Abstract
Aging is associated with increased prevalence and severity of pathogenic outcomes of periodontal disease, including soft tissue degeneration and bone loss around the teeth. Although lipopolysaccharide (LPS) derived from the key periodontal pathogen Porphyromonas gingivalis (Pg) plays an important role in the promotion of inflammation and osteoclastogenesis via toll-like receptor (TLR)4 signaling, its pathophysiological role in age-associated periodontitis remains unclear. This study investigated the possible effects of Pg-LPS on RANKL-primed osteoclastogenesis and ligature-induced periodontitis in relation to aging using young (2 months old) and aged (24 months old) mice. To the best of our knowledge, our results indicated that expression of TLR4 was significantly diminished on the surface of osteoclast precursors isolated from aged mice compared with that of young mice. Furthermore, our data demonstrated that the TLR4 antagonist (TAK242) dramatically decreased the numbers of tartrate-resistant acid phosphatase positive (TRAP+) osteoclasts differentiated from RANKL-primed young osteoclast precursors (OCPs) compared with those isolated from aged mice in response to Pg-LPS. In addition, using a ligature-induced periodontitis mouse model, we demonstrated that Pg-LPS elevated (1) secretion of senescence-associated secretory phenotype (SASP) markers, including the pro-inflammatory cytokines TNF-α, IL-6, and IL-1β, as well as osteoclastogenic RANKL, and (2) the number of OCPs and TRAP+ osteoclasts in the periodontal lesion induced in young mice. In contrast, Pg-LPS had little, or no, effect on the promotion of periodontitis inflammation induced in aged mice. Altogether, these results indicated that periodontal disease in older mice occurs in a manner independent of canonical signaling elicited by the Pg-LPS/TLR4 axis.
Keywords: Aging, Porphyromonas gingivalis lipopolysaccharide, Periodontitis, Osteoclastogenesis, Bone loss
Introduction
Periodontal disease is one of the most prevalent chronic age-associated infectious diseases affecting humans. Pathologic manifestations are characterized by soft tissue degeneration and alveolar bone loss around the teeth in association with oral microbial dysbiosis [11]. Alarming epidemiological data indicates that around 70% of senior patients at age over 65 years old suffer from some type of periodontal disease, compared with 40% of that incidence in the young and middle-aged population [15, 16].
It is commonly accepted that elevated alveolar bone loss in aged patients as well as in various animal models of bone osteolysis is associated with enhanced formation osteoclast derived from the hematopoietic progenitors of monocytes/macrophages and serves as the principal bone resorptive cells [24]. Osteoclast formation is critically induced by the receptor activators of nuclear factor-κB ligand (RANKL). It is true that one of the main characteristics of elevated alveolar bone loss, as well as other osteolytic lesions, e.g., osteoporosis, in aged patients is the heightened concentrations of RANKL compared with those in young patients [17]. In addition to RANKL, a distinct panel of pro-inflammatory cytokines, including, for example, tumor necrosis factor alpha (TNF-α), interleukin-1β (IL-1β), and IL-6, collectively termed senescence-associated secretory phenotype (SASP) elevates osteoclast activity, particularly in states of age-associated periodontitis [8, 37].
Although bacterial dysbiosis is thought to be linked to the pathogenesis of periodontitis, the role played by oral bacteria in age-associated periodontitis is not clearly understood. Porphyromonas gingivalis, an oral Gram-negative pathogen that colonizes in the oral cavity, is a keystone pathogen that elicits dysbiosis-mediated inflammation, resulting in the development of chronic periodontitis [23, 35, 36]. Furthermore, it was demonstrated that aging increases the colonization of P. gingivalis and reduces bacterial diversity in a mouse model of periodontitis [46]. Accumulated lines of evidence indicate that the lipopolysaccharide (LPS) located in the outer membrane of P. gingivalis (Pg-LPS) is considered one of the main pathogenic factors eliciting immune-inflammatory responses leading to elevated local production of osteogenic RANKL and osteoclast differentiation in the periodontitis lesions [20, 25, 41] via its ligation with toll-like receptor-(TLR)4 [31, 48]. In addition, the inflammatory contribution from Pg-LPS is connected to other age-associated diseases such as atherosclerosis and Alzheimer’s disease [40, 42].
Although chronic periodontitis is considered to be an age-associated disease, most of the published pre-clinical studies for Pg-LPS-elicited inflammation are performed using cells isolated from either young mice (less than 12 weeks old) or healthy human individuals younger than 20 years old [21, 31]. We, however, recently demonstrated that the expression of TLR4 is significantly reduced on the surface of peritoneal macrophages isolated from aged mice compared with that of young mice [47]. Thus, this study aimed to evaluate the age-related impact of Pg-LPS on RANKL-primed osteoclastogenesis in vitro, as well as in an experimental model of ligature-induced periodontitis using young (2 months old and aged 24 months old) mice.
Our results indicated that the expression of TLR4 on the cell surface of osteoclast precursors (OCPs) diminished along with the aging of mice. Furthermore, we demonstrated that Pg-LPS significantly elevated RANKL-mediated osteoclastogenesis as well as increased the number of OCPs and TRAP+ cells in the periodontal lesions induced in young mice compared with those in aged mice.
Material and methods
Animals
Young (2 months old) and aged (24 months old) CSF1r-eGFP knock-in (KI) mice (Jackson Laboratory) and their wild-type (WT) C57BL/6 mice were used in this study. WT mice in this study were obtained from the National Institute on Aging Aged Rodent Colony. The mice were kept on a 12-h light-dark cycle at constant temperature, with free access to food and water. This study was conducted in strict accordance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health, and the experimental procedures were approved by the Institutional Animal Care and Use Committee at Forsyth Institute and Nova Southeastern University.
