Abstract
The present study reports effect of various drying and storage conditions on physical, bioactive compounds, and antioxidant properties of doum (H. thebaica) fruit for the first time. Three types of fruit are used such as fresh, dried from tree, and dried fruits purchased from local market. Pulp of fresh fruit was dried using sun, shade and oven at different temperatures and stored under different conditions for three months. Samples were analysed before and after drying and storage. The results showed significantly (p < 0.01) higher dry matter (98.73 g/100g), pH (7.09), tannins (27.64 mg/g), flavonoids (19.90 mg/g) and total polyphenols (7.13 mg/g) contents in pericarp than other parts of fruit. The pulp without pericarp exhibited higher ash, amino acids, proteins and vitamin C contents, however pulp of whole fresh fruit had higher Ca (1.67 mg/g), Na (640.26 mg/g) and Zn (11.63 μg/g). Pulp of fruit purchased from local market showed significantly stronger antioxidant activities (DPPH and ABTS). All parameters evaluated were significantly (p < 0.01) affected in comparison with pulp of fresh fruit but varied with respect to drying methods and storage conditions. The shade-dried samples showed higher proteins, amino acids, vitamin C, ash, fibres and acidity contents than other drying methods. Polyphenols and antioxidant activities are higher in oven-dried samples at low temperature (40 °C) as compared to others. The highest DPPH and ABTS scavenging activities are observed after drying and storage conditions for three months. Significant and positive (p < 0.01) correlations are found between antioxidant compounds and antioxidant activities. Thus, drying methods and storage conditions can preserve pulp of doum fruit with high physicochemical, bioactive compounds and antioxidant capacities for human well-being up to three months, however shade-drying followed by oven-drying are highly promising process that must be considered as suitable drying methods for doum fruits.
Keywords: Doum (Hyphaene thebaica) fruit, Drying, Storage, Nutritional, Bioactive compounds, Antioxidant activity
Doum (Hyphaene thebaica) fruit, drying, storage, nutritional, bioactive compounds, antioxidant activity.
1. Introduction
The doum (Hyphaene thebaica) is a common palm tree with edible fruit which belongs to the family Arecaceae. It is reported as native to many regions of the world such as North Africa, sub-Saharan Africa, West India and Saudi Arabia (Aboshora et al., 2014). It names differently such as doum palm, doom palm, gingerbread palm, zembaba, mkoma, arkobkobai and kambash (Orwa et al., 2009; Auwal et al., 2012). Many researchers reported that doum fruits are very rich in vitamins, minerals, carbohydrates, proteins, fibres, volatile compounds and polyphenols, and have a good anticancer and antimicrobial properties (Aboshora et al., 2014, 2017; Reda and Aamer, 2015). Doum fruit is also a good natural source of natural antioxidants (Hsu et al., 2006; Atito et al., 2019). It was used in different countries for the treatment of diabetes, obesity, hypertension, dyslipidaemia and to reduce cardiovascular diseases (Hsu et al., 2006; Hetta and Yassin, 2006; Salib et al., 2013). However, antioxidant compounds and their antioxidant activities varied according to the seasonal variations and the type of phenolics content in samples (Laya and Koubala, 2020). In Cameroon, doum fruit is known as edible wild fruit and consumed in various regions, specially in the Far North region. The population use the roots of doum palm to treat bilharzias, to control hypertension and diabetes. Doum fruits are consumed in dry, fresh form or cut off to slices before drying and powdering for food products formulation. Also, the fresh fruits are transformed in traditional beverages, juice, jelly or puree. All these different food products are either consumed or exported to the Southern region of Cameroon. However, various factors such as drying methods, temperature and storage conditions may affect the nutritional composition, bioactive compounds, hence the antioxidant properties which may result into reduced benefits to consumers. Fact, drying is a process used by our ancestors to preserve and extend shelf life of food products. Dev and Raghavan (2012) reported that a range of drying techniques used for dehydration have advantages and disadvantages over the others, include air, sun, oven and freeze drying. Also, many researchers reported the effects of drying on chemical and phytochemical changes which can significantly affect nutritional value and antioxidant properties (Maskan, 2000; Di Scala and Crapiste, 2008; Mphahlele et al., 2016). Furthermore, previous studies on fruits such as banana, Van palm (Borassus aethiopum), apricot and mango (Mangifera indica) revealed the effect of drying on the physicochemical and antioxidant properties (Maskan, 2000; Sogi et al., 2013; Tiho et al., 2017). In addition, Eze and Akubor (2012) have reported the effect of drying and storage on the physicochemical properties such as polyphenols of okra. For H. thebaica fruit there have been many studies reported on the physicochemical, functional, nutritional and antioxidant properties, and volatile compounds (Aboshora et al., 2014, 2017). However, there was no information documented on the effect of drying methods and storage conditions on the polyphenolic compounds, nutritional and antioxidant properties of doum fruit. Thus, the present study aims to investigate for the first time the effects of drying and storage conditions on the physical properties, nutritional, bioactive compounds and antioxidant properties of doum (Hyphaene thebaica) fruit in order to select the best drying method and storage condition to maximally preserve the nutritional quality and bioactive properties of doum fruits in order to be used as a functional ingredient.
2. Material and methods
2.1. Samples of doum (H. thebaica) fruits
Fresh and dry fruits were harvested in November 2019 and February 2020, respectively from the same H. thebaica plant tree in Hougno, Wina Sub-division of Mayo-Danay Department, Cameroon. For each harvest, the fruits were packed in polystyrene bags and transported to the Laboratory of Biochemistry and Biological Chemistry (LabBBC) of the University of Maroua, Cameroon. The identification and authentication of fruits were done in the Biological Department of Sciences, University of Maroua. The dried fruits purchased from a popular local market “Marché Abattoir “were transported to the laboratory and were stored in box.
2.2. Samples preparation and fruit treatments
Thirty fruits of fresh form without any physical defects were randomly selected and washed many times with tap water and the pulps of fruit samples were separated from kernels and pericarps or epicarps using stainless steel knives (Koch Messer, Germany). The obtained pulp was divided in two parts. One part of pulp was treated with ascorbate (1 %) and bisulfite solution (1 %) and another part was untreated. Then, each pulp part was divided into three parts. One part of pulp was dried in an air convection oven (Binder, USA) at various temperature range such as 40 °C for 8 days, 50 °C for 6 days, 60 °C for 5 days and 70 °C for 4 days, and another part of pulp was dried in open shade for 4 days at 26–30 °C and open sun for 3 days at 24–45 °C, maximum temperature. However, the whole (with seed) fresh fruit samples were sun-dried for 15 days at 24–45 °C and 12–41 % relative humidity. While, the open shade-dried whole fresh fruits was dried for 21 days at 26–30 °C and 27–40% relative humidity. All these samples were dried until equilibrium was reached. Then, dried samples were powdered using a mortar and they were sieved (200 μm). Further, powder samples were divided and sealed in special polystyrene bags according to the storage conditions such as the presence of light and oxygen, presence of light and absence of oxygen, absence of light and presence of oxygen and absence of light and oxygen under ambient conditions, however the light was lightened during storage time (night and day). The different samples were analysed before (at zero month) and after three months of storage conditions (light, oxygen).
2.3. Reagents and chemicals
Orcinol, BSA, Ninhydrin, Alanine, Gallic acid, Quercetin, Vanillin, Catechin, ABTS, DPPH, FRAP and Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid) were purchased through Sigma-Aldrich Chemical Company (Mumbai, India). Oxalic acid, L-ascorbic acid, formaldehyde and all others were obtained through Fisher commercial source (New Jersey, USA).
2.4. Evaluation of physicochemical composition of samples
Dry matter, pH, ash and lipid contents were evaluated according to AOAC (2000 and 2005) methods and titrable acidity was determined as described by Ranganna (1979). The total soluble amino acid was determined as reported by Michel (1968). Total soluble sugar and reducing sugar contents were evaluated according to Miller (1972) method. For total carbohydrates content, there were evaluated as described by Dubois et al. (1956). The total protein content was determined as described by Devani et al. (1989) with some modifications. Briefly, samples were previously digested using Kjeldahl method. Then, the resulting mineralisate was treated with ammonia and acetyl acetone/formaldehyde reagent in order to determine the nitrogen content. The yellow complexes (3, 5-diacetyl-1, 4-dihydrolutidin) formed were showed a maximum absorption at 412 nm. The protein content was calculated using conversion factor of 6.25 and the fibres content were determined according to AOAC (2005) method. Vitamin C content was determined based on Barros et al. (2007), and Barrett and Lloyd (2012) methods using potassium permanganate as reagent at 525 nm. It becomes dark-violet when in an acid medium and colorless when it reacts with ascorbic acid. Minerals content were determined as described by Jones and Case (1990). The samples were digested with 6 mL of nitric acid for 4 h on a block digester before analysis by ICP-OES (Inductively Coupled Plasma-Optical Emission Spectroscopy).
