Abstract
The airborne transmission of SARS-CoV-2, the etiologic agent of the current COVID-19 pandemic, has been hypothesized as one of the primary routes of transmission. Current data suggest a low probability of airborne transmission of the virus in open environments and a higher probability in closed ones, particularly in hospitals or quarantine facilities. However, the potential diffusion of the virus in open environments, especially using particulate matter (PM) as a transport carrier, generated concern in the exposed populations. Several authors found a correlation between the exceeding of the PM10 concentration limits in some Italian cities and the prevalence of Covid-19 cases detected in those areas. This study investigated the potential presence of SARS-COV-2 RNA on a representative series of PM samples collected in the province of Padua in Northeastern Italy during the first wave of COVID pandemic. Forty-four samples of PM2.5 and PM10 were collected between February 24 and March 9, 2020 and analyzed with RT-qPCR for SARS-CoV-2 RNA. The experimental results did not indicate the presence of SARS-CoV-2 RNA in the outdoor PMs, thus confirming the low probability of virus airborne transmission through PM.
Keywords: SARS-CoV-2, COVID-19, Particulate matter, Airborne spread, Transport carrier
Graphical abstract
1. Introduction
Airborne transmission has been recognized as one of the primary routes of conveyance of etiologic agents such as respiratory viruses, including the Severe Acute Respiratory Syndrome (SARS) and the Middle East Respiratory Syndrome coronaviruses (Booth et al., 2005; Yu et al., 2004; Tellier et al., 2019). SARS-CoV-2, the cause of the current COVID-19 pandemic, also falls into this category (Lewis, 2020; National Research Council, 2020; WHO, 2020; Prather et al., 2020). Recently, 239 scientists from 32 countries have written an open letter to the World Health Organization (WHO) emphasizing the importance of preventing its airborne transmission (Morawska and Milton, 2020).
Most credited SARS-CoV-2 transmission pathway is by respiratory droplets as small as 5 μm or larger, generated by sneezes, coughs, or breaths during normal speaking (Lewis, 2020; National Research Council, 2020; Yu et al., 2018; WHO, 2020). The airborne lifetime of the droplets and the range of transmission (e.g. more than 1 m) remains unclear (Anderson et al., 2020; Morawska and Cao, 2020). The mechanisms underlying the airborne transport of SARS-CoV-2 have not been fully elucidated. Also, the influence of the carrier typology (e.g., droplets and aerosols including particulate matter, PM), the role of environmental conditions (e.g., wind speed, temperature, humidity, UV radiations, seasonal allergens such as pollens and spores), and air pollutant concentrations, remain unclear.
A recent study suggests a low probability of airborne virus transmission in open environments and a higher one in closed ones, especially in hospitals or quarantine facilities (Contini and Costabile, 2020). However, the experimental evidence supporting the statement above is weak. It mainly focuses on aerosols and droplets produced by infected patients through coughing, sneezing, speaking, and breathing. The presence of SARS-CoV-2 in the aerosols sampled inside two Hospitals of Wuhan during pandemic peaks was observed by Liu et al. (2020). Santarpia et al. (2020) reported similar findings concerning 13 isolation rooms for COVID-19 patients in the Nebraska University Hospital. Frequent room ventilation and extended permanence in open spaces were also indicated as effective measures for reducing virus diffusion. Md Nor et al. (2020) assessed the presence of SARS-CoV-2 RNA on indoor PM2.5 in hospital wards with infected patients in Kuala Lumpur, Malaysia. In contrast with these findings, Faridi et al. (2020) detected the absence of SARS-CoV-2 in the air sampled in hospital rooms in a range of 2 to 5 m from the beds of symptomatic COVID-19 patients.
The concern about the diffusion of the virus in open environments, particularly using PMs as carriers, is still widespread in the population. Some studies (Cascetta et al., 2021; Coccia, 2020; Bontempi, 2020; Setti et al., 2020a) found a correlation between the exceeding of the PM10 concentration limits in some Italian cities and the number of Covid-19 cases. Despite this limited evidence and bearing in mind that correlation is not causation (Altman and Krzywinski, 2015), the cause-and-effect relationship between PM concentration and COVID-19 prevalence and symptom severity remains controversial (Anand et al., 2021).
In this context, PMs may act as physical carriers of the virus, as possible infection boosting factors (Comunian et al., 2020; Paital and Agrawal, 2020), or as a combination of both. These possibilities require further investigation and proper experimental studies.
Preliminary research on the relationship between PMs and virus transmission was carried out by Setti et al. (2020b). It reported a first preliminary detection of the presence of SARS-COV-2 RNA on the PM from examining 34 PM10 samples collected from an industrial site in the province of Bergamo in Northern Italy. On the other hand, other outdoor air samples were simultaneously collected in Venice in Northeastern Italy and Lecce in Southern Italy in May 2020 and they were tested negative for SARS-CoV-2 RNA (Chirizzi et al., 2021). In these works, the hypothesized mechanism is that virus-laden aerosol could interact with the pre-existing atmospheric particles creating clusters of carriers (Belosi et al., 2021).
Due to the contradictory results previously mentioned and the lack of studies on this topic, this project aims to further investigate the potential presence of SARS-CoV-2 RNA on a representative series of PM collected in the Province of Padua in Northeastern Italy, an area severely affected by the first wave of the COVID-19 pandemic. The methodological issues related to the extraction and detection of viral RNA are also analyzed and discussed.
