Abstract
Traumatic brain injury (TBI) was shown to lead to the development of cerebral microbleeds (CMBs), which are associated with long term cognitive decline and gait disturbances in patients. The elderly is one of the most vulnerable parts of the population to suffer TBI. Importantly, ageing is known to exacerbate microvascular fragility and to promote the formation of CMBs. In this overview, the effect of ageing is discussed on the development and characteristics of TBI-related CMBs, with special emphasis on CMBs associated with mild TBI. Four cases of TBI-related CMBs are described to illustrate the concept that ageing exacerbates the deleterious microvascular effects of TBI and that similar brain trauma may induce more CMBs in old patients than in young ones. Recommendations are made for future prospective studies to establish the mechanistic effects of ageing on the formation of CMBs after TBI, and to determine long-term consequences of CMBs on clinically relevant outcome measures including cognitive performance, gait and balance function.
Keywords: Microbleed, Cerebral microhaemorrhage, Brain trauma, Mild traumatic brain injury, Ageing, Vascular changes
Introduction
Traumatic brain injury (TBI) is a serious health problem worldwide [1, 2]. In addition to its acute clinical significance, TBI was shown to lead to chronic neurological dysfunction (including long-term impairment of gait and cognition) and to promote psychiatric disorders [3, 4]. After the direct neuronal damage caused by the impact, a divergent process is initiated resulting in secondary injury of neuroglial tissue [2, 3, 5–9]. Injury of cerebral vessels and cerebrovascular dysfunction play a central role in the pathological processes of secondary injury [7, 8, 10, 11]. After mild, moderate and severe TBI mitochondrial dysfunction, oxidative stress and redox-dependent activation of matrix metalloproteinases (MMP) are enhanced, contributing to the damage of the microvascular wall and to the development of blood-brain barrier (BBB) dysfunction [2, 6, 10, 12–15]. These pathological processes contribute to the formation of microhaemorrhages around brain microvessels [7, 16, 17]. Cerebral microhaemorrhages, also referred as cerebral microbleeds (CMBs), are small hemosiderin deposits (less than 5 to 10 mm in diameter) resulting from bleeding from injured small arteries, cerebral arterioles or capillaries [9, 16, 18–20]. CMBs determine clinical outcome of patients; they are associated with the development of cognitive impairment indicated by attenuated processing speed, defective attention and executive dysfunction [21–24]. They also promote psychiatric disorders such as major depressive episodes [3, 4, 9, 16, 22]. Presence of CMBs is linked to dysfunction in gait coordination and balance: shorter stride length and decreased general functionality [25–28].
The elderly population is prone to suffer TBI [1]. The most frequent cause of trauma among the elderly is unintentional fall [1, 29], usually due to orthostathic hypotension and dehydration. The possibility of falls is exacerbated by impaired balance due to decreased muscular strength and different types of neuropathies [1, 30]. The prognosis of older patients after TBI is worse than middle aged or young individuals [9, 29, 31] indicated by increased mortality, longer hospital stay and higher need for rehabilitation [1, 30, 31]. Rehabilitation is also less effective in the elderly than in young patients [1, 30].
There is growing evidence that ageing is an independent risk factor for the development of CMBs [16, 20, 32–34]. The number of CMBs increases approximately by 20–40% in individuals aged 65 years and older [9, 16, 18, 34]. Strong evidence suggests that CMBs are causally linked to cognitive decline and gait disturbances in the elderly [16, 17, 20, 23, 34]. There is also evidence available that various age-related vascular processes, such as oxidative stress, increased MMP activity, modification of collagen and elastin content of the cerebrovascular wall, and increased fragility of aged cerebral vessels contribute to exacerbation of CMBs in the aged brain [16, 17]. Recent data suggest that in these processes, age-related endocrine changes, especially decline in circulating level of the vasoprotective hormone insulin like growth factor 1 (IGF-1), play a central role [16, 35–37]. Importantly, the prevalence of hypertension significantly increases with age, and hypertension and ageing interact to induce cerebrovascular dysfunction and the formation of cerebral microbleeds [16, 17, 34]. Preclinical studies show that the mechanisms by which hypertension and ageing interact to increase microvascular fragility and to promote the genesis of CMBs include increased oxidative stress, vascular lipohyalinosis, induction of MMP activation and extracellular matrix remodelling [16, 17]. As mentioned above, most of these mechanisms are also induced in TBI [6, 12, 13, 15, 38]. In the following, we present 4 cases of young and aged patients with and without mild TBI and discuss the possible mechanistic interaction between ageing and TBI to induce the formation of CMBs.
Cases
This study was approved by the Regional Ethic Committee of the University of Pecs, Medical School (7270-PTE 2018). We retrospectively analysed the medical history and susceptibility weighted (SWI) MRI (Siemens Magnetom Prisma Fit 3T) series of images of two patients (40-year and 60-year-old males) who suffered mild TBI and were referred to the Department of Neurosurgery, Medical School, University of Pecs, Hungary (Table 1, Figs. 1 and 2). We also evaluated the MRI images of two patients without brain trauma (31-year and 64-year-old males) (Table 1, Fig. 3). SWI MRI was demonstrated to be more proficient to detect CMBs compared to T2* gradient echo (GRE) [19, 32]. This is due to post-processing and the augmentation of the magnetic resonance signal with signal pulse shift [19, 39, 40]. Demonstrated by SWI sequences CMBs are round- or ovoid-shaped hypointense lesions with the dimensions of 5–10 mm, encircled by cerebral parenchyma (in whole or in part) [20, 32, 40]. Exclusion criteria were the presence of any of the following: epilepsy, previous stroke, transient ischemic attack, cerebral amyloid angiopathy, chronic hypertensive encephalopathy, acute haemorrhagic leukoencephality, CADASIL, cerebral vasculitis, cerebral metastases, intracranial infections, intracranial embolism, posterior reversible encephalopathy syndrome, or any types of neurodegenerative diseases [16, 19, 20, 32]. Severity of TBI was defined by the initial Glasgow Coma Scale (GCS): mild 14–15, moderate 8–13 and severe < 8 [30]. Two independent radiologists evaluated the number and distribution of CMBs on SWI series of patients, blinded to the medical history of the cases. Location was described by the Adams Classification system and the Microbleed Anatomical Rating Scale (MARS) system [20, 41, 42].
Table 1.
Summary of the main characteristics of the presented four patients
| Young TBI (YT) | Aged TBI (AT) | Young control (YC) | Aged control (AC) | |
|---|---|---|---|---|
| Age at trauma | 40 | 60 | N/A | N/A |
| Age at MR | 40 | 60 | 35 | 65 |
| Sex | Male | Male | Male | Male |
| Cause of trauma | Traffic accident | Fall | N/A | N/A |
| GCS | 15 | 15 | N/A | N/A |
| LOC | 3 min | None | N/A | N/A |
| PTA | None | None | N/A | N/A |
| Number of CMBs | 2 | 9 | 0 | 1 |
| Location of CMBs according to MARS | Lobar | Lobar, deep | N/A | Lobar |
| Adam’s grade | Grade I | Grade III | N/A | Grade I |
| Comorbidity | None | Hypertension | None | None |
Fig. 1.