2.5. Extraction of polyphenolic compounds of doum (H. thebaica) fruit
Briefly, 0.2 g powder sample was mixed in 10 mL of 80 % methanol and stirred for 24 h at room temperature. Then, the mixture was filtered with whatman No.4 filter paper before washing two times and the supernatants were combined. The residues were re-extracted again according to the method described by Laya and Koubala (2020). The residues were mixed in 4 mL of 2 M of NaOH and incubated for 15 min in a water bath at 80 °C. The mixture was cooled at room temperature and 2.5 mL HCl (2 M) were added before incubating in a water bath for 45 min at 80 °C. Then, 2.5 mL of 95 % MeOH were added in order to wash and stirred for 15 min before filtering through the whatman No. 4 filter paper and washed once more with 2.5 mL (95 %) methanol. The two filtrates were combined and kept at -20 °C until analysis.
2.6. Determination of polyphenolic compounds and antioxidant properties of doum (H. thebaica) fruit
2.6.1. Determination of polyphenolic compounds
Total phenolic, total flavonoid and total tannins content of methanolic extract were evaluated as described by Singleton et al. (1999), Brainbridge et al. (1992) and Gaytan-Martínez et al. (2017), respectively. The gallic, quercetin and catechin were used as standard and the results were expressed as milligrams of Gallic acid, Quercetin and Catechin equivalents per 100 g of dry weight sample (mg GAE/g dw, mg QE/g dw and mg CE/g dw, respectively).
2.6.2. Evaluation of antioxidant activities
DPPH (2.2-diphenyl-1-picyhydrazyl) radical scavenging activity, ABTS (2,2-azino-bis(3-ethylbenzylthiozoline-6-sulphonic acid)) radical scavenging and FRAP (Ferric reducing antioxidant potential) activity were carried out as described by Sun et al. (2002), Re et al. (1999) and Benzie and Strain (1996) respectively. The calibration curve was plotted with Trolox and the results were expressed in milligram Trolox equivalent per 100 g of dry weight sample (mg TE/100 g dw).
2.7. Statistical analysis
Data were analysed using one-way analysis of variance (ANOVA) performed by SPSS 20.0 Statistical Package for Windows (SPSS Inc., Chicago, IL, USA), Graph Pad Prism. 5.03. and XLSTAT (version 16). Data were assessed using Tukey's tests and significance was accepted at p < 0.01. Pearson's correlation and principal component analysis (PCA) were done to establish the relationship between evaluated parameters and the total variance of traits. Analysis of each sample was done in four replicates and the results were expressed as Mean ± Standard deviation.
3. Results and discussion
3.1. Physicochemical composition and antioxidant activity of pulp from doum (Hyphaene thebaica) fruit before various drying methods and storage conditions
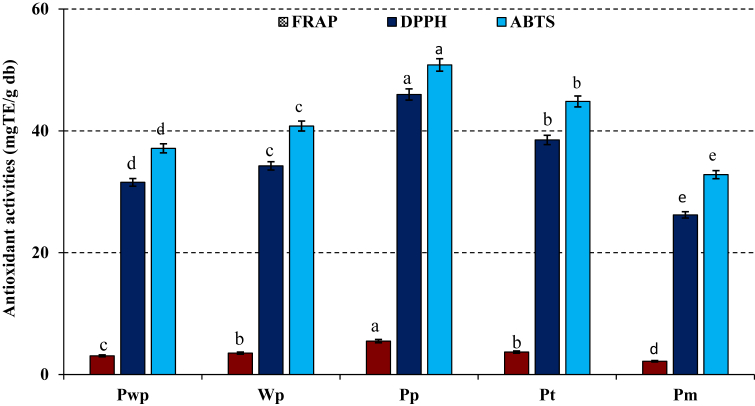
The values of proximate composition of doum (H. thebaica) fruit before different drying and storage conditions are presented in Table 1. The pericarp has significantly (p < 0.01) higher values of dry matter (98.73 g/100 g), pH (7.09), tannins (27.64 mg/g), flavanoids (19.90 mg/g) and total polyphenols content (7.13 mg/g) than other parts, however this portion shows the lowest values of ash, proteins and vitamin C contents (Table 1). Atito et al. (2019) also found that doum fruits parts had significantly variable total polyphenols and flavonoids content. The pulp from the fruit purchased in the local market showed the lowest values of all these parameters with the exception of amino acids, proteins and fibres (Table 1). However, pulp without pericarp showed the highest contents of ash (6.55 g/100 g), amino acids, proteins and vitamin C (4.97 mg/g), whereas pulp of dried fruit on the tree has significantly higher value of titrable acidity than others. The results were similar to those found by Aboshora et al. (2014), who reported higher values of vitamin C (1.15 mg/100 g), and ash (6.64 %) in pulp than epicarp (4.00 %). Table 2 shows a significant (p < 0.01) variation of the minerals content of different pulps from doum fruits. The pulp of whole fresh fruit showed the highest values of Ca (1.67 mg/g), Na (640.26 μg/g) and Zn (11.63 μg/g), however this sample exhibited significantly (p < 0.01) lower values of all other minerals except Cu. While, pulp of fruit purchased from the local market is significantly (p < 0.01) rich in Mg and microminerals such as Cu, Fe and Mn than others (Table 2). The pulp of fruit dried on the tree showed significantly (p < 0.01) higher value of K (27.57 mg/g) compared to others as shown in Table 2. Aboshora et al. (2014) also reported that doum fruits are very rich in essentials minerals. Total carbohydrates, total soluble sugars and reducing soluble sugars content were significantly (p < 0.01) varied from one parts of doum fruit to another as presented in Figure 1. Pulp without pericarp is significantly rich in total carbohydrates (88.67g/100 g), total soluble sugars (20.49g/100 g) and reducing soluble sugars (32.06 g/100 g) followed by whole pulp of fresh fruit with the value of 84.65, 19.86 and 28.54 g/100 g, respectively, while pulp of fruit purchased from the market has significantly the lowest contents of all these parameters (Figure 1). The highest value of carbohydrates content in pulp than other parts of the fruit was also observed with the results obtained by Aboshora et al. (2014), who found higher carbohydrates content in pulp than epicarp of doum fruit. For antioxidant activities of different portions parts of fruits from various source, activity varied significantly (p < 0.01) among portion parts and fruits, however pulp of fruit purchased from the market showed stronger antioxidant activities evaluated by DPPH and ABTS methods with the value of 26.20 and 32.81 mg TE/g, respectively, while this portion part of fruit had significantly the lowest antioxidant potential. This result may be due to the different mechanisms of both lipophilic and hydrophilic compounds which are different from one assay to another. Except FRAP activity (5.48 mg TE/g) is higher than other parts, pericarp part had significantly (p < 0.01) lower antioxidant activity than others (Figure 2). This variation of antioxidant activities of doum fruits parts was also reported by Atito et al. (2019). The highest FRAP activity obtained in this part may be justified by the highest amounts of polyphenols.
Table 1.
Dry matter (DM), pH, titrable acidity (TA), ash, amino acids (AA), proteins, fibres tannins, flavonoids, total polyphenols (TP) and vitamin C contents of doum palm (H. thebaica) fruit. Values are expressed on dry weight basis.
| Parameter | Samples |
||||
|---|---|---|---|---|---|
| Pwp | Wp | Pp | Pt | Pm | |
| DM (g/100g) | 97.65 ± 0.26b | 46.23 ± 0.28d | 98.73 ± 0.36a | 93.91 ± 0.26c | 97.58 ± 0.36b |
| pH | 4.78 ± 0.03c | 5.53 ± 0.03b | 7.09 ± 0.04a | 5.51 ± 0.06b | 4.63 ± 0.04d |
| TA (mg/g) | 10.88 ± 0.29b | 9.31 ± 0.23c | 3.09 ± 0.09d | 13.31 ± 0.27a | 10.04 ± 0.35c |
| Ash (g/100g) | 6.55 ± 0.09a | 5.90 ± 0.07b | 4.44 ± 0.09e | 5.52 ± 0.08c | 5.23 ± 0.07d |
| AA (mg/g) | 20.73 ± 0.17a | 16.20 ± 0.10b | 4.43 ± 0.08d | 15.92 ± 0.11b | 8.93 ± 0.07c |
| Proteins (g/100g) | 7.57 ± 0.05a | 6.83 ± 0.05b | 4.34 ± 0.08e | 6.54 ± 0.05c | 5.38 ± 0.04d |
| Fibres (g/100g) | 10.20 ± 0.09b | 10.43 ± 0.11b | 10.94 ± 0.11a | 9.85 ± 0.07c | 9.07 ± 0.14d |
| Tannin (mg/g) | 16.87 ± 0.22c | 20.19 ± 0.15b | 27.64 ± 0.11a | 19.79 ± 0.29b | 15.01 ± 0.20d |
| Flavonoids (mg/g) | 12.88 ± 0.18d | 16.19 ± 0.15b | 19.90 ± 0.13a | 14.38 ± 0.18c | 11.26 ± 0.16e |
| TP (mg/g) | 4.69 ± 0.12c | 4.88 ± 0.11 bc | 7.13 ± 0.09a | 5.01 ± 0.09b | 2.06 ± 0.05d |
| Vitamin C (mg/g) | 4.97 ± 0.09a | 4.41 ± 0.08b | 1.60 ± 0.03d | 4.79 ± 0.08a | 3.51 ± 0.06c |
Values are means ± standard deviation of three replicates (n = 3). In the same line, values followed by different superscript letters are significantly different (p < 0.01). Pwp = pulp without pericarp of fresh fruit; Wp = whole pulp of fresh fruit; Pp = pericarp; Pt = pulp of fruit dried from the tree; Pm = Pulp of fruit purchased from the local market.
