Abstract
Aging is associated with declines in cognitive performance, which are mediated in part by neuroinflammation, characterized by astrocyte activation and higher levels of pro-inflammatory cytokines; however, the upstream drivers are unknown. We investigated the potential role of the gut microbiome–derived metabolite trimethylamine N-oxide (TMAO) in modulating neuroinflammation and cognitive function with aging. Study 1: In middle-aged and older humans (65 ± 7 years), plasma TMAO levels were inversely related to performance on NIH Toolbox Cognition Battery tests of memory and fluid cognition (both r2 = 0.07, p < 0.05). Study 2: In mice, TMAO concentrations in plasma and the brain increased in parallel with aging (r2 = 0.60), suggesting TMAO crosses the blood-brain barrier. The greater TMAO concentrations in old mice (27 months) were associated with higher brain pro-inflammatory cytokines and markers of astrocyte activation vs. young adult mice (6 months). Study 3: To determine if TMAO independently induces an “aging-like” decline in cognitive function, young mice (6 months) were supplemented with TMAO in chow for 6 months. Compared with controls, TMAO-supplemented mice performed worse on the novel object recognition test, indicating impaired memory and learning, and had increased neuroinflammation and markers of astrocyte activation. Study 4: Human astrocytes cultured with TMAO vs. control media exhibited changes in cellular morphology and protein markers consistent with astrocyte activation, indicating TMAO directly acts on these cells. Our results provide translational insight into a novel pathway that modulates neuroinflammation and cognitive function with aging, and suggest that TMAO might be a promising target for prevention of neuroinflammation and cognitive decline with aging.
Electronic supplementary material
The online version of this article (10.1007/s11357-020-00257-2) contains supplementary material, which is available to authorized users.
Keywords: Cognitive impairment, Inflammation, Astrocyte activation, Brain, Memory
Introduction
Aging is associated with a gradual decline in cognitive function [29], primarily in the domains of memory and learning, attention/processing speed, and executive function [45]. Some declines in these cognitive domains are expected (sometimes referred to as “normal cognitive aging”) [29] but, nevertheless, may reduce quality of life and directly increase risk of chronic neurodegenerative disorders, including Alzheimer’s disease [32]. The mechanisms mediating cognitive decline with aging are multifactorial and incompletely understood, but systemic inflammation, which manifests in the brain as neuroinflammation, appears to play a key role in cognitive aging by impairing neuronal function [46, 49]. One important cause of neuroinflammation with normal cognitive aging is activation of glial cells (e.g., astrocytes and microglia) [12]. In particular, astrocytes, which can assume a toxic “reactive” state in response to immune-related stressors, are increasingly recognized as key drivers of brain aging and disease ([14, 28]; Supplementary Reference 1). However, the upstream drivers of these processes have not been elucidated.
Increased circulating levels of trimethylamine N-oxide (TMAO), a gut microbiome–derived metabolite, represent a novel potential upstream driver of systemic inflammation and neuroinflammation with aging. TMAO is produced through gut microbe–dependent conversion of ingested precursors (e.g., choline and l-carnitine) into trimethylamine (TMA), which is subsequently absorbed into the circulation and converted to TMAO by hepatic flavin monooxygenase 3 (FMO3) [58]. Once in the circulation, TMAO has been reported to activate pro-inflammatory signaling pathways [10, 48]. Indeed, increased plasma TMAO has been linked to several inflammatory disorders, including cardiovascular disease, in both mouse models and humans [36, 53, 54, 58]. We and others have reported that circulating concentrations of TMAO increase with aging in mice [9, 35] and humans [8, 59]. Furthermore, TMAO is detectible in cerebrospinal fluid from patients with dementia [16] and has been associated with cognitive decline in Alzheimer’s disease patients [62].
Using a combination of translational research approaches in cells, mice, and healthy human subjects, we investigated the potential modulatory effects of TMAO on cognitive aging, neuroinflammation, and astrocyte activation. First, in healthy middle-aged to older (MA/O) adults, we observed that plasma TMAO concentrations are inversely related to cognitive function, particularly memory. Second, we showed that plasma and brain concentrations of TMAO increase in parallel with aging in mice, along with neuroinflammation, suggesting that TMAO can cross the blood-brain barrier to directly access and potentially influence brain function. Third, we demonstrated that chronic dietary supplementation with TMAO induced an “aging-like” impairment in spatial memory and learning in young mice that was associated with neuroinflammation. Lastly, we showed that TMAO directly activates astrocytes, causing them to take on a reactive, pro-inflammatory phenotype. Collectively, our findings support the hypothesis that higher circulating and brain concentrations of TMAO with aging induce astrocyte activation and neuroinflammation associated with declines in cognitive function.
Methods
Assessment of cognitive function in middle-aged to older adults
All procedures involving human subjects were approved by the Institutional Review Board at the University of Colorado Boulder (protocol nos. B5094, B6310, B6398, B6545, B6549, 15-0402, 15-0518, and 16-0633) and were conducted in accordance with the Declaration of Helsinki. All subjects provided oral and written informed consent prior to participation in previously conducted studies by our laboratory. Subjects were aged 18–27 years (young reference group; N = 22) or 50–79 years (MA/O adults; N = 103), healthy (free from overt clinical disease) based on medical history and physical examination, and sedentary to recreationally active. MA/O adults had undergone incremental treadmill testing with ECG and blood pressure to ensure the absence of overt heart disease. Physical activity was estimated using both the modifiable activity questionnaire (MAQ; estimates MET-hours per week of leisure-based physical activity) [37] and the Community Healthy Activities Model Program for Seniors (CHAMPS) questionnaire (estimates energy expenditure in kcal due to exercise-related activity) [51]. Maximal oxygen consumption (VO2max) was determined during incremental treadmill exercise to exhaustion (Balke protocol) via open-circuit spirometry [19] on a separate day and used as a measure of maximal aerobic exercise capacity (cardiorespiratory fitness). Body mass and height were determined by anthropometry, and body mass index was calculated.
All subjects were studied ~ 2 h after their last meal and following > 20 h without alcohol or vigorous physical activity; caffeine consumption comparable to subjects’ habitual intake was allowed to avoid effects of caffeine withdrawal. Cognitive function was assessed with the NIH Toolbox Cognition Battery Test [60], which includes seven standardized computerized tests that assess five major subdomains of cognitive function, including working memory (List Sorting Test), episodic memory (Picture Sequence Memory Test), processing speed (Pattern Comparison Processing Speed Test), executive function (Flanker Inhibitory Control and Attention Test; Dimensional Change Card Sort Test), and language (Picture Vocabulary Test; Oral Reading Recognition Test). The Fluid Cognition Composite Score is a combination of the NIH Toolbox tests assessing working memory, episodic memory, attention, processing speed, and executive function. The Crystallized Cognition Composite Score is a combination of the tests assessing language. The Total Cognitive Function Score, a measure of general cognitive function, is a composite score of all tests. Fluid cognition is primarily dependent on dynamic biological processes and declines with advancing age [29]. In contrast, crystallized cognition is influenced primarily by knowledge acquired through the lifetime and is expected to either not change with age or increase slightly [29]. For each test, the NIH Toolbox Cognition Battery Test provides a raw score (composite scores are standardized to a normative mean of 100 with standard deviation of 15), an age-adjusted percentile score (relative to all people in the USA within each age group [binned by year] enrolled in the Toolbox National Norming Study) [4], and a fully corrected T-score (adjusted for age, sex, race, ethnicity, and highest level of education). In addition to the NIH Toolbox Cognition Battery Test, the Trail Making Test parts A and B were administered to determine processing speed and executive function, respectively [56].
On a separate day (within 2 weeks of cognitive function testing), following an 8–12-h overnight fast and > 20 h without alcohol, caffeine, or vigorous physical activity, blood pressure was assessed in triplicate over the brachial artery during seated rest using a semi-automated device (Dinamap XL; Johnson & Johnson, New Brunswick, NJ) and venous blood was collected. Blood was analyzed for lipid, lipoprotein, and glucose concentrations in a CLIA-certified laboratory (Boulder Community Hospital Clinical Laboratory, Boulder, CO), and additional aliquots were stored for later analysis of TMAO and related metabolites.
Animals
All animal procedures were approved by the University of Colorado Boulder Institutional Committee for the Care and Use of Animals and complied with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. A total of 50 male C57BL/6N mice were obtained for this study from Charles River at 8 weeks of age (N = 34) or from the National Institute on Aging colony maintained by Charles River at 20–24 months of age (N = 16). Of the young mice, 10 were studied to assess effects of aging on TMAO levels and neuroinflammation (data obtained from N = 9), and 24 were used for the TMAO supplementation intervention (see below; data obtained from N = 20). Mice were housed in a conventional facility on a 12-h light/dark cycle, given ad libitum access to rodent chow and drinking water, and allowed to acclimate to these conditions for at least 4 weeks prior to the start of experiments. Mice were single-housed to prevent mouse-to-mouse effects on the microbiome. These mice were part of a larger study of the role of TMAO on cardiovascular aging (see [8]). As such, only male mice were used as C57Bl/6 male, but not female, mice are an established model of cardiovascular aging in humans [21, 39, 50]. Importantly, we do not feel this is a limitation for the present work because (1) male C57Bl/6 mice are also a well-established model of cognitive/brain aging and neuroinflammation [2, 20] and (2) we observed no sex differences in concentrations of TMAO nor effects of TMAO on cognitive function in our human cohort.
All mice were sacrificed by exsanguination while maintained under anesthesia (inhaled isoflurane). Heparinized blood collected by cardiac venipuncture was separated by centrifugation, and plasma was stored at − 80 °C until analysis for TMAO and related metabolites by LC-MS, as described above. Whole brains were collected and flash-frozen until analysis.
Chronic TMAO supplementation and assessment of cognitive function in young adult mice
Beginning at 6 months of age, mice were fed a defined low-choline diet (0.07%; to control for precursors of TMAO, but sufficient to avoid choline deficiency) that was either not supplemented (control) or supplemented with 0.12% TMAO [58, 66] (customized diets from Envigo, Madison, WI; TMAO, Sigma-Aldrich Corp.) for 6 months, and sacrificed at 12 months of age. All other macronutrients and micronutrients were consistent with standard rodent chow, and there were no differences in food intake across groups (see Table 4).
