Abstract
Introduction
Spinal meningiomas represent 25–45% of intradural spinal tumors and ~2% of meningiomas of the central nervous system (CNS), and their occurrence during pregnancy is unusual. We present an updated literature review.
Case report
A 36-year-old woman, at 32.6 weeks of gestation, was hospitalized for urinary tract infection and urinary retention. One month earlier, she had decreased strength in lower limbs, and this weakness rapidly progressed to flaccid paraplegia without sphincters control. Magnetic resonance imaging (MRI) revealed a well-defined intradural extramedullary lesion in T3-T4. Using a posterolateral approach, the tumor was completely removed; however, there was no clinical improvement, and the patient was discharged with an impairment scale (AIS) grade A. Histopathology examination indicated a psammomatous meningioma.
Discussion
Meningiomas are benign tumors that are slowly progressive; however, the hemodynamic and hormonal changes of pregnancy are related to their accelerated growth. Reports show that the onset of the symptoms during the third trimester of pregnancy, including early neurological symptoms or signs of spinal cord compression, can be easily attributed to those of pregnancy by both the patient and the doctor. The time to diagnosis and medulla compression time are thus prolonged, which can be further compounded in middle-high income countries due to limitations in obtaining images for evaluation. Although rare, spinal meningiomas should be considered in the differential diagnosis of patients with neurological symptoms during pregnancy. Their early recognition is important to avoid irreversible neurological damage.
Subject terms: Cancer in the nervous system, Oncogenesis, Spinal cord, Physical examination, Reproductive signs and symptoms
Introduction
Intradural spinal tumors are rare lesions, with an incidence of 3–10 per 100,000 people [1]. They are classified as extramedullary (70%) and intramedullary (30%), and extramedullary tumors are predominantly meningiomas, schwannomas, and neurofibromas [1–3]. Meningiomas are benign slow-growing tumors that arise from arachnoid cells, are mostly sporadic, and occur more frequently in women, with a ratio of ~2:1 for intracranial tumors and 10:1 for spinal tumors [4]. Their incidence increases with age, reaching its peak in the seventh and eighth decades of life [5, 6]. Spinal meningiomas represent 25–45% of intradural spinal tumors and ~2% of central nervous system meningiomas [5]. The occurrence of spinal meningiomas during pregnancy is unusual. In a review by Roelvink et al. [7] on brain and spinal tumors in pregnancy, 5 out of 62 (8%) meningiomas that occurred were spinal. Meng et al. [8] and Han et al. [9] reported 21 and 10 cases, respectively, of spinal tumors during pregnancy, and no meningiomas were found. Another study reported 122 spinal meningiomas, of which 2 (1.5%) occurred during pregnancy. The authors performed a literature review in which they reported six cases [10]. This review includes 5 cases for a total of 11 reported to date, including the case presented (see Table 1).
Table 1.
Literature review, characteristics of spinal meningiomas reported during pregnancy.
| Author(s) | Age (years) | Pregnancy (gravidity) | Trimester symptoms began | Level | Symptoms | Surgery before (B) or after (A) delivery | Histopathology | Outcome |
|---|---|---|---|---|---|---|---|---|
| Bailey et al. [13] | Unknown | Unknown | Second | Cervical | Quadriparesis, sensitive symptoms not reported | A | Unknown | Good |
| O’Connell [20] | 24 | Second | Unknown | T7 | Weakness in both lower limbs, right chest pain, urinary incontinence | A | Psammomatous | Well at 3 years |
| Rath et al. [18] | 20 | Unknown | Third | C3 and T8a | Weakness in both lower limbs, midthoracic pain | A | Meningothelial and psammomatous | Well at 1 month |
| Mealey and Carter [16] | 25 | First | Third | T1 | Weakness and unsteadiness in both lower limbs, numb feeling in soles of the feet | B | Transitional or psammomatous | Well at 2 weeks |
| Cioffi et al. [10] | 23 | Unknown | Third | T1-T2 | Ataxia, weakness and unsteadiness in both lower limbs, no sensitive disturbances | A | Fibrous | Well at 1 year |
| Cioffi et al. [10] | 28 | Unknown | Third | T2 | Weakness in the right lower limb, right side mammary paresthesia | A | Meningothelial | Well at 6 months |
| Pardo et al. [19] | 25 | Unknown | Third | T6-T8 | Ataxia, weakness in both lower limbs, urinary incontinence | B | Fibrous | Good |
| Horn et al. [15] | 35 | Unknown | Second | T1-T3b | Ataxia, numbness and weakness in both lower limbs | A | Unknown | Well at 3 days |
| Clark et al. [14] | 21 | Second | Third | T9 | Left lower limb swelling, bowel and bladder incontinence, bilateral lower extremity paresis | A | Unknown | Well at 6 weeks |
| Pikis et al. [17] | 32 | Unknown | Third | T4 | Nocturnal back pain, bilateral hip numbness, an electric shock-like sensation radiating to both lower limbs | A | Transitional | Well at 3 months |
| This case report | 36 | Fifth | Third | T3-T4 | Ataxia, weakness in both lower limbs, urinary retention | A | Psammomatous | Bad |
Results obtained from a bibliographic review performed with the keywords “pregnancy,” “tumor spinal,” and “meningioma.” This search was performed in PubMed and Google Scholar.
