Abstract
Young-onset and late-onset Alzheimer’s disease has different clinical presentations with late-onset presenting most often with memory deficits while young-onset often presents with a non-amnestic syndrome. However, it is unknown whether there are differences in presentation and progression of neuropsychiatric symptoms in young- versus late-onset Alzheimer’s disease. We aimed to investigate differences in the prevalence and severity of neuropsychiatric symptoms in patients with young- and late-onset Alzheimer’s disease longitudinally with and without accounting for the effect of medication usage. Sex differences were also considered in these patient groups. We included 126 young-onset and 505 late-onset Alzheimer’s disease patients from National Alzheimer’s Coordinating Center–Uniform Data Set (NACC-UDS) and Alzheimer’s Disease Neuroimaging Initiative (ADNI). We investigated the prevalence and severity of neuropsychiatric symptoms using the Neuropsychiatric Inventory–Questionnaire over 4 visits with 1-year intervals, using a linear mixed-effects model. The prevalence of depression was significantly higher in young-onset than late-onset Alzheimer’s disease over a 4-year interval when antidepressant usage was included in our analyses. Our findings suggest that neuropsychiatric symptom profiles of young- and late-onset Alzheimer’s disease differ cross-sectionally but also display significant differences in progression.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11357-020-00304-y.
Keywords: Alzheimer’s disease (AD), Young-onset Alzheimer’s disease (YOAD), Late-onset Alzheimer’s disease (LOAD), Neuropsychiatric symptoms (NPS), Neuropsychiatric Inventory–Questionnaire (NPI-Q), Dementia
Introduction
Worldwide, 50 million people are affected with dementia with 60–70% of the cases due to Alzheimer’s disease (AD) [1]. Late-onset AD (LOAD) accounts for most AD cases and its prevalence increases with age [2]. Young-onset Alzheimer’s disease (YOAD) is diagnosed when someone develops AD before the age of 65 and represents up to 10% of all AD cases. The age of 65 for diagnosing YOAD versus LOAD was selected based on social factors such as the traditional retirement age [3] since the underlying pathology of amyloid and tau are similar between YOAD and LOAD. There are, however, important differences between the two as YOAD patients present with more severe gray matter atrophy [4], more abundant senile plaques, neurofibrillary tangles, and synaptic loss [5], as well as greater deficits in acetylcholine [6] than LOAD patients. YOAD presents more often with non-amnestic syndromes such as impaired language, attention, and visuospatial function, compared with LOAD who often present with more memory deficits [7]. Differences in the rate of progression have also been reported but while some studies revealed a faster rate of cognitive decline in younger patients [5, 7–9], others found no association between age of onset and rate of decline [10, 11]. YOAD is also considered to be more aggressive, with a lower life expectancy [9, 12].
In addition to the differences in cognitive deficits, there are also reports of differences in neuropsychiatric symptoms (NPS) between the YOAD and LOAD groups [13, 14]. NPS are common in AD as the disease progresses but can also be present at the onset [15, 16]. More than 80% of patients with AD develop at least one NPS over the course of the disease [17]. These symptoms include, but are not limited to, delusions, hallucinations, depression, anxiety, and apathy [18]. NPS can complicate the course of the disease and impact both patients and their caregivers as they are associated with early institutionalization [19, 20], increased mortality [21], and increased caregiver burden [22, 23]. NPS in YOAD can delay the diagnosis as these patients are often misdiagnosed with a psychiatric condition rather than a neurodegenerative disease [24, 25]. It has been suggested that NPS frequency and severity differ in YOAD versus LOAD due to psychosocial factors. For example, being diagnosed with AD at a younger age may have a more dramatic impact on an individual’s independence than at a later age [26, 27].
Some studies reported a lower prevalence of agitation, disinhibition, and delusions in YOAD [28], while others reported a higher prevalence of anxiety [29] or severe depression in the YOAD group [30]. The comparison of NPS frequency and severity between YOAD and LOAD has largely been explored in cross-sectional studies so the evolution of NPS in these 2 groups remains unknown. We hypothesized that the trajectory of NPS would differ between the two groups longitudinally and investigated the difference over 4 years.
Methods and materials
Participants
We included participants from the National Alzheimer’s Coordinating Center–Uniformed Data Set (NACC-UDS) and Alzheimer’s Disease Neuroimaging Initiative (ADNI). The data from NACC were collected between 1999 and 2017. The description of the UDS of NACC, including demographics, medical history, family history, behavioral and functional assessments, and a neuropsychological battery, was previously published [31]. Some data was obtained from the ADNI database (adni.loni.usc.edu). The ADNI was launched in 2003 as a public-private partnership, led by principal investigator Michael W. Weiner, MD. The primary goal of ADNI has been to test whether serial magnetic resonance imaging (MRI), positron emission tomography, other biological markers, and clinical and neuropsychological assessment can be combined to measure the progression of mild cognitive impairment and early AD.
We included the patients who were diagnosed with either YOAD or LOAD, had an MRI to exclude other pathologies, and whose clinical assessments included the Neuropsychiatric Inventory–Questionnaire (NPI-Q), a measure that assesses the presence/absence of dementia-related neuropsychiatric symptoms and their severity [32]. The longitudinal data included 4 visits with 1-year intervals. Age, sex, and Clinical Dementia Ratings (CDR® Dementia Staging Instrument) [33] scores of subjects were also collected from the databases.
