Abstract
Background
Over the past 20 years, insights from human and mouse genetics have illuminated the central role of the brain leptin-melanocortin pathway in controlling mammalian food intake, with genetic disruption resulting in extreme obesity, and more subtle polymorphic variations influencing the population distribution of body weight. At the end of 2020, the U.S. Food and Drug Administration (FDA) approved setmelanotide, a melanocortin 4 receptor agonist, for use in individuals with severe obesity due to either pro-opiomelanocortin (POMC), proprotein convertase subtilisin/kexin type 1 (PCSK1), or leptin receptor (LEPR) deficiency.
Scope of review
Herein, we chart the melanocortin pathway's history, explore its pharmacology, genetics, and physiology, and describe how a neuropeptidergic circuit became an important druggable obesity target.
Major conclusions
Unravelling the genetics of the subset of severe obesity has revealed the importance of the melanocortin pathway in appetitive control; coupling this with studying the molecular pharmacology of compounds that bind melanocortin receptors has brought a new obesity drug to the market. This process provides a drug discovery template for complex disorders, which for setmelanotide took 25 years to transform from a single gene into an approved drug.
Keywords: Melanocortin, Hypothalamus, Obesity, Genetics, Pharmacology, Therapy
1. Introduction
Obesity is associated with comorbidities such as type 2 diabetes, cardiovascular disease, and certain cancers and is arguably the greatest public health threat of the twenty-first century. While its increasing prevalence worldwide has clearly been driven by our changing lifestyle, a powerful genetic component underlies the large variations in body weight within this modern environment. Twin and adoption studies have revealed the heritability of the body mass index (BMI; weight in kg/height in m2) to be between 40% and 70% [1,2]. Over the past two decades, studies of human and mouse genetics have uncovered a number of circuits within the brain that play a central role in modulating mammalian appetitive behaviour and metabolism. The best characterised is the hypothalamic leptin-melanocortin signalling pathway, genetic disruption of which causes the majority of monogenic severe obesity disorders in both mice and humans.
The melanocortin system refers to a set of hormonal and neuropeptidergic pathways that are comprised of three main components: pro-peptide proopiomelanocortin (POMC), which is post-translationally processed by prohormone convertases into a number of biologically active moieties, including α-melanocyte stimulating hormone (MSH), β-MSH, γ-MSH, and adrenocorticotrophin (ACTH) [3], the eponymous melanocortin peptides; the five G protein-coupled melanocortin receptors, MC1R-MC5R, that mediate their actions [4]; and endogenous antagonists of those receptors, agouti and agouti-related protein (AgRP). The system is responsible for a dizzying array of functions, from melanogenesis and adrenal development, to energy homeostasis and sexual behaviour. We focus on the melanocortin pathway's role in regulating food intake and body weight in particular. At the end of 2020, the U.S. the Food and Drug Administration (FDA) approved the MC4R agonist setmelanotide for chronic weight management in adult and paediatric obesity patients due to POMC, proprotein convertase subtilisin/kexin type 1 (PCSK1), or leptin receptor (LEPR) deficiency [5]. We chart the pathway's history, explore its pharmacology, genetics, and physiology, and describe how a neuropeptidergic circuit became an important druggable obesity target.
2. History and cloning of melanocortin receptors
The effects of ACTH and MSH on the adrenal gland and pigmentation, respectively, were well known before direct effects of melanocortin peptides on the brain were described in the 1950s [6]. In the late 1970s, the discovery of melanocortin peptide immunoreactivity in the rat brain, coupled with cloning the POMC gene and the subsequent demonstration that it was expressed in the hypothalamus in the early 1980s indicated the existence of a brain melanocortin system [7]. The arcuate nucleus of the hypothalamus (ARC) and the nucleus of the tractus solitarius (NTS) are the main sites of POMC expression in the brain. However, it took until the late 1980s before binding sites for melanocortins could be demonstrated in the brain [8]. By that time, various effects were described of MSH and ACTH fragments on rodent behaviours [9], but the prominent effects of melanocortins on feeding and body weight regulation remained unknown. There were only a handful of studies describing the effect on feeding [10].
In 1992, receptors for the two “original” melanocortin peptides, α-MSH and ACTH, were cloned [11]. MSH was so named because of the peptide's ability to stimulate the synthesis of melanin by melanocytes, and its receptor, initially called MSH-R, was cloned from a melanoma sample known to be able to bind high levels of MSH. Given that α-MSH represents the first 13 amino acids of ACTH, literally being a part of the longer 39 amino acid peptide, it is unsurprising that their receptors share a great deal of sequence homology, which was how the ACTH-R was cloned. Similar methods leveraging sequence homology were then used to clone three additional melanocortin receptors, two of which were expressed primarily in the brain and the other in peripheral tissues. Their eventual names reflected the order in which they were cloned, with MSH-R and ACTH-R being renamed MC1R and MC2R, respectively; the two brain receptors followed as MC3R [12,13] and MC4R [12] and finally the peripheral MC5R was cloned [14].
3. The link between the melanocortin pathway and energy balance
POMC is expressed in hair follicles, the skin, pituitary cells, and neurons in the hypothalamus and brain stem [3]. The precursor peptide of POMC is cleaved by peptidases including PCSK1, proprotein convertase subtilisin/kexin type 2 (PCSK2), carboxypeptidase E (CPE), peptidyl-a-amidating monooxygenase (PAM), N-acetyltransferase (N-AT), and prolyl carboxypeptidase into different products in different cell populations, yielding, for example, ACTH in the pituitary corticotrophs vs α- and β-MSH in neurons localised to the arcuate nucleus (ARC) of the hypothalamus. Within the pituitary, POMC is expressed in excitable cells that produce ACTH and cells in the intermediate lobe that express α-MSH [3]. While agonists stimulate the production of cyclic adenosine monophosphate (cAMP) and recruitment of β-arrestin, they regulate a variety of physiological effects [4]. These include skin and hair pigmentation (MC1R), steroidogenesis (MC2R), appetite, satiety, energy homeostasis (MC3R/MC4R), and exocrine gland function (MC5R) in mice.
The linking of the melanocortin system to the regulation of body weight came with the study of a bright yellow mouse with severe obesity. Because the coat colour of animals is easily observed, pigmentation genetics became popular, including the hunt for genetic loci affecting coat colour. One of the classical genetic loci that influenced coat colour and pigmentation in several mammalian species was the extension locus, which encoded MC1R [15]. Yet another was the agouti locus, shown to be upstream of extension or MC1R. Agouti is normally expressed in the skin and acts as an antagonist on MC1R [16], blocking the production of darker melanin and producing lighter phaeomelanin, which in mice is a yellow-orange colour. Agouti mice have a mutation within the promotor that results in constitutive and ectopic production of the agouti peptide [17,18]. However, in addition to altered pigmentation, because agouti, which is normally expressed only in the skin, when ectopically expressed in the brain also antagonises MC4R [16,19], the mice are also hyperphagic and severely obese. The demonstration that Agouti also acts as an antagonist for the MC4R was a hallmark finding that launched the awareness that the melanocortin system plays a role in body weight regulation [16]. Based on its homology to agouti, agouti-related protein (AgRP) was cloned and shown to be expressed in the hypothalamus as natural antagonists of MC3R and MC4R [20], but not MC1R. Thus, transgenically overexpressing AgRP in mice resulted in hyperphagia and obesity, but not the characteristic coat colouration seen in agouti mice. We now know that a key physiological role of MC4R neurons within the paraventricular nucleus of the hypothalamus (PVN) is sensing the balance of orexigenic AgRP and anorexigenic melanocortin signals and to regulate feeding behaviour and energy expenditure [21] (Figure 1).
Figure 1.
Schematic illustrating POMC (red) and AgRP (blue) neuronal projections from the arcuate nucleus of the hypothalamus (ARH) to the lateral hypothalamus (LH), paraventricular nucleus of the hypothalamus (PVH), central nucleus of the amygdala (CEA), bed nucleus of the stria terminalis (BST), lateral parabrachial nucleus (LPB), reticular formation (RET), dorsal motor nucleus of the vagus (DMV), area postrema (AP), and nucleus of the solitary tract (NTS). POMC and AgRP neurons arise in the ARH adjacent to the median eminence (ME) and project widely. POMC neurons in the NTS have a more restricted set of projections. Both sets of neurons appear able to receive neural and endocrine signals from the body.
4. POMC neurons: Key regulators of energy expenditure and satiety
Within the ARC, POMC neurons are found adjacent to the medium eminence/tubero-infundibular zone at the base of the third ventricle, just above the pituitary stalk. Most of these neurons co-express cocaine- and amphetamine-related transcript (CART) peptide and project widely throughout the brain [22]. In the brainstem, NTS POMC neurons are thought to respond to, among other signals, gut-secreted cholecystokinin (CCK) [23] and adipocyte-derived leptin [24].
Over the past 20 years, the role of ARC POMC neurons in energy homeostasis, particularly in response to the adipocyte-derived hormone leptin, has been extensively studied [22]. Various peptide products of the POMC precursor have different receptors, so one needs to be cautious about fully ascribing POMC neuron actions with a discrete peptide receptor. Nevertheless, melanocortin peptides acting through central MC4R have a clear role in regulating appetite, energy expenditure, and body weight [22]. Because much of the initial characterisation of POMC peptides was performed in rodents that crucially lack cleavage sites to produce β-MSH, α-MSH was thought to be the key effector with respect to energy homeostasis. However, genetic evidence from humans [25] and dogs [26] point to β-MSH having at least as important a role as α-MSH, particularly in signalling through MC4R. Additionally, melanocortin peptides have been shown to regulate glucose homeostasis [27], erectile function [28], and cardiovascular tone [29], and peripherally to regulate skin and coat colouration, inflammation, skeletal muscle glucose uptake, and gut function [4].
POMC neurons are central mediators of endocrine signals. A significant proportion of ARC POMC neurons are activated by leptin expressing both STAT-3 and the immediate early gene c-fos. They are also activated by increasing blood glucose [30] by GLP-1R agonists [31] and inhibited by ghrelin [32]. It remains unclear if POMC neurons are activated by PYY3-36 [33,34]. POMC neurons are implicated in several models of cachexia [35,36]. It is unclear what the effect of insulin is on these neurons [37,38,39]. In general, POMC neurons are more active in post-nutritional repletion states than in fasting states [40].
Endocrine factors that activate POMC neurons cause anorexia and weight loss. These effects are mimicked by local application of α-MSH and are diminished in mice that lack MC4R [41], while food intake is supressed in mice lacking MC3R [42]. This shows that α-MSH signalling through the MC4R axis is at least partly responsible for some of these endocrine effects. In general, anorectic actions of melanocortin signalling at MC4R are considered to occur through neurons in the PVN [43,44,45], but neurons in other areas as the amygdala, DMH, and MPO [46] also contribute to the effects of melanocortins on appetite [44,45,47,48,49]. MC4R expressed on sympathetic preganglionic cholinergic neurons mediate actions on energy expenditure and glucose homeostasis by increasing sympathetic nervous system activity [50]. Sympathetic activation also mediates the effects of some melanocortins on heart rate and blood pressure, although the site of these receptors or their precise identity (MC3R vs MC4R) has not been determined [28]. Lateral hypothalamic MC3R-expressing neurons also modulate locomotor and energy expenditure activity [51].
Our understanding of the central melanocortin system has increased but the precise neural mechanisms by which it regulates appetite and energy expenditure are incompletely understood. The POMC system remains an intriguing drug target because of its importance in maintaining energy homeostasis and its capacity to influence several physiological processes.
5. AgRP neurons: An integrated controller of feeding and beyond
Soon after the discovery of AgRP, it was clear that while its overexpression resulted in hyperphagia and obesity, deleting the gene in mice did not result in a reduced body weight phenotype. It was not until four groups independently ablated the neurons expressing AgRP in adult mice, leading to an acute reduction in food intake to the point of starvation, that the necessity of those neurons to sustain feeding became clear [52,53,54]. Further experiments showed that chemogenetic and optogenetic stimulation of AgRP neurons increased food intake, demonstrating that AgRP neurons are also sufficient to engage feeding behaviour [55,56]. The fast-acting neurotransmitter GABA, which is co-released by AgRP neurons [57], was identified as a critical component of the rapid effects following AgRP neuron stimulation on food intake. Intriguingly, starvation induced by AgRP neuron ablation could not be rescued by chronic antagonism of MC3R and MC4R, whereas delivery of the GABA mimetic bretazenil to the parabrachial nucleus (PBN), where AgRP neurons densely project, could restore feeding and preserve body weight [55,58,59]. These results suggest that some behavioural and metabolic responses initiated by AgRP neuron activation might be independent of AgRP's role as an antagonist at melanocortin receptors. It was later shown that chemogenetic and optogenetic stimulation of AgRP neuron cell bodies or specific post-synaptic projections triggered a rapid feeding response that relies on GABA and NPY release, while AgRP release promoted a delayed and prolonged feeding response [60,61]. These results indicate that the kinetics of neuropeptide and other neurotransmitter release are paramount to both acute behavioural responses triggered by AgRP neurons and the neuromodulatory effect they promote at post-synaptic targets.
Since the original studies mapping the diversity of AgRP neuron projections to hypothalamic and extrahypothalamic regions [62], tracing methods combined with direct stimulation subsets of AgRP axons at post-synaptic targets have allowed the unravelling of redundant and parallel neural circuits through which AgRP neurons control food intake, metabolism, and cognitive-related responses. These approaches made possible the ability to functionally distinguish the circuits through which AgRP neuronal activity promotes or maintains food intake. As we previously discussed, acute loss of GABAergic input from AgRP neurons to the PBN appears to be the primary mechanism by which AgRP neuron ablation leads to starvation [58,59].
While direct stimulation of ARC AgRP neuronal → PBN projections failed to increase feeding [63], genetic or pharmacological inhibition of the PBN prevents the starvation that results from ablation of all AgRP neurons [58]. In the PBN, calcitonin gene-related peptide (CGRP)-expressing neurons, a critical integrative population of viscero-sensitive excitatory inputs from cholecystokinin (CCK)-expressing neurons in the NTS and catecholaminergic dopamine β-hydroxylase (Dbh)-expressing neurons in the raphe nucleus [64], were identified as a primary relay for ARC AgRP neuronal → PBN connections. Additionally, activation of PBN CGRP neurons projecting to the central nucleus of the amygdala (CeA) suppresses feeding [65], with activation of PBN-induced meal termination [66] sufficient to mimic conditioned taste aversions caused by malaise or nausea [67]. Moreover, it was shown that inhibition of CGRP neurons through direct stimulation of fibres projecting from AgRP neurons onto PBN CGRP neurons (ARCAgRP → PBNCGRP) resulted in increased feeding, even with exposure to anorectic signals LiCl, amylin, or CCK, but not when exposed to the inflammatory-related lipopolysaccharide signal (LPS). This study underscored a role of the ARCAgRP → PBNCGRP network in gating feeding response during homeostatic needs [68].
Conversely, for the PBN, direct stimulation of ARC AgRP projections to the periaqueductal grey area (PAG) or CeA failed to promote feeding, while [60] a positive response was observed after stimulation of AgRP neuron projections to the paraventricular thalamus (ARC AgRP → PVT), the anterior division of the bed nucleus of the stria terminalis (ARCAgRP → aBNST), the lateral hypothalamus (ARCAgRP → LH) [60] or the PVN (ARCAgRP → PVN). In fact, functional circuit mapping has identified a critical role of ARC AgRP neuron synapsing onto PVN oxytocin-producing neurons (ARCAgRP → PVNOXT) for a rapid feeding response evoked by both GABA and NPY release [61,63], while inhibition of PVN MC4R-bearing neurons also demonstrated to be instrumental for AgRP neuron-mediated feeding through PVNMC4R → LPBN circuits [44]. Deletion and re-expression of MC4R restricted to the PVN allowed the glutamatergic nature of PVNMC4R neurons [45] and prodynorphin-expressing PVN neurons (PVNPDYN) as two independent and additive circuits mediating anorexia through direct projection onto the PBN to be uncovered [45]. ARC → PVN projections from glutamatergic neurons expressing the OXY receptor, but independent from AGRP or POMC neurons, were discovered to promote a rapid anorectic response on a scale comparable to the rapid orexigenic response achieved through AgRP neuron chemo/optogenetic activation [48].
