Abstract
Background & Aims
Autoimmune Hepatitis (AIH) is a chronic, inflammatory disease of the liver with increasing prevalence. However, limited epidemiological data exists for prevalence of AIH in the United States. We used a large database to describe the prevalence of AIH in the USA and the autoimmune diseases associated with it.
Approach & Results
Data was collected from a commercial database (Explorys Inc, Cleveland, OH), an aggregate of Electronic Health Record data from 26 major integrated healthcare systems in the US. We identified a cohort of patients with a diagnosis of AIH from April 2014 to April 2019 based on a Systemized Nomenclature of Medicine- Clinical Terms and calculated the prevalence of AIH. Of the 37,161,280 individuals active in the database from April 2014 to 2019, we identified 11,600 individuals with a diagnosis of AIH with an overall prevalence rate of 31.2/100,000. The prevalence of AIH was increased in females compared to males [OR 3.21, p<0.0001], elderly (aged >65years) compared to adults (aged 18–65years) and children (aged <18years) [OR 2.51, p<0.0001] and Caucasians compared to African- Americans, Asians and Hispanics [OR 1.12, p<0.0001]. Moreover, patients with AIH were more likely to have Sjögren’s syndrome, systemic lupus erythematosus, ulcerative colitis, celiac disease, rheumatoid arthritis, Crohn’s disease and autoimmune thyroiditis as compared to patients without AIH.
Conclusions
We found that the estimated prevalence of AIH in the USA is 31.2/100,000, which is comparable to the reported prevalence of AIH in Europe. We confirmed that AIH has a strong association with other autoimmune diseases studied in the literature.
Keywords: prevalence, database, autoimmune diseases, symptoms, liver cirrhosis
Introduction
Autoimmune hepatitis (AIH) is a rare inflammatory disorder of the liver that can progress to hepatic failure or liver cirrhosis1. Diagnostic scores comprise mostly non disease specific findings which include autoantibodies, hypergammaglobulinemia (specifically elevated IgG levels), histologic findings of interface hepatitis, exclusion of other liver diseases and treatment response to steroids1–3. Although these criteria are available, often the diagnosis of AIH is made clinically.
AIH can be diagnosed in patients of all ages, sexes and races4. The epidemiologic data varies worldwide and the incidence appears to be increasing recently3. A nationwide registry-base cohort study in Denmark showed a prevalence of 23.9/100,000 during 1994–20125. van Gerven et al. reported the point prevalence of AIH in Netherland to 18.3/100,006. Moreover, prevalence was reported to be 23.4/100,000 in Japan8 and 4.8/100,000 in South Korea9. In the United States, AIH point prevalence was reported to be 42.9/100,000 individuals in Alaskan native population7. However, to date there is no large population-based epidemiologic study of AIH across the United States.
Given the increasing disease burden, we sought to describe the prevalence of AIH in the United States and among various age, gender and race based subgroups utilizing a large population-based database. These data will help increase our understanding of AIH demographics in the US and help guide the management of this condition.
Methods
Database
We performed a retrospective analysis of a large population- based, commercial database (Explorys Inc, Cleveland, OH) that contains electronic health record (EHR) data from 26 major integrated healthcare systems in the USA from 1999 to present and includes community as well as academic practices. The health institutions cover all 50 states and span the East, Midwest, South, Central and West divisions of the US13, thus providing a broad regional and climatic distribution of the source population. Explorys contains deidentified patient data from the participating institutions and uses a health data gateway (HDG) server behind the firewall of each participating healthcare organization that uses billing inquiries to collect the data. Data are then standardized and normalized by Explorys. Diagnoses, findings and procedures are mapped into the Systematized Nomenclature of Medicine Clinical Terms (SNOMED CT) hierarchy while prescription drug orders are mapped into SNOMED (to represent the pharmacological class) and RxNorm (to represent the drug itself). Each participating healthcare institution has access to Explorys online (password protected), which provides for browsing of the data from all participating healthcare institutions. Explorys data are automatically updated at least once every 24 hours. Explorys is a Health Insurance Portability and Accountability Act (HIPAA)—complaint platform—and thus Institutional Review Board (IRB) is not required13.
Patient Selection
Using the Explorys search tool, we identified an aggregated patient cohort of eligible patients with AIH at any point between April 2014 and April 2019. AIH patients were defined as those having a SNOMED-CT diagnosis of “autoimmune hepatitis”. Patients with a SNOMED-CT diagnosis of “acute viral hepatitis”, “chronic viral hepatitis”, “alcoholic liver damage”, “drug-induced hepatitis” and “drug-induced cirrhosis of liver” were excluded from the cohort to ensure that the patients with a diagnosis of AIH did not have any of these concurrent diagnoses.
Controls were defined as individuals without having a SNOMED-CT diagnosis of AIH and any of the aforementioned five SNOMED-CT diagnoses.
We determined the number of patients who had a SNOMED-CT diagnosis of “primary biliary cholangitis”. Moreover, to increase the specificity of AIH diagnosis, we sought to evaluate the number of patients with AIH who had a liver biopsy as a part of their work-up. We also determined the number of AIH patients who were on specific steroids and/or immunomodulator therapy. This search was done using the RxNorm names of the drugs. Medications included were “Prednisone”, “Budesonide”, “Azathioprine” and “Mycophenolate Mofetil”.
We further evaluated the number of patients with AIH (viral, alcoholic or drug-induced hepatitis excluded in the search) who were also found to have a diagnosis of liver cirrhosis and hepatocellular carcinoma (HCC). The SNOMED- clinical term used to search for patients with HCC was “primary malignant neoplasm of liver”. Lastly, we determined the number patients with AIH who received an orthotopic liver transplant (OLT).
