Highlights
-
•
IBS and Ulcerative Colitis (UC) patients have changes in brain network organization.
-
•
Changes in brain network organization correlate with disease symptomatology.
-
•
In UC, brain network organization changes correlate with disease duration.
-
•
Chronic gut inflammation as well as pain have neuroplastic effects on the brain.
Abbreviations: BGM, brain-gut-microbiome; BSQ, bowel symptom questionnaire; CNS, central nervous system; DWI, diffusion weighted imaging; EC, eigenvector centrality; FDR, false discovery rate; GI, gastrointestinal; GLM, general linear model; HADS, hospital anxiety and depression scale, HCs, healthy controls; IBD, inflammatory bowel disease; IBS, irritable bowel syndrome; MNI, montreal neurological institute; MRI, magnetic resonance imaging; PILL, pennebaker inventory of limbic lambiguity; PSS, perceived stress scale; PTI, powell tuck index; ROIs, regions of interest; SD, standard deviation; SII, secondary somatosensory cortex; STAI, state-trait anxiety inventory; UC, ulcerative colitis; VSI, visceral sensitivity index
Keywords: IBD, Brain imaging, Ulcerative colitis, Centrality, Graph theory, Diffusion weighted imaging
Abstract
Objective
We aimed to identify differences in network properties of white matter microstructure between asymptomatic ulcerative colitis (UC) participants who had a history of chronic gut inflammation, healthy controls (HCs) and a disease control group without gut inflammation (irritable bowel syndrome; IBS).
Design
Diffusion weighted imaging was conducted in age and sex-matched participants with UC, IBS, and HCs (N = 74 each), together with measures of gastrointestinal and psychological symptom severity. Using streamline connectivity matrices and graph theory, we aimed to quantify group differences in brain network connectivity. Regions showing group connectivity differences were correlated with measures showing group behavioral and clinical differences.
Results
UC participants exhibited greater centrality in regions of the somatosensory network and default mode network, but lower centrality in the posterior insula and globus pallidus compared to HCs (q < 0.05). Hub analyses revealed compromised hubness of the pallidus in UC and IBS compared to HCs which was replaced by increased hubness of the postcentral sulcus. Surprisingly, few differences in network matrices between UC and IBS were identified. In UC, centrality measures in the secondary somatosensory cortex were associated with depression (q < 0.03), symptom related anxiety (q < 0.04), trait anxiety (q < 0.03), and symptom duration (q < 0.05).
Conclusion
A history of UC is associated with neuroplastic changes in several brain networks, which are associated with symptoms of depression, trait and symptom-related anxiety, as well as symptom duration. When viewed together with the results from IBS subjects, these findings suggest that chronic gut inflammation as well as abdominal pain have a lasting impact on brain network organization, which may play a role in symptoms reported by UC patients, even when gut inflammation has subsided.
1. Introduction
Ulcerative colitis (UC) is one of the inflammatory bowel diseases (IBD) which, combined with Crohn’s disease, affects an estimated 1.3% of US. Adults (Dahlhamer et al., 2016). UC is characterized by chronically recurring inflammation of colonic mucosa, with symptoms of altered bowel habits, rectal bleeding, and abdominal pain (Bielefeldt et al., 2009). Acute inflammation in UC has been shown to cause visceral, peripheral, and central sensitization resulting in hyperalgesia (Mawe et al., 2009, Mayer and Gebhart, 1994) present during but not in between periods of active inflammation (Chang et al., 2000, V., L.-B., A.M., M., S., S., , 1989). Chronic gut inflammation in UC has recently been shown to increase the risk of cognitive decline, supporting the hypothesis of chronic gut inflammation affecting brain networks (Zhang et al., 2020). Irritable bowel syndrome (IBS), the most common functional gastrointestinal (GI) disorder, affects 7–21% of the general population (Chey et al., 2015, Drossman, 2006). Like UC, IBS presents as recurring abdominal pain associated with altered bowel habits (Longstreth et al., 2006). However, in IBS, there is a consensus that these symptoms occur in the absence of GI inflammation (Mayer and Bushnell, 2015) as a consequence of altered brain-gut interactions (Mayer and Brunnhuber, 2012, Mayer and Tillisch, 2011).
Despite the presence of GI mucosal inflammation, UC patients report less severe abdominal pain and preoccupation than IBS patients. However, both groups report increased pain and visceral sensitivity compared with healthy controls (HCs)(Chang et al., 2000, Mayer et al., 2005). Based on these differences in perceptual sensitivity together with reported variations in the activation of brain regions involved in endogenous pain modulation, we have previously proposed that these patients differ in the central processing of chronic visceral pain (Keltner, 2006, Piché et al., 2010, Porro et al., 2002), presumably by engagement of endogenous pain facilitation systems (Grupe and Nitschke, 2013, Paulus and Stein, 2006, Porro et al., 2002, Song et al., 2006, Straube et al., 2009, Wiech et al., 2008). Psychiatric comorbidities, frequently present in UC and IBS patients, are associated with greater symptom severity and more frequent disease flares (Lackner et al., 2018, Mittermaier et al., 2004, Shah et al., 2014). Both groups have higher rates of anxiety and depression than HCs (Gracie et al., 2018, Graff et al., 2009, Henningsen et al., 2012, Panara et al., 2014), with UC patients traditionally experiencing fewer lifetime psychiatric diagnoses than IBS patients (Walker et al., 1990).
Changes in the central nervous system (CNS), specifically in brain regional activity associated with rectal distension, and in grey matter volume, have been demonstrated in both UC and IBS, however disease related network alterations have only been reported in IBS (Hong et al., 2014, Icenhour et al., 2017, Qi et al., 2016, Yuan et al., 2003). Correlations between symptom duration and gray matter thickness have been seen in UC, consistent with neuroplastic changes secondary to chronically recurring abdominal pain and peripheral inflammation (Hong et al., 2014). In addition to a growing body of neuroimaging evidence, the effectiveness of cognitive therapies in IBS and IBD (Hall et al., 2018, Macer et al., 2017, Mikocka-Walus et al., 2012), further point to the vital role of the brain in the experience and expression of these chronic pain conditions.
We performed advanced brain network analysis using graph theory, focusing on white matter structural connectivity, and compared the centrality of regions in the salience, somatosensory, basal ganglia, and default mode networks. By assessing structural centrality in regions within these networks in UC, compared to two control populations (HCs and IBS), we aimed to test two non-exclusive hypotheses: (1) compared to HCs, asymptomatic UC patients with a longstanding history of gut inflammation and abdominal pain show alterations in white matter connectivity and network architecture, suggesting long lasting neuroplastic effects of gut inflammation that persist in the absence of symptomatic mucosal inflammation, and (2) compared to IBS, UC patients possess common brain alterations, particularly in the basal ganglia and somatosensory networks, suggesting a shared, long lasting influence of chronic abdominal pain on these regions.
2. Methods
2.1. Study participants
Seventy-four (39 female) participants with UC with a history of greater than one year of active, symptomatic disease who were symptom-free at the time of the study, 74 participants with IBS, in clinical remission with mild subjective symptoms at the time of the imaging study, (39 female) and 74 HCs (39 female) were recruited from the University of California Los Angeles (UCLA) and the wider Los Angeles community, as well as the University of Manitoba (UM) (Bernstein et al., 2006). IBS and HCs were age and sex matched to UC. All participants were right-handed. UCLA participants were recruited through advertisements circulated through online social media websites, local newspapers, university and hospital community list serves and mailing lists, and flyers were posted in the greater Los Angeles area and on the UCLA campus. The UM participants were recruited through the UM Registry, developed using an administrative definition of IBD to identify all individuals in Manitoba with IBD. Individuals in the Registry agree to be contacted about research initiatives, but participation is voluntary.
Exclusion criteria were substance abuse or tobacco dependence (smoked half a package of cigarettes or more daily), abdominal surgery, extremely strenuous exercise (more than 8 h/week of continuous exercise), active corticosteroid use, claustrophobia, metal implants, medical or neurological conditions, age greater than 50, and presence of past or current psychiatric disorders, as determined by the Mini International Neuropsychiatric Interview (Sheehan et al., 1998).
All procedures complied with the principles of the Declaration of Helsinki and all UCLA participants were approved by the Institutional Review Board at UCLA’s Office of Protection for Research Subjects and UM participants by the University of Manitoba Research Ethics Board. All participants provided written informed consent prior to the beginning of the experiment.
2.2. Behavioral measures
UC and IBS participants were administered the Bowel Symptom Questionnaire (BSQ), a validated questionnaire assessing self-reported symptom severity of GI symptoms, bloating, and abdominal pain in the past week on a scale from 0 to 20 (Talley et al., 1989). A score of zero denotes no complaints and the highest score refers to severe symptom experience. Other relevant measures include age of symptom onset, flare frequency and how long the patient is usually symptom-free.
The Powell Tuck Index (PTI) score was used to measure symptom severity in UC participants, with scores increasing with symptom severity. A PTI < 5 was used as a measure of remission (Powell-Tuck et al., 1978). Several measures of self-reported symptom severity were assessed. These measures included; 1) the Abdominal Symptom Intensity and Unpleasantness (24 h); 2) the Visceral Sensitivity Index (VSI), a 15-item questionnaire designed to measure fear, anxiety, and hypervigilance accompanying visceral sensations (Labus et al., 2004); and 3) the Perceived Stress Scale (PSS), the most widely used psychological instrument for measuring stress perception (Cohen et al., 1983).
Several measures of mood, behavior, and attribution framework were assessed. These included 1) The Pennebaker Inventory of Limbic Languidness (PILL) questionnaire, used to measure general sensory perception, including visceral and somatic sensations (Pennebaker, 1982); 2) The State-Trait Anxiety Inventory (STAI), a 20-item tool scored from 20 to 80; scores greater than 40 were considered clinical anxiety cases (Spielberger, 1983) 3) The Hospital Depression and Anxiety Scale (HADS), used to assess depression and anxiety in the past week (Zigmond and Snaith, 1983).
2.3. Imaging acquisition
Using a Siemens Magnetom Trio 3T at UCLA and Siemens Magnetom Verio 3T at UM, whole brain structural, and diffusion weighted imaging (DWI) was performed. Noise reducing headphones were used.
2.4. Structural MRI
A high resolution T1-structural image was acquired for each participant for registration purposes with a Magnetization-Prepared Rapid Gradient-Echo (MPRAGE) sequence, repetition time (TR) = 2200 ms, echo time (TE) = 3.26 ms, acquisition time (TA) = 5mins 12 s, slice thickness = 1 mm, 176 slices, 256*256 voxel matrix, 1 mm voxel size.
2.5. Diffusion weighted MRI (DWI):
Diffusion-weighted images (DWIs) were acquired according to two comparable acquisition protocols. Specifically, DWIs were acquired in either 61 or 64 non-collinear directions with b = 1000 s/mm2, with 8 or 1b = 0 s/mm2 images, respectively. Both protocols had a TR = 9400 ms, TE = 88 ms, and field of view (FOV) = 256 mm with an acquisition matrix of 128x128, and a slice thickness of 2 mm to produce 2 × 2 × 2 mm3 isotropic voxels.
2.6. Structural MRI preprocessing
After acquiring the raw imaging data, preprocessing for structural images were done in SPM12 (Penny et al., 2007). This included bias-field correction, co-registration, motion correction, spaitial normalization, tissue segmentation and fourier transformation. Structural images were included based on compliance with acquisition protocol, full brain coverage, minimal motion (<2 mm in all directions), absence of flow/zipper, and minor atrophy/vascular degeneration.
