Dear Editor,
In the context of the COVID-19 pandemic, frequent thrombosis were reported, with up to 43% of clinically relevant thrombotic events in critically patients [1]. Several abnormalities in coagulation parameters, endothelial cells activation and multiple cytokines production have been described resulting in a procoagulant state [2]. A prolonged activated partial-thromboplastin time (aPTT) was identified in a significant number of patients, associated with the presence of a Lupus Anticoagulant (LA) [[3], [4], [5]]. Besides, studies showed an increased prevalence of other antiphospholipid antibodies (aPLs) with different targets and/or isotypes in infected patients [6,7]. aPLs appeared associated with thrombosis and severity of COVID-19 [6,8,9] and were demonstrated as pathogenic in a murine model with induced vascular injury [7]. Recent findings raise the question of whether LA and other aPLs persist over time and thereby whether COVID-19 with thrombosis and aPLs could be considered as an unusual, induced antiphospholipid syndrome (APS). Several concerns arise from the current literature on COVID-19. LA positivity could be difficult to interpret as many patients did not have LA detection with aPTT and diluted Russell's Viper Venom Time (dRVVT) at admission in the hospital. Moreover, the inflammatory context, coagulation disorders or anticoagulant therapy could influence LA detection [3,10]. Siguret et al. showed LA as labile when measured a few days after first identification [11] but there is to date no systematic follow-up of the patients who presented COVID-19 infection and LA and/or aPLs. In the present study we aimed to describe the prevalence and persistence over time of criteria aPLs in a cohort of hospitalized COVID-19 patients who were tested positive for LA and assess their association with thrombosis.
Included patients were at least 18 years of age, had RT-PCR confirmed SARS-CoV-2 severe to critical infection according to WHO guidelines [12] and were positive for LA, between March 03rd and April 11th 2020. Study was approved by the Institutional Board of Strasbourg University Hospital (CE-2020-85). Data were collected from routine care. LA activity of patient plasma was assessed by the dRVVT (STA®-Staclot dRVV Screen and confirm reagent, Stago), and LA sensitive aPPT (STA®-PPT LA reagent, Stago) according to ISTH guidelines [13]. IgM and IgG anticardiolipin antibodies (aCLs) were evaluated through a fluorescence enzyme immunoassay (FEIA) designed as a sandwich assay on a Phadia 250 (Thermofisher, Phadia Uppsala Sweden). Anti-β2GPI antibodies were assessed using a sandwich ELISA (Inova Diagnostic, San Diego, CA, USA). Non-criteria aPLs titers were determined by ELISA. Screening for antinuclear antibodies (ANAs) was performed on HEp-2 cells (Zeus Scientific, USA). Fisher's exact test was used for categorial variables and Mann Whitney or unpaired t-test were used for quantitative variables according to variable distribution. Multivariate analysis including variable with P value <0.10 on univariate analysis was performed using multiple logistic regression. Statistical analysis was performed with JMP software version 7.10 (SAS institute, USA).
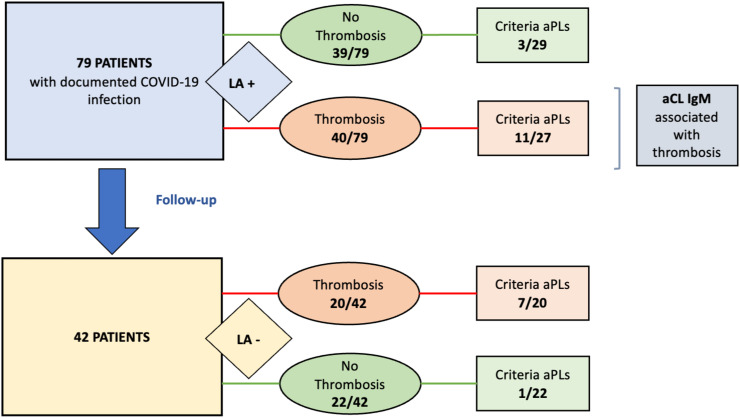
We identified 79 patients with COVID-19 and LA (Fig. 1 ). Patients characteristics were concordant with previously published data [14,15]. Patients had severe to critical COVID-19 with hospitalization for a median duration of 23 days [5–59] and 66 (83.5%) required mechanical ventilation (Table 1 ). Computed tomography (CT) quantification of pneumonia was performed for 69 patients with at least 25% involvement in the majority of cases (85.5%). All patients received standard of care as needed. Among the 79 LA positive patients, 50.6% displayed one thrombosis with 7.59% having at least one recurrence. Regarding the vascular distribution of thrombosis, 75% had venous thrombosis, 25% had arterial thrombosis and 25% had a catheter or ECMO oxygenator or Renal Replacement Therapy circuit clotting. Importantly, 82.5% of patients with thrombosis received a prophylaxis either with low molecular weight heparin (LMWH) or unfractionated heparin (UFH) before the first thrombotic event. The median highest CRP was 286 mg/l [16.7–492] and the median highest D-Dimer level was 7200 μg/l [730–20,000].
