Abstract
Although patients with diabetes mellitus mostly present with enlarged or normal-sized kidneys throughout their life, a small proportion of patients have small kidneys. This longitudinal study enrolled 83 diabetic patients treated with peritoneal dialysis (PD) between 2015 and 2019. Patients were stratified into two groups, those with enlarged or normal (n = 67) or small (n = 16) kidneys, based on their kidney sizes before dialysis. Patients with small kidney size were not only older (76.63 ± 10.63 vs. 68.03 ± 11.26 years, P = 0.007), suffered longer duration of diabetes mellitus (272.09 ± 305.09 vs. 151.44 ± 85.31 month, P = 0.006) and predominantly female (75.0 vs. 41.8%, P = 0.017), but also had lower serum levels of creatinine (9.63 ± 2.82 vs. 11.74 ± 3.32 mg/dL, P = 0.022) and albumin (3.23 ± 0.67 vs. 3.60 ± 0.47 g/dL, P = 0.010) than patients with enlarged or normal kidney size. At the end of analysis, 14 (16.9%) patients died. Patients with small kidney size demonstrated higher all-cause (50.0 vs. 9.0%, P < 0.001) and infection-related (43.8 vs. 7.5%, P < 0.001) mortality than patients with enlarged or normal kidney size. In a multivariate-logistic-regression model, small kidney size was a powerful predictor of mortality (odds ratio 6.452, 95% confidence interval 1.220–34.482, P = 0.028). Diabetic patients with small kidney size at the beginning of PD carry a substantial risk for mortality.
Subject terms: Endocrinology, Nephrology
Introduction
It is not uncommon to find enlarged kidneys in patients with diabetes mellitus, and the kidneys could remain large even in the advanced stage of the disease. In a Sardinian population cohort study, Piras et al.1 presented their data that younger age, female sex, diabetes mellitus, obesity, tall height, high waist-to-hip ratio and lower serum creatinine were significant predictors of larger kidney size. The presence of diabetes mellitus was associated with a 1.723-fold risk of having a large kidney. Moreover, Rigalleau et al.2 reported that large kidneys predicted poor kidney outcome in patients with diabetes and chronic kidney disease. In the second manifestations of arterial disease (SMART) study3, large kidney length was found to be associated with higher risk of cardiovascular events and mortality in high-risk patients, irrespective of estimated glomerular filtration rate.
Nevertheless, small kidneys could be observed in a small number of diabetic patients with chronic kidney disease. Majdan et al.4 reported that most of their type 2 diabetes mellitus patients with chronic kidney disease had small kidneys. Notably, Habib5 proposed that both kidney hypertrophy and atrophy can occur in diabetes mellitus. The early changes in diabetic kidneys are mainly due to tubular basement membrane thickening, which leads to kidney hypertrophy. On the other hand, various tubulointerstitial diseases can induce apoptosis in proximal tubular cells, causing tubular atrophy and fibrosis and ultimately kidney atrophy5. Furthermore, atherosclerosis and related ischemic diseases can decrease the blood supply to the kidneys, resulting in kidney atrophy5.
Chronic kidney disease is endemic in Taiwan; indeed, the incidence and prevalence of end-stage kidney disease (ESKD) are higher in Taiwan than in any other country6. In 2016, Taiwan, the United States, and the Jalisco region of Mexico reported the highest incidences of treated ESKD, with rates of 493, 378, and 355 patients per million general population, respectively. Notably, diabetes is the primary cause of ESKD in 46% of incident dialysis patients in Taiwan. Nevertheless, the majority of uremic patients in Taiwan are treated with hemodialysis rather than peritoneal dialysis (PD)7.
The rationale for this study was based on an important, but as yet unanswered, question that arose for many diabetic patients treated with PD in our hospital. The majority of diabetic patients had enlarged or normal kidneys when entering dialysis, whereas few diabetic patients were found to have small kidneys at the time of uremia. Therefore, this raises the question of what the impact of kidney size before dialysis on the outcomes of these patients is. Perhaps one of the potential clinical applications of small kidneys is to remind clinicians about diabetic patient care. For example, if small kidney size at the beginning of PD is found to be associated with a greater risk for infection-cause mortality, physicians should be alert of the possibility of occurrence of infection and consider early initiation of antimicrobial therapy in case of infectious symptoms and signs.
Diabetes mellitus is the most important cause of ESKD worldwide, but no work has been performed to compare outcomes of diabetic patients with enlarged or normal versus small kidney size, which initiated our interest in this research. Therefore, this study attempted to survey kidney size in diabetic PD patients before the commencement of dialysis and to analyze the association of kidney size with outcomes and laboratory biomarkers.
Results
This study included 83 diabetic patients receiving long-term PD at Chang Gung Memorial Hospital (Table 1). Most of the diabetic patients had enlarged or normal kidney size (n = 67, 80.7%) at the beginning of PD, but some patients had small kidney size (n = 16, 19.3%) when entering PD. The patients were aged 69.70 ± 11.57. These patients had been receiving PD for 42.82 ± 3.72 months. None of the patients were on immunosuppressive medications. Many patients suffered from hypertension (90.4%) and cardiovascular disease (41.0%). It was found that patients with small kidney size were not only older (76.63 ± 10.63 vs. 68.03 ± 11.26 years, P = 0.007) and suffered longer duration of diabetes mellitus (272.09 ± 305.09 vs. 151.44 ± 85.31 month, P = 0.006), but also more often female (75.0 vs. 41.8%, P = 0.017) than patients with enlarged or normal kidney size.
Table 1.