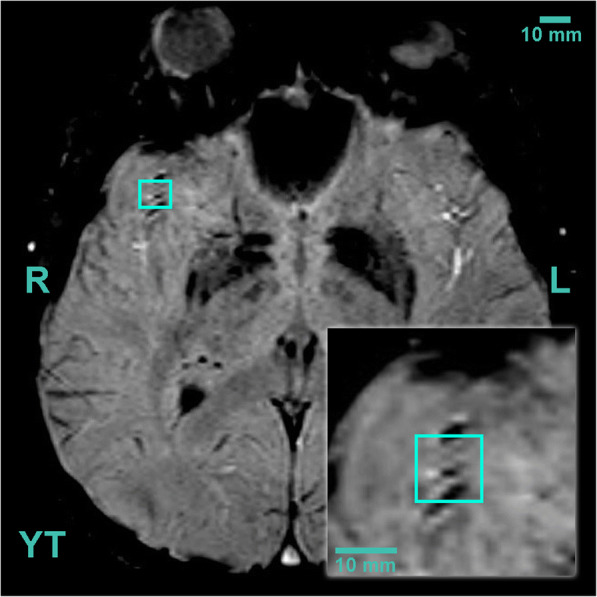
Blue square depicts a cerebral microbleed (CMB) in the right inferior longitudinal fasciculus of a young TBI patient (YT) (40-year-old male, mild TBI). On the axial susceptibility-weighted magnetic resonance image (SWI, obtained at 3 Tesla), the bleeding appears as an ovoid, hypointense lesion [20, 32, 33]
Fig. 2.
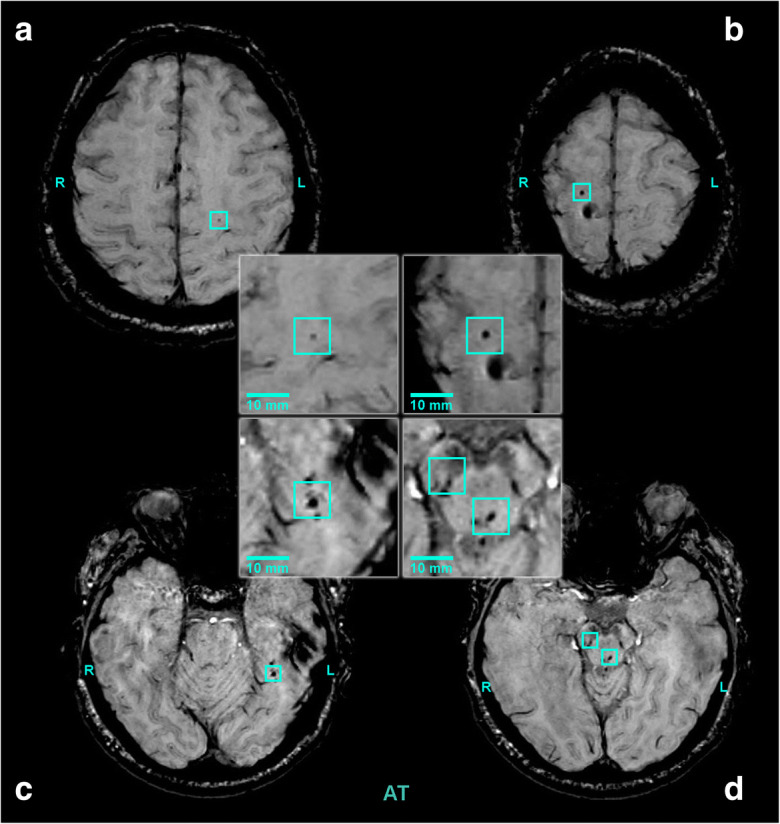
Axial susceptibility-weighted (SWI) magnetic resonance images (3 T) of an elderly patient (case 2) with mild traumatic brain injury (AT) (60-year-old, male). Cerebral microbleeds (CMBs) are highlighted by the blue boxes: A, left corona radiate; B, right corona radiate; C, left parahippocampal gyrus; D, crus cerebri, medial longitudinal fasciculus
Fig. 3.
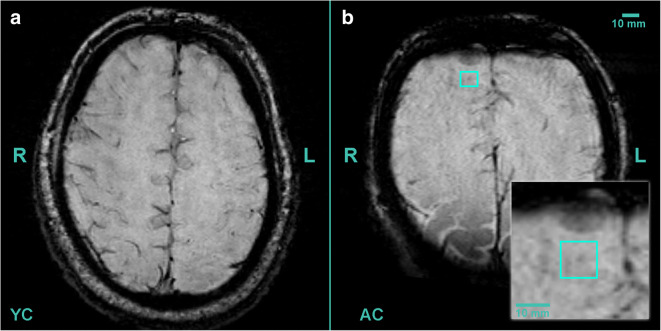
Axial (3 T) SWI MR images of aged and young control patients (without trauma). a Aged control (AC) patient presented one CMB lesion, located in right corona radiate, highlighted with blue square. b The young control (YC) patient had no cerebral lesion
Case 1
The 40-year-old male patient was admitted to the hospital because of a head trauma he had suffered in a road traffic accident. The initial GCS score was 15, no memory disturbances were documented, but following the accident, temporary loss of consciousness occurred for less than 3 min. Neurologic examination did not show any symptoms or signs. He had no comorbidities. CT scan showed no skull fractures or intracranial haemorrhage. On the patient’s MRI by MARS system, 2 CMBs were detected in the right temporal lobe, one was located in the cortical-subcortical border, the other one was located in the subcortical white matter (Fig. 1). According to Adam’s classification, the lesions are grade I.
Case 2
The second patient was a 60-year-old man, who was admitted to the hospital because of a fall. GCS score was 15 at the admittance, no neurological signs or symptoms could be detected. Hypertension had been known for 20 years with dilative cardiomyopathy. CT scan showed a cerebral contusion of 7 mm in diameter in the right parietal cortex and a minor parafalcin subdural haematoma. By MRI, multiple CMBs were detected (Fig. 2). According to MARS system, 7 lobar lesions were found (2 in the right frontal lobe, 1 in the left parietal lobe, 4 in the left temporal lobe) (Fig. 2). Further 2 lesions were found in the brainstem (Fig. 2). According to the Adam’s classification, he has grade III lesions (not affecting the corpus callosum).
Case 3
The patient was a 35-year-old man who attended the clinic with bilateral upper limb numbness. His medical anamnesis is negative for any significant pathology. Laboratory test showed no alterations. The MRI did not reveal any intracranial abnormalities, which could explain the symptoms. On the SWI images, no cerebral microbleeds were found (Fig. 3).
Case 4
The 65-year-old male patient was presented to the clinic with back pain, without any traumatic brain injury in his history. Spondylosis was diagnosed. On the SWI images, 1 cerebral microbleed was found in left frontal lobe in the subcortical white matter (Adams’ grade I lesion) (Fig. 3).
Cerebral microbleeds in TBI and ageing: possible mechanisms
In the presented elderly patient with mild TBI, multiple CMBs were found, which is representative to the imaging findings in patients in this age group. In the young trauma patient, the number of CMBs was markedly less, consistent with findings reported in TBI patients of this age group. It has to be noted that majority of the lesions in the older TBI patient were located in typical brain areas for traumatic CMBs (corona radiata, longitudinal fasciculus) (Fig. 2); however, two lesions were detected in the brainstem, an atypical location for traumatic microbleeds [3, 19, 23, 25, 34, 43]. Cerebral microbleeds in deep cerebral areas are thought to be due to cerebral angiopathy induced by hypertension [33, 43]. The presented cases support the hypothesis that ageing and TBI may interact to promote the development of CMBs.