2. Material and methods
2.1. Experimental design and sampling strategy
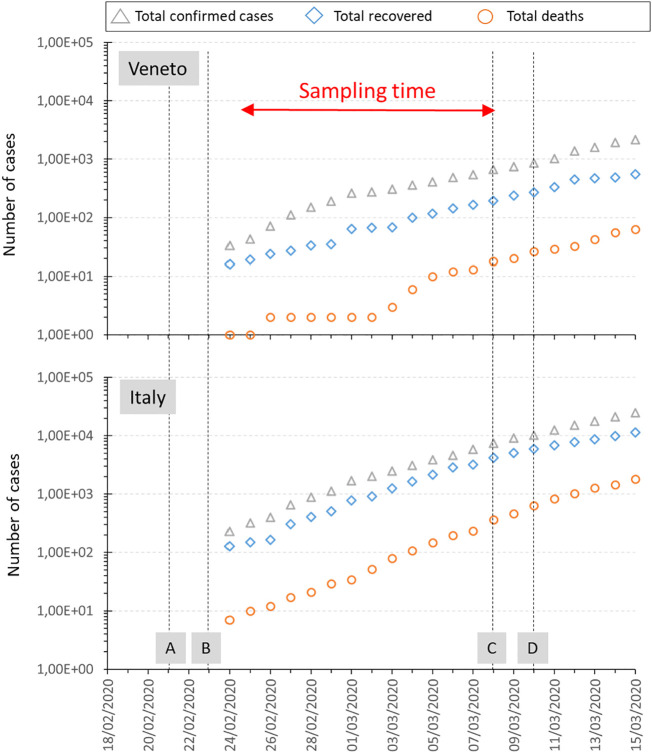
Since the initial spreading of the pandemic wave (February–March 2020), Italy has been recognized as one of the most affected countries. In response to the uncontrolled increase of COVID-19 cases (Fig. 1 ), the Italian government imposed several restrictions (lockdown, compulsory usage of sanitary masks, etc.). Finally, on May 17, 2020, the nationwide lockdown ended, and less strict measures were adopted locally.
Fig. 1.
The development of COVID-19 in Italy and the Veneto Region when the PM samples were collected. The graph was based on Gatto et al. (2020). Time marks (A, B, C, and D) represent the most critical epidemiological events and measures for both mobility and contact restrictions at each time point: A) On February 21, 2020 (day 1), “patient one” was officially confirmed as a case of COVID-19 by the “Ospedale Sacco” in Milan; by the end of the day, other 14 cases in Lombardy and 2 cases in Veneto were confirmed.
B) On February 23, 2020 (day 3), evidence for local transmission from “patient one” increased and new cases of infections was discovered in the municipality of Vo’ (Province of Padua). Ten municipalities in Lombardy and one in the Providence of Padua, identified as hotspots, were maintained under strict lockdown (i.e., labeled as critical red areas), while some preventive restrictions (e.g., temporary closure of schools and universities) were enforced in some regions.
C) On March 8, 2020 (day 17), the whole of Lombardy and 14 Italian provinces (including the Province of Padua) were set under lockdown by the application of the Prime Ministerial Decree (DPCM) of August 03, 2020. Social distancing measures were implemented in the whole country.
D) On March 10, 2020 (day 19), the lockdown area was extended to the whole nation by the application of the Prime Ministerial Decree (DPCM) of March 09, 2020; progressive restrictive limitations on mobility and social distance were also instituted.
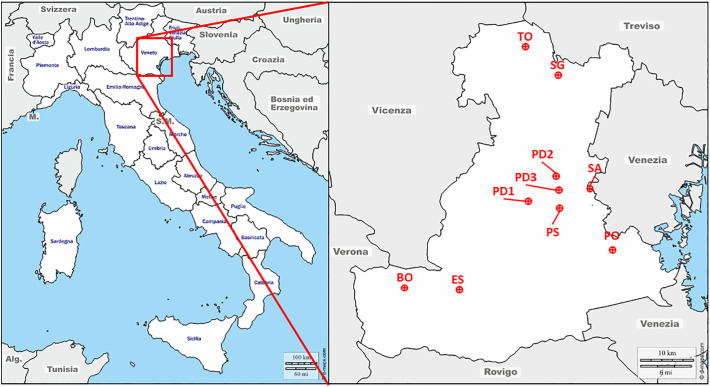
PM sampling was performed in the Province of Padua (Fig. 2 ) with the frequency reported in Table 1 between February 24 and March 9, 2020, i.e. the two weeks before lockdown. The sampling sites are described in Table 2 and classified according to the European Directive 2008/50/EC. During the sampling period (14 days), the meteorological conditions were registered from dedicated stations installed directly in the PM samplers or from the closest stations (Table 1). Considering the collected samples, the average daily temperature was 7.9 °C (Standard Deviation, SD = 1.0); the average daily irradiation was 99.7 W/m2 (SD = 62.6); the average daily wind density was 1.2 m/s (SD = 0.5). Precipitations were observed only for 14 samples.
Fig. 2.
Map of the area investigated in this study. The red dots indicate the locations of PM sampling in Padova Province. BO: Borgo Veneto-Piazza Della Vittoria; ES: Este-Via Stazie Bragadine; PD1: Padova-Mandria; PD2: Padova-Via Carli; PD3: Padova-Internato Ignoto; PS: Ponte San Nicolò-Via Garibaldi; SG: S. Giustina In Colle; SA: Saonara-Via Villanova; and TO: Tombolo (maps from: http://d-maps.com). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Table 1.
Information of the PM samples: date of sampling; sample code (sample code used in the regional monitoring network); sampling site, including the referred codes used in Fig. 2; meteorological conditions; PM typology (PM2.5 or PM10); PM concentration.
