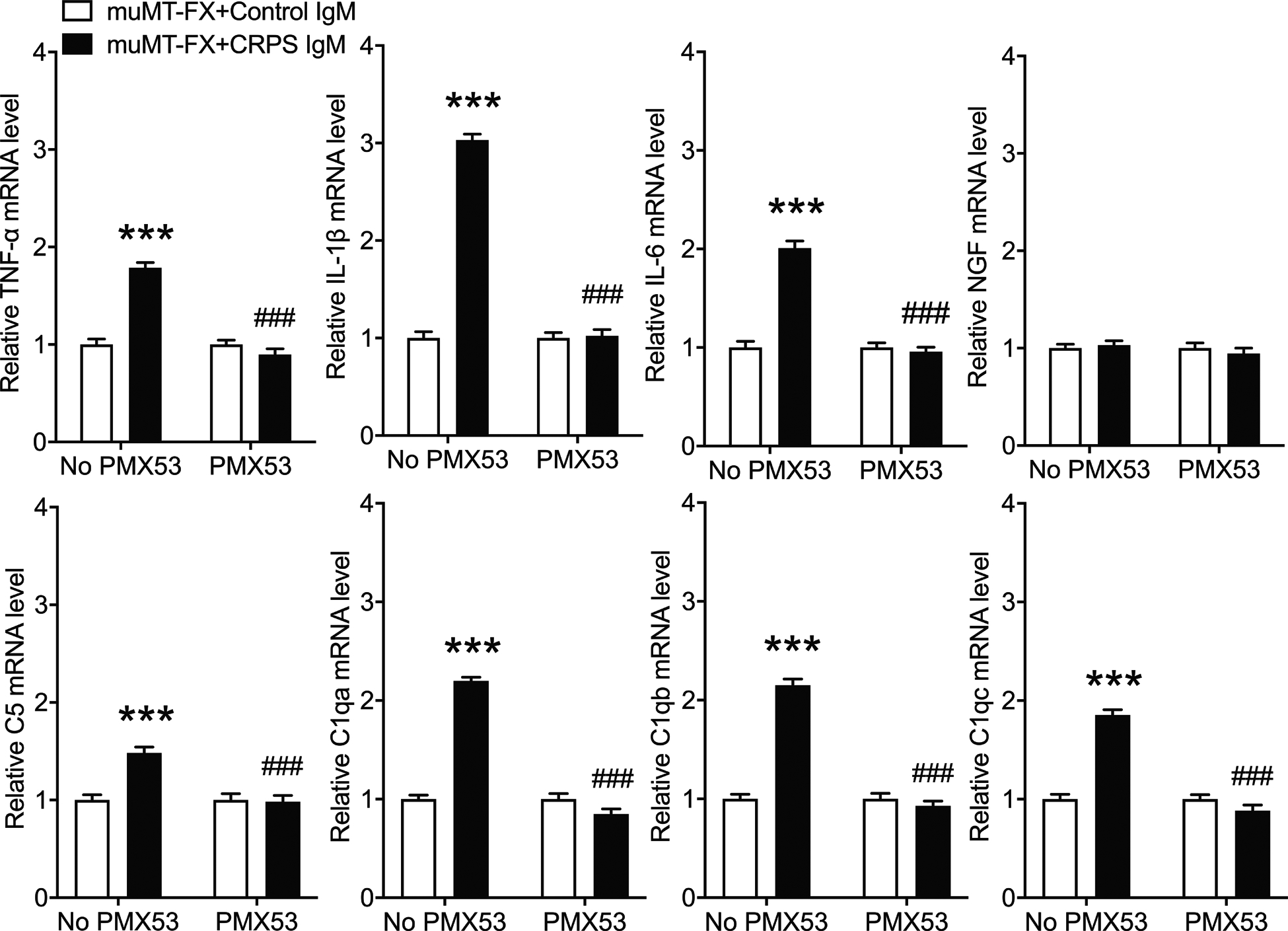
PCR was used to measure plantar cutaneous gene expression of TNF, IL-1, IL-6, NGF, C5, C1qa, C1qb, and C1qc in the fracture hindpaw at 1 hour after intraplantar IgM injection in 4 week post fracture muMT mice lacking IgM. Intraplantar injection of early CRPS patient IgM (5ug/5ul, IPL) caused up-regulated plantar cutaneous expression of TNF, IL-1, IL-6, C1qa, C1qb, and C1qc when compared to expression in mice intradermally injected with normal subject control IgM (5ug/5ul, IT). A one-way analysis of variance was used to test the effects of each treatment group on the dependent variables, using a Sidak correction test for post hoc contrasts. Data are expressed as mean values ± SEM, n = 10 per cohort. *P < 0.05, ** P < 0.01, and *** P < 0.001 for differences between treatment groups. CRPS: complex regional pain syndrome, IPL: intradermal plantar injection, TNF: tumor necrosis factor, IL-1: interleukin 1, IL-6: interleukin 6, NGF: nerve growth factor, C5: complement 5, C1qa, C1qb, C1qc: a, b, and c isoforms of complement 1q
Figure 6. Intraplantar injection of CRPS IgM into muMT fracture mice increased epidermal C5b-9 complement deposition.
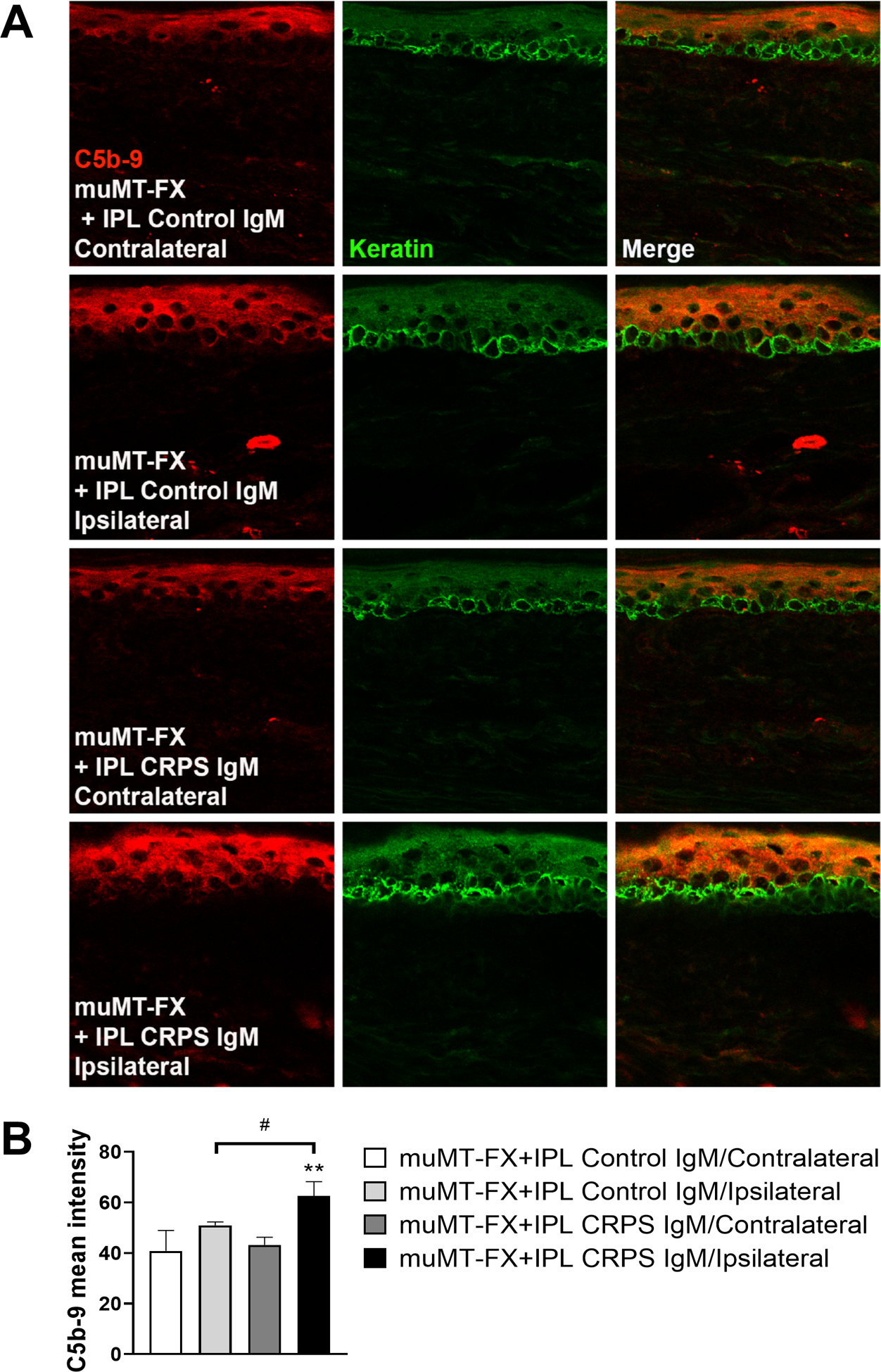
Changes in hind paw skin C5b-9 membrane attack complex protein levels in muMT fracture mice (FX) after intradermal plantar injection with either normal subject IgM (Control IgM) or early CRPS patient IgM (CRPS IgM). (A) Representative C5b9 protein (red), keratin (green, a keratinocyte marker), and merged images from contralateral and ipsilateral hindpaw skin for each of the treatment conditions are displayed. Top panels are images (magnification, 63x) from a contralateral hindpaw skin in a muMT FX mouse treated with Control IgM, second panels are from the ipsilateral hindpaw skin in the muMT FX mouse treated with Control IgM, third panels are from a contralateral hindpaw skin in a muMT FX mouse treated with CRPS IgM, and bottom panels are from the ipsilateral hindpaw skin in the muMT FX mouse treated with CRPS IgM. CRPS IgM treatment leads to increased C5b-9 deposition in the ipsilateral hindpaw skin to fracture. (B) Quantitation of C5b9 mean intensity in the hindpaw skin. Data are expressed as mean values ± SD.**p < 0.01 for FX-CRPS IgM/Ipsilateral (n = 4) vs FX-CRPS IgM/Contralateral (n = 4) values, # p < 0.05 for FX-CRPS IgM/Ipsilateral (n = 4) vs FX-Control IgM/Ipsilateral (n = 4). FX: fracture, muMT: mice lacking B cells and immunoglobulin, IPL: intraplantar injection.