Mouse models of periodontitis
To induce periodontal bone loss, a mouse model of ligature-induced periodontitis was employed as we described earlier [30, 45]. Briefly, young and aged male mice were randomly divided into three groups (n = 6 mice/group): (I) control, no ligature, (II) group receiving ligation alone, followed by a local injection of phosphate-buffered saline (PBS) solution containing ultrapure Pg-LPS (10 μg/μl; InVivoGen) into palatal gingival tissue between the maxillary first and second molars, and (III) group receiving ligation, followed by a local injection of PBS solution only. To induce bone loss, animals from the groups II and III were anesthetized by an intraperitoneal (i.p.) injection of KX cocktail to minimize discomfort and pain, and then a silk ligature (5.0 size; Johnson & Johnson) was placed and a knot made by tying the ligatured ends at the palatal site of the upper left second molar with assistant of Dumont forceps assistance (Fine Science Tools). Then, a solution of Pg-LPS (group III) in PBS or control PBS alone (groups I and II) was injected once every other day using a 10-μl NanoFill syringe with attached 33G needle (WPI). The injection of each solution was performed by the same operator in the morning between 8:00 and 10:00 am. At day 8, mice were euthanized, and tissues were collected for analysis of cytokine production and immunohistochemistry.
Preparation of gingival tissue homogenates for enzyme-linked immunosorbent assay
Gingival tissues were homogenized with a Dounce glass homogenizer in PBS supplemented with 0.05% Tween20 in the presence of 10 μg/ml of ethylenediaminetetraacetic acid (EDTA)-free protease inhibitor cocktail (Thermo Scientific™). The suspension of homogenized tissue was centrifuged at 1500 rpm and supernatant was harvested. Concentrations of RANKL, TNF-α, IL-1β, and IL-6 proteins were measured in the collected supernatant using commercial sandwich enzyme-linked immunosorbent assay (ELISA) kits (R&D) according to the manufacturer’s instructions.
Histological staining of periodontal tissue sections
Maxilla bone collected from the euthanized mice was fixed in 10% formaldehyde and then decalcified in 10% EDTA solution. Decalcified samples were embedded in paraffin and sectioned (6-μm thickness). Tartrate-resistant acid phosphatase (TRAP) staining was performed as previously described [29]. Briefly, tissue sections were first incubated in 0.2 M acetate buffer containing 50 mM L-(+)-tartaric acid (Sigma) at room temperature and then in TRAP staining solution (0.2 M acetate buffer, 50 mM L-(+)-tartaric acid, 0.5 mg/ml naphthol AS-MX phosphate, and 1.1 mg/ml Fast Red ASTR salt; Sigma) at 37 °C. Finally, the tissue sections were counterstained with hematoxylin solution (Sigma) at room temperature.
Multicolor flow cytometry of osteoclast precursors
Our recently published observations indicated that macrophages, as a source of OCPs, isolated from aged mice [47], show reduced expression of TLR4 compared with that of young mice. We also showed that CD11b+CSF1r-eGFP+ cells isolated from the spleen of Csf1r-eGFP-KI mice could be characterized as OCPs [29], CD11b+ CSF1r-eGFP+ OCPs were FACS sorted from the spleen of young and aged CSF1r-eGFP-KI mice as we described elsewhere [29]. Therefore, mononuclear cells collected from the spleen of young and aged CSF1r-eGFP-KI mice were stained with anti-mouse CD16/32 (Fc-blocking antibody, BioLegend) and anti-CD11b conjugated to Pacific blue. Then, the CD11b+CSF1r-eGFP+ cells were FACS sorted using the BD FACSAria™ II Cell Sorter. To characterize the age-dependent expressions of TLR2 and TLR4 on the surface of OCPs, cells were additionally incubated with anti-CD282-PE and anti-CD284-Alexa 647 (BioLegend). To quantify the number of CD11b+CSF1r-eGFP+ OCPs in the periodontal lesion, young and aged CSF1r-eGFP-KI mice were euthanized 72 h after the ligature placement. Then, whole maxillae with gingival tissue were dissected and incubated in digestion buffer (1 mg/ml of Collagenase D and 25 U/ml DNase I, both from Roche) in MEM-α supplemented with 10% FBS at 37 °C for 60 min. Mononuclear cells were separated from blood and tissue by Ficoll histopaque-1083 (Sigma) density gradient and then the cell interface (buffy coat) was washed with cold PBS. Finally, the cells were incubated with anti-mouse CD16/32 followed by staining with anti-mouse CD11b Ab conjugated with Pacific blue (BioLegend). Data were acquired on the BD FACSAria™ II Cell Sorter (BD Biosciences) and analyzed using the FlowJo (ver. 10) software (Tree Star, Ashland, OR).
RANKL-induced osteoclastogenesis and TRAP staining in vitro
FACS-sorted CD11b+CSF1r-eGFP+ OCPs were seeded in 96-well plates at a density of 1 × 104 cells/well in α-MEM medium (Life Technologies) containing 10% FBS (Atlanta Biologicals) and 30 ng/ml of M-CSF and 5 ng/ml of RANKL (BioLegend) recombinant proteins in the presence or absence of various concentrations of ultrapure Pg-LPS (1, or 10 ng/ml; InvivoGen), CU-CPT22 (1 and 10 μM; TLR2 antagonist; Cayman Chemical), and TAK242 (1 and 10 μM; TLR4 antagonist; Cayman Chemical). Five days later, cells were stained for tartrate-resistant acid phosphatase (TRAP) using a leukocyte acid phosphatase kit (Sigma). TRAP-positive (TRAP+) cells with more than three nuclei were considered mature osteoclasts and were microscopically counted. The results were expressed as numbers per well.
Statistical analysis
The collected data were analyzed using the Student’s t test for the comparison between two groups, or a one-way ANOVA with post hoc Tukey’s test for the comparisons among different groups. A p < 0.05 was considered statistically significant. Data were analyzed using the Sigma Plot v.14 software.