Assessments
The NPI-Q was used to evaluate NPS in both the NACC-UDS and ADNI. The NPI is based on scripted questions administered to the participants’ caregivers or an informant familiar with the participant and used to evaluate the presence, severity, and frequency of twelve commonly encountered NPS in dementia: delusions, hallucinations, agitation, depression, anxiety, elation, apathy or indifference, disinhibition, irritability, and aberrant motor behavior [32]. We focused on the presence or absence of each symptom and its severity on a 3-point scale (1, mild; 2, moderate; 3, severe).
The CDR was used to assess disease severity in AD patients. Six domains including memory, orientation, judgment, problem-solving, community affairs, and home/hobbies/personal care were assessed and then, a Global CDR and a CDR Sum of boxes score were calculated [33].
Many patients are prescribed medications to manage their NPS. Antidepressants, anxiolytics, and antipsychotics are used for relieving symptoms including depressions, anxiety, and delusions/hallucinations/agitations, respectively. Since medications can eliminate or reduce the symptoms, caregivers of patients on medications may report no such neuropsychiatric symptoms. To avoid this confound and capture the true patient population who exhibit neuropsychiatric symptoms, new measures “[NPI-Q]inc[MED]” were created. For this new measure, patients who were on medications to treat NPS were included in the positive NPS group even if their caregivers reported no NPS on the NPI-Q. This new categorization resulted in the DEPincANTIDEP group which combined patients using antidepressants and patients who were positive for depression on NPI-Q; ANXincANXIOL group which combined patients using anxiolytics and patients who were positive for anxiety on NPI-Q; and DELincANTIPSY, HALincANTIPSY, and AGIincANTIPSY groups which combined patients using antipsychotics and patients with the presence of delusions/hallucinations/agitation on NPI-Q, respectively. We then constructed linear mixed-effects models (LMMs) and calculated contrasts at each visit for comparing the true presence and progression of neuropsychiatric symptoms in YOAD vs. LOAD considering medication usage.
Statistical analysis
All analyses were performed using R version 3.5.2 [34]. We constructed repeated-measure LMMs to evaluate the change in prevalence and severity of NPI-Q in patients with YOAD (N = 128) or LOAD (N = 509) over 4 visits, with 1-year intervals. We conducted a retrospective power analysis using the Powerlmm R package [35] for a longitudinal linear mixed-effects model with a large effect size (d = 0.80) and an alpha level of 0.05. Results showed that a sample size of 120 with equal-sized patient groups was required to achieve a power of 1.00. Both prevalence and severity of each NPI-Q domain on each visit were investigated independently from each other. We fit these models to every NPS domain separately. The mean response represented the absence/presence of particular neuropsychiatric symptoms and its severity was defined as a linear combination of fixed and random effects. The fixed effects included the group identity of YOAD or LOAD, visit number, the interaction between visit number-group identity, and visit number-CDR sum of boxes. Random effects described the individual differences in the trajectories of prevalence and severity of neuropsychiatric symptoms. We then computed the estimated contrasts of prevalence or severity of neuropsychiatric symptoms between patients with YOAD versus LOAD [35]. The group identity accounts for age because being diagnosed with YOAD versus LOAD relies on whether the patient is below or above 65. To support this, we constructed separate linear models for YOAD and LOAD patient groups to investigate whether age was predictive of NPI-Q prevalence or severity.
To evaluate whether the characteristics of patients who withdrew from the study had any effects on the results, we compared the prevalence and severity of NPS in patients whose data was available for four visits versus those who had data for only 3 years, in both YOAD and LOAD groups.
Results
Patient demographics
Our study consisted of 631 patients (145 from NACC-UDS, 486 from ADNI); 126 (F = 64, M = 62) with YOAD and 505 (F = 219, M = 286) with LOAD at the baseline assessments (Table 1). There was no significant difference in sex distribution. As expected, the patients with LOAD were significantly older than the YOAD (p < 0.0001, Fisher’s exact test; Table 1). CDR sum of boxes and global were not significantly different between the two patient groups (Table 1). At baseline, a significantly higher number of patients with YOAD were prescribed antidepressants than the LOAD (45% versus 24% respectively, p < 0.0001, Fisher’s exact test; Table 1). Ten percent of YOAD patients and 4% of LOAD patients were prescribed anxiolytics (p < 0.05, Fisher’s exact test). There was no significant difference in the prescription of acetylcholinesterase inhibitor (AChEI), memantine, antipsychotics, or mood-stabilizers between YOAD and LOAD patients.
Table 1.