To further add to the overall complexity of hunger-activated circuits, transcriptomic studies and functional tracing revealed the diversity among AgRP neurons based on projection sites and specific receptor equipment for circulating metabolic signals [60,63,69]. Hence, an ARCAgRP → PBNCGRP → CeA circuit appears to be critical to prevent various aspects of food avoidance or aversion, ARCAgRP → aBNST, PVN, LH, and PVT seem rather engaged in the positive regulation of feeding.
Aside from their ability to integrate circulating signals of hunger and satiety, in vivo recording of calcium-based fluorescence in AgRP neurons, used as proxy of neuronal activity, revealed that AgRP neurons can also rapidly process food-related cues and interoceptive signals. The increase in AgRP neuronal activity induced by food deprivation is rapidly dampened by the sensory detection of food, even before calories are ingested [70,71,72], while stimulation of intestinal mechanosensory information routed by the vagal nerve can durably inhibit AgRP neuron activity [73,74,75,76]. Hence, AgRP neurons critically influence a broad array of behavioural and physiological responses that, beyond the strict consummatory sequence, ensures the optimisation of strategies (prediction, caloric evaluation, sensory, and interoceptive signals) for foraging food and conserving body weight. This includes the role of AgRP neurons in non-consummatory behaviour involved in decision-making when balancing the need for food and the risk associated with food retrieval. Thus, the AgRP neuronal network functions as a core hub integrating the constellation of responses required for the adaptive response to food availability.
6. Genetics of the melanocortin system
The discovery that mutation of the leptin gene explained the phenotype of the obese/obese mouse in 1994 [77] heralded the modern era of genetic and mechanistic obesity studies, making clear that regulating food intake, and hence body weight, went beyond simply “will-power.” In 1997 came the report of two young cousins with severe early onset obesity harbouring leptin mutations [78], and in 1998, that of three sisters with similar obesity with mutations in their leptin receptors [79], confirming that this system was relevant across mammalian species. We now know that the role of leptin, which is secreted by adipocytes into the circulation in proportion to fat mass [80], is to inform the brain of long-term energy stores and turn on the starvation response when levels drop beyond a certain threshold, pointing to a role of leptin in controlling the neuroendocrine system during starvation [81]. Crucially, the melanocortin pathway is one of the key effector mechanisms of leptin signalling in the brain, certainly in terms of appetite regulation.
The first direct genetic evidence of the melanocortin pathway in energy homeostasis came in 1997 from the targeted disruption of MC4R in mice [82], which resulted in hyperphagia, severe obesity and hyperinsulinaemia. Over the next few years, torrents of genetic findings were reported, in both humans and mice, that delineated most of the central melanocortin pathway's key components. As previously mentioned, generating melanocortin peptides from POMC depends on a series of enzymes. The first step is affected by proconvertase 1 (PC1), followed by proconvertase 2 (PC2) and carboxypeptidase E (CPE). The same year, loss-of-function mutations in the PCSK1 gene that encodes PC1 were identified in human cases of early onset obesity [83]. In 1998 came the identification of mutations in both POMC [84] and MC4R [85,86] that were linked to severe human obesity. In 2000, the deletion of a single allele of Sim-1, a gene encoding a transcription factor necessary for the normal development of the PVN produce obesity in mice [87] and later in humans [88].
The one component of the central melanocortin pathway that when disrupted does not clearly cause severe obesity is MC3R. Mc3r −/− mice have a complicated phenotype, showing changes in body composition with increased fat mass but reduced lean mass compared to wild-type littermates despite being “hypophagic” and maintaining normal metabolic rates [42,89]. However, mice lacking both MC3R and MC4R become significantly heavier than Mc4r−/− mice [42], indicating that they serve non-redundant roles in regulating energy homeostasis. No human MC3R mutations to date have been conclusively linked to severe obesity, even if multiple smaller studies have reported possible associations of MC3R variations with increased body weight [90,91,92,93], although nowhere near the effect size of that seen with mutations in MC4R. Taking into account all of the available evidence, MC3R is less likely to be related to food intake and more likely to revolve around nutrient partitioning. MC3R may thus serve a critical role in ensuring the delicate balance between energy expenditure and fat storage in the body [94].
The shared phenotype seen in monogenic non-syndromic obesity includes severe early onset obesity (before 5 years of age) with major hyperphagia. Depending on the gene involved, patients can often be affected by a number of endocrine abnormalities that include hypogonadism, growth hormone deficiency, hypothyroidism (for LEP and LEPR), and corticotropic insufficiency (for POMC). A review reported that across 88 patients with LEPR mutations, 100% had early onset obesity (<5 years), 96% had hyperphagia, and 34% had one or more pituitary hormone deficiencies [95]. In POMC variants, patients display modifications in hair colour, adrenal dysfunction, and obesity related to the absence of POMC-derived ligands for the melanocortin receptors MC1R (αMSH), MC2R (ACTH), and MC4R (α and β MSH). Since the proconvertase 1 (PC1) (encoded by PCSK1) enzyme is involved in processing POMC, insulin, and proglucagon, some patients also exhibit post-prandial hypoglycaemia and adrenal insufficiency [84].
This list was recently extended with the discovery of variants in genes that either regulate or are proposed to regulate the melanocortin system. They include the MC4R regulator melanocortin receptor accessory protein 2 (MRAP2) [96,97], adenylate cyclase 3 (ADCY3) [98,99], and steroid coreceptor activator-1 (SRC-1) [100]. Other variants in genes acting on leptin/melanocortin signalling were reported including brain-derived neurotrophic factor (BDNF) [101] and its receptor neurotrophic tyrosine kinase, receptor type 2 (NTRK2) [102], or SH2B adaptor protein 1 (SH2B1) [103].
Hundreds of MC4R variants have been identified, leading to many functional alterations that are sometimes inconsistent across subtypes [104]. Heterozygous variants in MC4R are the most common and frequently result in obesity with variable severity occurring at a later age than in homozygous variant carriers. MC4R variants are found in approximately 2%–5% of childhood and adult obesity [105] depending on the population background and in up to 1% of the general population with a BMI > 30 [106]. It cannot be excluded that due to underdiagnoses, the frequency could increase to 30% in some populations such as those with a high level of consanguinity [107]. Additionally, while most MC4R functional variants caused a reduction in activity, a subset actually result in a gain of function (GoF), and these GoF MC4R variants are associated with a lower risk of obesity and its cardiometabolic complications in the general population [108]. Of note, the degree to which a mutant receptor is dysfunctional as measured by in vitro cAMP accumulation assays can actually predict the amount of food eaten by the patient carrying that particular mutation at a test meal [109]. All of this implies that MC4R, and hence the melanocortin pathway more broadly, does not act in a binary on/off fashion, but is a “tuneable” system.
As it turns out, the rheostatic nature of the melanocortin pathway in regulating feeding behaviour is highly conserved, with naturally occurring mutations in the pathway identified in a wide range of different species depending on their selection pressure. For example, studies in Labrador retrievers, which are known to be more food motivated than other dog breeds, have found that anywhere from 20 to 25% carry a 14bp deletion in POMC that disrupts the β-MSH and β-endorphin-coding sequences and is associated with greater food motivation and increased body weight [26]. Certain pig breeds have also been shown to carry MC4R missense mutations that are associated with fatness, growth, and food intake traits [110]. MC4R mutations have even contributed to the adaption and survival of blind Mexican cavefish to nutrient-poor conditions found in underwater caves [111].
Crucially, it is increasingly recognised that monogenic and polygenic forms of obesity are not discrete entities but instead are on a spectrum sharing at least some common biology, with the melanocortin pathway being a prime exemplar. As genome-wide association studies have continued to discover more obesity-associated loci, an increasing number of these loci harbour genes that sit within the pathway, including MC4R [112,113], POMC [114], PCSK1 [115], SH2B1 [116,117], and BDNF [116] among others. Thus, while genetic disruption of the pathway results in severe obesity, subtle variations in or near these same genes in the pathway influence where one might sit in the population distribution of BMI [118].
7. Melanocortin ligands
We summarise efforts to explore potential therapeutic applications for treating obesity resulting from efforts to target the melanocortin pathway. At the core of the pathway are the five G-protein coupled receptors through which melanocortin peptides signal to affect their diverse functions. Soon after their cloning, they were immediately attractive “druggable” targets, and attempts to create more potent, selective, and stable compounds resulted in the discovery of many high-affinity ligands with differing specificities for the five receptors.
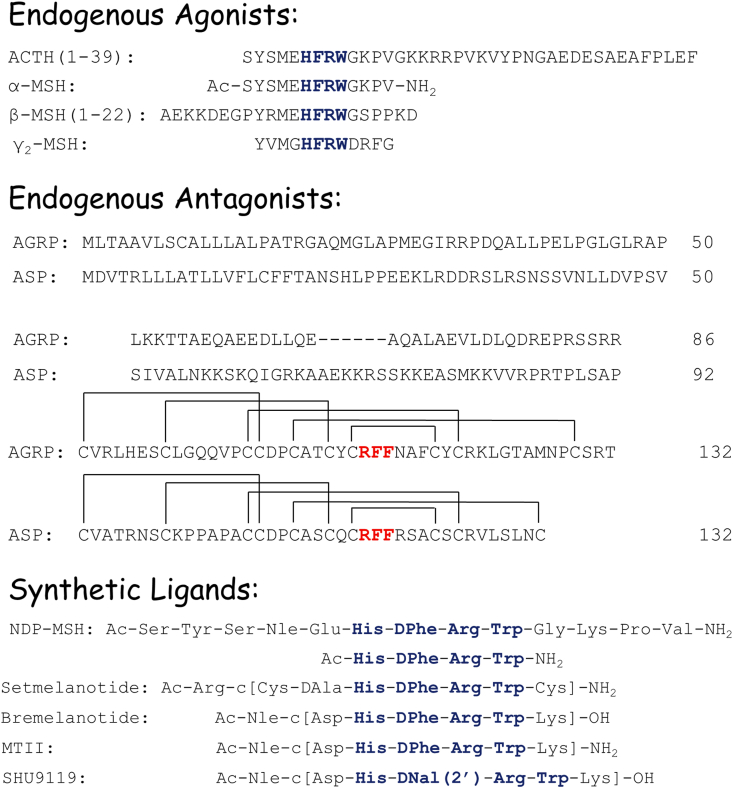
The toolbox of melanocortin ligands (Figure 2) include the endogenous peptide agonists (ACTH, α-MSH, ß-MSH, and γ2-MSH) and naturally occurring antagonists (agouti and AgRP) as well as classic synthetic peptides (MTII, SHU9119, and Ac-His-DPhe-Arg-Trp-NH2), clinically approved melanocortin ligands (α-NDP-MSH/afamelanotide and bremelanotide), and setmelanotide, the FDA-approved agonist currently undergoing further clinical trials. While melanocortin receptors have been reported to signal through many different pathways, we focus on ligands’ effects on intracellular cAMP levels, as this was the signal transduction pathway that discovery of most ligands was based upon. Table 1, Table 2 list reported binding and functional activities, respectively, of these melanocortin ligands in cloned murine and human MC1R, MC3R, MC4R, and MC5R. These are not exhaustive lists, but are meant to illustrate the reported data. Since MC2R only interacts with ACTH [119], MC2R is not further discussed. The pharmacology of β-MSH, ACTH, and agouti is further separated based upon the sequence used in the reported assays.
Figure 2.
Structures of the synthetic and endogenous human melanocortin ligands. The commonly hypothesised active sequences are highlighted in blue (endogenous agonists and synthetic ligands) and red (endogenous antagonists).
Table 1.
Binding affinities of melanocortin ligands in cloned murine and human melanocortin receptors.
| Peptide | Murine receptors (nM)a |
Human receptors (nM) |
||||||
|---|---|---|---|---|---|---|---|---|
| MC1R | MC3R | MC4R | MC5R | MC1R | MC3R | MC4R | MC5R | |
| α-MSH | 3.68 [165] | 6.67(r) [166] 9.4(r) [167] 30.2 [168] 52(r) [169] |
9.17(r) [167] 61.2(r) [166] |
62.5 [170] ∼80 [171] |
0.0334 [172] 0.092 [173] 0.230 [174] 0.321 [175] 1.5 [176] 3.9 [177] 5.97 [178] |
15.5 [175] 19 [177] 20.7 [172] 21.2 [166] 31.5 [174] 46 [176] 50.04 [178] |
19 [177] 26 [176] 38.7 [178] 41.4 [175] 522 [166] 641 [179] 900 [174] |
120 [177] 150 [176] 332 [175] 557 [178] 928 [180] 7160 [174] 8240 [172] |
| β-MSHb | 34.6(r) [166] | 27.2(r) [166] | 0.449 [173] 0.864 [175] 1.17 [172] |
13.4 [172] 15.1 [166] 23.2 [175] |
19.9 [175] 76 [179] 387 [166] |
306 [175] 1750 [180] 14,400 [172] |
||
| β-MSH(5–22) | 19.4 [168] | 212 [170] | 2.29 [181] | 39.46 [181] | 22.74 [181] | 301.5 [181] | ||
| β-MSH(1–22) | 0.89 [182] | 10.64 [182] | 8.18 [182] | 76.90 [182] | ||||
| γ2-MSH | 6.7 [168] 44(r) [169] |
1270 [170] | 11.2 [172] 20.8 [175] |
17.7 [172] 57.2 [175] 72 [183] |
770 [183] 6250 [175] 100,000 [179] |
2500 [183] 3250 [175] >100,000 [172] |
||
| ACTH(1–24) | 8.15(r) [166] | 35.6(r) [166] | 32.8 [166] | 755 [166] | ||||
| ACTH(1–39) | 21.1 [168] | 236 [170] ∼300 [171] |
0.170 [173] 2.50 [172] |
86.9 [172] | 693 [179] | 929 [180] 17,000 [172] |
||
| Setmelanotide | 2.7(r) [185] | 5.5 [184],e 3.9 [175,185] |
10 [175,185] 18 [184],e |
2.1 [175,185] 5.0 [184],e |
430 [175,185] 1910 [184],e |
|||
| Bremelanotide | 3.4 [184],e | 72.07 [186] 220 [184],e |
10 [187] 19.25 [186] 29 [184],e |
166.8 [186],e 190 [184] |
||||
| MTII | 0.22 [188]c | 1.85 [188]c 4.77(r) [167] 67.8(r) [166] |
1.74(r) [167] 6.22(r) [166] 0.50 [188,189]c |
28.4 [188]c | 0.20 [184],e 0.268 [175] 0.36 [177] 0.686 [174] 6.4 [176] |
1.6 [190] 3.1 [177] 20 [184],e 34.1 [174] 24.0 [175] 52.6 [166] 53 [176] |
0.07 [190] 0.18 [177] 0.25 [176] 1.5 [184],e 2.66 [175] 6.60 [174] 39.7 [166] |
0.89 [190] 1.2 [177] 17 [176] 23 [184],e 23.1 [175] 46.1 [174] |
| Ac-His-DPhe-Arg-Trp-NH2 | 388 [191] | 60%@100 μM [191] | 214 [191] | 615 [178] | >10 μM [178] | 1153 [178] | >10 μM [178] | |
| NDP-MSH | 0.29 [188] 0.79 [165] 0.31 [191] |
0.479(r) [166] 1.19(r) [167] 1.4 [168] 1.45 [188] 4.18 [191] 10(r) [169] |
0.417(r) [166] 0.88 [188,189] 1.09 [191] 3.14(r) [167] |
1.1 [170] 27.6 [188] |
0.023 [173] 0.0231 [172] 0.046 [176] 0.109 [175,192] 0.39 [193] 0.51 [178] |
0.224 [172] 0.319 [166] 0.469 [174] 0.680 [175] 0.78 [176,193] 1.17 [178] 2.9 [183] |
0.30 [176] 0.620 [175] 1.16 [178] 1.6 [193] 1.96 [166] 2.16 [179] 2.93 [174] 3.3 [183] |
0.27 [193] 0.48 [176] 0.86 [178] 0.89 [183] 0.891 [175] 2.39 [172] 5.18 [180] 5.50 [174] |
| SHU9119 | 0.879(r) [167] 1.68(r) [166] |
0.238(r) [167] 0.38 [189]c 0.583(r) [166] |
0.62 [177] 0.714 [170] |
0.23 [177,194] 1.20 [174] 1.65 [166] |
0.06 [194] 0.07 [177] 0.360 [174] 0.689 [166] |
0.065 [177] 0.09 [194] 1.12 [174] |
||
| Agoutid | 2.6(B16F10) [195] | 190 [195] | 54 [195] | 1200 [195] | 23 [195] | 112 [196] 140 [195] |
12 [196] 70 [195] |
>1000 [195] |
| AgoutiYY | 15.3 [197] | 14.1 [197] | 1.8 [197] | |||||
| AGRP | 2.8 [198]c | 0.66 [189]c 3.0 [198]c |
None [199]c | 1.1 [200] 4.5 [201] 11.2 [199]c |
0.5 [200] 3.5 [201] 9.0 [199]c 24.7 [202]c |
25.6 [199]c >40 [200] |
||
Values labelled with a (r) represent data from cloned rat receptors. Unlabelled numbers represent mouse receptors.
