Abstract
Patients with mutations in Cyclin M2 (CNNM2) suffer from hypomagnesaemia, seizures, and intellectual disability. Although the molecular function of CNNM2 is under debate, the protein is considered essential for renal Mg2+ reabsorption. Here, we used a Cnnm2 knock out mouse model, generated by CRISPR/Cas9 technology, to assess the role of CNNM2 in Mg2+ homeostasis. Breeding Cnnm2+/− mice resulted in a Mendelian distribution at embryonic day 18. Nevertheless, only four Cnnm2−/− pups were born alive. The Cnnm2−/− pups had a significantly lower serum Mg2+ concentration compared to wildtype littermates. Subsequently, adult Cnnm2+/− mice were fed with low, control, or high Mg2+ diets for two weeks. Adult Cnnm2+/− mice showed mild hypomagnesaemia compared to Cnnm2+/+ mice and increased serum Ca2+ levels, independent of dietary Mg2+ intake. Faecal analysis displayed increased Mg2+ and Ca2+ excretion in the Cnnm2+/− mice. Transcriptional profiling of Trpm6, Trpm7, and Slc41a1 in kidneys and colon did not reveal effects based on genotype. Microcomputed tomography analysis of the femurs demonstrated equal bone morphology and density. In conclusion, CNNM2 is vital for embryonic development and Mg2+ homeostasis. Our data suggest a previously undescribed role of CNNM2 in the intestine, which may contribute to the Mg2+ deficiency in mice and patients.
Subject terms: Kidney, Neurogenesis
Introduction
Hypomagnesaemia is defined by a serum magnesium (Mg2+) level below < 0.7 mmol/L and is associated with hypertension, muscle cramps, diabetes mellitus type II, epilepsy, and cardiac arrhythmias1. To maintain a physiological blood Mg2+ concentration, the intestine absorbs Mg2+ from the diet and can subsequently be stored in soft tissues and bone. The kidneys are considered as major regulators of the blood Mg2+ concentration. Here, Mg2+ is largely reabsorbed via paracellular transport in the proximal tubule and thick ascending limb of Henle (TAL). Fine-tuning of Mg2+ reabsorption take place in the distal convoluted tubule (DCT), where transport is an active transcellular process2.
In the DCT, apical Mg2+ transport is realised by the luminal divalent cation channel transient receptor potential melastatin type 6 and 7 (TRPM6/7)3–7. Mutations in TRPM6 cause hypomagnesaemia with secondary hypocalcaemia (HOMG1/HSH; MIM# 602014). At the basolateral side, several proteins have been considered to facilitate Mg2+ extrusion including solute carrier 41a1 (SLC41A1) and Cyclin M2 (CNNM2)8. Although the definite role of this latter protein has not been elucidated on a molecular level, patient studies have shown the importance of CNNM2 in renal Mg2+ handling8–11. Mutations in this gene are causative for hypomagnesaemia, seizures, and intellectual disability (HSMR) syndrome (HSMR; MIM# 616418)12–14. In addition to renal Mg2+ wasting, patients suffer from seizures, motor skills difficulties, intellectual disability, and deficits in speech12,13. Only patients affected by recessive mutations have been demonstrated to show structural brain anomalies, such as demyelination or enlarged supratentorial outer cerebrospinal liquor spaces13. Of note, genome wide associates studies (GWAS) have linked the CNNM2 locus with grey matter morphology, schizophrenia, increased body mass index, and hypertension15–19.
Although CNNM2 plays an important role in renal Mg2+ homeostasis and brain function, the underlying molecular mechanisms and their implications for patients suffering from HSMR syndrome have not been elucidated. Therefore, the aim of this study was to investigate the role of CNNM2-mediated Mg2+ homeostasis in greater detail by generating novel CNNM2 mouse models.
Methods
Generation of CNNM2 deficient mice
Cnnm2−/− mice were generated by CRISPR/Cas9 mediated genomic editing in murine zygotes20. In brief, guide RNAs were cloned into pX330 (pX330 was a gift from Feng Zhang (Addgene plasmid 42230) and Cas9 into pTLN21. For the generation of Indels in Cnnm2 the target sequence was 5′-GTCCTGCAAGCAGCTGCGGGC-3′, for insertion of the loxP sites 5′-CTGTAGTAGATCCTCGCGCG-3′ and 5′-GCATCCCGCAGGGTAGATTA-3′. A T7-promoter was added to the sgRNA sequence by PCR and the Cas9 plasmid was linearized using MluI. The PCR product and the linearized plasmid were in vitro transcribed using MEGA shortscript and mMessage Machine KIT (Life Technologies, United States of America (USA)). Both Cas9 mRNA and the sgRNAs were purified using MEGAclear kit (Life Technologies) and eluted in RNase-free water. For the generation of the Indel allele, Cas9 mRNA and sgRNA, for the generation of the floxed allele Cas9 mRNA, sgRNAs and a plasmid containing a floxed exon 1 of Cnnm2 were injected into fertilized zygotes. These zygotes were kept overnight (O/N) at 37 °C and then transferred to pseudopregnant foster mice. Founders were identified by PCR and Sanger sequencing. Mice were bred to C57Bl/6N for two generations. The deleted allele was obtained by crossing mice with the floxed allele to a Cre deleter strain (Tg(CMV-cre)1Cgn; JAX #006054).
Animals and ethics
Two animal studies were performed: a study with embryos and newborn with both Cnnm2+/+, Cnnm2+/− and Cnnm2−/− mice and was performed in Charité, Berlin. The other study was performed in the Radboud Institute for Molecular Life Sciences, Nijmegen and used 30 Cnnm2+/+ mice (15 female, 15 male) and 36 Cnnm2+/− mice (18 female. 18 male). These mice subsequently divided over three experimental groups (Cnnm2+/+ 10 per group, 5 male/female and Cnnm2+/− 16 mice per group, 6 male/female).
Mice were maintained according to institutional guidelines of Charité Berlin. After timed mating of the mothers, embryos were isolated on day 18.5 of gestation. Newborns were culled directly after birth. Genotyping of the allele containing the deletion was performed using the oligonucleotides 5′-CCGGGTGGGAAGGATGAAGC-3′ and 5′- GGGACCACGTCTCGTTGTTG-3′, and of the floxed allele using the oligonucleotides 5′-AGTCCGGCTCTGGTGCTC-3′ (KO), 5′-AAGCCCAAAACTGCCATTAC-3′ (WT), 5′-TTCTGCCAAAACCACACTTG-3′ (WT/KO). Studies on mice were performed at the Charité Universitätsmedizin Berlin approved by the Animal Care and Use Committee from Berlin:LAGeSo. The study using Mg2+ diets in Cnnm2+/+ and Cnnm2+/− mice was performed at the Radboud Institute for Molecular Life Sciences, Nijmegen, the Netherlands. The study was ethically approved by the Local Ethical Committee of the Radboud University Nijmegen (RU DEC 2015-0112) and by the Dutch Central Commission for Animal Experiments (CCD, AVD103002016382). This study was performed in compliance with the ARRIVE guidelines.
