Summary
We show that C. elegans nematodes learn to associate food with a combination of proprioceptive cues and information on the structure of their surroundings (maze), perceived through mechanosensation. By using the custom-made Worm-Maze platform, we demonstrate that C. elegans young adults can locate food in T-shaped mazes and, following that experience, learn to reach a specific maze arm. C. elegans learning inside the maze is possible after a single training session, it resembles working memory, and it prevails over conflicting environmental cues. We provide evidence that the observed learning is a food-triggered multisensory behavior, which requires mechanosensory and proprioceptive input, and utilizes cues about the structural features of nematodes' environment and their body actions. The CREB-like transcription factor and dopamine signaling are also involved in maze performance. Lastly, we show that the observed aging-driven decline of C. elegans learning ability in the maze can be reversed by starvation.
Subject areas: Behavioral Neuroscience, Biological Sciences, Neuroscience
Highlights
-
•
C. elegans can be trained to reach a target arm in a T-shaped maze
-
•
Learning requires the contribution of tactile and proprioceptive cues
-
•
C. elegans follow a kind of response learning strategy in the maze environment
-
•
Learning is short-term and sensitive to distraction
Behavioral Neuroscience; Biological Sciences; Neuroscience
Introduction
Despite having only 302 neurons, C. elegans hermaphrodites demonstrate non-associative learning in the form of habituation or sensitization (Rankin and Broster, 1992; Rankin et al., 1990), as well as associative learning (Ardiel and Rankin, 2010; Rankin et al., 1990; Stein and Murphy, 2014), where nematodes learn to make positive or negative associations (Zhang et al., 2005; Torayama et al., 2007). Imprinting may also occur during their early developmental stages, when C. elegans' behavior can be modified by food-derived odors (Remy and Hobert, 2005). All these types of learning have been extensively studied in C. elegans, mainly in the context of chemical cues.
With their nervous system mapped (White et al., 1986; Varshney et al., 2011; Cook et al., 2019) and many of the neuronal connections characterized (Towlson et al., 2013; Arnatkeviciute et al., 2018), C. elegans have been successfully used to dissect key decision-making neuronal circuits (Faumont et al., 2012; Ghosh et al., 2016; Jarrell et al., 2012; Tanimoto et al., 2017). Indeed, the rich behavioral palette these nematodes exhibit is particularly suitable for studying the neurobiology of behavior. Interestingly, even though C. elegans actively explore their environment, i.e., when foraging, their spatial orientation, navigation, learning, and decision-making have been sparsely studied. This has been achieved primarily in assays where C. elegans avoid or prefer moving toward unpleasant or favored odorants (Bargmann, 2006; Klein et al., 2017), osmolarity (Culotti and Russell, 1978) and temperature (Garrity et al., 2010). These studies reveal behavioral and survival strategies adopted by C. elegans, sometimes accentuated through conditioning. However, the question as to whether C. elegans can learn to navigate in a structured environment toward a targeted location or whether C. elegans are capable of spatial learning remains unanswered.
Mazes and maze-like platforms have been broadly used to investigate the ability of animals to learn space-related tasks (Morris, 1984; Derdikman and Knierim, 2014) and to decipher their decision-making (Bardgett et al., 2009; Olton, 1979; Vorhees and Williams, 2014). Y-shaped mazes have been used even to assess plant behavior (Gagliano et al., 2016). Despite being highly informative as a tool, mazes have been employed in surprisingly few C. elegans studies, to tangentially evaluate their ability to locate food (Oliver et al., 2016) or to explore worms' decision making (Pandey et al., 2011). The only targeted attempt to date in which a maze-like platform is used to explore C. elegans behavior (Qin and Wheeler, 2007) provides intriguing indications of improved performance after repeated training. However, even though a dopamine-poor mutant, cat-2, was tested, the observed behavior was not thoroughly characterized, neither was the steering mechanism explored.
Recently, Han and colleagues (Han et al., 2017) demonstrated that C. elegans can distinguish between spatial variations in their environment and show clear preferences. The conclusion that mechanosensation plays a key role to spatial selectivity (Han et al., 2017) fits nicely to what is known about limitations in some of C. elegans' other senses (Ward et al., 2008; Goodman, 2006). It also raises the question of whether mechanosensation could contribute to nematodes' spatial navigation and learning. In parallel, the finding of proprioceptive neurons in C. elegans (Li et al., 2006) offers an additional option to the feedback these roundworms may receive from their bodies (Schafer, 2006), besides tactile input, when interacting with their environment. Proprioception, along with mechanosensation, has been reported as one of the non-visual sensory modalities that contribute to spatial memory formation (Yamamoto and Shelton, 2005; Waller et al., 2004; Klatzky, 1998; Chance et al., 1998).
It is therefore notable that in the rich literature which deals with C. elegans' diverse behavior, their remarkable learning abilities, and their fascinating sensory biology, there is a gap regarding their spatial and navigational learning capability. This opening exists even though for behavioral studies in other organisms, informative tools, such as simple mazes, have been developed, that could be adjusted for use with C. elegans.
Here, we introduce a custom-made T-shaped maze platform, namely the Worm-Maze, to explore C. elegans' navigation skills, and to establish worms' ability to learn a maze-related task. We characterize this behavior in relation to diverse environmental cues, and we investigate the role of selected genes, involved in chemosensation, mechanosensation, and proprioception. We also report the contribution of dopamine signaling and of the CREB-homolog transcription factor. Lastly, we demonstrate that C. elegans learning strategy includes the usage of information about the structural traits of the animals' surroundings and their body actions. We suggest that C. elegans learn to associate food/reward with a combination of proprioceptive cues about their body posture and actions, and mechanosensation-perceived cues on the structural features of their environment. Lastly, we show that the Worm-Maze can be used to study maze performance and learning in the context of aging or physiological manipulations, e.g., starvation.
Results
Determination of C. elegans learning in the maze environment
Simple T-shaped mazes (Figure 1) were used to run a basic control experiment in the absence of food, as reference. These mazes had rough floor surfaces (Figure S1D), providing nematodes with continuous tactile information. A nematode was placed at the maze starting point (Figure 1B) and was allowed to travel. The worm was recorded until it reached one of the two ends of the maze, either on the left or on the right maze side (Figure 1D, see Methods for description, and Table S1). In a population of 70 worms scored, 52% reached the left end of the maze and 48% reached the right end of the maze, scoring a decision index DIBasic = 0.03 (Figures 1F and Tables S1 and S2; calculations of all indices are described in the Methods section and details on statistics are provided in Methods and in supplemental information). Thus, there is almost equal probability for a naïve worm to reach either arm of a simple T-maze, provided that it reaches any of the maze arms (Oliver et al., 2016).
Figure 1.
The T-maze arena and the basic (reference) Control and Training/Testing experiments
(A): The 3D-printed mold, as designed in SolidWorks software. Nine copies of individual maze molds are included, 5-7mm apart, with the supporting arms of the entire structure branching out in an X-like configuration (see also Figure S1). The square-shaped plate prevents liquid agar from forming a meniscus around each maze mold (Figure S1). Scale bar: 5mm.
(B): Schematic showing the dimensions of each maze in mm; wavy green: the functional corridors of the maze, light green: circular auxiliary areas, allowing for extra room to pipette the food in (left and right maze ends) and placing the worm (maze bottom—starting point). The depth of the actual maze is ~5mm.
(C): Actual T-maze in an NGM plate. The maze has continuously rough surface. Scale bar: 2.5mm.
(D): Basic Control experiment, schematic of the experimental steps.
(E): Basic Training/Testing experiment, schematic of the experimental steps, (F) food (E. coli OP50).
(F): % of worms scored in Basic Control and Training/Testing experiments (left y axis). Diamond shapes indicate the preference indices (right y axis): for the Control maze, Decision Index DIBasic, , where worms that reached the left arm of the maze, worms that reached the right arm of the maze; for the Training maze, Chemotaxis Index: CIBasic, ; and for the Testing maze, Learning Index: LIBasic, (see also Methods, Tables S1 and S2). Index error bars represent the standard deviation of the index (see supplemental information), dashed line illustrates the 50% and index = 0 level, p values of binomial probability test provided above bars. See also Video S1. Training_1., Video S2. Testing_1., Video S3. Training_2., Video S4. Testing_2..
As a next step, we tested whether the presence of food at one end of the maze introduces a bias in C. elegans' behavior. To this end, the basic training/testing experiment was conducted (Figure 1E, see Methods). From 106 worms scored in the training maze, 85% reached the side where food was placed (left side of the maze), and 15% reached the side without food (right side of the maze), scoring a chemotaxis index CIBasic = 0.69 (Figures 1F and Table S2). Hence, when food is placed at one end of the maze, there is a strongly biased navigational outcome.
Next, we tested whether nematodes that locate the food in the training maze maintain a bias in their behavior when placed in a similar environment for a second time. To this end, every worm that reached the food-containing left side of the training maze remained in the area for ~3 min (see Methods) and then was transferred into a new, similar maze that contained no food, namely the testing maze (Figure 1E). From 70 worms scored in the testing maze, 73% reached the left side of the maze, whereas 27% reached the right side of the maze, scoring LIBasic = 0.42 (Figures 1F, Table S2). Therefore, the majority of worms in the testing maze reached the side of the maze that had previously held food (Figure 1). We consider this biased behavior, which comes as a result of C. elegans prior experience, as an indication of learning.