Results
Aging impaired the expressions of TLR4 receptor on the surface OCPs in vitro
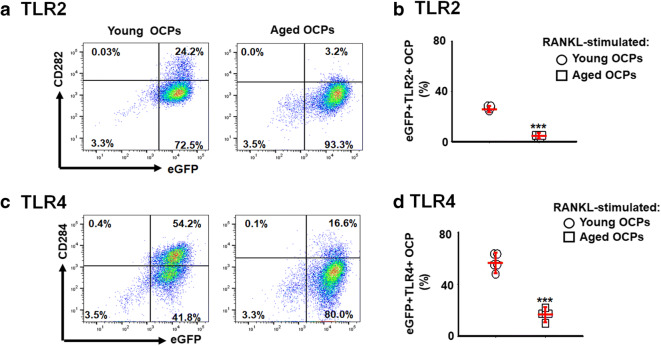
Because it was reported that toll-like receptor (TLR)2 and TLR4 both play an essential role in periodontitis [1, 10, 22], we first assessed the age-dependent expressions of the TLR2 and TLR4 receptors on the surface of FACS-sorted CD11b+CSF1r-eGFP+ OCPs using a flow cytometer. The expressions of TLR2 and TLR4 were significantly diminished on OCPs isolated from aged CSF1r-eGFP-KI mice compared with those of young mice (Fig. 1a–d). These results indicated that the expressions of TLR2 and TLR4 on OCPs decline with aging, which may negatively impact the engagement of the Pg-LPS in periodontal bone loss associated with aging.
Fig. 1.
Age-dependent expressions of Pg-LPS receptors, TLR2, and TLR4, on the surface of CD11b+CSF1r-eGFP+ OCPs. Osteoclast precursors (OCPs) were FACS sorted from the spleen of young (2 months old) and aged (24 months old) CSF1r-eGFP-KI mice and then were stimulated with macrophage colony-stimulating factor (M-CSF) (30 ng/ml) and RANKL (5 ng/ml). After 24 h of stimulation, the expression of TLR2 (a and b) and TLR4 (c and d) was evaluated by multicolor flow cytometry. ***p < 0.001
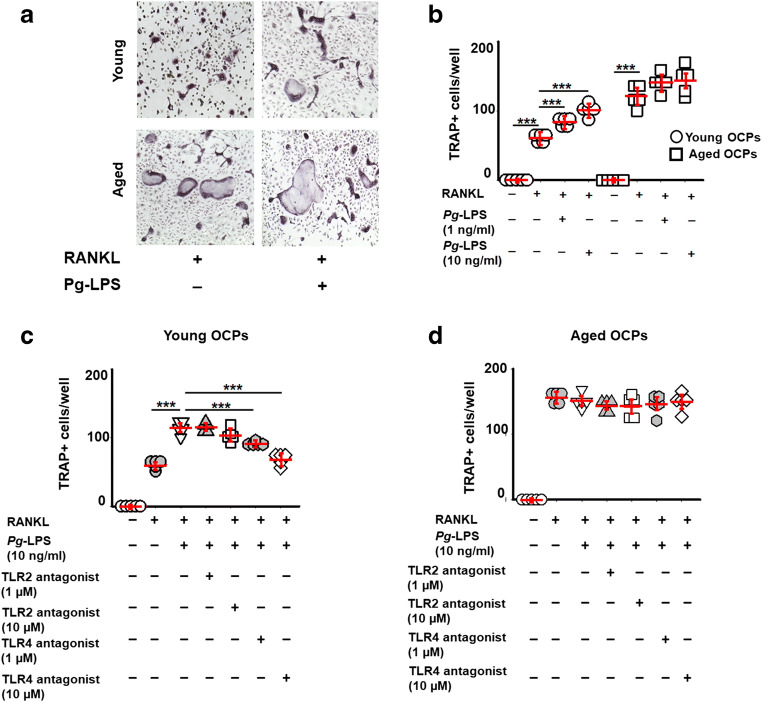
Aging diminished effects of Pg-LPS on RANKL-induced osteoclastogenesis in vitro
Some studies reported that the level of pathogenic bone loss is positively correlated with the age in various experimental models of periodontitis, as well as in patients with this disease [15, 46]. Therefore, we next examined whether Pg-LPS elicits osteoclastogenesis in relation to aging using RANKL-stimulated OCPs isolated from the spleen of young and aged CSF1r-eGFP-KI mice. We observed that Pg-LPS significantly enhanced the formation of TRAP+ cells in vitro from the RANKL-primed young OCPs when compared with aged OCPs (Fig. 2a and b). Furthermore, among the tested TLR2 and TLR4 cell signal inhibitors, only the TLR4 antagonist TAK242 dramatically decreased the number of TRAP+ osteoclasts differentiated from Pg-LPS-stimulated young OCPs compared with that isolated from aged mice, in response to priming by RANKL (Fig. 2c and d). In contrast, the TLR2 antagonist (CU-CPT22) had no effect on the osteoclast differentiation of RANKL-primed young and aged OCPs exposed to Pg-LPS (Fig. 2c and d). These observations implied that the ligation of Pg-LPS with TLR4 promotes osteoclastogenesis induced in the young OCPs but not aged mice, in vitro.
Fig. 2.