Experimental design Mg2+ diet intervention
Mice were held in standard cages with bedding and kept in a temperature- and light-controlled room. Standard pellet chow and autoclaved tap water were available ad libitum. After genotyping, 36 Cnnm2+/− mice and 30 Cnnm2+/+ (wild-type) mice of age week 8–10 were evenly divided between females and males, and blood was drawn via submandibular puncture. The mice were then kept in metabolic cages individually for 48 h. Faeces and urine were sampled for 24 h. By genotype and sex, mice were subsequently randomly divided in three diet groups, fed with low 0.02% (w/w) Mg2+, normal 0.23% (w/w) Mg2+, or enriched 0.48% Mg2+ (w/w) diets. After two weeks, mice were housed again in metabolic cages for faeces and urine sampling for 48 h in total and subsequently euthanised by cervical dislocation under 5% (v/v) isofluorance anaesthesia, followed by dissecting the organs.
Electrolyte measurements
Serum Mg2+ and Ca2+ concentration of newborn animals were determined by colorimetric assays (QuantiChrom, BioAssay Systems, USA) according to the manufacturer’s instruction).
The total Mg2+ concentration in serum, urine, and faeces in adult mice was determined by a xylidyl blue colorimetric assay kit according to the manufacturer's protocol (Roche/Hitachi, Japan) and measured at 600 nm on a BioRad plate reader (BioRad, California, USA). Total Ca2+ was measured using an o-cresolphthalein complexone method and read at 570 nm, as described previously22. Faeces were dissolved in nitric acid (> 65%) and incubated at 65 °C for 15 min prior to measurements. Sodium and potassium were determined by the Radboudumc clinical lab using an automated analysis system (Abbott Diagnostics, the Netherlands).
Antibodies
The hexa-His-tagged intracellular carboxyterminus of murine CNNM2 (aa 593-875) was expressed in BL-21 bacteria and purified using a Nickel-nitrilotriacetic acid-column (QIAGEN, Germany). The hexa-His-tag was removed by cleavage with Tobacco Etch Virus protease (Genscript, the Netherlands). Guinea pigs were immunized with the purified protein (Pineda, Germany). Serum was collected after 60 days and 3 boosts and purified by affinity chromatography using the carboxy-terminus of murine CNNM2. Rabbit anti-NCC was a kind gift from Dr. D.H. Ellison. For western blot analyses, rabbit anit-β-actin (A2228, Sigma-Aldrich, USA) and rabbit anti-CNNM2 (ab111351, Abcam, UK) were used.
SDS-PAGE and Western blot analysis
Tissues were homogenised in 140 mmol/L NaCl, 20 mmol/L Tris, 5 mmol/L EDTA, adjusted with Tris to pH 7.4, with protease inhibitors by Ultra Turrax-T25 followed by Dounce homogeniser. Samples were centrifuged 10 min 1000 g at 4 °C to remove nuclei. Membranes were pelleted by centrifugation at 100,000 g at 4 °C for 30 min and resuspended in lysis buffer (50 mmol/L Tris-HCl pH 6.8, 5 mmol/L EDTA, 2% (w/v) SDS, with protease inhibitors). For SDS-PAGE, 25 µg protein was loaded, followed by transfer to a polyvinylidene fluoride membrane. Membranes were blocked with 5% (w/v) non-fat dry milk in phosphate buffered solution (PBS) for 1 h at room temperature (RT) and incubated overnight with blocking buffer supplemented with primary antibody at 4 °C. The next day, membranes were washed with PBS and incubated with appropriate peroxidase-conjugated secondary antibodies (Roche, Mannheim, Germany) and visualized by enhanced chemiluminescence.
Histology
Paraffin-embedded brains from adult mice and E17.5 embryos were processed into 5 µm-thick sections and mounted on slides for Nissl staining. Briefly, slides were deparaffinized in xylene for 10 min followed by 5 min incubation in the graded ethanol series (100, 95, 75, 50%). Slides were incubated in 0,1% (w/v) cresyl violet solution prepared in distilled water for 8 mi,n followed by a 2 min differentiation step in 95% ethanol and dehydrated in 100% ethanol for 2 min. Slides were then cleared in xylene and mounted with permanent mounting medium.
Immunohistochemistry
Kidney cryosections (10 µm) of Cnnm2+/+ and Cnnm2−/− embryos were fixed with 4% (w/v) paraformaldehyde in PBS for 8 min. Blocking was performed in 5% (v/v) donkey serum (Biozol, Germany), 0.3% (v/v) Triton X-100 in PBS for 1 h at RT. Sections were then incubated in blocking solution supplemented with guinea pig anti-CNNM2 antibody (1:500), and rabbit anti-NCC (1:500) at RT for 2.5 h followed by secondary antibody (AlexaFluor488 donkey-anti-rabbit and AlexaFluor594 donkey-anti-guinea-pig (Thermofisher Scientific, Germany)) incubation for 1 h at RT. Finally, sections were mounted by using ProLong Diamond antifade reagent (Invitrogen, Germany) with DAPI and imaged on Nikon Laser Scanning Microscope A1R (AMBIO core facility, Charité Universtitätsmedizin, Berlin, Germany).
RNA isolation and real-time quantitative polymerase chain reaction (RT-qPCR)
Total RNA was isolated from kidneys and colon using TriZol (Invitrogen, the Netherlands) and treated with DNAse (1 U/µg RNA, Promega, USA) Subsequently, cDNA was synthesised from 1.5 µg total RNA by Moloney Murine Leukaemia Virus reverse transcriptase (Invitrogen) at 37 °C for 1 h. IQ SYBR Green Mix (Bio-Rad) was used to determine gene expression levels. Relative expression was determined by the Livak method and shown as fold-change compared to the control group (wild-type mice, 0.23% (w/w) Mg2+ diet) and corrected for Gapdh expression. All primers used for gene expression analysis were equally efficient (see Supplementary Table 1 for primer sequences).
Structural analysis by the microcomputed tomography
Femurs were dissected and fixed in 4% (v/v) formalin for 24 h and subsequently kept in 70% (v/v) ethanol. Femurs were scanned using the Skyscan 1076 in vivo X-ray computed tomography (Bruker microCT, Kontich, Belgium) with a voxel size of 8.88 μm and 2300 ms exposure time in line with the guidelines for the assessment of bone microstructure23. The following settings were used: X-ray power of 40 kV and tube current of 250 mA. Beam hardening (20%) was reduced using a 1 mm aluminum filter, ring-artefacts was set at 5 and an average of three photos (frame averaging) at each angle (0.8°) were taken to generate the final images. For the analysis of trabecular bone parameters, the distal metaphysis was scanned (a scan area of 1.35 mm from the distal growth plate towards femoral center). Analysis of the cortical bone parameters was performed in the diaphyseal cortex, which comprised a scan area of 0.45 mm in the femoral center. 3D reconstruction and data analysis were performed with using manufacturer-provided software (NRecon, Data viewer, CT analyzer; Bruker microCT).
