Abstract
Forty-three female African green monkeys (Chlorocebus aethiops sabaeus) were selected to represent young adult to advanced geriatric ages (7–24 years) to exhibit a wide range of obesity status (8–53% body fat) and diverse metabolic syndrome criteria such as diabetes, dyslipidemia, and hypertension. Subcutaneous and visceral adipose tissues were collected and evaluated for the presence of senescence cells in both whole tissue and single-cell isolates from subcutaneous sources, utilizing senescence-associated β-galactosidase (SAβ-gal) staining. Plasma samples were analyzed for selected metabolic and inflammatory biomarkers related to the senescence-associated secretory profile. Our results indicated that tissue staining scores did not differ between subcutaneous and intra-abdominal visceral depots and were highly related within individuals. Tissue staining was significantly associated with chronological age; however, no associations with fatness or metabolic syndrome criteria were observed. Associations with age were unchanged when obesity status was included in regression models. Isolated cell staining did positively relate to age but not tissue staining, suggesting some of the SAβ-gal-positive cells were stromal vascular cells or small adipocytes, but that mature large adipocytes, filtered out in the cell isolation process, are also likely to exhibit positive SAβ-gal staining. Plasminogen activator inhibitor-1 (PAI-1) concentration in circulation was the sole inflammation-related biomarker that positively associated with age and is considered to be a marker of senescent cell burden. Our study is the largest, most comprehensive assessment of adipose SAβ-gal staining in a relevant animal model of human aging, and confirms that this senescence-associated biomarker specifically indicates an age-related process.
Keywords: Adipose, Aging, Senescence, Non-human primate, Plasminogen activator inhibitor 1, Senescence-associated β-galactosidase
Introduction
Cellular senescence is the process in which cells undergo irreversible proliferative arrest and exhibit distinctive phenotypic alterations (van Deursen 2014; Hickson et al. 2019; Palmer et al. 2019). Senescent cells are known to accumulate in adipose tissue located beneath the skin (subcutaneous adipose tissue (SQAT)) or around vital organs (visceral adipose tissue (VAT)) (Justice et al. 2019, Palmer et al. 2019. Throughout life, adipocyte distribution and turnover occur naturally and continuously. Recent studies have suggested that accumulation of senescent cells in adipose tissue could be the root cause of adipose metabolic dysfunction in obesity. This dysfunction of adipose tissue may accelerate the process of age-related metabolic diseases, chronic inflammation, and cancer (Cartwright et al. 2010, Tchkonia et al. 2010, Ghosh et al. 2019). While these cells remain in cell cycle arrest and fail to respond to growth or death stimuli, they are still metabolically active and secrete pro-inflammatory cytokines, chemokines, and proteases referred to senescence-associated secretory phenotype (SASP) (Xu et al. 2018; Hickson et al. 2019).
SASP markers such as interleukin (IL)-6, IL-1, monocyte chemoattractant protein (MCP)-1, and plasminogen activator inhibitor (PAI)-1 increase with senescent-induced factors such as aging, chronic diseases, and radiation or chemotherapy (Xu et al. 2018; Zeng et al. 2018). These cytokines result in local and remote tissue dysfunction which accumulate with age and have led senescence to be included as one of the biological pillar processes of aging (Lopez-Otin et al. 2013). Senolytics, drugs that selectively induce apoptosis in senescent cells, have recently been developed and used for senescent-associated diseases. The “senolytic cocktail” dasatinib and quercetin (D+Q) disables the senescence-associated anti-apoptotic pathways that defend against the pro-apoptotic microenvironment and SASP (Xu et al. 2018; Hickson et al. 2019; Justice et al. 2019). D+Q treatment of aged mice resulted in decreased SASP inflammatory cytokine secretion by VAT. In addition, mice treated biweekly with D+Q had higher median post-treatment life spans and significantly lower mortality than the control group, substantiating that senolytics increase life span without extending morbidity in aged mice (Xu et al. 2018). During the first human clinical trial of D+Q, this treatment was given to patients with idiopathic pulmonary fibrosis, a fatal cellular senescence-associated disease. Results showed evidence of significantly alleviated physical dysfunction in the patients (Justice et al. 2019). Hickson et al. (2019) also completed a study with D+Q in patients with diabetic kidney disease and reported decreases in p16Ink4a+ and p21Cip1+ expressing cells, circulating SASP factors (IL-6, MMPs-9), and senescence-associated β-galactosidase (SAβ-gal) activity.
In vivo methods for definitively detecting senescent cells are challenging. Quantifying SASP cytokines is a straightforward approach, but no single biomarker has been validated as exclusively senescence associated. Measuring p16Ink4a, a tumor suppressor and aging biomarker, is used to detect in situ senescent cell abundance via luciferase activity. Studies of p16Ink4a levels measured in people confirm an association with both age and function (Lawrence et al. 2018) but the cellular source remained unidentified. Palmer et al. (2019) showed that VAT senescent cell abundance increased in diet-induced obese (DIO) mice as measured by luciferase activity. Their results also indicated that macrophage abundance correlated with p16lnk4a expressing cells in the VAT of DIO mice. Many mouse studies suggest that VAT has a higher senescence abundance and macrophage content than SQAT. The contribution of macrophages by tissue depot is of interest as macrophages are the only cell type known that can become senescent and then return to their M-phenotypes as their microenvironments change (Vernon 2003; Yokoi et al. 2006; Cramer et al. 2010; Zhu et al. 2014; Palmer et al. 2015; Kirkland and Tchkonia 2017; Xu et al. 2018; Uchida et al. 2019). Shirakawa et al. (2019) investigated the increase of chronic inflammation and senescence-associated T cells in VAT of DIO mice. Their results showed a 20% increase in VAT chronic inflammation, including specific increases in senescence-associated T cells. After the mice were switched to normal chow at 30 weeks of age, VAT senescence-associated T cells decreased. Translation of rodent findings to higher species, such as primates, cannot be assumed as significant gaps exist regarding adipose tissue functions and distributions as well as immune cell types (Birsoy et al. 2013).
