Abstract
A combination of freeze-dried powder of disproportionating enzyme (D-enzyme)-containing potato tuber and β-amylase-containing ramie leaf was used to improve the gluten-free (GF) bread, and its physicochemical properties were characterized. The presence of D-enzyme and β amylase in the potato tuber and ramie leaf was confirmed. Sixty five percent of partially gelatinized rice flour and 20% corn starch was combined with 10% freeze-dried potato tuber and 1% ramie leaf powder, and baked. The specific volume increased by 23% compared to the control with improved internal characteristics. Texture profile analysis revealed that retrogradation of the bread was retarded when stored for 90 h at 4 °C. The bread crumb amylose content was reduced from 14 to 9% and amylopectin branch chain-length distribution was rearranged, whereby the proportions of the branch chains with Degree of polymerization (DP) < 9 and DP > 19 decreased. The results suggest that D-enzyme and β-amylase cooperatively altered amylose/amylopectin ratio and amylopectin structure.
Keywords: Disproportionating enzyme, Gluten-free bread, Β-amylase, Ramie leaf, Amylose, Amylopectin
Introduction
Gluten-free (GF) products are in increasingly high demand among people who suffer from the gluten-related disorder celiac disease, since the uptake of gluten and gluten-like proteins from wheat, rye, and barley causes inflammation in the small intestine. However, a satisfactory substitute for gluten in wheat flour bread has proven elusive in the food industry, because other flour proteins and starch cannot sustain the gas bubbles in the product, and are inadequate in the role that gluten plays in network formation (Horstmann et al., 2017).
The main starch sources used in GF breads are corn, tapioca, potato, rice, and GF wheat (Masure et al., 2016). Rice-based breads are popular in many countries where rice is a staple food. Cereal starch plays a key role in the formulation of GF products, because it can form a matrix in which gas bubbles are entrapped, increasing the gas-holding capacity of dough or batter (Horstmann et al., 2016). Abdel-Aal (2009) suggested that starch is instrumental in the formation of hydrogen bonds, enhancing crumb softness and maintaining the consistency of dough during the mixing and baking process. Various starches derived from naturally GF sources, such as corn, cassava, potato, and rice, have been used in GF product formulation. Of these, rice starch is most commonly used as a basic ingredient in GF bread because it does not contain gluten and lacks gluten and contains easily digested carbohydrates, while starch of corn and tapioca may cause some difficulties due to taste to the product. (Gallagher et al., 2002). However, the low protein content and lack of elastic–plastic properties, which are intrinsic to wheat gluten and are essential for bread making, limit the use of rice flour in bread (Furlán et al., 2015; Renzetti and Rossel, 2016; Wang et al., 2017). Moreover, breads made of rice flour tend to retrograde and become crumbly within a few days when stored at low temperatures. It has been suggested that the amylose–amylopectin interaction in rice starch may account for the rapid retrogradation of rice bread (Araki et al., 2016).
Yano et al. (2017) and Aoki et al. (2020) examined the baking properties of pure rice bread with different amylose contents and amylopectin architectures. They showed that the physicochemical properties of rice bread were caused by the amylose content and amylopectin architecture, while bread expansion was attributed to the unwinding of the double helices. Rice bread made from high-amylose cultivars was less concave in shape, with a higher specific loaf volume (Aoki et al., 2020). Amylose content was also positively correlated with bread hardness, and crumb structure maintenance. Therefore, the amylose content of rice starch should be considered in the balance between retrogradation and crumb structure. Furthermore, rice bread made from cultivars with a higher proportion of amylopectin long branch chains yielded a harder texture than those with lower proportions of amylopectin long branch chains (Aoki et al., 2012). Regarding the retrogradation process, it has been reported that the reorganization of amylose dominates the early stages of bread aging, while the recrystallization of amylopectin occurs at a later stage (Hug-Iten et al., 2003). Consequently, the efficacy of enzymes as anti-staling agents may be attributable to the modification of both amylose and amylopectin structures. Therefore, the application of enzymes suitable for the modification of both amylose and amylopectin in rice starch is essential in the development of GF rice bread (Buwalda et al., 2014; Hug-Iten et al., 2003). As a potential means of achieving the optimum effect, Park et al. (2018) proposed the combination of two or more enzymes, or the application of enzyme treatments using chemical/ physical methods that have been proven to be effective for starch modification.
D-enzyme (disproportionating enzyme; 4-alpha-glucanotransferase) transfers the glucan moiety of amylose to amylopectin, resulting not only in the reduction of amylose content but also in the redistribution of amylopectin branch chain length (Van der Maarel and Leemhuis, 2013). However, β-amylase preferentially attacks amylopectin molecules and shortens the branch chains by releasing maltose from the non-reducing end of the branch chains (Dicko et al., 1999). Thus, the combination of these two enzymes can confer synergetic effects that hinder the retrogradation of GF rice bread.