| N. | Date | Sample code | Sampling site | Meteorological conditionsa | PM typology | Concentration (μg/m3) |
|---|---|---|---|---|---|---|
| 1 | 24/02/2020 | 734202 | PONTE SAN NICOLO' - VIA GARIBALDI (PS) | P = 0.0 mm; W = 76.1 W/m2; T = 6.6 °C; I = 1.0 m/s | PM 10 | 86 |
| 2 | 24/02/2020 | 734209 | PIOVE DI SACCO - VIA LONGHENA (PO) | P = 0.0 mm; 76.1 W = W/m2; T = 7.1 °C; I = 0.8 m/s | PM 10 | 94 |
| 3 | 24/02/2020 | 734218 | S.GIUSTINA IN COLLE (SG) | P = 0.0 mm; W = 65.0 W/m2; T = 8.4 °C; I = 1.4 m/s | PM 10 | 68 |
| 4 | 24/02/2020 | 735504 | BORGO VENETO - PIAZZA DELLA VITTORIA (BO) | P = 0.0 mm; W = 116.6 W/m2; T = 8.7 °C; I = 1.2 m/s | PM2.5 | 74 |
| 5 | 25/02/2020 | 735430 | S.GIUSTINA IN COLLE (SG) | P = 0.0 mm; W = 55.0 W/m2; T = 9.8 °C; I = 0.9 m/s | PM 10 | 91 |
| 6 | 26/02/2020 | 735506 | BORGO VENETO - PIAZZA DELLA VITTORIA (BO) | P = 0.0 mm; W = 139.5 W/m2; T = 9.7 °C; I = 1.9 m/s | PM2.5 | 24 |
| 7 | 27/02/2020 | 735415 | PIOVE DI SACCO - VIA LONGHENA (PO) | P = 10 mm; W = 130 W/m2; T = 6.8 °C; I = 1.1 m/s | PM 10 | 21 |
| 8 | 27/02/2020 | 735432 | S.GIUSTINA IN COLLE (SG) | P = 0.0 mm; W = 132.3 W/m2; T = 7.5 °C; I = 1.6 m/s | PM 10 | 24 |
| 9 | 27/02/2020 | 735700 | PONTE SAN NICOLO’ - VIA GARIBALDI (PS) | P = 0.0 mm; W = 130.0 W/m2; T = 7.3 °C; I = 1.2 m/s | PM 10 | 17 |
| 10 | 27/02/2020 | 735507 | BORGO VENETO - PIAZZA DELLA VITTORIA (BO) | P = 0.0 mm; W = 143.1 W/m2; T = 7.3 °C; I = 1.0 m/s | PM2.5 | 18 |
| 11 | 28/02/2020 | 735508 | BORGO VENETO - PIAZZA DELLA VITTORIA (BO) | P = 0.0 mm; W = 185.0 W/m2; T = 9.4 °C; I = 1.6 m/s | PM2.5 | 13 |
| 12 | 29/02/2020 | 735434 | S.GIUSTINA IN COLLE (SG) | P = 0.0 mm; W = 87.8 W/m2; T = 7.3 °C; I = 1.0 m/s | PM 10 | 37 |
| 13 | 29/02/2020 | 735509 | BORGO VENETO - PIAZZA DELLA VITTORIA (BO) | P = 0.0 mm; W = 84.3 W/m2; T = 8.2 °C; I = 1.3 m/s | PM2.5 | 29 |
| 14 | 01/03/2020 | 735411 | SAONARA - VIA VILLANOVA (SA) | P = 15 mm; W = 17.3 W/m2; T = 7.6 °C; I = 1.4 m/s | PM 10 | 25 |
| 15 | 01/03/2020 | 735418 | PIOVE DI SACCO - VIA LONGHENA (PO) | P = 14 mm; W = 6.0 W/m2; T = 6.4 °C; I = 1.3 m/s | PM 10 | 29 |
| 16 | 01/03/2020 | 735445 | TOMBOLO (TO) | P = 15.0 mm; W = 17.3 W/m2; T = 7.6 °C; I = 1.4 m/s | PM 10 | 20 |
| 17 | 01/03/2020 | 735703 | PONTE SAN NICOLO' - VIA GARIBALDI (PS) | P = 2.0 mm; W = 6.0 W/m2; T = 6.7 °C; I = 1.1 m/s | PM 10 | 24 |
| 18 | 01/03/2020 | 735446 | TOMBOLO (TO) | P = 15.0 mm; W = 17.3 W/m2; T = 7.6 °C; I = 1.4 m/s | PM2.5 | 18 |
| 19 | 01/03/2020 | 735467 | PADOVA - MANDRIA (PD1) | P = 2.0 mm; W = 6.0 W/m2; T = 6.7 °C; I = 1.1 m/s | PM2.5 | 23 |
| 20 | 01/03/2020 | 735472 | PADOVA - VIA CARLI (PD2) | P = 2.0 mm; W = 6.0 W/m2; T = 6.7 °C; I = 1.0 m/s | PM2.5 | 20 |
| 21 | 01/03/2020 | 735477 | PADOVA - INTERNATO IGNOTO (PD3) | P = 2.0 mm; W = 6.0 W/m2; T = 6.7 °C; I = 1.2 m/s | PM2.5 | 16 |
| 22 | 01/03/2020 | 735510 | BORGO VENETO - PIAZZA DELLA VITTORIA (BO) | P = 1.0 mm; W = 19.3 W/m2; T = 7.3 °C; I = 2.0 m/s | PM2.5 | 19 |
| 23 | 02/03/2020 | 735436 | S.GIUSTINA IN COLLE (SG) | P = 22.0 mm; W = 20.9 W/m2; T = 6.8 °C; I = 3.3 m/s | PM 10 | 28 |
| 24 | 02/03/2020 | 736758 | BORGO VENETO - PIAZZA DELLA VITTORIA (BO) | P = 0.0 mm; W = 23.7 W/m2; T = 6.7 °C; I = 2.3 m/s | PM2.5 | 27 |
| 25 | 03/03/2020 | 736175 | S.GIUSTINA IN COLLE (SG) | P = 4.0 mm; W = 62.4 W/m2; T = 9.0 °C; I = 1.6 m/s | PM 10 | 16 |
| 26 | 03/03/2020 | 736759 | BORGO VENETO - PIAZZA DELLA VITTORIA (BO) | P = 0.0 mm; W = 50.1 W/m2; T = 8.1 °C; I = 1.1 m/s | PM2.5 | 13 |
| 27 | 04/03/2020 | 735706 | PONTE SAN NICOLO' - VIA GARIBALDI (PS) | P = 0.0 mm; W = 127.4 W/m2; T = 7.1 °C; I = 0.7 m/s | PM 10 | 17 |
| 28 | 04/03/2020 | 736183 | PIOVE DI SACCO - VIA LONGHENA (PO) | P = 0.0 mm; W = 127.4 W/m2; T = 7.9 °C; I = 1.1 m/s | PM 10 | 18 |
| 29 | 04/03/2020 | 736760 | BORGO VENETO - PIAZZA DELLA VITTORIA (BO) | P = 0.0 mm; W = 111.3 W/m2; T = 8.4 °C; I = 0.9 m/s | PM2.5 | 12 |
| 30 | 05/03/2020 | 736177 | S.GIUSTINA IN COLLE (SG) | P = 5.0 mm; W = 84.2 W/m2; T = 6.9 °C; I = 1.1 m/s | PM 10 | 27 |
| 31 | 05/03/2020 | 736761 | BORGO VENETO - PIAZZA DELLA VITTORIA (BO) | P = 0.0 mm; W = 101.8 W/m2; T = 6.9 °C; I = 1.3 m/s | PM2.5 | 21 |
| 32 | 06/03/2020 | 736178 | S.GIUSTINA IN COLLE (SG) | P = 13.0 mm; W = 142.7 W/m2; T = 8.5 °C; I = 2.0 m/s | PM 10 | 14 |