2.7. Diffusion weighted MRI (DWI) preprocessing
All diffusion images were corrected for eddy current-induced distortions and movement using the eddy_correct tool in FSL (Andersson and Sotiropoulos, 2016). Images, b-vectors and b-values were then converted from FSL to Camino data formats using fsl2scheme and image2voxel in Camino. (Cook et al., 2006) A diffusion tensor was fit on voxel-order data in Camino using wdtfit using weighted linear least squares regression. (Cook et al., 2006) Whole-brain deterministic tractography was then performed using the track command in Camino euler algorithm with a step size of 0.5 and curve threshold of 76. Connectivity matrices were the constructed using the conmat command in Camino, which computes a matrix counting the number of streamlines connecting each pair of ROIs created using the FreeSurfer pipeline. Preprocessing for DWI included visually checking for artifacts and motion on the raw diffusion weighted and b0 images, visual assessment of FA and mean diffusivity (MD) map quality, as well checking for physiologically feasible FA and MD values (FA of 0–0.1 and MD of 3–4 μm2/ms in ventricles, and FA of 0.6–0.9 and MD of 0.6–0.9 μm2/ms in splenium of corpus callosum). Maximum relative motion thresholds for translation and rotation for each direction (x, y, and z) were set at 2 mm and 2°, respectively.
2.8. Anatomical network construction
The fundamental step for brain network analysis is to define the nodes (i.e., brain regions) and edges (i.e., number of fiber tracts connecting regions). Gray matter was parceled into 165 regions based on the Destrieux (Destrieux et al., 2010) and Harvard-Oxford Subcortical atlases (Desikan et al., 2006), all of which served as network nodes. We investigated specific regions of interest (ROIs) based on prior neuroimaging research on IBS (Bhatt et al., 2019a, Ellingson et al., 2013a, Ellingson et al., 2013b, Mayer et al., 2015), IBD (Kornelsen et al., 2020), and chronic pain participants (Bhatt et al., 2019b, Gupta et al., 2019, Woodworth et al., 2015). The ROIs and their associated functional networks included: somatosensory network (precentral gyrus and sulcus [M1], supplementary motor area [SMA/M2], postcentral gyrus and sulcus [S1], thalamus, posterior insula [pINS]); basal-ganglia network (nucleus accumbens [NAcc], putamen [Pu], globus pallidus [Pal] and caudate nucleus [CaN]); salience network (anterior insula [aINS], anterior midcingulate cortex [aMCC], orbitofrontal cortex [OFC]), and default mode network (precuneus [PrCun], posterior dorsal part of the cingulate gyrus [PosDCgG], angular gyrus [AngG], transverse frontopolar gyri and sulci [TrFPoG_S], middle temporal gyrus [MTG], lateral aspect of the superior temporal gyrus [SupTGLp]).
Regional parcellation and tractography results were combined to produce a weighted, undirected connectivity matrix. White matter connectivity for each participant was estimated between the 165 brain regions using DWI fiber tractography (Irimia and Van Horn, 2013), performed via the Fiber Assignment by Continuous Tracking (FACT) algorithm using TrackVis (http://trackvis.org). The final estimate of white matter connectivity between each brain region was determined based on the number of fiber tracts intersecting each region. Connection weights were then expressed as the absolute fiber count divided by the individual volumes of the two interconnected regions (Irimia et al., 2012).
2.9. Computing network metrics
The Graph Theory GLM toolbox (GTG) (www.nitrc.org/projects/metalab_gtg) and in-house MATLAB scripts were applied to the participant-specific anatomical brain networks indexing ROI centrality. Measures of centrality are the most common measures of global connectedness identifying brain regions most likely to participate in integrative processing. Three centrality metrics were selected: 1) Strength reflects the weighted version of the number of connections to a given node. 2) Betweenness centrality describes the degree to which a region lies on the shortest path between two other regions, and thus its role in controlling information flow. 3) Eigenvector centrality reflects the number of highly connected brain regions to which a brain region is connected (Rubinov and Sporns, 2010).
2.10. Hub analysis
Hub nodes. Nodes with high centrality are often referred to as hub nodes. Hub node disruptions significantly reduce the global brain network efficiency and have been implicated in several disorders (Crossley et al., 2016, Crossley et al., 2014). We performed a hub analysis to determine whether UC participants showed differences in hub regions when compared to IBS and HCs. Hub regions were defined as nodes with eigenvector centrality (EC) at least one standard deviation (SD) greater than the mean EC across all nodes, i.e., EC(i) > mean(EC) + std(EC) (Gong et al., 2012, 2009). Major classes of neural responses to perturbation can be inferred from these analyses (Fornito et al., 2015).
Centrality disruption. We performed a centrality disruption analysis to measure how the nodes in each network are reorganized in UC compared to disease groups (Achard et al., 2012). First the mean EC of 165 nodes in the three groups were calculated. Second, each nodal EC in HCs (x-axis) versus [disease group – HCs] (y-axis) was plotted to evaluate the slope of the fitted line. A negative slope indicates a nodal EC higher in HCs and lower in disease groups. A positive slope indicates a nodal EC higher in disease groups and lower in the HCs. If the slope is zero, it means HCs and disease groups have the same nodal EC. Centrality disruption will be interpreted as a positive or negative slope, indicating a statistically significant difference in the nodal EC between groups.
2.11. Experimental design and statistical analysis
Planned contrast analyses based in the general linear model (GLM) for independent groups was applied to test for group differences in clinical variables and the centrality of ROIs, while controlling for age and sex in MATLAB. Three linear contrasts were specified: (1) UC participants vs IBS participants; (2) UC participants vs. HCs; (3) IBS participants vs. HCs. A false-discovery rate (FDR) was applied to determine statistical significance at 5% (Chumbley et al., 2010). This correction was performed within each contrast and centrality measure, by the number of regions in each network (basal ganglia, salience, somatosensory, default mode), and by laterality (left vs. right). GLMs were run to evaluate between-group differences in overall network density, or the fraction of present connections (i.e. non-zero connections in the connectome matrix) to all possible connections between each ROI (Rubinov and Sporns, 2010). Significance was set at FDR corrected p-value (q < 0.05). The effect size Cohen’s d was computed for each contrast. To determine the associations between the disease-related alterations in network metrics and clinical variables, partial correlations were performed while controlling for age and sex. Significance was set at FDR corrected q-value < 0.05.
Additionally, one way ANOVAs were run to determine if there were differences in network metrics in UC patients who had symptoms across different locations in the colon (Supplementary Material).
3. Results
3.1. Demographics, clinical and behavioral variables
Mean scores on the clinical questionnaires are presented in (Table 1).
Table 1.
Descriptive Statistics for Participants with Ulcerative Colitis, Irritable Bowel Syndrome, and Healthy Controls.
| UC | IBS | HCs | |||||||
|---|---|---|---|---|---|---|---|---|---|
| N = 74 (39F, 35 M) | N = 74 (39F, 35 M) | N = 74 (39F, 35 M) | |||||||
| Measurement | Mean(SD) | Range | N | Mean(SD) | Range | N | Mean(SD) | Range | N |
| Age (years) | 32.34 (10.64) | 42 | 74 | 32.8 (10.48) | 39 | 74 | 32.32 (10.28) | 39 | 74 |
| Symptom Severity | |||||||||
| Abdominal Symptom Intensity 24 h | 4.53 (4.14) | 14 | 70 | 8.87 (3.88) | 16 | 72 | 0.692 (1.44) | 5 | 13 |
| Abdominal Symptom Unpleasantness 24 h | 3.89 (3.47) | 13 | 69 | 7.69 (3.24) | 14 | 72 | 0.54 (1.20) | 4 | 13 |
| Symptom Duration | 11.61 (8.81) | 49 | 74 | 13.29 (10.38) | 50 | 72 | N/A | N/A | N/A |
| Psychological | |||||||||
| VSI Score | 25.55 (16.03) | 59 | 70 | 36.47 (16.19) | 72 | 74 | 7.32 (3.88) | 17 | 71 |
| PSS Score | 13.87 (6.47) | 29 | 70 | 17.12 (6.93) | 31 | 61 | 9.48 (5.42) | 23 | 71 |
| PILL Score | 13.66 (7.62) | 31 | 56 | 14.96 (7.65) | 34 | 69 | 4.72 (5.27) | 21 | 54 |
| HADS Anxiety | 5.94 (3.14) | 12 | 57 | 7.49 (4.15) | 16 | 74 | 3.12 (3.14) | 13 | 74 |
| HADS Depression | 2.96 (2.98) | 13 | 57 | 3.3 (2.82) | 11 | 74 | 1.23 (2.41) | 14 | 74 |
| STAI Trait Anxiety | 48.81 (9.13) | 37 | 69 | 56.93 (12.72) | 46 | 60 | 44.17 (8.44) | 38 | 71 |
| STAI State Anxiety | 45.53 (8.9) | 40 | 74 | 49.56 (8.86) | 35 | 54 | 41.88 (7.39) | 36 | 66 |
KEY: Groups: UC – ulcerative colitis, IBS – irritable bowel syndrome, HCs – healthy control
Questionnaires: VSI – Visceral Sensitivity Index, PSS – Perceived Stress Scale, PILL – Pennebaker Inventory of Limbic Languidness, HADS – Hospital Anxiety and Depression Scale, STAI – State Trait Anxiety Inventory
UC patients had an average disease duration of 12 years (range 1–50 years); 23% of the patients had pancolitis, 5% had subtotal colitis, 32% had left sided, and 21% had rectal or rectosigmoid disease and in the remaining 19% the information was not available. IBS patients had an average disease duration of 13 years (range 1–51). Nineteen IBS participants were constipation predominant, 32 were diarrhea predominant, and the remaining 23 participants experienced mixed constipation and diarrhea.
The mean age for each group was 32 years. Medication use is presented in (Table 2). Of the patients with UC, fourty-five were on mesalamine therapy, ten were on thiopurine agents, and six were on immunosuppressive agents. All UC patients had a history of steroid use, but none were on steroids at the time of enrollment or during the study. Seventeen UC participants were taking analgesics, compared to 7 IBS participants.
Table 2.
Patient Medication Use.
| HC | IBS | UC | Total | |
|---|---|---|---|---|
| N = 74 | N = 74 | N = 74 | N = 222 | |
| Medication | Number of Patients | |||
| Acetaminophen | 7 | 7 | 17 | 31 |
| Antidepressant (SNRI) | 0 | 0 | 2 | 2 |
| Antidepressant (SSRI) | 0 | 5 | 1 | 6 |
| Antidepressant (Tricyclic) | 0 | 1 | 0 | 1 |
| Antidiarrheal | 0 | 9 | 4 | 13 |
| Antihistamine | 8 | 7 | 5 | 20 |
| Benzodiazepine | 0 | 3 | 0 | 3 |
| Cannabis | 2 | 2 | 4 | 8 |
| Birth Control Pills | 8 | 10 | 10 | 28 |
| Immunosuppressants | 0 | 0 | 16 | 16 |
| Mesalamine | 0 | 0 | 45 | 45 |
| NSAID | 10 | 17 | 11 | 38 |
| Opioid | 0 | 0 | 3 | 3 |
| Probiotic | 3 | 8 | 5 | 16 |
| Supplement | 25 | 28 | 36 | 89 |
Medications actively used by patients at time of enrollment and throughout duration of study.