Statistics
Data is represented as mean ± SEM. Comparisons were made by a One- or- Two-way ANOVA, followed by a Tukey’s post-hoc test. An alpha of P < 0.05 was considered statistically significant. Statistical analysis is described in detail in the figure legends.
Results
Cnnm2−/− mice are embryonically lethal and display exencephaly
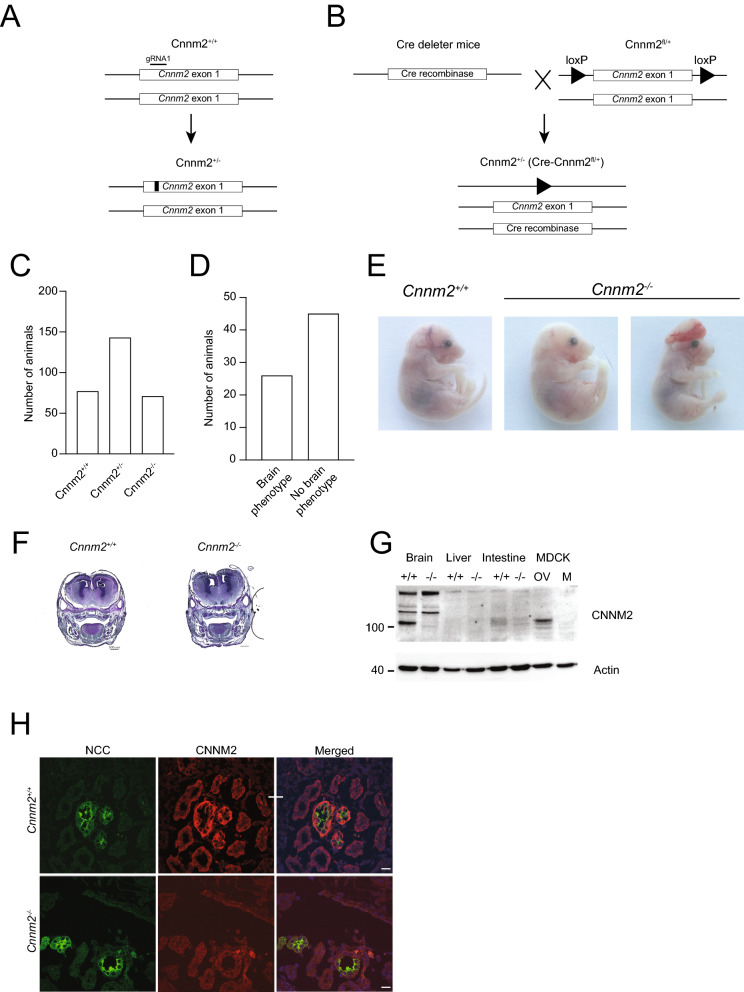
Cnnm2−/− mice were developed using CRISPR/Cas9 technology. In a first approach using Cas9 and a single guideRNA (sgRNA), deletions were introduced in exon 1 of the Cnnm2 locus. Two independent lines were established with either a deletion of 55 bp resulting in a frameshift at position 28 (p.A28D fsX9) or a deletion of 37 bp resulting in a frameshift at position 40 (p.V40H fsX3) (Fig. 1A). In a second approach using Cas9, two sgRNAs and a donor plasmid, LoxP sites were introduced upstream and downstream of exon 1, which was removed by cre mediated excision after crossing the mice with the floxed allele to a cre deleter strain (Fig. 1B). At E18, embryos were present at Mendelian ratio (Cnnm2+/+: 27%, Cnnm2+/−: 49% Cnnm2−/−: 24%) (Fig. 1C). However, the Cnnm2−/− offspring all died within the first day after birth in the three investigated lines. Consequently, Cnnm2−/− animals were analysed at embryonic day 18 (E18). 36% of Cnnm2−/− embryos displayed exencephaly, which may contribute to the mortality of these mice (Fig. 1D,E). The Cnnm2−/− embryos (E17.5) that did not show exencephaly also did not show obvious morphological neurological differences, as demonstrated with a Nissl staining (Fig. 1F). Both lines were used to generate mice homozygous for the deleted Cnnm2 allele and resulted in early postnatal lethality and a similar penetrance of exencephaly.
Figure 1.
Generation and verification of a Cnnm2 knock-out mouse model. Schematic overview of the strategy used for genomic deletion of Cnnm2 by CRISPR/Cas9 mediated deletions (A) or by CRISPR/Cas9 mediated insertion of loxP sites followed by Cre recombinase mediated excision (B). (C) Distribution of the genotype of embryos at E18.5. (D) Distribution of Cnnm2−/− mice exhibiting exencephaly. (E) Representative pictures of embryos at E18.5, in which exencephaly is observed in Cnnm2−/− mice. (F) Van Nissl staining of E17.5 Cnnm2+/+ and Cnnm2+/− embryos showing no apparent differences in morphology in the absence of exencephaly. (G) Western blot of brain membrane preparation from Cnnm2+/+ and Cnnm2−/− mice. Madin-Darby canine kidney cells transfected with murine Cnnm2 (OV) or mock (M) served as a control for antibody specificity. (H) Immunohistochemistry of kidney cryo-sections of E18.5 Cnnm2+/+ and Cnnm2−/− stained for CNNM2 (red) and Na+/Cl− co-transporter (NCC) (green). Scale bar depict 50 µm.
Absence of CNNM2 protein was confirmed by Western blot analysis of embryonic brains and specificity of the antibody was confirmed using CNNM2 overexpressing Madin-Darby canine kidney (MDCK) cells (Fig. 1G). In addition, immunohistochemistry verified that CNNM2 expression in the DCT, defined by Na+/Cl− co-transporter (NCC) positive tubules, was absent in kidney sections of Cnnm2−/− pups (Fig. 1H).
Cnnm2+/− mice exhibit low serum Mg2+ levels
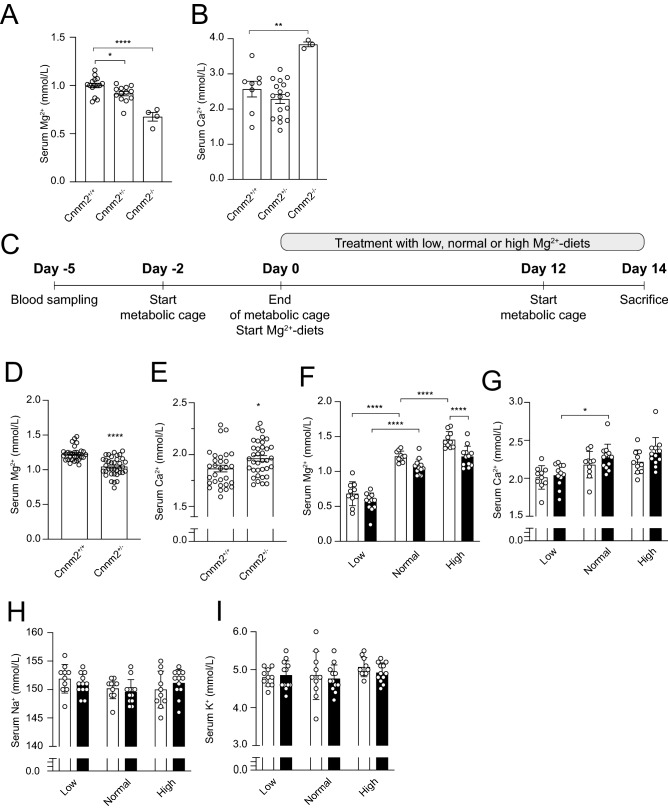
To determine the effects of CNNM2 depletion on electrolyte homeostasis, serum Mg2+ and Ca2+ levels were determined in pups within the first hours of life (≤ 8 h). The Cnnm2−/− mice had a significant decreased serum Mg2+ concentration compared to both Cnnm2+/− mice and Cnnm2+/+ mice (0.68 ± 0.05 mmol/L versus 0.92 ± 0.02 mmol/L and 1.00 ± 0.02 mmol/L, respectively, P < 0.05) (Fig. 2A). Determination of the serum Ca2+ levels revealed a significant 50% increase in Cnnm2−/− mice whereas similar Ca2+ levels were observed in Cnnm2+/− mice (Fig. 2B).