D-enzyme has been detected in large proportions in potato tuber (Takaha et al., 1993). Furthermore, the retrogradation rate was significantly slowed in enzyme-treated breads stored at 4 °C (Buwalda et al., 2014; Kim et al., 2013; Nguyen et al., 2014; Park et al., 2007a; Park et al., 2007b; Park et al., 2018). In contrast, β-amylase showed only minor effects on bread firmness, but showed significant potential in maintaining elastic recovery, because the enzyme was inactive during the earlier stages of the baking process before the starch gelatinization due to the low thermal stability of plant-originated β-amylase (55–60 °C) (Hug-Iten et al., 2003). Conversely, ramie leaf exhibits high β-amylase activity with high thermostability (65–75 °C) (He et al., 2017). Thus, the addition of ramie leaf β-amylase with D-enzyme to GF rice bread can achieve a synergetic effect associated with the combination of the two enzymes. Furthermore, potato tuber and ramie leaf enzymes can be preferentially applied to bread-making processes as safe food ingredients of plant origin (Park et al., 2018).
This study constitutes the first investigation of the combined effects of potato tuber and ramie leaf extracts containing D-enzyme and β-amylase, respectively, on the quality of GF rice bread.
Materials and methods
Materials
Brown rice, a Vietnamese cultivar with a 16.4% amylose content, was milled and sieved through 0.25-mm mesh screens. Fresh potato tubers were purchased from local markets in My Tho, Vietnam, and Seoul, Korea. Fresh ramie leaf was harvested from a farm, at Seocheon, Korea. The bread improver was kindly donated by the SPC Group (Seoul, Korea). Maltotriose (Glc3) and the standard maltooligosaccharides were kindly provided by Professor Park Jong-Tae, Choong Nam National University, Korea.
Preparation of potato tuber and ramie leaf powder for enzyme treatment
Freshly peeled potato was sliced into 2–3-mm thick pieces, frozen at – 60 °C in a deep freezer, and freeze-dried. Ramie leaf was frozen at – 60 °C in a deep freezer immediately after harvest and freeze-dried. The freeze-dried leaves were ground, sieved through 100-mesh screens, and stored in a vacuum desiccator.
Determination of enzyme activity of potato tuber and ramie leaf powder
Crude enzyme preparation of freeze-dried potato tuber (0.5–1.0 mL) was incubated with 1.0 mL of Glc3 or soluble starch, at pH 6.0 and 40 °C for 5–48 h with mild shaking; 0.5 mL of the reaction mixture was extracted at each reaction for 0, 5, 24, and 48 h. The enzyme reaction was stopped by boiling for 10 min. After centrifugation, the supernatant was analyzed using thin-layer chromatography (TLC) and high-performance anion-exchange chromatography (HPAEC).
The activity and thermostability of ramie leaf β-amylase was previously determined (He et al., 2017).
Reaction product analysis by TLC
An aluminium plate (TLC Silica gel 60, Merck, Darmstadt, Germany) was used for analysis. Prepared samples were spotted on a plate using a pipette; the plate was placed in a TLC chamber containing a solvent mixture of isopropyl alcohol/ethyl acetate/water (3:1:1, vol./vol./vol.) and developed at room temperature. The plate was dried, visualized by dipping into a solution containing 0.3% N-(1-naphthyl)-ethylenediamine and 5% sulfuric acid in methanol, dried, and heated for 10 min at 110 °C.
Partial gelatinization of starches and bread baking
To make the GF rice bread, half of the total rice flour (32.5 g) was mixed with boiling water (half of the total water: 50 mL) and mixed for 5 min to produce a dough. The dough was left to rest until the temperature had decreased to 40 °C. The yeast, which had been placed in warm water (35 °C), the remaining flours (30 g corn flour and 5 g soybean flour), and all other ingredients (10 g sugar, 1.25 g salt, 6 g plant oil, 12 g egg, 25 mL fresh milk, and 15% maltodextrin (Degree of Equivalent (DE) = 19)) were mixed with 1.0 mL of supplementary SPC-bread improver in a Bear mixer. For the rice/potato bread, 10 g of freeze-dried potato tuber powder was added and the mixture was kneaded manually for 30 min at 45 °C. In accordance with the recipe for the rice/potato/ramie bread, 1 g freeze-dried ramie leaf powder was added to the rice/potato formula, and 100 g of the resulting dough was placed in a greased bread pan for a 35-min fermentation at room temperature. Finally, the fermented dough was baked at 175 °C for 35 min. All control breads were prepared by replacing the freeze-dried potato powder with commercial enzyme-inactive potato powder. The recipes for the experimental breads are summarized in Table 1. After baking, the loaves were removed from the pans and cooled at room temperature. After 1 h of cooling, the specific volumes of the bread samples were determined. Texture profile analysis (TPA) was carried out after a 2-h rest at room temperature using a CT3 Texture Analyzer (Brookfield Engineering, Middleboro, MA, USA). The batches were prepared in three replicates.
Table 1.