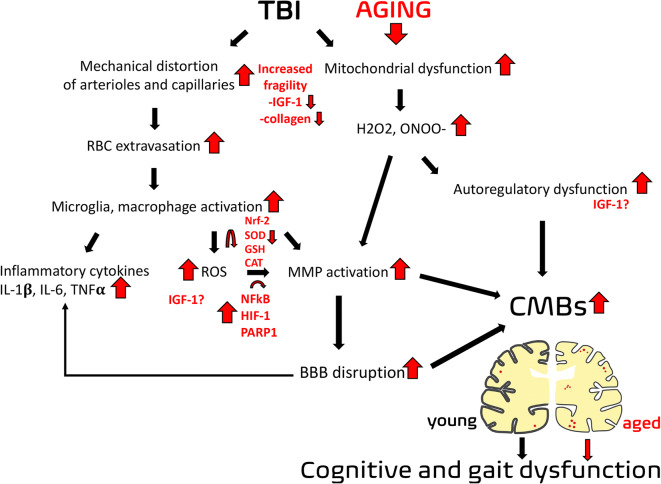
In the following section, the possible mechanisms by which ageing promotes TBI-induced CMBs and exacerbates CMB-related neuronal dysfunction are discussed (Fig. 4). Sudden accelerating and decelerating shearing forces during head trauma likely play a central role in the development of CMBs, which accompanies TBI-related diffuse axonal injury [7, 9, 25, 44]. Mechanical distortion of endothelial cells leads to disruption of the BBB and capillary damage, provoking blood extravasation and the formation of small haemorrhagic lesions [8, 44–46]. Traumatic microbleeds are characteristic in the vicinity of small cerebral arteries, arterioles, capillaries and bridging veins [8, 44, 46]. Collagen (mainly I and III) plays an important role in vascular stiffness and tissue repair [16, 36, 47]. During ageing, vascular collagen is modified due to age-related mineralisation [16, 36]. The mineralised and modified collagen is more fragile; thus, the aged vessels are more susceptible to be injured after trauma [16, 48–50]. In addition to the increased vascular stiffness due to age-related enhanced collagen content of the cerebrovascular wall, ageing promotes the structural modification of elastin leading to impaired elasticity of the vessels [16, 36, 47–49]. These age-related changes in biomechanical properties of cerebral vessels most likely exacerbate the abovementioned TBI-related mechanical injury. Interestingly, in animal models, both mild and severe TBIs were shown to lead to a decrease in cerebrovascular stiffness indicated by attenuated modulus of rigidity, as well as an increase in the radius of the vessels in the affected cerebral tissue. This potentially contributes to reactive local hyperperfusion [51–54]. One can hypothesise that this hydrostatic burden may exacerbate TBI-related vascular injury in the elderly. After cerebral vessels become leaky, extravasated erythrocytes and plasma triggers activation of microglia and macrophages, migration of neutrophils and increased production of cytokines [7–9, 55]. This inflammatory reaction contributes to neuronal damage and dysfunction as shown by demyelination, loss of neuropil, impaired fluid removal in perivascular spaces, impaired neurogenesis and differentiation [7, 33, 45, 55, 56]. These are most likely exacerbated in ageing, as the number of activated microglia is increased in the aged brain, being responsible for excessive and prolonged expression of inflammatory cytokines IL-1, IL-6, IL-12 and TNF α [56–58].
Fig. 4.
Possible mechanisms of the synergistic effect of traumatic brain injury and ageing on the formation of cerebral microbleeds. Please see detailed description in the text. Traumatic brain injury (TBI) leads to mechanical distortion of cerebral vessels, which may directly lead to injury of the vascular wall and formation of microhemorrhages around cerebral arterioles and capillaries. This mechanism may be enhanced by ageing via age-related changes of the cerebrovascular wall leading to increased fragility of the vessels. TBI-induced mitochondrial oxidative stress and production of reactive oxygen species (ROS) and inflammatory mediators in activated microglia and macrophages following TBI may be exacerbated by ageing due to the age-related decreased antioxidant cellular mechanisms. In addition to the direct damage of the cerebrovascular wall, TBI-induced autoregulatory dysfunction may contribute to the development of cerebral microbleeds by placing increased hydrostatic burden on the cerebral microcirculation due to lack of proximal protection against blood pressure. Autoregulatory dysfunction may be exacerbated by age-related deficiency of circulating insulin-like growth factor 1 (IGF-1). These mechanisms converge on the disruption of the blood-brain barrier (BBB) and formation of cerebral microbleeds and consequent cognitive and gait dysfunction following TBI. We posit that enhanced vascular fragility, increased cerebrovascular oxidative stress and autoregulatory dysfunction in the elderly result in the formation of more cerebral microbleeds and more severe impairment of cognitive and gait function compared to young patients
TBI induces mitochondrial dysfunction and excessive production of mitochondria-derived free radicals (mostly hydrogen peroxide and peroxynitrite), which are further exacerbated by accumulation of hemosiderin, heme and free iron in the cerebral parenchyma and in endothelial cells [7, 45, 55, 59]. This results in further BBB disruption and formation of vasogenic and cytotoxic oedema, leading to a vicious cycle [7, 8, 10, 45]. The aforementioned cascade is thought to be more critical in the elderly. For example, in ageing, TBI-induced microglial proliferation is more pronounced than in young patients because of age-related decreased phagocytic activity, increased ROS production and enhanced leukocyte activation [11, 60, 61]. In ageing, cerebrovascular oxidative stress is increased compared to younger individuals, partly due to impaired antioxidant defence mechanisms (including dysfunction of the Nrf2-dependent cytoprotective pathways, decreased level and activity of antioxidant enzymes as superoxide dismutase (SOD), catalase and the glutathione system (GSH)) as well as up-regulation of NADPH oxidases [16, 17, 50, 58, 61–72]. One of the main sources of ROS in the cerebrovasculature are mitochondria [17, 62, 71, 73]. Mitochondrial oxidative stress has been shown to be increased after TBI as well as in ageing [2, 6, 7, 11, 12, 58, 60, 62]. Importantly, mitochondrial DNA is more prone to damage caused by reactive oxygen substances, which is also exaggerated in ageing [62, 63]. It is of note that mitochondrial oxidative stress was shown to contribute to autoregulatory dysfunction following TBI, which may result in downstream injury of the cerebral microcirculation due to pressure and volume overload [10, 12]. This hydrostatic burden contributes to the development of both BBB disruption and formation of microhaemorrhages [7, 10, 15, 16]. This pathophysiological mechanism is likely enhanced by ageing and should be studied in the future [35].
Matrix metalloproteinases (MMPs) play a central role in structural microvascular damage and BBB disruption after TBI [10, 13–15]. Importantly, ageing results in increased MMP activity in the brain [74]. MMPs are activated by age-related crosslinking of vascular collagen and are induced by age-related oxidative stress and by decreased activity of protease inhibitors [16, 17, 36, 48, 49, 74]. TBI can induce MMP activity via activating transcription factors such as hypoxia-inducible factor 1 alpha (HIF1α), NF-kB and poly(ADP-ribose) polymerase-1 (PARP-1) [7, 11, 49, 73]. These transcription factors are found to be induced in the elderly [17, 61]. Age-related activation of NF-kB and HIF1 α alters mitochondrial and cellular repair function, as well, augmenting inflammatory mechanisms and further potentiating TBI-induced secondary injury [61].
It is important to note that age-related endocrine changes, specifically, the age-related decline in IGF-1 may play a central role in the development of age-related, hypertension-induced formation of microbleeds [35, 75, 76]. It was recently demonstrated that deficiency of circulating IGF-1 after genetic knock-down of hepatic production of the hormone in mice exacerbates the formation of cerebral microbleeds in response to hypertension, mimicking the ageing phenotype [35, 76]. IGF-1 is also known to confer multifaceted neuroprotective effects [75]. Important in this regard is that the GH/IGF-1 axis is the most sensitive to be impaired following TBI [77, 78]. Shearing forces acting on the hypothalamo-hypopituitary system, TBI-related increased intracranial pressure, haemorrhage and oedema formation and consequent local circulatory deficit have been suggested to contribute to the impairment of the GH/IGF-1, which affects approximately 10–20% of TBI patients [77, 79, 80]. GH/IGF-1 deficiency can last for years following TBI and has been suggested to significantly contribute to chronic cognitive decline, as well as to decrease quality of life of TBI patients [75, 77, 81, 82]. It is logical to posit that TBI-related attenuation of IGF-1 production may be exacerbated in aged subjects. Future studies should determine the contribution of age-related IGF-1 deficiency to the genesis of CMBs and/or exacerbated CMB-induced neuronal damage in older adults.
