1. Introduction
Calcitonin gene-related peptide (CGRP) is abundantly expressed in unmyelinated C-fiber primary afferent neurons in trigeminal ganglia (TG, [46; 49]) and plays an important role in the pathophysiology of migraine [51]. CGRP is released from TG neurons during migraine attacks [27]. Infusion of CGRP to migraine patients induces delayed migraine-like headaches [36]. In rodents, dural application of CGRP produces female-specific behavioral hyper-sensitivity [5], and peripheral administration of CGRP induces spontaneous pain-like behaviors [50]. Antibodies against CGRP or its canonical receptor significantly reduces migraine frequency in some patients [19], likely through blocking CGRP signaling pathways at peripheral sites [34; 45]. Physiological studies suggest that the antibodies’ mechanism of action is unlikely through prevention of arterial dilatation [52], but rather through inhibition of the thinly myelinated Aδ- but not unmyelinated C-fiber meningeal nociceptors [42]. In rat and human TG and dura tissues, both calcitonin receptor-like receptor and receptor activity modifying protein 1 (RAMP1), the obligatory subunits of the canonical CGRP receptor, are expressed in the Aδ- but not C-fiber sensory neurons [20; 21], suggesting that endogenous CGRP activates/sensitizes Aδ nociceptors via paracrine signaling.
On the other hand, CGRP binds to the amylin-1 (AMY1) receptor with the same affinity as to the canonical CGRP receptor [31], and AMY1 receptor subunits RAMP1 and calcitonin receptor are expressed in some small- and medium-sized TG neurons [61]. In cultured TG neurons, gold-labeled CGRP binds to neurons that express CGRP [54], and CGRP upregulates CGRP mRNA levels [65], suggesting the presence of CGRP autocrine signaling in TG neurons. These studies are conducted either in naïve animals or in models of episodic migraine. Whether the paracrine and autocrine CGRP signaling in TG neurons undergo dynamic changes under chronic migraine state is not clear. We investigated this question in the current study.
To identify CGRP-expressing TG neurons with fluorescent signal, we modeled chronic migraine with repeated administration of nitroglycerin (NTG, a reliable migraine trigger in migraineurs) in heterozygous CGRPαEGFPf/+ mice which express farnesylated enhanced green fluorescent protein (EGFPf) at one Calca/Cgrpa locus [40]. Since the activation of both the canonical CGRP receptor and AMY1 receptor increase the intracellular free Ca2+ ([Ca2+]in) in addition to stimulating the synthesis of cyclic adenosine monophosphate in many cells [17; 33], we used CGRP-evoked [Ca2+]in increase to define neurons that exhibit functional CGRP receptors under normal and chronic migraine-like states. We investigated whether chronic migraine state alters the number of TG neurons that respond to CGRP, and if yes, whether the change occurs in EGFP+, CGRP-expressing neurons. We also examined the overlap between neurons that respond to CGRP and to pituitary adenylate cyclase-activating polypeptide (PACAP), another neuropeptide that is implicated in migraine pathophysiology [1; 38; 53; 59; 63]. Since peripheral CGRP signaling also contributes to the development of behaviors related to post-traumatic headache (PTH) in animal models [9; 44], we examined whether similar changes occur in a mouse model of mild traumatic brain injury (mTBI)-induced PTH; and whether they can be blocked by low-dose interleukin-2 (ld-IL2) treatment that reverses chronic migraine- and PTH-related behaviors.
2. Materials and Methods
2.1. Mice
All procedures were carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health and were approved by the Institutional Animal Care and Use Committee at Washington University in St. Louis. To avoid social isolation stress, all mice were group housed (2-5 per cage, same sex) in the animal facility of Washington University in St. Louis on a 12-hour light–dark cycle with constant temperature (23-24°C), humidity (45-50%), and food and water ad libitum. All experiments were performed during the light phase (9 am to 4 pm). Adult mice (6-12 weeks old, both male and female) were used in the experiments. Adult Swiss Webster mice were purchased from Charles River. Adult C57BL/6J mice were purchased from the Jackson Laboratory. Breeders of CGRPαEGFPf/+ mice on C57BL/6J background, which express EGFPf at one Calca/Cgrpa locus and CGRP at the other allele, were purchased from the Mutant Mouse Resource & Research Center (MMRRC_036773_UNC). The genotype was determined by polymerase chain reaction of tail DNA as described [40].
2.2. Mouse models of chronic migraine, mTBI-induced PTH and drug treatment
To model chronic migraine, mice received repetitive intraperitoneal (i.p.) injections of NTG (10 mg/kg in saline with 1% propylene glycol) every 2 days for 5 or more times as described previously [64]. The control mice received vehicle (saline with 1% propylene glycol, 10 ml/kg) injections. To reveal hyperalgesic priming, mice received daily low-dose NTG (0.1 mg/kg in saline with 0.01% propylene glycol) after the facial mechanical threshold returned to baseline level. NTG (SDM27, Copperhead Chemical, Tamaqua, PA) was freshly diluted from the stock (10% in propylene glycol, aliquoted in airtight glass vials and stored at room temperature and kept in darkness) with saline for every injection.
To reverse NTG-induced sensitization, mice received daily i.p. injections of 1 μg IL2 in 100 μl saline, starting 1 day after the 2nd NTG injection [64]. The control mice received daily i.p. injections of 100 μl saline. Recombinant mouse IL2 (carrier-free; Biolegend, San Diego, CA) was freshly diluted from the stock (0.5-1 mg/ml aliquots at −80°C) every day.
To induce mTBI, male Swiss Webster mice (8-10 weeks old) were anesthetized with 3% isoflurane for 90 seconds and were placed chest down on a foam sponge (4 cm thickness, 0.062 g/cm3 density) directly underneath a hollow cylindrical tube (1.5 cm inner diameter) placed approximately 1 cm vertically over the mouse’s head. A 30 g weight (1.3 cm diameter, 3.4 cm height) was dropped through the tube from a height of 80 cm, striking the center point between the ears once [64]. All mice regained righting reflex within 2 minutes (min). No mortality, skull fracture or motor deficit was observed in any mice. The reduction of facial mechanical threshold between 3 and 28 days post-mTBI is mechanistically related to acute PTH, as it can be alleviated by anti-migraine drugs sumatriptan and anti-CGRP antibodies [9; 44]. Sham mice were anesthetized with 3% isoflurane for 90 seconds but not subjected to the weight drop. One day after the mTBI procedure, some mice received daily ld-IL2 treatment for 6 days. TG tissues were collected for primary culture the next day.
2.3. Behavioral tests – withdrawal responses to facial mechanical stimuli
Wild-type and CGRPα knockout (KO) mice were generated by crossing heterozygous CGRPαEGFPf/+ breeders. Mice were extensively handled by the experimenters for 2 weeks and were well-habituated to the test room and the test apparatus before each experiment. The experimenters were blinded to the genotype and the treatments mice received during data collection and analysis.
The hair on mouse forehead (above and between two eyes) was shaved the day before testing. On the test day, the experimenter gently held the mouse on the palm with minimal restraint and applied the calibrated von Frey filament perpendicularly to the shaved skin, causing the filament to bend for 5 seconds. A positive response was determined by the following criteria as previously described: mouse vigorously stroked its face with the forepaw, head withdrawal from the stimulus, or head shaking [64]. The up-down paradigm was used to determine the 50% withdrawal threshold [12]. The withdrawal thresholds to facial von Frey stimuli were measured at baseline and two days after each vehicle or NTG injection.
2.4. Primary culture of mouse TG and dorsal root ganglion (DRG) neurons
TG and lumbar DRG (L3-L5) tissues were collected and were treated with 2.5 mg/ml collagenase IV for 10 min followed by 2.5 mg/ml trypsin at 37°C for 15 min, respectively. Cells were dissociated by triturating with fire-polished glass pipettes, resuspended in MEM-based culture medium containing 5% fetal bovine serum, 25 ng/ml nerve growth factor (NGF, R&D) and 10 ng/ml glial cell-derived neurotrophic factor (GDNF, R&D), and seeded on Matrigel-coated coverslips. Ca2+ imaging were performed in neurons 2 days in vitro. In some experiments, neurons were cultured in the absence of NGF and GDNF and were used within 24 h after plating. Each set of experiment contained neurons from at least 3 batches of culture.
2.5. Ratiometric Ca2+ imaging
Coverslips containing cultured TG or DRG neurons were incubated with HBSS/HEPES solution containing 2.5 μM fura-2 AM and 10% Pluronic F-68 (all from Molecular Probe) at 37°C for 45 min to load the ratiometric Ca2+ indicator. De-esterification of the dye was carried out by washing the coverslips 3 times with HBSS/HEPES solution and incubating the coverslips in HBSS/HEPES solution in the dark for an additional 15 min at 37°C. Neurons were used for Ca2+ imaging experiments within 1 hour after Fura-2 loading.
Coverslips with fura-2 loaded neurons were placed in a flow chamber mounted on a Nikon TE2000S inverted epifluorescent microscope and were perfused with room temperature Tyrode’s solution (1 ml/min) containing (in mM): 130 NaCl, 2 KCl, 2 CaCl2, 2 MgCl2, 25 Hepes, 30 glucose, pH 7.3-7.4 with NaOH, and 310 mosmol/kgH2O. Differential interference contrast (DIC) images of neurons were captured to calculate soma diameters from cross-sectional areas off-line. Healthy neurons were chosen based on their morphology under DIC. In a pilot test, all selected neurons (> 100) exhibited robust Ca2+ influx in response to a depolarization stimulus (extracellular solution containing 50 mM KCl). Fura-2 was alternately excited by 340 and 380 nm light (Sutter Lambda LS) and the emission was detected at 510 ± 20 nm by a UV-transmitting 20x objective (N.A. 0.75) and a CoolSnapHQ2 camera (Photometrics). The frame capture period was 50 milliseconds at 1.5 seconds interval. SimplePCI software (Hamamatsu) was used for controlling and synchronizing the devices as well as image acquisition and analysis. Regions of interest (ROIs) encompassing individual neurons were defined a priori. The ratio of fluorescence excited by 340 nm divided by fluorescence excited by 380 nm (R340/380) was determined on a pixel-by-pixel basis and was averaged for each ROI. An additional background area was recorded in each field for off-line subtraction of background fluorescence. Peak responses were determined by calculating the relative increase in R340/380 above baseline (F0, the average R340/380 during the 2-3 min baseline measurement). A ΔF/F0 > 20% was set as the threshold for a response.
After a 2-3 min baseline measurement in Tyrode’s solution, neurons were perfused with 50 nM PACAP1-38 (Tocris, 1136) for 1 min followed by washing with Tyrode’s for 4 min. Subsequently, the coverslip was incubated with 3 μM human αCGRP (Tocris, 3012) for 1 min followed by washing with Tyrode’s for 4 min, 1 min perfusion of 1 μM capsaicin (Sigma 21750), and 5 min Tyrode’s wash. At the end of each experiment, neurons were stained with 3 μg/ml Fluorescein isothiocyanate-conjugated isolectin B4 (FITC-IB4, Sigma) for 5 min. The FITC fluorescence on soma membrane was detected after 5 min perfusion with Tyrode’s solution to wash off unbound FITC-IB4. CGRP, CGRP8-37 (Tocris, 1181), PACAP, vasoactive intestinal peptide (VIP, Tocris 1911) and capsaicin were freshly diluted from the stock (aliquots at −80°C) in Tyrode’s solution every day.
2.6. Immunohistochemistry
TG cultures from naïve CGRPαEGFPf/+ mice were fixed by 4% formaldehyde in phosphate-buffered saline (pH 7.2) at 4°C for 10 min. Coverslips were incubated with primary antibodies (chicken anti-EGFP, AVES lab, GFP-1020, 1:1000 and rabbit anti-CGRP, Peninsula laboratories, T-4032, 1:1000) at 4°C overnight. After washing off the primary antibodies, neurons were stained with AlexaFluor 488-conjugated goat anti-chicken and AlexaFluor 568-conjugated goat anti-rabbit secondary antibodies (Invitrogen, 1:1000) at room temperature for 2 hours. Immunofluorescence was observed through a 20x objective on a Nikon TE2000S inverted epifluorescence microscope and random, non-overlapping images were captured with a CoolSnapHQ2 camera and analyzed with the SimplePCI software. A total of 2589 TG neurons from 3 mice were analyzed. Representative images were adjusted for contrast and brightness using the same parameter within individual experiments. No other manipulations were made to the images. Image analysis was done with experimenters blinded to the experimental groups.
To test the effects of lower concentration of CGRP, we incubated TG cultures from vehicle- and NTG-treated female Swiss Webster mice with 100 nM CGRP for 30 min as in a previous study [62]. Coverslips were then fixed and incubated with rabbit anti-pCREB (phosphorylated cAMP response element-binding protein, Cell Signaling Technology, #9198, 1:500) and rabbit anti-pp38 (phosphorylated p38 MAP kinase, Cell Signaling Technology, #4511, 1:500) at 4°C overnight. The percentages of neurons that contained pCREB and pp38 immunoreactivities were quantified.
2.7. Statistical analysis
For behavioral experiments, power analysis was conducted to estimate sample size with > 80% power to show an effect size of 0.8, alpha (two-sided) of 0.05, and a simple covariance structure for repeated measures. The experimenters were blinded to the genotypes and the treatments mice received. For Ca2+ imaging, all groups were tested in parallel in individual experiments. Each experiment contained neurons from at least 3 batches of culture. For immunohistochemistry, sample size was estimated based on our previous experience.
All data were reported as mean ± standard error of the mean. Shapiro–Wilk test showed that all data were normally distributed. Statistical significance between or within experimental groups was assessed by two-tailed t-test, one-way repeated-measures analysis of variance (RM ANOVA) with post hoc Dunnett’s test, and two-way RM ANOVA with post hoc Student–Newman–Keuls test where appropriate, using Origin and Statistica softwares (from OriginLab and StatSoft, respectively). Fisher’s exact test and χ2 test with post hoc Fisher’s exact test with Bonferroni correction were used to compare the distribution of TG subpopulations between various treatments and/or genotypes. Differences with p < 0.05 were considered statistically significant. The statistical analysis for individual experiments was described in figure legends.