3.5. Fracture increased C5a receptor expression in microglia the ipsilateral lumbar cord
At 3 weeks post fracture ipsilateral lumbar spinal cord sections were collected and immunostained for C5aR, Iba-1 (an activated microglial marker), and NeuN (a neuronal marker). Fracture caused a 2.6-fold increase in C5aR labeled activated microglia and a 1.3 fold increase in C5aR labeled neurons in the dorsal horn when compared to intact controls (Fig. 7).
Figure 7. Fracture caused an increase in C5aR expressing microglia in the lumbar spinal cord.
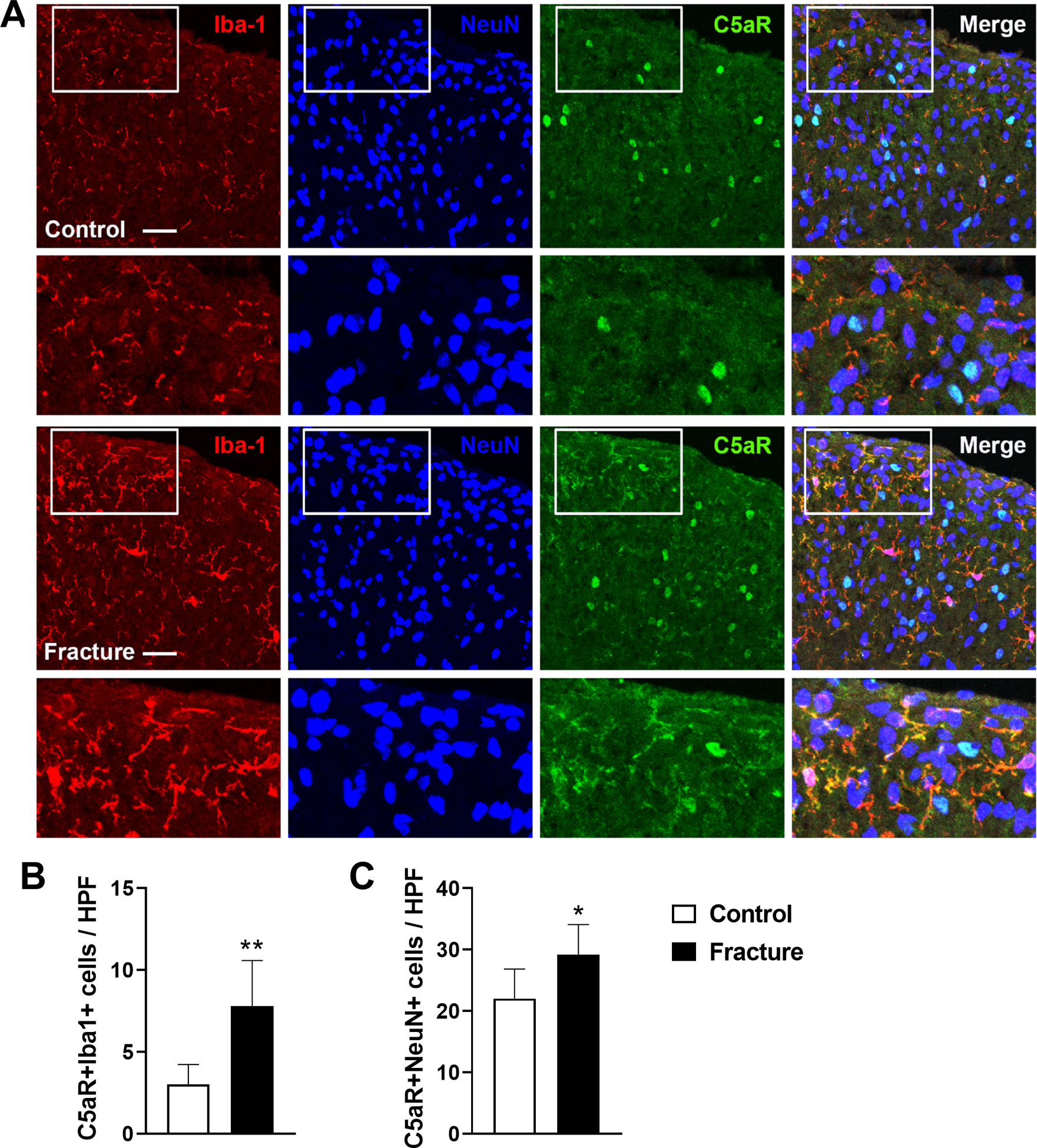
Immunohistochemistry was used to quantify C5aR cellular expression in the ipsilateral lumbar spinal cord of fracture and control mice. (A) Representative confocal fluorescent images of Iba1 (red, a microglial marker), NeuN (blue, a neuronal marker), and C5aR1 (green). The top row of images are from an intact control mouse, the second row are the enlargements of the box regions of the first row, the third row of images are from a 3 weeks post fracture mouse, and the bottom row panels are the enlargements of the box regions of the third row. Triple labeling demonstrated a 2.6 fold increase in C5aR-positive microglia and a 1.3 fold increase in C5aR-positive neurons in the lumbar cord after fracture. Scale bar = 25 μm. Bar graphs present the average number of C5aR+Iba1+ (B) and C5aR+NeuN +(C) double-labeled cells per high-power field (HPF) in the lumbar cord of fracture and intact control mice. An unpaired student’s t-test was used to test differences between treatment groups. Data are expressed as mean values ± SEM, n = 5 per cohort. *p<0.05, **p<0.01 for fracture vs controls.
3.6. Pronociceptive C5a signaling in the fracture mouse spinal cord requires immunoglobulin
Intrathecal injection of PMX53 (200ng/5ul) into 3 weeks post fracture wildtype mice partially reversed allodynia and unweighting between 0.5–6 hours post injection, but had no effect in muMT fracture mice, indicating that immunoglobulin is required for the post fracture development of C5a receptor mediated pronociceptive effects (Fig. 8 A,B). Intrathecal injection of C5a (200ng/5ul) into 3 weeks post fracture muMT mice caused increased allodynia and unweighting lasting for 3 hours, but had no effect in muMT nonfracture mice, indicating that the pronociceptive effects of activating C5a receptors in the hindpaw skin are dependent on hindlimb fracture trauma 3 weeks prior to testing (Fig. 8 C,D). Interestingly, at 3 weeks post fracture there was an increase in the mRNA expression of C5aR1 and C5aR2 receptors in the spinal cord of muMT fracture mice (Fig. 8 E,F), potentially amplifying the C5a signal after fracture. Furthermore, C5a protein levels increased in the lumbar spinal cord of wildtype mice after fracture (Fig. 8 G), a possible explanation for the analgesic effectiveness of PMX53 intrathecal injections in wildtype fracture mice (Fig. 8 A,B).
