Abstract
BACKGROUND AND PURPOSE:
MMD has been shown to result in impairment of executive functioning in adults. The purpose of this study was to correlate presurgical neuropsychological assessments with the severity of primary MMD as measured by CBF and CVR and with secondary damage from MMD as estimated by cortical stroke and WMD.
MATERIALS AND METHODS:
A retrospective analysis of 31 adult patients with MMD was performed. Xe-CT was used to obtain CBF and CVR, and MRI was reviewed to grade cortical stroke and WMD. Two tests of executive functioning (FAS and TMT-B) were correlated with imaging findings. A multiple regression analysis was performed.
RESULTS:
There was a significant overall positive relationship between mean CBF and FAS (P = .038) and TMT-B scores (P = .014). A significant negative relationship was present between the WMD score and the FAS (P = .009) and TMT-B scores (P = .015). Per-region analysis demonstrated that FAS and TMT-B scores were significantly decreased by the presence of a posterior stroke (P < .0001 and P = .001) or WMD (P = .006 and P = .004). All patients with posterior parieto-occipital WMD or stroke also had secondary disease in the anterior regions.
CONCLUSIONS:
Impaired executive functioning in adults with MMD is most strongly associated secondary damage in the form of WMD or cortical stroke. The effect is most profound with parieto-occipital lobe involvement, likely a reflection of overall disease severity. Increasing global WMD burden may be a better indicator of cognitive decline than cortical infarction. Patients with higher baseline CBF seem to have better cognitive functioning.
MMD is a rare cerebrovascular disease first described by Suzuki and Takaku in 1969.1 The disease is characterized by progressive stenosis or occlusion of the supraclinoid internal carotid arteries and the anterior and middle cerebral arteries with the development of irregular collateral “Moyamoya vessels” near the occluded or stenotic regions. While MMD more commonly affects the anterior circulation, it can also involve the posterior circulation.2 The cause of the disease remains unknown, but a genetic component has been suggested, given its predominance in Japanese populations. Several studies have found associations between MMD and chromosome 17q25.3,4
MMD in North America is more common in women and appears to have a bimodal age of onset, most commonly being diagnosed in the first decade or the third and fourth decades of life.5 MMD typically presents acutely with various neurologic events, including intracranial hemorrhage, TIAs, cerebral infarction, or seizures. Neuropsychological sequelae from MMD have been most extensively studied in Japan and have focused on intelligence testing in pediatric populations.6,7 Neuropsychological studies in adults have been more limited. Karzmark et al8 was the first group to evaluate the effect of MMD on cognition in a large series of adults. They found relative sparing of intelligence and memory in adult populations; however, approximately half of the patients had impairment in executive functioning such as initiation and mental set-shifting efficiency, raising the possibility that adult MMD most severely affects frontal lobe function. The incidence of discrete cerebrovascular events in the study sample was not evaluated. Therefore, it is unknown whether cognitive impairment in adult patients is related to changes in CBF dynamics related to MMD itself or due to secondary parenchymal damage by ischemic stroke.
The purpose of the current study was to further evaluate whether the effect of MMD on cognition was related to primary MMD itself or to secondary parenchymal damage. Measurements of primary MMD were defined as baseline CBF and CVR. CVR is calculated as the percentage increase in CBF after a vasodilatory stimulus relative to baseline CBF and is a reliable predictor of critically reduced perfusion.9 “Secondary damage” from MMD was defined as cortical cerebral infarction and/or white matter disease. We also sought to determine whether any region of the brain is more affected in patients with decreased executive functioning.
Materials and Methods
Patients
The participants included 31 adult patients (12 men and 19 women) with neurodiagnostically confirmed MMD who all underwent baseline neuropsychological assessment, Xe-CT examination with acetazolamide vasodilatory challenge, and MR imaging for presurgical evaluation. The mean age was 37.3 ± 11.5 years; range, 19–61 years. Patients were excluded from the study for the following reasons: age younger than 18 years, lack of proficiency in English (Wechsler Adult Intelligence Scale-Third Edition vocabulary score below 7), the presence of major psychopathology (psychosis, major depression, substance abuse), the presence of a motor deficit that precluded administration of the neuropsychological tests, the presence of neurologic comorbidity (congenital malformations, traumatic brain injury, intraparenchymal hemorrhage), low intelligence (full-scale intelligence quotient below 70), and incomplete Xe-CT or MR imaging examinations.