Clinical importance
Gait dysfunction
Posture and gait necessitate coordinated operation of cortical (motor cortex) and subcortical areas (basal ganglia, thalamus, cerebellum, the limbic system, midbrain, pons, medulla and spinal locomotor network) [16, 83]. Development of CMBs results in gait dysfunction by damaging these centres and disrupting the communicating pathways between them [27, 28, 58]. Accordingly, CMBs in temporal and frontal lobes, in basal ganglia and corona radiata (independent of white matter lesions) showed a significant correlation with poor gait function in elderly patients [20, 27]. In the elderly, gait disturbance manifests as impaired stride length, double support time, cadence and decreased performance on the timed up and go tests [27, 28, 84]. Interestingly, only one case series investigated the effect of traumatic microbleeds on gait dysfunction [85]. This study showed that SWI positive TBI patients developed vestibular or balance abnormalities [85]. It is logical to postulate that TBI exacerbates gait dysfunction in the elderly, and gait disturbance of the elderly most likely is a central factor in the increased incidence of TBI amongst them due to increased propensity to fall. Future clinical studies are evidently needed to clarify the possible interactions between ageing and TBI on gait function and the possible role of cerebral microbleeds.
Cognitive dysfunction
The association between the number and distribution of CMBs of different etiologies and cognitive decline has been widely analysed; however, the underlying mechanism is not fully understood. It is proposed that in development of cognitive decline, cumulative effects of the lesions as well as damage in specific anatomical locations are critical [23, 24, 33, 65]. For example, microstructural damage of fronto-subcortical circuits linking prefrontal areas to basal ganglia is associated with impairment in executive function of healthy individuals in all age groups of patients with vascular disease, whereas disarrangement of pathways from the mentioned areas projecting to thalamus results in memory disturbances [23, 24, 33, 86, 87].
In non-demented healthy elderly patients, presence of deep, subcortical CMBs was related to deterioration of global cognitive performance, particularly affecting executive function, memory and information processing, while strictly lobar CMBs resulted in executive dysfunction, decreased processing speed and gait disturbances, as well [21, 33, 83, 88].
Despite the aforementioned association between brain trauma and the development of microbleeds, limited information is available regarding the effect of traumatic CMBs on cognitive outcome [3, 9, 44]. A single case study proposed a connection between traumatic CMBs following mild TBI and decline in cognitive performance of a previously healthy 57-year-old male patient [5]. This was further substantiated by studies showing that in mild TBI patients’ number of traumatic CMBs correlated with altered neurocognitive function, impaired short-term memory, concentration difficulties and depression [3, 4, 44, 87]. Interestingly, the number of lesions in the acute stage predicted the progress of post-concussion syndrome and decline in processing speed a year after the injury [87]. Although it seems logical to posit that (even mild) brain trauma results in enhanced cognitive disturbances in elderly individuals, to our best knowledge, no studies have tested this hypothesis. Thus, future clinical research should investigate the synergistic effect of ageing and TBI-related formation cerebral microbleeds on cognitive decline.
Conclusion and perspective
Traumatic brain injury in older adults is associated with the development of multiple CMBs (Fig. 2); however, our case report does not provide statistically relevant data to support the synergistic effect of ageing and TBI on the formation of cerebral microbleeds after brain trauma. Future clinical studies should determine the predictive value of CMBs in older TBI patients in order to estimate long-term outcome, including detailed characterization of their effect on gait and cognitive function.
Specific cellular and molecular mechanisms should be identified that could be targeted pharmacologically to prevent the development of CMBs and/or limit their deleterious effects on neuronal survival and function in the elderly after brain trauma. Testing the role of different factors involved in the synergistic pathways between ageing and TBI in formation of microbleeds could be tested by applying brain trauma of different severity on preclinical models of accelerated vascular ageing. For example, the specific role of IGF-1 deficiency in the development of traumatic microbleeds could be tested by studying the development of cerebral microhemorrhages after brain trauma in mice with viral knockdown of hepatic production of IGF-1, and the protective effect of IGF-1 supplementation could be tested [76]. As outlined above, age-related increased oxidative stress is a likely factor enhancing the formation of microbleeds following TBI. In this regard, age-dependent decreased antioxidant mechanisms play a pivotal role. Thus, it would be logical to study the formation and characterize TBI-induced CMBs in the previously used Nrf2-deficient mice [68]. Applying a similar theoretical approach, TBI-induced formation of cerebral microbleeds should also be studied in mice overexpressing mitochondrial catalase, which was previously demonstrated to effectively attenuate cerebrovascular mitochondrial oxidative stress [72, 73]. Various pharmacological interventions have been shown to decrease cerebrovascular oxidative stress and its consequences. For example, hypertension- and ageing-induced development of microhaemorrhages was prevented by treatment the animals with resveratrol, and the mitochondrial antioxidant peptide SS-31 [17, 67]. The potential positive effect of these compounds should be tested on the formation and development of TBI-induced cerebral microbleeds in ageing. Based on recent results showing that restoring cellular NAD+ levels in aged mice by treatment with nicotinamide mononucleotide (NMN), a key NAD+ intermediate, rescues neurovascular function, increases cerebral blood flow, and improves performance on cognitive tasks, we posit that NMN treatment likely prevent the formation of TBI-induced formation of cerebral microbleeds in both young and aged laboratory animals, as well [66]. Future studies should verify the preventive/protective effects of dietary intake of the NAD+ boosting compounds quercetin and luteolin in patients after TBI [89]. Other possible neuroprotective pathways should be studied, as well. For example, neurotrophins, such as brain-derived neurotrophic factor (BDNF) acting on tropomyosin receptor kinase B (TrK/B) receptors, have a significant role in neuronal survival, synaptic plasticity and neurogenesis under various pathological conditions [90–94]. Following TBI, the level of BDNF is temporarily increased to exert neuroprotection [91–93]. Interestingly, the level of BDNF significantly decrease with age, and it is also attenuated in chronic cardiac failure being associated with ageing, as well [90, 93–97]. Therefore, ageing presumably limits the protective increase in BDNF after TBI. This hypothesis and the role of BDNF in age- and TBI-related neuronal dysfunction should be tested in the future.
Author contributions
LT, AC, PH, BK and NS performed literature search and collected patient data; LT, AC and NS generated figures; LT, AC and PT wrote the manuscript; LT, AC, PH, BK, NS, AS, ZU, AB and TP edited and revised the manuscript.
Funding
Open access funding provided by University of Pécs. This work was supported by grants from the National Research, Development and Innovation Office to PT (NKFI-FK123798), the Hungarian Academy of Sciences Bolyai Research Scholarship to PT, Higher Education Institutional Excellence Programme of the Ministry of Human Capacities –FIKP II/5, EFOP-3.6.2.-16-2017-00008, GINOP-2.3.2-15-2016-00048 and GINOP-2.3.3-15-2016-00032 to PT and AB, National Institute of Health R01-AG055395, R01-NS100782, R01-AT006526 to ZU.
Availability of data and material
The manuscript has not been published previously.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
Compliant to all relevant ethical standards. The study was approved by the Regional Ethic Committee of the University of Pecs, Medical School (7270-PTE 2018).
Consent to participate
Compliant to all relevant ethical standards. For this type of study, formal consent is not required.
Consent for publication
Consent to submit has been received from all co-authors and responsible authorities at the institute where the work has been carried out before the work is submitted.