Baseline characteristics of peritoneal dialysis patients stratified by kidney size (n = 83).
| Variable | All patients (n = 83) | Diabetic patients with enlarged or normal kidney size (n = 67) | Diabetic patients with small kidney size (n = 16) | P value |
|---|---|---|---|---|
| Demographics | ||||
| Age, years | 69.70 ± 11.57 | 68.03 ± 11.26 | 76.63 ± 10.63 | 0.007** |
| Female sex, n (%) | 40 (48.2) | 28 (41.8) | 12 (75.0) | 0.017* |
| Biopsy-proved glomerulonephritis, n (%) | 1 (1.2) | 1 (1.5) | 0 (0.0) | 1.000 |
| Polycystic kidney disease, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1.000 |
| Hypertension, n (%) | 75 (90.4) | 61 (91.0) | 14 (87.5) | 0.666 |
| Cardiovascular disease, n (%) | 34 (41.0) | 26 (38.8) | 8 (50.0) | 0.413 |
| Chronic liver disease, n (%) | 5 (6.0) | 3 (4.5) | 2 (12.5) | 0.226 |
| Hepatitis B virus carrier, n (%) | 10 (12) | 9 (13.4) | 1 (6.3) | 0.428 |
| Hepatitis C virus carrier, n (%) | 6 (7.2) | 5 (7.5) | 1 (6.3) | 0.866 |
| Body mass index, kg/m2 | 25.47 ± 3.71 | 25.77 ± 3.59 | 24.29 ± 4.09 | 0.154 |
| Smoking habit, n (%) | 15 (18.1) | 13 (19.4) | 2 (12.5) | 0.519 |
| Alcohol consumption, n (%) | 7 (8.4) | 6 (9.0) | 1 (6.3) | 0.726 |
| Immunosuppressive medications | 0 (0) | 0 (0) | 0 (0) | 1.000 |
| Duration of peritoneal dialysis, month | 42.82 ± 3.72 | 39.16 ± 32.22 | 59.14 ± 35.33 | 0.701 |
| Duration of diabetes mellitus, month | 174.69 ± 1158.67 | 151.44 ± 85.31 | 272.09 ± 305.09 | 0.006** |
| Hypoglycemic therapy | 0.455 | |||
| Oral hypoglycemic agents, n (%) | 59 (71.1) | 47 (70.1) | 12 (75.0) | |
| Insulin, n (%) | 18 (21.7) | 16 (23.9) | 2 (12.5) | |
| Oral hypoglycemic agents and insulin, n (%) | 6 (0.72) | 4 (0.60) | 2 (12.5) | |
*P < 0.05; **P < 0.01.
Compared with patients with enlarged or normal kidney size (Table 2), patients with small kidney size had lower serum levels of creatinine (9.63 ± 2.82 vs. 11.74 ± 3.32 mg/dL, P = 0.022) and albumin (3.23 ± 0.67 vs. 3.60 ± 0.47 g/dL, P = 0.010).
Table 2.
Laboratory findings of peritoneal dialysis patients stratified by kidney size (n = 83).
| Variable | All patients (n = 83) | Diabetes patients with enlarged or normal kidney size (n = 67) | Diabetes patients with small kidney size (n = 16) | P value |
|---|---|---|---|---|
| Blood urea nitrogen, mg/dL | 70.66 ± 21.58 | 72.25 ± 21.64 | 63.65 ± 19.33 | 0.149 |
| Creatinine, mg/dL | 11.32 ± 3.35 | 11.74 ± 3.32 | 9.63 ± 2.82 | 0.022* |
| Uric acid, mg/dL | 6.73 ± 1.87 | 6.93 ± 1.94 | 5.93 ± 1.24 | 0.052 |
| Sodium, mEq/L | 135.11 ± 3.44 | 135.10 ± 3.46 | 135.13 ± 3.30 | 0.983 |
| Potassium, mEq/L | 4.12 ± 1.35 | 4.25 ± 1.43 | 3.58 ± 0.66 | 0.071 |
| Corrected calcium, mg/dL | 9.75 ± 0.83 | 9.69 ± 0.85 | 10.01 ± 0.70 | 0.162 |
| Inorganic phosphorus, mg/dL | 5.52 ± 1.63 | 5.70 ± 1.68 | 4.81 ± 1.31 | 0.050 |
| Fasting glucose, mg/dL | 163.06 ± 71.87 | 164.03 ± 75.18 | 156.88 ± 53.52 | 0.721 |
| Glycated hemoglobin, % | 7.20 ± 1.47 | 7.19 ± 1.48 | 7.23 ± 1.35 | 0.939 |
| Albumin, g/dL | 3.5 ± 0.53 | 3.60 ± 0.47 | 3.23 ± 0.67 | 0.010* |
| Total cholesterol, mg/dL | 180.41 ± 54.90 | 183.45 ± 56.45 | 166.69 ± 44.32 | 0.272 |
| Triglyceride, mg/dL | 231.45 ± 257.20 | 231.31 ± 276.30 | 220.81 ± 132.98 | 0.883 |
| Aspartate aminotransferase, U/L | 27.65 ± 15.18 | 26.94 ± 14.03 | 30.38 ± 19.23 | 0.417 |
| Alanine aminotransferase, U/L | 25.23 ± 20.45 | 25.04 ± 21.46 | 27.00 ± 14.54 | 0.731 |
| Intact parathyroid hormone, pg/mL | 283.77 ± 239.04 | 287.71 ± 237.40 | 250.16 ± 238.26 | 0.572 |
| Iron, ug/dL | 66.54 ± 27.88 | 67.87 ± 29.31 | 61.50 ± 22.09 | 0.418 |
| Total iron binding capacity, ug/dL | 267.41 ± 62.95 | 272.81 ± 63.20 | 247.31 ± 58.54 | 0.146 |
| Ferritin, ng/mL | 538.06 ± 601.00 | 528.38 ± 637.81 | 554.53 ± 384.32 | 0.876 |
| White blood cell count, 1000/µL | 8.22 ± 2.32 | 8.07 ± 1.92 | 8.92 ± 3.54 | 0.188 |
| Red blood cell count, million/dL | 3.90 ± 3.76 | 4.02 ± 4.13 | 3.38 ± 0.48 | 0.453 |
| Hemoglobin, g/dL | 9.97 ± 1.40 | 10.07 ± 1.46 | 9.53 ± 1.27 | 0.179 |
| Hematocrit, % | 30.15 ± 4.18 | 30.43 ± 4.38 | 28.79 ± 3.87 | 0.172 |