Table 2.
Minerals content of doum palm (H. thebaica) fruit. Values are in μg/g or mg/g of dry weight basis.
| Minerals | Samples |
||
|---|---|---|---|
| Wp | Pt | Pm | |
| Ca (mg/g) | 1.67 ± 0.01a | 0.45 ± 0.01c | 0.62 ± 0.02b |
| Mg (mg/g) | 1.09 ± 0.01c | 1.60 ± 0.01b | 1.70 ± 0.02a |
| K (mg/g) | 18.53 ± 0.08c | 27.57 ± 0.09a | 25.34 ± 0.16b |
| Na (μg/g) | 640.26 ± 2.10a | 385.70 ± 2.31b | 321.16 ± 1.15c |
| Zn (μg/g) | 11.63 ± 0.03a | 10.66 ± 0.03b | 10.77 ± 0.06b |
| Cu (μg/g) | 9.77 ± 0.02b | 9.77 ± 0.03b | 9.86 ± 0.03a |
| Fe (μg/g) | 12.20 ± 0.01c | 14.83 ± 0.04b | 17.58 ± 0.04a |
| Mn (μg/g) | 7.94 ± 0.03c | 10.43 ± 0.04b | 11.34 ± 0.06a |
Values are means ± standard deviation of three replicates (n = 3). In the same line, values followed by different superscript letters are significantly different (p < 0.01). Wp = whole pulp of fresh fruit; Pt = pulp of fruit dried from the tree; Pm = Pulp of fruit purchased from the market. Cu: Copper; Zn: Zinc; Mn: Magnesium; Fe: Iron; Ca: Calcium; Mg: Manganese; K: Potassium; Na: Sodium.
Figure 1.
Total carbohydrates (TC), total soluble sugars (TSS) and reducing soluble sugars (RSS) content of pulp of doum palm (H. thebaica) fruit. Values are means ± standard deviation of three repetitions (n = 3).Values are expressed on dry weight basis. Pwp = pulp without pericarp of fresh fruit; Wp = whole pulp of fresh fruit; Pp = pericarp; Pt = pulp of fruit dried from the tree; Pm = Pulp of fruit purchased from the market. n = 3; Bars followed by different lowercase letters for each parameter are significantly different (p < 0.01).
Figure 2.
Antioxidant activities of different portions of doum palm (H. thebaica) fruit. Ferric reducing antioxidant potential (FRAP). DPPH and ABTS radical scavenging activities. Values are in dry weight basis. Values are means ± standard deviation of three repetitions (n = 3). Pwp = pulp without pericarp of fresh fruit; Wp = whole pulp of fresh fruit; Pp = pericarp; Pt = pulp of fruit dried from the tree; Pm = Pulp of fruit purchased from the market. n = 3; Bars followed by different lowercase letters for each parameter are significantly different (p < 0.01).
3.2. Effects of drying and storage conditions on physical and nutritional composition of doum plam (H. thebaica) fruit
Table 3 shows proximate composition of fruit samples after various drying methods and storage under different conditions for three months. Dry matter content of samples are significantly (p < 0.01) varied in respect to drying methods and storage conditions with values ranged from 93.61 (swol) to 99.16 % (Otol) after shade and oven-drying (70 °C), respectively. However, general trend shows that effect was slightly significant among samples dried by the same method, while a significant (p < 0.01) difference was observed for the samples dried by different methods. The values obtained were higher when compared to the whole pulp of fresh fruit. The pH of Otol sample was higher after oven-drying at 70 °C compared to the other samples, however sample treated, OTOL had lower value of pH at 40 °C. Here, there was slight decrease of pH values after three months of storage, While, sTOL sample shade-dried showed the highest value of titrable acidity (15.77 mg/g) followed by sTOl (15.76 mg/g), whereas the lowest value was obtained in Owl sample after high temperature oven-dried (70 °C) compared to the others (Table 3). The changes of acidity of samples treated are also observed with others. The general trends showed an increase of titrable acidity of different samples compared to the whole pulp of fresh fruit, however the decrease was observed when compared to the pulp from tree dried. An increase of titrable acidity during storage was consistent with the results of Wani et al. (2018), who found significant increase of titrable acidity of frozen apricots during storage of 90 days. Mohammed et al. (2020) have also reported an increase of acidity in mango and pineapples dried in various methods.
Table 3.
Effect of drying and storage condition on dry matter (DM) content, pH, titrable acidity (TA), ash content, total soluble amino acids (TSAA), total proteins content (TPC) and fibres content of doum palm (H. thebaica) fruit. Values are expressed on dry weight basis.
| Drying methods | Samples | DM (g/100 g) | pH | TA (mg/g) | Ash (g/100 g) | TSAA (mg/100 g) | TPC (mg/100 g) | Fibres (g/100 g) |
|---|---|---|---|---|---|---|---|---|
| Sun(25–45 °C) | Swol | 96.70 ± 0.05j | 5.11 ± 0.04c | 9.68 ± 0.09n | 6.16 ± 0.06c | 13.61 ± 0.15fg | 6.40 ± 0.02a | 9.59 ± 0.10c |
| StOL | 97.60 ± 0.04c | 5.09 ± 0.06c | 9.59 ± 0.10n | 5.89 ± 0.04f | 11.99 ± 0.06np | 6.16 ± 0.04c | 9.89 ± 0.10a | |
| StOl | 97.69 ± 0.03c | 5.10 ± 0.04c | 9.64 ± 0.15n | 5.86 ± 0.05f | 12.04 ± 0.08n | 6.18 ± 0.04c | 9.58 ± 0.10c | |
| StoL | 97.01 ± 0.09f | 5.24 ± 0.04b | 9.82 ± 0.24mn | 5.87 ± 0.08f | 13.17 ± 0.04h | 6.16 ± 0.05c | 9.58 ± 0.08c | |
| Stol | 96.88 ± 0.17f | 5.22 ± 0.03b | 13.45 ± 0.29d | 5.87 ± 0.06f | 13.14 ± 0.07h | 6.16 ± 0.03c | 9.60 ± 0.09c | |
| STOL | 96.56 ± 0.09g | 4.88 ± 0.03e | 13.47 ± 0.30d | 5.75 ± 0.04f | 12.04 ± 0.10n | 6.21 ± 0.01c | 9.62 ± 0.05c | |
| STOl | 97.24 ± 0.09e | 4.84 ± 0.02e | 13.32 ± 0.16d | 5.77 ± 0.05f | 12.07 ± 0.08n | 6.22 ± 0.01c | 9.58 ± 0.02c | |
| SToL | 97.43 ± 0.08c | 4.87 ± 0.03e | 13.73 ± 0.04c | 5.78 ± 0.05f | 12.48 ± 0.06kl | 6.20 ± 0.01c | 9.56 ± 0.07c | |
| STol | 96.91 ± 0.11f | 4.88 ± 0.02e | 11.62 ± 0.23i | 5.82 ± 0.04f | 12.36 ± 0.10kl | 6.21 ± 0.02c | 9.57 ± 0.05c | |
| Shade(25–30 °C) | swol | 93.61 ± 0.07k | 4.95 ± 0.05d | 8.63 ± 0.04q | 6.33 ± 0.09a | 15.18 ± 0.10c | 6.46 ± 0.01a | 9.60 ± 0.09c |
| stOL | 95.99 ± 0.12i | 5.24 ± 0.03b | 8.86 ± 0.06p | 6.13 ± 0.08c | 12.39 ± 0.16kl | 6.22 ± 0.03c | 9.76 ± 0.14b | |
| stOl | 96.13 ± 0.05h | 5.27 ± 0.07b | 8.62 ± 0.05q | 6.13 ± 0.09c | 12.32 ± 0.12lm | 6.24 ± 0.04c | 9.77 ± 0.07ab | |