Sensitivity Analysis
To evaluate the demographics in patients who had a diagnosis of AIH confirmed on a liver biopsy, a sensitivity analysis was performed. We identified the number of patients with a SOMED-CT diagnosis of “autoimmune hepatitis” and excluded patients with a diagnosis of “acute viral hepatitis”, “chronic viral hepatitis”, “alcoholic liver damage”, “drug-induced hepatitis” and “drug-induced cirrhosis of liver” between February 2015 and February 2020. Patients within this cohort who underwent a liver biopsy were identified using the SNOMED-CT procedure code as “biopsy of liver”. Similar demographical data and comorbidities in the aforementioned cohort were then analyzed.
Associated Gastrointestinal Symptoms and Findings
We evaluated the prevalence of symptoms commonly seen in patients with AIH. These included non-specific generalized symptoms such as abdominal pain, nausea, vomiting, malaise and fatigue. We also evaluated the prevalence of signs and complications of advanced liver diseases in our patient cohort including jaundice, hepatic encephalopathy (HE), ascites, hepato-renal syndrome (HRS), hepatic failure, esophageal varices and upper and lower gastrointestinal hemorrhage (UGIH, LGIH). Lastly, we evaluated the difference in these complications based on gender.
Associated Autoimmune Diseases
We identified autoimmune diseases associated with AIH that have been suggested by prior studies14–20. Data were extracted by using SNOMED-clinical terms for these conditions. The specific autoimmune diseases included were autoimmune thyroiditis, rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), type 1 diabetes mellitus, ulcerative colitis (UC), Crohn’s disease (CD), celiac disease, Sjögren’s syndrome and psoriasis.
Statistical Analysis
For patients with AIH, demographics and associated diseases were characterized by descriptive statistics. Univariate analysis was performed to assess the differences in the prevalence of associated medical conditions in patients with AIH and in controls by calculating odds ratios (OR) and 95% confidence intervals (CI).
For calculation of overall period prevalence, we identified all patients in the database with AIH (excluding viral, alcoholic and drug-induced hepatitis) between April 2014 and April 2019. We then divided this number by the total number of patients in the database during the same time period, thus making sure that all the patients in the denominator (population at risk) had an equal opportunity of being diagnosed with AIH. Similarly, age-based, gender-based and race-based prevalence rates were calculated. The odds ratio (OR), its standard error, and 95% confidence interval were calculated according to Altman, 1991, using the MedCalc Statistical Software with a case–control design21,22. To compare the signs and symptoms in our patient cohort to the controls a chi-square test was performed using SPSS software23.
Explorys rounds cell counts to the nearest 10 and treats all cell counts between 0 and 10 as equivalent in order to protect the identities of patients.
Results
A total of 37,161,280 individuals in the Explorys database from April 2014 to April 2019 comprised the source population. Of these, 11,600 patients (0.0312%) had the SNOMED-CT diagnosis of AIH without a concomitant diagnosis of acute and chronic viral hepatitis, alcoholic liver damage, drug-induced hepatitis or drug-induced cirrhosis of the liver (Table 1).
Table 1:
Demographic characteristics of AIH
| AIH Cases | |
|---|---|
| Overall, n | 11600 |
| Gender | |
| Male, n (%) | 2300 (20) |
| Female, n (%) | 9300 (80) |
| Age group (years old) | |
| Children (<18), n (%) | 130 (1) |
| Adults (18–65), n (%) | 6630 (57) |
| Elderly (> 65), n (%) | 4880 (42) |
| Race | |
| Caucasian, n (%) | 8720 (75) |
| African American, n (%) | 1320 (13) |
| Asian, n (%) | 270 (2) |
| Hispanic, n (%) | 160 (1) |
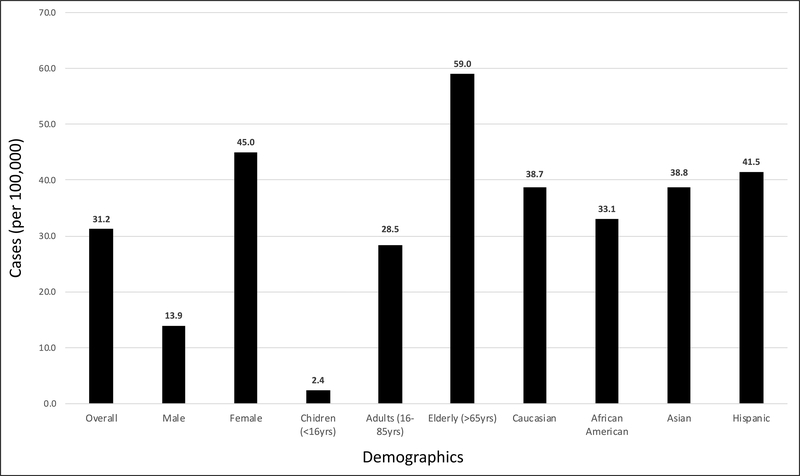
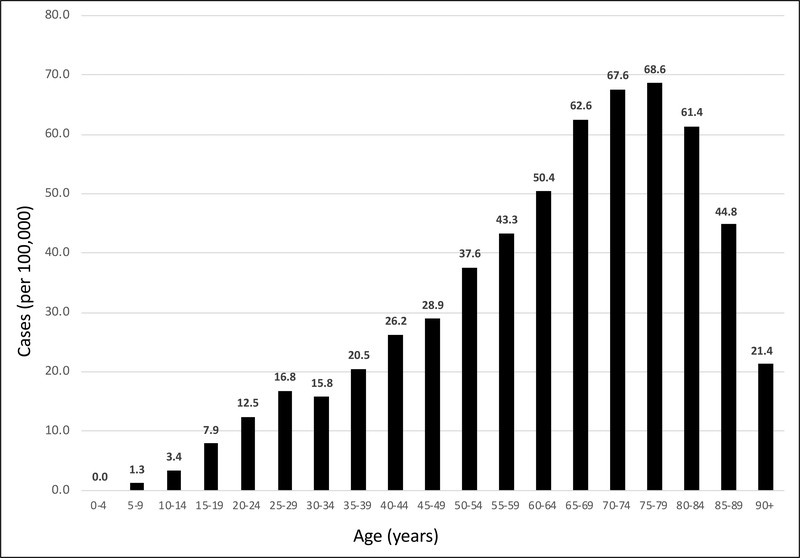