| 33 | 06/03/2020 | 736762 | BORGO VENETO - PIAZZA DELLA VITTORIA (BO) | P = 0.0 mm; W = 174.8 W/m2; T = 7.8 °C; I = 2.2 m/s | PM2.5 | 18 |
| 34 | 07/03/2020 | 736186 | PIOVE DI SACCO - VIA LONGHENA (PO) | P = 0.0 mm; W = 133.0 W/m2; T = 9.5 °C; I = 1.1 m/s | PM10 | 23 |
| 35 | 07/03/2020 | 736763 | BORGO VENETO - PIAZZA DELLA VITTORIA (BO) | P = 0.0 mm; W = 162.5 W/m2; T = 10.2 °C; I = 1.3 m/s | PM2.5 | 23 |
| 36 | 08/03/2020 | 736083 | ESTE - VIA STAZIE BRAGADINE (ES) | P = 0.0 mm; W = 189.3 W/m2; T = 9.7 °C; I = 0.5 m/s | PM 10 | 13 |
| 37 | 08/03/2020 | 736108 | PADOVA - MANDRIA (PD1) | P = 0.0 mm; W = 176.0 W/m2; T = 8.4 °C; I = 0.8 m/s | PM 10 | 13 |
| 38 | 08/03/2020 | 736118 | PADOVA - INTERNATO IGNOTO (PD3) | P = 0.0 mm; W = 176.0 W/m2; T = 8.4 °C; I = 0.8 m/s | PM 10 | 14 |
| 39 | 08/03/2020 | 736127 | PADOVA - VIA CARLI (PD2) | P = 0.0 mm; W = 176.0 W/m2; T = 8.4 °C; I = 0.8 m/s | PM 10 | 13 |
| 40 | 08/03/2020 | 736180 | S.GIUSTINA IN COLLE (SG) | P = 0.0 mm; W = 170.9 W/m2; T = 9.0 °C; I = 1.2 m/s | PM 10 | 15 |
| 41 | 08/03/2020 | 736109 | PADOVA - MANDRIA (PD1) | P = 0.0 mm; W = 176.0 W/m2; T = 8.4 °C; I = 0.8 m/s | PM2.5 | 11 |
| 42 | 08/03/2020 | 736119 | PADOVA - INTERNATO IGNOTO (PD3) | P = 0.0 mm; W = 176.0 W/m2; T = 8.4 °C; I = 0.8 m/s | PM2.5 | 12 |
| 43 | 08/03/2020 | 736128 | PADOVA - VIA CARLI (PD2) | P = 0.0 mm; W = 176.0 W/m2; T = 8.4 °C; I = 0.8 m/s | PM2.5 | 10 |
| 44 | 09/03/2020 | 736181 | S.GIUSTINA IN COLLE (SG) | P = 0.0 mm; W = 125.0 W/m2; T = 8.0 °C; I = 0.8 m/s | PM 10 | 39 |
Meteorological conditions were registered from dedicated stations installed directly in the PM samplers or from the closest stations. The data are average daily measures of precipitation (P) in mm, solar irradiation (W) in W/m2, temperature (T) in °C and wind intensity (I) in m/s.
Table 2.
Description of the sample sites.
| Code | Place | Geographical coordinatesa | Type of station | Population density per km2b |
|---|---|---|---|---|
| BO | Borgo Veneto - Piazza Della Vittoria | X: 1698916; Y:5011095 | Urban backround | 178 |
| ES | Este - Via Stazie Bragadine | X: 1709338; Y:5011647 | Industrial (suburban) | 505 |
| PD1 | Padova - Mandria | X: 1722487; Y: 5028105 | Urban backround | 2216 |
| PD2 | Padova - Via Carli | X: 1727511; Y: 5033159 | Industrial (Urban) | 2216 |
| PD3 | Padova - Internato Ignoto | X: 1726463; Y: 5053899 | Industrial (Urban) | 2216 |
| PO | Piove Di Sacco - Via Longhena | X: 1738075; Y: 5019476 | Urban backround | 534 |
| PS | Ponte San Nicolò - Via Garibaldi | X: 1728628; Y: 5027768 | Urban backround | 979 |
| SA | Saonara - Via Villanova | X: 1732825; Y: 5030147 | Urban backround | 742 |
| SG | S. Giustina In Colle | X: 1726463; Y: 5053899 | Rural background | 397 |
| TO | Tombolo | X: 1719591; Y: 5057855 | Industrial (suburban) | 747 |
Reference coordinates: Gauss Boaga West Corner.
Density of the Municipality as reported in ISTAT (2021).
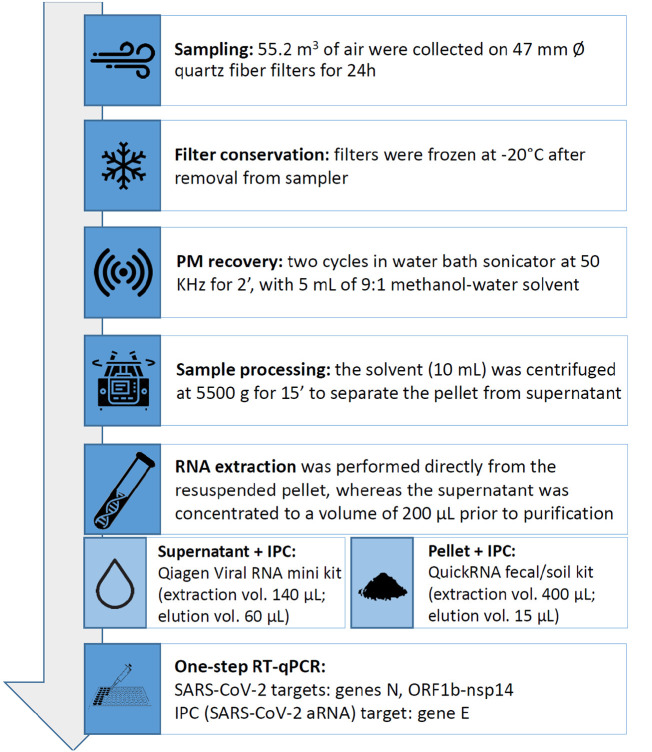
PM (PM10, PM2.5) samples were collected on quartz fiber filters (47 mm Ø, Whatman QMA, GE Healthcare, USA) using the low-volume sampling setting according to the European standard EN 12341:2014 at a nominal flow of 2.3 m3 h−1 for 24 h, starting at midnight. The filters have a retention efficiency higher than 99.95% for particles with an aerodynamic diameter of 0.3 μm. Before reaching the laboratory, the samples remained at the sampling station from 3 to 4 days in containers kept in the dark and at 20 °C. Then, the filters were conditioned for gravimetric analysis for 48 h in a chamber with constant temperature 20 ± 1 °C and relative humidity 50 ± 5% (Emerson S05KA Emerson Network Power, Italy). The filters were then weighed twice with an analytical balance with a sensitivity of 0.0001 mg (Sartorius series Genius, mod. SE2, Germany). The final weight was calculated as the average of the two measurements. Finally, the samples were frozen in clean Petri slides at −20 °C for the subsequent analysis. Laboratory testing was performed by the BSL-2 Research Laboratory of Hygiene and Applied Microbiology of the Padua University (Italy). The laboratory implements updated OECD Good Laboratory Practices and adopted fundamental precautions for the correct handling of RNA samples.