Affinity β-MSH values were split depending on the sequence used. If a sequence was not provided in the paper, values are listed in the β-MSH row.
The radiolabels [125I]MTII, [125I]SHU9119, and [125I]AGRP were used at their respective ligands as indicated in place of [125I]NDP-MSH.
A mouse cell line natively expressing the MC1R was used.
Values shown are IC50 values converted from pKi and pIC50 values reported in the source publication.
Table 2.
Functional pharmacology of the melanocortin ligands in cloned murine and human melanocortin receptors.
| Peptide | Murine receptorsa |
Human receptors |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MC1R |
MC3R |
MC4R |
MC5R |
MC1R |
MC3R |
MC4R |
MC5R |
|||||||
| EC50 (nM) | EC50 (nM) | pA2 | EC50 (nM) | pA2 | EC50 (nM) | EC50 (nM) | pA2 | EC50 (nM) | pA2 | EC50 (nM) | pA2 | EC50 (nM) | pA2 | |
| α-MSH | 0.039 [203] 0.15 [191] 0.23 [189] 0.55 [204] 0.70 [205] |
0.73 [189] 0.76 [191] 0.79 [204] 1.21 [168] 2.10 [205] 3.8(r) [169] |
2.6 [205] 3.07 [189] 4.0 [191] 5.37 [204] 18(r) [206] |
0.41 [189] 0.44 [204] 0.58(r) [207] 0.59 [191] 1.07 [170] 2.00 [205] |
0.0022 [203] 0.091 [157] 1.01 [175] |
0.669 [157] ∼1 [12] 1.04 [175] 16.7 [208] |
0.210 [157] 0.88 [202] 1.34 [209] 1.5 [210] 2.56 [181] 4.69 [175] 9.2 [208] |
0.807 [157] 10.5 [175] 35.3 [208] 57 [211] |
||||||
| β-MSHb | 12(r) [207] | 4.24 [175] | 1.59 [175] | 6.62 [175] | 23.7 [175] 209 [211] |
|||||||||
| β-MSH(5–22) | 1.1 [168] | 6.5 [170] | 9.33 [181] | |||||||||||
| β-MSH(1–22) | 0.21 [203] | 3.8(r) [169] | 0.013 [203] | ∼10 [12] | 0.24 [202] 0.30 [209] 3.24 [181] |
|||||||||
| γ2-MSH | >100 [203] 680 [205] 1090 [191] |
0.64 [168] 3.8(r) [169] 34.6 [191] 38 [205] |
420 [205] 869 [191] |
35.1 [191] 42 [205] 42.9 [170] |
0.082 [203] 3.05 [175] 300 [212] |
0.487 [175] 5.9 [183] 700 [212] |
93.7 [175] >100 [210] 107 [209] 170 [202] 260 [183] 710 [212] |
490 [183] 500 [175] 550 [212] 2700 [205] |
||||||
| ACTH(1–24) | 0.46(r) [207] | 0.86 [202] 1.63 [209] |
30 [211] | |||||||||||
| ACTH(1–39) | 0.37 [203] | 3.3 [168] 3.8(r) [169] |
24(r) [206] | 6.02 [170] 6.2(r) [207] |
0.0083 [203] | ∼1 [12] | 0.68 [210] | |||||||
| Setmelanotide | 0.28(r) [185] | 0.26 [184],d 5.8 [175,185] |
0.69 [184],d 5.3 [175,185] 30.9 [208] |
<0.032 [184],d 0.27 [175,185] 1.7(L) [184],c,d 5.7 [208] |
109.7 [208] 1600 [175,185] | |||||||||
| Bremelanotide | 0.095 [184],d | 2.4 [184],d | 0.25 [184],d 4.1(L) [184],c,d |
|||||||||||
| MTII | 0.020 [204] 0.037 [213] 0.06 [214] |
0.011 [213] 0.16 [204] 0.18 [214] |
0.083 [213] 0.087 [204] 0.15 [214] |
0.14 [214] 0.16 [204] 0.17 [213] |
<0.032 [184],d 0.199 [175] 0.30 [215] |
0.204 [184],d 0.512 [175] 0.75 [216] 1.3 [215] 33.9 [208] |
0.011 [209] 0.017 [202] <0.032 [184],d 0.0542 [175] 0.19 [216] 0.35(L) [184],c,d 2.9 [215] 8.6 [208] |
2.8 [216] 3.3 [215] 5.33 [175] 33.9 [208] |
||||||
| Ac-His-DPhe-Arg-Trp-NH2 | 14.1 [191] 25.6 [189] |
55.5 [191] 195 [189] |
10.2 [189] 13.7 [191] |
3.46 [189] 9.8 [191] |
4.3 [217] 228 [178] |
7.1 [217] | 0.57 [209] 0.72 [202] 548 [217] 793 [178] |
3.9 [217] | ||||||
| NDP-MSH | 0.0015 [203] 0.030 [218,219] 0.038 [204] 0.04 [214] 0.21 [218] 1.0 [205] |
0.09 [205] 0.098 [204] 0.10 [189] 0.19 [218] 0.23 [214] 0.24 [191] 0.43 [168] 1.6(r) [169] |
0.10 [205] 0.21 [204] 0.32 [189] 0.41 [220] 0.46 [191] 0.47 [214] |
0.050 [170] 0.071 [204] 0.086 [189] 0.18 [220,221] 0.22 [205] 0.31 [191] 1.0(r) [207] |
0.0016 [203] 0.023 [157] 0.462 [175] |
0.109 [175] 0.132 [157] 0.24 [183] 6.8 [208] |
0.011 [210] 0.017 [157] 0.022 [202] 0.023 [209] 0.0752 [175] 0.20 [183] 0.4 [208] |
0.23 [183] 0.253 [175] 0.53 [205] 1.1 [208] 1.8 [211] |
||||||
| SHU9119 | 0.64 [213] 0.98 [214] |
Partial agonist [214] | 8.8 [214] 9.5 [213] |
9.3 [214] 10.4 [213] |
Partial agonist [214] 2.31 [213] |
0.036 [157] 1.5 [215] |
Partial agonist [157] | 8.3 [157] 8.7 [216] |
9.1 [216] 9.3 [157] |
0.25 [216] 0.434 [157] 1.2 [215] |
||||
| Agouti | 9.3 [222] | 8.2 [222] | 9.9 [222] | 8.9 [222] | ||||||||||
| AgoutiYY | 8.4 [197] | 8.6 [197] | 9.3 [197] | |||||||||||
| AGRP (87–132) | >100,000 [204] | 8.7 [220] 8.9 [204] |
8.7 [220] 9.4 [204] |
>100,000 [204] | 8.4 [199] | 8.20 [202] 8.6 [199] 8.76 [209] |
||||||||
Values labelled with a (r) represent data from cloned rat receptors. Unlabelled numbers represent mouse receptors.
Potency EC50 β-MSH values were split depending on the sequence used. If a sequence was not provided in the paper, values are listed in the β-MSH row.
Values labelled with a (L) were reported using a low receptor expression.
Values shown were converted from pEC50 values reported in the source publication.
8. Structure-activity relationships of endogenous ligands
While melanocortin peptides are initially generated through cleavage by prohormone convertases, the final processing amidation and acetylation steps are crucial for their conversion into biologically active moieties. The amidation process of peptides/hormones is not limited to the processing of POMC so, as with proconvertase enzymes, this cannot be regarded as being specific to POMC, and any associations with a biological phenomenon are pleiotropic. However, acetylation is relatively more specific to POMC processing. Acetylation is a common modification of intracellular soluble proteins but is not common for secreted peptides. Thus, acetylation of α-MSH represents a special case in which the peptide's activity can be modified [120,121]. The N-terminus of α-MSH is acetylated by adding N- or N–O-acetyl groups, with the non-acetylated form termed des-acetyl-α-MSH (des-α-MSH), and acetylated forms, being mature forms of the peptide, are termed α-MSH. This may be either mono-acetylated to form N-acetyl-α-MSH or di-acetylated (N,O-diacetyl-α-MSH) (Figure 2). Acetylation of α-MSH is hypothesised to enhance activity through increased biological stability. When the role of α-MSH as a brain peptide was recognised 40 years ago, it was reported that it was more effective than its deacetylated form [122]. It was suggested that the difference in potency could be because α-MSH is more resistant to degradation than the deacetylated form.
Endogenous peptides share the tetrapeptide sequence His-Phe-Arg-Trp (Figure 2, highlighted in blue). When the N-terminal of this tetrapeptide is acetylated and the C-terminal is carboxyamidated, we obtain a minimal sequence with binding and functional activity using classic frog and lizard skin bioassays [123,124]. The full length of ACTH is 39 amino acids long, although ACTH(1–24) is hypothesised to be the functional domain [125]. ACTH is the only known endogenous peptide for MC2R, making it the only ligand that can stimulate all five MCRs [119]. The first 13 residues of the N-terminal domain of ACTH are cleaved and modified through N-terminal acetylation and C-terminal carboxyamidation to produce α-MSH [122,126]. In MC1R, MC3R, MC4R, and MC5R, α-MSH potencies range from nanomolar to sub-nanomolar (Table 2). Unlike α-MSH, the β-MSH terminals are unmodified. The binding affinities of β-MSH follow the general trend of MC1R > MC3R ≥ MC4R > MC5R, with nanomolar to sub-nanomolar binding affinity at MC1R (Table 1). Three different forms of γ-MSH have been described: γ3-MSH (N-glycosylated 23 amino acid N-terminal), γ2-MSH (first 12 amino acids of γ3-MSH), and γ1-MSH (first 11 amino acids of γ3-MSH with a carboxyamidated C-terminal) [127,128]. Although γ2-MSH is selective for hMC3R over hMC4R and hMC5R [129,130], γ2-MSH is not selective for mMC3R over mMC5R [131] (Table 2).
Distinctive to the melanocortin receptors compared to other G protein-coupled receptors is the presence of two endogenous antagonist peptides, agouti and AgRP. Agouti and AgRP contain a tripeptide Arg-Phe-Phe sequence postulated to be important for binding and functional activity located in their C-terminal domains [20,132,133]. Agouti and AgRP possess five C-terminal disulphide bonds, creating a similar cysteine knot conformation [20,133,134]. While in humans both are synthesised as 132 amino acid precursors, the active form of agouti is agouti [[23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50]], [[51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69], [70]], [[71], [72], [73], [74], [75], [76], [77], [78], [79], [80], [81], [82], [83], [84], [85], [86], [87], [88], [89], [90], [91], [92], [93], [94], [95], [96], [97], [98], [99], [100]], [[101], [102], [103], [104], [105], [106], [107], [108], [109], [110], [111], [112], [113], [114], [115], [116], [117], [118], [119], [120], [121], [122], [123], [124], [125], [126], [127], [128], [129], [130], [131], [132]] and AgRP is the shorter AgRP (87-132) [[135], [136], [137]]. AgRP is centrally expressed in the arcuate nucleus (partially overlapping with the location of POMC neurons) and active at MC3R and MC4R with nanomolar potencies, with a more variable response at the MC5R depending on the sample's purity, experimental conditions, and concentrations assayed (Table 1) [16,20,138,139]. While agouti was first described as an antagonist at MC1R, it also interacts with MC3R and MC4R with nanomolar potencies and with micromolar affinity at MC5R (Table 1) [132,140,[141], [142], [143], [144]]. AgRP also acts as an inverse agonist at MC4R [132,[140], [141], [142], [143], [144]]. MC4R displays constitutive receptor activity, meaning that in the absence of an endogenous agonist, it signals and affects, for instance, the gating of ion channels [145], which is inhibited by AgRP. In POMC-null mice, administration of AgRP has long-lasting effects on energy balance and increases food intake, confirming its role in the absence of an agonist [146]. Besides being an inverse agonist, AgRP also acts as a biased agonist that activates the MAPK signalling pathway [147].
9. Synthetic ligands
Synthetic ligands for MCRs have been discovered from structure–activity relationship studies of endogenous melanocortin ligands. The conversion of Met(4) into norleucine or Nle(4) and Phe(7) to DPhe(7) in α-MSH results in the peptide [Nle4,DPhe7]α-MSH (Figure 2), also known as NDP-MSH, melanotan I (MTI), and afamelanotide (approved to treat erythropoietic protoporphyria) [148]. First reported in 1980 [149], NDP-MSH has nanomolar to sub-nanomolar potencies in MC1R, MC3R, MC4R, and MC5R (Table 2). The tetrapeptide Ac-His-DPhe-Arg-Trp-NH2 is the minimum NDP-MSH sequence that maintains nanomolar agonist potency in mouse MC1R, MC3R, MC4R, and MC5R [150] (Table 2). Melanotan II (MTII) maintains Nle and DPhe substitutions of NDP-MSH, but cycles through a lactam bridge between substituted Glu(5) to Asp(5) and Gly(10) to Lys(10) of NDP-MSH and truncations of residues 1–3 and 11–13 (NDP-MSH numbering; Figure 2) [151,152]. MTII is a non-selective melanocortin agonist with nanomolar to sub-nanomolar potencies [151,152] (Table 2). Bremelanotide (PT-141) is a cyclic lactam that differs from MTII by one functional group (a C-terminal carboxylic acid vs carboxamide) reported to possess similar agonist potency compared to MTII (Table 2) and is approved for treating hypoactive sexual desire disorder [153,154]. While it is well known that MC4R agonists acting centrally can trigger sexual arousal behaviour in males and females, the details of pathway mechanisms mediating this response still remain largely unknown [155,156]. Within the MTII scaffold, substituting DPhe(7) with D-2′-naphthylalanine or DNal(2′)(7) results in SHU9119 [157] (Figure 2). SHU9119 is a nanomolar to sub-nanomolar agonist in MC1R and MC5R with antagonist activity in MC3R and MC4R [157] (Table 2). SHU9119 also possesses partial agonist efficacy in MC3R. In 2020, the 2.8 Å crystal structure of hMC4R bound to the SHU9119 cyclic peptide antagonist was reported [158], revealing the importance of the Ca+2 ion for melanocortin receptor function as initially observed in the 1980s [159].
10. Targeting the melanocortin system as an obesity therapy
For over 20 years, oral small molecules and injectable peptide agonists of MC4R have been clinically evaluated for treating obesity (oral small molecule MK-0493 (Merck) and peptide agonists LY2112688 (Eli Lilly), MC4-NN-0453 (Novo Nordisk), AZD2820 (AstraZeneca), and setmelanotide (Rhythm Pharmaceuticals)). Most of these compounds produced to develop therapeutics for treating obesity failed in the clinic despite preclinical efficacy. Table 3 provides a list of these compounds along with the dose ranges explored in clinical studies and the observed side effects. Failure of most of these MC4R agonist drug candidates has been variably ascribed to the: i) lack of human efficacy, ii) significant cardiovascular adverse side effects, iii) occurrences of nausea and vomiting, and iv) stimulation of sexual arousal.
Table 3.