Figure 8. C5aR spinal signaling contributed to post fracture nociceptive sensitization in wildtype mice, but not in muMT mice lacking IgM.
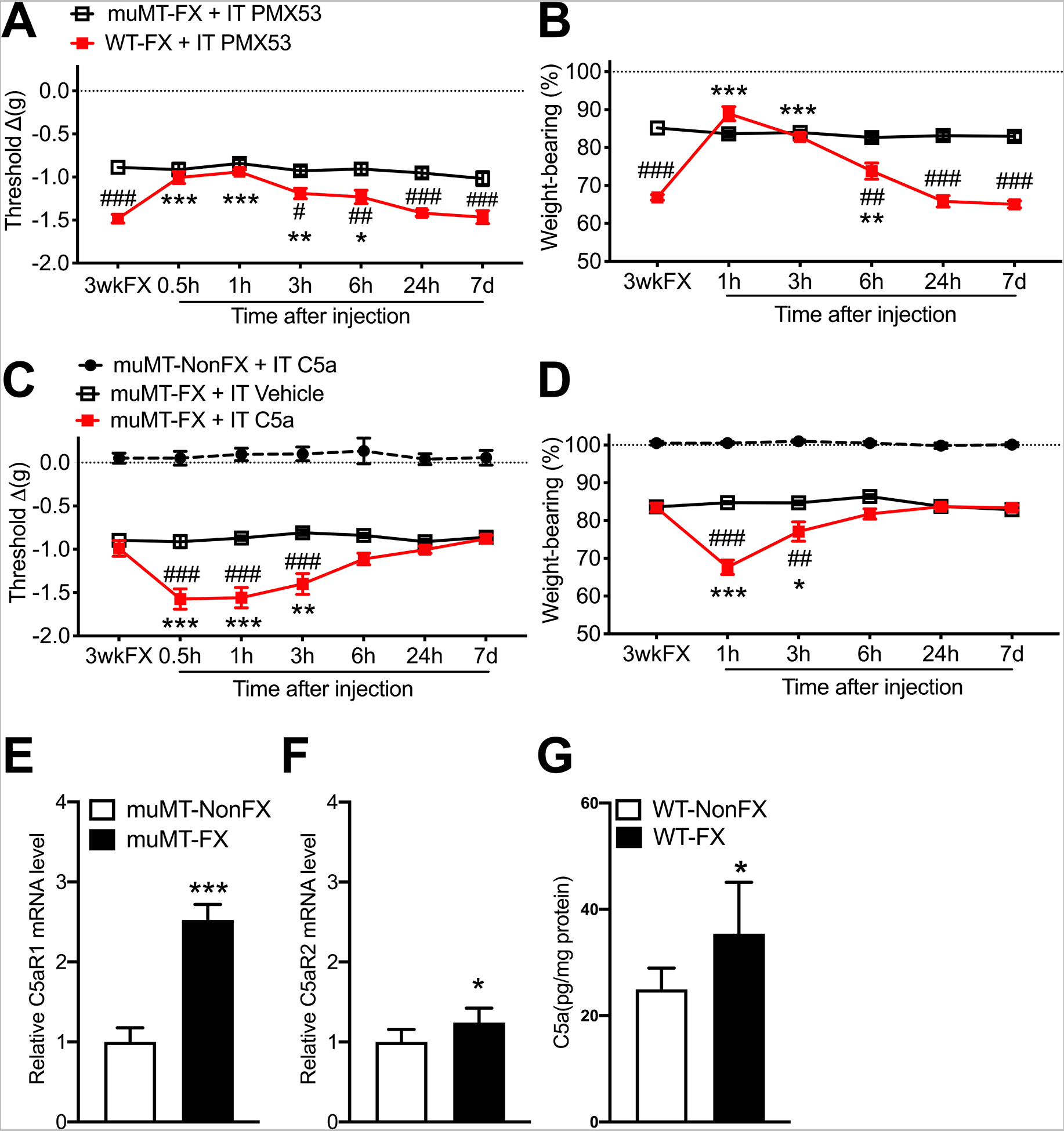
By 3 weeks post fracture (3wkFX) wildtype (WT) mice developed robust hindpaw von Frey allodynia (A) and unweighting (B), but 3wkFX muMT mice lacking IgM exhibited attenuated post FX hindpaw allodynia and unweighting. Intrathecal injection of a C5aR antagonist (PMX53, 200ng/5ul, IT) transiently reversed allodynia (A) and unweighting (B) in WT FX mice, but not in muMT FX mice lacking IgM. Intrathecal injection of C5a (200 ng/5ul, IT) into muMT mice exacerbated post FX allodynia (C) and unweighting (D), and these pronociceptive effects were restricted to the FX limb (data not shown) and were not observed when C5a was intrathecally injected into nonfracture muMT mice. Fracture induced increased C5aR1 (E) and C5aR2 (F) mRNA expression in the lumbar cord of muMT mice and increased C5a protein levels in the lumbar cord of WT mice (G). A 2-way repeated measures analysis of variance was used to test the effects of each treatment group on the dependent variables over time, using a Sidak correction test for post hoc contrasts. Data are expressed as mean values ± SEM, n = 7–8 per cohort. #P < 0.05, ## P < 0.01, and ### P < 0.001 for differences between the treatment groups, *P < 0.05, ** P < 0.01, and *** P < 0.001 for differences over time from 3wkFX values (A-D)or for differences between NonFX and FX mice (E-G). muMT: mice lacking B cells and immunoglobulin, WT: wildtype C57BL/6 mice, FX: fracture, NonFX: no fracture, IT: intrathecal injection, PMX53: C5aR antagonist, 3wkFX: 3 weeks after fracture, C5a: complement 5a
3.7. Intrathecal injection of CRPS patient IgM induced C5a and cytokine mediated inflammatory pronociceptive effects in fracture mouse lumbar spinal cord
Intrathecal injection of control subject IgM (5ug/5ul) into 3 weeks post fracture muMT mice, with or without the co-injection of PMX53 (200ng/5ul), had no effect on hindpaw allodynia and unweighting (Fig. 9 A,B). Intrathecal injection of CRPS patient IgM (5ug/5ul) in muMT fracture mice caused increased hindpaw allodynia and unweighting lasting for 6 hours, and this pronociceptive antibody effect was completely blocked by PMX53 co-injection (Fig. 9 C,D), indicating that CRPS IgM causes nociceptive sensitization in the muMT fracture mouse and this pronociceptive effect is dependent on C5a signaling in the spinal cord. Similarly, pretreatment with the global cytokine inhibitor pentoxifylline (200mg/k, PO daily) for 2 days prior also blocked CRPS IgM pronociceptive effects in the spinal cord (Fig. 9 E,F). Another experiment observed that intrathecal injection of CRPS patient IgM (5ug/5ul) into muMT fracture mice evoked increased lumbar cord mRNA expression of TNF and IL-1 relative to the effects of intrathecal injection of control subject IgM (Fig.10). There was no increase in IL-6, NGF, C5, C1qa, C1qb, and C1qc expression after CRPS IgM injection. Intrathecal injection of CRPS IgM with PMX53 (200ng/5ul) completely blocked the spinal cord proinflammatory effects of CRPS IgM (Fig. 10). Collectively, these results support the hypothesis that CRPS IgM binds to post fracture neoantigens in the lumbar cord, activating the classical complement cascade generating C5a that activates C5aR receptors on spinal microglia, triggering inflammatory cytokine up-regulation and subsequent nociceptive sensitization.
Figure 9. The pronociceptive effects of CRPS patient IgM antibodies in the spinal cord of muMT fracture mice were mediated by C5aR and cytokine signaling.