Results
CGRPα KO mice do not develop NTG-induced acute or persistent sensitization.
Migraine headache can be triggered by multiple mechanisms, one of which is the activation of the nitric oxide (NO) signaling pathway. Infusion of NTG, a NO donor, to migraine patients triggers a delayed, migraine-like headache [47]. In rodents, administration of NO donors induces acute cutaneous hyper-sensitivity that can be inhibited by migraine abortive drugs such as sumatriptan and CGRP signaling blockers [2; 6; 15]. Repeated administration of NTG have been used to mimic the triggering of recurring migraine episodes in chronic migraine patients [14; 48; 56; 58; 64]. This results in a progressive and sustained cutaneous mechanical hyper-sensitivity in mice that can be blocked by the migraine preventive drugs topiramate and propranolol [8; 14; 48], validating that these behavioral changes are mechanistically related to the episodic and chronic migraine state in humans, respectively. We conducted pilot studies to determine the dose of NTG that reliably induces long-lasting behavioral sensitization in mice. After measuring the baseline mechanical thresholds at the periorbital region, naïve female Swiss Webster mice were injected with 1, 3 and 10 mg/kg NTG (i.p.) every two days for 5 times. Facial mechanical thresholds were assessed two days after each NTG injection. The lowest dose (1 mg/kg) of NTG did not significantly alter facial mechanical thresholds (Fig. S1A-B). The intermediate dose (3 mg/kg) of NTG resulted in persistent facial mechanical hyper-sensitivity that fully recovered 4 days after the cessation of NTG (Fig. S1C). However, subsequent injections of low-dose NTG (0.1 mg/kg, i.p.) did not alter mechanical thresholds (Fig. S1D), indicating that this dose of NTG is not sufficient to induce hyperalgesic priming. Repeated administration of 10 mg/kg NTG resulted in robust and sustained reduction of facial mechanical thresholds that returned to baseline level 11 days after the cessation of NTG (Fig. S1E). Subsequent injections of low-dose NTG re-established facial mechanical hyper-sensitivity (Fig. S1F), revealing the hyperalgesic priming resulting from the previous high-dose NTG administration. Notably, the hyperalgesic priming persisted for a week after the facial mechanical thresholds returned to baseline level (Fig. S1F). We therefore used repeated injection of 10 mg/kg NTG in subsequent experiments, as it induces long-lasting behavioral sensitization and hyperalgesic priming.
To confirm that the endogenous CGRP signaling contributes to the establishment of chronic migraine-like state in the mouse model used in the current study, we compared the behaviors of wild-type and CGRPα KO mice in response to repeated administration of 10 mg/kg NTG (Fig. 1A). In female wild-type mice, the mechanical threshold at the periorbital region was significantly reduced 3 hours after a single injection of NTG (Fig. 1B). The facial mechanical hyper-sensitivity persisted for at least 2 days (Fig. 1C, day 3), and was sustained by the subsequent NTG injections (Fig. 1C, day 5-11). In contrast, CGRPα KO mice did not exhibit facial mechanical hyper-sensitivity in response to either single or repeated NTG injections (Fig. 1B-C), indicating that the endogenous CGRP signaling is required for the development of NTG-induced episodic and chronic migraine-related behaviors.
Figure 1. CGRPα KO mice do not develop NTG-induced acute or persistent sensitization.
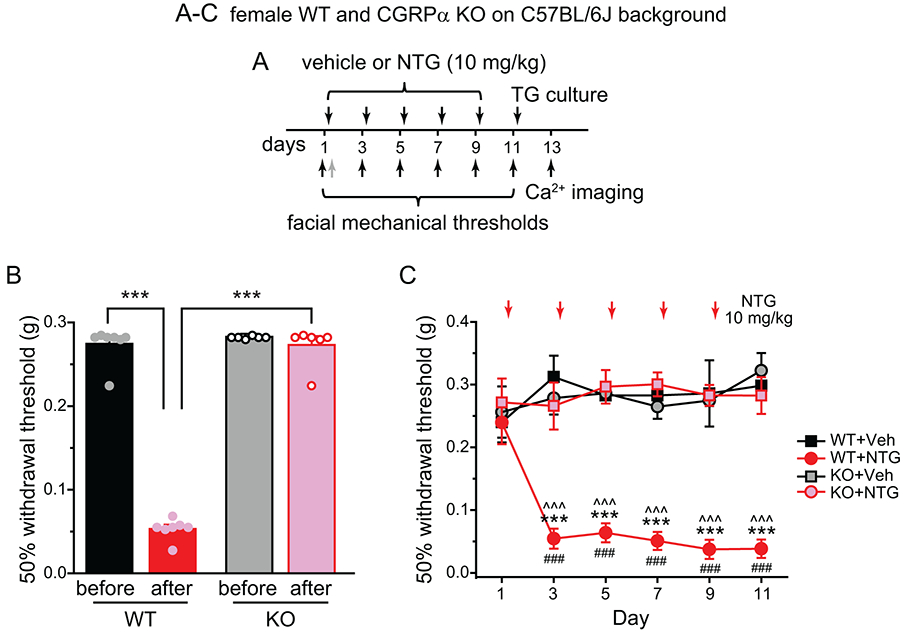
(A) Time line of experiments in (B) and (C). Note that vehicle (Veh) or NTG was always injected after the completion of behavioral tests on the same day. In some mice, the facial mechanical thresholds were also measured 2 hours after the 1st NTG injection as indicated by the grey arrow.
(B) The 50% withdrawal thresholds to von Frey filaments at the periorbital region of female wild-type (WT) and CGRPα KO mice on C57BL/6J background before and 2 hours after the first NTG injection (n = 7 mice/group, respectively). Two-way ANOVA: p < 0.001 for genotype (F[1, 27] = 52.16), treatment (F[1, 27] = 30.79), and genotype x treatment interaction (F[1, 27] = 54.21); post hoc Bonferroni test: ***p < 0.001.
(C) The effect of repeated NTG administration on facial mechanical thresholds in female WT and CGRPα KO mice (n = 5-7 mice/group). Two-way RM ANOVA: p < 0.001 for group (F[3, 572] = 38.31) and group x time interaction (F[15, 572] = 4.17; post hoc Student–Newman–Keuls test: ***p < 0.001, between the corresponding WT+NTG and KO+NTG groups; ^^^p < 0.001, between the corresponding WT+Veh and WT+NTG groups; ###p < 0.001, compared with the baseline (day 1) threshold in the WT+NTG group.
The numbers of CGRP-responsive (CGRP-R) and PACAP-responsive (PACAP-R) TG neurons are significantly increased by the repeated NTG administration.
First, we conducted pilot studies to determine the concentrations of CGRP and PACAP that allows us to identify CGRP-R and PACAP-R neurons in TG culture. Very few TG neurons from naïve female Swiss Webster mice responded to the bath application of 1 μM αCGRP with > 20% increase in [Ca2+]in from baseline (Fig. S2A). Bath application of 3 μM αCGRP evoked significant [Ca2+]in increase in about 15% of neurons (Fig. S2A). This was consistent with the previously reported CGRP-evoked Ca2+ transients in mouse and rat TG cultures [11; 22; 54]. Co-application of 3 μM αCGRP with 15 μM CGRP8-37 substantially reduced the percentage of CGRP-R neurons to 3% (2 of 65 neurons, Fig. S2A), validating that CGRP-induced Ca2+ transients results from the activation of CGRP-family receptors [31]. We therefore used bath application of 3 μM αCGRP to define CGRP-R neurons in subsequent experiments.
We used perfusion of PACAP1-38 to identify PACAP-R neurons in TG cultures from naïve mice, as it is more abundantly expressed in tissues than the truncated PACAP1-27 [60]. Since both 50 nM and 100 nM PACAP1-38 evoked Ca2+ transients in about 25% of TG neurons (Fig. S2B), we used 50 nM PACAP1-38 to define PACAP-R neurons in subsequent experiments. At this concentration, PACAP1-38 activates the PACAP-preferring type 1 (PAC1) receptor as well as VIP receptor type 1 and type 2 (VPAC1 and VPAC2), but a similar concentration of VIP preferentially activates VPAC1 and VPAC2 receptors [30; 60]. Only 3.6% of TG neurons responded to the perfusion of 100 nM VIP (Fig. S2C, Veh group), suggesting that PACAP1-38 induces Ca2+ transients in TG neurons mainly through the activation of PAC1 receptors, despite the fact that the mRNAs and proteins of all three receptors are shown to be expressed in TG neurons [13; 29; 35].
The CGRPαEGFPf/+ mice we planned to use to identify autocrine and paracrine CGRP signaling is on inbred C57BL/6J background. Here, we used the outbred female Swiss Webster mice to test the effects of repeated NTG administration. This allows us to examine whether the effects of repeated NTG is mouse strain dependent. We injected female Swiss Webster mice with vehicle or 10 mg/kg NTG every two days for 5 times. Two days after the last injection, we cultured TG neurons and measured CGRP- and PACAP-induced [Ca2+]in increase in individual neurons (Fig. 2A). In TG cultures from NTG-treated mice, about 40% of neurons were CGRP-R, significantly higher than that from vehicle-treated mice (Fig. 2B). Repeated NTG administration also doubled the numbers of PACAP-R neurons in TG cultures (Fig. 2B) without altering the percentage of VIP-responsive (VIP-R) neurons (Fig. S2C, p = 0.31 between the Veh and NTG groups, two-tailed t-test), suggesting that repeated NTG preferentially increases the number of TG neurons that express functional PAC1 receptors. To exclude the possibility that pre-exposure to PACAP may affect neurons’ responses to CGRP, we measured CGRP-induced Ca2+ transients alone in another experiment and observed a similar NTG-induced increase in CGRP-R TG neurons (Fig. 2C). The percentages of CGRP-R and PACAP-R TG neurons were comparable between TG cultures from mice that received repeated saline and vehicle (1% propylene glycol in saline) injections (Fig. S2D), indicating that vehicle does not contribute to the increase in CGRP-R and PACAP-R TG neurons in NTG-treated mice.
Figure 2. The numbers of CGRP-R and PACAP-R TG neurons were significantly increased by the repeated NTG administration.
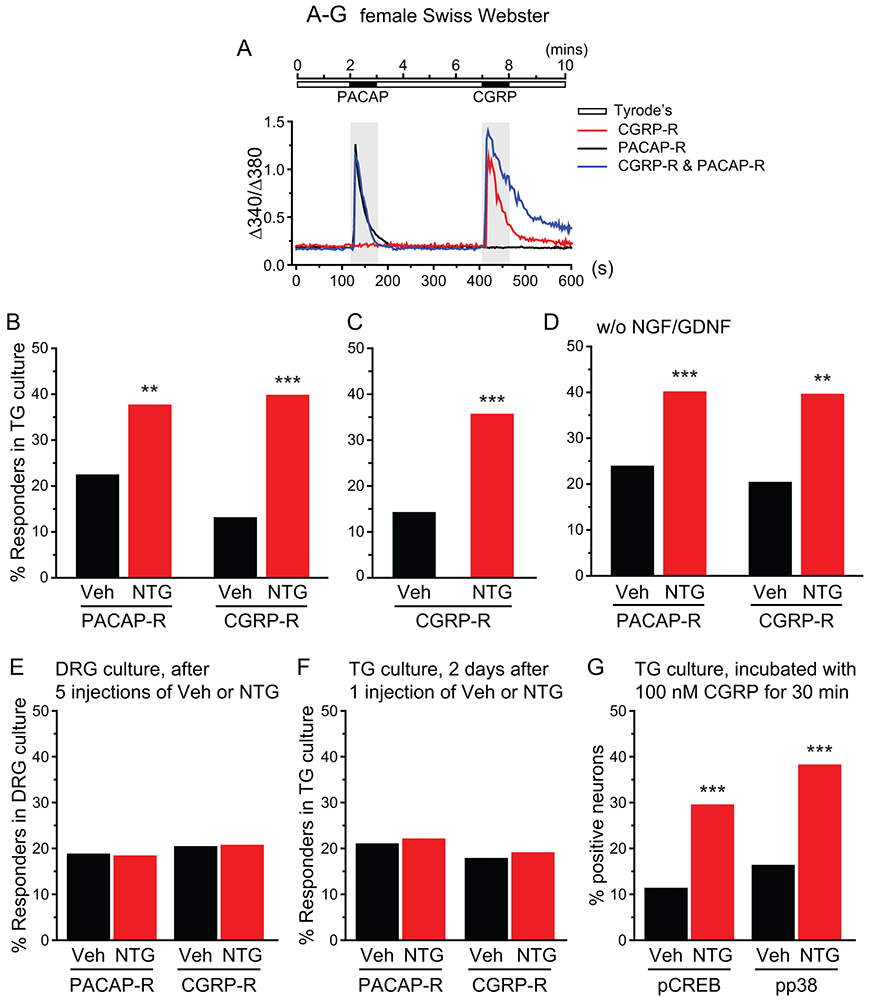
(A) The protocol to measure PACAP- and CGRP-evoked [Ca2+]in increase and the representative traces of PACAP- and CGRP-induced Ca2+ transients in TG neurons.
(B) The percentages of PACAP-R and CGRP-R neurons in TG cultures from female Swiss Webster mice after 5 repetitive i.p. injections of Veh or NTG (n = 4 mice/group; a total of 137 and 140 neurons were measured in vehicle and NTG groups, respectively). **p < 0.01, ***p < 0.001, Fisher’s exact test between Veh and NTG groups.