| Mean corpuscular volume, % | 87.20 ± 6.18 | 86.89 ± 7.21 | 88.48 ± 6.83 | 0.427 |
| Mean corpuscular hemoglobin, pg/cell | 28.90 ± 2.51 | 28.75 ± 2.69 | 29.51 ± 2.42 | 0.303 |
| Mean corpuscular hemoglobin concentration, g Hb/dL | 22.15 ± 1.14 | 33.10 ± 1.17 | 33.36 ± 1.08 | 0.408 |
| Red blood cell distribution width, % | 15.29 ± 2.08 | 15.20 ± 1.91 | 15.83 ± 2.99 | 0.298 |
| Platelet count, 1000/µL | 233.87 ± 90.32 | 238.43 ± 89.70 | 212.75 ± 91.55 | 0.308 |
| High-sensitivity C-reactive protein, mg/L | 20.22 ± 18.05 | 20.93 ± 44.85 | 16.48 ± 19.21 | 0.649 |
| Log (high sensitivity C-reactive protein), mg/L | 0.83 ± 0.58 | 0.80 ± 0.6 | 0.97 ± 0.51 | 0.301 |
The data represented the last laboratory values prior to the patients being started on PD. *P < 0.05.
Table 3 shows that there were no significant differences between the groups in terms of peritoneal transporter characteristics, dialysis adequacy, residual kidney function or cardiothoracic ratio.
Table 3.
Dialysis-related data of peritoneal dialysis patients stratified by kidney size (n = 83).
| Variable | All patients (n = 83) | Diabetic patients with enlarged or normal kidney size (n = 67) | Diabetic patients with small kidney size (n = 16) | P value |
|---|---|---|---|---|
| Erythropoietin dose, unit/month | 17,108.43 ± 6248.93 | 16,716.42 ± 6630.52 | 18,750.00 ± 4057.91 | 0.245 |
| Dialysate/plasma creatinine | 0.69 ± 0.11 | 0.69 ± 0.013 | 0.68 ± 0.029 | 0.822 |
| Peritoneal equilibration test | 0.517 | |||
| High, n (%) | 12 (14.5) | 10 (14.9) | 2 (12.5) | |
| High average, n (%) | 46 (55.4) | 38 (56.7) | 8 (50.0) | |
| Low average, n (%) | 21 (25.3) | 15 (22.4) | 6 (37.5) | |
| Low, n (%) | 4 (4.8) | 4 (6.0) | 0 (0.0) | |
| Weekly Kt/Vurea | 2.01 ± 0.04 | 2.00 ± 0.39 | 2.06 ± 0.39 | 0.896 |
| Weekly creatinine clearance rate, L/1.73 m2 | 59.52 ± 15.53 | 60.67 ± 16.48 | 54.71 ± 9.67 | 0.170 |
| Normalized protein nitrogen appearance, g/kg/day | 1.03 ± 0.03 | 1.04 ± 0.03 | 0.99 ± 0.06 | 0.426 |
| Residual kidney function, mL/min | 10.66 ± 15.41 | 11.98 ± 16.56 | 5.15 ± 7.26 | 0.112 |
| Cardiothoracic ratio | 0.51 ± 0.06 | 0.51 ± 0.06 | 0.51 ± 0.05 | 0.854 |
Residual kidney function was determined by urinary creatinine clearance using a 24-h urine collection method. Cardiothoracic ratio was measured by dividing the biggest transverse diameter of the heart silhouette by the length between the internal margins of the ribs at the level of right hemidiaphragm.
At the end of analysis, 14 (16.9%) patients died (Table 4). Patients with small kidney size demonstrated a higher mortality rate than patients with enlarged or normal kidney size (50.0 vs. 9.0%, P < 0.001). Moreover, there was more infection-related mortality in the patients with small than in those with enlarged or normal kidney size (43.8 vs. 7.5%, P < 0.001). The sources of infection-related mortalities were three PD-related peritonitis, three nosocomial pneumonia, two pressure sore infection, one fulminant clostridium difficile colitis, one cellulitis, one diabetic foot wound infection and one pulmonary tuberculosis.
Table 4.
Outcomes of peritoneal dialysis patients stratified by kidney size (n = 83).
| Variable | All patients (n = 83) | Diabetic patients with enlarged or normal kidney size (n = 67) | Diabetic patients with small kidney size (n = 16) | P value |
|---|---|---|---|---|
| Follow-up duration, month | 28.98 ± 13.22 | 29.76 ± 12.69 | 25.69 ± 15.25 | 0.271 |
| All-cause mortality, n (%) | 14 (16.9) | 6 (9.0) | 8 (50.0) | < 0.001*** |
| Infection-related mortality, n (%) | 12 (14.5) | 5 (7.5) | 7 (43.8) | < 0.001*** |
| Cardiovascular-cause mortality, n (%) | 2 (2.4) | 1 (1.5) | 1 (6.3) | 0.265 |
| Technical failure, n (%) | 30 (36.1) | 24 (35.8) | 6 (37.5) | 0.900 |
| Peritonitis, episode/100 months | 0.90 ± 2.06 | 0.82 ± 2.23 | 1.07 ± 1.12 | 0.706 |
***P < 0.001.
In a multivariate logistic regression model (Table 5), it was revealed that small kidney size was a significant risk factor associated with mortality (odds ratio 6.452, 95% confidence interval 1.220–34.482, P = 0.028).