Demographic characteristics of the study population at baseline including age, sex, CDR scores, and the percentage of patients on medications and their comparison between YOAD and LOAD patients
| YOAD (N = 126) mean ± SD or N (%) | LOAD (N = 505) mean ± SD or N (%) | p value | |
|---|---|---|---|
| Age | 60.81 ± 2.71 | 79.72 ± 2.90 | p < 0.0001*† |
| CDR sum of boxes | 2.18 ± 2.27 | 2.19 ± 2.67 | p = 0.96† |
| CDR global | 0.55 ± 0.33 | 0.47 ± 0.44 | p = 0.06† |
| Education (years) | 16.17 ± 2.68 | 15.76 ± 4.81 | p = 0.21† |
| Sex | |||
| Male | 62 (49%) | 286 (57%) | p = 0.59‡ |
| Female | 64 (51%) | 219 (43%) | p = 0.66‡ |
| Medications for NPS | |||
| Antidepressant | 57 (45%) | 121 (24%) | p < 0.0001*‡ |
| Antipsychotic | 2 (2%) | 3 (1%) | p = 0.26‡ |
| Anxiolytic | 12 (10%) | 19 (4%) | p < 0.05*‡ |
| Other medications | |||
| AChEI | 46 (37%) | 154 (30%) | p = 0.20‡ |
| Memantine | 14 (11%) | 73 (14%) | p = 0.39‡ |
| Mood | 1 (1%) | 7 (1%) | p = 1.00‡ |
AChEI, acetylcholinesterase inhibitor
†Student t test
‡Fisher’s exact test
*Significant p values at the alpha level of 0.05 or 0.001
Prevalence of neuropsychiatric symptoms at baseline
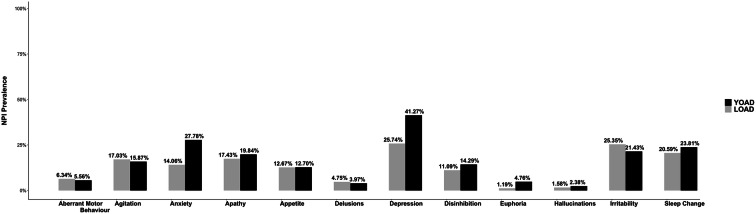
Depression was the most common NPS in both YOAD (41%) and LOAD (26%) but statistically higher in YOAD (p < 0. 001, Fisher’s exact test; Table.A.1, Fig. 1). A significantly higher number of YOAD patients exhibited anxiety (28% versus 14% respectively, p < 0.0001, Fisher’s exact test; Table.A.1) and euphoria (5% versus 1% respectively, p < 0.05, Fisher’s exact test; Table.A.1). The prevalence of the other NPI-Q symptoms was not different between the groups.
Fig. 1.
Prevalence of NPI-Q symptoms in patients with YOAD and LOAD at baseline
We repeated the same analyses for the newly created measures; patients on antidepressants were considered positive for depression, those on anxiolytic were considered positive for anxiety, and those on antipsychotics were considered positive for delusions, hallucinations, and agitation on NPI-Q. Considering DEPincANTIDEP and ANXincANXIOL groups, the patients with YOAD reported a significantly higher prevalence of depression (64% versus 41%, p < 0.001, Fisher’s exact test) and anxiety (33% versus 17%, p < 0.001, Fisher’s exact test) than the patients with LOAD (Table.A.2).
Progression of neuropsychiatric symptom prevalence
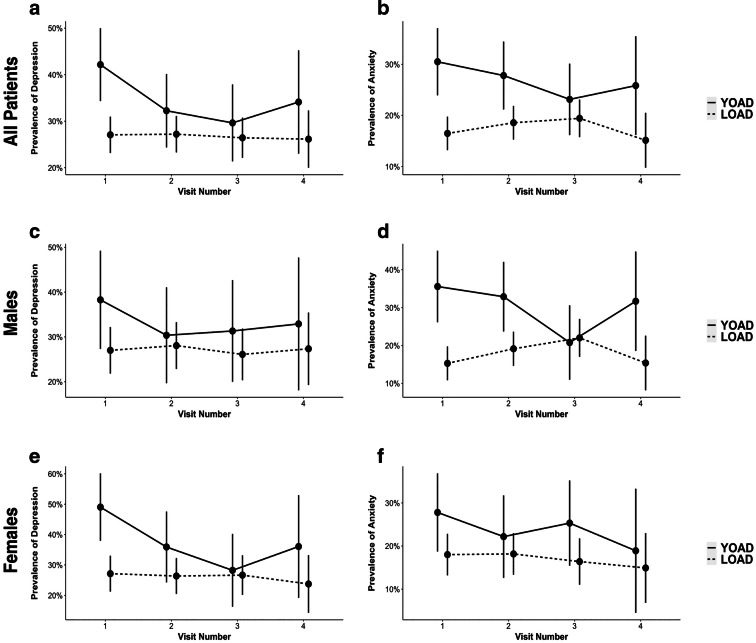
The LMM showed that the estimated prevalence of depression (β = 0.15, CI95% = [0.01, 0.29], p < 0.05) and anxiety (β = 0.14, CI95% = [0.03, 0.25], p < 0.05) in YOAD was significantly higher than in LOAD at baseline (Fig. 2a, b, Table 1). However, these significant differences were not observed in the follow-up visits because of the prevalence of depression and anxiety decreased at each visit in YOAD patients (Fig. 2a, b). The rest of the NPS were not significantly different between the two groups at baseline or longitudinally (Fig.A.1).
Fig. 2.
Prevalence of depression and anxiety in YOAD versus LOAD. Patients with YOAD had higher a depression and b anxiety at the first visit. c The prevalence of depression was not significantly different between YOAD and LOAD males. d Male YOAD patients had significantly higher anxiety than male LOAD. e Female YOAD patients had significantly higher depression than the female LOAD group, but no difference in f Anxiety was observed. Horizontal lines represent 95% confidence intervals
We constructed separate LMMs for YOAD and LOAD patient groups with age as a fixed effect and the prevalence of NPI-Q domains, specifically depression and anxiety, as a mean response. Age was not predictive of the prevalence of depression or anxiety in YOAD or LOAD patient groups.