(C) The percentages of CGRP-R neurons in TG cultures from Veh- and NTG-treated female Swiss Webster mice (n = 3 mice/group; a total of 95 and 131 neurons were measured in Veh and NTG groups, respectively). **p < 0.001, Fisher’s exact test.
(D) The percentages of PACAP-R and CGRP-R neurons in acutely dissociated TG neurons from female Swiss Webster mice without NGF and GDNF exposure (n = 5-6 mice/group; a total of 146 and 192 neurons were measured in Veh and NTG groups, respectively). **p < 0.01, ***p < 0.001, Fisher’s exact test between vehicle and NTG groups.
(E) The percentages of PACAP-R and CGRP-R neurons in DRG cultures from female Swiss Webster mice after 5 repetitive i.p. injections of Veh or NTG (n = 3-4 mice/group; a total of 306 and 430 neurons were measured in Veh and NTG groups, respectively).
(F) The percentages of PACAP-R and CGRP-R neurons in TG cultures from female Swiss Webster mice that received 1 Veh or NTG injection (n = 6 mice/group; a total of 190 and 199 neurons were measured in Veh and NTG groups, respectively).
(G) The percentages of neurons that exhibit CGRP-induced phosphorylation of CREB and p38 proteins in TG cultures from female Swiss Webster mice after 5 repetitive i.p. injections of Veh or NTG. Neurons were incubated with 100 nM CGRP for 30 min (n = 4 mice/group; a total of 1418 and 1372 neurons were quantified in Veh and NTG groups for pCREB staining; a total of 1216 and 1065 neurons were quantified in Veh and NTG groups for pp38 staining, respectively). ***p < 0.001, Fisher’s exact test between Veh and NTG groups.
Consistent with the dose-dependent effects of NTG on behavioral sensitization, repeated injections of 1 mg/kg NTG did not alter the percentages of CGRP-R and PACAP-R TG neurons (Fig. S2E), whereas repeated administration of 3 mg/kg NTG increased in the numbers of CGRP-R and PACAP-R TG neurons, although not as robust as 10 mg/kg NTG (Fig. S2F).
We also tested whether the abundance of CGRP-R and PACAP-R TG neurons depends on culture conditions. Instead of incubating neurons in media containing NGF and GDNF for 2 days, we measured CGRP- and PACAP-induced [Ca2+]in increase in acutely dissociated TG neurons plated in media without NGF and GDNF. Once again, the percentages of CGRP-R and PACAP-R neurons were significantly higher in TG cultures from NTG-treated mice than those from vehicle controls (Fig. 2D), indicating that the difference between vehicle- and NTG-treated groups is independent of the culture conditions. Although repeated NTG administration results in hindpaw mechanical hyper-sensitivity [48; 64], the percentages of CGRP-R or PACAP-R neurons in DRG culture were not altered by the repeated NTG administration (Fig. 2E). A recent study reported differences between the translatomes from TG and DRG nociceptors [41]. Translational efficiency in mechanistic target of rapamycin-related genes is higher in TG, whereas AMP-activated protein kinase-associated genes have higher translational efficiency in DRG [41]. It is possible that repeated migraine episodes differentially sensitizes TG and DRG neurons, preferentially increasing the numbers of CGRP-R and PACAP-R neurons in TG but not DRG.
Interestingly, we found no change in the abundance of CGRP-R or PACAP-R neurons in TG cultures from mice that received a single NTG injection (Fig. 2F). To explore how increase in CGRP-R and PACAP-R TG neurons may contribute to NTG-induced behavioral sensitization, we compared the responses to single and repeated NTG in greater detail. In mice that received a single 10 mg/kg NTG injection, the facial mechanical hyper-sensitivity lasted till 2 days post-NTG and fully recovered 4 to 6 days post-NTG (Fig. S2G, day 1-7). Subsequent daily injections of low-dose NTG (0.1 mg/kg, i.p.) did not alter facial mechanical thresholds, indicating that a single injection of 10 mg/kg NTG is not sufficient to establish hyperalgesic priming (Fig. S2G, day 7-11). In mice that received repeated NTG injections (10 mg/kg, i.p.), the facial mechanical threshold did not return to baseline level until 11 days after the cessation of NTG (Fig. S2H, day 1-20). Subsequent low-dose NTG administration revealed the presence of hyperalgesic priming (Fig. S2H, day 20-27). We speculate that the increase in CGRP-R and PACAP-R TG neurons after repeated NTG may contribute to the delayed recovery of facial mechanical threshold and the development of hyperalgesic priming.
The concentration of CGRP used here was comparable to what had previously been used to evoke Ca2+ transients in TG cultures [11; 22; 54], but was much higher than what had been used in biochemical assays [31; 61; 62; 65] . This may largely result from the inherent differences between the biochemical assays and Ca2+-imaging analysis. In biochemical assays, CGRP was applied for 5-30 min, and the readouts were a summation of the activation of functional receptors in all cells during this period of time. Ca2+-imaging analysis, on the other hand, detected signals from individual neurons that peaked within 10-30 seconds of ligand application (Fig. 2A). A higher concentration of CGRP would be required to activate sufficient numbers of functional receptors in individual neurons within a short period of time.
To confirm the physiological relevance of our results, we incubated TG cultures from vehicle- and NTG-treated mice with 100 nM CGRP for 30 min to induce the phosphorylation of CREB and p38 proteins in TG neurons as reported in a previous study [62]. In TG cultures from vehicle-treated mice, 10-15% of neurons exhibited pCREB and pp38 immunoreactivities (Fig. 2G), similar to the percentage of CGRP-R neurons in vehicle control (Fig. 2B-C). In TG cultures from NTG-treated mice, 30-40% of neurons exhibited pCREB and pp38 immunoreactivities (Fig. 2G), similar to the percentage of CGRP-R neurons in NTG group (Fig. 2B-C). These results indicate that the number of TG neurons with functional CGRP receptors identified in Ca2+-imaging experiments with 3 μM CGRP was comparable to the number identified in the pCREB and pp38 assays using 100 nM CGRP, validating the physiological relevance of the results from Ca2+-imaging experiments.
Repeated NTG administration enhances both autocrine and paracrine CGRP signaling in TG neurons.
We proceeded to use TG cultures from heterozygous CGRPαEGFPf/+ mice expressing EGFPf at one Calca/Cgrpa locus to study the relationship between CGRP-expressing, CGRP-R and PACAP-R TG neurons under normal and chronic migraine-like conditions. First, we confirmed that there was a substantial overlap between the EGFP-immunoreactivity (EGFP-ir) and CGRP-immunoreactivity (CGRP-ir) in TG cultures from naïve CGRPαEGFPf/+ mice (Fig. S3A). More than 80% EGFP-ir neurons contained CGRP-ir, regardless of soma size (Fig. S3B). Likewise, 80% of small-diameter (< 25 μm) CGRP-ir neurons also contained EGFP-ir; whereas the percentage was lower in medium-sized CGRP-ir neurons (64%, Fig. S3C). We conclude that the EGFP signal corresponds well with the endogenous αCGRP expression in TG neurons from CGRPαEGFPf/+ mice.
Next, we treated both male and female CGRPαEGFPf/+ mice with vehicle or NTG every two days for 5 times and cultured TG neurons two days after the last injection. This allowed us to test whether repeated NTG exerts sex dimorphic effects on TG neurons. The percentage of EGFP+ neurons was not altered by the repetitive NTG injections in either male or female mice (Table 1), suggesting that the number of TG neurons expressing CGRP is not changed during migraine chronification.
Table 1.
The effects of repeated NTG administration on neurochemical properties of TG neurons.
| Male CGRPαEGFPf/+ | Female CGRPαEGFPf/+ | |||
|---|---|---|---|---|
| vehicle | NTG | vehicle | NTG | |
| EGFP+ | 34.9% | 38.1% | 31.6% | 31.2% |
| Capsaicin-Responsive | 45.1% | 53.0%* | 46.8% | 50.1% |
| Small IB4− | 46.5% | 52.6% | 50.6% | 52.1% |
| Small IB4+ | 33.9% | 31.0% | 28.6% | 31.0% |
| Medium-sized | 19.6% | 16.4% | 20.8% | 16.9% |
| Total TG neurons analyzed | 490 | 481 | 395 | 490 |
| Number of mice | 12 | 14 | 11 | 13 |
p < 0.05, Fisher’s exact test between the vehicle and NTG groups in male CGRPαEGFPf/+ mice.
Small IB4−: small-diameter (< 25 μm) neurons that do not bind to isolectin B4.
Small IB4+: small-diameter neurons that bind to isolectin B4.
Repeated NTG administration has been shown to increase αCGRP mRNA level in TG tissues [23; 28], and NO donor enhances αCGRP promotor activity in TG neurons [7]. This may result from more TG neurons expressing αCGRP mRNA and/or the upregulation of αCGRP mRNA in individual neurons. Our results support the latter possibility, as the percentage of EGFP+, αCGRP-expressing TG neurons was not altered by the repeated NTG administration in either male or female mice. That said, increase in the number of TG neurons exhibiting CGRP-ir has been reported in rats several hours after NTG infusion [7; 16; 55]. This may result from the upregulation of βCGRP, which can be detected by the anti-CGRP antibodies but not by the EGFP+ signal in CGRPαEGFPf/+ mice.
The percentages of CGRP-R neurons in TG cultures from both male and female, vehicle- and NTG-treated CGRPαEGFPf/+ mice were similar to those of wild-type Swiss Webster females (Fig. 3A-B), indicating that loss of one functional Calca allele does not affect the abundance of CGRP-R TG neurons, and repeated NTG-induced increase in CGRP-R TG neurons is independent of mouse sex and strain. In TG cultures from vehicle-treated CGRPαEGFPf/+ mice, only 4% of neurons (36 of 885 total TG neurons) were both EGFP+ and CGRP-R (EGFP+CGRP-R, Fig. 3C, D, E), suggesting that they can respond to endogenous CGRP through autocrine mechanism. Conversely, more than 10% of neurons were EGFP− and CGRP-R (EGFP−CGRP-R, Fig. 3C-E), therefore could only engage in paracrine CGRP signaling. These data indicate that TG neurons respond to CGRP mainly through paracrine mechanism under normal condition, in line with previous reports [20; 21; 42].
Figure 3. Repeated NTG administration increases the number of CGRP-R neurons in both EGFP+ and EGFP− TG subpopulations in CGRPαEGFPf/+ mice.
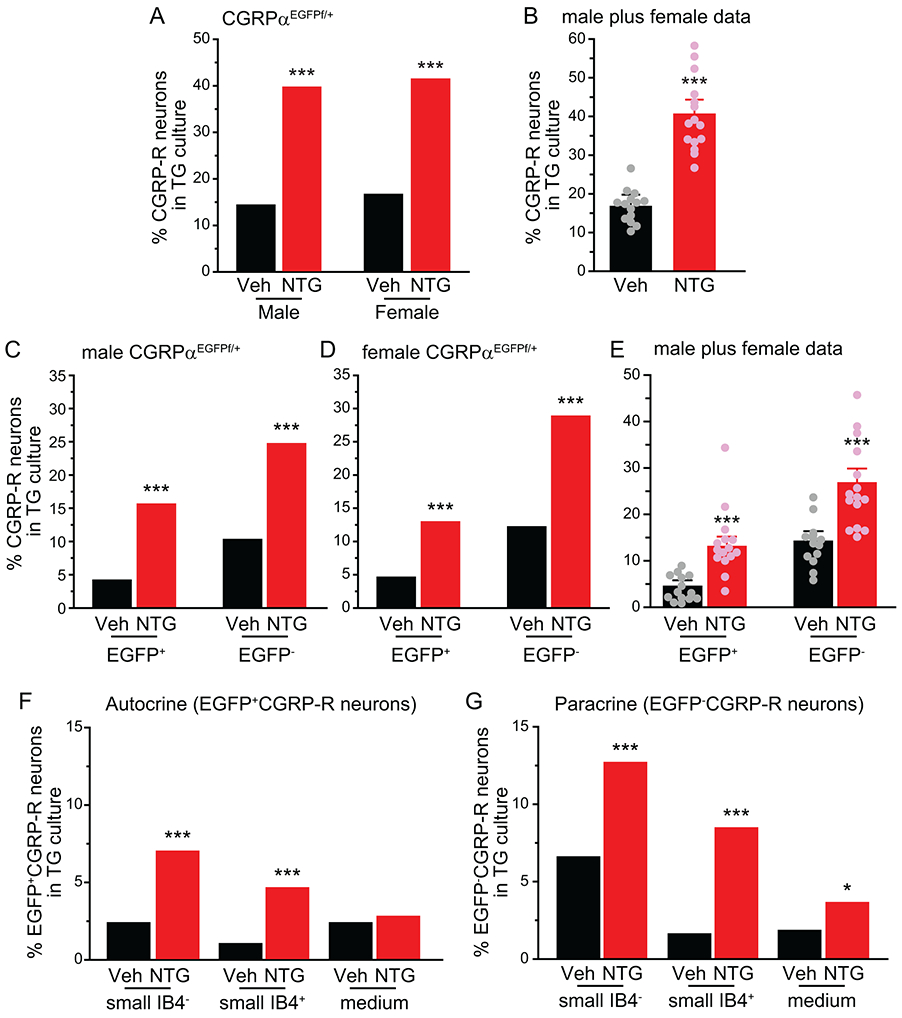
(A) The percentages of CGRP-R neurons in TG cultures from CGRPαEGFPf/+ mice on C57BL/6J background after 5 repeated injections of Veh or NTG (n = 12 and 14 mice/group in male mice; a total of 490 and 481 neurons were measured in Veh and NTG groups; n = 11 and 13 mice/group in female mice; a total of 395 and 490 neurons were measured in Veh and NTG groups, respectively). ***p < 0.001, Fisher’s exact test between vehicle and NTG groups.
(B) The percentages of CGRP-R neurons in TG culture. Data from male and female mice in A are pooled and are grouped by the number of experiments (n = 14). Each experiment included 1-2 Veh- and NTG-treated littermates of the same sex. ***p < 0.001, two-tailed t-test.