Table 4.
Animal characteristics and concentrations of precursors of TMAO in Control and TMAO-supplemented mice
| Control | TMAO | |
|---|---|---|
| Body mass (g) | 40 ± 2 | 42 ± 2 |
| Food intake (g/day) | 3.7 ± 0.1 | 3.8 ± 0.1 |
| Mass of key organs | ||
| Brain mass (mg) | 458 ± 9 | 456 ± 7 |
| Heart mass (mg) | 158 ± 8 | 160 ± 4 |
| Liver mass (g) | 1.89 ± 0.14 | 2.09 ± 0.14 |
| Kidney mass (mg) | 206 ± 10 | 216 ± 7 |
| Visceral fat mass (g) | 2.16 ± 0.23 | 2.15 ± 0.19 |
| Plasma concentrations of TMAO precursors | ||
| Choline (μM) | 25 ± 3 | 28 ± 4 |
| Betaine (μM) | 11.9 ± 1.2 | 13.6 ± 1.7 |
| l-Carnitine (μM) | 23.8 ± 5.9 | 19.7 ± 2.9 |
| γ-Butyrobetaine (μM) | 1.0 ± 0.2 | 1.0 ± 0.2 |
| Brain concentrations of TMAO precursors | ||
| Choline (μM) | 129 ± 10 | 120 ± 6 |
| Betaine (μM) | 5.6 ± 0.6 | 6.4 ± 0.8 |
| l-Carnitine (μM) | 18.2 ± 1.9 | 17.1 ± 1.8 |
| γ-Butyrobetaine (μM) | 0.9 ± 0.3 | 1.3 ± 0.2 |
Data are mean ± SEM. N = 8–11/group
TMAO trimethylamine N-oxide
Cognitive function
Spatial memory and learning were assessed using the novel object recognition test [3, 27]. Testing was conducted over 4 consecutive days at the same time of day (9AM to 11AM) in a subsection of the vivarium in which animals were housed. All tests were performed by one investigator (V.E.B.). Once mice were placed in the arena and the test commenced, the investigator left the room until the end of each test. A custom-built open field apparatus was used for all tests. The apparatus consisted of two side-by-side arenas (40 cm long × 40 cm wide × 30 cm high each) with white-matte finish, an overhead diffuse lighting source, and a top-mounted video recorder for offline multi-arena video tracking (EthoVision XT; Noldus Information Technology, Leesburg, VA). The experimental apparatus and all objects placed in the arena were cleaned with 70% ethanol between testing each mouse.
On day 1, mice completed a 5-min open field test [34] that served to both measure voluntary locomotion and habituate mice to the arena (no objects present). Locomotion was quantified as total distance traveled during the 5-min period.
On days 2 and 3, two identical objects (either two stoppers or two small Erlenmeyer flasks) were placed 3 cm from the arena walls in adjacent quadrants of the arena. The objects and which quadrants the objects were placed in were counterbalanced across mice and kept consistent for each mouse across both days. Mice were given 10 min per day to explore the objects.
On day 4, one of the familiar objects was replaced (same location) with a novel object (a yellow octopus action figure). Mice were given 5 min to explore the objects. Interaction with each object was defined as nose pointed in the direction of the object within 2 cm of the object. Incidental contact with the object from the tail or body while vertically rearing or grooming was not considered an interaction. The number of interactions with each object and the duration of time spent interacting with each object were quantified. In addition, the latency (duration of time in seconds) until the first interaction with each object was recorded.
Assessment of TMAO and related metabolites in humans and mice
TMAO and its precursors, TMA, choline, betaine, and l-carnitine, were quantified in plasma from human subjects (treated with EDTA) and mice (heparinized) and in whole-brain lysates from mice by isocratic ultra-performance liquid chromatography tandem mass spectrometry using a stable isotope dilution method against internal standards [6, 7, 59].
Brain inflammatory cytokines and nuclear factor kappa B p65
Concentrations of the inflammatory cytokines interleukin (IL)-1β, IL-6, tumor necrosis factor (TNF)-α, and IL-10 were assessed in aliquots of whole-brain lysate containing 40 μg of protein using a commercially available multiplex ELISA kit (Ciraplex® Mouse Cytokine Array 1; Aushon BioSystems, Inc., Billerica, MA) according to the manufacturer’s instructions. Protein abundance of phosphorylated and total nuclear factor kappa B (NF-κB) (p65 subunit) were determined in whole-brain lysates using capillary electrophoresis Western detection with use of a WES instrument (ProteinSimple, San Jose, CA). Aliquots of whole-brain lysate containing 0.20 μg/μl of protein were separated by capillary electrophoresis. Electrophoretic separation and immunodetection were performed automatically using the default settings, except the primary antibody incubation phase was extended to 150 min. Samples, blocking reagent, wash buffer, primary antibodies, secondary antibodies, and chemiluminescent substrate were aliquoted by the instrument. Resulting data were analyzed with the installed Compass software (ProteinSimple). Primary antibodies used were anti-phosphorylated NF-κB p65 at Ser536 (1:300; Cell Signaling Technology, Danvers, MA; Cat # 3036S), anti-total NF-κB p65 (1:100, Cell Signaling Technology, Danvers, MA; Cat # C22B4), and anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (1:200, Cell Signaling Technology, Cat # 14C10). Relative intensity was normalized against intensity of GAPDH signal in lysates. Electropherograms in figures are represented as pseudo-blots generated using Compass software.
Markers of astrocyte activation in mouse brains
Protein abundance of lipocalin 2 (LCN2) was determined in whole-brain lysates by traditional Western immunoblotting. Briefly, 20 μg of protein was loaded onto SDS gels, separated by electrophoresis, and then transferred onto nitrocellulose membranes that were incubated for ≥ 1 h in blocking buffer (Tris-buffered saline with Tween, TBS-T, with 5% w/v dry milk powder). Membranes were incubated at 4 °C overnight with primary antibodies for either LCN2 (1:1000; Abcam, Cambridge, UK; Cat # ab63929) or GAPDH (1:1000, Cell Signaling Technology, Cat # 14C10), washed 3 times with TBS-T, incubated with the appropriate horseradish peroxidase–conjugated secondary antibody, and washed 3 times with TBS-T. Target proteins were then detected by chemiluminescent imaging after incubation in ECL Western Blotting Substrate (Thermo Fisher Scientific, Inc., Waltham, MA). Membranes were stripped with Restore Stripping Buffer (Thermo Fisher Scientific) between probing for LCN2 and that for GADPH. LCN2 abundance of each sample was measured as relative intensity normalized against the GAPDH intensity.
Human astrocyte cell culture
Primary human astrocytes were obtained from Lonza (Basel, Switzerland) and grown on poly-l-lysine-coated plates in astrocyte-specific medium (ScienCell Research Laboratories, Carlsbad, CA) with 10% FBS and penicillin-streptomycin in a humidified incubator at 37 °C and 5% CO2. All experiments were performed on passage 2–3 cells. Treatments were applied by adding fresh medium containing reagents, and cells were harvested at 90% confluency. All experiments were repeated 3–5 times. For immunofluorescence analyses, medium was removed and cells were washed once in DPBS then fixed in 4% paraformaldehyde, washed and permeabilized in 0.25% Triton X, and then blocked in 3% fetal bovine serum and 3% normal goat serum. Primary antibodies for either CD44 (1:500; Novus Biologicals, Littleton, CO; Cat # 47386) or LCN2 (1:100; Abcam, Cat # ab63929) in 5% normal goat serum were added, and samples were incubated at 4 °C overnight. Cells were next washed in DPBS with 0.1% Tween, and then incubated with secondary antibodies (1:1000 dilutions in 5% normal goat serum) for 1 h. DAPI was added at 1 μg/ml in DPBS, and cells were washed again before mounting with ProLong mounting medium for imaging on a Zeiss Axioskop fluorescence microscope.
Statistical analyses
Data presented in human subjects are mean ± SD, as group sample sizes differ between young and MA/O adults and demographic data are provided. Data presented in mice and cells are mean ± SEM to facilitate comparison across groups. Statistical significance was set to a two-sided α value of 0.05.
Young vs. MA/O subject comparisons in humans
Differences in the following variables between young and MA/O adults were compared using Student’s unpaired t test: plasma concentrations of TMAO and related metabolites, subject characteristics, and all scores on tests of cognitive function.
Human cognitive function and plasma TMAO linear regression analysis
Data from 77 MA/O subjects were used for regression analyses. Due to skewness, plasma TMAO values were naturally log-transformed (Shapiro-Wilk test: p < 0.0001). First, we performed simple linear (unadjusted) regression between Ln plasma TMAO and T-scores for each test of cognitive function. T-scores were used to reduce variability related to the level of educational attainment; age and sex are also adjusted for in these scores. Next, T-scores for the 3 tests of cognitive function that were significantly related to TMAO (working memory, episodic memory, and fluid cognition) were regressed onto Ln plasma TMAO with (1) the following covariates added to the model in order to adjust for markers of physical fitness: VO2max, MET-hours per week related to leisure activity (estimated by MAQ), and kcal per week energy expenditure related to physical activity (estimated by CHAMPS questionnaire); (2) the following covariates added to the model in order to adjust for traditional risk factors for chronic disease: body mass index, systolic blood pressure (SBP), serum total cholesterol and serum LDL cholesterol, and fasted blood glucose; and (3) all physical activity and risk factor–related covariates included. p values for the main effect of TMAO in each model were calculated. To determine the independent effect of TMAO after accounting for all covariates, we determined multivariable partial relations (partial r2 of TMAO) by calculating the correlation between the residuals of TMAO and the residuals of each of the 3 tests of cognitive function in the multiple regression.
Effects of aging and TMAO supplementation in mice
Differences in the following variables between young vs. old mice and control vs. TMAO-supplemented mice were compared using Student’s unpaired t test: body mass, food intake, mass of key organs, plasma and brain concentrations of TMAO and related metabolites, parameters on the novel object recognition test, locomotion, brain concentrations/abundance of inflammatory cytokines, phosphorylated and total NF-κB, and LCN2. The correlation between plasma and brain concentrations of TMAO in young and old mice was determined using simple linear regression.