Table 5.
Analysis of mortality using a multivariate logistic regression model (n = 83).
| Variable | Univariate analysis | P value | Multivariate analysis | P value |
|---|---|---|---|---|
| Odds ratio (95% confidence interval) | Odds ratio (95% confidence interval) | |||
| Age (per 1-year increase) | 1.072 (1.013–1.136) | 0.017* | 1.029 (0.949–1.115) | 0.489 |
| Alanine aminotransferase (per 1 U/L increase) | 1.009 (0.986–1.033) | 0.442 | ||
| Albumin (per 1 g/dL increase) | 0.132 (0.038–0.463) | 0.002** | 0.589 (0.090–3.861) | 0.581 |
| Alcohol consumption (yes) | 1.238 (0.137–11.169) | 0.849 | ||
| Aspartate aminotransferase (per 1 U/L increase) | 1.041 (1.006–1.077) | 0.021* | 1.018 (0.964–1.076) | 0.520 |
| Blood urea nitrogen (per 1 mg/dL increase) | 0.995 (0.968–1.022) | 0.696 | ||
| Body mass index (per 1 kg/m2 increase) | 1.018 (0.871–1.190) | 0.821 | ||
| Calcium (per 1 mg/dL increase) | 0.782 (0.429–1.423) | 0.421 | ||
| Cardiovascular disease (no) | 0.316 (0.095–1.046) | 0.059 | ||
| Chronic liver disease (no) | 0.273 (0.041–1.809) | 0.178 | ||
| Corrected calcium (per 1 mg/dL increase) | 1.401 (0.685–2.867) | 0.356 | ||
| Creatinine (per 1 mg/dL increase) | 0.689 (0.541–0.877) | 0.003** | 0.780 (0.556–1.095) | 0.151 |
| Duration of peritoneal dialysis (per 1-month increase) | 1.004 (0.987–1.021) | 0.674 | ||
| Fasting glucose (per 1 mg/dL increase) | 1.006 (0.999–1.013) | 0.118 | ||
| Female sex (yes) | 0.308 (0.088–1.078) | 0.065 | ||
| Ferritin (per 1 ng/mL increase) | 1.001 (1.000–1.001) | 0.197 | ||
| Glycated hemoglobin (per 1% increase) | 1.295 (0.887–1.890) | 0.181 | ||
| Hemoglobin (per 1 g/dL increase) | 0.593 (0.373–0.944) | 0.028* | 0.701 (0.387–1.270) | 0.701 |
| Hepatitis B virus carrier (no) | 0.414 (0.093–1.849) | 0.248 | ||
| Hepatitis C virus carrier (no) | 0.369 (0.061–2.246) | 0.279 | ||
| High-density lipoprotein (per 1 mg/dL increase) | 0.985 (0.937–1.036) | 0.555 | ||
| Hypertension (yes) | 3.491 (0.728–16.743) | 0.118 | ||
| High-sensitivity C-reactive protein (per 1 mg/L increase) | 1.013 (0.985–1.043) | 0.364 | ||
| Inorganic phosphorus (per 1 mg/dL increase) | 0.776 (0.534–1.127) | 0.182 | ||
| Intact parathyroid hormone (per 1 pg/mL decrease) | 0.998 (0.995–1.001) | 0.212 | ||
| Iron (per 1 µg/dL increase) | 0.995 (0.974–1.017) | 0.667 | ||
| Mean corpuscular hemoglobin (per 1 pg/cell increase) | 1.180 (0.919–1.516) | 0.195 | ||
| Mean corpuscular hemoglobin concentration (per 1 g Hb/dL increase) | 1.394 (0.822–2.366) | 0.218 | ||
| Mean corpuscular volume (per 1% increase) | 1.040 (0.953–1.134) | 0.384 | ||
| Peritonitis (per 1 episode/100 months increase) | 1.401 (0.961–2.042) | 0.080 | ||
| Platelet count (each increase of 11,000/µL) | 0.989 (0.981–0.997) | 0.006** | 0.990 (0.980–1.001) | 0.064 |
| Red blood cell count (per 1 million/dL increase) | 0.330 (0.103–1.061) | 0.063 | ||
| Small kidney size (yes) | 10.204 (2.801–37.037) | < 0.001*** | 6.452 (1.220–34.482) | 0.028* |
| Smoking habit (yes) | 3.309 (0.398–27.483) | 0.268 | ||
| Sodium (per 1 mEq/L increase) | 0.886 (0.741–1.060) | 0.185 | ||
| Total cholesterol (per 1 mg/dL increase) | 0.997 (0.986–1.008) | 0.556 | ||
| Total iron binding capacity (per 1 µg/dL increase) | 0.995 (0.985–1.005) | 0.347 | ||
| Triglyceride (per 1 mg/dL increase) | 1.001 (0.999–1.003) | 0.441 | ||
| Uric acid (per 1 mg/dL increase) | 1.005 (0.737–1.369) | 0.977 | ||
| White blood cell (per 11,000/µL increase) | 1.276 (0.993–1.638) | 0.056 |
*P < 0.05; **P < 0.01; *P < 0.001.