Properties of gluten-free rice breads prepared with potato/ramie enzymes
| Breads | Enzyme Treatments | Loaf weight(g)/ 100 g batter | Amylose contents/dry basis bread (%) | Specific volume(cm3/g) | Loaf hardness after baking(N) |
|---|---|---|---|---|---|
| Rice/Potato | Rice starch treated with freeze dried potato tuber powder (D-enzyme active) | 82.3 ± 0.6NS1) | 9.0 ± 0.8a2) | 3.6 ± 0.1c | 1.8 ± 0.26a |
| -Control | Replaced with potato tuber powder (enzyme inactive) from market | 81.8 ± 1.5 | 14.0 ± 1.2b | 2.5 ± 0.1a | 4.9 ± 0.3b |
| Rice/Potato/Ramie | Rice/Potato plus freeze dried ramie powder as a β-amylase source | 80.7 ± 0.6 | 9.0 ± 0.9a | 3.7 ± 0.1c | 1.8 ± 0.1a |
| -Control | Replaced with potato tuber powder (enzyme inactive) from market | 83.6 ± 1.6 | 14.0 ± 1.3b | 3.0 ± 0.2b | 4.6 ± 0.0b |
| Wheat/Potato | Rice flour of was replaced with wheat flour | 82.1 ± 1.9 | 10.1 ± 0.9a | 4.2 ± 0.2d | 1.8 ± 0.2a |
| -Control | Replaced with potato tuber powder (enzyme inactive) from market | 82.2 ± 2.0 | 13.0 ± 1.0b | 2.4 ± 0.1a | 5.4 ± 0.4c |
1) NS: Values in a column are not significantly different at p < 0.05
2) a-d: Means with the different superscript in a column are significantly different at p < 0.05
Statistical analysis: The data were expressed as means of triplicate determinations with standard deviation
Analysis of variance (ANOVA) was performed with Duncan’s multiple range test (p < 0.05) using SPSS (ver. 19 for Windows, Chicago, IL, USA)
Specific volume measurement
Bread loaf volume (cm3) and weight (g) were determined after a 60-min cooling period. Loaf volume was measured using a displacement method: sesame seeds were poured into a container of known volume until the bottom was covered. The loaf was placed inside the container, which was then filled to the top with more seeds. The extra sesame seeds, equal to the loaf volume, were measured in a graduated cylinder. The specific loaf volume was calculated as volume per weight (mL/g).
TPA of the bread products
TPA was performed using a CT3 Texture Analyzer (Brookfield). Single 25-mm-thick slices or two 12.5-mm-thick slices were placed under a 38.1-mm-diameter cylindrical probe (TA4; Brookfield). For the latter, TPA of the crumb was conducted at a constant speed of 2.0 mm/s (pre-test speed, test speed, and post-test speed) over a distance of 10 mm. The wait time between the first and the second compression cycles was 5 s, and the trigger force was 10 g. Triplicate measurements were taken for each loaf. The peak force of compression was reported as hardness, in accordance with the American Association of Clinical Chemistry method 74–09 (AACC, 2000).
Determination of branch chain length of starch by HPAEC
The samples were dissolved with 100 mM sodium acetate buffer (pH 5.0). For the hydrolysis of the α-1,6 linkage, The mixture (0.2%, w/v) was reacted with 0.72 units of pullulanase (Megazyme, Wicklow, Ireland) and 0.1 units of isoamylase (Megazyme, Wicklow, Ireland) at 40 °C for 48 h. Chain length distribution of each sample was analyzed with a High-Performance Anion-Exchange Chromatography (HPAEC; ICS-5000+, Dionex, Sunnyvale, CA, USA) and a Carbopac PA-1 column (4 × 250 mm; Dionex, Sunnyvale, CA, USA) was used. The reaction mixtures were boiled for 10 min and centrifuged at 12,000 g for 10 min, then the supernatants were filtered with membrane filter (0.45 μm; Hyundai micro, Korea). Samples were eluted with a 0.6 M sodium acetate in 0.15 M NaOH at a flow rate of 1.0 mL/min. Gradients of 0.6 M sodium acetate were applied up to 64%.
Statistical analysis
All data was obtained with triplicate analyses. Statistically significant differences in mean values were determined by one-way analysis of variance (ANOVA) using Duncan’s multiple range test (p < 0.05) and SPSS (ver. 19 for windows, Chicago, IL, USA).