| stoL | 96.70 ± 0.08f | 5.35 ± 0.05b | 9.14 ± 0.14o | 6.06 ± 0.06c | 13.51 ± 0.10g | 6.50 ± 0.03a | 9.89 ± 0.14a | |
| stol | 96.51 ± 0.14f | 5.29 ± 0.10b | 15.67 ± 0.22a | 6.10 ± 0.06c | 13.57 ± 0.12fg | 6.52 ± 0.04a | 9.75 ± 0.04b | |
| sTOL | 96.13 ± 0.08h | 4.63 ± 0.05f | 15.77 ± 0.09a | 5.88 ± 0.04f | 14.86 ± 0.07d | 6.36 ± 0.03b | 9.83 ± 0.12a | |
| sTOl | 96.14 ± 0.06h | 4.52 ± 0.01f | 15.76 ± 0.11a | 5.89 ± 0.04f | 15.11 ± 0.10c | 6.20 ± 0.03c | 9.80 ± 0.21a | |
| sToL | 95.79 ± 0.08j | 4.66 ± 0.05f | 13.93 ± 0.22b | 5.95 ± 0.10e | 15.94 ± 0.08b | 6.47 ± 0.04a | 9.75 ± 0.06b | |
| sTol | 96.38 ± 0.06f | 4.80 ± 0.05e | 10.48 ± 0.20j | 5.94 ± 0.08e | 16.36 ± 0.09a | 6.45 ± 0.02a | 9.75 ± 0.15b | |
| Owol | 97.22 ± 0.06e | 5.16 ± 0.04c | 6.20 ± 0.30r | 6.11 ± 0.10c | 10.30 ± 0.06s | 5.85 ± 0.02d | 9.76 ± 0.06b | |
| Oven (70 °C) | Otol | 99.16 ± 0.05a | 5.88 ± 0.04a | 13.06 ± 0.07e | 6.04 ± 0.07d | 9.40 ± 0.13u | 5.70 ± 0 .02d | 9.02 ± 0.10g |
| OTol | 98.50 ± 1.12b | 4.89 ± 0.03e | 10.65 ± 0.11j | 5.92 ± 0.04e | 9.22 ± 0.10u | 5.68 ± 0.02d | 8.96 ± 0.14h | |
| Owol | 97.19 ± 0.07e | 4.68 ± 0.02e | 9.94 ± 0.12m | 6.22 ± 0.13b | 10.53 ± 0.13r | 5.91 ± 0.05d | 8.96 ± 0.06h | |
| Oven (60 °C) | Otol | 98.80 ± 0.04ab | 5.00 ± 0.04d | 13.57 ± 0.30c | 6.04 ± 0.07d | 9.95 ± 0.05t | 5.75 ± 0.02d | 9.16 ± 0.17f |
| OTol | 98.70 ± 0.05b | 4.60 ± 0.04f | 10.13 ± 0.19l | 5.92 ± 0.04e | 11.75 ± 0.14pq | 5.76 ± 0.04d | 9.06 ± 0.14f | |
| Owol | 96.44 ± 0.07f | 5.09 ± 0.03c | 9.61 ± 0.25n | 6.22 ± 0.03b | 12.53 ± 0.09jk | 6.11 ± 0.05d | 9.07 ± 0.08f | |
| Oven (50 °C) | Otol | 98.69 ± 0.05b | 4.98 ± 0.03d | 13.09 ± 0.31e | 6.01 ± 0.02d | 12.69 ± 0.08ij | 5.87 ± 0.05d | 9.40 ± 0.17e |
| OTol | 98.12 ± 0.07b | 4.44 ± 0.03g | 10.26 ± 0.13k | 5.98 ± 0.07e | 12.16 ± 0.13mn | 6.09 ± 0.02d | 9.38 ± 0.04e | |
| Owol | 97.20 ± 0.07d | 4.97 ± 0.02d | 10.87 ± 0.11j | 6.26 ± 0.07b | 8.51 ± 0.11w | 6.23 ± 0.02c | 9.41 ± 0.43e | |
| Oven (40 °C) | OwOl | 97.11 ± 0.10e | 5.12 ± 0.01c | 10.27 ± 0.02k | 6.19 ± 0.04b | 8.49 ± 0.10w | 6.34 ± 0.02b | 9.49 ± 0.13d |
| OwoL | 97.20 ± 0.08d | 5.10 ± 0.02c | 10.27 ± 0.02k | 6.22 ± 0.04bc | 12.75 ± 0.14i | 6.49 ± 0.01a | 9.57 ± 0.04c | |
| Owol | 97.31 ± 0.83d | 4.99 ± 0.03d | 9.54 ± 0.19n | 6.17 ± 0.04c | 12.75 ± 0.14i | 6.46 ± 0.02a | 9.53 ± 0.31d | |
| OtOL | 98.22 ± 0.06b | 5.16 ± 0.04bc | 10.05 ± 0.13l | 6.11 ± 0.04c | 11.96 ± 0.09no | 6.25 ± 0.03c | 9.60 ± 0.01c | |
| OtOl | 97.66 ± 0.08c | 5.01 ± 0.03d | 9.98 ± 0.08j | 6.13 ± 0.04c | 11.98 ± 0.58no | 6.19 ± 0.01c | 9.60 ± 0.11c | |
| OtoL | 98.13 ± 0.15b | 5.20 ± 0.04b | 9.88 ± 0.10m | 6.10 ± 0.06c | 13.72 ± 0.16ef | 6.35 ± 0.04b | 9.56 ± 0.07c | |
| Otol | 97.65 ± 0.09c | 5.23 ± 0.05b | 10.45 ± 0.11k | 6.08 ± 0.07c | 13.85 ± 0.08e | 6.35 ± 0.04b | 9.60 ± 0.05c | |
| OTOL | 97.12 ± 0.12e | 4.25 ± 0.04h | 12.54 ± 0.23f | 5.94 ± 0.05e | 12.09 ± 0.14n | 6.34 ± 0.04b | 9.55 ± 0.05d | |
| OTOl | 97.81 ± 0.07c | 4.28 ± 0.04h | 12.31 ± 0.22g | 5.92 ± 0.04e | 12.06 ± 0.13n | 6.41 ± 0.14a | 9.55 ± 0.02dc | |
| OToL | 97.40 ± 0.05d | 4.39 ± 0.03g | 12.05 ± 0.06h | 5.91 ± 0.03e | 11.79 ± 0.16nop | 6.38 ± 0.07a | 9.59 ± 0.21c | |
| OTol | 97.14 ± 0.09e | 4.50 ± 0.03f | 12.12 ± 0.14h | 5.92 ± 0.04e | 11.78 ± 0.19pq | 6.42 ± 0.54a | 9.59 ± 0.04c |
Values are means ± standard deviation of three repetitions (n = 3). In the same column, values followed by different superscript letters are significantly different (p < 0.01). T = pulp treated with antioxydant/antimicrobial; t = untreated pulp; O/o = pulp powder stored in the presence (O) or absence of oxygen (o); L/l = pulp powder stored in the presence (L) or absence of oxygen (l) of light. Pulps or whole fruits (w) were sun-dried (S), shade-dried (s) or oven-dried (O) at 40, 60, 50 and 70 °C.
The ash contents varied significantly among different drying method, samples and storage conditions (Table 3). Also, swol sample shade-dried has higher value of ash content, however STOL (5.75 g/100 g) followed by SToL, STol and STOL, sun-dried had lower ash content (Table 3), suggesting significant effect of drying methods on ash content. The results show an increase of ash of almost samples analysed compared to the pulp from tree dried and whole pulp of fresh fruit. Total amino acid content (16.36 mg/100 g) of sTol sample shade-dried was significantly (p < 0.01) higher than other samples followed by sToL sample (15.94 mg/100 g), whereas OwOl sample oven-dried at 40 °C had lower content (8.49 mg/100 g) presented in Table 3. Comparing the results obtained before various drying and storage conditions, there was significant (p < 0.01) decrease of contents of amino acids in all samples which may be due to enzymatic or chemical oxidation during storage. As observed in the case of total amino acids, stol sample shade-dried shows higher contents of total protein (6.52 mg/100 g), while OTol sample oven-dried has the lowest value of total proteins (5.68 mg/100 g), which was not significantly different with Otol sample (5.70 mg/100 g). The results show also significant (p < 0.01) reduction of proteins concentration after applying different drying and storage conditions of samples, suggesting negative effects of drying temperature and storage conditions on proteins content. Drying methods cause some denaturation of protein in dried samples (Hassan et al., 2007). The negative effect of different drying methods on nutritional components have been reported by Badmus et al. (2019). Total proteins content and amino acids decreased during storage. This may be attributed to Maillard browning that probably occurred during fruit drying at high temperature. Fact, Maillard reaction which are naturally produced in food during thermal processing and storage by interaction between available reducing sugars and amino acids. The results showed that both treatment and storage conditions affected amino acids and proteins content of samples.