Kaplan–Meier analysis revealed that patients with small kidney size had lower cumulative survival than did patients with enlarged or normal kidney size (Fig. 1, log-rank test, chi-squared = 15.614, P < 0.001).
Figure 1.
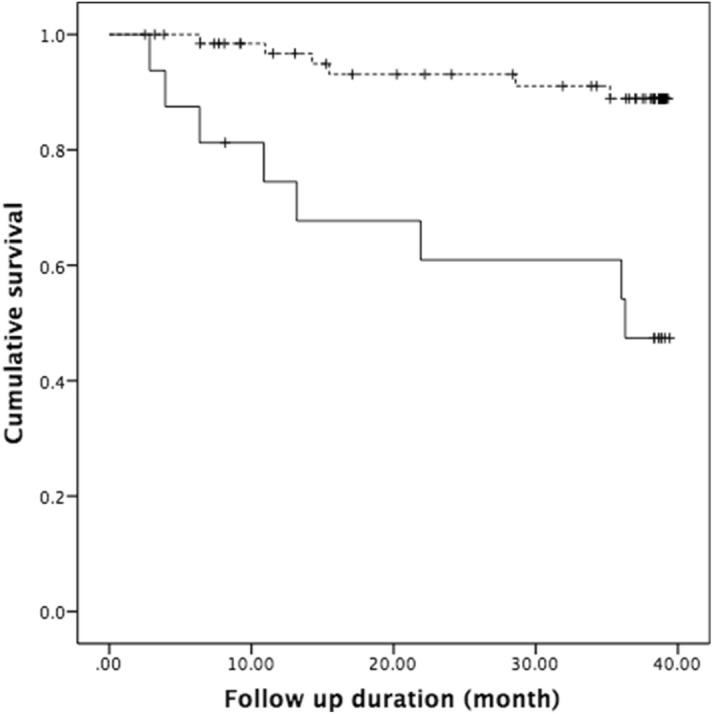
Kaplan–Meier analysis. Patients with small kidney size at the initiation of dialysis (solid line) had lower cumulative survival than patients with enlarged or normal kidney size (dashed line) (log-rank test, chi-squared = 15.614, P < 0.001).
Discussion
The analytical data from this study suggested that small kidney size at the commencement of dialysis could be a risk factor for mortality in the diabetic PD population. This finding adds new knowledge to the existing dialysis literature. There was no clear explanation for the observation. Nevertheless, patients with small kidney size were not only older (P = 0.007), suffered longer duration of diabetes mellitus (P = 0.006), but also had lower serum levels of creatinine (P = 0.022) and albumin (P = 0.010) than patients with enlarged or normal kidney size. The abovementioned variables, for example older age, longer duration of diabetes mellitus, as well as malnutrition (lower blood creatinine and albumin level) could attribute to increased mortality.
Many traditional risk factors for mortality have been described in diabetes patients receiving PD. In a 2-year nationwide cohort study, Abe et al.8 found that glycated albumin ≥ 20.0% was associated with a decrease in survival in diabetic patients on PD. In a study of 118 diabetic PD patients, Coronel et al.9 disclosed that age and cardiovascular comorbidity are the factors associated with mortality. In a retrospective study, Chung et al.10 revealed that old age, female sex, the presence of cardiovascular disease or protein-energy wasting and low residual kidney function were predictors of mortality in diabetic PD patients. In a study of 2798 diabetic PD patients, Duong et al.11 reported that poor glycemic control was associated incrementally with higher mortality. In a study of 61 diabetic PD patients, Koc et al.12 showed that hypoparathyroidemia, hypocalcemia and hypoalbuminemia were risk factors associated with mortality. In a 5-year cohort study of 809 diabetic PD patients, Yang et al.13 reported that older age and the presence of cardiovascular disease, hyperglycemia, anemia and hypoalbuminemia were important risk factors for mortality. In another study of 200 diabetic PD patients, Peng et al.14 showed that increased glycated hemoglobin and decreased albumin-corrected glycated serum protein were associated with mortality in diabetic PD patients.
Patients with small kidney size were older than patients with enlarged or normal kidney size (P = 0.007). Many groups have confirmed such a positive association between decreasing kidney size and aging15–18. Hommos et al.16 reported that aging could be associated with considerable changes in kidney parenchymal structure, even in the absence of age-related comorbidities. Gross pathology examination of aged kidneys found a decrease in kidney cortical volume, an increase in surface roughness, and an increase in the number and size of simple kidney cysts with aging. Histopathology examination also found an increase in nephrosclerosis (arteriosclerosis/arteriolosclerosis, global glomerulosclerosis, interstitial fibrosis, and tubular atrophy) associated with the aging process.
Patients with small kidney size were more often female than patients with enlarged or normal kidney size (P = 0.017). This positive association between small kidney size and female sex has been confirmed by many independent studies17,18. In a study of 665 adult volunteers, Emamina et al.17 reported a sex difference in kidney size in adults; the median kidney lengths were 11.0 cm (women) and 11.5 (men) on the left side and 10.7 cm (women) and 11.2 cm (men) on the right side. Therefore, the female kidney was usually smaller than the male kidney. On the other hand, Piras et al.1 revealed that female sex was associated with a 2.035-fold risk of having a large kidney. Finally, it was unclear whether the diabetic females had more history of analgesic use than diabetic males, although there were no medical records for this, and the recall history was subjected to memory bias. A questionnaire survey in Norway revealed19 that women used both more nonprescription analgesics and more prescription analgesics than men.