(C-E) The percentages of EGFP+CGRP-R and EGFP−CGRP-R neurons in TG cultures from (C) male and (D) female CGRPαEGFPf/+ mice after 5 repeated injections of Veh or NTG (same neurons as in A). C-D: ***p< 0.001, Fisher’s exact test between Veh and NTG groups. E: Data from male and female mice in C and D are pooled and are grouped by the number of experiments (n = 14). ***p < 0.001, two-tailed t-test.
(F) The percentages of small IB4−, small IB4+ and medium-sized EGFP+CGRP-R neurons in TG cultures (same neurons as in A). ***p < 0.001, Fisher’s exact test between Veh and NTG groups.
(G) The percentages of small IB4−, small IB4+ and medium-sized EGFP−CGRP-R neurons in TG cultures (same neurons as in A). *p < 0.05, ***p < 0.001, Fisher’s exact test between Veh and NTG groups.
After repeated NTG administration, the percentage of EGFP+CGRP-R TG neurons increased more than 3-fold, from 4 ± 1% to 13 ± 2% in cultures from CGRPαEGFPf/+ mice, and the percentage of EGFP−CGRP-R TG neurons increased 2-fold, from 14 ± 2% to 26 ± 3% (Fig. 3C-E, red bars). Thus, under chronic migraine-like condition, the number of TG neurons that can mediate autocrine and paracrine CGRP signaling both increases significantly, and the paracrine signaling still predominates. Upon further analysis, we found that repeated NTG increased the proportion of EGFP+CGRP-R neurons in both IB4+ and IB4− small-diameter neurons but not in medium-sized TG subpopulation (Fig. 3F). The percentages of EGFP−CGRP-R neurons were higher in both small and medium TG neurons after repeated NTG injections, with the small IB4+ subpopulation exhibiting the biggest (5-fold) increase (Fig. 3G).
More CGRP-expressing TG neurons respond to both CGRP and PACAP after repeated NTG administration.
The percentages of PACAP-R neurons in TG cultures from both female and male, vehicle- and NTG-treated CGRPαEGFPf/+ mice (Fig. 4A-B) were similar to those of wild-type Swiss Webster females (Fig. 2B), indicating that loss of one functional Calca allele does not affect the abundance of PACAP-R TG neurons, and repeated NTG-induced increase in PACAP-R TG neurons is independent of mouse sex and strain. When comparing the overlap between CGRP-R and PACAP-R neurons in TG culture, we found that repeated NTG administration significantly increased the abundance of TG neurons that respond to both CGRP and PACAP, from less than 4% to more than 15% in both male and female mice (Fig. 4C, p < 0.001, Fisher’s exact test between individual vehicle and NTG groups). The percentage of TG neurons that responded to CGRP but not to PACAP was also significantly increased by repeated NTG (Fig. 4C, p < 0.01, Fisher’s exact test between individual vehicle and NTG groups). On the contrary, the percentage of TG neurons that responded to PACAP but not to CGRP remained steady after repeated NTG administration (Fig. 4C).
Figure 4. The number of TG neurons that respond to both CGRP and PACAP is increased by repeated NTG administration.
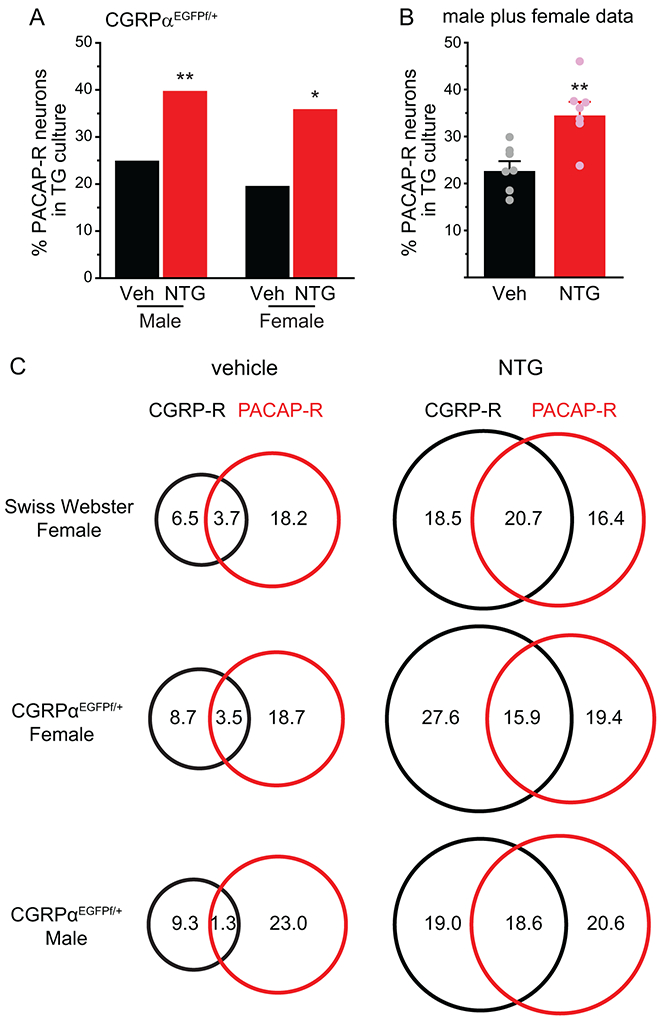
(A) The percentages of PACAP-R neurons in TG culture from CGRPαEGFPf/+ mice after 5 repeated injections of vehicle or NTG (n = 5 and 6 mice/group in male mice; a total of 226 and 237 neurons were measured in vehicle and NTG groups; n = 5 and 6 mice/group in female mice; a total of 171 and 232 neurons were measured in vehicle and NTG groups, respectively). *p < 0.05, **p < 0.01, Fisher’s exact test between vehicle and NTG groups.
(B) The percentages of PACAP-R neurons in TG culture. Data from male and female mice in A are pooled and grouped by the number of experiments (n = 7). Each experiment included 1-2 vehicle- and NTG-treated littermates of the same sex. ***p < 0.001, two-tailed t-test.
(C) Venn diagrams of the percentages of CGRP-R and PACAP-R neurons in TG culture from female Swiss Webster (same neurons as in Figure 1A) as well as female and male CGRPαEGFPf/+ mice (same neurons as in A).
To examine whether PACAP-R TG neurons express endogenous αCGRP, we pooled data from male and female CGRPαEGFPf/+ mice (Fig. 5A). In both vehicle- and NTG-treated mice, about half of PACAP-R neurons (56% and 52%, respectively) were EGFP+, suggesting that they express αCGRP (Fig. 5A). Within the EGFP+ TG subpopulation, the abundance of neurons that respond to both CGRP and PACAP (CGRP-R&PACAP-R) increased more than 7 fold after repeated NTG administration (Fig. 5B). This was accompanied by a concomitant reduction of neurons that did not respond to either CGRP or PACAP (CGRP-NR&PACAP-NR, Fig. 5B). Overall, less than 50% of CGRP-expressing TG neurons respond to either CGRP or PACAP under normal condition. This number increased to 70% in chronic migraine-like state, and 28% of CGRP-expressing neurons can be activated by both CGRP and PACAP. In EGFP− neurons that do not express αCGRP, repeated NTG administration not only increased the number of neurons that respond to both CGRP and PACAP, but also those that respond to CGRP but not PACAP (CGRP-R&PACAP-NR, Fig. 5C).
Figure 5. Repeated NTG administration preferentially increases the number of neurons that respond to both CGRP and PACAP in the TG subpopulation that express αCGRP.
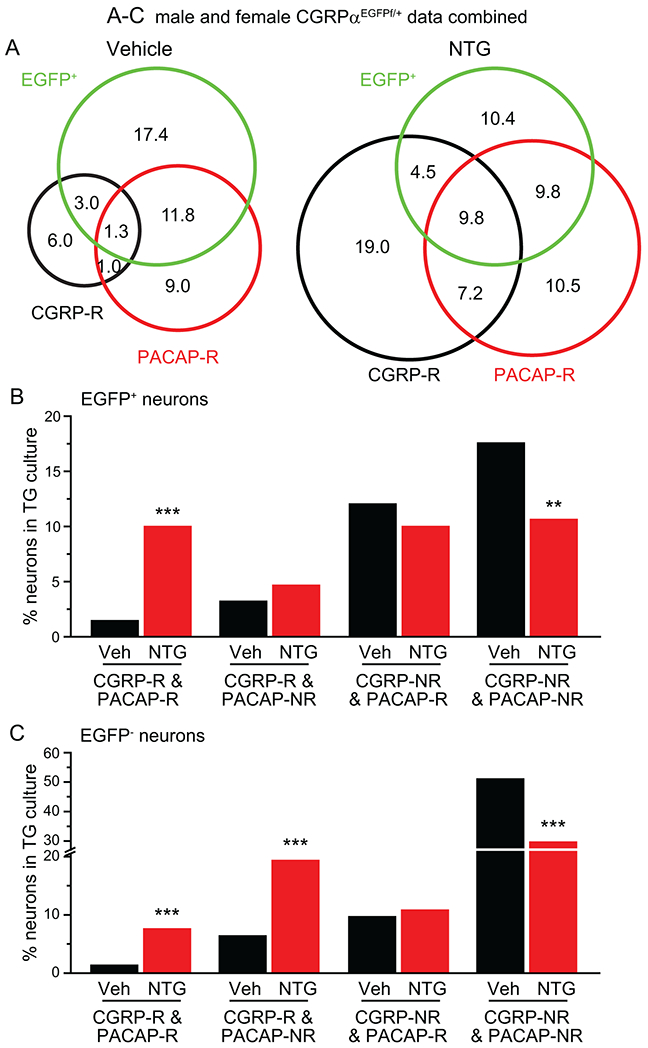
(A) Venn diagrams of the percentages of CGRP-R, PACAP-R and EGFP+ neurons in TG culture from CGRPαEGFPf/+ mice after 5 repeated injections of vehicle or NTG (same neurons as in Figure 4A. Data from male and female mice are combined).
(B-C) The percentages of EGFP+ (B) and EGFP− (C) neurons that are CGRP-R&PACAP-R, CGRP-R&PACAP-NR, CGRP-NR&PACAP-R or CGRP-NR&PACAP-NR in TG culture (same neurons as in A). **p < 0.01, ***p < 0.001, Fisher’s exact test between the corresponding vehicle and NTG groups.
To further characterize TG neurons that respond to both CGRP and PACAP, we exposed TG cultures to 1 μM capsaicin, the selective agonist of the transient receptor potential cation channel subfamily V member 1 (TRPV1) channel, and measured capsaicin-induced Ca2+ transient after washing out CGRP. Interestingly, more than 80% of CGRP-R&PACAP-R neurons in both vehicle and NTG groups were capsaicin-responsive (Cap-R, Fig. 6A-B). The few capsaicin non-responsive (cap-NR) CGRP-R&PACAP-R neurons from NTG-treated mice all had medium-sized diameter somata (Fig. 6A). The majority (> 70%) of neurons that respond to PACAP but not CGRP (CGRP-NR&PACAP-R) were also cap-R (Fig. 6A-B). On the contrary, the majority of neurons that respond to CGRP but not PACAP were cap-NR in both vehicle- and NTG-treated mice (Fig. 6A-B). Thus, most PACAP-R neurons, regardless of whether they respond to CGRP, belong to unmyelinated C-fiber nociceptors that express functional TRPV1 channels.
Figure 6. The majority of PACAP-R TG neurons express functional TRPV1 channels.
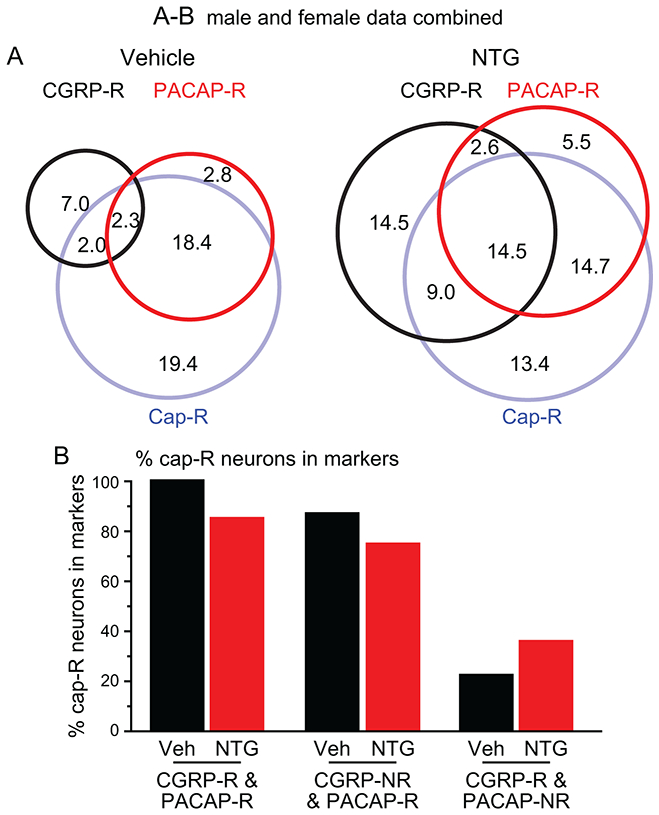
(A) Venn diagrams of the percentages of CGRP-R, PACAP-R and Cap-R neurons in TG culture from vehicle- and NTG-treated CGRPαEGFPf/+ mice (same neurons as in Figure 4A. Data from male and female mice are combined).
(B) The percentages of cap-R neurons in CGRP-R&PACAP-R, CGRP-NR&PACAP-R and CGRP-R&PACAP-NR neurons (same neurons as in A).