For fiber content, there was also significant effect of drying and storage condition, however value is higher in StOL sample (9.89 g/100 g) sun-dried and lower in OTol and Owol samples oven-dried at 60–70 °C (Table 3). Similarly, Eze and Akubor (2012) have reported the effect of drying and storage on the fiber of okra. Figure 3 displays values of carbohydrates content of different samples affected significantly (p < 0.01) by drying methods and according to storage conditions. For samples sun-dried, the value of total carbohydrates varied significantly among samples with respect to untreated and treated with values ranged from 80.20 to 83.01 g/100 g in StOl and Swol, respectively. However, values of total soluble sugars varied with a significant difference between 18.15 and 19.35 g/100 g with respect in StOL and Swol samples, while contents of reducing soluble sugars ranged from 26.80 to 28.51 g/100 g showed by StOL and STol samples, respectively presented in Figure 3A. The samples shade-dried show that value of carbohydrates content varied between 80.95 and 83.37 g/100 g showed by stOL and swol, respectively, however total soluble sugars ranged from 18.21 to 19.49 g/100 g and for the reducing soluble sugars, the values ranged from 27.73 to 29.79 g/100 g with the same samples (Figure 3B). The total carbohydrates content of samples oven-dried at 40 °C varied between 81.34 and 83.14 g/100 g in OTOL and owol, however soluble sugars content ranged from 18.20 to 19.02 g/100 g in otOl and oTol, respectively, whereas reducing soluble sugars of samples ranged from 27.33 to 28.63 g/100 g with respect of OTOL and owol (Figure 3C). Figure 3D shows higher variation of total carbohydrates content with values ranged from 78.02 to 81.01 g/100 g in otol and owol dried at 70 °C and 50 °C, respectively, however the highest values of total soluble sugars varied between 17.34 and 18.40 g/100 g in the same samples as observed in the case of total carbohydrates, respectively. While, reducing soluble sugars ranged from 26.65 to 27.99 g/100 g in otol and owol dried at 60 °C and 50 °C, respectively (Figure 3D). Comparing with the values found in different samples among drying and storage conditions, general trend show that sample (swol) shade-dried had higher values of total carbohydrates, total soluble sugars and reducing soluble sugars content, however when compared to the pulp of fresh fruit and pulp of fruit dried from tree, there were decreases except reducing sugars which was slightly higher than pulp of fresh fruit. These decreases may be due to non-enzymatic browning reactions occurring during thermal processing of fruit samples. Mohammed et al. (2020) found also a variation of total carbohydrates and sugars content when mango and pineapples were subjected to different drying methods. The results were similar to those observed by Wani et al. (2018), who reported a decrease of sugars content in dried sample of apricots fruit throughout storage period. The increase of reducing sugars content found in samples after three months of storage also was reported by Muzzaffar et al. (2016) in pumpkin candy. Contrary to the present study, Wani et al. (2018) reported a decrease of reducing sugars content suggesting that the effect of treatments and species of fruits used may be important for biochemical variation. In general trend, most samples did not show significant differences in measured nutritional parameters as a result of effect of light and oxygen storage conditions.
Figure 3.
Effect of drying and storage condition on total carbohydrates (TC), total soluble sugars (TSS) and reducing soluble sugars (RSS) of pulp of doum palm (H. thebaica) fruit. Pulp sun-dried (A), shade-dried (B) and oven-dried at 40 °C (C), 50 °C, 60 °C and 70 °C (D). Values are means ± standard deviation of three repetitions (n = 3).T = pulp treated with antioxydant/antimicrobial; t = untreated pulp; O/o = pulp powder stored in the presence (O) or absence of oxygen (o); L/l = pulp powder stored in the presence (L) or absence of oxygen (l) of light. Pulps or whole fruits (w) were sun-dried (S), shade-dried (s) or oven–dried (O) at 40, 60, 50 and 70 °C.
3.3. Effects of drying and storage conditions on polyphenols, vitamin C contents and antioxidant activities of pulp from doum plam (H. thebaica) fruit
The analyses were carried out after three months storage under different conditions. Tannins content of samples after drying and storage conditions varied significantly (p < 0.01) between 17.86 and 23.06 mg/g dry weight in Owol sample oven-dried at 70 °C and 40 °C, respectively (Table 4). The amount of tannins decrease in samples oven-dried at temperatures situated between 50 and 70 °C, however there was an increase with sample oven-dried at 40 °C compared to the whole pulp of fresh fruit and pulp from local market. This result suggest the negative effect of higher temperature on tannins in different samples dried. Also, contents of flavonoid (14.92 mg/g) was significantly higher in OTol sample treated oven-dried at low temperature (40 °C), whereas at high temperature (70 °C), lower was the amount in Otol sample (12.62 mg/g) followed by Owol sample (12.69 mg/g) in comparison with others. These results show the decrease of flavonoids content when compared to the whole pulp of fresh fruit values before subjecting to different drying and storage conditions. This lower amount may due to the effect of high temperature which degraded flavonoids. Total polyphenols content also are significantly (p < 0.01) affected according to the drying and storage conditions presented in Table 4. These results were similar to those reported by Lin et al. (2011). The OTol sample shows the highest content (4.85 g/100 g) which was not significantly (p > 0.01) different with OToL sample both treated and oven-dried at 40 °C, however the lowest value (3.09 g/100 g) was observed in StOL sample sun-dried in comparison with others. This result also reveals that total polyphenol (TP) contents decrease at high temperature due to the losses of heat-labile phenolics compounds, and when compared to whole pulp of fresh fruit and pulp from tree dried, however there is an increase in TP contents when compared to the pulp from local market. This may be due of drying method used as well storage time and temperature of the fruits purchased from the local market. Similarly, a significant decrease of total phenolics content of apricots fruit throughout the storage period was observed by Wani et al. (2018). Also, Mohammed et al. (2020) reported a reduction of total phenolics content from mango and pineapples dried by different methods. This reduction could be also due to an increase of oxidative degradation under oxygen and ultraviolet radiations during the peeling and treatment process of fruits. Fact, at high temperature as well as a polyphenol enzymatic degradation may occur during hot-air drying as well as photo-oxidation of some polyphenols due to the presence of oxygen during sun drying (Volf et al., 2014; Papoutsis et al., 2018). The vitamin C was affected by different drying and storage conditions as shown in Table 4, however the values ranged from 3.50 to 5.87 mg/g with respect to Otol and sTOl samples oven-dried (60 °C) and shade-dried (treated), respectively. Compared to the pulp from local market, amounts of vitamin C is higher, however slight decrease was observed depending on storage conditions or treatments of samples (Table 4). This result was similar to that observed by Dereje and Abera (2018), who found that vitamin C in dried mango slices increase or decrease according to the drying methods. It has been reported that at ascorbic acid undergoes degradation at relatively lower temperatures contributing to non-enzymatic browning even with or without available oxygen (Nursten, 2005). Also, it has been established in various foods that ascorbic acid is negatively impacted upon exposure to light and higher temperature, a lowering of ascorbic acid is also seen on storage of food and food products owing to various biochemical reactions (Pham et al., 2019; Mahnot et al., 2019). Marcin and Julita (2020) reported the concentration of total vitamin C in apple affected significantly by drying methods. Except, tannins content higher contents of total phenolic, flavonoid and vitamin C were found in treated samples suggesting during storage the effect of oxidation and polymerisation of phenolics compounds was minimised by antioxidants which avoid the formation of melanins from the enzymatic browning reactions. The decrease of phenolics compounds were consistent with the results obtained by Badmus et al. (2019).
