Abstract
Endoplasmic reticulum (ER) stress occurs when protein folding or maturation is disrupted. A malfunction in the ER stress response can lead to cell death and has been observed in many neurological diseases. However, how the ER stress response is regulated in neuronal cells remains largely unclear. Here, we studied an E3 ubiquitin ligase named neural precursor cell expressed developmentally downregulated protein 4-like (Nedd4-2). Nedd4-2 is highly expressed in the brain and has a high affinity toward ubiquitinating membrane-bound proteins. We first utilized unbiased proteomic profiling with ultra-performance liquid chromatography tandem mass spectrometry of isolated membrane fractions from mouse whole brains to identify novel targets of Nedd4-2. Through this screen, we found that the expression and ubiquitination of ribosomal proteins are regulated by Nedd4-2 and confirmed an association between Nedd4-2 and ribosomes through ribosome sedimentation and polysome profiling. Further, we utilized immunoprecipitation and western blotting to show that induction of ER stress promotes an association between Nedd4-2 and ribosomal proteins, which is mediated through dephosphorylation of Nedd4-2 at serine-342. This increased interaction between Nedd4-2 and ribosomal proteins in turn mediates ER stress associated translational suppression. In summary, the results of this study demonstrate a novel regulatory mechanism underlying the ER stress response and a novel function of Nedd4-2 in translational control. Our findings may shed light on neurological diseases in which the ER stress response or the function of Nedd4-2 is dysregulated.
Keywords: Nedd4-2, ubiquitination, ribosome, translation, stress
INTRODUCTION
The endoplasmic reticulum (ER) is a fundamental eukaryotic organelle which plays an important role in a variety of cellular processes, including calcium regulation, lipid synthesis, and protein synthesis (Schwarz and Blower 2016). Because of the importance of these processes in proper cellular function, it is necessary for cells to be able to respond quickly and efficiently to disturbances in ER homeostasis. To counteract these disturbances, the cell utilizes a set of evolutionarily conserved mechanisms known as the unfolded protein response (UPR), which leads to global translational suppression and controlled degradation of misfolded proteins (Hetz 2012; Guan et al. 2014; Kocaturk and Gozuacik 2018). Importantly, it is known that the controlled degradation of proteins during ER stress requires coordination with the ubiquitin proteasome system (UPS) (Cybulsky 2013).
Ubiquitination is a post-translational modification which regulates many cellular processes. Ubiquitination occurs through a series of enzymatic cascades involving an E1 ubiquitin activating enzyme, an E2 ubiquitin conjugating enzyme, and an E3 ubiquitin ligase (Nandi et al. 2006). The result of this enzymatic cascade is the covalent attachment of ubiquitin to its substrate protein. Importantly, the fate of ubiquitinated proteins is determined by the pattern of ubiquitination (Woelk et al. 2007). Mono-ubiquitination often leads to changes in the activity or cellular localization of the protein, while poly-ubiquitination leads to targeted degradation by the proteasome or other intracellular trafficking events (Song and Luo 2019). Despite our knowledge of the general ubiquitination process and its role in protein degradation during ER stress, the specific E3 ligases involved remain understudied.
The neural precursor cell expressed developmentally downregulated protein 4-like (Nedd4-2) is a HECT-type E3 ligase, which belongs to the Nedd4 family of ubiquitin ligases (Donovan and Poronnik 2013). Members of the Nedd4 family have a characteristic protein structure. At the amino terminus, there is a Ca2+-phospholipid binding (C2) domain, which mediates the affinity of Nedd4-2 towards membrane bound proteins. Each member of the Nedd4 family contains two to four WW domains. Nedd4-2 contains four WW domains which mediate its interactions between substrate and adaptor proteins. At the carboxyl terminus, there is a HECT (homologous to the E6-AP C-terminus) domain, which catalyzes the addition of ubiquitin to substrate proteins. Nedd4-2 has a high affinity toward binding and ubiquitinating membrane bound proteins, which are often targeted for endocytosis and lysosomal/proteasomal degradation (Zhou et al. 2007; Fotia et al. 2004). Interestingly, Nedd4-2 has been implicated in a variety of neurological diseases including epilepsy and stroke (Dibbens et al. 2007; Lackovic et al. 2012). While an altered ER stress response has also been observed in these diseases (Ni et al. 2018; Liu et al. 2013; Nakka et al. 2010), whether Nedd4-2 functions to facilitate the ER stress response has not been tested.
In this study, we conducted a proteomic screen and biochemical experiments to show that the expression and ubiquitination of ribosomal proteins are regulated by Nedd4-2 in the brain. Upon induction of ER stress, we showed that Nedd4-2 exhibits enhanced association with ribosomal proteins and is required for global translational suppression during ER stress. Mechanistically we found that these effects result from decreased phosphorylation of Nedd4-2 at serine residue 342. Altogether, our findings uncover a novel function of Nedd4-2 in translational control during ER stress and suggest a potential pathophysiological deficit surrounding Nedd4-2 in diseases where Nedd4-2 is genetically deficient or mutated.
MATERIALS AND METHODS
This study was not pre-registered. All experiments using animal data followed the guidelines of Animal Care and Use provided by the Illinois Institutional Animal Care and Use Committee (IACUC) and the guidelines of the Euthanasia of Animals provided by the American Veterinary Medical Association (AVMA) to minimize animal suffering and the number of animals used. This study was performed under approved IACUC animal protocols at the University of Illinois at Urbana-Champaign (#17075 and #20049 to N.-P. Tsai).
Animals
Wild-type (WT) (RRID: IMSR JAX: 000664), Ribotag (RRID: IMSR JAX: 011029) and Emx1-Cre (RRID: IMSR JAX: 022762) mice in C57BL/6J background were obtained from The Jackson Laboratory. Nedd4-2 floxed mice were obtained from Dr. Hiroshi Kawabe (Max Planck Institute, Göttingen, Germany). The animals were housed in standard cages on a 12hr light: 12hr dark cycle with ad libitum access to food and water. For primary cortical neuron cultures, newborn mice at postnatal (P) day 0-1 were anesthetized by hypothermia and then decapitated. For immunoprecipitation experiments, we utilized brains from mice at P19-P24. These mice were anesthetized by isofluorane inhalation and then decapitated. Genotyping for the Nedd4-2 loxP allele and Emx1-Cre were performed using PCR as described previously (Zhu et al. 2019). The primers used to detect the Ribotag allele are: 5’-GGGAGGCTTGCTGGATATG-3’ and 5’-TTTCCAGACACAGGCTAAGTACAC-3’. The PCR program is as follows: 2 min at 94°C; 10 cycles of 20 s at 94°C, 15 s at 65°C (with 0.5°C per cycle decrease), 10 s at 68°C; 28 cycles of 15 s at 94°C, 15 s at 60°C, 10 s at 72°C; and 2 min at 72°C.