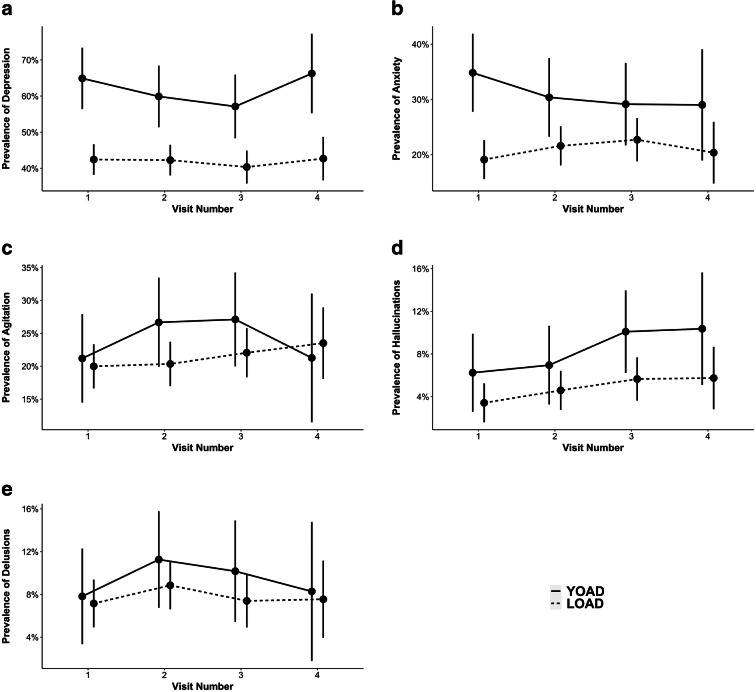
We repeated the same longitudinal analyses with LMMs after reclassifying patients who are on medications (antidepressant, anxiolytic, or antipsychotic) as positive. Reclassification of depression as the DEPincANTIDEP group, analyses revealed a significantly higher number of YOAD patients with depression at baseline (β = 0.22, CI95% = [0.08, 0.37], p < 0.001) and on the follow-up visits (Fig. 3, Table.A.3). Reclassification of anxiety as the ANXincANXIOL group, the prevalence of anxiety was still significantly higher (β = 0.16, CI95% = [0.03, 0.28], p < 0.05) in the YOAD group than in the LOAD at baseline, but not in the follow-up visits (Fig. 3, Table.A.3). Reclassification of hallucinations, agitation, and delusions as the AGIincANTIPSY, HALincANTIPSY, and DELincANTIPSY groups, there was no significant difference between YOAD and LOAD patients in terms of the prevalence of hallucinations, agitation, or delusions (Fig. 3).
Fig. 3.
Prevalence of NPI-Q symptom in YOAD versus LOAD groups including a depression, b anxiety, c agitation, d hallucinations, e delusions while accounting for medication usage. Comparison of NPI-Q between patients with YOAD versus LOAD for a DEPincANTIDEP, b ANXincANXIOL, c AGIincANTIPSY, d HALincANTIPSY, e DELincANTIPSY. Horizontal lines represent 95% confidence intervals
Severity of neuropsychiatric symptoms at baseline and its progression
The severity of NPI-Q symptoms was not significantly different between the YOAD and LOAD patients at baseline or longitudinally (Fig.A.2).
Sex differences in prevalence and severity of neuropsychiatric symptoms
The prevalence of anxiety was significantly higher (β = 0.20, CI95% = [0.04, 0.36], p < 0.05) in male patients with YOAD than LOAD at baseline (Fig. 2d). When the medication was taken into account, the prevalence of anxiety (β = 0.22, CI95% = [0.05, 0.39], p < 0.01) remained higher in male YOAD groups while a higher number of male YOAD patients also reported experiencing depression (β = 0.21, CI95% = [0.01, 0.41], p < 0.05) than LOAD at baseline. There were no differences between the male YOAD and LOAD patients in the follow-up visits with or without medications. The prevalence of depression (β = 0.22, CI95% = [0.02, 0.41], p < 0.05) was higher in female YOAD patients than LOAD at baseline (Fig. 2e). When the medication was included, the prevalence of depression (β = 0.24, CI95% = [0.03, 0.46], p < 0.05) remained higher in the female YOAD group than in LOAD. The analyses with or without medications showed that there were no differences between the female YOAD and LOAD patients in the follow-up visits. We also did not observe any sex differences in NPS severity between the YOAD and LOAD patient groups.
Drop-out comparisons
Eighty-five percent of YOAD and 76% of LOAD patients stayed in the study for the first three visits (3 years). Of these, 49% of YOAD and 45% of LOAD patients stayed for the last visit. In both YOAD and LOAD, there was no significant difference between the patients who withdrew from the study after the third visit and those that remained for the last visit, in terms of prevalence or severity of NPS at baseline or longitudinally.
Discussion
NPS are common in AD although there have been inconsistent findings on the prevalence and severity of such symptoms in YOAD versus LOAD. Our study compared the prevalence and severity of NPS in YOAD versus LOAD patients over 4 visits with 1-year intervals. A significantly higher number of YOAD patients experienced anxiety and depression at baseline, compared to the LOAD group. When accounting for medication use (i.e., antidepressants, anxiolytic, and antipsychotics), the prevalence of depression and anxiety was significantly different between YOAD and LOAD groups throughout all four visits. This was not apparent if medication usage was not considered. Interestingly, at baseline, there was a difference in the neuropsychiatric symptom profile with depression being higher in YOAD females compared with LOAD females and anxiety significantly higher in male YOAD patients compared to LOAD.
We report that the prevalence and severity of anxiety, depression, disinhibition, hallucinations, euphoria, and appetite change were generally higher in patients with YOAD than LOAD. The most common NPS in YOAD were anxiety and depression; in LOAD, it was depression and irritability. Our results of anxiety and depression being more common in YOAD are in line with previous research [29, 30, 36, 37]. In keeping with some of the literature [38, 39], we also report delusions and hallucinations as the least common NPS in both the YOAD and LOAD groups. There are others, however, who have reported a lower prevalence of delusions, agitation, depression, anxiety, apathy, irritability, and aberrant motor behavior in YOAD [40] or a higher prevalence of anxiety in the LOAD group [28].