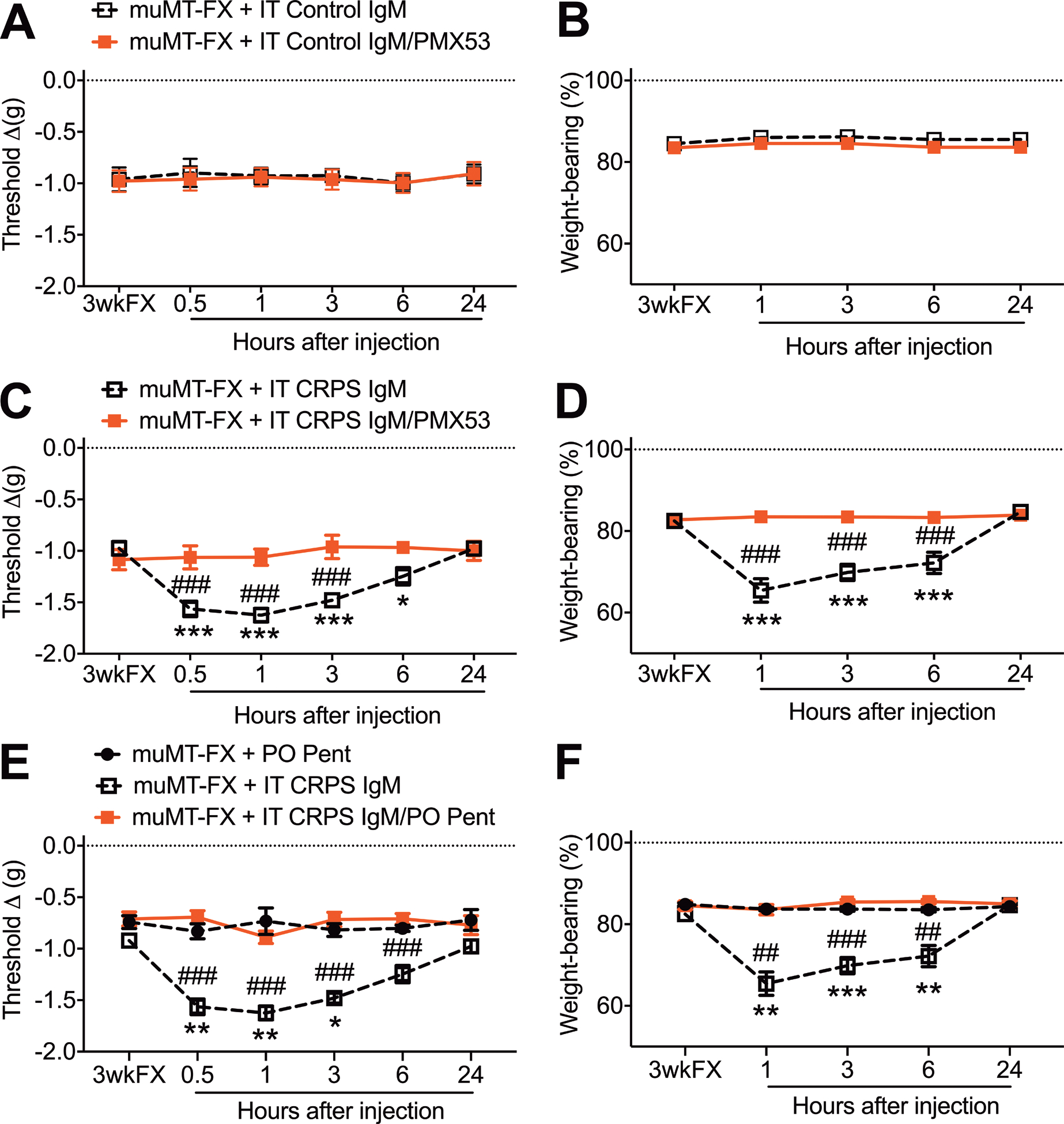
Intrathecal injection of normal subject Control IgM (5ug/5ul, IPL), with or without a C5aR antagonist (PMX53, 30ug/5ul, IPL), into 3 weeks post fracture (3wkFX) muMT mice lacking IgM had no effect on hindpaw von Frey allodynia (A) and unweighting (B). Intrathecal early CRPS patient IgM (5ug/5ul, IPL) injection had pronociceptive effects in muMT FX mice, evoking increased hindpaw allodynia (C) and unweighting (D) over a 6 hour post injection period, and these pronociceptive effects were restricted to the FX limb (data not shown). Intrathecal injection of CRPS patient IgM with co-injection of a C5aR antagonist (PMX53, 30ug/5ul, IPL) blocked IgM pronociceptive effects on allodynia (C) and unweighting (D) in muMT FX mice. Similarly, pretreatment with the global cytokine inhibitor pentoxifylline (200mg/kg, PO daily) for 2 days also blocked IgM pronociceptive effects on allodynia (E) and unweighting (F) in muMT FX mice. A 2-way repeated measures analysis of variance was used to test the effects of each treatment group on the dependent variables over time, using a Sidak correction test for post hoc contrasts. Data are expressed as mean values ± SEM, n = 7–8 per cohort. ## P < 0.01, and ### P < 0.001 for differences between the treatment groups, *P < 0.05, ** P < 0.01, and *** P < 0.001 for differences over time from 3wkFX values. muMT: mice lacking B cells and immunoglobulin, FX: fracture, IT: intrathecal injection, PMX53: C5aR antagonist, 3wkFX: 3 weeks after fracture, PO Pent: oral pentoxifylline
Figure 10. CRPS patient IgM induced the spinal expression of TNF and IL-1 in muMT fracture mice.
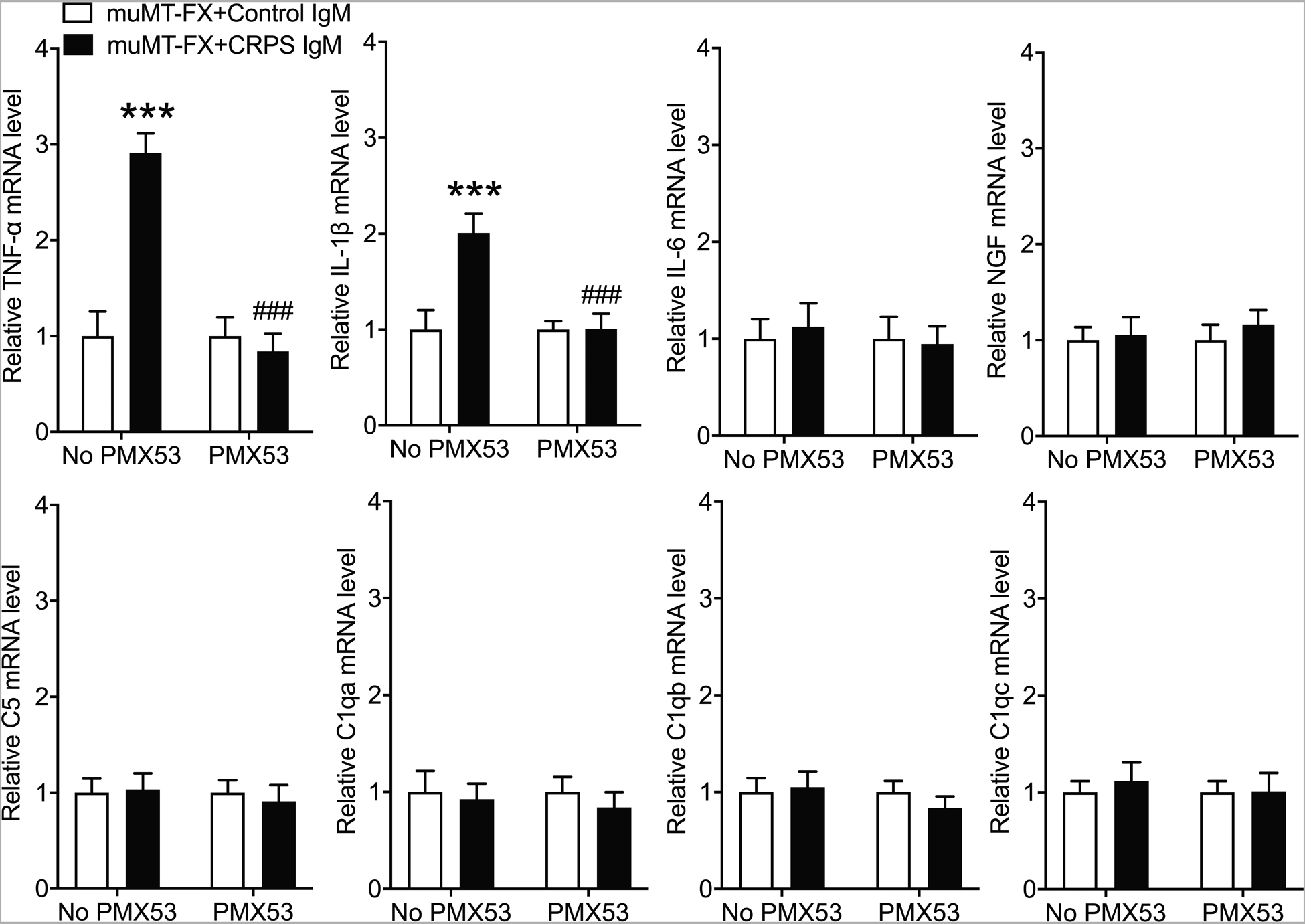
PCR was used to measure lumbar spinal cord gene expression of TNF, IL-1, IL-6, NGF, C5, C1qa, C1qb, and C1qc at 1 hour after intrathecal injection of early CRPS patient IgM into 3 week post fracture muMT mice lacking B cells and IgM. Intrathecal injection of IgM (5ug/5ul, IT) caused up-regulated spinal expression of TNF and IL-1, when compared to spinal cord expression in mice intrathecally injected with normal subject control IgM (5ug/5ul, IT). A one-way analysis of variance was used to test the effects of each treatment group on the dependent variables, using a Sidak correction test for post hoc contrasts. Data are expressed as mean values ± SEM, n = 10 per cohort. *P < 0.05, ** P < 0.01, and *** P < 0.001 for differences between treatment groups. CRPS: complex regional pain syndrome, IT: intrathecal injection, TNF: tumor necrosis factor, IL-1: interleukin 1, IL-6: interleukin 6, NGF: nerve growth factor, C5: complement 5, C1qa, C1qb, C1qc: a, b, and c isoforms of complement 1q
3.8. Chronic CRPS IgM had pronociceptive effects after intrathecal injection
Figure 11 illustrates the development of increased hindpaw von Frey allodynia and unweighting after intrathecally injecting 5ug of chronic CRPS patient IgM into muMT fracture mice. Intraplantar injection of 5ug of chronic CRPS patient IgM had no effect in the muMT fracture mice. We also tested the effects of an intraplantar injection with a higher dose (10ug) of control subject IgM in the muMT fracture mice, but unfortunately this was pronociceptive, thus we were unable to increase the intraplantar injection dosage for chronic CRPS IgM. Intrathecal or intraplantar injection with 5ug of resolved CRPS patient IgM or with chronic CRPS patient IgG had no pronociceptive effects. Previously we observed pronociceptive effects after intraperitoneal sera (0.5ml) injections in just 2/20 chronic CRPS sera; these 2 patients had disease durations of 15 and 24 months and the systemic injection of their sera only increased von Frey allodynia in the muMT mice, with no effect on hindpaw unweighting.