Neuropsychological Assessment
All subjects were administered a comprehensive neuropsychological assessment battery previously described.8 Because previous research indicated that adult patients with MMD had deficits in executive function, neuropsychological tests of executive ability were selected for the current study. These were the TMT-B, a measure of mental flexibility, and the FAS, a measure of verbal fluency. The neuropsychological tests were administered to all subjects in accordance with manual instructions. Subjects were evaluated in a single 3-hour session.
Radiologic Imaging
All patients underwent a baseline Xe-CT examination of the brain with acetazolamide vasodilatory challenge as part of their presurgical evaluation on a 16-section scanner (GE CT Lightspeed Ultra, GE Healthcare, Milwaukee, Wisconsin), equipped with a system to administer xenon gas and a software package for processing CBF data (XeCT-NT; Diversified Diagnostic Products, Houston, Texas). Immediately following the initial Xe-CT study, patients were administered 1 g of acetazolamide intravenously. Twenty minutes following drug administration, the Xe-CT examination was repeated. Patients who were unable to complete a postacetazolamide examination due to a contraindication (sulfa allergy, glaucoma, recent acute ischemic event) or an inability to cooperate were not included in the study.
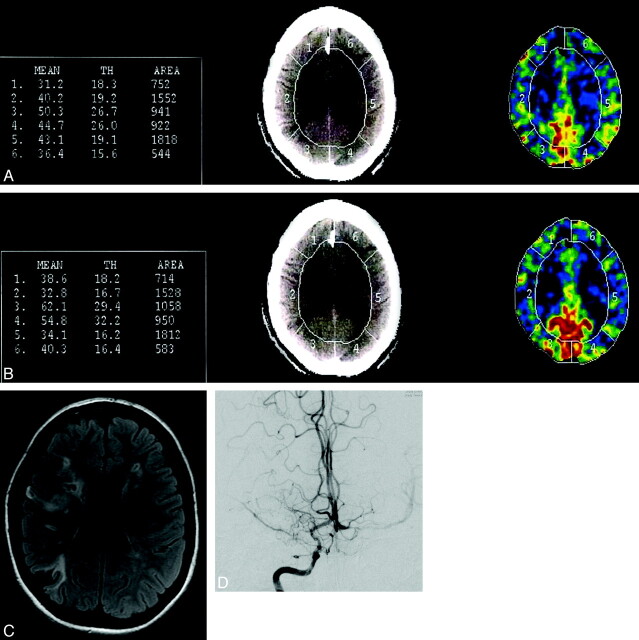
CBF was measured at 4 different levels in each patient. Due to slight variations in the levels acquired in some patients, only 3 levels were selected for analysis that corresponded to the level of the basal ganglia through the top of the lateral ventricles for both pre- and postacetazolamide examinations. Mean CBF data at each level were calculated for 6 contiguous ROIs aligned along the outer cortical ribbon of both hemispheres with a total of 18 ROIs for each patient (Fig 1A, -B). The diameter or depth of the ROI was approximately 2 cm, to optimize inclusion of both gray and white matter.
Fig 1.
Calculation of CBF by Xe-CT is shown both pre- (A) and postacetazolamide (B) on a per-region basis with 6 regions per section. Regions 2 and 5 show a decrease in CBF following the acetazolamide challenge, consistent with vascular steal. C, The corresponding section level on MR imaging shows areas of WMD in the bilateral frontal regions and cortical infarcts in the right frontal and parietal lobes, corresponding to regions 2 and 3 on the Xe-CT. D, The right internal carotid artery injection on the cerebral angiogram shows moderate narrowing of the supraclinoid internal carotid artery and severe narrowing of the M1 segment of the right middle cerebral artery, with decreased filling of the distal branches and numerous Moyamoya collateral vessels.
All patients underwent MR imaging of the brain on a 1.5T scanner (GE MR Signa Excite, GE Healthcare). FLAIR, diffusion, and gradient- recalled-echo MR imaging sequences were blindly reviewed without knowledge of the CBF findings by a neuroradiologist (M.A.M). MR imaging ROIs corresponding to approximately the same ROIs on the Xe-CT examination given differences in section angle were evaluated for evidence of a cortical stroke, WMD, acute stroke, or hemorrhage (Fig 1C). Each ROI was assigned a grade for each of the above abnormalities as follows: 0 = no pathology present, 1 = focal abnormality (involves <50% of the ROI), and 2 = confluent abnormality (involves >50% of the ROI). The WMD score and stroke score, therefore, reflect the total number of regions with the presence of WMD and chronic cortical stroke, as well as the severity of involvement in each region.