MC4R agonists studied in human clinical studies for various indications.
| MC4R agonist, sponsor | Molecule type | Indication | Route of administration | Clinical efficacy | Dose administered | Side effects | Current status | Reference |
|---|---|---|---|---|---|---|---|---|
| Melanotan II, University of Arizona |
7-amino acid cyclic peptide | Male erectile dysfunction | Subcutaneous | Human efficacy demonstrated | 0.25 mg/kg, bolus | Increased cardiovascular (CV) activity, nausea, and vomiting | Development discontinued | [223] |
| Bremelanotide (PT141), Palatin Inc |
7-amino acid cyclic peptide | Male erectile dysfunction (MED) and pre-menopausal hypoactivity sexual desire disorder (HSDD) | Subcutaneous | Efficacious in both indications | HSDD: 175 mg, bolus | MED: Increased CV activity, nausea, and vomiting HSAD: Therapeutic index established against cardiovascular side effects |
Marketed for HSDD (trade name Vyleesi) | [224,231] |
| PF-00446687, Pfizer Inc |
Small molecule | MED and post-menopausal HSDD | Oral | Efficacious in both indications | MED: 200 mg HSDD: 200 mg |
Not disclosed | Unknown | [225] |
| MK-0493, Merck |
Small molecule | Obesity | Oral | No efficacy observed | 200–1000 mg | None; well tolerated | Development discontinued | [226] |
| LY2112688, Eli Lilly |
8-amino acid cyclic peptide | Obesity | Subcutaneous infusion | No efficacy observed | 0.5, 0.15, 0.45, and 1 mg infused over 24 h 0.15, 0.45, 1, and 2 mg/day for 7 days |
Increased CV and erectile activity | Development discontinued | [227] |
| MC4-NN-0453, Novo Nordisk |
12 amino acid peptide with a modified lipid chain | Obesity | Subcutaneous | Lack of weight loss | Single dose study: 0.3–15 mg/kg Multi-dose study: 0.75–30 mg/kg |
Skin tanning and sexual arousal disturbances | Development discontinued | [228] |
| AZD2820a, AstraZeneca |
Undisclosed | Obesity | Subcutaneous | Undisclosed | Undisclosed | Serious adverse events related to allergic reactions | Development discontinued | [232] |
| Setmelanotide, Rhythm Pharmaceuticals |
8-amino acid cyclic peptide | Genetic obesity | Subcutaneous | Efficacy established in completed phase II and phase III studies | 1–3 mg bolus per day | No increase in CV activity. Selected common side effects can include hyperpigmentation, nausea/vomiting, penile erection, and injection site reactions | Currently under evaluation for treating obesity-associated MC4R pathway deficiencies | [5,229,230,233,234] |
Partial agonist in MC4R.
The exception to this to date has been a cyclic octapeptide setmelanotide, which received FDA approval in 2020 for treating obesity caused by genetic defects in POMC, LEPR, or PCSK1. Setmelanotide did not elicit undesirable cardiovascular effects [5,104,160,161,162]. Minor adverse effects that were well tolerated are listed in Table 3
Interestingly, an older study demonstrated that in obese controls not known to have these mutations, setmelanotide showed initial evidence of moderate weight loss over a 28-day treatment period (0.99 kg/wk; P =< 0.0001). In this study, weight loss in subjects with heterozygous MC4R deficiency leading to only one remaining wild-type MC4R allele as the target of setmelanotide tended to have significant weight loss (0.6 kg/wk; P = 0.09) [104]. The efficacy of setmelanotide was more pronounced in subjects with biallelic POMC and LEPR deficiency obesity, with 80% of POMC-deficient subjects and 45% of LEPR-deficient subjects losing >10% of their weight in approximately one year. This weight loss was also accompanied by a significant decrease in hunger scores (−27.1%, P = 0.0005 in POMC deficiency and −43.7%, P =< 0.0001 in LERP deficiency) [5]. Based on these evaluations, it has become clear that potency at MC4R associated with clinical anti-obesity benefits can be attained by peptide ligands as exemplified by setmelanotide and that nano-molar plasma concentrations suffice to elicit clinical benefits (setmelanotide EC50 at MC4R is ∼0.3 nM). One of the possible reasons setmelanotide may differ from other clinically tested MC4R agonists is that its activated MC4R signal transduction pathway differs from α-MSH. GoF MC4R variants that were associated with significantly lower BMI, for instance, exhibit a signalling bias toward β-arrestin recruitment and increased mitogen-activated protein kinase pathway activation [108]. Setmelanotide also potently induces PLC-β activation through MC4R, likely via Gαq activation. In the PLC assay (through the NFAT reporter gene) that captures this pathway's activity, 100 nM of AgRP could not antagonise setmelanotide-stimulated PLC activation while stimulation by α-MSH was antagonised. MC4R displays biased agonism, whereby setmelanotide also potently activates the PLC pathway. Determining NFAT signalling after MC4R stimulation revealed an approximately 100-fold higher efficacy of setmelanotide compared with the endogenous agonist. These insights may help advance the ability to target the MC4R pathway and continue to develop next-generation MC4R agonists for treating obesity.
Regarding treating more common types of obesity, Wilding et al. recently described the therapeutic benefits of a once-weekly injection of the GLP-1R agonist semaglutide to treat general obesity [163]. Interestingly, in rodent experiments, the GLP-1R agonist liraglutide and setmelanotide were shown to have additive effects on glycaemic control, weight loss, and cholesterol metabolism, indicating their independent metabolic effects [164]. Clinical studies also demonstrated the potential to activate the MC4R pathway for weight loss in the general obese population, although more clinical research is needed [104].
11. Concluding remarks
The discovery of MC4R drugs to treat obesity exemplifies how today's molecular neuroscience and circuit neurophysiology contributes to the development of circuit-targeted pharmaceuticals. The MC4R agonist setmelanotide received FDA approval in 2020 for treating subjects with specific genetic defects upstream of MC4R, resulting in obesity. Unravelling the genetics of a disorder, dissecting the precise role of genes in a physiological process, and studying the molecular pharmacology of drug targets brought a new drug to the market for a complex disorder. This drug discovery process is one of the first examples of a new era in finding drugs for complex disorders such as brain diseases, which in the case of setmelanotide took 25 years to go from identifying critical genes associated with clinical symptoms to an approved drug.
Funding
G.S.H.Y. is supported by the UK Medical Research Council (MRC Metabolic Diseases Unit (MC_UU_00014/1) and MRC project grant (MR/S026193/1). A.M.S. is supported by a UK MRC project grant (MR/S026193/1). S.L is supported by the Centre National la Recherche Scientifique (CNRS), the Université de Paris, the Modern Diet and Physiology Research Center (MDPRC), the National Research Agency National Research Agency (ANR-15-CE14-0030-01: Nutritpathos) and the Foundation pour la Recherche Médicale (FRM). D.H.M.C receives support the National Research Agency National Research Agency (ANR-15-CE14-0030-01: Nutritpathos). K.C. is supported by the Fondation pour la Recherche médicale (FRM). C.H.L, Z.M.K, M.D.E and C.M.L are funded by the NIH NIDDK (1R01DK108893). S.E.S and M.A.C. are funded by an Australian NHMRC grant (1163341). R.A.H.A. is supported by the European Union Seventh Framework Programme (grant number 607310; Nudge-it), the Netherlands Organisation for Scientific Research (grant numbers ALWOP.137 and OCENW.KLEIN.071), the Swedish Research Council (2018–02588), and ERA-NET NEURON (MiGBAN).
Author contributions
G.S.H.Y. and R.A.H.A. conceived and assembled this consortium review. All of the other authors drafted their respective sections and commented on the final manuscript.
Contributor Information
Giles S.H. Yeo, Email: gshy2@cam.ac.uk.
Daniela Herrera Moro Chao, Email: klctnn@gmail.com.
Anna-Maria Siegert, Email: ams299@medschl.cam.ac.uk.
Zoe M. Koerperich, Email: koerp005@umn.edu.
Mark D. Ericson, Email: erics063@umn.edu.
Stephanie E. Simonds, Email: Stephanie.Simonds@monash.edu.
Courtney M. Larson, Email: lars5005@umn.edu.
Serge Luquet, Email: serge.luquet@u-paris.fr.
Iain Clarke, Email: iain.clarke@unimelb.edu.au.
Shubh Sharma, Email: ssharma@rhythmtx.com.
Karine Clément, Email: karine.clement@aphp.fr.
Michael A. Cowley, Email: michael.cowley@monash.edu.
Carrie Haskell-Luevano, Email: chaskell@umn.edu.
Lex Van Der Ploeg, Email: lex@riffitnow.com.
Roger A.H. Adan, Email: R.A.H.Adan@umcutrecht.nl.
Conflict of interest
Shubh Sharma is an employee and shareholder of Rhythm Pharmaceuticals. Lex Van der Ploeg served as CSO of Rhythm Pharmaceuticals from 2011 to 2019 and since 2019 has been an advisor to Rhythm Pharmaceuticals and shareholder. Rhythm Pharmaceuticals is a NASDAQ publicly traded company (RYTM). KC is a primary investigator for several setmelanotide trials funded by Rhythm Pharmaceuticals in France, but this does not alter this review's content. All of the other authors have no conflicts of interest to declare.
References
- 1.Stunkard A.J., Foch T.T., Hrubec Z. A twin study of human obesity. Journal of the American Medical Association. 1986;256(1):51–54. [PubMed] [Google Scholar]
- 2.Stunkard A.J., Harris J.R., Pedersen N.L., McClearn G.E. The body-mass index of twins who have been reared apart. New England Journal of Medicine. 1990;322(21):1483–1487. doi: 10.1056/NEJM199005243222102. [DOI] [PubMed] [Google Scholar]
- 3.Bertagna X. Proopiomelanocortin-derived peptides. Endocrinology and Metabolism Clinics of North America. 1994;23(3):467–485. [PubMed] [Google Scholar]
- 4.Cone R.D. Studies on the physiological functions of the melanocortin system. Endocrine Reviews. 2006;27(7):736–749. doi: 10.1210/er.2006-0034. [DOI] [PubMed] [Google Scholar]
- 5.Clément K., van den Akker E., Argente J., Bahm A., Chung W.K., Connors H. Efficacy and safety of setmelanotide, an MC4R agonist, in individuals with severe obesity due to LEPR or POMC deficiency: single-arm, open-label, multicentre, phase 3 trials. The Lancet Diabetes & Endocrinology. 2020;8(12):960–970. doi: 10.1016/S2213-8587(20)30364-8. [DOI] [PubMed] [Google Scholar]
- 6.Ferrari W. Behavioural changes in animals after intracisternal injection with adrenocorticotrophic hormone and melanocyte-stimulating hormone. Nature. 1958;181(4613):925–926. doi: 10.1038/181925a0. [DOI] [PubMed] [Google Scholar]
- 7.Gee C.E., Chen C.L., Roberts J.L., Thompson R., Watson S.J. Identification of proopiomelanocortin neurones in rat hypothalamus by in situ cDNA-mRNA hybridization. Nature. 1983;306(5941):374–376. doi: 10.1038/306374a0. [DOI] [PubMed] [Google Scholar]
- 8.Tatro J.B. Melanotropin receptors in the brain are differentially distributed and recognize both corticotropin and alpha-melanocyte stimulating hormone. Brain Research. 1990;536(1–2):124–132. doi: 10.1016/0006-8993(90)90016-5. [DOI] [PubMed] [Google Scholar]
- 9.Dewied D. Influence of anterior pituitary on avoidance learning and escape behavior. American Journal of Physiology. 1964;207:255–259. doi: 10.1152/ajplegacy.1964.207.1.255. [DOI] [PubMed] [Google Scholar]
- 10.Vergoni A.V., Poggioli R., Bertolini A. Corticotropin inhibits food intake in rats. Neuropeptides. 1986;7(2):153–158. doi: 10.1016/0143-4179(86)90091-0. [DOI] [PubMed] [Google Scholar]
- 11.Mountjoy K.G., Robbins L.S., Mortrud M.T., Cone R.D. The cloning of a family of genes that encode the melanocortin receptors. Science. 1992;257(5074):1248–1251. doi: 10.1126/science.1325670. [DOI] [PubMed] [Google Scholar]
- 12.Gantz I., Konda Y., Tashiro T., Shimoto Y., Miwa H., Munzert G. Molecular cloning of a novel melanocortin receptor. Journal of Biological Chemistry. 1993;268(11):8246–8250. [PubMed] [Google Scholar]
- 13.Roselli-Rehfuss L., Mountjoy K.G., Robbins L.S., Mortrud M.T., Low M.J., Tatro J.B. Identification of a receptor for gamma melanotropin and other proopiomelanocortin peptides in the hypothalamus and limbic system. Proceedings of the National Academy of Sciences of the United States of America. 1993;90(19):8856–8860. doi: 10.1073/pnas.90.19.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gantz I., Shimoto Y., Konda Y., Miwa H., Dickinson C.J., Yamada T. Molecular cloning, expression, and characterization of a fifth melanocortin receptor. Biochemical and Biophysical Research Communications. 1994;200(3):1214–1220. doi: 10.1006/bbrc.1994.1580. [DOI] [PubMed] [Google Scholar]
- 15.Robbins L.S., Nadeau J.H., Johnson K.R., Kelly M.A., Roselli-Rehfuss L., Baack E. Pigmentation phenotypes of variant extension locus alleles result from point mutations that alter MSH receptor function. Cell. 1993;72(6):827–834. doi: 10.1016/0092-8674(93)90572-8. [DOI] [PubMed] [Google Scholar]
- 16.Lu D., Willard D., Patel I.R., Kadwell S., Overton L., Kost T. Agouti protein is an antagonist of the melanocyte-stimulating-hormone receptor. Nature. 1994;371(6500):799–802. doi: 10.1038/371799a0. [DOI] [PubMed] [Google Scholar]
- 17.Bultman S.J., Michaud E.J., Woychik R.P. Molecular characterization of the mouse agouti locus. Cell. 1992;71(7):1195–1204. doi: 10.1016/s0092-8674(05)80067-4. [DOI] [PubMed] [Google Scholar]
- 18.Miller M.W., Duhl D.M., Vrieling H., Cordes S.P., Ollmann M.M., Winkes B.M. Cloning of the mouse agouti gene predicts a secreted protein ubiquitously expressed in mice carrying the lethal yellow mutation. Genes & Development. 1993;7(3):454–467. doi: 10.1101/gad.7.3.454. [DOI] [PubMed] [Google Scholar]
- 19.Fan W., Boston B.A., Kesterson R.A., Hruby V.J., Cone R.D. Role of melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature. 1997;385(6612):165–168. doi: 10.1038/385165a0. [DOI] [PubMed] [Google Scholar]
- 20.Ollmann M.M., Wilson B.D., Yang Y.K., Kerns J.A., Chen Y., Gantz I. Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science. 1997;278(5335):135–138. doi: 10.1126/science.278.5335.135. [DOI] [PubMed] [Google Scholar]
- 21.Cowley M.A., Pronchuk N., Fan W., Dinulescu D.M., Colmers W.F., Cone R.D. Integration of NPY, AGRP, and melanocortin signals in the hypothalamic paraventricular nucleus: evidence of a cellular basis for the adipostat. Neuron. 1999;24(1):155–163. doi: 10.1016/s0896-6273(00)80829-6. [DOI] [PubMed] [Google Scholar]
- 22.Cone R.D. Anatomy and regulation of the central melanocortin system. Nature Neuroscience. 2005;8(5):571–578. doi: 10.1038/nn1455. [DOI] [PubMed] [Google Scholar]
- 23.Fan W., Ellacott K.L.J., Halatchev I.G., Takahashi K., Yu P., Cone R.D. Cholecystokinin-mediated suppression of feeding involves the brainstem melanocortin system. Nature Neuroscience. 2004;7(4):335–336. doi: 10.1038/nn1214. [DOI] [PubMed] [Google Scholar]
- 24.Ellacott K.L., Halatchev I.G., Cone R.D. Characterization of leptin-responsive neurons in the caudal brainstem. Endocrinology. 2006;147(7):3190–3195. doi: 10.1210/en.2005-0877. [DOI] [PubMed] [Google Scholar]