Fig. 1.
Flowchart of the study.
Table 1.
Characteristics and biological parameters of SARS-CoV-2 infected patients with LA and association with thrombotic events.
| Thrombosis N = 40 |
No Thrombosis N = 39 |
Univariate analysis p* |
Multivariate analysis p** |
|
|---|---|---|---|---|
| Age1 | 65 [29–81] | 64 [24–86] | 0.51 | |
| Sex ratio (F/M) | 9/31 | 7/32 | 0.78 | |
| BMI2 | 28 [21–41] | 29 [22−31] | 0.46 | |
| Co-existing conditions (n, %) | ||||
| HTA | 19(48) | 16(41) | 0.65 | |
| Diabetes | 14(35) | 10(26) | 0.46 | |
| Smoker | 3(8) | 3(8) | 0.99 | |
| Respiratory disease | 4(10) | 6(15) | 0.85 | |
| Cardiac arrhythmia | 2(5) | 0 | 0.49 | |
| Past medical history (n, %) | ||||
| Thrombotic events | 1(3) | 2(5) | 0.61 | |
| Malignancy | 3(8) | 1(3) | 0.61 | |
| Therapy on arrival (n, %) | ||||
| Long term LDA treatment | 8(20) | 3(8) | 0.11 | 0.11 |
| Long term anticoagulant | 1(3) | 0 | 0.99 | |
| Thromboprophylaxis | 22(55) | 27(69) | 0.11 | 0.16 |
| LMWH | 19(48) | 25(64) | 0.11 | |
| UFH | 3(8) | 2(5) | 0.99 | |
| Thrombotic events Recurrence >1/>2 |
40(51) 6/2 |
|||
| Delay between COVID-19 first symptoms and thrombosis3 | 19 [4–38] | |||
| Delay between inflammatory peak and thrombosis3 | 6.5 [0−23] | |||
| Anticoagulation before first thrombosis | 33(83) | |||
| Venous thrombosis | 30(75) | |||
| Pulmonary thrombosis | 27(68) | |||
| Deep or superficial vein thrombosis | 5(13) | |||
| Arterial thrombosis | 10(25) | |||
| Acute cerebral infarction | 9(23) | |||
| Mesenteric infarction | 1(3) | |||
| Myocardial infarction | 0 | |||
| Catheter thrombosis | 5(13) | |||
| ECMO or RRT circuit Clotting | 5(13) | |||
| Covid-19 therapy | ||||
| Lopinavir-Ritonavir | 24(60) | 20(51) | 0.43 | |
| Hydroxychloroquine | 7(18) | 10(26) | 0.38 | |
| Remdesivir | 2(5) | 1(3) | 0.57 | |
| Interferon beta | 2(5) | 0 | 0.16 | |
| Anakinra | 1(3) | 0 | 0.32 | |
| Tocilizumab | 1(3) | 0 | 0.32 | |
| Imaging features | ||||
| Infiltrates <25% on CT | 5/34(15) | 5/35(14) | 0.99 | |
| Infiltrates >50% on CT | 22/34(65) | 21/35(60) | 0.80 | |
| Outcome | ||||
| ICU admission (n, %) | 33(83) | 34(87) | 0.76 | |
| Invasive mechanical ventilation | 32(80) | 34(87) | 0.39 | |
| Time to hospital discharge3 | 25 [8–59] | 22 [5–49] | 0.31 | |
| Death | 4(10) | 3(8) | ||
| Laboratory findings during hospitalization | ||||
| Criteria aPLs (n/assessed, %) | 11/27(41) | 3/29(10) | 0.01 | |
| aCL IgM | 11/27(41) | 2/29(7) | 0.004 | 0.09 |
| aCL IgG | 1/27 | 0 | 0.48 | |
| Anti-β2GPI IgM | 2/27(7) | 1/29(3) | 0.60 | |
