Abstract
Purpose
To report two cases of thyroid eye disease (TED) associated compressive optic neuropathy (CON) that resolved after treatment with teprotumumab.
Observation
Two patients presented with active TED resulting in mild CON with the typical corresponding visual field (VF) defects. Both patients were initiated on intravenous (IV) corticosteroid therapy but despite treatment had persistent VF defects. Both patients were then treated with teprotumumab and demonstrated marked clinical improvement and complete resolution of TED-CON VF defects early in their infusion course.
Conclusions and importance
These cases suggest that teprotumumab can be a rapid and effective treatment for TED-CON, and raises the question of whether it may be superior to IV corticosteroid therapy.
Keywords: Thyroid eye disease, Graves' disease, Compressive optic neuropathy, Teprotumumab, Visual field defect
1. Introduction
Thyroid eye disease (TED) is an autoimmune disorder that causes inflammation, expansion, and fibrosis of the extraocular muscles and connective tissue of the orbit. The underlying pathogenic mechanism has not been fully elucidated but evidence suggests involvement of autoantibody-mediated upregulation of thyroid stimulating hormone receptor (TSHR) and insulin-like growth factor receptor 1 (IGF-1R) complex in orbital fibroblasts.1 This phenotypically heterogenous disease can lead to a range of disfiguring and debilitating ocular manifestations including proptosis, diplopia, and in its more severe form, compressive optic neuropathy (CON). Traditional treatment options for sight threatening TED-CON include intravenous (IV) or oral corticosteroid therapy or surgical orbital decompression, each of which has variable efficacy in treatment and the potential for significant adverse side effects and complications.2,3
Teprotumumab, a fully human monoclonal antibody directed against IGF-1R, was recently approved by the United States Food and Drug Administration (FDA) to address the need for an effective and targeted medical therapy for patients with TED. The teprotumumab randomized clinical trials demonstrated promising results for the treatment of moderate and severe TED; however, patients with CON were excluded from the trials.4,5 In this report, we describe two cases of TED with mild CON and typical visual field (VF) defects that persisted despite IV corticosteroid therapy, but resolved completely shortly after initiation of treatment with teprotumumab. The research in this manuscript is compliant with the Declaration of Helsinki and Health Insurance Portability and Accountability Act regulations.
2. Case 1
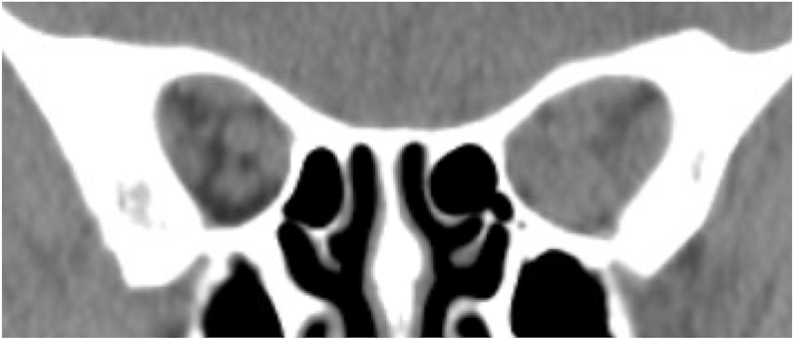
A 68-year-old Caucasian female with Graves’ disease treated with radioactive iodine therapy eleven years prior was referred for evaluation of periorbital swelling, proptosis, pain with extraocular movements, and binocular diplopia for 3 months. The patient was taking levothyroxine with serum thyroid hormone levels in the normal range and was a never-smoker. On examination, visual acuity (VA) was 20/25 in both eyes (OU) with no relative afferent pupillary defect (rAPD). Ishihara color testing was 8 out of 8 plates OU, but the patient reported subjective red desaturation in the left eye (OS). Extraocular motility was moderately restricted in supraduction and abduction OS. The intraocular pressures (IOP) were 22 mm Hg right eye (OD) and 24 mm Hg OS. Hertel exophthalmometry measured 24 mm OD and 27 mm OS. She had severe bilateral eyelid edema and erythema, conjunctival injection and chemosis. The optic discs appeared normal OU. The Clinical Activity Score (CAS) was 6 out of 7. Computed tomography (CT) of the orbits revealed enlarged extraocular muscles, left greater than right, with apical crowding, raising concern for CON OS (Fig. 1). Humphrey visual field (HVF) testing was full OD and showed a Stage 1a small inferior paracentral hemifield defect6 with a mean deviation (MD) of −0.83 dB OS (Fig. 2A). The patient was started on weekly 500 mg IV solumedrol, but following a discussion with her hepatologist the treatment was discontinued after four doses due to elevated liver enzymes. Alternative treatment options were discussed including orbital decompression and teprotumumab. The patient opted for teprotumumab; however, there was a predictable delay in initiating treatment due to insurance and infusion center logistical details.