Data Analysis
CBF and CVR (CVR = [CBF (postacetazolamide) − CBF (baseline)/ CBF (baseline) × 100] were calculated as a mean across all 18 ROIs and also as a mean across anterior ROIs (1, 2, 5, and 6) versus posterior ROIs (3 and 4) for each patient, roughly corresponding to the frontotemporal-versus-parieto-occipital lobes; however, brain parenchyma corresponding to the occipital lobes was not completely included on the routine Xe-CT examination, and the anterior parietal lobe brain parenchyma was included in the anterior ROIs (2 and 5). Lesions were not characterized by vascular territory involvement because the territories involved in patients with MMD change considerably due to proximal vessel occlusion and the development of Moyamoya vessels. Univariate Pearson linear correlations among FAS and TMT-B scores and CBF, CVR, WMD scores, cortical stroke scores, and age were computed. Effects of CBF, CVR, WMD scores, and cortical stroke scores on the FAS and TMT-B scores were tested by multivariate regression analysis, including age as a covariate and adjusted for clustering of observations within patients. All statistical analyses were performed by using STATA, release 9.2 (StataCorp, College Station, Texas).
Results
Review of MR imaging in the regions of the frontotemporal and parieto-occipital lobes, corresponding to the ROIs on Xe-CT, demonstrated no acute infarcts in any of the patients. Only 4 of the 31 patients had minor areas of hemosiderin staining associated with cortical strokes or WMD. Twenty-three of the 31 patients had at least 1 region of WMD on MR imaging. Eight of the 31 patients had MR imaging evidence of an old cortical stroke in at least 1 region. Eight of the 31 patients had no evidence of WMD or cortical stroke in the examined regions.
Per-patient mean CBF across all sections and regions was 45.4 ± 10.0 mL/100 g/min with a range of 25.3–76.2 mL/100 g/min. Per-patient mean percent change in CVR was 10.2% ± 14.2%, with a range of −13.9%–44.5%.
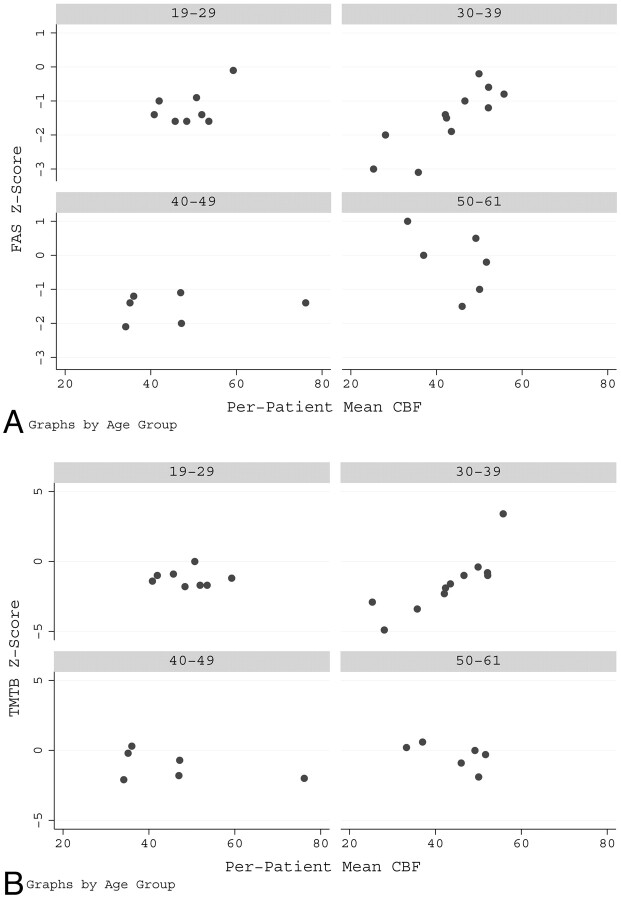
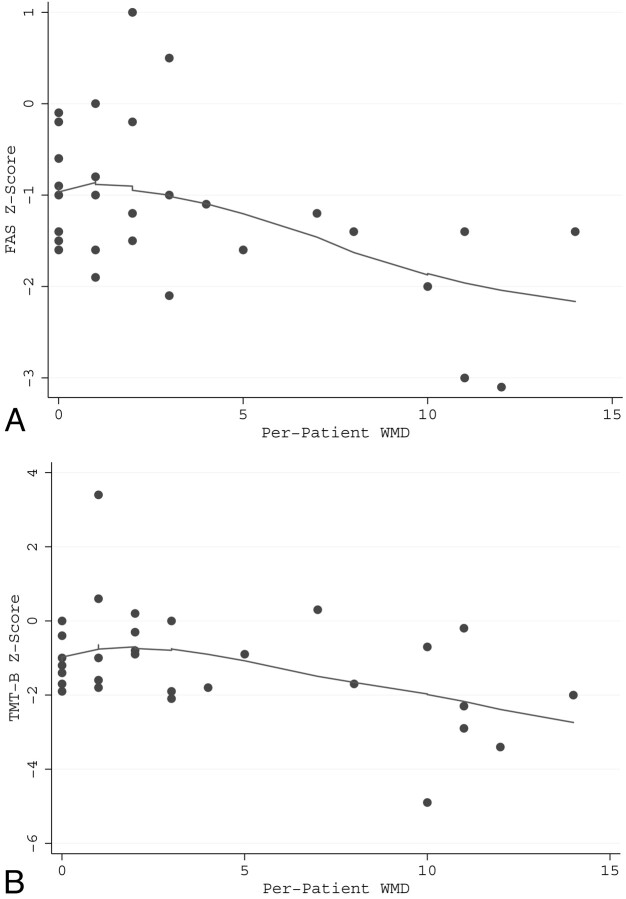
FAS and TMT-B scores were moderately positively correlated across patients (r = 0.60, P = .0003). There was a significant overall positive relationship between mean baseline CBF and FAS score (P = .038) and TMT-B score (P = .014), which was most strongly seen in the 30- to 39-year-old age range (Fig 2A, -B). A significant negative relationship was present between the WMD score and both the FAS score (P = .009) and the TMT-B score (P = .015) (Fig 3A, -B). There was a significant U-shaped relationship (P = .007) between FAS score and age and a significant positive linear relationship (P = .018) between TMT-B score and age.
Fig 2.
A significant positive relationship is present between CBF and the FAS score (P = .038) (A) and the TMT-B score (P = .014) (B). This relationship was most strongly seen in the 30- to 39-year-old age group.
Fig 3.
A significant negative relationship is present between the WMD score (Burden) and the FAS score (P = .009) (A) and the TMT-B score (P = .015) (B). The line is a locally weighted regression smoothed to indicate trend.