The cause for increased reporting of anxiety and depression in YOAD is likely multifactorial. Firstly, the challenges for YOAD are different than for LOAD as many YOAD may still have large responsibilities within their families such as raising children and holding down a job. This may result in financial and emotional hardships in addition to the usual suffering seen in LOAD [41], which may result in greater depression and anxiety. In addition, patients with YOAD may be more aware of their deficits, which may contribute to greater depression and anxiety [42]. We have also investigated the severity of 12 NPI-Q domains in patients with YOAD versus LOAD in our longitudinal study. There was no significant difference in severity between YOAD and LOAD in any domains either at baseline or on follow-up visits.
Inconsistent findings on the prevalence of NPS in YOAD and LOAD have been reported across different studies [28–30, 36, 37, 40]. This may be due to small sample sizes, differences in sex distribution, or ignoring medication usage. When we compared the NPS of female and male patients separately, we found that depression was significantly higher in female YOAD patients compared to female LOAD patients while anxiety was more common in male YOAD patients compared to male LOAD patients, in line with previous research [36]. Men with dementia have been documented to be more anxious than women [43]. Further work is required to investigate the relationship between sex and NPS in YOAD versus LOAD. The use of certain medications such as antidepressant, anxiolytic, or antipsychotic may help resolve some of the NPS symptoms such as depressions, anxiety, agitation, hallucinations, and delusions, and if medication usage is not considered, this can obscure the actual NPS prevalence. For example, a patient who is on antidepressant can no longer appear depressed and so the caregiver may not report depression symptoms on NPI-Q. In our analyses, we addressed this confound by redoing the comparison analysis and considered patients who took certain medications for a symptom as having that NPS. This approach allowed us to capture the true prevalence of NPS and with this, a higher prevalence of anxiety and depression was observed in YOAD compared to LOAD across all visits.
Growing evidence shows that NPS in AD are associated with neurodegeneration in specific neural pathways and need to be considered as presenting symptoms, not secondary to the illness [45]. AD pathology may manifest as NPS in an early stage of AD or even years preceding cognitive decline [45, 46]. For example, neurofibrillary tangles in the brainstem evident even in the earlier stages of AD have been reported to be correlated with increased risk of agitation, depression, anxiety, and appetite loss [46]. NPS in cognitively normal individuals have been reported to be predictive of dementia and cognitive decline over time as well as the incidence of non-amnestic mild cognitive impairment [47–49]. Even independently of mild cognitive impairment, the presence of NPS such as delusions, hallucinations, depression, anxiety, and aberrant motor behavior was shown to be associated with an increased risk of developing dementia [50]. The understanding that NPS may reflect AD pathophysiology may help develop better diagnostic tools and effective treatments for AD that can be implemented even before the cognitive decline. Increased awareness that NPS are more frequent in YOAD would facilitate an earlier diagnosis in this population.
One of the limitations of this study was that some patients did not have data for their fourth annual visit either because they withdrew or missed their annual follow-up visit. To assess its possible confounding effect on our results, we compared the prevalence and severity of NPS between the patients who withdrew after the third visit and those who came to their 4th visit. There was no significant difference in any NPI-Q domains or severity between those that withdrew and those that remained and this in both YOAD and LOAD. There is always a concern that patients who have the most symptoms or more severe symptoms become too unwell to participate. These analyses increase the robustness of our findings as they show that our results were not driven by a particular type of patient dropping out. Another limitation includes a lack of information on the length and amount of medication usage. Socioeconomic was not used in the analysis and could have an impact on some NPS. This should be explored further.
In this study, the absence/presence and the severity of NPS were reported by caregivers, which is subjective, and no accurate diagnoses were made by a psychiatrist. Furthermore, caregivers can sometimes habituate to certain symptoms over time and so, they may report them less and as less severe. However, large sample size and longer follow-up times increased the robustness of our results. Age was not used as a covariate in this analysis because 65 years were used as a threshold and an equal follow-up period of 4 years was available in YOAD and LOAD, making it unnecessary to use age as another factor. A similar approach was previously used in investigating NPS profiles of patients with YOAD versus LOAD [37].
In conclusion, we show that the prevalence of anxiety and depression in patients with YOAD is greater than in LOAD and that this difference persists over time if include medication usage in the analysis. This study also suggests that male patients with YOAD might be at greater risk for anxiety than in LOAD while depression was more common in female YOAD patients. This has diagnostic implications given that young-onset patients usually encounter a delay in diagnosis [44]. The cause of differences in NPS prevalence between YOAD and LOAD as well as the influence of sex differences requires further research as there may be pathophysiological causes in addition to socioeconomic ones. Ultimately, understanding the progression of NPS and how medications influence such symptoms will help us better understand the true differences between YOAD and LOAD and provide more targeted therapy for each patient group.
Supplementary information
(DOCX 552 kb)
Acknowledgments
Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education and the study is coordinated by the Alzheimer’s Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
Authors’ contributions
M.G. analyzed, interpreted the data, and wrote the first draft of the manuscript. N.M. obtained the data from the databases and selected participants. M.L.M. assisted with the analysis and the model. M.C.T. provided advice throughout the process and supervised the project.