- 25.Lee Y.S., Challis B.G., Thompson D.A., Yeo G.S.H., Keogh J.M., Madonna M.E. A POMC variant implicates beta-melanocyte-stimulating hormone in the control of human energy balance. Cell Metabolism. 2006;3(2):135–140. doi: 10.1016/j.cmet.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 26.Raffan E., Dennis R.J., O'Donovan C.J., Becker J.M., Scott R.A., Smith S.P. A deletion in the canine POMC gene is associated with weight and appetite in obesity-prone labrador retriever dogs. Cell Metabolism. 2016;23(5):893–900. doi: 10.1016/j.cmet.2016.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berglund E.D., Vianna C.R., Donato J., Jr., Kim M.H., Chuang J., Lee C.E. Direct leptin action on POMC neurons regulates glucose homeostasis and hepatic insulin sensitivity in mice. Journal of Clinical Investigation. 2012;122(3):1000–1009. doi: 10.1172/JCI59816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dunbar J.C., Lu H. Proopiomelanocortin (POMC) products in the central regulation of sympathetic and cardiovascular dynamics: studies on melanocortin and opioid interactions. Peptides. 2000;21(2):211–217. doi: 10.1016/s0196-9781(99)00192-8. [DOI] [PubMed] [Google Scholar]
- 29.Faulkner L.D., Dowling A.R., Stuart R.C., Nillni E.A., Hill J.W. Reduced melanocortin production causes sexual dysfunction in male mice with POMC neuronal insulin and leptin insensitivity. Endocrinology. 2015;156(4):1372–1385. doi: 10.1210/en.2014-1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parton L.E., Dowling A.R., Stuart R.C., Nillni E.A., Hill J.W. Glucose sensing by POMC neurons regulates glucose homeostasis and is impaired in obesity. Nature. 2007;449(7159):228–232. doi: 10.1038/nature06098. [DOI] [PubMed] [Google Scholar]
- 31.Secher A., Jelsing J., Baquero A.F., Hecksher-Sørensen J., Cowley M.A., Dalbøge L.S. The arcuate nucleus mediates GLP-1 receptor agonist liraglutide-dependent weight loss. Journal of Clinical Investigation. 2014;124(10):4473–4488. doi: 10.1172/JCI75276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cowley M.A., Smith Ro.G., Diano S., Tschöp M., Pronchuk N., Grove K.L. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron. 2003;37(4):649–661. doi: 10.1016/s0896-6273(03)00063-1. [DOI] [PubMed] [Google Scholar]
- 33.Batterham R.L., Cowley M.A., Small C.J., Herzog H., Cohen M.A., Dakin C.L. Gut hormone PYY(3-36) physiologically inhibits food intake. Nature. 2002;418(6898):650–654. doi: 10.1038/nature00887. [DOI] [PubMed] [Google Scholar]
- 34.Ghamari-Langroudi M., Colmers W.F., Cone R.D. PYY3-36 inhibits the action potential firing activity of POMC neurons of arcuate nucleus through postsynaptic Y2 receptors. Cell Metabolism. 2005;2(3):191–199. doi: 10.1016/j.cmet.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 35.Marks D.L., Ling N., Cone R.D. Role of the central melanocortin system in cachexia. Cancer Research. 2001;61(4):1432–1438. [PubMed] [Google Scholar]
- 36.Scarlett J.M., Bowe D.D., Zhu X., Batra A.K., Grant W.F., Marks D.L. Genetic and pharmacologic blockade of central melanocortin signaling attenuates cardiac cachexia in rodent models of heart failure. Journal of Endocrinology. 2010;206(1):121–130. doi: 10.1677/JOE-09-0397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dodd G.T., Michael N.J., Lee-Young R.S., Mangiafico S.P., Pryor J.T., Munder A.C. Insulin regulates POMC neuronal plasticity to control glucose metabolism. Elife. 2018;7 doi: 10.7554/eLife.38704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hill J.W., Elias C.F., Fukuda M., Williams K.W., Berglund E.D., Holland W.L. Direct insulin and leptin action on pro-opiomelanocortin neurons is required for normal glucose homeostasis and fertility. Cell Metabolism. 2010;11(4):286–297. doi: 10.1016/j.cmet.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Plum L., Schubert M., Bruning J.C. The role of insulin receptor signaling in the brain. Trends in Endocrinology and Metabolism. 2005;16(2):59–65. doi: 10.1016/j.tem.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 40.Wu Q., Lemus M.B., Stark R., Bayliss J.A., Reichenbach A., Lockie S.H. The temporal pattern of cfos activation in hypothalamic, cortical, and brainstem nuclei in response to fasting and refeeding in male mice. Endocrinology. 2014;155(3):840–853. doi: 10.1210/en.2013-1831. [DOI] [PubMed] [Google Scholar]
- 41.Chen A.S., Metzger J.M., Trumbauer M.E., Guan X.M., Yu H., Frazier E.G. Role of the melanocortin-4 receptor in metabolic rate and food intake in mice. Transgenic Research. 2000;9(2):145–154. doi: 10.1023/a:1008983615045. [DOI] [PubMed] [Google Scholar]
- 42.Chen A.S., Marsh D.J., Trumbauer M.E., Frazier E.G., Guan X.M., Yu H. Inactivation of the mouse melanocortin-3 receptor results in increased fat mass and reduced lean body mass. Nature Genetics. 2000;26(1):97–102. doi: 10.1038/79254. [DOI] [PubMed] [Google Scholar]
- 43.Balthasar N., Dalgaard L.T., Lee C.E., Yu J., Funahashi H., Williams T. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell. 2005;123(3):493–505. doi: 10.1016/j.cell.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 44.Garfield A.S., Li C., Madara J.C., Shah B.P., Webber E., Steger J.S. A neural basis for melanocortin-4 receptor-regulated appetite. Nature Neuroscience. 2015;18(6):863–871. doi: 10.1038/nn.4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shah B.P., Vong L., Olson D.P., Koda S., Krashes M.J., Ye C. MC4R-expressing glutamatergic neurons in the paraventricular hypothalamus regulate feeding and are synaptically connected to the parabrachial nucleus. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(36):13193–13198. doi: 10.1073/pnas.1407843111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim M.S., Rossi M., Abusnana S., Sunter D., Morgan D.G., J Small C. Hypothalamic localization of the feeding effect of agouti-related peptide and alpha-melanocyte-stimulating hormone. Diabetes. 2000;49(2):177–182. doi: 10.2337/diabetes.49.2.177. [DOI] [PubMed] [Google Scholar]
- 47.de Backer M.W., la Fleur S.E., Brans M.A.D., van Rozen A.J., Luijendijk M.C.M., Merkestein M. Melanocortin receptor-mediated effects on obesity are distributed over specific hypothalamic regions. International Journal of Obesity. 2011;35(5):629–641. doi: 10.1038/ijo.2010.169. [DOI] [PubMed] [Google Scholar]
- 48.Fenselau H., Campbell J.N., Verstegen A.M.J., Madara J.C., Xu J., Shah B.P. A rapidly acting glutamatergic ARC->PVH satiety circuit postsynaptically regulated by alpha-MSH. Nature Neuroscience. 2017;20(1):42–51. doi: 10.1038/nn.4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kwon E., Jo Y.H. Activation of the ARC(POMC)->MeA projection reduces food intake. Frontiers in Neural Circuits. 2020;14:595783. doi: 10.3389/fncir.2020.595783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rossi J., Balthasar N., Olson D., Scott M., Berglund E., Lee C.E. Melanocortin-4 receptors expressed by cholinergic neurons regulate energy balance and glucose homeostasis. Cell Metabolism. 2011;13(2):195–204. doi: 10.1016/j.cmet.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pei H., Patterson C.M., Sutton A.K., Burnett K.H., Myers M.G., Jr., Olson D.P. Lateral hypothalamic Mc3R-expressing neurons modulate locomotor activity, energy expenditure, and adiposity in male mice. Endocrinology. 2019;160(2):343–358. doi: 10.1210/en.2018-00747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bewick G.A., Gardiner J.V., Dhillo W.S., Kent A.S., White N.E., Webster Z. Post-embryonic ablation of AgRP neurons in mice leads to a lean, hypophagic phenotype. The FASEB Journal. 2005;19(12):1680–1682. doi: 10.1096/fj.04-3434fje. [DOI] [PubMed] [Google Scholar]
- 53.Gropp E., Shanabrough M., Borok E., Xu A.W., Janoschek R., Buch T. Agouti-related peptide-expressing neurons are mandatory for feeding. Nature Neuroscience. 2005;8(10):1289–1291. doi: 10.1038/nn1548. [DOI] [PubMed] [Google Scholar]
- 54.Luquet S., Perez F.A., Hnasko T.S., Palmiter R.D. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science. 2005;310(5748):683–685. doi: 10.1126/science.1115524. [DOI] [PubMed] [Google Scholar]
- 55.Aponte Y., Atasoy D., Sternson S.M. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nature Neuroscience. 2011;14(3):351–355. doi: 10.1038/nn.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Krashes M.J., Koda S., Ye C.P., Rogan S.C., Adams A.C., Cusher D.S. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. Journal of Clinical Investigation. 2011;121(4):1424–1428. doi: 10.1172/JCI46229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Horvath T.L., Bechmann I., Naftolin F., Kalra S.P., Leranth C. Heterogeneity in the neuropeptide Y-containing neurons of the rat arcuate nucleus: GABAergic and non-GABAergic subpopulations. Brain Research. 1997;756(1–2):283–286. doi: 10.1016/s0006-8993(97)00184-4. [DOI] [PubMed] [Google Scholar]
- 58.Wu Q., Boyle M.P., Palmiter R.D. Loss of GABAergic signaling by AgRP neurons to the parabrachial nucleus leads to starvation. Cell. 2009;137(7):1225–1234. doi: 10.1016/j.cell.2009.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu Q., Howell M.P., Cowley M.A., Palmiter R.D. Starvation after AgRP neuron ablation is independent of melanocortin signaling. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(7):2687–2692. doi: 10.1073/pnas.0712062105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Betley J.N., Cao Z.F.H., Ritola K.D., Sternson S.M. Parallel, redundant circuit organization for homeostatic control of feeding behavior. Cell. 2013;155(6):1337–1350. doi: 10.1016/j.cell.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Krashes M.J., Shah B.P., Koda S., Lowell B.B. Rapid versus delayed stimulation of feeding by the endogenously released AgRP neuron mediators GABA, NPY, and AgRP. Cell Metabolism. 2013;18(4):588–595. doi: 10.1016/j.cmet.2013.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Broberger C., Johansen J., Johansson C., Schalling M., Hökfelt T. The neuropeptide Y/agouti gene-related protein (AGRP) brain circuitry in normal, anorectic, and monosodium glutamate-treated mice. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(25):15043–15048. doi: 10.1073/pnas.95.25.15043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Atasoy D., Betley J.N., Su H.H., Sternson S.M. Deconstruction of a neural circuit for hunger. Nature. 2012;488(7410):172–177. doi: 10.1038/nature11270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wu Q., Clark M.S., Palmiter R.D. Deciphering a neuronal circuit that mediates appetite. Nature. 2012;483(7391):594–597. doi: 10.1038/nature10899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Carter M.E., Soden M.E., Zweifel L.S., Palmiter R.D. Genetic identification of a neural circuit that suppresses appetite. Nature. 2013;503(7474):111–114. doi: 10.1038/nature12596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Campos C.A., Bowen A.J., Schwartz M.W., Palmiter R.D. Parabrachial CGRP neurons control meal termination. Cell Metabolism. 2016;23(5):811–820. doi: 10.1016/j.cmet.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Carter M.E., Han S., Palmiter R.D. Parabrachial calcitonin gene-related peptide neurons mediate conditioned taste aversion. Journal of Neuroscience. 2015;35(11):4582–4586. doi: 10.1523/JNEUROSCI.3729-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Essner R.A., Smith A.G., Jamnik A.A., Ryba A.R., Trutner Z.D., Carter M.E. AgRP neurons can increase food intake during conditions of appetite suppression and inhibit anorexigenic parabrachial neurons. Journal of Neuroscience. 2017;37(36):8678–8687. doi: 10.1523/JNEUROSCI.0798-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Henry F.E., Sugino K., Tozer A., Branco T., Sternson S.M. Cell type-specific transcriptomics of hypothalamic energy-sensing neuron responses to weight-loss. Elife. 2015;4 doi: 10.7554/eLife.09800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Betley J.N., Xu S.J., Cao Z.F.H., Gong R., Magnus C.J., Yu Y. Neurons for hunger and thirst transmit a negative-valence teaching signal. Nature. 2015;521(7551):180–185. doi: 10.1038/nature14416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen Y., Lin Y.C., Kuo T.W., Knight Z.A. Sensory detection of food rapidly modulates arcuate feeding circuits. Cell. 2015;160(5):829–841. doi: 10.1016/j.cell.2015.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mandelblat-Cerf Y., Ramesh R.N., Burgess C.R., Patella P., Yang Z.F., Lowell B.B. Arcuate hypothalamic AgRP and putative POMC neurons show opposite changes in spiking across multiple timescales. Elife. 2015;4 doi: 10.7554/eLife.07122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bai L., Mesgarzadeh S., Ramesh K.S., Huey E.L., Liu Y., Gray L.A. Genetic identification of vagal sensory neurons that control feeding. Cell. 2019;179(5):1129–1143. doi: 10.1016/j.cell.2019.10.031. e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Beutler L.R., Chen Y.M., Ahn J.S., Lin Y.C., Essner R.A., Knight Z.A. Dynamics of gut-brain communication underlying hunger. Neuron. 2017;96(2):461–475. doi: 10.1016/j.neuron.2017.09.043. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Goldstein N., McKnight A.D., Carty J.R.E., Arnold M., Betley J.N., Alhadeff A.L. Hypothalamic detection of macronutrients via multiple gut-brain pathways. Cell Metabolism. 2021 doi: 10.1016/j.cmet.2020.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Su Z., Alhadeff A.L., Betley J.N. Nutritive, post-ingestive signals are the primary regulators of AgRP neuron activity. Cell Reports. 2017;21(10):2724–2736. doi: 10.1016/j.celrep.2017.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang Y., Proenca R., Maffei M., Barone M., Leopold L., Friedman J.M. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372(6505):425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 78.Montague C.T., Farooqi I.S., Whitehead J.P., Soos M.A., Rau H., Wareham N.J. Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature. 1997;387(6636):903–908. doi: 10.1038/43185. [DOI] [PubMed] [Google Scholar]
- 79.Clement K., Vaisse C., Lahlou N., Cabrol S., Pelloux V., Cassuto D. A mutation in the human leptin receptor gene causes obesity and pituitary dysfunction. Nature. 1998;392(6674):398–401. doi: 10.1038/32911. [DOI] [PubMed] [Google Scholar]
- 80.Friedman J.M., Halaas J.L. Leptin and the regulation of body weight in mammals. Nature. 1998;395(6704):763–770. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- 81.Ahima R.S., Prabakaran D., Mantzoros C., Qu D., Lowell B., Maratos-Flier E. Role of leptin in the neuroendocrine response to fasting. Nature. 1996;382(6588):250–252. doi: 10.1038/382250a0. [DOI] [PubMed] [Google Scholar]
- 82.Huszar D., Lynch C.A., Fairchild-Huntress V., Dunmore J.H., Fang Q., Berkemeier L.R. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell. 1997;88(1):131–141. doi: 10.1016/s0092-8674(00)81865-6. [DOI] [PubMed] [Google Scholar]