Per-region analysis with multivariate regression demonstrated that the FAS score was significantly decreased by the presence of a posterior cortical stroke (P < .0001) or WMD (P = .006) but not an anterior cortical stroke or WMD (P = .149 and .132, respectively). The same was true for the TMT-B score for posterior stroke and WMD (P = .001 and .004, respectively), and anterior cortical stroke or WMD (P = .209 and .804, respectively). All patients with posterior parieto-occipital (3 and 4) region WMD or cortical stroke also had secondary disease burden in the frontal regions (P = .041 by the 1-sided Fisher exact test) (Table and On-line Fig 1).
Frequency of WMD and/or cortical stroke in anterior and posterior regions
| Posterior WMD or Cortical Stroke | Anterior WMD or Cortical Stroke |
Total | |
|---|---|---|---|
| No | Yes | ||
| No | 8 (36%) | 14 (64%) | 22 (71%) |
| Yes | 0 (0%) | 9 (100%) | 9 (29%) |
| Total | 8 (26%) | 23 (74%) | 31 (100%) |
Discussion
Neuropsychological sequelae from MMD has been documented in both children and adults; however, the research on cognitive effects associated with MMD has been limited in scope, especially in adult patients. Karzmark et al8 were the first to document that MMD in adults can impair cognition primarily affecting executive functioning. It was unknown whether impaired cognition in this patient population was a reflection of low cerebral blood perfusion related to primary MMD or whether it was due to secondary damage from the underlying MMD such as cortical strokes or WMD visualized on MR imaging.
Executive functioning as measured by FAS and TMT-B scores in adult MMD is significantly decreased with the presence of a cortical stroke and WMD in the posterior (parieto-occipital) regions. This is likely due to overall increased secondary MMD burden because all patients with posterior region involvement also had evidence of cortical strokes or WMD in the anterior (frontotemporal) regions. Kim et al10 found that adult MMD severity, as measured by degree of intracranial arterial involvement on cerebral angiography, increased in patients with ischemic lesions involving the cortical regions and the posterior part of the brain. This finding may be related to later involvement of the posterior cerebral arteries in advanced stages of MMD. While the current study did not examine angiographic findings, it is presumed that hemodynamic compromise is responsible for ischemic lesions in this patient population. It is not surprising then that patients with posterior involvement have more severe disease burden; however, to our knowledge, there has been no previous publication reporting whether this is also associated with decreased executive functioning. Patients with cortical strokes and WMD isolated to the anterior regions appear to have relatively preserved cognitive functioning. This finding was somewhat unexpected because executive functioning is generally attributed to the frontal lobes. Our findings suggest that the relationship between executive functioning and parenchymal involvement by secondary disease in MMD is more complex, and patients are able to compensate until the disease severity involves both anterior and posterior circulation territories. In addition, our results showed a significant decrease in executive functioning with increasing global WMD burden, suggesting that this may be a better measurement for cognitive decline than evidence of cortical infarction.