Of the 11,600 AIH patients, the majority were females (80%) (Table 1). The overall prevalence of AIH was estimated as 31.2/100,000 (Fig 1). The prevalence of AIH was higher in females at 45.0/100,000 vs. males at 13.9/100,000 [OR 3.21, 95% CI 3.07 to 3.36, p<0.0001]. Based on age, AIH prevalence was highest in the 7th decade of life after which there was a decline in prevalence rates (Fig. 2). Specifically, AIH prevalence in the elderly (defined as age > 65 years) was 59.0/100,000 vs. adults (age 18–65 years) at 28.5/100,00 and children (age < 18 years) at 2.4/100,000 [OR: 2.51, 95% CI: 2.42 to 2.60, p<0.0001]. Based on ethnicity, the AIH prevalence in Caucasians was 38.7/100,000 vs. African Americans at 33.1/100,000, Asians at 38.8/100,000 and Hispanics at 41.5/100,000 [OR 1.12, 95% CI 1.06 to 1.17, p<0.0001].
Figure 1:
Prevalence (per 100,000) of Autoimmune Hepatitis in the United States between 2014 and 2019 overall and among different age-based, race-based and gender-based groups
Figure 2:
5-year Age Interval Based Prevalence (per 100,000) of AIH in the United States between 2014 and 2019
Of the 11, 600 patient with AIH, 810 (7.0%) of the patients were found to have a diagnosis of primary biliary cholangitis. Furthermore, of the 11,600 patients with AIH, 3880 (33.4%) had a liver biopsy. Of the 2300 male patients with AIH, 810 (35.2%) had a liver biopsy and, of the 9300 female patients with AIH, 3060 (32.9%) had a liver biopsy. Upon evaluation of medications used for treatment of patients with AIH, we found that 7020 (60.5%) were either on Prednisone or Budesonide (Table 2). We also determined that 4150 (35.8%) of the patients were on Azathioprine.
Table 2:
Treatment medications for patients with AIH
| Medications | AIH Cases (n) |
|---|---|
| Prednisone | 5630 |
| Budesonide | 1390 |
| Azathioprine | 4150 |
| Mycophenolate Mofetil | 1010 |
Among patients with AIH, 4080 (35.2%) patients had a diagnosis of cirrhosis. Upon further evaluation of patient with AIH and liver cirrhosis, we found that the prevalence of cirrhosis in male patients (34%) was similar to the prevalence in female patients at 36% with no significant difference (p=0.22). Furthermore, 110 out of 11,600 (0.95%) patients with AIH had an OLT.
Lastly, we performed sensitivity analysis and found that in patients with AIH and liver cirrhosis, the prevalence of primary neoplasm of the liver was 1.4%.
Sensitivity Analysis
In order to further validate the results of our study, we performed a sensitivity analysis to evaluate the demographical data in the patients with a diagnosis of AIH who also had a liver biopsy. Similar to our original patient cohort, majority of the patients with AIH confirmed on liver biopsy were females (79%) (Table 3). Among the age groups, 61% were noted to be adults and 37% were elderly. Similar racial distribution was also present, with majority of the patients being Caucasians (75%).
Table 3:
Demographic characteristics of patients with AIH confirmed on biopsy
| AIH with Biopsy Cases | |
|---|---|
| Overall, n | 4430 |
| Gender | |
| Male, n (%) | 920 (21) |
| Female, n (%) | 3510 (79) |
| Age group (years old) | |
| Children (<18), n (%) | 110 (2) |
| Adults (18–65), n (%) | 2730 (61) |
| Elderly (> 65), n (%) | 1630 (37) |
| Race | |
| Caucasian, n (%) | 3310 (75) |
| African American, n (%) | 620 (14) |
| Asian, n (%) | 120 (3) |
| Hispanic, n (%) | 70 (2) |
Moreover, among patients with AIH on confirmed biopsies, 1930 (43.6%) patients had a diagnosis of cirrhosis and 2900 (66%) patients were found to have a prescription for corticosteroids given after a biopsy was done. We also found that 12% of the patients had hepatic failure. These results are comparable to the results obtained from total patients diagnosed with AIH (with and without biopsies).
Associated Gastrointestinal Symptoms and Findings
As shown in Table 4, 23 to 44% of our patient cohort had non-specific generalized symptoms including abdominal pain, malaise and nausea. Moreover, approximately 10% of the patients had complications such as jaundice, ascites and esophageal varices.