aMultiple meningiomas.
bRecurrent meningioma.
Case report
A 36-year-old patient, gravidity 5 and parity 4, with no significant medical or family history, was hospitalized at 32.6 weeks of pregnancy for urinary tract infection and urinary retention that required a bladder catheter, and 1 month earlier, she had reported decreased strength in the lower limbs. Upon physical examination, the uterine height was 32 cm, and the fetal heart rate was 146 bpm. Uroanalysis, urine culture, ultrasound of the urinary tract, and consultation for gynecology and maternal-fetal medicine were requested. During her hospital stay, the patient’s weakness in the lower limbs progressed rapidly to prevent walking. During her consultation with neurology, the patient was alert, oriented with spastic paraparesis, bilateral Babinski sign, inexhaustible Achilles tendon clonus, neurogenic spastic bladder and bowel, and a bilateral T8 sensory level, no neurological deficit at cranial nerves, or upper limbs. Extrinsic medullary compression was suspected; therefore, simple dorsal magnetic resonance imaging (MRI) was requested.
The uroanalysis indicated pathological results, the urine culture reported Pseudomonas aeruginosa and extended-spectrum β-lactamase-producing Klebsiella positivity, ultrasound of the urinary tract did not show alterations, and fetal well-being tests determined viability. Antibiotic therapy was initiated after consultation with the infectious disease department (ceftriaxone 1 g intravenously (IV) every 12 h, meropenem 1 g IV every 12 h, vaginal clotrimazole). At 37.1 weeks of gestation, fetal placental alteration (Doppler type II) was detected, and an emergency cesarean was performed with good perinatal results, with a male newborn weighing 2749 g with Apgar scores of 8, 10, and 10 at 1, 5, and 10 min, respectively.
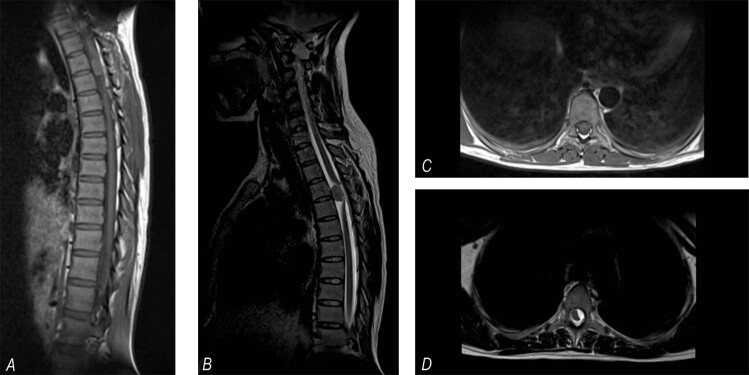
Approximately 1 month after the request, MRI was performed, which showed a left intradural extramedullary lesion at the T3-T4 level with severe compression and posterior lateral displacement of the spinal cord (see Fig. 1). The preoperative neurological examination of the patient showed flaccid paraplegia with areflexia and without sphincter control with a T4 sensory level. Impairment scale (AIS) grade A, 4 weeks before her neurologic classification was AIS grade C. Because of an uncertain prognosis due to evolution time of the compression, on the seventh day postpartum, a T1-5 posterolateral laminectomy was performed, and the attachments of the denticulate ligaments to the dura mater were divided. A grayish and ischemic medulla was observed, as well a solid pinkish ventrolateral tumor. Simpson grade III dissection was achieved. The histopathology results revealed grade I meningioma, according to the World Health Organization (WHO) classification [4], with a psammomatous subtype (see Fig. 2). The patient had no postsurgical complications or neurological improvement and was discharged with a T6 neurological level and AIS grade A [11]. No information is available on her long-term neurological assessment.
Fig. 1. Simple dorsal MRI.