Code availability
Not applicable.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Krishnamoorthy V, Distelhorst JT, Vavilala MS, Thompson H. Traumatic brain injury in the elderly: burden, risk factors, and prevention. J Trauma Nurs. 2015;22(4):204–208. doi: 10.1097/jtn.0000000000000135. [DOI] [PubMed] [Google Scholar]
- 2.Ng SY, Lee AYW. Traumatic brain injuries: pathophysiology and potential therapeutic targets. Front Cell Neurosci. 2019;13:528. doi: 10.3389/fncel.2019.00528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Niogi SN, Mukherjee P, Ghajar J, Johnson C, Kolster RA, Sarkar R, Lee H, Meeker M, Zimmerman RD, Manley GT, McCandliss BD. Extent of microstructural white matter injury in postconcussive syndrome correlates with impaired cognitive reaction time: a 3T diffusion tensor imaging study of mild traumatic brain injury. AJNR Am J Neuroradiol. 2008;29(5):967–973. doi: 10.3174/ajnr.A0970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang X, Wei XE, Li MH, Li WB, Zhou YJ, Zhang B, Li YH. Microbleeds on susceptibility-weighted MRI in depressive and non-depressive patients after mild traumatic brain injury. Neurol Sci. 2014;35(10):1533–1539. doi: 10.1007/s10072-014-1788-3. [DOI] [PubMed] [Google Scholar]
- 5.Bigler ED. Neuropsychological results and neuropathological findings at autopsy in a case of mild traumatic brain injury. J Int Neuropsychol Soc. 2004;10(5):794–806. doi: 10.1017/s1355617704105146. [DOI] [PubMed] [Google Scholar]
- 6.Eastman CL, D'Ambrosio R, Ganesh T. Modulating neuroinflammation and oxidative stress to prevent epilepsy and improve outcomes after traumatic brain injury. Neuropharmacology. 2019:107907. 10.1016/j.neuropharm.2019.107907. [DOI] [PMC free article] [PubMed]
- 7.Glushakova OY, Johnson D, Hayes RL. Delayed increases in microvascular pathology after experimental traumatic brain injury are associated with prolonged inflammation, blood-brain barrier disruption, and progressive white matter damage. J Neurotrauma. 2014;31(13):1180–1193. doi: 10.1089/neu.2013.3080. [DOI] [PubMed] [Google Scholar]
- 8.Griffin AD, Turtzo LC, Parikh GY, Tolpygo A, Lodato Z, Moses AD, Nair G, Perl DP, Edwards NA, Dardzinski BJ, Armstrong RC, Ray-Chaudhury A, Mitra PP, Latour LL. Traumatic microbleeds suggest vascular injury and predict disability in traumatic brain injury. Brain. 2019;142(11):3550–3564. doi: 10.1093/brain/awz290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Irimia A, Van Horn JD, Vespa PM. Cerebral microhemorrhages due to traumatic brain injury and their effects on the aging human brain. Neurobiol Aging. 2018;66:158–164. doi: 10.1016/j.neurobiolaging.2018.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Szarka N, Pabbidi MR, Amrein K, Czeiter E, Berta G, Pohoczky K, Helyes Z, Ungvari Z, Koller A, Buki A, Toth P. Traumatic brain injury impairs myogenic constriction of cerebral arteries: role of mitochondria-derived H(2)O(2) and TRPV4-dependent activation of BK(ca) channels. J Neurotrauma. 2018;35(7):930–939. doi: 10.1089/neu.2017.5056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ritzel RM, Doran SJ, Glaser EP, Meadows VE, Faden AI, Stoica BA, Loane DJ. Old age increases microglial senescence, exacerbates secondary neuroinflammation, and worsens neurological outcomes after acute traumatic brain injury in mice. Neurobiol Aging. 2019;77:194–206. doi: 10.1016/j.neurobiolaging.2019.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khatri N, Thakur M, Pareek V, Kumar S, Sharma S, Datusalia AK. Oxidative stress: major threat in traumatic brain injury. CNS Neurol Disord Drug Targets. 2018;17(9):689–695. doi: 10.2174/1871527317666180627120501. [DOI] [PubMed] [Google Scholar]
- 13.Suehiro E, Fujisawa H, Akimura T, Ishihara H, Kajiwara K, Kato S, Fujii M, Yamashita S, Maekawa T, Suzuki M. Increased matrix metalloproteinase-9 in blood in association with activation of interleukin-6 after traumatic brain injury: influence of hypothermic therapy. J Neurotrauma. 2004;21(12):1706–1711. doi: 10.1089/neu.2004.21.1706. [DOI] [PubMed] [Google Scholar]
- 14.Pijet B, Stefaniuk M, Kaczmarek L. MMP-9 contributes to dendritic spine remodeling following traumatic brain injury. Neural Plasticity. 2019;2019:3259295–3259212. doi: 10.1155/2019/3259295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trivedi A, Noble-Haeusslein LJ, Levine JM, Santucci AD, Reeves TM, Phillips LL. Matrix metalloproteinase signals following neurotrauma are right on cue. Cell Mole Life Sci. 2019;76(16):3141–3156. doi: 10.1007/s00018-019-03176-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ungvari Z, Tarantini S, Kirkpatrick AC, Csiszar A, Prodan CI. Cerebral microhemorrhages: mechanisms, consequences, and prevention. Am J Physiol Heart Circ Physiol. 2017;312(6):H1128–H1h43. doi: 10.1152/ajpheart.00780.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Toth P, Tarantini S, Springo Z, Tucsek Z, Gautam T, Giles CB, Wren JD, Koller A, Sonntag WE, Csiszar A, Ungvari Z. Aging exacerbates hypertension-induced cerebral microhemorrhages in mice: role of resveratrol treatment in vasoprotection. Aging Cell. 2015;14(3):400–408. doi: 10.1111/acel.12315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Altmann-Schneider I, Trompet S, de Craen AJ, van Es AC, Jukema JW, Stott DJ, et al. Cerebral microbleeds are predictive of mortality in the elderly. Stroke. 2011;42(3):638–644. doi: 10.1161/STROKEAHA.110.595611. [DOI] [PubMed] [Google Scholar]
- 19.Yates PA, Villemagne VL, Ellis KA, Desmond PM, Masters CL, Rowe CC. Cerebral microbleeds: a review of clinical, genetic, and neuroimaging associations. Front Neurol. 2014;4:205. doi: 10.3389/fneur.2013.00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yakushiji Y. Cerebral microbleeds: detection, associations and clinical implications. Front Neurol Neurosci. 2015;37:78–92. doi: 10.1159/000437115. [DOI] [PubMed] [Google Scholar]
- 21.Akoudad S, Wolters FJ, Viswanathan A, de Bruijn RF, van der Lugt A, Hofman A, Koudstaal PJ, Ikram MA, Vernooij MW. Association of cerebral microbleeds with cognitive decline and dementia. JAMA Neurol. 2016;73(8):934–943. doi: 10.1001/jamaneurol.2016.10172526492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu R, Feng C, Zhao Y, Jin A-P, Fang M, Liu X. A meta-analysis of association between cerebral microbleeds and cognitive impairment. Med Sci Monit. 2014;20:2189–2198. doi: 10.12659/MSM.891004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Werring DJ, Gregoire SM, Cipolotti L. Cerebral microbleeds and vascular cognitive impairment. J Neurol Sci. 2010;299(1–2):131–135. doi: 10.1016/j.jns.2010.08.034S0022-510X(10)00401-6. [DOI] [PubMed] [Google Scholar]
- 24.Werring DJ, Frazer DW, Coward LJ, Losseff NA, Watt H, Cipolotti L, et al. Cognitive dysfunction in patients with cerebral microbleeds on T2*-weighted gradient-echo MRI. Brain. 2004;127(10):2265–2275. doi: 10.1093/brain/awh253. [DOI] [PubMed] [Google Scholar]