Fig. 1.
Coronal computed tomography of the orbits without contrast demonstrated enlarged extraocular muscles, left greater than right, with apical crowding.
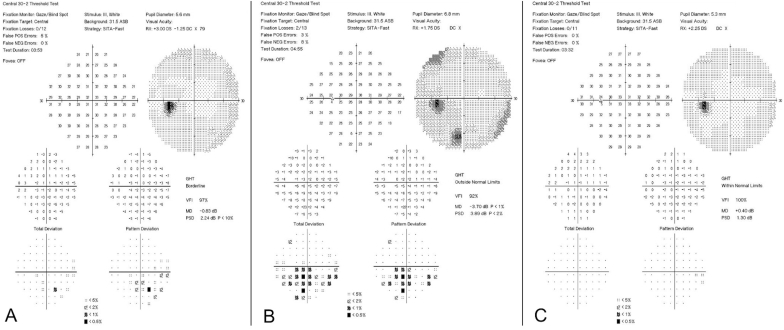
Fig. 2.
A, HVF 30-2 OS on initial visit showed a Stage 1a small inferior paracentral hemifield defect with MD = −0.83 dB. B, HVF 30-2 OS after IV solumedrol demonstrated a Stage 1b large inferior paracentral hemifield defect with MD = −3.70 dB. C, HVF 30-2 OS after teprotumumab demonstrated a full field with MD = +0.40 dB.
HVF, Humphrey visual field; OS, left eye; MD, mean deviation.
Two months after discontinuation of IV solumedrol, just prior to receiving teprotumumab, she presented urgently with a subjective decrease in color vision OS, worsening pain with extraocular movement, and progressive diplopia. On examination, VA was 20/25 OU with no rAPD. Ishihara color testing was 8 out of 8 plates OU with persistent subjective red desaturation OS. Extraocular motility demonstrated interval worsening with severe restriction in supraduction and new restriction in infraduction and adduction OS. The IOP was 25 mm Hg OD and 26 mm Hg OS. Hertel exophthalmometry measured 24.5 mm OD and 26.5 mm OS. The eyelid and conjunctival inflammation persisted bilaterally. The CAS was 7 out of 10. Repeat HVF testing remained full OD and there was progression to a Stage 1b large inferior paracentral hemifield defect6 with the MD worsening to −3.70 dB OS (Fig. 2B). The patient opted for careful monitoring until initiation of teprotumumab and was started on timolol OU. She received her first infusion of teprotumumab two weeks later.
A few days after the first dose of teprotumumab, she reported complete resolution of retrobulbar pain, eyelid edema and erythema, and near complete resolution of diplopia. After her third infusion of teprotumumab, VA was 20/25 OU with no rAPD, Ishihara color testing was 8 out of 8 plates OU, and there was resolution of the subjective red desaturation OS. Extraocular motility improved in all directions of gaze. The IOP improved to 16 mm Hg OD and 17 mm Hg OS while remaining on timolol. Hertel exophthalmometry measured 20 mm OD and 23.5 mm OS (an improvement of 4.5 mm OD and 3 mm OS). There was resolution of eyelid inflammation and chemosis, and improvement in conjunctival injection bilaterally. The CAS was 1 out of 10. HVF testing revealed complete resolution of the previous defect with MD of +0.40 dB OS (Fig. 2C).
3. Case 2
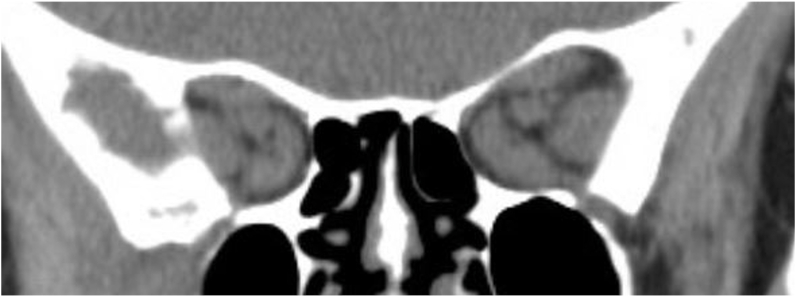
A 68-year-old Caucasian female with Graves’ disease diagnosed seven years prior presented with blurry vision OD and binocular diplopia for 6 months that had acutely worsened over the past weeks. The patient was taking methimazole with serum thyroid hormone levels in the normal range and was a never-smoker. On examination, VA was 20/30 OD and 20/25 OS with an rAPD OD. Ishihara color testing was 8 out of 8 plates OU, but with slower response OD. Extraocular motility was severely restricted in supraduction and moderately restricted in abduction and adduction OU. The IOP was 25 mm Hg OD and 21 mm Hg OS. Hertel exophthalmometry measured 20.5 mm OD and 21 mm OS. She had moderately severe eyelid edema and mild chemosis OU. The optic discs appeared normal OU. The CAS was 3 out of 7. CT of the orbits revealed enlarged extraocular muscles and apical crowding concerning for CON, right greater than left (Fig. 3). HVF testing showed a Stage 2a pattern with advancement of the inferior altitudinal defect above the horizontal midline into the superior temporal field6 with MD of −3.61 dB OD (Fig. 4A) and full field OS. The patient was started on weekly 500 mg IV solumedrol until teprotumumab therapy could be initiated.