UC participants. The PTI showed scores of zero for all UC participants, consistent with clinical remission, and the Bowel Symptom Questionnaire showed overall low symptoms (overall symptom severity: mean = 5.42, SD = 3.58; abdominal pain = 5.17(1.15); bloating = 4.92(4.27) on a scale from 0 to 20). Since chronic bowel symptoms were absent in HCs, no comparison was performed for bowel symptom scores between HCs and UC.
3.2. Ulcerative colitis vs healthy controls
Psychological Measures. UC participants had significantly higher symptom-related anxiety and somatic focus (p’s < 0.01), significantly higher anxiety (HADS and STAI State and Trait), and depression (p’s < 0.01). However, all scores were within the normal range of values for these surveys, indicating subclinical anxiety and depression. Table 3.
Table 3.
Differences Between Groups on Clinical and Behavioral Measurements.
| UC vs. HCs | UC vs. IBS | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Measurement | F-value | df | β | p-value | Cohen's d | Interpretation | F-value | df | β | p-value | Cohen's d | Interpretation |
| Age (years) | 0.000062 | (1, 219) | 0.01 | 0.99 | −0.0019 | – | 0.07 | (1, 219) | −0.46 | 0.79 | 0.04 | – |
| Symptom Severity | ||||||||||||
| Abdominal Symptom Intensity 24 h | 10.77 | (1, 152) | 3.84 | 0.001 | −1.24 | UC > HC | 44.61 | (1, 152) | −4.34 | 4.25E-10 | 1.08 | IBS > UC |
| Abdominal Symptom Unpleasantness 24 h | 11.66 | (1, 151) | 3.34 | 0.001 | −1.29 | UC > HC | 48.85 | (1, 151) | −3.81 | 8.30E-11 | 1.13 | IBS > UC |
| Psychological | ||||||||||||
| VSI Score | 102.51 | (1, 212) | 22.81 | 6.60E-20 | −1.56 | UC > HC | 24 | (1, 212) | −10.93 | 2.00E-06 | 0.68 | IBS > UC |
| PSS Score | 17.3 | (1, 199) | 4.39 | 4.80E-05 | −0.74 | UC > HC | 8.73 | (1, 199) | −3.25 | 0.004 | 0.48 | IBS > UC |
| PILL Score | 44.71 | (1, 176) | 8.94 | 2.92E-10 | −1.26 | UC > HC | 1.06 | (1, 176) | −1.3 | 0.3 | 0.17 | – |
| HADS Anxiety | 20.46 | (1, 202) | 2.82 | 1.00E-05 | −0.9 | UC > HC | 6.14 | (1, 202) | −1.55 | 0.014 | 0.42 | IBS > UC |
| HADS Depression | 13.17 | (1, 202) | 1.74 | 3.79E-04 | −0.64 | UC > HC | 0.48 | (1, 202) | −0.332 | 0.49 | 0.12 | – |
| STAI Trait Anxiety | 7.35 | (1, 197) | 4.64 | 0.007 | −0.53 | UC > HC | 20.64 | (1, 197) | −8.12 | 1.00E-05 | 0.73 | IBS > UC |
| STAI State Anxiety | 6.57 | (1, 191) | 3.65 | 0.011 | −0.45 | UC > HC | 7.17 | (1, 191) | −4.03 | 0.008 | 0.45 | IBS > UC |
KEY: Groups: UC – ulcerative colitis, IBS – irritable bowel syndrome, HCs – healthy control; Questionnaires: VSI – Visceral Sensitivity Index, PSS – Perceived Stress Scale, PILL – Pennebaker Inventory of Limbic Languidness, HADS – Hospital Anxiety and Depression Scale, STAI – State Trait Anxiety Inventory; Statistics: F – F statistic, df – degrees of freedom, β - unstandardized beta
3.3. Ulcerative colitis vs irritable bowel syndrome
Psychological Measures. UC participants had significantly lower symptom-related anxiety (p’s < 0.01), HADS (p = 0.01) and STAI State and Trait anxiety (p < 0.01). Table 3.
3.4. Irritable bowel syndrome vs healthy controls
Psychological Measures. IBS participants had significantly greater symptom related anxiety and somatic focus (p’s < 0.01), and significantly greater HADS anxiety and depression and STAI State and Trait anxiety (p’s < 0.01) (Supplementary Material).
3.5. Diffusion weighted brain imaging
3.5.1. Ulcerative colitis versus healthy controls
Significant group differences in centrality were seen in basal ganglia, somatosensory, and default mode networks; nodes with statistically significant metric differences are displayed in Fig. 1A, 2A, 3. (Fig. 1A, 2A, 3, Table 4). No differences were seen in network density (p = 0.21).
Fig. 1.
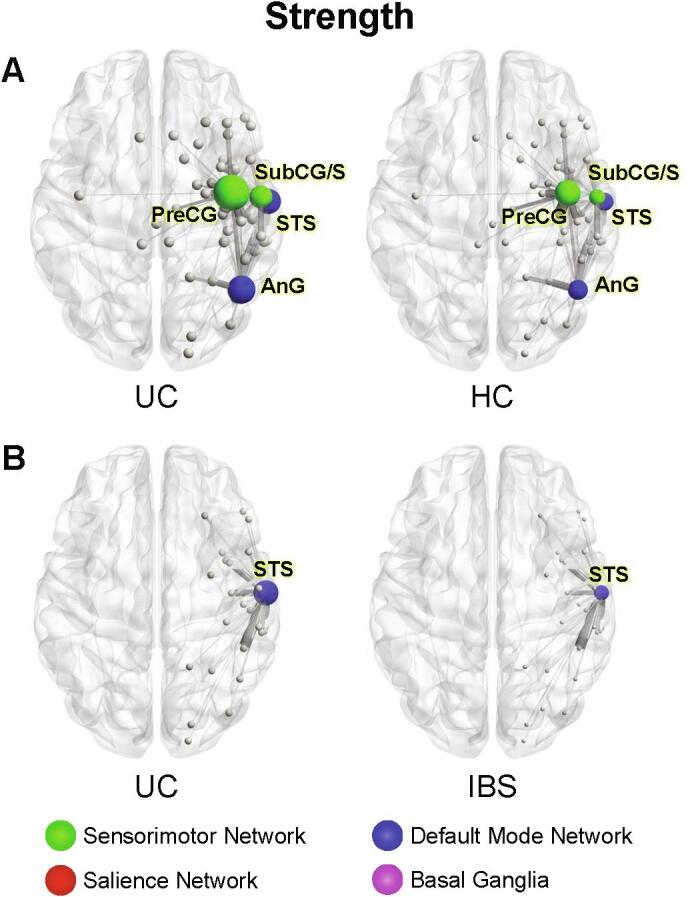
Significant group differences in nodal strength A. UC compared to HCs Image illustrates greater strength in the precentral gyrus (PreCG) and subcentral gyrus and sulcus (SubCG/S) of the sensorimotor network in UC compared to HCs, and greater strength in the superior temporal sulcus (STS) and angular gyrus (AnG) of the default mode network in UC compared with HCs. B. UC compared to IBS Image illustrates greater strength in the superior temporal sulcus (STS) of UC compared with IBS. Circle size represents relative node centrality. Lines represent connections to other nodes. Only statistically significant differences in network metrics are shown. STS: Superior Temporal Sulcus, AnG: Angular Gyrus, PreCG: Precentral Gyrus, SubCG/S: Subcentral gyrus and sulcus, UC: Ulcerative Colitis, HCs: Healthy Controls; IBS Irritable bowel syndrome.
Fig. 2.
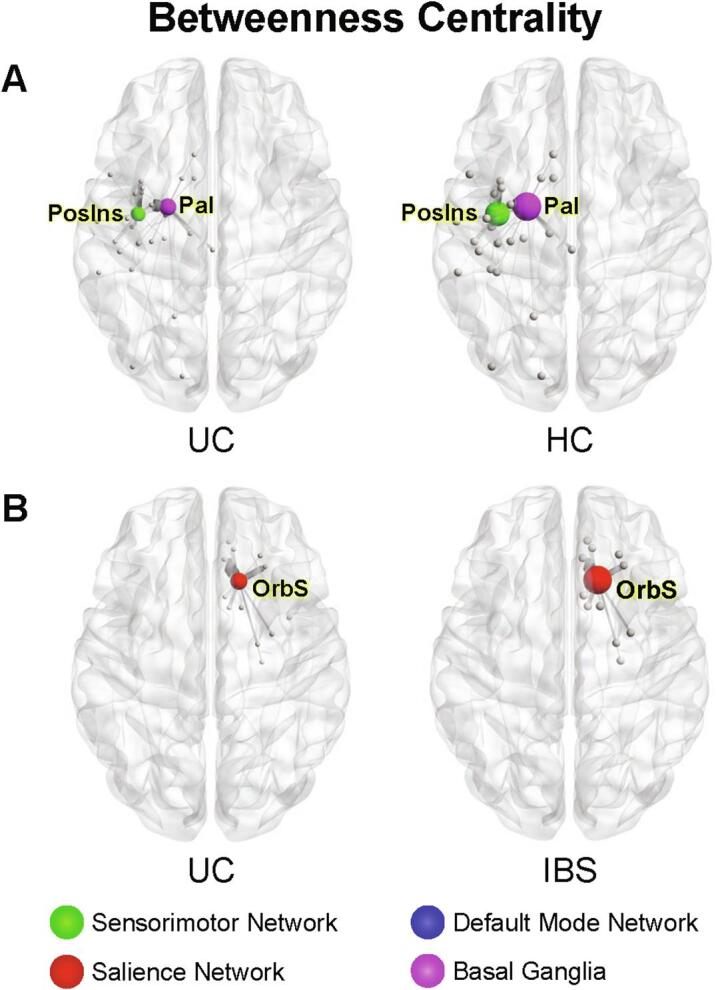
Significant group differences in betweenness centrality A. UC compared to HCs Image represents lower betweenness centrality in posterior insula (PosIns) of the sensorimotor network and globus pallidus (Pal) of the basal ganglia network of UC compared to HCs. B. UC compared to IBS Image represents lower betweenness centrality in the orbital sulcus (OrbS) of the salience network in UC compared to IBS. Circle size represents relative node centrality. Lines represent connections to other nodes. Only statistically significant differences in network metrics are shown. PosIns: Posterior insula, Pal: Globus pallidus, UC: Ulcerative Colitis, HCs: Healthy Controls, OrbS: Orbital sulcus.
Fig. 3.
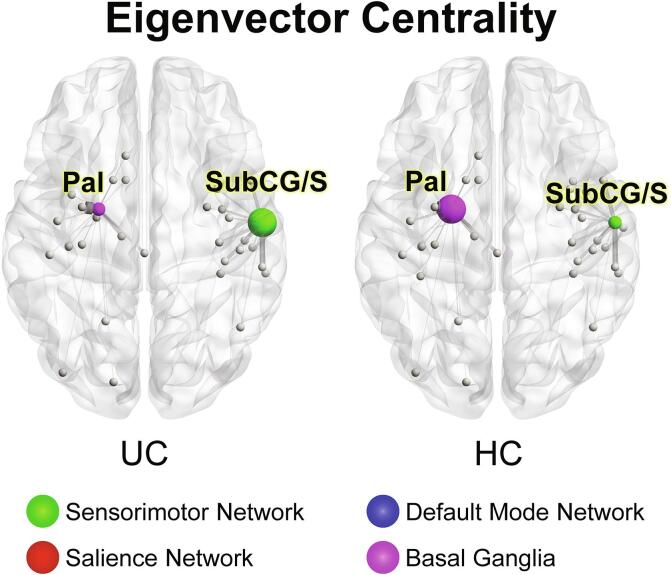
Significant group differences in eigenvector centrality in UC compared to HCs Demonstrating lower eigenvector centrality in the globus pallidus (Pal) of the basal ganglia network and subcentral gyrus and sulcus (SubCG/S) of the sensorimotor network in UC compared to HCs. Circle size represents relative node centrality. Lines represent connections to other nodes. Only statistically significant differences in network metrics are shown.SubCG/S: Subcentral gyrus and sulcus, Pal: Globus pallidus, UC: Ulcerative Colitis, HCs: Healthy Controls.