Reagents
Protein A/G beads were from Santa Cruz Biotechnology (RRID: AB_10201400). Bovine Serum Albumin (BSA) was from Fisher Scientific (catalog: BP9706-100). Dimethyl sulfoxide (DMSO) was from Fisher Scientific (catalog: BP231-100). Saline was from Hanna Pharmaceutical (catalog: NC9054335). Thapsigargin was from Adipogen (catalog: AG-CN2-0003). Puromycin was from MP Biomedicals (catalog: 100552). The antibodies used in this study were purchased from ProteinTech (anti-Gapdh, RRID: AB_2107436), Millipore (anti-puromycin, RRID: AB_2566826), Abcam (anti-PABPC1 [RRID: AB_777008] and anti-phospho-S448 Nedd4-2 [RRID: AB_2801582]), and Cell Signaling (anti-Nedd4-2 [RRID: AB_1904063], anti-phospho-S342 Nedd4-2 [RRID: AB_2797829], anti-hemagluttinin (HA) [RRID: 10691311], anti-ubiquitin [RRID: AB_331292], anti-CHOP [RRID: AB_2089254] and anti-N-cadherin [RRID: AB_2798427]). The Nedd4-2 WT, S342A and C962A expression constructs were from Addgene (Gao et al. 2009) . The Nedd4-2 S342D expression construct was generated by site-directed mutagenesis provided by Applied Biological Materials. Human embryonic kidney (HEK 293) cells were from ATCC, Manassas, VA, USA (ATCC #CRL-1573, RRID: CVCL_0045). The HEK 293 cell line is not listed as a commonly misidentified cell line by the International Cell Line Authentication Committee (ICLAC) and was not authenticated in this study. HEK cells were cultured in Dulbecco’s Modified Eagle Medium (Sigma, St Louis, MO, USA, catalog: 10017CV) with 10% Fetal Bovine Serum (JM Bioscience, catalog:100-500, San Diego, CA, USA). Cells were used between passages 3 and 30 and kept at 37°C in a humidified incubator with 5% CO2.
Proteomics
The proteomic screening was conducted using total membrane fractions of Nedd4-2 WT (Nedd4-2f/f Cre−) and Nedd4-2 conditional knock out (Nedd4-2f/f Cre+) mouse brains with label-free analyses provided by Bioproximity. The membrane protein extraction assay was conducted by the Mem-PER Plus Membrane Protein Extraction Kit (Thermo Scientific; catalog: 89842) and according to the manufacturer’s protocols with minor modification as described previously (Zhu et al. 2019). Following membrane extraction, trypsin was added at a ratio of 1:50 to the samples which were then incubated at 37°C overnight. The peptides were then extracted, lyophilized, and resuspended in 2-20 μL of 0.1% formic acid. Ultra-performance liquid chromatography – tandem mass spectrometry (UPLC-MS/MS) was then carried out by the Easy-nLC1200 and HF-X Hybrid Quadrupole-Orbitrap Mass Spectrometer. The relative protein abundance was determined by chromatographic peak intensity measurements, which were done by aligning the chromatographic peaks of precursor ions. The relative intensity of each identified protein from each sample set was then normalized to the intensity of β-actin. The mean, and subsequent standard deviation from the mean used to create heat maps, was derived from the pooled average of individual genes from all samples. The raw data files were analyzed and searched against Uniprot-Mus musculus protein databases.
Isolation of Total Ribosomes
Ribosomes were isolated according to a published protocol (Seimetz et al., 2019). In brief, whole brains were lysed using a glass dounce homogenizer in 1 mL polysome buffer [(20 mM Tris-HCl, pH 7.5; 15 mM MgCl2; 200 mM KCl; and 1% Triton-X-100) with cycloheximide (100 μg/mL), dithiothreitol (DTT, 2 mM), and heparin (1 mg/mL) added fresh]. Homogenates were spun in a microfuge at 13,000g at 4°C for 20 minutes. The supernatant was carefully removed into a new tube, taking care not to disturb the cell debris pellet. Fresh polysome buffer was added to the lysate to a final volume of 1.1 mL. 1 mL of diluted supernatant was carefully layered onto 2.5 mL of sucrose cushion [(20 mM Tris-HCl, pH 7.5; 15 mM MgCl2; 200 mM KCl; 1% Triton-X-100; 25% w/v Sucrose) with cycloheximide (100 μg/mL); DTT (2 mM); and heparin (1 mg/mL) added fresh] in an ultracentrifuge tube (Beckman Coulter, #349622); the remaining 100 uL of supernatant was saved as an input (total lysate). Samples were subjected to ultracentrifugation at 149,000g at 44°C for 2 hours in a Beckman TLA-100.3 rotor. Following ultracentrifugation, supernatant was recovered and transferred to a fresh ultracentrifuge tube taking care to maintain the solution distribution. The resulting polysome pellet was resuspended in 100 μL of polysome buffer. The supernatant was subjected to further ultracentrifugation at 100,000g at 4°C for 24 hours in a Beckman TLA-100.3 rotor. Following the second ultracentrifugation, the supernatant was recovered and saved as the soluble protein fraction. All resulting samples (total lysate, ribosome fraction, and ultracentrifugation supernatant) were subjected to western blotting.
Polysome Profiling
Whole brains were extracted, immediately frozen in liquid nitrogen, and stored at −80°C. Brains were pulverized under liquid nitrogen using a previously cooled, RNAse free mortar and pestle. The powder obtained was transferred to a 10-cm plate on dry ice while subsequent brains were pulverized. Afterward, 1 mL of lysis buffer (10 mM Tris-HCl at pH 8.0; 150 mM NaCl; 5 mM MgCl2; 1 mg/mL heparin; 1% Nonidet-P40; 0.5% deoxycholate; 40 mM DTT; 1 U/mL SUPERaseIn RNAse inhibitor [Thermo Fisher]; and 150 μg/mL cycloheximide) was added to the tissue powder. Next, re-suspended powder was scraped from the plate and transferred to a microcentrifuge tube with pipetting 10 times to lyse the cells. The cell nuclei and cell debris were removed by centrifugation (2,000g, 10 min, at 4°C). The supernatant was transferred to a fresh tube and then centrifuged again at 16,000g for 7.5 minutes at 4°C. 400 μL of supernatant was layered onto a 10 mL linear sucrose gradient (15-45% sucrose [w/v] made using a Biocomp Gradient Master) and centrifuged in a SW41Ti rotor (Beckman) for 125 minutes at 38,000 rpm at 4°C with the brake off. Polysome profiles were recorded using a UA-6 absorbance (ISCO) detector at 254 nm and fractions were collected along the gradient. Fractions were used for subsequent western blot analysis.
Primary Neuron Cultures
Primary cortical neuron cultures were made from mixed-sex WT, Nedd4-2 WT, and Nedd4-2 cKO mice at P0 or P1. Briefly, cortices were dissected, isolated, and triturated, and cells were plated with Dulbecco’s Modified Eagle Medium (DMEM). Four to six hours later, the DMEM was removed and neurons were subsequently maintained in NeuralA basal medium (Thermo Fisher, catalog: 10888022, Waltham, MA, USA) supplemented with B27 supplement (Invitrogen, catalog: 17504001, Carlsbad, CA, USA), GlutaMax (2 mM; Invitrogen, catalog: 35050061), Penicilin/Streptomycin (100 IU/ml of Penicillin and 100 ug/ml of Streptomycin; Thermo Fisher, catalog: 30002CI, Waltham, MA, USA) and Cytosine ß-D-arabinofuranoside (AraC, 2 μM; Sigma, catalog: C1768-100MG). Half the culture media was replaced with fresh media every three to four days thereafter until the experiments on days-in-vitro (DIV) 14. Each experiment in this study was performed with at least 3 independent litters and cultures.
HEK Cell Transfection, Immunoprecipitation, and Western Blotting
Transfection in HEK cells was performed using Lipofectamine 3000 (Invitrogen, catalog: L3000015). Mock transfected HEK cells received all aspects of the transfection process with the exception of a plasmid. In instances where transfected HEK cells were subsequently treated with DMSO or thapsigargin, each set of transfected HEK cells was split into two plates 24 to 48 hours after transfection in order to ensure similar expression of the transfected proteins. For immunoprecipitation (IP), whole brain lysate was obtained after homogenization in an IP buffer (50 mM Tris, pH 7.4; 120 mM NaCl; 0.5% Triton-X-100). Following homogenization, the samples were centrifuged twice at 5000 rpm for 5 minutes. 80 μg of total protein lysate was mixed with 2 μL of primary antibodies for 2 hours at 4°C. Protein A/G agarose beads were added for another hour followed by washing with IP buffer two times. Immunoprecipitated samples or input control lysates were mixed in an SDS buffer (40% glycerol; 240 mM Tris-HCl, pH=6.8; 8% sodium dodecyl sulfate; 0.04% bromophenol blue; and 5%-β-mercaptoethanol) and boiled for 5 minutes. After cooling on ice, the samples were loaded onto 8% or 10% sodium dodecyl sulfate-polyacrylaminde gel electrophoresis gels.