The average age of patients in this study was 69.70 ± 11.57 years. This figure is close to that of the Japanese study. In Japan, patients are approximately 69 years of age at the initiation of dialysis20. Currently, there has been a trend of shifting from hospital-based care of older patients to home-based care. Assisted PD has been promoted as an alternative method for older patients with ESKD21. Older patients are susceptible to many physiological changes related to aging and problems such as anxiety, depression, dementia, visual impairment, and cognitive impairment, all of which impede self-performance of PD. Assistance from home-care nurses or assistance from a younger family member may be the solution. In a study of 128 Japanese PD patients, Sakai and Nihei20 reported that the older group (≥ 70 years of age) did not show higher rates of technical failure, but their survival was shorter than that of the younger group (< 70 years of age). In Taiwan, most families are large families, with three generations living in the same household. Younger family members can take care of their older parents and assist them in performing PD therapy. This may explain the higher age of PD in this study.
Patients with small kidney size patients suffered from lower serum levels of creatinine (P = 0.022) and albumin (P = 0.010) than patients with enlarged or normal kidney size. Albumin is the most abundant protein in human serum22. Albumin is produced by the liver and is an indicator of malnutrition. On the other hand, creatinine is the end product of creatine, is mainly present in skeletal muscle and is used as a surrogate measurement of muscle mass23. Creatinine generation is reduced in the setting of low skeletal muscle mass, such as in older patients. Since patients with small kidney size were older and with a predominance of females, it was not surprising that these patients had lower serum levels of creatinine and albumin than the enlarged or normal kidney size group.
Patients with small kidney size demonstrated higher all-cause (P < 0.001) and infection-related (P < 0.001) mortality than patients with enlarged or normal kidney size. Diabetics generally have an increased propensity to develop infections. The increased occurrence of infections in diabetic patients is caused by the hyperglycemic milieu, which supports immune dysfunction (such as the impairment of neutrophil function, the suppression of antioxidant system, and humoral immunity), angiopathy, neuropathy, a decrease in the antibacterial activity of urine, gastrointestinal and urinary dysmotility, and an increased need for medical interventions24. Diabetes impairs antibacterial defenses and increases the risk of infection25. Furthermore, a high concentration of glucose degradation products in PD solutions could accelerate leucocyte apoptosis and damage peritoneal antibacterial defense26. Previous studies27,28 have reported diabetes mellitus as a risk factor for PD-associated peritonitis. According to a large retrospective cohort study in China29, peritonitis was always associated with a higher risk of mortality in PD patients, and its influence on mortality was more significant in patients with longer PD durations. The assessment of the immune defense system of the diabetic patients with small kidney size will need further investigation.
The limitations of this study included small sample size and short follow up duration. In addition, the relationship between small kidneys and mortality was simply a correlation, rather than causal relationship. Since this study involves retrospective review of existing data, it is impossible to make a causal relationship conclusion. The pathophysiological mechanism by which small kidneys confer increased infection risk cannot be answered by this study. Although patients with small kidney size were older and suffered longer duration of diabetes mellitus than patients with enlarged or normal kidney size, there were no difference in blood sugar control between both groups, which could confer increased infection risk. This study is also limited by lacking protocol kidney biopsy. Since approximately half of cases of ESKD in Taiwan are due to diabetic nephropathy, it is possible that half of this cohort may have had other kidney diseases. All patients in this study are diabetes, but the etiologies of renal failure are varied. Since kidney biopsy remains an invasive procedure, it is usually not considered a routine in patients with diabetes mellitus unless suspicion of non-diabetic renal disease. As shown in Table 1, only one patient received kidney biopsy and the pathology report revealed co-existing glomerulonephritis. Further prospective studies are warranted to confirm this finding.
Conclusion
In summary, small kidney size at the beginning of dialysis carries a substantial risk for mortality in diabetic PD patients. These patients demonstrated higher all-cause and infection-related mortality than patients with enlarged or normal kidney size.
Methods
Inclusion and exclusion criteria
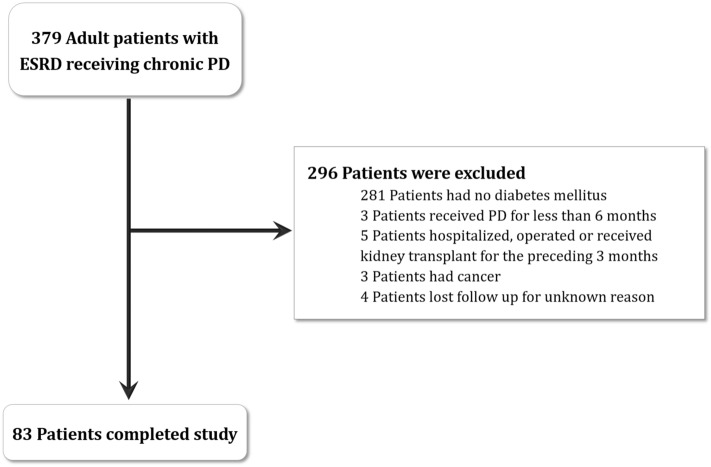
All diabetic patients aged 18 years and above receiving chronic PD at Chang Gung Memorial Hospital between 2015 and 2019 were included in this study. Patients who had been receiving PD for less than 6 months; those who had been hospitalized or operated on or who had received a kidney transplant in the preceding 3 months; and those with cancer were excluded from the study (Fig. 2).
Figure 2.
Flow chart. Diagram shows the enrolment and status of patients. ESKD end-stage kidney disease, PD peritoneal dialysis.
Groups
Patients who met the inclusion criteria were divided into two groups according to their kidney size when entering PD. All patients received ultrasonographic evaluation of kidney size and echogenicity, and a small kidney was defined as when the kidney length was less than 9.0 cm4.