Funding
This work was supported by Toronto Western & General Foundation. The NACC database is funded by NIA/NIH Grant U01 AG016976. NACC data are contributed by the NIA-funded ADCs: P30 AG019610 (PI Eric Reiman, MD), P30 AG013846 (PI Neil Kowall, MD), P30 AG062428-01 (PI James Leverenz, MD), P50 AG008702 (PI Scott Small, MD), P50 AG025688 (PI Allan Levey, MD, PhD), P50 AG047266 (PI Todd Golde, MD, PhD), P30 AG010133 (PI Andrew Saykin, PsyD), P50 AG005146 (PI Marilyn Albert, PhD), P30 AG062421-01 (PI Bradley Hyman, MD, PhD), P30 AG062422-01 (PI Ronald Petersen, MD, PhD), P50 AG005138 (PI Mary Sano, PhD), P30 AG008051 (PI Thomas Wisniewski, MD), P30 AG013854 (PI Robert Vassar, PhD), P30 AG008017 (PI Jeffrey Kaye, MD), P30 AG010161 (PI David Bennett, MD), P50 AG047366 (PI Victor Henderson, MD, MS), P30 AG010129 (PI Charles DeCarli, MD), P50 AG016573 (PI Frank LaFerla, PhD), P30 AG062429-01(PI James Brewer, MD, PhD), P50 AG023501 (PI Bruce Miller, MD), P30 AG035982 (PI Russell Swerdlow, MD), P30 AG028383 (PI Linda Van Eldik, PhD), P30 AG053760 (PI Henry Paulson, MD, PhD), P30 AG010124 (PI John Trojanowski, MD, PhD), P50 AG005133 (PI Oscar Lopez, MD), P50 AG005142 (PI Helena Chui, MD), P30 AG012300 (PI Roger Rosenberg, MD), P30 AG049638 (PI Suzanne Craft, PhD), P50 AG005136 (PI Thomas Grabowski, MD), P30 AG062715-01 (PI Sanjay Asthana, MD, FRCP), P50 AG005681 (PI John Morris, MD), P50 AG047270 (PI Stephen Strittmatter, MD, PhD). Data collection and sharing for this project was funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd. and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada.
Data availability
The data is available at National Alzheimer’s Coordinating Center–Uniform Data Set (NACC-UDS) and Alzheimer’s Disease Neuroimaging Initiative (ADNI) databases.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval and consent to participate
Ethics approval and consent were obtained at the site by National Alzheimer’s Coordinating Center–Uniform Data Set (NACC-UDS) and Alzheimer’s Disease Neuroimaging Initiative (ADNI).
Consent for publication
Not applicable.
Footnotes
Data used in the preparation of this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in the analysis or writing of this report. A complete listing of ADNI investigators can be found at http://adni.loni.usc.edu/wp-content/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Global action plan on the public health response to dementia 2017–2025. Geneva: World Health Organization; 2017.
- 2.Scheltens P, Blennow K, Breteler MMB, de Strooper B, Frisoni GB, Salloway S, van der Flier WM. Alzheimer’s disease. Lancet. 2016;388(10043):505–517. doi: 10.1016/S0140-6736(15)01124-1. [DOI] [PubMed] [Google Scholar]
- 3.Eriksson H, Fereshtehnejad S-M, Falahati F, Farahmand B, Religa D, Eriksdotter M. Differences in routine clinical practice between early and late onset Alzheimer’s disease: data from the Swedish Dementia Registry (SveDem) J Alzheimers Dis JAD. 2014;41(2):411–419. doi: 10.3233/JAD-132273. [DOI] [PubMed] [Google Scholar]
- 4.Frisoni GB, Testa C, Sabattoli F, Beltramello A, Soininen H, Laakso MP. Structural correlates of early and late onset Alzheimer’s disease: voxel based morphometric study. J Neurol Neurosurg Psychiatry. 2005;76(1):112–114. doi: 10.1136/jnnp.2003.029876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sakai M, Hanyu H, Kume K, Sato T, Hirao K, Kanetaka H, Abe S, Kanaya K, Sakurai H, Iwamoto T. Rate of progression of Alzheimer’s disease in younger versus older patients: a longitudinal single photon emission computed tomography study. Geriatr Gerontol Int. 2013;13(3):555–562. doi: 10.1111/j.1447-0594.2012.00934.x. [DOI] [PubMed] [Google Scholar]
- 6.Nochlin D, van Belle G, Bird TD, Sumi SM. Comparison of the severity of neuropathologic changes in familial and sporadic Alzheimer’s disease. Alzheimer Dis Assoc Disord. 1993;7(4):212–222. [PubMed] [Google Scholar]
- 7.Koss E, Edland S, Fillenbaum G, Mohs R, Clark C, Galasko D, Morris JC. Clinical and neuropsychological differences between patients with earlier and later onset of Alzheimer’s disease: a CERAD analysis, Part XII. Neurology. 1996;46(1):136–141. doi: 10.1212/wnl.46.1.136. [DOI] [PubMed] [Google Scholar]
- 8.Seltzer B, Sherwin I. A comparison of clinical features in early- and late-onset primary degenerative dementia. One entity or two? Arch Neurol. 1983;40(3):143–146. doi: 10.1001/archneur.1983.04050030037006. [DOI] [PubMed] [Google Scholar]
- 9.Jacobs D, Sano M, Marder K, Bell K, Bylsma F, Lafleche G, Albert M, Brandt J, Stern Y. Age at onset of Alzheimer’s disease: relation to pattern of cognitive dysfunction and rate of decline. Neurology. 1994;44(7):1215–1220. doi: 10.1212/wnl.44.7.1215. [DOI] [PubMed] [Google Scholar]