Table 4:
GI symptoms and findings noted in patients with AIH and chi-square test results for findings in AIH patients as compared to patients without AIH
| Reported Symptoms and Findings | AIH cases (n) | % cases | Chi-square Test |
|---|---|---|---|
| Non-specific symptoms | |||
| Abdominal pain | 5110 | 44.1 | X2 (1) = 5270. P<0.001 |
| Nausea | 3020 | 26.0 | X2 (1) = 4101, p<0.001 |
| Vomiting | 2400 | 20.7 | X2 (1) = 2308, p<0.001 |
| Malaise | 3510 | 30.3 | X2 (1) = 5185, p<0.001 |
| Fatigue | 2700 | 23.3 | X2 (1) = 4691, p<0.001 |
| Liver-specific symptoms | |||
| Ascites | 1260 | 10.9 | X2 (1) = 36550, p<0.001 |
| Jaundice | 1180 | 10.2 | X2 (1) = 20414, p<0.001 |
| Esophageal Varices | 1160 | 10.0 | X2 (1) = 126466, p<0.001 |
| Hepatic Failure | 970 | 8.4 | X2 (1) = 70745, p<0.001 |
| LGIH | 740 | 6.4 | X2 (1) = 1103, p<0.001 |
| HE | 650 | 5.6 | X2 (1) = 88448, p<0.001 |
| UGIH | 540 | 4.7 | X2 (1) = 2381, p<0.001 |
| HRS | 140 | 1.2 | X2 (1) = 18820, p<0.001 |
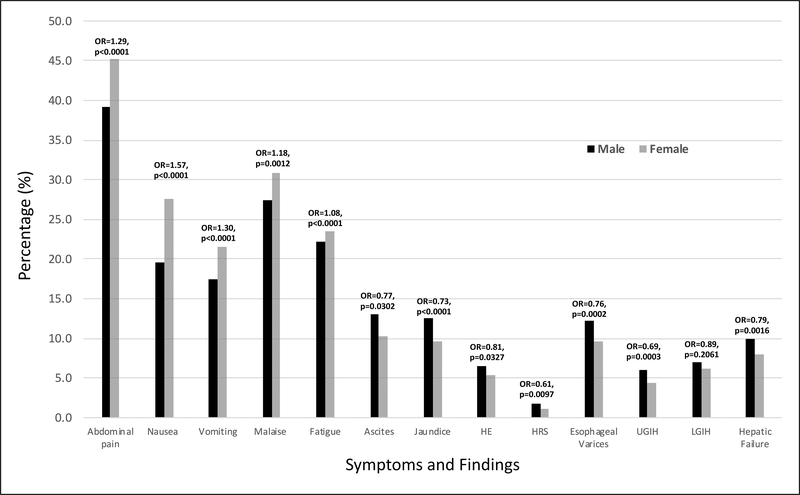
Females were more likely to complain of generalized symptoms (abdominal pain, nausea, vomiting, malaise and fatigue) while complications associated with liver diseases (jaundice, HE, ascites, HRS, HPS, varices, GI hemorrhage, hepatic failure) were more commonly seen in male patients (Figure 3).
Figure 3:
GI symptoms and findings in female and male patients with AIH. Odd ratios for findings in female patients as compared to male patients indicated on the top of the bars
Associated Autoimmune Diseases
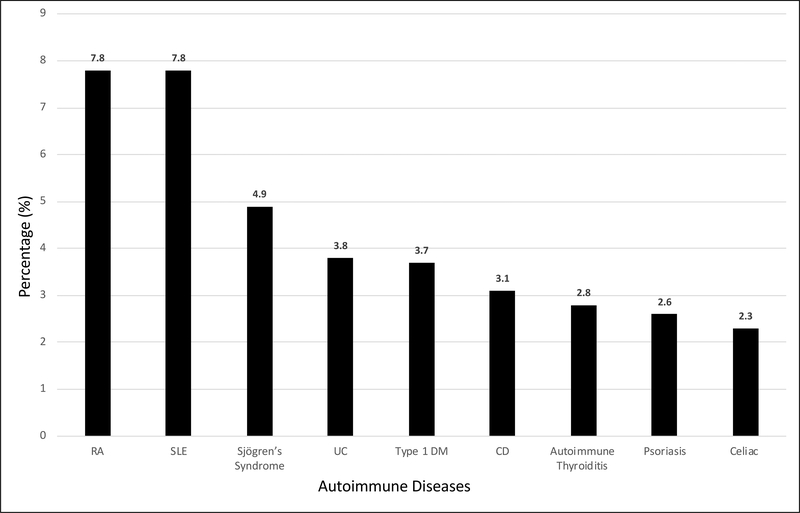
Compared to the general population, individuals with AIH were more likely to have a history of Sjogren’s syndrome, SLE, ulcerative colitis, celiac disease, rheumatoid arthritis, Crohn’s disease, autoimmune thyroiditis, psoriasis and type 1 diabetes (Figure 4, Table 5).