Cultured human astrocytes
Differences in LCN2 and CD44 were determined between TMAO-treated and control cells using Student’s unpaired t test.
Results
Plasma TMAO is inversely related to cognitive function in middle-aged to older adults
Plasma TMAO
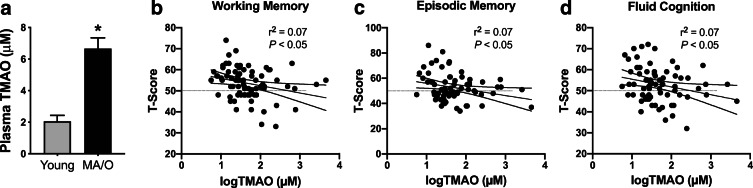
Fasted plasma concentrations of TMAO and related metabolites were measured in N = 103 MA/O adults (aged 64 ± 7 years [range 50–79]; 44 M/59 F) and a reference group of N = 22 young adults (aged 22 ± 2 years [range 20–27]; 11 M/11 F) and previously reported elsewhere [8]. All subjects were sedentary to recreationally active and healthy (no overt clinical disease). Plasma concentrations of TMAO were higher in MA/O vs. young adults (p < 0.01; Fig. 1a). MA/O adults also had higher plasma concentrations of the TMAO precursor choline (MA/O: 13.9 ± 6.1 vs. young: 7.9 ± 3.2 μM, p < 0.0001), but plasma concentrations of other TMAO precursors like betaine (MA/O: 20.9 ± 12.0 vs. young: 19.3 ± 11.0, p = 0.58) and l-carnitine (MA/O: 57 ± 31 vs. young: 47 ± 15, p = 0.14) were not different between age groups. Within MA/O adults, there was no significant difference in plasma TMAO concentrations between men (7.4 ± 7.4 μM) and women (6.2 ± 6.6 μM) (p = 0.39). Men had higher plasma l-carnitine levels than women (64.6 ± 5.5 vs. 51.9 ± 3.4 μM, p < 0.05), but there were no other sex differences in plasma levels of TMAO precursors.
Fig. 1.
Plasma TMAO is higher in middle-aged to older (MA/O) adults and associated with lower memory and fluid cognition. a Plasma concentrations of TMAO in young (20–27 years; N = 22) and MA/O (50–79 years; N = 103) adults. Data are mean ± standard deviation (SD). *p < 0.05 vs. young adults. b–d Within MA/O adults (N = 77), plasma concentrations of TMAO (naturally log-transformed due skewness) were inversely and independently related to performance on National Institutes of Health Toolbox Cognition Battery assessments of working memory (b), episodic memory (c), and fluid cognition (d; composite of working and episodic memory, attention, processing speed, and executive function). Results of unadjusted linear regression models are shown with 95% confidence intervals
Cognitive function
In a subset of subjects, cognitive function was measured using the NIH Toolbox Cognition Battery Test and the Trail Making Test (N = 77 MA/O adults and N = 14 young adults). Subject characteristics are provided in Table 1. Compared with young adults, MA/O adults had higher blood pressure (both systolic and diastolic, p < 0.05), higher serum total cholesterol (p < 0.0001), higher fasted blood glucose (p < 0.05), lower cardiorespiratory fitness (VO2max; p < 0.0001), and lower physical activity, as estimated by the CHAMPS questionnaire (p < 0.05). There were no other differences across age groups.
Table 1.
Subject characteristics for subjects included in cognitive function analyses
| Young | MA/O | |
|---|---|---|
| N | 14 | 77 |
| Age (years) | 22 ± 3 | 65 ± 7* |
| Male/female | 7/7 | 28/49 |
| Body mass (kg) | 70 ± 12 | 68 ± 13 |
| Height (cm) | 171 ± 10 | 170 ± 9 |
| Body mass index (kg/m2) | 24 ± 3 | 23 ± 3 |
| Systolic BP (mmHg) | 111 ± 12 | 121 ± 16* |
| Diastolic BP (mmHg) | 63 ± 9 | 72 ± 9* |
| Heart rate (beats/min) | 61 ± 10 | 58 ± 8 |
| Clinical laboratory blood tests | ||
| Total cholesterol | 146 ± 23 | 181 ± 29* |
| LDL cholesterol (mg/dl) | 73 ± 19 | 105 ± 26 |
| HDL cholesterol (mg/dl) | 54 ± 12 | 58 ± 16 |
| Triglycerides (mg/dl) | 75 ± 23 | 87 ± 44 |
| Fasted glucose (mg/dl) | 82 ± 5 | 86 ± 7* |
| Physical activity | ||
| VO2max (ml/min/kg) | 46 ± 10 | 31 ± 6* |
| MAQ leisure activity (MET-hours/week) | 63 ± 33 | 48 ± 32 |
| CHAMPS energy expenditure (kcal/week) | 9559 ± 7577 | 5556 ± 3712* |
Data are mean ± SD
*p < 0.05 vs. young
Average raw scores, age-adjusted percentiles, and fully adjusted T-scores (adjusted for age, sex, race, ethnicity, and highest level of education) for each test of cognitive function in young and MA/O adults are provided in Table 2. Young and MA/O adults scored slightly above average but similarly within their respective normative age-specific ranges of performance—group average percentile scores ranged from 52 to 92%. MA/O adults scored higher for their age (percentile) on the test of working memory compared with young adults (p < 0.05) and had slightly lower fully adjusted Fluid Cognition Composite scores (p < 0.05), but there were no significant differences between young and MA/O adults in percentiles or T-scores for any other tests.
Table 2.
Cognitive function in young and middle-aged to older (MA/O) adults
| Subdomain of cognitive function and test | Raw (unadjusted) score | Age-adjusted percentile | Fully adjusted T-score | Relation to TMAO in MA/O adults | ||||
|---|---|---|---|---|---|---|---|---|
| Young | MA/O | Young | MA/O | Young | MA/O | r2 | p value | |
| Working memory | ||||||||
| NIHTBList Sorting | 19.4 ± 1.9 | 17.1 ± 2.3* | 54 ± 26 | 70 ± 22* | 50 ± 8 | 54 ± 7 | 0.07 | 0.02* |
| Episodic memory | ||||||||
| NIHTBPicture Sequence Memory | 638 ± 92 | 480 ± 85* | 72 ± 30 | 61 ± 28 | 59 ± 12 | 53 ± 11 | 0.07 | 0.03* |
| Processing speed | ||||||||
| NIHTBPattern Comparison | 128 ± 16 | 102 ± 12* | 77 ± 25 | 63 ± 30 | 61 ± 11 | 53 ± 11 | < 0.01 | 0.55 |
| TTrail Making Test part A (s) | 17 ± 4 | 28 ± 7* | - | - | - | - | < 0.01 | 0.93 |
| Executive function | ||||||||
| NIHTBFlanker Inhibitory Control and Attention | 9.1 ± 0.7 | 8.2 ± 0.5* | 52 ± 36 | 54 ± 21 | 51 ± 14 | 48 ± 6 | < 0.01 | 0.44 |
| NIHTBDimensional Change Card Sort | 9.2 ± 0.9 | 8.0 ± 0.7* | 68 ± 36 | 71 ± 20 | 58 ± 12 | 54 ± 8 | < 0.01 | 0.92 |
| TTrail Making Test part B (s) | 41 ± 15 | 55 ± 17* | - | - | - | - | < 0.01 | 0.93 |
| Language | ||||||||
| NIHTBPicture Vocabulary | 110 ± 9 | 129 ± 12* | 78 ± 17 | 86 ± 19 | 56 ± 7 | 62 ± 11 | < 0.01 | 0.73 |
| NIHTBOral Reading Recognition | 114 ± 6 | 128 ± 11* | 87 ± 17 | 88 ± 15 | 65 ± 7 | 61 ± 8 | < 0.01 | 0.96 |
| Cognitive function composite scores | ||||||||
| NIHTBFluid | 121 ± 11 | 100 ± 9* | 69 ± 28 | 68 ± 23 | 59 ± 13 | 53 ± 8* | 0.07 | 0.03* |
| NIHTBCrystallized | 111 ± 5 | 131 ± 12* | 87 ± 11 | 92 ± 12 | 61 ± 5 | 65 ± 10 | < 0.01 | 0.50 |
| NIHTBTotal | 118 ± 6 | 120 ± 14 | 83 ± 11 | 85 ± 18 | 61 ± 7 | 61 ± 11 | 0.02 | 0.20 |
Age-adjusted percentiles are the percent of people from the US Toolbox Norming Study within each subject’s age bracket (by year) with a lower raw (unadjusted) score. Standardized scores are adjusted for age, sex, race, ethnicity, and highest level of education. Test score data are mean ± SD. R2 regression coefficients and p values are from unadjusted linear regression models (no covariates included) between log-transformed TMAO and T-scores, just within MA/O subjects
*p < 0.05 vs. young
NIHTBNIH Toolbox Cognition Battery measure (higher scores indicate better cognitive function)
TTrail Making tests (faster times/lower number of seconds indicate better cognitive function)
Consistent with normal cognitive aging, MA/O adults had lower raw (unadjusted) scores on NIH Toolbox Cognition Battery tests of working memory (despite scoring in a higher percentile for their age), episodic memory, attention, processing speed, and executive function (all p < 0.001) than young adults, which resulted in a lower Fluid Cognition Composite Score (calculated based on these subdomains; p < 0.00001). MA/O adults were also slower to complete Trail Making Test parts A and B than young adults (both p < 0.01), indicating declines in processing speed and executive function, respectively. Scores on NIH Toolbox Cognition Battery tests of language, i.e., a subdomain not typically affected by aging and considered to reflect general life experience and knowledge [1, 29], were higher in MA/O compared with young adults (both p < 0.0001). As a result, the Crystallized Cognition Composite Score was higher in MA/O vs. young adults (p < 0.0001). This resulted in no age group difference in Total Cognition Composite Score (p = 0.51), as calculated from the average of the Fluid and Crystallized Composite scores.