Figure 2.
CNNM2 depletion affects Mg2+ and Ca2+ homeostasis. (A) Serum Mg2+ is significantly reduced in newborn CNNM2 deficient mice compared to wild type littermates. Data are presented as mean ± SEM (n = 4–15) (B) Serum Ca2+ in newborn Cnnm2−/− mice is significantly increased compared to Cnnm2+/+ mice (n = 3–17) (C) Cnnm2+/+ or Cnnm2+/− mice were treated with low (0.02%), normal (0.23%), or high (0.48%) (w/w) Mg2+ diets for two weeks, followed by housing in metabolic cages. (D,F) Cnnm2+/− mice (black bars) have lower serum Mg2+ levels and higher compared to wild type mice (white bars), independent of the Mg2+ diet. (E,G) Cnnm2+/− mice have lower serum Mg2+ levels and an overall increase in serum Ca2+ levels compared to wild type littermates, independent of the Mg2+ diet. Serum Na+ (H) and K+ (I) levels in Cnnm2+/− mice are unchanged (n = 10–12 per group). Data are presented as mean ± SEM. Significance determined with two-tailed Student’s T-test or Two-way ANOVA followed by a Tukey post-hoc test. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
As Cnnm2−/− mice died shortly after birth, further studies to determine the role of CNNM2 in Mg2+ homeostasis were performed in the adult Cnnm2+/− mice. Adult Cnnm2+/− had comparable brain morphology with wild type mice (Supplementary Fig. 2).
To delineate the role of CNNM2 in Mg2+ further, Cnnm2+/+ and Cnnm2+/− adult mice were fed with a low, normal, or high Mg2+ diet for two weeks (Fig. 2C). Similar to newborn Cnnm2−/− mice, adult Cnnm2+/− mice displayed significantly decreased Mg2+ levels in the blood compared to Cnnm2+/+ mice (1.05 ± 0.02 versus 1.23 ± 0.02 mmol/L, respectively, P < 0.05) (Fig. 2D). When exposed to a low, normal, or high Mg2+ diet for two weeks, serum Mg2+ levels were consistently low post-diet in Cnnm2+/− mice compared to Cnnm2+/+ (Fig. 2E). No differences were observed in weight gain between mice of different genotypes or when fed on a particular diet (Table 1). In adult Cnnm2+/− mice, a slight increase of serum Ca2+ concentration was measured compared to Cnnm2+/+ mice (Fig. 2F,G). Blood Na+ and K+ levels remained unaffected upon exposure to the diets (Fig. 2H,I).
Table 1.
Metabolic parameters of Cnnm2+/− mice upon exposure to different Mg2+ diets.
| Diet | Cnnm2+/+ | Cnnm2+/− | ||||
|---|---|---|---|---|---|---|
| Low | Normal | High | Low | Normal | High | |
| Body weight M (g) | 23.7 ± 0.3 | 22.8 ± 0.3 | 24.4 ± 0.3 | 23.3 ± 0.4 | 23.7 ± 0.8 | 23.7 ± 0.5 |
| Body weight F (g) | 19.5 ± 0.8 | 19.5 ± 0.5 | 19.7 ± 0.4 | 20.1 ± 0.4 | 20.3 ± 0.2 | 20.7 ± 0.3 |
| Food intake (g) | 3.5 ± 0.1 | 3.0 ± 0.2 | 3.4 ± 0.2 | 3.5 ± 0.2 | 3.5 ± 0.2 | 3.2 ± 0.3 |
| Water intake (mL) | 10 ± 2.4 | 9.7 ± 1.8 | 10 ± 1.7 | 10 ± 1.6 | 10 ± 1.3 | 7.9 ± 0.9 |
| Urine excretion (mL/24 h) | 3.4 ± 0.9 | 2.3 ± 0.4 | 3.8 ± 0.7 | 3.7 ± 0.7 | 3.3 ± 0.4 | 3.5 ± 0.6 |
| Faecal (mg/24 h) | 384 ± 21 | 429 ± 40 | 450 ± 36 | 391 ± 40 | 442 ± 38 | 428 ± 35 |
Cnnm2+/+ and Cnnm2+/− mice were kept in metabolic cages exposed at low (0.02%), normal (0.21%) and high (0.48%) (w/w) Mg2+ diets for 14 days. The last 48 h, mice were kept in metabolic cages for the collection of urine and faeces. Values represent the latter 24 h (mean ± SEM). M male, F female.