[14] The chronic CRPS IgM used in the current study was derived from the pooled sera of 5 patients (average CRPS duration of 4.4 years, range of 2.5–7 years) whose individual sera had no pronociceptive effects after intraperitoneal injections (0.5ml) into muMT fracture mice.[14] The resolved CRPS patient IgM used in this study was derived from the pooled sera of 2 patients who had previously met the diagnostic criteria for early (<12 months duration) CRPS and whose sera was initially pronociceptive when intraperitoneally injected into muMT fracture mice, but when these same patients were reevaluated several years later they no longer met the diagnostic criteria for CRPS and their sera had no pronociceptive effects after intraperitoneal injection into the muMT fracture mice.[14]
Figure 11. Chronic CRPS patient IgM (but not IgG) is pronociceptive when injected intrathecally into muMT fracture mice.
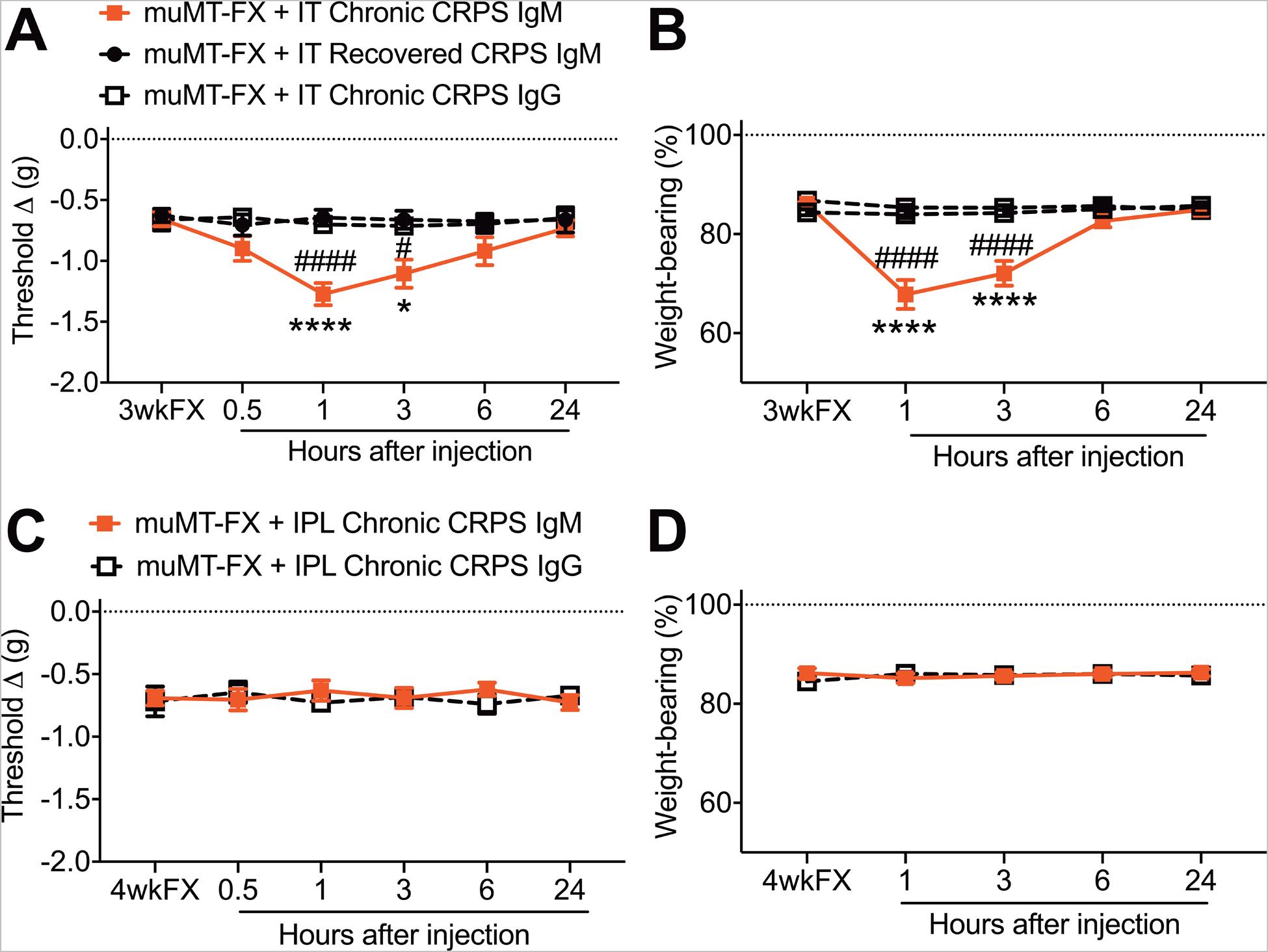
Intrathecal injection of chronic (> 12 months duration) CRPS patient IgM (5ug, IT) had pronociceptive effects in 3 weeks post fracture (3wkFX) muMT mice, evoking increased hindpaw allodynia (A) and unweighting (B) over a 6 hour post injection period, and these pronociceptive effects were restricted to the FX limb (data not shown). Intrathecal injection of recovered CRPS patient IgM (5ug, IT) or chronic (>12 months duration) CRPS patient IgG (5ug, IT) into muMT mice lacking IgM had no effect on hindpaw allodynia and unweighting. Intraplantar injections of chronic CRPS patient IgM (5ug, IPL) or IgG (5ug, IPL) into muMT FX mice had no effect on allodynia (C) and unweighting (D). A 2-way repeated measures analysis of variance was used to test the effects of each treatment group on the dependent variables over time, using a Sidak correction test for post hoc contrasts. Data are expressed as mean values ± SEM, n = 7–8 per cohort. #P < 0.01, and ### P < 0.001 for differences between the treatment groups. muMT: mice lacking B cells and immunoglobulin, FX: fracture, IT: intrathecal injection, IPL: intraplantar, FX: 3 weeks after fracture
4. Discussion
Figure 12 presents a schematic of the autoimmune signaling pathways mediating the pronociceptive effects CRPS patient IgM in mice. C1 complement binding to Fc fragments of antibody-antigen immune complexes initiates the classical complement pathway, with formation of C5a complement proteins activating C5aRs on dermal macrophages and spinal microglia to induce the production and secretion of IL-1, IL-6, TNF, and NGF.[28,33,36,37] Previously we demonstrated increased C5b-9 complement levels in fracture limb skin and nerve,[22] consistent with activation of the complement cascade, and observed spinal microglia activation and increased expression of spinal cord inflammatory mediators contributing to allodynia and unweighting in the injured limb.[23,32] When these inflammatory mediators (IL-1, IL-6, TNF, and NGF) were injected intraplantarly into hindpaw skin they each individually evoked mechanical allodynia,[20,24] consistent with literature demonstrating rapid direct local sensitizing effects on sensory nociceptive neurons and delayed long lasting sensitizing effects of NGF cutaneous injection in the dorsal horn neurons.[2,27,30,31]
Figure 12. Overview of the hypothesis that autoimmune signaling pathways mediate the pronociceptive effects CRPS patient IgM in muMT fracture mice lacking immunoglobulin.