Table 4.
UC vs HCs Network Metrics.
| UC (1) vs. HC (-1) | ||||||
|---|---|---|---|---|---|---|
| DTI | ||||||
| Region of Interest | β value | t-value | SE | p-value | q -value | Interpretation |
| Somatosensory Network | ||||||
| Betweenness Centrality | ||||||
| Left long insular gyrus and central sulcus of the insula | -128.12058 | -3.6573565 | 35.0309236 | 0.00093995 | 0.01926895 | UC<HC |
| Eigenvector Centrality | ||||||
| Right subcentral gyrus and sulci | 0.00892303 | 3.45087547 | 0.00258573 | 0.00201514 | 0.02686857 | UC>HC |
| Strength | ||||||
| Right Subcentral gyrus and sulci | 0.0214594 | 3.46627059 | 0.00619092 | 0.00183254 | 0.02443388 | UC>HC |
| Right precentral gyrus | 0.02778443 | 3.29630373 | 0.00842897 | 0.0032364 | 0.03236402 | UC>HC |
| Salience Network | ||||||
| Betweenness Centrality | ||||||
| No significant results found | ||||||
| Eigenvector Centrality | ||||||
| No significant results found | ||||||
| Strength | ||||||
| No significant results found | ||||||
| Basal Ganglia Network | ||||||
| Betweenness Centrality | ||||||
| Left Pallidum | -449.47871 | -3.8432992 | 116.951266 | 0.00046745 | 0.01916534 | UC<HC |
| Eigenvector Centrality | ||||||
| Left pallidum | -0.0153934 | -3.7997455 | 0.00405118 | 0.00052961 | 0.02171416 | UC<HC |
| Strength | ||||||
| No significant results found | ||||||
| Default Mode Network | ||||||
| Betweenness Centrality | ||||||
| No significant results found | ||||||
| Eigenvector Centrality | ||||||
| No significant results found | ||||||
| Strength | ||||||
| Right lateral aspect of the superior temporal gyrus | 0.00995621 | 4.00649075 | 0.00248502 | 0.00025778 | 0.00896518 | UC>HC |
| Right angular gyrus | 0.01852886 | 3.84846218 | 0.00481461 | 0.00044826 | 0.00896518 | UC>HC |
KEY: Groups: UC – ulcerative colitis, HCs – healthy control; Statistics: β – unstandardized beta, t – t statistic, SE – standard error, p – p value, q – FDR corrected p value
3.5.1.1. Basal ganglia network
Betweenness centrality (q = 0.019) and eigenvector centrality (q = 0.022), two measures of the importance of a brain region in controlling information flow, were lower in the left globus pallidus of UC participants.
3.5.1.2. Somatosensory network
In UC participants betweenness centrality was lower in the left posterior insula (long insular gyrus and central sulcus) (q = 0.019), while eigenvector centrality was greater in the right secondary somatosensory cortex (SII) (subcentral gyrus and sulci) (q = 0.027).
Strength, a measure of the overall number of connections to a given brain region, was greater in the right SII (subcentral gyrus and sulci) (q = 0.024) and the right primary motor cortex (precentral gyrus) (q = 0.032).
3.5.1.3. Default mode network
Strength was greater in the right lateral aspect of the superior temporal gyrus (q = 0.009) and right inferior parietal lobule (angular gyrus) (q = 0.009).
3.5.2. Ulcerative colitis versus irritable bowel syndrome
Significant group differences in centrality and strength measures were seen in both the salience and default mode networks; nodes with statistically significant metric differences are displayed in Fig. 1B and 2B (Fig. 1B, 2B, Table 5). No between-group differences in network density were seen (p = 0.05).
Table 5.
UC vs IBS Network Metrics.
| UC (1) vs. IBS (-1) | ||||||
|---|---|---|---|---|---|---|
| DTI | ||||||
| Region of Interest | β value | t-value | SE | p-value | q -value | Interpretation |
| Somatosensory Network | ||||||
| Betweenness Centrality | ||||||
| No significant results found | ||||||
| Eigenvector Centrality | ||||||
| No significant results found | ||||||
| Strength | ||||||
| No significant results found | ||||||
| Salience Network | ||||||
| Betweenness Centrality | ||||||
| Right Medial orbital sulcus | -175.35278 | -3.5881348 | 48.8701758 | 0.00120826 | 0.04833052 | UC<IBS |
| Eigenvector Centrality | ||||||
| No significant results found | ||||||
| Strength | ||||||
| No significant results found | ||||||
| Basal Ganglia Network | ||||||
| Betweenness Centrality | ||||||
| No significant results found | ||||||
| Eigenvector Centrality | ||||||
| No significant results found | ||||||
| Strength | ||||||
| No significant results found | ||||||
| Default Mode Network | ||||||
| Betweenness Centrality | ||||||
| No significant results found | ||||||
| Eigenvector Centrality | ||||||
| No significant results found | ||||||
| Strength | ||||||
| Right lateral aspect of Superior Temporal Gyrus | 0.0200973 | 3.80152487 | 0.00528664 | 0.00054973 | 0.02198913 | UC>IBS |
KEY: Groups: UC – ulcerative colitis, IBS – irritable bowel syndrome; Statistics: β – unstandardized beta, t – t statistic, SE – standard error, p – p value, q – FDR corrected p value
3.5.2.1. Salience network
Betweenness centrality was lower in the right orbitofrontal cortex (medial orbital sulcus) of UC participants (q = 0.048).
3.5.2.2. Default mode network
Strength was greater in the right lateral aspect of the superior temporal gyrus of UC participants (q = 0.022).
3.5.2.3. Irritable bowel syndrome versus healthy controls
Significant group differences in centrality and strength measures were seen in the salience network (Supplementary Material). No between group differences in network density were seen (p = 0.05).
3.5.2.4. Salience network
Eigenvector centrality (q = 0.043) and strength (q = 0.036) were greater in the right medial orbital sulcus of IBS participants.
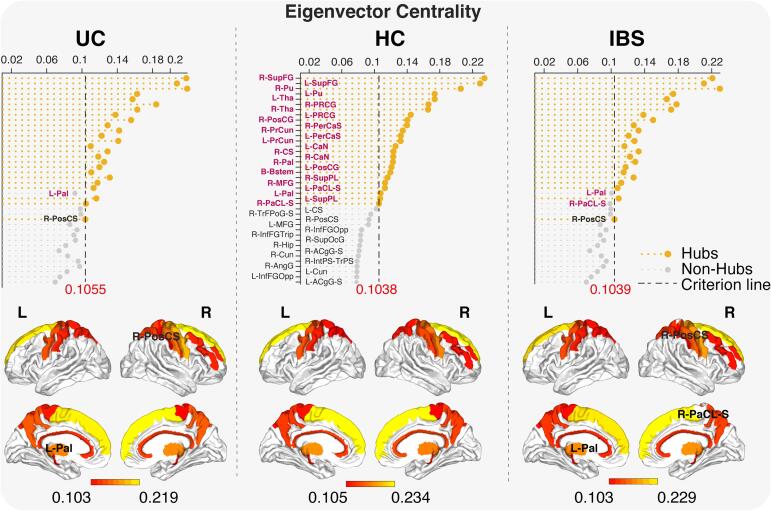
3.6. Hub analysis
Twenty-five hubs were identified in UC and HCs, and 24 in IBS (Fig. 4). Hubs are regions particularly important in controlling information flows, based on their eigenvector centrality. There were 23 common hubs among the three groups which were mainly located in the basal ganglia, frontal and parietal cortices, consistent with previous studies across psychiatric conditions and HCs (Gollo et al., 2018, Gong et al., 2009, Hagmann et al., 2008). While the globus pallidus was a hub in HCs, it lost this role in UC, and was replaced by the right postcentral sulcus. In IBS, both the left globus pallidus and the right paracentral lobule no longer functioned as hubs, and were replaced in this function by the right postcentral sulcus.
Fig. 4.
Hub distribution Units on the X-axis represent eigenvector centrality. The dashed line represents the eigenvector centrality cutoff value to classify the region as a hub, which is one standard deviation above the mean. The left globus pallidus is compromised in UC and IBS participants compared to HCs. This is compensated by the right postcentral sulcus in both disease groups.
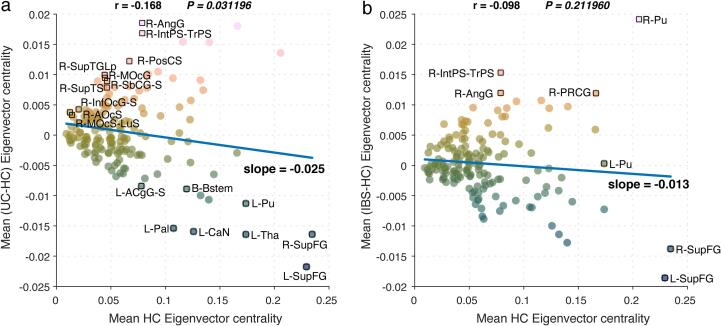
3.7. Centrality disruption of microstructural networks in ulcerative colitis and irritable bowel syndrome compared to HCs
As shown in Fig. 5, the analysis comparing UC participants to HCs, but not IBS showed a significant centrality disruption index (slope = -0.025, p = 0.031), where some regions showed higher, and others lower hubness. Nodes that were hubs in HCs, such as the bilateral superior frontal gyri, left thalamus and left putamen had lower eigenvector centrality in UC participants. Nodes that were not hubs in HCs, such as the right angular gyrus, right intraparietal/transverse parietal sulcus, and right postcentral sulcus, had higher eigenvector centrality in UC participants.
Fig. 5.
Centrality disruption analysis The analysis comparing UC participants to HCs showed a significant centrality disruption index (slope = -0.025, p = 0.031). Nodes that are hubs in HCs, such as the bilateral superior frontal gyri, left thalamus and left putamen are abnormally lower in eigenvector centrality (EC) in participants with UC. Nodes that are not hubs in HCs, such as the right angular gyrus, right intraparietal/transverse parietal sulcus, and right postcentral sulcus, are abnormally higher in EC in UC.
3.8. Network metric correlations with symptoms
3.8.1. UC vs HCs
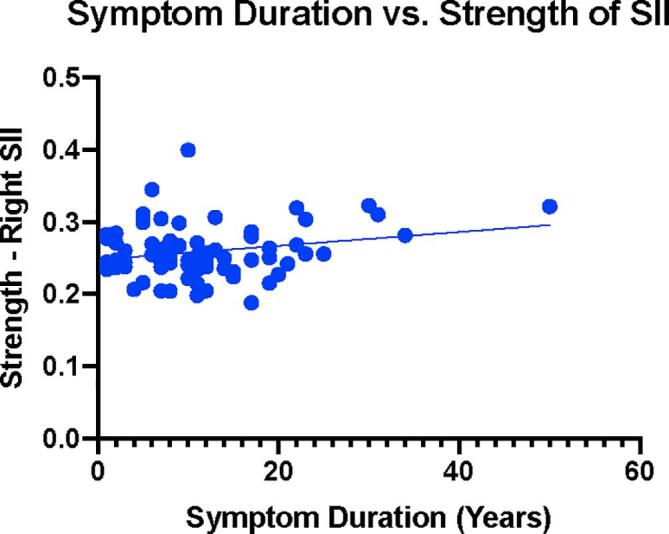
Multiple significant correlations between symptoms and alterations in network metrics of centrality were identified (Fig. 6, Table 6) in the UC group compared to HCs, specifically in the somatosensory network. The eigenvector centrality of the right SII correlated with greater HADS Depression (r(53) = 0.368, q = 0.024). Strength in this region correlated with greater STAI Trait Anxiety (r(65) = 0.325, q = 0.021) along with greater symptom related anxiety (VSI; r(66) = 0.314, q = 0.036), and greater symptom duration (r(72) = 0.233, q = 0.046) (Fig. 7). Betweenness centrality in the posterior insula was associated with greater symptom duration (r(72) = 0.295, q = 0.011) (Fig. 8).