Results and discussion
Presence of D-enzyme as the active carbohydrate enzyme in potato tuber
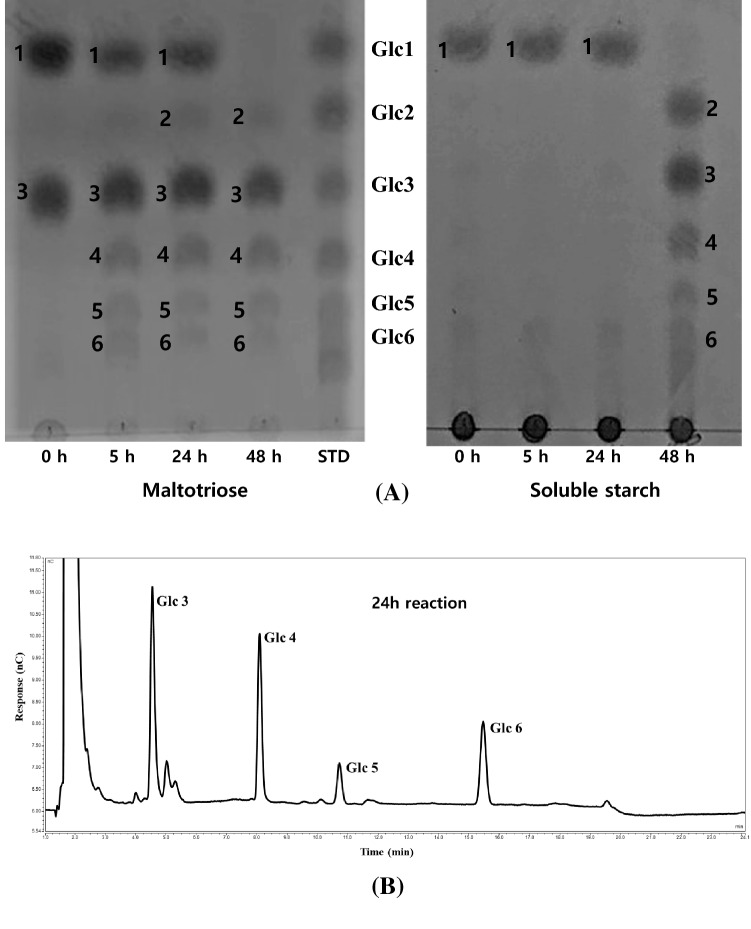
The freeze-dried potato tuber powder was incubated with Glc3 and soluble starch at the enzyme’s optimum temperature of 45 °C and the reaction products were analyzed by TLC (Fig. 1A). The reaction products were also incubated with Glc3 for 24 h and identified by HPAEC (Fig. 1B). Substantial amounts of maltose (Glc2)– maltohexaose (Glc6) (2–6) were produced from Glc3 (3) within an incubation time of 5–24 h, while the reaction of the mixture with starch yielded various maltodextrins of Glc2– maltoheptaose (Glc7) (2–6) after a 48-h reaction time. A dense spot (1) in the Glc1 region appeared to included potato tuber pigments. The results indicated that the D-enzyme of the potato tuber extract catalyzed the transfer of the glucan moiety (e.g., Glc2 from Glc3 to another acceptor molecule), thereby creating various maltodextrins (Glc2–Glc6). Therefore, the potato tuber extract prepared in this study appeared to contain high-activity D-enzyme as its predominant enzyme. Consequently, freeze-dried potato tuber can be used as a major source of plant D-enzyme. D-enzyme from potato tuber was isolated by ion exchange chromatography, and was characterized by its transfer reaction activity (Takaha et al., 1993). In contrast, β-amylase showed less activity in potato tuber due to dormancy while being active in ramie leaf (Akoumianakis et al., 2016; Sergeeva et al., 2012). This result supports the premise that β-amylase activity decreases with the onset of dormancy and increases again after the initiation of sprouting (Akoumianakis et al., 2016; Sergeeva et al., 2012).
Fig. 1.
(A) TLC analysis of freeze-dried potato tuber powder reacted with G3 and soluble starch, (B) HPAEC analysis of freeze-dried potato tuber powder reacted with G3 and soluble starch
GF rice bread formula and baking process for optimum enzyme reaction
In the preliminary study of GF rice bread formulation, the incorporation of corn starch flour (30 wt.%) and soybean flour (5 wt.%) into rice flour (65 wt.%) significantly improved the bread's flavor and appearance. To achieve an efficient enzymatic reaction, the rice flour was partially gelatinized by the addition boiling water before dough mixing, in which 10–15% of the starch was gelatinized (Nguyen et al., 2015). The mixture containing partially gelatinized rice flour and freeze-dried potato tuber was continuously mixed, allowing D-enzyme to interact with rice starch near the optimum temperature of 40–45 °C. At this temperature, which is higher than the glass-transition temperature of zein protein (Tg = 35 °C), the zein protein in corn starch can most effectively incorporate with other starches and proteins in the mixture, leading to a fine texture of the product (Tandazo, 2013). Efficient ramie β-amylase activity can also be achieved at a dough temperature of 65–80 °C, near starch gelatinization during the baking process, due to the high thermostability of the enzyme (He et al., 2017).
Effects of potato tuber D-enzyme on GF rice bread properties
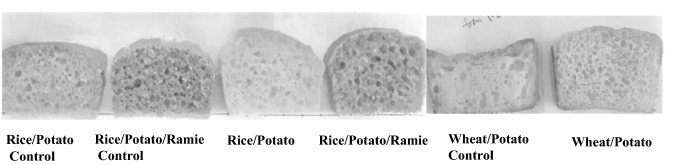
To investigate the effect of potato tuber D-enzyme, the properties of breads prepared with freeze-dried potato powder (rice/potato and wheat/potato) were evaluated and compared with those of commercial potato powder treatment (rice/potato (control) and wheat/potato (control)) as enzyme-inactive controls, in which enzymes were completely inactive during thermal processing. The specific loaf volume of the rice/potato bread was higher (3.6 cm3/g) than that of the rice/potato (control) bread (2.5 cm3/g) (Table 1, Fig. 2). Likewise, the wheat/potato bread showed a substantial increase in volume, from 2.4 to 4.2 cm3/g, compared to the wheat/potato bread (control). The potato enzymes had a notable effect, both in the rice-based (rice/potato) and wheat-based (wheat/potato) formulas, mainly via starch modification by D-enzyme, indicating the positive correlation of potato tuber D-enzyme with specific loaf volume.