Cnnm2 deficiency leads to mild urinary Mg2+ wasting
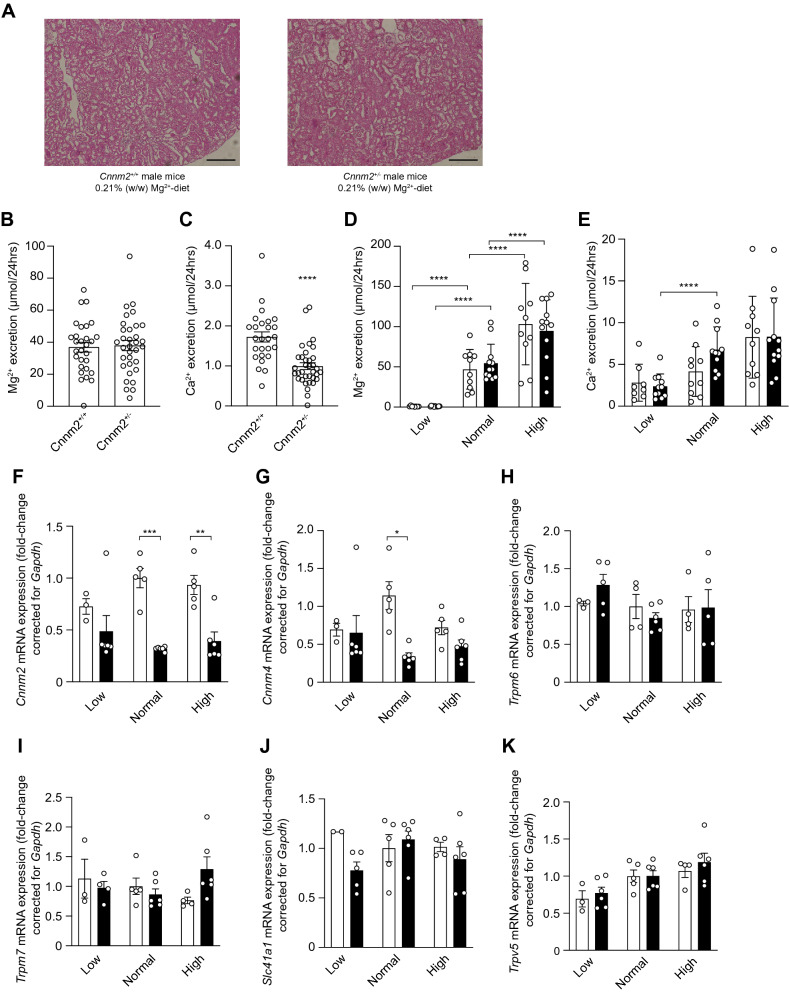
To pinpoint the cause of the low serum Mg2 + levels in the Cnnm2+/− mice, the 24 h urinary Mg2+ excretion and kidney mRNA expression of genes involved in Mg2+ reabsorption were assessed. Morphology of the kidneys was similar in both genotypes (Fig. 3A). Cnnm2+/− deficient mice did not show a significant decrease in urinary Mg2+ excretion (38 ± 3.2 versus 37 ± 3.0 mmol/L/24hrs, respectively) in the urine compared to wild type mice across the different diets (Fig. 3B,C), despite having decreased serum Mg2+ concentration, suggestive for a small renal reabsorption defect. Interestingly, Cnnm2+/− displayed Ca2+ retention at the baseline urine measurement (1.0 ± 0.1 vs 1.7 ± 0.1 μmol/24hrs, respectively, P < 0.0001) (Fig. 3D), although this effect was not observed at the final day of the diets (Fig. 3E). Gene expression analysis showed significantly decreased renal expression of Cnnm2 in Cnnm2+/− mice on the control and high Mg2+ diet (Fig. 3F). Expression of the family member Cnnm4 was decreased 3.6-fold in Cnnm2+/− mice fed on the normal Mg2+-diet (Fig. 3G). Levels of magnesiotropic genes Trpm6, Trpm7, Slc41a1 were measured in the kidney, but did not show any genotype-specific effect (Fig. 3H–J). The expression of the epithelial Ca2+ channel Trpv5 showed an overall significant increase due the diets, but no differences were observed between the Cnnm2+/− compared to wild type mice (Fig. 3K).
Figure 3.
Cnnm2 deficiency does not affect overall renal Mg2+ reabsorption. (A) Representative pictures of kidney morphology by H&E staining. Scale bar depicts 50 μm. (B,D) Cnnm2+/− mice do not show altered Mg2+ excretion via the urine, but retain more Ca2+ (n = 30–36 per group). (C–E) Lower Mg2+ intake is associated with lower renal Mg2+ excretion, not with Ca2+, independent of genotype in Cnnm2+/− mice (black bars) (n = 8–12 per group). (F) Renal Cnnm2 expression is lowered in Cnnm2+/− mice, Cnnm4 (G), Trpm6 (H), Trpm7 (I), Slc41a1 (J), and Trpv5 (K) remain majorly unaltered (n = 3–6 mice per group). Data are presented as mean ± SEM. Significance determined with two-tailed Student’s T-test or Two-way ANOVA followed by a Tukey post-hoc test. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Cnnm2+/− mice have perturbed intestinal Mg2+ absorption
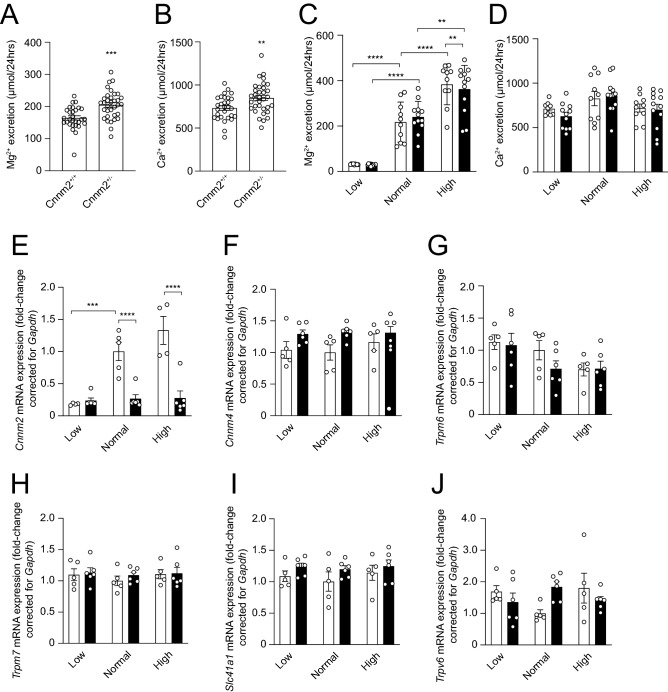
Before the start of the diets Cnnm2+/− mice displayed a 17.5% higher faecal Mg2+ content compared to Cnnm2+/+ mice (Fig. 4A,B). This was similar to Ca2+ levels, which were also significantly increased (Fig. 4C,D). Furthermore, we investigated the mRNA expression of key magnesiotropic genes present in the distal part of the colon, where transcellular Mg2+ absorption takes place. Similar to the observation in the kidney, Cnnm2 transcript levels were significantly reduced in the Cnnm2+/− mice compared to control mice. Yet there was an overall increase of Trpm6 expression and Cnnm4 expression based on diet or genotype, respectively (Two-Way ANOVA). No diet or genotype dependent changes were observed for other magnesiotropic genes (Fig. 4E–I). The expression of the epithelial Ca2+ channel Trpv6 was not significantly different among all mice groups (Fig. 4J).
Figure 4.