The effects of Pg-LPS and TLR’s antagonists on RANKL-induced osteoclastogenesis in relation to aging. Microscopic evaluation (a) of the TRAP staining and quantification of the number of TRAP+ multinucleated cells (b) in the RANKL-stimulated young and aged OCPs exposed to Pg-LPS in vitro. Effects of TLR4 (CAS 243984-11-4) and TLR2 antagonists (CU-CPT22) on young (c) and aged (d) RANKL-stimulated OCPs exposed to Pg-LPS. OCPs were FACS sorted from the spleen of young and aged mice and then were stimulated with RANKL in the presence or absence of various concentrations of Pg-LPS as well as TLR2 and TLR4 antagonists. The cells were evaluated at day 6 after stimulation. **p < 0.01, ***p < 0.001
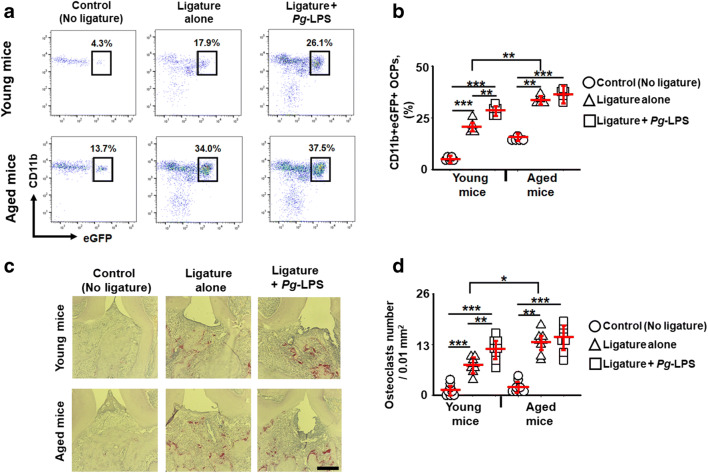
No effects of Pg-LPS on the infiltration of OCPs and the number of TRAP+ osteoclasts on periodontal lesions induced in aged mice
An essential advantage of the ligature placement around a mouse molar is that it facilitates biofilm accumulation leading to disruption of tissue homeostasis and alveolar bone loss [28]. Consequently, we next evaluated the impact of local injection of Pg-LPS on the ligature-induced alveolar bone loss in young and aged CSF1r-eGFP-KI mice. Using a multicolor flow cytometry assay, the number of infiltrating CD11b+CSF1r-eGFP+ OCPs in the experimental periodontitis lesions in relation to aging was quantified. To the best of our knowledge, the ligature placement significantly elevated recruitment of OCPs to periodontal lesions induced in aged mice compared with that of young mice (Fig. 3a and b). On the other hand, locally injected Pg-LPS significantly increased the infiltration of OCPs in the ligature-induced periodontal lesion of young CSF1r-eGFP-KI mice compared with the groups of aged mice (Fig. 3a and b), suggesting that migration of OCPs to periodontal lesions was upregulated by local injection with Pg-LPS only in the group of young CSF1r-eGFP-KI mice, but not in the group of aged mice.
Fig. 3.
Age-dependent impact of Pg-LPS on the incidence of CD11b+CSF1r-eGFP+ osteoclast precursors (OCPs) and the number of TRAP+ multinucleated cells in periodontal lesions induced in young versus aged CSF1r-eGFRP-KI mice. Representative contour plots and percentage of CD11b+CSF1r-eGFP+ OCPs (a and b) and histological evaluation of TRAP+ osteoclasts (c and d) in periodontal lesions of young and aged CSF1r-eGFP-KI mice that received: control (no ligature), ligature alone, and ligature/local gingival injection of Pg-LPS. *p < 0.05, **p < 0.01, ***p < 0.001
Then, we evaluated the number of TRAP+ osteoclasts in the ligature-induced periodontal lesions of young and aged CSF1r-eGFP-KI mice exposed to Pg-LPS. According to the histological images, we observed that the numbers of TRAP+ cells in the ligature-induced periodontal lesions of aged mice were remarkably higher than those seen in the young group (Fig. 3c and d). However, the number of TRAP+ cells was significantly elevated in the periodontal lesions of young mice that received additional local injection with Pg-LPS (Fig. 3c and d). By contrast, the local injection of Pg-LPS to the ligatured site showed no significant effect on the number of TRAP+ cells in the periodontal lesions of the aged mice (Fig. 3c and d). Therefore, in the ligature-induced periodontitis model, these results strongly suggest that locally administered Pg-LPS elevated migration of OCPs and number of TRAP+ osteoclasts in young, but not aged mice.
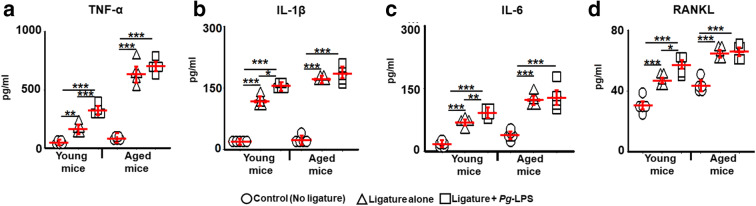
No effects of Pg-LPS on release of senescence-associated secretory phenotype markers and osteoclastogenic RANKL on periodontal lesions of aged mice
In order to establish the age-dependent role of Pg-LPS in the secretion of senescence-associated secretory phenotype (SASP), factors, including TNF-α, IL-6, and IL-1β cytokines, as well as, osteogenic factor, RANKL, in the homogenates of gingival tissue isolated from experimental mice euthanized at day 8 were evaluated using ELISA. In response to placing the ligature followed by local injections of Pg-LPS, the concentrations of TNF-α, IL-6, and IL-1β in the gingival tissue were significantly elevated in the young mouse groups (Fig. 4a–c). However, no significant increase in those cytokine levels was detected in the gingival tissue of aged mice that received a ligature placement followed by Pg-LPS injection. Further, only young mice showed a significantly elevated amount of RANKL in the ligature-induced periodontal lesions followed by local injections with Pg-LPS (Fig. 4d). On the other hand, the Pg-LPS injection showed no effect on the RANKL production in the periodontal lesions of aged mice (Fig. 4d). Therefore, as shown in the concentrations of SASP cytokines, osteogenic RANKL, and the number of OCPs and TRAP+ osteoclasts in periodontal lesions, these results indicate the presence of age-dependent differences in the effects of Pg-LPS. The results also suggest that the elevated periodontal inflammation in aged mice, compared with that of young mice, occurs in a manner independent from the canonical signaling elicited by Pg-LPS/TLR4 activation.