Fig. 2.
Finished bakery products using freeze-dried potato powder and commercial potato powder
The amylose contents (i.e., percent amylose of dry bread crumb) of the rice/potato bread and rice/potato/ramie bread were reduced from 14 to 9.0%, while that of the wheat/potato bread decreased from 13.0 to 10.1%; the corresponding controls were unchanged (Table 1).
Because D-enzyme catalyzes the transfer of the glucan moiety of amylose to the branch chain of amylopectin, D-enzyme likely plays a major role in reducing the amylose content, changing the amylose/amylopectin ratio, which is one of the key factors affecting starch retrogradation.
The amylose content of rice flour affects bread quality, including factors such as shape and hardness. Bread made from high-amylose rice cultivars exhibited a less-concave shape and a higher specific volume when compared to bread made from medium amylose rice that had been blended with 20% active wheat gluten (Araki et al., 2016; Lee et al., 2001).
There was a significant positive correlation between amylose content and specific loaf volume. Interestingly, the amylose content was also positively correlated with bread hardness. In this study, we found that the specific volume of the enzyme –treated bread increased whereas the amylose content of the bread (on a dry basis of the whole loaf) was reduced from 14 to 9% by the enzyme treatments. Unlike breads enriched with active wheat gluten (Araki et al., 2016; Lee et al., 2001), in this experiment the GF bread was prepared without wheat gluten. Discrepancy in the results may be attributed to GF-bread formula in which the presence or absence of wheat appeared to be critical criteria.
Moreover, the potato powder enzyme treatments improved the rheological properties, such as hardness. The hardness values, which signified the onset of retrogradation, increased slowly in the rice/potato and wheat/potato breads during storage in a refrigerator for 4 days. In contrast, the hardness values of the rice/potato (control) and wheat/potato (control) breads treated with commercial enzyme-inactive potato powder from the market increased rapidly (Fig. 3). These results revealed that potato tuber D-enzyme modified the starch structure of rice, resulting in slower retrogradation of bread during storage.
Fig. 3.
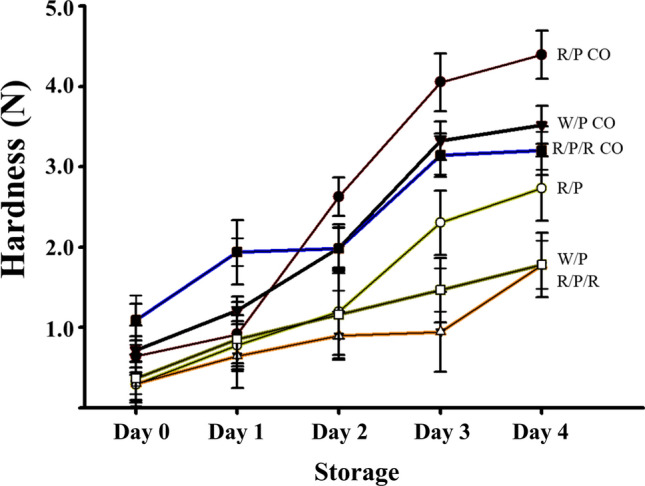
Hardness of the rice/potato/ramie bread during a 4-day storage
The specific loaf volume values obtained in these experiments were higher than those of previously reported rice breads of 1.9 cm3/g (Kim et al., 2015), 2.3 cm3/g (Masure et al., 2018), and 1.9 cm3/g for whole rice bread (Kadan et al., 2001).
Combined effects of potato tuber and ramie leaf enzymes on GF rice bread properties
To investigate the combined effects of potato tuber and ramie leaf enzymes containing D-enzyme and β-amylase, respectively, the properties of GF rice bread prepared with freeze-dried potato tuber and ramie leaf (rice/potato/ramie) were evaluated. The rice/potato/ramie bread had a high specific loaf volume of 3.7 cm3/g, which was higher than that of the corresponding control (3.0 cm3/g), but similar to that of rice/potato bread. The results indicated that the thermostable β-amylase of ramie leaf did not show any significant effect on the loaf volume increase of the GF bread. Notably, the rice/potato/ramie bread showed a smooth and round loaf top surface, without any significant structural collapse, while the loaf top surface of the rice/potato bread had a concave-like shape (Fig. 2). In general, substantial collapse of GF breads presents a problem that must be avoided during baking (Masure et al., 2018). The presence of ramie leaf powder appears to limit such collapses. This effect may be attributed to the fact that potato D-enzyme and ramie β-amylase not only reduce the amylose content but also modify the amylopectin architecture to promote the network formation and stabilization of gas cells, retaining the crumb structure. In addition, we postulate that phospholipids in ramie leaf may involve in gas cell stabilization (Kwon et al., 1994).