Which side of the maze contains the food reward is not critical for the generation of a biased behavior. When food was placed at the right end of the training maze, instead of the left, C. elegans developed a bias toward the right maze side (Figure S2B). Moreover, unlike nematodes that reached the food-containing side of the training maze, worms that reached the food-lacking side of the training maze showed unbiased behavior in the testing maze (Figure S2C). Similarly, when nematodes were placed in a second, empty maze after experiencing an empty first (training) maze, the behavior of all worms in the second maze was unbiased (Figure S2D). Therefore, food location is required for learning in the maze, and if nematodes experience an empty maze, no bias is generated.
Characterization of C. elegans learning in mazes
Next, we asked whether C. elegans learn to reach a target maze arm based on the cardinal points, north, east, south, and west. To this end, the training/testing maze experiment was repeated, with the testing maze now rotated by 180° (Figure S3A), e.g., having the left end of the maze pointing north instead of south, opposite to the experiments described above (Figure 1). Rotating the testing maze did not affect C. elegans learning since 69% of worms that reached the left side of training maze also reached the same side in the testing maze, with LI=0.35 (Figure 2A, Table S2). Therefore, worms that learn do not migrate based on cardinal directions.
Figure 2.
Characterization of C. elegans learning in the maze environment
(A): The effect of maze orientation. Experimental steps are similar to Figure 1E schematic, with testing mazes rotated by 180° (Figure S3), using mazes shown in Figure 1C.
(B): The effect of conflicting environmental cues. Experimental steps are similar to Figure 1E, and an angled maze is shown in inset.
(C): The effect of tactile input. Experimental steps are similar to Figure 1E, with the use of smooth mazes, shown in Figure S1D. All top panels: % of worms scored in Control and Training/Testing experiments (left y axis). Diamond shapes indicate the preference indices (right y axis): for the Control maze Decision Index, for the Training maze Chemotaxis Index, and for the Testing maze Learning Index (Methods). Index error bars represent the standard deviation of the index; dashed line illustrates the 50% and index = 0 level, above bars is indicated the number of nematodes that were scored (), see also Table S2. All bottom panels: p values (binomial distribution probability) for comparisons between each case and basic Control or basic Training/Testing experiment. ControlBasic refers to the basic control experiment (Figures 1D and 1F) and TrainBasic, TestBasic refer to basic Training/Testing experiment (Figures 1E and 1F).
To investigate the worms' behavior in the presence of conflicting environmental cues, T-mazes with arms forming 60°/120° angles were used (Figure 2B, inset), instead of the basic T-shaped design of 90°/90° angles (Figure 1). The configuration was chosen based on previous studies (Pandey et al., 2011), which report a C. elegans' preference for obtuse over acute angles in two-option junctions. As shown in Figure 2B inset, the left arm presented the acute angle (60°) and the right arm presented the obtuse angle (120°). Of all the nematodes scored in an empty 60°/120°-angled maze (control experiment), 68% reached the right, obtuse-angled arm. Only 32% of the worms reached the left, acute-angled maze arm (DI60°/120° = -0.25; Figures 2B, Table S2). This is consistent with previous findings (Pandey et al., 2011) on worms navigating in microfluidic devices. However, when food was added to the left, acute-angled maze arm, 70% of the worms reached the left end of the training maze, with CI 60°/120° = 0.48 (Figure 2B), indicating that nematodes overcame their aversion of acute angles in the presence of food. Intriguingly, from these worms, 73% reached again the left, acute-angled arm when placed in the testing maze, with LI60°/120°=0.71 (Figure 2B, Table S2), even though no food was present this time. Thus, C. elegans learning is enough to reverse their inherent bias, even when the triggering stimulus (food) is no longer present.
The mazes used in all the above experiments had a continuously rough floor surface (Figure S1D), which was produced by selecting a low printing resolution during mold fabrication in the 3D printer (see Methods). We hypothesized that the rough surface, being rich in tactile stimuli, is important for worms' navigation and learning. To test this hypothesis, we fabricated mazes with smooth floor surfaces (Figure S1D), using molds generated in a higher resolution 3D printer (see Methods).
First, we ran a control experiment with an empty smooth maze, in which 53% of the scored worms reached the left side of the maze and 47% of the worms reached the right side of the maze, with DISmooth=0.06 (Figure 2C, Table S2). This finding is similar to control experiments ran on rough surfaces (DIBasic = 0.03, Figure 2). Next, we performed a training/testing experiment using smooth mazes (Figure 2C), where 68% of scored worms located food effectively and reached the left, food-containing side of the smooth training maze, scoring CISmooth = 0.34 (Figure 2C, Table S2). Interestingly, only 55% of them reached the left side of the smooth testing maze, with LISmooth = 0.08 (Figure 2C, Table S2), indicating that the smooth texture of the maze surface significantly reduces worms’ learning.
Timeframe of retention and retrieval of learned information
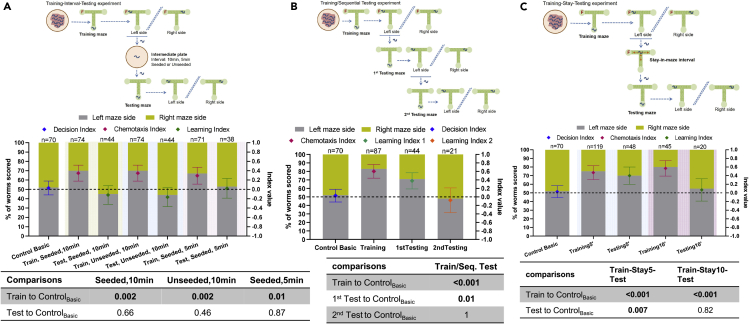
To define how long C. elegans retain their learning after a single training session, we ran three series of experiments (Figure 3). In the first scheme, namely the training-interval-testing experiment, after training worms were placed in an intermediate plate for 5 or 10 min before being transferred into the testing maze (Methods, Figure 3A). The intermediate plate was seeded with OP50 in the first version of the experiment, and unseeded in the second. In the 10 min interval experiment, 70% of scored worms reached the food-containing, left side of the training maze, scoring CISeeded10 = 0.35 and CIUnseeded10 = 0.35. However, from them only 45% reached the left side of the testing maze when the intermediate plate was seeded (LISeeded10 = -0.12), and 44% when the intermediate plate was unseeded (LIUnseeded10 = -0.16), as shown in Figure 3A and Table S2. Similarly, in the 5 min interval experiment, 67% of scored worms reached the left side of the training maze (CISeeded5 = 0.29), but only 53% of them reached the left side of the testing maze (LISeeded5 = 0.02, Figure 3A, Table S2). Therefore, placing the worms in an intermediate plate for 5 or 10 min resulted in no learning, 8-13 min after having reached the food in the training maze, regardless of the intermediate plate being seeded with food or unseeded. The 8-13 min period is calculated given that worms spend ~ 3 min in the food-containing area of the training maze (see Methods), plus the intermediate plate time.
Figure 3.
Time frame of C. elegans learning in the maze environment
(A): Training-interval-Testing experiment.
(B): Training-sequential Testing experiment.
(C): Training-stay-Testing experiment. (A),(B),(C), top panels: schematic of experimental steps; middle panels: % of worms scored in Basic Control and Training/Testing experiments (left y axis). Diamond shapes indicate the preference indices (right y axis): for the Control maze Decision Index, for the Training maze Chemotaxis Index, and for the Testing maze Learning Index (Methods). Indices error bars represent the standard deviation of the index; above bars is indicated the number of nematodes that were scored (n), see also Table S2; dashed line illustrates the 50% and index = 0 level, bottom panels: p values (binomial distribution probability) for comparisons between each case and basic Control experiment. ControlBasic refers to the basic Control experiment (Figures 1D and 1F) and TrainBasic, TestBasic refer to basic Training/Testing experiment (Figures 1E and 1F). Shaded background indicates data from separate experiments.
In the second scheme, namely the training-sequential testing experiment, nematodes that located food effectively in the training maze (83%, CITrainseq = 0.60, Figure 3B) were placed sequentially in two testing mazes, to test for how long or how many times they can retrieve the learned information. In the first testing maze, 71% of scored worms reached the left side of the maze again, scoring LITest1seq = 0.38 (Figure 3B). These worms, after allowed to spend ~3 min in the target destination area (see Methods), were placed in a second testing maze. In there, only 48% of scored worms reached the left side of the maze again, scoring LITest2seq = -0.08 (Figure 3B). Therefore, C. elegans do not retrieve the learned information a second time. Given that in order to reach the left side of the 1st testing maze the scored worms needed 0.83-14.3 min, with a mean of 3.66 min, we can add that the learned information is not retrieved twice if worms are asked to repeat the task after > 7 min (3min at the food-containing area of the training maze+3.66min to traverse the 1st testing maze). If we consider also the time spent in the destination area of the 1st testing maze, then it could be said that worms are not able to recall the learned information ~ 10 min after they reach the food in the training maze (3min+3.66min+ another 3min). Hence, the time passed since locating food in the training maze (~10 min) results in loss of the ability to retrieve the learned information, or the learned information cannot be retrieved twice within a 7 min period. Alternatively, the intervening experience of the 1st testing, empty maze may result in worms “unlearning” or “overwriting” the information registered during training.