Fig. 6.
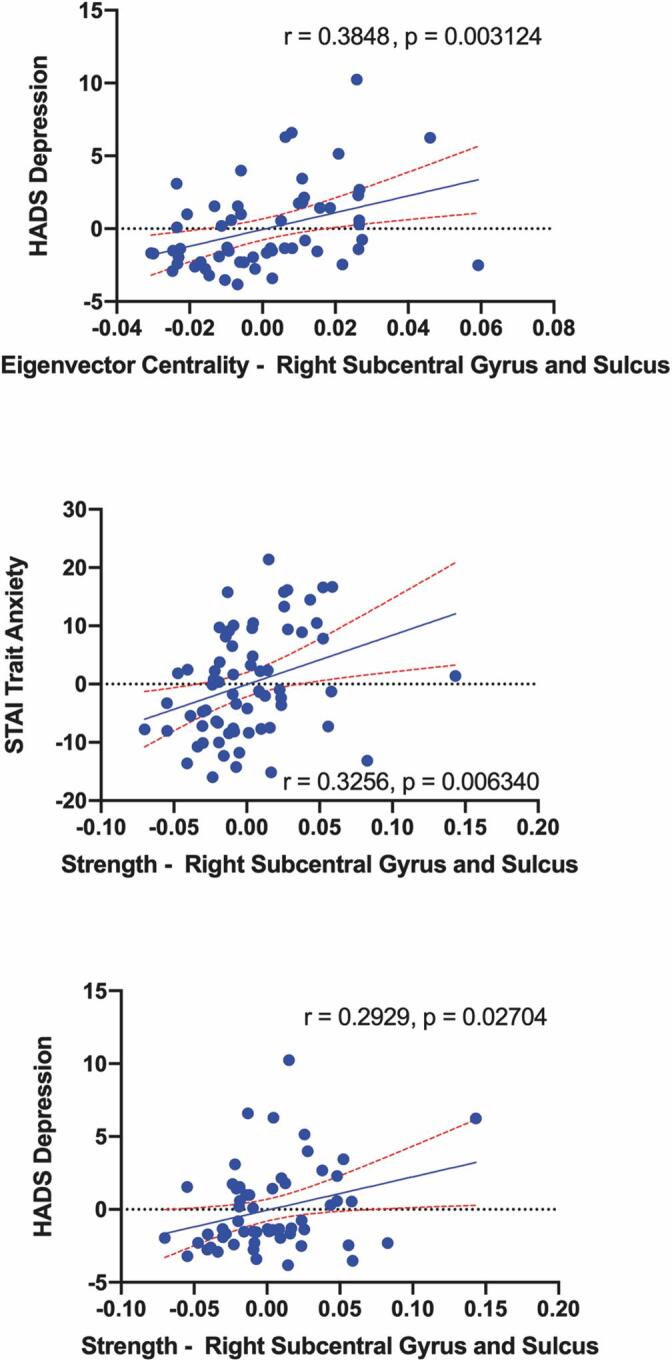
Significant associations between nodal measures of centrality and behavioral/clinical measures in UC. Blue dots represent UC. Red dashed lines around best-fit line represents 95% confidence interval. HADS: Hospital Anxiety and Depression Scale, STAI: State-Trait Anxiety Inventory, VSI: Visceral Sensitivity Index. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Table 6.
UC Correlations.
| Somatosensory Network | ||||||
|---|---|---|---|---|---|---|
| Brain Region | Clinical Variable | Correlation | Significance | q value | df | GLM Finding |
| Betweenness Centrality | ||||||
| No correlations found | ||||||
| Eigenvector Centrality | ||||||
| Right Subcentral Gyrus and Sulcus | HAD Depression | 0.368 | 0.006 | 0.024 | 53 | UC>HC |
| Strength | ||||||
| Right Subcentral Gyrus and Sulcus | STAI Tanxiety | 0.325 | 0.007 | 0.021 | 65 | UC>HC |
| Right Subcentral Gyrus and Sulcus | VSI Score | 0.314 | 0.009 | 0.036 | 66 | UC>HC |
| Right Subcentral Gyrus and Sulcus | HAD Depression | 0.317 | 0.018 | 0.072 | 53 | UC>HC |
| Salience Network | ||||||
| Betweenness Centrality | ||||||
| No correlations found | ||||||
| Eigenvector Centrality | ||||||
| No correlations found | ||||||
| Strength | ||||||
| No correlations found | ||||||
| Basal Ganglia Network | ||||||
| Betweenness Centrality | ||||||
| No correlations found | ||||||
| Eigenvector Centrality | ||||||
| Left pallidum | STAI Sanxiety | 0.282 | 0.017 | 0.068 | 70 | UC<HC |
| Strength | ||||||
| No correlations found | ||||||
| Default Mode Network | ||||||
| Betweenness Centrality | ||||||
| No correlations found | ||||||
| Eigenvector Centrality | ||||||
| No correlations found | ||||||
| Strength | ||||||
| Right Lateral Aspect of Superior Temporal Gyrus | PILL_Score_noGIQs | 0.269 | 0.049 | 0.19 | 52 | UC>HC |
| Right Lateral Aspect of Superior Temporal Gyrus | STAI Tanxiety | -0.267 | 0.043 | 0.553 | 56 | UC>HC |
KEY: Groups: UC – ulcerative colitis; Questionnaires: VSI – Visceral Sensitivity Index, HADS – Hospital Anxiety and Depression Scale, STAI – State Trait Anxiety Inventory; Statistics: df – degrees of freedom.
Fig. 7.
Symptom Duration versus Betweenness Centrality of Posterior Insula. Blue dots represent UC. Units on the X-axis represent symptom duration in years. Units on the Y-axis represent betweenness centrality in the Left Posterior Insula. BWC: Betweenness Centrality, pINS: Posterior Insula.
Fig. 8.
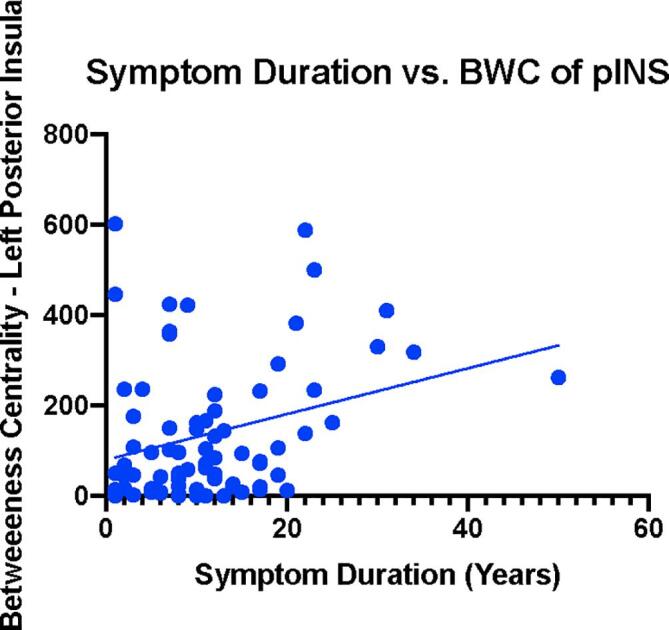
Symptom Duration versus Strength of SII. Blue dots represent UC. Units on the X-axis represent symptom duration in years. Units on the Y-axis represent strength in the Right SII. SII: Secondary Somatosensory Cortex.
3.8.2. UC vs IBS
No significant correlations between symptoms and alterations in network centrality were identified in UC compared to IBS or in IBS compared to HCs.
3.9. Differences of network metrics in patients with UC across different locations of the colon
There were no sigficant differences across any of the network metrics across different sites of disease location in the colon in patients with UC (Supplementary Material).
4. Discussion
We evaluated disease related, neuroplastic brain network alterations in white matter connectivity in asymptomatic individuals with a longstanding history of gut inflammation and recurrent abdominal pain, and compared them with both HCs and IBS participants in clinical remission. Despite the fact that UC participants were in clinical remission at the time of the study (based on subjective ratings), they showed extensive network architecture and connectivity differences compared to HCs, with few differences and several similarities compared to IBS. Both disease groups showed elevated behavioral parameter measures, with fewer abnormalities in UC compared with IBS.
Our findings support our primary hypothesis that UC participants in clinical remission show widespread alterations in white matter organization compared to HCs, some of which related to recurrent bouts of inflammation. This conclusion is based on the fact that the duration of symptoms in UC but not in IBS participants was correlated with the observed white matter changes in the secondary somatosensory cortex and posterior insula, two key regions of the somatosensory network. The same regions have previously been shown to have reduced cortical thickness (Hong et al., 2014). They also support our secondary hypothesis that some of these alterations are a consequence of the chronic abdominal pain characteristic for both disease groups. Importantly, the neuroanatomical alterations seen in UC correlated primarily with anxiety, somatic focus and depression, as participants did not report significant abdominal symptoms at the time of the study, despite the fact that twice as many UC participants (n = 17) indicated the use of analgesics than IBS participants (n = 7). This implies a connection in both UC and IBS participants between brain network alterations and subjective disease experience. To our knowledge, this is the first report demonstrating such network alterations associated with symptom-related fear, anxiety and depression in patients with longstanding gut inflammation, which may play a role in a subgroup of UC patients with persistent symptoms and reduced quality of life despite mucosal healing (Colombel et al., 2017).
4.1. UC network architecture differs from HCs
UC participants had lower centrality in the left globus pallidus and posterior insula, a finding also reflected in the hub analysis. The basal ganglia play an important role in integrating, modifying and modulating the pain experience (Borsook et al., 2010). Pain pathways from the spinal cord to the globus pallidus have been reported (Braz et al., 2005), and the globus pallidus’ role in sensory integration to higher-order structures is well established. The globus pallidus has high structural connectivity to the posterior insula (Ghaziri et al., 2018), the primary interoceptive cortex with a key role in sensory processing (Craig, 2003, Craig, 2002, Evrard, 2019, Icenhour et al., 2017, Mayer et al., 2015, Uddin et al., 2017). Deep brain stimulation of the globus pallidus has been shown to relieve persistent chronic pain (Loher et al., 2002). Our results suggest that the experience of chronic visceral pain, seen in both UC and IBS participants, results in a reduction in globus pallidus centrality. As it is strongly connected with the amygdala and emotional brain networks, neuroplastic alterations in this region may result in a reduction in the globus pallidus’ GABAergic, inhibitory influences on these emotional and somatosensory brain mechanisms (Albin et al., 1989, Nóbrega-Pereira et al., 2010, Sian et al., 1999). This decreased inhibition may result in the increased anxiety reported in both UC and IBS.