Moreover, the hardness of the rice/potato/ramie bread was substantially lower than that of the rice/potato bread during a 4-day storage period. Among all products, the combined potato tuber and ramie leaf powder treatment resulted in the lowest hardness value along with the wheat/potato bread during the 4-day storage period. The results clearly demonstrated that the combination of D-enzyme and β-amylase of potato tuber and ramie leaf, respectively, have a synergistic effect on starch retrogradation in the GF bread as an anti-staling agent. In addition, components such as phospholipids and fiber in ramie leaf powder might influence the rheological properties like hardness increase of bread (Kwon et al., 1994).
Structural changes of starches by reaction of potato tuber D-enzyme and ramie leaf β-amylase during GF rice bread making
Figure 4 presents the branch chain-length distribution of the GF bread compared to bread both with and without potato tuber enzymes. When the number of branch chains of the rice/potato and rice/potato control breads were compared, the proportion of short branch chains with DP < 10 in the rice/potato control bread was greater than that of the rice/potato bread, while the proportion of the intermediate branch chains with 19 > DP > 11 in the rice/potato bread was higher than the control. The proportion of long branch chains with DP > 19 in the rice/potato/ramie bread was lowest among all three samples. The results demonstrated that the thermostable β-amylase from ramie leaf was instrumental in shortening branch chains with DP > 19, thereby reducing the number of longer branch chains and producing shorter branch chains. Thus, the branch chain-length distribution clearly demonstrated that amylopectin architecture is modified not only by potato D-enzyme but also by ramie leaf β-amylase. Furthermore, the number of branch chains in the range of DP = 11–16 was increased in both the rice/potato and rice/potato/ramie breads. The results indicate that the high proportion of intermediate branch chains of amylopectin was generated by the combination reaction of D-enzyme and β-amylase. The bread made from rice with intermediate branch chain amylopectin yielded a high expansion with a moderate unwinding rate of double helices (Yano et al., 2017). Among high-amylose cultivars, those with a higher proportion of amylopectin long chains yield breads that were harder in texture than those made from cultivars with lower proportions of amylopectin long chains, regardless of the slight difference in amylose content (Aoki et al., 2012; Araki et al., 2016).
Fig. 4.
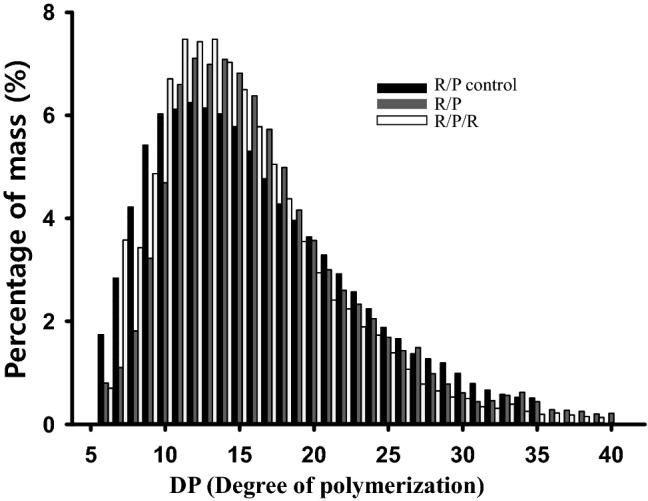
Branch chain-length distribution of the GF bread with/without potato tuber enzymes
Overall, the change in amylopectin architecture caused by β-amylase instigated a synergic anti-staling effect with D-enzyme. This observation supports the proposal that amylopectin recrystallization is effectively hindered as a consequence of the partial degradation of its side chains (Hug-Iten et al., 2003). Moreover, rice bread made from cultivars with a higher proportion of amylopectin long branch chains yielded a harder texture than those with lower proportions of amylopectin long branch chains (Aoki et al., 2012).
β-amylase often shows only a slight effect due to its low thermostability (Hug-Iten et al., 2003). However, ramie leaf β-amylase is highly thermostable up to 65–75 °C (He et al., 2017) and may be actively involved in shortening the branch chains of amylopectin around the gelatinization temperature of rice and corn starches. Hug-Iten et al. (2003) suggested that β-amylase with a high temperature optimum may represent a suitable anti-staling enzyme that can readily attack amylopectin molecules around the gelatinization temperature of starch. Thermostable ramie leaf β-amylase, which is active up to 70–75 °C, may contribute to modifying the amylopectin of rice starch (He et al., 2017). However, D-enzyme also catalyzes the intramolecular transfer reaction between branch chains, whereby the glucan moiety is transferred between branch chains within amylopectin molecules or undergoes cyclization. In the present study, the presence of cyclized amylopectin in GF rice bread was negligible. Therefore, intramolecular transfer may contribute to the rearrangement of amylopectin branch chains.