We also observed a small but significant increase in the percentage of cap-R neurons in TG cultures from NTG-treated male CGRPαEGFPf/+ mice (Table 1), and the increase preferentially occurred in the small IB4− subpopulation (Fig. S4A). Repeated NTG did not alter the percentage of cap-R neurons in female CGRPαEGFPf/+ mice (Table 1, Fig. S4B) or female Swiss Webster mice (50% [68/137] and 55% [77/140] in vehicle and NTG groups, respectively, p = 0.4, Fisher’s exact test).
Endogenous CGRP signaling is required for NTG-induced increase in CGRP-R but not PACAP-R TG neurons.
We used the CGRPα KO mice to test whether the endogenous CGRP signaling plays a role in NTG-induced increase in CGRP-R and PACAP-R TG neurons. The percentages of CGRP-R, PACAP-R as well as CGRP-R&PACAP-R neurons in TG cultures from vehicle-treated CGRPα KO mice were similar to those of vehicle-treated wild-type mice (Fig. 7A-C). However, repeated NTG administration did not alter the number of CGRP-R TG neurons in CGRPα KO mice (Fig. 7A), whereas the magnitude of PACAP-R neuron increase was comparable between NTG-treated wild-type and CGRPα KO mice (Fig. 7B). The percentage of neurons that respond to both CGRP and PACAP was not significantly changed in NTG-treated KO mice either (Fig. 7C). We also compared the overlap between CGRP-R, PACAP-R and EGFP+ TG neurons in KO mice (Fig. 7D). Unlike what we observed in heterozygous CGRPαEGFPf/+ mice, in EGFP+ neurons from KO mice, the percentage of neurons that respond to both CGRP and PACAP was not altered by the repeated NTG administration (Fig. 7E). Neither was there a reduction of CGRP-NR&PACAP-NR neurons (Fig. 7F). We conclude that activation of the endogenous CGRP signaling pathway is required for NTG-induced increase in CGRP-R neurons in TG, whereas the increase in PACAP-R TG neurons was independent of the release of endogenous αCGRP.
Figure 7. Repeated NTG administration increases the number of PACAP-R TG neurons but not the number of CGRP-R neurons in CGRPα KO mice.
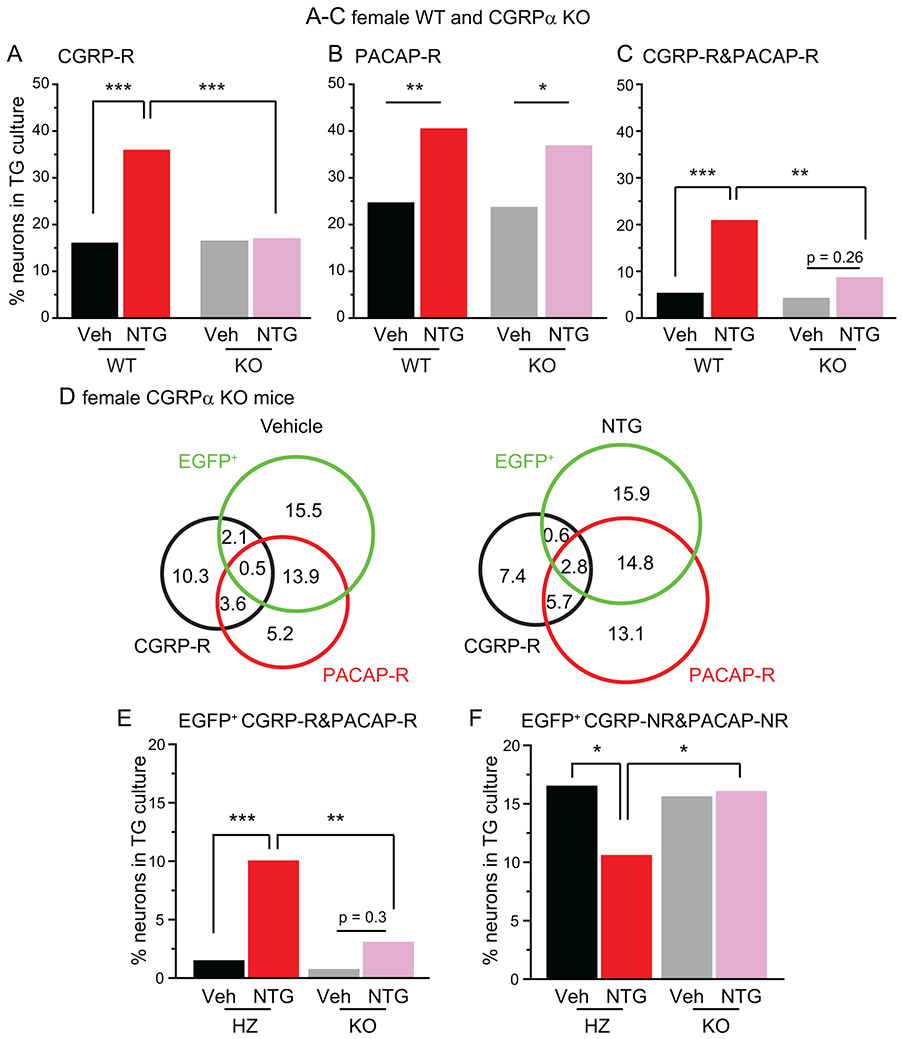
(A-C) The percentages of CGRP-R (A), PACAP-R (B) and CGRP-R&PACAP-R (C) neurons in TG cultures from female WT or CGRPα KO mice on C57BL/6J background after 5 repeated injections of Veh or NTG (n = 3-6 mice/group, a total of 116, 130,194 and 176 neurons were measured in WT+Veh, WT+NTG, KO+Veh and KO+NTG groups, respectively). *p < 0.05, **p < 0.01, ***p < 0.001, χ2 test followed by post hoc Fisher’s exact test with Bonferroni correction.
(D) Venn diagrams of the percentages of CGRP-R, PACAP-R and EGFP+ neurons in TG cultures from Veh- or NTG-treated CGRPα KO mice (same neurons as in A-C).
(E-F) The percentages of EGFP+ neurons that are CGRP-R&PACAP-R (E) or CGRP-NR&PACAP-NR (F) in TG culture from heterozygous (HZ) CGRPαEGFPf/+ mice (same neurons as in Figure 5B) and CGRPα KO mice (same neurons as in A-C). *p < 0.05, **p < 0.01, ***p < 0.001, χ2 test followed by post hoc Fisher’s exact test with Bonferroni correction.
Ld-IL2 treatment blocks NTG-induced increase in CGRP-R and PACAP-R TG neurons.
In a previous study, we reported that daily ld-IL2 treatment completely reversed NTG-induced persistent behavioral sensitization in mice [64]. In male C57BL/6J mice that received 5 NTG injections (10 mg/kg, i.p.), facial mechanical hyper-sensitivity returned to baseline level 11 days after the cessation of NTG (Fig. 8A, NTG+saline group, day 1-20). Subsequent daily injections of low-dose NTG (0.1 mg/kg, i.p.) re-established facial mechanical hyper-sensitivity (Fig. 8A, NTG+saline group, day 20-28), revealing the hyperalgesic priming resulting from the previous high-dose NTG administration. Ld-IL2 treatment not only accelerated the reversal of high-dose NTG-induced facial mechanical hyper-sensitivity (Fig. 8A, NTG+IL2 group, day 1-20), but also completely blocked the effect of low-dose NTG (Fig. 8A, NTG+IL2 group, day 20-28), indicating that ld-IL2 prevents the development of hyperalgesic priming. Since the therapeutic effect of ld-IL2 is likely mediated by the regulatory T cells at peripheral sites [64], we investigated whether ld-IL2 treatment reduces NTG-induced increase in CGRP-R and PACAP-R TG neurons in female Swiss Webster mice (Fig. 8B). Ld-IL2 treatment alone had no effect on the percentages of CGRP-R, PACAP-R or CGRP-R&PACAP-R neurons in TG cultures (Fig. 8C, D, E, Veh+saline versus Veh+IL2 groups). However, it completely blocked NTG-induced increase in CGRP-R and PACAP-R TG neurons (Fig. 8C-E), suggesting that one of ld-IL2’s mechanisms of action may be through normalizing the strength of CGRP and PACAP signaling pathways in primary afferent neurons.
Figure 8. Ld-IL2 treatment blocks NTG-induced increase in CGRP-R and PACAP-R TG neurons.
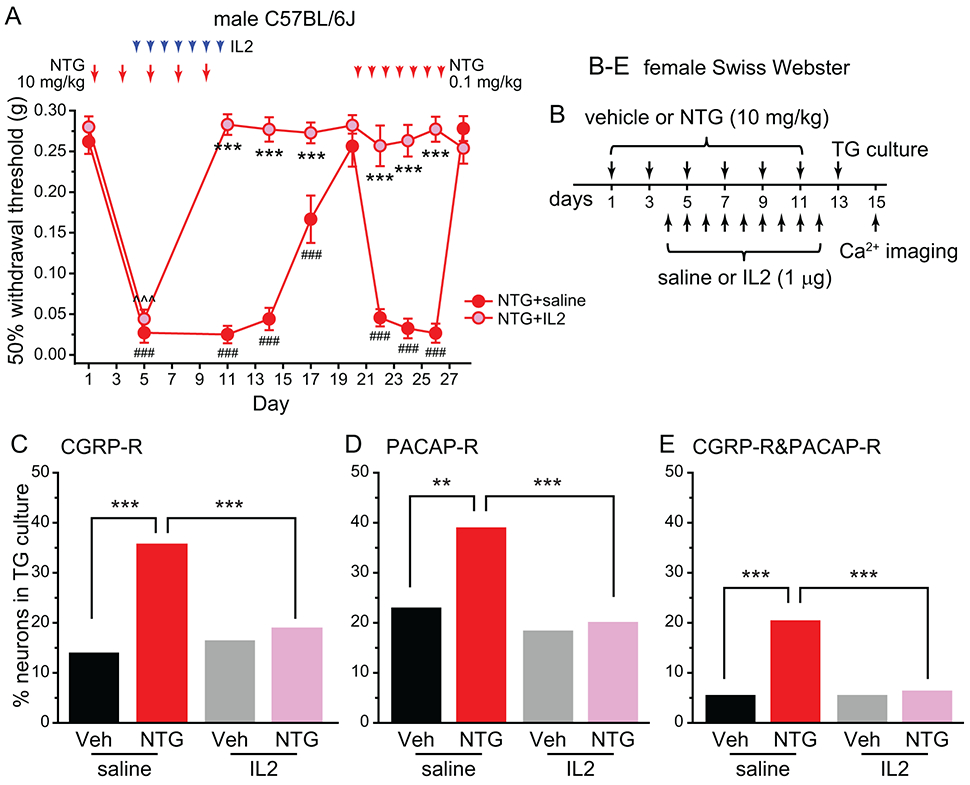
(A) The effects of repeated NTG and/or ld-IL2 on the 50% withdrawal thresholds to von Frey filaments at the periorbital region of male C57BL/6J mice (n = 6/group). Note that IL2 and NTG were always injected after the completion of behavioral tests on the same day. Two-way RM ANOVA: p < 0.001 for group (F[1, 238] = 381.1), time (F[9, 238] = 61.4) and group x time interaction (F[9, 238] = 36.8); post hoc Student–Newman–Keuls test: ***p < 0.001, between the corresponding NTG+saline and NTG+IL2 groups; ###p < 0.001, compared with the baseline (day 1) threshold in the NTG+saline group; ^^^p < 0.001, compared with the baseline (day 1) threshold in the NTG+IL2 group.
(B) Time line of the experiment in C-E.
(C-E) The percentages of CGRP-R (C), PACAP-R (D) and CGRP-R&PACAP-R (E) neurons in TG cultures from Veh-, NTG- and/or ld-IL2-treated female Swiss Webster mice (n = 6-15 mice/group, a total of 366, 463,197 and 533 neurons were measured in Veh+saline, NTG+saline, Veh+IL2 and NTG+IL2 groups, respectively). **p < 0.01, ***p < 0.001, χ2 test followed by post hoc Fisher’s exact test with Bonferroni correction.
The number of TG neurons that respond to both CGRP and PACAP is significantly increased in a mouse model of PTH.
PTH is the most common medical consequence of mTBI and often resembles migraine headache [3]. Peripheral CGRP signaling contributes to the expression of mTBI-induced acute PTH-related behaviors as well as the establishment of chronic PTH-related hyperalgesic priming in animal models [9; 10; 44]. To test whether mTBI enhances CGRP and/or PACAP signaling in TG neurons, we subjected male Swiss Webster mice to a mTBI paradigm which resulted in a prolonged facial mechanical hyper-sensitivity as well as the development of hyperalgesic priming in our previous study [64]. Seven days after mTBI, when facial mechanical hyper-sensitivity reached its peak, we cultured TG neurons and measured their responses to CGRP and PACAP (Fig. 9A). The percentages of CGRP-R and PACAP-R TG neurons were significantly increased by mTBI (Fig. 9B-C). This largely resulted from the increase in the number of neurons that respond to both CGRP and PACAP (Fig. 9D); whereas the number of neurons that respond to CGRP only or to PACAP only was not significantly altered by mTBI (Fig. 9E).
Figure 9. The number of TG neurons that respond to both CGRP and PACAP is significantly increased after mTBI.