Figure 4:
Percentage of patients with AIH and other autoimmune diseases
Table 5:
Odd ratios for presence of autoimmune diseases in patients with AIH as compared to patients without AIH
| Autoimmune Disease | AIH cases (n) | Odd Ratios | 95% Confidence Interval |
|---|---|---|---|
| Sjögren’s Syndrome | 570 | 26.24 | 24.11 to 28.55, p<0.0001 |
| Systemic Lupus Erythematosus | 920 | 23.49 | 21.95 to 25.13, p<0.0001 |
| Ulcerative Colitis | 440 | 10.50 | 9.55 to 11.55, p<0.0001 |
| Celiac Disease | 270 | 8.63 | 7.65 to 9.74, p<0.0001 |
| Rheumatoid Arthritis | 900 | 7.39 | 6.90 to 7.91, p<0.0001 |
| Crohn’s Disease | 360 | 7.20 | 6.48 to 7.99, p<0.0001 |
| Autoimmune Thyroiditis | 330 | 7.19 | 6.44 to 8.02, p<0.0001 |
| Psoriasis | 300 | 3.51 | 3.13 to 3.94, p<0.0001 |
| Diabetes Mellitus Type 1 | 430 | 3.13 | 2.84 to 3.44, p<0.0001 |
Discussion
In this study, we evaluated the prevalence of autoimmune hepatitis in the Explorys database between 2014 and 2019. To our knowledge this is the largest study to date that estimates the prevalence of autoimmune hepatitis in the US at the national level. This is also the first large study to describe race-, age- and sex-based prevalence of AIH in the US from national-level data. We examined associated GI symptoms and findings as well as symptoms and complications associated with advanced liver disease and liver failure in patients with AIH, and further evaluated those findings in male and female patients. Additionally, we also examined the prevalence of other associated autoimmune medical conditions in patients with AIH.
We estimated the 5-year prevalence rate of AIH between 2014 and 2019 to be 31.2/100,000. Wong RJ et al.25 conducted a retrospective cohort analysis of AIH within a large hepatology clinic network in Northern California and Nevada. One hundred and eighty three patients with AIH were identified but a point prevalence was not estimated. Hurkburt et al.7 described the epidemiology of AIH in an Alaskan population using the patient database in the Alaska Native Medical Center and state-wide hepatology and internal medicine clinics from 1983 to 2000. Forty nine patients with a diagnosis of AIH were identified, and the overall 17-year point prevalence was estimated to be 42.9/100,000. Similarly, Gronbaek et al.5 conducted a population-based study in Denmark using the nationwide health registries for all Danish citizens diagnosed with autoimmune hepatitis in 1994–2001 and identified 1721 individuals with AIH. The point prevalence was noted to be 23.9/1000,000. Ngu et al.11 estimated the prevalence of AIH in Canterbury, New Zealand as 24.5/100, 000. These prevalence estimates are comparable to the findings in our study. Given that in our study, we had larger numbers of autoimmune hepatitis cases and total number of individuals in the database, our estimate of prevalence may be more precise than prior estimates. However, a direct comparison with previous studies is not possible due to differences in the time period, methodology, and geographic locations.
Previous studies have reported female predilection of the disease. Gronbaek et al.5 evaluated the prevalence of AIH as 34.6 per 100,000 for women and 13.0 per 100,000 for men in Denmark. Similarly, the Ngu et al.11 study from Canterbury, New Zealand reported the prevalence as 35.3 per 100,000 in women and 13.2 per 100,000 in men. Our study showed comparable results with AIH prevalence of 45.0 per 100,000 in females and 14.0 per 100,000 in males.
Early reports describing the epidemiology of autoimmune hepatitis have suggested a bimodal age distribution, with the first peak between the ages of 10–30 years, a second between 40 and 60 years; however, recent data have showed that the disease has been increasingly recognized in all age-groups3. Ngu et al.11 reported a peak age of presentation in the sixth decade of life. Similarly, in Puustinen’s epidemiological study from Finland, the highest prevalence rates were from the 45–84 age group10. Our study determined the prevalence in contrast to incidence and demonstrated the highest prevalence in elderly (>65 years) population and an increase in AIH prevalence rates until the seventh decade of life (Figure 2). Increase in prevalence rates in older age groups can be associated with the highly effective treatment options available for AIH, which successfully help achieve and maintain remission.
Furthermore, Ngu et al.11 showed that 96% of the patient population were Caucasians. Wong et al.25 identified 183 patients from the database in Northern California with AIH out of which 79.8% of the patients were Caucasian. de Boer YS et al.33 did a multicenter study in Europe and showed that 897 out of 985 patients with a diagnosis of AIH were white patients as compared to 88 black patients. Wen et al.34 conducted a prevalence study using the National Inpatient Sample database in the US, and 57% of the patients admitted with a primary diagnosis of AIH were whites. Similar to these studies, our patient cohort had majority of Caucasians (75%) and, the prevalence rate was significantly higher in Caucasians when compared to Asians, Hispanics and African Americans.
To further verify the robustness of our diagnosis of AIH in the patient cohort, we estimated the number of patients who had a liver biopsy since histological finding of interface hepatitis is a part of the simplified criteria for AIH diagnosis. Moreover, both AASLD and EASL practice guidelines for AIH recommends that patients have a liver biopsy at the time of presentation to establish diagnosis and to guide the treatment decisions24. Interestingly, we found that approximately one-third of our patients with AIH had a liver biopsy, which could indicate the approach to AIH diagnosis could vary among multiple health centers in the US. We performed sensitivity analysis on the patients with AIH confirmed on biopsies and found that the demographical data was similar to the data obtained from the total number of patients with AIH, which further validates our findings obtained from overall patient cohort.
Prednisone or prednisolone alone or in combination with azathioprine remains the mainstay therapy for autoimmune hepatitis12. AASLD recommends that immunosuppressive treatment should be started in patients with markedly elevated AST, ALT and serum globulin levels and/or with certain histological features and that treatment should be individualized for adult patients without symptoms or with mild laboratory and histological changes. Moreover, treatment should not be instituted in patients with minimal or no disease activity24. The majority of our patient cohort (61%) were either on Prednisone or Budesonide. We speculated that 40% of the patient cohort who did not have a prescription for either of the steroids likely had minimal disease activity or were in clinical remission on steroid sparing agents or immunomodulators. Our results showed that 35.8% of our patient cohort was on Azathioprine.