Relations between circulating TMAO and cognitive function in MA/O adults
We used linear regression analysis to determine the relation between TMAO and cognitive function within our cohort of MA/O adults; data are summarized in Table 2. As young adults were excluded from this analysis, T-scores on tests of cognitive function were used in order to reduce variability related to educational attainment; however, when significant relations were observed, regressions were rerun using raw (unadjusted) scores to confirm the strength of relation. In all models, plasma concentrations of TMAO were natural log-transformed to account for skewness.
In unadjusted models, circulating TMAO was inversely related to working memory, episodic memory, and fluid cognition (all p < 0.05; Fig. 1b–d) but was not significantly related to scores for any other subdomain of cognitive function (all p > 0.50). The relations between plasma TMAO and working memory, episodic memory, and fluid cognition remained significant when the raw (unadjusted) scores were used.
Cardiorespiratory fitness and physical activity are independent factors related to cognitive function in some cohorts of older adults [18, 31, 67]. As such, we assessed whether circulating TMAO remained inversely related to T-scores of working memory, episodic memory, and fluid cognition when linear regression models were adjusted for VO2max and physical activity. In these models, a significant independent effect of TMAO on cognitive function remained after accounting for these factors (partial r2 for an independent effect of TMAO on working memory [r2 = 0.07], episodic memory [r2 = 0.06], and fluid cognition [r2 = 0.06]; all p < 0.05), meaning that TMAO explains ~ 7% of the overall variance in cognitive function that cannot be explained by any other factor.
Cognitive aging can also be modulated by traditional risk factors for chronic diseases [15, 26]. In linear regression models adjusted for body mass index, systolic blood pressure, serum total and LDL cholesterol, and fasted blood glucose (age and sex already accounted for by using T-scores), TMAO remained inversely related to T-scores of working memory and fluid cognition (partial r2 for independent effect of TMAO, both r2 = 0.07, p < 0.05). However, the relation between TMAO and episodic memory was weakened and no longer significant after accounting for these factors (partial r2 = 0.05, p = 0.07). Linear regression models adjusting for all factors (those related to risk factors and physical activity) also remained significant for an independent effect of TMAO on working memory and fluid cognition (both partial r2 = 0.06, p < 0.05), but not episodic memory (partial r2 = 0.03, p = 0.15).
Overall, these data indicate that circulating concentrations of TMAO predict working memory and fluid cognition independent of cardiorespiratory fitness, physical activity, and traditional risk factors. However, effects of TMAO on age-related declines in episodic memory appear to be driven, at least in part, by cardiovascular risk.
Aging increases TMAO levels in the brain and markers of neuroinflammation
To investigate direct effects of TMAO on cognitive function and the underlying mechanisms, we used a mouse model that demonstrates many clinical aspects of human aging, including cognitive dysfunction [2, 20, 22, 23]. First, we sought to establish the effects of aging on TMAO concentrations and markers of neuroinflammation.
TMAO levels
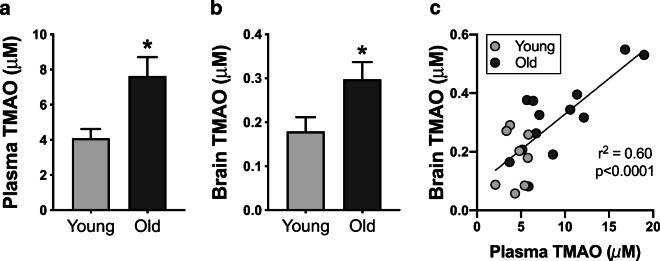
To determine if TMAO is present in the brains of older animals and in proportion to levels in circulation, which would indicate that TMAO could have a direct effect on the brain and cognitive function, we measured TMAO and its precursors by LC-MS/MS in plasma and whole-brain lysates from young adult (age 6 months; N = 9) and older (age 27 months; N = 14) C57BL/6N mice. Both plasma and brain concentrations of TMAO were higher in old vs. young animals (Fig. 2a, b; both p < 0.05). Furthermore, plasma and brain TMAO levels were highly correlated (Fig. 2c; r2 = 0.60, p < 0.0001), suggesting that TMAO likely crosses the blood-brain barrier. Plasma and brain concentrations of the three primary precursors of TMAO—choline, betaine, and l-carnitine—are presented in Table 3. There were differences in concentrations of all three precursors between young and old animals, in both plasma and brain lysates (all p < 0.05). However, there were no relations between plasma and brain concentrations for any precursor (all r2 ≤ 0.02, p ≥ 0.53). These data are consistent with another report indicating that TMAO may cross the blood-brain barrier to a greater extent than other compounds/metabolites [57].
Fig. 2.
Aging proportionally increases plasma and brain TMAO levels. Concentrations of TMAO in plasma (a) and whole-brain lysates (b) from young (6 months; N = 9) and old (27 months; N = 14) mice. Data are mean ± standard error of the mean (SEM). *p < 0.05 vs. young. c Plasma and brain concentrations of TMAO are highly correlated
Table 3.
Concentrations of precursors of trimethylamine N-oxide (TMAO) in young (6 months, N = 8) and old (27 months, N = 12) mice
| Young | Old | |
|---|---|---|
| Plasma | ||
| Choline (μM) | 19 ± 1 | 77 ± 18* |
| Betaine (μM) | 164.8 ± 18.2 | 55.4 ± 12.4* |
| l-Carnitine (μM) | 20.3 ± 0.5 | 30.9 ± 2.6* |
| Whole-brain lysates | ||
| Choline (μM) | 210 ± 34 | 90 ± 7* |
| Betaine (μM) | 4.9 ± 0.6 | 9.1 ± 1.3* |
| l-Carnitine (μM) | 20.0 ± 2.6 | 29.7 ± 2.5* |
Data are mean ± SEM
*p<0.05 vs. young
Neuroinflammation and astrocyte activation
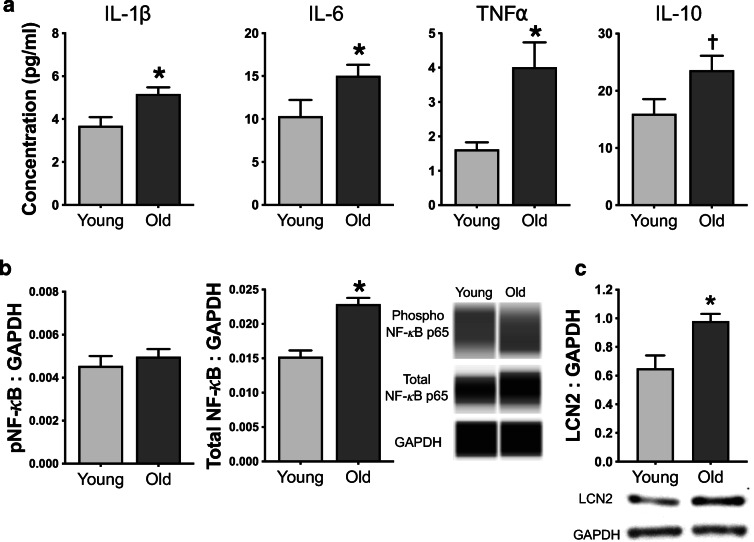
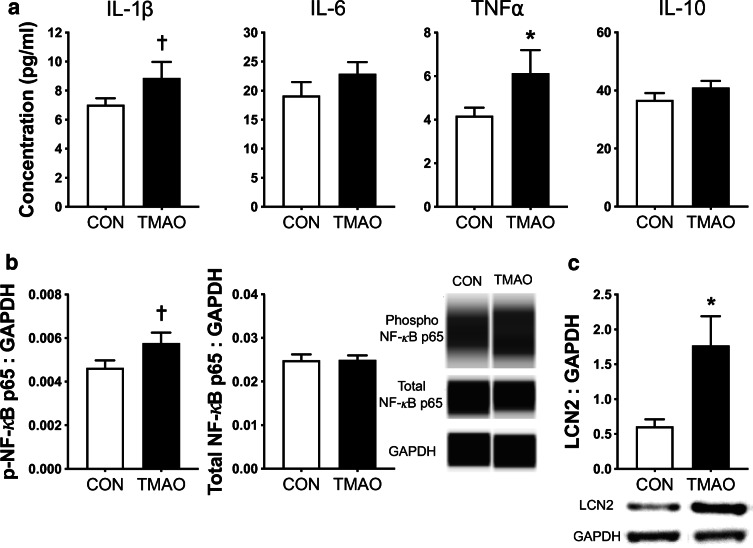
Concentrations of pro-inflammatory cytokines commonly associated with neuroinflammation (IL1-β, IL-6, and TNF-α) and the anti-inflammatory cytokine IL-10 were measured in whole-brain lysates from young and old mice and are presented in Fig. 3a. Whole-brain concentrations of pro-inflammatory cytokines were all ~ 50–100% higher in old vs. young mice (all p < 0.05). Old mice also tended to have (presumably compensatory) higher brain IL-10 compared to young mice (p = 0.07). Next, we measured the pro-inflammatory transcription factor nuclear factor kappa B (NF-κB), which is a key regulator of pro-inflammatory cytokine signaling. Although abundance of phosphorylated (i.e., activated) NF-κB was similar between brains from young and old mice (p = 0.49), old mice had ~ 50% higher abundance of total NF-κB (p < 0.001; Fig. 3b), suggesting a greater capacity to upregulate pro-inflammatory cytokine production. Finally, LCN2, an established marker of reactive astrocytes involved in the innate immune response to bacteria [5, 63], was higher in brain lysates from old compared to young mice (p < 0.05; Fig. 3c). Together, these observations in mice indicate that aging is associated with astrocyte immune activation and neuroinflammation.
Fig. 3.