Fig. 4.
Age-dependent role of Pg-LPS on secretion of some pro-inflammatory SASP cytokines, including TNF-α (a), IL-1β (b), IL-6 (c), and osteoclastogenic factor, RANKL (d) in periodontal lesions of young and aged wild-type mice. Gingival tissues were sampled from the inflamed periodontal sites of young and aged mice and then were evaluated by ELISA. *p < 0.05, **p < 0.01; ***p < 0.001
Discussion
In this study, we aimed to examine the age-dependent differences of P. gingivalis lipopolysaccharide (Pg-LPS) on osteoclastogenesis in vitro as well as on periodontal inflammation induced in young and aged mice. Our results indicate that Pg-LPS significantly promotes the secretion of SASP cytokines (TNF-α, IL-1β, and IL-6), and osteoclastogenic RANKL as well as the infiltration of OCPs and TRAP+ cells in the periodontal lesions of young mice compared with those in aged mice. To the best of our knowledge, no previous studies have compared the role of Pg-LPS as a contributing factor to the pathogenesis of ligature-induced periodontitis in relation to aging. In this study, we also demonstrated that both young and aged mice show elevated periodontal bone loss in response to the ligature placement in a manner independent of Pg-LPS indicating that other virulence factors produced by oral bacteria may also be associated with age-dependent periodontal bone loss.
Published observations indicate that LPS is a major constituent of the outer membrane of the key periodontal pathogen P. gingivalis, which plays a crucial role in mediating periodontal inflammation and is predominately mediated by toll-like receptor (TLR)4 signaling [3, 31, 48]. Furthermore, some studies have demonstrated that Pg-LPS can also trigger pro-inflammatory signaling via TLR2 [3]. Although the role of TLR4 as well as that of TLR2 in periodontitis has been the focus of study for several decades, their role in the Pg-LPS-elicited signaling in the context of age-dependent deteriorated pathogenesis of periodontitis remains controversial. In the current study, we clearly demonstrated the age-dependent decline of TLR2 and TLR4 expressions on the surface of OCPs (Fig. 1). These data agree with earlier published observations indicating that expression of both TLR2 and TLR4 is diminished in the various immune cells, including macrophages and fibroblasts, isolated from the aged mice compared with that in young mice [5, 13, 14, 38, 39, 47]. By contrast, a study reported the absence of age-dependent differences in the mRNA and protein expressions of both TLR2 and TLR4 on macrophages [43]. However, a heightened expression of TLR2 was observed in the aged macrophages upon activation with live P. gingivalis compared with that of the control young macrophages [27]. In this study, we also reported that ligation of Pg-LPS with its TLR4 receptor, but not TLR2, exacerbates signaling in the young OCPs compared with that of aged OCPs in vitro (Fig. 2). The present study also demonstrated that neither TLR2 nor TLR4 inhibitors had effects on the Pg-LPS-stimulated OCPs isolated from aged mice in vitro (Fig. 2), indicating no, or only minimal, effects of Pg-LPS on aged OCPs in vitro (Fig. 2). Furthermore, according to in vivo experiments, Pg-LPS upregulated the recruitment of OCPs as well as the number of TRAP+ osteoclasts in the ligature-induced periodontal lesions in young mice, but not aged mice (Fig. 3) suggesting that the Pg-LPS-mediated promotion of osteoclastogenesis is diminished in aged individuals because of the impaired function of TLR4 signaling. Thus, this study, for the first time, demonstrated the possible different impact of Pg-LPS/TLR4 axis on alveolar bone loss in the context of aging.
Although aging increased colonization of P. gingivalis [46], it was demonstrated that Pg-LPS is not prevalent in the diseased periodontal tissues of patients [32]. By contrast, a complex of dihydroceramide sphingolipids isolated from P. gingivalis was abundantly detected in the inflamed human periodontal tissues [33]. We recently reported that P. gingivalis-derived sphingolipids promote RANKL-primed osteoclastogenesis of bone marrow-derived macrophages isolated from TLR2/TLR4-double-knock-out mice, indicating that ceramides may represent an alternative TLR-independent virulence factor produced by P. gingivalis in the pathogenesis of age-associated periodontitis. Since the property of sphingolipids as well as other virulence factors produced by oral pathogens in age-associated periodontitis is outside of the scope of the present study, this issue will be addressed in the future studies.
Besides the role of Pg-LPS in the promotion of periodontal bone loss, several studies reported that its persistent exposure promotes cellular senescence in dental pulp [18, 19] and alveolar osteocytes [2]. It is true that the major beneficial effects of senescence cells involve preparing the tissue microenvironment for regeneration and wound healing and promoting cancer suppression. On the other hand, the accumulation of senescence cells in the tissue leads to the pathologies associated with aging [7]. It is important to mention that life-long elimination of senescent cells can prevent the development of certain age-related pathologies in a mouse model of segmental accelerated aging [4]. Thus, it is plausible that the cellular senescence phenomenon shows the beneficial effects at young ages, but detrimental effects at older ages [6]. A key feature of the senescent cells is the secretion of a complex of pro-inflammatory cytokines, particularly tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and IL-6 collectively termed as a senescence-associated secretory phenotype (SASP) [8]. It should also be noted that the acquisition of the SASP turns senescent cells into pro-inflammatory cells that alter tissue structure and function [9]. Several studies reported that Pg-LPS can mediate inflammation via accumulation of SASPs including TNF-α, IL-1β, and IL-6 as well as osteoclastogenic RANKL [12, 25]. It was also demonstrated that the concentrations of pro-inflammatory SASPs and RANKL are positively correlated with the age-dependently increased levels of alveolar bone loss induced in the experimental models of periodontitis, among the young, middle-aged, and aged mice used in those experiments [10, 26, 44, 46]. Furthermore, it was recently suggested that pro-inflammatory SASPs may contribute to pathogenic bacterial overgrowth in the periodontal pocket leading to alveolar bone loss [2]. Findings from this study confirmed that Pg-LPS exacerbates the local productions of TNF-α, IL-1β, and IL-6 as well as RANKL in the ligature-induced periodontal lesions of young mice compared with those produced in the aged mice (Fig. 4), corresponding to the report which showed that Pg-LPS increases more TNF-α release from young macrophages rather than from the aged cells [43]. However, it is important to mention that LPS isolated from the periodontal pathogen Campylobacter rectus significantly raised the production of SASP cytokines from aged gingival fibroblasts compared with that of young fibroblasts [34]. Thus, it is plausible that LPS from different Gram-negative bacterial species may display obvious differences in the promotion of periodontal inflammation in relation to aging which may be important for successful therapeutic treatment and preventive regimen of periodontitis in aged patients.