Right: A sagittal section shows an extra-axial mass at T3-T4, isointense T1 (a) and hypointense T2 signals (b), and displacement of the spinal cord posteriorly. Left: Axial section showing a T1 hypointense left ventrolateral mass (c) and a T2 hyperintense mass (d) displacing the spinal cord to the right and posteriorly.
Fig. 2. Hematoxylin-eosin staining (100×).
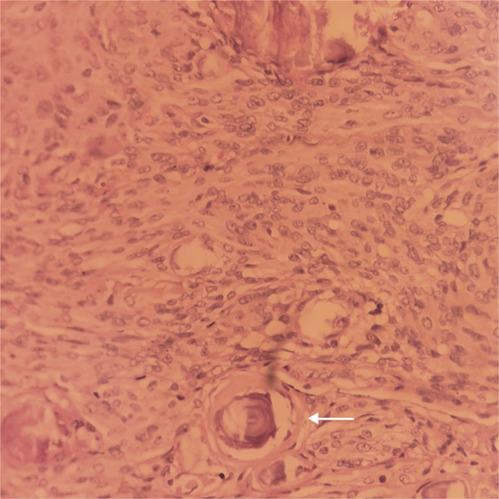
Hematoxylin-eosin staining shows a psammomatous meningioma formed by psammoma bodies (arrow).
Discussion
Spinal meningiomas are less common in younger patients, and during pregnancy, they occur more frequently in middle-aged patients. The presence of spinal meningiomas with neurofibromatosis type 2 has been reported in young patients [5]. Spinal meningiomas, including those with benign histological characteristics, are associated with an aggressive clinical course in younger patients [12].
Two hypotheses have been proposed to explain rapid tumor growth during pregnancy: hormonal action and hemodynamic changes. Factors that indicate hormonal regulation of tumor growth include female prevalence, frequent expression of progesterone receptors in ~70% of meningiomas [4–6], and epidemiological evidence of associations between meningioma and pregnancy [7, 10, 13–20], breast cancer, and the use of exogenous hormones [6, 21, 22]. Recently, in a study by Peyre et al. [23], the molecular basis of progestin-associated meningiomas was explored for the first time, and a high frequency of PIK3CA mutations in patients with continuous exposure to progesterone agonist was found. In addition, meningiomas induced by progestogens, such as PIK3CA mutants, were associated with younger age. Hemodynamic changes are linked to the onset of symptoms during the last trimester of pregnancy. When hydrodynamic changes are prominent, meningiomas may be explained by the redistribution of increase in blood flow through the vertebral venous plexus secondary to compression of the vena cava through the gravid uterus [8, 10, 16]. The onset of symptoms is observed more frequently during the third trimester of pregnancy [7, 10, 14, 16–19], and its clinical presentation is related to the size and location in the spine, usually consisting of radicular pain and/or myelopathy if compression of the cord occurs [1, 2, 24]. During pregnancy, the symptoms can be difficult to recognize because the early neurological syndrome can mimic the fatigue, weakness, and urinary symptoms observed due to increased uterine size, ligament tension, and lumbosacral nerve irritation [10, 16]. The majority of spinal meningiomas are intradural, located mainly in the thoracic region and ventrolaterally in relation to the spinal cord [1, 5, 12, 24]. Of the cases reviewed, eight were dorsal, one was cervical, and one included multiple tumors at dorsal and cervical locations. MRI is the diagnostic method of choice; generally, spinal meningiomas are isointense to hypointense on T1-weighted MRI images and slightly hypertensive on T2-weighted images [1, 2, 5]. They can show a dural tail sign or a linear enhancement of the adjacent dura mater after administration of gadolinium, similar to intracranial meningiomas, which allows them to be distinguished from other intradural tumors [5]. MRI of the patient was performed without contrast due to contraindication during pregnancy [8].
Approximately 92.8 % of spinal meningiomas are benign [1, 4–6], and low-grade meningiomas (WHO) [4] develop three times more often in women during reproductive years. The meningothelial or psammomatous subtypes predominate in young patients [12, 24]. In our review, four of the eight cases which reported with histopathological information were psammomatous. It is not clear whether a correlation exists between neurological restoration and histopathological parameters. Schaller found that the resection of psammomatous meningiomas of the spine was associated with a less favorable neurological outcome after the operation compared to the other histological subtypes [24].