For western blotting, after gel electrophoresis, the gel was transferred onto a polyvinylidene fluoride membrane. The membrane was blocked with 1% Bovine Serum Albumin solution in Tris-buffered saline Tween-20 buffer (TBST; [20 mM Tris, pH 7.5; 150 mM NaCl, 0.1% Tween-20]) and then incubated overnight with primary antibody at 4°C. The next day, the membrane was washed three times for 10 minutes with TBST, followed by incubation with an HRP-conjugated secondary antibody (anti-mouse IgG from Cell Signaling, RRID: AB_330924; anti-rabbit IgG from Jackson Immuno Research, RRID: AB_ 10015282) in 5% non-fat milk in TBST for 1 hour at room temperature, followed by three 10-minute washes with TBST. The membrane was developed with an enhanced chemiluminescence reagent. To quantify the ubiquitin signal, we measured the entire area of the ubiquitin smear (which spans from slightly above 50 kDa to 250 kDa). We then normalized this value relative to the HA-signal obtained from the immunoprecipitation, before comparing the fold change relative to the control genotype or treatment. In order to confirm the specificity of the anti-HA antibody used throughout our study, we conducted control immunoprecipitation experiments with Ribotag mouse brains with the anti-HA-antibody as well as a non-target antibody (Supplemental Figure S1).
In-vitro Reconstitution
Ribosomes were immunoprecipitated from Ribotag mouse whole brains, as described above with only one wash with IP buffer after incubation with protein A/G agarose beads. Following this wash, 20 μg protein from transfected HEK cell lysates were added to the ribosomes and incubated together for 2 hours. Following this incubation, the samples were washed two times with IP buffer and mixed with an SDS buffer. These samples were subjected to western blotting.
Experimental Design and Statistical Analysis
Specific information regarding the sample number for each experiment is included in the figure legends. Although sample size calculations were not performed, at least 3 independent litters were used in each experiment as established by previous work in our lab (Zhu et al. 2019). For experiments utilizing cortical neuron cultures, each experiment was performed and analyzed using sister cultures made from the same litter. For experiments utilizing mice, littermate controls were used as appropriate. Due to the design of our research, no blinding was performed. The data presented in this study have been tested for normality using Kolmogorov-Smirnov Test. ANOVA with post-hoc Tukey HSD (Honest Significant Differences) tests were used for multiple comparisons between treatments or genotypes. Student’s t-test was used for western blotting results when two conditions were compared. Outliers were determined using GraphPad Outlier calculator, which performs the Grubbs’ test. Specific sample numbers are indicated in the figure legends. Differences are considered significant at the level of p < 0.05.
RESULTS
Unbiased proteomic screening reveals potential new functions of Nedd4-2 in translational control.
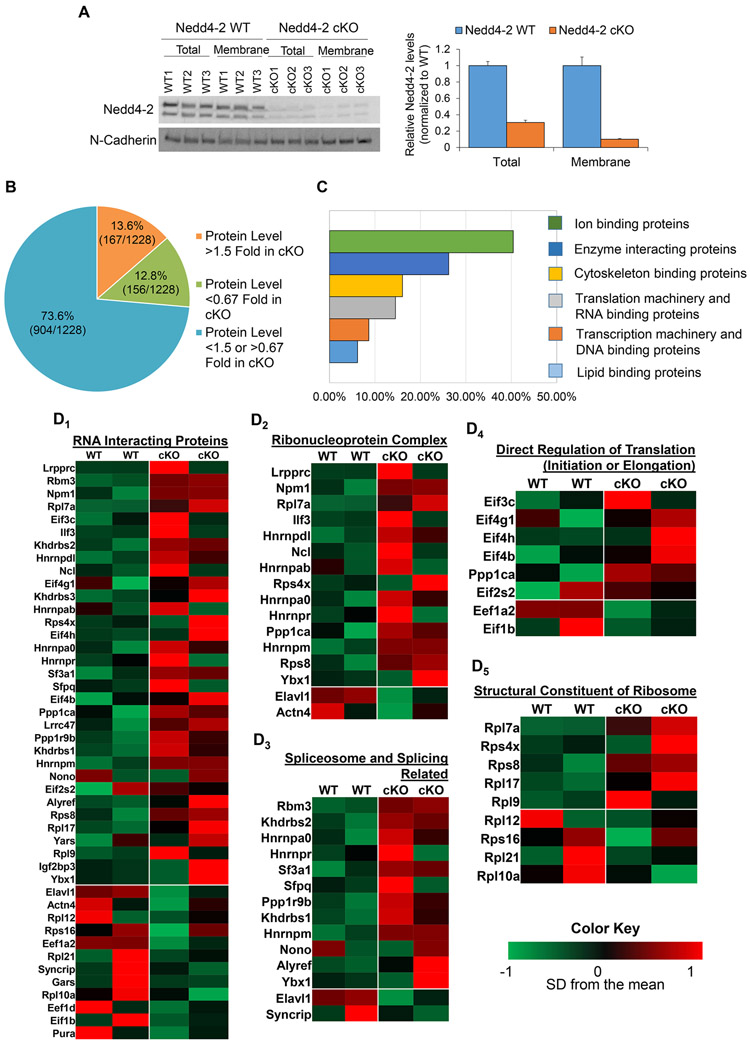
We utilized an unbiased proteomic approach to identify novel targets of Nedd4-2, with the hypothesis that any proteins which were identified as upregulated in the screen may be targets of ubiquitination leading to proteasomal degradation by Nedd4-2. To this end, we obtained total membrane fractions, using Mem-PER Plus Membrane Protein Extraction reagents from whole brains of Nedd4-2 WT (Nedd4-2f/f Emx1-Cre−) and Nedd4-2 cKO (Nedd4-2f/f Emx1-Cre+) mice for quantitative proteomic profiling. We focused on membrane fractions because Nedd4-2 is known to interact with and ubiquitinate membrane-bound proteins (Zhu et al. 2019). Emx1-Cre allows for reduction of Nedd4-2 in the cortex and hippocampus beginning at embryonic day 10.5 (Gorski et al. 2002), and western blotting shows an 80% reduction of Nedd4-2 in the whole brain (Figure 1A). Using littermate controls for each genotype, we obtained quantitative measurements for a total of 1228 proteins. Of these proteins, 167 exhibited at least a 50% increase (1.5 fold) and 156 exhibited at least a 33% reduction (0.67 fold) in Nedd4-2 cKO compared to Nedd4-2 WT (Figure 1B and Supplemental Table 1). Due to the large number of hits from our proteomics screen, we chose to investigate whether the proteins identified as upregulated were enriched in any specific functions in order to guide us toward a specific phenomenon to study. Analysis of the function of these identified proteins using a gene ontology program (GO Term Mapper) showed an enrichment in six functions: ion binding, enzyme binding, cytoskeleton binding, translation machinery and RNA binding, transcription machinery and DNA binding, and lipid binding (Figure 1C). We decided to focus on translation machinery and RNA binding because Nedd4-2 has not been shown to participate in post-transcriptional gene regulation. Upon further functional dissection of the RNA binding proteins, we saw a trend towards upregulation of several classes of proteins including those directly involved in translation initiation and elongation, as well as those considered to be structural constituents of the ribosome (Figure 1D). Taken together, this suggests a potential role for Nedd4-2 in translational control.