In the third scheme, the training-stay-testing experiment, nematodes that located food in the training maze are allowed to remain in the maze for up to an additional 5 or 10 min period (training-stay5-testing and training-stay10-testing experiment, respectively) before they are transferred to the testing maze (Figure 3C). This corresponds to 3 min stay time in the food-containing area, applied in all training sessions of all experiments, plus 5 or 10 additional minutes, respectively. In the training-stay5-testing experiment, 75% of scored worms reached the food-containing left side of the training maze (CITrainStay5 = 0.46), and from them 70% reached the left side of the testing maze (Figure 3C), as well (LIStayTest5=0.35). Therefore, worms were able to retain and retrieve the learned information after ~ 8 min (3 min + 5 min). In contrast, in the training-stay10-testing experiment, 80% of scored worms reached the food-containing left side of the training maze (CITrainStay10=0.57), and from them only 55% reached the left side of the testing maze (Figure 3C), as well (LIStayTest10=0.07). This means that worms did not retain and/or retrieve the learned information after ~ 13 min (3 min + 10 min). These findings combined suggest that time passed alone, without any change of environment, does not result in loss of the learned information in the case of ≤ 8 min, but after ≤ 13 min the retention or retrieval ability is lost.
Investigation of learning mechanism
Next, we explored the participation of selected genes in the learning mechanism (Figure 4). First, to identify sensory modalities that are potentially involved, we examined strains with mutations in genes involved in chemosensation, mechanosensation, and proprioception. To this end, we ran the basic training/testing experiment using day 1 adults carrying mutations related to chemosensation, strains odr-3(n2150) and odr-10(ky32), mechanosensation, strains mec-4(e1611) and mec-10(e1515), and proprioception, strains trp-4(ok1605) and trp-1(sy690) trp-2(sy691).
Figure 4.
Role of sensory modalities and selected biochemical pathways
(A–E) (A): chemosensation impaired strains, (B): mechanosensation impaired strains, (C): proprioception impaired strains, (D): strains lacking the CREB-like transcription factor (crh-1) or the dopamine-3 receptor (dop-3); (A), (B), (C), (D): % of scored worms in Training/Testing experiments (left y axis). Diamond shapes: preference indices (right y axis), for the Training maze Chemotaxis Index, and for the Testing maze Learning Index. Indices error bars: standard deviation of the index; above bars: number of scored nematodes (n), dashed line: 50% and index = 0 level; (E): p values (binomial distribution probability) for comparisons between each mutant strain and basic control or basic training/testing experiment. Experimental steps as in Figure 1. See also Tables S2 and S3, and Figure S6. TrainN2, TestN2 refer to basic Training/Testing esxperiment (Figures 1E and 1F).
In chemosensation-defective mutants, only 55% of scored odr-3 and 52% of scored odr-10 C. elegans reached the food-containing left side of the training maze, with CIodr-3=0.06 and CIodr-10=0.03 (DIodr-3=0.03, DIodr-10=0.00), and from them, 45% of odr-3 and 51% of odr-10 worms also reached the left side in the testing maze, with LIodr-3=-0.13 and LIodr-10=0.02 (Figure 4A and Table S2). Therefore, impaired chemosensation has detrimental effects on C. elegans food location ability and subsequently to overall maze performance.
Experiments with mechanosensation-defective strains showed that 52% of scored mec-4, and 62% of scored mec-10 nematodes reached the food-containing left arm of the training maze, scoring CImec-4=0.28 and CImec-10=0.14 (DImec-4=-0.14, DImec-10=0.11), and from them 49% of mec-4 and 55% of mec-10 worms reached the left arm also in the testing maze, with LImec-4=0.12 and LImec-10=0.00 (Figure 4B and Table S2). Therefore, impaired mechanosensation significantly reduces C. elegans food location ability and consequently compromises overall maze performance.
Lastly, when animals defective in proprioception were tested, 76% of scored trp-4(ok1605) reached the food-containing left side of the training maze, scoring CItrp-4=0.72 (DItrp-4=-0.02), and from them, only 39% reached the left side of the testing maze, as well, with LItrp-4=-0.54 (Figure 4C and Table S2). In addition, 77% of scored trp-1(sy690) trp-2(sy691) reached the left, food-containing arm of the training maze, scoring CItrp1;2=0.50 (DItrp1;2=0.02), but from them only 52% reached the left arm of the testing maze, with LItrp1;2=0.007 (Figure 4C and Table S2). Therefore, it seems that trp-4(ok1605) maintain their ability to locate food effectively because even though the difference compared to N2 animals (Figure 1) appears to be borderline statistically significant with p=0.042 (Figure 4E), the 76% reaching the food in training shows that the food location ability is not importantly compromised. However, trp-4(ok1605) is not able to learn (Figure 4C). Similarly, trp-1(sy690) trp-2(sy691) maintain their food location ability, as 77% of them reach the food in training (Figure 4C), despite the borderline p = 0.041 when compared to N2 (Figure 4E), but they are not capable of learning. Note that both the 76% of trp-4 mutants and the 77% of the trp-1;trp-2 mutants scored during training fall well within the range of N2 performance in training (Figure S2A), and are close to the mean score of 75%. Therefore, proprioception is revealed to be of critical importance for C. elegans learning in the maze, but not for food location.
CREB (cyclic AMP-response element binding protein) is known to be required for associative learning in C. elegans (Kauffman et al., 2010). In addition, dopamine plays a key role in C. elegans neuronal plasticity and mechanosensation-mediated learning (Kindt et al., 2007; Sanyal et al., 2004), and particularly the D2-like dopamine receptor DOP-3 is involved in touch-dependent dopamine signaling (Han et al., 2017). Based on the above, we investigated the behavior of crh-1(tz2) and dop-3(vs106) strains, which lack an ortholog of CREB, and a homolog of mammalian D2 dopamine receptor, respectively. 62% percent of scored crh-1 and 52% of scored dop-3 worms reached the food-containing left side of the training maze, with CIcrh-1=0.25 and CIdop-3=0.10 (DIcrh-1=-0.02, DIdop-3=-0.06, Figure 4D and Table S2). From them, 43% of crh-1 and 38% of dop-3 worms reached the left side of the testing maze, as well, with LIcrh-1=-0.11 and LIdop-3=-0.15 (Figure 4D and Table S2). Therefore, the CREB-ortholog and the dopamine receptor homolog are involved in C. elegans performance in the maze environment.
In control experiments, nematodes of all mutant strains reached the left or the right side of an empty T-maze with almost equal probability (Figure 4, all panels).
Investigation of learning strategy
C. elegans travel by crawling either primarily on the floor or primarily on the walls of the mazes (with the exception of ~0-5% of scored worms that crawl on the maze top covers, see Methods). To further explore their learning strategy, we analyzed the recordings of selected training/testing experiments (Figures 1, 2C, and 4), focusing on whether C. elegans repeat in the testing maze the wall or floor choice they made in training. Indeed, most of the N2 wild type nematodes (74%) repeat their wall or floor choice in testing (Figure 5A), with the percentage of nematodes that make the same floor or wall choice rising to 75% among worms that reached the same maze side (Figure 5A, last bar), namely have learned. Interestingly, this is not the case when C. elegans traverse smooth mazes, neither when they have impaired mechanosensation, as in mec-4 and mec-10 strains, (Figure 5B, last four bars). However, nematodes with impaired proprioception (trp-4 and trp-1;trp-2 strains) still repeat the wall or floor choice, as do N2 nematodes (Figure 5C, last four bars).
Figure 5.
C elegans' interaction with structural features of the maze
Graphs show % of nematodes scored in the Testing maze, with respect to their wall or floor route choice.
(A): N2 wild type experiments (Figure 1). For the first two bars the % is calculated over the total number of nematodes that were scored in the Testing maze. For the last two bars the % is calculated over the number of nematodes that reached the same maze side.
(B): Smooth mazes (Figure 2A) and mec-4 and mec-10 strains (Figure 4B) experiments. For the first two sets of bars the % is calculated over the total number of nematodes that were scored in the Testing maze. For the last two sets of bars the % is calculated over the number of nematodes that reached the same maze side.
(C): trp-4 and trp-;trp-2 strains (Figure 4C). For the first two sets of bars the % is calculated over the total number of nematodes that were scored in the Testing maze. For the last two sets of bars the % is calculated over the number of nematodes that reached the same maze side.
All panels: Same side: nematodes that reached the same side in Training and Testing; Same F/W: nematodes that traversed both mazes crawling mainly on the floor, or on the wall; Same side-Dfrnt F/W: Nematodes that reached the same side in Training and Testing, but did not traverse both mazes crawling mainly on the floor, or on the wall; Same side/Same F/W: Nematodes that reached the same side in Training and Testing, and traversed both mazes crawling mainly on the floor or on the wall. Nematodes that reached the same side are the worms that are considered to have learned. Dashed line indicates 50% level. Error bars: standard deviation, above bars: number of scored nematodes (n), asterisks indicate p values (binomial distribution probability), ∗: p < 0.05, ∗∗: p < 0.01, ∗∗∗: p < 0.001, ∗∗∗∗: p < 0.0001. All comparisons are made against the respective N2 result. Nematodes that crawled on the maze ceiling (cover agar pads), varying between 0 and 5%, are not included.