Cnnm2 deficiency decreases faecal Mg2+ absorption. (A,C) Cnnm2+/− mice display significantly increased Mg2+ and Ca2+ excretion via the faeces (n = 29–34 per group). (B,D) Lower Mg2+ intake is associated with lower intestinal Mg2+ excretion, not with Ca2+, independent of genotype in Cnnm2+/+ (white bars) and Cnnm2+/− mice (black bars) (n = 8–12 per group) (E) Cnnm2 expression is significantly diminished in Cnnm2+/− mice, Cnnm4 (G), Trpm6 (H), Trpm7 (I), Slc41a1 (J), and Trpv6 (K) remain unaltered (n = 3–6 per group). Data are presented as mean ± SEM. Significance determined with two-tailed Student’s T-test or Two-way ANOVA followed by a Tukey post-hoc test. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
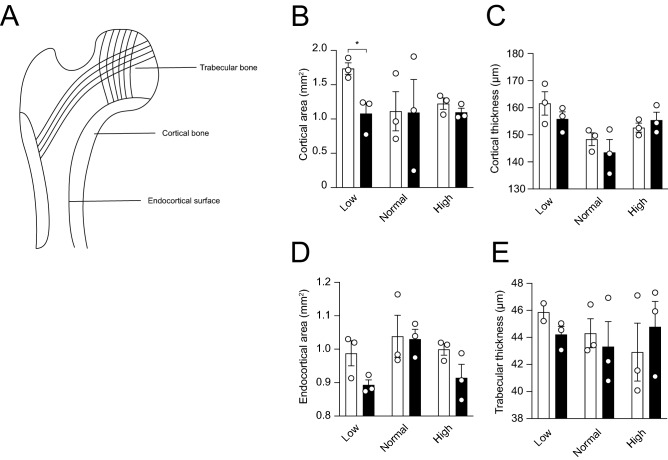
Cnnm2+/− mice display normal bone morphology
The femurs of three male mice were subjected to micro-computed tomography analysis. Both the cortical and trabecular bone microarchitecture were not significantly affected by genotype nor diet, except in Cnnm2+/− mice on a low Mg2+ diet (Fig. 5A–E).
Figure 5.
CNNM2 depletion does not affect bone morphology. Micro-CT analysis of femurs of which the cortical area (A) and thickness (B) were determined, endocortical area (C) and trabecular thickness (D). (n = 3 per group) Data are presented as mean ± SEM. Significance determined with two-tailed Student’s T-test or Two-way ANOVA followed by a Tukey post-hoc test. *p < 0.05.
Discussion
In this study, we used several genetic mouse models to investigate the role of CNNM2 in mice. Cnnm2−/− mice exhibited maldevelopment of the brain, prominently consisting of exencephaly. The Cnnm2−/− newborns displayed hypomagnesaemia concomitant with increased serum Ca2+ levels. Cnnm2+/− adult mice showed a normal development, but similarly suffered from a low serum Mg2+ concentration, which could be partially explained by faecal and renal Mg2+ loss. Our study emphasises the role of CNNM2 in embryonic development and overall Mg2+ handling, and disclosed a novel role in intestinal Mg2+ absorption.
Impaired renal Mg2+ reabsorption and urinary Mg2+ wasting have been considered as the primary defect of HSMR patients as renal Mg2+ excretion remains similar despite lowered serum Mg2+ levels13,14. In line with the observation in patients, Cnnm2+/− mice displayed low serum Mg2+ levels when compared to Cnnm2+/+ mice. Yet, no significant increase in urinary Mg2+ excretion was observed. The Cnnm2+/− mice displayed lowered urinary Mg2+ excretion upon exposure to lowered Mg2+ diet, similarly to wild type mice. This demonstrates the compensatory capacity of Mg2+ reabsorption in these mice. Yet, Cnnm2+/− mice showed decreased serum Mg2+ levels. The fact that this low serum value is not associated with a diminished urinary Mg2+ excretion can be interpreted as a sign of a renal Mg2+ leak. This has previously been reported in other mouse models of hypomagnesemia, e.g. Slc41a3−/− mice and high-fat diet-fed mice24,25. The role of CNNM2 in the kidney has been substantiated by studies of kidney-specific knockout mice of Cnnm2 which suffer from hypomagnesemia, demonstrating that CNNM2 regulates renal Mg2+ handling19.
Interestingly, intestinal malabsorption may also play a role in the Mg2+ deficiency observed in the Cnnm2+/− mice. At basal conditions, faecal Mg2+ excretion was increased in Cnnm2+/− mice, indicative of Mg2+ malabsorption. Although CNNM2 has been primarily studied in the kidney, our current and previous studies demonstrate that Cnnm2 is expressed in the distal colon8. Indeed, several recent single cell RNA sequencing datasets in colon and rectum confirm that CNNM2 is particularly expressed in enterocytes26,27. Of interest, CNNM4, a close family member of CNNM2, is present in the basolateral membrane of the colon, where it orchestrates Mg2+ efflux28–30. We have previously shown an interaction of CNNM2 with CNNM4 upon expression in HEK293 cells, suggesting that these proteins could form a complex8. Indeed, a significant increase of Cnnm4 expression in the colon was observed in Cnnm2+/− mice, suggesting that CNNM4 compensates for the loss of CNNM2. However, a CNNM4-independent function of CNNM2 cannot be excluded. Although malabsorption of Mg2+ was not previously reported in Cnnm2+/− mice or patients19, it should be noted that faecal Mg2+ excretion is often not detected or even determined in mice, although also in intestinal-specific Trmp6−/− and Trpm7−/− mice decreased serum Mg2+ levels and increased faecal Mg2+ excretion can be observed6,31. On average, 30% of dietary Mg2+ is absorbed and foremost ends in the faeces, making it challenging to detect changes in absorption. However, Mg2+ supplementation was proven difficult in patients with CNNM2 mutations, which potentially indicates a reduced ability to absorb Mg2+13,14. Our findings suggest that oral Mg2+ supplementation in patient with HSMR syndrome depends on paracellular Mg2+ transport in the duodenum, as transcellular Mg2+ absorption in the colon may be reduced by CNNM2 mutations.
In addition to the Mg2+ disturbances, Cnnm2+/− mice displayed renal Ca2+ retention resulting in an increased serum Ca2+ concentration. Decreased serum Mg2+ levels could trigger the TAL to increase its paracellular Mg2+ transport. In addition, this would lead to increased Ca2+ reabsorption and decreased urinary Ca2+ levels, which is coordinated via Claudins 10, 14, 16 and 1932–36. This compensatory capacity may be specific for mice, as patients with mutated CNNM2 have significant urinary Mg2+ wasting. In line with this, 24 h urinary Ca2+ levels are normal in patients with CNNM2 mutations. Interestingly, a few patients with CNNM2 mutations have been reported with impaired Ca2+ and phosphate homeostasis. Whether this is a common phenotype in these patients remains to be determined, as the patients are consanguineous or have other genetic defects, such as mutations in the vitamin D receptor (VDR)12,37. However, the role of TAL in CNNM2-associated physiology should be interpreted with caution, as direct effects of CNNM2 on Ca2+ homeostasis cannot be excluded.
Our study demonstrates that CNNM2 is essential for brain development and early life survival. Until embryonic day 18.5, the Cnnm2−/− embryos were present at Mendelian ratios, but newborn Cnnm2−/− mice died shortly after birth. Although lethality of Cnnm2−/− mice was reported earlier19, we are the first to report the presence of exencephaly, which suggests that neural tube defects contribute to the brain phenotype. It is known that defects in the closure of the cranial neural tube result in protrusion of the neuroepithelium outside the developing brain, inevitably disturbing normal neurodevelopment38. Although previous studies suggested an important role of CNNM2 in brain function, the exact consequences of CNNM2 deficiency may be species-dependent. Patients with recessive CNNM2 mutations exhibit structural brain deformities, such as demyelination, failure of opercularisation, and cerebral cortical atrophy, often concomitant with motor skill defects, epileptic seizures, and intellectual disability12,13. Moreover, knockdown of cnnm2 in zebrafish resulted in maldevelopment of the mid-hindbrain boundary13.