- 10.Katzman R. Alzheimer’s disease as an age-dependent disorder. CIBA Found Symp. 1988;134:69–85. doi: 10.1002/9780470513583.ch6. [DOI] [PubMed] [Google Scholar]
- 11.Bowler JV, Munoz DG, Merskey H, Hachinski V. Factors affecting the age of onset and rate of progression of Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 1998;65(2):184–190. doi: 10.1136/jnnp.65.2.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Panegyres PK, Chen H-Y. Differences between early and late onset Alzheimer’s disease. Am J Neurodegener Dis. 2013;2(4):300–306. [PMC free article] [PubMed] [Google Scholar]
- 13.Toyota Y, Ikeda M, Shinagawa S, Matsumoto T, Matsumoto N, Hokoishi K, Fukuhara R, Ishikawa T, Mori T, Adachi H, Komori K, Tanabe H. Comparison of behavioral and psychological symptoms in early-onset and late-onset Alzheimer’s disease. Int J Geriatr Psychiatry. 2007;22(9):896–901. doi: 10.1002/gps.1760. [DOI] [PubMed] [Google Scholar]
- 14.Ferran J, Wilson K, Doran M, Ghadiali E, Johnson F, Cooper P, et al. The early onset dementias: a study of clinical characteristics and service use. Int J Geriatr Psychiatry. 1996;11(10):863–869. [Google Scholar]
- 15.Petrovic M, Hurt C, Collins D, Burns A, Camus V, Liperoti R, Marriott A, Nobili F, Robert P, Tsolaki M, Vellas B, Verhey F, Byrne EJ. Clustering of behavioural and psychological symptoms in dementia (BPSD): a European Alzheimer’s Disease Consortium (EADC) study. Acta Clin Belg. 2007;62(6):426–432. doi: 10.1179/acb.2007.062. [DOI] [PubMed] [Google Scholar]
- 16.Smits LL, Pijnenburg YAL, Koedam ELGE, van der Vlies AE, Reuling IEW, Koene T, Teunissen CE, Scheltens P, van der Flier WM. Early onset Alzheimer’s disease is associated with a distinct neuropsychological profile. J Alzheimers Dis JAD. 2012;30(1):101–108. doi: 10.3233/JAD-2012-111934. [DOI] [PubMed] [Google Scholar]
- 17.Lyketsos CG, Lopez O, Jones B, Fitzpatrick AL, Breitner J, DeKosky S. Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: results from the cardiovascular health study. JAMA. 2002;288(12):1475–1483. doi: 10.1001/jama.288.12.1475. [DOI] [PubMed] [Google Scholar]
- 18.Kiely KM, Mortby ME, Anstey KJ. Differential associations between sensory loss and neuropsychiatric symptoms in adults with and without a neurocognitive disorder. Int Psychogeriatr. 2018;30(2):261–272. doi: 10.1017/S1041610217001120. [DOI] [PubMed] [Google Scholar]
- 19.Cuijpers P. Depressive disorders in caregivers of dementia patients: a systematic review. Aging Ment Health. 2005;9(4):325–330. doi: 10.1080/13607860500090078. [DOI] [PubMed] [Google Scholar]
- 20.de Vugt ME, Nicolson NA, Aalten P, Lousberg R, Jolle J, Verhey FRJ. Behavioral problems in dementia patients and salivary cortisol patterns in caregivers. J Neuropsychiatry Clin Neurosci. 2005;17(2):201–207. doi: 10.1176/jnp.17.2.201. [DOI] [PubMed] [Google Scholar]
- 21.Peters ME, Schwartz S, Han D, Rabins PV, Steinberg M, Tschanz JT, Lyketsos CG. Neuropsychiatric symptoms as predictors of progression to severe Alzheimer’s dementia and death: the Cache County Dementia Progression Study. Am J Psychiatry. 2015;172(5):460–465. doi: 10.1176/appi.ajp.2014.14040480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.González-Salvador MT, Arango C, Lyketsos CG, Barba AC. The stress and psychological morbidity of the Alzheimer patient caregiver. Int J Geriatr Psychiatry. 1999;14(9):701–710. doi: 10.1002/(sici)1099-1166(199909)14:9<701::aid-gps5>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 23.Chiao C-Y, Wu H-S, Hsiao C-Y. Caregiver burden for informal caregivers of patients with dementia: a systematic review. Int Nurs Rev. 2015;62(3):340–350. doi: 10.1111/inr.12194. [DOI] [PubMed] [Google Scholar]
- 24.Bature F, Guinn B, Pang D, Pappas Y. Signs and symptoms preceding the diagnosis of Alzheimer’s disease: a systematic scoping review of literature from 1937 to 2016. BMJ Open. 2017;7(8):e015746. doi: 10.1136/bmjopen-2016-015746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Woolley JD, Khan BK, Murthy NK, Miller BL, Rankin KP. The diagnostic challenge of psychiatric symptoms in neurodegenerative disease; rates of and risk factors for prior psychiatric diagnosis in patients with early neurodegenerative disease. J Clin Psychiatry. 2011;72(2):126–133. doi: 10.4088/JCP.10m06382oli. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reed J, Cantley C, Clarke CL, Stanley D. Services for younger people with dementia: problems with differentiating needs on the basis of age. Dementia. 2002;1(1):95–112. [Google Scholar]
- 27.Rosness TA, Barca ML, Engedal K. Occurrence of depression and its correlates in early onset dementia patients. Int J Geriatr Psychiatry. 2010;25(7):704–711. doi: 10.1002/gps.2411. [DOI] [PubMed] [Google Scholar]