Table 4.
Effect of drying and storage condition on tannins, flavonoids, total polyphenols (TP), vitamin C content, ferric reducing antioxidant potential (FRAP), DPPH and ABTS radical scavenging activities of pulp of doum palm (H. thebaica) fruit. Values are expressed on dry weight basis.
| Drying methods | Samples | Tannins (mg/g) | Flavonoids (mg/g) | TP (g/100g) | Vitamin C (mg/g) | FRAP (mgTE/g) | DPPH (mgTE/g) | ABTS (mgTE/g) |
|---|---|---|---|---|---|---|---|---|
| Sun(25–45 °C) | Swol | 19.47 ± 0.18f | 13.73 ± 0.14h | 3.94 ± 0.03g | 4.32 ± 0.02h | 3.29 ± 0.09d | 28.45 ± 0.15i | 32.86 ± 0.29m |
| StOL | 19.33 ± 0.18f | 12.84 ± 0.10gh | 3.09 ± 0.05l | 3.72 ± 0.07l | 2.50 ± 0.12g | 26.91 ± 0.14m | 39.47 ± 0.20h | |
| StOl | 19.35 ± 0.19f | 12.77 ± 0.07h | 3.15 ± 0.04k | 3.92 ± 0.06k | 2.66 ± 0.07g | 27.03 ± 0.12l | 38.43 ± 0.14k | |
| StoL | 19.35 ± 0.21f | 13.62 ± 0.13i | 3.65 ± 0.03i | 4.05 ± 0.05j | 2.90 ± 0.06f | 27.04 ± 0.11l | 38.51 ± 0.16k | |
| Stol | 19.36 ± 0.21f | 13.71 ± 0.16h | 3.66 ± 0.03i | 4.16 ± 0.02i | 3.06 ± 0.03d | 27.26 ± 0.17k | 38.76 ± 0.11k | |
| STOL | 19.24 ± 0.13g | 13.96 ± 0.10g | 3.99 ± 0.05g | 5.50 ± 0.02c | 3.61 ± 0.06c | 28.78 ± 0.32i | 39.30 ± 0.30i | |
| STOl | 19.20 ± 0.14g | 14.04 ± 0.11f | 4.01 ± 0.02f | 5.70 ± 0.15b | 3.72 ± 0.05c | 29.39 ± 0.12e | 40.36 ± 0.28f | |
| SToL | 19.29 ± 0.17g | 14.03 ± 0.07f | 4.18 ± 0.04d | 5.66 ± 0.12b | 3.74 ± 0.07c | 29.41 ± 0.14e | 41.19 ± 0.12c | |
| STol | 19.33 ± 0.13f | 14.16 ± 0.03e | 4.23 ± 0.04d | 5.80 ± 0.06a | 3.80 ± 0.08c | 29.67 ± 0.11d | 41.81 ± 0.15ab | |
| Shade(25–30 °C) | swol | 22.06 ± 0.22d | 13.89 ± 0.07g | 4.32 ± 0.01c | 4.58 ± 0.01g | 3.44 ± 0.04d | 30.77 ± 0.27c | 41.94 ± 0.13a |
| stOL | 21.91 ± 0.15e | 13.25 ± 0.07j | 3.94 ± 0.06g | 4.07 ± 0.07j | 2.96 ± 0.06f | 29.20 ± 0.56f | 39.99 ± 0.19g | |
| stOl | 21.90 ± 0.24e | 13.42 ± 0.13i | 4.02 ± 0.02f | 4.03 ± 0.06j | 3.15 ± 0.06e | 29.05 ± 0.13h | 39.04 ± 0.06j | |
| stoL | 22.16 ± 0.82d | 13.74 ± 0.04h | 4.12 ± 0.03e | 4.29 ± 0.04h | 3.27 ± 0.05d | 29.24 ± 0.13g | 39.32 ± 0.11i | |
| stol | 21.99 ± 0.15e | 13.88 ± 0.12g | 4.13 ± 0.03e | 4.37 ± 0.04h | 3.28 ± 0.05d | 29.45 ± 0.30e | 39.60 ± 0.07g | |
| sTOL | 22.02 ± 0.03d | 14.17 ± 0.01e | 4.21 ± 0.03d | 5.66 ± 0.03b | 3.75 ± 0.07c | 30.93 ± 0.12c | 39.85 ± 0.12g | |
| sTOl | 21.97 ± 0.08e | 14.27 ± 0.11d | 4.22 ± 0.03d | 5.87 ± 0.06a | 3.83 ± 0.04c | 31.39 ± 0.09b | 40.79 ± 0.16e | |
| sToL | 21.81 ± 0.24e | 14.38 ± 0.06d | 4.35 ± 0.04c | 5.74 ± 0.25ab | 3.90 ± 0.08c | 31.67 ± 0.13b | 40.91 ± 0.13e | |
| sTol | 21.82 ± 0.18e | 14.53 ± 0.11cd | 4.38 ± 0.05c | 5.88 ± 0.13a | 3.91 ± 0.05c | 31.92 ± 0.12a | 41.27 ± 0.11c | |
| Owol | 17.86 ± 0.15i | 12.69 ± 0.12m | 3.59 ± 0.03i | 3.75 ± 0.02l | 3.26 ± 0.04d | 27.44 ± 0.18j | 41.46 ± 0.15b | |
| Oven (70 °C) | Otol | 17.89 ± 0.16i | 12.62 ± 0.13m | 3.35 ± 0.40j | 3.62 ± 0.02lm | 3.08 ± 0.06e | 26.78 ± 0.13m | 38.60 ± 0.04k |
| OTol | 18.23 ± 0.07i | 12.88 ± 0.06l | 3.55 ± 0.05i | 4.82 ± 0.05f | 3.68 ± 0.07c | 27.91 ± 0.11j | 37.63 ± 0.23l | |
| Owol | 18.11 ± 0.14i | 13.84 ± 0.18g | 3.66 ± 0.06i | 3.64 ± 0.04l | 3.29 ± 0.02d | 27.03 ± 0.19l | 39.05 ± 0.15j | |
| Oven (60 °C) | Otol | 17.80 ± 0.24i | 13.30 ± 0.12k | 3.49 ± 0.04i | 3.50 ± 0.02m | 3.10 ± 0.04e | 25.21 ± 0.18s | 39.29 ± 0.20i |
| OTol | 17.94 ± 0.16i | 13.91 ± 0.16g | 3.61 ± 0.06i | 5.12 ± 0.01e | 3.69 ± 0.05c | 26.60 ± 0.25n | 38.85 ± 0.21k | |
| Owol | 19.85 ± 0.18f | 13.97 ± 0.14g | 4.08 ± 0.04e | 3.87 ± 0.02k | 3.31 ± 0.06d | 25.36 ± 0.30p | 39.56 ± 0.29h | |
| Oven (50 °C) | Otol | 19.05 ± 0.17h | 13.51 ± 0.11i | 3.74 ± 0.05h | 3.64 ± 0.02l | 3.09 ± 0.03e | 24.68 ± 0.21r | 43.52 ± 0.11a |
| OTol | 19.33 ± 0.19f | 14.28 ± 0.22d | 4.03 ± 0.03f | 5.30 ± 0.3d | 3.87 ± 0.04c | 26.48 ± 0.19n | 38.57 ± 0.25l | |
| Owol | 21.50 ± 0.31e | 14.32 ± 0.05d | 4.36 ± 0.03c | 3.96 ± 0.02k | 3.44 ± 0.08d | 25.64 ± 0.10p | 39.10 ± 0.20j | |
| Oven (40 °C) | OwOl | 21.79 ± 0.09e | 14.41 ± 0.05d | 4.38 ± 0.03c | 4.06 ± 0.06j | 3.53 ± 0.09d | 25.88 ± 0.11o | 39.62 ± 0.09h |
| OwoL | 22.61 ± 0.16c | 14.44 ± 0.06d | 4.49 ± 0.01bc | 4.02 ± 0.05j | 3.40 ± 0.07d | 26.35 ± 0.10n | 39.78 ± 0.12h | |
| Owol | 23.06 ± 0.36a | 14.51 ± 0.10c | 4.57 ± 0.05b | 4.13 ± 0.01i | 3.53 ± 0.05d | 26.76 ± 0.15m | 39.99 ± 0.03g | |
| OtOL | 22.32 ± 0.20c | 14.36 ± 0.19d | 3.99 ± 0.06g | 3.86 ± 0 .01k | 3.15 ± 0.05e | 24.95 ± 0.30r | 41.39 ± 0.22b | |
| OtOl | 22.32 ± 0.19c | 14.19 ± 0.09e | 4.02 ± 0.06f | 3.95 ± 0.03k | 3.20 ± 0.04e | 24.91 ± 0.05r | 39.30 ± 0.11i | |
| OtoL | 22.71 ± 0.02c | 14.45 ± 0.08c | 4.13 ± 0.01e | 4.08 ± 0.06i | 3.32 ± 0.07d | 25.19 ± 0.14q | 39.39 ± 0.17i | |
| Otol | 22.78 ± 0.05b | 14.53 ± 0.11c | 4.16 ± 0.02e | 4.12 ± 0.02i | 3.37 ± 0.07d | 25.52 ± 0.20p | 39.91 ± 0.21g | |
| OTOL | 22.44 ± 0.28c | 14.79 ± 0.13b | 4.53 ± 0.08b | 5.24 ± 0.03d | 4.33 ± 0.04a | 26.44 ± 0.23n | 40.49 ± 0.14e | |
| OTOl | 22.18 ± 0.05c | 14.82 ± 0.12ab | 4.57 ± 0.04b | 5.12 ± 0.01e | 4.44 ± 0.12a | 27.29 ± 0.12k | 40.40 ± 0.24e | |
| OToL | 22.51 ± 0.11b | 14.88 ± 0.11a | 4.83 ± 0.07a | 5.28 ± 0.05d | 4.07 ± 0.06b | 27.30 ± 0.11k | 40.83 ± 0.16d | |
| OTol | 22.67 ± 0.07b | 14.92 ± 0.10a | 4.85 ± 0.27a | 5.42 ± 0.02c | 4.21 ± 0.06b | 27.67 ± 0.13j | 41.22 ± 0.08c |
Values are means ± standard deviation of three repetitions (n = 3). In the same column, values followed by different superscript letters are significantly different (p < 0.01). T = pulp treated with antioxydant/antimicrobial; t = untreated pulp; O/o = pulp powder stored in the presence (O) or absence of oxygen (o); L/l = pulp powder stored in the presence (L) or absence of oxygen (l) of light. Pulps or whole fruits (w) were sun-dried (S), shade-dried (s) or oven-dried (O) at 40, 60, 50 and 70 °C.
Table 4 shows significant (p < 0.01) effects of different drying and storage conditions of three months on antioxidant activities of dried fruits. FRAP activity is significantly (p < 0.01) lower in StOL sample (2.50 mg TE/g) followed by StOl (2.66 mg TE/g) sun-dried than others, however the activity is high in OTOl sample (4.44 TEg/g), treated and oven-dried at low temperature (40 °C) presented in Table 4. When compared to the whole pulp of fresh fruit or pulp dried from the tree, antioxidant potential decreased with the drying and storage treatments used except treated samples such as OTol, OTOl, OTOL, and OToL oven-dried at 40 °C, which their FRAP activity increased with a significant difference, suggesting that positive effect of treatments (ascorbate/bisulphite) on heat-labile phenolics compounds and also a release of bound phenolics for higher activity during oven-drying at low temperature. Wani et al. (2018) reported highest FRAP activity of apricots fruit after storage period compared to initial fruit. For DPPH and ABTS radical scavenging activities, they were significantly (p < 0.01) higher in OtOL sample (24.94 mg TE/g) followed by OtOl sample (24.95 mg TEg/g) oven-dried both at 40 °C, however the lowest activity was showed by sTol sample shade-dried, and when compared to the whole pulp of fresh fruit activity, there is almost an increase of DPPH radical scavenging activity with the exception of pulp from local market, which the activity was lower. This result was similar to those reported by Fernandes et al. (2018), who found that DPPH radical scavening activities of petals varied according to the drying method, and also the activity was higher in dried samples than the fresh petals. The increased antioxidant capacity of the sample could be due to the release of the bound polyphenols, which are hightly active, as well as to the formation of Maillard reaction products, which also are constituents with antioxidant capacities.