To explore whether ventral/dorsal body orientation plays a role in maze learning, we repeated the training/testing experiment (schematic in Figure 1) using the Pmyo-3::mcherry::unc-54 strain, which has fluorescently tagged body wall and vulva muscles (Figure 6A). This allows for distinguishing between the ventral and dorsal side, of nematodes that crawl on the maze floor (Figure 6A). From the worms scored in the testing maze, 73% reached the same maze side with the food-containing one in Training (Figures 6B and 6C first bar), corroborating learning. In addition, 70% of all worms scored in testing repeated the floor or wall choice, independently of side choice (Figure 6C, second bar), similarly to N2 (Figure5A, second bar). When the ventral/dorsal orientation of only the floor-crawling worms is examined, it is found that only 43% maintains the same ventral/dorsal orientation in both mazes (Figure 6D, first bar), and only 38% has the same body orientation and reaches the same maze side (Figure 6D, second bar). Therefore, maintaining the same dorsal/ventral orientation is not critical for C. elegans learning in the maze, in contrast with the floor/wall interaction. Note that in order to call the V/D body orientation of a nematode, emphasis was given on the body orientation at the time of the final bend(s) at the maze junction (Figure 6A, ventral and dorsal bends).
Figure 6.
C. elegans' body orientation during Training and Testing
Training/Testing experiment, using Pmyo-3:mcherry:unc-54 strain. Experimental steps as in Figure 1.
(Α): Images of Pmyo-3:mcherry:unc-54 strain nematodes. Vulva muscles are visible and dorsal/ventral body sides are distinguishable. Nematodes taking a ventral, or a dorsal side bend are depicted.
(Β): Training/Testing experiment on Pmyo-3:mcherry:unc-54 strain. Graph shows % of scored nematodes in Training and Testing mazes (left y axis). Diamond shapes indicate the preference indices (right y axis): Chemotaxis Index for Training maze, Learning Index for Testing maze. Indices error bars: standard deviation of the index; dashed line: 50% and index = 0 level, above bars: number of scored nematodes (n), see also Table S2; table shows p values (binomial distribution probability) for comparisons to basic Control or basic Training/Testing experiment (Figure 1).
(C): % of nematodes that reached the same maze side (Same side) or repeated the floor- or wall-dominant choice in both Training and Testing (Same F/W), over the total number of scored worms.
(D): % of nematodes that maintained the same ventral/dorsal orientation (Same V/D) or maintained the same ventral/dorsal orientation and reached the same maze side in training and testing (Same side V/D), over the number of worms that crawled on the floor. Panels C and D: Dashed line indicates 50% level. Error bars: standard deviation, above bars: number of scored nematodes (n). Note that nematodes marked as Same V/D and Same Side V/D are only floor crawlers, since it not possible to call a V/D orientation in worms crawling on the walls. See also Videos S5 and S6.
The effect of aging and starvation
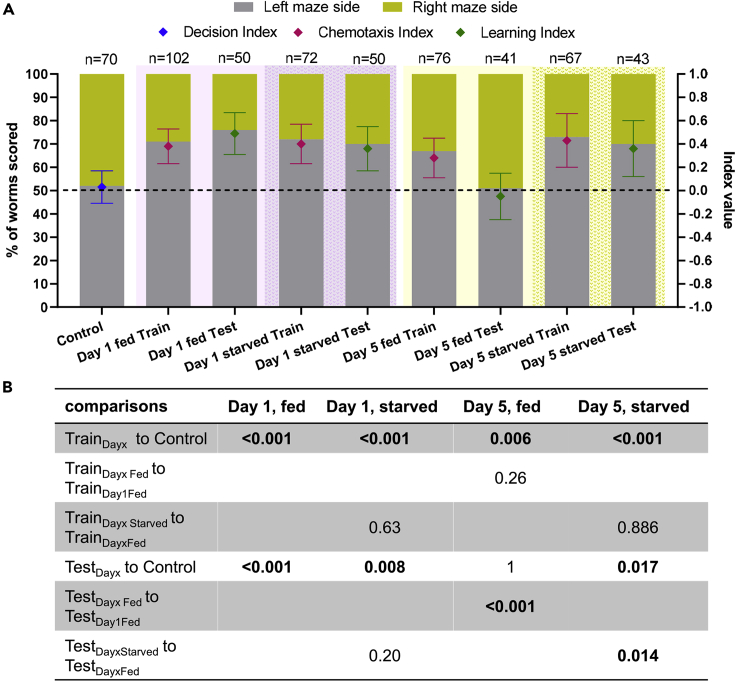
We asked whether the learning ability observed in day 1 adult C. elegans is retained in older animals. To this end, we ran the training/testing experiment on day 5 adult worms (Figure 7), which are considered middle-aged individuals. In this experiment, OP50 was mixed with L-lysine (see Methods), to enhance its appeal to nematodes, in anticipation of potentially reduced chemosensation of older animals (Collins et al., 2008) (the effect of L-lysine alone as an attractant in a simple T-maze is shown in Figure S3B). We found that 67% of scored day 5 adult C. elegans reached the left side of the training maze, with CIDay5=0.29 (Figure 7 and Table S2), and from them only 51% reached the left side of the testing maze as well, with LIDay5=-0.04 (Figure 7 and Table S2). Therefore, the learning observed in young day 1 adults is completely lost in middle-aged day 5 adult C. elegans.
Figure 7.
The effect of aging and starvation on C elegans performance in the maze environment
(A): % of scored nematodes in Training/Testing experiments, of two different age groups, being either fully fed or starved for 24 hr (left y axis). Diamond shapes indicate the preference indices (right y axis): for the Control experiment Decision Index, for the Training maze Chemotaxis Index, and for the Testing maze Learning Index (Methods). Indices error bars represent the standard deviation of the index; dashed line illustrates the 50% and index = 0 level, above bars is indicated the number of scored nematodes (n), see also Table S2.
(B): p values (binomial distribution probability) for comparisons between each age cohort and Control, or Training/Testing experiment of the fully fed respective age cohort. Experimental steps are the same as basic Training/Testing experiment (Figure 1). Shaded background indicates data from separate age cohorts and feeding conditions.
Next, we explored the effect of starvation (Kaeberlein et al., 2006) on young and middle-aged animals. For this purpose, we ran the training/testing experiment using Ν2 wild-type nematodes in days 1 and 5 of their adult life that had been starved for 24 hours prior to experiment (see Methods). When starved day 5 adult C. elegans are challenged with the Worm-Maze, 73% of scored worms located the food in the training maze (CIDay5Starved=0.43) and from them, 70% reached the same side of the testing maze (LIDay5Starved=0.36), indicating significant improvement in their learning ability (Figure 7, and Table S2). When day 1 adult C. elegans were starved for 24 hours, there was no detectable effect of starvation, as 72% of scored nematodes reached the food-containing arm in the training maze (CIDay1Starved=0.4), and 70% reached the same maze arm in the testing maze (LIDay1Starved=0.36). Consequently, 24hrs starvation improves learning in the maze environment in middle-aged C. elegans.
Worm-Maze can be used to evaluate C. elegans locomotion
Although the Worm-Maze allows for assessment of behavior without measuring locomotion features, it can be used to evaluate C. elegans locomotive behavior, if desired. For example, it is possible to quantify the worms’ traveling speed, i.e. time needed to reach one end of the maze. Indeed, worms need more time to reach the maze end in the testing than in the training maze (Figure S4A), suggesting that to travel toward a target location based on learned information might take longer than being driven by a sensory input. Moreover, trained and naïve worms have a different distribution of traveling time frequencies (Figure S4B). Indicatively, the 75% percentile lies at 5.8 min for the training maze, at 8.7 min for the testing maze and at 6.4 min for the control maze (Figure S4C), revealing a more similar distribution between control and training, than between control and testing.
We highlight that all conclusions presented in this manuscript are based on the final behavioral outcome of the worms, i.e., which maze arm they reach, as well as on the nematodes’ interaction with maze walls and floor. No conclusions presented here require details on the animals’ locomotion (reversals, omega turns, pirouettes, etc.).
Range and variability in maze performance
Behavioral responses are known to be subjected to significant variability (Yemini et al., 2013; Tervo et al., 2014; Schwarz et al., 2015). Conforming with that, C. elegans performance in the Worm-Maze varies among experiments. As illustrated in Figure S2A, young adult N2 wild type C. elegans reached the food in the Training maze in percentages ranging between 85% (Figure 2A) to 67% (Figure 7A, day 1 adult fully fed animals) among experiments, with the mean at 75%. Variation in the Testing maze was also detected among experiments, although in a lesser degree (Figure S2A), with a range between 78% (Figure 1) and 69% (Figure 7A, day 1 adult fully fed animals) and the mean at 73%. Importantly, all differences derived by genetic or environmental manipulations presented in Figures 2, 3, 4, 5, 6, and 7 that result in loss of learning extend beyond the range of the experimental variation in question.