| Anti-β2GPI IgG | 0 | 0 | ||
| Non-criteria aPLs | 6/26(23) | 7/27(26) | 0.99 | |
| PE | 1/26(4) | 0 | 0.61 | |
| PS | 0 | 1/27(4) | 0.99 | |
| PT | 5/26(19) | 5/27(19) | 0.99 | |
| AV | 0 | 1/27(4) | 0.99 | |
| ANAs | 18/27(67) | 16/26(62) | 0.78 | |
| High-sensitivity CRP at the peak (mg/l) | 290 [16–437] | 285 [121–492] | 0.09 | 0.46 |
| Laboratory findings at follow-up4 |
Thrombosis N = 20 |
No Thrombosis N = 22 |
||
| Criteria aPLs (n/assessed, %) | 7/20(35) | 1/22(5) | ||
| aCL IgM | 5/20(25) | 0 | ||
| aCL IgG | 1/20(5) | 1/22(5) | ||
| Anti-β2GPI IgM | 0 | 0 | ||
| Anti-β2GPI IgG | 1/20(5) | 0 | ||
| Non-criteria aPLs | 5/20(25) | 0 | ||
| PE | 0 | 0 | ||
| PS | 1/20(5) | 0 | ||
| PT | 5/20(25) | 0 | ||
| AV | 0 | 0 | ||
| ANAs | 15/20(75) | 7/22(32) |
Note: results are given in median [Range], n(%) or n/N(%), where N is the total number of patients with available data. 1 Age is expressed in years; 2 BMI is expressed in kg/m2; 3 Delays between COVID-19 first symptoms/inflammatory peak and thrombosis and time to hospital discharge are expressed in days; 4 Patients were followed up 3 to 6 months after first LA identification. * Difference using Fisher's exact test for categorical variables and Mann Whitney or unpaired t-test according to distribution for quantitative variables. **Using multiple logistic regression. Abbreviations: BMI, Body Mass Index; LDA, Low Dose Aspirine; UFH, Unfractionated Heparin; LMWH, Low Molecular Weight Heparin; ECMO, Extra Corporeal Membrane Oxygenation; RRT, Renal Replacement Therapy; CT, Computed Tomography; ICU, Intensive Care Unit; aPLs, antiphospholipid antibodies (Ab); aCL, anticardiolipin Ab; PS, Anti-Phosphatidylserine Ab; PE, anti-Phosphatidyl-ethanolamine Ab; PT, anti-prothrombin Ab; AV, anti-annexin V Ab; ANAs, antinuclear Abs.
Fifty-six patients with LA were further explored for other criteria aPLs with 14 being positive according to laboratory values (Table 1). Three had anti-β2GPI IgM, 13 had aCL IgM and 1 had aCL IgG. Fifty-three patients were explored for non-criteria aPLs and 20 were positive for at least one non criteria aPL among anti-Phosphatidylserine (PS), anti-Phosphatidyl-ethanolamine (PE), anti-prothrombin (PT), anti-annexin V (AV). Altogether, 29 were positive for criteria or non-criteria aPLs. Additionally, 53 patients with LA were explored for antinuclear antibodies (ANAs). Noteworthy, 33 (62.3%) tested patients were positive for ANAs at a titer >1/80 and 15 (28.3%) above 1/320 dilution.