To address gaps in knowledge about senescence burden in adipose tissues, we measured markers of senescent cell abundance in both SQAT and VAT deposits, along with circulatory SASP, in non-human primates of various ages and body fatness. We additionally hypothesized that adipose single-cell isolate SAβ-gal staining, a population enriched in stromal vascular cells, would be reflected by whole tissue SAβ-gal, with little impact of obesity, and that SQAT SAβ-gal whole tissue scoring is our suggested in vivo approach for estimating senescent burden. Our results generated from the highly translational primate model support targeting with senolytics to address core aging biological processes.
Methods
Animals and procedures
All animal procedures were conducted under a Wake Forest University Institutional Animal Care and Use Committee approved protocol according to recommendations in the Guide for Care and Use of Laboratory Animals and in compliance with the USDA Animal Welfare Act and Animal Welfare Regulations.
Female African green monkeys (Chlorocebus aethiops sabaeus; n = 43) ranging from ages 7 to 24 were evaluated. These animals were housed in the Wake Forest University School of Medicine Vervet Research Colony. The animals selected represented diversity in age, health, and obesity status. Adulthood was defined as 4 years and older, and geriatric as being 17 years and older. Our age range included adults to late geriatric life stages, as the median age at death of this species in captivity is approximately 22 years old. Obesity was defined as a waist circumference of > 40 cm and metabolic syndrome criteria were measured as previously described (Kavanagh et al. 2017). Body fat distribution was determined by computerized tomography scanning as previously described (Bacarella et al. 2020). Plasma cytokines, interleukin (IL)-6, IL-1β, macrophage chemoattractant protein 1 (MCP1), adiponectin, and plasminogen activator inhibitor-1 (PAI-1) were measured by ELISA (R&D Systems, Minneapolis, MN).
SQAT and VAT tissues were surgically collected from the umbilical region of the abdomen. Extracted adipose tissue size of each sample was ~ 250 mg and stored in Dulbecco’s modified Eagle media (DMEM) for further processing.
Whole tissue and adipocyte processing and staining
Whole adipose tissue
Adipose tissue samples were cut into 50 mg (± 2 mg) sections and washed in 1× phosphate-buffered saline (PBS) 3 times for 3 s per wash. The tissues were fixed and stained according to the Cell Signaling Technologies Senescence B-galactosidase Staining Kit (Danvers, MA). The SAβ-gal activity assay utilizes a small piece of fresh adipose tissue to be lightly fixed and stained with the SAβ-gal solution containing X-gal at a pH of 6. The same process was applied to single-cell isolates created from the collected tissue samples as described below.
Tissues were incubated in a non-CO2 incubator for 16–18 h. A tissue piece exposed to pH greater than 6 was used as a negative control, and a pH of less than 6 was used as a positive control. Following incubation, the tissues were washed in 1× PBS 3 times for 3 s per wash, and images of each tissue were captured. Stained whole tissue was then evaluated with the naked eye and scored by four blinded individuals; a mean of their scores was used in the final data analysis. Scores ranged from 0 to 4: 0 = no blue color (bright yellow), 1–3 = gradual blue color development, and 4 = dark blue color, respectively (Fig. 1). A higher score was considered indicative of a greater senescent cell abundance.
Fig. 1.
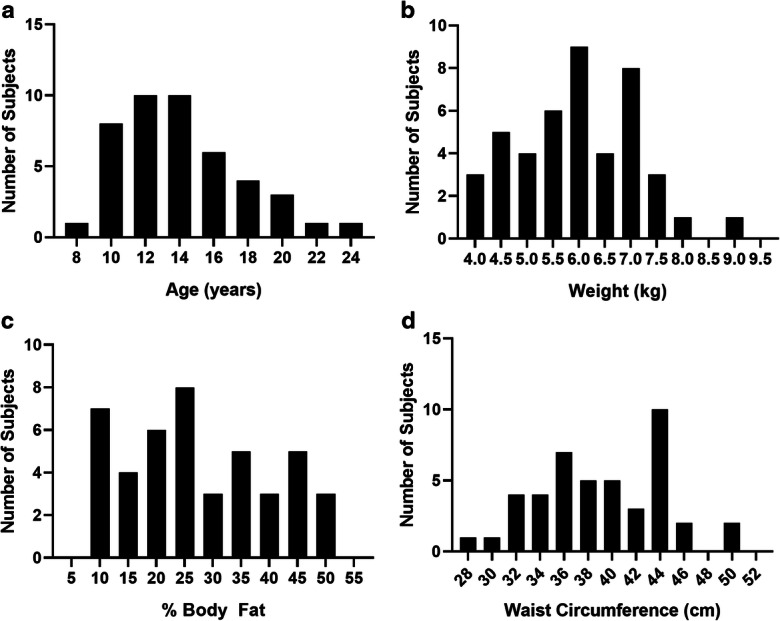
Frequency histograms depicting the non-human primate (NHP) cohort characteristics. a Age ranges were selected to represent adulthood through to the oldest. b Body weights. c Percentages of body fat as measured by computed tomography image analysis. d Waist circumferences were used to indicate obesity status of the cohort. Animals were selected to represent a wide range so as to enable examination of both an age- and weight-diverse group of NHPs. Bodyweight is an imperfect estimate for obesity; thus, we used additional screening measures to be sure a wide range was captured
Dissociated adipocytes
Prior to generating single-cell isolates from 13 of the 43 SQAT whole adipose explants, 6-well plates were coated with 1 mL of poly d-lysine and poly l-lysine solution (R&D Systems, Minneapolis, MN) or human fibronectin solution (Millipore Sigma, St. Louis, MO) and incubated overnight in a CO2 incubator. The pre-coated plates were then rinsed with 1 mL of 1× PBS three times. The adipose tissue was suspended into single-cell isolates using the Miltenyi Biotec (Bergisch Gladbach, Germany) gentle MACS single-cell dissociation protocol. One gram of each tissue type was used for isolation (> 1 × 106 live cells/mL at completion of protocol). Cell isolates were re-suspended in growth media (DMEM), added to the pre-coated 6-well plate, and incubated in CO2 for 24 h. After 24 h, the DMEM were removed from adherent cells, and the plate was rinsed once with 1 mL of 1× PBS. The tissues were fixed and stained according to manufacturer’s instructions, covered with parafilm, and incubated in a non-CO2 incubator for a further 16–18 h. With tissue staining, a pH greater than 6 was used as a negative control and a pH of less than 6 was used as a positive control. Following incubation, the cells were imaged using the Invitrogen (Carlsbad, CA) Evos XL Core microscope. Blue-stained senescent cells were quantified and documented per image using the naked eye. A cell was deemed positive for senescence when the entire cell exhibited a blue color. The total number of cells (median numbers of cells counted = 223, mean number of cells counted = 249) in each image was obtained using a customized cell counting application created in Visiopharm software (Hoersholm, Denmark) and a percentage then calculated for each image.