We found that 35% of our patients also had a diagnosis of liver cirrhosis. Similar to our results, Wong et al.25 reported that 33.9% of 183 patients with AIH in their study were found to have liver cirrhosis as well. Additionally, in our study we found that 110 out of 11,600 patients (0.95%) with AIH had an orthotopic liver transplant. Campsen et al.26 reported that 1032 OLTs were performed on adult patients at the University of Colorado Health Science Center during an 18 year period (1988 to 2006) and only 66 patients (6.4%) were transplanted for AIH.
With regards to signs and symptoms commonly seen in patients with AIH, we found that 23–44% of our patient cohort complained of non-specific symptoms that included abdominal pain (44.1%), malaise (30.3%), nausea (26%) and vomiting (20.7%). Moreover, 10% were also found to have liver-specific complications which included jaundice, ascites and esophageal varices. These complications were noted to be significantly associated with AIH when compared to patients without AIH. We further evaluated gender differences in patient symptoms. We found that in both gender groups, abdominal pain was the most common non-specific symptom. Ascites and jaundice (~10–11%) were the most common liver-specific findings. Interestingly, we found that female patients were more likely to have non-specific symptoms while findings of advanced liver disease were more commonly seen in males. There are limited data on the association of gender with clinical manifestations of AIH. Czaja et al.27 reported that there was no difference in age, laboratory indices and serological markers between two gender groups at the time of presentation. Similarly, a study conducted by Al-Chalabi et al.28 investigated the difference in clinical outcomes between 51 male and 187 female patients with AIH and concluded that there was no significant difference in clinical manifestations (jaundice, encephalopathy, arthralgia/flu like symptoms/malaise/lethargy, ascites or stigmata of chronic liver disease) at the time of presentation between males and females. On the contrary, our study had 9300 female and 2300 male patients, making our estimates more precise. However, a limitation of our study is that we are unable to determine the signs and symptoms at the time of initial presentation. These differences seen between gender groups can be attributed to differences in health-care seeking behavior - where men seek professional help less frequently than women29. Hence, it may be extrapolated from our study that females were more likely to seek care when they had milder symptoms, while male patients sought care when they had advanced symptoms. Another possible explanation for differences in disease complications amongst gender can be attributed to the fact the AIH conventionally has been viewed as a disease of young women and hence, healthcare professionals may not suspect the diagnosis in men. This could be delay the initiation of appropriate treatment and therefore, lead to disease progression. It can also be postulated that AIH may have a more aggressive disease course in male patients.
Lastly, we looked at the prevalence of other autoimmune diseases in patients with AIH. It has been reported in previous studies that patients with AIH are also more likely to have a concurrent diagnosis of other autoimmune diseases 14–20. In our study we found that approximately 8% of our patient had a diagnosis of RA and/or SLE (Figure 4). The odds of having a concurrent diagnosis of Sjögren’s syndrome was the highest (26.24), and both SLE and ulcerative colitis were also strongly associated with AIH (Table 5). These results were comparable to prior studies as well.
There are certain limitations that need to be acknowledged with this study. We were unable to determine the incidence of the autoimmune hepatitis from the Explorys database. Secondly, only one-third of the patients diagnosed with AIH were noted to have a liver biopsy. We suspect that these numbers could be underestimated if the liver biopsies were done at a different health care center not covered by the Explorys database hence, un-coded in the system. The working group for AIH have suggested that the histology is essential for the diagnosis of AIH, since it is required to exclude other disease entities as well2. Given our limitation that not every patient in the Explorys database with a diagnosis of AIH had a documented liver biopsy, we ensured that the patient cohort with AIH also did not have other concurrent diagnoses (DILI, viral hepatitis or alcoholic liver damage) which, as per guidelines, should be excluded using serological or histological markers. To further validate our results obtained from the AIH patient cohort, we performed sensitivity analysis to evaluate the demographical data. After evaluating the patients with AIH who underwent a liver biopsy, we found that the majority of the patients were females, adults (aged 18 to 65 years) and Caucasians (Table 3). We also evaluated the prevalence of liver cirrhosis and hepatic failure. These results are similar to the data obtained from the original patient cohort with a diagnosis of AIH.
There are a few limitations to this study which are unique to the Explorys database that should be acknowledged. As we are using SNOMED-CT for the diagnosis of diseases, not all patients with autoimmune hepatitis may have been captured and others may have been misclassified. Validation of the SNOMED-CT diagnostic code for autoimmune hepatitis was not possible because the patient information in the database is de-identified. However, by excluding the patients diagnosed with other causes of liver damage, our search was made more precise. Although the ICD-9 and SNOMED-CT are both medical terminology systems for recording medical diagnoses and concepts, SNOMED-CT has many more concepts to be coded per clinical document than the ICD-930 that makes it more accurate in terms of enlisting pertinent clinical information. Another limitation of this database is the inability to capture information that is unavailable in the Explorys database. Pertinent information to this study were autoantibody titers, IgG levels and pathology reports from liver biopsies, which are also the components of diagnostic criteria. Moreover, even though we were able to determine that patients were prescribed with corticosteroids and/or immunomodulators, information regarding monotherapy vs. combination therapy and treatment response is unavailable in Explorys.