Fig. 3.
Coronal computed tomography of the orbits without contrast demonstrated enlarged extraocular muscles bilaterally with more severe apical crowding on the right.
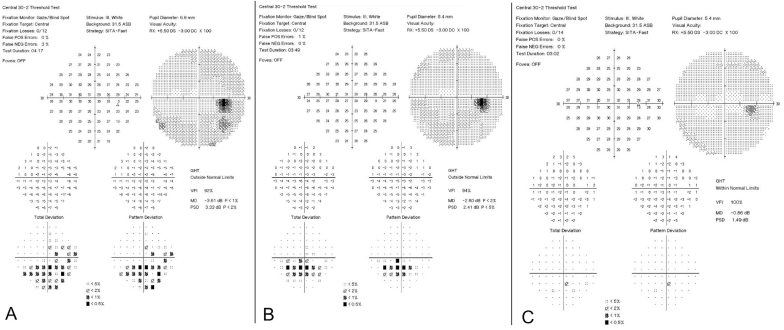
Fig. 4.
A, HVF 30-2 OD on initial visit showed a Stage 2a pattern with advancement of the inferior altitudinal defect above the horizontal midline into the superior temporal field with MD = −3.61 dB. B, HVF 30-2 OD after IV solumedrol demonstrated a Stage 1b inferior paracentral hemifield defect with MD = −2.80 dB. C, HVF 30-2 OD showed a full field with MD = −0.86 dB.
HVF, Humphrey visual field; OD, right eye; MD, mean deviation.
After the fourth weekly dose of 500 mg IV solumedrol, the patient continued to report blurry vision OD and binocular diplopia. On exam, VA was 20/25 OU with resolution of the rAPD OD. Ishihara color testing was 8 out of 8 plates OU, although the response was still slower OD. Extraocular motility restriction was unchanged. IOP was 21 mm Hg OD and 23 mm Hg OS. Hertel exophthalmometry measured 20 OU. She had mild improvement in eyelid edema and resolution of chemosis OU. The optic discs remained normal OU. The CAS was 1 out of 10. HVF testing showed slight improvement, now with a Stage 1b inferior paracentral hemifield defect6 with MD of −2.80 dB OD (Fig. 4B).
After the sixth weekly dose of 500 mg IV solumedrol, teprotumumab therapy was initiated.
She was evaluated one week after the second teprotumumab infusion. She reported complete resolution of diplopia several days after the first infusion of teprotumumab. On examination, VA was stable at 20/25 OU with no rAPD. Ishihara color testing was a brisk 8 out of 8 plates OU. Extraocular motility remained restricted in supraduction OU and improved in all other directions of gaze. IOP was 18 mm Hg OD and 19 mm Hg OS. Hertel exophthalmometry measured 15mm OD and 14mm OS (an improvement in proptosis of 5 mm OD and 6 mm OS). The CAS was 0 out of 10. HVF testing revealed complete resolution of the previous defect with MD of −0.86 dB OD (Fig. 4C).
4. Discussion
TED is a poorly-understood, phenotypically heterogenous autoimmune condition that in severe forms can progress to vision threatening CON. Advanced cases of TED-CON have historically required aggressive medical or surgical treatment, often with variable improvement of physical and visual symptoms and the potential for significant adverse side effects and surgical complications. Teprotumumab is the first FDA-approved treatment for TED, with Phase 2 and 3 clinical trials demonstrating a favorable safety profile and promising results for the treatment of active moderate to severe disease.4,5 The primary endpoint for the phase 2 trial was a response as measured by a reduction of ≥ 2 points in CAS, and a reduction of ≥ 2 mm in proptosis at week 24, and the primary endpoint in the phase 3 trial was a reduction of proptosis of ≥ 2mm at week 24. Secondary endpoints were based on participant reported effects of TED on quality of life, the CAS score, mean change in proptosis, and a diplopia response. Patients with TED-CON were excluded from these trials.