Data analysis
All data were examined and log transformed (TG, IL-6, PAI-1, percentage of isolated cells positively stained) if required to achieve normal distributions. Within-individual tissue scores were compared by paired t tests. Associations and adjusted associations were calculated using Pearson’s correlation and partial correlation analyses, respectively. All statistical analyses were performed using Statistica V.13 (StatSoft Inc., Tulsa, OK). Significance was set as α ≤ 0.05.
Results
We aimed to explore relationships between SAβ-gal staining and age, obesity, and adipose tissue source. We carefully selected 43 non-human primates to represent a very wide range in age, obesity, and metabolic health (Fig. 1, Table 1). Our cohort included individuals that were morbidly obese, diabetic, and represented the oldest. We saw a range of tissue scores exhibited naturally in both SQAT and VAT explants, and positively stained cells were clearly identified in our single-cell isolates (Table 1, Fig. 2). SQAT tissue scores across the entire cohort ranged from 0 to 4, and VAT tissues similarly scored with mean values ranging from 0.1 to 4. Average scores did not differ significantly between fat depots (SQAT 1.75 vs. VAT 1.53, p = 0.31), although there was variability. The scores between these two tissue sources were strongly correlated (r = 0.56, p < 0.001; Fig. 3); thus, we conclude that tissue scoring of SQAT adequately represents the senescent cell burden using SAβ-gal staining as an estimate. The percentage of cells staining positive in single-cell isolates was generally low with a median value of 1.75% (IQR 1.29–3.66%; Table 1). There was no correlation between the tissue score and positive cell abundance in SQAT (r = − 0.11, p = 0.71). Single-cell isolates are enriched in stromal vascular cells, immune cells, and small adipocytes, which suggests that larger mature adipocytes, which are filtered out in the cell separation process, may be a source of SAβ-gal-positive cells, and assessing stromal vascular fractions or isolated cells is not an accurate approach for quantitating this biomarker in situ.
Table 1.
Descriptive data for non-human primate cohort demographics and study outcomes
| Mean | Median | Minimum | Maximum | Interquartile range | |
|---|---|---|---|---|---|
| Age (years) | 14.15 | 13.66 | 8.91 | 23.02 | 11.53–16.15 |
| Body weight (kg) | 5.96 | 5.85 | 4.04 | 9.12 | 5.18–6.86 |
| Waist circumference (cm) | 38.98 | 39.00 | 28.00 | 50.50 | 35.50–43.13 |
| Fasting blood glucose (mg/dL) | 99.36 | 73.00 | 45.00 | 356.00 | 64.75–98.75 |
| Insulin (mU/L) | 25.23 | 16.18 | 0.54 | 108.87 | 8.26–27.30 |
| HOMA score (AU) | 6.10 | 2.72 | 0.10 | 39.62 | 1.59–6.42 |
| High-density lipoprotein cholesterol (mg/dL) | 95.76 | 74.47 | 38.54 | 205.81 | 54.15–138.80 |
| Triglycerides (mg/dL) | 70.99 | 58.60 | 33.40 | 181.54 | 47.66–77.82 |
| IL-6 (pg/mL) | 7.58 | 5.38 | 1.34 | 40.64 | 3.86–7.99 |
| IL-1ß (pg/mL) | 3.00 | 2.09 | 0.03 | 10.41 | 0.63–4.59 |
| MCP-1 (pg/mL) | 323.81 | 287.65 | 76.96 | 901.19 | 230.18–391.93 |
| Adiponectin (ng/mL) | 77.87 | 80.20 | 3.27 | 162.14 | 49.22–105.59 |
| PAI-1 (ng/mL) | 6.61 | 4.42 | 1.03 | 25.17 | 1.98–7.14 |
| Fatness (% body weight) | 27.37 | 25.26 | 8.22 | 52.00 | 17.96–37.90 |
| Visceral:subcutaneous adipose ratio | 2.64 | 2.36 | 0.64 | 8.62 | 1.56–3.22 |
| Liver attenuation (HU) | 58.32 | 59.85 | 28.00 | 72.67 | 53.33–64.13 |
| Subcutaneous adipose SAβ-gal score | 1.75 | 1.88 | 0.00 | 4.00 | 0.94–2.44 |
| Visceral adipose SAβ-gal score | 1.53 | 1.50 | 0.13 | 4.00 | 0.84–1.91 |
| SAβ-gal positive cell isolates (%) | 4.07 | 1.75 | 0.75 | 24.65 | 1.29–3.66 |
Abbreviations: HOMA, homeostasis model assessment for insulin resistance; IL, interleukin; MCP, monocyte chemoattractant protein; PAI, plasminogen activator inhibitor; SAβ-gal, senescence-associated beta-galactosidase; AU, arbitrary units; HU, Hounsfield units
Fig. 2.