Complete resection of meningiomas is generally possible with a favorable result [1]. Reports have shown that Simpson grades I and II resection can be achieved in 82–100% of cases [5]. In our patient, a total resection was achieved, and no dural coagulation was required. In all reported cases except ours, neurological function was recovered. The prognostic factors of patients with severe motor deficit and spinal meningioma include the location, degree of resection, and preoperative neurological status [24]. Mehta et al. [1] in a retrospective study spanning 10 years found that postoperative neurological deficits occurred more frequently in tumors located ventrally in the upper thoracic spine. This finding could be associated with the greater relationship between the cord and the canal, as well as the weak vascular supply in this medullary region. A ventral or ventrolateral location, large tumors occupying 75% or more of the spinal canal, and poor preoperative functional status were associated with poor functional outcome at the 1-year follow-up [5]. Other studies have shown functional recovery even with severe neurological deficits [1, 25]. Timely surgery plays an important role in the postoperative functional result, possibly because the outcome of the patients is affected by prolonged time of spinal compression. Long-term compression of the spinal cord causes damage to nerve tissues by mechanical and vascular mechanisms. When compression is not at an advanced stage, the self-regulation of spinal arterial circulation is still maintained, and mechanical factors and vascular insufficiency can be compounded, causing a greater disorder [26]. The recurrence rate of spinal meningiomas is low, with recurrence and progression rates for spinal meningiomas of 0% and 13%, respectively, which are lower than those found for intracranial meningiomas [5]. Cohen-Gadol et al. reported higher recurrence rates in young patients [12].
Spinal meningiomas during pregnancy have been reported on rare occasions, and their early symptoms or signs of spinal cord compression can be easily attributed to those typical of pregnancy by both the patient and by the doctor. The diagnosis and therefore the time of spinal compression are thus prolonged, which can be further compounded in middle-high-income countries due to limitations in obtaining images for evaluation.
Although rare, spinal meningiomas should be considered in differential diagnosis of patients with neurological symptoms during pregnancy. Their early recognition is important to avoid irreversible neurological damage.
Supplementary information
Acknowledgements
The authors would like to thank Dr Patricia Cruz and Dr Héctor Santaella, Department of Pathology, Erasmo Meoz University Hospital, Cúcuta, Colombia.
Compliance with ethical standards
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Víctor Enrique Antolínez Ayala, María Daniela García Arias
Supplementary information
The online version of this article (10.1038/s41394-020-00368-0) contains supplementary material, which is available to authorized users.
References
- 1.Mehta AI, Adogwa O, Karikari IO, Thompson P, Verla T, Null UT, et al. Anatomical location dictating major surgical complications for intradural extramedullary spinal tumors: a 10-year single-institutional experience. J Neurosurg Spine. 2013;19:701–7. doi: 10.3171/2013.9.SPINE12913. [DOI] [PubMed] [Google Scholar]
- 2.Abul-Kasim K, Thurnher MM, McKeever P, Sundgren PC. Intradural spinal tumors: current classification and MRI features. Neuroradiology. 2008;50:301–14.. doi: 10.1007/s00234-007-0345-7. [DOI] [PubMed] [Google Scholar]
- 3.Traul DE, Shaffrey ME, Schiff D. Part I: spinal-cord neoplasms—intradural neoplasms. Lancet Oncol. 2007;8:35–45. doi: 10.1016/S1470-2045(06)71009-9. [DOI] [PubMed] [Google Scholar]
- 4.Commins DL, Atkinson RD, Burnett ME. Review of meningioma histopathology. Neurosurg Focus. 2007;23:E3. doi: 10.3171/FOC-07/10/E3. [DOI] [PubMed] [Google Scholar]
- 5.Maiti TK, Bir SC, Patra DP, Kalakoti P, Guthikonda B, Nanda A. Spinal meningiomas: clinicoradiological factors predicting recurrence and functional outcome. Neurosurg Focus. 2016;41:E6. doi: 10.3171/2016.5.FOCUS16163. [DOI] [PubMed] [Google Scholar]
- 6.Barnholtz-Sloan JS, Kruchko C. Meningiomas: causes and risk factors. Neurosurg Focus. 2007;23:E2. doi: 10.3171/FOC-07/10/E2. [DOI] [PubMed] [Google Scholar]