2.2. PM recovery from filters and preliminary sample processing
Recovery of PM from quartz fiber filters reprised the procedure described by Roper et al. (2015). Filters were placed with the PM face down in 100 mL glass beakers containing 5 mL of a 9:1 methanol/sterile distilled water solvent. Beakers were then sonicated for 2 min in a water bath sonicator at 50 KHz (Labsonic LBS1, Falc Instruments, Italy). Reported PM removal efficiency following sonication is of 98.0 ± 1.4%. The solvent was then collected in a 15 mL Falcon® conical centrifuge tube and the filter was sonicated again, repeating the described step. After the second round of sonication, both filter and beaker were rinsed with 5 mL of clean solvent, that ultimately was also collected in the same 15 mL tube. Falcon tubes were then centrifuged at 5500g for 15 min (refrigerated centrifuge Allegra 21R, Beckman Coulter, California, USA) to separate solids from the liquid solvent. Subsequently, the two phases were processed in parallel to detect viral particles both complexed to the pellet PM or still suspended in the supernatant. As a matter of fact, if taking into account the hydrophilic nature of PM (Jiang et al., 2019), the liquid phases may in principle contain smaller and disaggregated PM particles and possibly non-complexed viruses. The pellet directly underwent RNA extraction, whereas the supernatant had to be concentrated prior to extraction. The supernatant was carefully removed from the tube and transferred into a concentration device (Amicon Ultra-15,100 KDa centrifugal filter, Merck-Millipore, Germany). It was then centrifuged at 2000g for 4 min (refrigerated swinging-bucket centrifuge PK131R, ALC, Italy), yielding a final volume of about 200 μL. Retention efficiency for particles with molecular weight similar to the SARS-CoV-2 virion is of >90%, as per manufacturer's specifications.
2.3. RNA extraction and molecular detection
RNA extraction from concentrated supernatant was carried out on a volume of 140 μL with a commercial kit (QIAamp viral RNA mini kit, Qiagen, Germany), following the manufacturer's instructions. RNA extraction from the pellet was performed with the same total RNA extraction kit (QuickRNA™ Fecal/Soil Microprep Kit R2040, Zymo Research, USA) used by Setti et al. (2020b). RNA extraction efficiency for both kits, in terms of RNA yield, is reported to be >90% by the respective manufacturers. The pellet was resuspended in 600 μL of the kit RNA lysis buffer. It was then transferred into the provided 2 mL bashing beads tube and thoroughly vortexed for 60 s. Apart from these minor modifications, extraction proceeded according to the manufacturer's protocol. An Internal Positive Control (IPC), i.e. 3 μL (9 × 104 gc/μL) of synthetic SARS-CoV-2 armored (i.e. encapsidated) RNA (2019-nCoV E gene aRNA kit cod. 001B-03886, EVAg-Protisvalor, France), was added to each sample before extraction as process indicator. IPC was also used to assess the presence of inhibitors of the quantitative reverse-transcription polymerase chain reaction (RT-qPCR).
Two WHO-shared One-step RT-qPCR assays were chosen for the molecular detection of SARS-Cov-2 RNA, targeting genes N (screening) and ORF1b-nsp14 (confirm) (Chu et al., 2020). Primers and dual-labeled probes were provided from Thermo Fisher (USA). Synthetic dsDNA fragments were used as positive controls and were also purchased by GeneArt/Thermo Fisher. In each PCR run, 2 replicates were loaded for each extract. Moreover, 2 positive and 2 negative controls were included. Amplification of the IPC was carried out with the dedicated assay (i.e. primers and dual-labeled probe), also provided with the aRNA kit, following the manufacturer's instructions. PCR runs were carried out on a StepOne-Plus™ Real-Time PCR System (Applied Biosystems, USA). Positivity was attributed only to reactions with cycle threshold (Ct) <40. The limit of detection (LOD) of the implemented assays was determined using DNA plasmids as positive standards and found to be below 10 genome copies (gc) per reaction (i.e. sample volume/well = 4 μL), that is. 2.5 gc/μL. Nevertheless, some authors suggested a possible differential performance between the N and the Orf1b assay, with N showing a 10× sensitivity in both clinical and environmental samples (Chu et al., 2020; Baldovin et al., 2020). The above described processes are graphically represented in the flow chart of Fig. 3 .
Fig. 3.
Flow chart of the process of RNA extraction and molecular detection.
3. Results and discussion
RNA extraction and molecular detection of SARS-CoV-2 nucleic acid was carried out on 88 individual samples (i.e. 44 paired pellets and supernatants). No successful amplification of the N gene nor the ORF1b-nsp14 was detected in any of the tested samples. However, IPC amplification was achieved for all samples, thus excluding the presence of PCR inhibitors. Moreover, an average delta of 3 cycles was observed for the Ct of IPC in supernatant vs pellet extracts, suggesting a better extraction power for the commercial kit used for the liquid phase. As reference, in a 100% efficient PCR reaction, a ten-fold dilution of the target gene should fall 3.3 cycles apart.