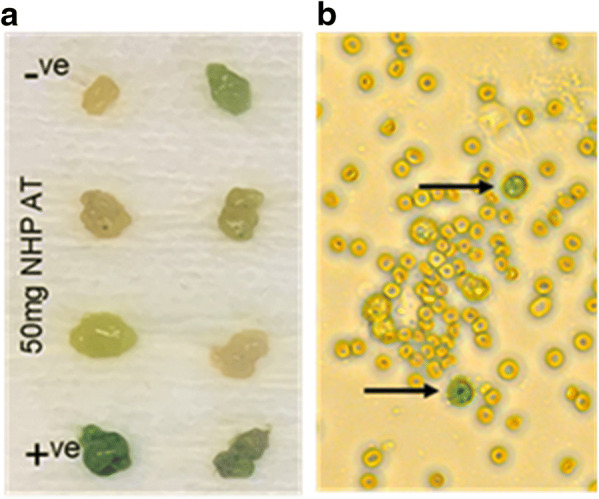
a Tissue staining and scoring. Tissues were scored by multiple blinded observers using a scale of 0–4. Tissue controls (positive, +; negative, −) were included to verify assay integrity. Negative staining resulted in a white/yellow adipose color and positive staining resulted in a dark blue color. b Cell staining: Single-cell isolates from whole adipose tissue were plated, stained, and images captured. Senescent cells, indicated by arrows, were deemed as positive due to whole cell staining with a blue color. Positive cells were counted manually by blinded observers, and an image analysis application was used to count total numbers of cells plated as the denominator used in calculating final percentages
Fig. 3.
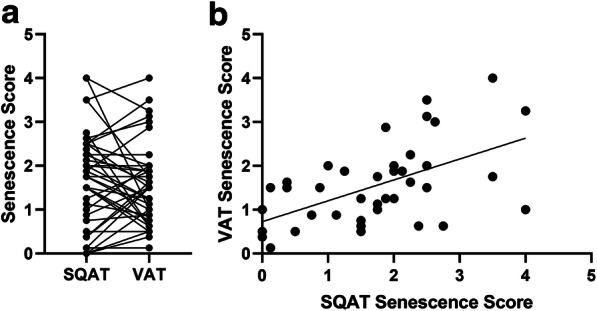
a Tissue scores for senescence-associated β-galactosidase (SAβ-gal) staining did not differ within individual subjects’ subcutaneous (SQAT) and visceral (VAT) adipose samples collected (p = 0.31). b Tissue scores calculated for SQAT and VAT were highly associated (r = 0.56, p < 0.001)
SQAT tissue score was positively associated with age (r = 0.303, p < 0.05; Fig. 4a), whereas VAT did not associate with age (r = − 0.15, p = 0.37). More importantly, age was singular in its relationship with SQAT SAβ-gal staining scores (Table 2). VAT also did not associate with measures of health or obesity. When the relationship with age was adjusted for fatness (fat % BW as measured from CT), the strength of the relationship did not change (partial correlation r = 0.29), which was expected as age and obesity measures were also unassociated (example: age vs. BW r = 0.06, p = 0.70). There were no associations with metabolic syndrome criteria, further supporting markers of senescence being a fundamentally age-associated process.
Fig. 4.
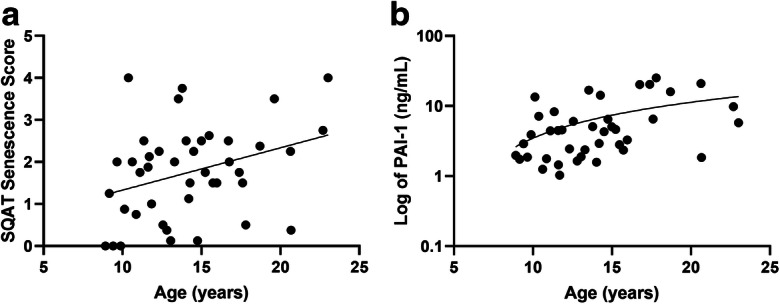
a Tissue scores for senescence-associated β-galactosidase (SAβ-gal) staining in SQAT significantly and positively associated with chronological age (r = 0.31, p < 0.05). b Circulating plasminogen activator inhibitor 1 (PAI-1), a proposed biomarker of senescence, was significantly and positively associate with chronological age (r = 0.39, p = 0.01)
Table 2.
Association outcomes (R) between senescence-associated beta-galactosidase scores calculated for subcutaneous adipose tissue (SQAT), visceral adipose tissue (VAT), and demographic and inflammatory variables
| Age (years) | Waist (cm) | A1c (%) | Fatness (% BW) | SQ:VIS adipose (AU) | Fasting glucose (mg/dL) | Insulin (mU/L) | HOMA (AU) | SBP (mmHg) | DBP (mmHg) | HDLC (mg/dL) | TG (mg/dL) | IL-6 (pg/mL) | IL-1ß (pg/mL) | MCP-1 (pg/mL) | Adiponectin (ng/mL) | PAI-1 (ng/mL) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SQAT score R | 0.303 | − 0.022 | 0.079 | − 0.032 | 0.23 | 0.049 | − 0.13 | − 0.12 | 0.19 | 0.045 | 0.021 | 0.041 | − 0.056 | 0.19 | 0.029 | − 0.16 | 0.14 | |
| N | 43 | 43 | 43 | 43 | 43 | 43 | 41 | 41 | 43 | 43 | 43 | 43 | 43 | 37 | 43 | 43 | 41 | |
| p value | 0.04 | 0.89 | 0.61 | 0.84 | 0.14 | 0.76 | 0.41 | 0.44 | 0.22 | 0.77 | 0.90 | 0.80 | 0.72 | 0.26 | 0.85 | 0.31 | 0.37 | |
| VAT score R | − 0.15 | 0.055 | − 0.091 | .013 | 0.020 | 0.053 | − 0.026 | − 0.063 | 0.097 | − 0.021 | 0.22 | − 0.101 | − 0.007 | − 0.16 | 0.19 | 0.028 | − 0.068 | |
| N | 40 | 40 | 40 | 40 | 40 | 40 | 38 | 40 | 40 | 40 | 40 | 40 | 40 | 34 | 40 | 40 | 38 | |
| p value | 0.37 | 0.74 | 0.58 | 0.94 | 0.90 | 0.75 | 0.88 | 0.71 | 0.55 | 0.90 | 0.17 | 0.54 | 0.96 | 0.37 | 0.23 | 0.87 | 0.68 | |
| Cell isolate % R | 0.39 | 0.24 | 0.48 | 0.44 | − 0.52 | 0.47 | 0.15 | 0.47 | − 0.62 | − 0.44 | 0.53 | 0.36 | − 0.34 | 0.404 | − 0.017 | − 0.29 | 0.29 | |
| N | 14 | 14 | 14 | 14 | 14 | 14 | 14 | 14 | 14 | 14 | 14 | 14 | 14 | 11 | 14 | 14 | 14 | |
| p value | 0.16 | 0.40 | 0.08 | 0.12 | 0.06 | 0.09 | 0.60 | 0.09 | 0.02 | 0.11 | 0.05 | 0.21 | 0.23 | 0.22 | 0.95 | 0.31 | 0.32 |
Abbreviations: A1c, glycosylated hemoglobin chain A1c; HOMA, homeostasis model assessment for insulin resistance; HDLC, high-density lipoprotein cholesterol; TG, triglyceride; IL, interleukin; MCP, monocyte chemoattractant protein; PAI, plasminogen activator inhibitor; SBP, systolic blood pressure; DBP, diastolic blood pressure
The isolated adipocyte cell fraction was underpowered to detect relationships between health variables (Table 2), but it is noteworthy that the direction and strength of the relationship with age were similar to those of SQAT whole tissue staining, although it was not significant. The cell isolates were from adult and geriatric age categories (n = 7/age group) and it is worthy of note that geriatric animals had > 4 times higher percentage of positive cells which neared statistical significance and is supportive that cells in this fraction become more senescent with increasing age (1.61% vs. 6.54%, p = 0.06). Additionally, unlike whole tissue, the isolated cell staining frequency was generally positively related to metabolic health parameters such as glycemia-related measures and obesity.