Finally, although Explorys uses a master–patient identifier to match the same patient across different healthcare institutions and combine the data, some patients may have received care in multiple institutions within Explorys healthcare partners and thus could have been counted multiple times31,32. However, this is countered by the fact that Explorys uses a robust patient matching algorithm and thus the effect of this error might be minimal and may affect the AIH and control groups equally.
In conclusion, this is the largest study to date that evaluates the epidemiology of autoimmune hepatitis in the US. We estimated the 5-year prevalence rate of AIH at 31.2 per 100,000 persons. We found the prevalence of AIH to be higher in females, elderly and Caucasian patients. Furthermore, this is the also the largest study to compare the associated signs and symptoms of AIH in female and male patients. We found that while females are more likely to have non-specific generalized symptoms, males more often have signs of advanced liver disease (such as ascites, jaundice and varices). We also found 35% of the patients with AIH had a diagnosis of liver cirrhosis and 2% of the patients had a diagnosis of primary neoplasm of the liver. The prevalence of OLT in patients with AIH was approximately 1%. Lastly, we determined that AIH is strongly associated with other autoimmune diseases, and individuals with AIH are more likely to have Sjögren’s syndrome, SLE, ulcerative colitis, celiac disease and rheumatoid arthritis as compared to patients without AIH.
Acknowledgments
Financial support: None
Abbreviations: Listed in order of appearance in manuscript
- AIH
Autoimmune Hepatitis
- USA
United States of America
- HER
Electronic Health Record
- HDG
Health data gateway
- SNOMED-CT
Systematized Nomenclature Of Medicine—Clinical Terms
- HIPAA
Health Insurance Portability and Accountability Act
- IRB
International Review Board
- HCC
Hepatocellular carcinoma
- OLT
Orthotopic liver transplant
- HE
Hepatic Encephalopathy
- HRS
Hepato-renal syndrome
- UGIH
Upper gastrointestinal hemorrhage
- LGIH
Lower gastrointestinal hemorrhage
- RA
Rheumatoid Arthritis
- SLE
Systemic Lupus Erythematosus
- UC
Ulcerative colitis
- CD
Crohn’s disease
- OR
Odds ratio
- CI
Confidence interval
- AASLD
American Association for the Study of Liver Diseases
- EASL
European Association for the Study of the Liver
- ICD-9
International Classification of Diseases- 9th Revision
Footnotes
Conflicts of interest: There are no potential conflicts (financial, professional, or personal) to disclose by all the authors (Nahel A. Tunio MD, Emad Mansoor MD, Mohammed Z. Sheriff, Gregory S. Cooper MD, Seth N. Sclair MD, Stanley M. Cohen MD).
References
- 1.Sebode M, Hartl J, Vergani D, et al. ; International Autoimmune Hepatitis Group (IAIHG). Autoimmune hepatitis: From current knowledge and clinical practice to future research agenda. Liver Int. 2018. January;38(1):15–22. [DOI] [PubMed] [Google Scholar]
- 2.Hennes EM, Zeniya M, Czaja AJ, et al. ; International Autoimmune Hepatitis Group. Simplified criteria for the diagnosis of autoimmune hepatitis. Hepatology. 2008. July;48(1):169–76. [DOI] [PubMed] [Google Scholar]
- 3.Heneghan MA, Yeoman AD, Verma S, et al. Autoimmune hepatitis. Lancet. 2013. October 26;382(9902):1433–44. doi: 10.1016/S0140-6736(12)62163-1. Epub 2013 Jun 14. Review. [DOI] [PubMed] [Google Scholar]
- 4.Gatselis NK, Zachou K, Koukoulis GK, et al. Autoimmune hepatitis, one disease with many faces: etiopathogenetic, clinico-laboratory and histological characteristics. World J Gastroenterol. 2015. January 7;21(1):60–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grønbæk L, Vilstrup H, Jepsen P. Autoimmune hepatitis in Denmark: incidence, prevalence, prognosis, and causes of death. A nationwide registry-based cohort study. J Hepatol. 2014. March;60(3):612–7. [DOI] [PubMed] [Google Scholar]
- 6.van Gerven NM, Verwer BJ, Witte BI, et al. ; Dutch Autoimmune hepatitis STUDY group. Epidemiology and clinical characteristics of autoimmune hepatitis in the Netherlands. Scand J Gastroenterol. 2014. October;49(10):1245–54. [DOI] [PubMed] [Google Scholar]
- 7.Hurlburt KJ, McMahon BJ, Deubner H, et al. Prevalence of autoimmune liver disease in Alaska Natives. Am J Gastroenterol. 2002; 97: 2402–07. [DOI] [PubMed] [Google Scholar]
- 8.Yoshizawa K, Joshita S, Matsumoto A, et al. Incidence and prevalence of autoimmune hepatitis in the Ueda area, Japan. Hepatol Res. 2016. August;46(9):878–83. [DOI] [PubMed] [Google Scholar]
- 9.Kim BH, Choi HY, Ki M, et al. Population-based prevalence, incidence, and disease burden of autoimmune hepatitis in South Korea. PLoS One. 2017. August 3;12(8):e0182391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Puustinen L, Barner-Rasmussen N, Pukkala E, et al. Incidence, prevalence,and causes of death of patients with autoimmune hepatitis: A nationwide register-based cohort study in Finland. Dig Liver Dis. 2019. February 10. [DOI] [PubMed] [Google Scholar]