Interestingly, exencephaly and other neural tube defects have been more often observed in mouse models of hypomagnesemia. Trmp6 knockout mice display embryonic lethality, which is accompanied by the presence of exencephaly and spina bifida5,39. Similarly, the absence of Trpm7 expression in mice during embryonic development, a close homologue of TRPM6, has been associated with neural tube defects40,41. Although this homology suggests that neural tube defects may be the direct result of hypomagnesaemia, it should be noted that neural tube defects are uncommon in patients with hypomagnesaemia1. Consequently, it remains to be determined whether this is a Mg2+ dependent or independent effect. Of note: as only a subset of Cnnm2−/− mice displayed exencephaly, neural tube defects may only partially explain the lethality.
The absence of a clear urinary Mg2+ wasting in Cnnm2+/− mice shows that renal Mg2+ reabsorption may be different in mice and men, as HSMR syndrome show clear renal Mg2+ wasting13. In line with this, kidney-specific Trpm6 knock out mice were reported to have normal serum Mg2+ levels and without renal Mg2+ leak, unlike patients with Trpm6 mutations (HOMG1/HSH; MIM# 602014)31. Similarly, Kcnj10 and Fxyd2 knock out animals have normal urinary Mg2+ levels, in contrast to patients with mutations (OMIM: 612780 & 154020)42–46. Additionally, a mice model deficient in solute carrier family 41 member 3 (Slc41a3) expression, a putative Mg2+ transporter and highly enriched in DCT, was associated with hypomagnesemia without renal Mg2+ wasting24. Altogether, these studies show that mice are able to compensate better for impaired DCT Mg2+ reabsorption compared to men.
In conclusion, CNNM2 regulates the systemic Mg2+ balance and is essential for neurodevelopment. Our study points toward a putative role of CNNM2 in intestinal Mg2+ absorption.
Supplementary Information
Acknowledgements
This work was financially supported by grants from the Netherlands Organization for Scientific Research (NWO Veni 016.186.012. Vici 016.130.668), and the European Joint Program for Rare Diseases (EJPRD2019-40). M.S. and D.M. were supported by the Deutsche Forschungsgemeinschaft (DFG), Research Training Group GRK 2318.
Author contributions
G.F., M.S., C.B., R.B., J.H., D.M, T.B., and J.d.B. designed the research studies, G.F., M.S., C.B., L.S., T.B., and J.d.B. conducted experiments and/or analysed data, G.F. and J.d.B. wrote the manuscript. All authors corrected the manuscript and approved the final version.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Gijs A. C. Franken and Murat Seker.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-87548-6.
References
- 1.de Baaij JH, Hoenderop JG, Bindels RJ. Magnesium in man: implications for health and disease. Physiol. Rev. 2015;95(1):1–46. doi: 10.1152/physrev.00012.2014. [DOI] [PubMed] [Google Scholar]
- 2.Quamme GA, Dirks JH. Magnesium transport in the nephron. Am. J. Physiol. 1980;239(5):F393–401. doi: 10.1152/ajprenal.1980.239.5.F393. [DOI] [PubMed] [Google Scholar]
- 3.Groenestege WM, et al. The epithelial Mg2+ channel transient receptor potential melastatin 6 is regulated by dietary Mg2+ content and estrogens. J. Am. Soc. Nephrol. 2006;17(4):1035–1043. doi: 10.1681/ASN.2005070700. [DOI] [PubMed] [Google Scholar]
- 4.Thebault S, et al. EGF increases TRPM6 activity and surface expression. J. Am. Soc. Nephrol. 2009;20(1):78–85. doi: 10.1681/ASN.2008030327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Woudenberg-Vrenken TE, et al. Transient receptor potential melastatin 6 knockout mice are lethal whereas heterozygous deletion results in mild hypomagnesemia. Nephron Physiol. 2011;117(2):11–19. doi: 10.1159/000320580. [DOI] [PubMed] [Google Scholar]
- 6.Mittermeier L, et al. TRPM7 is the central gatekeeper of intestinal mineral absorption essential for postnatal survival. Proc. Natl. Acad. Sci. 2019;116(10):4706–4715. doi: 10.1073/pnas.1810633116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li M, Jiang J, Yue L. Functional characterization of homo- and heteromeric channel kinases TRPM6 and TRPM7. J. Gen. Physiol. 2006;127(5):525–537. doi: 10.1085/jgp.200609502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Baaij JHF, et al. Membrane topology and intracellular processing of cyclin M2 (CNNM2) J. Biol. Chem. 2012;287(17):13644–13655. doi: 10.1074/jbc.M112.342204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arjona FJ, de Baaij JHF. CrossTalk opposing view: CNNM proteins are not Na(+) /Mg(2+) exchangers but Mg(2+) transport regulators playing a central role in transepithelial Mg(2+) (re)absorption. J. Physiol. 2018;596(5):747–750. doi: 10.1113/JP275249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arjona FJ, de Baaij JHF. Rebuttal from Francisco J. Arjona and Jeroen H. F. de Baaij. J. Physiol. 2018;596(5):753–754. doi: 10.1113/JP275705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sponder G, et al. Human CNNM2 is not a Mg(2+) transporter per se. Pflugers Arch. 2016;468(7):1223–1240. doi: 10.1007/s00424-016-1816-7. [DOI] [PubMed] [Google Scholar]
- 12.Accogli A, et al. CNNM2 homozygous mutations cause severe refractory hypomagnesemia, epileptic encephalopathy and brain malformations. Eur. J. Med. Genet. 2019;62(3):198–203. doi: 10.1016/j.ejmg.2018.07.014. [DOI] [PubMed] [Google Scholar]
- 13.Arjona FJ, et al. CNNM2 mutations cause impaired brain development and seizures in patients with hypomagnesemia. PLoS Genet. 2014;10(4):e1004267. doi: 10.1371/journal.pgen.1004267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stuiver M, et al. CNNM2, encoding a basolateral protein required for renal Mg2+ handling, is mutated in dominant hypomagnesemia. Am. J. Hum. Genet. 2011;88(3):333–343. doi: 10.1016/j.ajhg.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li C, et al. Genome-wide association study meta-analysis of long-term average blood pressure in East Asians. Circ. Cardiovasc. Genet. 2017;10(2):e001527–e001527. doi: 10.1161/CIRCGENETICS.116.001527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lv W-Q, et al. Novel common variants associated with body mass index and coronary artery disease detected using a pleiotropic cFDR method. J. Mol. Cell. Cardiol. 2017;112:1–7. doi: 10.1016/j.yjmcc.2017.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ohi K, et al. The impact of the genome-wide supported variant in the cyclin M2 gene on gray matter morphology in schizophrenia. Behav. Brain Funct. BBF. 2013;9:40–40. doi: 10.1186/1744-9081-9-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rose EJ, et al. Effects of a novel schizophrenia risk variant rs7914558 at CNNM2 on brain structure and attributional style. Br. J. Psychiatry. 2014;204(2):115–121. doi: 10.1192/bjp.bp.113.131359. [DOI] [PubMed] [Google Scholar]