Figure 1. Translational machinery and RNA interacting proteins are upregulated in membrane fractions of Nedd4-2 cKO brains.
(A) Representative western blot showing the efficiency of Nedd4-2 reduction in whole brains and isolated membrane fractions. The quantification is on the right. (B) A pie chart summarizing the results of the proteomics screen of the membrane isolated fractions from Nedd4-2 WT and Nedd4-2 cKO mouse brains. (C) A bar graph identifying the top cellular functions of the proteins identified in the proteomics screen. (D) Heat maps showing the upregulation of RNA interacting proteins in Nedd4-2 cKO brains (D1), as well as heat maps of these proteins based on further functional subclassification (D2-D5).
Nedd4-2 interacts with ribosomes.
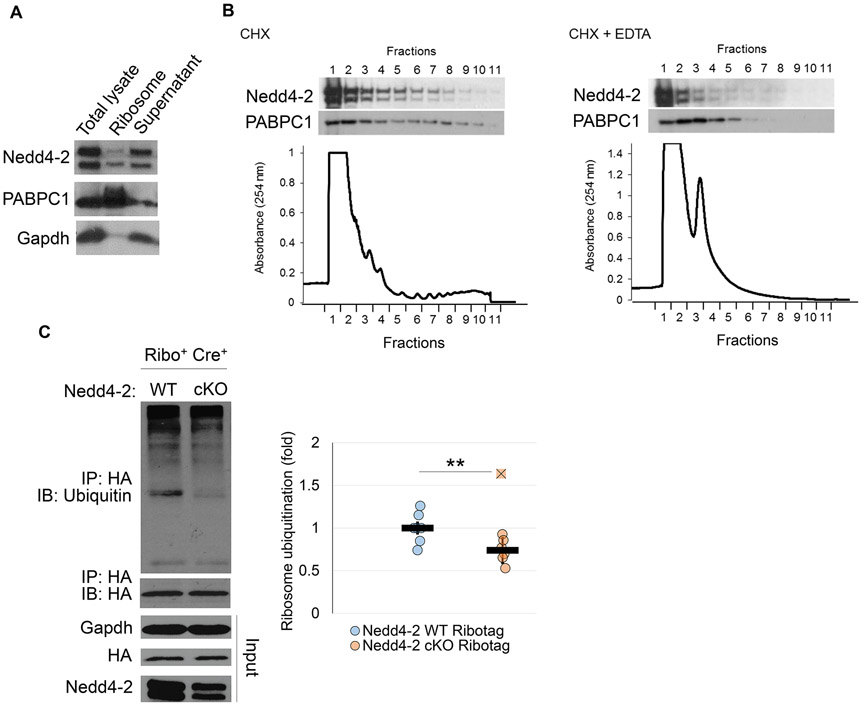
In order to determine whether ribosomal proteins are targets of Nedd4-2, we first confirmed whether Nedd4-2 associates with intact ribosomes. Using sucrose density ultracentrifugation to isolate ribosomes from the brains of 3-week old WT mice, we found that Nedd4-2 is present in the ribosome pellet, suggesting an interaction between Nedd4-2 and the ribosomes (Figure 2A). Following this observation, we aimed to determine the distribution of Nedd4-2 in ribosomes through polysome profiling by sucrose gradient sedimentation of mouse brain homogenates. Utilizing this technique, we observed that Nedd4-2 is enriched in the monosome fractions (fractions 2-4) and remains present in the polysome fractions (fractions 4-10). Addition of EDTA successfully dissociated the polysome fractions and shifted both Nedd4-2 and poly(A) binding protein cytoplasmic 1 (PABPC1) to the lighter monosome fractions. Taken together, these data indicate an interaction between Nedd4-2 and ribosomes.
Figure 2. Nedd4-2 interacts with ribosomes.
(A) Representative western blots of Nedd4-2, poly(A) binding protein cytoplasmic 1 (PABPC1), and Gadph after sucrose density ultracentrifugation of WT whole brains. PABPC1 and Gapdh serve as positive and negative controls, respectively, for ribosomes. (B) Representative western blots of Nedd4-2 and PABPC1, as well as polysome analysis, from WT brains. (C) Representative western blots of ubiquitin after immunoprecipitation with an anti-HA antibody from Nedd4-2 WT Ribotag (n=7 whole brains from 4 independent litters) and Nedd4-2 cKO Ribotag (n=7 whole mouse brains from 5 independent litters) mouse whole brains. Quantification is on the right. Ubiquitin values were normalized to HA-Tag. The fold change is relative to WT controls. Data were analyzed using Students t-test and are represented as mean ± SEM, with **p<0.01.
After establishing that Nedd4-2 and the ribosomes interact, we aimed to determine whether the ubiquitination of ribosomal proteins can be regulated by Nedd4-2. To answer this question, we utilized the Ribotag mouse in which the ribosomal protein Rpl22 is fused with a Cre-inducible hemagluttinin (HA)-Tag (Sanz et al. 2009), which allows for co-immunoprecipitation of ribosomes and ribosomal proteins with an anti-HA antibody. We crossbred Ribotag mice with our Emx1-Cre and Nedd4-2 floxed mice in order to obtain the following genotypes: Nedd4-2+/+ Ribotag+/− Cre+ (Nedd4-2 WT Ribotag) and Nedd4-2f/f Ribotag+/− Cre+ (Nedd4-2 cKO Ribotag). For the purposes of our study, the Ribotag mice are advantageous as they allow confidence that the ribosomes and ribosomal proteins obtained through immunoprecipitation are derived from the same population of neurons with and without Nedd4-2 expression. At three weeks of age, we harvested whole brains from male mice and conducted immunoprecipitation with an anti-HA antibody in order to isolate ribosomal proteins. Following immunoprecipitation, we conducted western blotting against ubiquitin to measure ubiquitination of ribosomal proteins (Figure 2C). We found that upon genetic reduction of Nedd4-2, ubiquitination of ribosomal proteins is decreased, suggesting that ribosomal proteins could be a target of Nedd4-2 ubiquitination. Although the reduction in the ubiquitination of ribosomal proteins is not robust, we attribute that to the likelihood that Nedd4-2 likely ubiquitinates only select ribosomal proteins.