PD prescription
PD prescriptions were based on the peritoneal membrane characteristics determined by the peritoneal equilibration test30. Intermittent therapy was prescribed to patients with high membrane transport and continuous therapy and to those with average or low membrane transport. Low-calcium (1.5 or 1.25 mmol/L), icodextrin-based (7.5 g/dL) or standard dialysates containing glucose (sodium, 135 mmol/L; lactate, 35 mmol/L; calcium, 1.75 mmol/L) were used according to the patients' peritoneal transport characteristics and serum calcium levels to maintain adequate ultrafiltration and enlarged or normal calcium levels. Dialysis prescription aimed at obtaining a total Kt/V of at least 1.8 per week.
Laboratory analysis
The data represented the last laboratory values prior to the patients being started on PD. All laboratory values, including blood cell count, biochemical data, dialysate/plasma creatinine ratio, peritoneal transport characteristics, weekly creatinine clearance and weekly Kt/Vurea, were surveyed by automated and standardized methods. All blood samples were collected in the morning after at least 10 h of fasting. Serum levels of calcium, phosphate and intact parathyroid hormone were also surveyed, and the corrected serum calcium level was calculated as calcium (mg/dL) = [0.8 (4.0 − albumin[g/dL])]. All other markers were surveyed via standard laboratory methods using an automatic analyzer.
Statistical analysis
The continuous variables are presented as the means ± the standard deviations for the numbers of observations, whereas the categorical variables are presented as numbers (percentages)31. For comparison between two groups, Student's t-test was used for quantitative variables, whereas the chi-squared or Fisher's exact test was used for categorical variables. Survival data were analyzed with the Kaplan–Meier method and tested for significance using the log-rank test. A univariate binary logistic regression analysis was performed to compare the frequency of potential risk factors associated with mortality. To control for confounders, a stepwise backward multivariate binary logistic regression analysis was performed to analyze the variables that were significant on univariate analysis. The criterion for significance to reject the null hypothesis was a 95% confidence interval. The statistical analyses were performed using IBM SPSS Statistics Version 25 for Mac (IBM corporation, Armonk, NY, USA).
Ethical statement
This longitudinal observational study complied with the guidelines of the Declaration of Helsinki and was approved by the Medical Ethics Committee of Chang Gung Memorial Hospital. Because this study was a retrospective review of existing data, Institutional Review Board approval was obtained, but without specific informed consent from the patients. The Institutional Review Board of Chang Gung Memorial Hospital specifically waived the need for consent. The Institutional Review Board number assigned to the study was 202000663B0.
Acknowledgements
T.-H.Y. is funded by a research grant from Chang Gung Memorial Hospital (CORPG3K0109, CMRPG3K2021). F.W.K.T. is supported by the Ken and Mary Minton Chair of Renal Medicine, the Royal Society International Exchange Grant, United Kingdom (IEC\R3\183057), and the National Institute for Health Research Imperial Biomedical Research Centre, United Kingdom (RDA28).
Author contributions
C.-H.C. performed study and wrote manuscript, C.-Y.C., Y.-H.C. C.-H.W. ,W.-H.H., C.-W.H. managed patient, M.-C.Y., J.-F.F., Y.-C.H., I.-K.W., F.W.K.T. analyzed data, T.-H.Y. designed and supervised study.
Competing interests
The authors declare no competing interests. FWKT has received research project grants from AstraZeneca Limited, Baxter Biosciences, Boehringer Ingelheim, MedImmune, and Rigel Pharmaceuticals. He has consultancy agreements with Baxter Biosciences, Novartis and Rigel Pharmaceuticals.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Piras D, et al. Kidney size in relation to ageing, gender, renal function, birthweight and chronic kidney disease risk factors in a general population. Nephrol. Dial. Transplant. 2018 doi: 10.1093/ndt/gfy270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rigalleau V, et al. Large kidneys predict poor renal outcome in subjects with diabetes and chronic kidney disease. BMC Nephrol. 2010;11:3. doi: 10.1186/1471-2369-11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van der Sande NGC, et al. Relation between kidney length and cardiovascular and renal risk in high-risk patients. Clin. J. Am. Soc. Nephrol. 2017;12:921–928. doi: 10.2215/CJN.08990816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Majdan M, Kurowska M, Orlowska-Kowalik G, Drop A. Ultrasonographic evaluation of kidneys in type-2 diabetes patients without overt nephropathy and with chronic renal failure. Wiad Lek. 2005;58:25–28. [PubMed] [Google Scholar]
- 5.Habib SL. Kidney atrophy vs hypertrophy in diabetes: which cells are involved? Cell Cycle. 2018;17:1683–1687. doi: 10.1080/15384101.2018.1496744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johansen KL, et al. US renal data system 2020 annual data report: epidemiology of kidney disease in the United States. Am. J. Kidney Dis. 2021;77:A7–A8. doi: 10.1053/j.ajkd.2021.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang IK, et al. Risk of new-onset diabetes in end-stage renal disease patients undergoing dialysis: analysis from registry data of Taiwan. Nephrol. Dial. Transplant. 2018;33:670–675. doi: 10.1093/ndt/gfx250. [DOI] [PubMed] [Google Scholar]