- 19.Funato Y, Yamazaki D, Miki H. Renal function of cyclin M2 Mg2+ transporter maintains blood pressure. J. Hypertens. 2017;35(3):585–592. doi: 10.1097/HJH.0000000000001211. [DOI] [PubMed] [Google Scholar]
- 20.Wang H, et al. One-step generation of mice carrying mutations in multiple genes by CRISPR/Cas-mediated genome engineering. Cell. 2013;153(4):910–918. doi: 10.1016/j.cell.2013.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lorenz C, Pusch M, Jentsch TJ. Heteromultimeric CLC chloride channels with novel properties. Proc. Natl. Acad. Sci. U. S. A. 1996;93(23):13362–13366. doi: 10.1073/pnas.93.23.13362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoenderop JGJ, et al. Calcitriol controls the epithelial calcium channel in kidney. J. Am. Soc. Nephrol. 2001;12(7):1342. doi: 10.1681/ASN.V1271342. [DOI] [PubMed] [Google Scholar]
- 23.Bouxsein ML, et al. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J. Bone Miner. Res. 2010;25(7):1468–1486. doi: 10.1002/jbmr.141. [DOI] [PubMed] [Google Scholar]
- 24.de Baaij JH, et al. Identification of SLC41A3 as a novel player in magnesium homeostasis. Sci. Rep. 2016;6:28565. doi: 10.1038/srep28565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kurstjens S, et al. Renal phospholipidosis and impaired magnesium handling in high-fat-diet–fed mice. FASEB J. 2019;33(6):7192–7201. doi: 10.1096/fj.201801778RR. [DOI] [PubMed] [Google Scholar]
- 26.Parikh K, et al. Colonic epithelial cell diversity in health and inflammatory bowel disease. Nature. 2019;567(7746):49–55. doi: 10.1038/s41586-019-0992-y. [DOI] [PubMed] [Google Scholar]
- 27.Wang, Y. et al. Single-cell transcriptome analysis reveals differential nutrient absorption functions in human intestine.J. Exp. Med. 217(2) (2019). [DOI] [PMC free article] [PubMed]
- 28.Funato Y, et al. Membrane protein CNNM4-dependent Mg2+ efflux suppresses tumor progression. J. Clin. Investig. 2014;124(12):5398–5410. doi: 10.1172/JCI76614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamazaki D, et al. Cnnm4 deficiency suppresses Ca2+ signaling and promotes cell proliferation in the colon epithelia. Oncogene. 2019;38(20):3962–3969. doi: 10.1038/s41388-019-0682-0. [DOI] [PubMed] [Google Scholar]
- 30.Yamazaki D, et al. Basolateral Mg2+ extrusion via CNNM4 mediates transcellular Mg2+ transport across epithelia: a mouse model. PLoS Genet. 2013;9(12):e1003983. doi: 10.1371/journal.pgen.1003983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chubanov V, et al. Epithelial magnesium transport by TRPM6 is essential for prenatal development and adult survival. Elife. 2016;5:e20914. doi: 10.7554/eLife.20914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Breiderhoff T, et al. Deletion of claudin-10 (Cldn10) in the thick ascending limb impairs paracellular sodium permeability and leads to hypermagnesemia and nephrocalcinosis. Proc. Natl. Acad. Sci. U. S. A. 2012;109(35):14241–14246. doi: 10.1073/pnas.1203834109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dimke H, et al. Activation of the Ca(2+)-sensing receptor increases renal claudin-14 expression and urinary Ca(2+) excretion. Am. J. Physiol. Renal Physiol. 2013;304(6):F761–F769. doi: 10.1152/ajprenal.00263.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gong Y, et al. Claudin-14 regulates renal Ca++ transport in response to CaSR signalling via a novel microRNA pathway. Embo J. 2012;31(8):1999–2012. doi: 10.1038/emboj.2012.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hou J, et al. Claudin-16 and claudin-19 interaction is required for their assembly into tight junctions and for renal reabsorption of magnesium. Proc. Natl. Acad. Sci. U. S. A. 2009;106(36):15350–15355. doi: 10.1073/pnas.0907724106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ohta H, et al. Restricted localization of claudin-16 at the tight junction in the thick ascending limb of Henle's loop together with claudins 3, 4, and 10 in bovine nephrons. J. Vet. Med. Sci. 2006;68(5):453–463. doi: 10.1292/jvms.68.453. [DOI] [PubMed] [Google Scholar]
- 37.García-Castaño A, et al. Novel variant in the CNNM2 gene associated with dominant hypomagnesemia. PLoS ONE. 2020;15(9):e0239965. doi: 10.1371/journal.pone.0239965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Copp AJ, Greene NDE. Neural tube defects–disorders of neurulation and related embryonic processes. Wiley Interdiscip. Rev. Dev. Biol. 2013;2(2):213–227. doi: 10.1002/wdev.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Walder RY, et al. Mice defective in Trpm6 show embryonic mortality and neural tube defects. Hum. Mol. Genet. 2009;18(22):4367–4375. doi: 10.1093/hmg/ddp392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jin J, et al. The channel kinase, TRPM7, is required for early embryonic development. Proc. Natl. Acad. Sci. U. S. A. 2012;109(5):E225–E233. doi: 10.1073/pnas.1120033109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu W, et al. TRPM7 regulates gastrulation during vertebrate embryogenesis. Dev. Biol. 2011;350(2):348–357. doi: 10.1016/j.ydbio.2010.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reichold M, et al. KCNJ10 gene mutations causing EAST syndrome (epilepsy, ataxia, sensorineural deafness, and tubulopathy) disrupt channel function. Proc. Natl. Acad. Sci. U. S. A. 2010;107(32):14490–14495. doi: 10.1073/pnas.1003072107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arystarkhova E, et al. Paradoxical activation of the sodium chloride cotransporter (NCC) without hypertension in kidney deficient in a regulatory subunit of Na, K-ATPase, FXYD2. Physiol. Rep. 2014;2(12):e12226. doi: 10.14814/phy2.12226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.de Baaij JH, et al. Recurrent FXYD2 p.Gly41Arg mutation in patients with isolated dominant hypomagnesaemia. Nephrol. Dial Transplant. 2015;30(6):952–957. doi: 10.1093/ndt/gfv014. [DOI] [PubMed] [Google Scholar]
- 45.Jones DH, et al. Na, K-ATPase from mice lacking the gamma subunit (FXYD2) exhibits altered Na+ affinity and decreased thermal stability. J. Biol. Chem. 2005;280(19):19003–19011. doi: 10.1074/jbc.M500697200. [DOI] [PubMed] [Google Scholar]
- 46.Meij IC, et al. Dominant isolated renal magnesium loss is caused by misrouting of the Na(+), K(+)-ATPase gamma-subunit. Nat. Genet. 2000;26(3):265–266. doi: 10.1038/81543. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.