Collectively, therefore, the findings from this study, along with published lines of evidence, indicate that Pg-LPS shows diminished effects on production of SASP cytokines and RANKL from periodontitis lesions in relation to aging, which is a novel observation.
Conclusion
This study, for the first time, demonstrated that Pg-LPS dramatically increased the degree of RANKL-primed osteoclastogenesis in young OCPs in vitro and that it elevated the number of OCPs and mature osteoclasts in the periodontitis lesions induced in young mice. However, Pg-LPS had no effect on the promotion of periodontal inflammation in aged mice. While comprehensive assessment of the roles of virulence factors produced by different oral pathogens in age-dependent periodontitis is required in the future studies, these results clearly indicated that the elevated periodontal inflammation in aged individuals can be promoted in a manner independent of Pg-LPS.
Funding information
This work was supported by a Nova Southeastern University President Faculty Research Development Grant and NIH Grants R03 AG-053615, R01 AG-064003, R03 DE-027153, R15 DE-028699 (A.M.), and R15 DE-027851 (T.K.).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Agosto LM, Hirnet JB, Michaels DH, Shaik-Dasthagirisaheb YB, Gibson FC, Viglianti G, Henderson AJ. Porphyromonas gingivalis-mediated signaling through tlr4 mediates persistent HIV infection of primary macrophages. Virology. 2016;499:72–81. doi: 10.1016/j.virol.2016.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aquino-Martinez R, Rowsey JL, Fraser DG, Eckhardt BA, Khosla S, Farr JN, Monroe DG. Lps-induced premature osteocyte senescence: implications in inflammatory alveolar bone loss and periodontal disease pathogenesis. Bone. 2020;132:115220. doi: 10.1016/j.bone.2019.115220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bainbridge BW, Darveau RP. Porphyromonas gingivalis lipopolysaccharide: an unusual pattern recognition receptor ligand for the innate host defense system. Acta Odontol Scand. 2001;59(3):131–138. doi: 10.1080/000163501750266710. [DOI] [PubMed] [Google Scholar]
- 4.Baker DJ, Wijshake T, Tchkonia T, LeBrasseur NK, Childs BG, van de Sluis B, Kirkland JL, van Deursen JM. Clearance of p16ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011;479(7372):232–236. doi: 10.1038/nature10600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boehmer ED, Meehan MJ, Cutro BT, Kovacs EJ. Aging negatively skews macrophage tlr2- and tlr4-mediated pro-inflammatory responses without affecting the il-2-stimulated pathway. Mech Ageing Dev. 2005;126(12):1305–1313. doi: 10.1016/j.mad.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 6.Campisi J. Cancer and ageing: rival demons? Nat Rev Cancer. 2003;3(5):339–349. doi: 10.1038/nrc1073. [DOI] [PubMed] [Google Scholar]
- 7.Campisi J. Aging, cellular senescence, and cancer. Annu Rev Physiol. 2013;75:685–705. doi: 10.1146/annurev-physiol-030212-183653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coppe JP, Desprez PY, Krtolica A, Campisi J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol. 2010;5:99–118. doi: 10.1146/annurev-pathol-121808-102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coppe JP, Rodier F, Patil CK, Freund A, Desprez PY, Campisi J. Tumor suppressor and aging biomarker p16(ink4a) induces cellular senescence without the associated inflammatory secretory phenotype. J Biol Chem. 2011;286(42):36396–36403. doi: 10.1074/jbc.M111.257071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Darveau RP, Pham TT, Lemley K, Reife RA, Bainbridge BW, Coats SR, Howald WN, Way SS, Hajjar AM. Porphyromonas gingivalis lipopolysaccharide contains multiple lipid a species that functionally interact with both toll-like receptors 2 and 4. Infect Immun. 2004;72(9):5041–5051. doi: 10.1128/IAI.72.9.5041-5051.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dietrich T, Garcia RI. Associations between periodontal disease and systemic disease: evaluating the strength of the evidence. J Periodontol. 2005;76(11 Suppl):2175–2184. doi: 10.1902/jop.2005.76.11-S.2175. [DOI] [PubMed] [Google Scholar]
- 12.Diya Z, Lili C, Shenglai L, Zhiyuan G, Jie Y. Lipopolysaccharide (lps) of porphyromonas gingivalis induces il-1beta, tnf-alpha and il-6 production by thp-1 cells in a way different from that of Escherichia coli lps. Innate Immun. 2008;14(2):99–107. doi: 10.1177/1753425907088244. [DOI] [PubMed] [Google Scholar]
- 13.Domon H, Tabeta K, Nakajima T, Yamazaki K. Age-related alterations in gene expression of gingival fibroblasts stimulated with Porphyromonas gingivalis. J Periodontal Res. 2014;49(4):536–543. doi: 10.1111/jre.12134. [DOI] [PubMed] [Google Scholar]
- 14.Dunston CR, Griffiths HR. The effect of ageing on macrophage toll-like receptor-mediated responses in the fight against pathogens. Clin Exp Immunol. 2010;161(3):407–416. doi: 10.1111/j.1365-2249.2010.04213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eke PI, Dye BA, Wei L, Thornton-Evans GO, Genco RJ, Cdc Periodontal Disease Surveillance workgroup: James Beck GDRP Prevalence of periodontitis in adults in the United States: 2009 and 2010. J Dent Res. 2012;91(10):914–920. doi: 10.1177/0022034512457373. [DOI] [PubMed] [Google Scholar]