SASP and inflammatory markers resulted in only PAI-1 having a moderately strong relationship with age (r = 0.39, p = 0.01; Fig. 4b). No SASP marker or cytokine was significantly associated with tissue scores or any measure of body composition, and only IL-6 was positively associated with the percentage of SAβ-gal staining isolated cells (r = 0.65, p = 0.01). This may result from adipose tissue scores being unable to reflect all systemic sources of PAI-1 secretion, and/or that this marker is related to aging-associated senescent cells and increases in other stimuli for PAI-1 release. Adipose does secrete PAI-1 so even though adiposity measures and PAI-1 were unrelated, we adjusted the association of age with PAI-1 by percentage of body fat which did attenuate the relationship with chronological age (r = 0.29, p = 0.06) and indicated there may be an important interaction between aging and obesity in senescence abundance. The abundance of SAβ-gal-positive cells, unlike age, was related to higher IL-6 levels in our generally healthy aging non-human primate population (Table 2) and IL-6 levels were not related to adiposity.
Discussion
Senescence is a cell state characterized by primarily irreversible replicative arrest through either the p53-p21 or p16Ink4a –pRB pathways, secretion of pro-inflammatory cytokines, pro-fibrotic factors, and growth factors that negatively impact local and systemic environments (Zhu et al. 2014; Kirkland and Tchkonia 2017; Justice et al. 2019). SAβ-gal staining is associated with non-proliferative adipose cell senescence and reflects the pH-dependent hydrolase activity that is enhanced in the lysosomes of senescent cells (Lee et al. 2006). We have conducted the largest investigation to date of primate adipose SAβ-gal staining, utilizing a method currently in wide use as a biomarker of senescence. We also present the first comparison between adipose depots and evaluate fractionated adipose for consistency in results. Our results support the ongoing use of SAβ-gal staining of adipose tissue as an aging-related biomarker, and we conclude that sampling of the more readily accessible subcutaneous fat source is the preferred substrate for further senescence-related investigations. Single-cell isolates of adipose show similar staining properties and increase with age as well as with metabolic syndrome criteria. The poor correlation between cells and tissue staining could suggest that primarily mature post-mitotic adipocytes become senescent, which is a concept increasingly being discussed and accepted across multiple post-mitotic cell types.
Senescent cell accumulation is proposed to increase adipose inflammation, and therefore contribute to tissue damage and dysfunction that increases with age. Illustrative of this is an in vivo relationship between senescent cell abundance in adipose and physical function in older female humans (Justice et al. 2018). The presence of senescent cells in tissue leads to SASP and is a sufficient paracrine effect to initiate further senescent cell development (Xu et al. 2018), thus promoting a vicious cycle in aging and tissue dysfunction. This effect has been specifically shown in adipose tissue (Xu et al. 2015) where senescence inhibits adipocyte differentiation and limits the ability for adipose to accumulate lipid and perform normal biological regulation of energy metabolism. High glucose induces premature cellular senescence in endothelial cells, adipocyte progenitors, and fibroblasts (Cramer et al. 2010; Palmer et al. 2015; Yokoi et al. 2006). Aging and dysregulated energy metabolism often co-occur, so dissecting whether senescence primarily contributes to metabolic disease or metabolic disease drives senescence is a difficult question to answer. In our NHP model, which spontaneously displays a range of metabolic health status (Kavanagh et al. 2017), the data suggests that aging primarily increases senescence across all cells in adipose tissue, but also that metabolic health may be related to senescence in the stromal vascular fraction. Senescent cells are an important target for metabolic syndrome as they accumulate in human AT with aging and obesity, both of which contribute to metabolic disease development (Kirkland and Tchkonia 2017). Studies have not determined how clearing senescent cells impacts surviving macrophages; however, preliminary evidence suggests removal of senescent cells ameliorates inflammation and indices of health and life spans (Baker et al. 2016; Ghosh et al. 2019; Palmer et al. 2019).