Moreover, greater SII centrality in both UC and IBS participants compared to HCs suggests that greater anxiety, somatic focus, and depression may also be due to reduced inhibition from the globus pallidus, especially since the hub analysis showed that the globus pallidus was replaced in its function as a hub by the postcentral sulcus (Albin et al., 1989). Past studies have found altered microstructural connectivity in the globus pallidus across visceral pain populations, where lower connectivity has been associated with greater symptom severity and duration (Ellingson et al., 2013a, Ellingson et al., 2013b, Ford and Kensinger, 2014, Woodworth et al., 2015). In a clinical setting, GABA agonists have been shown to reduce visceral pain through central mechanisms (Davis, 2012, Kannampalli and Sengupta, 2015). When viewed together, one may speculate that the observed alterations in the left globus pallidus result in a reduction of GABAergic input to SII, resulting in a disinhibition and increased centrality of this region. Previous studies have demonstrated SII’s role in the modulation of visceral sensitivity and processing of painful stimuli, emotion regulation, sensorimotor integration (Eickhoff et al., 2006, Kragel and LaBar, 2016, Orenius et al., 2017, Timmermann et al., 2001). Our findings reinforce the role of SII in emotional modulation and symptom-related anxiety in individuals with IBD (Kropf et al., 2019).
4.2. UC network architecture differs from IBS
More microstructural differences were seen between UC compared to HCs than between UC and IBS participants, supporting our hypothesis that in addition to a history of chronic abdominal pain, regardless of etiology, a history of chronic recurrent inflammation, contributes to alterations in brain network organization and plays a role in disease symptomatology (Harte et al., 2018). UC participants had lower betweenness centrality in the right medial orbital sulcus compared with IBS. This region is known to play a role in sensory processing and decision making (Kringelbach, 2005, Zald and b, Kim, S.W., , 1996) and has rich connections to the amygdala and thalamus (Zald and Kim, 1996). UC participants also exhibited greater strength in a default mode network region (right lateral aspect of the superior temporal gyrus), compared to both IBS and HCs. This region was traditionally recognized as the auditory cortex but a growing number of studies highlight its role in social processing and fear-related behaviors (De Bellis et al., 2002, Quirk et al., 1997).
Even though no significant correlations between symptom duration and network alterations involving the right medial orbital sulcus and the right superior temporal gyrus were observed, one can speculate that the observed differences between UC and IBS may be related to a history of chronic gut inflammation in UC only. Such chronic inflammation induced brain changes are consistent with previous preclinical studies highlighting the reported persistent impact of transient gut inflammation on the brain (Gray and Holtmann, 2017, Hong et al., 2014), and a recent study showing a greater risk for dementia related cognitive deficits in UC (Zhang et al., 2020).
4.3. Limitations
Our cross-sectional study design does not allow us to clearly differentiate the brain effects of chronic GI symptoms (present in both UC and IBS) from those caused by chronic gut inflammation. However, the fact that symptom duration was correlated with key brain changes in UC in this and our previous study (Hong et al., 2014), but not in IBS, suggests that the exposure of the brain to inflammatory signals from the gut plays an important role in the observed neuroplasticity. The fact that the extent of colonic involvement (e.g. distal vs pancolitis) did not show such a correlation, argues against a simple dose response relationship between inflammatory signaling and brain changes. Our results provide a plausible mechanism linking a history of chronically recurring abdominal pain and inflammation to affective symptoms which have been observed previously in UC patients (Graff et al., 2009, Mittermaier et al., 2004, Walker et al., 1990). As UC participants did not have colonic biopsies or fecal calprotecin measures at the time of the brain imaging study, we cannot rule out that even though asymptomatic, some participants may have had active GI inflammation at the time of the study since symptoms and inflammation do not always correlate (Targownik et al., 2015). A hallmark of UC, even when in a deep clinical remission is chronic mucosal architectural changes which may also be associated with chronic neural alterations. Furthermore, a small but significant number of UC participants were on anti-inflammatory, immune modulating drugs at the time of study, suggesting the presence of active inflammation at some time prior to the study. However, the goal of this study was to characterize the effect of a history of chronic intermittent gut inflammation on the brain, and not the influence of active inflammation.
4.4. Clinical implications
Thirty-one percent of UC patients in clinical remission report IBS-like symptoms possibly triggered by anxiety and somatic focus (Gracie and Ford, 2015, Ishihara et al., 2018). It is clear that both UC and IBS, traditionally viewed to primarily affect the gut, also have specific, symptom related effects on the brain. In addition, our findings demonstrate persistent neuroplastic changes in microstructural brain connectivity in asymptomatic UC participants with longstanding history of gut inflammation which may underlie some symptom reports. Previous studies have demonstrated that brain changes observed in chronic pain can be reversed with treatment (Seminowicz et al., 2011). Our findings provide potential central mechanisms that could be used to study the effectiveness of therapies like tricyclic antidepressants (Hall et al., 2018, Macer et al., 2017, Mikocka-Walus et al., 2012), cognitive behavioral therapy (Craske et al., 2011, Lackner et al., 2018) and hypnotherapy (Peters et al., 2015). A better understanding of the brain mechanisms involved in such changes is essential for guiding the development of targeted treatment strategies, and it is conceivable that such strategies may be effective in reducing the risk of cognitive decline (Zhang et al., 2020).
4.5. Directions for future research
Future longitudinal studies in UC and IBS participants will be needed to better establish a causal link between mucosal disease activity, symptoms and the brain. These studies should include an objective assessment of inflammation from colonic biopsies or fecal inflammatory markers to better differentiate the effect of chronic from active GI inflammation on brain networks and function. As many UC participants with active inflammation report little or no pain, such studies would also address the role of endogenous pain inhibition systems in UC. Finally, it will be of interest to evaluate the effect of IBD therapies on structural and functional brain alterations.
5. Grant Support
This research was supported by grants from the National Institutes of Health including R01 DK048351 (EAM), K23106528 (AG), China Scholarship Council 201,906,070,121 (HW). These funders played no role in study design, or the collection, analysis, and interpretation of the data.
6. Disclosures
EAM is a scientific advisory board member of Danone, Axial Biotherapeutics, Viome, Amare, Mahana Therapeutics, UBiome, Pendulum, Bloom Biosciences, APC Microbiome Ireland. CNB is on the advisory boards for Abbvie Canada, Amgen Canada, Bristol Myers Squibb Canada, Janssen Canada, Roche Canada, Sandoz Canada, Takeda Canada, and Pfizer Canada. CNB is also a consultant for Mylan Pharmaceuticals. CNB has received educational grants from Abbvie Canada, Janssen Canada, Takeda Canada and Pfizer Canada. CNB has received research funding from Abbvie Canada. CNB is on the speaker’s bureau for Abbvie Canada, Janssen Canada, Takeda Canada and Medtronic Canada. No other authors have anything to disclose.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2021.102613.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Achard S., Delon-Martin C., Vértes P.E., Renard F., Schenck M., Schneider F., Heinrich C., Kremer S., Bullmore E.T. Hubs of brain functional networks are radically reorganized in comatose patients. Proc. Natl. Acad. Sci. USA. 2012 doi: 10.1073/pnas.1208933109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albin R.L., Young A.B., Penney J.B. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989 doi: 10.1016/0166-2236(89)90074-X. [DOI] [PubMed] [Google Scholar]
- Andersson J.L.R., Sotiropoulos S.N. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage. 2016 doi: 10.1016/j.neuroimage.2015.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein C.N., Rawsthorne P., Cheang M., Blanchard J.F. A population-based case control study of potential risk factors for IBD. Am. J. Gastroenterol. 2006 doi: 10.1111/j.1572-0241.2006.00381.x. [DOI] [PubMed] [Google Scholar]
- Bhatt R.R., Gupta A., Labus J.S., Zeltzer L.K., Tsao J.C., Shulman R.J., Tillisch K. Altered brain structure and functional connectivity and its relation to pain perception in girls with irritable bowel syndrome. Psychosom. Med. 2019 doi: 10.1097/PSY.0000000000000655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R.R. Bhatt A. Gupta E.A. Mayer L.K. Zeltzer Chronic pain in children: structural and resting-state functional brain imaging within a developmental perspective 2019 Res Pediatr 10.1038/s41390-019-0689-9. [DOI] [PMC free article] [PubMed]
- Bielefeldt K., Davis B., Binion D.G. Pain and inflammatory bowel disease. Inflamm. Bowel Dis. 2009;15:778–788. doi: 10.1002/ibd.20848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D. Borsook J. Upadhyay E.H. Chudler L. Becerra A Key Role of the Basal Ganglia in Pain and Analgesia - Insights Gained through Human Functional Imaging Mol. Pain 6 2010 1744–8069-6–27 10.1186/1744-8069-6-27. [DOI] [PMC free article] [PubMed]
- Braz J.M., Nassar M.A., Wood J.N., Basbaum A.I. Parallel “pain” pathways arise from subpopulations of primary afferent nociceptor. Neuron. 2005 doi: 10.1016/j.neuron.2005.08.015. [DOI] [PubMed] [Google Scholar]
- Chang L., Munakata J., Mayer E.A., Schmulson M.J., Johnson T.D., Bernstein C.N., Saba L., Naliboff B., Anton P.A., Matin K. Perceptual responses in patients with inflammatory and functional Bowel disease. Gut. 2000 doi: 10.1136/gut.47.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- W.D. Chey J. Kurlander S. Eswaran Irritable bowel syndrome: A clinical review 2015 Am. Med. Assoc JAMA - J 10.1001/jama.2015.0954. [DOI] [PubMed]
- Chumbley J., Worsley K., Flandin G., Friston K. Topological FDR for neuroimaging. Neuroimage. 2010 doi: 10.1016/j.neuroimage.2009.10.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S., Kamarck T., Mermelstein R. A Global Measure of Perceived Stress. J. Health Soc. Behav. 1983 doi: 10.2307/2136404. [DOI] [PubMed] [Google Scholar]
- Colombel J.F., Keir M.E., Scherl A., Zhao R., de Hertogh G., Faubion W.A., Lu T.T. Discrepancies between patient-reported outcomes, and endoscopic and histological appearance in UC. Gut. 2017 doi: 10.1136/gutjnl-2016-312307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- P. Cook a, Bai, Y., Seunarine, K.K., Hall, M.G., Parker, G.J., Alexander, D.C., Camino: Open-Source Diffusion-MRI Reconstruction and Processing. 14th Sci. Meet. Int. Soc Magn. Reson. Med. 2006.