- 25.de Haan S, de Groot JC, Jacobs B, van der Naalt J. The association between microhaemorrhages and post - traumatic functional outcome in the chronic phase after mild traumatic brain injury. Neuroradiology. 2017;59(10):963–969. doi: 10.1007/s00234-017-1898-8. [DOI] [PubMed] [Google Scholar]
- 26.Yamaguchi T, Takai H, Hirai S, Yokosuka K, Toi H, Kuwayama K, et al. Microbleeds as a prognostic factor for acute subdural hematoma. Neurol Med Chir. 2013;53(5):318–322. doi: 10.2176/nmc.53.318. [DOI] [PubMed] [Google Scholar]
- 27.Srikanth V, Phan TG, Chen J, Beare R, Stapleton JM, Reutens DC. The location of white matter lesions and gait--a voxel-based study. Ann Neurol. 2010;67(2):265–269. doi: 10.1002/ana.21826. [DOI] [PubMed] [Google Scholar]
- 28.de Laat KF, van den Berg HA, van Norden AG, Gons RA, Olde Rikkert MG, de Leeuw FE. Microbleeds are independently related to gait disturbances in elderly individuals with cerebral small vessel disease. Stroke. 2011;42(2):494–497. doi: 10.1161/STROKEAHA.110.596122. [DOI] [PubMed] [Google Scholar]
- 29.Cheng PL, Lin HY, Lee YK, Hsu CY, Lee CC, Su YC. Higher mortality rates among the elderly with mild traumatic brain injury: a nationwide cohort study. Scandi J Trauma Resuscitation Emerg Med. 2014;22:7. doi: 10.1186/1757-7241-22-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Susman M, DiRusso SM, Sullivan T, Risucci D, Nealon P, Cuff S, et al. Traumatic brain injury in the elderly: increased mortality and worse functional outcome at discharge despite lower injury severity. J Trauma. 2002;53(2):219–223. doi: 10.1097/00005373-200208000-00004. [DOI] [PubMed] [Google Scholar]
- 31.LeBlanc J, de Guise E, Gosselin N, Feyz M. Comparison of functional outcome following acute care in young, middle-aged and elderly patients with traumatic brain injury. Brain Inj. 2006;20(8):779–790. doi: 10.1080/02699050600831835. [DOI] [PubMed] [Google Scholar]
- 32.Greenberg SM, Vernooij MW, Cordonnier C, Viswanathan A, Al-Shahi Salman R, Warach S, et al. Cerebral microbleeds: a guide to detection and interpretation. Lancet Neurol. 2009;8(2):165–174. doi: 10.1016/S1474-4422(09)70013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ding J, Sigurethsson S, Jonsson PV, Eiriksdottir G, Meirelles O, Kjartansson O, et al. Space and location of cerebral microbleeds, cognitive decline, and dementia in the community. Neurology. 2017;88(22):2089–2097. doi: 10.1212/wnl.0000000000003983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vernooij MW, van der Lugt A, Ikram MA, Wielopolski PA, Niessen WJ, Hofman A, Krestin GP, Breteler MMB. Prevalence and risk factors of cerebral microbleeds: the Rotterdam scan study. Neurology. 2008;70(14):1208–14. doi: 10.1212/01.wnl.0000307750.41970.d9. [DOI] [PubMed] [Google Scholar]
- 35.Toth P, Tucsek Z, Tarantini S, Sosnowska D, Gautam T, Mitschelen M, Koller A, Sonntag WE, Csiszar A, Ungvari Z. IGF-1 deficiency impairs cerebral myogenic autoregulation in hypertensive mice. J Cereb Blood Flow Metab. 2014;34(12):1887–1897. doi: 10.1038/jcbfm.2014.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Freitas-Rodriguez S, Folgueras AR, Lopez-Otin C. The role of matrix metalloproteinases in aging: Tissue remodeling and beyond. Biochim Biophys Acta, Mol Cell Res. 2017;1864(11 Pt A):2015–2025. doi: 10.1016/j.bbamcr.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 37.Tarantini S, Giles CB, Wren JD, Ashpole NM, Valcarcel-Ares MN, Wei JY, Sonntag WE, Ungvari Z, Csiszar A. IGF-1 deficiency in a critical period early in life influences the vascular aging phenotype in mice by altering miRNA-mediated post-transcriptional gene regulation: implications for the developmental origins of health and disease hypothesis. AGE. 2016;38(4):239–258. doi: 10.1007/s11357-016-9943-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hannon MJ, Sherlock M, Thompson CJ. Pituitary dysfunction following traumatic brain injury or subarachnoid haemorrhage - in “Endocrine Management in the Intensive Care Unit”. Best Pract Res Clin Endocrinol Metab. 2011;25(5):783–798. doi: 10.1016/j.beem.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 39.Akiyama Y, Miyata K, Harada K, Minamida Y, Nonaka T, Koyanagi I, et al. Susceptibility-weighted magnetic resonance imaging for the detection of cerebral microhemorrhage in patients with traumatic brain injury. Neurol Med Chir. 2009;49(3):97–99. doi: 10.2176/nmc.49.97. [DOI] [PubMed] [Google Scholar]
- 40.Toth A, Kovacs N, Perlaki G, Orsi G, Aradi M, Komaromy H, Ezer E, Bukovics P, Farkas O, Janszky J, Doczi T, Buki A, Schwarcz A. Multi-modal magnetic resonance imaging in the acute and sub-acute phase of mild traumatic brain injury: can we see the difference? J Neurotrauma. 2013;30(1):2–10. doi: 10.1089/neu.2012.2486. [DOI] [PubMed] [Google Scholar]
- 41.Gregoire SM, Chaudhary UJ, Brown MM, Yousry TA, Kallis C, Jager HR, Werring DJ. The Microbleed Anatomical Rating Scale (MARS): reliability of a tool to map brain microbleeds. Neurology. 2009;73(21):1759–1766. doi: 10.1212/WNL.0b013e3181c34a7d73/21/1759. [DOI] [PubMed] [Google Scholar]
- 42.Izzy S, Mazwi NL, Martinez S, Spencer CA, Klein JP, Parikh G, Glenn MB, Greenberg SM, Greer DM, Wu O, Edlow BL. Revisiting grade 3 diffuse axonal injury: not all brainstem microbleeds are prognostically equal. Neurocrit Care. 2017;27(2):199–207. doi: 10.1007/s12028-017-0399-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Imaizumi T, Miyata K, Inamura S, Kohama I, Nyon KS, Nomura T. The difference in location between traumatic cerebral microbleeds and microangiopathic microbleeds associated with stroke. J Neuroimaging. 2011;21(4):359–364. doi: 10.1111/j.1552-6569.2011.00593.x. [DOI] [PubMed] [Google Scholar]
- 44.Huang YL, Kuo YS, Tseng YC, Chen DY, Chiu WT, Chen CJ. Susceptibility-weighted MRI in mild traumatic brain injury. Neurology. 2015;84(6):580–585. doi: 10.1212/wnl.0000000000001237. [DOI] [PubMed] [Google Scholar]
- 45.Lok J, Leung W, Murphy S, Butler W, Noviski N, Lo EH. Intracranial hemorrhage: mechanisms of secondary brain injury. Acta Neurochir Suppl. 2011;111:63–69. doi: 10.1007/978-3-7091-0693-8_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fazekas F, Kleinert R, Roob G, Kleinert G, Kapeller P, Schmidt R, Hartung HP. Histopathologic analysis of foci of signal loss on gradient-echo T2*-weighted MR images in patients with spontaneous intracerebral hemorrhage: evidence of microangiopathy-related microbleeds. AJNR Am J Neuroradiol. 1999;20(4):637–642. [PMC free article] [PubMed] [Google Scholar]
- 47.Kalaria RN, Hase Y. Neurovascular ageing and age-related diseases. Subcell Biochem. 2019;91:477–499. doi: 10.1007/978-981-13-3681-2_17. [DOI] [PubMed] [Google Scholar]