- 83.Jackson R.S., Creemers J.W., Ohagi S., Raffin-Sanson M.L., Sanders L., Montague C.T. Obesity and impaired prohormone processing associated with mutations in the human prohormone convertase 1 gene. Nature Genetics. 1997;16(3):303–306. doi: 10.1038/ng0797-303. [DOI] [PubMed] [Google Scholar]
- 84.Krude H., Biebermann H., Luck W., Horn R., Brabant G., Grüters A. Severe early-onset obesity, adrenal insufficiency and red hair pigmentation caused by POMC mutations in humans. Nature Genetics. 1998;19(2):155–157. doi: 10.1038/509. [DOI] [PubMed] [Google Scholar]
- 85.Vaisse C., Clement K., Guy-Grand B., Froguel P. A frameshift mutation in human MC4R is associated with a dominant form of obesity. Nature Genetics. 1998;20(2):113–114. doi: 10.1038/2407. [DOI] [PubMed] [Google Scholar]
- 86.Yeo G.S.H., Farooqi I.S., Aminian S., Halsall D.J., Stanhope R.G., O'Rahilly S. A frameshift mutation in MC4R associated with dominantly inherited human obesity. Nature Genetics. 1998;20(2):111–112. doi: 10.1038/2404. [DOI] [PubMed] [Google Scholar]
- 87.Michaud J.L., Boucher F., Melnyk A., Gauthier F., Goshu E., Lévy E. Sim1 haploinsufficiency causes hyperphagia, obesity and reduction of the paraventricular nucleus of the hypothalamus. Human Molecular Genetics. 2001;10(14):1465–1473. doi: 10.1093/hmg/10.14.1465. [DOI] [PubMed] [Google Scholar]
- 88.Ramachandrappa S., Raimondo A., Cali A.M.G., Keogh J.M., Henning El., Saeed S. Rare variants in single-minded 1 (SIM1) are associated with severe obesity. Journal of Clinical Investigation. 2013;123(7):3042–3050. doi: 10.1172/JCI68016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Butler A.A., Kesterson R.A., Khong K., Cullen M.J., Pelleymounter M.A., Dekoning J. A unique metabolic syndrome causes obesity in the melanocortin-3 receptor-deficient mouse. Endocrinology. 2000;141(9):3518–3521. doi: 10.1210/endo.141.9.7791. [DOI] [PubMed] [Google Scholar]
- 90.Demidowich A.P., Jun J.Y., Yanovski J.A. Polymorphisms and mutations in the melanocortin-3 receptor and their relation to human obesity. Biochimica et Biophysica Acta - Molecular Basis of Disease. 2017;1863(10 Pt A):2468–2476. doi: 10.1016/j.bbadis.2017.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lee Y.S., Poh L.K., Loke K.Y. A novel melanocortin 3 receptor gene (MC3R) mutation associated with severe obesity. Journal of Clinical Endocrinology & Metabolism. 2002;87(3):1423–1426. doi: 10.1210/jcem.87.3.8461. [DOI] [PubMed] [Google Scholar]
- 92.Mencarelli M., Dubern B., Alili R., Maestrini S., Benajiba L., Tagliaferri M. Rare melanocortin-3 receptor mutations with in vitro functional consequences are associated with human obesity. Human Molecular Genetics. 2011;20(2):392–399. doi: 10.1093/hmg/ddq472. [DOI] [PubMed] [Google Scholar]
- 93.Zegers D., Beckers S., de Freitas F., Peeters Ar.V., Mertens I.L., Verhulst St.L. Identification of three novel genetic variants in the melanocortin-3 receptor of obese children. Obesity. 2011;19(1):152–159. doi: 10.1038/oby.2010.127. [DOI] [PubMed] [Google Scholar]
- 94.Ghamari-Langroudi M., Cakir I., Lippert R.N., Sweeney P., Litt M.J., Ellacott K.L.J. Regulation of energy rheostasis by the melanocortin-3 receptor. Science Advances. 2018;4(8) doi: 10.1126/sciadv.aat0866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kleinendorst L., Abawi O., van der Kamp H.J., Alders M., Meijers-Heijboer H.E.J., van Rossum E.F.C. Leptin receptor deficiency: a systematic literature review and prevalence estimation based on population genetics. European Journal of Endocrinology. 2020;182(1):47–56. doi: 10.1530/EJE-19-0678. [DOI] [PubMed] [Google Scholar]
- 96.Asai M., Ramachandrappa S., Joachim M., Shen Y., Zhang R., Nuthalapati N. Loss of function of the melanocortin 2 receptor accessory protein 2 is associated with mammalian obesity. Science. 2013;341(6143):275–278. doi: 10.1126/science.1233000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Baron M., Maillet J., Huyvaert M., Dechaume A., Boutry R., Loiselle H. Loss-of-function mutations in MRAP2 are pathogenic in hyperphagic obesity with hyperglycemia and hypertension. Nature Medicine. 2019;25(11):1733–1738. doi: 10.1038/s41591-019-0622-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Grarup N., Moltke I., Andersen M.K., Dalby M., Vitting-Seerup K., Kern T. Loss-of-function variants in ADCY3 increase risk of obesity and type 2 diabetes. Nature Genetics. 2018;50(2):172–174. doi: 10.1038/s41588-017-0022-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Saeed S., Bonnefond A., Tamanini F., Mirza M.U., Manzoor J., Janjua Q.M. Loss-of-function mutations in ADCY3 cause monogenic severe obesity. Nature Genetics. 2018;50(2):175–179. doi: 10.1038/s41588-017-0023-6. [DOI] [PubMed] [Google Scholar]
- 100.Yang Y., van der Klaauw A.A., Zhu L.G., Cacciottolo T.M., He Y.L., Stadler L.K.J. Steroid receptor coactivator-1 modulates the function of Pomc neurons and energy homeostasis. Nature Communications. 2019;10(1):1718. doi: 10.1038/s41467-019-08737-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gray J., Yeo G.S.H., Cox J.J., Morton J., Adlam A.L.R., Keogh J.M. Hyperphagia, severe obesity, impaired cognitive function, and hyperactivity associated with functional loss of one copy of the brain-derived neurotrophic factor (BDNF) gene. Diabetes. 2006;55(12):3366–3371. doi: 10.2337/db06-0550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yeo G.S.H., Hung C.C.C., Rochford J., Keogh J., Gray J., Sivaramakrishnan S. A de novo mutation affecting human TrkB associated with severe obesity and developmental delay. Nature Neuroscience. 2004;7(11):1187–1189. doi: 10.1038/nn1336. [DOI] [PubMed] [Google Scholar]
- 103.Doche M.E., Bochukova E.G., Su H.Wen., Pearce L.R., Keogh J.M., Henning E. Human SH2B1 mutations are associated with maladaptive behaviors and obesity. Journal of Clinical Investigation. 2012;122(12):4732–4736. doi: 10.1172/JCI62696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Collet T.H., Dubern B., Mokrosinski J., Connors H., Keogh J.M., de Oliveira E.M. Evaluation of a melanocortin-4 receptor (MC4R) agonist (Setmelanotide) in MC4R deficiency. Molecular Metabolism. 2017;6(10):1321–1329. doi: 10.1016/j.molmet.2017.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kuhnen P., Krude H., Biebermann H. Melanocortin-4 receptor signalling: importance for weight regulation and obesity treatment. Trends in Molecular Medicine. 2019;25(2):136–148. doi: 10.1016/j.molmed.2018.12.002. [DOI] [PubMed] [Google Scholar]
- 106.Alharbi K.K., Spanakis E., Tan K., Smith M.J., Aldahmesh M.A., O'Dell S.D. Prevalence and functionality of paucimorphic and private MC4R mutations in a large, unselected European British population, scanned by meltMADGE. Human Mutation. 2007;28(3):294–302. doi: 10.1002/humu.20404. [DOI] [PubMed] [Google Scholar]
- 107.Saeed S., Bonnefond A., Manzoor J., Shabbir F., Ayesha H., Philippe J. Genetic variants in LEP, LEPR, and MC4R explain 30% of severe obesity in children from a consanguineous population. Obesity. 2015;23(8):1687–1695. doi: 10.1002/oby.21142. [DOI] [PubMed] [Google Scholar]
- 108.Lotta L.A., Mokrosiński J., de Oliveira E.M., Li C., Sharp S.J., Luan J. Human gain-of-function MC4R variants show signaling bias and protect against obesity. Cell. 2019;177(3):597–607. doi: 10.1016/j.cell.2019.03.044. e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Farooqi I.S., Keogh J.M., Yeo G.S.H., Lank E.J., Cheetham T., O'Rahilly S. Clinical spectrum of obesity and mutations in the melanocortin 4 receptor gene. New England Journal of Medicine. 2003;348(12):1085–1095. doi: 10.1056/NEJMoa022050. [DOI] [PubMed] [Google Scholar]
- 110.Kim K.S., Larsen N., Short T., Plastow G., Rothschild M.F. A missense variant of the porcine melanocortin-4 receptor (MC4R) gene is associated with fatness, growth, and feed intake traits. Mammalian Genome. 2000;11(2):131–135. doi: 10.1007/s003350010025. [DOI] [PubMed] [Google Scholar]
- 111.Aspiras A.C., Rohner N., Martineau B., Borowsky R.L., Tabin C.J. Melanocortin 4 receptor mutations contribute to the adaptation of cavefish to nutrient-poor conditions. Proceedings of the National Academy of Sciences of the United States of America. 2015;112(31):9668–9673. doi: 10.1073/pnas.1510802112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chambers J.C., Elliott P., Zabaneh D., Zhang W.H., Li Y., Froguel P. Common genetic variation near MC4R is associated with waist circumference and insulin resistance. Nature Genetics. 2008;40(6):716–718. doi: 10.1038/ng.156. [DOI] [PubMed] [Google Scholar]
- 113.Loos R.J., Lindgren C.M., Li S.X., Wheeler E., Zhao J.H., Prokopenko I. Common variants near MC4R are associated with fat mass, weight and risk of obesity. Nature Genetics. 2008;40(6):768–775. doi: 10.1038/ng.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Speliotes E.K., Willer C.J., Berndt S.I., Monda K.L., Thorleifsson G., Jackson A.U. Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nature Genetics. 2010;42(11):937–948. doi: 10.1038/ng.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kichaev G., Bhatia G., Loh P.R., Gazal S., Burch K., Freund M.K. Leveraging polygenic functional enrichment to improve GWAS power. The American Journal of Human Genetics. 2019;104(1):65–75. doi: 10.1016/j.ajhg.2018.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Thorleifsson G., Walters G.B., Gudbjartsson D.F., Steinthorsdottir V., Sulem P., Helgadottir A. Genome-wide association yields new sequence variants at seven loci that associate with measures of obesity. Nature Genetics. 2009;41(1):18–24. doi: 10.1038/ng.274. [DOI] [PubMed] [Google Scholar]
- 117.Willer C.J., Speliotes E.K., Loos R.J.F., Li S.X., Lindgren C.M., Heid I.M. Six new loci associated with body mass index highlight a neuronal influence on body weight regulation. Nature Genetics. 2009;41(1):25–34. doi: 10.1038/ng.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ayers K.L., Glicksberg B.S., Garfield A.S., Longerich S., White J.A., Yang P.W. Melanocortin 4 receptor pathway dysfunction in obesity: patient stratification aimed at MC4R agonist treatment. Journal of Clinical Endocrinology & Metabolism. 2018;103(7):2601–2612. doi: 10.1210/jc.2018-00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Schioth H.B., Chhajlani V., Muceniece R., Klusa V., Wikberg J.E. Major pharmacological distinction of the ACTH receptor from other melanocortin receptors. Life Sciences. 1996;59(10):797–801. doi: 10.1016/0024-3205(96)00370-0. [DOI] [PubMed] [Google Scholar]
- 120.Glembotski C.C. Acetylation of alpha-melanotropin and beta-endorphin in the rat intermediate pituitary. Subcellular localization. Journal of Biological Chemistry. 1982;257(17):10493–10500. [PubMed] [Google Scholar]
- 121.Smith A.I., Funder J.W. Proopiomelanocortin processing in the pituitary, central nervous system, and peripheral tissues. Endocrine Reviews. 1988;9(1):159–179. doi: 10.1210/edrv-9-1-159. [DOI] [PubMed] [Google Scholar]
- 122.O'Donohue T.L., Handelmann G.E., Chaconas T., Miller R.L., Jacobowitz D.M. Evidence that N-acetylation regulates the behavioral activity of alpha-MSH in the rat and human central nervous system. Peptides. 1981;2(3):333–344. doi: 10.1016/s0196-9781(81)80126-x. [DOI] [PubMed] [Google Scholar]
- 123.Castrucci A.M., Hadley M.E., Sawyer T.K., Wilkes B.C., al-Obeidi F., Staples D.J. Alpha-melanotropin: the minimal active sequence in the lizard skin bioassay. General and Comparative Endocrinology. 1989;73(1):157–163. doi: 10.1016/0016-6480(89)90066-x. [DOI] [PubMed] [Google Scholar]
- 124.Hruby V.J., Wilkes B.C., Hadley M.E., Al-Obeidi F., Sawyer T.K., Staples D.J. alpha-Melanotropin: the minimal active sequence in the frog skin bioassay. Journal of Medicinal Chemistry. 1987;30(11):2126–2130. doi: 10.1021/jm00394a033. [DOI] [PubMed] [Google Scholar]
- 125.Schwyzer R. ACTH: a short introductory review. Annals of the New York Academy of Sciences. 1977;297:3–26. doi: 10.1111/j.1749-6632.1977.tb41843.x. [DOI] [PubMed] [Google Scholar]
- 126.Guo L., Münzberg H., Stuart R.C., Nillni E.A., Bjørbaek C. N-acetylation of hypothalamic alpha-melanocyte-stimulating hormone and regulation by leptin. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(32):11797–11802. doi: 10.1073/pnas.0403165101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Oki S., Nakao K., Tanaka I., Kinoshita F., Naki Y., Imura H. Characterization of gamma-melanotropin-like immunoreactivity and its secretion in an adrenocorticotropin-producing mouse pituitary tumor cell line. Endocrinology. 1982;111(2):418–424. doi: 10.1210/endo-111-2-418. [DOI] [PubMed] [Google Scholar]
- 128.van Strien F.J., Devreese B., Van Beeumen J., Roubos E.W., Jenks B.G. Biosynthesis and processing of the N-terminal part of proopiomelanocortin in Xenopus laevis: characterization of gamma-MSH peptides. Journal of Neuroendocrinology. 1995;7(10):807–815. doi: 10.1111/j.1365-2826.1995.tb00718.x. [DOI] [PubMed] [Google Scholar]
- 129.Grieco P., Balse P.M., Weinberg D., MacNeil T., Hruby V.J. D-Amino acid scan of gamma-melanocyte-stimulating hormone: importance of Trp(8) on human MC3 receptor selectivity. Journal of Medicinal Chemistry. 2000;43(26):4998–5002. doi: 10.1021/jm000211e. [DOI] [PubMed] [Google Scholar]
- 130.Grieco P., Balse-Srinivasan P., Han G., Weinberg D., MacNeil T., Van der Ploeg L.H.T. Synthesis and biological evaluation on hMC3, hMC4 and hMC5 receptors of gamma-MSH analogs substituted with L-alanine. The Journal of Peptide Research. 2002;59(5):203–210. doi: 10.1034/j.1399-3011.2002.01966.x. [DOI] [PubMed] [Google Scholar]
- 131.Joseph C.G., Yao H., Scott J.W., Sorensen N.B., Marnane R.N., Mountjoy K.G. gamma(2)-Melanocyte stimulation hormone (gamma(2)-MSH) truncation studies results in the cautionary note that gamma(2)-MSH is not selective for the mouse MC3R over the mouse MC5R. Peptides. 2010;31(12):2304–2313. doi: 10.1016/j.peptides.2010.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kiefer L.L., Veal J.M., Mountjoy K.G., Wilkison W.O. Melanocortin receptor binding determinants in the agouti protein. Biochemistry. 1998;37(4):991–997. doi: 10.1021/bi971913h. [DOI] [PubMed] [Google Scholar]
- 133.Tota M.R., Smith T.S., Mao C., MacNeil T., Mosley R.T., Van der Ploeg L.H. Molecular interaction of Agouti protein and Agouti-related protein with human melanocortin receptors. Biochemistry. 1999;38(3):897–904. doi: 10.1021/bi9815602. [DOI] [PubMed] [Google Scholar]
- 134.Willard D.H., Bodnar W., Harris C., Kiefer L., Nichols J.S., Blanchard S. Agouti structure and function: characterization of a potent alpha-melanocyte stimulating hormone receptor antagonist. Biochemistry. 1995;34(38):12341–12346. doi: 10.1021/bi00038a030. [DOI] [PubMed] [Google Scholar]
- 135.Creemers J.W., Pritchard L.E., Gyte A., Le Rouzic P., Meulemans S., Wardlaw S.L. Agouti-related protein is posttranslationally cleaved by proprotein convertase 1 to generate agouti-related protein (AGRP)83-132: interaction between AGRP83-132 and melanocortin receptors cannot be influenced by syndecan-3. Endocrinology. 2006;147(4):1621–1631. doi: 10.1210/en.2005-1373. [DOI] [PubMed] [Google Scholar]