We compared the patients with thrombosis and without thrombosis among the 79 patients with LA (Table 1). Groups were similar for age, sex and BMI, cardiovascular risk factors and previous history of thrombotic events. There was no difference regarding COVID severity and anticoagulant therapies. We found a strong association between thrombosis and positivity of aCLs IgM (11/27 [41%] patients with thrombosis vs 2/29 [7%] patients without thrombosis, p = 0.004, OR = 9.28 IC95 2.0 to 44.4).
Forty-two patients were followed-up and screened for antiphospholipid antibodies and ANAs at least 3 months and up to 6 months after first LA identification (Table 1). LA was found negative in all of 42 patients. The presence of aCLs was noted in 7/42 (16.7%) patients, mostly IgM aCLs. Anti-β2-GPI were found in 1/42 (2.38%) patients. Non-criteria aPLs were found in 5/42 (11.9%) patients, mostly anti-prothrombin. Overall, 10/42 (23.8%) patients had at least one positive aPL. The association between thrombosis and positivity of aCLs IgM was confirmed (5/20 [25%] patients with thrombosis vs 0/22 patients without thrombosis). Of note, 22/42 (52.4%) patients still remained positive for ANAs with a titer above 1/80 and 11 of 42 (26.2%) above 1/320 dilution. None of the 42 patients had a new episode of thrombosis during follow-up and none of them presented autoimmune manifestations.
In conclusion, LA is frequent in COVID-19 patients in the acute phase, but its signification is controversial. Herein we show that LA is transient. Based on our study and others, LA with aCLs of IgM or IgG isotype is strongly associated with the occurrence of thrombosis during the acute phase of COVID-19 infection [9]. In these situations, reinforced preventive anticoagulation is mandatory. Interestingly aCLs including of IgM isotype, can persist, in association with ANAs, suggesting that COVID-19 promotes a durable breakdown of tolerance and questioning the needed duration of follow-up in these patients. Viral infections are known to stimulate autoreactive B-cells and patients can transiently produce non-pathogenic aPLs, especially of IgM isotype, which are usually not associated with thrombosis. Thus, potentially thrombogenic aPLs in COVID-19 is an uncommon situation. COVID-19 infection is associated with coagulation disorders and a strong inflammatory context which could favor this breakdown of tolerance [16]. aPLs may be generated via molecular mimicry with some viral proteins [17,18]. Recent studies describe a SARS-CoV-2 induced vasculopathy and endothelial damage which could increase the pathogenicity of aPLs, or PLs and their cofactor exposition [19]. Therefore, considering the two-hit hypothesis, widely accepted as mechanism of thrombus formation in APS, COVID-19 may provide both first (aPLs emergence) and second hit (vascular damage) necessary for thrombosis [20]. Further experimental settings about the specificity and avidity of COVID-19 induced aPLs are likely required.
Fundings
None.
Disclosure of conflicts of interest
None.
Acknowledgments
We thank the European reference Networks (ERNs) RITA and ReCONNET.