- 48.Fonck E, Feigl GG, Fasel J, Sage D, Unser M, Rufenacht DA, et al. Effect of aging on elastin functionality in human cerebral arteries. Stroke. 2009;40(7):2552–2556. doi: 10.1161/STROKEAHA.108.528091. [DOI] [PubMed] [Google Scholar]
- 49.Duca L, Blaise S, Romier B, Laffargue M, Gayral S, El Btaouri H, et al. Matrix ageing and vascular impacts: focus on elastin fragmentation. Cardiovasc Res. 2016;110(3):298–308. doi: 10.1093/cvr/cvw061. [DOI] [PubMed] [Google Scholar]
- 50.Ungvari Z, Tarantini S, Donato AJ, Galvan V, Csiszar A. Mechanisms of vascular aging. Circ Res. 2018;123(7):849–867. doi: 10.1161/CIRCRESAHA.118.311378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bell ED, Sullivan JW, Monson KL. Subfailure overstretch induces persistent changes in the passive mechanical response of cerebral arteries. Front Bioeng Biotechnol. 2015;3:2. doi: 10.3389/fbioe.2015.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Qiu S, Jiang W, Alam MS, Chen S, Lai C, Wang T, Li X, Liu J, Gao M, Tang Y, Li X, Zeng J, Feng Y. Viscoelastic characterization of injured brain tissue after controlled cortical impact (CCI) using a mouse model. J Neurosci Methods. 2020;330:108463. doi: 10.1016/j.jneumeth.2019.108463. [DOI] [PubMed] [Google Scholar]
- 53.Alfasi AM, Shulyakov AV, Del Bigio MR. Intracranial biomechanics following cortical contusion in live rats. J Neurosurg. 2013;119(5):1255–1262. doi: 10.3171/2013.7.Jns121973. [DOI] [PubMed] [Google Scholar]
- 54.Feng Y, Gao Y, Wang T, Tao L, Qiu S, Zhao X. A longitudinal study of the mechanical properties of injured brain tissue in a mouse model. J Mech Behav Biomed Mater. 2017;71:407–415. doi: 10.1016/j.jmbbm.2017.04.008. [DOI] [PubMed] [Google Scholar]
- 55.Rosidi NL, Zhou J, Pattanaik S, Wang P, Jin W, Brophy M, Olbricht WL, Nishimura N, Schaffer CB. Cortical microhemorrhages cause local inflammation but do not trigger widespread dendrite degeneration. PLoS One. 2011;6(10):e26612. doi: 10.1371/journal.pone.0026612PONE-D-11-18489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Whitney NP, Eidem TM, Peng H, Huang Y, Zheng JC. Inflammation mediates varying effects in neurogenesis: relevance to the pathogenesis of brain injury and neurodegenerative disorders. J Neurochem. 2009;108(6):1343–1359. doi: 10.1111/j.1471-4159.2009.05886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.DiSabato DJ, Quan N, Godbout JP. Neuroinflammation: the devil is in the details. J Neurochem. 2016;139(Suppl 2):136–153. doi: 10.1111/jnc.13607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Allen RG. Oxidative stress and superoxide dismutase in development, aging and gene regulation. AGE. 1998;21(2):47–76. doi: 10.1007/s11357-998-0007-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yan S, Sun J, Chen Y, Selim M, Lou M. Brain iron deposition in white matter hyperintensities: a 3-T MRI study. AGE. 2013;35(5):1927–1936. doi: 10.1007/s11357-012-9487-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Anson RM, Bohr VA. Mitochondria, oxidative DNA damage, and aging. J Am Aging Assoc. 2000;23(4):199–218. doi: 10.1007/s11357-000-0020-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Das UN. Ageing: is there a role for arachidonic acid and other bioactive lipids? A review. J Adv Res. 2018;11:67–79. doi: 10.1016/j.jare.2018.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Grimm A, Eckert A. Brain aging and neurodegeneration: from a mitochondrial point of view. J Neurochem. 2017;143(4):418–431. doi: 10.1111/jnc.14037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Garbarino VR, Orr ME, Rodriguez KA, Buffenstein R. Mechanisms of oxidative stress resistance in the brain: lessons learned from hypoxia tolerant extremophilic vertebrates. Arch Biochem Biophys. 2015;576:8–16. doi: 10.1016/j.abb.2015.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Droy-Lefaix MT. Effect of the antioxidant action of Ginkgo biloba extract (EGb 761) on aging and oxidative stress. AGE. 1997;20(3):141–149. doi: 10.1007/s11357-997-0013-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kohama SG, Rosene DL, Sherman LS. Age-related changes in human and non-human primate white matter: from myelination disturbances to cognitive decline. AGE. 2012;34(5):1093–1110. doi: 10.1007/s11357-011-9357-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tarantini S, Valcarcel-Ares MN, Toth P, Yabluchanskiy A, Tucsek Z, Kiss T, Hertelendy P, Kinter M, Ballabh P, Süle Z, Farkas E, Baur JA, Sinclair DA, Csiszar A, Ungvari Z. Nicotinamide mononucleotide (NMN) supplementation rescues cerebromicrovascular endothelial function and neurovascular coupling responses and improves cognitive function in aged mice. Redox Biol. 2019;24:101192. doi: 10.1016/j.redox.2019.101192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tarantini S, Valcarcel-Ares NM, Yabluchanskiy A, Fulop GA, Hertelendy P, Gautam T, et al. Treatment with the mitochondrial-targeted antioxidant peptide SS-31 rescues neurovascular coupling responses and cerebrovascular endothelial function and improves cognition in aged mice. Aging Cell. 2018;17(2). 10.1111/acel.12731. [DOI] [PMC free article] [PubMed]
- 68.Tarantini S, Valcarcel-Ares MN, Yabluchanskiy A, Tucsek Z, Hertelendy P, Kiss T, et al. Nrf2 deficiency exacerbates obesity-induced oxidative stress, neurovascular dysfunction, blood brain barrier disruption, neuroinflammation, amyloidogenic gene expression and cognitive decline in mice, mimicking the aging phenotype. J Gerontol A Biol Sci Med Sci. 2018; in press. [DOI] [PMC free article] [PubMed]
- 69.Springo Z, Tarantini S, Toth P, Tucsek Z, Koller A, Sonntag WE, Csiszar A, Ungvari Z. Aging exacerbates pressure-induced mitochondrial oxidative stress in mouse cerebral arteries. J Gerontol A Biol Sci Med Sci. 2015;70:1355–1359. doi: 10.1093/gerona/glu244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ungvari Z, Bailey-Downs L, Sosnowska D, Gautam T, Koncz P, Losonczy G, et al. Vascular oxidative stress in aging: a homeostatic failure due to dysregulation of Nrf2-mediated antioxidant response. Am J Phys Heart Circ Phys. 2011;301(2):H363–H372. doi: 10.1152/ajpheart.01134.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ungvari Z, Bailey-Downs L, Gautam T, Sosnowska D, Wang M, Monticone RE, et al. Age-associated vascular oxidative stress, Nrf2 dysfunction and NF-kB activation in the non-human primate Macaca mulatta. J Gerontol A Biol Sci Med Sci. 2011;66(8):866–875. doi: 10.1093/gerona/glr092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schriner SE, Linford NJ. Extension of mouse lifespan by overexpression of catalase. AGE. 2006;28(2):209–218. doi: 10.1007/s11357-006-9010-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Islam MT. Oxidative stress and mitochondrial dysfunction-linked neurodegenerative disorders. Neurol Res. 2017;39(1):73–82. doi: 10.1080/01616412.2016.1251711. [DOI] [PubMed] [Google Scholar]