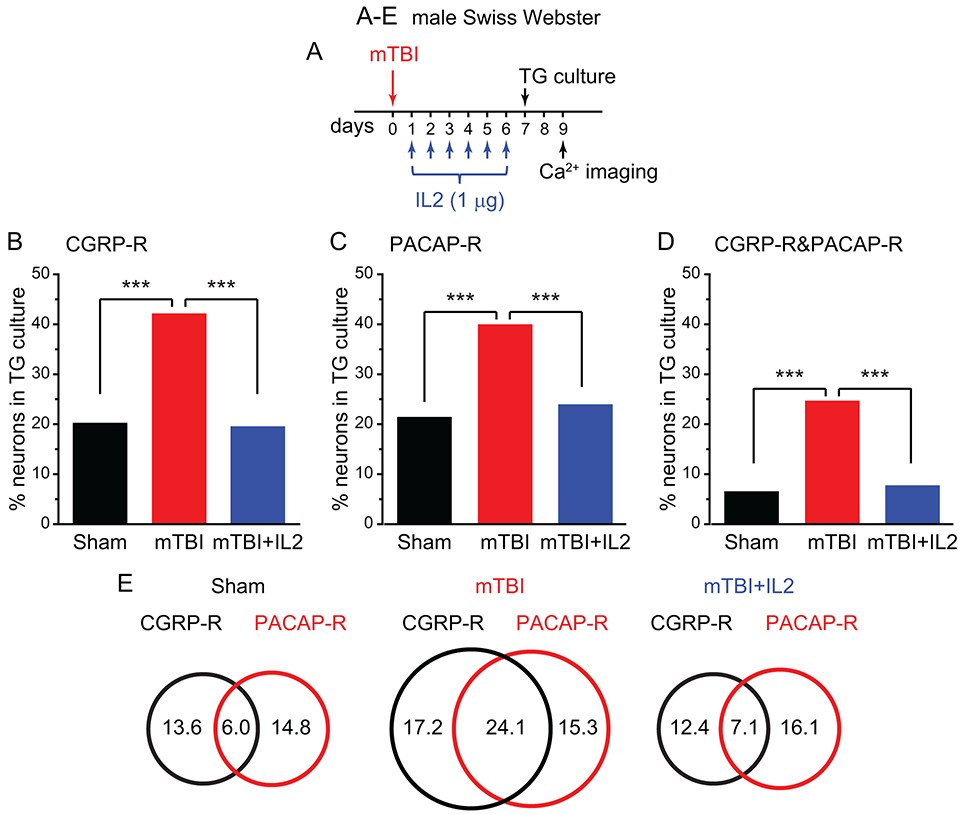
(A) Time line of the experiment in B-E.
(B-D) The percentages of CGRP-R (B), PACAP-R (C) and CGRP-R&PACAP-R (D) neurons in TG cultures from male Swiss Webster mice that received sham procedure, mTBI, and mTBI with ld-IL2 treatment (n = 6-10 mice/group, a total of 336, 378 and 224 neurons were measured in sham, mTBI and mTBI+IL2 groups, respectively). ***p < 0.001, χ2 test followed by post hoc Fisher’s exact test with Bonferroni correction.
(E) Venn diagrams of the percentages of CGRP-R and PACAP-R neurons in TG cultures from mice in the sham, mTBI and mTBI+IL2 groups (same neurons as in B-D).
We have shown previously that starting daily ld-IL2 treatment 1 day after mTBI completely prevents the development of acute PTH-related behaviors as well as the hyperalgesic priming related to chronic PTH [64]. Here, we treated mice with ld-IL2 between day 1 and day 6 post-mTBI, and then cultured TG neurons to measure how they respond to CGRP and PACAP (Fig. 9A). Similar to what we observed in the chronic migraine model, ld-IL2 treatment completely blocked mTBI-induced increase in CGRP-R&PACAP-R TG neurons (Fig. 9B-E), suggesting that blocking CGRP and PACAP signaling simultaneously in TG neurons may prevent the development of chronic migraine and PTH. Lastly, mTBI also resulted in a small but significant increase in the percentage of cap-R TG neurons (Fig. S4C), which was also blocked by ld-IL2 treatment (Fig. S4C).
Discussion
In this study, we used EGFP signal in CGRPαEGFPf/+ mice to identify neurons expressing endogenous αCGRP and ligand-induced [Ca2+]in increase to define CGRP-R and PACAP-R neurons in TG culture. This allowed us to reveal a previously undescribed relationship between CGRP-R, PACAP-R and αCGRP-expressing neurons in mouse TG under normal and chronic migraine-like states. Under normal condition, most CGRP-R TG neurons did not express αCGRP, indicating that paracrine CGRP signaling predominates. This is consistent with previous studies showing that there is little overlap between neurons that express CGRP and those that express the canonical CGRP receptor subunits [20; 21], and anti-CGRP antibody selectively inhibits the activity of Aδ but not C-fiber meningeal nociceptors [42].
After repeated NTG administration, there was a significant increase in CGRP-R neurons in mouse TG but not DRG, supporting its relevance to chronic migraine. Both NTG-induced behavioral sensitization and the increase in CGRP-R TG neurons were absent in CGRPα KO mice, indicating that both behavioral and cellular changes require the release of endogenous CGRP. NTG-induced increase in CGRP-R neurons was more prominent in EGFP+, αCGRP-expressing neurons than EGFP− neurons (3-fold versus 2-fold). Consequently, the percentage of CGRP-R neurons that could engage in autocrine signaling increased from 24% to 32%, suggesting that a larger fraction of CGRP signaling in TG neurons may be mediated through the autocrine mechanism in some chronic migraine patients, especially those with migraine episodes resulting from the activation of NO signaling pathway.
NTG infusion in rats increases the number of TG neurons that express RAMP1 [55], the obligatory subunit for both canonical CGRP receptor and AMY1 receptor, supporting a possible upregulation of both receptor subtypes under pathological conditions. The concentration of CGRP (3 μM) used in this study could potentially activate the high-affinity canonical CGRP receptor, AMY1 receptor as well as some other CGRP-family receptors with lower-affinity to CGRP [31; 62]. This served the purpose of identifying all the αCGRP-expressing neurons that could possibly respond to CGRP, as autocrine CGRP signaling likely occurs through both high- and low-affinity receptors due to the high effective concentration of locally released CGRP in the immediate vicinity of cell-surface receptors. Conversely, paracrine CGRP signaling is likely mediated by the high-affinity receptors, as the concentration of CGRP diffuses to those neurons would be much lower. Thus, our experimental condition would allow us to accurately identify the number of TG neurons with autocrine CGRP signaling, but might lead to over-estimation of the number of neurons with paracrine CGRP signaling. If this is indeed the case, the contribution of autocrine CGRP signaling during chronic migraine may be even greater than we reported here. It is tempting to propose a working model that the release of endogenous CGRP during migraine episodes increases the number of functional CGRP receptors in TG neurons. This, in turn, sensitizes TG neurons, leading to more CGRP release and prolonged headache. Future study is warranted to determine which types of CGRP-family receptors mediate the autocrine and paracrine responses under normal and chronic migraine conditions, which receptor subunits are upregulated by the repeated NTG administration, whether any receptor subunits undergo post-translational modification and/or translocation, and lastly, what is the relative contributions of autocrine versus paracrine CGRP signaling in TG neurons to migraine chronification.
A large body of work, including the current study, indicate that blocking CGRP signaling prevents NTG-induced acute and persistent behavioral sensitization ([15; 43] and references within), supporting the importance of CGRP signaling in migraine pathophysiology. Antibodies against CGRP or its canonical receptor successfully reduce migraine frequency in many patients, but 30-40% of patients remain unresponsive to antibody therapy [4; 19]. In light of our findings, it is possible that the anti-CGRP antibodies are less effective in patients with enhanced autocrine CGRP signaling in TG neurons. Mathematical modeling has predicted that, to effectively inhibit autocrine signaling, the concentration of anti-ligand antibody needs to be 4-8 orders of magnitude greater than the equilibrium dissociation constant for ligand binding to surface receptors [25]. The model also predicts that the anti-receptor antibody is far more effective than the anti-ligand antibody in inhibiting autocrine signaling [26]. However, the efficacy of erenumab, the antibody against the canonical CGRP receptor, is not significantly higher than those of anti-CGRP antibodies [4; 19], suggesting that the autocrine CGRP signaling may be mediated through other receptors. Indeed, anatomical studies show little overlap between the expression of CGRP and the canonical CGRP receptor subunits in TG neurons [20; 21; 37]. It is likely that the autocrine CGRP signaling in TG is mediated by AMY1 receptor and other CGRP-family receptors that are not blocked by erenumab [61; 65].
In addition to CGRP signaling, the role of neuropeptide PACAP in migraine pathophysiology has also been rigorously pursued. CGRP and PACAP are co-expressed in some TG neurons [18], and PACAP is also released during migraine attacks [59; 63]. Infusion of PACAP induces migraine-like headache in humans [53] as well as the activation and sensitization of central trigeminovascular neurons in rats [1]. Antibody against PAC1 receptor effectively inhibits stimulus-evoked activity in central trigeminovascular neurons [32], suggesting that migraine episodes may be prevented by blocking the peripheral PACAP signaling pathway. We found that 40% of EGFP+ TG neurons were PACAP-R but not VIP-R in control mice, suggesting that PACAP signaling through PAC1 receptor may facilitate the release of endogenous CGRP. In EGFP+ TG neurons, repeated NTG administration preferentially increased the number of neurons that were not only CGRP-R but also PACAP-R (Fig. 5B). The majority (> 70%) of these neurons belonged to unmyelinated C-fiber nociceptors that express functional TRPV1 channels (Fig. 6). Although more experiments are needed to elucidate the contribution of this TG subpopulation to migraine chronification, it is reasonable to speculate that CGRP can be released from these neurons by the activation of autocrine CGRP signaling [54; 65], PAC1 receptors as well as TRPV1 channels. Thus, even if the autocrine CGRP signaling can be effectively inhibited, PACAP- and/or TRPV1-mediated sensitization and CGRP release would remain intact in some chronic migraine patients. This provides another explanation for the lack of efficacy of antibodies targeting CGRP signaling in migraine prevention.
Interestingly, repeated NTG still increased the number of PACAP-R neurons in TG of CGRPα KO mice, despite the absence of behavioral sensitization or the change of CGRP-R neurons (Fig. 7). This by no means negates the physiological relevance of PACAP signaling, but rather implicates that PACAP signaling contributes to migraine chronification through the release of endogenous CGRP. It also raises the possibility of two parallel feedforward loops under chronic migraine conditions – the release of endogenous CGRP and PACAP may lead to the increase in CGRP-R and PACAP-R TG neurons, respectively. Future in-depth studies are needed to test these possibilities.
Although NTG administration has been widely used to establish migraine-like state in rodents [2; 6; 15; 24; 39; 48; 56-58; 64], it only models one of the many mechanisms underlying migraine pathophysiology. Systemic injection of NTG increases the number of CD3+ T cells, CGRP-R neurons and PACAP-R neurons in mouse TG but not DRG (Fig. 2, [64]), suggesting that the effects of NTG in mice are likely headache-relevant. That said, the dose of NTG required to establish persistent behavioral sensitization and hyperalgesic priming in naïve mice is much higher than that used to trigger migraine-like headache in migraine patients [47]. More work is warranted to investigate whether the increase in CGRP-R and PACAP-R TG neurons occurs in chronic migraine states generarted through other mechanisms. In a mouse model of mTBI-induced PTH, we observed a similar increase in TG neurons that respond to both CGRP and PACAP, suggesting that both signaling pathways may contribute to the sensitization of these neurons and the chronification of migraine and PTH. This is consistent with recent work revealing that both peripheral CGRP-dependent and peripheral CGRP-independent mechanisms underlie the development of PTH- and chronic migraine-related behaviors. Early and prolonged treatment of anti-CGRP antibodies ameliorates acute PTH-related behaviors in male but not female rats [9; 10]. Once mTBI-induced central sensitization is established, anti-CGRP antibodies become less effective in attenuating chronic PTH-related behaviors [10; 44]. The canonical CGRP receptor antagonist olcegepant also fails to block chronic migraine-related behaviors induced by repeated exposure to NO donor isosorbide dinitrate [14].
Taken together, our study suggests that the expansion of unmyelinated C-fiber nociceptors in TG that express CGRP as well as the functional receptors for both CGRP and PACAP may play an important role in chronic migraine and mTBI-induced PTH. This not only supports the notion that both peripheral CGRP and PACAP signaling pathways are targets for chronic migraine and PTH therapy, but also predicts that inhibiting both peripheral CGRP and PACAP signaling may be necessary for patients that are not responsive to drugs targeting individual pathways. In this regard, the robust efficacy of ld-IL2 in reversing established chronic migraine- and PTH-related behavioral sensitization in male and female, inbred and outbred strains of mice [64] may result from its ability to block the increase in CGRP-R, PACAP-R as well as cap-R neurons, thereby breaking the vicious circle of CGRP/PACAP/TRPV1-induced CGRP release from TG neurons.
Supplementary Material
Figure S3. Substantial overlap between CGRP-ir and EGFP-ir in TG neurons from CGRPα-GFP+/− mice.
(A) Representative images EGFP-ir and CGRP-ir in TG culture from naïve female CGRPαEGFPf/+ mice.
(B) The percentages of small- and medium-sized EGFP+ TG neurons that contain CGRP-ir. A total of 2589 TG neurons from 3 mice were counted.
(C) The percentages of small- and medium-sized CGRP-ir TG neurons that contain EGFP-ir (same neurons as in B).
Figure S4. Repeated NTG increases the percentage of small IB4−cap-R neurons in TG culture from male CGRPαEGFPf/+ mice.
(A-B) The percentages of small IB4+, small IB4− and medium-sized cap-R neurons in TG cultures from male (A) and female (B) CGRPαEGFPf/+ mice after 5 repeated injections of vehicle or NTG (same neurons as in Table 1). *p < 0.05, Fisher’s exact test between vehicle and NTG groups.
(C) The percentages of cap-R neurons in TG cultures from male Swiss Webster mice that received sham procedure, mTBI, and mTBI with ld-IL2 treatment (same neurons as in Figure 9B-D). *p < 0.05, **p < 0.01, χ2 test followed by post hoc Fisher’s exact test with Bonferroni correction.
Figure S1. Pilot studies to determine the dose of NTG that reliably induces long-lasting behavioral sensitization in mice.
(A) Repeated i.p. administration of 1 mg/kg NTG does not significantly alter facial mechanical thresholds in female Swiss Webster mice (n = 6). One-way RM ANOVA: p = 0.08 (F[5, 35] = 2.43).