Discussion
C. elegans can learn to reach a target location in a T-maze
T-shaped mazes have been widely used to assess spatial memory in rodents (Shoji et al., 2012; Wenk, 2001) and odor preference in invertebrate models (Davies et al., 2019), like Drosophila melanogaster (Tully and Quinn, 1985; Yamaguchi et al., 2010). Surprisingly, they have been used in very few studies on C. elegans navigational ability (Pandey et al., 2011) and overall behavior (Oliver et al., 2016; Qin and Wheeler, 2007). On the other hand, there are several studies that thoroughly illustrate C. elegans learning to avoid or favor specific odors, temperatures and osmolarities (Ardiel and Rankin, 2010; Mcewan and Rankin, 2013; Sasakura and Mori, 2013; Amano and Maruyama, 2011; Rankin et al., 1990). In these cases, nematodes' biased motion toward or away from these stimuli is not claimed to indicate memory of spatial or navigational information but is considered the behavioral expression of olfactory or temperature-related memory.
The notion that C. elegans can transform sensory representation to motor representation has been proposed (Luo et al., 2014). Authors explain how a complex experience-dependent behavior can be encoded in the small nervous system of C. elegans (Luo et al., 2014), and estimate that their navigational circuits are more sophisticated than previously recognized. Here, we present evidence that C. elegans are capable of learning to reach a target location in a structured maze environment, as a result of their prior experience.
The presence of food is enough motivation to change C. elegans’ behavior in a maze (Figure 1). Following that experience, they maintain a significantly higher probability of reaching the same side the second time they traverse a similar but empty maze, than reaching any side randomly. We consider this a strong indication of C. elegans’ learning in the maze environment, that goes beyond remembering an attractive odor. In addition, (Figure S2) C. elegans do not show an inherent side preference in a simple T-maze, which implies the absence of innate “handedness”.
It is noted that most nematodes, although they spend some time at the junction area, they do not visit (do not sample) both sides of the maze before reaching one of the two maze arms. Therefore, they have practically no way of knowing how the non-selected arm feels or what lies at the arm they do not visit, or even that there is a second arm. Hence, their learning is not based on a choice between two experienced options, but rather stems from learning to repeat a behavior that lead them to a reward location.
To eliminate the possibility of out-of-the-maze influence, experiments were run by multiple experimenters, in multiple lab locations (Figure S5). No correlation was found to specific researchers or lab conditions (light, ventilation, traffic). In addition, the chemical consistency or surface texture do not differ, at least consistently, between the two maze sides (Figure S5).
Contradictory cues and time frame of retention
Learning in the maze is sufficient to make C. elegans overcome an inherent bias (Figure 2B), as it can shift nematodes’ behavioral outcome despite the actual environmental input. This could be indicative of evolutionary prioritization of needs, since learning of a potential food location might be considered high priority in a given environment.
In mazes that provide less tactile input due to their smooth surface (Figure S1D), C. elegans can still locate food (Figure 2C). This ability is reduced compared to basic training/testing experiment (Figure 1), although the percentage of worms that locate food (68%) is at the lower bound of the observed training range (69%, Figure S2A). The time needed for worms to reach the target maze arm of a smooth maze is similar to the respective travel time in a rough maze (Figure S4), which means that locomotion in the smooth mazes is not impeded. Hence, navigation toward successful food location in the maze environment may be enhanced by tactile input. These findings are supported by results on mec-10 strain, whereas mec-4 mutants, the mechanosensory neurons of which are degenerated (Driscoll and Chalfie, 1991), do not locate food in the maze (Figure 4B, and below). Multisensory food location has not been reported in chemotaxis on NGM plates, the standard arenas for assessing C. elegans behavior. It is possible that to detect the contribution of tactile cues in food location a more complex environment than NGM plates is needed. The 3-dimensional Worm-Maze platform is such an arena.
C. elegans tested on smooth mazes do not show any learning (Figure 2C). This suggests that maze learning requires tactile input (as well as proprioceptive, as explained below). This is intuitively in agreement with the argument of the multisensory nature of food location in the maze. Recent work has established C. elegans’ multisensory integration (Ghosh et al., 2016, 2017) through cellular mechanisms and neuronal circuit cross-talk.
We find that time passed in combination with change of environment results in loss of learning (Figure 3A). Moreover, C. elegans do not retrieve the learned information a second time (Figure 3B). The experience in the first testing maze may lead to worms “unlearning” or forgetting what they previously learned, or the training maze information might be “overwritten” by this new experience. Alternatively, the experience of the empty second testing maze could result in extinction of the previously learned information (Hartley and Phelps, 2012) or this could be simply due to single training session resulting in short-term learning.
Confirming the above, time passed without change of environment does not result in abolishing learning (Figure 3C), probably because C. elegans might revisit the food-containing area during that time, thus enhancing (“refreshing”) the achieved learning. When C. elegans stay in the same maze for longer, learning is lost (Figure 3C), probably because revisiting the food-containing area from a different route may result in “forgetting” or “overwriting”. The findings that learning is very short term and sensitive to distraction imply resemblance to a form of working memory (Cowan, 2008; Van Asselen et al., 2006).
Sensory modalities and maze learning
When odr-3 and odr-10 strains (Sengupta et al., 1996; Roayaie et al., 1998) were tested, loss of C. elegans' ability to locate food in the maze was observed (Figure 4A). Subsequently, no learning was observed (Figure 4A). Chemosensory genes are known to be required for odor-driven associative learning (Bargmann, 2006). In addition, the ODR-10 olfactory receptor is required for response to the odorant diacetyl (Sengupta et al., 1996), and loss of ODR-10 impairs food taxis (Ryan et al., 2014). We speculate that for the food-driven learning to occur, it is necessary for the worms to first receive and then act upon the triggering stimulus. If no food is detected, then food location fails, and no learning occurs. This implies that learning is activated only when there is a motive for the animal to learn.
Mechanosensation is important in the maze environment (mec-4 and mec-10 mutants, Figure 4B) (Driscoll and Chalfie, 1991; Suzuki et al., 2003). This is in line with results in smooth mazes (Figure 2C), suggesting that tactile input contributes to successful navigation in the maze environment. Remarkably, mechanosensory pathways are found to participate in sensing non-mechanical stimuli (Russell et al., 2014) in worms, supporting the hypothesis of multisensory integration or multi-pathway cross-talk.
Proprioception plays a distinct role in C. elegans learning inside the maze, as it is the only sensory modality investigated that does not affect food location but is required for learning (trp-4 and trp-1;trp-2 strains, Figure 4C) (Li et al., 2006; Kubanek et al., 2018) (Yeon et al., 2018). Therefore, sensation of their own body's posture and actions is of key importance. Indeed, it is known that spatial information can be acquired from nonvisual modalities (Yamamoto and Shelton, 2005; King, 2009), and proprioception and mechanosensation are major among them (Chance et al., 1998; Klatzky et al., 1998; Waller et al., 2004; Klatzky, 1999; Klatzky and Lederman, 2003; Shelton and Mcnamara, 2001). Especially in the absence of detailed visual cues (Ward et al., 2008), C. elegans would have to rely on their other, sharper sensory modalities to navigate their environment and learn their way around food sources, pathogens or predators.
Selected biochemical pathways, aging, and starvation
Dopamine is pivotal for C. elegans behavioral plasticity (Kindt et al., 2007; Sanyal et al., 2004), and the D2-like dopamine receptor DOP-3 is involved in C. elegans spatial pattern selectivity (Han et al., 2017). When dop-3 mutants that lack the dopamine receptor DOP-3 (Ezak and Ferkey, 2010; Mcdonald et al., 2007) were tested, they did not locate food effectively and consequently showed no learning (Figure 4D). This supports the hypothesis of “no food location, no learning”, to which reward-driven, dopamine-mediated modulation of neuronal receptors (Voglis and Tavernarakis, 2008; Kindt et al., 2007) could contribute. The exact reason why dop-3 have reduced food location ability in the maze is not known, although it could be related to the multiple food-modulated behaviors in which the dopamine pathway is involved (Ezcurra et al., 2011; Oranth et al., 2018).
CREB is involved in associative learning in C. elegans (Kauffman et al., 2010) and other species (Kandel, 2012). The crh-1 strain tested here lacks a homolog of CREB (Kauffman et al., 2010), and worms retain the ability, although significantly reduced, to locate food (Figure 4D). Behavioral defect of crh-mutants has been reported in the past (Nishida et al., 2011). Moreover, crh-1 mutants do not show any learning (Figure 4D). It is unlikely that CREB contributes to maze learning through remodeling of neurons (Middei et al., 2012), yet other aspects of CREB function might be involved (Chen et al., 2010). Interestingly, CRH-1 is also required in the C. elegans AFD thermosensory neuron for memory-related behavior (Nishida et al., 2011).
Cognitive behaviors, especially memory and learning, deteriorate with aging in many organisms, spanning nematodes to humans (Kausler, 1994; Kauffman et al., 2010). Here, day 5 adult middle-aged C. elegans (Figure 7) do not show any learning (Figure 7). Similar aging-driven decline has been reported for other types of learning in C. elegans (Arey et al., 2018). Interestingly, we find that a well-established life span extending dietary regimen (dietary restriction through food deprivation) (Kaeberlein et al., 2006), can significantly delay this process.