- 16.Eke PI, Dye BA, Wei L, Slade GD, Thornton-Evans GO, Borgnakke WS, Taylor GW, Page RC, Beck JD, Genco RJ. Update on prevalence of periodontitis in adults in the United States: Nhanes 2009 to 2012. J Periodontol. 2015;86(5):611–622. doi: 10.1902/jop.2015.140520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fazzalari NL, Kuliwaba JS, Atkins GJ, Forwood MR, Findlay DM. The ratio of messenger RNA levels of receptor activator of nuclear factor kappab ligand to osteoprotegerin correlates with bone remodeling indices in normal human cancellous bone but not in osteoarthritis. J Bone Miner Res. 2001;16(6):1015–1027. doi: 10.1359/jbmr.2001.16.6.1015. [DOI] [PubMed] [Google Scholar]
- 18.Feng X, Feng G, Xing J, Shen B, Tan W, Huang D, Lu X, Tao T, Zhang J, Li L, Gu Z. Repeated lipopolysaccharide stimulation promotes cellular senescence in human dental pulp stem cells (dpscs) Cell Tissue Res. 2014;356(2):369–380. doi: 10.1007/s00441-014-1799-7. [DOI] [PubMed] [Google Scholar]
- 19.Feng G, Zheng K, Cao T, Zhang J, Lian M, Huang D, Wei C, Gu Z, Feng X. Repeated stimulation by lps promotes the senescence of dpscs via tlr4/myd88-nf-kappab-p53/p21 signaling. Cytotechnology. 2018;70(3):1023–1035. doi: 10.1007/s10616-017-0180-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao A, Wang X, Yu H, Li N, Hou Y, Yu W. Effect of porphyromonas gingivalis lipopolysaccharide (pg-lps) on the expression of epha2 in osteoblasts and osteoclasts. In Vitro Cell Dev Biol Anim. 2016;52(2):228–234. doi: 10.1007/s11626-015-9965-0. [DOI] [PubMed] [Google Scholar]
- 21.Golz L, Memmert S, Rath-Deschner B, Jager A, Appel T, Baumgarten G, Gotz W, Frede S. Lps from p. Gingivalis and hypoxia increases oxidative stress in periodontal ligament fibroblasts and contributes to periodontitis. Mediat Inflamm. 2014;2014:986264. doi: 10.1155/2014/986264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hirschfeld M, Weis JJ, Toshchakov V, Salkowski CA, Cody MJ, Ward DC, Qureshi N, Michalek SM, Vogel SN. Signaling by toll-like receptor 2 and 4 agonists results in differential gene expression in murine macrophages. Infect Immun. 2001;69(3):1477–1482. doi: 10.1128/IAI.69.3.1477-1482.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holt SC, Ebersole JL. Porphyromonas gingivalis, treponema denticola, and tannerella forsythia: the “red complex”, a prototype polybacterial pathogenic consortium in periodontitis. Periodontology 2000. 2005;38:72–122. doi: 10.1111/j.1600-0757.2005.00113.x. [DOI] [PubMed] [Google Scholar]
- 24.Jin Z, Wei W, Yang M, Du Y, Wan Y. Mitochondrial complex i activity suppresses inflammation and enhances bone resorption by shifting macrophage-osteoclast polarization. Cell Metab. 2014;20(3):483–498. doi: 10.1016/j.cmet.2014.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kassem A, Henning P, Lundberg P, Souza PP, Lindholm C, Lerner UH. Porphyromonas gingivalis stimulates bone resorption by enhancing rankl (receptor activator of nf-kappab ligand) through activation of toll-like receptor 2 in osteoblasts. J Biol Chem. 2015;290(33):20147–20158. doi: 10.1074/jbc.M115.655787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kato C, Mikami M. Effect of aging on bcg immunostimulation of Porphyromonas gingivalis infection in mice. Biomed Res. 2011;32(1):45–54. doi: 10.2220/biomedres.32.45. [DOI] [PubMed] [Google Scholar]
- 27.Liang S, Domon H, Hosur KB, Wang M, Hajishengallis G. Age-related alterations in innate immune receptor expression and ability of macrophages to respond to pathogen challenge in vitro. Mech Ageing Dev. 2009;130(8):538–546. doi: 10.1016/j.mad.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marchesan J, Girnary MS, Jing L, Miao MZ, Zhang S, Sun L, Morelli T, Schoenfisch MH, Inohara N, Offenbacher S, Jiao Y. An experimental murine model to study periodontitis. Nat Protoc. 2018;13(10):2247–2267. doi: 10.1038/s41596-018-0035-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Movila A, Ishii T, Albassam A, Wisitrasameewong W, Howait M, Yamaguchi T, Ruiz-Torruella M, Bahammam L, Nishimura K, Van Dyke T, et al. Macrophage migration inhibitory factor (mif) supports homing of osteoclast precursors to peripheral osteolytic lesions. J Bone Miner Res. 2016;31(9):1688–1700. doi: 10.1002/jbmr.2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Movila A, Kajiya M, Wisitrasameewong W, Stashenko P, Vardar-Sengul S, Hernandez M, Thomas Temple H, Kawai T. Intravital endoscopic technology for real-time monitoring of inflammation caused in experimental periodontitis. J Immunol Methods. 2018;457:26–29. doi: 10.1016/j.jim.2018.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nativel B, Couret D, Giraud P, Meilhac O, d’Hellencourt CL, Viranaicken W, Da Silva CR. Porphyromonas gingivalis lipopolysaccharides act exclusively through tlr4 with a resilience between mouse and human. Sci Rep. 2017;7(1):15789. doi: 10.1038/s41598-017-16190-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nichols FC. Distribution of 3-hydroxy ic17:0 in subgingival plaque and gingival tissue samples: relationship to adult periodontitis. Infect Immun. 