Nedd4-2 is required for ER stress-induced translational suppression
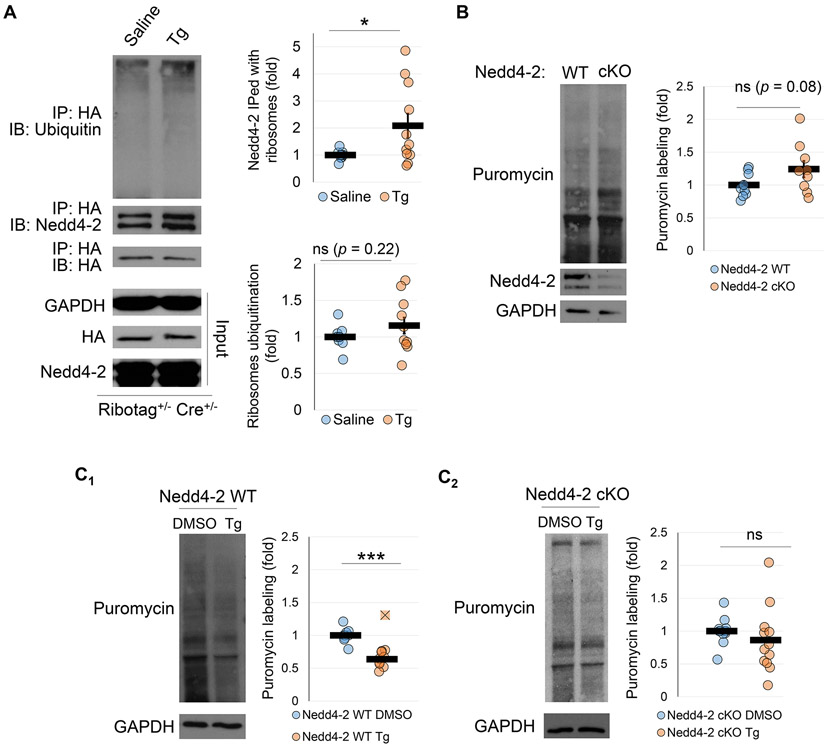
A previous study has demonstrated that ER stress leads to ubiquitination of ribosomal proteins (Higgins et al. 2015). Based on our results showing a potential role of Nedd4-2 in the ubiquitination of ribosomal proteins, we asked whether interaction between Nedd4-2 and ribosomal proteins may be promoted upon induction of ER stress. To this end, we intraperitoneally injected Ribotag mice (Ribotag+/− Cre+/−) with thapsigargin (Tg; 2 mg/kg body weight), a drug which induces ER stress by inhibiting the sarco/endoplasmic reticulum calcium ATPase (SERCA) (Liu et al. 2019). We confirmed by measuring the expression of an ER stress marker, CHOP, that intraperitoneal injection of Tg was sufficient to induce ER stress in the brain (Supplemental Figure S2). This effect has also been demonstrated previously by our lab and others (Liu et al. 2019; Kim et al. 2017). Four hours after injection, the whole brains were collected and the ribosomal proteins were obtained through co-immunoprecipitation with an anti-HA antibody, followed by western blotting against ubiquitin and Nedd4-2 (Figure 3A). Interestingly, we found a significant increase in the interaction between Nedd4-2 and the ribosomal proteins following injection of Tg. However, we did not observe a significant increase in the ubiquitination of ribosomal proteins. These data suggest that, while Nedd4-2 can regulate ubiquitination of ribosomal proteins, it may exert its effects on ribosomes and their associated proteins through a ubiquitination-independent mechanism during ER stress.
Figure 3. Nedd4-2 is required for ER stress induced translational suppression.
(A) Representative western blots of ubiquitin and Nedd4-2 after immunoprecipitation with an anti-HA antibody in Ribotag mice treated with saline (n=10 mouse brains from 6 independent litters) or thapsigargin (Tg, n=11 mouse brains from 6 independent litters). Ubiquitin values were normalized to HA-Tag. The fold change is relative to saline treated controls. (B) Representative western blots of basal puromycin labeling in Nedd4-2 WT and Nedd4-2 cKO cortical neuron cultures (n=9 cultures from 4 independent litters for both genotypes). Puromycin labeling was normalized to Gapdh. The fold change is relative to Nedd4-2 WT values. (C) Representative western blots of puromycin labeling in Nedd4-2 WT (C1; n=10 cultures from 7 independent litters) and Nedd4-2 cKO (C2; n=12 cultures from 8 independent litters) cortical neuron cultures after treatment with DMSO or Tg. Puromycin labeling was normalized to Gapdh. The fold change is relative to DMSO treated controls. Data were analyzed using Students t-test and are represented as mean ± SEM, with *p<0.05, ***p<0.001, and ns: non-significant.
We were then interested to determine whether Nedd4-2 plays any role in translation basally or upon induction of ER stress. To this end, we utilized primary cortical neuron cultures derived from Nedd4-2 WT and Nedd4-2 cKO mice. At days-in-vitro 14 (DIV14), we measured basal translation by treating the neurons with puromycin (10 μg/mL) for 1 hour to label newly synthesized proteins. Our results demonstrated a trend toward increased basal translation in the Nedd4-2 cKO neuron cultures, suggesting a potential role of Nedd4-2 in repressing basal translation (Figure 3B). To investigate the role of Nedd4-2 in ER stress induced translational suppression, we treated Nedd4-2 WT and Nedd4-2 cKO cultures with Tg (1 μM) for four hours and labeled newly synthesized proteins with puromycin during the final hour. As expected, the Nedd4-2 WT cultures showed a dramatic decrease in translation upon treatment with Tg (Figure 3C1). However, this effect on translation was impaired in the Nedd4-2 cKO cultures (Figure 3C2). Taken together, these data suggest a role for Nedd4-2 in mediating translation, particularly during ER stress, likely through increased interaction with ribosomes.
Dephosphorylation of Nedd4-2 at serine-342 increases the association between Nedd4-2 and ribosomes.
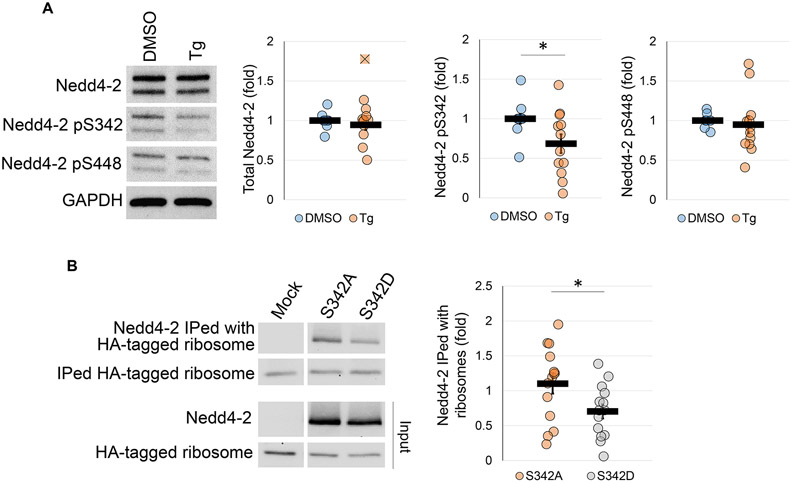
We next asked what molecular regulation may be underlying Nedd4-2’s effect on translation. To answer this question, we treated WT primary cortical neuron cultures with Tg (1 μM) for 4 hours. Following treatment, we conducted western blotting of total Nedd4-2, as well as phosphorylated Nedd4-2 at serine-342 and serine-448. Importantly, it is known that phosphorylation of Nedd4-2 at these serine residues mediates its substrate interaction and specificity (Ichimura et al. 2005). Through this experiment, we found that although the total expression of Nedd4-2 does not change during ER stress, there is a significant decrease in the phosphorylation at serine-342 (Figure 4A).
Figure 4. Dephosphorylation of Nedd4-2 at serine-342 during ER stress promotes association with ribosomal proteins.
(A) Representative western blots showing total Nedd4-2, as well as phosphorylated Nedd4-2 at serine sites-342 and 448 after treatment with DMSO or Tg in WT cortical neuron cultures (for total Nedd4-2 and p-Nedd4-2 S448, n=12 cultures from 10 independent litters; for p-Nedd4-2 S342, n=11 cultures from 9 independent litters). Nedd4-2 values were normalized to Gapdh. Phosphorylation at serine-342 and serine-448 is relative to total Nedd4-2. Fold change values are relative to DMSO treated controls. (B) Representative western blots showing interaction between the Nedd4-2 variants and partially purified ribosomes and ribosomal proteins (n=14 with 7 individual mouse brains and 7 individual sets of HEK cell transfections). Interaction was determined by normalizing to total Nedd4-2 expression. Data were analyzed using Students t-test and are represented as mean ± SEM, with *p<0.05 and ns: non-significant.