Aging is accompanied by neuroinflammation and astrocyte activation. In whole-brain lysates from young (6 months; N = 9) and old (27 months; N = 14) mice, a concentrations of pro-inflammatory cytokines (interleukin (IL)-1β, IL-6, and tumor necrosis factor (TNF)α) and anti-inflammatory cytokine (IL-10); b protein abundance of phosphorylated and total nuclear factor kappa B (NF-κB) normalized to GAPDH, with representative Western blot images generated from WES electropherograms shown on the right; and c protein abundance of lipocalin 2 (LCN2), an established marker of astrocyte activation, with representative Western blot images shown below, were determined. Data are mean ± SEM. *p < 0.05 vs. young; †p < 0.10 vs. young
Dietary supplementation of TMAO increases plasma and whole-brain TMAO and impairs cognitive function in young mice
TMAO concentrations
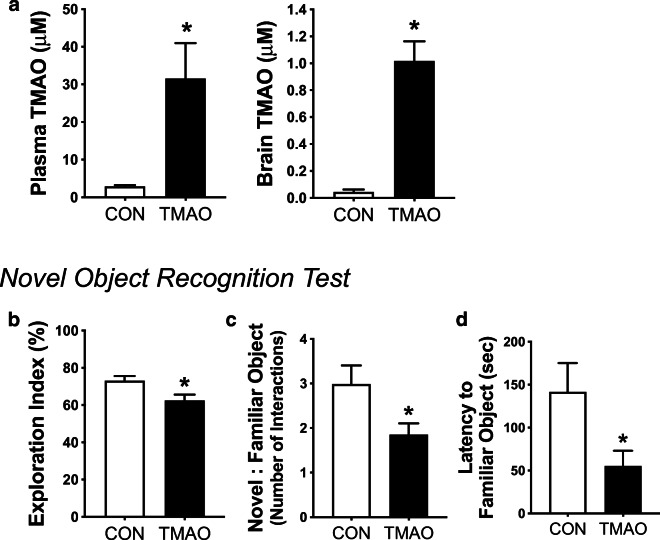
To investigate if increases in circulating and/or brain concentrations of TMAO induce aging-like impairments in cognitive function, young mice were fed a choline-defined diet (0.07% choline; to control for precursors of TMAO) that was either not supplemented (controls; N = 9) or supplemented with 0.12% TMAO [58, 66] (N = 11) for 6 months. TMAO was greater in both plasma and whole-brain lysates in TMAO-supplemented mice vs. controls (both p < 0.01; Fig. 4a). There were no effects of TMAO supplementation on plasma or brain precursors of TMAO, including choline, betaine, and l-carnitine (all p > 0.27; Table 4). TMAO supplementation did not affect body mass, food intake, or mass of key organs (all p > 0.27; Table 4).
Fig. 4.
Chronic supplementation with TMAO in young mice impairs cognitive function. In young mice fed control chow (CON; N = 8–9) or chow supplemented with 0.12% TMAO (N = 10–11) for 6 months, a concentrations of TMAO in plasma (left) and whole-brain lysates (right), b exploration index (percent of time out of the 5-min test spent exploring the novel vs. both objects), c the ratio of interactions with the novel vs. familiar object, and d the latency time until the first interaction with the familiar object on the novel object recognition test of spatial memory and learning were determined. Data are mean ± SEM. *p < 0.05 vs. CON
Cognitive function
On the novel object recognition test, an established measure of memory and spatial learning [3, 27], young mice supplemented with TMAO spent more time during the 5-min test exploring the familiar object than control mice, presented both as the percentage of time spent with the novel object vs. both objects (i.e., exploration index; p < 0.05; Fig. 4b) and as a ratio of the number of interactions with the novel vs. both objects (p < 0.05; Fig. 4c). Additionally, mice that had been supplemented with TMAO interacted with the familiar object just as quickly into the test as they did with the novel object (latency of time to first interaction, familiar object [55 ± 18 s] vs. novel object [65 ± 26 s], p = 0.58), whereas control mice did not interact with the familiar object until much later into the test (familiar object latency [142 ± 34 s, p < 0.05] vs. TMAO; Fig. 4d; latency to novel object for control mice [58 ± 28 s, p < 0.05] vs. latency to familiar object). Overall, these results show that TMAO-supplemented mice did not recognize the familiar object as well as control mice, indicating that TMAO impaired memory and spatial learning. Lastly, locomotion, assessed as total distance traveled during the 5-min open field test (day 1), was not different across groups (control [1540 ± 98] vs. TMAO [1645 ± 139], p = 0.56), suggesting that the TMAO-induced impairments on the novel object recognition test were not influenced by ability to move around the arena.
TMAO supplementation in young mice induces neuroinflammation and astrocyte activation
Concentrations of pro-inflammatory cytokines IL-1β and TNF-α were ~ 30% (p = 0.09) and ~ 50% (p < 0.05) higher, respectively, in whole-brain lysates from TMAO-supplemented mice compared with controls (Fig. 5a), whereas there were no significant differences in IL-6 or anti-inflammatory IL-10 (both p ≥ 0.22). Abundance of phosphorylated (i.e., activated) NF-κB was higher in brain lysates from TMAO-supplemented vs. control mice (p < 0.10), but total brain NF-κB was comparable (p = 0.97) (Fig. 5b). These findings are consistent with the idea of TMAO-induced neuroinflammation. Finally, abundance of the reactive astrocyte marker LCN2 was ~ 3-fold higher in brain lysates from TMAO-supplemented vs. control mice (p < 0.01; Fig. 5c), suggesting that TMAO-induced neuroinflammation was associated with glial cell activation.
Fig. 5.
Chronic supplementation with TMAO in young mice induces neuroinflammation and astrocyte activation. In whole-brain lysates from young mice fed control chow (CON) or chow supplemented with 0.12% TMAO for 6 months, a concentrations of pro-inflammatory cytokines (interleukin (IL)-1β, IL-6, and tumor necrosis factor (TNF)-α) and anti-inflammatory cytokine (IL-10); b protein abundance of phosphorylated and total nuclear factor kappa B (NF-κB) normalized to GAPDH, with representative Western blot images generated from WES electropherograms shown on the right; and c protein abundance of lipocalin 2 (LCN2), an established marker of astrocyte activation, with representative Western blot images shown below, were determined. Data are mean ± SEM. *p < 0.05 vs. CON. †p < 0.10 vs. CON
TMAO directly activates cultured human astrocytes
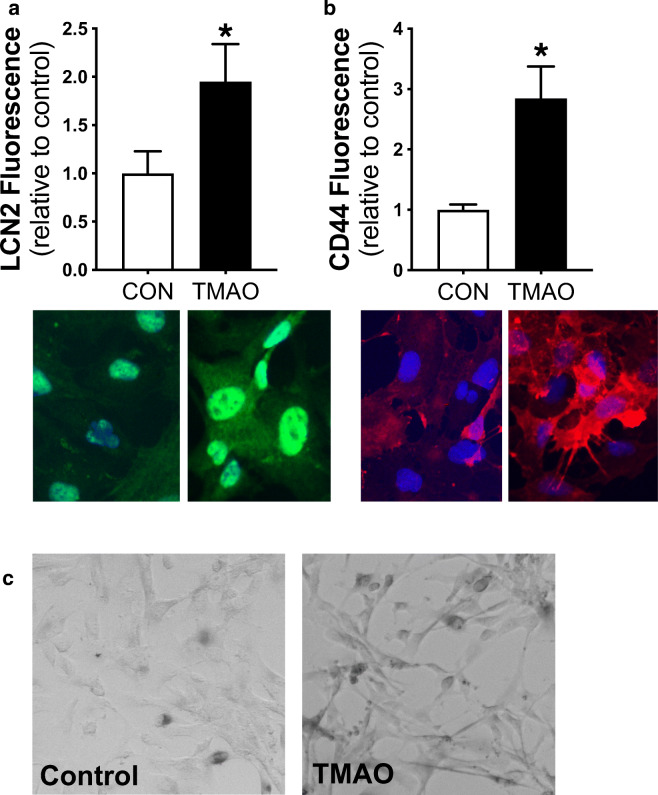
To determine if TMAO directly induces astrocyte activation, we treated primary human astrocytes with 100 μM TMAO, a dose previously reported to induce inflammation in cultured endothelial cells [48, 52], or control media for 24 h. We found that TMAO treatment activated astrocytes, as indicated by a ~ 2-fold increase in LCN2 (Fig. 6a) and a ~ 3-fold increase in CD44 (Fig. 6b), a pro-inflammatory intercellular adhesion protein characteristic of reactive astrocytes [10]. We did not observe any cell death across either condition, but TMAO was associated with slight changes in cellular morphology (“stellation”) that is typical of activated astrocytes [40] (Fig. 6c).
Fig. 6.
TMAO induces astrocyte activation. Protein abundance of the reactive astrocyte markers (a lipocalin 2, LCN2, and b CD44) in human astrocytes treated with 100 μM TMAO vs. control (CON) media for 24 h. Representative immunofluorescence images are shown below. N = 3/condition. *p < 0.05 vs. CON. c Representative brightfield images of human astrocytes showing differences in morphology (especially stellation) in cells cultured for 24 h with 100 μM TMAO vs. control media
Discussion
Although expected and “normal” on the one hand, declines in cognitive function with aging may limit quality of life and, importantly, increase the risk of age-related neurogenerative diseases [32, 61]. As such, a better understanding of the mechanisms underlying cognitive aging may provide new opportunities to prevent, slow, or delay age-related cognitive impairments, and perhaps the development of age-related neurodegenerative diseases and dementia. Our findings add to a growing body of literature that neuroinflammation and reactive astrocytes contribute to cognitive aging [17, 46], and we identify a novel potential upstream mediator of these processes—elevated levels of TMAO. Specifically, across cells, mice, and people, we showed that increases in plasma and brain levels of TMAO, induced by either natural aging or supplementation, are accompanied by astrocyte activation and neuroinflammation and are associated with declines in cognitive function, particularly in the subdomain of memory. To our knowledge, this is the first study to comprehensively assess the role of TMAO in cognitive aging and to investigate the potential mechanisms linking TMAO to declines in cognitive function, particularly neuroinflammation.
We and others have previously shown that TMAO increases in circulation with aging in both mice [9, 42] and humans [8, 59], likely due to a combination of greater abundance of gut microbes that produce TMA and greater capacity to convert TMA into TMAO in the liver [9]. Here, we show that TMAO also increases in the brain with aging in parallel with increases in plasma concentrations. As FMO3, the enzyme needed to convert TMA to TMAO, is not detectibly expressed in the brain [33], these data suggest that TMAO crosses the blood-brain barrier and may therefore have direct contact with brain cells such as astrocytes and neurons. In support of this claim, others have detected TMAO in cerebrospinal fluid from patients with Alzheimer’s disease and other neurological disorders [16]. Importantly, our observations here indicate that plasma TMAO could be measured as a surrogate for brain TMAO, allowing for non-invasive study of these pathways in humans.