References
- 1.Helms J., Tacquard C., Severac F., Leonard-Lorant I., Ohana M., Delabranche X., et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46:1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Connors J.M., Levy J.H. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135:2033–2040. doi: 10.1182/blood.2020006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reyes Gil M., Barouqa M., Szymanski J., Gonzalez-Lugo J.D., Rahman S., Billett H.H. Assessment of lupus anticoagulant positivity in patients with coronavirus disease 2019 (COVID-19) JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.17539. [DOI] [PubMed] [Google Scholar]
- 4.Bowles L., Platton S., Yartey N., Dave M., Lee K., Hart D.P., et al. Lupus anticoagulant and abnormal coagulation tests in patients with Covid-19. N Engl J Med. 2020;383:288–290. doi: 10.1056/NEJMc2013656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harzallah I., Debliquis A., Drénou B. Lupus anticoagulant is frequent in patients with Covid-19. J Thromb Haemost JTH. 2020;18:2064–2065. doi: 10.1111/jth.14867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bertin D., Brodovitch A., Beziane A., Hug S., Bouamri A., Mege J.L., et al. Anti-cardiolipin IgG autoantibodies are an independent risk factor of COVID-19 severity. Arthritis Rheumatol Hoboken NJ. 2020 doi: 10.1002/art.41409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zuo Y., Estes S.K., Ali R.A., Gandhi A.A., Yalavarthi S., Shi H., et al. Prothrombotic autoantibodies in serum from patients hospitalized with COVID-19. Sci Transl Med. 2020 doi: 10.1126/scitranslmed.abd3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pineton de Chambrun M., Frere C., Miyara M., Amoura Z., Martin-Toutain I., Mathian A., et al. High frequency of antiphospholipid antibodies in critically ill COVID-19 patients: a link with hypercoagulability? J Intern Med. 2020 doi: 10.1111/joim.13126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Le Joncour A., Frere C., Martin-Toutain I., Gougis P., Ghillani-Dalbin P., Maalouf G., et al. Antiphospholipid antibodies and thrombotic events in COVID-19 patients hospitalized in medicine ward. Autoimmun Rev. 2020;102729 doi: 10.1016/j.autrev.2020.102729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Platton S., Bowles L., Pasi K.J. Lupus anticoagulant in patients with Covid-19. Reply N Engl J Med. 2020;383:1893–1894. doi: 10.1056/NEJMc2027508. [DOI] [PubMed] [Google Scholar]
- 11.Siguret V., Voicu S., Neuwirth M., Delrue M., Gayat E., Stépanian A., et al. Are antiphospholipid antibodies associated with thrombotic complications in critically ill COVID-19 patients? Thromb Res. 2020;195:74–76. doi: 10.1016/j.thromres.2020.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clinical management of COVID-19 2021. https://www.who.int/publications-detail-redirect/clinical-management-of-covid-19 (accessed February 3, 2021)
- 13.Pengo V., Tripodi A., Reber G., Rand J.H., Ortel T.L., Galli M., et al. Update of the guidelines for lupus anticoagulant detection. Subcommittee on lupus anticoagulant/antiphospholipid antibody of the scientific and standardisation committee of the international society on thrombosis and haemostasis. J Thromb Haemost JTH. 2009;7:1737–1740. doi: 10.1111/j.1538-7836.2009.03555.x. [DOI] [PubMed] [Google Scholar]
- 14.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet Lond Engl. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaeuffer C., Le Hyaric C., Fabacher T., Mootien J., Dervieux B., Ruch Y., et al. Clinical characteristics and risk factors associated with severe COVID-19: prospective analysis of 1,045 hospitalised cases in North-Eastern France, March 2020. Euro Surveill Bull Eur Sur Mal Transm Eur Commun Dis Bull. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.48.2000895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ehrenfeld M., Tincani A., Andreoli L., Cattalini M., Greenbaum A., Kanduc D., et al. Covid-19 and autoimmunity. Autoimmun Rev. 2020;19:102597. doi: 10.1016/j.autrev.2020.102597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shoenfeld Y., Blank M., Cervera R., Font J., Raschi E., Meroni P. Infectious origin of the antiphospholipid syndrome*. Ann Rheum Dis. 2006;65:2–6. doi: 10.1136/ard.2005.045443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kanduc D., Shoenfeld Y. Molecular mimicry between SARS-CoV-2 spike glycoprotein and mammalian proteomes: implications for the vaccine. Immunol Res. 2020:1–4. doi: 10.1007/s12026-020-09152-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Magro C.M., Mulvey J., Kubiak J., Mikhail S., Suster D., Crowson A.N., et al. Severe COVID-19: a multifaceted viral vasculopathy syndrome. Ann Diagn Pathol. 2021;50:151645. doi: 10.1016/j.anndiagpath.2020.151645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Noureldine M.H.A., Nour-Eldine W., Khamashta M.A., Uthman I. Insights into the diagnosis and pathogenesis of the antiphospholipid syndrome. Semin Arthritis Rheum. 2019;48:860–866. doi: 10.1016/j.semarthrit.2018.08.004. [DOI] [PubMed] [Google Scholar]