We next investigated whether this dephosphorylation promotes increased interactions with ribosomes. To this end, we performed an in vitro reconstitution experiment using HEK cells in which no endogenous Nedd4-2 is expressed (Lee et al. 2018). Briefly, total cell lysate was collected from HEK cells transfected with Nedd4-2 S342A (de-phospho-mimetic) or Nedd4-2 S342D (phospho-mimetic) and then incubated with partially purified ribosomal proteins co-immunoprecipitated from brains of the Ribotag mice using the anti-HA antibody. Following incubation, we conducted western blotting to measure whether and how the Nedd4-2 phospho-variants co-precipitated with the ribosomes. Through this experiment, we found that Nedd4-2 S342A associated more with immunoprecipitated ribosomal proteins than Nedd4-2 S342D (Figure 4B). Taken together with the previous result, this suggests that dephosphorylation of Nedd4-2 during ER stress promotes its interaction with ribosomal proteins and prompted us to determine how Nedd4-2 dephosphorylation may regulate translation.
Dephosphorylated Nedd4-2 mediates ER stress-induced translational suppression via ubiquitination-independent mechanisms.
Because Nedd4-2 dephosphorylation increases interaction with ribosomal proteins, we questioned whether this had any effect on translation during ER stress. We transfected HEK cells with WT Nedd4-2, Nedd4-2 S342A or Nedd4-2 S342D. Following transfection, we treated the HEK cells with Tg (5 μM) for 4 hours and with puromycin (10 μg/mL) for the final hour. Following treatment, we conducted western blotting using an anti-puromycin antibody, as well as total Nedd4-2 and phosphorylated Nedd4-2 at serine-342 and serine-448 in order to validate the mutations, as well as the specificity of the mutations to serine-342 (Figure 5A). The mutations were additionally validated through sequencing because our phospho-specific antibody did not recognize our phospho-mimetic protein, likely due the change in the amino acid sequence (Cowen et al. 2019). As expected, the mock transfected HEK cells showed a dramatic decrease in puromycin incorporation following Tg treatment. This effect was mimicked in the Nedd4-2 S342A transfected HEK cells. However, the translational attenuation upon ER stress was impaired in those HEK cells transfected with Nedd4-2 WT and Nedd4-2 S342D. Importantly, when we transfect HEK cells with Nedd4-2, the transfected Nedd4-2 protein always becomes highly phosphorylated (Lee et al. 2018). Therefore, we have interpreted our data to suggest that effective dephosphorylation of Nedd4-2 at serine-342 during ER stress allows for translational suppression.
Figure 5. ER stress associated translational suppression is mediated by Nedd4-2 dephosphorylation but is independent of catalytic activity of HECT domain.
(A) Representative images showing puromycin incorporation in HEK cells transfected with various phospho-mutants of Nedd4-2 (n=6 independent sets of HEK cell transfections). (B) Representative images showing puromycin incorporation in HEK cells transfected with various dephospho-mimetic and catalytically inactive mutants of Nedd4-2 (n=3 independent sets of HEK cell transfections). In both A and B, fold change was determined by directly comparing puromycin incorporation in the Tg treated HEK cells to control DMSO treated HEK cells. Data was analyzed using one-way ANOVA with post-hoc Tukey HSD test, and are represented as mean ± SEM, with *p<0.05 and ns: non-significant.
Following this observation, we wondered whether the E3 ligase activity of Nedd4-2 was required to mediate its effect on translation. To this end, we utilized a catalytically inactive Nedd4-2 mutant, Nedd4-2 C962A, as well as a Nedd4-2 construct containing both the S342A and C962A mutations. HEK cells were transfected with each of these Nedd4-2 variants, and then treated with Tg and puromycin as described above. Western blotting with an anti-puromycin antibody demonstrated, surprisingly, that all transfected HEK cells responded similarly in suppressing translation to mock transfected HEK cells upon induction of ER stress (Figure 5B). Although plasmid DNA can influence the stress response of cells, the data presented here show similar ER stress induced translational suppression in the presence or absence of a plasmid, ruling out the interference from plasmid DNA. Together, these data suggest that the ability of dephosphorylated Nedd4-2 to allow for translation suppression is not dependent on its E3 ligase activity. This conclusion also supports our previous observation (Figure 3A) in which induction of ER stress increases the association between Nedd4-2 and ribosomal proteins but does not promote ubiquitination of ribosomal proteins.
DISCUSSION
This current study aimed to identify novel functions of Nedd4-2 mediated ubiquitination. Through our work, we identified and confirmed the interaction between Nedd4-2 and ribosomes and the role of Nedd4-2 in ER stress induced translational suppression (Figure 6). It is tempting to speculate that this interaction is related to the trend toward increased translation observed in the Nedd4-2 cKO cultures. However, further studies are necessary to confirm whether ubiquitination of ribosomal proteins by Nedd4-2 mediates this observed effect. Specifically, it will be necessary to confirm the specific ribosomal proteins which may be subject to Nedd4-2 ubiquitination, such as those identified in our proteomics screen, and the specific lysine residues within those proteins which are ubiquitinated. Mutating these residues to create mutants unable to be ubiquitinated by Nedd4-2 will allow for greater understanding of the mechanism by which translation is elevated in Nedd4-2 cKO cultures. Further, our study demonstrated an increased interaction between Nedd4-2 and ribosomal proteins during ER stress. This increased interaction appears to be mediated by decreased phosphorylation at serine-342. Importantly, when dephosphorylation is impaired at serine-342, ER stress associated translational suppression is impaired. However, this effect does not appear to be mediated by ubiquitination as mutation of the catalytically active domain of Nedd4-2 does not cause deficits in translational suppression.
Figure 6. A working model for the role of Nedd4-2 mediated translational control.
(A) In undisturbed neurons, Nedd4-2 interacts transiently with ribosomal proteins to mediate basal translation rates. (B) During conditions in which neurons experience ER stress, Nedd4-2 becomes dephosphorylated at serine-342. This dephosphorylation mediates increased affinity towards ribosomal proteins and acts to suppress translation through an unknown ubiquitination independent mechanism. In the absence of this mechanism, as is the case in Nedd4-2 cKO neurons, translational suppression is impaired upon induction of ER stress.
Several questions remain regarding the specific mechanism by which Nedd4-2 mediates ER stress associated translational suppression. To begin, this study investigated whether Nedd4-2 ubiquitinates ribosomal proteins in order to suppress translation upon induction of ER stress. It is important to note that there are several other well-known pathways by which translation is regulated during ER stress. One of those pathways is mediated by decreased Akt phosphorylation during ER stress leading to a reduction in mTORC1 signaling (Guan et al. 2014). The other, and perhaps best known pathway, leading to translational suppression during ER stress is phosphorylation of eIF2a mediated by PERK activation (Sprenkle et al. 2017). Our proteomics screen identified multiple translational regulators of these pathways as potential substrates of Nedd4-2. Therefore, while it is beyond the scope of this current study, an important future direction would be to investigate whether Nedd4-2 is able to modulate these well-known pathways either directly or indirectly.
In addition to the question of what molecular pathway, or pathways, Nedd4-2 may be modulating to suppress translation, it is also important to further investigate how changes to the phosphorylation status of Nedd4-2 impact its activity during ER stress. First, it is possible that dephosphorylation at serine-342 renders Nedd4-2 inactive, which may explain the lack of effect of the Nedd4-2 S342A C962A mutant in ER stress associated translational suppression. While phosphorylation is known to mediate changes in interaction between Nedd4-2 and its substrate and adaptor proteins, the functional impact of these changes in interaction is quite variable. For instance, in the kidney, phosphorylation of Nedd4-2 increases its interaction with 14-3-3, which in turn impairs its ability to ubiquitinate the epithelial sodium channel (Nagaki et al. 2006) . Conversely, in the brain, interaction between 14-3-3 and Nedd4-2 appears to promote the ability of Nedd4-2 to ubiquitinate the GluA1 subunit of the AMPA receptor (Zhu et al. 2017). Thus, the possibility remains open that dephosphorylation of Nedd4-2 at serine-342 promotes interaction with a protein that inhibits the action of Nedd4-2. Second, it is possible that phosphorylated Nedd4-2 may actively maintain translation through ubiquitination. Although our study did not test this possibility directly, we suspect this may be the case because we observed translational suppression comparable to mock transfected HEK cells in those transfected with Nedd4-2 C962A (Figure 5B). Importantly, this transfected mutant was still extensively phosphorylated, which was observed to cause impairments in translational suppression during ER stress in our previous experiment (Figure 5A). However, it would be necessary to perform the same experiment with HEK cells transfected with Nedd4-2 S342D and Nedd4-2 S342D C962A mutants to provide additional evidence supporting this hypothesis.