Growing evidence indicates that neuroinflammation is an important mediator of normal cognitive aging [46, 49]. In the present study, we observed higher levels of NF-κB and inflammatory cytokines in brains from old mice compared with young adult mice, consistent with previous studies in multiple species that have demonstrated increased expression of NF-κB in the aged brain and greater steady-state levels of NF-κB gene targets, including IL-6, IL-1β, and TNF-α [24, 25, 55]. Although acute increases in these cytokines mediate the appropriate inflammatory response to pathogens and/or injury, chronically elevated pro-inflammatory cytokines, particularly in the aging brain, are known to potentiate glial cell reactivity, leading to synaptic loss and neuronal dysfunction/damage [47]. Importantly, we found that dietary supplementation with TMAO induced aging-like increases in brain NF-κB and pro-inflammatory cytokine levels in young adult animals. The magnitude of these increases (compared with that in mice fed control chow) was on average ~ 50% of the difference between young and old mice, which represents a substantial increase in pro-inflammatory signaling attributable to just one metabolite. Although it is probable that multiple processes work in concert to induce neuroinflammation with natural aging, our data indicate that increased circulating TMAO may play an important modulatory role.
As identified by our experiments in mice and cultured human astrocytes, one mechanism by which TMAO may induce neuroinflammation could involve activation of the innate immune system—the network of pattern recognition receptors that detect bacteria/viruses/other pathogens and activate pro-inflammatory signaling, recruitment of immune cells, and other immunoreactive processes [30, 38]. Astrocytes are highly abundant immune-active cells in the brain that can assume a toxic “reactive” state in response to immune-related stressors, and recent reports demonstrate that reactive astrocytes accumulate in the aging brain [12]. Stressors that typically initiate astrocyte activation and neuroinflammation include central nervous trauma and, notably, infection [13]. It is not clear how TMAO might signal astrocyte activation in the brain, as no cellular receptor has been identified. It is possible that, as a bacterium-derived metabolite, TMAO is recognized by astrocytes as being pathogenic, thereby initiating innate immune signaling and inflammation. Furthermore, astrocytes can undergo cellular senescence with aging, including development of a pro-inflammatory senescence–associated secretory phenotype [12, 14], which has been associated with cognitive decline in older adults (Supplementary Reference 1). Because TMAO accelerates vascular endothelial cell senescence in mice [35], one mechanism by which TMAO contributes to cognitive decline with aging may be through promoting cellular senescence in astrocytes.
Our results indicate that TMAO-induced neuroinflammation and astrocyte activation are associated with declines in cognitive function. Indeed, we observed in both mice and humans that elevated circulating (and brain) concentrations of TMAO were inversely related to cognitive function, particularly in the subdomain of memory, consistent with results from a recent study in young, senescence-accelerated mice [43]. In humans, higher circulating concentrations of TMAO predicted cognitive impairment post stroke [68]; however, to our knowledge, this is the first study to investigate of role of TMAO in modulating cognitive aging in healthy humans. Importantly, TMAO predicted working memory and fluid cognition, i.e., a composite score of all subdomains of cognitive function that typically decline with aging (memory, processing speed, attention, and executive function), independent of physical activity levels, cardiorespiratory fitness, and other traditional risk factors for chronic diseases. On the other hand, the relation between TMAO and episodic memory per se was weakened and no longer significant when traditional risk factors were included in the model. It is possible that TMAO may have both direct (e.g., via neuroinflammation) and indirect effects on the brain in humans. The latter may be related to effects on the cardiovascular system, as we and others have shown that elevated circulating TMAO impairs vascular endothelial function [8, 41] and is predictive of future cardiovascular diseases [54, 66].
Limitations
Another type of glial cell involved in neuroinflammation is microglia. We focused on astrocyte rather than microglial reactivity given the growing evidence of a role for toxic astrocytes in brain aging and disease progression [28] and the technical challenges associated with detecting microglial reactivity. However, others have shown that TMAO supplementation exacerbates hippocampal activation of microglia and neuroinflammation induced by major abdominal surgery [44] and exposure to the anesthetic sevoflurane [65] in rats. Thus, it is possible that TMAO induces neuroinflammation with aging via both astrocyte and microglial activation. In addition, although the plasma concentrations of TMAO attained with dietary supplementation in young mice (~ 30 μM) were higher than average plasma levels observed in older mice and humans, those values were not outside of the physiological range, as plasma TMAO ranged from ~ 1 to 45 μM in our MA/O subjects. The concentration of TMAO that we used for astrocyte cell culture (100 μM) was also higher than circulating levels with aging. In preliminary experiments, we also studied 25 μM TMAO (to match plasma levels observed in mice with dietary supplementation) but did not observe any effects. Because cells were incubated with TMAO for just 24 h, it is likely that a higher concentration is needed over this brief period in vitro to induce similar effects as what would be seen with lower levels of TMAO over a duration of months in vivo.
Conclusion and perspectives
Overall, our findings provide broad translational evidence for TMAO as a key gut microbiome–derived metabolite that contributes to neuroinflammatory and astrocyte-mediated modulation of cognitive performance with normal aging. The upstream mediators of cognitive aging have been largely unknown, which has hindered the development of effective interventions. Our results identify TMAO as a potentially novel target for preventing and/or treating neuroinflammation and declines in cognitive performance with aging in humans. Because age-related declines in cognitive function may reduce the quality of life and increase the risk of mild cognitive impairment and neurodegenerative diseases in some adults [32], interventions to reduce circulating and brain concentrations of TMAO with aging may be effective in promoting healthy cognitive aging.
Finally, our results also suggest TMAO could be considered a “pro-geronic” factor. Increasing evidence indicates that gut microbiome composition is altered with aging and that these changes can affect physiological function [9, 11, 64]. Moreover, differences in microbiomic signatures have been reported between healthy and non-healthy adults > 70 years of age, as discerned by discordant occurrence of major clinical diseases (Supplementary Reference 2). In light of these findings, we note that our data show a large range of plasma TMAO concentrations within MA/O adults. Those with higher TMAO exhibit poorer cognitive performance, as well as impaired vascular endothelial function, compared to individuals with lower circulating TMAO (present study; [8]). Taken together, these observations suggest that older adults with higher circulating concentrations of TMAO are at greater risk of developing age-related clinical diseases and that lower circulating concentrations of TMAO may be a marker (predictor) of successful physiological aging.
Electronic supplementary material
(DOCX 14 kb)
Acknowledgments
The authors would like to sincerely thank Brian Ziemba, James Richey, Danijel Djukovic, Lauren Cuevas, Christopher J. Angiletta, Laura E. Griffin, and Patrick K. Gonzales for their assistance with the data collection and analysis.
Authors’ contributions
V.E.B., T.J.L., C.D.L., and D.R.S. conceived and designed the experiments. V.E.B., T.J.L., A.E.B., Z.J.S., J.M-D., R.A.G.-R., and A.P.N. collected and analyzed the data. V.E.B., T.J.L., C.D.L., and D.R.S. interpreted the data. V.E.B. wrote the initial draft of the manuscript. All authors revised the manuscript critically for intellectual content, approved the final version, and agree to be accountable for all aspects of the work.
Funding
This work was supported by the National Institutes of Health awards R01 HL143887 (D.R.S.), F32 HL140875 (V.E.B.), and R01 NS063964 (C.D.L.); Administrative Supplement to NS063964 (T.J.L.) and Colorado CTSA UL1 TR002535; and the Hatch Program of the National Institute of Food and Agriculture, US Department of Agriculture (A.P.N.).
Data availability
All data will be made freely available upon reasonable request to the corresponding author.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
All procedures involving human subjects were approved by the Institutional Review Board at the University of Colorado Boulder and were conducted in accordance with the Declaration of Helsinki. All animal procedures were approved by the University of Colorado Boulder Institutional Animal Care and Use Committee and complied with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Consent to participate
All human subjects provided oral and written informed consent prior to participation.