Senescent cell accumulation alters the AT microenvironment and likely influences macrophage polarization. In obesity, macrophages can account for a significant proportion of cells in adipose tissue, with some estimates being as high as 40% of all cells (Weisberg et al. 2003). Resistance and aerobic exercise training in senescence-accelerated-prone mice decreased pro-inflammatory cytokine mRNA expression in AT, attenuated AT classically activated M1-type macrophage infiltration, and M1/M2 (alternatively activated) macrophage polarization (Uchida et al. 2019). Senescent macrophages respond to environmental stimuli and can then return to an M-phenotype that has polarized accordingly (Vernon 2003). Senolytics, or senescent cell clearing agents, alleviate AT inflammation and improve AT function in in vivo models (Palmer et al. 2015; Xu et al. 2018; Justice et al. 2019). Deployment of the tyrosine kinase inhibitor D+Q (48-h treatment) on omental adipose explants from obese patients decreased pro-inflammatory cytokine secretion, including monocyte chemoattractant protein-1 (MCP-1) (Xu et al. 2018). Single treatment in obese people reduced SQAT SAβ-gal staining as well as macrophage numbers and crown-like structures (Hickson et al. 2019). The frequency of positive staining cells in this study of SQAT people was approximately 3% (median value) when whole tissue was evaluated (Hickson et al. 2019) as opposed to a median value of 1.75% in our single-cell isolate assessments. This corroborates our interpretation that a significant number of mature adipocytes are SAβ-gal positive, leading to differences in tissue staining versus fractionating out the smaller cells in adipose. Treatment of diet-induced obese mice with D+Q reduced total VAT macrophage infiltration and improved glucose uptake (Palmer et al. 2015). D+Q treatment has not been deployed in healthy aged humans or NHPs, but determining the effects on health span and life span would be a large translational step towards determining if clearing senescent cells will prevent age-related development of metabolic disease and organ dysfunction.
Evaluation of selected cytokines indicated that none was highly related to Saβ-gal staining levels although PAI-1, which was age related, had non-significant positive relationships in SQAT and its isolated cells. This circulatory biomarker has been purported to be a senescence indicator (Vaughan et al. 2017), but much of the evidence has been related to endothelial cell senescence (Xu et al. 2000) and cardiovascular disease progression. PAI-1 is a serine protease inhibitor and functions as the principal inhibitor of tissue plasminogen activators (t-PA) and urokinase plasminogen activator in mammals. By directly inhibiting t-PA, PAI-1 promotes fibrosis in several tissues, leading to the onset and progression of many age-related diseases such as arteriosclerosis and diabetes (Vaughan et al. 2017). Recent studies also show a strong correlation between increased PAI-1 secretion and accelerated aging in mice (Eren et al. 2014; Omer et al. 2018), and PAI-1 levels are higher in fibroblasts from aged human fibroblast cultures compared with those from young sources (Goldstein et al. 1994). Notably, PAI-1 was validated as a mediator of cellular senescence as PAI-1-deficient fibroblasts were resistant to senescence and exhibited prolonged replication compared with wild-type cells (Kortlever et al. 2006). These studies along with many others reveal that PAI-1 may directly relate to whole-body senescent cell burden. As these circulatory values increased with age and not obesity or metabolic disease measures, it is possible that the circulatory concentration reflects senescence in the vascular and other cellular compartments. In our analyses, this biomarker appears to be complementary and should be considered alongside other prioritized biomarkers of aging (Justice et al. 2018) that are proposed for geroscience interventional studies.
Our study has a number of limitations; most notably, sex as a biological variable was not addressed (all subjects were female), and differences in relationships in male subjects may exist. However, in human clinical studies of senolytics, sex differences have not been noted although sample sizes have been small (Justice et al. 2018, 2019). Senescence-associated βgal activity increases in aged tissues, making it a useful biomarker for the detection of senescent cells in vivo in primates (Itahana, Itahana et al. 2013). Moreover, many studies combine assays for in vitro and in vivo markers, such as p16Ink4a expression and SA-βgal activity, which can be very informative but still have their limitations. Both SA-βgal and p16Ink4a expression can be elevated in macrophages, and p16Ink4a is not consistently elevated in cells considered to be senescent. As such, both of these markers are neither 100% sensitive nor specific for detection of senescence (Khosla et al. 2020). Many pro-inflammatory cells such as macrophages, osteoclasts, and cancer cells exhibit a “senescence-like” phenotype, and all can arguably be considered a true senescent cell but not specifically associated with advancing age (Khosla et al. 2020). Furthermore, senescence phenotypes have been identified in neurons and were reported in mouse retinal ganglia cells under ischemic conditions (von Zglinicki et al. 2020). Additionally, the SA-βgal assay can produce false positive and negatives if the pH is off by ± 0.2 units from 6.0. Therefore, careful attention, to include assay controls using relevant tissues or cells in order to draw concrete conclusions of cellular senescence as well as combinations of cellular and tissue assays, is paramount.
NHP models of aging have proven useful to evaluate the translational relevance of many biological pathways considered critical (Lopez-Otin et al. 2013; Colman et al. 2014; Chichester et al. 2015). In the current report, we take advantage of their comparative biology to confirm the presence of increased senescence with chronological aging. We also note that, despite variability, such increase appears linearly across early adulthood through to advanced geriatric stages and without any large changes in trajectory or plateaus across the life span in this model. In conclusion, SA-βgal staining of adipose tissues and circulatory PAI-1 relate to age and may be important methods for evaluation of therapies intended to reverse aging in NHPs, and thus, perhaps translational to human intervention.
Acknowledgments
The authors wish to acknowledge the funding source listed below, all technicians involved in the care and evaluation of these animals, and the wonderful non-human primates that made this study possible.
Code availability
N/A
Author contributions
All authors participated in tissue sample collections, senescence scoring, and final manuscript review. KK is the Primary Investigator and the NIH grant recipient for this study, and performed all statistical evaluations. CS was the primary editor for the manuscript and created all figures and tables. AR and RV participated in ß-gal tissue staining. MB participated in histological evaluations. AO was the senior author of this manuscript, creating original drafts of each section, performed histological evaluations, and was the primary technician for ß-gal tissue staining, adipocyte isolation, and histological evaluations.
Funding information
Funding for this project was provided by the National Institute Health grant number R01HL142930.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
All animal procedures were conducted on a Wake Forest University Institutional Animal Care and Use Committee approved protocol according to recommendations in the Guide for Care and Use of Laboratory Animals and in compliance with the USDA Animal Welfare Act and Animal Welfare Regulations.