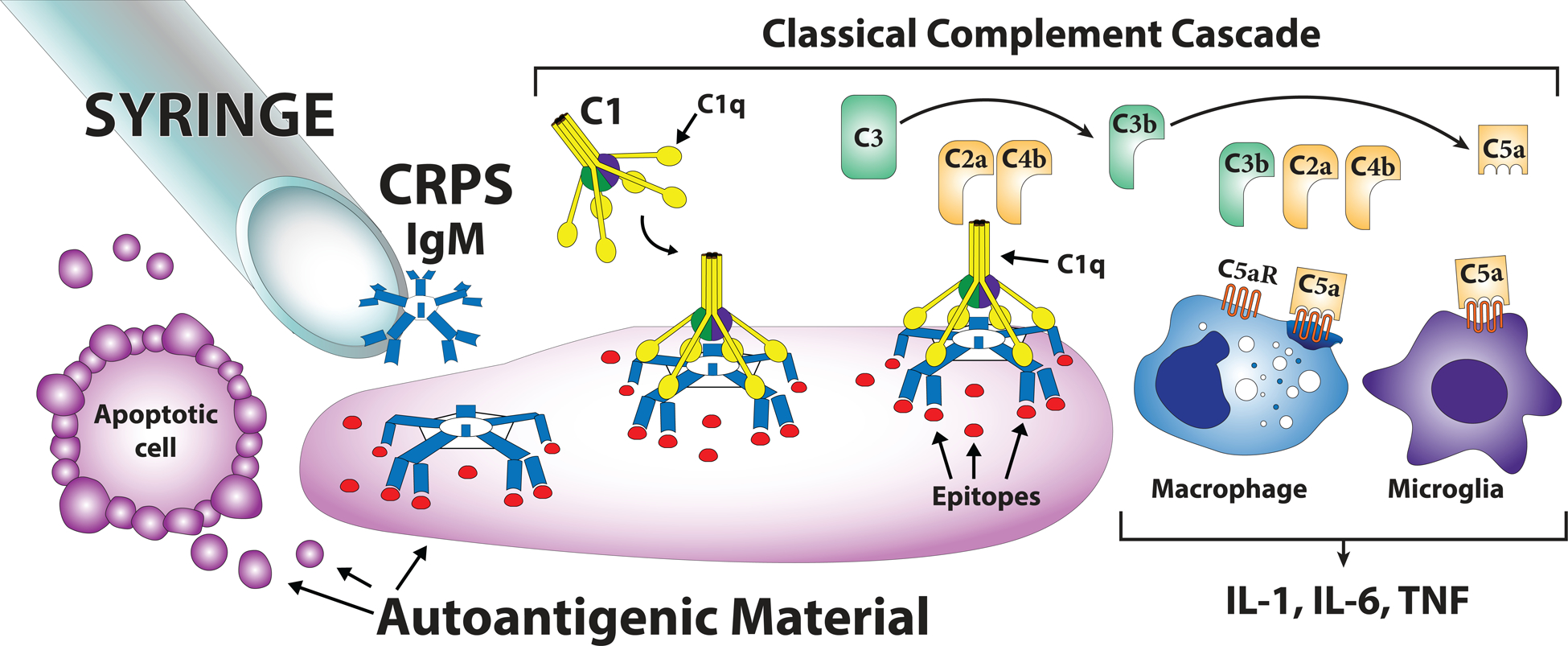
When CRPS patient IgM is injected intraplantarly or intrathecally into 3-week post fracture muMT mice it attaches to autoantigenic material released from apoptotic cells in the skin or spinal cord. When pentameric IgM binds to antigenic material larger than itself it can induce the conformational changes required to allow C1q binding. C1q, together with C1r and C1s, form the C1 complex that is activated when C1q binds to the antigen-antibody complex. Activation of the C1 complex initiates the classical complement pathway, thus generating the C5a complement components capable of activating the C5aR receptors expressed by skin macrophages and spinal cord microglia and inducing the release of the proinflammatory cytokines (IL-1, IL-6, and TNF) contributing to nociceptive sensitization in the fracture model of CRPS.
The current study demonstrated that systemic treatment with the C5aR antagonist PMX53 reduced post fracture allodynia and unweighting in WT mice and that mice lacking C5aR developed attenuated post fracture nociceptive sensitization (Fig.1). After fracture C5a levels in the skin increased and dermal macrophages expressed C5aRs (Figs.2,3). Intraplantar injection of PMX53 reduced nociceptive sensitization in wildtype fracture mice, but not in muMT fracture mice lacking B cells and immunoglobulin, evidence that antibody pronociceptive effects are C5a dependent (Fig.3A,B). Intraplantar injection of CRPS patient IgM (but not control subject IgM) caused increased allodynia and unweighting in the fracture limb hindpaw of muMT mice (Fig.4C,D), reaching the same levels of nociceptive sensitization as observed in wildtype fracture mice (Fig.1A,B). When CRPS IgM was co-injected intraplantarly with PMX53 it had no pronociceptive effect in muMT fracture mice, indicating that C5a signaling was required for CRPS IgM pronociceptive effects (Fig.4C,D). C5a intraplantar injection into nonfractured muMT mice had no effect, conversely, after fracture C5a intraplantar injection was pronociceptive (Fig.3 C,D). Fracture caused an increase in the hindpaw skin expression of C5aR in muMT mice (Fig.3 E,F) and increased hindpaw C5a protein levels in WT fracture mice (Fig.3 G), suggesting that exaggerated C5a/C5aR cutaneous signaling contributed to the pronociceptive effects of CRPS IgM.