Again, the strongest activitiy showed by ABTS method was observed in SwOl sample (32.86 mg TE/g) sun-dried but lower activity was observed in Otol sample oven-dried at 50 °C (Table 4). The results show an increase of ABTS radical scavenging activity when compared to the pulp dried on tree but lower than pulp from local market. The results may suggest the reduction of phenolics compounds quality to scavenge radicals in higher manner after exposure of sample fruits to UV radiations of light and high temperature. In the present study, a trend showed that samples did not show significant differences in measured antioxidant compounds as a result of effect of light and oxygen storage conditions.
3.4. Correlation between total polyphenols, total flavonoids, total tannins, vitamin C and antioxidant activities
The Pearson correlation between antioxidant compounds and antioxidant capacities are evaluated. There was significant positive correlation between vitamin C and FRAP (p < 0.01, r = 0.331) suggesting the important role of this compounds in the antioxidant potential, however a significant (p < 0.05, r = -0.180) and negative association was found with flavonoids. While, tannins are not showing any significant association between parameters evaluated. This positive correlation indicates that the highest total polyphenols or flavonoid contents lead to a high total antioxidant activity. Significant positive relationships were also found with flavonoids, TPC (p < 0.01, r = 0.814), FRAP (p < 0.01, r = 0.690), ABTS (p < 0.01, r = 0.832) and DPPH (p < 0.01, r = 0.600). Total polyphenols content (TPC) had significant and positive association with FRAP (p < 0.01, r = 0.616), ABTS (p < 0.01, r = 0.750) and DPPH (p < 0.01, r = 0.592). These results demonstrate that flavonoids and TPC are the most contributors in antioxidant activities in fruits. Fact, a positive correlation between polyphenols content and total antioxidant activity has been reported for fruits and vegetables such as raspberries, common vegetables, fruits and leaves, respectively (Chu et al., 2002; Liu et al., 2002; Sun et al., 2002; Saikia et al., 2016; Laya and Koubala, 2020). FRAP presented a significant and positive correlation with ABTS (p < 0.01, r = 0.753) and DPPH (p < 0.01, r = 0.572). Similarly, significant positive correlation was found also between ABTS and DPPH (p < 0.01, r = 0.681) suggesting their common effects for scavenging radical.
3.5. Principal component analysis
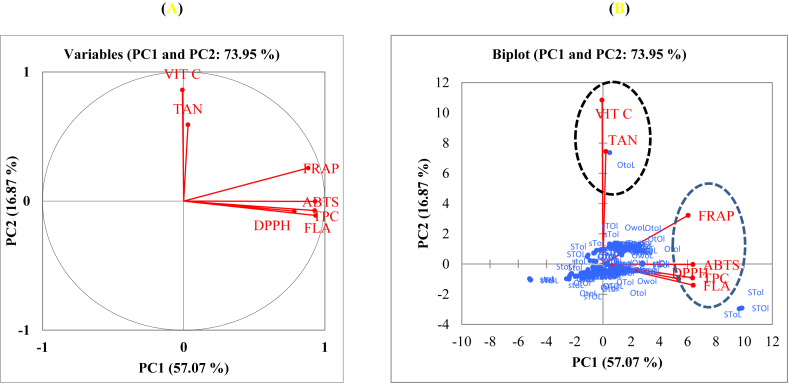
Figure 4 shows the scores of the first two principal components for the type of fruit and samples subjected to the different drying and storage conditions. The first two principal components explained 73.95 % of the total variation (PC1: 57.07 % and PC2: 16.87 %, respectively). PC1 was mainly correlated positively to the flavonoids, total polyphenols, ABTS, DPPH and FRAP values of the reducing power. PC2 was highly correlated to the tannins and vitamin C, however it was weakly correlated with FRAP value (Figure 4a). Figure 4b shows biplot of antioxidant compounds, antioxidant activities and contribution of different samples regarding activity. In relation with the antioxidant compounds and their activity, there was a formation of two clusters presented in Figure 4b. Sample Otol was marked alone with its high content in tannins content, while samples such as Stol, SToL and STOl are in the right side, due to their values in antioxidant compounds and activities.
Figure 4.
Principal component analysis (PCA) mean showing the relationship among total polyphenols, total flavonoids, total tannins and antioxidant activities. (A) Correlation between variables and factors and (B) biplot of samples distribution according to their polyphenolic contents and antioxidant activities. VIT C: vitamin C; TAN: tannin; TPC: total phenolic content; FLA: flavonoids.
4. Conclusion
The proximate composition of portions parts of doum (H. thebaica) fruit before different drying, treatments and storage conditions vary significantly, however the pericarp has significantly (p < 0.01) higher values of dry matter, pH, tannins, flavanoids and total polyphenols content than other parts. The pulp without pericarp has the highest values of ash (6.55 g/100 g), amino acids, proteins and vitamin C content, however the pulp of fruit purchased from the local market is significantly (p < 0.01) rich in Mg and microminerals such as Cu, Fe and Mn. Pulp of fruit purchased from the market showed significantly stronger antioxidant activities. Results reveals also that proximate composition and antioxidant activities of the various dried pulp fruits are significantly (p < 0.01) affected both by the drying methods and storage conditions used in comparison with the pulp of fresh fruit. The shade-dried samples have higher proteins, amino acids, vitamin C and ash contents. Polyphenols and antioxidant activity are high in oven-dried samples at low temperature, 40 °C. The effects of oxygen and light under storage conditions on phenolics, antioxidant activities and nutritional value vary with respect of treatment and drying methods. Significant and positive correlations are found between antioxidant compounds and antioxidant activities. In addition, principal component analysis explains 73.95 % of the total variance. The present study reveals that pulp samples of doum fruits retain most of its nutritional, bioactive compounds and high antioxidant capacities by various drying methods and storage conditions up to 3 months. The doum fruit can be powder as flour and could be used to overcome malnutrition. The shade and oven-dried samples stored in the absence of oxygen and light would be better for food formulations or food industry.
Declarations
Author contribution statement
Kolla M. C.: Performed the experiments; Wrote the paper.
Laya A.: Analyzed and interpreted the data; Wrote the paper.
Bayang J. P.: Analyzed and interpreted the data.
Koubala B. B.: Conceived and designed the experiments.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Aboshora W., Idriss S.E., Omar K.A., Hassanin H.A.M., Yu J., Abdalla M., Raza H., Zhang L. Volatile flavor compounds of peel and pulp from doum (Hyphaene thebaica L.) fruit. Am. J. Food Sci. Nutri Res. 2017;4(5):165–169. http://www.openscienceonline.com/journal/fsnr [Google Scholar]
- Aboshora W., Lianfu Z., Dahir M., Gasmalla M.A.A., Musa A., Omer E., Thapa M. Physicochemical, nutritional and functional properties of the epicarp, flesh and pitted sample of doum fruit (Hyphaene thebaica) J. Food Nutri. Res. 2014;2(4):180–186. [Google Scholar]
- AOAC . Published by the Association of Official Analytical Chemists International, Suite 400 2200 Wilson Boulevard, Arlington, Virginia, USA. 2000. Official methods of analysis of the association of official analytical chemists international 17th ed; pp. 22201–23301. [Google Scholar]
- AOAC . In: Official Methods of Analysis of AOAC International. eighteenth ed. Horwitz W., Latimer G., editors. AOAC International; Gaithersburg, MD: 2005. Arlington, Virginia, USA. [Google Scholar]
- Atito E., Moustafa M.F., Siddiqui S., El-Sayed M. Antioxidant, anti-α-amylase and antimicrobial activities of doum (Hyphaene thebaica) and argun (Medemia argun) fruit parts. Int. J. Pharmacol. 2019;15(8):953–961. [Google Scholar]
- Auwal M.S., Mairiga I.A., Shuaibu A., Ibrahim A., Gulani I.A., Wampana B., Lateefat G.I., Lawan F.A., Sanda K.A., Thaluvwa A.B., Njobdi A.N., Yagana K.Z. Preliminary phytochemical and in vitro antibacterial evaluation of the crude pericarp extract of Hyphaene thebaica (doum palm) JMPHTR. 2013;1:1–7. [Google Scholar]
- Badmus O.U., Taggart A.M., Boyd G.K. The effect of different drying methods on certain nutritionally. J. Appl. Phycol. 2019;31:3883–3897. [Google Scholar]
- Barrett D.M., Lloyd B. Advanced preservation methods and nutrient retention in fruits and vegetables. J. Sci. Food Agric. 2012;92(1):7–22. doi: 10.1002/jsfa.4718. [DOI] [PubMed] [Google Scholar]
- Barros L., Ferreira M.-J., Queiros B., Ferreira I.C.F.R., Baptista P. Total phenols, ascorbic acid, b-carotene and lycopene in Portuguese wild edible mushrooms and their antioxidant activities. Food Chem. 2007;103:413–419. [Google Scholar]