(B) The facial mechanical thresholds before and one day after two low-dose NTG injections (0.1 mg/kg, i.p., day 11 and 12, same mice as in A).
(C) Repeated i.p. administration of 3 mg/kg NTG results in sustained facial mechanical hyper-sensitivity in female Swiss Webster mice (n = 6). One-way RM ANOVA: p < 0.05 (F[6, 41] = 2.94), post hoc Dunnett’s test: *p < 0.05, compared with the baseline threshold on day 1.
(D) The facial mechanical thresholds before and one day after two low-dose NTG injections (0.1 mg/kg, i.p., day 13 and 14, same mice as in C).
(E) Repeated i.p. administration of 10 mg/kg NTG results in robust and persistent facial mechanical hyper-sensitivity in female Swiss Webster mice (n = 7). One-way RM ANOVA: p < 0.001 (F[8, 62] = 33.91), post hoc Dunnett’s test: **p < 0.01, ***p < 0.001, compared with the baseline threshold on day 1.
(F) Mice received repeated 10 mg/kg NTG exhibit hyperalgesic priming that lasts for a week. Mice (same as in E) received 2 daily low-dose NTG injections (0.1 mg/kg, i.p.) 0, 7 and 14 days after the facial mechanical thresholds returned to baseline level (day 0 here is day 20 in E). ***p < 0.01, two-tailed paired t-test.
Figure S2. Pilot studies to determine the concentrations of CGRPα and PACAP1-38 to define CGRP-R and PACAP-R neurons in TG culture.
(A) The percentages of CGRP-R neurons in TG cultures from naïve female Swiss Webster mice. CGRP8-37 was co-applied with αCGRP. On average, 80 neurons were measure under each condition.
(B) The percentages of PACAP-R neurons in TG cultures from naïve female Swiss Webster mice. On average, 102 neurons were measure under each condition.
(C) The percentages of VIP-R neurons in TG cultures from female Swiss Webster mice that received repeated Veh or NTG injections. On average, 162 neurons were measure under each condition.
(D) The percentages of PACAP-R and CGRP-R neurons in TG cultures from female Swiss Webster mice after 5 repeated i.p. injections of saline (Sal) or Veh (n = 6 mice/group; a total of 268 and 232 neurons were measured in Sal and Veh groups, respectively).
(E) The percentages of PACAP-R and CGRP-R neurons in TG cultures from female Swiss Webster mice after 5 repeated i.p. injections of Veh or 1 mg/kg NTG (n = 6 mice/group; a total of 218 and 293 neurons were measured in Veh and NTG groups, respectively).
(F) The percentages of PACAP-R and CGRP-R neurons in TG cultures from female Swiss Webster mice after 5 repeated i.p. injections of Veh or 3 mg/kg NTG (n = 6 mice/group; a total of 190 and 265 neurons were measured in Veh and NTG groups, respectively). *p < 0.05, **p < 0.01, Fisher’s exact test between Veh and NTG groups.
(G) The effect of a single NTG injection on the 50% withdrawal thresholds to von Frey filaments at the periorbital region of female Swiss Webster mice (n = 8). One-way RM ANOVA: p < 0.001 (F[5, 47] = 19.6), post hoc Dunnett’s test: ***p < 0.001, compared with the baseline (day 1) threshold.
(H) The effects of repeated NTG on the 50% withdrawal thresholds to von Frey filaments at the periorbital region of female Swiss Webster mice (n = 7, same mice as in Fig. S1E-F). Note that NTG were always injected after the completion of behavioral tests on the same day. One-way RM ANOVA: p < 0.001 (F[11, 83] = 20.09), post hoc Dunnett’s test: *p < 0.05, ***p < 0.001, compared with the baseline threshold on day 1.
Acknowledgements
The authors thank members of Cao lab for valuable comments on the manuscript. This study is supported by the National Institute of Neurological Disorders and Stroke grant NS103350-02S1 and the Department of Defense Investigator-Initiated Research Award W81XWH1810627 (to YQC). The authors report no conflict of interests.
References:
- [1].Akerman S, Goadsby PJ. Neuronal PAC1 receptors mediate delayed activation and sensitization of trigeminocervical neurons: Relevance to migraine. Sci Transl Med 2015;7(308):308ra157. [DOI] [PubMed] [Google Scholar]
- [2].Akerman S, Karsan N, Bose P, Hoffmann JR, Holland PR, Romero-Reyes M, Goadsby PJ. Nitroglycerine triggers triptan-responsive cranial allodynia and trigeminal neuronal hypersensitivity. Brain 2019;142(1):103–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ashina H, Porreca F, Anderson T, Amin FM, Ashina M, Schytz HW, Dodick DW. Post-traumatic headache: epidemiology and pathophysiological insights. Nat Rev Neurol 2019;15(10):607–617. [DOI] [PubMed] [Google Scholar]
- [4].Ashina M The most important advances in headache research in 2018. Lancet Neurol 2019;18(1):5–6. [DOI] [PubMed] [Google Scholar]
- [5].Avona A, Burgos-Vega C, Burton MD, Akopian AN, Price TJ, Dussor G. Dural Calcitonin Gene-Related Peptide Produces Female-Specific Responses in Rodent Migraine Models. J Neurosci 2019;39(22):4323–4331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bates EA, Nikai T, Brennan KC, Fu YH, Charles AC, Basbaum AI, Ptacek LJ, Ahn AH. Sumatriptan alleviates nitroglycerin-induced mechanical and thermal allodynia in mice. Cephalalgia : an international journal of headache 2010;30(2):170–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Bellamy J, Bowen EJ, Russo AF, Durham PL. Nitric oxide regulation of calcitonin gene-related peptide gene expression in rat trigeminal ganglia neurons. The European journal of neuroscience 2006;23(8):2057–2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ben Aissa M, Tipton AF, Bertels Z, Gandhi R, Moye LS, Novack M, Bennett BM, Wang Y, Litosh V, Lee SH, Gaisina IN, Thatcher GR, Pradhan AA. Soluble guanylyl cyclase is a critical regulator of migraine-associated pain. Cephalalgia : an international journal of headache 2018;38(8):1471–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bree D, Levy D. Development of CGRP-dependent pain and headache related behaviours in a rat model of concussion: Implications for mechanisms of post-traumatic headache. Cephalalgia : an international journal of headache 2018;38(2):246–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Bree D, Mackenzie K, Stratton J, Levy D. Enhanced post-traumatic headache-like behaviors and diminished contribution of peripheral CGRP in female rats following a mild closed head injury. Cephalalgia : an international journal of headache 2020;40(7):748–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ceruti S, Villa G, Fumagalli M, Colombo L, Magni G, Zanardelli M, Fabbretti E, Verderio C, van den Maagdenberg AM, Nistri A, Abbracchio MP. Calcitonin gene-related peptide-mediated enhancement of purinergic neuron/glia communication by the algogenic factor bradykinin in mouse trigeminal ganglia from wild-type and R192Q Cav2.1 Knock-in mice: implications for basic mechanisms of migraine pain. J Neurosci 2011;31(10):3638–3649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. Journal of neuroscience methods 1994;53(1):55–63. [DOI] [PubMed] [Google Scholar]
- [13].Chaudhary P, Baumann TK. Expression of VPAC2 receptor and PAC1 receptor splice variants in the trigeminal ganglion of the adult rat. Brain research Molecular brain research 2002;104(2):137–142. [DOI] [PubMed] [Google Scholar]
- [14].Dallel R, Descheemaeker A, Luccarini P. Recurrent administration of the nitric oxide donor, isosorbide dinitrate, induces a persistent cephalic cutaneous hypersensitivity: A model for migraine progression. Cephalalgia : an international journal of headache 2018;38(4):776–785. [DOI] [PubMed] [Google Scholar]
- [15].Demartini C, Greco R, Zanaboni AM, Sances G, De Icco R, Borsook D, Tassorelli C. Nitroglycerin as a comparative experimental model of migraine pain: From animal to human and back. Progress in neurobiology 2019;177:15–32. [DOI] [PubMed] [Google Scholar]
- [16].Dieterle A, Fischer MJ, Link AS, Neuhuber WL, Messlinger K. Increase in CGRP- and nNOS-immunoreactive neurons in the rat trigeminal ganglion after infusion of an NO donor. Cephalalgia : an international journal of headache 2011;31(1):31–42. [DOI] [PubMed] [Google Scholar]
- [17].Drissi H, Lasmoles F, Le Mellay V, Marie PJ, Lieberherr M. Activation of phospholipase C-beta1 via Galphaq/11 during calcium mobilization by calcitonin gene-related peptide. The Journal of biological chemistry 1998;273(32):20168–20174. [DOI] [PubMed] [Google Scholar]
- [18].Edvinsson JCA, Grell AS, Warfvinge K, Sheykhzade M, Edvinsson L, Haanes KA. Differences in pituitary adenylate cyclase-activating peptide and calcitonin gene-related peptide release in the trigeminovascular system. Cephalalgia : an international journal of headache 2020:333102420929026. [DOI] [PubMed] [Google Scholar]
- [19].Edvinsson L, Haanes KA, Warfvinge K, Krause DN. CGRP as the target of new migraine therapies - successful translation from bench to clinic. Nat Rev Neurol 2018;14(6):338–350. [DOI] [PubMed] [Google Scholar]
- [20].Eftekhari S, Salvatore CA, Calamari A, Kane SA, Tajti J, Edvinsson L. Differential distribution of calcitonin gene-related peptide and its receptor components in the human trigeminal ganglion. Neuroscience 2010;169(2):683–696. [DOI] [PubMed] [Google Scholar]
- [21].Eftekhari S, Warfvinge K, Blixt FW, Edvinsson L. Differentiation of nerve fibers storing CGRP and CGRP receptors in the peripheral trigeminovascular system. The journal of pain : official journal of the American Pain Society 2013;14(11):1289–1303. [DOI] [PubMed] [Google Scholar]
- [22].Fabbretti E, D’Arco M, Fabbro A, Simonetti M, Nistri A, Giniatullin R. Delayed upregulation of ATP P2X3 receptors of trigeminal sensory neurons by calcitonin gene-related peptide. J Neurosci 2006;26(23):6163–6171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Farajdokht F, Mohaddes G, Shanehbandi D, Karimi P, Babri S. Ghrelin attenuated hyperalgesia induced by chronic nitroglycerin: CGRP and TRPV1 as targets for migraine management. Cephalalgia : an international journal of headache 2018;38(11):1716–1730. [DOI] [PubMed] [Google Scholar]
- [24].Farkas S, Bolcskei K, Markovics A, Varga A, Kis-Varga A, Kormos V, Gaszner B, Horvath C, Tuka B, Tajti J, Helyes Z. Utility of different outcome measures for the nitroglycerin model of migraine in mice. Journal of pharmacological and toxicological methods 2016;77:33–44. [DOI] [PubMed] [Google Scholar]
- [25].Forsten KE, Lauffenburger DA. Autocrine ligand binding to cell receptors. Mathematical analysis of competition by solution “decoys”. Biophys J 1992;61(2):518–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Forsten KE, Lauffenburger DA. Interrupting autocrine ligand-receptor binding: comparison between receptor blockers and ligand decoys. Biophys J 1992;63(3):857–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Goadsby PJ, Edvinsson L, Ekman R. Vasoactive peptide release in the extracerebral circulation of humans during migraine headache. Ann Neurol 1990;28(2):183–187. [DOI] [PubMed] [Google Scholar]
- [28].Greco R, Demartini C, Zanaboni AM, Tassorelli C. Chronic and intermittent administration of systemic nitroglycerin in the rat induces an increase in the gene expression of CGRP in central areas: potential contribution to pain processing. The journal of headache and pain 2018;19(1):51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Han X, Ran Y, Su M, Liu Y, Tang W, Dong Z, Yu S. Chronic changes in pituitary adenylate cyclase-activating polypeptide and related receptors in response to repeated chemical dural stimulation in rats. Molecular pain 2017;13:1744806917720361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Harmar AJ, Fahrenkrug J, Gozes I, Laburthe M, May V, Pisegna JR, Vaudry D, Vaudry H, Waschek JA, Said SI. Pharmacology and functions of receptors for vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide: IUPHAR review 1. British journal of pharmacology 2012;166(1):4–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Hay DL, Garelja ML, Poyner DR, Walker CS. Update on the pharmacology of calcitonin/CGRP family of peptides: IUPHAR Review 25. British journal of pharmacology 2018;175(1):3–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Hoffmann J, Miller S, Martins-Oliveira M, Akerman S, Supronsinchai W, Sun H, Shi L, Wang J, Zhu D, Lehto S, Liu H, Yin R, Moyer BD, Xu C, Goadsby PJ. PAC1 receptor blockade reduces central nociceptive activity: new approach for primary headache? Pain 2020;161(7):1670–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Huang Y, Fischer JE, Balasubramaniam A. Amylin mobilizes [Ca2+]i and stimulates the release of pancreatic digestive enzymes from rat acinar AR42J cells: evidence for an exclusive receptor system of amylin. Peptides 1996;17(3):497–502. [DOI] [PubMed] [Google Scholar]
- [34].Johnson KW, Morin SM, Wroblewski VJ, Johnson MP. Peripheral and central nervous system distribution of the CGRP neutralizing antibody [(125)I] galcanezumab in male rats. Cephalalgia : an international journal of headache 2019;39(10):1241–1248. [DOI] [PubMed] [Google Scholar]
- [35].Knutsson M, Edvinsson L. Distribution of mRNA for VIP and PACAP receptors in human cerebral arteries and cranial ganglia. Neuroreport 2002;13(4):507–509. [DOI] [PubMed] [Google Scholar]
- [36].Lassen LH, Haderslev PA, Jacobsen VB, Iversen HK, Sperling B, Olesen J. CGRP may play a causative role in migraine. Cephalalgia : an international journal of headache 2002;22(1):54–61. [DOI] [PubMed] [Google Scholar]