Learning strategy
C. elegans repeat in the testing maze the interaction they had with structural features in the training maze, namely walls and floor (Figures 5A and 6C). Slight differences in the roughness of walls and floors in both smooth and rough mazes, due to the 3D-printing mold fabrication process, could allow nematodes to distinguish the two surfaces inside a maze. When a nematode is inserted in the maze, it is placed on the floor of the starting area (Figure 1), where it wonders briefly before it starts its journey. Therefore, all nematodes have experienced the floor, even briefly. Repeating the wall or floor interaction is not observed in smooth mazes (less overall tactile input, Figure 5B) or in the touch sensation impaired mutants (mec-4, mec-10, Figure 5B). This suggests that touch sensation mediates input from maze structural features, especially when they provide cues through rich texture.
In addition, proprioception-impaired mutants (trp-4, trp-1;trp-2) maintain the floor/wall preference (Figure 5C). This implies that proprioception does not mediate input related to the structural features of C. elegans environment, although it is required for learning in the maze (Figure 4C), by contributing cues on the body's actions. Moreover, tactile input from multiple body areas when nematodes crawl where the walls meet the floor or during sharp bends as they make their final turn at the T-junction while crawling on the wall, might contribute to registering key events of their trip.
At the same time, C. elegans ventral/dorsal body orientation with respect to the maze sides is not maintained the same in training and in testing (Figure 6). This suggests that nematodes do not associate body sides with maze sides. Moreover, nematodes do not learn based on absolute directions, i.e., east/west or north/south (Figure 2A). This means that they either learn the location or physical position of their goal based on landmarks of the maze environment (place learning) or they learn to perform a specific series of movements (response learning) (Wang, 2012; Marchette et al., 2011; Shettleworth, 2010).
The maze environment lacks landmarks that point to one side or the other, since all features are symmetrical, continuous, and present throughout the maze, with no consistent difference between the two arms (Figure S5). Note that the training maze plates and the testing maze plates used in each experiment were not made from the same mold. This minimized the probability of consistently present textural differences, generated during the mold 3D-printing process, between training and testing mazes experienced by the same nematode. At the same time, impaired proprioception results in abolished learning (Figure 4C), and interactions between the worm's body and walls/floor of the maze are important for learning (Figure 5). Therefore, a type of response learning, mediated by proprioception and mechanosensation, is likely to constitute the strategy in the maze environment (Iachini et al., 2014). This does not mean that C. elegans “memorize” their entire maze trip; rather, they associate proprioception-mediated cues on key body actions (e.g. bends) (Bures and Fenton, 2000) combined with mechanosensation-mediated experience of wall/floor edges and surfaces, with the presence of food-reward.
Since C. elegans learn to associate a navigational behavior with the reward of food location, the observed learning may be considered a form of basic operational (instrumental) learning (Staddon and Cerutti, 2003).
To conclude, we methodically portray C. elegans learning in a maze environment, a previously uncharacterized behavior. Nematodes learn to associate food with a combination of proprioceptive cues and mechanosensation-provided information on their interaction with their surroundings (Figure S6), to navigate and reach a target location.
Limitations of the study
Successful food location in the training maze requires the contribution of chemical and tactile input (Figures 4A, 4B, and 2C). In the current assay it is not possible to distinguish between food detection and subsequent food location. When successfully trained worms are placed in a second, similar, empty maze (testing maze), they express a biased behavior, and the majority of them reach the same maze side with the one that contained food previously. The current assay cannot distinguish between learning formation and learning expression (retrieval) processes. Moreover, the current experimental setting does not allow tracking of the nematodes throughout their journey, especially when they crawl on the maze walls. This limits our ability to extract information about locomotion features that are affected by learning and possibly change after successful training.
Resource availability
Lead contact
Information and requests for resources should be directed to and will be fulfilled by the lead contact, Ao-Lin Hsu aolinhsu@umich.edu.
Materials availability
This study did not generate any new reagents.
Data and code availability
All data supporting findings of this work are provided within the manuscript and its supplemental information section. Video recordings in addition to the ones provided in the supplemental information of the electronic version are available upon reasonable request.
Methods
All methods can be found in the accompanying transparent methods supplemental file.
Acknowledgments
We are grateful to Joy Alcedo for her advice on mutant strains, data analysis, and feedback on the manuscript, to Scott Pletcher for multiple critical discussions, and to Geoff Murphy, and members of the Pletcher Lab, for their remarks on data interpretation. We are thankful to Marie-Beatrix Kruth, Emily Branch, and Allison LaMonica for help with experiments, Steel Cardoza for improvements on the mold design, Surojit Sural for extended feedback, Abrielle Fretz for help with crosses, Jaimee Moline for assistance with video analysis, and Tuhin Chakraborty for comments on the manuscript. Some maze molds were printed at a 3D printer of the Pletcher lab; we thank David Paris for his assistance. Smooth maze molds were printed at the University of Michigan 3D Lab, Duderstadt Center, College of Engineering; we thank Shawn O'Grady for his assistance. Strains were provided by the CGC (Caenorhabditis Genetics Center), which is funded by National Institutes of Health (United States) Office of Research Infrastructure Programs (P40 OD010440). We thank Andrés Vidal-Gadea and Kyuhyung Kim for kindly sharing strains. Finally, we thank Ryan Oliver for discussions at the early stages of this work, and Carol Mousigian for technical assistance. Research was supported by ALH's National Institutes of Health-National Institute on Aging (United States) R01-AG051439 grant and by the Ministry of Science and Technology, Taiwan, 107-2628-B-010-012 grant. EG is the recipient of a National Institutes of Health-National Institute on Aging (United States) K01-AG057833 award. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author contributions
E.G. and A.L.H. conceived the idea and designed experiments. E.G., K.A., A.G., and C.C. ran experiments. E.G. designed and built mazes, analyzed data, and wrote the manuscript. E.G. and A.L.H. interpreted data. A.L.H. edited the manuscript. All authors reviewed and approved the manuscript prior to publication.
Declaration of interests
The authors have no competing interests.
Published: April 23, 2021
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2021.102284.
Contributor Information
Eleni Gourgou, Email: egourgou@umich.edu.
Ao-Lin Hsu, Email: aolinhsu@umich.edu.
Supplemental information
References
- Amano H., Maruyama I.N. Aversive olfactory learning and associative long-term memory in Caenorhabditis elegans. Learn. Mem. 2011;18:654–665. doi: 10.1101/lm.2224411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardiel E.L., Rankin C.H. An elegant mind: learning and memory in Caenorhabditis elegans. Learn Mem. 2010;17:191–201. doi: 10.1101/lm.960510. [DOI] [PubMed] [Google Scholar]
- Arey R.N., Stein G.M., Kaletsky R., Kauffman A., Murphy C.T. Activation of gαq signaling enhances memory consolidation and slows cognitive decline. Neuron. 2018;98:562–574.e5. doi: 10.1016/j.neuron.2018.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnatkeviciute A., Fulcher B.D., Pocock R., Fornito A. Hub connectivity, neuronal diversity, and gene expression in the Caenorhabditis elegans connectome. PLoS Comput. Biol. 2018;14 doi: 10.1371/journal.pcbi.1005989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardgett M.E., Depenbrock M., Downs N., Points M., Green L. Dopamine modulates effort-based decision-making in rats. Behav. Neurosci. 2009;123:242–251. doi: 10.1037/a0014625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargmann C.I. Chemosensation in C. elegans. In: WormBook, editor. WormBook. The C. elegans Research Community; 2006. pp. 1–29.http://www.wormbook.org [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bures J., Fenton A.A. Neurophysiology of spatial cognition. Physiology. 2000;15:233–240. doi: 10.1152/physiologyonline.2000.15.5.233. [DOI] [PubMed] [Google Scholar]
- Chance S.S., Gaunet F., Beall A.C., Loomis J.M. Locomotion mode affects the updating of objects encountered during travel: the contribution of vestibular and proprioceptive inputs to path integration. Presence. 1998;7:168–178. [Google Scholar]
- Chen G., Zou X., Watanabe H., Van Deursen J.M., Shen J. CREB binding protein is required for both short-term and long-term memory formation. J. Neurosci. 2010;30:13066–13077. doi: 10.1523/JNEUROSCI.2378-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins J.J., Huang C., Hughes S., Kornfeld K. WormBook; 2008. The measurement and analysis of age-related changes in Caenorhabditis elegans; pp. 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook S.J., Jarrell T.A., Brittin C.A., Wang Y., Bloniarz A.E., Yakovlev M.A., Nguyen K.C.Q., Tang L.T.H., Bayer E.A., Duerr J.S. Whole-animal connectomes of both Caenorhabditis elegans sexes. Nature. 2019;571:63–71. doi: 10.1038/s41586-019-1352-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan N. What are the differences between long-term, short-term, and working memory? Prog. Brain Res. 2008;169:323–338. doi: 10.1016/S0079-6123(07)00020-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culotti J.G., Russell R.L. Osmotic avoidance defective mutants of the nematode Caenorhabditis elegans. Genetics. 1978;90:243–256. doi: 10.1093/genetics/90.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies R., Gagen M.H., Bull J.C., Pope E.C. Maze learning and memory in a decapod crustacean. Biol. Lett. 2019;15:20190407. doi: 10.1098/rsbl.2019.0407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derdikman D., Knierim J.J. Springer; 2014. Space,Time and Memory in the Hippocampal Formation. [Google Scholar]