- 136.He L., Gunn T.M., Bouley D.M., Lu X.Y., Watson S.J., Schlossman S.F. A biochemical function for attractin in agouti-induced pigmentation and obesity. Nature Genetics. 2001;27(1):40–47. doi: 10.1038/83741. [DOI] [PubMed] [Google Scholar]
- 137.Ollmann M.M., Lamoreux M.L., Wilson B.D., Barsh G.S. Interaction of Agouti protein with the melanocortin 1 receptor in vitro and in vivo. Genes & Development. 1998;12(3):316–330. doi: 10.1101/gad.12.3.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Fong T.M., Mao C., MacNeil T., Kalyani R., Smith T., Weinberg D. ART (protein product of agouti-related transcript) as an antagonist of MC-3 and MC-4 receptors. Biochemical and Biophysical Research Communications. 1997;237(3):629–631. doi: 10.1006/bbrc.1997.7200. [DOI] [PubMed] [Google Scholar]
- 139.Yang Y.K., Thompson D.A., Dickinson C.J., Wilken J., Barsh G.S., Kent S.B. Characterization of Agouti-related protein binding to melanocortin receptors. Molecular Endocrinology. 1999;13(1):148–155. doi: 10.1210/mend.13.1.0223. [DOI] [PubMed] [Google Scholar]
- 140.Kiefer L.L., Ittoop O.R., Bunce K., Truesdale A.T., Willard D.H., Nichols J.S. Mutations in the carboxyl terminus of the agouti protein decrease agouti inhibition of ligand binding to the melanocortin receptors. Biochemistry. 1997;36(8):2084–2090. doi: 10.1021/bi962647v. [DOI] [PubMed] [Google Scholar]
- 141.McNulty J.C., Jackson P.J., Thompson D.A., Chai B.X., Gantz I., Barsh G.S. Structures of the agouti signaling protein. Journal of Molecular Biology. 2005;346(4):1059–1070. doi: 10.1016/j.jmb.2004.12.030. [DOI] [PubMed] [Google Scholar]
- 142.Shutter J.R., Graham M., Kinsey A.C., Scully S., Lüthy R., Stark K.L. Hypothalamic expression of ART, a novel gene related to agouti, is up-regulated in obese and diabetic mutant mice. Genes & Development. 1997;11(5):593–602. doi: 10.1101/gad.11.5.593. [DOI] [PubMed] [Google Scholar]
- 143.Haskell-Luevano C., Monck E.K. Agouti-related protein functions as an inverse agonist at a constitutively active brain melanocortin-4 receptor. Regulatory Peptides. 2001;99(1):1–7. doi: 10.1016/s0167-0115(01)00234-8. [DOI] [PubMed] [Google Scholar]
- 144.Nijenhuis W.A., Oosterom J., Adan R.A. AgRP(83-132) acts as an inverse agonist on the human-melanocortin-4 receptor. Molecular Endocrinology. 2001;15(1):164–171. doi: 10.1210/mend.15.1.0578. [DOI] [PubMed] [Google Scholar]
- 145.Agosti F., Gonzalez S.C., Damonte V.M., Tolosa M.J., Di Siervi N., Schioth H.B. Melanocortin 4 receptor constitutive activity inhibits L-type voltage-gated calcium channels in neurons. Neuroscience. 2017;346:102–112. doi: 10.1016/j.neuroscience.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 146.Tolle V., Low M.J. In vivo evidence for inverse agonism of Agouti-related peptide in the central nervous system of proopiomelanocortin-deficient mice. Diabetes. 2008;57(1):86–94. doi: 10.2337/db07-0733. [DOI] [PubMed] [Google Scholar]
- 147.Mo X.L., Tao Y.X. Activation of MAPK by inverse agonists in six naturally occurring constitutively active mutant human melanocortin-4 receptors. Biochimica et Biophysica Acta. 2013;1832(12):1939–1948. doi: 10.1016/j.bbadis.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 148.Luger T.A., Bohm M. An alpha-MSH analog in erythropoietic protoporphyria. Journal of Investigative Dermatology. 2015;135(4):929–931. doi: 10.1038/jid.2015.16. [DOI] [PubMed] [Google Scholar]
- 149.Sawyer T.K., Sanfilippo P.J., Hruby V.J., Engel M.H., Heward C.B., Burnett J.B. 4-Norleucine, 7-D-phenylalanine-alpha-melanocyte-stimulating hormone: a highly potent alpha-melanotropin with ultralong biological activity. Proceedings of the National Academy of Sciences of the United States of America. 1980;77(10):5754–5758. doi: 10.1073/pnas.77.10.5754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Haskell-Luevano C., Holder J.R., Monck E.K., Bauzo R.M. Characterization of melanocortin NDP-MSH agonist peptide fragments at the mouse central and peripheral melanocortin receptors. Journal of Medicinal Chemistry. 2001;44(13):2247–2252. doi: 10.1021/jm010061n. [DOI] [PubMed] [Google Scholar]
- 151.Al-Obeidi F., Castrucci A.M., Hadley M.E., Hruby V.J. Potent and prolonged acting cyclic lactam analogues of alpha-melanotropin: design based on molecular dynamics. Journal of Medicinal Chemistry. 1989;32(12):2555–2561. doi: 10.1021/jm00132a010. [DOI] [PubMed] [Google Scholar]
- 152.Al-Obeidi F., Hruby V.J., Castrucci A.M., Hadley M.E. Design of potent linear alpha-melanotropin 4-10 analogues modified in positions 5 and 10. Journal of Medicinal Chemistry. 1989;32(1):174–179. doi: 10.1021/jm00121a032. [DOI] [PubMed] [Google Scholar]
- 153.Molinoff P.B., Shadiack A.M., Earle D., Diamond L.E., Quon C.Y. T-141: a melanocortin agonist for the treatment of sexual dysfunction. Annals of the New York Academy of Sciences. 2003;994:96–102. doi: 10.1111/j.1749-6632.2003.tb03167.x. [DOI] [PubMed] [Google Scholar]
- 154.Pfaus J.G., Shadiack A., Van Soest T., Tse M., Molinoff P. Selective facilitation of sexual solicitation in the female rat by a melanocortin receptor agonist. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(27):10201–10204. doi: 10.1073/pnas.0400491101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kingsberg S.A., Clayton A.H., Portman D., Williams L.A., Krop J., Jordan R. Bremelanotide for the treatment of hypoactive sexual desire disorder: two randomized phase 3 trials. Obstetrics & Gynecology. 2019;134(5):899–908. doi: 10.1097/AOG.0000000000003500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Van der Ploeg L.H., Martin W.J., Howard A.D., Nargund R.P., Austin C.P., Guan X.M. A role for the melanocortin 4 receptor in sexual function. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(17):11381–11386. doi: 10.1073/pnas.172378699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hruby V.J., Lu D., Sharma S.D., Castrucci A.L., Kesterson R.A., al-Obeidi F.A. Cyclic lactam alpha-melanotropin analogues of Ac-Nle4-cyclo[Asp5, D-Phe7,Lys10] alpha-melanocyte-stimulating hormone-(4-10)-NH2 with bulky aromatic amino acids at position 7 show high antagonist potency and selectivity at specific melanocortin receptors. Journal of Medicinal Chemistry. 1995;38(18):3454–3461. doi: 10.1021/jm00018a005. [DOI] [PubMed] [Google Scholar]
- 158.Yu J., Gimenez L.E., Hernandez C.C., Wu Y., Wein A.H., Han G.W. Determination of the melanocortin-4 receptor structure identifies Ca(2+) as a cofactor for ligand binding. Science. 2020;368(6489):428–433. doi: 10.1126/science.aaz8995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Hadley M.E., Anderson B., Heward C.B., Sawyer T.K., Hruby V.J. Calcium-dependent prolonged effects on melanophores of [4-norleucine, 7-D-phenylalanine]-alpha-melanotropin. Science. 1981;213(4511):1025–1027. doi: 10.1126/science.6973820. [DOI] [PubMed] [Google Scholar]
- 160.Chen K.Y., Muniyappa R., Abel B.S., Mullins K.P., Staker P., Brychta R.J. RM-493, a melanocortin-4 receptor (MC4R) agonist, increases resting energy expenditure in obese individuals. Journal of Clinical Endocrinology & Metabolism. 2015;100(4):1639–1645. doi: 10.1210/jc.2014-4024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Clement K., Biebermann H., Farooqi I.S., Van der Ploeg L., Wolters B., Poitou C. MC4R agonism promotes durable weight loss in patients with leptin receptor deficiency. Nature Medicine. 2018;24(5):551–555. doi: 10.1038/s41591-018-0015-9. [DOI] [PubMed] [Google Scholar]
- 162.Kuhnen P., Clément K., Wiegand S., Blankenstein O., Gottesdiener K., Martini L.L. Proopiomelanocortin deficiency treated with a melanocortin-4 receptor agonist. New England Journal of Medicine. 2016;375(3):240–246. doi: 10.1056/NEJMoa1512693. [DOI] [PubMed] [Google Scholar]
- 163.Wilding J.P.H., Batterham R.L., Calanna S., Davies M., Van Gaal L.F., Lingvay I. Once-weekly semaglutide in adults with overweight or obesity. New England Journal of Medicine. 2021 doi: 10.1056/NEJMc2106918. [DOI] [PubMed] [Google Scholar]
- 164.Clemmensen C., Finan B., Fischer K., Tom R.Z., Legutko B., Sehrer L. Dual melanocortin-4 receptor and GLP-1 receptor agonism amplifies metabolic benefits in diet-induced obese mice. EMBO Molecular Medicine. 2015;7(3):288–298. doi: 10.15252/emmm.201404508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lu D., Vage D.I., Cone R.D. A ligand-mimetic model for constitutive activation of the melanocortin-1 receptor. Molecular Endocrinology. 1998;12:592–604. doi: 10.1210/mend.12.4.0091. [DOI] [PubMed] [Google Scholar]
- 166.Schioth H.B., Bouifrouri A.A., Rudzish R., Muceniece R., Watanobe H., Wikberg J.E.S. Pharmacological comparison of rat and human melanocortin 3 and 4 receptors in vitro. Regulatory Peptides. 2002;106:7–12. doi: 10.1016/s0167-0115(02)00025-3. [DOI] [PubMed] [Google Scholar]
- 167.Adan R.A., Szklarczyk A.W., Oosterom J., Brakkee J.H., Nijenhuis W.A., Schaaper W.M. Characterization of melanocortin receptor ligands on cloned brain melanocortin receptors and on grooming behavior in the rat. European Journal of Pharmacology. 1999;378:249–258. doi: 10.1016/s0014-2999(99)00465-3. [DOI] [PubMed] [Google Scholar]
- 168.Desarnaud F., Labbe O., Eggerickx D., Vassart G., Parmentier M. Molecular cloning, functional expression and pharmacological characterization of a mouse melanocortin receptor gene. Biochemical Journal. 1994;299(Pt 2):367–373. doi: 10.1042/bj2990367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Roselli-Rehfuss L., Mountjoy K.G., Robbins L.S., Mortrud M.T., Low M.J., Tatro J.B. Identification of a receptor for gamma melanotropin and other proopiomelanocortin peptides in the hypothalamus and limbic system. Proceedings of the National Academy of Sciences of the U S A. 1993;90:8856–8860. doi: 10.1073/pnas.90.19.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Labbe O., Desarnaud F., Eggerickx D., Vassart G., Parmentier M. Molecular cloning of a mouse melanocortin 5 receptor gene widely expressed in peripheral tissues. Biochemistry. 1994;33:4543–4549. doi: 10.1021/bi00181a015. [DOI] [PubMed] [Google Scholar]
- 171.Gantz I., Shimoto Y., Konda Y., Miwa H., Dickinson C.J., Yamada T. Molecular cloning, expression, and characterization of a fifth melanocortin receptor. Biochemical and Biophysical Research Communications. 1994;200:1214–1220. doi: 10.1006/bbrc.1994.1580. [DOI] [PubMed] [Google Scholar]
- 172.Schioth H.B., Muceniece R., Wikberg J.E., Chhajlani V. Characterisation of melanocortin receptor subtypes by radioligand binding analysis. European Journal of Pharmacology. 1995;288:311–317. doi: 10.1016/0922-4106(95)90043-8. [DOI] [PubMed] [Google Scholar]
- 173.Chhajlani V., Wikberg J.E. Molecular cloning and expression of the human melanocyte stimulating hormone receptor cDNA. FEBS Letters. 1992;309:417–420. doi: 10.1016/0014-5793(92)80820-7. [DOI] [PubMed] [Google Scholar]
- 174.Schioth H.B., Müceniece R., Mutulis F., Prusis P., Lindeberg G., Sharma S.D. Selectivity of cyclic [D-Nal7] and [D-Phe7] substituted MSH analogues for the melanocortin receptor subtypes. Peptides. 1997;18:1009–1013. doi: 10.1016/s0196-9781(97)00079-x. [DOI] [PubMed] [Google Scholar]
- 175.Kievit P., Halem H., Marks D.L., Dong J.Z., Glavas M.M., Sinnayah P. Chronic treatment with a melanocortin-4 receptor agonist causes weight loss, reduces insulin resistance, and improves cardiovascular function in diet-induced obese rhesus macaques. Diabetes. 2013;62:490–497. doi: 10.2337/db12-0598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Conde-Frieboes K., Thøgersen H., Lau J.F., Sensfuss U., Hansen T.K., Christensen L. Identification and in vivo and in vitro characterization of long acting and melanocortin 4 receptor (MC4-R) selective alpha-melanocyte-stimulating hormone (alpha-MSH) analogues. Journal of Medicinal Chemistry. 2012;55:1969–1977. doi: 10.1021/jm201489a. [DOI] [PubMed] [Google Scholar]
- 177.Bednarek M.A., MacNeil T., Tang R., Fong T.M., Cabello M.A., Maroto M. Potent and selective peptide agonists of alpha-melanocyte stimulating hormone (alphaMSH) action at human melanocortin receptor 5; their synthesis and biological evaluation in vitro. Chemical Biology & Drug Design. 2007;69:350–355. doi: 10.1111/j.1747-0285.2007.00513.x. [DOI] [PubMed] [Google Scholar]
- 178.Haskell-Luevano C., Hendrata S., North C., Sawyer T.K., Hadley M.E., Hruby V.J. Discovery of prototype peptidomimetic agonists at the human melanocortin receptors MC1R and MC4R. Journal of Medicinal Chemistry. 1997;40:2133–2139. doi: 10.1021/jm960840h. [DOI] [PubMed] [Google Scholar]
- 179.Schioth H.B., Muceniece R., Wikberg J.E. Characterisation of the melanocortin 4 receptor by radioligand binding. Pharmacology & Toxicology. 1996;79:161–165. doi: 10.1111/j.1600-0773.1996.tb00261.x. [DOI] [PubMed] [Google Scholar]
- 180.Chhajlani V., Muceniece R., Wikberg J.E. Molecular cloning of a novel human melanocortin receptor. Biochemical and Biophysical Research Communications. 1993;195:866–873. doi: 10.1006/bbrc.1993.2125. [DOI] [PubMed] [Google Scholar]
- 181.Yan L.Z., Hsiung H.M., Heiman M.L., Gadski R.A., Emmerson P.J., Hertel J. Structure-activity relationships of beta-MSH derived melanocortin-4 receptor peptide agonists. Current Topics in Medicinal Chemistry. 2007;7:1052–1067. doi: 10.2174/156802607780906591. [DOI] [PubMed] [Google Scholar]
- 182.Mayer J.P., Hsiung H.M., Flora D.B., Edwards Pa., Smith D.P., Zhang X.Y. Discovery of a beta-MSH-derived MC-4R selective agonist. Journal of Medicinal Chemistry. 2005;48:3095–3098. doi: 10.1021/jm0501432. [DOI] [PubMed] [Google Scholar]
- 183.Grieco P., Balse P.M., Weinberg D., MacNeil T., Hruby V.J. D-Amino acid scan of gamma-melanocyte-stimulating hormone: importance of Trp(8) on human MC3 receptor selectivity. Journal of Medicinal Chemistry. 2000;43:4998–5002. doi: 10.1021/jm000211e. [DOI] [PubMed] [Google Scholar]
- 184.Durek T., Cromm P.M., White A.M., Schroeder C.I., Kaas Q., Weidmann J. Development of novel melanocortin receptor agonists based on the cyclic peptide framework of sunflower trypsin inhibitor-1. Journal of Medicinal Chemistry. 2018;61:3674–3684. doi: 10.1021/acs.jmedchem.8b00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Kumar K.G., Sutton G.M., Dong J.Z., Roubert P., Plas P., Halem H.A. Analysis of the therapeutic functions of novel melanocortin receptor agonists in MC3R-and MC4R-deficient C57BL/6J mice. Peptides. 2009;30:1892–1900. doi: 10.1016/j.peptides.2009.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Blood C.H., Shadiack A.M., Bernstein J.K., Herbert G.H. U.S. patent; 2001. Compositions and methods for treatment of sexual dysfunction. [Google Scholar]