Consent to participate
N/A
Consent for publication
N/A
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Bacarella N, Ruggiero A, Davis AT, et al. Whole body irradiation induces diabetes and adipose insulin resistance in nonhuman primates. Int J Radiat Oncol Biol Phys. 2020;106(4):878–86. 10.1016/j.ijrobp.2019.11.034 [DOI] [PubMed]
- Baker DJ, Childs BG, Durik M, Wijers ME, Sieben CJ, Zhong J, Saltness RA, Jeganathan KB, Verzosa GC, Pezeshki A, Khazaie K, Miller JD, van Deursen JM. Naturally occurring p16(Ink4a)-positive cells shorten healthy lifespan. Nature. 2016;530(7589):184–189. doi: 10.1038/nature16932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birsoy K, Festuccia WT, Laplante M. A comparative perspective on lipid storage in animals. J Cell Sci. 2013;126(Pt 7):1541–1552. doi: 10.1242/jcs.104992. [DOI] [PubMed] [Google Scholar]
- Cartwright MJ, Schlauch K, Lenburg ME, Tchkonia T, Pirtskhalava T, Cartwright A, Thomou T, Kirkland JL. Aging, depot origin, and preadipocyte gene expression. J Gerontol A Biol Sci Med Sci. 2010;65(3):242–251. doi: 10.1093/gerona/glp213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chichester L, Wylie AT, Craft S, Kavanagh K. Muscle heat shock protein 70 predicts insulin resistance with aging. J Gerontol A Biol Sci Med Sci. 2015;70(2):155–162. doi: 10.1093/gerona/glu015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman RJ, Beasley TM, Kemnitz JW, Johnson SC, Weindruch R, Anderson RM. Caloric restriction reduces age-related and all-cause mortality in rhesus monkeys. Nat Commun. 2014;5:3557. doi: 10.1038/ncomms4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer C, Freisinger E, Jones RK, Slakey DP, Dupin CL, Newsome ER, Alt EU, Izadpanah R. Persistent high glucose concentrations alter the regenerative potential of mesenchymal stem cells. Stem Cells Dev. 2010;19(12):1875–1884. doi: 10.1089/scd.2010.0009. [DOI] [PubMed] [Google Scholar]
- Eren M, Boe AE, Murphy SB, Place AT, Nagpal V, Morales-Nebreda L, Urich D, Quaggin SE, Budinger GR, Mutlu GM, Miyata T, Vaughan DE. PAI-1-regulated extracellular proteolysis governs senescence and survival in Klotho mice. Proc Natl Acad Sci U S A. 2014;111(19):7090–7095. doi: 10.1073/pnas.1321942111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh AK, O’Brien M, Mau T, Qi N, Yung R. Adipose tissue senescence and inflammation in aging is reversed by the young milieu. Journals of Gerontology Series a-Biological Sciences and Medical Sciences. 2019;74(11):1709–1715. doi: 10.1093/gerona/gly290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein S, Moerman EJ, Fujii S, Sobel BE. Overexpression of plasminogen activator inhibitor type-1 in senescent fibroblasts from normal subjects and those with Werner syndrome. J Cell Physiol. 1994;161(3):571–579. doi: 10.1002/jcp.1041610321. [DOI] [PubMed] [Google Scholar]
- Hickson LJ, Langhi Prata LGP, Bobart SA, Evans TK, Giorgadze N, Hashmi SK, Herrmann SM, Jensen MD, Jia Q, Jordan KL, Kellogg TA, Khosla S, Koerber DM, Lagnado AB, Lawson DK, LeBrasseur NK, Lerman LO, McDonald KM, McKenzie TJ, Passos JF, Pignolo RJ, Pirtskhalava T, Saadiq IM, Schaefer KK, Textor SC, Victorelli SG, Volkman TL, Xue A, Wentworth MA, Wissler Gerdes EO, Zhu Y, Tchkonia T, Kirkland JL. Senolytics decrease senescent cells in humans: preliminary report from a clinical trial of dasatinib plus quercetin in individuals with diabetic kidney disease. EBioMedicine. 2019;47:446–456. doi: 10.1016/j.ebiom.2019.08.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itahana K, Itahana Y, Dimri GP. Colorimetric detection of senescence-associated beta galactosidase. Methods Mol Biol. 2013;965:143–156. doi: 10.1007/978-1-62703-239-1_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justice JN, Gregory H, Tchkonia T, LeBrasseur NK, Kirkland JL, Kritchevsky SB, Nicklas BJ. Cellular senescence biomarker p16INK4a+ cell burden in thigh adipose is associated with poor physical function in older women. J Gerontol A Biol Sci Med Sci. 2018;73(7):939–945. doi: 10.1093/gerona/glx134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justice JN, Nambiar AM, Tchkonia T, LeBrasseur NK, Pascual R, Hashmi SK, Prata L, Masternak MM, Kritchevsky SB, Musi N, Kirkland JL. Senolytics in idiopathic pulmonary fibrosis: results from a first-in-human, open-label, pilot study. EBioMedicine. 2019;40:554–563. doi: 10.1016/j.ebiom.2018.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavanagh K, Davis AT, Peters DE, LeGrand AC, Bharadwaj MS, Molina AJ. Regulators of mitochondrial quality control differ in subcutaneous fat of metabolically healthy and unhealthy obese monkeys. Obesity (Silver Spring) 2017;25(4):689–696. doi: 10.1002/oby.21762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khosla S, Farr JN, Tchkonia T, Kirkland JL. The role of cellular senescence in ageing and endocrine disease. Nat Rev Endocrinol. 2020;16(5):263–275. doi: 10.1038/s41574-020-0335-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkland JL, Tchkonia T. Cellular senescence: a translational perspective. EBioMedicine. 2017;21:21–28. doi: 10.1016/j.ebiom.2017.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortlever RM, Higgins PJ, Bernards R. Plasminogen activator inhibitor-1 is a critical downstream target of p53 in the induction of replicative senescence. Nat Cell Biol. 2006;8(8):877–884. doi: 10.1038/ncb1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence I, Bene M, Nacarelli T, Azar A, Cohen JZ, Torres C, Johannes G, Sell C. Correlations between age, functional status, and the senescence-associated proteins HMGB2 and p16(INK4a) Geroscience. 2018;40(2):193–199. doi: 10.1007/s11357-018-0015-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BY, Han JA, Im JS, Morrone A, Johung K, Goodwin EC, Kleijer WJ, DiMaio D, Hwang ES. Senescence-associated beta-galactosidase is lysosomal beta-galactosidase. Aging Cell. 2006;5(2):187–195. doi: 10.1111/j.1474-9726.2006.00199.x. [DOI] [PubMed] [Google Scholar]
- Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153(6):1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omer A, Patel D, Lian XJ, Sadek J, Di Marco S, Pause A, et al. Stress granules counteract senescence by sequestration of PAI-1. EMBO Rep. 2018;19(5). [DOI] [PMC free article] [PubMed]
- Palmer AK, Tchkonia T, LeBrasseur NK, Chini EN, Xu M, Kirkland JL. Cellular senescence in type 2 diabetes: a therapeutic opportunity. Diabetes. 2015;64(7):2289–2298. doi: 10.2337/db14-1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer AK, Xu M, Zhu Y, Pirtskhalava T, Weivoda MM, Hachfeld CM, Prata LG, van Dijk TH, Verkade E, Casaclang-Verzosa G, Johnson KO, Cubro H, Doornebal EJ, Ogrodnik M, Jurk D, Jensen MD, Chini EN, Miller JD, Matveyenko A, Stout MB, Schafer MJ, White TA, Hickson LJ, Demaria M, Garovic V, Grande J, Arriaga EA, Kuipers F, von Zglinicki T, LeBrasseur NK, Campisi J, Tchkonia T, Kirkland JL. Targeting senescent cells alleviates obesity-induced metabolic dysfunction. Aging Cell. 2019;18(3):e12950. doi: 10.1111/acel.12950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirakawa K, Yano W, Inoue K, Katsumata Y, Endo J, Sano M. Influence of long term administration of tofogliflozin on chronic inflammation of visceral adipose tissue in mice with obesity induced by a high-fat diet. PLoS One. 2019;14(1):e0211387. doi: 10.1371/journal.pone.0211387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchkonia T, Morbeck DE, Von Zglinicki T, Van Deursen J, Lustgarten J, Scrable H, Khosla S, Jensen MD, Kirkland JL. Fat tissue, aging, and cellular senescence. Aging Cell. 2010;9(5):667–684. doi: 10.1111/j.1474-9726.2010.00608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida M, Horii N, Hasegawa N, Fujie S, Oyanagi E, Yano H, Iemitsu M. Gene expression profiles for macrophage in tissues in response to different exercise training protocols in senescence mice. Front Sports Act Living. 2019;1(50). 10.3389/fspor.2019.00050. [DOI] [PMC free article] [PubMed]
- van Deursen JM. The role of senescent cells in ageing. Nature. 2014;509(7501):439–446. doi: 10.1038/nature13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan DE, Rai R, Khan SS, Eren M, Ghosh AK. Plasminogen activator inhibitor-1 is a marker and a mediator of senescence. Arterioscler Thromb Vasc Biol. 2017;37(8):1446–1452. doi: 10.1161/ATVBAHA.117.309451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernon RGFDJ. Structure and function of white adipose tissue. Encyclopedia of Food Sciences and Nutrition, Second Edition. B. Caballero, Elsevier Science Ltd, 2003;23–29.
- von Zglinicki T, Wan T, Miwa S. Senescence in post-mitotic cells: a driver of aging? Antioxid Redox Signal 2020. [DOI] [PMC free article] [PubMed]
- Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Investig. 2003;112(12):1796–1808. doi: 10.1172/JCI200319246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D, Neville R, Finkel T. Homocysteine accelerates endothelial cell senescence. FEBS Lett. 2000;470(1):20–24. doi: 10.1016/S0014-5793(00)01278-3. [DOI] [PubMed] [Google Scholar]
- Xu M, Palmer AK, Ding H, Weivoda MM, Pirtskhalava T, White TA, Sepe A, Johnson KO, Stout MB, Giorgadze N, Jensen MD, LeBrasseur NK, Tchkonia T, Kirkland JL. Targeting senescent cells enhances adipogenesis and metabolic function in old age. Elife. 2015;4:e12997. doi: 10.7554/eLife.12997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Pirtskhalava T, Farr JN, Weigand BM, Palmer AK, Weivoda MM, Inman CL, Ogrodnik MB, Hachfeld CM, Fraser DG, Onken JL, Johnson KO, Verzosa GC, Langhi LGP, Weigl M, Giorgadze N, LeBrasseur NK, Miller JD, Jurk D, Singh RJ, Allison DB, Ejima K, Hubbard GB, Ikeno Y, Cubro H, Garovic VD, Hou X, Weroha SJ, Robbins PD, Niedernhofer LJ, Khosla S, Tchkonia T, Kirkland JL. Senolytics improve physical function and increase lifespan in old age. Nat Med. 2018;24(8):1246–1256. doi: 10.1038/s41591-018-0092-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoi T, Fukuo K, Yasuda O, Hotta M, Miyazaki J, Takemura Y, Kawamoto H, Ichijo H, Ogihara T. Apoptosis signal-regulating kinase 1 mediates cellular senescence induced by high glucose in endothelial cells. Diabetes. 2006;55(6):1660–1665. doi: 10.2337/db05-1607. [DOI] [PubMed] [Google Scholar]
- Zeng S, Shen WH, Liu L. Senescence and cancer. Cancer Transl Med. 2018;4(3):70–74. doi: 10.4103/ctm.ctm_22_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Armstrong JL, Tchkonia T, Kirkland JL. Cellular senescence and the senescent secretory phenotype in age-related chronic diseases. Curr Opin Clin Nutr Metab Care. 2014;17(4):324–328. doi: 10.1097/MCO.0000000000000065. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article.