- Driscoll M., Chalfie M. The mec-4 gene is a member of a family of Caenorhabditis elegans genes that can mutate to induce neuronal degeneration. Nature. 1991;349:588–593. doi: 10.1038/349588a0. [DOI] [PubMed] [Google Scholar]
- Ezak M.J., Ferkey D.M. The C. elegans D2-like dopamine receptor DOP-3 decreases behavioral sensitivity to the olfactory stimulus 1-octanol. PLoS One. 2010;5:e9487. doi: 10.1371/journal.pone.0009487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezcurra M., Tanizawa Y., Swoboda P., Schafer W.R. Food sensitizes C. elegans avoidance behaviours through acute dopamine signalling. EMBO J. 2011;30:1110–1122. doi: 10.1038/emboj.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faumont S., Lindsay T.H., Lockery S.R. Neuronal microcircuits for decision making in C. elegans. Curr. Opin. Neurobiol. 2012;22:580–591. doi: 10.1016/j.conb.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagliano M., Vyazovskiy V.V., Borbély A.A., Grimonprez M., Depczynski M. Learning by association in plants. Sci. Rep. 2016;6:38427. doi: 10.1038/srep38427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrity P.A., Goodman M.B., Samuel A.D., Sengupta P. Running hot and cold: behavioral strategies, neural circuits, and the molecular machinery for thermotaxis in C. elegans and Drosophila. Genes Dev. 2010;24:2365–2382. doi: 10.1101/gad.1953710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh D.D., Nitabach M.N., Zhang Y., Harris G. Multisensory integration in C. elegans. Curr. Opin. Neurobiol. 2017;43:110–118. doi: 10.1016/j.conb.2017.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh D.D., Sanders T., Hong S., Mccurdy L.Y., Chase D.L., Cohen N., Koelle M.R., Nitabach M.N. Neural architecture of hunger-dependent multisensory decision making in C. elegans. Neuron. 2016;92:1049–1062. doi: 10.1016/j.neuron.2016.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman M.B. The C. Elegans Research Community. Wormbook; 2006. Mechanosensation.www.wormbook.org [DOI] [Google Scholar]
- Han B., Dong Y., Zhang L., Liu Y., Rabinowitch I., Bai J. Dopamine signaling tunes spatial pattern selectivity in C. elegans. eLife. 2017;6:e22896. doi: 10.7554/eLife.22896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley A.C., Phelps E.A. Extinction. In: Seel Norbert., editor. Encyclopedia of the Sciences of Learning. Springer US; 2012. p. 867. [Google Scholar]
- Iachini T., Ruggiero G., Ruotolo F. Does blindness affect egocentric and allocentric frames of reference in small and large scale spaces? Behav. Brain Res. 2014;273:73–81. doi: 10.1016/j.bbr.2014.07.032. [DOI] [PubMed] [Google Scholar]
- Jarrell T.A., Wang Y., Bloniarz A.E., Brittin C.A., Xu M., Thomson J.N., Albertson D.G., Hall D.H., Emmons S.W. The connectome of a decision-making neural network. Science. 2012;337:437–444. doi: 10.1126/science.1221762. [DOI] [PubMed] [Google Scholar]
- Kaeberlein T.L., Smith E.D., Tsuchiya M., Welton K.L., Thomas J.H., Fields S., Kennedy B.K., Kaeberlein M. Lifespan extension in Caenorhabditis elegans by complete removal of food. Aging Cell. 2006;5:487–494. doi: 10.1111/j.1474-9726.2006.00238.x. [DOI] [PubMed] [Google Scholar]
- Kandel E.R. The molecular biology of memory: cAMP, PKA, CRE, CREB-1, CREB-2, and CPEB. Mol. Brain. 2012;5:14. doi: 10.1186/1756-6606-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauffman A.L., Ashraf J.M., Corces-Zimmerman M.R., Landis J.N., Murphy C.T. Insulin signaling and dietary restriction differentially influence the decline of learning and memory with age. Plos Biol. 2010;8:e1000372. doi: 10.1371/journal.pbio.1000372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kausler B. Academic Press; 1994. Learning and Memory in Normal Aging. [Google Scholar]
- Kindt K.S., Quast K.B., Giles A.C., De S., Hendrey D., Nicastro I., Rankin C.H., Schafer W.R. Dopamine mediates context-dependent modulation of sensory plasticity in C. elegans. Neuron. 2007;55:662–676. doi: 10.1016/j.neuron.2007.07.023. [DOI] [PubMed] [Google Scholar]
- King A.J. Visual influences on auditory spatial learning. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2009;364:331–339. doi: 10.1098/rstb.2008.0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klatzky R.L. Allocentric and egocentric spatial representations: definitions, distinctions, and interconnections. In: Freksa, Habel, Wender, editors. Spatial Cognition: An Interdisciplinary Approach to Representing and Processing Spatial Knowledge. Springer Berlin Heidelberg; 1998. [Google Scholar]
- Klatzky R.L. Path completion after haptic exploration without vision: implications for haptic spatial representations. Percept. Psychophys. 1999;61:220–235. doi: 10.3758/bf03206884. [DOI] [PubMed] [Google Scholar]
- Klatzky R.L., Lederman S.J. Representing spatial location and layout from sparse kinesthetic contacts. J. Exp. Psychol. Hum. Percept. Perform. 2003;29:310–325. doi: 10.1037/0096-1523.29.2.310. [DOI] [PubMed] [Google Scholar]
- Klatzky R.L., Loomis J.M., Beall A.C., Chance S.S., Golledge R.G. Spatial updating of self-position and orientation during real, imagined, and virtual locomotion. Psychol. Sci. 1998;9:293–298. [Google Scholar]
- Klein M., Krivov S.V., Ferrer A.J., Luo L., Samuel A.D.T., Karplus M. Exploratory search during directed navigation in C. elegans and Drosophila larva. eLife. 2017;6:30503. doi: 10.7554/eLife.30503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubanek J., Shukla P., Das A., Baccus S.A., Goodman M.B. Ultrasound elicits behavioral responses through mechanical effects on neurons and ion channels in a simple nervous system. J. Neurosci. 2018;38:3081–3091. doi: 10.1523/JNEUROSCI.1458-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Feng Z., Sternberg P.W., Xu X.Z. A C. elegans stretch receptor neuron revealed by a mechanosensitive TRP channel homologue. Nature. 2006;440:684–687. doi: 10.1038/nature04538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo L., Wen Q., Ren J., Hendricks M., Gershow M., Qin Y., Greenwood J., Soucy E., Klein M., Smith-Parker H.K. Dynamic encoding of perception, memory and movement in a C. elegans chemotaxis circuit. Neuron. 2014;82:1115–1128. doi: 10.1016/j.neuron.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchette S.A., Bakker A., Shelton A.L. Cognitive mappers to creatures of habit: differential engagement of place and response learning mechanisms predicts human navigational behavior. J. Neurosci. 2011;31:15264–15268. doi: 10.1523/JNEUROSCI.3634-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcdonald P.W., Hardie S.L., Jessen T.N., Carvelli L., Matthies D.S., Blakely R.D. Vigorous motor activity in Caenorhabditis elegans requires efficient clearance of dopamine mediated by synaptic localization of the dopamine transporter DAT-1. J. Neurosci. 2007;27:14216–14227. doi: 10.1523/JNEUROSCI.2992-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcewan A.H., Rankin C.H. Vol. 22. 2013. pp. 91–111. (Mechanosensory Learning and Memory in Caenorhabditis elegans). [Google Scholar]
- Middei S., Spalloni A., Longone P., Pittenger C., O'mara S.M., Marie H., Ammassari-Teule M. CREB selectively controls learning-induced structural remodeling of neurons. Learn. Mem. 2012;19:330–336. doi: 10.1101/lm.025817.112. [DOI] [PubMed] [Google Scholar]
- Morris R. Developments of a water-maze procedure for studying spatial memory in the rat. J. Neurosci. Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Nishida Y., Sugi T., Nonomura M., Mori I. Identification of the AFD neuron as the site of action of the CREB protein in Caenorhabditis elegans thermotaxis. EMBO Rep. 2011;12:855–862. doi: 10.1038/embor.2011.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver C.R., Gourgou E., Bazopoulou D., Chronis N., Hart A.J. On-demand isolation and manipulation of C. elegans by in vitro maskless photopatterning. PLoS One. 2016;11:e0145935. doi: 10.1371/journal.pone.0145935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olton D.S. Mazes, maps, and memory. Am. Psychol. 1979;34:583–596. doi: 10.1037//0003-066x.34.7.583. [DOI] [PubMed] [Google Scholar]
- Oranth A., Schultheis C., Tolstenkov O., Erbguth K., Nagpal J., Hain D., Brauner M., Wabnig S., Steuer Costa W., Mcwhirter R.D. Food sensation modulates locomotion by dopamine and neuropeptide signaling in a distributed neuronal network. Neuron. 2018;100:1414–1428.e10. doi: 10.1016/j.neuron.2018.10.024. [DOI] [PubMed] [Google Scholar]
- Pandey S., Joseph A., Lycke R., Parashar A. Decision-making by nematodes in complex microfluidic mazes. Adv. Biosci. Biotechnol. 2011;02:409–415. [Google Scholar]
- Qin J., Wheeler A.R. Maze exploration and learning in C. elegans. Lab Chip. 2007;7:186–192. doi: 10.1039/b613414a. [DOI] [PubMed] [Google Scholar]
- Rankin C.H., Beck C.D., Chiba C.M. Caenorhabditis elegans: a new model system for the study of learning and memory. Behav. Brain Res. 1990;37:89–92. doi: 10.1016/0166-4328(90)90074-o. [DOI] [PubMed] [Google Scholar]
- Rankin C.H., Broster B.S. Factors affecting habituation and recovery from habituation in the nematode Caenorhabditis elegans. Behav. Neurosci. 1992;106:239–249. doi: 10.1037//0735-7044.106.2.239. [DOI] [PubMed] [Google Scholar]
- Remy J.-J., Hobert O. An interneuronal chemoreceptor required for olfactory imprinting in C. elegans. Science. 2005;309:787–790. doi: 10.1126/science.1114209. [DOI] [PubMed] [Google Scholar]
- Roayaie K., Crump J.G., Sagasti A., Bargmann C.I. The gα protein ODR-3 mediates olfactory and nociceptive function and controls cilium morphogenesis in C. elegans olfactory neurons. Neuron. 1998;20:55–67. doi: 10.1016/s0896-6273(00)80434-1. [DOI] [PubMed] [Google Scholar]
- Russell J., Vidal-Gadea A.G., Makay A., Lanam C., Pierce-Shimomura J.T. Humidity sensation requires both mechanosensory and thermosensory pathways in Caenorhabditis elegans. Proc. Natl. Acad. Sci. U S A. 2014;111:8269–8274. doi: 10.1073/pnas.1322512111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan D.A., Miller R.M., Lee K., Neal S.J., Fagan K.A., Sengupta P., Portman D.S. Sex, age, and hunger regulate behavioral prioritization through dynamic modulation of chemoreceptor expression. Curr. Biol. 2014;24:2509–2517. doi: 10.1016/j.cub.2014.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanyal S., Wintle R.F., Kindt K.S., Nuttley W.M., Arvan R., Fitzmaurice P., Bigras E., Merz D.C., Hébert T.E., Van Der Kooy D. Dopamine modulates the plasticity of mechanosensory responses in Caenorhabditis elegans. EMBO J. 2004;23:473–482. doi: 10.1038/sj.emboj.7600057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasakura, H. & Mori, I. 2013. Thermosensory Learning in Caenorhabditis elegans. 22, 124-139.