The experimental results suggest that SARS-CoV-2 RNA is not present, or either that the viral load falls below the detectability threshold (1.2 gc/m3), in any of the 44 samples of PM10 and PM2.5 collected from February the 24th and March the 3rd 2020 in Padua province during the first pandemic wave. The average detectability threshold was calculated taking into account the LOD of molecular assays (i.e. 2.5 gc/μL), the recovery efficiency of each analytical procedure (i.e. PM removal from filters 98%, concentration 90% and RNA extraction 90%) and total air volume sampled over 24 h (55.2 m3). A higher threshold (i.e. no detectable concentration < 3 gc/m3) has been described by Belosi et al. (2021), whereas Chirizzi et al. (2021) reported a lower one (0.8 gc/m3), but presumably the latter can be explained by a stated higher LOD for their molecular assay (i.e. 10 gc/μL = 50 gc/reaction). Setti et al. (2020b) did not report a detectability threshold, but an inferred value (1.5 gc/m3) is proposed in Table 3 .
Table 3.
Comparison from the current study and the ones of Chirizzi et al. (2021) and Setti et al. (2020b).
| Operative conditions | Current study | Chirizzi et al. (2021) | Setti et al. (2020b) |
|---|---|---|---|
| Sampling size | 44 samples from 10 sites | 60 samples from 2 sites | 34 samples from 2 sites |
| Positive samples | 0 samples | 0 samples | 20 samples |
| Period of sampling | From February 24th to March 9th 2020 (14 days) | From 13th to 27th of May 2020 (14 days) | From February 21st to March 13th 2020 (21 days) |
| Location of sampling | Padua province (NE Italy) | two different Italian regions: Veneto (NE Italy) and Apulia (in the SE Italy) | Bergamo Province (North Italy) |
| Typology of sampling point | Different: urban and rural background sites; traffic and industrial sites | Urban background site | Industrial site |
| Filter used | Quartz fiber filters (47 mm Ø, Whatman QMA, GE Healthcare, USA) | Quartz fiber filters | Quartz fiber filters |
| Sampler typology | Two different samplers were used: Low-volume aerosol sampler (Skypost PM-TCR Tecora) equipped with a sequential sampler (Charlie) that operates at flow rate of 38.3 L min−1 for 24 h; it was used for the following sites: BO, TO, ES, PO, SA. Low volume aerosol sampler (SWAM 5a Dual Channel Monitor-FAI Instruments) that operates at flow rate of 38,3 L min−1 for 24 h; it was used for the following sites: PD1, PD2, PD3, SG. |
Two different samplers were used per site: In Veneto: low volume aerosol sampler (Skypost PM-TCR Tecora) equipped with a sequential sampler (Charlie) that operates at flow rate of 38.3 L min−1 for 48 h; model 110 MOUDI cascade impactor with an average flow of 30 L min−1 for about 6 d. In Apulia: low volume aerosol sampler (SWAM 5a Dual Channel Monitor-FAI Instruments) that operates at flow rate of 38 L min−1 for 48 h; a rotating model 120 MOUDI-II™ cascade impactor, operating at 30 L min−1 for about 6 d. |
Low-volume gravimetric air sampler (38.3 L/min for 24 h) |
| Average air collected per sample | 55.2 m3 | 110 m3 or 250 m3 | 55.2 m3 |
| Particulate matter retention | >99.95% for particles with an aerodynamic diameter of 0.3 μm | Not reported | 99.9% |
| Sampling procedure | EN 12341:2014 | Not reported | EN 12341:2014 |
| Meteorological conditions | Temperature; irradiation; precipitation; wind intensity | Temperature; relative humidity; precipitation | Temperature; relative humidity; irradiance |
| Solid phase extraction | Quick-RNA™ Fecal/Soil Microbe Microprep Kit, Zymo Research, USA |
Total RNA Purification Kit, Norgen Biotek Corp. | Quick-RNA™ Fecal/Soil Microbe Microprep Kit, Zymo Research, USA |
| Internal Positive Control | SARS-CoV-2 (E gene) aRNA, EVA, Marseille, France. | Not reported | Not reported |
| RT-PCR reference protocol | Chu et al., 2020 | Corman et al., 2020 | Corman et al., 2020 |
| RT-PCR oligos | Custom oligos, Thermofisher | Diatheva commercial kit | Not reported |
| RT-PCR molecular targets | Genes N and Orf1b-14nsp | Genes RdRp and E | Genes E, RdRP and N |
| Limit of Detection | 2.5 gc/μL | 10 gc/μL | Not reported |
| Detection threshold | 1.2 gc m−3 | <0.8 gc m−3 | 1.5 gc m−3a |
Detection threshold for the method of Setti et al. (2020b) was calculated assuming a LOD for their molecular assay of 2 gc/μL (Corman et al., 2020) and that the RNA extraction protocol strictly followed the kit manufacturer's instructions, with a 90% purification efficiency.
These results agree with Chirizzi et al. (2021), who reported the absence of SARS-CoV-2 RNA in any of the 60 collected air samples. On the other hand, these results contradict the finding by Setti et al. (2020b) that reported 20 positive results for at least one of the three SARS-CoV-2 marker genes in 34 samples even if, due to the lack of enough PM materials, the simultaneous positivity for all the 3 markers was not demonstrated. A comparative analysis of the current and cited investigations is reported in Table 3.
Overall, based on the scientific evidence of this and of other studies, we are reasonably convinced of the low probability of detecting SARS-CoV-2 RNA in airborne samples. This depends on the occurrence of various events such as: a) the probability that the virus-laden aerosol emitted outside may impact pre-existing particulates to form a cluster or a complex; b) the probability that the RNA structure of the virus may remain intact (nucleic acid persistence) during and after the formation of the cluster, the sampling procedure, the transport and storage phase until the molecular analysis is performed.
Regarding the first aspect, Belosi et al. (2021) estimated a very low average outdoor concentration of SARS-CoV-2 RNA, at less than 1 RNA gc/m3, in the uncrowded public areas in the Lombardy Region, even in the worst-case scenario with an infection rate of up to 25% of the local population. These results are comparable with those found experimentally in an outdoor residential area in Wuhan, China, during the COVID pandemic (Liu et al., 2020).