It is intriguing that the actions of Nedd4-2 are not mediated by an increase in total Nedd4-2 protein, but rather through changes in its phosphorylation status. Nedd4-2 is known to be targeted for phosphorylation by a variety of proteins including Serum/Glucocorticoid Regulated Kinase 1 (SGK1), Protein Kinase B (Akt), 5' AMP-activated protein kinase (AMPK), c-Jun N-terminal kinase 1 (JNK1), and IκB Kinase β (IKKβ) (Bhalla et al. 2005; Lee et al. 2007; Bhalla et al. 2006; Hallows et al. 2010; Edinger et al. 2009). Importantly, amongst these kinases, Akt activity has been observed to decrease during ER stress, either through down-regulation or decreased phosphorylation (Hosoi et al. 2007; Zhao et al. 2016). Alternatively, increased phosphatase activity may mediate the decrease in Nedd4-2 phosphorylation. Previous research has demonstrated that inhibition of Protein phosphatase 1 (PP1) and Protein phosphatase 2 (PP2A) increases the basal phosphorylation of Nedd4-2 (Ichimura et al. 2005). Further, these phosphatases are known to be involved in the ER stress response (Cnop et al. 2017; Christen et al. 2007). Another phosphatase, pyridoxal-5'-phosphate phosphatase/chronophin (PLPP/CIN), has also been shown to dephosphorylate Nedd4-2, although at serine-448 instead of serine-342 (Kim et al. 2019). This current study did not investigate the specific mechanism behind Nedd4-2 dephosphorylation, but it is an important direction for future study.
A previous study has demonstrated that Nedd4-2 is upregulated in surviving cortical neurons following ischemic stroke (Lackovic et al. 2012), suggesting that it plays a protective role against neuronal death. However, it remains unclear what Nedd4-2 may be targeting during these conditions which mediates its protective effect. Because ER stress and subsequent translational suppression are well known to occur during ischemia (Xin et al. 2014; Mengesdorf et al. 2002), it is possible that Nedd4-2 is mediating its protective effect through its interactions with ribosomes during ER stress. Future studies should be conducted to determine whether ischemia promotes an interaction between Nedd4-2 and the ribosome. These results may also translate to other neurological diseases in which ER stress pathways are activated and proper function of Nedd4-2 is disrupted.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Tiffany Jong, Bailey Metcalf, and Jack Gerling for their technical assistance. This work is supported by the startup fund provided by the School of Molecular and Cellular Biology, University of Illinois at Urbana-Champaign (to N-P.T.), by the National Institute of Health (R01NS105615 and R03NS120516 to N-P.T., and R01HL126845 to A.K.) and by the American Heart Association Predoctoral Fellowship (20PRE35210705 to D.E.E. and 17PRE33670030 to J.S.).
Abbreviations:
- AMPK
5' AMP-activated protein kinase
- Arac
Cytosine ß-D-arabinofuranoside
- cKO
conditional knockout
- DIV
days-in-vitro
- DMSO
Dimethyl sulfoxide
- DTT
Dithiothreitol
- ER
endoplasmic reticulum
- HA
hemagluttinin
- HECT
homologous to the E6-AP C-terminus
- HEK
human embryonic kidney
- IKKβ
IκB Kinase β
- IP
immunoprecipitation
- JNK1
c-Jun N-terminal kinase 1
- Nedd4-2
neural precursor cell expressed developmentally downregulated protein 4-like
- PABPC1
poly(A) binding protein cytoplasmic 1
- PCR
polymerase chain reaction
- PP1
protein phosphatase 1
- PP2A
protein phosphatase 2
- RRID
Research Resource Identifier (see scicrunch.org)
- SERCA
sarco/endoplasmic reticulum calcium ATPase
- SGK1
Serum/Glucocorticoid Regulated Kinase 1
- Tg
Thapsigargin
- UPR
unfolded protein response
- UPS
ubiquitin proteasome system
- WT
wild-type
Footnotes
CONFLICT OF INTERST STATEMENT
The authors declare no competing financial interests.
REFERENCES
- Bhalla V, Daidié D, Li H, Pao AC, LaGrange LP, Wang J, Vandewalle A, Stockand JD, Staub O, Pearce D (2005) Serum- and glucocorticoid-regulated kinase 1 regulates ubiquitin ligase neural precursor cell-expressed, developmentally down-regulated protein 4-2 by inducing interaction with 14-3-3. Mol. Endocrinol 19, 3073–3084. [DOI] [PubMed] [Google Scholar]
- Bhalla V, Oyster NM, Fitch AC, Wijngaarden MA, Neumann D, Schlattner U, Pearce D, Hallows KR (2006) AMP-activated kinase inhibits the epithelial Na + channel through functional regulation of the ubiquitin ligase Nedd4-2. J. Biol. Chem 281, 26159–26169. [DOI] [PubMed] [Google Scholar]
- Christen V, Treves S, Duong FHT, Heim MH (2007) Activation of endoplasmic reticulum stress response by hepatitis viruses up-regulates protein phosphatase 2A. Hepatology 46, 558–565. [DOI] [PubMed] [Google Scholar]
- Cnop M, Toivonen S, Igoillo-Esteve M, Salpea P (2017) Endoplasmic reticulum stress and eIF2α phosphorylation: The Achilles heel of pancreatic β cells. Mol. Metab 6, 1024–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowen LE, Luo H, Tang Y (2019) Characterization of SMG7 14-3-3-like domain reveals phosphoserine binding-independent regulation of p53 and UPF1. Sci. Rep 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cybulsky AV (2013) The intersecting roles of endoplasmic reticulum stress, ubiquitin-proteasome system, and autophagy in the pathogenesis of proteinuric kidney disease. Kidney Int. 84, 25–33. [DOI] [PubMed] [Google Scholar]
- Dibbens LM, Ekberg J, Taylor I, Hodgson BL, Conroy SJ, Lensink IL, Kumar S, et al. (2007) Nedd4-2 as a potential candidate susceptibility gene for epileptic photosensitivity. Genes, Brain Behav. 6, 750–755. [DOI] [PubMed] [Google Scholar]
- Donovan P, Poronnik P (2013) Nedd4 and Nedd4-2: Ubiquitin ligases at work in the neuron. Int. J. Biochem. Cell Biol 45, 706–710. [DOI] [PubMed] [Google Scholar]
- Edinger RS, Lebowitz J, Li H, Alzamora R, Wang H, Johnson JP, Hallows KR (2009) Functional regulation of the epithelial Na + channel by IκB kinase-β occurs via phosphorylation of the ubiquitin ligase Nedd4-2. J. Biol. Chem 284, 150–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fotia AB, Ekberg J, Adams DJ, Cook DI, Poronnik P, Kumar S (2004) Regulation of neuronal voltage-gated sodium channels by the ubiquitin protein ligases Nedd4 and Nedd4-2. J. Biol. Chem 279, 28930–28935. [DOI] [PubMed] [Google Scholar]