- 187.Molinoff P.B., Shadiack A.M., Earle D., Diamond L.E., Quon C.Y. PT-141: a melanocortin agonist for the treatment of sexual dysfunction. Annals of the New York Academy of Sciences. 2003;994:96–102. doi: 10.1111/j.1749-6632.2003.tb03167.x. [DOI] [PubMed] [Google Scholar]
- 188.Haskell-Luevano C., Monck E.K., Wan Y.P., Schentrup A.M. The agouti-related protein decapeptide (Yc[CRFFNAFC]Y) possesses agonist activity at the murine melanocortin-1 receptor. Peptides. 2000;21:683–689. doi: 10.1016/s0196-9781(00)00194-7. [DOI] [PubMed] [Google Scholar]
- 189.Haskell-Luevano C., Cone R.D., Monck E.K., Wan Y.P. Structure activity studies of the melanocortin-4 receptor by in vitro mutagenesis: identification of agouti-related protein (AGRP), melanocortin agonist and synthetic peptide antagonist interaction determinants. Biochemistry. 2001;40:6164–6179. doi: 10.1021/bi010025q. [DOI] [PubMed] [Google Scholar]
- 190.Bednarek M.A., Macneil T., Kalyani R.N., Tang R., Van der Ploeg L.H., Weinberg D.H. Analogs of MTII, lactam derivatives of alpha-melanotropin, modified at the N-terminus, and their selectivity at human melanocortin receptors 3, 4, and 5. Biochemical and Biophysical Research Communications. 1999;261:209–213. doi: 10.1006/bbrc.1999.0981. [DOI] [PubMed] [Google Scholar]
- 191.Lensing C.J., Freeman K.T., Schnell S.M., Adank D.N., Speth R.C., Haskell-Luevano C. An in vitro and in vivo investigation of bivalent ligands that display preferential binding and functional activity for different melanocortin receptor homodimers. Journal of Medicinal Chemistry. 2016;59:3112–3128. doi: 10.1021/acs.jmedchem.5b01894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Schioth H.B., Müceniece R., Mutulis F., Prusis P., Lindeberg G., Sharma S.D. Selectivity of cyclic [D-Nal(7)] and [D-Phe(7)] substituted MSH analogues for the melanocortin receptor subtypes. Peptides. 1997;18:1009–1013. doi: 10.1016/s0196-9781(97)00079-x. [DOI] [PubMed] [Google Scholar]
- 193.Bednarek M.A., Macneil T., Tang R., Fong T.M., Cabello M.A., Maroto M. Analogs of alpha-melanocyte stimulating hormone with high agonist potency and selectivity at human melanocortin receptor 1b: the role of Trp(9) in molecular recognition. Biopolymers. 2008;89:401–408. doi: 10.1002/bip.20863. [DOI] [PubMed] [Google Scholar]
- 194.Bednarek M.A., MacNeil T., Kalyani R.N., Tang R., Van der Ploeg L.H., Weinberg D.H. Selective, high affinity peptide antagonists of α-melanotropin action at human melanocortin receptor 4: their synthesis and biological evaluation in vitro. Journal of Medicinal Chemistry. 2001;44:3665–3672. doi: 10.1021/jm010165y. [DOI] [PubMed] [Google Scholar]
- 195.Kiefer L.L., Ittoop O.R., Bunce K., Truesdale A.T., Willard D.H., Nichols J.S. Mutations in the carboxyl terminus of the agouti protein decrease agouti inhibition of ligand binding to the melanocortin receptors. Biochemistry. 1997;36:2084–2090. doi: 10.1021/bi962647v. [DOI] [PubMed] [Google Scholar]
- 196.Oosterom J., Garner K.M., den Dekker W.K., Nijenhuis W.A., Gispen W.H., Burbach J.P. Common requirements for melanocortin-4 receptor selectivity of structurally unrelated melanocortin agonist and endogenous antagonist, Agouti protein. Journal of Biological Chemistry. 2001;276:931–936. doi: 10.1074/jbc.M007261200. [DOI] [PubMed] [Google Scholar]
- 197.McNulty J.C., Jackson P.J., Thompson D.A., Chai B.X., Gantz I., Barsh G.S. Structures of the agouti signaling protein. Journal of Molecular Biology. 2005;346:1059–1070. doi: 10.1016/j.jmb.2004.12.030. [DOI] [PubMed] [Google Scholar]
- 198.Wilczynski A., Wang X.S., Bauzo R.M., Xiang Z.M., Shaw A.M., Millard W.J. Structural characterization and pharmacology of a potent (Cys101-Cys119, Cys110-Cys117) bicyclic agouti-related protein (AGRP) melanocortin receptor antagonist. Journal of Medicinal Chemistry. 2004;47:5662–5673. doi: 10.1021/jm049620r. [DOI] [PubMed] [Google Scholar]
- 199.Yang Y.K., Thompson D.A., Dickinson C.J., Wilken J., Barsh G.S., Kent S.B. Characterization of Agouti-related protein binding to melanocortin receptors. Molecular Endocrinology. 1999;13:148–155. doi: 10.1210/mend.13.1.0223. [DOI] [PubMed] [Google Scholar]
- 200.Fong T.M., Mao C., MacNeil T., Kalyani R., Smith T., Weinberg D. ART (protein product of agouti-related transcript) as an antagonist of MC-3 and MC-4 receptors. Biochemical and Biophysical Research Communications. 1997;237:629–631. doi: 10.1006/bbrc.1997.7200. [DOI] [PubMed] [Google Scholar]
- 201.Tota M.R., Smith T.S., Mao C., MacNeil T., Mosley R.T., Van der Ploeg L.H. Molecular interaction of Agouti protein and Agouti-related protein with human melanocortin receptors. Biochemistry. 1999;38:897–904. doi: 10.1021/bi9815602. [DOI] [PubMed] [Google Scholar]
- 202.Proneth B., Xiang Z.M., Pogozheva I.D., Litherland S.A., Gorbatyuk O.S., Shaw A.M. Molecular mechanism of the constitutive activation of the L250Q human melanocortin-4 receptor polymorphism. Chemical Biology & Drug Design. 2006;67:215–229. doi: 10.1111/j.1747-0285.2006.00362.x. [DOI] [PubMed] [Google Scholar]
- 203.Mountjoy K.G. The human melanocyte stimulating hormone receptor has evolved to become "super-sensitive" to melanocortin peptides. Molecular and Cellular Endocrinology. 1994;102:R7–R11. doi: 10.1016/0303-7207(94)90113-9. [DOI] [PubMed] [Google Scholar]
- 204.Wilczynski A., Wang X.S., Joseph C.G., Xiang Z.M., Bauzo R.M., Scott J.W. Identification of putative agouti-related protein(87-132)-melanocortin-4 receptor interactions by homology molecular modeling and validation using chimeric peptide ligands. Journal of Medicinal Chemistry. 2004;47:2194–2207. doi: 10.1021/jm0303608. [DOI] [PubMed] [Google Scholar]
- 205.Joseph C.G., Yao H., Scott J.W., Sorensen N.B., Marnane R.N., Mountjoy K.G. gamma(2)-Melanocyte stimulation hormone (gamma(2)-MSH) truncation studies results in the cautionary note that gamma(2)-MSH is not selective for the mouse MC3R over the mouse MC5R. Peptides. 2010;31:2304–2313. doi: 10.1016/j.peptides.2010.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Alvaro J.D., Tatro J.B., Quillan J.M., Fogliano M., Eisenhard M., Lerner M.R. Morphine down-regulates melanocortin-4 receptor expression in brain regions that mediate opiate addiction. Molecular Pharmacology. 1996;50:583–591. [PubMed] [Google Scholar]
- 207.Griffon N., Mignon V., Facchinetti P., Diaz J., Schwartz J.C., Sokoloff P. Molecular cloning and characterization of the rat fifth melanocortin receptor. Biochemical and Biophysical Research Communications. 1994;200:1007–1014. doi: 10.1006/bbrc.1994.1550. [DOI] [PubMed] [Google Scholar]
- 208.Palmer D., Gonçalves J.P.L., Hansen L.V., Wu B.Q., Hald H., Schoffelen S. Click-chemistry-mediated synthesis of selective melanocortin receptor 4 agonists. Journal of Medicinal Chemistry. 2017;60:8716–8730. doi: 10.1021/acs.jmedchem.7b00353. [DOI] [PubMed] [Google Scholar]
- 209.Xiang Z., Proneth B., Dirain M.L., Litherland S.A., Haskell-Luevano C. Pharmacological characterization of 30 human melanocortin-4 receptor polymorphisms with the endogenous proopiomelanocortin-derived agonists, synthetic agonists, and the endogenous agouti-related protein antagonist. Biochemistry. 2010;49:4583–4600. doi: 10.1021/bi100068u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Mountjoy K.G., Mortrud M.T., Low M.J., Simerly R.B., Cone R.D. Localization of the melanocortin-4 receptor (MC4-R) in neuroendocrine and autonomic control circuits in the brain. Molecular Endocrinology. 1994;8:1298–1308. doi: 10.1210/mend.8.10.7854347. [DOI] [PubMed] [Google Scholar]
- 211.Fathi Z., Iben L.G., Parker E.M. Cloning, expression, and tissue distribution of a fifth melanocortin receptor subtype. Neurochemical Research. 1995;20:107–113. doi: 10.1007/BF00995160. [DOI] [PubMed] [Google Scholar]
- 212.Cai M., Stankova M., Muthu D., Mayorov A., Yang Z.H., Trivedi D. An unusual conformation of gamma-melanocyte-stimulating hormone analogues leads to a selective human melanocortin 1 receptor antagonist for targeting melanoma cells. Biochemistry. 2013;52:752–764. doi: 10.1021/bi300723f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Haskell-Luevano C., Lim S., Yuan W., Cone R.D., Hruby V.J. Structure activity studies of the melanocortin antagonist SHU9119 modified at the 6, 7, 8, and 9 positions. Peptides. 2000;21:49–57. doi: 10.1016/s0196-9781(99)00167-9. [DOI] [PubMed] [Google Scholar]
- 214.Tala S.R., Schnell S.M., Haskell-Luevano C. Microwave-assisted solid-phase synthesis of side-chain to side-chain lactam-bridge cyclic peptides. Bioorganic & Medicinal Chemistry Letters. 2015;25:5708–5711. doi: 10.1016/j.bmcl.2015.10.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Qu H., Cai M.Y., Mayorov A.V., Grieco P., Zingsheim M., Trivedi D. Substitution of arginine with proline and proline derivatives in melanocyte-stimulating hormones leads to selectivity for human melanocortin 4 receptor. Journal of Medicinal Chemistry. 2009;52:3627–3635. doi: 10.1021/jm801300c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Grieco P., Han G., Weinberg D., Van der Ploeg L.H., Hruby V.J. Design and synthesis of highly potent and selective melanotropin analogues of SHU9119 modified at position 6. Biochemical and Biophysical Research Communications. 2002;292:1075–1080. doi: 10.1006/bbrc.2002.6739. [DOI] [PubMed] [Google Scholar]
- 217.Mowlazadeh Haghighi S., Zhou Y., Dai J.X., Sawyer J.R., Hruby V.J., Cai M.Y. Replacement of Arg with Nle and modified D-Phe in the core sequence of MSHs, Ac-His-D-Phe-Arg-Trp-NH2, leads to hMC1R selectivity and pigmentation. The European Journal Medicinal Chemistry. 2018;151:815–823. doi: 10.1016/j.ejmech.2018.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Haskell-Luevano C., Holder J.R., Monck E.K., Bauzo R.M. Characterization of melanocortin NDP-MSH agonist peptide fragments at the mouse central and peripheral melanocortin receptors. Journal of Medicinal Chemistry. 2001;44:2247–2252. doi: 10.1021/jm010061n. [DOI] [PubMed] [Google Scholar]
- 219.Lensing C.J., Freeman K.T., Schnell S.M., Adank D.N., Speth R.C., Haskell-Luevano C. An in vitro and in vivo investigation of bivalent ligands that display preferential binding and functional activity for different melanocortin receptor homodimers. Journal of Medicinal Chemistry. 2016;59:3112–3128. doi: 10.1021/acs.jmedchem.5b01894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Ericson M.D., Freeman K.T., Schnell S.M., Fleming K.A., Haskell-Luevano C. Structure-Activity relationship studies on a macrocyclic Agouti-related protein (AGRP) scaffold reveal Agouti signaling protein (ASP) residue substitutions maintain melanocortin-4 receptor antagonist potency and result in inverse agonist pharmacology at the melanocortin-5 receptor. Journal of Medicinal Chemistry. 2017;60:8103–8114. doi: 10.1021/acs.jmedchem.7b00856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Tala S.R., Schnell S.M., Haskell-Luevano C. Microwave-assisted solid-phase synthesis of side-chain to side-chain lactam-bridge cyclic peptides. Bioorganic & Medicinal Chemistry Letters. 2015;25:5708–5711. doi: 10.1016/j.bmcl.2015.10.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Yang Y.K., Ollmann M.M., Wilson B.D., Dickinson C., Yamada T., Barsh G.S. Effects of recombinant agouti-signaling protein on melanocortin action. Molecular Endocrinology. 1997;11:274–280. doi: 10.1210/mend.11.3.9898. [DOI] [PubMed] [Google Scholar]
- 223.Wessells H., Gralnek D., Dorr R., Hruby V.J., Hadley M.E., Levine N. Effect of an alpha-melanocyte stimulating hormone analog on penile erection and sexual desire in men with organic erectile dysfunction. Urology. 2000;56:641–646. doi: 10.1016/s0090-4295(00)00680-4. [DOI] [PubMed] [Google Scholar]
- 224.Clayton A.H., Althof S.E., Kingsberg S., DeRogatis L.R., Kroll R., Goldstein I. Bremelanotide for female sexual dysfunctions in premenopausal women: a randomized, placebo-controlled dose-finding trial. Women's Health. 2016;12:325–337. doi: 10.2217/whe-2016-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Lansdell M.I., Hepworth D., Calabrese A., Brown A.D., Blagg J., Burring D.J. Discovery of a selective small-molecule melanocortin-4 receptor agonist with efficacy in a pilot study of sexual dysfunction in humans. Journal of Medicinal Chemistry. 2010;53:3183–3197. doi: 10.1021/jm9017866. [DOI] [PubMed] [Google Scholar]
- 226.Krishna R., Gumbiner B., Stevens C., Musser B., Mallick M., Suryawanshi S. Potent and selective agonism of the melanocortin receptor 4 with MK-0493 does not induce weight loss in obese human subjects: energy intake predicts lack of weight loss efficacy. Clinical Pharmacology & Therapeutics. 2009;86:659–666. doi: 10.1038/clpt.2009.167. [DOI] [PubMed] [Google Scholar]
- 227.Greenfield J.R., Miller J.W., Keogh J.M., Henning E., Satterwhite J.H., Cameron G.S. Modulation of blood pressure by central melanocortinergic pathways. New England Journal of Medicine. 2009;360:44–52. doi: 10.1056/NEJMoa0803085. [DOI] [PubMed] [Google Scholar]
- 228.Royalty J.E., Konradsen G., Eskerod O., Wulff B.S., Hansen B.S. Investigation of safety, tolerability, pharmacokinetics, and pharmacodynamics of single and multiple doses of a long-acting alpha-MSH analog in healthy overweight and obese subjects. The Journal of Clinical Pharmacology. 2014;54:394–404. doi: 10.1002/jcph.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Eneli I., Xu J.Y., Webster M., McCagg A., Van Der Ploeg L., Garfield A.S. Tracing the effect of the melanocortin-4 receptor pathway in obesity: study design and methodology of the TEMPO registry. The Application of Clinical Genetics. 2019;12:87–93. doi: 10.2147/TACG.S199092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Kuhnen P., Clément K., Wiegand S., Blankenstein O., Gottesdiener K., Martini L.L. Proopiomelanocortin deficiency treated with a melanocortin-4 receptor agonist. New England Journal of Medicine. 2016;375:240–246. doi: 10.1056/NEJMoa1512693. [DOI] [PubMed] [Google Scholar]
- 231.FDA approves new treatment for hypoactive sexual desire disorder in premenopausal women, June 21, 2019. https://www.fda.gov/news-events/press-announcements/fda-approves-new-treatment-hypoactive-sexual-desire-disorder-premenopausal-women Press Release, Source.
- 232.AstraZeneca PLC-palatin technologies halts phase I obesity trial, June 20, 2012. https://wwwbiospacecom/article/releases/astrazeneca-plc-palatin-technologies-halts-phase-i-obesity-trial-/ Press release: Accessed from:
- 233.Clement K., Biebermann H., Farooqi I.S., Van der Ploeg L., Wolters B., Poitou C. MC4R agonism promotes durable weight loss in patients with leptin receptor deficiency. Nature Medicine. 2018;24(5):551–555. doi: 10.1038/s41591-018-0015-9. [DOI] [PubMed] [Google Scholar]
- 234.Haws R., Brady S., Davis E., Fletty K., Yuan G.J., Gordon G. Effect of setmelanotide, a melanocortin-4 receptor agonist, on obesity in Bardet-Biedl syndrome. Diabetes, Obesity and Metabolism. 2020;22(11):2133–2140. doi: 10.1111/dom.14133. Epub 2020 Jul 22. PMID: 32627316; PMCID: PMC7689750. [DOI] [PMC free article] [PubMed] [Google Scholar]