- 7.Roelvink NCA, Kamphorst W, Van Alphen HAM, Rao BR. Pregnancy-related primary brain and spinal tumors. Arch Neurol. 1987;44:209–15.. doi: 10.1001/archneur.1987.00520140069020. [DOI] [PubMed] [Google Scholar]
- 8.Meng T, Yin H, Li Z, Li B, Zhou W, Wang J, et al. Therapeutic strategy and outcome of spine tumors in pregnancy: a report of 21 cases and literature review. Spine. 2015;40:E146–53. doi: 10.1097/BRS.0000000000000719. [DOI] [PubMed] [Google Scholar]
- 9.Han IH, Kuh SU, Kim JH, Chin DK, Kim KS, Yoon YS, et al. Clinical approach and surgical strategy for spinal diseases in pregnant women: a report of ten cases. Spine. 2008;33:E614–9. doi: 10.1097/BRS.0b013e31817c6c7d. [DOI] [PubMed] [Google Scholar]
- 10.Cioffi F, Buric J, Carnesecchi S, Romoli S, Conti P. Spinal meningiomas in pregnancy: report of two cases and review of the literature. Eur J Gynaecol Oncol. 1996;17:384–8. [PubMed] [Google Scholar]
- 11.Kirshblum SC, Waring W, Biering-Sorensen F, Burns SP, Johansen M, Schmidt-Read M, et al. Reference fot the 2011 revision of the International Standards for Neurological Classification of Spinal Cord Injury. J Spinal Cord Med. 2011;3:547–54.. doi: 10.1179/107902611X13186000420242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen-Gadol AA, Zikel OM, Koch CA, Scheithauer BW, Krauss WE. Spinal meningiomas in patients younger than 50 years of age: A 21-year experience. J Neurosurg. 2003;98:258–63.. doi: 10.3171/spi.2003.98.3.0258. [DOI] [PubMed] [Google Scholar]
- 13.Bailey P, Bucy P. Tumors of the spinal canal. Surg Clin N Am. 1930;10:233–57. [Google Scholar]
- 14.Clark A, Digiovanni N, Hart S, Russo M, Bui C. Epidural anesthesia for cesarean delivery in a morbidly obese parturient with spinal meningioma. Ochsner J. 2012;12:145–8. [PMC free article] [PubMed] [Google Scholar]
- 15.Horn EM, Deshmukh VR, Lekovic GP, Dickman CA. Durectomy and reconstruction for the treatment of a recurrent spinal meningioma: case report. J Neurosurg Spine. 2006;5:76–8. doi: 10.3171/spi.2006.5.1.76. [DOI] [PubMed] [Google Scholar]
- 16.Mealey J, Carter JE. Spinal cord tumor during pregnancy. Obstet Gynecol. 1968;32:204–9. [PubMed] [Google Scholar]
- 17.Pikis S, Cohen JE, Rosenthal G, Barzilay Y, Kaplan L, Shoshan Y, et al. Spinal meningioma becoming symptomatic in the third trimester of pregnancy. J Clin Neurosci. 2013;20:1797–9. doi: 10.1016/j.jocn.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 18.Rath S, Mathai KV, Chandy J. Multiple meningiomas of the spinal canal: case report. J Neurosurg. 1967;26:639–40.. doi: 10.3171/jns.1967.26.6.0639. [DOI] [PubMed] [Google Scholar]
- 19.Pardo CG, León RH, Silva AS, Gutiérrez MFG, Alfornso CE, González JL, et al. Meningioma dorsal en el embarazo. Rev Cuba Obstet Ginecol. 2009;35:1–8. [Google Scholar]
- 20.O’Connell J. Neurosurgical problems in pregnancy. Proc R Soc Med. 1962;55:577–82. [PubMed] [Google Scholar]
- 21.Wigertz A, Lönn S, Mathiesen T, Ahlbom A, Hall P, Feychting M. Risk of brain tumors associated with exposure to exogenous female sex hormones. Am J Epidemiol. 2006;164:629–36.. doi: 10.1093/aje/kwj254. [DOI] [PubMed] [Google Scholar]
- 22.Custer BS, Koepsell TD, Mueller BA. The association between breast carcinoma and meningioma in women. Cancer. 2002;94:1626–35.. doi: 10.1002/cncr.10410. [DOI] [PubMed] [Google Scholar]
- 23.Peyre M, Gaillard S, de Marcellus C, Giry M, Bielle F, Villa C, et al. Progestin-associated shift of meningioma mutational landscape. Ann Oncol. 2018;29:681–6. doi: 10.1093/annonc/mdx763. [DOI] [PubMed] [Google Scholar]
- 24.Schaller B. Spinal meningioma: relationship between histological subtypes and surgical outcome? J Neurooncol. 2005;75:157–61.. doi: 10.1007/s11060-005-1469-4. [DOI] [PubMed] [Google Scholar]
- 25.Sandalcioglu IE, Hunold A, Müller O, Bassiouni H, Stolke D, Asgari S. Spinal meningiomas: critical review of 131 surgically treated patients. Eur Spine J. 2008;17:1035–41. doi: 10.1007/s00586-008-0685-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ciappetta P, Domenicucci M, Raco A. Spinal meningiomas: prognosis and recovery factors in 22 cases with severe motor deficits. Acta Neurol Scand. 1988;77:27–30. doi: 10.1111/j.1600-0404.1988.tb06969.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.