There was a significant positive relationship between baseline CBF and both the FAS and TMT-B score, most apparent for the 30- to 39-year-old age group, suggesting that patients with higher baseline CBF are less susceptible to cognitive deficits in executive functioning. A single case report in an adult patient with MMD did show global cognitive improvement after baseline CBF was increased by revascularization surgery, suggesting that subtle-yet-important cognitive deficits may be affected by hypoperfusion alone.11
Quantitative assessment of CVR by using the acetazolamide challenge is an additional measurement of hemodynamic impairment in patients with major cerebral artery occlusive disease.12 The risk of developing an ischemic event is known to be higher in patients with reduced CVR.13,14 The CVR, therefore, often aids in decision-making for treatment options and is used to follow the course of the disease.15 In our study, however, there was no significant relationship between CVR and measurements of executive functioning. This suggests that secondary damage caused by MMD is more strongly associated with neuropsychological impairment than primary MMD itself.
Patients presenting for presurgical evaluation at an older age had higher executive functioning. The explanation for this is likely multifactorial but may be related to a slower course of disease progression, allowing compensation, or higher baseline cognitive functioning that resulted in a later presentation of the disease.
Surgical revascularization is performed to augment blood flow to the affected hemisphere, either directly with extracranial-intracranial bypass surgery or indirectly via multiple different techniques. Most studies have shown clinical benefits and hemodynamic improvements after surgery,16,17 though there are few published cases of neuropsychological status pre- and postrevascularization in adults.11 Our findings demonstrate that patients with lower executive functioning pre-revascularization had more secondary damage as evidenced by WMD burden and/or cortical strokes. It is unclear what effect revascularization surgery has on executive functioning, and further investigation of the effect of revascularization surgery on neuropsychological status and imaging findings may be warranted.
The current study used FAS and TMT-B scores to measure cognitive deficits because these test results were found to be abnormal in approximately half of the adult patients in the original study of Karzmark et al.8 While these are well-accepted tests of executive functioning, the study is limited by the use of only 2 tests to detect cognitive deficits in the study population. Certain radiologic limitations were also present. Xe-CT only covers the most central portion of the supratentorial brain parenchyma, and MR imaging abnormalities were examined only in areas that corresponded to CBF data. Primary and secondary disease in the posterior fossa, basal ganglia, or most superior or inferior portions of the supratentorial brain was not evaluated. Newer MR imaging–based methods used to obtain quantitative cerebral hemodynamic data such as dynamic susceptibility contrast–weighted bolus tracking MR imaging and arterial spin-labeling cover the entire brain and may provide more accurate assessments in future studies.15 In addition, the MR imaging studies were reviewed and graded by the primary author only on 1 occasion without a second reader, limiting assessment of intra- or inter-reader reliability. Last, adult patients with MMD commonly present with intracranial hemorrhage. These patients were excluded from the current study, so the above findings cannot be generalized to adult patients with MMD presenting with intraparenchymal hemorrhage.
Conclusions
The current study indicates that decreased executive functioning seen in an adult population with MMD is most strongly associated with secondary damage to the brain parenchyma in the form of WMD or cortical stroke. The effect is most profound in patients with involvement of the parieto-occipital lobes, which likely reflects overall increase in disease severity because all of these patients also had disease in the frontal regions. Our results also suggest that increasing global WMD burden may be a better measurement for cognitive decline than evidence of cortical infarction. Most interesting, CVR has no correlation with cognitive deficits, but patients with higher baseline CBF seem to have better cognitive functioning.
Supplementary Material
ABBREVIATIONS:
- CVR
cerebral vascular reserve
- FAS
Controlled Oral Word-Association Test
- MMD
Moyamoya disease
- TMT-B
Trail Making Test, Part B
- WMD
white matter disease
- Xe-CT
133xenon-enhanced CT
Footnotes
Paper previously presented at: Annual Meeting of the American Society of Neuroradiology, May 15–20, 2010; Boston, Massachusetts.