Consent for publication
As part of the informed consent process, all human subjects consented to publication of all data resulting from their participation in the studies, including use of samples for publication of future studies.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Akshoomoff N, Beaumont JL, Bauer PJ, Dikmen SS, Gershon RC, Mungas D, Slotkin J, Tulsky D, Weintraub S, Zelazo PD, Heaton RK. VIII. NIH Toolbox Cognition Battery (CB): composite scores of crystallized, fluid, and overall cognition. Monogr Soc Res Child Dev. 2013;78:119–132. doi: 10.1111/mono.12038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ammassari-Teule M, Fagioli S, Rossi-Arnaud C. Radial maze performance and open-field behaviours in aged C57BL/6 mice: further evidence for preserved cognitive abilities during senescence. Physiol Behav. 1994;55:341–345. doi: 10.1016/0031-9384(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 3.Antunes M, Biala G. The novel object recognition memory: neurobiology, test procedure, and its modifications. Cogn Process. 2011;13:93–110. doi: 10.1007/s10339-011-0430-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beaumont JL, Havlik R, Cook KF, Hays RD, Wallner-Allen K, Korper SP, Lai J-S, Nord C, Zill N, Choi S, Yost KJ, Ustsinovich V, Brouwers P, Hoffman HJ, Gershon R. Norming plans for the NIH Toolbox. Neurology. 2013;80:S87–S92. doi: 10.1212/WNL.0b013e3182872e70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bi F, Huang C, Tong J, Qiu G, Huang B, Wu Q, Li F, Xu Z, Bowser R, Xia X-G, Zhou H. Reactive astrocytes secrete LCN2 to promote neuron death. Proc Natl Acad Sci U S A. 2013;110:4069–4074. doi: 10.1073/pnas.1218497110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boutagy NE, Neilson AP, Osterberg KL, Smithson AT, Englund TR, Davy BM, Hulver MW, Davy KP. Probiotic supplementation and trimethylamine-N-oxide production following a high-fat diet. Obesity. 2015;23:2357–2363. doi: 10.1002/oby.21212. [DOI] [PubMed] [Google Scholar]
- 7.Boutagy NE, Neilson AP, Osterberg KL, Smithson AT, Englund TR, Davy BM, Hulver MW, Davy KP. Short-term high-fat diet increases postprandial trimethylamine-N-oxide in humans. Nutr Res. 2015;35:858–864. doi: 10.1016/j.nutres.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 8.Brunt VE, Gioscia-Ryan RA, Casso AG, VanDongen NS, Ziemba BP, Sapinsley ZJ, Richey JJ, Zigler MC, Neilson AP, Davy KP, Seals DR. Trimethylamine-N-oxide promotes age-related vascular oxidative stress and endothelial dysfunction in mice and healthy humans. Hypertension. 2020;76:101–112. doi: 10.1161/HYPERTENSIONAHA.120.14759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brunt VE, Gioscia-Ryan RA, Richey JJ, Zigler MC, Cuevas LM, González A, Vázquez-Baeza Y, Battson ML, Smithson AT, Gilley AD, Ackermann G, Neilson AP, Weir T, Davy KP, Knight R, Seals DR. Suppression of the gut microbiome ameliorates age-related arterial dysfunction and oxidative stress in mice. J Physiol. 2019;597:2361–2378. doi: 10.1113/JP277336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen M-L, Zhu X-H, Ran L, Lang H-D, Yi L, Mi M-T. Trimethylamine-N-oxide induces vascular inflammation by activating the NLRP3 inflammasome through the SIRT3-SOD2-mtROS signaling pathway. J Am Heart Assoc. 2017;6:e006347. doi: 10.1161/JAHA.117.006347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Claesson MJ, Jeffery IB, Conde S, Power SE, O’Connor EM, Cusack S, Harris HMB, Coakley M, Lakshminarayanan B, O’Sullivan O, Fitzgerald GF, Deane J, O’Connor M, Harnedy N, O’Connor K, O’Mahony D, van Sinderen D, Wallace M, Brennan L, Stanton C, Marchesi JR, Fitzgerald AP, Shanahan F, Hill C, Ross RP, O’Toole PW. Gut microbiota composition correlates with diet and health in the elderly. Nature. 2012;488:178–184. doi: 10.1038/nature11319. [DOI] [PubMed] [Google Scholar]
- 12.Clarke LE, Liddelow SA, Chakraborty C, Münch AE, Heiman M, Barres BA. Normal aging induces A1-like astrocyte reactivity. Proc Natl Acad Sci U S A. 2018;115:E1896–E1905. doi: 10.1073/pnas.1800165115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Colombo E, Farina C. Astrocytes: key regulators of neuroinflammation. Trends Immunol. 2016;37:608–620. doi: 10.1016/j.it.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 14.Csipo T, Lipecz A, Ashpole NM, Balasubramanian P, Tarantini S. Astrocyte senescence contributes to cognitive decline. Geroscience. 2020;42:51–55. doi: 10.1007/s11357-019-00140-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Debette S, Seshadri S, Beiser A, Au R, Himali JJ, Palumbo C, Wolf PA, DeCarli C. Midlife vascular risk factor exposure accelerates structural brain aging and cognitive decline. Neurology. 2011;77:461–468. doi: 10.1212/WNL.0b013e318227b227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Del Rio D, Zimetti F, Caffarra P, Tassotti M, Bernini F, Brighenti F, Zini A, Zanotti I. The gut microbial metabolite trimethylamine-N-oxide is present in human cerebrospinal fluid. Nutrients. 2017;9:1053. doi: 10.3390/nu9101053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Di Benedetto S, Müller L, Wenger E, Düzel S, Pawelec G. Contribution of neuroinflammation and immunity to brain aging and the mitigating effects of physical and cognitive interventions. Neurosci Biobehav Rev. 2017;75:114–128. doi: 10.1016/j.neubiorev.2017.01.044. [DOI] [PubMed] [Google Scholar]
- 18.Erickson KI, Hillman C, Stillman CM, Ballard RM, Bloodgood B, Conroy DE, Macko R, Marquez DX, Petruzzello SJ, Powell KE, 2018 Physical Activity Guidelines Advisory Committee Physical activity, cognition, and brain outcomes: a review of the 2018 physical activity guidelines. Med Sci Sports Exerc. 2019;51:1242–1251. doi: 10.1249/MSS.0000000000001936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Evans SL, Davy KP, Stevenson ET, Seals DR. Physiological determinants of 10-km performance in highly trained female runners of different ages. J Appl Physiol. 1995;78:1931–1941. doi: 10.1152/jappl.1995.78.5.1931. [DOI] [PubMed] [Google Scholar]
- 20.Fahlström A, Zeberg H, Ulfhake B. Changes in behaviors of male C57BL/6J mice across adult life span and effects of dietary restriction. Age (Dordr) 2012;34:1435–1452. doi: 10.1007/s11357-011-9320-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fleenor BS, Sindler AL, Marvi NK, Howell KL, Zigler ML, Yoshizawa M, Seals DR. Curcumin ameliorates arterial dysfunction and oxidative stress with aging. Exp Gerontol. 2013;48:269–276. doi: 10.1016/j.exger.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Forster MJ, Dubey A, Dawson KM, Stutts WA, Lal H, Sohal RS. Age-related losses of cognitive function and motor skills in mice are associated with oxidative protein damage in the brain. Proc Natl Acad Sci U S A. 1996;93:4765–4769. doi: 10.1073/pnas.93.10.4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frick KM, Burlingame LA, Arters JA, Berger-Sweeney J. Reference memory, anxiety and estrous cyclicity in C57BL/6NIA mice are affected by age and sex. Neuroscience. 2000;95:293–307. doi: 10.1016/s0306-4522(99)00418-2. [DOI] [PubMed] [Google Scholar]
- 24.Gabuzda D, Yankner BA. Physiology: inflammation links ageing to the brain. Nature. 2013;497:197–198. doi: 10.1038/nature12100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Godbout JP, Johnson RW. Interleukin-6 in the aging brain. J Neuroimmunol. 2004;147:141–144. doi: 10.1016/j.jneuroim.2003.10.031. [DOI] [PubMed] [Google Scholar]
- 26.González HM, Tarraf W, Harrison K, Windham BG, Tingle J, Alonso A, Griswold M, Heiss G, Knopman D, Mosley TH. Midlife cardiovascular health and 20-year cognitive decline: Atherosclerosis Risk in Communities Study results. Alzheimers Dement. 2018;14:579–589. doi: 10.1016/j.jalz.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grayson B, Leger M, Piercy C, Adamson L, Harte M, Neill JC. Assessment of disease-related cognitive impairments using the novel object recognition (NOR) task in rodents. Behav Brain Res. 2015;285:176–193. doi: 10.1016/j.bbr.2014.10.025. [DOI] [PubMed] [Google Scholar]
- 28.Habib N, McCabe C, Medina S, Varshavsky M, Kitsberg D, Dvir-Szternfeld R, Green G, Dionne D, Nguyen L, Marshall JL, Chen F, Zhang F, Kaplan T, Regev A, Schwartz M. Disease-associated astrocytes in Alzheimer’s disease and aging. Nat Neurosci. 2020;169:1276. doi: 10.1038/s41593-020-0624-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harada CN, Natelson Love MC, Triebel KL. Normal cognitive aging. Clin Geriatr Med. 2013;29:737–752. doi: 10.1016/j.cger.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heneka MT, Kummer MP, Latz E. Innate immune activation in neurodegenerative disease. Nat Rev Immunol. 2014;14:463–477. doi: 10.1038/nri3705. [DOI] [PubMed] [Google Scholar]
- 31.Hörder H, Johansson L, Guo X, Grimby G, Kern S, Östling S, Skoog I. Midlife cardiovascular fitness and dementia: a 44-year longitudinal population study in women. Neurology. 2018;90:e1298–e1305. doi: 10.1212/WNL.0000000000005290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hou Y, Dan X, Babbar M, Wei Y, Hasselbalch SG, Croteau DL, Bohr VA. Ageing as a risk factor for neurodegenerative disease. Nat Rev Neurol. 2019;15:565–581. doi: 10.1038/s41582-019-0244-7. [DOI] [PubMed] [Google Scholar]
- 33.Janmohamed A, Hernandez D, Phillips IR, Shephard EA. Cell-, tissue-, sex- and developmental stage-specific expression of mouse flavin-containing monooxygenases (Fmos) Biochem Pharmacol. 2004;68:73–83. doi: 10.1016/j.bcp.2004.02.036. [DOI] [PubMed] [Google Scholar]