Intraplantar injection of CRPS IgM increased the expression of TNF, IL-1, IL-6, C5, and C1q in the fracture hindpaw skin in muMT mice and co-injection of PMX53 completely blocked this inflammatory effect (Fig. 5). Furthermore, intraplantar injection of IgM after pretreatment with the global cytokine inhibitor pentoxifylline blocked IgM pronociception (Fig. 4 E,F). These data suggest that CRPS IgM pronociceptive effects in the skin are mediated by forming antibody-antigen complexes that bind to C1q, thus activating the complement cascade producing C5 that is enzymatically cleaved into C5a and C5b fragments, subsequently activating C5a receptors on dermal macrophages, thus triggering the expression and secretion of pronociceptive TNF, IL-1, and IL-6 cytokines. C5b complement combines with C6, C7, C8, and C9 to generate membrane attack complex (C5b-9). Figure 6 illustrates increased epidermal C5b-9 complement deposition after intraplantar injection of CRPS IgM in muMT fracture mice, indicating IgM antibody activation of the complement cascade in the fracture hindpaw epidermis. Previously we observed increased IgM deposition in the fracture hindpaw skin that persisted from 3 to 20 weeks after fracture and then spontaneously resolved, corresponding to the time course of post fracture nociceptive sensitization.[11]
Fracture increased C5aR expressing activated microglia and neurons in the lumbar dorsal horns (Fig.7). Intrathecal injection of PMX53 reduced nociceptive sensitization in wildtype fracture mice, but not muMT fracture lacking B cells, indicating that immunoglobulin was required to initiate C5a pronociceptive effects (Fig. 8 A,B). Intrathecal CRPS patient IgM (but not control subject IgM) increased post fracture allodynia and unweighting in muMT mice (Fig. 9 C,D), reaching the same level of nociceptive sensitization as observed in wildtype fracture mice (Fig. 1 C,D). When CRPS IgM was co-injected with PMX53 it had no pronociceptive spinal effects in muMT fracture mice, indicating that CRPS IgM pronciception was mediated by C5a/C5aR signaling (Fig. 9C,D). Intrathecal injection of C5a into muMT nonfractured mice it had no effect, but intrathecal C5a injection into muMT fracture mice was pronociceptive (Fig.8 C,D). After fracture there was increased expression of C5aR mRNA in the lumbar spinal cord of muMT mice (Fig. 8 E,F), and a post traumatic increase in C5a protein levels in the lumbar cord of wildtype fracture mice (Fig. 8 G), suggesting that exaggerated C5a/C5aR spinal signaling contributed to the pronociceptive effects of CRPS IgM.
Other investigators have reported that C5a receptors in the spinal cord are expressed on microglia and that after peripheral nerve injuries only microglia express C1q, C3, and C4 in the spinal cord.[10] The presence of C5a receptors on microglia in the lumbar spinal cord of fracture mice supports the hypothesis that intrathecal CRPS IgM injection triggers C5a activation of microglia, with subsequent pronociceptive inflammatory mediator expression. Previously we observed that fracture increased TNF, IL-1, L-6, and NGF levels in the lumbar spinal cord and intrathecal injection of selective inhibitors for these inflammatory mediators partially reversed allodynia and unweighting in the fracture hindpaw.[32] In addition, fracture caused spinal microglia activation and proliferation, and intrathecal injection of the microglia inhibitor minocycline blocked microglia activation and partially reversed nociceptive sensitization.[23]
The intrathecal injection of CRPS IgM increased the expression of TNF and IL-1 in the lumbar spinal cord of muMT fracture mice and the co-injection of PMX53 completely blocked the expression of these cytokines (Fig. 10). Furthermore, intrathecal injection of IgM after pretreatment with the global cytokine inhibitor pentoxifylline blocked IgM pronociception (Fig. 9 E,F). These results suggest that CRPS IgM pronociceptive effects in the spinal cord are mediated by the formation of antibody-antigen complexes in the spine activating the complement cascade producing C5a, thus subsequently activating C5a receptors on microglia and triggering the expression and secretion of pronociceptive inflammatory mediators.
There is emerging evidence that C5a and its cell membrane receptor, C5aR, contribute to the development and maintenance of acute and chronic pain states, including data from post incisional, neuropathic, and inflammatory mouse pain models.[26] Previously we investigated the role of C5a/C5aR signaling in the hindpaw incision post-operative pain model, using local and systemic injections of PMX53 and utilizing C5aR deficient mice.[5,18,25] The concentration of C5a and expression of C5aR increased in the area surrounding the incision, as did the number of neutrophils and levels of IL-1 and NGF. Inhibition of C5a signaling in the incision model reduced peri-incisional nociceptive sensitization, edema, neutrophil migration, and IL-1 and NGF levels. Microarray expression studies of the spinal cord using multiple peripheral nerve injury mouse pain models have demonstrated that increased expression of the complement cascade is the most prominent spinal transcriptional change and that C5a, acting via C5a receptors expressed on microglia, was the complement component responsible for nociceptive sensitization in these neuropathic pain models.[10]
Systemic injections of PMX53 have been tested in inflammatory pain models using intraperitoneal injections of zymosan, carrageenan, or lipopolysaccharide to activate innate immunity in mice.[34,36] Blocking C5aR signaling reduced mechanical sensitization in all 3 models, but did not block the increase in TNF and IL-1 levels. Another study used ovalbumin and complete Freund’s adjuvant to prime an adaptive immune response to ovalbumin injection and demonstrated that PMX53 pretreatment reduced ovalbumin induced nociceptive sensitization, but inflammatory cytokine levels were not examined.[34]
Previously we observed that early (1–12 months post injury) CRPS patient (n=20) sera (0.5ml) were always pronociceptive after systemic injection into muMT fracture mice, and chronic (>12 months post injury) CRPS sera were rarely pronociceptive (2/20 patients), while sera from resolved CRPS patients (n=5), normal subjects (n=20) and from orthopedic trauma patients without CRPS (n=15) were never pronociceptive.[14] We now demonstrate that chronic CRPS patient IgM (but not IgG or resolved CRPS patient IgM) had pronociceptive effects when injected intrathecally into muMT fracture mice (Fig. 11 A,B). Neither chronic CRPS IgM or IgG had pronociceptive effects when injected intraplantarly (Fig. 11 C,D), suggesting that autoantibodies targeting peripheral neoantigens may slowly decline over time in CRPS patients, which could explain why systemic injections of chronic CRPS sera were usually not pronociceptive. Previous investigators have observed pronociceptive effects after repeated systemic injections of chronic CRPS patient IgG into the mouse hindpaw incision model of post surgical pain, effects possibly involving IL-1 signaling and nociceptor sensitization.[6,16] The discrepancy between these studies and our negative results may be attributable to differences in the injury model utilized, IgG doses, or injection methods.
In summary, one of the most convincing proofs of autoimmune disease is the demonstration of pathogenic autoantibodies. The current study demonstrated that CRPS IgM immunoglobulin had pronociceptive effects in the fracture limb skin and corresponding spinal cord mediated by C5a signaling, inflammatory cytokine expression and subsequent nociceptive sensitization. Interestingly, B cell (rituximab) and C5aR (avacopan [1,17]) immunosuppressive drugs are or will be soon available for off-label clinical trials in CRPS patients. The data presented in this study advance our understanding of potential CRPS autoimmune mechanisms and may contribute to the development of more effective target specific treatments for this debilitating chronic pain state.
Supplementary Material
Acknowledgements
This study was supported by the National Institutes of Health grants NS072143 and NS094438, and the Department of Veterans Affairs, Rehabilitation Research and Development Merit grant I01RX001475. Drs. Birklein and Escolano were supported by the European Commission FP-7 Health-2013-Innovation, Grant no. 602133. The authors do not have financial or other relationships that might lead to conflict of interest.