- Gao S, Alarcón C, Sapkota G, Rahman S, Chen PY, Goerner N, Macias MJ, Erdjument-Bromage H, Tempst P, Massagué J (2009) Ubiquitin Ligase Nedd4L Targets Activated Smad2/3 to Limit TGF-β Signaling. Mol. Cell 36, 457–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorski JA, Talley T, Qiu M, Puelles L, Rubenstein JLR, Jones KR (2002) Cortical excitatory neurons and glia, but not GABAergic neurons, are produced in the Emx1-expressing lineage. J. Neurosci 22, 6309–6314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan BJ, Krokowski D, Majumder M, Schmotzer CL, Kimball SR, Merrick WC, Koromilas AE, Hatzoglou M (2014) Translational control during endoplasmic reticulum stress beyond phosphorylation of the translation initiation factor eif2. J. Biol. Chem 289, 12593–12611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallows KR, Bhalla V, Oyster NM, Wijngaarden MA, Lee JK, Li H, Chandran S, et al. (2010) Phosphopeptide screen uncovers novel phosphorylation sites of Nedd4-2 that potentiate its inhibition of the epithelial Na+ channel. J. Biol. Chem 285, 21671–21678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetz C (2012) The unfolded protein response: Controlling cell fate decisions under ER stress and beyond. Nat. Rev. Mol. Cell Biol 13, 89–102. [DOI] [PubMed] [Google Scholar]
- Higgins R, Gendron JM, Rising L, Mak R, Webb K, Kaiser SE, Zuzow N, et al. (2015) The Unfolded Protein Response Triggers Site-Specific Regulatory Ubiquitylation of 40S Ribosomal Proteins. Mol. Cell 59, 35–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosoi T, Hyoda K, Okuma Y, Nomura Y, Ozawa K (2007) Akt up- and down-regulation in response to endoplasmic reticulum stress. Brain Res. 1152, 27–31. [DOI] [PubMed] [Google Scholar]
- Ichimura T, Yamamura H, Sasamoto K, Tominaga Y, Taoka M, Kakiuchi K, Shinkawa T, Takahashi N, Shimada S, Isobe T (2005) 14-3-3 Proteins modulate the expression of epithelial Na+ channels by phosphorylation-dependent interaction with Nedd4-2 ubiquitin ligase. J. Biol. Chem 280, 13187–13194. [DOI] [PubMed] [Google Scholar]
- Kim E, Sakata K, Liao F-F (2017) Bidirectional interplay of HSF1 degradation and UPR activation promotes tau hyperphosphorylation. PLOS Genet. 13, e1006849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JE, Lee DS, Kim MJ, Kang TC (2019) PLPP/CIN-mediated Nedd4-2 S448 dephosphorylation regulates neuronal excitability via GluA1 ubiquitination. Cell Death Dis. 10, 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocaturk NM, Gozuacik D (2018) Crosstalk between mammalian autophagy and the ubiquitin-proteasome system. Front. Cell Dev. Biol 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lackovic J, Howitt J, Callaway JK, Silke J, Bartlett P, Tan SS (2012) Differential regulation of Nedd4 ubiquitin ligases and their adaptor protein Ndfip1 in a rat model of ischemic stroke. Exp. Neurol 235, 326–335. [DOI] [PubMed] [Google Scholar]
- Lee IH, Dinudom A, Sanchez-Perez A, Kumar S, Cook DI (2007) Akt mediates the effect of insulin on epithelial sodium channels by inhibiting Nedd4-2. J. Biol. Chem 282, 29866–29873. [DOI] [PubMed] [Google Scholar]
- Lee KY, Jewett KA, Chung HJ, Tsai N-P (2018) Loss of fragile X protein FMRP impairs homeostatic synaptic downscaling through tumor suppressor p53 and ubiquitin E3 ligase Nedd4-2. Hum. Mol. Genet 27, 2805–2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D-C, Eagleman DE, Tsai N-P (2019) Novel roles of ER stress in repressing neural activity and seizures through Mdm2- and p53-dependent protein translation. PLOS Genet. 15, e1008364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu GL, Wang KY, Guo H, Zhao SJ, Shen Y, Zhao YB (2013) Inositol-requiring protein 1α signaling pathway is activated in the temporal cortex of patients with mesial temporal lobe epilepsy. Neurol. Sci 34, 357–364. [DOI] [PubMed] [Google Scholar]
- Mengesdorf T, Proud CG, Mies G, Paschen W (2002) Mechanisms underlying suppression of protein synthesis induced by transient focal cerebral ischemia in mouse brain. Exp. Neurol. 177, 538–546. [DOI] [PubMed] [Google Scholar]
- Nagaki K, Yamamura H, Shimada S, Saito T, Hisanaga SI, Taoka M, Isobe T, Ichimura T (2006) 14-3-3 mediates phosphorylation-dependent inhibition of the interaction between the ubiquitin E3 ligase Nedd4-2 and epithelial Na+ channels. Biochemistry 45, 6733–6740. [DOI] [PubMed] [Google Scholar]
- Nakka VP, Gusain A, Raghubir R (2010) Endoplasmic reticulum stress plays critical role in brain damage after cerebral ischemia/reperfusion in rats. Neurotox. Res 17, 189–202. [DOI] [PubMed] [Google Scholar]
- Nandi D, Tahiliani P, Kumar A, Chandu D (2006) The Ubiquitin-Proteasome System. J. Biosci 31, 137–155. [DOI] [PubMed] [Google Scholar]
- Ni H, Rui Q, Li D, Gao R, Chen G (2018) The Role of IRE1 Signaling in the Central Nervous System Diseases. Curr. Neuropharmacol 16, 1340–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz E, Yang L, Su T, Morris DR, McKnight GS, Amieux PS (2009) Cell-type-specific isolation of ribosome-associated mRNA from complex tissues. Proc. Natl. Acad. Sci. U. S. A 106, 13939–13944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz DS, Blower MD (2016) The endoplasmic reticulum: Structure, function and response to cellular signaling. Cell. Mol. Life Sci 73, 79–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seimetz J, Arif W, Bangru S, Hernaez M, Kalsotra A (2019) Cell-type specific polysome profiling from mammalian tissues. Methods. 155, 131–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song L, Luo ZQ (2019) Post-translational regulation of ubiquitin signaling. J. Cell Biol 218, 1776–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprenkle NT, Sims SG, Sánchez CL, Meares GP (2017) Endoplasmic reticulum stress and inflammation in the central nervous system. BioMed Central Ltd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woelk T, Sigismund S, Penengo L, Polo S (2007) The ubiquitination code: a signalling problem. Cell Div. 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin Q, Ji B, Cheng B, Wang C, Liu H, Chen X, Chen J, Bai B (2014) Endoplasmic reticulum stress in cerebral ischemia. Neurochem. Int 68, 18–27. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Han Y, Bu DF, Zhang J, Li QR, Jin HF, Du JB, Qin J (2016) Reduced AKT phosphorylation contributes to endoplasmic reticulum stress-mediated hippocampal neuronal apoptosis in rat recurrent febrile seizure. Life Sci. 153, 153–162. [DOI] [PubMed] [Google Scholar]
- Zhou R, Patel SV, Snyder PM (2007) Nedd4-2 catalyzes ubiquitination and degradation of cell surface ENaC. J. Biol. Chem 282, 20207–20212. [DOI] [PubMed] [Google Scholar]
- Zhu J, Lee KY, Jewett KA, Man HY, Chung HJ, Tsai NP (2017) Epilepsy-associated gene Nedd4-2 mediates neuronal activity and seizure susceptibility through AMPA receptors. PLoS Genet. 13, e1006634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Lee KY, Jong TT, Tsai N (2019) C2-lacking isoform of Nedd4-2 regulates excitatory synaptic strength through GluA1 ubiquitination-independent mechanisms. J. Neurochem 151, 289–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.