GF rice bread was developed using a mixture of potato tuber and ramie leaf powder containing D-enzyme and β-amylase, respectively. The combination of these two enzymes exerted a synergic effect on the modification of the bread starches. D-enzyme was instrumental in altering the amylose/amylopectin ratio via the transfer reaction, whereas β-amylase shortened the branch chain of amylopectin. The enzymatically modified GF rice bread exhibited excellent anti-staling characteristics during storage, demonstrating that the structural changes of rice starch can inhibit the retrogradation of bread starch. The results suggest that the physicochemical properties of GF rice bread depend on the amylose content and the branch chain-length distribution of amylopectin. Consequently, combined enzyme treatment is instrumental in the modification of starch, whereby each enzyme preferentially targets amylose and amylopectin molecules, respectively. Furthermore, enzymes of plant origin can be used widely because they are safe and even beneficial for human health. Future studies should further optimize the combined enzyme treatment process in terms temperature and time, dough formula, and enzyme concentrations.
Declarations
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Thi Kim Le Loan, Email: lethikimloan@tgu.edu.vn.
Nguyen Minh Thuy, Email: nmthuy@ctu.edu.vn.
Quang Le Tri, Email: lequantri_1973@yahoo.com.
Park Sunghoon, Email: parksh@spc.co.kr.
References
- Abdel-Aal ESM. Functionality of starches and hydrocolloids in gluten-free foods. In: Gallagher E, editor. Gluten-Free Food Science and Technology. Oxford, UK: Wiley-Blackwell; 2009. pp. 200–224. [Google Scholar]
- Akoumianakis KA, Alexios Alexopoulos, Karapanos IC, Kalatzopoulos K, Aivalakis G, Passam HC. Carbohydrate metabolism and tissue differentiation during potato tuber initiation, growth and dormancy induction. Australian Journal of Crop Science. 2016;10:185–192. [Google Scholar]
- American Association of Clinical Chemistry method 74–09 (AACC, 2000)
- Aoki N, Umemoto T, Hamada S, Suzuki K, Suzuki Y. The Amylose content and amylopectin structure affect the shape and hardness of rice bread. Journal of Applied Glycoscience. 2012;59:75–82. doi: 10.5458/jag.jag.JAG-2011_013. [DOI] [Google Scholar]
- Aoki N, Kataoka T, Nishiba Y. Crucial role of amylose in the rising of gluten- and additive-free rice bread. Journal of Cereal Science. 2020;92:102905. doi: 10.1016/j.jcs.2019.102905. [DOI] [Google Scholar]
- Araki E, Ashida K, Aoki N, Takahashi M, Hamada S. Characteristics of rice flour suitable for the production of rice flour bread containing gluten and methods of reducing the cost of producing rice flour. Japan Agricultural Research Quarterly. 2016;50:23–31. doi: 10.6090/jarq.50.23. [DOI] [Google Scholar]
- Buwalda PL, Melenhorst VHJ, Mastenbroek J. Starch composition and method to produce a baked product. US patent, 8697170B2 (2014)
- Dicko M, Searle-van Leeuwen M, Beldman G, Ouedraogo O, Hilhorst R, Traore A. Purification and characterization of β-amylase from Curculigo Pilosa. Applied Microbiology and Biotechnology. 1999;52:802–805. doi: 10.1007/s002530051595. [DOI] [Google Scholar]
- Furlán LTR, Padilla AP, Campderrós ME. Improvement of gluten-free bread properties by the incorporation of bovine plasma proteins and different saccharides into the matrix. Food Chemistry. 2015;170:257–264. doi: 10.1016/j.foodchem.2014.08.033. [DOI] [PubMed] [Google Scholar]
- Gallagher E, Polenghi O, Gormley TR. Novel rice starches in gluten-free bread. In Proceedings International Association for Cereal Science and Technology Conference., Budapest, Hungary, 26–29 May 2002
- He L, Park SH, Nguyen DHD, Duong HX, Duong THC, Tran PL, Park JT, Ni L, Park KH. Characterization and thermal inactivation kinetics of highly thermostable ramie leaf β-amylase. Enzyme and Microbial Technology. 2017;101:17–23. doi: 10.1016/j.enzmictec.2017.02.011. [DOI] [PubMed] [Google Scholar]
- Horstmann S, Belz M, Heitmann M, Zannini E, Arendt E. Fundamental study on the impact of gluten-free starches on the quality of gluten-free model breads. Foods. 2016;5:30. doi: 10.3390/foods5020030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horstmann SW, Lynch KM, Arendt EK. Starch characteristics linked to gluten-free products. Foods. 2017;6:29. doi: 10.3390/foods6040029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hug-Iten S, Escher F, Conde-Petit B. Staling of bread: role of amylose and amylopectin and influence of starch-degrading enzymes. Cereal Chemistry. 2003;80:654–661. doi: 10.1094/CCHEM.2003.80.6.654. [DOI] [Google Scholar]