- 28.van Vliet D, de Vugt ME, Aalten P, Bakker C, Pijnenburg YAL, Vernooij-Dassen MJFJ, Koopmans RTCM, Verhey FRJ. Prevalence of neuropsychiatric symptoms in young-onset compared to late-onset Alzheimer’s disease - part 1: findings of the two-year longitudinal NeedYD-study. Dement Geriatr Cogn Disord. 2012;34(5–6):319–327. doi: 10.1159/000342824. [DOI] [PubMed] [Google Scholar]
- 29.Porter VR, Buxton WG, Fairbanks LA, Strickland T, O’Connor SM, Rosenberg-Thompson S, et al. Frequency and characteristics of anxiety among patients with Alzheimer’s disease and related dementias. J Neuropsychiatry Clin Neurosci. 2003;15(2):180–186. doi: 10.1176/jnp.15.2.180. [DOI] [PubMed] [Google Scholar]
- 30.Lawlor BA, Ryan TM, Schmeidler J, Mohs RC, Davis KL. Clinical symptoms associated with age at onset in Alzheimer’s disease. Am J Psychiatry. 1994;151(11):1646–1649. doi: 10.1176/ajp.151.11.1646. [DOI] [PubMed] [Google Scholar]
- 31.Besser L, Kukull W, Knopman DS, Chui H, Galasko D, Weintraub S, Jicha G, Carlsson C, Burns J, Quinn J, Sweet RA, Rascovsky K, Teylan M, Beekly D, Thomas G, Bollenbeck M, Monsell S, Mock C, Zhou XH, Thomas N, Robichaud E, Dean M, Hubbard J, Jacka M, Schwabe-Fry K, Wu J, Phelps C, Morris JC, Neuropsychology Work Group, Directors, and Clinical Core leaders of the National Institute on Aging-funded US Alzheimer’s Disease Centers Version 3 of the National Alzheimer’s Coordinating Center’s Uniform Data Set. Alzheimer Dis Assoc Disord. 2018;32(4):351–358. doi: 10.1097/WAD.0000000000000279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The neuropsychiatric inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44(12):2308–2314. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- 33.Morris JC. The clinical dementia rating (CDR): current version and scoring rules. Neurology. 1993;43(11):2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 34.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 2018. Available from: https://www.R-project.org/
- 35.Makowski D. The psycho package: an efficient and publishing-oriented workflow for psychological science. J Open Source Softw. 2018;3(22):470. [Google Scholar]
- 36.Kaiser NC, Liang L-J, Melrose RJ, Wilkins SS, Sultzer DL, Mendez MF. Differences in anxiety among patients with early- versus late-onset Alzheimer’s disease. J Neuropsychiatry Clin Neurosci. 2014;26(1):73–80. doi: 10.1176/appi.neuropsych.12100240. [DOI] [PubMed] [Google Scholar]
- 37.Panegyres PK, Chen HY. Early-onset Alzheimer’s disease: a global cross-sectional analysis. Eur J Neurol. 2014;21(9):1149–1e65. doi: 10.1111/ene.12453. [DOI] [PubMed] [Google Scholar]
- 38.Zhao Q-F, Tan L, Wang H-F, Jiang T, Tan M-S, Tan L, Xu W, Li JQ, Wang J, Lai TJ, Yu JT. The prevalence of neuropsychiatric symptoms in Alzheimer’s disease: systematic review and meta-analysis. J Affect Disord. 2016;190:264–271. doi: 10.1016/j.jad.2015.09.069. [DOI] [PubMed] [Google Scholar]
- 39.Ferreira M d C, Abreu MJ, Machado C, Santos B, Machado Á, Costa AS. Neuropsychiatric profile in early versus late onset Alzheimer’s disease. Am J Alzheimers Dis Other Dement. 2018;33(2):93–99. doi: 10.1177/1533317517744061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hori K, Oda T, Asaoka T, Yoshida M, Watanabe S, Oyamada R, et al. First episodes of behavioral symptoms in Alzheimer’s disease patients at age 90 and over, and early-onset Alzheimer’s disease: comparison with senile dementia of Alzheimer’s type. Psychiatry Clin Neurosci. 2005;59(6):730–735. doi: 10.1111/j.1440-1819.2005.01444.x. [DOI] [PubMed] [Google Scholar]
- 41.Svanberg E, Spector A, Stott J. The impact of young onset dementia on the family: a literature review. Int Psychogeriatr. 2011;23(3):356–371. doi: 10.1017/S1041610210001353. [DOI] [PubMed] [Google Scholar]
- 42.Mendez MF. Early-onset Alzheimer’s disease: nonamnestic subtypes and type 2 AD. Arch Med Res. 2012;43(8):677–685. doi: 10.1016/j.arcmed.2012.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Calleo J, Kunik ME, Reid D, Kraus-Schuman C, Paukert A, Regev T, et al. Characteristics of generalized anxiety disorder in patients with dementia. Am J Alzheimers Dis Other Dement. 2011;26(6):492–497. doi: 10.1177/1533317511426867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kuruppu DK, Matthews BR. Young-Onset Dementia. Semin Neurol. 2013;33(4):365–385. doi: 10.1055/s-0033-1359320. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 552 kb)
Data Availability Statement
The data is available at National Alzheimer’s Coordinating Center–Uniform Data Set (NACC-UDS) and Alzheimer’s Disease Neuroimaging Initiative (ADNI) databases.