Regarding the second aspect, the considerable atmospheric residence time (days to weeks) of PM before sampling dominates the nucleic acid persistence because, in this period, the cluster of particulate and virus could be primarily influenced by meteorological parameters, such as UV radiation, temperature, and oxidizing agents like NOX and ozone. This scenario is particularly relevant in the Province of Padua, which is characterized by low wind speed accompanied by long periods of stable conditions with shallow mixing layers, especially during the winter period. Therefore, it is also unlikely that the virus will stay viable in these conditions.
Moreover, considering other parameters, such as SARS-CoV-2's viability, infectivity, and infective dose, which remain unclear (Barakat et al., 2020), it can be concluded that the outdoor airborne transmission is much less probable than the indoor route.
In conclusion, based on the experimental results and the above-reported observations, we believe that monitoring for the presence of SARS-CoV-2 RNA in outdoor particulates is not suitable for an efficient early indicator of SARS-Cov-2 diffusion or/and an early indicator of a recurrence of the pandemic.
CRediT authorship contribution statement
Pivato A. Conceptualization, Methodology, Data Curation, Writing - Review & Editing
Baldovin T. Conceptualization, Methodology, Laboratory investigation, Formal analysis, Writing - Review & Editing
Formenton G. Methodology, Resources, Field investigation, Data Curation, Writing - Review & Editing
Amoruso I. Methodology, Laboratory investigation, Data Curation, Writing - Review & Editing
Di Maria F. Methodology, Writing - Review & Editing
Bonato T. Methodology, Resources, Writing - Review & Editing
Vanin S. Data Curation, Visualization, Writing - Review & Editing
Marion A. Visualization, Formal analysis, Writing - Review & Editing
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We express our kind appreciation to A. Corazzina, E. Ravazzolo and M. Riondato, support staff of the LIMA Laboratory (University of Padua, DCTV) for their precious technical assistance.
Editor: Damia Barcelo
References
- Altman N., Krzywinski M. Points of significance: association, correlation and causation. Nat. Methods. 2015;12:899–900. doi: 10.1038/nmeth.3587. [DOI] [PubMed] [Google Scholar]
- Anand U., Adelodun B., Pivato A., Suresh S., Indari O., Jakhmola S., Chandra H., Kumar P., Tripathi V., Di Maria F. A review of the presence of SARS-CoV-2 RNA in wastewater and airborne particulates and its use for virus spreading surveillance. Environ. Res. 2021;196 doi: 10.1016/j.envres.2021.110929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson E.L., Turnham P., Griffin J.R., Clarke C.C. Consideration of the aerosol transmission for COVID-19 and public health. Risk Anal. 2020;40(5):902–907. doi: 10.1111/risa.13500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldovin T., Amoruso I., Fonzo M., Buja A., Baldo V., Cocchio S., Bertoncello C. SARS-CoV-2 RNA detection and persistence in wastewater samples: an experimental network for COVID-19 environmental surveillance in Padua, Veneto Region (NE Italy) Sci. Total Environ. 2020 doi: 10.1016/j.scitotenv.2020.143329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barakat T., Muylkens B., Su B.-L. 2020. Is Particulate Matter of Air Pollution a Vector of Covid-19 Pandemic? [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belosi F., Conte M., Gianelle V., Santachiara G., Contini D. On the concentration of SARS-CoV-2 in outdoor air and the interaction with pre-existing atmospheric particles. Environ. Res. 2021;193 doi: 10.1016/j.envres.2020.110603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bontempi E. First data analysis about possible COVID-19 virus airborne diffusion due to air particulate matter (PM): the case of Lombardy (Italy) Environ. Res. 2020;186 doi: 10.1016/j.envres.2020.109639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth T.F., Kournikakis B., Bastien N., Ho J., Kobasa D., Stadnyk L., Li Y., Spence M., Paton S., Henry B., Mederski B., White D., Low D.E., McGeer A., Simor A., Vearncombe M., Downey J., Jamieson F.B., Tang P., Plummer F. Detection of airborne severe acute respiratory syndrome (SARS) coronavirus and environmental contamination in SARS outbreak units. J. Infect. Dis. 2005;191:1472–1477. doi: 10.1086/429634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascetta E., Henke I., Di Francesco L. The effects of air pollution, sea exposure and altitude on covid-19 hospitalization rates in Italy. Int. J. Environ. Res. Public Health. 2021;18:1–11. doi: 10.3390/ijerph18020452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirizzi D., Conte M., Feltracco M., Dinoi A., Gregoris E., Barbaro E., La Bella G., Ciccarese G., La Salandra G., Gambaro A., Contini D. SARS-CoV-2 concentrations and virus-laden aerosol size distributions in outdoor air in north and south of Italy. Environ. Int. 2021;146 doi: 10.1016/j.envint.2020.106255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu D.K.W., Pan Y., Cheng S.M.S., Hui K.P.Y., Krishnan P., Liu Y., Ng D.Y.M., Wan C.K.C., Yang P., Wang Q., Peiris M., Poon L.L.M. Molecular diagnosis of a novel coronavirus (2019-nCoV) causing an outbreak of pneumonia. Clin. Chem. 2020;555:549–555. doi: 10.1093/clinchem/hvaa029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccia M. Factors determining the diffusion of COVID-19 and suggested strategy to prevent future accelerated viral infectivity similar to COVID. Sci. Total Environ. 2020;729 doi: 10.1016/j.scitotenv.2020.138474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comunian S., Dongo D., Milani C., Palestini P. Air pollution and Covid-19: the role of particulate matter in the spread and increase of Covid-19’s morbidity and mortality. Int. J. Environ. Res. Public Health. 2020;17(12):4487. doi: 10.3390/ijerph17124487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contini D., Costabile F. Does air pollution influence COVID-19 outbreaks? Atmosphere. 2020;1:19–23. doi: 10.3390/atmos11040377. [DOI] [Google Scholar]
- Corman V.M., Landt O., Kaiser M., Molenkamp R., Meijer A., Chu D.K.W., Bleicker T., Brünink S., Schneider J., Schmidt M.L., Mulders D.G.J.C., Haagmans B.L., Van Der Veer B., Van Den Brink S., Wijsman L., Goderski G., Romette J.L., Ellis J., Zambon M., Peiris M., Goossens H., Reusken C., Koopmans M.P.G., Drosten C. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Eurosurveillance. 2020;25:1–8. doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faridi S., Niazi S., Sadeghi K., Naddafi K., Yavarian J., Shamsipour M., Jandaghi N.Z.S., Sadeghiniiat K., Nabizadeh R., Yunesian M., Momeniha F., Mokamel A., Hassanvand M.S., MokhtariAzad T. A field indoor air measurement of SARSCoV-2 in the patient rooms of the largest hospital in Iran. Sci. Total Environ. 2020;725 doi: 10.1016/j.scitotenv.2020.138401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatto M., Bertuzzo E., Mari L., Miccoli S., Carraro L., Casagrandi R., Rinaldo A. Spread and dynamics of the COVID-19 epidemic in Italy: effects of emergency containment measures. Proc. Natl. Acad. Sci. 2020 doi: 10.1073/pnas.2004978117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISTAT ASC Atlante Statistico dei Comuni. 2021. http://asc.istat.it/ASC/ URL.