- A.D. Craig Interoception: The sense of the physiological condition of the body 2003 Opin. Neurobiol Curr 10.1016/S0959-4388(03)00090-4. [DOI] [PubMed]
- A.D. Craig How do you feel? Interoception: The sense of the physiological condition of the body Nat. Rev. Neurosci. 2002 10.1038/nrn894. [DOI] [PubMed]
- M.G. Craske K.B. Wolitzky-Taylor J. Labus S. Wu M. Frese E.A. Mayer B.D. Naliboff A cognitive-behavioral treatment for irritable bowel syndrome using interoceptive exposure to visceral sensations 2011 Res. Ther Behav 10.1016/j.brat.2011.04.001. [DOI] [PMC free article] [PubMed]
- N.A. Crossley A. Mechelli C. Ginestet M. Rubinov E.T. Bullmore P. Mcguire Altered hub functioning and compensatory activations in the connectome: A meta- Analysis of functional neuroimaging studies in schizophrenia 2016 Bull Schizophr 10.1093/schbul/sbv146. [DOI] [PMC free article] [PubMed]
- Crossley N.A., Mechelli A., Scott J., Carletti F., Fox P.T., Mcguire P., Bullmore E.T. The hubs of the human connectome are generally implicated in the anatomy of brain disorders. Brain. 2014 doi: 10.1093/brain/awu132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlhamer J.M., Zammitti E.P., Ward B.W., Wheaton A.G., Croft J.B. Morb. Mortal. Wkly; Rep: 2016. Prevalence of inflammatory bowel disease among adults aged ≥18 years — United States, 2015. https://doi.org/10.15585/mmwr.mm6542a3. [DOI] [PubMed] [Google Scholar]
- M.P. Davis Drug management of visceral pain: Concepts from basic research 2012 Treat Pain Res 10.1155/2012/265605. [DOI] [PMC free article] [PubMed]
- M.D. De Bellis M.S. Keshavan H. Shifflett S. Iyengar R.E. Dahl D.A. Axelson B. Birmaher J. Hall G. Moritz N.D. Ryan Superior temporal gyrus volumes in pediatric generalized anxiety disorder 2002 Psychiatry Biol 10.1016/S0006-3223(01)01375-0. [DOI] [PubMed]
- Desikan R.S., Ségonne F., Fischl B., Quinn B.T., Dickerson B.C., Blacker D., Buckner R.L., Dale A.M., Maguire R.P., Hyman B.T., Albert M.S., Killiany R.J. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006 doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Destrieux C., Fischl B., Dale A., Halgren E. Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. Neuroimage. 2010 doi: 10.1016/j.neuroimage.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drossman D.A. The Functional Gastrointestinal Disorders and the Rome III Process. Gastroenterology. 2006 doi: 10.1053/j.gastro.2006.03.008. [DOI] [Google Scholar]
- Eickhoff S.B., Lotze M., Wietek B., Amunts K., Enck P., Zilles K. Segregation of visceral and somatosensory afferents: An fMRI and cytoarchitectonic mapping study. Neuroimage. 2006 doi: 10.1016/j.neuroimage.2006.01.023. [DOI] [PubMed] [Google Scholar]
- Ellingson Benjamin M., Mayer E., Harris R.J., Ashe-Mcnally C., Naliboff B.D., Labus J.S., Tillisch K. Diffusion tensor imaging detects microstructural reorganization in the brain associated with chronic irritable bowel syndrome. Pain. 2013 doi: 10.1016/j.pain.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellingson Benjamin M, Mayer E., Harris R.J., Ashe-McNally C., Naliboff B.D., Labus J.S., Tillisch K. Diffusion tensor imaging detects microstructural reorganization in the brain associated with chronic irritable bowel syndrome. Pain. 2013;154:1528–1541. doi: 10.1016/j.pain.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- H.C. Evrard The organization of the primate insular cortex 2019 Neuroanat Front 10.3389/fnana.2019.00043. [DOI] [PMC free article] [PubMed]
- J.H. Ford E.A. Kensinger The relation between structural and functional connectivity depends on age and on task goals 2014 Hum. Neurosci Front 10.3389/fnhum.2014.00307. [DOI] [PMC free article] [PubMed]
- A. Fornito A. Zalesky M. Breakspear The connectomics of brain disorders 2015 Rev. Neurosci Nat 10.1038/nrn3901. [DOI] [PubMed]
- J. Ghaziri A. Tucholka G. Girard O. Boucher J.C. Houde M. Descoteaux S. Obaid G. Gilbert I. Rouleau D.K. Nguyen Subcortical structural connectivity of insular subregions 2018 Rep Sci 10.1038/s41598-018-26995-0. [DOI] [PMC free article] [PubMed]
- L.L. Gollo J.A. Roberts V.L. Cropley M.A. Di Biase C. Pantelis A. Zalesky M. Breakspear Fragility and volatility of structural hubs in the human connectome 2018 Neurosci Nat 10.1038/s41593-018-0188-z. [DOI] [PubMed]
- Gong G., He Y., Chen Z.J., Evans A.C. Convergence and divergence of thickness correlations with diffusion connections across the human cerebral cortex. Neuroimage. 2012 doi: 10.1016/j.neuroimage.2011.08.017. [DOI] [PubMed] [Google Scholar]
- G. Gong Y. He L. Concha C. Lebel D.W. Gross A.C. Evans C. Beaulieu Mapping anatomical connectivity patterns of human cerebral cortex using in vivo diffusion tensor imaging tractography 2009 Cortex Cereb 10.1093/cercor/bhn102. [DOI] [PMC free article] [PubMed]
- Gracie D.J., Ford A.C. IBS-like symptoms in patients with ulcerative colitis. Clin. Exp. Gastroenterol. 2015;8:101–109. doi: 10.2147/CEG.S58153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracie D.J., Guthrie E.A., Hamlin P.J., Ford A.C. Bi-directionality of Brain-Gut Interactions in Patients With Inflammatory Bowel Disease. Gastroenterology. 2018;154:1635–1646.e3. doi: 10.1053/J.GASTRO.2018.01.027. [DOI] [PubMed] [Google Scholar]
- Graff L.A., Walker J.R., Bernstein C.N. Depression and anxiety in inflammatory bowel disease: A review of comorbidity and management. Inflamm. Bowel Dis. 2009;15:1105–1118. doi: 10.1002/ibd.20873. [DOI] [PubMed] [Google Scholar]
- Gray M., Holtmann G. Gut Inflammation: More Than a Peripheral Annoyance. Dig. Dis. Sci. 2017;62:2205–2207. doi: 10.1007/s10620-017-4587-x. [DOI] [PubMed] [Google Scholar]
- D.W. Grupe J.B. Nitschke Uncertainty and anticipation in anxiety: An integrated neurobiological and psychological perspective 2013 Rev. Neurosci Nat 10.1038/nrn3524. [DOI] [PMC free article] [PubMed]
- Gupta A., Bhatt R.R., Naliboff B.D., Kutch J.J., Labus J.S., Vora P.P., Alaverdyan M., Schrepf A., Lutgendorf S., Mayer E.A. Impact of early adverse life events and sex on functional brain networks in patients with urological chronic pelvic pain syndrome (UCPPS): A MAPP Research Network study. PLoS ONE. 2019 doi: 10.1371/journal.pone.0217610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagmann P., Cammoun L., Gigandet X., Meuli R., Honey C.J., Van Wedeen J., Sporns O. Mapping the structural core of human cerebral cortex. PLoS Biol. 2008 doi: 10.1371/journal.pbio.0060159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall B.J., Hamlin P.J., Gracie D.J., Ford A.C. The Effect of Antidepressants on the Course of Inflammatory Bowel Disease. Can. J. Gastroenterol. Hepatol. 2018;2018:1–11. doi: 10.1155/2018/2047242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harte S.E., Harris R.E., Clauw D.J. The neurobiology of central sensitization. J. Appl. Biobehav. Res. 2018 doi: 10.1111/jabr.12137. [DOI] [Google Scholar]
- Henningsen P., Zimmermann T., Sattel H. Medically unexplained physical symptoms, anxiety, and depression: a meta-analytic review. Psychosom. Med. 2012;65:528–533. doi: 10.1097/01.psy.0000075977.90337.e7. [DOI] [PubMed] [Google Scholar]
- Hong J.-Y., Labus J.S., Jiang Z., Ashe-Mcnalley C., Dinov I., Gupta A., Shi Y., Stains J., Heendeniya N., Smith S.R., Tillisch K., Mayer E.A. Regional neuroplastic brain changes in patients with chronic inflammatory and non-inflammatory visceral pain. PLoS ONE. 2014;9 doi: 10.1371/journal.pone.0084564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Icenhour A., Witt S.T., Elsenbruch S., Lowén M., Engström M., Tillisch K., Mayer E.A., Walter S. Brain functional connectivity is associated with visceral sensitivity in women with Irritable Bowel Syndrome. NeuroImage Clin. 2017;15:449–457. doi: 10.1016/j.nicl.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irimia A., Chambers M.C., Torgerson C.M., Van Horn J.D. Circular representation of human cortical networks for subject and population-level connectomic visualization. Neuroimage. 2012 doi: 10.1016/j.neuroimage.2012.01.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irimia A., Van Horn J.D. The structural, connectomic and network covariance of the human brain. Neuroimage. 2013 doi: 10.1016/j.neuroimage.2012.10.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara, S., Kawashima, K., Fukuba, N., Tada, Y., Kotani, S., Mishima, Y., Oshima, N., Kinoshita, Y., 2018. Irritable bowel syndrome-like symptoms in ulcerative colitis patients in clinical remission: Association with residual colonic inflammation, in: Digestion. https://doi.org/10.1159/000494412. [DOI] [PubMed]
- Kannampalli P., Sengupta J.N. Role of principal ionotropic and metabotropic receptors in visceral pain. J. Neurogastroenterol. Motil. 2015 doi: 10.5056/jnm15026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keltner J.R. Isolating the Modulatory Effect of Expectation on Pain Transmission: A Functional Magnetic Resonance Imaging Study. J. Neurosci. 2006 doi: 10.1523/JNEUROSCI.4463-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornelsen J., Wilson A., Labus J.S., Witges K., Mayer E.A., Bernstein C.N. Brain Resting-State Network Alterations Associated With Crohn’s Disease. Front. Neurol. 2020;11:48. doi: 10.3389/fneur.2020.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- P.A. Kragel K.S. LaBar Somatosensory representations link the perception of emotional expressions and sensory experience. eNeuro 2016 10.1523/ENEURO.0090-15.2016. [DOI] [PMC free article] [PubMed]
- M.L. Kringelbach The human orbitofrontal cortex: Linking reward to hedonic experience 2005 Rev. Neurosci Nat 10.1038/nrn1747. [DOI] [PubMed]
- E. Kropf S.K. Syan L. Minuzzi B.N. Frey From anatomy to function: the role of the somatosensory cortex in emotional regulation 2019 Bras. Psiquiatr Rev 10.1590/1516-4446-2018-0183. [DOI] [PMC free article] [PubMed]
- Labus J.S., Bolus R., Chang L., Wiklund I., Naesdal J., Mayer E.A., Naliboff B.D. The Visceral Sensitivity Index: development and validation of a gastrointestinal symptom-specific anxiety scale. Aliment. Pharmacol. Ther. 2004;20:89–97. doi: 10.1111/j.1365-2036.2004.02007.x. [DOI] [PubMed] [Google Scholar]
- Lackner J.M., Jaccard J., Keefer L., Brenner D.M., Firth R.S., Gudleski G.D., Hamilton F.A., Katz L.A., Krasner S.S., Ma C.X., Radziwon C.D., Sitrin M.D. Improvement in Gastrointestinal Symptoms After Cognitive Behavior Therapy for Refractory Irritable Bowel Syndrome. Gastroenterology. 2018;155:47–57. doi: 10.1053/j.gastro.2018.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loher T.J., Burgunder J.M., Weber S., Sommerhalder R., Krauss J.K. Effect of chronic pallidal deep brain stimulation on off period dystonia and sensory symptoms in advanced Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry. 2002 doi: 10.1136/jnnp.73.4.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longstreth G.F., Thompson W.G., Chey W.D., Houghton L.A., Mearin F., Spiller R.C. Functional Bowel Disorders. Gastroenterology. 2006;130:1480–1491. doi: 10.1053/J.GASTRO.2005.11.061. [DOI] [PubMed] [Google Scholar]
- Macer B.J.D., Prady S.L., Mikocka-Walus A. Antidepressants in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2017;23:534–550. doi: 10.1097/MIB.0000000000001059. [DOI] [PubMed] [Google Scholar]
- G.M. Mawe D.S. Strong K.A. Sharkey Plasticity of enteric nerve functions in the inflamed and postinflamed gut 2009 Motil Neurogastroenterol 10.1111/j.1365-2982.2009.01291.x. [DOI] [PMC free article] [PubMed]
- Mayer E.A., Berman S., Suyenobu B., Labus J., Mandelkern M.A., Naliboff B.D., Chang L. Differences in brain responses to visceral pain between patients with irritable bowel syndrome and ulcerative colitis. Pain. 2005;115:398–409. doi: 10.1016/j.pain.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Mayer E.A., Brunnhuber S. Gastrointestinal disorders. Handbook of Clinical Neurology. 2012:607–631. doi: 10.1016/B978-0-444-52002-9.00036-X. [DOI] [PubMed] [Google Scholar]
- E.A. Mayer M.C. Bushnell Functional pain syndromes: Presentation and pathophysiology 2015 Presentation and Pathophysiology Functional Pain Syndromes 10.1136/gut.2009.187542.