1994;62(9):3753–3760. doi: 10.1128/iai.62.9.3753-3760.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nichols FC, Yao X, Bajrami B, Downes J, Finegold SM, Knee E, Gallagher JJ, Housley WJ, Clark RB. Phosphorylated dihydroceramides from common human bacteria are recovered in human tissues. PLoS One. 2011;6(2):e16771. doi: 10.1371/journal.pone.0016771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Okamura H, Yamaguchi M, Abiko Y. Enhancement of lipopolysaccharide-stimulated pge2 and il-1beta production in gingival fibroblast cells from old rats. Exp Gerontol. 1999;34(3):379–392. doi: 10.1016/s0531-5565(99)00006-6. [DOI] [PubMed] [Google Scholar]
- 35.Ozmeric N, Preus NR, Olsen I. Genetic diversity of porphyromonas gingivalis and its possible importance to pathogenicity. Acta Odontol Scand. 2000;58(4):183–187. doi: 10.1080/000163500429190. [DOI] [PubMed] [Google Scholar]
- 36.Paster BJ, Boches SK, Galvin JL, Ericson RE, Lau CN, Levanos VA, Sahasrabudhe A, Dewhirst FE. Bacterial diversity in human subgingival plaque. J Bacteriol. 2001;183(12):3770–3783. doi: 10.1128/JB.183.12.3770-3783.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Salminen A, Kauppinen A, Kaarniranta K. Emerging role of nf-kappab signaling in the induction of senescence-associated secretory phenotype (sasp) Cell Signal. 2012;24(4):835–845. doi: 10.1016/j.cellsig.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 38.Shaik-Dasthagirisaheb YB, Huang N, Weinberg EO, Shen SS, Genco CA, Gibson FC., 3rd Aging and contribution of myd88 and trif to expression of tlr pathway-associated genes following stimulation with Porphyromonas gingivalis. J Periodontal Res. 2015;50(1):89–102. doi: 10.1111/jre.12185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shaw AC, Panda A, Joshi SR, Qian F, Allore HG, Montgomery RR. Dysregulation of human toll-like receptor function in aging. Ageing Res Rev. 2011;10(3):346–353. doi: 10.1016/j.arr.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Singhrao SK, Olsen I. Are porphyromonas gingivalis outer membrane vesicles microbullets for sporadic Alzheimer’s disease manifestation? J Alzheimer’s Dis Rep. 2018;2(1):219–228. doi: 10.3233/ADR-180080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Suda K, Udagawa N, Sato N, Takami M, Itoh K, Woo JT, Takahashi N, Nagai K. Suppression of osteoprotegerin expression by prostaglandin e2 is crucially involved in lipopolysaccharide-induced osteoclast formation. J Immunol. 2004;172(4):2504–2510. doi: 10.4049/jimmunol.172.4.2504. [DOI] [PubMed] [Google Scholar]
- 42.Suh JS, Kim S, Bostrom KI, Wang CY, Kim RH, Park NH. Periodontitis-induced systemic inflammation exacerbates atherosclerosis partly via endothelial-mesenchymal transition in mice. Int J Oral Sci. 2019;11(3):21. doi: 10.1038/s41368-019-0054-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sun Y, Li H, Yang MF, Shu W, Sun MJ, Xu Y. Effects of aging on endotoxin tolerance induced by lipopolysaccharides derived from Porphyromonas gingivalis and Escherichia coli. PLoS One. 2012;7(6):e39224. doi: 10.1371/journal.pone.0039224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang M, Shakhatreh MA, James D, Liang S, Nishiyama S, Yoshimura F, Demuth DR, Hajishengallis G. Fimbrial proteins of porphyromonas gingivalis mediate in vivo virulence and exploit tlr2 and complement receptor 3 to persist in macrophages. J Immunol. 2007;179(4):2349–2358. doi: 10.4049/jimmunol.179.4.2349. [DOI] [PubMed] [Google Scholar]
- 45.Wisitrasameewong W, Kajiya M, Movila A, Rittling S, Ishii T, Suzuki M, Matsuda S, Mazda Y, Torruella MR, Azuma MM, Egashira K, Freire MO, Sasaki H, Wang CY, Han X, Taubman MA, Kawai T. Dc-stamp is an osteoclast fusogen engaged in periodontal bone resorption. J Dent Res. 2017;96(6):685–693. doi: 10.1177/0022034517690490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu Y, Dong G, Xiao W, Xiao E, Miao F, Syverson A, Missaghian N, Vafa R, Cabrera-Ortega AA, Rossa C, Jr, Graves DT. Effect of aging on periodontal inflammation, microbial colonization, and disease susceptibility. J Dent Res. 2016;95(4):460–466. doi: 10.1177/0022034515625962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yamada C, Beron-Pelusso C, Algazzaz N, Heidari A, Luz D, Rawas-Qalaji M, Toderas I, Mascarenhas AK, Kawai T, Movila A. Age-dependent effect between MARCO and TLR4 on pmma particle phagocytosis by macrophages. J Cell Mol Med. 2019;23(8):5827–5831. doi: 10.1111/jcmm.14494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang J, Yu C, Zhang X, Chen H, Dong J, Lu W, Song Z, Zhou W. Porphyromonas gingivalis lipopolysaccharide induces cognitive dysfunction, mediated by neuronal inflammation via activation of the tlr4 signaling pathway in c57bl/6 mice. J Neuroinflammation. 2018;15(1):37. doi: 10.1186/s12974-017-1052-x. [DOI] [PMC free article] [PubMed] [Google Scholar]