- Jiang X., Xu F., Qiu X., Shi X., Pardo M., Shang Y., Wang J., Rudich Y., Zhu T. Hydrophobic organic components of ambient fine particulate matter (PM2.5) associated with inflammatory cellular response. Environ. Sci. Technol. 2019;53(17):10479–10486. doi: 10.1021/acs.est.9b02902. [DOI] [PubMed] [Google Scholar]
- Lewis D. Is the coronavirus airborne? Experts can’t agree. Nature. 2020;580:175. doi: 10.1038/d41586-020-00974-w. [DOI] [PubMed] [Google Scholar]
- Liu Yuan, Ning Z., Chen Y., Guo M., Liu Yingle, Gali N.K., Sun L., Duan Y., Cai J., Westerdahl D., Env D., Liu X., Ho K.-F., Kan H., Fu Q., Lan K. Aerodynamic characteristics and RNA concentration of SARS-CoV-2 aerosol in Wuhan hospitals during COVID-19 outbreak. bioRxiv. 2020 doi: 10.1101/2020.03.08.982637. [DOI] [Google Scholar]
- Md Nor N.S., Wai Y.C., Ibrahim N., Rashid Z.Z., Mustafa N., Hamid H.H.A., Latif M.T., Er S.P., Yik L.C., Alhasa K.M., Hashim J.H., nadzir mohd shahrul mohd. Particulate matter (PM2.5) as a potential SARS-CoV-2 carrier. Sci. Rep. 2020;1–6 doi: 10.21203/rs.3.rs-33354/v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morawska L., Cao J. Airborne transmission of SARS-CoV-2: the world should face the reality. Environ. Int. 2020;139 doi: 10.1016/j.envint.2020.105730. (2020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morawska L., Milton D.K. It is time to address airborne transmission of COVID-19. Clin. Infect. Dis. 2020:1–23. doi: 10.1093/cid/ciaa939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council . The National Academies Press. National Academies Press; Washington, D.C: 2020. Rapid Expert Consultation on the Possibility of Bioaerosol Spread of SARS-CoV-2 for the COVID-19 Pandemic (April 1, 2020) [DOI] [Google Scholar]
- Paital B., Agrawal P.K. Air pollution by NO2 and PM2.5 explains COVID-19 infection severity by overexpression of angiotensin-converting enzyme 2 in respiratory cells: a review. Environ. Chem. Lett. 2020;19:25–42. doi: 10.1007/s10311-020-01091-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prather K.A., Prather Kimberly A., Wang C.C., Schooley R.T. Reducing transmission of SARS-CoV-2. 2020;368(6498):1422–1424. doi: 10.1126/science.abc6197. [DOI] [PubMed] [Google Scholar]
- Roper C., Chubb L.G., Cambal L., Tunno B., Clougherty J.E., Mischler S.E. Characterization of ambient and extracted PM2.5 collected on filters for toxicology applications. Inhal. Toxicol. 2015;27(13):673–681. doi: 10.3109/08958378.2015.1092185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarpia J.L., Rivera D.N., Herrera V., Morwitzer M.J., Creager H., Santarpia G.W., Crown K.K., Brett-Major D., Schnaubelt E., Broadhurst M.J., Lawler J.V., Reid S.P., Lowe J.J. 2020. Transmission Potential of SARS-CoV-2 in Viral Shedding Observed at the University of Nebraska Medical Center. medRxiv 2020.03.23.20039446. [DOI] [Google Scholar]
- Setti L., Passarini F., De Gennaro G., Di Gilio A., Palmisani J., Buono P., Fornari G., Perrone M., Piazzalunga A., Barbieri P., Rizzo E., Miani A. SIMA; 2020. Evaluation of the Potential Relationship Between Particulate Matter (PM) Pollution and COVID-19 Infection Spread in Italy. [Google Scholar]
- Setti L., Passarini F., Gennaro G. De, Barbieri P., Perrone M.G., Borelli M., Palmisani J., Gilio A. Di, Torboli V., Pallavicini A., Piscitelli P., Miani A. SARS-Cov-2 RNA found on particulate matter of Bergamo in northern Italy: first preliminary evidence. Environ. Res. 2020;188:109754. doi: 10.1016/j.envres.2020.109754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tellier R., Li Y., Cowling B., Tang J. Recognition of aerosol transmission of infectious agents: a commentary. BMC Infect. Dis. 2019;19:101. doi: 10.1186/s12879-019-3707-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO Modes of transmission of virus causing COVID-19: implications for IPC precaution recommendations. Sci. Br. 2020:1–3. doi: 10.1056/NEJMoa2001316.5. [DOI] [Google Scholar]
- Yu I.T.S., Li Y., Wong T.W., Tam W., Chan A.T., Lee J.H.W., Leung D.Y.C., Ho T. Evidence of airborne transmission of the severe acute respiratory syndrome virus. N. Engl. J. Med. 2004;350:1731–1739. doi: 10.1056/NEJMoa032867. [DOI] [PubMed] [Google Scholar]
- Yu H., Afshar-Mohajer N., Theodore A.D., Lednicky J.A., Fan Z.H., Wu C.Y. An efficient virus aerosol sampler enabled by adiabatic expansion. J. Aerosol Sci. 2018;117:74–84. doi: 10.1016/j.jaerosci.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]