References
- 1. Suzuki J, Takaku A. Cerebrovascular “Moyamoya” disease: disease showing abnormal net-like vessels in base of brain. Arch Neurol 1969;20:288–99 [DOI] [PubMed] [Google Scholar]
- 2. Miyamoto S, Kikuchi H, Karasawa J, et al. Study of the posterior circulation in Moyamoya disease: clinical and neuroradiological evaluation. J Neurosurg 1984;61:1032–37 [DOI] [PubMed] [Google Scholar]
- 3. Mineharu Y, Liu W, Inoue K, et al. Autosomal dominant Moyamoya disease maps to chromosome 17q25.3. Neurology 2008;70:2357–63. Epub 2008 May 7 [DOI] [PubMed] [Google Scholar]
- 4. Yamauchi T, Tada M, Houkin K, et al. Linkage of familial Moyamoya disease (spontaneous occlusion of the circle of Willis) to chromosome 17q25. Stroke 2000;31:930–35 [DOI] [PubMed] [Google Scholar]
- 5. Suzuki J, Kodama N. Moyamoya disease–a review. Stroke 1983;14:104–09 [DOI] [PubMed] [Google Scholar]
- 6. Matsushima Y, Aoyagi M, Masaoka H, et al. Mental outcome following encephaloduroarteriosynangiosis in children with Moyamoya disease with the onset earlier than 5 years of age. Childs Nerv Syst 1990;6:440–43 [DOI] [PubMed] [Google Scholar]
- 7. Sato H, Sato N, Tamaki N, et al. Chronic low-perfusion state in children with Moyamoya disease following revascularization. Childs Nerv Syst 1990;6:166–71 [DOI] [PubMed] [Google Scholar]
- 8. Karzmark P, Zeifert PD, Tan S, et al. Effect of Moyamoya disease on neuropsychological functioning in adults. Neurosurgery 2008;62:1048–51, discussion 1051–52 [DOI] [PubMed] [Google Scholar]
- 9. Vagal AS, Leach JL, Fernandez-Ulloa M, et al. The acetazolamide challenge: techniques and applications in the evaluation of chronic cerebral ischemia. AJNR Am J Neuroradiol 2009;30:876–84. Epub 2009 Feb 26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kim JM, Lee SH, Roh JK. Changing ischaemic lesion patterns in adult Moyamoya disease. J Neurol Neurosurg Psychiatry 2009;80:36–40 [DOI] [PubMed] [Google Scholar]
- 11. Jefferson AL, Glosser G, Detre JA, et al. Neuropsychological and perfusion MR imaging correlates of revascularization in a case of Moyamoya syndrome. AJNR Am J Neuroradiol 2006;27:98–100 [PMC free article] [PubMed] [Google Scholar]
- 12. Rim NJ, Kim HS, Shin YS, et al. Which CT perfusion parameter best reflects cerebrovascular reserve? Correlation of acetazolamide-challenged CT perfusion with single-photon emission CT in Moyamoya patients. AJNR Am J Neuroradiol 2008;29:1658–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kuroda S, Houkin K, Kamiyama H, et al. Long-term prognosis of medically treated patients with internal carotid or middle cerebral artery occlusion: can acetazolamide test predict it? Stroke 2001;32:2110–16 [DOI] [PubMed] [Google Scholar]
- 14. Ogasawara K, Ogawa A, Yoshimoto T. Cerebrovascular reactivity to acetazolamide and outcome in patients with symptomatic internal carotid or middle cerebral artery occlusion: a xenon-133 single-photon emission computed tomography study. Stroke 2002;33:1857–62 [DOI] [PubMed] [Google Scholar]
- 15. Lee M, Zaharchuk G, Guzman R, et al. Quantitative hemodynamic studies in Moyamoya disease: a review. Neurosurg Focus 2009;26:E5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ikezaki K, Matsushima T, Kuwabara Y, et al. Cerebral circulation and oxygen metabolism in childhood Moyamoya disease: a perioperative positron emission tomography study. J Neurosurg 1994;81:843–50 [DOI] [PubMed] [Google Scholar]
- 17. Touho H, Karasawa J, Ohnishi H. Preoperative and postoperative evaluation of cerebral perfusion and vasodilatory capacity with 99mTc-HMPAO SPECT and acetazolamide in childhood Moyamoya disease. Stroke 1996;27:282–89 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.