- [37].Lennerz JK, Ruhle V, Ceppa EP, Neuhuber WL, Bunnett NW, Grady EF, Messlinger K. Calcitonin receptor-like receptor (CLR), receptor activity-modifying protein 1 (RAMP1), and calcitonin gene-related peptide (CGRP) immunoreactivity in the rat trigeminovascular system: differences between peripheral and central CGRP receptor distribution. The Journal of comparative neurology 2008;507(3):1277–1299. [DOI] [PubMed] [Google Scholar]
- [38].Markovics A, Kormos V, Gaszner B, Lashgarara A, Szoke E, Sandor K, Szabadfi K, Tuka B, Tajti J, Szolcsanyi J, Pinter E, Hashimoto H, Kun J, Reglodi D, Helyes Z. Pituitary adenylate cyclase-activating polypeptide plays a key role in nitroglycerol-induced trigeminovascular activation in mice. Neurobiol Dis 2012;45(1):633–644. [DOI] [PubMed] [Google Scholar]
- [39].Marone IM, De Logu F, Nassini R, De Carvalho Goncalves M, Benemei S, Ferreira J, Jain P, Li Puma S, Bunnett NW, Geppetti P, Materazzi S. TRPA1/NOX in the soma of trigeminal ganglion neurons mediates migraine-related pain of glyceryl trinitrate in mice. Brain 2018;141(8):2312–2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].McCoy ES, Taylor-Blake B, Zylka MJ. CGRPalpha-expressing sensory neurons respond to stimuli that evoke sensations of pain and itch. PloS one 2012;7(5):e36355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Megat S, Ray PR, Tavares-Ferreira D, Moy JK, Sankaranarayanan I, Wanghzou A, Fang Lou T, Barragan-Iglesias P, Campbell ZT, Dussor G, Price TJ. Differences between Dorsal Root and Trigeminal Ganglion Nociceptors in Mice Revealed by Translational Profiling. J Neurosci 2019;39(35):6829–6847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Melo-Carrillo A, Strassman AM, Nir RR, Schain AJ, Noseda R, Stratton J, Burstein R. Fremanezumab-A Humanized Monoclonal Anti-CGRP Antibody-Inhibits Thinly Myelinated (Adelta) But Not Unmyelinated (C) Meningeal Nociceptors. J Neurosci 2017;37(44):10587–10596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Messlinger K, Russo AF. Current understanding of trigeminal ganglion structure and function in headache. Cephalalgia : an international journal of headache 2019;39(13):1661–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Navratilova E, Rau J, Oyarzo J, Tien J, Mackenzie K, Stratton J, Remeniuk B, Schwedt T, Anderson T, Dodick D, Porreca F. CGRP-dependent and independent mechanisms of acute and persistent post-traumatic headache following mild traumatic brain injury in mice. Cephalalgia : an international journal of headache 2019:333102419877662. [DOI] [PubMed] [Google Scholar]
- [45].Noseda R, Schain AJ, Melo-Carrillo A, Tien J, Stratton J, Mai F, Strassman AM, Burstein R. Fluorescently-labeled fremanezumab is distributed to sensory and autonomic ganglia and the dura but not to the brain of rats with uncompromised blood brain barrier. Cephalalgia : an international journal of headache 2020;40(3):229–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].O’Connor TP, van der Kooy D. Enrichment of a vasoactive neuropeptide (calcitonin gene related peptide) in the trigeminal sensory projection to the intracranial arteries. J Neurosci 1988;8(7):2468–2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Olesen J The role of nitric oxide (NO) in migraine, tension-type headache and cluster headache. Pharmacology & therapeutics 2008;120(2):157–171. [DOI] [PubMed] [Google Scholar]
- [48].Pradhan AA, Smith ML, McGuire B, Tarash I, Evans CJ, Charles A. Characterization of a novel model of chronic migraine. Pain 2014;155(2):269–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Price TJ, Flores CM. Critical evaluation of the colocalization between calcitonin gene-related peptide, substance P, transient receptor potential vanilloid subfamily type 1 immunoreactivities, and isolectin B4 binding in primary afferent neurons of the rat and mouse. The journal of pain : official journal of the American Pain Society 2007;8(3):263–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Rea BJ, Wattiez AS, Waite JS, Castonguay WC, Schmidt CM, Fairbanks AM, Robertson BR, Brown CJ, Mason BN, Moldovan-Loomis MC, Garcia-Martinez LF, Poolman P, Ledolter J, Kardon RH, Sowers LP, Russo AF. Peripherally administered calcitonin gene-related peptide induces spontaneous pain in mice: implications for migraine. Pain 2018;159(11):2306–2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Russo AF. Calcitonin gene-related peptide (CGRP): a new target for migraine. Annu Rev Pharmacol Toxicol 2015;55:533–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Schain AJ, Melo-Carrillo A, Stratton J, Strassman AM, Burstein R. CSD-Induced Arterial Dilatation and Plasma Protein Extravasation Are Unaffected by Fremanezumab: Implications for CGRP’s Role in Migraine with Aura. J Neurosci 2019;39(30):6001–6011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Schytz HW, Birk S, Wienecke T, Kruuse C, Olesen J, Ashina M. PACAP38 induces migraine-like attacks in patients with migraine without aura. Brain 2009;132(Pt 1):16–25. [DOI] [PubMed] [Google Scholar]
- [54].Segond von Banchet G, Pastor A, Biskup C, Schlegel C, Benndorf K, Schaible HG. Localization of functional calcitonin gene-related peptide binding sites in a subpopulation of cultured dorsal root ganglion neurons. Neuroscience 2002;110(1):131–145. [DOI] [PubMed] [Google Scholar]
- [55].Seiler K, Nusser JI, Lennerz JK, Neuhuber WL, Messlinger K. Changes in calcitonin gene-related peptide (CGRP) receptor component and nitric oxide receptor (sGC) immunoreactivity in rat trigeminal ganglion following glyceroltrinitrate pretreatment. The journal of headache and pain 2013;14:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Sufka KJ, Staszko SM, Johnson AP, Davis ME, Davis RE, Smitherman TA. Clinically relevant behavioral endpoints in a recurrent nitroglycerin migraine model in rats. The journal of headache and pain 2016;17:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Tassorelli C, Greco R, Cappelletti D, Sandrini G, Nappi G. Comparative analysis of the neuronal activation and cardiovascular effects of nitroglycerin, sodium nitroprusside and L-arginine. Brain Res 2005;1051(1–2):17–24. [DOI] [PubMed] [Google Scholar]
- [58].Tipton AF, Tarash I, McGuire B, Charles A, Pradhan AA. The effects of acute and preventive migraine therapies in a mouse model of chronic migraine. Cephalalgia : an international journal of headache 2016;36(11):1048–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Tuka B, Helyes Z, Markovics A, Bagoly T, Szolcsanyi J, Szabo N, Toth E, Kincses ZT, Vecsei L, Tajti J. Alterations in PACAP-38-like immunoreactivity in the plasma during ictal and interictal periods of migraine patients. Cephalalgia : an international journal of headache 2013;33(13):1085–1095. [DOI] [PubMed] [Google Scholar]
- [60].Vaudry D, Gonzalez BJ, Basille M, Yon L, Fournier A, Vaudry H. Pituitary adenylate cyclase-activating polypeptide and its receptors: from structure to functions. Pharmacological reviews 2000;52(2):269–324. [PubMed] [Google Scholar]
- [61].Walker CS, Eftekhari S, Bower RL, Wilderman A, Insel PA, Edvinsson L, Waldvogel HJ, Jamaluddin MA, Russo AF, Hay DL. A second trigeminal CGRP receptor: function and expression of the AMY1 receptor. Annals of clinical and translational neurology 2015;2(6):595–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Walker CS, Raddant AC, Woolley MJ, Russo AF, Hay DL. CGRP receptor antagonist activity of olcegepant depends on the signalling pathway measured. Cephalalgia : an international journal of headache 2018;38(3):437–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Zagami AS, Edvinsson L, Goadsby PJ. Pituitary adenylate cyclase activating polypeptide and migraine. Annals of clinical and translational neurology 2014;1(12):1036–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Zhang J, Czerpaniak K, Huang L, Liu X, Cloud ME, Unsinger J, Hotchkiss RS, Li D, Cao YQ. Low-dose interleukin-2 reverses behavioral sensitization in multiple mouse models of headache disorders. Pain 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Zhang Z, Winborn CS, Marquez de Prado B, Russo AF. Sensitization of calcitonin gene-related peptide receptors by receptor activity-modifying protein-1 in the trigeminal ganglion. J Neurosci 2007;27(10):2693–2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S3. Substantial overlap between CGRP-ir and EGFP-ir in TG neurons from CGRPα-GFP+/− mice.
(A) Representative images EGFP-ir and CGRP-ir in TG culture from naïve female CGRPαEGFPf/+ mice.
(B) The percentages of small- and medium-sized EGFP+ TG neurons that contain CGRP-ir. A total of 2589 TG neurons from 3 mice were counted.
(C) The percentages of small- and medium-sized CGRP-ir TG neurons that contain EGFP-ir (same neurons as in B).
Figure S4. Repeated NTG increases the percentage of small IB4−cap-R neurons in TG culture from male CGRPαEGFPf/+ mice.
(A-B) The percentages of small IB4+, small IB4− and medium-sized cap-R neurons in TG cultures from male (A) and female (B) CGRPαEGFPf/+ mice after 5 repeated injections of vehicle or NTG (same neurons as in Table 1). *p < 0.05, Fisher’s exact test between vehicle and NTG groups.
(C) The percentages of cap-R neurons in TG cultures from male Swiss Webster mice that received sham procedure, mTBI, and mTBI with ld-IL2 treatment (same neurons as in Figure 9B-D). *p < 0.05, **p < 0.01, χ2 test followed by post hoc Fisher’s exact test with Bonferroni correction.
Figure S1. Pilot studies to determine the dose of NTG that reliably induces long-lasting behavioral sensitization in mice.
(A) Repeated i.p. administration of 1 mg/kg NTG does not significantly alter facial mechanical thresholds in female Swiss Webster mice (n = 6). One-way RM ANOVA: p = 0.08 (F[5, 35] = 2.43).
(B) The facial mechanical thresholds before and one day after two low-dose NTG injections (0.1 mg/kg, i.p., day 11 and 12, same mice as in A).
(C) Repeated i.p. administration of 3 mg/kg NTG results in sustained facial mechanical hyper-sensitivity in female Swiss Webster mice (n = 6). One-way RM ANOVA: p < 0.05 (F[6, 41] = 2.94), post hoc Dunnett’s test: *p < 0.05, compared with the baseline threshold on day 1.
(D) The facial mechanical thresholds before and one day after two low-dose NTG injections (0.1 mg/kg, i.p., day 13 and 14, same mice as in C).
(E) Repeated i.p. administration of 10 mg/kg NTG results in robust and persistent facial mechanical hyper-sensitivity in female Swiss Webster mice (n = 7). One-way RM ANOVA: p < 0.001 (F[8, 62] = 33.91), post hoc Dunnett’s test: **p < 0.01, ***p < 0.001, compared with the baseline threshold on day 1.
(F) Mice received repeated 10 mg/kg NTG exhibit hyperalgesic priming that lasts for a week. Mice (same as in E) received 2 daily low-dose NTG injections (0.1 mg/kg, i.p.) 0, 7 and 14 days after the facial mechanical thresholds returned to baseline level (day 0 here is day 20 in E). ***p < 0.01, two-tailed paired t-test.
Figure S2. Pilot studies to determine the concentrations of CGRPα and PACAP1-38 to define CGRP-R and PACAP-R neurons in TG culture.
(A) The percentages of CGRP-R neurons in TG cultures from naïve female Swiss Webster mice. CGRP8-37 was co-applied with αCGRP. On average, 80 neurons were measure under each condition.
(B) The percentages of PACAP-R neurons in TG cultures from naïve female Swiss Webster mice. On average, 102 neurons were measure under each condition.
(C) The percentages of VIP-R neurons in TG cultures from female Swiss Webster mice that received repeated Veh or NTG injections. On average, 162 neurons were measure under each condition.
(D) The percentages of PACAP-R and CGRP-R neurons in TG cultures from female Swiss Webster mice after 5 repeated i.p. injections of saline (Sal) or Veh (n = 6 mice/group; a total of 268 and 232 neurons were measured in Sal and Veh groups, respectively).
(E) The percentages of PACAP-R and CGRP-R neurons in TG cultures from female Swiss Webster mice after 5 repeated i.p. injections of Veh or 1 mg/kg NTG (n = 6 mice/group; a total of 218 and 293 neurons were measured in Veh and NTG groups, respectively).
(F) The percentages of PACAP-R and CGRP-R neurons in TG cultures from female Swiss Webster mice after 5 repeated i.p. injections of Veh or 3 mg/kg NTG (n = 6 mice/group; a total of 190 and 265 neurons were measured in Veh and NTG groups, respectively). *p < 0.05, **p < 0.01, Fisher’s exact test between Veh and NTG groups.
(G) The effect of a single NTG injection on the 50% withdrawal thresholds to von Frey filaments at the periorbital region of female Swiss Webster mice (n = 8). One-way RM ANOVA: p < 0.001 (F[5, 47] = 19.6), post hoc Dunnett’s test: ***p < 0.001, compared with the baseline (day 1) threshold.
(H) The effects of repeated NTG on the 50% withdrawal thresholds to von Frey filaments at the periorbital region of female Swiss Webster mice (n = 7, same mice as in Fig. S1E-F). Note that NTG were always injected after the completion of behavioral tests on the same day. One-way RM ANOVA: p < 0.001 (F[11, 83] = 20.09), post hoc Dunnett’s test: *p < 0.05, ***p < 0.001, compared with the baseline threshold on day 1.