In this report we describe two patients with active, severe TED resulting in mild CON with corresponding HVF loss who opted for treatment with Teprotumumab after lack of improvement with IV solumedrol. Both patients reported marked improvement in their TED symptoms within days after their first dose of teprotumumab. Clinical evaluation with formal VF testing was not performed until their scheduled follow-up visits after their third and second infusions, respectively. On examination, both patients demonstrated notable clinical improvement with complete resolution of their previous TED-CON VF defects.
The rapid rate of TED-CON resolution in these two patients paralleled the rapid clinical improvement of the outcome measures in the Phase 2 and 3 clinical trials. Both patients also exhibited marked improvement in their periorbital signs and symptoms as reported in the clinical trials. In patient 1, there had been no clinical or HVF improvement with IV solumedrol. In patient 2, an improvement in periorbital signs was noted after initation of IV solumedrol; however, there was no improvement in her proptosis OU and her VF defect persisted OD. Both patients demonstrated complete resolution of the VF defects only after treatment with teprotumumab.
Although the role of teprotumumab in the treatment of TED-CON has yet to be evaluated in a randomized clinical trial, the rapid and complete resolution of VF defects in these two patients suggests that tetromumab is effective in treating Stages 1 and 2 VF defects in TED-CON in which the duration of VF loss is up to several months. Although this report is limited by the small number of patients, lack of controls and short follow-up period, the rapid response to teprotumumab after a poor response to weekly IV corticosteroid therapy raises the question of whether Teprotumumab may be superior to IV corticosteroid therapy in the treatment of TED-CON. This study adds to the emerging literature supporting teprotumumab as a viable treatment option for this subset of patients.7,8
5. Conclusion
These cases demonstrate that teprotumumab can be a rapid and effective treatment for patients with TED-CON, and may be considered as an alternative to steroid therapy or orbital decompression.
Patient consent
This report does not contain any personal information that could lead to the identification of the patient.
Funding sources
Heed Ophthalmic Foundation Fellowship to Edith R. Reshef, MD.
Disclosures
The following authors have no financial disclosures: C.A.C. and E.R.R. Suzanne K Freitag is a consultant and on the advisory board of Horizon Therapeutics USA, Inc.
All authors attest that they meet the current ICMJE criteria for Authorship.
Acknowledgements
None.
References
- 1.Douglas R.S. Teprotumumab, an insulin-like growth factor-1 receptor antagonist antibody, in the treatment of active thyroid eye disease: a focus on proptosis. Eye. 2019;33:183–190. doi: 10.1038/s41433-018-0321-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartalena L., Krassas G.E., Wiersinga W. Efficacy and safety of three different cumulative doses of intravenous methylprednisolone for moderate to severe and active Graves' orbitopathy. J Clin Endocrinol Metab. 2012;97:4454–4463. doi: 10.1210/jc.2012-2389. [DOI] [PubMed] [Google Scholar]
- 3.Strianese D. Efficacy and safety of immunosuppressive agents for thyroid eye disease. Ophthalmic Plast Reconstr Surg. 2018;34:S56–S59. doi: 10.1097/IOP.0000000000001131. [DOI] [PubMed] [Google Scholar]
- 4.Douglas R.S., Kahaly G.J., Patel A. Teprotumumab for the treatment of active thyroid eye disease. N Engl J Med. 2020;382:341–352. doi: 10.1056/NEJMoa1910434. [DOI] [PubMed] [Google Scholar]
- 5.Smith T.J., Kahaly G.J., Ezra D.G. Teprotumumab for thyroid-associated ophthalmopathy. N Engl J Med. 2017;376:1748–1761. doi: 10.1056/NEJMoa1614949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Freitag S.K., Tanking T. A nomenclature to describe the sequence of visual field defects in progressive thyroid eye disease–compressive optic neuropathy (an American ophthalmological society thesis) Am J Ophthalmol. 2020;213:293–305. doi: 10.1016/j.ajo.2019.12.005. [DOI] [PubMed] [Google Scholar]
- 7.Sears C.M., Azad A.D., Dosiou C., Kossler A.L. Teprotumumab for dysthyroid optic neuropathy. Ophthalmic Plast Reconstr Surg. 2020 Sep 22 doi: 10.1097/IOP.0000000000001831. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 8.Slentz D.H., Smith T.J., Kim D.S., Joseph S.S. Teprotumumab for optic neuropathy in thyroid eye disease. JAMA Ophthalmol. 2021;139:244. doi: 10.1001/jamaophthalmol.2020.5296. [DOI] [PubMed] [Google Scholar]