- 8.Abe M, et al. Glycemic control and survival in peritoneal dialysis patients with diabetes: a 2-year nationwide cohort study. Sci. Rep. 2019;9:3320. doi: 10.1038/s41598-019-39933-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coronel F, Cigarran S, Herrero JA. Morbidity and mortality in diabetic patients on peritoneal dialysis. Twenty-five years of experience at a single centre. Nefrologia. 2010;30:626–632. doi: 10.3265/Nefrologia.pre2010.Jul.10553. [DOI] [PubMed] [Google Scholar]
- 10.Chung SH, et al. Risk factors for mortality in diabetic peritoneal dialysis patients. Nephrol. Dial. Transplant. 2010;25:3742–3748. doi: 10.1093/ndt/gfq233. [DOI] [PubMed] [Google Scholar]
- 11.Duong U, et al. Glycemic control and survival in peritoneal dialysis patients with diabetes mellitus. Clin. J. Am. Soc. Nephrol. 2011;6:1041–1048. doi: 10.2215/CJN.08921010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koc Y, Unsal A, Ahbap E, Sakaci T, Yilmaz M. Clinical outcome of diabetic peritoneal dialysis patients and evaluation of factors affecting mortality: a single centre's experience from Turkey. J. Ren. Care. 2011;37:94–100. doi: 10.1111/j.1755-6686.2011.00218.x. [DOI] [PubMed] [Google Scholar]
- 13.Yang X, et al. Clinical outcome and risk factors for mortality in Chinese patients with diabetes on peritoneal dialysis: a 5-year clinical cohort study. Diabetes Res. Clin. Pract. 2013;100:354–361. doi: 10.1016/j.diabres.2013.03.030. [DOI] [PubMed] [Google Scholar]
- 14.Peng F, et al. The effect of glycated hemoglobin and albumin-corrected glycated serum protein on mortality in diabetic patients receiving continuous peritoneal dialysis. Perit Dial Int. 2015;35:566–575. doi: 10.3747/pdi.2014.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang X, et al. Age, kidney function, and risk factors associate differently with cortical and medullary volumes of the kidney. Kidney Int. 2014;85:677–685. doi: 10.1038/ki.2013.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hommos MS, Glassock RJ, Rule AD. Structural and functional changes in human kidneys with healthy aging. J. Am. Soc. Nephrol. 2017;28:2838–2844. doi: 10.1681/ASN.2017040421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Emamian SA, Nielsen MB, Pedersen JF, Ytte L. Kidney dimensions at sonography: correlation with age, sex, and habitus in 665 adult volunteers. AJR. 1993;160:83–86. doi: 10.2214/ajr.160.1.8416654. [DOI] [PubMed] [Google Scholar]
- 18.Krairittichai U, Leehacharoenkul S, Dowreang J. Length of normal kidneys in Thai adults. J. Med. Assoc. Thai. 2011;94(Suppl 2):S23–28. [PubMed] [Google Scholar]
- 19.Samuelsen PJ, Slordal L, Mathisen UD, Eggen AE. Analgesic use in a Norwegian general population: change over time and high-risk use—The Tromso Study. BMC Pharmacol. Toxicol. 2015;16:16. doi: 10.1186/s40360-015-0016-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sakai K, Nihei H. Peritoneal dialysis in elderly patients. Contrib. Nephrol. 2018;196:141–147. doi: 10.1159/000485714. [DOI] [PubMed] [Google Scholar]
- 21.Dimkovic N, Oreopoulos DG. Assisted peritoneal dialysis as a method of choice for elderly with end-stage renal disease. Int. Urol. Nephrol. 2008;40:1143–1150. doi: 10.1007/s11255-008-9427-7. [DOI] [PubMed] [Google Scholar]
- 22.Keller U. Nutritional laboratory markers in malnutrition. J. Clin. Med. 2019 doi: 10.3390/jcm8060775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thongprayoon C, Cheungpasitporn W, Kashani K. Serum creatinine level, a surrogate of muscle mass, predicts mortality in critically ill patients. J. Thorac. Dis. 2016;8:E305–311. doi: 10.21037/jtd.2016.03.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Casqueiro J, Casqueiro J, Alves C. Infections in patients with diabetes mellitus: a review of pathogenesis. Indian J. Endocrinol. Metab. 2012;16(Suppl 1):S27–36. doi: 10.4103/2230-8210.94253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dalai P, Misra M. Improving the care of diabetic patients on peritoneal dialysis. Contrib. Nephrol. 2012;178:271–277. doi: 10.1159/000337890. [DOI] [PubMed] [Google Scholar]
- 26.Santamaria B, et al. Peritoneal defence–lessons learned which apply to diabetes complications. Nephrol. Dial. Transplant. 2006;21(2):ii12–ii15. doi: 10.1093/ndt/gfl185. [DOI] [PubMed] [Google Scholar]
- 27.Nessim SJ, Bargman JM, Austin PC, Nisenbaum R, Jassal SV. Predictors of peritonitis in patients on peritoneal dialysis: results of a large, prospective Canadian database. Clin. J. Am. Soc. Nephrol. 2009;4:1195–1200. doi: 10.2215/CJN.00910209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nadeau-Fredette AC, et al. Center-specific factors associated with peritonitis risk—a multi-center registry analysis. Perit. Dial. Int. 2016;36:509–518. doi: 10.3747/pdi.2015.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ye H, et al. The impact of peritoneal dialysis-related peritonitis on mortality in peritoneal dialysis patients. BMC Nephrol. 2017;18:186. doi: 10.1186/s12882-017-0588-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang PC, et al. Torus palatinus in Taiwan patients receiving peritoneal dialysis and hemodialysis: a prospective observational study. J. Multidiscip. Healthc. 2020;13:373–379. doi: 10.2147/JMDH.S252013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chang ST, et al. Acute kidney injury and the risk of mortality in patients with methanol intoxication. BMC Nephrol. 2019;20:205. doi: 10.1186/s12882-019-1404-0. [DOI] [PMC free article] [PubMed] [Google Scholar]