- Schafer W.R. Proprioception: a channel for body sense in the worm. Curr. Biol. 2006;16:R509–R511. doi: 10.1016/j.cub.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Schwarz R.F., Branicky R., Grundy L.J., Schafer W.R., Brown A.E.X. Changes in postural syntax characterize sensory modulation and natural variation of C. elegans locomotion. PLoS Comput. Biol. 2015;11:e1004322. doi: 10.1371/journal.pcbi.1004322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta P., Chou J.H., Bargmann C.I. odr-10 encodes a seven transmembrane domain olfactory receptor required for responses to the odorant diacetyl. Cell. 1996;84:899–909. doi: 10.1016/s0092-8674(00)81068-5. [DOI] [PubMed] [Google Scholar]
- Shelton A.L., Mcnamara T.P. Visual memories from nonvisual experiences. Psychol. Sci. 2001;12:343–347. doi: 10.1111/1467-9280.00363. [DOI] [PubMed] [Google Scholar]
- Shettleworth S.J. Oxford University Press; 2010. Cognition, Evolution, and Behavior. [Google Scholar]
- Shoji H., Hagihara H., Takao K., Hattori S., Miyakawa T. T-maze forced alternation and left-right discrimination tasks for assessing working and reference memory in mice. J. Vis. Exp. 2012;60:3300. doi: 10.3791/3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staddon J.E.R., Cerutti D.T. Operant conditioning. Annu. Rev. Psychol. 2003;54:115–144. doi: 10.1146/annurev.psych.54.101601.145124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein G.M., Murphy C.T. C. elegans positive olfactory associative memory is a molecularly conserved behavioral paradigm. Neurobiol. Learn. Mem. 2014;0:86–94. doi: 10.1016/j.nlm.2014.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H., Kerr R., Bianchi L., Frokjaer-Jensen C., Slone D., Xue J., Gerstbrein B., Driscoll M., Schafer W.R. In vivo imaging of C. elegans mechanosensory neurons demonstrates a specific role for the MEC-4 channel in the process of gentle touch sensation. Neuron. 2003;39:1005–1017. doi: 10.1016/j.neuron.2003.08.015. [DOI] [PubMed] [Google Scholar]
- Tanimoto Y., Yamazoe-Umemoto A., Fujita K., Kawazoe Y., Miyanishi Y., Yamazaki S.J., Fei X., Busch K.E., Gengyo-Ando K., Nakai J. Calcium dynamics regulating the timing of decision-making in C. elegans. eLife. 2017;6:e21629. doi: 10.7554/eLife.21629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tervo Dougal g.R., Proskurin M., Manakov M., Kabra M., Vollmer A., Branson K., Karpova Alla y. Behavioral variability through stochastic choice and its gating by anterior cingulate cortex. Cell. 2014;159:21–32. doi: 10.1016/j.cell.2014.08.037. [DOI] [PubMed] [Google Scholar]
- Torayama I., Ishihara T., Katsura I. Caenorhabditis elegans integrates the signals of butanone and food to enhance chemotaxis to butanone. J. Neurosci. 2007;27:741–750. doi: 10.1523/JNEUROSCI.4312-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towlson E.K., Vértes P.E., Ahnert S.E., Schafer W.R., Bullmore E.T. The rich club of the C. elegans neuronal connectome. J. Neurosci. 2013;33:6380–6387. doi: 10.1523/JNEUROSCI.3784-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tully T., Quinn W.G. Classical conditioning and retention in normal and mutantDrosophila melanogaster. J. Comp. Physiol. A. 1985;157:263–277. doi: 10.1007/BF01350033. [DOI] [PubMed] [Google Scholar]
- Van Asselen M., Kessels R.P., Neggers S.F., Kappelle L.J., Frijns C.J., Postma A. Brain areas involved in spatial working memory. Neuropsychologia. 2006;44:1185–1194. doi: 10.1016/j.neuropsychologia.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Varshney L.R., Chen B.L., Paniagua E., Hall D.H., Chklovskii D.B. Structural properties of the Caenorhabditis elegans neuronal network. PLoS Comput. Biol. 2011;7:e1001066. doi: 10.1371/journal.pcbi.1001066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voglis G., Tavernarakis N. A synaptic DEG/ENaC ion channel mediates learning in C. elegans by facilitating dopamine signalling. EMBO J. 2008;27:3288–3299. doi: 10.1038/emboj.2008.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorhees C.V., Williams M.T. Assessing spatial learning and memory in rodents. ILAR J. 2014;55:310–332. doi: 10.1093/ilar/ilu013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waller D., Loomis J.M., Haun D.B.M. Body-based senses enhance knowledge of directions in large-scale environments. Psychon. Bull. Rev. 2004;11:157–163. doi: 10.3758/bf03206476. [DOI] [PubMed] [Google Scholar]
- Wang R.F. Theories of spatial representations and reference frames: what can configuration errors tell us? Psychon. Bull. Rev. 2012;19:575–587. doi: 10.3758/s13423-012-0258-2. [DOI] [PubMed] [Google Scholar]
- Ward A., Liu J., Feng Z., Shawn Xu X.Z. Light-sensitive neurons and channels mediate phototaxis in C. elegans. Nat. Neurosci. 2008;11:916–922. doi: 10.1038/nn.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenk G.L. Assessment of spatial memory using the T maze. Curr. Protoc. Neurosci. 2001;8:Unit 8.5B. doi: 10.1002/0471142301.ns0805bs04. [DOI] [PubMed] [Google Scholar]
- White J.G., Southgate E., Thomson J.N., Brenner S. The structure of the nervous system of the nematode Caenorhabditis elegans. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1986;314:1–340. doi: 10.1098/rstb.1986.0056. [DOI] [PubMed] [Google Scholar]
- Yamaguchi S., Desplan C., Heisenberg M. Contribution of photoreceptor subtypes to spectral wavelength preference in Drosophila. Proc. Natl. Acad. Sci. U S A. 2010;107:5634–5639. doi: 10.1073/pnas.0809398107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto N., Shelton A.L. Visual and proprioceptive representations in spatial memory. Mem. Cognit. 2005;33:140–150. doi: 10.3758/bf03195304. [DOI] [PubMed] [Google Scholar]
- Yemini E., Jucikas T., Grundy L.J., Brown A.E.X., Schafer W.R. A database of C. elegans behavioral phenotypes. Nat. Methods. 2013;10:877–879. doi: 10.1038/nmeth.2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeon J., Kim J., Kim D.-Y., Kim H., Kim J., Du E.J., Kang K., Lim H.-H., Moon D., Kim K. A sensory-motor neuron type mediates proprioceptive coordination of steering in C. elegans via two TRPC channels. PLoS Biol. 2018;16:e2004929. doi: 10.1371/journal.pbio.2004929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Lu H., Bargmann C.I. Pathogenic bacteria induce aversive olfactory learning in Caenorhabditis elegans. Nature. 2005;438:179–184. doi: 10.1038/nature04216. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data supporting findings of this work are provided within the manuscript and its supplemental information section. Video recordings in addition to the ones provided in the supplemental information of the electronic version are available upon reasonable request.