- 34.Justice JN, Carter CS, Beck HJ, Gioscia-Ryan RA, McQueen M, Enoka RM, Seals DR. Battery of behavioral tests in mice that models age-associated changes in human motor function. Age (Dordr) 2013;36:583–595. doi: 10.1007/s11357-013-9589-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ke Y, Li D, Zhao M, Liu C, Liu J, Zeng A, Shi X, Cheng S, Pan B, Zheng L, Hong H. Gut flora-dependent metabolite trimethylamine-N-oxide accelerates endothelial cell senescence and vascular aging through oxidative stress. Free Radic Biol Med. 2018;116:88–100. doi: 10.1016/j.freeradbiomed.2018.01.007. [DOI] [PubMed] [Google Scholar]
- 36.Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, Britt EB, Fu X, Wu Y, Li L, Smith JD, DiDonato JA, Chen J, Li H, Wu GD, Lewis JD, Warrier M, Brown JM, Krauss RM, Tang WHW, Bushman FD, Lusis AJ, Hazen SL. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19:576–585. doi: 10.1038/nm.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kriska AM, Knowler WC, LaPorte RE, Drash AL, Wing RR, Blair SN, Bennett PH, Kuller LH. Development of questionnaire to examine relationship of physical activity and diabetes in Pima Indians. Diabetes Care. 1990;13:401–411. doi: 10.2337/diacare.13.4.401. [DOI] [PubMed] [Google Scholar]
- 38.Labzin LI, Heneka MT, Latz E. Innate immunity and neurodegeneration. Annu Rev Med. 2018;69:437–449. doi: 10.1146/annurev-med-050715-104343. [DOI] [PubMed] [Google Scholar]
- 39.LaRocca TJ, Gioscia-Ryan RA, Hearon CM, Seals DR. The autophagy enhancer spermidine reverses arterial aging. Mech Ageing Dev. 2013;134:314–320. doi: 10.1016/j.mad.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.LaRocca TJ, Mariani A, Watkins LR, Link CD. TDP-43 knockdown causes innate immune activation via protein kinase R in astrocytes. Neurobiol Dis. 2019;132:104514. doi: 10.1016/j.nbd.2019.104514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li T, Chen Y, Gua C, Li X. Elevated circulating trimethylamine N-oxide levels contribute to endothelial dysfunction in aged rats through vascular inflammation and oxidative stress. Front Physiol. 2017;8:350. doi: 10.3389/fphys.2017.00350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li T, Gua C, Wu B, Chen Y. Increased circulating trimethylamine N-oxide contributes to endothelial dysfunction in a rat model of chronic kidney disease. Biochem Biophys Res Commun. 2018;495:2071–2077. doi: 10.1016/j.bbrc.2017.12.069. [DOI] [PubMed] [Google Scholar]
- 43.Li D, Ke Y, Zhan R, Liu C, Zhao M, Zeng A, Shi X, Ji L, Cheng S, Pan B, Zheng L, Hong H. Trimethylamine-N-oxide promotes brain aging and cognitive impairment in mice. Aging Cell. 2018;49:e12768. doi: 10.1111/acel.12768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meng F, Li N, Li D, Song B, Li L. The presence of elevated circulating trimethylamine N-oxide exaggerates postoperative cognitive dysfunction in aged rats. Behav Brain Res. 2019;368:111902. doi: 10.1016/j.bbr.2019.111902. [DOI] [PubMed] [Google Scholar]
- 45.Miquel S, Champ C, Day J, Aarts E, Bahr BA, Bakker M, Bánáti D, Calabrese V, Cederholm T, Cryan J, Dye L, Farrimond JA, Korosi A, Layé S, Maudsley S, Milenkovic D, Mohajeri MH, Sijben J, Solomon A, Spencer JPE, Thuret S, vanden Berghe W, Vauzour D, Vellas B, Wesnes K, Willatts P, Wittenberg R, Geurts L. Poor cognitive ageing: vulnerabilities, mechanisms and the impact of nutritional interventions. Age Res Rev. 2018;42:40–55. doi: 10.1016/j.arr.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 46.Ownby RL. Neuroinflammation and cognitive aging. Curr Psychiatry Rep. 2010;12:39–45. doi: 10.1007/s11920-009-0082-1. [DOI] [PubMed] [Google Scholar]
- 47.Ransohoff RM. How neuroinflammation contributes to neurodegeneration. Science. 2016;353:777–783. doi: 10.1126/science.aag2590. [DOI] [PubMed] [Google Scholar]
- 48.Seldin MM, Meng Y, Qi H, Zhu W, Wang Z, Hazen SL, et al. Trimethylamine N-oxide promotes vascular inflammation through signaling of mitogen-activated protein kinase and nuclear factor-κB. J Am Heart Assoc. 2016;5. 10.1161/JAHA.115.002767. [DOI] [PMC free article] [PubMed]
- 49.Simen AA, Bordner KA, Martin MP, Moy LA, Barry LC. Cognitive dysfunction with aging and the role of inflammation. Ther Adv Chronic Dis. 2011;2:175–195. doi: 10.1177/2040622311399145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sindler AL, Fleenor BS, Calvert JW, Marshall KD, Zigler ML, Lefer DJ, Seals DR. Nitrite supplementation reverses vascular endothelial dysfunction and large elastic artery stiffness with aging. Aging Cell. 2011;10:429–437. doi: 10.1111/j.1474-9726.2011.00679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stewart AL, Mills KM, King AC, Haskell WL, Gillis D, Ritter PL. CHAMPS physical activity questionnaire for older adults: outcomes for interventions. Med Sci Sports Exerc. 2001;33:1126–1141. doi: 10.1097/00005768-200107000-00010. [DOI] [PubMed] [Google Scholar]
- 52.Sun X, Jiao X, Ma Y, Liu Y, Zhang L, He Y, Chen Y. Trimethylamine N-oxide induces inflammation and endothelial dysfunction in human umbilical vein endothelial cells via activating ROS-TXNIP-NLRP3 inflammasome. Biochem Biophys Res Commun. 2016;481:63–70. doi: 10.1016/j.bbrc.2016.11.017. [DOI] [PubMed] [Google Scholar]
- 53.Tang WHW, Wang Z, Fan Y, Levison B, Hazen JE, Donahue LM, Wu Y, Hazen SL. Prognostic value of elevated levels of intestinal microbe-generated metabolite trimethylamine-N-oxide in patients with heart failure: refining the gut hypothesis. J Am Coll Cardiol. 2014;64:1908–1914. doi: 10.1016/j.jacc.2014.02.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tang WHW, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, Wu Y, Hazen SL. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. 2013;368:1575–1584. doi: 10.1056/NEJMoa1109400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tha KK, Okuma Y, Miyazaki H, Murayama T, Uehara T, Hatakeyama R, Hayashi Y, Nomura Y. Changes in expressions of proinflammatory cytokines IL-1beta, TNF-alpha and IL-6 in the brain of senescence accelerated mouse (SAM) P8. Brain Res. 2000;885:25–31. doi: 10.1016/s0006-8993(00)02883-3. [DOI] [PubMed] [Google Scholar]
- 56.Tombaugh TN. Trail Making Test A and B: normative data stratified by age and education. Arch Clin Neuropsychol. 2004;19:203–214. doi: 10.1016/S0887-6177(03)00039-8. [DOI] [PubMed] [Google Scholar]
- 57.Vernetti L, Gough A, Baetz N, Blutt S, Broughman JR, Brown JA, Foulke-Abel J, Hasan N, in J, Kelly E, Kovbasnjuk O, Repper J, Senutovitch N, Stabb J, Yeung C, Zachos NC, Donowitz M, Estes M, Himmelfarb J, Truskey G, Wikswo JP, Taylor DL. Functional coupling of human microphysiology systems: intestine, liver, kidney proximal tubule, blood-brain barrier and skeletal muscle. Sci Rep. 2017;7:42296–42215. doi: 10.1038/srep42296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, Feldstein AE, Britt EB, Fu X, Chung Y-M, Wu Y, Schauer P, Smith JD, Allayee H, Tang WHW, DiDonato JA, Lusis AJ, Hazen SL. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang Z, Levison BS, Hazen JE, Donahue L, Li X-M, Hazen SL. Measurement of trimethylamine-N-oxide by stable isotope dilution liquid chromatography tandem mass spectrometry. Anal Biochem. 2014;455:35–40. doi: 10.1016/j.ab.2014.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weintraub S, Dikmen SS, Heaton RK, Tulsky DS, Zelazo PD, Bauer PJ, Carlozzi NE, Slotkin J, Blitz D, Wallner-Allen K, Fox NA, Beaumont JL, Mungas D, Nowinski CJ, Richler J, Deocampo JA, Anderson JE, Manly JJ, Borosh B, Havlik R, Conway K, Edwards E, Freund L, King JW, Moy C, Witt E, Gershon RC. Cognition assessment using the NIH Toolbox. Neurology. 2013;80:S54–S64. doi: 10.1212/WNL.0b013e3182872ded. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xia X, Jiang Q, McDermott J, Han J-DJ. Aging and Alzheimer’s disease: comparison and associations from molecular to system level. Aging Cell. 2018;17:e12802. doi: 10.1111/acel.12802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xu R & Wang Q. Towards understanding brain-gut-microbiome connections in Alzheimer’s disease. BMC Syst Biol. 2016;1–9. [DOI] [PMC free article] [PubMed]
- 63.Yim HCH, Williams BRG. Protein kinase R and the inflammasome. J Interf Cytokine Res. 2014;34:447–454. doi: 10.1089/jir.2014.0008. [DOI] [PubMed] [Google Scholar]
- 64.Zhang X, Yang Y, Su J, Zheng X, Wang C, Chen S, Liu J, Lv Y, Fan S, Zhao A, Chen T, Jia W, Wang X. Age-related compositional changes and correlations of gut microbiome, serum metabolome, and immune factor in rats. Geroscience. 2020;25:1234–1217. doi: 10.1007/s11357-020-00188-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhao L, Zhang C, Cao G, Dong X, Li D, Jiang L. Higher circulating trimethylamine N-oxide sensitizes sevoflurane-induced cognitive dysfunction in aged rats probably by downregulating hippocampal methionine sulfoxide reductase A. Neurochem Res. 2019;44:2506–2516. doi: 10.1007/s11064-019-02868-4. [DOI] [PubMed] [Google Scholar]
- 66.Zhu W, Gregory JC, Org E, Buffa JA, Gupta N, Wang Z, Li L, Fu X, Wu Y, Mehrabian M, Sartor RB, McIntyre TM, Silverstein RL, Tang WHW, DiDonato JA, Brown JM, Lusis AJ, Hazen SL. Gut microbial metabolite TMAO enhances platelet hyperreactivity and thrombosis risk. Cell. 2016;165:111–124. doi: 10.1016/j.cell.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhu N, Jacobs DR, Schreiner PJ, Yaffe K, Bryan N, Launer LJ, Whitmer RA, Sidney S, Demerath E, Thomas W, Bouchard C, He K, Reis J, Sternfeld B. Cardiorespiratory fitness and cognitive function in middle age: the CARDIA study. Neurology. 2014;82:1339–1346. doi: 10.1212/WNL.0000000000000310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhu C, Li G, Lv Z, Li J, Wang X, Kang J, Zhan C. Association of plasma trimethylamine-N-oxide levels with post-stroke cognitive impairment: a 1-year longitudinal study. Neurol Sci. 2020;41:57–63. doi: 10.1007/s10072-019-04040-w. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 14 kb)
Data Availability Statement
All data will be made freely available upon reasonable request to the corresponding author.