- Benzie I.F.F., Strain J.J. Ferric reducing ability of plasma (FRAP) a measure of antioxidant power: the FRAP assay. Anal. Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Braindbridge Z., Tomlins K., Westby A. Analysis of condensed tannins using acidified vanillin. J. Food Sc. Agri. 1996;29:77–79. [Google Scholar]
- Chu Y.-F., Sun J., Wu X., Liu R.H. Antioxidant and antiproliferative activities of common vegetables. J. Agric. Food Chem. 2002;50:6910–6916. doi: 10.1021/jf020665f. [DOI] [PubMed] [Google Scholar]
- Dereje B., Abera S. Effect of pretreatments and drying methods on the quality of dried mango (Mangifera Indica L.) slices. Cog Food. Agric. 2020;6(1):1747961. [Google Scholar]
- Dev S.A.R., Raghavan V.G.S. Advancement in drying techniques for food, fiber, and fuel. Dry. Technol. 2012;30:1147–1159. [Google Scholar]
- Devani M.B., Shishoo C.J., Shal S.A., Suhagia B.N. Spectrophotometric method for microdetermination of nitrogen in Kjedahl digest. J. Associ Official. Anal. Chem. 1989;72(6):953–956. [Google Scholar]
- Di Scala K., Crapiste G. Drying kinetics and quality changes during drying of red pepper. LWT - Food Sci. Technol. (Lebensmittel-Wissenschaft -Technol.) 2008;41:789–795. [Google Scholar]
- Dubois M., Gilles K.A., Hamilton J.K., Rebers P.A., Smith F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956;28:350–356. [Google Scholar]
- Eze J.I., Akubor P.I. Effect of drying methods and storage on the physicochemical properties of okra. Food Process Technol. 2012;3:177. [Google Scholar]
- Fernandes L., Casal S., Pereira J.A., Saraiva J.A., Ramalhosa E. Effects of different drying methods on the bioactive compounds and antioxidant properties of edible Centaurea (Centaurea cyanus) petals. Braz. J. Food Technol. 2018;21 Campinas. [Google Scholar]
- Gaytan-Martínez M., Cabrera-Ramírez A.H., Morales-Sanchez E., Ramírez-Jimenez A.K., Cruz-Ramírez J., Campos-Vega R., Velazquez G., Loarca-Pina G., Mendoza S. Effect of nixtamalization process on the content and composition of phenolic compounds and antioxidant activity of two sorghums varieties. J. Cereal. Sci. 2017;77:1–8. [Google Scholar]
- Hassan S.W., Umar R.A., Matazu I.K., Maishanu H.M., Abbas A.Y., Sani A.A. The effect of drying method on the nutrients and nonnutrients composition of leaves of Leptadenia hastata (Asclipiadaceae) Asian J. Biochem. 2007;2:188–192. [Google Scholar]
- Hetta M.H., Yassin N.Z. Comparative studies on hypocholesterolenic effect of different fractions of Hypanae thebaica (Doum) in experimental animals. Pharmazie. 2006;61:230–232. [PubMed] [Google Scholar]
- Hsu B., Coupar I.M., Ng K. Antioxidant activity of hot water extracts from the fruit of the doum palm (Hyphaene thebaica) Food Chem. 2006;98:317–328. [Google Scholar]
- Jones J.B., Case V.W. Sampling, handling and analyzing plant tissue samples. In: Westerman R.L., editor. third ed. Vol. 3. SSSA Book Series No.; 1990. (Soil Testing and Plant Analysis). [Google Scholar]
- Laya A., Koubala B.B. Polyphenols in cassava leaves (Manihot esculenta Crantz) and their stability in antioxidant potential after in vitro gastrointestinal digestion. Heliyon. 2020;6 doi: 10.1016/j.heliyon.2020.e03567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S.-D., Sung J.-M., Chen C.-L. Effect of drying and storage conditions on caffeic acid derivatives and total phenolics of Echinacea purpurea grown in Taiwan. Food Chem. 2011;125:226–231. [Google Scholar]
- Liu M., i X.Q., Weber C., Liu R.H. Antioxidant and antiproliferative activities of raspberries. J. Agric. Food Chem. 2002;50:2926–2930. doi: 10.1021/jf0111209. [DOI] [PubMed] [Google Scholar]
- Mahnot N.K., Mahanta C.L.M., Farkas B.E., Kevin M., Keener K.M., Misra N.N. Atmospheric cold plasma inactivation of Escherichia coli and Listeria monocytogenes in tender coconut water: inoculation and accelerated shelflife studies. Food Contr. 2019;106:106678. [Google Scholar]
- Marcin K., Julita G. Bioactive compounds, antioxidant activity, and sensory qualities of red-fleshed apples dried by different methods. LWT - Food Sci. Tech. 2020 [Google Scholar]
- Maskan M. Microwave/air and microwave finish drying of banana. J. Food Eng. 2000;44:71–85. [Google Scholar]
- Michel M.C. Dosage des acides aminés et amines par la ninhydrine. Amélioration pratique. Ann. Biol. Anim. Biochim. Biophys. 1968;8:557–563. [Google Scholar]
- Miller G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugars. Anal. Chem. 1972;31:426–428. [Google Scholar]
- Mohammed S., Edna M., Siraj K. The effect of traditional and improved solar drying methods on the sensory quality and nutritional composition of fruits: a case of mangoes and pineapples. Heliyon. 2020;6 doi: 10.1016/j.heliyon.2020.e04163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mphahlele R.R., Fawole O.A., Makunga N.P., Umezuruike L. Opara. Effect of drying on the bioactive compounds, antioxidant, antibacterial and antityrosinase activities of pomegranate peel. BMC Compl. Alternative Med. 2016;16:143. doi: 10.1186/s12906-016-1132-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzzaffar S., Baba W.N., Nazir N., Masoodi F.A., Bhat M.M., Bazaz R. Effect of storage on physicochemical, microbial and antioxidant properties of pumpkin (Cucurbita moschata) candy. Cogent Food. Agric. 2016;2:1163650. [Google Scholar]
- Nursten H. Royal Soci .Chem; Cambridge, U.K: 2005. The Maillard Reaction: Chemistry, Biochemistry and Implications (1st ed.) p. 226. [Google Scholar]
- Orwa C., Mutua A., Kindt R., Jamnadass R., Simons A. Agroforestree Database: a tree reference and selection guide version 4. 2009. http://www.worldagroforestry.org/af/treedb/ [Google Scholar]
- Papoutsis K., Vuong Q.V., Golding J.V., Hasperué J.H., Pristijono P., Bowyer M.C., Scarlett C.J., Stathopoulos C.E. Pretreatment of citrus by-products affects polyphenol recovery: a review. Food Rev. Int. 2018;1:27. [Google Scholar]
- Pham H.T.T., Bazmawe M., Kebede B., Buvé C., Hendrickx M.E., Van Loey A.M. Changes in the soluble and insoluble compounds of shelf-stable orange juice in relation to non-enzymatic browning during storage. J. Agric. Food Chem. 2019;67:12854–12862. doi: 10.1021/acs.jafc.9b05014. [DOI] [PubMed] [Google Scholar]
- Ranganna S. 1979. Détermination de l’acidité titrable. Manual of analysis of food. 7th, Churchille living stone. [Google Scholar]
- Re R., Pellegrini N., Proteggente A., Pannala A., Yang M., Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999;26(9-10):1231–1237. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Reda A., Aamer Physicohemical properties of doum (Hyphaene thebaica) fruits and utilization of its flour in formulating some functional foods. Alex. J. Fd. Sci. Technol. 2015;12(2):29–39. [Google Scholar]
- Saikia S., Mahnot N.K., Mahanta C.L. Phytochemical content and antioxidant activities of thirteen fruits of Assam, India. Food Biosci. 2016;13:15–22. [Google Scholar]
- Salib J.Y., Michael H.N., Eskande E.F. Antidiabetic properties of flavonoid compounds isolated from Hyphaene thebaica epicarp on alloxan induced diabetic rats. Pharmacogn. Res. 2013;5(1):22–29. doi: 10.4103/0974-8490.105644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton V.L., Orthofer R., Lamuela-Raventos R. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999;299:152–178. [Google Scholar]
- Sogi D.S., Siddiq M., Greiby I., Dolan K.D. Total phenolics, antioxidant activity, and functional properties of ‘Tommy Atkins’ mango peel and kernel as affected by drying methods. Food Chem. 2013;141:2649–2655. doi: 10.1016/j.foodchem.2013.05.053. [DOI] [PubMed] [Google Scholar]
- Sun J., Chu Y.F., Wu X.Z., Liu R.H. Antioxidant and antiproliferative activities of common fruits. J. Agric. Food Chem. 2002;50:7449–7454. doi: 10.1021/jf0207530. [DOI] [PubMed] [Google Scholar]
- Tiho T., Yao N.J.C., Brou Y.C., Adima A.A. Drying temperature effect on total phenols and flavonoids contents, and antioxidant activity of Borassus aethiopum Mart ripe fruits’pulp. J. Food Res. 2017;6:2. [Google Scholar]
- Volf I., Ignat I., Neamtu M., Popa V.I. Thermal stability, antioxidant activity, and photo-oxidation of natural polyphenols. Chem. Pap. 2014;68:121–129. [Google Scholar]
- Wani S.M., Masoodi F.A., Ahmad M., Mir S.A. Processing and storage of apricots: effect on physicochemical and antioxidant properties. J. Food Sci. Technol. 2018;55(11):4505–4514. doi: 10.1007/s13197-018-3381-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.