Reference
- [1].Bekker P, Dairaghi D, Seitz L, Leleti M, Wang Y, Ertl L, Baumgart T, Shugarts S, Lohr L, Dang T, Miao S, Zeng Y, Fan P, Zhang P, Johnson D, Powers J, Jaen J, Charo I, Schall TJ. Characterization of Pharmacologic and Pharmacokinetic Properties of CCX168, a Potent and Selective Orally Administered Complement 5a Receptor Inhibitor, Based on Preclinical Evaluation and Randomized Phase 1 Clinical Study. PloS one 2016;11(10):e0164646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Binshtok AM, Wang H, Zimmermann K, Amaya F, Vardeh D, Shi L, Brenner GJ, Ji RR, Bean BP, Woolf CJ, Samad TA. Nociceptors are interleukin-1beta sensors. J Neurosci 2008;28(52):14062–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Birklein F, Ibrahim A, Schlereth T, Kingery WS. The Rodent Tibia Fracture Model: A Critical Review and Comparison With the Complex Regional Pain Syndrome Literature. The journal of pain : official journal of the American Pain Society 2018;19(10):1102 e1–02 e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 1994;53(1):55–63. [DOI] [PubMed] [Google Scholar]
- [5].Clark JD, Qiao Y, Li X, Shi X, Angst MS, Yeomans DC. Blockade of the complement C5a receptor reduces incisional allodynia, edema, and cytokine expression. Anesthesiology 2006;104(6):1274–82. [DOI] [PubMed] [Google Scholar]
- [6].Cuhadar U, Gentry C, Vastani N, Sensi S, Bevan S, Goebel A, Andersson DA. Autoantibodies produce pain in complex regional pain syndrome by sensitizing nociceptors. Pain 2019;160(12):2855–65. [DOI] [PubMed] [Google Scholar]
- [7].David Clark J, Tawfik VL, Tajerian M, Kingery WS. Autoinflammatory and autoimmune contributions to complex regional pain syndrome. Molecular pain 2018;14:1744806918799127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].de Mos M, de Bruijn AG, Huygen FJ, Dieleman JP, Stricker BH, Sturkenboom MC. The incidence of complex regional pain syndrome: a population-based study. Pain 2007;129(1–2):12–20. [DOI] [PubMed] [Google Scholar]
- [9].Goebel A Autoantibody pain. Autoimmunity reviews 2016;15(6):552–7. [DOI] [PubMed] [Google Scholar]
- [10].Griffin RS, Costigan M, Brenner GJ, Ma CH, Scholz J, Moss A, Allchorne AJ, Stahl GL, Woolf CJ. Complement induction in spinal cord microglia results in anaphylatoxin C5a-mediated pain hypersensitivity. J Neurosci 2007;27(32):8699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Guo TZ, Shi X, Li WW, Wei T, Clark JD, Kingery WS. Passive transfer autoimmunity in a mouse model of complex regional pain syndrome. Pain 2017;158(12):2410–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Guo TZ, Shi X, Li WW, Wei T, Clark JD, Kingery WS. Sex differences in the temporal development of pronociceptive immune responses in the tibia fracture mouse model. Pain 2019;160(9):2013–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Guo TZ, Wei T, Shi X, Li WW, Hou S, Wang L, Tsujikawa K, Rice KC, Cheng K, Clark DJ, Kingery WS. Neuropeptide deficient mice have attenuated nociceptive, vascular, and inflammatory changes in a tibia fracture model of complex regional pain syndrome. Molecular pain 2012;8:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Guo TZ, Wei T, Tajerian M, Clark JD, Birklein F, Goebel A, Li WW, Sahbaie P, Escolano FL, Herrnberger M, Kramer HH, Kingery WS. Complex regional pain syndrome patient immunoglobulin M has pronociceptive effects in the skin and spinal cord of tibia fracture mice. Pain 2020;161(4):797–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Harden RN, Bruehl S, Stanton-Hicks M, Wilson PR. Proposed new diagnostic criteria for complex regional pain syndrome. Pain medicine 2007;8(4):326–31. [DOI] [PubMed] [Google Scholar]
- [16].Helyes Z, Tekus V, Szentes N, Pohoczky K, Botz B, Kiss T, Kemeny A, Kornyei Z, Toth K, Lenart N, Abraham H, Pinteaux E, Francis S, Sensi S, Denes A, Goebel A. Transfer of complex regional pain syndrome to mice via human autoantibodies is mediated by interleukin-1-induced mechanisms. Proceedings of the National Academy of Sciences of the United States of America 2019;116(26):13067–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Horiuchi T, Tsukamoto H. Complement-targeted therapy: development of C5- and C5a-targeted inhibition. Inflammation and regeneration 2016;36:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Jang JH, Liang D, Kido K, Sun Y, Clark DJ, Brennan TJ. Increased local concentration of complement C5a contributes to incisional pain in mice. Journal of neuroinflammation 2011;8:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kumar V, Lee JD, Clark RJ, Woodruff TM. Development and validation of a LC-MS/MS assay for pharmacokinetic studies of complement C5a receptor antagonists PMX53 and PMX205 in mice. Scientific reports 2018;8(1):8101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Li WW, Guo TZ, Li XQ, Kingery WS, Clark JD. Fracture induces keratinocyte activation, proliferation, and expression of pro-nociceptive inflammatory mediators. Pain 2010;151(3):843–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Li WW, Guo TZ, Shi X, Birklein F, Schlereth T, Kingery WS, Clark JD. Neuropeptide regulation of adaptive immunity in the tibia fracture model of complex regional pain syndrome. Journal of neuroinflammation 2018;15(1):105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Li WW, Guo TZ, Shi X, Czirr E, Stan T, Sahbaie P, Wyss-Coray T, Kingery WS, Clark JD. Autoimmunity contributes to nociceptive sensitization in a mouse model of complex regional pain syndrome. Pain 2014;155(11):2377–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Li WW, Guo TZ, Shi X, Sun Y, Wei T, Clark DJ, Kingery WS. Substance P spinal signaling induces glial activation and nociceptive sensitization after fracture. Neuroscience 2015;310:73–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Li WW, Sabsovich I, Guo TZ, Zhao R, Kingery WS, Clark JD. The role of enhanced cutaneous IL-1beta signaling in a rat tibia fracture model of complex regional pain syndrome. Pain 2009;144(3):303–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Liang DY, Li X, Shi X, Sun Y, Sahbaie P, Li WW, Clark JD. The complement component C5a receptor mediates pain and inflammation in a postsurgical pain model. Pain 2012;153(2):366–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Quadros AU, Cunha TM. C5a and pain development: An old molecule, a new target. Pharmacological research 2016;112:58–67. [DOI] [PubMed] [Google Scholar]
- [27].Richter F, Natura G, Loser S, Schmidt K, Viisanen H, Schaible HG. Tumor necrosis factor causes persistent sensitization of joint nociceptors to mechanical stimuli in rats. Arthritis Rheum 2010;62(12):3806–14. [DOI] [PubMed] [Google Scholar]
- [28].Ristoiu V Contribution of macrophages to peripheral neuropathic pain pathogenesis. Life sciences 2013;93(23):870–81. [DOI] [PubMed] [Google Scholar]
- [29].Sandroni P, Benrud-Larson LM, McClelland RL, Low PA. Complex regional pain syndrome type I: incidence and prevalence in Olmsted county, a population-based study. Pain 2003;103(1–2):199–207. [DOI] [PubMed] [Google Scholar]
- [30].Schaible HG. Nociceptive neurons detect cytokines in arthritis. Arthritis research & therapy 2014;16(5):470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Schmelz M, Mantyh P, Malfait AM, Farrar J, Yaksh T, Tive L, Viktrup L. Nerve growth factor antibody for the treatment of osteoarthritis pain and chronic low-back pain: mechanism of action in the context of efficacy and safety. Pain 2019;160(10):2210–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Shi X, Guo TZ, Wei T, Li WW, Clark DJ, Kingery WS. Facilitated spinal neuropeptide signaling and upregulated inflammatory mediator expression contribute to postfracture nociceptive sensitization. Pain 2015;156(10):1852–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Shutov LP, Warwick CA, Shi X, Gnanasekaran A, Shepherd AJ, Mohapatra DP, Woodruff TM, Clark JD, Usachev YM. The Complement System Component C5a Produces Thermal Hyperalgesia via Macrophage-to-Nociceptor Signaling That Requires NGF and TRPV1. J Neurosci 2016;36(18):5055–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Ting E, Guerrero AT, Cunha TM, Verri WA, Jr., Taylor SM, Woodruff TM, Cunha FQ, Ferreira SH. Role of complement C5a in mechanical inflammatory hypernociception: potential use of C5a receptor antagonists to control inflammatory pain. British journal of pharmacology 2008;153(5):1043–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Wei T, Sabsovich I, Guo TZ, Shi X, Zhao R, Li W, Geis C, Sommer C, Kingery WS, Clark DJ. Pentoxifylline attenuates nociceptive sensitization and cytokine expression in a tibia fracture rat model of complex regional pain syndrome. European journal of pain 2009;13(3):253–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Yan C, Zhu M, Staiger J, Johnson PF, Gao H. C5a-regulated CCAAT/enhancer-binding proteins beta and delta are essential in Fcgamma receptor-mediated inflammatory cytokine and chemokine production in macrophages. The Journal of biological chemistry 2012;287(5):3217–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Zhang X, Kimura Y, Fang C, Zhou L, Sfyroera G, Lambris JD, Wetsel RA, Miwa T, Song WC. Regulation of Toll-like receptor-mediated inflammatory response by complement in vivo. Blood 2007;110(1):228–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