- 74.Csipo T, Lipecz A, Ashpole NM, Balasubramanian P, Tarantini S. Astrocyte senescence contributes to cognitive decline. GeroScience. 2020;42(1):51–55. doi: 10.1007/s11357-019-00140-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sonntag WE, Deak F, Ashpole N, Toth P, Csiszar A, Freeman W, et al. Insulin-like growth factor-1 in CNS and cerebrovascular aging. Front Aging Neurosci. 2013;5:27. 10.3389/fnagi.2013.00027. [DOI] [PMC free article] [PubMed]
- 76.Tarantini S, Valcarcel-Ares NM, Yabluchanskiy A, Springo Z, Fulop GA, Ashpole N, Gautam T, Giles CB, Wren JD, Sonntag WE, Csiszar A, Ungvari Z. Insulin-like growth factor 1 deficiency exacerbates hypertension-induced cerebral microhemorrhages in mice, mimicking the aging phenotype. Aging Cell. 2017;16(3):469–479. doi: 10.1111/acel.12583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Popovic V. GH deficiency as the most common pituitary defect after TBI: clinical implications. Pituitary. 2005;8(3–4):239–243. doi: 10.1007/s11102-006-6047-z. [DOI] [PubMed] [Google Scholar]
- 78.Agha A, Rogers B, Sherlock M, O'Kelly P, Tormey W, Phillips J, et al. Anterior pituitary dysfunction in survivors of traumatic brain injury. J Clin Endocrinol Metab. 2004;89(10):4929–4936. doi: 10.1210/jc.2004-0511. [DOI] [PubMed] [Google Scholar]
- 79.Behan LA, Phillips J, Thompson CJ, Agha A. Neuroendocrine disorders after traumatic brain injury. J Neurol Neurosurg Psychiatry. 2008;79(7):753–759. doi: 10.1136/jnnp.2007.132837. [DOI] [PubMed] [Google Scholar]
- 80.Lithgow K, Chin A, Debert CT, Kline GA. Utility of serum IGF-1 for diagnosis of growth hormone deficiency following traumatic brain injury and sport-related concussion. BMC Endocr Disord. 2018;18(1):20. doi: 10.1186/s12902-018-0247-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Farias Quipildor GE, Mao K, Hu Z, Novaj A, Cui MH, Gulinello M, Branch CA, Gubbi S, Patel K, Moellering DR, Tarantini S, Kiss T, Yabluchanskiy A, Ungvari Z, Sonntag WE, Huffman DM. Central IGF-1 protects against features of cognitive and sensorimotor decline with aging in male mice. Geroscience. 2019;41(2):185–208. doi: 10.1007/s11357-019-00065-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kazanis I, Giannakopoulou M, Philippidis H, Stylianopoulou F. Alterations in IGF-I, BDNF and NT-3 levels following experimental brain trauma and the effect of IGF-I administration. Exp Neurol. 2004;186(2):221–234. doi: 10.1016/j.expneurol.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 83.Takakusaki K. Functional Neuroanatomy for Posture and Gait Control. J Mov Disord. 2017;10(1):1–17. doi: 10.14802/jmd.16062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Choi P, Ren M, Phan TG, Callisaya M, Ly JV, Beare R, Chong W, Srikanth V. Silent infarcts and cerebral microbleeds modify the associations of white matter lesions with gait and postural stability: population-based study. Stroke. 2012;43(6):1505–1510. doi: 10.1161/STROKEAHA.111.647271. [DOI] [PubMed] [Google Scholar]
- 85.Gattu R, Akin FW, Cacace AT, Hall CD, Murnane OD, Haacke EM. Vestibular, balance, microvascular and white matter neuroimaging characteristics of blast injuries and mild traumatic brain injury: four case reports. Brain Inj. 2016;30(12):1501–1514. doi: 10.1080/02699052.2016.1219056. [DOI] [PubMed] [Google Scholar]
- 86.Tekin S, Cummings JL. Frontal-subcortical neuronal circuits and clinical neuropsychiatry: an update. J Psychosom Res. 2002;53(2):647–654. doi: 10.1016/s0022-3999(02)00428-2. [DOI] [PubMed] [Google Scholar]
- 87.Studerus-Germann AM, Gautschi OP, Bontempi P, Thiran JP, Daducci A, Romascano D, von Ow D, Hildebrandt G, von Hessling A, Engel DC. Central nervous system microbleeds in the acute phase are associated with structural integrity by DTI one year after mild traumatic brain injury: a longitudinal study. Neurol Neurochir Pol. 2018;52(6):710–719. doi: 10.1016/j.pjnns.2018.08.011. [DOI] [PubMed] [Google Scholar]
- 88.Cohen JA, Verghese J, Zwerling JL. Cognition and gait in older people. Maturitas. 2016;93:73–77. doi: 10.1016/j.maturitas.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 89.Lionetti V, Tuana BS, Casieri V, Parikh M, Pierce GN. Importance of functional food compounds in cardioprotection through action on the epigenome. Eur Heart J. 2019;40(7):575–582. doi: 10.1093/eurheartj/ehy597. [DOI] [PubMed] [Google Scholar]
- 90.Agrimi J, Spalletti C, Baroni C, Keceli G, Zhu G, Caragnano A, Matteucci M, Chelko S, Ramirez-Correa GA, Bedja D, Casieri V, di Lascio N, Scalco A, Beltrami AP, Paolocci N, Caleo M, Lionetti V. Obese mice exposed to psychosocial stress display cardiac and hippocampal dysfunction associated with local brain-derived neurotrophic factor depletion. EBioMedicine. 2019;47:384–401. doi: 10.1016/j.ebiom.2019.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wurzelmann M, Romeika J, Sun D. Therapeutic potential of brain-derived neurotrophic factor (BDNF) and a small molecular mimics of BDNF for traumatic brain injury. Neural Regen Res. 2017;12(1):7–12. doi: 10.4103/1673-5374.198964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Stephan JS, Sleiman SF. Exercise factors as potential mediators of cognitive rehabilitation following traumatic brain injury. Curr Opin Neurol. 2019;32(6):808–814. doi: 10.1097/wco.0000000000000754. [DOI] [PubMed] [Google Scholar]
- 93.Bathina S, Das UN. Brain-derived neurotrophic factor and its clinical implications. Arch Med Sci. 2015;11(6):1164–1178. doi: 10.5114/aoms.2015.56342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zheng T, Liu H, Qin L, Chen B, Zhang X, Hu X, Xiao L, Qin S. Oxidative stress-mediated influence of plasma DPP4 activity to BDNF ratio on mild cognitive impairment in elderly type 2 diabetic patients: results from the GDMD study in China. Metabolism. 2018;87:105–112. doi: 10.1016/j.metabol.2018.03.014. [DOI] [PubMed] [Google Scholar]
- 95.Pal R, Singh SN, Chatterjee A, Saha M. Age-related changes in cardiovascular system, autonomic functions, and levels of BDNF of healthy active males: role of yogic practice. Age (Dordr) 2014;36(4):9683. doi: 10.1007/s11357-014-9683-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Erickson KI, Miller DL, Roecklein KA. The aging hippocampus: interactions between exercise, depression, and BDNF. Neuroscientist. 2012;18(1):82–97. doi: 10.1177/1073858410397054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Siuda J, Patalong-Ogiewa M, Żmuda W, Targosz-Gajniak M, Niewiadomska E, Matuszek I, Jędrzejowska-Szypułka H, Lewin-Kowalik J, Rudzińska-Bar M. Cognitive impairment and BDNF serum levels. Neurol Neurochir Pol. 2017;51(1):24–32. doi: 10.1016/j.pjnns.2016.10.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The manuscript has not been published previously.