- 11.Ngu JH, Bechly K, Chapman BA, et al. Population-based epidemiology study of autoimmune hepatitis: a disease of older women? J Gastroenterol Hepatol. 2010. October;25(10):1681–6. [DOI] [PubMed] [Google Scholar]
- 12.Czaja AJ. Diagnosis and Management of Autoimmune Hepatitis: Current Status and Future Directions. Gut Liver. 2016. March;10(2):177–203.Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Explorys Team. We unlock the power of BIG DATA to improve healthcare for everyone. Explorys 2017. https://www.explorys.com/about-us.html. Accessed November 25, 2017
- 14.Halling ML, Kjeldsen J, Knudsen T, et al. Patients with inflammatory bowel disease have increased risk of autoimmune and inflammatory diseases. World J Gastroenterol. 2017. September 7;23(33):6137–6146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Teufel A, Weinmann A, Kahaly GJ, et al. Concurrent autoimmune diseases in patients with autoimmune hepatitis. J Clin Gastroenterol. 2010. March;44(3):208–213. [DOI] [PubMed] [Google Scholar]
- 16.Fogel R, Comerford M, Chilukuri P, et al. Extrahepatic Autoimmune Diseases are Prevalent in Autoimmune Hepatitis Patients and Their First-Degree Relatives: Survey Study. Interact J Med Res 2018;7(2):e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo L, Zhou L, Zhang N, et al. Extrahepatic Autoimmune Diseases in Patients with Autoimmune Liver Diseases: A Phenomenon Neglected by Gastroenterologists. Gastroenterol Res Pract. 2017;2017:2376231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wong GW, Yeong T, Lawrence D, et al. Concurrent extrahepatic autoimmunity in autoimmune hepatitis: implications for diagnosis, clinical course and long-term outcomes. Liver Int 2017. March;37(3):449–457. [DOI] [PubMed] [Google Scholar]
- 19.Muratori P, Fabbri A, Lalanne C, et al. Autoimmune liver disease and concomitant extrahepatic autoimmune disease. Eur J Gastroenterol Hepatol. 2015. October;27(10):1175–9. [DOI] [PubMed] [Google Scholar]
- 20.Mirzaagha F, Azali SH, Islami F, et al. Coeliac disease in autoimmune liver disease: a cross-sectional study and a systematic review. Dig Liver Dis. 2010. September;42(9):620–3. [DOI] [PubMed] [Google Scholar]
- 21.Vollset SE. Confidence intervals for a binomial proportion. Stat Med. 1993;12:809–824. [DOI] [PubMed] [Google Scholar]
- 22.MedCalc Software Team. Odds ratio calculator. MedCalc 2018. https://www.medcalc.org/calc/odds_ratio.php. Accessed November 2, 2018.
- 23:IBM Corp. Released 2015. IBM SPSS Statistics for Windows, Version 23.0. Armonk, NY: IBM Corp. Accessed April 6, 2020. [Google Scholar]
- 24.Manns MP, Czaja AJ, Gorham JD, Krawitt EL, Mieli-Vergani G, Vergani D, Vierling JM; American Association for the Study of Liver Diseases. Diagnosis and management of autoimmune hepatitis. Hepatology. 2010. June;51(6):2193–213. [DOI] [PubMed] [Google Scholar]
- 25.Wong RJ, Gish R, Frederick T, et al. The impact of race/ethnicity on the clinical epidemiology of autoimmune hepatitis. J Clin Gastroenterol 2012. February;46(2):155–61. [DOI] [PubMed] [Google Scholar]
- 26.Campsen J, Zimmerman MA, Trotter JF, et al. Liver transplantation for autoimmune hepatitis and the successof aggressive corticosteroid withdrawal. Liver Transpl. 2008. September;14(9):1281–6. [DOI] [PubMed] [Google Scholar]
- 27.Czaja AJ, Donaldson PT. Gender effects and synergisms with histocompatibility leukocyte antigens in type 1 autoimmune hepatitis. Am J Gastroenterol. 2002. August;97(8):2051–7. [DOI] [PubMed] [Google Scholar]
- 28.Al-Chalabi T, Underhill JA, Portmann BC, et al. Impact of gender on the long-term outcome and survival of patients with autoimmune hepatitis. J Hepatol. 2008. January;48(1):140–7. [DOI] [PubMed] [Google Scholar]
- 29.Addis ME, Mahalik JR. Men, masculinity, and the contexts of help seeking. Am Psychol. 2003. Jan;58(1):5–14. [DOI] [PubMed] [Google Scholar]
- 30.Nadkarni PM, Darer JA. Migrating existing clinical content from ICD-9 to SNOMED. Journal of the American Medical Informatics Association: JAMIA. 2010;17:602–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mansoor E, Saleh MA, Cooper GS. Prevalence of eosinophilic gastroenteritis and colitis in a population-based study, from 2012 to 2017. Clin Gastroenterol Hepatol. 2017;15:1733–1741. [DOI] [PubMed] [Google Scholar]
- 32.Kaelber DC, Foster W, Gilder J, et al. Patient characteristics associated with venous thromboembolic events: a cohort study using pooled electronic health record data. J Am Med Inform Assoc. 2012;19:965–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Boer YS, Gerussi A, van den Brand FF, et al. Association Between Black Race and Presentation and Liver-Related Outcomes of Patients With Autoimmune Hepatitis. Clin Gastroenterol Hepatol. 2019. July;17(8):1616–1624. [DOI] [PubMed] [Google Scholar]
- 34.Wen JW, Kohn MA, Wong R, et al. Hospitalizations for Autoimmune Hepatitis Disproportionately Affect Black and Latino Americans. Am J Gastroenterol. 2018. February;113(2):243–253. [DOI] [PMC free article] [PubMed] [Google Scholar]