- Kadan RS, Robinson MG, Thibodeaux DP, Pepperman AB., Jr Texture and other physicochemical properties of whole rice bread. Journal of Food Science. 2001;66:940–944. doi: 10.1111/j.1365-2621.2001.tb08216.x. [DOI] [Google Scholar]
- Kim YL, Mun S, Park KH, Shim JY, Kim YR. Physicochemical functionality of 4-α-glucanotransferase-treated rice flour in food application. International Journal of Biological Macromolecules. 2013;60:422–426. doi: 10.1016/j.ijbiomac.2013.04.032. [DOI] [PubMed] [Google Scholar]
- Kim MS, Yun YK, Jeong YH. Effects of corn, potato, and tapioca starches on the quality of gluten-free rice bread. Food Science and Biotechnology. 2015;24:913–919. doi: 10.1007/s10068-015-0118-8. [DOI] [Google Scholar]
- Kwon MR, Park CS, Auh JH, Cho BM, Yang NS, Park KH. Phospholipid hydrolysate and antistaling amylase effects on retrogradation of starch in bread. Journal of Food Science. 1994;59:1072–1076. doi: 10.1111/j.1365-2621.1994.tb08193.x. [DOI] [Google Scholar]
- Lee MR, Swanson BG, Baik BK. Influence of amylose content on properties of wheat starch and breadmaking quality of starch and gluten blends. Cereal Chemistry. 2001;78:701–706. doi: 10.1094/CCHEM.2001.78.6.701. [DOI] [Google Scholar]
- Masure HG, Fierens E, Delcour JA. Current and forward looking experimental approaches in gluten-free bread making research. Journal of Cereal Science. 2016;67:92–111. doi: 10.1016/j.jcs.2015.09.009. [DOI] [Google Scholar]
- Masure HG, Wouters AGB, Fierens E, Delcour JA. Electrical resistance oven baking as a tool to study crumb structure formation in gluten-free bread. Food Research International. 2018;63:55–61. doi: 10.1016/j.foodres.2018.09.029. [DOI] [PubMed] [Google Scholar]
- Nguyen DHD, Tran PL, Li D, Han JA, Hwang JY, Hong WS, Lee JS, Kim YR, Yoo SH, Park JT, Choi YJ, Lee SY, Park KH. Modification of rice grain starch for lump-free cooked rice using thermostable disproportionating enzymes. Food Research International. 2014;63:55–61. doi: 10.1016/j.foodres.2014.04.007. [DOI] [Google Scholar]
- Nguyen DHD, Tran PL, Ha HS, Lee JS, Hong WS, Le QT, Oh BC, Park SH. Characterization and utilization of ramie leaf β-amylase as an anti-staling additive for starch-based foods. Food Science and Biotechnology. 2015;24:37–40. doi: 10.1007/s10068-015-0006-2. [DOI] [Google Scholar]
- Park JH, Kim HJ, Kim YH, Cha H, Kim YW, Kim TJ, Kim YR, Park KH. The action mode Thermus aquaticus YT-1 4-alphaglucanotransferase and its chimeric enzymes introduced with starch-binding domain on amylose and amylopectin. Carbohydrate Polymers. 2007;67:164–173. doi: 10.1016/j.carbpol.2006.05.018. [DOI] [Google Scholar]
- Park JH, Park KH, Jane JL. Physicochemical properties of enzymatically modified maize starch using 4-alpha-glucanotransferase. Food Science and Biotechnology. 2007;16:902–909. [Google Scholar]
- Park SH, Na YR, Kim JW, Kang SD, Park KH. Properties and applications of starch modifying enzymes for use in the baking industry. Food Science and Biotechnology. 2018;27:299–312. doi: 10.1007/s10068-017-0261-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renzetti S, Rosell CM. Role of enzymes in improving the functionality of proteins in non-wheat dough systems. Journal of Cereal Science. 2016;67:35–45. doi: 10.1016/j.jcs.2015.09.008. [DOI] [Google Scholar]
- Sergeeva LI, Claassens MMJ, Jamar DCL, Van Der Plas LHW, Vreugdenhil D. Starch-related enzymes during potato tuber dormancy and sprouting. Russian Journal of Plant Physiology. 2012;56:556–564. doi: 10.1134/S1021443712040115. [DOI] [Google Scholar]
- Tandazo SA. Rheological properties of gluten free dough systems. https//docs.lib.purdue.edu/open-access-theses/104 (2013)
- Takaha T, Yanase M, Okada S, Smith SM. Disproportionating enzyme (4-α-Glucanotransferase; EC 2.4.1.25) of potato: Purification, molecular cloning, and potential role in starch metabolism. Journal of Biological Chemistry. 1993;268:1391–1396. doi: 10.1016/S0021-9258(18)54088-6. [DOI] [PubMed] [Google Scholar]
- Van der Maarel MJEC, Leemhuis H. Starch modification with microbial alpha-glucanotransferase enzymes. Carbohydrate Polymers. 2013;93:116–121. doi: 10.1016/j.carbpol.2012.01.065. [DOI] [PubMed] [Google Scholar]
- Wang K, Lu F, Li Z, Zhad L, Han C. Recent developments in gluten-free bread baking approaches: a review. Food Science and Technology. Campinas. 2017;37(Suppl. 1):1–9. [Google Scholar]
- Yano H, Koda T, Miyata T, Nishio T, Fujita N, Nishioka A. Effect of molecular architecture of rice starch on bread qualities and rheological properties of pure rice bread. The Journal of the Society of Rheology Japan. 2017;45:33–37. [Google Scholar]