- Mayer E.A., Gebhart G.F. Basic and clinical aspects of visceral hyperalgesia. Gastroenterology. 1994 doi: 10.1016/0016-5085(94)90086-8. [DOI] [PubMed] [Google Scholar]
- Mayer E.A., Labus J.S., Tillisch K., Cole S.W., Baldi P. Towards a systems view of IBS. Nat. Rev. Gastroenterol. Hepatol. 2015;12:592–605. doi: 10.1038/nrgastro.2015.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer E.A., Tillisch K. The Brain-Gut Axis in Abdominal Pain Syndromes. Annu. Rev. Med. 2011;62:381–396. doi: 10.1146/annurev-med-012309-103958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikocka-Walus A.A., Gordon A.L., Stewart B.J., Andrews J.M. A magic pill? A qualitative analysis of patients’ views on the role of antidepressant therapy in inflammatory bowel disease (IBD) BMC Gastroenterol. 2012;12:93. doi: 10.1186/1471-230X-12-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- C. Mittermaier C. Dejaco T. Waldhoer A. Oefferlbauer-Ernst W. Miehsler M. Beier W. Tillinger A. Gangl G. Moser Impact of Depressive Mood on Relapse in Patients with Inflammatory Bowel Disease: A Prospective 18-Month Follow-Up Study 2004 Med Psychosom 10.1097/01.PSY.0000106907.24881.F2. [DOI] [PubMed]
- Nóbrega-Pereira S., Gelman D., Bartolini G., Pla R., Pierani A., Marín O. Origin and molecular specification of globus pallidus neurons. J. Neurosci. 2010 doi: 10.1523/JNEUROSCI.4023-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orenius T.I., Raij T.T., Nuortimo A., Näätänen P., Lipsanen J., Karlsson H. The interaction of emotion and pain in the insula and secondary somatosensory cortex. Neuroscience. 2017 doi: 10.1016/j.neuroscience.2017.02.047. [DOI] [PubMed] [Google Scholar]
- Panara A.J., Yarur A.J., Rieders B., Proksell S., Deshpande A.R., Abreu M.T., Sussman D.A. The incidence and risk factors for developing depression after being diagnosed with inflammatory bowel disease: a cohort study. Aliment. Pharmacol. Ther. 2014;39:802–810. doi: 10.1111/apt.12669. [DOI] [PubMed] [Google Scholar]
- M.P. Paulus M.B. Stein An Insular View of Anxiety 2006 Psychiatry Biol 10.1016/j.biopsych.2006.03.042. [DOI] [PubMed]
- Pennebaker J.W. The psychology of physical symptoms. The Psychology of Physical Symptoms. 1982 doi: 10.1016/j.advengsoft.2010.04.001. [DOI] [Google Scholar]
- W. Penny K. Friston J. Ashburner S. Kiebel T. Nichols Statistical Parametric Mapping: The Analysis of Functional Brain Images 2007 The Analysis of Functional Brain Images Statistical Parametric Mapping 10.1016/B978-0-12-372560-8.X5000-1.
- S.L. Peters J.G. Muir P.R. Gibson Review article: Gut-directed hypnotherapy in the management of irritable bowel syndrome and inflammatory bowel disease 2015 Pharmacol. Ther Aliment 10.1111/apt.13202. [DOI] [PubMed]
- Piché M., Arsenault M., Poitras P., Rainville P., Bouin M. Widespread hypersensitivity is related to altered pain inhibition processes in irritable bowel syndrome. Pain. 2010;148:49–58. doi: 10.1016/j.pain.2009.10.005. [DOI] [PubMed] [Google Scholar]
- C.A. Porro P. Baraldi G. Pagnoni M. Serafini P. Facchin M. Maieron P. Nichelli Does anticipation of pain affect cortical nociceptive systems? J. Neurosci. https:// 2002 doi.org/20026310. [DOI] [PMC free article] [PubMed]
- J. Powell-Tuck R.L. Bown J.E. Lennard-Jones A comparison of oral prednisolone given as single or multiple daily doses for active proctocolitis 1978 J. Gastroenterol Scand 10.3109/00365527809182199. [DOI] [PubMed]
- R. Qi J. Ke U.J. Schoepf A. Varga-Szemes C.M. Milliken C. Liu Q. Xu F. Wang L.J. Zhang G.M. Lu Topological Reorganization of the Default Mode Network in Irritable Bowel Syndrome 2016 Neurobiol Mol 10.1007/s12035-015-9558-7. [DOI] [PubMed]
- Quirk G.J., Armony J.L., LeDoux J.E. Fear conditioning enhances different temporal components of tone-evoked spike trains in auditory cortex and lateral amygdala. Neuron. 1997 doi: 10.1016/S0896-6273(00)80375-X. [DOI] [PubMed] [Google Scholar]
- Rubinov M., Sporns O. Complex network measures of brain connectivity: Uses and interpretations. Neuroimage. 2010;52:1059–1069. doi: 10.1016/j.neuroimage.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Seminowicz D.A., Wideman T.H., Naso L., Hatami-Khoroushahi Z., Fallatah S., Ware M.A., Jarzem P., Bushnell M.C., Shir Y., Ouellet J.A., Stone L.S. Effective Treatment of Chronic Low Back Pain in Humans Reverses Abnormal Brain Anatomy and Function. J. Neurosci. 2011 doi: 10.1523/JNEUROSCI.5280-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah E., Rezaie A., Riddle M., Pimentel M. Ann; Gastroenterol: 2014. Psychological disorders in gastrointestinal disease: Epiphenomenon, cause or consequence? [PMC free article] [PubMed] [Google Scholar]
- Sheehan D.V., Lecrubier Y., Sheehan K.H., Amorim P., Janavs J., Weiller E., Hergueta T., Baker R., Dunbar G.C. The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10, in. J. Clin. Psychiatry. 1998 [PubMed] [Google Scholar]
- Sian, J., Youdim, M., Riederer, P., Gerlach, M., 1999. Biochemical Anatomy of the Basal Ganglia and Associated Neural Systems, in: Basic Neurochemistry : Molecular, Cellular, and Medical Aspects.
- Song G.H., Venkatraman V., Ho K.Y., Chee M.W.L., Yeoh K.G., Wilder-Smith C.H. Cortical effects of anticipation and endogenous modulation of visceral pain assessed by functional brain MRI in irritable bowel syndrome patients and healthy controls. Pain. 2006;126:79–90. doi: 10.1016/j.pain.2006.06.017. [DOI] [PubMed] [Google Scholar]
- Spielberger C.D. Manual for the State-Trait Anxiety Inventory (STAI Form Y) Consulting Psychologists Palo Alto. 1983 doi: 10.5370/JEET.2014.9.2.478. [DOI] [Google Scholar]
- Straube T., Schmidt S., Weiss T., Mentzel H.J., Miltner W.H.R. Dynamic activation of the anterior cingulate cortex during anticipatory anxiety. Neuroimage. 2009 doi: 10.1016/j.neuroimage.2008.10.022. [DOI] [PubMed] [Google Scholar]
- N.J. Talley S.F. Phillips L.J. Melton C. Wiltgen A.R. Zinsmeister A patient questionnaire to identify bowel disease 1989 Intern. Med Ann 10.7326/0003-4819-111-8-671. [DOI] [PubMed]
- Targownik L.E., Sexton K.A., Bernstein M.T., Beatie B., Sargent M., Walker J.R., Graff L.A. The relationship among perceived stress, symptoms, and inflammation in persons with inflammatory bowel disease. Am. J. Gastroenterol. 2015 doi: 10.1038/ajg.2015.147. [DOI] [PubMed] [Google Scholar]
- Timmermann L., Ploner M., Haucke K., Schmitz F., Baltissen R., Schnitzler A. Differential coding of pain intensity in the human primary and secondary somatosensory cortex. J. Neurophysiol. 2001 doi: 10.1152/jn.2001.86.3.1499. [DOI] [PubMed] [Google Scholar]
- Uddin L.Q., Nomi J.S., Hébert-Seropian B., Ghaziri J., Boucher O. Structure and Function of the Human Insula. J. Clin. Neurophysiol. 2017 doi: 10.1097/WNP.0000000000000377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- V., L.-B., A.M., M., S., S., Anorectal manometry in active and quiescent ulcerative colitis 1989 Am. J Gastroenterol. [PubMed]
- Walker E.A., Roy-Byrne P.P., Katon W.J., Li L., Amos D., Jiranek G. Psychiatric illness and irritable bowel syndrome: a comparison with inflammatory bowel disease. Am. J. Psychiatry. 1990;147:1656–1661. doi: 10.1176/ajp.147.12.1656. [DOI] [PubMed] [Google Scholar]
- K. Wiech M. Ploner I. Tracey Neurocognitive aspects of pain perception 2008 Sci Trends Cogn 10.1016/j.tics.2008.05.005. [DOI] [PubMed]
- Woodworth D., Mayer E., Leu K., Ashe-McNalley C., Naliboff B.D., Labus J.S., Tillisch K., Kutch J.J., Farmer M.A., Apkarian A.V., Johnson K.A., Mackey S.C., Ness T.J., Landis J.R., Deutsch G., Harris R.E., Clauw D.J., Mullins C., Ellingson B.M. Unique microstructural changes in the brain associated with urological chronic pelvic pain syndrome (UCPPS) revealed by diffusion tensor MRI, super-resolution track density imaging, and statistical parameter mapping: A MAPP network neuroimaging study. PLoS ONE. 2015 doi: 10.1371/journal.pone.0140250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y.-Z., Tao R.-J., Xu B., Sun J., Chen K.-M., Miao F., Zhang Z.-W., Xu J.-Y. Functional brain imaging in irritable bowel syndrome with rectal balloon-distention by using fMRI. World J. Gastroenterol. 2003;9:1356–1360. doi: 10.3748/wjg.v9.i6.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D.H. Zald b, Kim, S.W., Anatomy and function of the orbital frontal cortex 1996 Function and relevance to obsessive-compulsive disorder. J. Neuropsychiatry Clin. Neurosci II 10.1176/jnp.8.3.249. [DOI] [PubMed]
- Zhang B., Wang H.E., Bai Y.M., Tsai S.J., Su T.P., Chen T.J., Wang Y.P., Chen M.H. Inflammatory bowel disease is associated with higher dementia risk: a nationwide longitudinal study. Gut. 2020 doi: 10.1136/gutjnl-2020-320789. [DOI] [PubMed] [Google Scholar]
- A.S. Zigmond R.P. Snaith The Hospital Anxiety and Depression Scale 1983 Scand Acta Psychiatr 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.